Abstract
Background
The PROTECT‐AF (Watchman Left Atrial Appendage Closure Technology for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) trials demonstrated noninferiority of left atrial appendage closure (LAAC) to warfarin for the composite end point of stroke, systemic embolism, or cardiovascular death. This study aims to quantify the net clinical benefit (NCB) of LAAC versus warfarin, accounting for differences in clinical impact of different event types.
Methods and Results
We performed a post hoc analysis of the PROTECT‐AF and PREVAIL trials, which randomized atrial fibrillation patients to LAAC or warfarin in a 2:1 fashion. The trials enrolled patients in the United States and Europe between 2005 and 2012 with paroxysmal, persistent, or permanent atrial fibrillation and CHADS 2 risk scores ≥1. Relative to an index weight for death (1.0), events were assigned weights based on their disabling effect: (1) stroke event weights were based on modified Rankin scores in the base case analyses, and (2) major bleed (0.05) and pericardial effusion (0.05). NCB was calculated as the sum of weight‐adjusted events per 100 patient‐years. Among 1114 randomized subjects, the NCB of LAAC was 1.42% per year (95% CI 0.01–2.82, P=0.04) and a relative risk of 0.74 (95% CI 0.56–1.00). NCB point estimates favored warfarin early in follow‐up, but trended in favor of LAAC after 1 to 2 years. The benefit of LAAC was preserved across subgroups, with particular benefit observed in the subgroup of prior stroke and without diabetes mellitus.
Conclusions
This analysis demonstrates long‐term NCB of LAAC with Watchman over warfarin therapy, as the upfront risk of periprocedural events is counterbalanced over time by reduced bleeding events and mortality.
Clinical Trial Registration
UR: http://www.clinicaltrials.gov. Unique identifiers: NCT01182441 and NCT00129545.
Keywords: anticoagulation, atrial fibrillation, left atrial appendage
Subject Categories: Atrial Fibrillation, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
This study compared the net clinical benefit of left atrial appendage closure versus warfarin therapy using the composite cardiovascular end point of stroke, systemic embolism, or cardiovascular death, and accounting for the clinical impact of events based on the severity of postevent disability.
What Are the Clinical Implications?
Left atrial appendage closure had a statistically significant net clinical benefit over warfarin of 1.42% events per year.
In the first year after device implantation, there was a nonsignificant benefit of warfarin therapy because of periprocedural complications of left atrial appendage closure. However, the balance shifted between 1 to 2 years follow‐up in favor of left atrial appendage closure.
Introduction
Anticoagulation with warfarin, and more recently nonwarfarin oral anticoagulants (NOACs), has been the mainstay of therapy to prevent stroke in patients with atrial fibrillation (AF).1, 2, 3, 4 However, long‐term warfarin therapy confers an increased risk of major bleeding events including intracranial hemorrhage.5 In 2 randomized trials, PROTECT‐AF (Watchman Left Atrial Appendage Closure Technology for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy), left atrial appendage closure (LAAC) was compared with warfarin for stroke prevention and, in the most recent meta‐analysis of these studies, had similar overall risk for the primary composite end point of stroke, systemic embolism, and cardiac death.6, 7, 8 But the relative rates of the primary stroke subtypes favored opposite study arms: a trend toward higher risk of ischemic stroke in the LAAC arm was counterbalanced by a significantly reduced risk of hemorrhagic stroke.8
Clinicians are often in situations where they need to choose between therapies that result in multiple heterogeneous consequences. Net clinical benefit (NCB) analyses can be helpful to provide a more clinically relevant assessment of treatments by synthesizing multiple effects into a single scalar outcome. In a previous net clinical benefit (NCB) analysis, we used data from the PROTECT‐AF study and the CAP (Continued Access Protocol) device registry, specific weights were applied to account for disability caused by different events and demonstrated that LAAC conferred clinical benefit of 1.73% to 4.97% per year.9 However, at the time of that analysis, the PROTECT‐AF study was only approximately midway in its planned 5‐year follow‐up (≈28 months mean follow‐up); now, in addition to PROTECT‐AF completing 5‐year follow‐up, PREVAIL is also available (also with 5‐year follow‐up).
In this present analysis, we sought to provide clinical context about the long‐term impact of LAAC by incorporating patient‐level 5‐year data from both randomized trials of Watchman in the NCB analysis. In addition, we performed NCB analyses using alternative approaches to account for disability from stroke. Our goal was to provide a comprehensive estimate of the NCB of LAAC compared with warfarin therapy over time and to identify patient characteristics that maximize NCB.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study Design
The PROTECT‐AF trial enrolled 707 patients with nonvalvular AF at 59 sites in the United States and Europe between February 2005 and June 2008.7, 10 Patients aged 18 years or older with paroxysmal, persistent, or permanent AF and CHADS2 risk scores ≥1 were eligible. Exclusions included the following: absolute contraindications to warfarin, comorbidities other than AF requiring anticoagulation, LAA thrombus, patent foramen ovale with atrial septal aneurysm and right‐to‐left shunt, mobile aortic atheroma, and symptomatic carotid artery disease. In PREVAIL, 407 patients with nonvalvular AF were enrolled at 50 sites in the United States between November 2010 and July 2012. Eligibility criteria included CHADS2 score ≥2 or a CHADS2 score ≥1 with 1 of the following higher risk characteristics: female ≥75 years of age, baseline ejection fraction ≥30% but <35%, 65 to 74 years of age with either diabetes mellitus or coronary artery disease, and ≥65 years of age with heart failure. Exclusion criteria were similar to the PROTECT‐AF trial, except patients in whom clopidogrel therapy was indicated were excluded because of the potential confounding influence of this drug on efficacy outcome. Both studies were approved by the local institutional review boards and patients signed informed consent before enrollment.
Patients in both trials were randomly assigned to the intervention or control groups in a 2:1 ratio. After implantation, patients were treated with warfarin for 45 days to allow endothelialization of the device, followed by clopidogrel (75 mg daily) plus aspirin (81–325 mg daily) until completion of the 6‐month follow‐up visit, and aspirin alone thereafter. The control group was assigned chronic warfarin therapy with a target international normalized ratio of 2.5, range 2.0 to 3.0.
Outcome Assessment
The end points in this NCB analysis included all death events irrespective of cause (DE), ischemic stroke (IS), intracranial hemorrhage (ICH), major extracranial bleeding (MB), and the major procedural complication, pericardial effusion (PE). Assessment of the outcomes was identical in both studies and an independent Clinical Events Committee adjudicated all events. MB was defined as those bleeds that required transfusion of ≥2 units of packed red blood cells or surgical intervention. Patients underwent screening for PE by transthoracic/transesophageal echocardiography before and within 2 days after device implantation. PE was considered an end point event when it resulted either in hemodynamic compromise or drainage and included cardiac perforation causing cardiac tamponade.
IS was defined as the sudden onset of a focal neurological deficit in the distribution of a single brain artery with symptoms and/or signs persisting ≥24 hours or when ≤24 hours if accompanied by the evidence of tissue loss without hemorrhage based on computed tomography or magnetic resonance brain imaging. Diagnosis of ICH was based on the sudden onset of neurological deficit with computed tomography or magnetic resonance evidence of hemorrhage. Stroke events were assessed using the National Institutes of Health Stroke Scale at set intervals and within 48 hours after the onset of symptoms suggestive of stroke or transient ischemic attack. The modified Rankin score (mRS) and the Barthel index were measured at 6‐, 9‐, and 18‐month telephone follow‐up contacts, at all clinic visits, and within 90 days of stroke or transient ischemic attack. Patients were referred for neurological evaluation if there was an increase in the National Institutes of Health Stroke Scale score of ≥2 points, increase in the mRS ≥1 point, or increase in Barthel index ≥15 points. Per‐protocol patients were followed for a maximum duration of 5 years.
Net Clinical Benefit Calculation
The NCB analysis of LAAC versus warfarin used weights for each end point reflecting the severity of postevent disability relative to death. We defined the NCB of LAAC as the sum of the differences between the annualized rates of DE, ICH, IS, MB, and PE occurring in the LAAC versus the warfarin group, with weights for each event reflecting the severity of functional impact relative to DE (unity). To avoid double counting of events, for patients who experienced more than 1 event, the event with the highest weight was used. We used 2 alternate methods to calculate NCB that reflected different weighting schemes. In the base case, subject‐level stroke severity was incorporated into the model by utilizing the mRS values after IS or ICH in the following equation to calculate NCB:
where i=0 to 6, Weight0=0, Weight1=0.046 Weight2=0.212 Weight3=0.331 Weight4=0.652 Weight5=0.944 Weight6=1.11
Briefly, the disability weight (“Weight”) for each possible mRS score from 0 to 6 was derived from a World Health Organization Global Burden of Disease Project study of disability according to mRS that used a Delphi process among a 9‐member panel of stroke experts.11 The weights for death, PE, and MB were fixed at 1.0 (reference), 0.05, and 0.05, respectively, and were consistent with our previous analysis.9 The value of 0.05 represents a 5% decrement from a year of life with perfect health. We doubled and tripled the weights assigned to PE and MB in sensitivity analyses.
As a point of comparison with our prior NCB analysis based on the PROTECT‐AF trial, we also performed a sensitivity analysis using the equation from the previous study:
The difference compared with our base case analysis was that fixed weights were used for ICH (0.60) and IS (0.20)—consistent with the NCB analysis of the ACTIVE (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events Trials) that showed the adjusted hazard ratio for death after hemorrhagic stroke was 3.08 compared with ischemic stroke.12 Again, MB requiring transfusion and PEs requiring intervention were both assigned impacts of 0.05, consistent with our previous analysis.9 Lastly, we artificially lowered the incidence of PE because the implanters’ experience at the time of the PROTECT‐AF and PREVAIL trials was limited, since most implanters were still early in their learning/experience curve. The PE incidence for this sensitivity analysis was lowered from 3% to 1%, in line with the US postapproval study.13
Statistical Analysis
Baseline characteristics were presented as mean±SD or median (25th, 75th) depending on distribution, and dichotomous variables as number and percentage. NCB in weight‐adjusted events per 100 person‐years of follow‐up was presented as absolute risk differences and compared between the LAAC and warfarin arms using the Mantel–Haenszel χ2 test. NCB was additionally estimated for subgroups based on age, sex, prior ischemic stroke, hypertension, diabetes mellitus, heart failure, and CHADS2 score. Statistical significance was accepted at the 95% CI (2‐sided P≤0.05) without adjustment for multiple comparisons. Analyses were performed using the R and RStudio software, version 3.4.4 (R Foundation, Vienna, Austria).14
Results
Baseline Characteristics
Patients were enrolled across 79 hospitals in the United States and Europe between 2005 and 2012 (Table S1). The pooled cohort consisted of 1114 patients, of whom 732 were randomized to LAAC with Watchman and 382 were treated with warfarin (Table 1). The baseline characteristics were well balanced with a mean age of 73±8 years and 30% of the subjects were female. The median CHA2DS2‐VASc score was 2 (2, 3) and 252 (23%) patients had a stroke or transient ischemic attack before enrollment. The most common AF subtype was paroxysmal (45%), followed by permanent (28%) and persistent (24%). Patients in both trials were followed for a mean duration of 48 months adding up to a total 4343 patient years.
Table 1.
Baseline Characteristics
| Warfarin | LAAC | P Value | |
|---|---|---|---|
| N | 382 | 732 | |
| Age, y, mean (SD) | 73.47 (8.60) | 72.56 (8.38) | 0.09 |
| CHADS2 Score, median (25th, 75th) | 2.00 [2.00, 3.00] | 2.00 [1.00, 3.00] | 0.06 |
| Risk factors | |||
| Female, n (%) | 108 (28.3) | 224 (30.6) | 0.46 |
| Age >75 y, n (%) | 192 (50.3) | 330 (45.1) | 0.11 |
| Congestive heart failure, n (%) | 98 (25.7) | 187 (25.5) | 1 |
| Hypertension, n (%) | 354 (92.7) | 653 (89.2) | 0.08 |
| Prior TIA or stroke, n (%) | 90 (23.6) | 162 (22.1) | 0.64 |
| Diabetes mellitus, n (%) | 113 (29.6) | 204 (27.9) | 0.60 |
| Coronary artery disease, n (%) | 192 (50.3) | 325 (44.4) | 0.08 |
| Prior gastrointestinal bleed, n (%) | 41 (10.7) | 73 (10.0) | 0.77 |
| AF pattern | |||
| Paroxysmal | 170 (44.5) | 331 (45.2) | 0.82 |
| Persistent | 89 (23.3) | 182 (24.9) | 0.56 |
| Permanent | 115 (30.1) | 202 (27.6) | 0.38 |
| Unknown | 3 (0.8) | 10 (1.4) | 0.56 |
| Paced | 5 (1.3) | 7 (1.0) | 0.56 |
Baseline characteristics of the pooled cohorts of the PROTECT‐AF and PREVAIL studies. AF indicates atrial fibrillation; LAAC, left atrial appendage closure; TIA, transient ischemic attack.
Net Clinical Benefit
Table 2 presents the NCB estimates over the course of follow‐up using the combined PROTECT‐AF and PREVAIL data. In the base case analysis, which incorporated mRS‐based stroke severity and fixed weights for MB and PE at 0.05, patients in the LAAC arm had 4.11 weight‐adjusted events per 100 patient‐years during the overall follow‐up period, versus 5.53 weight‐adjusted events in the warfarin arm. This resulted in a NCB of 1.42% for LAAC over warfarin (95% CI 0.01–2.82%, P=0.04) and a relative risk of 0.74 (95% CI 0.56–1.00).
Table 2.
Clinical Benefit by mRS Stroke Score and Varying Impact Weights for PE and MB
| Scenario | Events Watchman | Events Warfarin | Absolute Risk Differencea (95% CI) | Incidence Rate Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Base case (mRS, MB/PE=0.05) | 4.11 | 5.53 | 1.42 (0.01, 2.82) | 0.74 (0.56, 1.00) | 0.04 |
| mRS, MB/PE=0.10 | 4.21 | 5.59 | 1.38 (0.04, 2.80) | 0.75 (0.57, 1.01) | 0.05 |
| mRS, MB/PE=0.15 | 4.32 | 5.73 | 1.41 (0.02, 2.85) | 0.75 (0.57, 1.00) | 0.05 |
MB indicates major bleed; mRS, modified Rankin Scale; PE, pericardial effusion.
Difference in rate of events per 100 patient‐years in Watchman group vs warfarin group.
Sensitivity Analyses
As a sensitivity analysis, when the weights of MB and PE were increased to 0.10 there were 4.21 weight‐adjusted events per 100 patient‐years in the LAAC arm and 5.59 in the warfarin arm; thus, the NCB continued to favor LAAC at 1.38% (0.04–2.80) and RR 0.75 (0.57–1.01), P=0.05. When the weights for MB and PE were further increased to 0.15, there were 4.32 weight‐adjusted events per 100 patient‐years in the LAAC arm and 5.73 in the warfarin arm, again resulting in a NCB of 1.41 (0.02–2.85) and RR of 0.75 (0.57–1.00), P=0.05.
Instead of a graded scale for stroke based on mRS scores, when we used fixed weights for IS (weight of 0.2) and ICH (weight of 0.6) events as per our prior NCB analysis, the results remained similar to the primary analysis (Figure 1): there were 4.18 weight‐adjusted events per 100 patient‐years in the LAAC arm and 5.66 in the warfarin arm, resulting in a NCB of 1.48% for LAAC over warfarin (95% CI 0.06–2.90%, P=0.04) and a relative risk of 0.74 (95% CI 0.56–0.99). When the weights for IS and ICH were changed to 0.10 and 0.30, respectively, the NCB remained positive at 1.45% (95% CI 0.05–2.85%, P=0.04) with 4.07 weight‐adjusted events per 100 patient‐years in the LAAC arm and 5.53 in the warfarin arm. The lowered incidence rate of PE from 3% to 1% resulted in a smaller advantage of warfarin over LAAC at 90 days follow‐up of 3.89% versus 4.47% original data (Figure S1), with a similar overall NCB for LAAC during the course of complete 5‐year follow‐up compared with the base case analysis.
Figure 1.
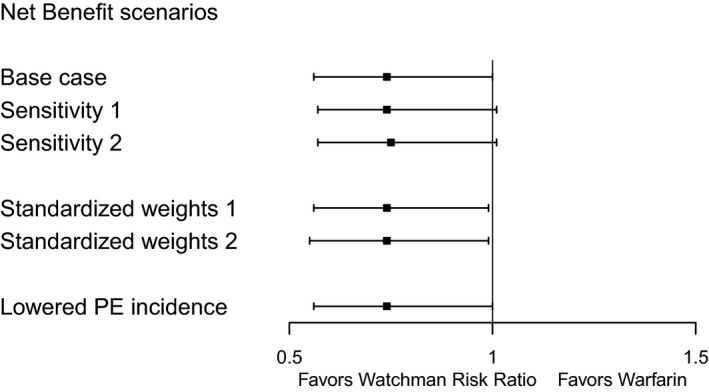
Net clinical benefit by different weight scenarios. Base case 1: mRS score for ischemic and hemorrhagic stroke are used and fixed weights for major bleed and PE of 0.05. Sensitivity 1: mRS score for ischemic and hemorrhagic stroke are used and fixed weights for major bleed and pericardial effusion of 0.10. Sensitivity 2: mRS score for ischemic and hemorrhagic stroke are used and fixed weights for major bleed and pericardial effusion of 0.15. Standardized weights 1: fixed weights for ischemic of 0.1, for hemorrhagic stroke of 0.3 and for major bleed and PE of 0.05. Standardized weights 2: fixed weights for ischemic of 0.2, for hemorrhagic stroke of 0.6, and for major bleed and pericardial effusion of 0.05. Lowered PE incidence: scenario where the PE incidence is artificially lowered to 1%. mRS score for ischemic and hemorrhagic stroke are used and fixed weights for major bleed and PE of 0.05. mRS indicates modified Rankin Scale; PE, pericardial effusion.
NCB Over Time
In the base case, the NCB during early follow‐up was nonsignificantly in favor of the warfarin arm, reflecting periprocedural events associated with LAAC (Figure 1). Between 1 and 2 years follow‐up, the NCB was neutral, but then shifted toward LAAC during long‐term follow‐up (Figure 2). In this clinical benefit analysis, death events were the primary driver of the overall result because they accounted for 179 (93%) out of 192 total weight‐adjusted events. This time dependence of the NCB was preserved in the various sensitivity analyses: varying the weights of MB and PE, and using fixed weights for IS and ICH (Figures S2 and S3).
Figure 2.
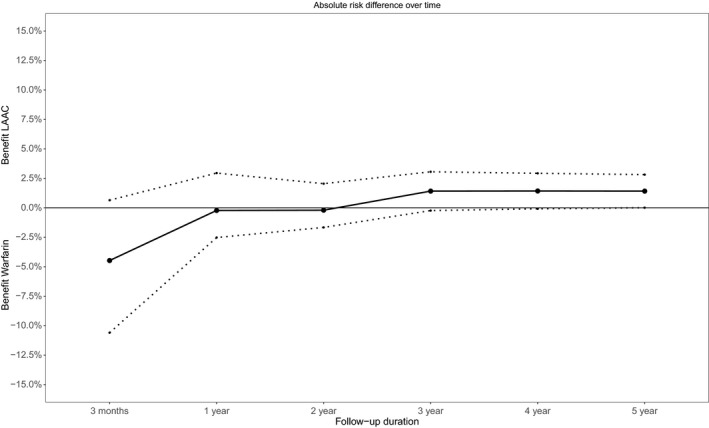
Net clinical benefit of LAAC compared with warfarin therapy over time. The figure presents the absolute risk difference at different time points during follow‐up. Below zero reflects a benefit of warfarin, whereas above zero reflects a benefit of LAAC. The dotted lines reflect the 95% CI. LAAC indicates left atrial appendage closure.
Subgroups
Although the 95% CIs were wide, the NCB of LAAC compared with warfarin was positive across subgroups (Figure 3) including both men and women. Patients with a history of stroke or transient ischemic attack comprised the subgroup with the largest NCB from LAAC, with a point estimate of the absolute risk reduction of 4.25% (95% CI 0.80–7.91%, P=0.02).
Figure 3.
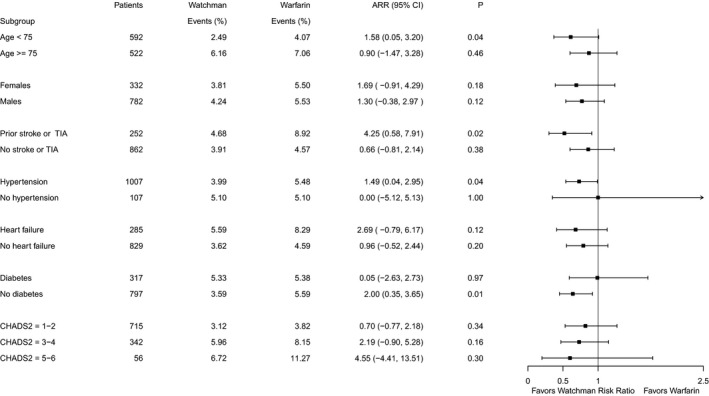
Forrest plot of subgroups. ARR, absolute risk reduction, events presented per 100 patient‐years follow‐up; TIA, transient ischemic attack.
Discussion
Main Findings
Through this NCB analysis, we incorporated the differential clinical impact of heterogeneous events to assess the overall effect of LAAC compared with warfarin therapy. This analysis utilizes 5‐year randomized trial data involving LAAC with the Watchman device and indicates that, in the long‐term, LAAC is likely to provide positive NCB compared with warfarin. Early on in follow‐up, there is a nonsignificant expected negative effect of LAAC because of periprocedural complications, but the balance shifts between 1 to 2 years follow‐up in favor of LAAC with Watchman.
Clinical Benefit Analysis
Clinical trials commonly use combined end points, recognizing that there are often more than 1 clinically relevant outcome type, while at the same time increasing the statistical power. However, it is widely acknowledged that the individual components of combined end points are clinically not equally important. In this NCB analysis, events were weighted based on the disability level after each event, recognizing that clinical importance of a PE resolved by drainage is different from a stroke resulting in severe disability or death. In order to reduce the subjectivity of assigning weights, stroke events were assigned weights based on stroke severity—that is, the difference between pre‐ and poststroke disability according to mRS scores.11 PE and MB were assigned relatively low weights (both 0.05) because of their transient nature and the fact that if they result in death, this would be captured in the death end point. All told, death events were the primary driver of this NCB analysis.
Periprocedural Complications
As shown in Figure 2, there is an initial increase in events during early follow‐up caused by periprocedural complications of LAAC, while there were few events immediately after initiation of long‐term warfarin therapy. Both trials represent the earliest experience of implanters with LAAC since they were Food and Drug Administration approval studies. But as expected, with additional experience, implanters have become more adept with the LAAC implant procedure, and several studies such as the EWOLUTION (Evaluating Real‐Life Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology) registry have demonstrated markedly reduced periprocedural complication rates, including a pericardial tamponade rate <1%.15 It is important to recognize that this reduction in periprocedural complications will result in clinical equivalence and benefit of LAAC earlier during follow‐up.
Long‐Term Outcomes
The concept of LAAC closure provides benefit during long‐term follow‐up for several reasons. There is no associated long‐term bleeding risk. It is well recognized that intracranial bleeding is associated with significantly greater morbidity than ischemic events. Also, there is no interaction with other drugs or therapeutic range that needs to be maintained.
Patient behavior and the ability to remain fully adherent to their medication regimen are important limitations of any long‐term preventive strategy. While patients in the NOAC trials were selected based on their ability to adhere to their drug regimen, the time in therapeutic range in the warfarin arm varied between 60% and 70%.16, 17, 18, 19 It has been shown in many studies that adherence decreases both over time and with each drug added to the medication regimen.20, 21, 22, 23 Given the fact that patients with AF often have a life expectancy of many years at the time of diagnosis and comorbidities such as hypertension, heart failure, and diabetes mellitus requiring drugs as well, long‐term adherence to warfarin is often suboptimal in clinical practice. The fact that the chronic efficacy of LAAC is not dependent on adherence issues may be a plausible explanation as to why the clinical benefit of LAAC continues to increase over time.
Limitations
Limitations of our analysis include the lack of data about NOACs. After the advent of the PROTECT‐AF and PREVAIL studies, NOACs have been widely adopted because of the reduced risk of intracranial hemorrhage and much simpler dosing. This rendered warfarin a suboptimal comparator to inform clinicians in many patients with AF. The relative efficacy of LAAC versus NOACs is currently unknown, and is the subject of ongoing clinical trials including PRAGUE‐17 (Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation) (NCT# 02426944), CLOSURE‐AF (Left Atrial Appendage CLOSURE in Patients With Atrial Fibrillation Compared to Medical Therapy) (NCT# 03463317), Occlusion‐AF (Left Atrial Appendage Occlusion Versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation) (NCT# 03642509) and STROKECLOSE (Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage) (NCT# 02830152), but these trials are somewhat limited by sample size.
A second limitation is that we did not account for uncertainty in the weights used for the events in our analysis. We did perform sensitivity analyses for the assigned weights and attempted to account for stroke based on actual measurements of disability associated with IS and ICH. These variations in the inputs for the NCB analysis did not materially change the conclusions.
Lastly, the implanters’ experience at the time of the PROTECT‐AF and PREVAIL trials was limited, since most implanters were still in their learning phase. This assumption is confirmed by the fact that since the PROTECT‐AF and PREVAIL trials, the periprocedural complication rate of Watchman implantation has come down substantially in registries such as the US postapproval registry and EWOLUTION.13, 15 In the current clinical benefit analysis, this would translate to smaller difference in the absolute difference in events rate between both groups and reversal of the clinical benefit earlier during follow‐up.
Conclusions
This clinical benefit analysis of the PROTECT‐AF and the PREVAIL studies demonstrates that when weights are used for each end point reflecting the severity of postevent disability, there is a significant long‐term benefit of LAAC with Watchman over warfarin therapy. However, in the first year after device implantation, there is a nonsignificant benefit of long‐term warfarin therapy because of periprocedural complications.
Sources of Funding
The PROTECT‐AF and PREVAIL clinical trials were funded by Boston Scientific, the manufacturer of the Watchman LAA closure device used in this trial (which acquired the company that initiated these trials, Atritech). Open access publication fee was covered by Boston Scientific. Role of the sponsors: The sponsor had no role in this study, except for providing the data.
Disclosures
Reddy has received research grant support from and has been a consultant to Boston Scientific, the manufacturer of the Watchman device. In addition, he has conflicts with manufacturers of other LAAC devices: grant support and consultant to Abbott and Biosense‐Webster, and equity ownership in Surecor Inc; Brouwer has received research grant support from Boston Scientific, the manufacturer of the Watchman device. Halperin has received consulting fees for advisory activities and studies involving anticoagulant drugs: Bayer HealthCare, Boehringer Ingelheim, Ortho‐McNeil‐Janssen Pharmaceuticals, and Medtronic. The remaining authors have no disclosures to report.
Supporting information
Table S1. List of Participating Hospitals in the Protect‐AF and PREVAIL Studies
Figure S1. Net clinical benefit of LAAC compared with warfarin therapy over time in the artificially lower PE incidence scenario.
Figure S2. Absolute risk difference using mRS weights for ischemic and hemorrhagic stroke and fixed weights of 0.10 for major bleed and pericardial effusion events.
Figure S3. Absolute risk difference using mRS weights for ischemic and hemorrhagic stroke and fixed weights of 0.15 for major bleed and pericardial effusion events.
Acknowledgments
The investigators acknowledge and thank the PROTECT‐AF and PREVAIL investigators and Boston Scientific Corp for conducting the trials and making the data available.
(J Am Heart Assoc. 2019;8:e013525 DOI: 10.1161/JAHA.119.013525.)
References
- 1. Miller PSJ, Andersson FL, Kalra L. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke. 2005;36:360–366. [DOI] [PubMed] [Google Scholar]
- 2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Esquivias GB, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart RG, Halperin JL, Pearce LA, Anderson DC, Kronmal RA, McBride R, Nasco E, Sherman DG, Talbert RL, Marler JR, Stroke Prevention in Atrial Fibrillation Investigators. Lessons from the Stroke Prevention in Atrial Fibrillation trials. Ann Intern Med. 2003;138:831–8. [DOI] [PubMed] [Google Scholar]
- 6. Holmes DR, Doshi SK, Kar S, Price MJ, Sanchez JM, Sievert H, Valderrabano M, Reddy VY. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient‐level meta‐analysis. J Am Coll Cardiol. 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 7. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, Holmes DR. 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 9. Gangireddy SR, Halperin JL, Fuster V, Reddy VY. Percutaneous left atrial appendage closure for stroke prevention in patients with atrial fibrillation: an assessment of net clinical benefit. Eur Heart J. 2012;33:2700–2708. [DOI] [PubMed] [Google Scholar]
- 10. Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 11. Hong K‐S, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke. 2009;40:3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connolly SJ, Eikelboom JW, Ng J, Hirsh J, Yusuf S, Pogue J, de Caterina R, Hohnloser S, Hart RG; ACTIVE (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events) Steering Committee and Investigators . Net clinical benefit of adding clopidogrel to aspirin therapy in patients with atrial fibrillation for whom vitamin K antagonists. Ann Intern Med. 2011;155:579–586. [DOI] [PubMed] [Google Scholar]
- 13. Reddy VY, Gibson DN, Kar S, O'Neill W, Doshi SK, Horton RP, Buchbinder M, Gordon NT, Holmes DR. Post‐approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–261. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 15. Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, Gori T, Meincke F, Protopopov AV, Betts T, Foley D, Sievert H, Mazzone P, De Potter T, Vireca E, Stein K, Bergmann MW. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1‐year follow‐up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. [DOI] [PubMed] [Google Scholar]
- 16. Giugliano R, Ruff C, Braunwald E, Murphy S, Wiviott S, Halperin J, Waldo A, Ezekowitz M, Weitz J, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher Y, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 17. Granger C, Alexander J, McMurray J, Lopes R, Hylek E, Hanna M, Al‐Khalidi H. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 18. Patel M, Mahaffey K, Garg J, Pan G, Singer D, Hacke W, Breithardt G, Halperin J, Hankey G, Piccini J, Becker R, Nessel C, Paolini J, Berkowitz S. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 19. Connolly S, Ezekowitz M, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly P, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 20. Yao X, Abraham NS, Caleb Alexander G, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074 DOI: 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chapman RH. Predictors of adherence with antihypertensive and lipid‐lowering therapy. Arch Intern Med. 2005;165:1147. [DOI] [PubMed] [Google Scholar]
- 22. Castellano JM, Bueno H, Fuster V. The cardiovascular polypill: clinical data and ongoing studies. Int J Cardiol. 2015;201(suppl):S8–S14. [DOI] [PubMed] [Google Scholar]
- 23. Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, Grobbee DE, Bots ML, Reddy KS, Cidambi R, Bompoint S, Billot L, Rodgers A. Effects of a fixed‐dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of Participating Hospitals in the Protect‐AF and PREVAIL Studies
Figure S1. Net clinical benefit of LAAC compared with warfarin therapy over time in the artificially lower PE incidence scenario.
Figure S2. Absolute risk difference using mRS weights for ischemic and hemorrhagic stroke and fixed weights of 0.10 for major bleed and pericardial effusion events.
Figure S3. Absolute risk difference using mRS weights for ischemic and hemorrhagic stroke and fixed weights of 0.15 for major bleed and pericardial effusion events.


