Abstract
Background
Traditional dietary recommendations for achieving optimal gestational weight gain are ineffective for pregnant women due to the lack of real-time communication and tedious consultation processes.
Objective
In this pilot study, we aimed to determine the feasibility of a novel food-coaching smartphone app for controlling gestational weight gain and macronutrient intake among overweight and obese pregnant women.
Methods
We designed a randomized controlled trial and recruited 30 overweight and obese pregnant women (1:1 ratio) during 18-20 weeks of gestation and followed them up after 4 and 8 weeks, respectively. Both groups received standard pregnancy dietary orientation at recruitment, while the intervention group received 8 weeks of real-time food coaching via a smartphone app. This food-coaching smartphone app (Glycoleap, Holmusk, Singapore) aimed to improve care and outcomes for people with diabetes. Pregnant women using this app were able to upload food images (eg, a picture of a meal, a drink, or a dessert) and received real-time and detailed food-coaching comments and guidance provided by professional dietitians during the day (8 AM to 8 PM). We recorded detailed characteristics during recruitment and examined anthropometry at all visits. We compared the mean differences of the 8-week gestational weight gain and macronutrient intake between the two groups.
Results
Upon study completion, three subjects dropped out from the intervention, and one gave birth prematurely in the control group. The acceptance rate of the smartphone app was 90%. More participants achieved optimal gestational weight gain per week in the intervention group (8/12, 67%) than in the control group (5/14, 36%). After the 8-week intervention, women in the intervention group appeared to have lower gestational weight gain (mean difference=–0.08 kg; 95% CI –1.80 to 1.63) and cholesterol intake (mean difference=–31.73 mg; 95% CI –102.91 to 39.45) than those in the control group.
Conclusions
Our findings showed that this food-coaching smartphone app is feasible and favorable for weight gain control and cholesterol intake control among overweight and obese pregnant women. Although our results were not significant (perhaps, attributed to the small sample size), it provided proof of concept for the feasibility of applying such technology in future randomized controlled trials with a larger sample size, an earlier intervention onset, and a longer follow-up for overweight and obese pregnant women.
Keywords: overweight, obesity, pregnant women; gestational weight gain; food diary; randomized controlled trial; smartphone app; food coaching; dietary recommendation; feasibility
Introduction
Overweight and obese pregnant women are often at an increased risk of a series of maternal and offspring adverse outcomes [1-3]. In developed countries, overweight and obese women accounted for 30%-50% of fertile women [4-6]. Therefore, the high prevalence of overweight and obesity among pregnant women signifies a substantial burden to public health welfare worldwide. Current clinical care management of overweight and obese women during pregnancy consists of information on healthy eating practices according to standard dietary guidelines both worldwide [7-10] and in Singapore [11,12], in terms of restricting daily energy intake; balancing the proportion of complex carbohydrate (33%-40%), protein (20%), and fat (40%); and lowering cholesterol intake However, the reported effects were equivocal due to limitations such as low compliance, delayed feedback, late initiation, and inefficient delivery of intervention [13,14].
In recent years, the use of mobile technology (such as smartphone apps) for patient care has been increasing. The feasibility and efficacy of such technology has been proven for weight management among pediatric obese patients [15] and for glucose control in mothers with gestational diabetes mellitus during pregnancy [16]. Furthermore, a systematic review summarized 12 studies using phone-based reporting interventions such as video call, phone calls, short messaging service (SMS) and smartphone apps and showed consistent evidence that although such approaches could help pregnant women control their gestational weight gain, they were not effective in preventing other pregnancy outcomes such as gestational diabetes mellitus [17]. Another systematic review of four randomized controlled trials (RCTs) did not draw any firm conclusions on the effects of mobile app interventions during pregnancy on maternal knowledge, behavior change, and perinatal health outcomes due to heterogeneity of interventions, comparators, and outcome measures across all RCTs [18]. In addition, based on the intervention evaluation alone, none of these mobile technologies provide a real-time communication between end users and medical workers or dietitians, thus likely affecting the compliance and efficacy of the phone-based intervention. Given the high prevalence of smartphone app usage among pregnant women, more rigorous studies are needed to optimize the study design and implementation of these technologies in improving maternal health outcomes. In this pilot randomized controlled trial study, we tested a food-coaching intervention program delivered through a smartphone app in overweight and obese pregnant subjects without gestational diabetes mellitus and examined its feasibility, acceptance, and preliminary utility during an 8-week follow-up in second trimester.
Methods
Study Design and Population
We conducted a prospective, two-arm, unblinded RCT in a subsidized clinic within a tertiary government hospital in Singapore (KK Women’s and Children’s Hospital [KKH]) between March and July 2018. We recruited pregnant women if they were Singapore citizens or permanent residents, overweight or obese (ie, prepregnancy or booking body mass index≥25 kg/m2), age≥21 years, between 18 and 20 weeks’ gestation at the time of recruitment, planning to deliver in KKH, capable of reading and writing in English, and able to download and use the smartphone app. We excluded women with special dietary restrictions due to medical conditions such as type 1 or type 2 diabetes, gestational diabetes mellitus, hypertension, and chronic kidney disease.
We conducted the study according to the tenets of the Declaration of Helsinki and obtained approval by the SingHealth Centralized Institutional Review Board and the National Health Group’s Domain Specific Review Board. We obtained written informed consent from all pregnant women at baseline recruitment. As the primary aim of this study is to test the feasibility of the smartphone app in the pilot phase, we only obtained the local institutional review board approval (CIRB 2017/2132), but did not register for an online RCT number.
Randomization and Procedures
Research coordinators randomly assigned pregnant women to the intervention (food-coaching smartphone app) or control group (standard pregnancy dietary orientation) in a 1:1 allocation ratio, using the envelope randomization method (Figure 1).
Figure 1.
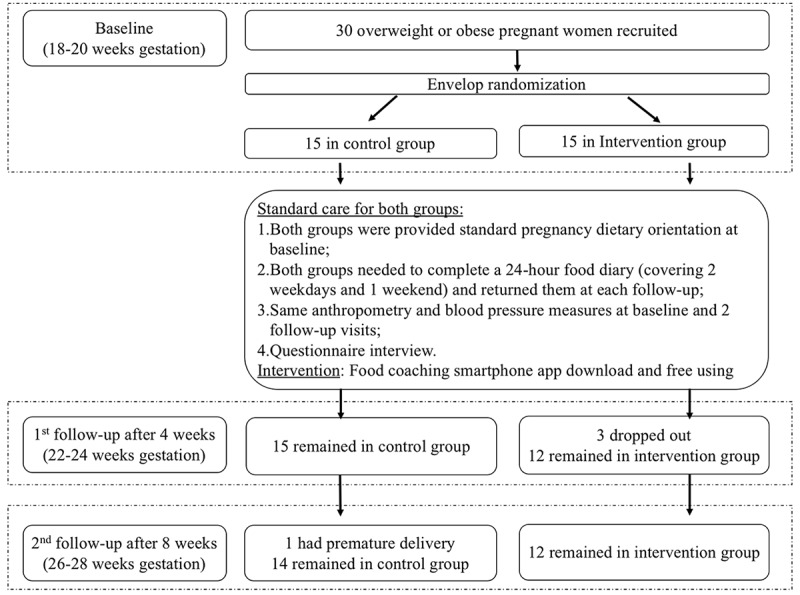
Study flow of the randomized controlled trial.
Standard Pregnancy Dietary Orientation
The same research coordinators administered the standard dietary orientation to women in the intervention and control groups using standardized materials, including basic nutrition principles of choosing a wide variety of nutrient-dense foods, limiting intake of high-fat and high-sugar food and beverages, and guiding food portion sizes, with reference to the local gestational dietary guidelines by the Health Promotion Board [19]. We informed participants in both groups about the recommended gestational weight gain, together with some simple physical activity pointers.
A Food-Coaching Smartphone App
The intervention group participants could download and use the food-coaching smartphone app (Glycoleap, Holmusk, Singapore) for free for up to 8 weeks during the RCT. The app aimed to improve care and outcomes of people with type 2 diabetes in terms of diet control. Pregnant women using this app were able to upload food images and received real-time and detailed food-coaching comments and guidance from professional dietitians during the day (8 AM to 8 PM). According to the local pregnancy guideline [20], food dietitians rated the food image from 1 to 5 (1=the least recommended score, 5=the most recommended scored) and provided feedback in terms of degree and balance of the food items and composition (Multimedia Appendix 1). Although this smartphone app was available on both Apple and Google store platforms, we did not think there was a high chance for the controls to obtain access to this app for two reasons: (1) This smartphone app requires an in-store purchase of up to SG $30 (US $22) per month, which is not subsidized by any local Singaporean medical insurance. All participants were recruited from the government tertiary hospital’s subsidized clinic; therefore, the chances for them to pay from their own pocket to afford additional medical care are low. (2) Only pregnant women randomized into the intervention group were informed of the name and company of the smartphone app, while those in the control group were not provided any information regarding the app. In addition, subjects in the intervention and control groups did not know of each other.
We recruited all subjects at baseline (18-20 weeks’ gestation) and followed them up after 4 weeks (22-24 weeks’ gestation) and 8 weeks (26-28 weeks’ gestation).
Outcomes
Based on the usage of the food-coaching smartphone app, we assessed the feasibility of the smartphone app by collecting participant feedback in the intervention group, using an evaluation form at the 8-week follow-up visit (Multimedia Appendix 2). In addition, we assessed the compliance in the intervention group via the times of log in for each participant in the first half and second half of the follow-up. We calculated the proportion of participants with optimal second trimester gestational weight gain based on the Institute of Medicine guidelines for overweight (0.23-0.33 kg/week) and obese women (0.17-0.27 kg/week) [9,21]. We assisted all pregnant subjects in quantifying their food and beverage intake. Such methods of assessing dietary data have been widely published [22-24]. We obtained data on 24-hour food recall at recruitment and collected self-administered 3-day food diary data (Multimedia Appendix 3) in both groups at two follow-ups. We analyzed the dietary records using an online nutrient analysis software [25], which was derived from locally available foods [20,26,27]. The Singapore Health Promotion Board online guidelines provided specific dietary instructions upon completion of the 3-day food diary and provided a picture compendium to each subject to facilitate identification of each type and quantity of food consumed. The compendium of food pictures consisted of photographs of individual food items coded to reflect the portion size of food items and standardize the estimated amounts eaten. The guidelines also used standard bowls, glasses, and spoons of varying sizes to estimate the volume of fluids or the amount of food consumed. Research coordinators entered each recorded food item into an online nutrient analysis database (Food Information and Nutrient Database system, Health Promotion Board, Singapore) based on a food composition database of locally available foods [25]. The daily food intake data were then summed up, and the total daily energy, macronutrients (carbohydrate, fiber, fat, and protein), calcium, cholesterol, and sodium intakes were averaged over each 3-day period and listed in kilocalories, grams, and percentages in the data summary form. The intake was compared between the intervention and control groups at the two follow-up visits.
Statistical Analysis
We applied the Fisher exact test and Student t test for categorical and continuous variables, respectively, to compare characteristics between the intervention and control groups. We used linear regression to examine the effect of the food-coaching smartphone app on weight gain control and macronutrient intake between two groups. Mean differences as an estimate referencing the control group were shown in linear regression. We performed statistical analysis using STATA (Version 14.0. STATA Corp, College Station, Texas), set a two-tailed P value for significance at .05, and provided the 95% CIs for all estimates.
Results
Among 30 pregnant women recruited at baseline with 1:1 allocation, 26 (12 in the intervention group and 14 in the control group) completed the 8-week RCT. The detailed RCT flow chart is shown in Figure 1. Based on the log-in records, the uptake of this smartphone app among our intervention subjects was up to 90% at the beginning (n=15) and 70% at the end of the study (n=12), respectively. Based on the 12 returned user evaluation forms, 75% (n=9) of the app users found that the app was easy to operate, more than 80% (n=10) thought the food-coaching guidance was acceptably fast, and 90% (n=11) were satisfied and reported that the app had somewhat or greatly improved their own diet. Therefore, they would recommend the smartphone app to family and friends. Furthermore, according to the backlog records from the smartphone app provider, we assessed compliance by calculating the average log-in frequency in the first and second half of the 8-week follow-up. Each of the 12 subjects in the intervention group logged into the smartphone app 8 times per week in the first 4 weeks, on an average, but this number reduced to 2 times per week in the subsequent 4 weeks.
Tables 1-3 show the baseline and follow-up characteristics between the intervention and control groups. More participants met the Institute of Medicine recommendation for optimal gestational weight gain per week in the intervention group (4-week follow-up: 7/12, 58%; 8-week follow-up: 8/12, 67%) than in the control group (4-week follow-up: 8/15, 53%; 8-week follow-up: 5/14, 36%).
Table 1.
Comparison of baseline characteristics between the intervention and control groups (baseline recruitment at 18-20 weeks’ gestation).
| Characteristics | Intervention group (n=15) | Control group (n=15) | P valuea | |
| Age (years), mean (SD) | 29.3 (4.4) | 30.7 (5) | .45 | |
| Ethnicity, n (%) |
|
.15 | ||
|
|
Chinese | 2 (13) | 6 (40) |
|
|
|
Malay | 12 (80) | 8 (53) |
|
|
|
Indian | 1 (7) | 0 (0) |
|
|
|
Others | 0 (0) | 1 (6) |
|
| Smoking history (yes), n (%) | 4 (27) | 1 (6) | .33 | |
| Parity, n (%) | .60 | |||
|
|
0 | 7 (47) | 5 (33) |
|
|
|
1 | 4 (27) | 7 (47) |
|
|
|
≥2 | 4 (27) | 3 (20) |
|
| Maternal college degree (yes), n (%) | 3 (20) | 5 (33) | .68 | |
| Paternal college degree (yes), n (%) | 1 (7) | 4 (27) | .33 | |
| Household income ≥US $6000/month, n (%) | 3 (20) | 6 (40) | .43 | |
| Past history of pregnancy outcomes | .16 | |||
|
|
Gestational diabetes mellitus, n (%) | 0 (0) | 3 (20) |
|
|
|
Hypertensive disorders during pregnancy, n (%) | 0 (0) | 1 (7) |
|
|
|
Prepregnancy weight (kg), mean (SD) | 78.5 (15.8) | 73.9 (6.8) | .31 |
|
|
Prepregnancy body mass index (kg/m2), mean (SD) | 31.0 (4.7) | 29.4 (2.2) | .27 |
| Gestational age at recruitment (weeks), mean (SD) | 19.2 (2.1) | 17.6 (2.8) | .08 | |
| Baseline body mass index (kg/m2), mean (SD) | 34.2 (4.3) | 31.3 (2.8) | .04 | |
| Baseline systolic blood pressure (mm Hg), mean (SD) | 118.6 (8.9) | 116.8 (9.4) | .59 | |
| Baseline diastolic blood pressure (mm Hg), mean (SD) | 69.4 (4.6) | 64.1 (7.9) | .03 | |
aStudent t test or Fisher exact test.
Table 3.
Comparison of follow-up measures between the intervention and control groups (8-week follow-up at 26-28 weeks’ gestation).
| Clinical measures | Intervention group (n=12) | Control group (n=14) | P valuea | ||||
| Anthropometric and blood pressure measures, mean (SD) | |||||||
|
|
Second follow-up body mass index (kg/m2) | 36.0 (4.6) | 32.4 (2.8) | .02 | |||
|
|
Second follow-up systolic blood pressure (mm Hg) | 120.2 (7.5) | 117.3 (7.3) | .33 | |||
|
|
Second follow-up diastolic blood pressure (mm Hg) | 69.6 (9.1) | 67.4 (5.8) | .47 | |||
|
|
Weight gain from baseline (kg) | 2.9 (1.9) | 3.0 (2.24) | .92 | |||
| Patients obtaining optimal weight gain per week, n (%) | 8 (67) | 5 (36) | .43 | ||||
| Dietary measures, mean (SD) | |||||||
|
|
Energy intake (kcal) | 1370.5 (359.4) | 1514.0 (362.6) | .44 | |||
|
|
Carbohydrate (g) | 1815.0) | 170.6 (49.4) | .61 | |||
|
|
Protein (g) | 56.7 (19.8) | 58.6 (15.8) | .79 | |||
|
|
Total fat (g) | 54.3 (20.7) | 43.5 (17.5) | .15 | |||
|
|
Cholesterol (mg) | 196.2 (89.3) | 220.6 (87.9) | .48 | |||
|
|
Calcium (g) | 461.0 (246.0) | 489.1 (222.9) | .76 | |||
|
|
Dietary fiber (g) | 13.0 (5.3) | 12.9 (4.5) | .99 | |||
|
|
Sodium (g) | 2.2 (0.7) | 2.4 (0.8) | .55 | |||
aStudent t test, Fisher exact test, or Wilcoxon signed rank test.
Table 2.
Comparison of follow-up measures between the intervention and control groups (4-week follow-up at 22-24 weeks’ gestation).
| Clinical measures | Intervention group (n=12) | Control group (n=15) | P valuea | ||||
| Anthropometric and blood pressure measures, mean (SD) | |||||||
|
|
First follow-up body mass index (kg/m2) | 36.4 (4.9) | 31.9 (2.6) | .003 | |||
|
|
First follow-up systolic blood pressure (mm Hg) | 121.9 (6.8) | 120.9 (6.4) | .73 | |||
|
|
First follow-up diastolic blood pressure (mm Hg) | 70.9 (7.5) | 66.5 (6.7) | .15 | |||
|
|
Weight gain from baseline (kg) | 1.3 (1.5) | 1.5 (1.6) | .83 | |||
| Patients obtaining optimal weight gain per week, n (%) | 7 (58) | 8 (53) | .67 | ||||
| Dietary measures, mean (SD) | |||||||
|
|
Energy intake (kcal) | 1370.5 (359.4) | 1514.0 (362.6) | .32 | |||
|
|
Carbohydrate (g) | 177.5 (55.3) | 189.5 (49.3) | .56 | |||
|
|
Protein (g) | 52.6 (15.0) | 65.1 (17.5) | .06 | |||
|
|
Total fat (g) | 49.4 (15.9) | 54.3 (17.3) | .46 | |||
|
|
Cholesterol (mg) | 182.0 (90.6) | 246.9 (109.7) | .11 | |||
|
|
Calcium (g) | 513.4 (320.8) | 513.3 (286.9) | .99 | |||
|
|
Dietary fiber (g) | 12.9 (4.3) | 12.9 (3.5) | .95 | |||
|
|
Sodium (g) | 2.4 (0.8) | 2.8 (0.8) | .18 | |||
aStudent t test, Fisher exact test, or Wilcoxon signed rank test.
Although not significant, we found a trend among women in the intervention group who tended to have less weight gain than those in the control group at the 4-week follow-up (mean difference=–0.15 kg; 95% CI –1.51 to 1.21) and 8-week follow-up (mean difference=–0.08 kg; –1.80 to 1.63; Table 4). In addition, women in the intervention group tended to consume less cholesterol than those in the control group at the 4-week follow-up (mean difference=–64.87 mg; 95% CI –146.04 to 16.31) and 8-week follow-up (mean difference=–31.73 mg; 95% CI –102.91 to 39.45).
Table 4.
Weight gain from baseline and dietary intake between the intervention and control groups.
| Dietary components | Mean difference between intervention and control (ref) groups | |||
|
|
4-week follow-up (22-24 weeks’ gestation)a | 8-week follow-up (26-28 weeks’ gestation)b | ||
|
|
β (95% CI) | P value | β (95% CI) | P value |
| Weight gain from baseline (kg) | –0.15 (–1.51 to 1.21) | .83 | –0.08 (–1.80 to 1.63) | .92 |
| Energy intake (kcal) | –143.55 (–431.66 to 144.56) | .32 | 123.99 (–222.74 to 470.72) | .47 |
| Carbo (g) | –12.05 (–53.54 to 29.44) | .56 | 9.35 (–33.62 to 52.31) | .66 |
| Protein (g) | –12.58 (–25.69 to 0.53) | .06 | –2.08 (–16.73 to 12.57) | .77 |
| Total fat (g) | –4.91 (–18.24 to 8.42) | .46 | 11.11 (–4.60 to 26.81) | .16 |
| Cholesterol (mg) | –64.87 (–146.04 to 16.31) | .11 | –31.73 (–102.91 to 39.45) | .37 |
| Calcium (g) | 0.04 (–241.08 to 241.16) | >.99 | –19.16 (–211.31 to 172.99) | .84 |
| Dietary fiber (g) | 0.10 (–2.97 to 3.17) | .95 | 0.20 (–3.82 to 4.22) | .92 |
| Sodium (g) | –413.16 (–1032.09 to 205.76) | .18 | –98.83 (–692.65 to 494.98) | .73 |
aIntervention group: n=12; control group: n=15.
bIntervention group: n=12; control group: n=14.
Discussion
In this in a small sample RCT, we used a smartphone app to guide overweight and obese pregnant women to eat heathier in order to obtain optimal weight gain. We noted a high uptake of the smartphone app, as described above, and found evidence that pregnant women in the intervention group were more likely to have optimal gestational weight gain and consume less cholesterol compared with women in the control group. Although our pilot results were not significant, it provided proof of concept for the feasibility of applying such technology in future RCTs with a larger sample size, an earlier intervention onset, and a longer follow-up for overweight and obese pregnant women.
Although the dietary intervention has been proven to be effective in terms of weight gain control among overweight and obese pregnant women [28,29], there are huge variations in the delivery of dietary recommendation such as timing of intervention initiation, intensity of intervention, and feedback availability [13,28-31]. In a recently published systematic review summarizing 12 phone-based intervention studies on gestational weight gain control outcomes, most of the studies used phone calls or SMS to provide weight control guidelines, encouraged physical activity, and provided educational information about healthy nutrition. Several studies suggested that telephone communication is one of the most cost-effective tools to keep track of pregnant women’s health [32]. However, phone-based interventions are typically initiated by health care providers and might not effectively motivate pregnant women to self-manage their behaviors. Given the high prevalence of smartphone app usage among pregnant women to improve their healthy behaviors, no firm conclusion has been drawn in terms of improving perinatal outcomes [18]. One of the major drawbacks is the lack of rigorous studies examining self-managing and self-regulatory behaviors in pregnant women. Our food-coaching app is more flexible and interactive and has greater variety of communication modalities, thus overcoming all aforementioned limitations in current technology. Given the high smartphone usage rate (up to 80%) among Singaporeans [33] and high prevalence (up to 30%) of overweight and obesity among pregnant women in tertiary hospital settings [4], use of a mobile app to promote healthy dietary intake is feasible and likely a promising intervention strategy. Our findings showed feasibility and acceptability of such a food-coaching smartphone app. For example, 75% of the app users found that the app was easy to operate, and more than 80% thought the food-coaching guidance was acceptably fast. Furthermore, 90% reported that the app had somewhat or greatly improved their own diet.
Interestingly, we did observe a trend of lower energy, carbohydrate, protein, total fat, and cholesterol intake in the first 4 weeks of the intervention, while most of the macronutrients did not maintain the same trend at 8 weeks of follow-up, except for cholesterol. This may be because pregnant subjects were more inclined to remember food items that are high in cholesterol (ie, saturated fat, red meat, full-fat dairy products) and were able to make an effort to avoid such food items compared with others. However, further studies are needed to verify our findings, as such findings with a small sample might be biased. Although the effect estimates are in the desired direction and supported the proof of concept as the primary focus in this pilot RCT, we still need to further verify the utility of such a smartphone app in a larger targeted population with an earlier phase intervention during pregnancy and a longer follow-up.
The strength of our study included a prospective RCT study design and the use of standard protocols in anthropometric measures and dietary assessments. However, our study has significant limitations regarding the loss to follow-up and a small sample for analysis.
In conclusion, our study provides proof of concept that smartphone technology is feasible and acceptable in clinical dietary guidance among overweight and obese pregnant women. In the future, we will adopt this food-coaching smartphone app and test its utility in a larger setting with a targeted population, earlier intervention, and longer follow-up throughout pregnancy.
Acknowledgments
This study is supported by Singapore National Medical Research Council Centre Grant (NMRC/CG/C008A/2017_KKH). L-JL is funded by Singapore National Medical Research Council Transition Award (NMRC TA/0027/2014) and (NMRC/CG/C008A/2017_KKH).
Abbreviations
- KKH
KK Women’s and Children’s Hospital
- RCT
randomized controlled trial
- SMS
short messaging service
Appendix
The electronic interface layout of the food-coaching smartphone app (Glycoleap).
Self-evaluation of acceptability on the GlycoLeap application during pregnancy.
Food diary form.
CONSORT‐EHEALTH checklist (V 1.6.1).
Footnotes
Conflicts of Interest: None declared.
Editorial notice: This randomized study was not prospectively registered. The editor granted an exception of ICMJE rules for prospective registration of randomized trials because the risk of bias appears low and the study was considered formative. However, readers are advised to carefully assess the validity of any potential explicit or implicit claims related to primary outcomes or effectiveness.
References
- 1.Torloni MR, Betrán A P, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009 Mar;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 2.Jolly MC, Sebire NJ, Harris JP, Regan L, Robinson S. Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003 Nov 10;111(1):9–14. doi: 10.1016/s0301-2115(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 3.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. http://dx.plos.org/10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong Y, Cai S, Lin H, Soh SE, Lee Y, Leow MK, Chan Y, Chen L, Holbrook JD, Tan K, Rajadurai VS, Yeo GS, Kramer MS, Saw S, Gluckman PD, Godfrey KM, Kwek K, GUSTO study group Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth. 2014 Oct 02;14:345. doi: 10.1186/1471-2393-14-345. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devlieger R, Benhalima K, Damm P, Van Assche A, Mathieu C, Mahmood T, Dunne F, Bogaerts A. Maternal obesity in Europe: where do we stand and how to move forward? A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG) Eur J Obstet Gynecol Reprod Biol. 2016 Jun;201:203–8. doi: 10.1016/j.ejogrb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell S, Shaw D. The worldwide epidemic of female obesity. Best Pract Res Clin Obstet Gynaecol. 2015 Apr;29(3):289–99. doi: 10.1016/j.bpobgyn.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists. [2018-11-06]. Challenges for Overweight and Obese Women https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Challenges-for-Overweight-and-Obese-Women.
- 8.Muktabhant Benja, Lawrie Theresa A, Lumbiganon Pisake, Laopaiboon Malinee. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015 Jun 15;(6):CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013 Jan;121(1):210–2. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. 2010. Jul, [2018-11-06]. Preparing for pregnancy: women with a BMI of 30 or more https://www.nice.org.uk/guidance/ph27/chapter/1-Recommendations#recommendation-1-preparing-for-pregnancy-women-with-a-bmi-of-30-or-more.
- 11.Lee YS, Biddle S, Chan MF, Cheng A, Cheong M, Chong YS, Foo LL, Lee CH, Lim SC, Ong WS, Pang J, Pasupathy S, Sloan R, Seow M, Soon G, Tan B, Tan TC, Teo SL, Tham KW, van Dam RM, Wang J. Health Promotion Board-Ministry of Health Clinical Practice Guidelines: Obesity. Singapore Med J. 2016 Jun;57(6):292–300. doi: 10.11622/smedj.2016103. https://sma.org.sg/UploadedImg/files/SMJ/5706/5706cpg1.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutrition During Pregnancy—Eating Right for Two. [2018-11-06]. HealthHub https://www.healthhub.sg/live-healthy/928/pregnancy-nutrition-during-pregnancy-eating-right-for-two.
- 13.Flynn AC, Dalrymple K, Barr S, Poston L, Goff LM, Rogozińska E, van Poppel MNM, Rayanagoudar G, Yeo S, Barakat Carballo R, Perales M, Bogaerts A, Cecatti JG, Dodd J, Owens J, Devlieger R, Teede H, Haakstad L, Motahari-Tabari N, Tonstad S, Luoto R, Guelfi K, Petrella E, Phelan S, Scudeller TT, Hauner H, Renault K, Sagedal LR, Stafne SN, Vinter C, Astrup A, Geiker NRW, McAuliffe FM, Mol BW, Thangaratinam S, i-WIP (International Weight Management in Pregnancy) Collaborative Group Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016 May;74(5):312–28. doi: 10.1093/nutrit/nuw005. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CJ, Walker RE, Blumfield ML, Gwini S, Ma J, Wang F, Wan Y, Dickinson H, Truby H. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2018 Jul;141:69–79. doi: 10.1016/j.diabres.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Chaplais E, Naughton G, Thivel D, Courteix D, Greene D. Smartphone Interventions for Weight Treatment and Behavioral Change in Pediatric Obesity: A Systematic Review. Telemed J E Health. 2015 Oct;21(10):822–30. doi: 10.1089/tmj.2014.0197. [DOI] [PubMed] [Google Scholar]
- 16.Borgen I, Garnweidner-Holme LM, Jacobsen AF, Bjerkan K, Fayyad S, Joranger P, Lilleengen AM, Mosdøl A, Noll J, Småstuen MC, Terragni L, Torheim LE, Lukasse M. Smartphone application for women with gestational diabetes mellitus: a study protocol for a multicentre randomised controlled trial. BMJ Open. 2017 Mar 27;7(3):e013117. doi: 10.1136/bmjopen-2016-013117. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=28348183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzandipour M, Nabovati E, Anvari S, Vahedpoor Z, Sharif R. Phone-based interventions to control gestational weight gain: a systematic review on features and effects. Inform Health Soc Care. 2018 Nov 27;:1–16. doi: 10.1080/17538157.2018.1540421. [DOI] [PubMed] [Google Scholar]
- 18.Daly LM, Horey D, Middleton PF, Boyle FM, Flenady V. The Effect of Mobile App Interventions on Influencing Healthy Maternal Behavior and Improving Perinatal Health Outcomes: Systematic Review. JMIR Mhealth Uhealth. 2018 Aug 09;6(8):e10012. doi: 10.2196/10012. http://mhealth.jmir.org/2018/8/e10012/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serné EH, de Jongh RT, Eringa EC, IJzerman RG, Stehouwer CDA. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007 Jul;50(1):204–11. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]
- 20.My Healthy Plate. 2010. [2018-11-06]. Health Promotion Board https://www.ntu.edu.sg/students/undergraduate/studentservices/healthandcounselling/documents/hpb_myhealthyplate_factsheet_fa(hires).pdf.
- 21.Gilmore LA, Redman LM. Weight gain in pregnancy and application of the 2009 IOM guidelines: toward a uniform approach. Obesity (Silver Spring) 2015 Mar;23(3):507–11. doi: 10.1002/oby.20951. doi: 10.1002/oby.20951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford PB, Obarzanek E, Morrison J, Sabry ZI. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J Am Diet Assoc. 1994 Jun;94(6):626–30. doi: 10.1016/0002-8223(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 23.De Keyzer Willem, Huybrechts I, De Vriendt Veerle, Vandevijvere S, Slimani N, Van Oyen Herman, De Henauw Stefaan. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr Res. 2011;55 doi: 10.3402/fnr.v55i0.7307. doi: 10.3402/fnr.v55i0.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, Schneider KL, Merriam PA, Hébert JR. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009 Aug;19(8):553–9. doi: 10.1016/j.annepidem.2009.04.010. http://europepmc.org/abstract/MED/19576535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Generate Nutrition Information Panel (NIP) Online. [2018-11-06]. Health Promotion Board https://focos.hpb.gov.sg/eservices/NIP/Add_Product.aspx.
- 26.Chong MF, Chia A, Colega M, Tint M, Aris IM, Chong Y, Gluckman P, Godfrey KM, Kwek K, Saw S, Yap F, van Dam RM, Lee YS, GUSTO Study Group Maternal Protein Intake during Pregnancy Is Not Associated with Offspring Birth Weight in a Multiethnic Asian Population. J Nutr. 2015 Jun;145(6):1303–10. doi: 10.3945/jn.114.205948. [DOI] [PubMed] [Google Scholar]
- 27.HealthHub. 2015. [2018-11-06]. Healthy start for your pregnancy https://www.healthhub.sg/live-healthy/840/healthy-start-for-your-pregnancy.
- 28.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012 May 10;10:47. doi: 10.1186/1741-7015-10-47. https://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, Kunz R, Mol BW, Coomarasamy A, Khan KS. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012 May 16;344:e2088. doi: 10.1136/bmj.e2088. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=22596383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008 Mar;32(3):495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- 31.Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009 Jun;101(6):569–77. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- 32.Chan KL, Chen M. Effects of Social Media and Mobile Health Apps on Pregnancy Care: Meta-Analysis. JMIR Mhealth Uhealth. 2019 Jan 30;7(1):e11836. doi: 10.2196/11836. https://mhealth.jmir.org/2019/1/e11836/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Digital influence lab. [2018-11-06]. Singapore Digital Marketing Statistics - 2015 https://digitalinfluencelab.com/singapore-digital-marketing-stats/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The electronic interface layout of the food-coaching smartphone app (Glycoleap).
Self-evaluation of acceptability on the GlycoLeap application during pregnancy.
Food diary form.
CONSORT‐EHEALTH checklist (V 1.6.1).


