Abstract
Purpose
Diabetic peripheral neuropathic pain (DPNP) is one of the most sufferings, disabling, and dominant complications of diabetes. Duloxetine (DLX) and Pregabalin (PGB) are among first-line therapy and the most prescribed drugs for DPNP relief. The effectiveness-risk profile of drugs may differ from region to region due to variations in genetic and health situation of populations. This study aims to evaluate the efficacy and safety of DLX and PGB in a sample of Iranian population with DPNP.
Methods
A double-blind, randomized clinical trial was conducted on 180 type-2 diabetic patients with DPNP≥40 mm according to Visual Analogue Scale (VAS), with other eligibility criteria throughout twelve weeks. We divided the patients randomly into two equal groups: DLX and PGB. Each patient received ten days placebo as a washout period, then blind capsules of DLX (group 1) or PGB (group 2). We assessed the efficacy and safety of drugs by VAS and recorded the Adverse Drug Reactions (ADRs) during the study.
Results
In the DLX group, sixty-six and the PGB group, seventy-eight patients completed the study. The intensity of patients’ pain was improved by both drugs significantly (p˂0.001), but there was no significant difference between the two groups. Average daily doses of DLX and PGB were 42.5 and 235.5 mg, respectively. In the DLX group, 74% of patients and the PGB group, 37% reported ADRs. The discontinuation rates due to ADRs were 19% and 7% correspondingly.
Conclusion
We found that in Iranian patients, the mean effective doses of these drugs are different in comparison with several other studies. Surprisingly intolerance and discontinuation of DLX in our patients were attributed to mild and severe Serotonin Syndrome, which had not much occurred in other studies. Accordingly, despite the same efficacy, PGB was better tolerated than DLX in our patients. Thus we would recommend PGB for DPNP treatment in Iranian patients.
Keywords: Adverse drug reactions (ADRs), Diabetes, Diabetic peripheral neuropathic pain (DPNP), Duloxetine (DLX), Pregabalin (PGB)
Introduction
Nearly 4.5 million Iranians were suffering from diabetes in 2011 [1], and this number is expected to rise to 9.5 million by 2030 [2]. The prevalence of DPNP in patients with diabetic neuropathy was reported about 11%, 20% and 50% from the United States, England, and the Middle East, respectively [3–5]. Some studies estimated that about 30% - 50% of Iranian diabetic patients have peripheral neuropathy [6, 7]. But we did not found any reports about the prevalence of DPNP in Iranian diabetic patients; however, regarding this high incidence of peripheral neuropathy, it can be estimated that a high proportion of Iranian diabetic patients are suffering from DPNP.
On the Other hand, DPNP has adverse effects on health and quality of life and also causes expensive costs for both patients and health systems of all countries, including Iran. Furthermore, in contrast to nociceptive pain, DPNP is a more frustrating pain, which leads to persistent inconvenience, depression, and abandonment of daily activities.
The characteristics of DPNP are three distinct types of pain, including dysesthesia, paresthesia, and muscle electrical shock-like pain. The patients may also experience allodynia and hyperalgesia and severe painful coldness. These symptoms begin at the lower extremities and develop to hands by disease progression and are known as the model of stocks and gloves. The pain gets worse during the night, and origins sleep problems. The patient’s activity decreases during the day by a sense of walking on the sand and rock. Consequently, the disturbances of night sleep and reduction of daily activity will deteriorate the patients’ blood glucose and progress diabetes [8].
There are some pharmacological and non-pharmacological methods for treating DPNP. In some cases, control of blood glucose can improve DPNP. In other patients, specific modes of electrical stimulation of nerves [9], acupuncture [10], and other non-pharmacological treatments may relief DPNP. However, the pharmacotherapy of DPNP contains serotonin-norepinephrine reuptake inhibitors such as DLX, alpha-2 delta calcium channel antagonists, like PGB, tricyclic antidepressants, opioids, and topical analgesics [11]. The FDA (Food and Drug Association of America) recommended either DLX or PGA as the first-line therapy of DPNP, and they are also approved for this usage in Iran. The effectiveness and risk profile of drugs may differ from region to region due to the variations in genetic and health situations of populations [12, 13].
There have not been any studies about the comparison of efficacy, dosage pattern, and safety of DLX and PGB in Iranian patients with DPNP. Consequently, we conducted this clinical trial to compare the effectiveness and safety of DLX and PGA for DPNP treatment in a sample of Iranian patients.
Materials and methods
Patients
This study was conducted according to the Helsinki Declaration and was approved by our University Ethics Committee and also was registered in the Iranian registry of clinical trials.
We selected the volunteers from two diabetic clinics of our University of Medical Sciences through some inclusion and exclusion criteria.
The inclusion criteria comprised of: 1- Type-2 diabetes (diagnosed and confirmed by an endocrinologist according to American Diabetes Association guideline 2017 [14]; 2- Diabetes duration≥5 years 3- DPNP identified by Michigan Neuropathy Screening Instrument (MNSI) examination which the previous studies validated it [15, 16]; 4- DPNP severity≥40 mm of 11 points (0 = no pain to 10 = severe pain) on Visual Analogue Scale (VAS); 5- pain duration ≥12 months; 6-age ≥ 40and ≤ 65 years; 7-signing informed consent form.
The exclusion criteria included: 1- MNSI examination score < 2; 2- history of any hypersensitivity to DLX or PGB; 3- any hepatic, heart and renal failure; 4- hemoglobin A1c > 11 mg%; 5- vision disability; 6- intellectual disorders; 7- usage of any other analgesics; 8- consumption of any anti-inflammatory or serotonergic drugs within 14 days prior to the beginning of the study; 9- uncontrolled hypertension; 10- smoking; 11- having severe depression and psychological disorders; 12- epilepsy or any other neuropathies; 13- pain attributable to different reasons.
Medications necessary for diabetes control and any other vital drugs such as antihypertensive and anti-dyslipidemia were almost unchanged during the study period.
Study design
We designed a controlled clinical trial with 12-weeks double-blind, randomized, parallel groups, and conducted it on 180 eligible patients from July 2017 to March 2018. We calculated the sample size by using formula suggested for randomized clinical trials, considering the type I error of 5% (α = 0.05), type II error of 20% (β = 0.2; power = 80%) plus 20% dropout. We divided the patients randomly into two equal groups of DLX and PGB.
We prepared some similar empty capsules and filled them with DLX or PGB or Starch as a placebo. At first, the patients in the DLX group received placebo capsules once daily for ten days as a washout period. Then they had a fixed dose of 30 mg/d of DLX in the first week of treatment and a different dose of 30 to 60 mg/d for the next eleven weeks based on drug efficacy and tolerability. The patients in the PGA group were given placebo twice daily (Bd) for ten days as a washout period, then a fixed dose of 75 mg/Bd in the first week and 75 to 150 mg/Bd for the last eleven weeks. After these twelve weeks and at the end of our study, according to the patients’ pain recovery and their tolerability, the drugs were continued or tapered and switched to other medications.
We assessed the efficacy and safety of the drugs by recording daily phone conversations with patients and monthly physical examinations. VAS measured the intensity of patients’ pain. The patients and also the physicians who determined the VAS value and the adverse drug reactions (ADRs) were both blinded to the kinds of drugs during the study. The rates of ADRs determined the safety of the drugs, and the discontinuation of the treatment was due to the severity of ADRs.
Statistical analysis
The SPSS software, version 19.0, was used for all statistical analyses, and the p value˂0.05 was considered significant. The Kolmogorov-Smirnov test assessed the normality of the data. The mean values of demographic and biochemical characteristics of patients, the monthly intensity of DPNP, and also the incidence of ADRs between the two groups were compared by t-test. The time × treatment interaction and the comparison of mean intensity in every month with the previous month in both groups were analyzed by repeated measure analysis. The quantitative and qualitative variables were reported respectively as mean ± SD, and pure frequency plus its percentage.
Results
The consort chart
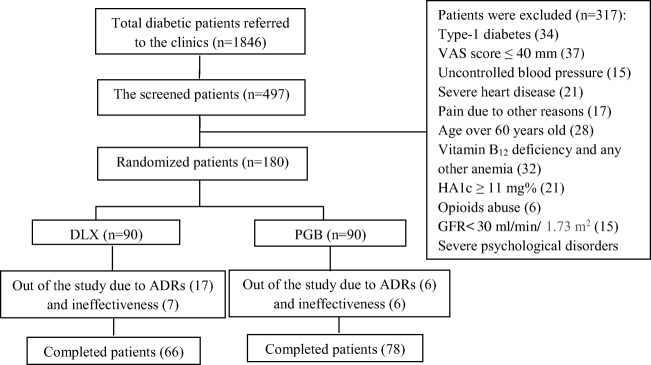
The flow diagram of studied patients is shown in Fig. 1. A total number of 1846 diabetic patients were interviewed, and 497 patients with peripheral pain were examined in two different diabetic clinics. From these screened patients, 317 were excluded. Finally, 180 patients (71 men and 109 women) were selected according to inclusion and exclusion criteria and randomly divided into two equal groups: DLX and PGB. In the DLX group, 66 patients (73%) and the PGB group, 78 patients (87%) completed the study (Fig. 1).
Fig. 1.
The consort chart of the patients: n: number, DLX: Duloxetine, PGB: Pregabalin, ADRs: Adverse Drug Reactions
The clinical characteristics of the patients
The demographic and primary clinical data of all patients in the two groups are shown in Table 1.
Table 1.
Mean demographic and clinical characteristics of patients
| Characteristics | Intervention groups Mean (SD) | p value | |
|---|---|---|---|
| DLX (N = 66) | PGB (N = 78) | ||
| Gender | |||
| Male (N) | 27 | 29 | |
| Female (N) | 39 | 49 | |
| Age (Y) | 54.93(3.70) | 54.03(4.46) | 0.388 |
| BMI (kg/m2) | 26.12(1.02) | 26.55(0.99) | 0.595 |
| HA1c (mg %) | 8.9 (1.20) | 8.7(1.72) | 0.655 |
| FBG (mg/dl) | 146.34 (13.39) | 144.74(19.44) | 0.699 |
| MNSI questionnaire | 6.65 (1.80) | 6.71(2.03) | 0.810 |
| MNSI examination | 2.69 (0.55) | 2.82(0.43) | 0.076 |
| VAS (mm) | 67.23(19.29) | 61.74(16.34) | 0.052 |
| Serum Creatinine (mg/dl) | 0.95(0.11) | 1.02(0.09) | 0.088 |
| Duration of Diabetes (Y) | 9.57(3.20) | 9.05(2.85) | 0.145 |
| Duration of DPNP (Y) | 3.55(1.66) | 4.09(2.02) | 0.067 |
SD, Standard Deviation; N, number of patients in groups that completed the study; Y, years; BMI, Body Mass Index; FBG, Fasting Blood Glucose; MNSI, Michigan Neuropathy Scale Instrument; VAS, Visual Analogue Scale
The dose profile of the drugs
We found the average daily doses of DLX and PGA during 12 weeks era of the study to be 42.5 and 235.5 mg, respectively.
The DPNP intensity in both groups
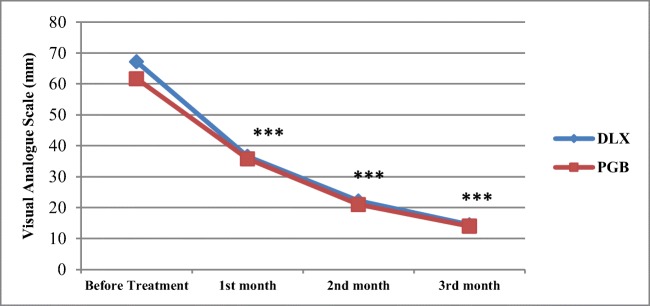
The mean DPNP intensities of both groups according to the VAS score, in different months, are presented in Fig. 2. Repeated measure analysis exhibited that in both drugs, the time × drug interaction in all time sections of the study was significant (P ˂ 0.001). The comparison of DPNP intensities in each group at monthly time sections of the study is shown in Table 2. In both DLX and PGB group, the differences between DPNP intensity at each month versus the previous month were significant (p˂0.001). But as it is evident in Fig. 2, there were no significant differences in DPNP intensities between the two groups in any time section of the study.
Fig. 2.
Visual Analogue Scale score at different time sections of the study in both groups (time× drug interaction curves), Mean VAS score of monthly time sections in each group *** p value˂0.001
Table 2.
Comparison of patients’ DPNP intensity, mean ± (SD) at time sections of the study
| DPNP mean by VAS (mm) | p- value | |
|---|---|---|
| DLX | ||
| Before treatment | 1st- month | |
| 67.2(19.3) | 32.4(8.5) | ˂0.001 |
| 1st-month | 2nd- month | |
| 32.4(8.5) | 22.3(6.4) | ˂0.001 |
| 2nd-month | 3rd-month | |
| 22.3(6.4) | 16.2(4.2) | ˂0.001 |
| PGA | ||
| Before treatment | 1st- month | |
| 61.7 (16.3) | 29.7(7.8) | ˂0.001 |
| 1st-month | 2nd-month | |
| 29.7(7.8) | 22.1(6.4) | ˂0.001 |
| 2nd-month | 3rd-month | |
| 22.3(6.4) | 16.0(5.5) | ˂0.001 |
SD: Standard Deviation; mm: millimeter
The adverse drug reactions (ADRs) in both groups
In DLX group sixty-seven (74%) of patients and PGA group thirty-four (37%) reported at least one ADR related to the medications. The rate of ADRs emerged in patients are demonstrated in Table 3. There are meaningful differences between the two groups regarding the incidence rate of some ADRs (p˂0.05, =0.01 & ˂0.001).
Table 3.
ADRs of DLX versus PGA in patients
| ADRs | DLX (N) | PGB (N) | p value |
|---|---|---|---|
| Anorexia | 22(25%) | 2(2%) | ˂0.001 |
| Nausea | 20(23%) | 2(2%) | ˂0.001 |
| Vomiting | 10(11%) | 0(0%) | ˂0.001 |
| Shivering | 16(18%) | 0(0%) | ˂0.001 |
| Agitation | 14(16%) | 0(0%) | ˂0.001 |
| Tremor | 14(16%) | 0(0%) | ˂0.001 |
| Muscle rigidity | 14(16%) | 0(0%) | ˂0.001 |
| Diaphoresis | 11(12%) | 0(0%) | ˂0.001 |
| Abdominal cramp | 14(16%) | 2(2%) | 0.02 |
| Diarrhea | 10(11%) | 3(3%) | 0.02 |
| Hyperthermia | 8(9%) | 0(0%) | ˂0.001 |
| Headache | 4 (5%) | 6(7%) | 0.75 |
| Hypertension | 10(11%) | 2(2%) | 0.01 |
| Tachycardia | 10(11%) | 2(2%) | 0.01 |
| Arrhythmia | 4(5%) | 0(0%) | 0.06 |
| Increased micturition | 8(9%) | 1(1%) | 0.01 |
| Weight gain | 0(0%) | 16(18%) | ˂0.001 |
| Dizziness | 4(5%) | 14 (15%) | 0.06 |
| Somnolence | 8(9%) | 18(20%) | 0.06 |
| Edema | 0(0%) | 11(12%) | ˂0.001 |
N, number of patients encountered ADRs
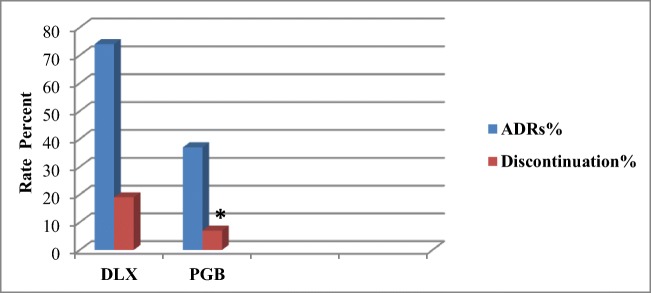
Several ADRs in each group were mild and somehow tolerable, but certain others were severe and intolerable that caused discontinuation of the drugs. The discontinuation rates due to ADRs in the DLX and PGA groups were 19% and 7% individually, which showed a significant difference between the two groups (p˂0.05). The percentages of ADRs and discontinuation rates attributable to ADRs in patients of both groups are exhibited in Fig. 3.
Fig. 3.
The Percentages of ADRs and discontinuation rates as a result of ADRs in both groups of DLX and PGB; *p < 0.05
Discussion
In this clinical trial, we compared the efficacy and safety of DLX and PGB in a sample of Iranian patients with DPNP for the first time. The monthly DPNP intensity of patients who tolerated the drugs in comparison to the previous month was significantly relieved in both groups, which are presented in Fig. 2. But according to the VAS score, there was no significant difference in efficacy of DLX compared with PGA along with the study. This result is dependable with several previous studies that examined this issue in other populations [17–24]. Among these studies, Boyle et al. [17], Tanenberg et al. [18], Devi et al. [19], compared the two drugs directly and the study of Quilici et al. [20] was a meta-analysis of nine types of research. In all these studies, parallel to our study, the DPNP relief effect of DLX and PGB did not differ significantly.
Furthermore, in our study, the prescribed dose of each drug was flexible via patients’ responsiveness. The average daily dose obtained for DLX in Iranian patients was 42.5 mg/d, which was beneath several related studies in other populations [17, 18, 20, 22, 25, 26]. The average daily therapeutic doses of DLX in two non-interventional Post-Hoc studies were 53.9 and 55.2 mg/d in German and American patients with DPNP [25, 26], which were higher than our study. Boyle et al. reported the higher doses of DLX (60 and 120 mg/d) on patients with DPNP in the United Kingdom, which both treatments were efficient without significant difference [17]. Tanenberg et al. conducted a similar study on white men from worldwide countries and reported a suitable dose of 60 mg/d for DLX [18].
Moreover, Devi et al. directed a clinical trial about the efficacy and safety of DLX and PGB in Indian patients with DPNP and prescribed flexible doses of 20 to 80 mg/d DLX via dose-responses [19]. In another clinical trial, Zakerkish et al. recommended flexible doses of 30 to 60 mg/d DLX in comparison to Nortryptilene for the patients with DPNP in Ahvaz-south of Iran [27]. Zakerkish et al. and Devi et al. did not report the average prescribed doses of drugs in their studies. The average dose of DLX in our study was almost similar to the study of Yasuda et al. in Japanese patients with DPNP that were given a fixed dose of 40 mg/d [21]. Another similarity between our study and Yasuda et al. was the average weight of patients (60 kg). Thus the resemblance of doses may be somehow related to the similarity of average weights. In the study of Raskin et al. which the average weight of patients (82 kg) was higher than our study, the effective dose of DLX was 120 mg/d that may be somewhat related to the higher weight of the patients [22]. But, in the other above mentioned articles, the average weight of patients had not been reported so we could not do further analysis of the correlation between weight and the dose of DLX.
The average daily dose of PGB in the present study was 235.5 mg/d, which is higher than 173.5 mg/d as reported by Happich et al. [25]. However, the average daily dose of PGB in our study in comparison with three other kinds of research [17, 18, 24] was lower. The PGB doses in the study of Devi et al. were flexible from 150 to 600 mg/d [19] but in the studies of Boyle et al. [17] and Tanenberg et al. [18] were fixed doses of 600/d and 300 mg/d respectively, which all are above the average dose of PGB in this study.
On the other hand, we found the ADRs of PGB to be almost in line with other previous studies. Some ADRs such as dizziness, weight gain, drowsiness, and edema emerged in PGB group was significantly more than the DLX group (Table 3). These ADRs were similar to previous studies [17–19], but surprisingly in this study, different results about kinds of ADRs of DLX were observed. In several various studies significant reported ADRs in DLX groups included anorexia, nausea, vomiting, and diaphoresis [17–22, 27], but tremor, shivering, muscle rigidity, agitation, hypertension, tachycardia that observed in a percentage of our patients were not reported that much by similar previous studies (Table 3). The emerged ADRs about DLX group in this clinical trial plus diaphoresis, hyperthermia, nausea, vomiting according to Sternbach and Hunter criteria are signs and symptoms of Serotonin Syndrome (SS). The Sternbach and Hunter criteria that we used to detect SS have been recognized as two standard procedures for assessing SS [28]. For differential diagnosis of SS, we excluded similar conditions such as neuroleptic malignant syndrome, malignant hyperthermia, sympathomimetic intoxication, anticholinergic intoxication, sedative-hypnotic withdrawal, meningitis, and encephalitis by the history of associated drugs use and clinical examination. The major causes of DLX discontinuation in our trial were moderate to severe SS. Although arrhythmia incidence in DLX group was not statistically more than PGB group (P = 0.06) but due to the importance of this ADR and the different incidence rates in two groups (5% in DLX vs. 0%in PGB), it would be clinically significant.
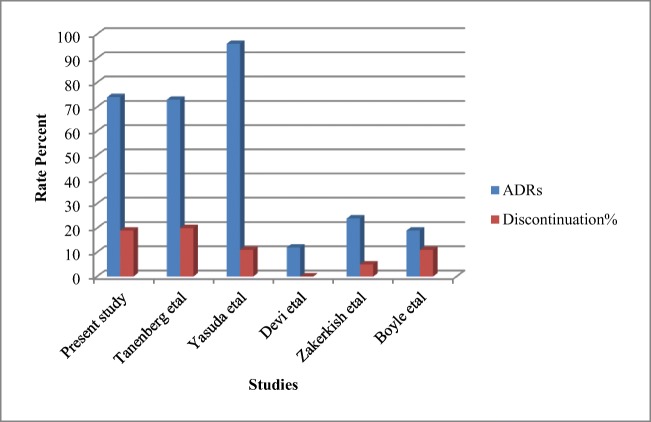
In addition to the kinds of ADRs, there were differences in rates of ADRs and discontinuation of DLX in our study as well as other studies. In this study, the ADRs and discontinuation rates were 74% and 19% about DLX, also 37% and 7% about PGB. But Devi et al. reported ADRs rates of DLX and PGB to be 9% entirely, and none of the patients discontinued the study for the reason of ADRs. According to Zakerkish et al., the ADRs of DLX versus Nortryptilene was negligible and did not result in the discontinuation of the trial [27]. Moreover, Yasuda et al. reported the rates of 96% ADRs and 22% discontinuation [21]. In the study of Boyle et al. [17], discontinuation rate of PGB group was higher than DLX group (22% vs. 11%) in contrast to our study (7% vs. 19%) and Tanenberg et al. study [18] was(10% vs. 19.6%), somehow similar to this study. The differences in rates of ADRs and discontinuation of DLX in different studies are presented in Fig. 4.
Fig. 4.
The rates of DLX Adverse Drug Reactions (ADR)% and Discontinuation % in the present study and some other studies
One of the possible explanations for different effective dose and safety of DLX in different studies may be the gene polymorphism. The specific pharmacodynamics and pharmacokinetic profiles of patients related to genotypic differences can cause diverse efficacy and safety drug profiles in various populations [29]. In 2007 in an animal model, Fox et al. reported that polymorphism in the serotonin transporter gene creates susceptibility to SS [30]. On the other hand, DLX elimination is chiefly done by hepatic metabolism. Two enzymes, CytochromeP1A2 (CYP1A2) and Cytochrome2D6 (CYP2D6), are involved in hepatic metabolism of DLX [31, 32]. In some reports, patients with CYP1A2 gene polymorphism suffered from severe ADRs of antipsychotic and anti-rheumatic drugs [33, 34]. There is a report that 24 % of Iranians have the specific gene of CYP2D6, which makes them relevant poor metabolizer. These patients suffer from ADRs of antipsychotics and antidepressants [35]. We can conclude that CYP1A2 and CYP2D6 gene polymorphism may exist in our patients who encountered ADRs.
The other factors such as social or natural environments, mental condition, nutrition, and hypoalbuminemia may also cause differences in dose-response and ADRs in different populations and regions [36, 37] which the future investigations will clarify the probable role of each one of these factors.
In conclusion, despite the equal efficacy of DLX and PGB, it seems that PGB is well tolerated than DLX for the treatment of DPNP in Iranian patients. In a different population, different dosage recommendations of DLX and PGB may be required for individual patients. Similarly, some clinical practices had recommended the lower doses of other certain drugs such as Tricyclic Antidepressants and Warfarin to reach a suitable effective dose in Iranian patients compared to Europeans and Americans [38]. Thus we highly recommend PGB rather than DLX for DPNP relief in Iranian diabetic patients, and in exceptional cases, if there is a force to use DLX, lower doses should be prescribed with extreme care for SS and probable arrhythmia.
Limitations and suggestions of the study
The Committee on Medical Ethics of our University objected having a long time placebo control group due to ethical issues in patients having pain. Thus we did not have a control group. However, each drug receiving group was considered as an active control group for another one.
As a recommendation, studies on the discovery of genetic polymorphism will help determine dosage regimens and ADRs before prescribing the medications.
Another suggestion is to design studies about the effect of other factors, such as social and natural environment, mental condition, and nutrition on efficacy and ADRs of drugs.
Acknowledgments
The present article is part of Dr. Moosareza Memari thesis for receiving Ph.D. degree in Pharmacology under the supervision of Dr. Khojasteh Joharchi at the Department of Pharmacology, School of Medicine, and was financially supported by “Research Department of the School of Medicine Shahid Beheshti University of Medical Sciences (SBUMS)” (Grant No 13/587). The authors would like to thank all faculty members in the pharmacology department of also Shohada and Mahdieh hospitals for their good corporations and also all the patients who participated in the study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of SBUMS (ethical code: IR.SBMU.MSP.REC.1395.41) and registered in the Iranian Registry of Clinical Trials website: http://www.irct.ir and the registration number is: IRCT2017012932277N1.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/13/2019
The article Efficacy and safety of duloxetine and Pregabalin in Iranian patients with diabetic peripheral neuropathic pain: a double-blind, randomized clinical trial, written by Khojasteh Joharchi, Moosareza Memari, Eznollah Azargashb, and Navid Saadat, was originally published.
Contributor Information
Khojasteh Joharchi, Email: joharchi-kh@sbmu.ac.ir.
Moosareza Memari, Email: reza_memari@sbmu.ac.ir.
References
- 1.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Noshad S, et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014;103(2):319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 2.Javanbakht M, Mashayekhi A, Baradaran HR, Haghdoost A, Afshin A. Projection of diabetes population size and associated economic burden through 2030 in Iran: evidence from micro-simulation Markov model and Bayesian meta-analysis. PLoS One. 2015;10(7):e0132505. doi: 10.1371/journal.pone.0132505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyck PJ, Kratz K, Karnes J, Litchy WJ, Klein R, Pach J, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817. doi: 10.1212/WNL.43.4.817. [DOI] [PubMed] [Google Scholar]
- 4.Abbott CA, Malik RA, Ernest R, Kulkarni J, Boulton AJ. Prevalence and Characteristics of Painful Diabetic Neuropathy in a Large Community-Based Diabetes Population in the UK. Diabetes care. 2011:DC_111108. [DOI] [PMC free article] [PubMed]
- 5.Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, et al. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res. 2011;39(2):366–377. doi: 10.1177/147323001103900204. [DOI] [PubMed] [Google Scholar]
- 6.Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, et al. Diabetes in Iran: prospective analysis from first nationwide diabetes report of National Program for prevention and control of diabetes (NPPCD-2016) Sci Rep. 2017;7(1):13461. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobhani S, Asayesh H, Sharifi F, Djalalinia S, Baradaran HR, Arzaghi SM, et al. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. Journal of Diabetes & Metabolic Disorders. 2014;13(1):97. doi: 10.1186/s40200-014-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakral G, Kim PJ, LaFontaine J, Menzies R, Najafi B, Lavery LA. Electrical stimulation as an adjunctive treatment of painful and sensory diabetic neuropathy. Los Angeles: SAGE Publications Sage CA; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrow AP, Xing M, Vere J, Verrall B, Wang L, Jude EB. Role of acupuncture in the management of diabetic painful neuropathy (DPN): a pilot RCT. Acupuncture in Medicine. 2014:acupmed-2013-010495. [DOI] [PubMed]
- 11.Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Therapeutic advances in chronic disease. 2015;6(1):15–28. doi: 10.1177/2040622314552071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agyeman AA, Ofori-Asenso R. Perspective: does personalized medicine hold the future for medicine? Journal of pharmacy & bioallied sciences. 2015;7(3):239. doi: 10.4103/0975-7406.160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maliepaard M, Nofziger C, Papaluca M, Zineh I, Uyama Y, Prasad K, et al. Pharmacogenetics in the evaluation of new drugs: a multiregional regulatory perspective. Nat Rev Drug Discov. 2013;12(2):103–115. doi: 10.1038/nrd3931. [DOI] [PubMed] [Google Scholar]
- 14.McCulloch DK, Hayward RA. Screening for type 2 diabetes mellitus. UpToDate UpToDate 2016.
- 15.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108(5):477–481. doi: 10.1016/j.clineuro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Herman W, Pop-Busui R, Braffett B, Martin C, Cleary P, Albers J, et al. Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle J, Eriksson ME, Gribble L, Gouni R, Johnsen S, Coppini DV et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes care. 2012:DC_120656. [DOI] [PMC free article] [PubMed]
- 18.Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V et al., editors. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clinic Proceedings; 2011: Elsevier. [DOI] [PMC free article] [PubMed]
- 19.Devi P, Madhu K, Ganapathy B, Sarma G, John L, Kulkarni C. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J Pharm. 2012;44(1):51. doi: 10.4103/0253-7613.91867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quilici S, Chancellor J, Löthgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9(1):6. doi: 10.1186/1471-2377-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda H, Hotta N, Kasuga M, Kashiwagi A, Kawamori R, Yamada T, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in J apanese adults with diabetic neuropathic pain: results from a randomized, 52-week, open-label study. Journal of Diabetes Investigation. 2016;7(1):100–108. doi: 10.1111/jdi.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: a 6-month open-label safety study. Pain Med. 2006;7(5):373–385. doi: 10.1111/j.1526-4637.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 23.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Parsons B, Argoff CE, Clair A, Emir B. Improvement in pain severity category in clinical trials of pregabalin. J Pain Res. 2016;9:779–785. doi: 10.2147/JPR.S102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Happich M, Schneider E, Boess FG, Wilhelm S, Schacht A, Birklein F, et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clin J Pain. 2014;30(10):875–885. doi: 10.1097/AJP.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 26.Sun P, Zhao Y, Zhao Z, Bernauer M, Watson P. Dosing pattern comparison between duloxetine and pregabalin among patients with diabetic peripheral neuropathic pain. Pain Practice. 2012;12(8):641–648. doi: 10.1111/j.1533-2500.2012.00537.x. [DOI] [PubMed] [Google Scholar]
- 27.Zakerkish M, Amiri F, Nasab NM, Ghorbani A. Comparative Efficacy of Duloxetine Versus Nortriptyline in Patients with Diabetic Peripheral Neuropathic Pain: A Double Blind Randomized Controlled Trial. Iranian Red Crescent Medical Journal. 2017;19(8).
- 28.Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533–540. [PMC free article] [PubMed] [Google Scholar]
- 29.Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Fox MA, Jensen CL, Gallagher PS, Murphy DL. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007;53(5):643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Lobo ED, Bergstrom RF, Reddy S, Quinlan T, Chappell J, Hong Q, et al. In vitro and in vivo evaluations of cytochrome P450 1A2 interactions with duloxetine. Clin Pharmacokinet. 2008;47(3):191–202. doi: 10.2165/00003088-200847030-00005. [DOI] [PubMed] [Google Scholar]
- 32.Skinner MH, Kuan HY, Pan A, Sathirakul K, Knadler MP, Gonzales CR, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003;73(3):170–177. doi: 10.1067/mcp.2003.28. [DOI] [PubMed] [Google Scholar]
- 33.Tay JK, Tan CH, Chong S-A, Tan E-C. Functional polymorphisms of the cytochrome P450 1A2 (CYP1A2) gene and prolonged QTc interval in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31(6):1297–1302. doi: 10.1016/j.pnpbp.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Grabar PB, Rozman B, Tomšič M, Šuput D, Logar D, Dolžan V. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur J Clin Pharmacol. 2008;64(9):871–876. doi: 10.1007/s00228-008-0498-2. [DOI] [PubMed] [Google Scholar]
- 35.Bagheri A, Kamalidehghan B, Haghshenas M, Azadfar P, Akbari L, Sangtarash MH, et al. Prevalence of the CYP2D6* 10 (C100T),* 4 (G1846A), and* 14 (G1758A) alleles among Iranians of different ethnicities. Drug Design, Development and Therapy. 2015;9:2627. doi: 10.2147/DDDT.S79709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi pharmaceutical journal. 2014;22(2):83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bushra R, Aslam N, Khan AY. Food-drug interactions. Oman Medical Journal. 2011;26(2):77–83. doi: 10.5001/omj.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy: a randomized, double-blind trial. Neurosciences. 2014;19(3):192. [PMC free article] [PubMed] [Google Scholar]