Abstract
Background/Purpose
Stratification of Gleason score (GS) into three categories (2-6, 7, and 8-10) may not fully utilize its prognostic discrimination, with Gleason Pattern 5 (GP5) previously identified as an independent adverse factor.
Materials/Methods
Patients treated on RTOG 9202 (n = 1292) or RTOG 9902 (n = 378) were pooled and assessed for association of GS and GP5 on biochemical failure (BF), local failure (LF), distant metastasis (DM), and overall survival (OS). Fine and Gray’s regression and cumulative incidence methods were used for univariate and multivariate analyses.
Results
With median follow-up of 9.4 years, patients with GS 8-10 with GP5 had worse outcome than GS 4+4 for DM on both RTOG9202 (p=0.038) and RTOG9902 (p<0.001) with a trend toward worse OS (p=0.059 and p=0.089, respectively), but without differences in BF or LF. At 10-years DM was higher by 11% (RTOG 9202) and 18% (RTOG 9902) with GP5 compared to GS 4+4. On multivariate analysis restricted to long-term androgen deprivation therapy the presence of GP5 substantially increased distant metastasis (HR=0.43, 95%CI: 0.24-0.76, p=0.0039) with a trend toward worse OS (HR:0.74, 95% CI:0.54-1.0, p=0.052) without association with LF (HR:0.55, 95%CI:0.28-1.09, p=0.085) or BF (HR:1.15, 95%CI:0.84-1.59, p=0.39). We did not observed substantial differences between Gleason 3+5, 5+3, or Gleason 9-10.
Conclusions
These results validate GP5 as an independent prognostic factor which is strongest for DM. As a result GP5 should be considered when stratifying patients with GS 8 and may be a patient population in which to evaluate newly approved systemic therapies or additional local treatments.
Keywords: Prostate Cancer, Gleason score, Distant Metastasis, Radiation Therapy
Background
Introduction
The Gleason score (GS) is key for counseling men about treatment, clinical trial enrollment, and comparison of treatment modalities.[1] However, growing evidence has suggested that the commonly utilized method of categorizing the GS into 3-groups (2-6, 7, 8-10) [2] over-simplifies stratification leading to heterogeneity, particularly in the Gleason 8-10 sub-group.[3–6] A 2014 consensus statement by the International Society of Urologic Pathologists (IUSP) adopted a modified grade grouping which considers Gleason 8-10 as two separate entities Gleason 8 and Gleason 9-10.[7] However, previous analyses are limited by differences in treatment which may have been influenced by the Gleason score and as such may limit the conclusions about the impact of Gleason score where grade potentially confounded treatment.
Therefore, we conducted a secondary analysis of two multi-institutional phase 3 trials performed in the 1990s with external beam radiation therapy (RT) for higher risk prostate cancers: NRG Oncology Radiation Therapy Oncology Group (RTOG) 9202 and RTOG 9902[8, 9].
Materials and Methods
Patient, Treatment, Follow-up
Eligible patients from RTOG 9202 and RTOG 9902 were analyzed[8, 9]. For RTOG 9202 patients had locally advanced prostate cancer (T2c-T4), and prostate-specific antigen (PSA) <150 ng/mL. All received external-beam RT to the whole pelvis (WP) followed by a boost to the prostate to a total dose of 67.5-70 Gy using fractions of 1.8-2.0 Gy. Patients were randomly assigned to receive short-term androgen deprivation therapy (STAD) for 4 months starting 2 months prior to RT or to the same with an additional 2 years of ADT (LTAD). For RTOG 9902 patients had localized prostate cancer without metastasis with either PSA 20-100 ng/mL and GS >= 7 (any T-stage) or clinical T-stage >=T2 and GS >= 8 (PSA<=100). All patients received WPRT followed by a boost to the prostate to a total dose of 70.2 Gy. Patients were randomly assigned to receive 24-months ADT or to these same with four cycles of Paclitaxel, Estramustine, and Etoposide starting 28 days after completion of RT.
Study Endpoints
Although not initially part of the planned protocols biochemical failure (BF) was defined here by the Phoenix definition: Nadir + 2 ng/mL, clinical progression (including metastasis), or initiation of salvage ADT [10]. Overall survival (OS) was defined as death by any cause. Local failure (LF) was defined as: tumor recurrence (positive re-biopsy at least 2 years after study entry) or by tumor re-growth by at least 50% or tumor never cleared by digital rectal exam. Distant metastasis (DM) was defined as the documentation of clinical evidence of lymph nodal, osseous or visceral spread. All event times were measured from the date of randomization.
Statistical Methods
The Chi-square test for categorical variables, and an ANOVA F-test for continuous variables was used to compare pretreatment.t characteristics across GS group while the Kaplan-Meier method [11] and log-rank test was used to estimate the rates for OS[12]. The cumulative incidence method [13] was used to estimate time to BF, LF, DM, and disease-specific survival (DSS) with Gray’s test utilized to compare the cumulative incidence rates over time between GS groups. [14] Death without an event was a competing risk for BF, LF, and DM while death from other causes was a competing risk for DSS. Multivariate analysis utilized Cox proportional hazard regression analyses[15] for OS and Fine and Gray’s regression analysis[16] for LF and DM. The following covariates were considered for the combined data of RTOG 9202 and 9902: Age (continuous), GS (2-6 (Reference Level [RL]),7,8 (without GP5),and 8-10 (with GP5)), PSA (continuous and dichotomized as ≤ 30 ng/mL (RL) vs. > 30 ng/mL), clinical stage (T1/T2 (RL) vs. > T3/4), comorbid illness (none (RL) vs. any), and Study (RTOG 9202 (RL) vs. RTOG 9902). All statistical comparisons were two-sided and a p-value<0.05 was statistically significant. All analyses were conducted using SAS ® Version 9.4 of the SAS System for Windows.
Results
Patient Characteristics
A total of 1670 patients are included in this analysis: RTOG 9202 ((77.4%) and RTOG 9902 (22.6%)). Median follow-up was 9.4-years (Range:0.04-22.0). There have been 1161 deaths of which 1021 (88%) were on RTOG 9202 and 140 (12%) were on RTOG 9902. On RTOG 9202 prostate cancer death increased with increasing Gleason grade: 12.6% (66/524) of Gleason 2-6 patients, 19.2% (86/449) for Gleason 7, 20.8% (21/101) for Gleason 8 (without GP5), and 34.9% (76/218) for Gleason 8-10 (with GP5). Cause of death was not ascertained on RTOG 9902.
Demographics are provided for the combined analysis (Table 1,Supplemental Tables 1–2). No difference was found for age or performance status based upon Gleason score. Mean PSA was 30.1 ng/mL (Range: 0.12-250) and was higher for those with GS 7 (35.6 (Standard deviation [STD]:25.8) than those with GS 2-6, 8 with No GP5, and 8-10 (with GP5) (26.6 STD:17.5, 28.2 STD:17.0, 27.6 STD:18.0, respectively; p<0.0001, Clinical tumor stage was T1-T2 in 51% and T3 in 46% with 3.5% of men with T4 disease and did differ by GS (p<0.001). GP 5 (either primary or secondary) was found in 22% (363/1670) men overall which was 17% (218/1292) for RTOG 9202 and 38% (145/378) for RTOG 9902. Combined pre-treatment characteristics are also provided based upon Gleason 4-4 (n=209), Gleason 3+5 (n=65), Gleason 5+3 (n=28), and Gleason 9-10, (n=270) (Supplemental Table 3) where there were no differences in any demographic features between these sub-groupings.
Table 1.
Combined RTOG 9202 and RTOG 9902 Pretreatment Characteristics
| Gleason Score |
|||||
|---|---|---|---|---|---|
| Characteristic | 2-6 (n=524) n (%) |
7 (n=574) n (%) |
8 (No GP5) (n=209) n (%) |
8 (GP5), 9-10 (n=363) n (%) |
Total (n=1670) n (%) |
| Age | |||||
| Mean | 69.5 | 68.6 | 67.9 | 68.1 | 68.7 |
| Standard Deviation | 6.5 | 6.8 | 7.5 | 7.5 | 7.0 |
| Min - Max | 44 - 86 | 44 - 87 | 43 - 88 | 42 - 88 | 42 - 88 |
| p-value* | 0.0074 | ||||
| PSA, ng/mL - Continuous | |||||
| Mean | 26.6 | 35.6 | 28.2 | 27.6 | 30.1 |
| Standard Deviation | 26.9 | 31.0 | 29.3 | 26.7 | 28.9 |
| Min - Max | 0.4 - 180.3 | 0.2 - 250 | 1.2 - 172.7 | 0.123 - 152 | 0.123 - 250 |
| p-value* | <0.0001 | ||||
| PSA - Dichotomized | |||||
| ≤ 30 ng/mL | 383 (73.1%) | 332 (57.8%) | 146 (69.9%) | 251 (69.1%) | 1112 (66.6%) |
| > 30 ng/mL | 141 (26.9%) | 242 (42.2%) | 63 (30.1%) | 112 (30.9%) | 558 (33.4%) |
| p-value** | >0.0001 | ||||
| KPS/Zubrod | |||||
| Zubrod 0, KPS 90-100 | 481 (91.8%) | 533 (92.9%) | 184 (88.0%) | 334 (92.0%) | 1532 (91.7%) |
| Zubrod 1, KPS 70-80 | 43 (8.2%) | 41 (7.1%) | 25 (12.0%) | 29 (8.0%) | 138 (8.3%) |
| p-value** | 0.1902 | ||||
| Clinical T-Stage | |||||
| T1 | 0 (0.0%) | 33 (5.7%) | 7 (3.3%) | 10 (2.8%) | 50 (3.0%) |
| T2 | 288 (55.0%) | 236 (41.1%) | 113 (54.1%) | 161 (44.4%) | 798 (47.8%) |
| T3 | 223 (42.6%) | 285 (49.7%) | 82 (39.2%) | 174 (47.9%) | 764 (45.7%) |
| T4 | 13 (2.5%) | 20 (3.5%) | 7 (3.3%) | 18 (5.0%) | 58 (3.5%) |
| p-value** | <0.0001 | ||||
| Study | |||||
| 9202 | 524 (100.0%) | 449 (78.2%) | 101 (48.3%) | 218 (60.1%) | 1292 (77.4%) |
| 9902 | 0 (0.0%) | 125 (21.8%) | 108 (51.7%) | 145 (39.9%) | 378 (22.6%) |
| p-value** | <0.0001 | ||||
Clinical End-Points as a Function of Gleason Score - Bivariate Analysis
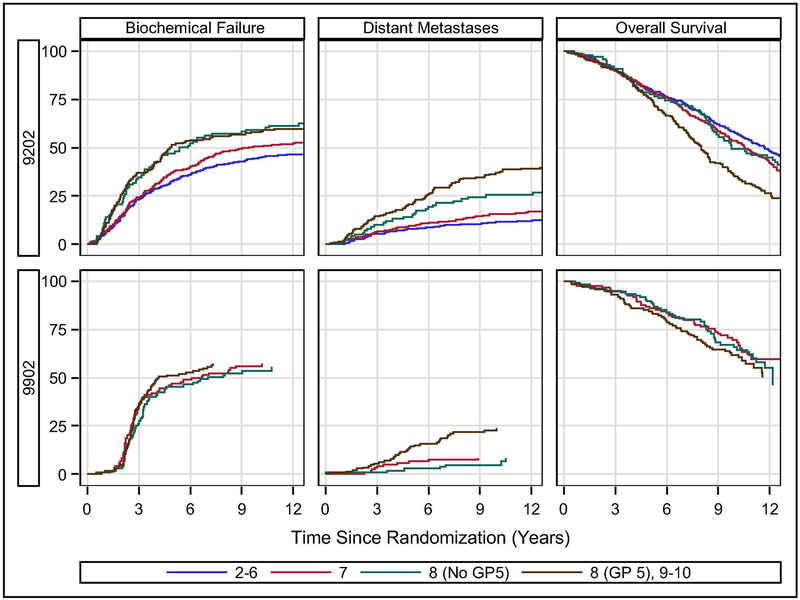
On RTOG 9202, higher GS associated with increased biochemical recurrence (p<0.0001, Figure 1A, Table 2); however, there was no difference between GS 8 (without GP5) vs. GS 8-10 (with GP5) with 10-year rates of BF 59.3% and 58.0%, respectively (HR:1.05, 95% Confidence Interval [CI]:0.78-1.43, p=0.73). Similarly, LF (Supplemental Figure 1A) was higher with GS 8-10 (25.1% without GP5 and 22.7% with GP5) compared to GS 2-6 (14.5%) or GS 7 (15.6%, p=0.01), but not based upon GP 5 (p=0.66). Unlike BF and LF, however, DM was greater both with increasing GS (p<0.0001) and with the presence of GP5 with 10-year rates of metastasis of 11.5%, 15.7%, 25.8%, and 37.0% for those with GS 2-6, 7, 8 (No GP5), and 8-10 (GP5), respectively (Figure 1C, Table 2). The absence of GP5 reduced the risk of DM in those with GS 8 prostate cancer by more than 30% (p=0.038, HR:0.64, 95%CI:0.41-0.98). On RTOG 9202, OS was associated with GS (p=0.0001) with 10-yr survival of 57.8%, 54.2%, 49.3%, and 34.6% as a function of increasing GS (2-6, 7, 8 (No GP5), 8-10 (GP5)), respectively, with the difference between those with and without GP5 approaching but not significant (p=0.059, HR:0.78, 95%CI:0.60-1.01; Figure 1E, Table 2). In addition, when broken down within Gleason 8-10 (4+4, 3+5, 5+3, or 9-10) there was no difference in any clinical end-point within those with GP5 (3+5, 5+3 or 9-10) (supplemental Tables 4–7). At 10-years DM was 25.4% (4+4), 36.8% (3+5), 35.3% (5+3), and 37.2% (Gleason 9-10) which was only statistically different for Gleason 4+4 (p<0.05) which paralleled OS at 10-years which was 49.3% (4+4), 36.1% (3+5), 41.2% (5+3), and 33.9% (Gleason 9-10) with only Gleason 4+4 being statistically different (p<0.05).
Figure 1:
Outcomes by study. Biochemical failure and distant metastasis plots are cumulative incidence estimator. Overall survival plot is Kaplan-Meier estimator.
Table 2.
Influece of Gleason Score and Gleason Pattern 5 on Clinical Events for RTOG 9202
| Biochemical Failure | Distant Metastasis | Local Failure | Overall Survival | |||||
|---|---|---|---|---|---|---|---|---|
| 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | |
| Gleason 2-6 | 32.8% (28.7-32.6) |
44.7% (40.3-49.0) |
7.7% (5.8-10.4) |
11.5% (8.9-14.4) |
12.7% (9.8-15.5) |
14.5% (11.5-17.5) |
80.7% (77.1-83.9) |
57.8% (53.4-62.2) |
| Gleason 7 | 37.4% (40.0-49.3) |
50.8% (46.0-55.4) |
9.4% (6.9-12.4) |
15.7% (12.3-19.1) |
13.0% (9.9-16.1) |
15.6% (12.2-19.0) |
79.5% (75.8-83.3) |
54.2% (49.4-59.0) |
| Gleason 8 (No GP5) | 47.0% (36.9-56.4) |
59.3% (48.8-68.3) |
14.1% (8.1-21.7) |
25.8% (17.3-34.4) |
23.0% (15.3-31.7) |
25.1% (17.0-34.0) |
78.7% (70.6-86.8) |
49.3% (39.0-58.8) |
| Gleason 8-10 (GP5) | 51.6% (44.7-58.0) |
58.0% (51.0-64.3) |
20.8% (20.3-32.0) |
37.0% (30.4-43.4) |
18.8% (14.0-24.3) |
22.7% (17.3-28.5) |
75.8% (70.1-81.5) |
34.6% (28.0-41.2) |
| p-value (Overall)* | <0.0001 | <0.0001 | 0.01 | 0.0001 | ||||
| p-value for Gleason pattern 5** | 0.73 HR: 1.05 (0.78-1.43) |
0.0382 HR: 0.64 (0.41-0.98) |
0.66 HR: 1.1 (0.69-1.79) |
0.0586 HR 0.78 (0.60-1.01) |
||||
Table 2: Cumulative Incidence (and 95% confidence intervals) of events at 5- and 10-years from randomization for RTOG 9202.
p-value from Gray’s test for the influence of Gleason score overall (2-6, 7, 8 (without Gleason pattern 5), or, 8-10 (with Gleason pattern 5) or
just when comparing Gleason 8-10 with or without Gleason pattern 5.
HR: hazard ratio (and 95% confidence interval); GP: Gleason pattern.
For RTOG 9902 GS 2-6 patients were not enrolled, and there was no difference in BF (p<0.76) or LF (p<0.29) as a function of GS (Figure 1B, Table 3). However, DM was strongly associated with GS (p=0.0002) with 10-yr DM rates of 8.4%, 4.7%, and 23.8% for GG 7, 8 (No GP5), and 8-10 (GP5), and a lack of GP5 was favorable (p=0.001, HR:0.26, 95% CI:0.12-0.58; Figure 1D, Table 3). Overall survival was not substantially worse based upon GS with 10-year OS of 69.5%, 65.3%, and 54.4% for GS 7, 8 (No GP5), and 8-10 (GP5), these rates were not different either overall (p=0.18) or in the subset analysis of GP5 (p=0.089)(Figure 1F, Table 3). In addition, when broken down within Gleason 8-10 there was no difference in any clinical end-point within those with GP5 (3+5, 5+3 or 9-10) except BF was higher in those with Gleason 5+3 (HR:2.3 (95%CI:1.4-4.1), p=0.002) when compared to those with Gleason 9-10. In addition, although DM did not differ by sub-groups within Gleason 8-10 the lack of differences may be due to small sample sizes with DM at 10-years of 4.7% (4+4), 13.3% (3+5), 10% (5+3), and 26.2% (Gleason 9-10) which was only statistically different for Gleason 4+4 (p=0.04) vs. Gleason 9-10 (supplemental Tables 4–7).
Table 3.
Influence of Gleason Score and Gleason Pattern 5 on Clinical Events for RTOG 9902
| Biochemical Failure | Distant Metastasis | Local Failure | Overall Survival | |||||
|---|---|---|---|---|---|---|---|---|
| 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | |
| Gleason 2-6 | NA | NA | NA | NA | NA | NA | NA | NA |
| Gleason 7 | 47.1% (33.0-50.5) |
55.8% (46.6-64.3) |
6.6% (3.1-12.0) |
8.4%% (4.3-14.2) |
6.7% (3.1-12.1) |
10.1% (5.5-16.4) |
85.9% (78.2-91.0) |
65.5% (53.5-77.4) |
| Gleason 8 (No GP5) | 45.4% (35.7-54.5) |
53.4% (43.4-62.5) |
2.8% (0.7-7.3) |
4.7% (1.7-10.0) |
3.7% (1.2-8.6) |
6.2% (2.5-12.3) |
89.7% (83.9-95.5) |
65.3% (49.1-81.4) |
| Gleason 8-10 (GP5) | 51.1% (42.5-9.1) |
57.2% (48.5-65.0) |
14.3% (9.1-20.6) |
23.8% (16.9-31.3) |
4.3% (1.8-8.6) |
10.3% (5.9-16.0) |
84.4% (78.4-90.4) |
54.4% (37.8-71.1) |
| p-value (Overall)* | <0.76 | 0.0002 | 0.32 | 0.18 | ||||
| p-value for Gleason pattern 5** | 0.49 HR: 0.89 (0.64-1.24) |
0.001 HR: 0.26 (0.12-0.58) |
0.12 HR: 0.48 (0.19-1.22) |
0.089 HR 0.84 (0.56-1.26) |
||||
Table 3: Cumulative Incidence (and 95% confidence intervals) of events at 5- and 10-years from randomization for RTOG 9902.
p-value from Gray’s test for the influence of Gleason score overall (2-6, 7, 8 (without Gleason pattern 5), or 8-10 (with Gleason pattern 5) or
just when comparing Gleason 8-10 with or without Gleason pattern 5.
HR: hazard ratio (and 95% confidence interval); GP: Gleason pattern.
Impact of Gleason Pattern 5 on RT plus Long-term ADT
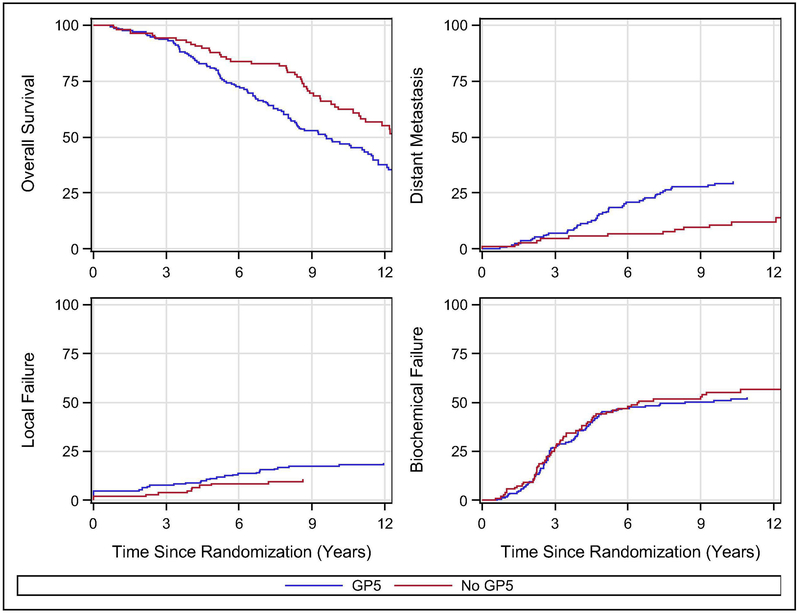
Given that LTAD has become the standard of care when added to RT in men with GS 8-10 prostate cancer an exploratory analysis was performed specifically looking at the impact of GP5 in those with GS 8-10 prostate cancers treated with LTAD. When pooling the experimental arm from RTOG 9202 (excluding those treated with STAD) with the standard arm of 9902 (excluding those treated with LTAD plus chemotherapy) there were a total of 279 men with GS 8-10 treated with LTAD on these two trials. In this group the presence of GP5 as compared to no GP5 had borderline association with OS (Fig 2A, 10-yr: 63.5% (95%CI:53.0-72.2) vs. 47.7% (95%CI:39.8-52.8); HR:0.74 (95% CI:0.54-1.0), p=0.052) and was associated with higher DM (Fig 2B, 10-yr: 10.7% (95%CI:5.6-17.6) vs. 29.1% (95%CI:23.1-36.2), HR=0.43 (95%CI: 0.24-0.76), p=0.0039). The presence of GP5 resulted in numerically higher but not statistically different rates of LF (Fig 2C, 10-yr: 10.5% (95%CI:5.6-17.4) vs. 17.3% (95%CI:12.0-23.4); HR:0.55 (95%CI:0.28-1.09), p=0.085) with no difference in BF (Fig 2D, 10-yr: 55.2%, (95%CI:45.0-64.3) vs. 50.8% (95%CI:42.9-58.2); HR:1.15, 95%CI:0.84-1.59, p=0.39).
Figure 2:
Outcomes for men with GS 8-10 who received the current standard of care, the experimental arm from RTOG 9202 (excluding those treated with STAD) with the standard arm of 9902 (excluding those treated with LTAD plus chemotherapy). Overall survival plot is Kaplan-Meier estimator. Biochemical failure, local failure, and distant metastasis plots are cumulative incidence estimator.
Clinical End-Points as a Function of Gleason Score - Multivariate Analysis
Finally, a pooled multivariate analysis was performed of both RTOG 9202 and RTOG 9902 as a function of the presence or absence of GP 5 (Table 4). After controlling for clinical variables those without GP5 had significantly lower BF (p=0.0038, HR: 0.78 (95%CI:0.66-0.92)), DM (p<0.0001, HR:0.38 (95%CI:0.30-0.48)), and all-cause mortality (p<0.0001, HR:0.73 (95% CI:0.63-0.85)). Younger age (p<0.0001), higher PSA (p<0.0001), and higher T-stage (p=0.0056) were all associated with an increased risk for BF, while comorbid illness (p=0.8) and study (p=0.54) were not. For DM, in addition to GP5 younger age (p<0.0001), higher PSA (p=0.015), higher T-stage (p<0.0001), and treatment on RTOG 9202 (p<0.0001) were each associated with increased DM while comorbid illness had no impact (p=0.19). Adverse risk factors for OS included older age (p<0.0001), pre-existing co-morbid illness (p=0.033), more advanced T-stage (p=0.018), and treatment on RTOG 9202 (p=0.01), but not PSA level (p=0.67).
Table 4.
Cox Proportional Hazards Models
| Overall Survival | Biochemical Failure | Distant Metastases | |||||
|---|---|---|---|---|---|---|---|
| Covariate | Comparison | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Gleason (grouped) | GP5 (RL) vs No GP5 | 0.73 (0.63, 0.85) |
<0.0001 | 0.78 (0.66, 0.92) |
0.0038 | 0.38 (0.30, 0.48) |
<0.0001 |
| Age | Continuous | 1.05 (1.04, 1.06) |
<0.0001 | 0.97 (0.96, 0.98) |
<0.0001 | 0.96 (0.94, 0.97) |
<0.0001 |
| PSA | < 30 (RL) vs ≥ 30 | 1.03 (0.91, 1.16) |
0.6673 | 1.49 (1.30, 1.71) |
<0.0001 | 1.33 (1.06, 1.66) |
0.0148 |
| Intercurrent disease | No (RL) vs Yes | 1.07 (1.01, 1.14) |
0.0326 | 0.99 (0.89, 1.09) |
0.7958 | 0.85 (0.67, 1.09) |
0.1947 |
| T-Stage | T1/T2 (RL) vs T3/T4 | 1.15 (1.02, 1.29) |
0.0181 | 1.21 (1.06, 1.38) |
0.0056 | 1.70 (1.35, 2.13) |
<0.0001 |
| Study | 9202 (RL) vs 9902 | 0.78 (0.64, 0.94) |
0.0100 | 0.95 (0.80, 1.12) |
0.5369 | 0.48 (0.34, 0.66) |
<0.0001 |
GP = Gleason pattern, RL = reference level, HR = hazard ratio, CI = confidence interval Patients with unknown intercurrent disease were excluded from the model.
Additional analyses were performed to more fully ascertain the prognostic significant of higher Gleason grade. First, in a pooled multivariate analysis Gleason. score was broken-up to assess potential differences between Gleason 4+4, 3+5, 5+3, or Gleason 9-10. These results mirrored those seen in the individual trials where compared to Gleason 9-10 there was reduce risk of all-cause mortality with Gleason 4+4 (HR:0.75 (95%CI:0.59-0.95, p=0.015) but not with either Gleason 3+5 (p=0.97) or Gleason 5+3 (p=0.21)(Supplemental Table 12). Similarly distant metastasis was lower with Gleason 4+4 (HR:0.49 (95%CI:0.33-0.72), p=0.003) but not with Gleason 3+5 (p=0.36) or Gleason 5+3 (p=0.38)(Supplemental Table 13). However, there was no difference in BF between any of the pathologic sub-groups contained within Gleason 8-10 (Supplemental Table 14). Finally, given the discordance between BF and DM, BF was further assessed based upon the PSA rise portion of the Phoenix definition with clinical failure (local or distant) or initiation of ADT considered competing events. Of 332 BF events in 572 men with Gleason 8-10 a rise in PSA alone was the cause in 88% (292/332) and there was no difference in BF by rising PSA within the Gleason 8-10 sub-groupings (Supplemental Table 15, all p>0.10).
Discussion
More than 50-years ago Donald Gleason proposed the eponymous grading system for prostate cancer which bears his name. [1] He identified 5 histologic patterns (from most well differentiated (Gleason Pattern 1) to least differentiated (GP 5)) and noted that when combined with stage this system was prognostic for overall survival. It is notable that Dr. Gleason suggested the use of this system in a continuous fashion without grouping. Over the years this system was largely adopted and different risk stratification schemes were later developed that incorporated the GS to facilitate comparisons between studies or treatment modalities. For ease of use most of these systems have historically grouped GS into 2-6 (low-risk), 7 (intermediate-risk), and 8-10 (high-risk) with other factors such as T-stage, PSA, and the volume of cancer often included. [2]
Studies suggested that this 3-tiered grouping does not capture the full stratification potential of the GS. Johns Hopkins noted after radical prostatectomy those with GS 9-10 where more than 2.8 fold more likely to have biochemical recurrence than those with GS of 8 [17, 18]. Sabolch et al studied men treated with dose-escalated EBRT and found substantially worse clinical outcome in those with GS 8-10 prostate cancer who had either primary or secondary GP5 with the 5-year prostate cancer mortality of 9% (+/−4%) in those with GS 8 prostate cancer (without GP5) compared to 36% (+/−7%) in those with GS 8-10 with GP5 (p<0.0001, HR:3.6 (95%CI:2.0-6.5)).[3] Population based data using the Surveillance Epidemiology and End Results (SEER) Program data also noted increasing prostate cancer specific mortality with increasing GS (2-6, 3+4, 4+3, 4+4, 8 with GP5, 9-10) both for patients treated with RT [19] and even in men with metastatic disease.[20] The finding in patients with metastasis at diagnosis is intriguing as it may suggest a shorter time to castrate resistance in those with GP5. Mahal et al also suggested that Gleason 5+3=8 should be considered differently from Gleason 3+5 with those with Gleason 5+3 having twice the risk of death from prostate cancer from Gleason 4+4 or 3+5 (p<0.001, HR:2.2 (95%CI:2.0-2.4)),[21] a finding mirrored by others.[22] These and similar reports led to the new ISUP Grade Grouping for Prostate Cancer that uses a 5 compartment model (Grade groups 1 through 5) instead of the more commonly utilized 3 compartment groupings [7]; although Grade Group 4 includes all Gleason 8 prostate cancers regardless of GP5. Notably we were not able to adopt the ISUP grade grouping approach (as primary and secondary GP was not always recorded for GS 7 prostate cancer) and looked at the presence of GP 5 (primary or secondary) as the primary variable of interest. Upon further analysis we were also not able to identify substantial differences between Gleason 3+5 or 5+3 as compared to Gleason 9-10 while Gleason 4+4 had lower rates of DM and all-cause mortality when compared to either any GP5 or explicitly to Gleason 9-10. Although exploratory and limited by sample size these results are suggestive that the presence of GP5 alone should be considered a significant risk factor and as such not all Gleason 8 scores (ISUP grade group 4) should be considered the same.
The pooled analysis of both studies also indicated that for high-risk prostate cancer a lack of GP5 led to decreased BF (HR: 0.78 (95%CI:0.66-0.92)), distant metastasis (HR:0.38 (95%CI:0.30-0.48)), and overall mortality (HR:0.73 (95%CI:0.63-0.85)) compared to those with GP with the greatest impact on distant metastasis. When BF was evaluated only as rising PSA (excluding local and distance failure as well as starting ADT prior to meeting the nadir+2 definition) there was no difference in BF between any of the sub-categories of Gleason 8-10 while Gleason 4+4 did have lower rates of DM and all-cause mortality compared to the groups including GP5. This would also be consistent with the finding that not all BF events bear equal weight and that some biochemical recurrences, such as earlier events, those happening coincident with metastasis, or with shorter PSA doubling time, may be associated with greater clinical risk.[23] The recent validation of DM as a surrogate for overall survival lends further impact to the adverse weight of GP5. [24].
The results of this analysis must also be interpreted in light of newer agents such as Docetaxel, Abiraterone, and Enzalutamide now playing a role in prostate cancer management. [25] Further, a recent meta-analysis of trials involving RT and ADT suggested that in those with GS 9-10 prostate cancer had greatest benefit from life-long ADT while in GS 8 prostate cancer the optimal duration might be long-term (but not lifelong) ADT.[26] In addition to systemic treatments, local control may still play a part even in those with the highest GS. The ASCENDE-RT trial recently demonstrated that permanent prostate implant with 125I added to external RT in addition to 12-months of ADT was shown to significantly decrease the risk of biochemical failure compared to dose-escalated RT plus ADT but without a significant difference in DM or OS.[27] However, additional retrospective or population based analyses have noted that the greatest benefit of adding a brachytherapy boost might be in those with Gleason 9-10 disease, perhaps as a means to prevent local recurrence which later leads to DM[28–30].
Finally, newer means to assess clinical risk based upon transcriptional or whole genomic profiles have also been developed.[31] These demonstrated added prognostic significance even when including GS. However, most have not independently evaluated GP 5 or GS 9-10 separate from GS 8. Therefore, it remains to be seen how much of these biologic differences might be captured with the more continuous use of GS as presented here or in the ISUP grade grouping scheme.
CONCLUSION
Given the potential impact of GP5 on the both risk-stratification and treatment it is paramount that design and analysis of future studies should be undertaken accounting for differences in clinical outcome (particularly DM and OS) between those with GS 8-10 prostate cancer without or without GP 5. Notably, for example, in those with high-risk prostate cancer without GP 5 treated as part of RTOG 9902 had a <10% risk of DM at 10-years which was >20% in those with GP pattern 5.
Supplementary Material
Highlights.
Gleason pattern 5 increased the risk of distant metastases compared to Gleason 8 (4+4).
This may impact treatment decisions and risk stratification.
Gleason 8-10 should not be utilized as a homogenous group given differences based upon GP5.
Acknowledgements
We gratefully acknowledge the contribution of the late Gerald E. Hanks, MD to this manuscript. Funding Source: This project was supported by grants NCORP (UG1CA189867), NRG SDMC (U10CA180822), NRG Ops (U10CA180868), and CTEP from the National Cancer Institute (NCI).
Role of the funding source: The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Disclosures: Dr(s). Dominello, D’Souza, Hartford, Horwitz, Jones, Lepor, Peters, Pienta, Pisansky, Rosenthal, and Zeitzer have nothing to disclose. Dr. Feng reports personal fees from Dendreon, personal fees from EMD Serono, personal fees from Janssen Oncology, personal fees from Ferring, personal fees from Sanofi, personal fees from Bayer, personal fees from Blue Earth Diagnostics, personal fees from Celgene, personal fees from Medivation/Astellas, personal fees from Clovis Oncology, other from PFS Genomics, grants from Zenith Epigenetics, grants from NRG Oncology, outside the submitted work; In addition, Dr. Feng has a patent EP3047037 A4 issued. Dr. Gomella reports grants from NCI, personal fees from Janssen, personal fees from Astellas, during the conduct of the study. Dr. Hall reports institutional research support from Elekta AB, Stockholm, Sweden, outside the submitted work. Dr. Hamstra reports grants and other from Augmenix, other from Boston Scientific, other from Medivation outside the submitted work. Dr. Pugh reports other from Millennium, other from Pfizer-Astellas, outside the submitted work. Dr. Sandler reports personal fees from Janssen, other from Radiogel, outside the submitted work Dr. Souhami reports other from VARIAN, other from JANSSEN, other from BAYER, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: NRG/RTOG 9202: & NRG/RTOG 9902:
References
- 1.Gleason DF, Classification of prostatic carcinomas. Cancer Chemother Rep, 1966. 50(3): p. 125–8. [PubMed] [Google Scholar]
- 2.D’Amico AV, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA, 1998. 280(11): p. 969–74. [DOI] [PubMed] [Google Scholar]
- 3.Sabolch A, et al. Gleason pattern 5 is the greatest risk factor for clinical failure and death from prostate cancer after dose-escalated radiation therapy and hormonal ablation. Int J Radiat Oncol Biol Phys, 2011. 81(4): p. e351–60. [DOI] [PubMed] [Google Scholar]
- 4.Chan TY, et al. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology, 2000. 56(5): p. 823–7. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, et al. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol, 2005. 23(13): p. 2911–7. [DOI] [PubMed] [Google Scholar]
- 6.Jackson W, et al. Gleason pattern 5 is the strongest pathologic predictor of recurrence, metastasis, and prostate cancer-specific death in patients receiving salvage radiation therapy following radical prostatectomy. Cancer, 2013. 119(18): p. 3287–94. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol, 2016. 40(2): p. 244–52. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz EM, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol, 2008. 26(15): p. 2497–504. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal SA, et al. A Phase 3 Trial of 2 Years of Androgen Suppression and Radiation Therapy With or Without Adjuvant Chemotherapy for High-Risk Prostate Cancer: Final Results of Radiation Therapy Oncology Group Phase 3 Randomized Trial NRG Oncology RTOG 9902. Int J Radiat Oncol Biol Phys, 2015. 93(2): p. 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roach M 3rd, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOGASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys, 2006. 65(4): p. 965–74. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL and Meier P, Nonparametric Estimation from Incomplete Observations Journal of the American Statistical Association, 1958. 53(282): p. 457–481. [Google Scholar]
- 12.Mantel N, Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep, 1966. 50(3): p. 163–70 [PubMed] [Google Scholar]
- 13.Kalbfleisch JD and Prentice RL, The Statistical analysis of failure time data. 1980, New York: John Wiley & Sons, Inc; 321. [Google Scholar]
- 14.Gray RJ, A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics, 1988. 16(3): p. 1141–1154. [Google Scholar]
- 15.Cox D, Regression models and life tables. . J R Stat Soc Series B 1972. 34: p. 187–229. [Google Scholar]
- 16.Fine J and Gray R, A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999. 94: p. 496–509. [Google Scholar]
- 17.Trock BJ, et al. Tertiary Gleason patterns and biochemical recurrence after prostatectomy: proposal for a modified Gleason scoring system. J Urol, 2009. 182(4): p. 1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashine K, et al. Tertiary Gleason pattern 5 and oncological outcomes after radical prostatectomy. Jpn J Clin Oncol, 2011. 41(4): p. 571–6. [DOI] [PubMed] [Google Scholar]
- 19.Rusthoven CG, et al. Gleason stratifications prognostic for survival in men receiving definitive external beam radiation therapy for localized prostate cancer. Urol Oncol, 2015. 33(2): p. 71 e11–9. [DOI] [PubMed] [Google Scholar]
- 20.Rusthoven CG, et al. The prognostic significance of Gleason scores in metastatic prostate cancer. Urol Oncol, 2014. 32(5): p. 707–13. [DOI] [PubMed] [Google Scholar]
- 21.Mahal BA, et al. Gleason score 5 + 3 = 8 prostate cancer: much more like Gleason score 9? BJU Int, 2016. 118(1): p. 95–101. [DOI] [PubMed] [Google Scholar]
- 22.Harding-Jackson N, et al. Outcome of Gleason 3 + 5 = 8 Prostate Cancer Diagnosed on Needle Biopsy: Prognostic Comparison with Gleason 4 + 4 = 8. J Urol, 2016. 196(4): p 1076–81. [DOI] [PubMed] [Google Scholar]
- 23.Hamstra DA, et al. Impact of biochemical failure classification on clinical outcome: a secondary analysis of Radiation Therapy Oncology Group 9202 and 9413. Cancer, 2015. 121(6): p. 844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie W, et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol, 2017. 35(27): p. 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komura K, et al. Current treatment strategies for advanced prostate cancer. Int J Urol, 2018. 25(3): p. 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishan AU, et al. Association of Gleason Grade With Androgen Deprivation Therapy Duration and Survival Outcomes: A Systematic Review and Patient-Level Meta-analysis. JAMA Oncol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris WJ, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High - and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys, 2017. 98(2): p. 275–285. [DOI] [PubMed] [Google Scholar]
- 28.Shilkrut M, et al. The addition of low-dose-rate brachytherapy and androgen-deprivation therapy decreases biochemical failure and prostate cancer death compared with dose-escalated external-beam radiation therapy for high-risk prostate cancer. Cancer, 2013. 119(3): p. 681–90. [DOI] [PubMed] [Google Scholar]
- 29.Kishan AU, et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9–10 Prostate Cancer. JAMA, 2018. 319(9): p. 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang M and Nguyen PL, Significant association of brachytherapy boost with reduced prostate cancer-specific mortality in contemporary patients with localized, unfavorable-risk prostate cancer. Brachytherapy, 2015. 14(6): p. 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornberg Z, et al. Genomic biomarkers in prostate cancer. Transl Androl Urol, 2018. 7(3): p. 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.