Abstract
Background
Although hypertension is an established risk factor for chronic kidney disease, less is known about the relationship of pulse pressure (PP), a measure of arterial stiffness, with chronic kidney disease. We investigated the association of systolic blood pressure (BP), diastolic BP, PP, and mean arterial pressure with the risk of end‐stage renal disease (ESRD) in the prospective population‐based Singapore Chinese Health Study.
Methods and Results
We used data from 30 636 participants who had BP measured at ages 46 to 85 years during follow‐up I interviews between 1999 and 2004. Information on lifestyle factors was collected at recruitment from 1993 to 1998, and selected factors were updated at follow‐up I. We identified 463 ESRD cases over an average 11.3 years of follow‐up I by linkage with the nationwide Singapore Renal Registry. Cox proportional hazards regression models were used to assess the relations between different BP indexes and ESRD risk. Each BP index was positively associated with ESRD when studied individually. However, when PP was included as a covariate, systolic and diastolic BP and mean arterial pressure were no longer associated with ESRD. Conversely, PP remained significantly associated with ESRD risk in a dose‐dependent manner (P trend<0.001) after adjusting for systolic or diastolic BP. Compared with the lowest group (<45 mm Hg) of PP, the hazard ratio was 5.25 (95% CI, 3.52–7.84) for the highest group (≥85 mm Hg). The association between hypertension and ESRD risk was attenuated and no longer significant after adjusting for PP.
Conclusions
Our findings provide a basis for targeting reduction of arterial stiffness to decrease ESRD risk.
Keywords: blood pressure, end‐stage renal disease, pulse pressure, Singapore Chinese Health Study, systolic blood pressure
Subject Categories: Epidemiology, Risk Factors
Clinical Perspective
What Is New?
Pulse pressure had the strongest association with the risk of end‐stage renal diseases compared with other blood pressure indexes.
The association between elevated systolic blood pressure in hypertension and end‐stage renal disease risk could be mediated by increased pulse pressure.
What Are the Clinical Implications?
Our findings provide novel evidence that supports the recommendations that the management of hypertension may require not only reducing systolic and diastolic blood pressure but also maintaining pulse pressure within a low range for renal protection.
Future studies are needed to evaluate potential therapies that target reduction of arterial stiffness to decrease the risk of end‐stage renal disease.
Introduction
End‐stage renal disease (ESRD), defined as the final stage of chronic kidney disease (CKD), is a severe irreversible decline in kidney function that requires costly lifelong sustaining treatment including dialysis or kidney transplantation. ESRD is a rising global public health threat associated with high morbidity and mortality rates.1, 2 According to the 2018 US Renal Data System annual report, Singapore is ranked fifth for incidence of treated ESRD in the world.3
Hypertension, defined as the elevation of systolic blood pressure (SBP) and/or diastolic blood pressure (DBP), has been well documented as an independent risk factor for the development and progression of CKD.4, 5, 6 Despite evidence from cohort studies showing SBP as a strong and independent risk factor for decline in estimated glomerular filtration rate7, 8 and risk of ESRD,9, 10 intensive SBP treatment (<120 mm Hg) in clinical trials has not resulted in benefits for CKD compared with standard treatment (<140 mm Hg) among patients with diabetes mellitus.11 Furthermore, SPRINT (Systolic Blood Pressure Intervention Trial) unexpectedly found >3‐fold incidence of CKD in the intensive SBP treatment group compared with the standard SBP treatment group at 3‐year follow‐up I.12
Mean arterial pressure (MAP) and pulse pressure (PP) are major indexes computed from SBP and DBP to reflect the hemodynamic components of pressure and flow. MAP is a measure of the steady component and is determined by cardiac output and peripheral vascular resistance. PP is a measure of the pulsatile component and arterial stiffness and is determined by stroke volume, aortic stiffness, and wave reflections.13 PP has been reported to be an independent predictor of cardiovascular mortality and morbidity,14 and the Framingham Heart Study showed that PP was superior to SBP and DBP in predicting coronary heart disease risk.15 Increasing evidence has recently highlighted that arterial stiffness may have a potentially deleterious effect on kidney function through hemodynamic and metabolic injuries.16, 17, 18 However, the effect of PP on renal function is relatively understudied compared with SBP and DBP.
In this study, we used data from the Singapore Chinese Health Study, a prospective cohort of middle‐aged and older Chinese adults in Singapore, to study the associations between various blood pressure (BP) indexes, namely, SBP, DBP, PP, and MAP, and the risk of ESRD. We also studied whether the association between hypertension and ESRD risk could be explained by PP.
Methods
The data that support the findings of this study and the analytic methods and study materials are available from the corresponding author on reasonable request and in compliance with National Institutes of Health guidelines.
Study Participants
The Singapore Chinese Health Study is a population‐based prospective cohort study consisting of 63 257 Chinese adults (35 298 women and 27 959 men) who were aged 45 to 74 years during recruitment between April 1993 and December 1998. The participants were citizens or permanent residents of Singapore and restricted to the 2 major dialect groups of Chinese in Singapore, Hokkien and Cantonese, who originated from the Fujian and Guangdong provinces, respectively, in the southern part of China.19 Follow‐up I interviews were conducted via telephone between 1999 and 2004. Among 52 322 participants who were recontacted successfully, 30 636 participants agreed to donate blood for research and had BP measurements taken before venepunction.20 The institutional review board of the National University of Singapore approved this study, and all enrolled participants gave written informed consent.
Ascertainment of BP and Covariates
At recruitment, the trained interviewers conducted the face‐to‐face interviews using a structured questionnaire and obtained information on demographics, height, weight, tobacco use, alcohol intake, habitual physical activity, sleep duration, incense use, and medical history, including physician‐diagnosed hypertension, diabetes mellitus, coronary artery disease, and stroke. Habitual diet was assessed using a validated 165‐item, semiquantitative food frequency questionnaire. Body mass index (kg/m2) was calculated by body weight in kilograms divided by height in square meters. Information on age, body mass index, smoking status, alcohol consumption, and medical history was updated during the follow‐up I interview.
BP was measured at home for all participants who consented to home visits for blood collection after follow‐up I interviews, and the protocol was described previously.20 Briefly, BP was measured in a seated position by trained staff using the Omron HEM‐705CP automatic digital BP monitor. Three measurements were obtained at 3‐minute intervals, and the average values of SBP and DBP were rounded to the next integer and used as the final measurements. PP is the arithmetic difference between SBP and DBP, and MAP is computed using the following formula: [(2×DBP)+SBP]/3. During the visit, participants were asked again about history of hypertension and the use of antihypertensive medication if they reported physician‐diagnosed hypertension.
Ascertainment of Incident ESRD Cases
We identified ESRD cases by linking the cohort database with the population‐based Singapore Renal Registry, which has been shown to be comprehensive in recording incident ESRD cases in Singapore since 1999. The registry used multiple sources to identify ESRD cases, including listings of patients on dialysis, laboratory records, and hospital records.21 ESRD cases were registered in renal registry if they met ≥1 of the following criteria: (1) serum creatinine level ≥500 μmol/L (10 mg/dL) since 2010, (2) estimated glomerular filtration rate <15 mL/min per 1.73 m2 (based on the Modification of Diet in Renal Disease Study equation, the Cockcroft–Gault equation, or 24‐hour creatinine clearance), (3) hemodialysis or peritoneal dialysis treatment, or (4) kidney transplant recipient. To qualify as ESRD cases, the first 3 criteria had to be persistent over 3 months.21 As of December 31, 2014, only 47 participants were unavailable for follow‐up I from our cohort because of migration or other reasons, suggesting that loss to follow‐up I was negligible.
Statistical Analysis
Among the 30 636 participants, we excluded those who developed ESRD before the visits for BP measurements (n=102) and those with unrealistic BP measurements (<80 or ≥250 mm Hg for SBP, <40 or ≥150 mm Hg for DBP, or <10 mm Hg for PP; n=17). A total of 30 517 participants remained in the current analysis. We compared the correlations between pairs of BP indexes using pairwise Pearson correlation coefficients. Distributions of the baseline characteristics were compared by the extreme categories of BP indexes. We counted person‐years from the date of BP measurement to the date of reported ESRD, loss to follow‐up I, death, or December 31, 2014, whichever came first. We created categories of BP indexes using cutoff values that were clinically useful, also guided by the distribution of BPs in the cohort to maximize statistical power: SBP <120, 120 to 129, 130 to 139, 140 to 149, 150 to 159 and ≥160 mm Hg; DBP <70, 70 to 79, 80 to 89, 90 to 99, and ≥100 mm Hg; PP <45, 45 to 54, 55 to 64, 65 to 74, 75 to 84, and ≥85 mm Hg; and MAP <90, 90 to 99, 100 to 109, 110 to 119, and ≥120 mm Hg. We used multivariable Cox proportional hazards regression models to compute the hazard ratios (HRs) and 95% CIs for risk of ESRD associated with higher categories of SBP, DBP, PP, and MAP, using the group with the lowest level of each index as the reference group. We selected potential confounders that were either established risk factors for ESRD or based on prior associations with risk of ESRD in our study population.22, 23, 24, 25, 26, 27 The Cox regression model for analysis was adjusted for the following factors: age (years) at follow‐up I; sex; dialect (Hokkien, Cantonese); education level (none, primary school, secondary school, or higher); body mass index at follow‐up I (kg/m2); smoking status at follow‐up I (never, ever); alcohol consumption at follow‐up I (none/monthly, weekly, daily); physical activity (defined as any weekly moderate activity, vigorous activity, or strenuous sports lasting at least 30 minutes; yes, no); sleep duration (<5, 6, 7, 8, ≥9 hours); total energy intake (kcal/day); total protein intake (g/day, in quartiles); red meat intake (g/day, in quartiles); coffee consumption (none to <1, 1, ≥2 cups/day); domestic incense use (current, noncurrent); self‐reported history of physician‐diagnosed diabetes mellitus, coronary artery disease, and stroke at follow‐up I (yes, no); and antihypertensive medication use at BP measurement (yes, no). Because we had transformed each BP index from a continuous variable to a categorical variable that was ordinal in nature, we were able to assign ordinal values to the categories, starting with 1 for the lowest category and 6 for the highest categories for SBP and PP (6 categories) and 5 for the highest categories for DBP and MAP (5 categories). The P for trend was based on a test for linear trend using the ordinal values of the BP categories in the model.
Restricted cubic spline analysis was used to examine the shape of the relationships between BP indexes and risk of ESRD. We selected the number of knots based on the values of Akaike information criteria to fit the best approximating model, chose either the first or second knot as reference, and tested for linearity by Wald test. To determine the independent effect of the different BP indexes, we also analyzed various dual BP components in the same model. The goodness of fit between the model with 1 BP index and the dual model with 2 BP indexes was compared using the likelihood ratio test. Because all BP indexes showed linear dose‐dependent responses above specific levels based on observations from restricted cubic spline analysis and risk estimates from ordinal BP categories, we calculated HRs and 95% CIs per 10‐mm Hg increment of each BP index beyond the level at which the risk could be observed to increase in a linear manner by restricting the analyses to participants whose BP measurements were above the cutoff values determined from the results.
We also performed stratified analysis by age (<65 versus ≥65 years); sex (men versus women); history of comorbidities comprising diabetes mellitus, coronary artery disease, and stroke (with at least 1 versus none of the 3 comorbidities); and antihypertensive medication use (yes versus no). The heterogeneity of the PP–ESRD associations by these factors was tested by adding an interaction term (product of PP and interaction factor) to the model. We classified the participants into 5 different BP categories following the 2017 high BP clinical practice guideline28—(1) normal BP (ie, SBP <120 mm Hg and DBP <80 mm Hg), (2) elevated BP (SBP 120–129 mm Hg and DBP <80 mm Hg), (3) stage 1 hypertension (SBP 130–139 mm Hg or DBP 80–89 mm Hg), (4) low stage 2 hypertension (SBP 140–149 mm Hg or DBP 90–99 mm Hg), and (5) high stage 2 hypertension (SBP ≥150 mm Hg or DBP ≥100 mm Hg)—and evaluated the risk of ESRD in each category using those with normal BP as the referent group. We then further adjusted for PP in the model to determine whether the observed BP–ESRD risk was explained by higher PP in the higher BP categories.
In a dual model that included 2 BP indexes in the same model, the covariate BP index was included as an ordinal variable in the main analysis and as a categorical variable in a sensitivity analysis. We excluded all participants with <5 years of follow‐up I in another sensitivity analysis to overcome the potential bias of reverse causality arising from the possibility that PP could be elevated by CKD that was already present at recruitment. In addition, we repeated the analysis by excluding 107 participants with PP <25 mm Hg. All analyses were performed using Stata statistical software, release 14.0 (StataCorp), and a 2‐sided P<0.05 was considered statistically significant.
Results
The mean age of the study participants was 63.0±7.8 years at BP measurement. After a mean follow‐up I of 11.3±3.1 years, we identified 463 incident ESRD cases and 7731 deaths unrelated to ESRD. Characteristics of participants in the highest and lowest groups for SBP, DBP, PP, and MAP are shown in Table 1. For all BP indexes, those in the highest groups were more likely to be ever smokers, daily alcohol drinkers, and current incense users and to have higher body mass index and lower education levels. Those in the highest groups for SBP, PP, and MAP were also older than those in lower groups and more likely to have a history of diabetes mellitus or cardiovascular disease and to have extreme sleep durations. Participants in the highest groups for SBP, DBP, and MAP were more likely to be men and to have higher coffee intake than participants in lower groups.
Table 1.
Baseline Characteristics According to the Extreme Categories of SBP, DBP, PP, and MAP in the Singapore Chinese Health Study
| Characteristic | SBP (mm Hg) | DBP (mm Hg) | PP (mm Hg) | MAP (mm Hg) | ||||
|---|---|---|---|---|---|---|---|---|
| <120 | ≥160 | <70 | ≥100 | <45 | ≥85 | <90 | ≥120 | |
| Participants | 7007 (23.0) | 4609 (15.1) | 4827 (15.8) | 1521 (5.0) | 7718 (25.3) | 1870 (6.1) | 7876 (25.8) | 2195 (7.2) |
| Deaths unrelated to ESRD | 1189 (17.0) | 1943 (42.4) | 1374 (28.5) | 496 (32.7) | 1070 (13.9) | 1042 (56.3) | 1679 (21.3) | 852 (39.0) |
| PP, mm Hg | 39.4±7.0 | 81.8±13.7 | 50.2±15.6 | 69.5±18.2 | 37.8±4.9 | 95.0±10.1 | 44.2±11.0 | 79.3±17.9 |
| Age, y | 59.8±7.0 | 67.0±7.6 | 63.4±8.3 | 62.9±7.3 | 58.8±6.4 | 70.1±6.9 | 61.4±7.7 | 65.0±7.6 |
| Body mass index (kg/m2) | 22.2±3.4 | 23.8±3.6 | 21.9±3.4 | 24.3±3.7 | 22.7±3.5 | 23.4±3.5 | 22.1±3.4 | 24.1±3.6 |
| Men | 2421 (34.6) | 2244 (48.7) | 1499 (31.1) | 910 (59.8) | 3107 (40.3) | 752 (40.2) | 2642 (33.5) | 1189 (54.2) |
| Dialect | ||||||||
| Cantonese | 3635 (51.9) | 2254 (48.9) | 2573 (53.3) | 706 (46.4) | 3962 (51.3) | 959 (51.3) | 4101 (52.1) | 1037 (47.2) |
| Hokkien | 3372 (48.1) | 2355 (51.1) | 2254 (46.7) | 815 (53.6) | 3756 (48.7) | 911 (48.7) | 3775 (47.9) | 1158 (52.8) |
| Higher educationa | 2746 (39.2) | 1047 (22.7) | 1486 (30.8) | 448 (29.5) | 3303 (42.8) | 308 (16.5) | 2818 (35.8) | 552 (25.2) |
| Ever smokers | 1800 (25.7) | 1746 (37.9) | 1380 (28.6) | 606 (39.8) | 2036 (26.4) | 695 (37.2) | 2127 (27.0) | 851 (38.8) |
| Daily alcohol drinkers | 157 (2.2) | 182 (4.0) | 111 (2.3) | 80 (5.3) | 177 (2.3) | 57 (3.1) | 178 (2.3) | 103 (4.7) |
| Coffee consumption, cups/d | ||||||||
| 0 to <1 | 2281 (32.6) | 1320 (28.6) | 1621 (33.6) | 429 (28.2) | 2477 (32.1) | 551 (29.5) | 2565 (32.6) | 625 (28.5) |
| 1 | 2460 (35.1) | 1696 (36.8) | 1668 (34.6) | 508 (33.4) | 2679 (34.7) | 699 (37.4) | 2747 (34.9) | 789 (36.0) |
| ≥2 | 2266 (32.3) | 1593 (34.6) | 1538 (31.9) | 584 (38.4) | 2562 (33.2) | 620 (33.2) | 2564 (32.6) | 781 (35.6) |
| Sleep, h/d | ||||||||
| <5 | 619 (8.8) | 524 (11.4) | 499 (10.3) | 154 (10.1) | 653 (8.5) | 238 (12.7) | 747 (9.5) | 246 (11.2) |
| 6–8 | 5943 (84.8) | 3756 (81.5) | 3999 (82.9) | 1276 (83.9) | 6583 (85.3) | 1499 (80.2) | 6617 (84.0) | 1803 (82.1) |
| ≥9 | 445 (6.4) | 329 (7.1) | 329 (6.8) | 91 (6.0) | 482 (6.3) | 133 (7.1) | 512 (6.5) | 146 (6.7) |
| Total protein intakeb | 60.0±10.0 | 58.7±10.2 | 59.8±9.6 | 58.4±10.6 | 59.8±10.1 | 58.9±9.8 | 59.8±9.9 | 58.6±10.6 |
| Red meat intakeb | 30.5±18.8 | 30.3±18.7 | 29.7±17.1 | 31.3±20.4 | 30.8±19.4 | 30.0±17.3 | 29.9±18.2 | 30.8±19.2 |
| Current daily incense users | 4995 (71.3) | 3631 (78.8) | 3479 (72.1) | 1193 (78.4) | 5552 (71.9) | 1455 (77.8) | 5637 (71.6) | 1726 (78.6) |
| Physical activityc | 2450 (35.0) | 1536 (33.3) | 1610 (33.4) | 567 (37.3) | 2842 (36.8) | 581 (31.1) | 2681 (34.0) | 756 (34.4) |
| History of diabetes mellitus | 552 (7.9) | 1081 (23.5) | 694 (14.4) | 213 (14.0) | 541 (7.0) | 586 (31.3) | 846 (10.7) | 409 (18.6) |
| History of CVD | 515 (7.4) | 708 (15.4) | 557 (11.5) | 157 (10.3) | 499 (6.5) | 363 (19.4) | 716 (9.1) | 281 (12.8) |
| Antihypertensive medication use | 1155 (16.5) | 2477 (53.7) | 1135 (23.5) | 734 (48.3) | 1537 (19.9) | 1140 (61.0) | 1558 (19.8) | 1139 (51.9) |
Data are shown as n (%) or mean±SD. CVD indicates cardiovascular disease; DBP, diastolic blood pressure; ESRD, end‐stage renal disease; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Secondary school or higher education level.
Red meat and total protein were reported as g/d.
Physical activity defined as at least 0.5 h/wk of moderate activity, vigorous activity, or strenuous sports.
The baseline characteristics of the overall cohort are presented in Table S1. Pearson correlation coefficients for pairwise comparisons of SBP, DBP, PP, and MAP are shown in Table S2. All indexes had moderate to strong correlations with one another. Among the indexes, SBP and MAP had the highest correlation (r=0.88), whereas DBP and PP had the lowest correlation (r=0.28). The correlation coefficient was 0.84 for SBP and PP.
After a mean follow‐up I duration of 11.3±3.1 years among 30 517 participants, 463 incident ESRD cases were documented. As shown in Table 2, positive and dose–response associations with the risk of ESRD were observed for all indexes in a multivariable‐adjusted model. Compared with the lowest categories, the multivariable‐adjusted HRs of ESRD for the highest categories were 2.50 (95% CI, 1.76–3.55) for SBP, 1.58 (95% CI, 1.05–2.39) for DBP, 5.25 (95% CI, 3.52–7.84) for PP, and 2.66 (95% CI, 1.90–3.72) for MAP (all P trend≤0.001).
Table 2.
HR (95% CI) for ESRD According to Categories of SBP, DBP, PP, and MAP in the Singapore Chinese Health Study
| n | Cases/Person‐Year | ESRD, HR (95% CI)a | |||
|---|---|---|---|---|---|
| Multivariate Modelb | Modelb+DBP | Modelb+PP | |||
| SBP, mm Hg | |||||
| <120 | 7007 | 45/81 310 | 1.00 | 1.00 | 1.00 |
| 120–129 | 5349 | 39/62 225 | 0.79 (0.51–1.21) | 0.84 (0.54–1.30) | 0.64 (0.41–0.99) |
| 130–139 | 5468 | 59/62 638 | 1.01 (0.68–1.50) | 1.12 (0.75–1.69) | 0.66 (0.43–1.01) |
| 140–149 | 4772 | 88/53 918 | 1.48 (1.03–2.15) | 1.73 (1.17–2.58) | 0.79 (0.51–1.23) |
| 150–159 | 3312 | 62/36 704 | 1.42 (0.95–2.11) | 1.71 (1.11–2.64) | 0.60 (0.36–0.99) |
| ≥160 | 4609 | 170/47 863 | 2.50 (1.76–3.55) | 3.24 (2.12–4.96) | 0.73 (0.42–1.29) |
| P for LRc | 0.04 | <0.001 | |||
| P for trend | <0.001 | <0.001 | 0.55 | ||
| Per 10 mm Hgd | 30 517 | 463/344 659 | 1.29 (1.22–1.37) | 1.50 (1.36–1.66) | 1.08 (0.97–1.21) |
| Modelb+SBP | Modelb+PP | ||||
| DBP, mm Hg | |||||
| <70 | 4827 | 61/53 008 | 1.00 | 1.00 | 1.00 |
| 70–79 | 10 169 | 122/115 662 | 0.83 (0.61–1.13) | 0.62 (0.45–0.86) | 0.77 (0.56–1.05) |
| 80–89 | 9886 | 154/112 609 | 0.97 (0.72–1.32) | 0.53 (0.38–0.75) | 0.80 (0.59–1.08) |
| 90–99 | 4114 | 86/46 722 | 1.30 (0.93–1.82) | 0.55 (0.37–0.83) | 0.94 (0.67–1.33) |
| ≥100 | 1521 | 40/16 659 | 1.58 (1.05–2.39) | 0.57 (0.35–0.93) | 1.03 (0.68–1.57) |
| P for LRc | <0.001 | <0.001 | |||
| P for trend | 0.001 | 0.05 | 0.47 | ||
| Per 10 mm Hge | 30 517 | 463/344 659 | 1.38 (1.12–1.70) | 0.78 (0.60–1.02) | 1.08 (0.84–1.38) |
| Modelb+SBP | Modelb+DBP | ||||
| PP, mm Hg | |||||
| <45 | 7718 | 39/91 291 | 1.00 | 1.00 | 1.00 |
| 45–54 | 7774 | 59/90 093 | 1.19 (0.79–1.79) | 1.21 (0.80–1.85) | 1.18 (0.78–1.77) |
| 55–64 | 6564 | 106/74 581 | 2.04 (1.40–2.97) | 2.12 (1.36–3.30) | 2.00 (1.37–2.93) |
| 65–74 | 4219 | 87/46 075 | 2.29 (1.54–3.39) | 2.43 (1.44–4.10) | 2.24 (1.51–3.34) |
| 75–84 | 2372 | 68/24 687 | 2.85 (1.88–4.33) | 3.08 (1.68–5.65) | 2.78 (1.82–4.24) |
| ≥85 | 1870 | 104/17 932 | 5.25 (3.52–7.84) | 5.72 (3.02–10.8) | 5.07 (3.36–7.66) |
| P for LRc | 0.74 | 0.49 | |||
| P for trend | <0.001 | <0.001 | <0.001 | ||
| Per 10 mm Hgf | 30 517 | 463/344 659 | 1.39 (1.30–1.48) | 1.28 (1.11–1.47) | 1.49 (1.33–1.66) |
| Modelb+SBP | Modelb+PP | ||||
| MAP, mm Hg | |||||
| <90 | 7876 | 64/89 615 | 1.00 | 1.00 | 1.00 |
| 90–99 | 8866 | 99/101 601 | 1.03 (0.75–1.41) | 0.67 (0.47–0.97) | 0.83 (0.60–1.15) |
| 100–109 | 7630 | 124/86 626 | 1.22 (0.89–1.66) | 0.52 (0.33–0.83) | 0.81 (0.58–1.12) |
| 110–119 | 3950 | 93/43 617 | 1.66 (1.20–2.31) | 0.50 (0.28–0.89) | 0.87 (0.60–1.26) |
| ≥120 | 2195 | 83/23 201 | 2.66 (1.90–3.72) | 0.71 (0.38–1.30) | 1.18 (0.79–1.76) |
| P for LRc | <0.001 | <0.001 | |||
| P for trend | <0.001 | 0.99 | 0.31 | ||
| Per 10 mm Hgg | 30 517 | 463/344 659 | 1.43 (1.30–1.57) | 0.70 (0.56–0.87) | 1.11 (0.98–1.26) |
DBP indicates diastolic blood pressure; ESRD, end‐stage renal disease; HR, hazard ratio; LR, likelihood ratio; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Blood pressure components were adjusted as ordinal variables in the dual model.
HRs were adjusted for age; sex; dialect; education level; body mass index; physical activity; smoking status; alcohol use; sleep duration; total energy intake; total protein intake; red meat consumption; coffee consumption; incense use; physician‐diagnosed diabetes mellitus, coronary artery disease, and stroke; and antihypertensive medication use.
Goodness of fit between the model with 1 blood pressure index and the dual model with 2 blood pressure indexes were compared using the LR test.
HR (95% CI) per 10‐mm Hg increment was generated only for SBP ≥140 mm Hg.
HR (95% CI) per 10‐mm Hg increment was generated only for DBP ≥90 mm Hg.
HR (95% CI) per 10‐mm Hg increment was generated only for PP ≥55 mm Hg.
HR (95% CI) per 10‐mm Hg increment was generated only for MAP ≥100 mm Hg.
Restricted cubic spline analysis showed a nonlinear relationship between all BP indexes (nonlinearity, P<0.001 for SBP, DBP, and MAP and P=0.003 for PP) and risk of ESRD (Figure), and the HRs were observed to increase in a linear dose‐dependent manner beyond a certain level for each index. The curves of SBP, DBP, PP, and MAP in relation to risk of ESRD were similar; however, there could be subtle differences at very low levels. The association with the risk of ESRD for SBP appeared to plateau at very low levels, whereas the risk of ESRD seemed to increase with decreasing DBP or PP at very low levels to create an overall J‐shaped curve. Combining the results from Table 2 and Figure, the risk of ESRD appeared to increase significantly beyond 140 mm Hg for SBP, 90 mm Hg for DBP, 55 mm Hg for PP, and 100 mm Hg for MAP. The HRs of ESRD risk for per‐10 mm Hg increment beyond the respective cutoff levels were 1.29 (95% CI, 1.22–1.37) for SBP, 1.38 (95% CI, 1.12–1.70) for DBP, 1.39 (95% CI, 1.30–1.48) for PP, and 1.43 (95% CI, 1.30–1.57) for MAP.
Figure 1.
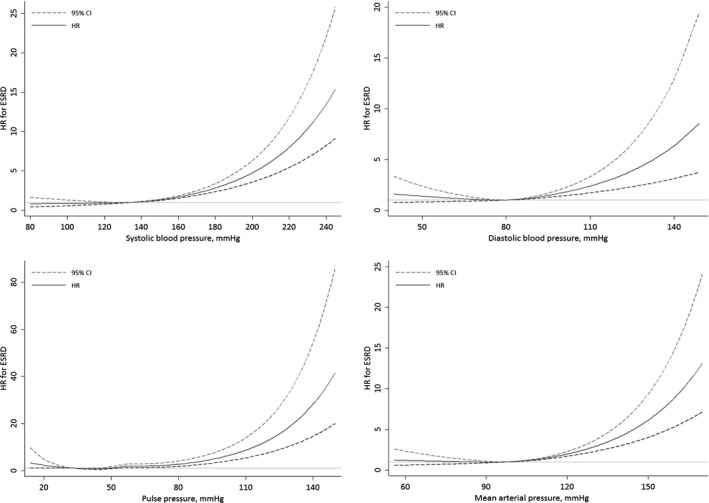
Multivariable adjusted hazard ratios (HRs) for risk of end‐stage renal disease (ESRD) according to systolic blood pressure, diastolic blood pressure, pulse pressure, and mean arterial pressure. HRs were adjusted for age; sex; dialect; education level; body mass index; physical activity; smoking status; alcohol use; sleep duration; total energy intake; total protein intake; red meat consumption; coffee consumption; incense use; physician‐diagnosed diabetes mellitus, coronary artery disease, and stroke; and antihypertensive medication use.
As shown in Table 2, the analysis that included dual BP components showed that the positive and dose‐dependent association between PP and ESRD risk remained essentially unchanged when further adjusted for SBP or DBP. However, the associations for SBP, DBP, and MAP were substantially attenuated and no longer significant when adjusted for PP. Results were similar between the models that included the covariate BP index as an ordinal variable (Table 2) and a categorical variable (data not shown). In the model that included both SBP and DBP, the risk estimates for the association between SBP and risk of ESRD rose, whereas increasing DBP was associated with reduced risk of ESRD. The likelihood ratio test did not show any improvement in goodness of fit for a model with PP and SBP or PP and DBP over the model with PP alone (likelihood ratio, P>0.05). In contrast, addition of PP significantly improved the goodness‐of‐fit for all models (likelihood ratio, all P<0.001; Table 2).
In stratified analyses, we did not find significant heterogeneity in the risk estimates between those with and without any of the 3 preexisting comorbidities or between women and men (interaction, all P≥0.22; Table 3). There were generally stronger associations for PP categories in the participants aged <65 years compared with their older counterparts (interaction, P=0.002) and in those who were not using antihypertensive medication compared with those on such medication (interaction, P=0.005). There were 2955 participants who reported having hypertension but were not using antihypertensive medication. The results of stratified analysis remained essentially unchanged after excluding these hypertensive patients who were not taking antihypertensive medication (data not shown).
Table 3.
HR (95% CI) for ESRD According to Categories of PP, Stratified by Comorbidities, Sex, Age, and Antihypertensive Medication Use
| PP Categories (mm Hg) | P for Trend | ||||||
|---|---|---|---|---|---|---|---|
| <45 | 45–54 | 55–64 | 65–74 | 75–84 | ≥85 | ||
| Any of the 3 comorbiditiesa | |||||||
| Cases/person‐year | 21/10 538 | 26/14 989 | 61/16 392 | 58/12 638 | 50/8170 | 74/6621 | |
| HR (95% CI)b | 1.00 | 0.91 (0.51–1.62) | 2.01 (1.21–3.32) | 2.59 (1.56–4.33) | 3.54 (2.09–6.00) | 6.67 (4.00–11.1) | <0.001 |
| No comorbidities | |||||||
| Cases/person‐year | 18/80 760 | 33/75 105 | 45/58 188 | 29/33 437 | 18/16 518 | 30/11 311 | |
| HR (95% CI)b | 1.00 | 1.36 (0.76–2.43) | 1.75 (1.00–3.07) | 1.63 (0.88–3.00) | 1.81 (0.91–3.60) | 3.61 (1.91–6.83) | <0.001 |
| P for factor interaction | 0.08 | 0.17 | 0.36 | 0.48 | 0.16 | ||
| P for interaction | 0.76 | ||||||
| Men | |||||||
| Cases/person‐year | 16/35 351 | 37/40 656 | 55/32 848 | 51/20 004 | 29/10 237 | 36/6726 | |
| HR (95% CI)b | 1.00 | 1.67 (0.93–3.01) | 2.63 (1.49–4.64) | 3.43 (1.92–6.14) | 3.23 (1.70–6.12) | 5.69 (3.04–10.7) | <0.001 |
| Women | |||||||
| Cases/person‐year | 23/55 939 | 22/49 438 | 51/41 733 | 36/26 071 | 39/14 451 | 68/11 205 | |
| HR (95% CI)b | 1.00 | 0.83 (0.46–1.49) | 1.64 (0.99–2.73) | 1.57 (0.91–2.72) | 2.54 (1.46–4.40) | 4.85 (2.86–8.23) | <0.001 |
| P for factor interaction | 0.11 | 0.36 | 0.09 | 0.82 | 0.99 | ||
| P for interaction | 0.22 | ||||||
| Age <65 y | |||||||
| Cases/person‐year | 28/76 348 | 29/64 342 | 58/42 561 | 41/20 084 | 28/8686 | 34/4287 | |
| HR (95% CI)b | 1.00 | 0.91 (0.54–1.53) | 2.08(1.31–3.32) | 2.43 (1.47–4.03) | 3.30 (1.90–5.73) | 6.00 (3.49–10.3) | <0.001 |
| Age ≥65 y | |||||||
| Cases/person‐year | 11/14 943 | 30/25 751 | 48/32 020 | 46/25 992 | 40/16 001 | 70/13 645 | |
| HR (95% CI)b | 1.00 | 1.50 (0.75–3.00) | 1.62 (0.84–3.14) | 1.79 (0.92–3.48) | 2.13 (1.09–4.19) | 4.07 (2.13–7.79) | <0.001 |
| P for factor interaction | 0.31 | 0.38 | 0.25 | 0.12 | 0.12 | ||
| P for interaction | 0.002 | ||||||
| Not using antihypertensive medication | |||||||
| Cases/person‐year | 11/73 414 | 15/63 162 | 30/44 776 | 22/24 572 | 14/12 010 | 17/7188 | |
| HR (95% CI)b | 1.00 | 1.32 (0.60–2.89) | 3.19 (1.56–6.51) | 3.74 (1.75–7.98) | 3.97 (1.72–9.16) | 7.99 (3.53–18.1) | <0.001 |
| Using antihypertensive medication | |||||||
| Cases/person‐year | 28/17 876 | 44/26 931 | 76/29 805 | 65/21 504 | 54/12 677 | 87/10 744 | |
| HR (95% CI)b | 1.00 | 1.02 (0.63–1.64) | 1.49 (0.96–2.31) | 1.68 (1.07–2.65) | 2.21 (1.38–3.56) | 4.08 (2.59–6.41) | <0.001 |
| P for factor interaction | 0.42 | 0.02 | 0.01 | 0.03 | 0.01 | ||
| P for interaction | 0.005 | ||||||
ESRD indicates end‐stage renal disease; HR, hazard ratio; PP, pulse pressure.
Comorbidities included of diabetes mellitus, coronary artery disease, and stroke.
HRs were adjusted for age; sex; dialect; education level; body mass index; physical activity; smoking status; alcohol use; sleep duration; total energy intake; total protein intake; red meat consumption; coffee consumption; incense use; physician‐diagnosed diabetes mellitus, coronary artery disease, and stroke; and antihypertensive medication use except the stratified factors.
PP increased with increasing BP in the categories defined by the 2017 high BP clinical practice guideline.28 Compared with those with normal BP, the elevated risk of ESRD was observed only in those with stage 2 hypertension; the highest HR was 2.26 (95% CI, 1.57–3.25), observed in those with SBP ≥150 mm Hg or DBP ≥100 mm Hg. However, when PP was included in the models as a covariate, all association between BP categories and the risk of ESRD became null (Table 4). Finally, the results in all sensitivity analyses were essentially the same as the results in the main analysis (data not shown).
Table 4.
HR (95% CI) for ESRD According to BP Categories
| BP Categories | |||||
|---|---|---|---|---|---|
| Normal | Elevated | Stage 1 Hypertension | Stage 2 Hypertension | ||
| Low | High | ||||
| Definition, mm Hg | SBP <120 and DBP <80 | SBP 120–129 and DBP <80 | SBP 130–139 or DBP 80–89 | SBP 140–149 or DBP 90–99 | SBP ≥150 or DBP ≥100 |
| Participants, n (%) | 6486 (21.3) | 3523 (11.5) | 7177 (23.5) | 5306 (17.4) | 8025 (26.3) |
| PP, mm Hg, mean±SD | 39.9±6.9 | 51.4±5.6 | 50.7±9.8 | 59.0±9.9 | 75.4±14.4 |
| Cases/person‐years | 37/74 993 | 33/40 398 | 70/83 065 | 90/60 414 | 233/85 789 |
| Adjusted HR (95% CI)a | 1.00 | 1.07 (0.67–1.72) | 1.09 (0.73–1.63) | 1.55 (1.05–2.30) | 2.26 (1.57–3.25) |
| Additionally adjusted HR (95% CI)b | 1.00 | 0.79 (0.47–1.34) | 0.74 (0.47–1.18) | 0.84 (0.51–1.38) | 0.77 (0.45–1.31) |
BP indicates blood pressure; DBP, diastolic blood pressure; ESRD, end‐stage renal disease; HR, hazard ratio; PP, pulse pressure; SBP, systolic blood pressure.
HRs were adjusted for age; sex; dialect; education level; body mass index; physical activity; smoking status; alcohol use; sleep duration; total energy intake; total protein intake; red meat consumption; coffee consumption; incense use; physician‐diagnosed diabetes mellitus, coronary artery disease, and stroke; and antihypertensive medication use.
HRs were additionally adjusted for PP.
Discussion
In this study, among middle‐aged and older Chinese adults from a population‐based cohort in Singapore, we showed that PP had the strongest association with ESRD risk compared with other BP indexes (SBP, DBP, and MAP). This observed PP–ESRD association was stronger in participants aged <65 years and in those who were not using antihypertensive medication. The association between hypertension and ESRD risk was attenuated and no longer significant after adjusting for PP.
In line with our findings, one cross‐sectional study that included 212 patients with isolated systolic hypertension suggested that increased PP was related to lower glomerular filtration rate independent of MAP among the patients aged >60 years.29 One cohort study with 4853 participants aged 60 years in the United States also showed that PP was associated with faster kidney function decline that was independent of SBP; this finding is consistent with our results.30 A secondary analysis of the RENAAL (Reduction of Endpoint in Non–Insulin‐Dependent Diabetes With the Angiotensin II Antagonist Losartan) study involving 1513 patients with diabetes mellitus and nephropathy showed that PP and SBP were independent and equally strong predictors for doubling of serum creatinine or development of ESRD; however, when ESRD and death were examined as the combined outcome, PP was a more powerful predictor than SBP.31 In addition, the Rotterdam Study, which included 3666 participants, investigated the association between arterial stiffness and kidney function by creating a genetic risk score with 10 single‐nucleotide polymorphisms associated with PP. The results showed that a higher PP genetic risk score was associated with steeper annual estimated glomerular filtration rate decline and higher risk of incident CKD and provided evidence of a causal relationship between PP and kidney function decline.18
PP and SBP are highly correlated in middle‐aged and older adults.13 Elevated SBP has long been associated with the development and progression of CKD.7, 8, 9, 10 Our findings initially also showed that SBP was strongly associated with ESRD in a linear and positive association, and this association was strengthened after adjusting for DBP in the dual model. However, in the dual model that adjusted for PP, all associations between increasing SBP categories and ESRD risk were radically inversed, and except for a borderline significance for SBP 150 to 159 mm Hg, the risk estimates and the P values for linear trend no longer reached statistical significance. Our findings suggest that the positive association between SBP and ESRD risk might be mediated through the widening of PP with increasing SBP, and this explains why the association became inverse and nonsignificant after controlling for PP in the same model. Conversely, an increase in DBP reduced PP, and this would explain the inverse association between DBP and ESRD risk after adjusting for SBP and the corresponding null association after adjusting for PP. Contrary to our findings, other studies have shown that SBP plays a more critical role in kidney function decline than PP. One such study included 2181 patients with isolated systolic hypertension and aged 65 years in the placebo arm of SHEP (Systolic Hypertension Elderly Program) and showed that SBP was a strong predictor of kidney function decline independent of PP after 5 years of follow‐up I.7 Another study involving 2772 CKD patients with a median follow‐up I of 4 years showed that PP was not associated with risk of ESRD after adjustment for SBP.9 However, although hypertension is a risk factor of CKD, secondary hypertension can also be a consequence of CKD. Therefore, the observed association between SBP and the deterioration in kidney function or development of ESRD after a relatively short period of follow‐up I in both studies could be explained by reverse causality. In a study that included 4365 older adults with an average age of 72.2 years in the United States, SBP had a stronger association with risk of rapid kidney function decline in comparison to PP.8 Nevertheless, results from the dual model that included both BP indexes showed that the risk estimate for SBP was still attenuated substantially after adjusting for PP, suggesting that the SBP‐related decline in kidney function was still partially explained by increased PP.
Another study that recruited 158 365 Chinese participants with an average age of 53 years in China and followed them for an average of 8.3 years for renal replacement therapy (n=121) or death from renal failure (n=259) showed that SBP was a strong predictor of ESRD independent of PP.10 In that study, glomerulonephritis was the most common underlying assigned cause of ESRD (35%), followed by diabetes mellitus (16%) and hypertension (9%). In contrast, in our cohort, diabetic nephropathy was the most common underlying cause for >60% of ESRD patients, and only 15% of ESRD cases were attributed to glomerulonephritis.24 We postulate that the heterogeneity between this study and our results may be explained by the difference in etiology of ESRD between the younger participants of this study in China and the older participants in our study. Because arterial stiffness is an early risk marker for diabetes mellitus,32 we hypothesize that PP, which is a measure of arterial stiffness, could be most strongly associated with risk of ESRD in populations where diabetes mellitus is the prevalent underlying cause. Diabetes mellitus has become the leading cause of ESRD in all developed and most developing countries, causing >50% of CKD and ESRD globally.3, 33 Consequently, our finding has implication for optimizing management of hypertension to reduce the risk of ESRD in the general population.
PP is a measure that has acquired a central position as an effective and technically simple surrogate measure of aortic stiffness that is dependent on cardiac output, the stiffness of elastic central arteries like the aorta, and wave reflection.34 The kidney is an organ subjected to high flow but low resistance; this renders it particularly sensitive to excessive pressure and flow pulsatility, and thus it is vulnerable to detrimental effects of aortic stiffness.35 As aortic stiffness increases, the kidneys are exposed to greater pressure fluctuations and wave reflection, resulting in excessive pressure and flow pulsatility into the microvascular beds of the kidneys that potentially lead to microvascular ischemia and renal tissue damage.35 Arterial stiffness and increased PP could lead to glomerular hypertrophy, hyperfiltration, and segmental glomerular sclerosis, which would eventually cause nephrosclerosis and fibrosis.16
In stratified analyses, we found more prominent ESRD risk associated with PP among participants aged <65 years compared with their older counterparts, suggesting that the impact of pulsatile stress on kidney function was more dramatic in younger participants. Our findings also showed a stronger risk associated with PP among participants who were not using antihypertensive medication compared with those who were. In addition to the protective effect on renal function by reducing BP, antihypertensive drugs may also have beneficial effects on intrarenal mechanisms directly.36 This hypothesis is supported by a meta‐analysis of 15 randomized controlled trials that have suggested antihypertensive medication could reduce arterial stiffness beyond the effect on BP control.37
The strengths of this study are its population‐based prospective design, large sample size, long follow‐up I, objective assessment of ESRD end points, and virtual completeness of follow‐up I by linkage with the nationwide Singapore Renal Registry.21 Several limitations should be acknowledged. First, we did not collect the information on types of antihypertensive medication. Because different classes of antihypertensive medicines could have different intrarenal effects besides BP reductions, we were unable to differentiate the renal protective effects of various antihypertensive medications on development of ERSD. Second, misclassification bias may exist because BP was a 1‐time measurement. However, because BP was measured prospectively before the onset of ESRD, the probability of participants being misclassified in the BP indexes could be expected to be similar between those who eventually developed ESRD and the rest of the cohort that did not have ESRD. Consequently, the misclassification bias is nondifferential, and such nondifferential misclassifications, in general, result in underestimation of the true association in epidemiologic studies. In addition, this single measurement of BP in this cohort has been found to be correlated with cardiovascular mortality in published studies.20, 38 Furthermore, some lifestyle factors collected at baseline but not at follow‐up I, such as diet, sleep, and physical activity, could have changed over time. However, we believe that any subsequent misclassification of these factors due to uncaptured changes would also be nondifferential in nature. Third, we cannot completely rule out residual confounding given the limitation of the observational study design. Fourth, we did not measure kidney function at recruitment, and thus we were unable to study the relation between PP and the rate of deterioration in renal function. Finally, our participants were middle‐aged and older adults of Chinese ancestry; therefore, our results may not be generalizable to the younger population or other ethnic groups.
Conclusions
PP is an independent and strong risk factor for the development of ESRD. Our findings provide novel evidence that supports the recommendations that the management of hypertension may require not only in reducing SBP and DBP but also maintaining PP within a low range for renal protection. Future studies are needed to evaluate potential therapies that target reduction of arterial stiffness to decrease the risk of ESRD.
Sources of Funding
This study was supported by the US National Institutes of Health(R01 CA144034 and UM1 CA182876).
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Participants in the Singapore Chinese Health Study
Table S2. Pearson Correlation Coefficients for Blood Pressure Indexes in the Singapore Chinese Health Study
Acknowledgments
We thank the Singapore Renal Registry for assistance with the identification of end‐state renal disease cases via database linkages. We are grateful to Siew‐Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Dr Renwei Wang for the maintenance of the cohort study database. We acknowledge the founding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu. W.‐P.K. and T.‐T.G. designed the study; W.‐P.K. and J.‐M.Y. supervised the data acquisition; T.‐T.G. and M.T. performed the statistical analysis; T.‐T.G. wrote the first draft, and W.‐P.K., T.H.J., M.T., and J.‐M.Y. participated in the interpretation of results and editing of the article. All authors approved the final draft, and W.‐P.K. has primary responsibility for its content.
(J Am Heart Assoc. 2019;8:e013282 DOI: 10.1161/JAHA.119.013282.)
References
- 1. Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population‐based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Nahas M. The global challenge of chronic kidney disease. Kidney Int. 2005;68:2918–2929. [DOI] [PubMed] [Google Scholar]
- 3. United States Renal Data System . 2018 USRDS annual data report: epidemiology of kidney disease in the United States. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garofalo C, Borrelli S, Pacilio M, Minutolo R, Chiodini P, De Nicola L, Conte G. Hypertension and prehypertension and prediction of development of decreased estimated GFR in the general population: a meta‐analysis of cohort studies. Am J Kidney Dis. 2016;67:89–97. [DOI] [PubMed] [Google Scholar]
- 5. Huang Y, Cai X, Zhang J, Mai W, Wang S, Hu Y, Ren H, Xu D. Prehypertension and Incidence of ESRD: a systematic review and meta‐analysis. Am J Kidney Dis. 2014;63:76–83. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Xia P, Xu L, Wang Y, Chen L. A meta‐analysis on prehypertension and chronic kidney disease. PLoS One. 2016;11:e0156575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol. 2002;13:2776–2782. [DOI] [PubMed] [Google Scholar]
- 8. Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, Newman AB, Siscovick DS, Peralta CA. Blood pressure components and decline in kidney function in community‐living older adults: the Cardiovascular Health Study. Am J Hypertens. 2013;26:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell EK, Gao L, Judd S, Glasser SP, McClellan W, Gutierrez OM, Safford M, Lackland DT, Warnock DG, Muntner P. Blood pressure indexes and end‐stage renal disease risk in adults with chronic kidney disease. Am J Hypertens. 2012;25:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, Duan X, Chen CS, Klag MJ, Whelton PK, He J. A population‐based, prospective study of blood pressure and risk for end‐stage renal disease in China. J Am Soc Nephrol. 2007;18:1928–1935. [DOI] [PubMed] [Google Scholar]
- 11. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, Cheung AK, Cohen D, Freedman BI, Hawfield AT, Killeen AA, Kimmel PL, Lash J, Papademetriou V, Rahman M, Rastogi A, Servilla K, Townsend RR, Wall B, Whelton PK. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med. 2017;167:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, O'Rourke M, Nichols WM. McDonald's Blood Flow in Arteries. London: CRC Press; 2011. [Google Scholar]
- 14. Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. [DOI] [PubMed] [Google Scholar]
- 15. Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study Circulation. 1999;100:354–360. [DOI] [PubMed] [Google Scholar]
- 16. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. [DOI] [PubMed] [Google Scholar]
- 17. Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens. 2015;28:561–569. [DOI] [PubMed] [Google Scholar]
- 18. Sedaghat S, Mattace‐Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015;10:2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. [DOI] [PubMed] [Google Scholar]
- 20. Talaei M, Hosseini N, Koh AS, Yuan JM, Koh WP. Association of “Elevated Blood Pressure” and “Stage 1 Hypertension” with cardiovascular mortality among an asian population. J Am Heart Assoc. 2018;7:e008911 DOI: 10.1161/JAHA.118.008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Registry of Diseases Office . Singapore Renal Registry Annual Registry Report 1999‐2013 (Preliminary). 2014;2018.
- 22. Jin A, Koh WP, Chow KY, Yuan JM, Jafar TH. Smoking and risk of kidney failure in the Singapore Chinese health study. PLoS One. 2013;8:e62962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, Koh WP. Red meat intake and risk of ESRD. J Am Soc Nephrol. 2017;28:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lew QJ, Jafar TH, Talaei M, Jin A, Chow KY, Yuan JM, Koh WP. Increased body mass index is a risk factor for end‐stage renal disease in the Chinese Singapore population. Kidney Int. 2017;92:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jafar TH, Jin A, Koh WP, Yuan JM, Chow KY. Physical activity and risk of end‐stage kidney disease in the Singapore Chinese Health Study. Nephrology (Carlton, Vic). 2015;20:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geng T‐T, Jafar TH, Yuan J‐M, Koh W‐P. Sleep duration and risk of end‐stage renal disease: the Singapore Chinese Health Study. Sleep Med. 2018;54:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geng TT, Jafar TH, Yuan JM, Koh WP. Long‐term incense use and the risk of end‐stage renal disease among Chinese in Singapore: the Singapore Chinese health study. BMC Nephrol. 2019;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 29. Verhave JC, Fesler P, du Cailar G, Ribstein J, Safar ME, Mimran A. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension. 2005;45:586–591. [DOI] [PubMed] [Google Scholar]
- 30. Peralta CA, Jacobs DR Jr, Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;59:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. [DOI] [PubMed] [Google Scholar]
- 32. Muhammad IF, Borne Y, Ostling G, Kennback C, Gottsater M, Persson M, Nilsson PM, Engstrom G. Arterial stiffness and incidence of diabetes: a population‐based cohort study. Diabetes Care. 2017;40:1739–1745. [DOI] [PubMed] [Google Scholar]
- 33. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. [DOI] [PubMed] [Google Scholar]
- 34. Bramwell JC, Hill AV. Velocity of transmission of the pulse‐wave and elasticity of arteries. Lancet. 1922;199:891–892. [Google Scholar]
- 35. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 36. Wenzel RR. Renal protection in hypertensive patients: selection of antihypertensive therapy. Drugs. 2005;65(suppl 2):29–39. [DOI] [PubMed] [Google Scholar]
- 37. Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, Boutouyrie P. Aortic stiffness is reduced beyond blood pressure lowering by short‐term and long‐term antihypertensive treatment: a meta‐analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–1042. [DOI] [PubMed] [Google Scholar]
- 38. Koh AS, Talaei M, Pan A, Wang R, Yuan JM, Koh WP. Systolic blood pressure and cardiovascular mortality in middle‐aged and elderly adults—the Singapore Chinese Health Study. Int J Cardiol. 2016;219:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Participants in the Singapore Chinese Health Study
Table S2. Pearson Correlation Coefficients for Blood Pressure Indexes in the Singapore Chinese Health Study


