Abstract
Patients with comorbidities are often excluded from clinical trials, limiting the evidence base for pulmonary arterial hypertension (PAH)-specific therapies. This review aims to discuss the effect of comorbidities on the diagnosis and management of PAH. The comorbidities discussed in this review (systemic hypertension, obesity, sleep apnoea, clinical depression, obstructive airway disease, thyroid disease, diabetes, and ischaemic cardiovascular event) were chosen based on their prevalence in patients with idiopathic PAH in the REVEAL registry (Registry to EValuate Early and Long-term PAH disease management). Comorbidities can mask the symptoms of PAH, leading to delays in diagnosis and also difficulty evaluating disease progression and treatment effects. Due to the multifactorial pathophysiology of pulmonary hypertension (PH), the presence of comorbidities can lead to difficulties in distinguishing between Group 1 PH (PAH) and the other group classifications of PH. Many comorbidities contribute to the progression of PAH through increased pulmonary artery pressures and cardiac output, therefore treatment of the comorbidity may also reduce the severity of PAH. Similarly, the development of one comorbidity can be a risk factor for the development of other comorbidities. The management of comorbidities requires consideration of drug interactions, polypharmacy, adherence and evidence-based strategies. A multidisciplinary team should be involved in the management of patients with PAH and comorbidities, with appropriate referral to supportive services when necessary. The treatment goals and expectations of patients must be managed in the context of comorbidities.
Keywords: Pulmonary arterial hypertension, Comorbidities, Diagnosis, Management, Treatment
Introduction
The definition of comorbidity was first proposed by Feinstein in 1970, as the presence of ‘any distinct additional clinical entity that has existed or that may occur during the clinical course of a patient who has the index disease under study’.1 The development of comorbidities can be driven by genetics, lifestyle and societal factors, and/or the interaction of both.2 Comorbidities in pulmonary arterial hypertension (PAH) may be present at the time of PAH diagnosis or may develop during the course of treatment for PAH. The concurrence of PAH and comorbidities increases the complexity of disease management for patients, who may require multiple pharmacological interventions to treat both PAH and the comorbidity.
At the current population level, approximately one in four adults lives with two or more chronic conditions.3 In PAH, approximately three quarters of patients have at least one comorbidity,4 with patients aged 65 years and over having a greater number of comorbidities.4,5 Current research suggests that the presence of comorbid conditions in patients with PAH negatively affects outcomes.6,7 To optimize patient care, it is important that the effects of comorbidities on PAH are well understood. The 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines recommend that assessments of patients with PAH should provide information on comorbidities.8 Physicians should assess patients on a regular basis to identify clinically relevant comorbidities.8
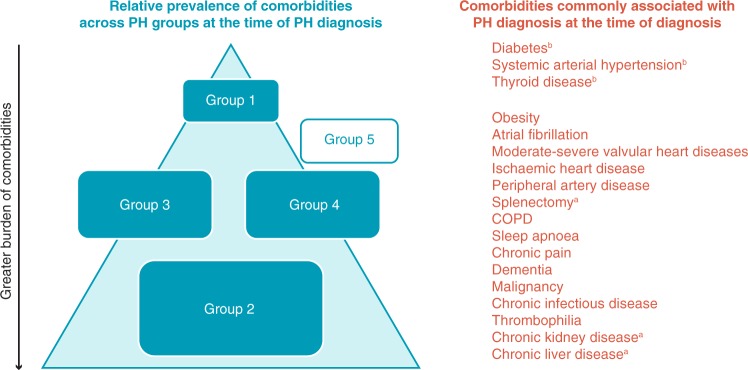
The aim of this review is to explore how comorbidities affect the diagnosis and management of PAH. There are multiple causes of PAH; however, for the purpose of this review, known associations of PAH with connective tissue disease (CTD), HIV infection, portal hypertension, congenital heart disease, and schistosomiasis,8 which can be classified as PAH sub-entities, are not considered comorbidities of PAH. Similarly, combinations of Group 1 pulmonary hypertension (PH) with Groups 2–5 PH are not discussed in detail, given these are classified as multiple causes of PH. Furthermore, pregnancy as an important transient comorbidity is not addressed here but is discussed elsewhere.9 Comorbidities discussed in this review were selected based on those with the greatest (>10%) prevalence in idiopathic PAH from the REVEAL Registry (Registry to EValuate Early and Long-term PAH disease management),10 which included patients from over 50 centres in the USA. These comorbidities were: systemic hypertension, obesity, sleep apnoea, clinical depression, obstructive airway disease, thyroid disease, diabetes mellitus, and ischaemic cardiovascular event.10 This is not an exhaustive list and there are many other comorbidities associated with PAH that have been discussed elsewhere, such as anaemia,11 chronic kidney disease,12 chronic liver disease,13 chronic pain,14,15 chronic muscle disease,16,17 frailty, peripheral vascular disease, cancer, and dementia. All comorbidities add a significant burden to PAH patients’ lives, as well as their caregivers, and presents a challenge to the treating physician. It is also important to note that, across all PH diagnoses, PAH (Group 1) patients typically present with the fewest comorbidities at the time of diagnosis (Figure 1). Summarizing the latest guidance on all of the above-listed comorbidities for all the PH groups is beyond the scope of this review, and condition-specific considerations are therefore limited to those with the greatest (>10%) prevalence in idiopathic PAH from the REVEAL Registry.
Figure 1.
The burden of comorbidities observed across pulmonary hypertension groups at the time of diagnosis in authors’ experience.18–21aComorbidities that are typically associated with pulmonary hypertension Group 5, but may affect all groups. bComorbidities that are typically associated with IPAH, but may affect all groups. This schematic is used to illustrate the typical scenarios the authors observe in clinic; it is not drawn to scale and is largely based on authors own experience rather than a robust body of evidence in the literature. The blue triangle and the rectangular ‘Group’ boxes represent the burden of comorbidity and thus, the placement of the pulmonary hypertension group boxes within the triangle, as well as their size, represent the relative prevalence of comorbidities in each group (with the lowest and largest group having the greatest prevalence). Group 5 pulmonary hypertension is shown outside the triangle because published data and the authors’ findings from the clinic are not sufficient to draw conclusions from; there is no consensus on the prevalence of comorbidities in Group 5 pulmonary hypertension at the time of diagnosis. COPD, chronic obstructive pulmonary disease; IPAH, idiopathic pulmonary arterial hypertension; PH, pulmonary hypertension.
The impact of comorbidities on diagnosing pulmonary arterial hypertension
Comorbidities in pulmonary arterial hypertension
The concurrence of PAH and comorbidities (Figure 2) increases the complexity of disease identification and management. Patients may require multiple pharmacological therapies or medical interventions for PAH and comorbidities, which can affect the choice of PAH-specific therapies due to drug interactions, dosing regimens, or contraindications. Similarly, patients may require care services to be co-ordinated and a multidisciplinary team to manage their conditions.22 However, patients with comorbidities are frequently excluded from clinical trials, and as such there is a paucity of data on the effectiveness of PAH-specific therapies in patients with multiple comorbid conditions.
Figure 2.
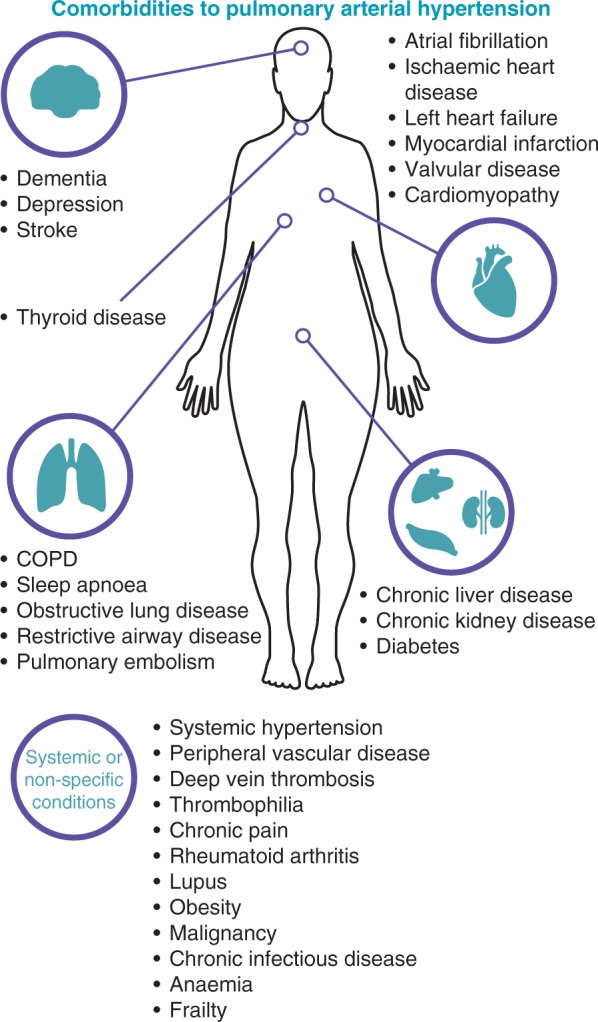
Comorbidities that have been reported in patients with idiopathic pulmonary arterial hypertension. Comorbidities are those reported in the REVEAL registry10 and supplemented by the authors’ clinical experience. COPD, chronic obstructive pulmonary disease.
In populations of patients with three or more of the following comorbidities: body mass index (BMI) ≥30 kg/m2; hypertension; diabetes; and coronary disease, patients experienced a similar treatment effect compared with those with two or fewer comorbidities. A post hoc analysis of the GRIPHON study revealed that selexipag was as effective in patients with three or more comorbidities (n = 99) as those with two or fewer (n = 653), and safety profiles for both subgroups were similar and consistent with the known profile of selexipag.23 In the AMBITION study, patients with three or more comorbidities (n = 105) experienced a similar treatment effect to first line combination therapy of ambrisentan and tadalafil compared with patients who had two or fewer comorbidities (n = 500), with good tolerability.24 Similar responses to treatment (World Health Organization functional class; exercise capacity and natriuretic peptide levels) were also observed after 12 months between patients (with ≥3 comorbidities, n = 139; with ≤2 comorbidities, n = 421) in an analysis of the COMPERA registry, who were receiving either an endothelin receptor antagonist (ERA), a phosphodiesterase-5 inhibitor (PDE-5), or a prostacyclin analogue or combination therapy.25
Differentiation of pulmonary hypertension groups
Pulmonary hypertension is a complex disease with several possible causes, which are not always mutually exclusive and management is made more difficult in cases where a patient’s diagnosis includes multiple PH groups. For example, there is high prevalence of left heart disease and/or interstitial lung disease in patients with CTD, thus it can be difficult to distinguish whether Group 1 (PAH associated with CTD), Group 2 (PH due to left heart disease), or Group 3 (PH due to lung diseases and/or hypoxia) is the leading or primary condition.8,26 It is important to identify the primary condition driving PH progression to ensure appropriate treatment.8 For example, the use of PAH-specific therapies is not recommended in patients with Groups 2 and 3 PH and there is a paucity of evidence regarding their efficacy in these patients.8
Common comorbidities such as ischaemic left heart disease, diabetes, or aortic valve stenosis increase pulmonary artery wedge pressure, which can lead to diagnostic uncertainty in the presence of precapillary disease. For example, severe aortic stenosis in a case of precapillary PH (such as Eisenmenger syndrome) can lead to combined pre- and post-capillary disease, for which the outcomes with PAH-approved therapies are uncertain.8
Confounding the diagnosis of pulmonary arterial hypertension
The presence of comorbidities can lead to delayed diagnosis by masking the symptoms of PAH,27 particularly due to difficultly identifying and differentiating between non-specific symptoms of PAH such as dyspnoea, fatigue, syncope and chest pain, and symptoms of common comorbidities. Research has shown that patients may experience symptoms for a number of years before receiving a diagnosis of PAH.28,29 Given the progressive nature of PAH, a delayed diagnosis and subsequent delay to treatment initiation can have severe consequences. The importance of screening patients for PAH to allow treatment to be started in a timely manner is discussed in more detail in a review focused on screening, also in this supplement.30 The presence of comorbidities, such as systemic hypertension, obstructive airway diseases, and obesity, can also confound the interpretation of prognostic tests for PAH such as the 6-min walk test, contributing to a delay in diagnosis. The diagnostic PAH sign of interventricular septum flattening may be mitigated by concurrent increase in left ventricular pressures, such as in the course of cardiomyopathy. It is therefore important that results are interpreted in the context of known comorbidities.6
One example of a population with a high prevalence of comorbidities is in the elderly. Among patients with idiopathic PAH, approximately a quarter of older patients (≥65 years) were found to have at least four of the following comorbidities: systemic hypertension, diabetes, ischaemic stroke, ischaemic heart disease, atrial fibrillation, obesity, or kidney dysfunction, compared with less than 7% of those aged under 65 years.4 Patients aged over 50 years with PAH were diagnosed with more advanced, severe disease compared with patients younger than 50 years in a review of the UK registry of patients with idiopathic, heritable, or anorexigen-induced PAH.5 Management considerations for an older population with PAH are discussed in more depth in a separate review in this supplement.31
Management considerations with comorbidities and pulmonary arterial hypertension
Patients with PAH and comorbidities are likely to be on complex pharmacological treatment regimens that need close monitoring and regular adjustment, with an overview by physicians from different specialties. A multidisciplinary team and network are therefore important when patients with PAH are also being treated for comorbidities. Patients with PAH and comorbidities may require different aspects of care including cardiac exercise rehabilitation (for peripheral artery disease, heart failure, myocardial infarction, and atrial fibrillation) and surgical interventions, in addition to multiple pharmacological therapies.32–37 Patients may also require additional support in their daily lives from social workers, occupational therapists, and psychotherapists. Treatment interventions, and expectations must be managed in the context of comorbidities,8 and the goals of patients with PAH may need to be reassessed following, or in preparation for, major surgical procedures required to manage a comorbidity.
The ESC/ERS treatment algorithm for treating patients with PAH8 was developed for patients with no significant cardiovascular comorbidities.38 An amended treatment algorithm that was endorsed by the Cologne Consensus Conference in 2018, takes into account patients with cardiopulmonary comorbidities, and recommended that oral monotherapy should be the first initiated treatment for PAH, regardless of risk category.38 Treatment should then be escalated in the case of an inadequate clinical response.38 This recommendation was made based on the lack of efficacy data in the population of patients with cardiovascular comorbidities, in addition to safety concerns arising from a high incidence of drug discontinuations attributed to adverse events.38
Condition-specific considerations
Systemic hypertension and ischaemic cardiovascular events
Systemic hypertension increases the risk of cardiovascular morbidity/mortality, including coronary events and stroke.39 Up to three-quarters of patients who experience a first stroke or myocardial infarction have concomitant systemic hypertension.40
Lifestyle modifications should be used to decrease the required dose levels of anti-hypertensive drugs, where appropriate.39 The use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and calcium channel blockers have all been found to cause a similar reduction in cardiovascular events on the basis of decreased blood pressure.39 Care must be taken when PAH-specific therapies are used concomitantly with anti-hypertensive medications due to the risk of excessive systemic hypotension.8 The use of beta-blockers as a first line anti-hypertensive drug should be avoided in PAH patients.
Calcium channel blockers can also be used in the treatment of PAH, and relatively high doses are needed to provide a benefit in idiopathic PAH following vasoreactivity testing.8 Patients with PAH who are treated with calcium channel blockers should be monitored closely, with complete reassessment (including right heart catheterization) after 3–4 months of therapy.8 Similarly, patients who have had an ischaemic stroke are likely to be taking anti-coagulants,41 such as warfarin. The potential benefits of anticoagulation therapy in idiopathic PAH are unclear due to contrasting evidence. Analyses of the REVEAL Registry and GRIPHON study did not demonstrate a survival benefit for patients with PAH taking anticoagulants, compared with those who were not.42,43 However, analysis of the COMPERA registry showed that during the 3-year follow-up, there was a significantly lower mortality rate in patients receiving anticoagulants compared with the non-anticoagulated group.44 Careful monitoring of patients using bosentan and warfarin is required, as bosentan increases warfarin metabolism and subsequent dose adjustments may be necessary.8
Obesity
The prevalence of obesity in patients with PAH is between 30% and 40%.10,45 Increased mortality has been observed in patients with BMI ≥40 kg/m2 with PAH aged under 65 years (hazard ratio = 3.01),45 and a higher BMI (≥30 kg/m2) has been associated with worse functional class.6 Not surprisingly, patients with obesity and PAH also had significantly lower 6-min walk distances than patients without obesity.46 These findings indicate that weight management is an important consideration in patients with PAH and who have obesity.45 The presence of obesity must be considered when using the 6-min walk test as a prognostic tool for PAH, as it may act as a confounding factor but may also contribute to PAH severity.47
Bariatric surgery for patients with obesity can positively affect PH with lower right ventricular systolic pressure (RVSP)48 and pulmonary artery pressure.49 In patients with PH and a pre-operative RVSP ≥35 mmHg who underwent bariatric surgery there was no mortality in the first 30 days following surgery, indicating that bariatric surgery can be performed safely in this population without the need for bridging therapies to improve PH.48
Obesity is linked to a number of other conditions such as sleep apnoea, insulin resistance, and obesity hypoventilation syndrome, all of which have been linked to the development of PH.50 Thus, reductions in body weight can lead to improvement in or resolution of these associated conditions as well as an improvement of PH.
Sleep apnoea and obstructive airway disease
In the REVEAL Registry, obstructive airway disease was defined as obstructive lung disease (including asthma and bronchiectasis), reactive airways disease, and chronic obstructive pulmonary disease (COPD).10 Most of these conditions, in addition to sleep apnoea, which was considered a separate comorbidity, are classified as a cause of Group 3 PH.8 Due to this, there is a paucity of research and evidence into the management of these comorbidities and PAH, despite their presence in approximately one-quarter of patients with PAH.10
When sleep apnoea is present in combination with comorbidities that also cause hypoxaemia, patients commonly present with more severe PH.50 Sleep apnoea is associated with increased pulmonary artery pressure, which can be reduced by treatment with continuous positive airway pressure.51 During the daytime, patients with PAH and sleep apnoea display lower arterial oxygen and higher arterial carbon dioxide tension compared with patients without PAH,52 which may necessitate supplemental oxygen therapy. There is limited evidence of a benefit of PAH-specific therapies in Group 3 PH (due to lung diseases and/or hypoxaemia),8 therefore, in our opinion, when patients with PAH present with or develop conditions such as sleep apnoea and COPD physicians should carefully monitor for the absence or loss of efficacy of PAH-specific therapies. The potential effects of PAH-specific therapies on the symptoms and progression of the lung disease must also be considered.8
Clinical depression
Clinical depression was observed in over 25% of patients with idiopathic PAH in the REVEAL Registry.10 However, there is some concern that the diagnosis of depression may be missed due to the overlapping symptoms of fatigue and apathy for PAH and depression.53 Furthermore, less than 25% of PH patients with a psychiatric diagnosis go on to receive psychiatric treatment.54 Given the progressive and debilitating nature of PAH, paired with the changes in lifestyle such as job loss and financial concerns, screening, identification, diagnosis and treatment of depression in this population should be prominent in PAH management.53
Despite the prevalence of depression in patients with PAH, optimal pharmacological approaches are not well defined.55 In the general population, psychotherapy is recommended in mild depression, with the addition of pharmacological therapies for moderate-to-severe depression. Selective serotonin reuptake inhibitors (SSRIs) have been associated with higher risk of mortality and clinical worsening in patients with PAH, compared with patients not taking SSRIs.56 Patients with PAH may also consider joining patient support groups to help them cope with the uncertainty associated with the disease.55,57
Thyroid disease
Thyroid disorders are classified as mechanisms for the development of Group 5 PH8; however, thyroid disease is also commonly observed concomitantly in patients with PAH (either present during the initial assessments or developing during the course of PAH).10,58 In both hypothyroidism and hyperthyroidism, cardiac output and pulmonary vascular resistance (PVR) are increased, which in turn can act as a driver for PH.59,60 As such, tests of thyroid function should be conducted as part of the investigation into PAH, and in established PAH, thyroid function tests should be conducted at least once a year or in cases of rapid deterioration.8,60
Early and aggressive treatment of patients with PAH and hyperthyroidism is critical, due to potentially fatal complications of thyroid disease.61 The increases in cardiac output and PVR can be reversed with beta-blockers and anti-thyroid medications such as propylthiouracil and saturated solutions of potassium iodide, and can return the patient to a euthyroid state.59,61 Beta-blockers are not ordinarily recommended for patients with PAH but can be used when necessitated by the presence of comorbidities.8 For example, there is the potential for a drug–drug interaction between sildenafil, which is metabolized by cytochrome (CYP)-3A4, and those beta-blockers that are CYP3A4 substrates.8 The concomitant use of PAH-specific therapies and beta-blockers should also be carefully managed to avoid excessive systemic hypotension.8
Diabetes
Glucose intolerance and insulin resistance are increasingly thought to influence both the pathogenesis and prognosis of PAH,62 and diabetes as a comorbid condition to idiopathic or heritable PAH reduces right ventricular function.63 Patients with PAH and diabetes have been shown to have significantly lower 10-year survival compared with PAH patients without diabetes.63 Identification and mitigation of modifiable risk factors for diabetes, and targeted treatment of diabetes if it develops, may delay PAH progression and improve patient quality of life.62 Patients should be educated about the risks of diabetes and PAH, and there should be regular testing for diabetes in these patients.62
Considerations regarding polypharmacy
Polypharmacy has been associated with poor treatment outcomes in the general population, which may be due to the adverse side effects of drugs, drug interactions, and/or non-adherence to the treatment regimen,64 and the impact of multiple medical conditions.65 In a study of 174 patients with PAH, the median number of drugs per patient was nine, and over 80% were taking five drugs or more.66 The side-effect profiles of PAH-specific therapies are well documented when used as monotherapy or in combination; however, new side effects may be observed when concomitantly administered with therapies for other conditions. Drug–drug interactions must also be considered (Table 1). For instance, PDE-5 inhibitors for PAH, such as sildenafil, cannot be concomitantly used with nitrates, which are used to treat a number of coronary conditions,70 and there are known interactions between anticoagulants and both prostacyclin analogues and ERAs, which contraindicate their concomitant use.71
Table 1.
Potentially significant drug interactions with drugs approved for PAH
| PAH drug | Mechanism of interaction | Interacting drug | Interaction |
|---|---|---|---|
| Ambrisentan | ? | Cyclosporine, Ketoconazole | Caution is required in the co-administration of ambrisentan with ketoconazole and cyclosporine. |
| Bosentan | CYP3A4 inducer | Sildenafil | Sildenafil levels fall 50%; bosentan levels increase 50%. May not require dose adjustments of either drug. |
| CYP3A4 substrate | Cyclosporine | Cyclosporine levels fall 50%; bosentan levels increase 4-fold. Combination contraindicated. | |
| CYP3A4 substrate | Erythromycin | Bosentan levels increase. May not require dose adjustment of bosentan during a short course. | |
| CYP3A4 substrate | Ketoconazole | Bosentan levels increase two-fold. | |
| CYP3A4 substrate + bile salt pump inhibitor | Glibenclamide | Increase incidence of elevated aminotransferases. Potential decrease of hypoglycaemic effect of glibenclamide. Combination contraindicated. | |
| CYP2C9 and CYP3A4 substrate | Fluconazole, amiodarone | Bosentan levels increase considerably. Combination contraindicated. | |
| CYP2C9 and CYP3A4 inducers | Rifampicin, phenytoin | Bosentan levels decrease by 58%. Need for dose adjustment uncertain. | |
| CYP2C9 inducer | HMG CoA reductase inhibitors | Simvastatin levels reduce 50%; similar effects likely with atorvastatin. Cholesterol level should be monitored. | |
| CYP2C9 inducer | Warfarin | Increase warfarin metabolism, may need to adjust warfarin dose. Intensified monitoring of warfarin recommended following initiation but dose adjustment usually unnecessary. | |
| CYP2C9 and CYP3A4 inducers | Hormonal contraceptives | Hormone levels decrease. Contraception unreliable. | |
| Macitentan | To be determined. | ||
| Selexipag | To be determined. | ||
| Sildenafil67 | CYP3A4 substrate | Bosentan | Sildenafil levels fall 50%; bosentan levels increase 50%. May not require dose adjustments of either drug. |
| CYP3A4 substrate | HMG CoA reductase inhibitors | May increase simvastatin/atorvastatin levels through competition for metabolism. Sildenafil levels may increase. Possible increased risk of rhabdomyolysis. | |
| CYP3A4 substrate | HIV protease inhibitors | Ritonavir and saquinovir increase sildenafil levels markedly. | |
| CYP3A4 inducer | Phenytoin | Sildenafil level may fall. | |
| CYP3A4 substrate | Erythromycin | Sildenafil levels increase. May not require dose adjustment for a short course. | |
| CYP3A4 substrate | Ketoconazole | Sildenafil levels increase. May not require dose adjustment. | |
| CYP3A4 substrate | Cimetidine | Sildenafil levels increase. May not require dose adjustment. | |
| cGMP | Nitrates, Nicorandil Molsidomine | Profound systemic hypotension, combination contraindicated. | |
| Tadalafil68 | CYP3A4 substrate | Bosentan | Tadalafil exposure decreases by 42%, no significant changes in bosentan levels.68 May not require dose adjustment. |
| cGMP | Nitrates, Nicorandil | Profound systemic hypotension, combination contraindicated. | |
| Riociguat69 | cGMP | Sildenafil, other PDE-5 inhibitors | Hypotension, severe side effects, combination contraindicated. |
| cGMP | Nitrates, Nicorandil | Profound systemic hypotension, combination contraindicated. |
cGMP, cyclic guanosine monophosphate; PDE-5, phosphodiesterase type-5; ?, unknown.
See also updated official prescribing information for each compound.
Reproduced from Galiè et al.8 with permission from Oxford University Press.
Adherence may reduce as the number of medications increases, and patients may not notice the short-term effects of occasional missed doses72; however, given the progressive nature of PAH, the loss of steady-state from repeated missed doses may have negative consequences for longer-term disease progression. Therefore, patient education through appropriate communication channels is important to maintain adherence.72 The dosing regimen of PAH-specific therapies should also be considered in patients who are taking multiple medications.73 For instance, ambrisentan, macitentan, and tadalafil are taken once per day, bosentan and selexipag are taken twice per day, and sildenafil and riociguat are taken three times daily. The pill burden of each therapy should be considered when deciding on medications for monotherapy or combination therapy, in addition to treatment regimens for comorbidities. Older adults with PAH are the most likely to have multiple comorbidities, and the administration of complex therapy regimens in these patients is more challenging.72 This is discussed in more detail in a specific review on management of PAH in older patients, which is also in this supplement.31
Conclusions
Comorbidities can mask the symptoms of PAH, which can lead to a delayed diagnosis and deleterious consequences for disease progression and survival. Similarly, the presence of comorbidities increases the difficulty of evaluating disease progression and treatment effects by confounding prognostic assessments. The management of comorbidities in addition to PAH should consider drug interactions, polypharmacy, adherence and evidence-based strategies. Thus, it is important that any and all comorbidities are identified and diagnosed so that each patient receives the optimal treatment regimen. A multidisciplinary team approach is essential in the management of patients with PAH and comorbidities. In addition to management by physicians of different specialties, patients may require social, financial, and psychological support. Furthermore, healthcare professionals must manage each patient’s treatment goals and expectations in the context of comorbidities.
Funding
Medical writing and editorial support were provided by Victoria Atess, Zoe Schafer and Richard McDonald of Watermeadow Medical, an Ashfield Company, funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Conflict of interest: I.M.L. has received grants, personal fees and non-financial support from Actelion Pharmaceuticals Ltd, and grants and personal fees from AOP Orphan Pharmaceuticals. M.P. has received grants, personal fees and non-financial support from Actelion Pharmaceuticals Ltd.
References
- 1. Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis 1970;23:455–468. [DOI] [PubMed] [Google Scholar]
- 2. Rappaport SM. Genetic factors are not the major causes of chronic diseases. PLoS One 2016;11:e0154387.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B.. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 4. Hjalmarsson C, Radegran G, Kylhammar D, Rundqvist B, Multing J, Nisell MD, Kjellstrom B.. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J 2018;51:1702310.. [DOI] [PubMed] [Google Scholar]
- 5. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, Fisher AJ, Lordan JL, Gaine S, Coghlan JG, Wort SJ, Gatzoulis MA, Peacock AJ.. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186:790–796. [DOI] [PubMed] [Google Scholar]
- 6. Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD.. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013;144:169–176. [DOI] [PubMed] [Google Scholar]
- 7. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD.. Predicting survival in pulmonary arterial hypertension. Circulation 2010;122:164–172. [DOI] [PubMed] [Google Scholar]
- 8. Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 9. Olsson KM, Channick R.. Pregnancy in pulmonary arterial hypertension. Eur Respir J 2016;25:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD.. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 11. Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, Sinclair-McGarvie M, Arnold J, Sheares KK, Morrell NW, Pepke-Zaba J.. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax 2011;66:326–332. [DOI] [PubMed] [Google Scholar]
- 12. Nickel NP, O’Leary JM, Brittain EL, Fessel JP, Zamanian RT, West JD, Austin ED.. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm Circ 2017;7:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken) 2014;4:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mensah KA, Yadav R, Trow TK, Brunet CM, Fares WH.. Lupus-associated pulmonary arterial hypertension: variable course and importance of prompt recognition. Case Rep Med 2015;2015:328435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pagani-Estévez GL, Swetz KM, McGoon MD, Frantz RP, Tointon SK, Karnyski AM, Durst LA, Watson JC.. Characterization of prostacyclin-associated leg pain in patients with pulmonary arterial hypertension. Ann Am Thorac Soc 2017;14:206–212. [DOI] [PubMed] [Google Scholar]
- 16. Bauer R, Dehnert C, Schoene P, Filusch A, Bartsch P, Borst MM, Katus HA, Meyer FJ.. Skeletal muscle dysfunction in patients with idiopathic pulmonary arterial hypertension. Respir Med 2007;101:2366–2369. [DOI] [PubMed] [Google Scholar]
- 17. Marra AM, Arcopinto M, Bossone E, Ehlken N, Cittadini A, Grunig E.. Pulmonary arterial hypertension-related myopathy: an overview of current data and future perspectives. Nutr Metab Cardiovasc Dis 2015;25:131–139. [DOI] [PubMed] [Google Scholar]
- 18. Grinnan D, Farr G, Fox A, Sweeney L.. The role of hyperglycemia and insulin resistance in the development and progression of pulmonary arterial hypertension. J Diabetes Res 2016;2016:2481659.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM, Archer SL.. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes 2018;11:e003973.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delcroix M, Howard L.. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev 2015;24:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, Heussel CP, Warth A, Kolb M, Herth FJ.. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS One 2016;11:e0151425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mercer SW, Smith SM, Wyke S, O'Dowd T, Watt GC.. Multimorbidity in primary care: developing the research agenda. Fam Pract 2009;26:79–80. [DOI] [PubMed] [Google Scholar]
- 23. Rosenkranz S, Channick R, Chin K, Jenner B, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Mclaughlin VV, Preiss R, Rubin LJ, Simonneau G, Sitbon O, Tapson V, Lang IM.. Efficacy and safety of selexipag in pulmonary arterial hypertension (PAH) patients with and without significant cardiovascular (CV) comorbidities. Eur Heart J 2019;40(Suppl.1):ehz746.0032. [Google Scholar]
- 24. Mclaughlin V, Galiè N, Barbera JA, Frost A, Ghofrani HA, Hoeper M, Peacock AJ, Simonneau G, Vachiéry J-L, Blair C, Gillies HC, Harris J, Langley J, Rubin LJ.. A comparison of characteristics and outcomes of patients with atypical and classical pulmonary arterial hypertension from the AMBITION trial. Am J Respir Crit Care Med 2015;191:A2196. [Google Scholar]
- 25. Opitz CF, Hoeper MM, Gibbs JSR, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grünig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S.. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016;68:368–378. [DOI] [PubMed] [Google Scholar]
- 26. Günther S, Jaïs X, Maitre S, Bérezné A, Dorfmüller P, Seferian A, Savale L, Mercier O, Fadel E, Sitbon O, Mouthon L, Simonneau G, Humbert M, Montani D.. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum 2012;64:2995–3005. [DOI] [PubMed] [Google Scholar]
- 27. Hoeper MM, Simon R.. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014;23:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, Frost AE, Liou TG, Turner M, Feldkircher K, Miller DP, Elliott CG.. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strange G, Gabbay E, Kermeen F, Williams T, Carrington M, Stewart S, Keogh A.. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 2013;3:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiely DG, Lawrie A, Humbert M.. Screening strategies for pulmonary arterial hypertension. Eur Heart J Suppl 2019;21:K9–K20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sitbon O, Howard L.. Management of pulmonary arterial hypertension in patients aged over 65 years. Eur Heart J Suppl 2019;21:K29–K36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keusch S, Turk A, Saxer S, Ehlken N, Grunig E, Ulrich S; On Behalf Of The Swiss Society Of Pulmonary Hypertension. Rehabilitation in patients with pulmonary arterial hypertension. Swiss Med Wkly 2017;147:w14462. [DOI] [PubMed] [Google Scholar]
- 33. Sahay S, Melendres-Groves L, Pawar L, Cajigas HR.. Pulmonary hypertension care center network: improving care and outcomes in pulmonary hypertension. Chest 2017;151:749–754. [DOI] [PubMed] [Google Scholar]
- 34. Martin B-J, Devrome A, Hauer T, Austford L, Arena R, Stone JA, Aggarwal S.. Abstract 18122: cardiac rehabilitation in subjects with peripheral arterial disease: a higher risk patient population who benefit from attendance. Circulation 2016;134(Suppl_1):A18122. [Google Scholar]
- 35. Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal HM, Lough F, Rees K, Singh S, Taylor RS.. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015;2:e000163.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piotrowicz R, Wolszakiewicz J.. Cardiac rehabilitation following myocardial infarction. Cardiol J 2008;15:481–487. [PubMed] [Google Scholar]
- 37. Younis A, Shaviv E, Nof E, Israel A, Berkovitch A, Goldenberg I, Glikson M, Klempfner R, Beinart R.. The role and outcome of cardiac rehabilitation program in patients with atrial fibrillation. Clin Cardiol 2018;41:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoeper MM, Apitz C, Grünig E, Halank M, Ewert R, Kaemmerer H, Kabitz H-J, Kähler C, Klose H, Leuchte H, Ulrich S, Olsson KM, Distler O, Rosenkranz S, Ghofrani HA.. Targeted therapy of pulmonary arterial hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272:37–45. [DOI] [PubMed] [Google Scholar]
- 39. Aronow WS. Treatment of systemic hypertension. Am J Cardiovasc Dis 2012;2:160–170. [PMC free article] [PubMed] [Google Scholar]
- 40. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y.. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:e21–e181. [DOI] [PubMed] [Google Scholar]
- 41. Fallah F. Recent strategies in treatment of pulmonary arterial hypertension, a review. Glob J Health Sci 2015;7:307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Preston IR, Roberts KE, Miller DP, Sen GP, Selej M, Benton WW, Hill NS, Farber HW.. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the registry to evaluate early and long-term pah disease management (REVEAL). Circulation 2015;132:2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tapson VF, Channick R, Chin K, Di Scala L, Farber H, Gaine S, Galiè N, Ghofrani HA, Lang I, McLaughlin V, Preiss R, Rubin LJ, Simonneau G, Sitbon O, Hoeper MM.. Anticoagulant therapy is not associated with long term outcome in patients with pulmonary arterial hypertension (PAH): insights from the GRIPHON Study. J Heart Lung Transplant 2016;35:S120. [Google Scholar]
- 44. Said K. Anticoagulation in pulmonary arterial hypertension: contemporary data from COMPERA registry. Glob Cardiol Sci Pract 2014;2014:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weatherald J, Huertas A, Boucly A, Guignabert C, Taniguchi Y, Adir Y, Jevnikar M, Savale L, Jais X, Peng M, Simonneau G, Montani D, Humbert M, Sitbon O.. Association between BMI and obesity with survival in pulmonary arterial hypertension. Chest 2018;154:872–881. [DOI] [PubMed] [Google Scholar]
- 46. Weatherald J, Huertas A, Boucly A, Sitbon O, Humbert M, Simonneau G, Montani D, Savale L, Jaïs X.. Is there an obesity paradox in pulmonary arterial hypertension? Eur Respir J 2017;50:PA3521. [Google Scholar]
- 47. Perrotta F, Nigro E, Mollica M, Costigliola A, D’Agnano V, Daniele A, Bianco A, Guerra G.. Pulmonary hypertension and obesity: focus on adiponectin. Int J Mol Sci 2019;20:912.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hanipah ZN, Mulcahy MJ, Sharma G, Punchai S, Steckner K, Dweik R, Aminian A, Schauer PR, Brethauer SA.. Bariatric surgery in patients with pulmonary hypertension. Surg Obes Relat Dis 2018;14:1581–1586. [DOI] [PubMed] [Google Scholar]
- 49. Sheu EG, Channick R, Gee DW.. Improvement in severe pulmonary hypertension in obese patients after laparoscopic gastric bypass or sleeve gastrectomy. Surg Endosc 2016;30:633–637. [DOI] [PubMed] [Google Scholar]
- 50. Friedman SE, Andrus BW.. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes 2012;2012:505274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sajkov D, McEvoy RD.. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009;51:363–370. [DOI] [PubMed] [Google Scholar]
- 52. Bady E, Achkar A, Pascal S, Orvoen-Frija E, Laaban J-P.. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax 2000;55:934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verma S, Cardenas-Garcia J, Mohapatra PR, Talwar A.. Depression in pulmonary arterial hypertension and interstitial lung diseases. N Am J Med Sci 2014;6:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lowe B, Grafe K, Ufer C, Kroenke K, Grunig E, Herzog W, Borst MM.. Anxiety and depression in patients with pulmonary hypertension. Psychosom Med 2004;66:831–836. [DOI] [PubMed] [Google Scholar]
- 55. Verma S, Sahni S, Vijayan VK, Talwar A.. Depression in pulmonary arterial hypertension: an undertreated comorbidity. Lung India 2016;33:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sadoughi A, Roberts KE, Preston IR, Lai GP, McCollister DH, Farber HW, Hill NS.. Use of selective serotonin reuptake inhibitors and outcomes in pulmonary arterial hypertension. Chest 2013;144:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Flattery MP, Pinson JM, Savage L, Salyer J.. Living with pulmonary artery hypertension: patients' experiences. Heart Lung 2005;34:99–107. [DOI] [PubMed] [Google Scholar]
- 58. Silva DR, Gazzana MB, John AB, Siqueira DR, Maia AL, Barreto SS.. Pulmonary arterial hypertension and thyroid disease. J Bras Pneumol 2009;35:179–185. [DOI] [PubMed] [Google Scholar]
- 59. Tudoran C, Tudoran M, Vlad M, Balas M, Pop GN, Parv F.. Echocardiographic evolution of pulmonary hypertension in female patients with hyperthyroidism. Anatol J Cardiol 2018;20:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marvisi M, Balzarini L, Mancini C, Mouzakiti P.. Thyroid gland and pulmonary hypertension. What's the link? Panminerva Med 2013;55:93–97. [PubMed] [Google Scholar]
- 61. Trapp CM, Elder RW, Gerken AT, Sopher AB, Lerner S, Aranoff GS, Rosenzweig EB.. Pediatric pulmonary arterial hypertension and hyperthyroidism: a potentially fatal combination. J Clin Endocrinol Metab 2012;97:2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Whitaker ME, Nair V, Sinari S, Dherange PA, Natarajan B, Trutter L, Brittain EL, Hemnes AR, Austin ED, Patel K, Black SM, Garcia JGN, Yuan Md PhD JX, Vanderpool RR, Rischard F, Makino A, Bedrick EJ, Desai AA.. Diabetes mellitus associates with increased right ventricular afterload and remodeling in pulmonary arterial hypertension. Am J Med 2018;131:702.e7–702.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Benson L, Brittain EL, Pugh ME, Austin ED, Fox K, Wheeler L, Robbins IM, Hemnes AR.. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ 2014;4:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, Hughes CM.. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5:e009235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maher RL, Hanlon J, Hajjar ER.. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014;13:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Suárez JA, Manzaneque A, Garcia NC, Creus MT, Mir JB.. DI-058 Risk of drug–drug interactions in a pulmonary arterial hypertension population. Eur J Hosp Pharm 2017;24(Suppl 1):A138. [Google Scholar]
- 67. Paul GA, Gibbs JS, Boobis AR, Abbas A, Wilkins MR.. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol 2005;60:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI.. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol 2008;48:610–618. [DOI] [PubMed] [Google Scholar]
- 69. Galiè N, Muller K, Scalise AV, Grunig E.. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J 2015;45:1314–1322. [DOI] [PubMed] [Google Scholar]
- 70. Kloner RA. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions. Am J Cardiol 2005;96:42m–46m. [DOI] [PubMed] [Google Scholar]
- 71. Ghofrani HA, Schermuly R, Weissmann N, Voswinckel R, Gall H, Seeger W, Grimminger F.. Drug interactions in pulmonary arterial hypertension and their implications. US Cardiol 2009;6:105–106. [Google Scholar]
- 72. Burks M, Stickel S, Galiè N.. Pulmonary arterial hypertension: combination therapy in practice. Am J Cardiovasc Drugs 2018;18:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grady D, Weiss M, Hernandez-Sanchez J, Pepke-Zaba J.. Medication and patient factors associated with adherence to pulmonary hypertension targeted therapies. Pulm Circ 2017;8:2045893217743616. [DOI] [PMC free article] [PubMed] [Google Scholar]