Summary
GacS/GacA is a conserved two‐component system that functions as a master regulator of virulence‐associated traits in many bacterial pathogens, including Pseudomonas spp., that collectively infect both plant and animal hosts. Among many GacS/GacA‐regulated traits, type III secretion of effector proteins into host cells plays a critical role in bacterial virulence. In the opportunistic plant and animal pathogen Pseudomonas aeruginosa, GacS/GacA negatively regulates the expression of type III secretion system (T3SS)‐encoding genes. However, in the plant pathogenic bacterium Pseudomonas syringae, strain‐to‐strain variation exists in the requirement of GacS/GacA for T3SS deployment, and this variability has limited the development of predictive models of how GacS/GacA functions in this species. In this work we re‐evaluated the function of GacA in P. syringae pv. tomato DC3000. Contrary to previous reports, we discovered that GacA negatively regulates the expression of T3SS genes in DC3000, and that GacA is not required for DC3000 virulence inside Arabidopsis leaf tissue. However, our results show that GacA is required for full virulence of leaf surface‐inoculated bacteria. These data significantly revise current understanding of GacS/GacA in regulating P. syringae virulence.
Keywords: GacS/GacA, plant–microbe interactions, Pseudomonas syringae, two‐component regulatory systems, type III secretion, virulence regulation
Many bacterial pathogens can survive outside of their hosts yet must transition into more virulent forms during initial stages of infection. Pseudomonas syringae is a Gram‐negative plant pathogenic bacterium that infects the interior of leaf tissue by swimming through natural openings or wounds in the leaf surface (Abramovitch et al., 2006; Xin et al., 2018). Once inside, P syringae switches from motile to sessile and begins secreting immunity‐suppressing effectors into host cells via a type III secretion system (T3SS), a lifestyle switch necessary for P. syringae to cause disease (Abramovitch et al., 2006; Schreiber and Desveaux, 2011; Xin et al., 2018). How motility and T3SS deployment, as well as other virulence‐associated traits, are coordinately regulated in P. syringae to mediate this lifestyle transition is poorly understood.
GacS/GacA is a highly conserved two‐component system in γ‐proteobacteria, and in many pathogenic species is a master regulator of virulence‐associated traits (Heeb and Haas, 2001). A role for GacS/GacA in regulating bacterial virulence was first established through studies of P. syringae over 25 years ago (Willis et al., 1990), and GacS/GacA homologues have since been shown to regulate the virulence of many pathogens, including Vibrio cholera (Wong et al., 1998), Salmonella typhimurium (Johnston et al., 1996), and the opportunistic plant and animal pathogen Pseudomonas aeruginosa (Heeb and Haas, 2001; Rahme et al., 2019). In P.aeruginosa, GacS/GacA negatively regulates T3SS deployment and motility, and positively regulates biofilm formation and type VI secretion, among other factors (Valentini et al., 2018). In P. syringae, GacS/GacA functions as a master regulator of multiple virulence traits, including T3SS deployment, toxin production and motility (Chatterjee et al., 2003; Heeb and Haas, 2001; Mole et al., 2007). However, significant strain‐to‐strain differences in phenotypes of gacS − and gacA − mutants have been reported. For instance, a gacS − mutant of the bean pathogen P. syringae pv. syringae B728a had decreased field fitness yet had sufficient levels of type III secretion to trigger a host defence response and was fully virulent in laboratory infections of host plants (Hirano et al., 1997; Willis et al., 1990). In contrast, a gacA − mutant of P. syringae pv. syringae DC3000, a pathogen of Arabidopsis and tomato, had decreased T3SS gene expression and was less virulent on host plants (Chatterjee et al., 2003; Vargas et al., 2013). Because DC3000 is one of the most intensively studied plant pathogens, these results largely established GacS/GacA as a positive regulator of T3SS deployment in P. syringae (Brencic and Winans, 2005; Mole et al., 2007; Tang et al., 2006). However, the apparently conflicting modes of GacS/GacA‐T3SS regulation between DC3000, B728a (Hirano et al., 1997; Willis et al., 1990; Yu et al., 2014) and other strains (Marutani et al., 2008) suggest that GacS/GacA functions have diversified at the level of individual P. syringae isolates, and this variability has complicated efforts to establish predictive species‐level models of how GacS/GacA regulates P. syringae virulence.
Here we re‐examined the virulence‐associated phenotypes of strain AC811, a DC3000 Tn5::gacA mutant (Chatterjee et al., 2003; Ferreiro et al., 2018; Vargas et al., 2013). Contrary to previous reports (Chatterjee et al., 2003; Vargas et al., 2013), we demonstrate that AC811 hyper‐expresses T3SS‐encoding genes in culture and during plant infection. We further show that GacA is dispensable for DC3000 virulence in the leaf interior but is required for virulence of leaf surface‐inoculated bacteria, most likely due to motility defects caused by loss of gacA. Together, these results significantly revise current understanding of how GacS/GacA functions to regulate type III secretion by P. syringae (Brencic and Winans, 2005; Lapouge et al., 2008; MacLean and Studholme, 2010; Mole et al., 2007; Tang et al., 2006). These data provide a solid framework for modelling and testing future hypotheses of how GacS/GacA regulates lifestyle switching of P. syringae during plant infection.
We initially assessed whether GacA is required for DC3000 to respond to plant‐derived metabolic signals that induce the expression of T3SS‐encoding genes in DC3000 (Anderson et al., 2014). To investigate, we introduced a transcriptional reporter consisting of the promoter of T3SS effector gene avrPto fused to green fluorescent protein (gfp) into DC3000 and AC811. We then cultured these reporter strains in minimal medium supplemented with T3SS‐inducing fructose and citric acid (Anderson et al., 2014; Huynh et al., 1989). AC811 expressed higher levels of avrPto in response to the bioactive metabolites (Fig. 1A). Increased avrPto expression occurred in metabolite‐treated AC811 cultures inoculated from King’s B (KB) agar (Fig. 1A) or KB broth cultures (Fig. S1). We confirmed these results by immunoblot detection of AvrPto produced by expression of endogenous avrPto (Fig. 1B) and by qRT‐PCR of avrPto mRNA levels (Fig. S2A). Over‐expression of gacA in AC811 fully restored avrPto expression back to DC3000 levels (Fig. S3). We also used allelic exchange to delete gacA in DC3000 (Fig. S4) and measured similar heightened avrPto expression in the resulting ΔgacA‐1 mutant strain (Fig. 1C), as well as significantly increased transcript levels of the T3SS master regulator hrpL (Fig. S2A).
Figure 1.
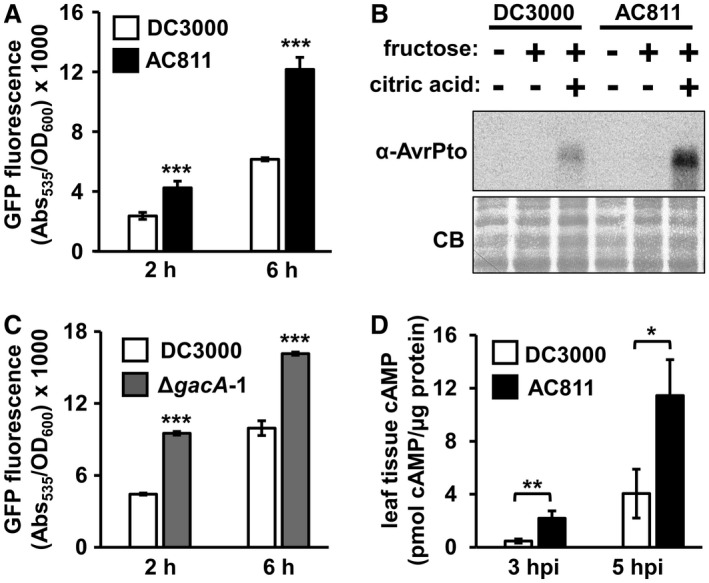
GacA negatively regulates type III secretion in Pseudomonas syringae pv. tomato DC3000. (A) GFP fluorescence of DC3000 and AC811 avrPtopromoter:gfp reporter strains. Bacteria were incubated in minimal medium (MM) with 10 mM fructose and 400 µM citric acid. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains, n = 4. Data are representative of three independent experiments. Asterisks denote significant difference between strains based on t‐test, ***P < 0.001. (B) AvrPto levels in DC3000 and AC811 incubated in MM supplemented with 200 µM citric acid and/or 10 mM fructose as indicated. Upper panel is immunoblot detection of AvrPto in treated bacteria after 5 h. Lower panel is Coomassie Brilliant Blue (CB) staining of blot as a loading control. (C) GFP fluorescence of DC3000 and ΔgacA‐1 carrying avrPto promoter:gfp reporter plasmids. Bacteria were incubated in MM with 10 mM fructose and 400 µM citric acid. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains, n = 4. Data are representative of three independent experiments. Asterisks denote significant difference between strains based on t‐test, ***P < 0.001. (D) cAMP levels in Arabidopsis leaves infected with DC3000 or AC811 strains carrying an AvrPto‐adenylate cyclase reporter (AvrPto‐CyaA). Graphed data are means ± SE of cAMP levels in infected tissue sampled at 3 and 5 h post‐infection (hpi), normalized to total protein content of samples. Data were pooled from three independent experiments, n = 9. **P < 0.01; *P < 0.05 based on t‐test.
In light of these results, we next sought to determine whether AC811 similarly hyper‐expresses its T3SS in planta. The hypersensitive response (HR) is a localized cell death phenotype caused by immune receptor‐mediated recognition of pathogen effectors inside plant cells (Dangl and Jones, 2001). Pseudomonas syringae mutants that cannot deliver effectors are unable to trigger an HR. In this regard, AC811 was reported to elicit a weaker HR in tobacco leaves (Chatterjee et al., 2003). We re‐examined this phenotype in tobacco using an ion leakage assay and observed that T3SS‐dependent HR cell death induced by AC811 and ΔgacA‐1 was at least equivalent to DC3000 (Fig. S5). To measure effector delivery in a more quantitative manner, we used an adenylate cyclase (CyaA) reporter assay to quantify the amount of AvrPto‐CyaA delivered by AC811 into plant cells during infection of Arabidopsis (Miao et al., 1999; Schechter et al., 2004). Consistent with the increased avrPto expression in cultured AC811, we measured significantly higher levels of AvrPto‐CyaA delivery in AC811‐infected leaf tissue (Fig. 1D). We conclude from these data that GacA negatively regulates T3SS deployment by DC3000 when cultured with defined T3SS‐inducing metabolite signals and during plant infection.
Increased expression and delivery of type III effectors by AC811 was unexpected based on the reported virulence defect of this strain. To re‐evaluate the role of GacA in regulating DC3000 virulence, we infiltrated DC3000, AC811 and ΔgacA‐1 into the interior, or apoplast, of Arabidopsis leaves. Three days post‐infection we observed a significant reduction in visible disease symptoms caused by AC811, as previously reported (Fig. 2A) (Chatterjee et al., 2003). We also measured a significant decrease in the number of bacteria in AC811‐infected tissue (Fig. 2B). However, ΔgacA‐1 did not phenocopy AC811 and instead caused disease symptoms and grew to levels comparable to those of DC3000 (Fig. 2A,B). To confirm that these conflicting results were not due to variation in laboratory strains of DC3000 (Landgraf et al., 2006), we deleted gacA in DC3000 isolates obtained from two other laboratories (Table S1) and observed no significant decrease in the virulence of these mutants (Fig. S6). Also, gacA over‐expression did not rescue the virulence defect of AC811 (Fig. 2C). Based on these results, we conclude that GacA is not required for DC3000 virulence within Arabidopsis leaf tissue, and that loss of GacA is not responsible for the virulence defect of AC811. In follow‐up experiments we discovered that a polar effect of Tn5::gacA on downstream uvrC expression and a nonsense mutation in cell wall recycling enzyme anmK are responsible for decreased virulence of AC811 (O'Malley et al., 2019).
Figure 2.
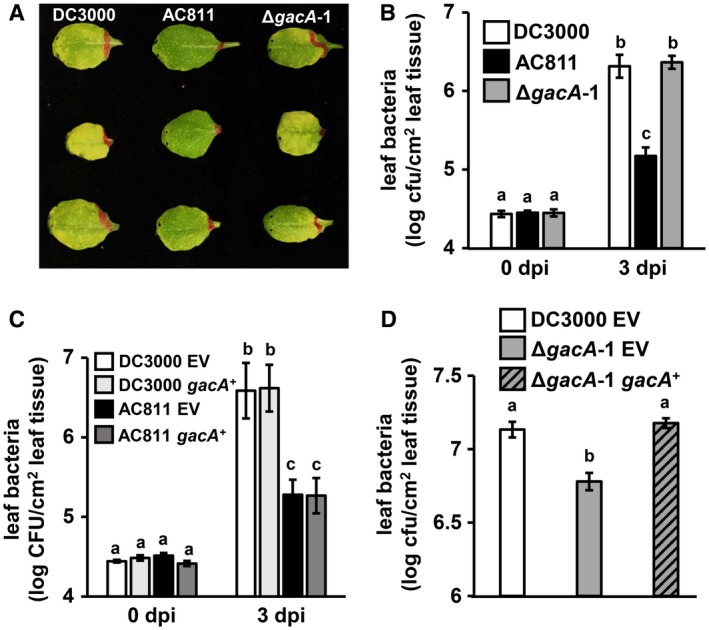
GacA is not required for virulence of DC3000 within the apoplast but is required for growth of leaf‐surface inoculated DC3000. (A) Leaves of 4‐week‐old Arabidopsis plants were syringe‐infiltrated with DC3000, AC811 or ΔgacA‐1. Shown is a photograph of disease symptoms on detached leaves 3 days post‐infection (dpi). (B) Growth of DC3000, AC811 and ΔgacA‐1 in leaves of 4‐week‐old plants infected by syringe‐infiltration. Graphed are means ± SE of colony‐forming units (cfus) in leaves based on serial dilution plating, n = 9. Data were pooled from three independent experiments. (C) DC3000 or AC811 carrying plasmids with gacA under native promoter control (gacA +) were syringe‐infiltrated into Arabidopsis Col‐0 leaves and bacterial levels measured by serial dilution plating. EV, empty vector. Graphed are means ± SE of cfus in leaves based on serial dilution plating, n = 3. Data are representative of three independent experiments. (D) Bacterial populations in 5‐week‐old leaves infection by spray‐inoculation. Graphed are means ± SE of bacterial levels 3 dpi based on serial dilution plating, n = 6. Data are representative of three independent experiments. Lower case letters in panels B to D denote statistical groups determined by ANOVA with multiple pairwise t‐test comparisons and Tukey’s post hoc HSD analysis, P < 0.05.
In addition to regulation of T3SS, GacS/GacA was previously reported to positively regulate DC3000 motility based on swimming and swarming defects of AC811 (Chatterjee et al., 2003; Ferreiro et al., 2018; Vargas et al., 2013). We tested both AC811 and our ΔgacA‐1 strain on swim agar plates and confirmed that GacA is required for swimming motility (Fig. S7). Because motility is necessary for invasion of leaf tissue by P. syringae, we reasoned that ΔgacA‐1 may be less virulent when inoculated onto a leaf surface. To investigate, we sprayed Arabidopsis leaves with suspensions of DC3000, ΔgacA‐1 or a gacA‐complemented ΔgacA‐1 strain. In contrast to apoplast infection, we measured a significant decrease in the population of bacteria on ΔgacA‐1‐infected leaves, and this phenotype was fully complemented by expression of gacA (Fig. 2D). We conclude that GacA is required for full virulence of leaf surface‐inoculated DC3000.
In summary, our results indicate that GacA functions as a negative regulator of type III secretion in DC3000. Our data also demonstrate that GacA is not required for virulence of DC3000 in the leaf apoplast, but is required for full virulence of DC3000 on the leaf surface, most likely due to its role in regulating motility. We propose a model in which GacS/GacA regulates a lifestyle switch of P. syringae by inversely regulating motility and T3SS during host infection. In this model, GacS/GacA is activated on the leaf surface, thereby promoting motility and dampening T3SS deployment, and deactivated during apoplast colonization, allowing for decreased motility and increased type III secretion to suppress host immunity. GacS/GacA may coordinately regulate additional virulence‐associated traits such as toxin production and biofilm formation in a similar manner. Testing this model will require closer examination of when, where and how GacS/GacA is activated during leaf infection, as well as examining signalling pathways downstream of GacS/GacA activation and how they may coordinately regulate both motility and T3SS.
Supporting information
Fig. S1 Hyper‐expression of avrPto occurs in AC811 cultured in KB broth prior to fructose and citric acid treatment. GFP fluorescence of DC3000 and AC811 strains carrying an avrPtopromoter:gfp reporter plasmid. Bacteria were first cultured overnight in KB broth then incubated in a minimal medium (MM) with 10 mM fructose and 400 µM citric acid. Graphed are means ± SE of GFP fluorescence at 6 h post‐inoculation normalized to OD600 and fluorescence from pProbe‐GT empty vector strains, n = 4. Asterisks denote significant difference based on t‐test, P < 0.001. Data are representative of three independent experiments.
Fig. S2 GacA negatively regulates the abundance of mRNA transcripts from T3SS‐associated genes. DC3000, AC811 and ΔgacA‐1 were incubated in minimal medium (MM) with 10 mM fructose and 400 µM citric acid for 2 h. The abundance of avrPto and hrpL transcripts in treated bacteria was measured by quantitative RT‐PCR. Transcript abundance was normalized to (A) gyrA or (B) ffh reference genes, followed by normalization to transcript levels in DC3000. Graphed are means ± SE from data pooled from three independent experiments, n = 10. Asterisks denote statistical significance as determined by pairwise t‐tests between DC3000 and indicated mutant strains. *P < 0.05; ***P < 0.001; n.s., no significant difference. (C) Threshold cycle (Ct) values from quantitative RT‐PCR of reference genes gyrA (left) and ffh (right). Graphed are means ± SE from data pooled from three independent experiments, n = 10. Statistical significance was determined by pairwise t‐tests; n.s., no significant difference based on P > 0.05.
Fig. S3 Expression of gacA complements the avrPto hyper‐expression phenotype of AC811. (A) Abundance of gacA transcripts measured by qRT‐PCR using gacA‐specific primers. gacA transcripts were normalized to transcripts from gyrA. Graphed are means ± SE, with data pooled from two independent experiments, n = 8. ***P < 0.001 based on two sample t‐test comparisons with DC3000. (B) GFP fluorescence of DC3000 and AC811 avrPtopromoter:gfp reporter strains carrying either a gacA‐complementing plasmid (gacA +) or empty vector (EV). Bacteria were incubated in minimal medium (MM) with 10 mM fructose and 400 µM aspartic acid. Graphed are means ± SE of GFP fluorescence at 12 h post‐inoculation normalized to OD600 and fluorescence from pProbe‐GT empty vector strains, n = 9. Data are pooled from three independent experiments. Asterisks denote significant difference between strains based on t‐test, P < 0.001.
Fig. S4 PCR genotyping of ΔgacA‐1 confirms deletion of gacA. A fragment of DNA containing the gacA open reading frame was PCR‐amplified from DC3000 or ΔgacA‐1 genomic DNA. Shown are PCR products separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
Fig. S5 Loss of gacA does not decrease the hypersensitive response in non‐host tobacco leaves. Leaves of Nicotiana tabacum cultivar KY21 were syringe‐infiltrated with DC3000 and DC3000‐derived mutants, including a T3SS‐deficient hrcC ‐ strain. (A) Photograph of a leaf 8 h post‐infiltration with 1 × 108 cfu/mL (left) or 1 × 107 cfu/mL (right) of bacteria. Labels are 1, 2, ΔhrcC; 3, 4, DC3000; 5, 6, AC811; 7, 8, ΔgacA‐1. Image is representative of three independent experiments. (B) Leaf disks (five disks/infected area) were taken at 8 h post‐infiltration and incubated in 5 mL of H2O for 1 h. Ion leakage from leaf tissue was quantified by conductivity meter. Graphed are means ± SE of conductivity measurements, n = 9. Data are pooled from three independent experiments. Lower case letters denote statistical groups determined by ANOVA with multiple pairwise t‐test comparisons and Tukey’s post hoc HSD analysis, P < 0.05.
Fig. S6 GacA is not required for virulence of DC3000 syringe‐infiltrated into Arabidopsis leaves. Growth of ΔgacA deletion mutants in Arabidopsis leaves infected by syringe infiltration. Solid colours indicate DC3000 obtained from G. Martin (Cornell) and its corresponding mutant ΔgacA‐2; dotted bars indicate DC3000 obtained from B. Kunkel (Wash U) and its corresponding mutant ΔgacA‐3. Graphed are means ± SE of colony‐forming units (cfus) in leaves based on serial dilution plating of leaf tissue extracts, n = 6. Data were pooled from two independent experiments. dpi, days post‐infection; ns, not significant based on ANOVA with multiple pairwise t‐test comparisons and Tukey’s post hoc HSD analysis, P < 0.05.
Fig. S7 GacA positively regulates motility of DC3000. DC3000, AC811 and ΔgacA‐1 were individually spotted onto King's B medium (KBM) agar plates containing 0.25% agar to detect swimming motility. (A) Photographs of bacteria on swimming motility plates after 24 h. White bars show scale of 1 cm. (B) Graphed are means ± SE of radii of bacterial spread measured after 24 h on swim plates, n = 4. Data are representative of three independent experiments.
Table S1 Sequences of oligonucleotide primers used in this study.
Table S2 List of bacterial strains used in this study.
Methods S1 Experimental Procedures.
Acknowledgments
We thank Dr Jeff Chang for helpful discussions and critical reading of this manuscript, and colleagues for providing strains. This work was funded by NSF grant IOS‐1557694 to J.C.A., and NSF grants IOS‐1051286 and IOS‐1456256 to S.C.P. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
References
- Abramovitch, R.B. , Anderson, J.C. and Martin, G.B. (2006) Bacterial elicitation and evasion of plant innate immunity. Nat. Rev. Mol. Cell Biol. 7, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J.C. , Wan, Y. , Kim, Y.‐M. , Pasa‐Tolic, L. , Metz, T.O. and Peck, S.C. (2014) Decreased abundance of type III secretion system‐inducing signals in Arabidopsis mkp1 enhances resistance against Pseudomonas syringae . Proc. Natl. Acad. Sci. USA, 111, 6846–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic, A. and Winans, S.C. (2005) Detection of and response to signals involved in host–microbe interactions by plant‐associated bacteria. Microbiol. Mol. Biol. Rev. 69, 155–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, A. , Cui, Y. , Yang, H. , Collmer, A. , Alfano, J.R. and Chatterjee, A.K. (2003) GacA, the response regulator of a two‐component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol. Plant–Microbe Interact. 16, 1106–1117. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L. and Jones, J.D.G. (2001) Plant pathogens and integrated defense responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Ferreiro, M.‐D. , Nogales, J. , Farias, G.A. , Olmedilla, A. , Sanjuán, J. and Gallegos, M.T. (2018) Multiple CsrA proteins control key virulence traits in Pseudomonas syringae pv. tomato DC3000. Mol. Plant–Microbe Interact. 31, 525–536. [DOI] [PubMed] [Google Scholar]
- Heeb, S. and Haas, D. (2001) Regulatory roles of the GacS/GacA two‐component system in plant‐associated and other Gram‐negative bacteria. Mol. Plant–Microbe Interact. 14, 1351–1363. [DOI] [PubMed] [Google Scholar]
- Hirano, S.S. , Ostertag, E.M. , Savage, S.A. , Baker, L.S. , Willis, D.K. and Upper, C.D. (1997) Contribution of the regulatory gene lemA to field fitness of Pseudomonas syringae pv. syringae . Appl. Environ. Microbiol. 63, 4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, T.V. , Dahlbeck, D. and Staskawicz, B.J. (1989) Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science, 245, 1374–1377. [DOI] [PubMed] [Google Scholar]
- Johnston, C. , Pegues, D.A. , Christoph, J. , Lee, C.A. and Miller, S.I. (1996) Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response‐regulator superfamily. Mol. Microbiol. 22, 715–727. [DOI] [PubMed] [Google Scholar]
- Landgraf, A. , Weingart, H. , Tsiamis, G. and Boch, J. (2006) Different versions of Pseudomonas syringae pv. tomato DC3000 exist due to the activity of an effector transposon. Mol. Plant Pathol. 7, 355–364. [DOI] [PubMed] [Google Scholar]
- Lapouge, K. , Schubert, M. , Allain, F.H.T. and Haas, D. (2008) Gac/Rsm signal transduction pathway of γ‐proteobacteria: From RNA recognition to regulation of social behaviour. Mol. Microbiol. 67, 241–253. [DOI] [PubMed] [Google Scholar]
- MacLean, D. and Studholme, D.J. (2010) A Boolean model of the Pseudomonas syringae hrp regulon predicts a tightly regulated system. PLoS ONE, 5, E9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutani, M. , Taguchi, F. , Ogawa, Y. , Hossain, M.M. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2008) Gac two‐component system in Pseudomonas syringae pv. tabaci is required for virulence but not for hypersensitive reaction. Mol. Genet. Genomics, 279, 313–322. [DOI] [PubMed] [Google Scholar]
- Miao, E.A. , Scherer, C.A. , Tsolis, R.M. , Kingsley, R.A. , Adams, L.G. , Bäumler, A.J. and Miller, S.I. (1999) Salmonella typhimurium leucine‐rich repeat proteins are targeted to the SPl1 and SPl2 type III secretion systems. Mol. Microbiol. 34, 850–864. [DOI] [PubMed] [Google Scholar]
- Mole, B.M. , Baltrus, D.A. , Dangl, J.L. and Grant, S.R. (2007) Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol. 15, 363–371. [DOI] [PubMed] [Google Scholar]
- O'Malley, M.R. , Weisberg, A.J. , Chang, J.H. and Anderson, J.C. (2019) Re‐evaluation of a Tn5::gacA mutant of Pseudomonas syringae pv. tomato DC3000 uncovers roles for uvrC and anmK in promoting virulence. BioRxiv. 10.1101/774711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme, L.G. , Stevens, E.J. , Wolfort, S.F. , Shao, J. , Ronald, G. , Rahme, L.G. , Stevens, E.J. , Wolfort, S.F. , Shao, J. , Tompkins, R.G. and Ausubelt, F.M. (2019) Common virulence factors for bacterial pathogenicity in plants and animals. Science, 268, 1899–1902. [DOI] [PubMed] [Google Scholar]
- Schechter, L.M. , Roberts, K.A. , Jamir, Y. , Alfano, J.R. and Collmer, A. (2004) Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, K.J. and Desveaux, D. (2011) AlgW regulates multiple Pseudomonas syringae virulence strategies. Mol. Microbiol. 80, 364–377. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Xiao, Y. and Zhou, J.M. (2006) Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant–Microbe Interact. 19, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Valentini, M. , Gonzalez, D. , Mavridou, D.A. and Filloux, A. (2018) Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa . Curr. Opin. Microbiol. 41, 15–20. [DOI] [PubMed] [Google Scholar]
- Vargas, P. , Farias, G.A. , Nogales, J. , Prada, H. , Carvajal, V. , Barón, M. , Rivilla, R. , Martín, M. , Olmedilla, A. and Gallegos, M.T. (2013) Plant flavonoids target Pseudomonas syringae pv. tomato DC3000 flagella and type III secretion system. Environ. Microbiol. Rep. 5, 841–850. [DOI] [PubMed] [Google Scholar]
- Willis, D.K. , Hrabak, E.M. , Rich, J.J. , Barta, T.M. and Panopoulos, N.J. (1990) Isolation and characterization of a Pseudomonas syringae pv. syringae mutant deficient in lesion formation in bean. Mol. Plant–Microbe Interact. 3, 149–156. [Google Scholar]
- Wong, S.M. , Carroll, P.A. , Rahme, L.G. , Ausubel, F.M. and Calderwood, S.B. (1998) Modulation of expression of the ToxR regulon in Vibrio cholerae by a member of the two‐component family of response regulators. Infect. Immun. 66, 5854–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, X.F. , Kvitko, B. and He, S.Y. (2018) Pseudomonas syringae: what it takes to be a pathogen. Nat. Rev. Microbiol. 16, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Lund, S.P. , Greenwald, J.W. , Records, A.H. , Scott, R.A. , Nettleton, D. , Lindow, S.E. , Gross, D.C. and Beattie, G.A. (2014) Transcriptional analysis of the global regulatory networks active in Pseudomonas syringae during leaf colonization. mBio, 110, E425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Hyper‐expression of avrPto occurs in AC811 cultured in KB broth prior to fructose and citric acid treatment. GFP fluorescence of DC3000 and AC811 strains carrying an avrPtopromoter:gfp reporter plasmid. Bacteria were first cultured overnight in KB broth then incubated in a minimal medium (MM) with 10 mM fructose and 400 µM citric acid. Graphed are means ± SE of GFP fluorescence at 6 h post‐inoculation normalized to OD600 and fluorescence from pProbe‐GT empty vector strains, n = 4. Asterisks denote significant difference based on t‐test, P < 0.001. Data are representative of three independent experiments.
Fig. S2 GacA negatively regulates the abundance of mRNA transcripts from T3SS‐associated genes. DC3000, AC811 and ΔgacA‐1 were incubated in minimal medium (MM) with 10 mM fructose and 400 µM citric acid for 2 h. The abundance of avrPto and hrpL transcripts in treated bacteria was measured by quantitative RT‐PCR. Transcript abundance was normalized to (A) gyrA or (B) ffh reference genes, followed by normalization to transcript levels in DC3000. Graphed are means ± SE from data pooled from three independent experiments, n = 10. Asterisks denote statistical significance as determined by pairwise t‐tests between DC3000 and indicated mutant strains. *P < 0.05; ***P < 0.001; n.s., no significant difference. (C) Threshold cycle (Ct) values from quantitative RT‐PCR of reference genes gyrA (left) and ffh (right). Graphed are means ± SE from data pooled from three independent experiments, n = 10. Statistical significance was determined by pairwise t‐tests; n.s., no significant difference based on P > 0.05.
Fig. S3 Expression of gacA complements the avrPto hyper‐expression phenotype of AC811. (A) Abundance of gacA transcripts measured by qRT‐PCR using gacA‐specific primers. gacA transcripts were normalized to transcripts from gyrA. Graphed are means ± SE, with data pooled from two independent experiments, n = 8. ***P < 0.001 based on two sample t‐test comparisons with DC3000. (B) GFP fluorescence of DC3000 and AC811 avrPtopromoter:gfp reporter strains carrying either a gacA‐complementing plasmid (gacA +) or empty vector (EV). Bacteria were incubated in minimal medium (MM) with 10 mM fructose and 400 µM aspartic acid. Graphed are means ± SE of GFP fluorescence at 12 h post‐inoculation normalized to OD600 and fluorescence from pProbe‐GT empty vector strains, n = 9. Data are pooled from three independent experiments. Asterisks denote significant difference between strains based on t‐test, P < 0.001.
Fig. S4 PCR genotyping of ΔgacA‐1 confirms deletion of gacA. A fragment of DNA containing the gacA open reading frame was PCR‐amplified from DC3000 or ΔgacA‐1 genomic DNA. Shown are PCR products separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
Fig. S5 Loss of gacA does not decrease the hypersensitive response in non‐host tobacco leaves. Leaves of Nicotiana tabacum cultivar KY21 were syringe‐infiltrated with DC3000 and DC3000‐derived mutants, including a T3SS‐deficient hrcC ‐ strain. (A) Photograph of a leaf 8 h post‐infiltration with 1 × 108 cfu/mL (left) or 1 × 107 cfu/mL (right) of bacteria. Labels are 1, 2, ΔhrcC; 3, 4, DC3000; 5, 6, AC811; 7, 8, ΔgacA‐1. Image is representative of three independent experiments. (B) Leaf disks (five disks/infected area) were taken at 8 h post‐infiltration and incubated in 5 mL of H2O for 1 h. Ion leakage from leaf tissue was quantified by conductivity meter. Graphed are means ± SE of conductivity measurements, n = 9. Data are pooled from three independent experiments. Lower case letters denote statistical groups determined by ANOVA with multiple pairwise t‐test comparisons and Tukey’s post hoc HSD analysis, P < 0.05.
Fig. S6 GacA is not required for virulence of DC3000 syringe‐infiltrated into Arabidopsis leaves. Growth of ΔgacA deletion mutants in Arabidopsis leaves infected by syringe infiltration. Solid colours indicate DC3000 obtained from G. Martin (Cornell) and its corresponding mutant ΔgacA‐2; dotted bars indicate DC3000 obtained from B. Kunkel (Wash U) and its corresponding mutant ΔgacA‐3. Graphed are means ± SE of colony‐forming units (cfus) in leaves based on serial dilution plating of leaf tissue extracts, n = 6. Data were pooled from two independent experiments. dpi, days post‐infection; ns, not significant based on ANOVA with multiple pairwise t‐test comparisons and Tukey’s post hoc HSD analysis, P < 0.05.
Fig. S7 GacA positively regulates motility of DC3000. DC3000, AC811 and ΔgacA‐1 were individually spotted onto King's B medium (KBM) agar plates containing 0.25% agar to detect swimming motility. (A) Photographs of bacteria on swimming motility plates after 24 h. White bars show scale of 1 cm. (B) Graphed are means ± SE of radii of bacterial spread measured after 24 h on swim plates, n = 4. Data are representative of three independent experiments.
Table S1 Sequences of oligonucleotide primers used in this study.
Table S2 List of bacterial strains used in this study.
Methods S1 Experimental Procedures.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


