Abstract
Background
Polycystic ovary syndrome (PCOS) is characterised by infrequent or absent ovulation, and high levels of androgens and insulin (hyperinsulinaemia). Hyperinsulinaemia occurs secondary to insulin resistance and is associated with an increased biochemical risk profile for cardiovascular disease and an increased prevalence of diabetes mellitus. Insulin‐sensitising agents such as metformin may be effective in treating PCOS‐related anovulation. This is an update of Morley 2017 and only includes studies on metformin.
Objectives
To evaluate the effectiveness and safety of metformin in combination with or in comparison to clomiphene citrate (CC), letrozole and laparoscopic ovarian drilling (LOD) in improving reproductive outcomes and associated gastrointestinal side effects for women with PCOS undergoing ovulation induction.
Search methods
We searched the following databases from inception to December 2018: Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO and CINAHL. We searched registers of ongoing trials and reference lists from relevant studies.
Selection criteria
We included randomised controlled trials of metformin compared with placebo, no treatment, or in combination with or compared with CC, letrozole and LOD for women with PCOS subfertility.
Data collection and analysis
Two review authors independently assessed studies for eligibility and bias. Primary outcomes were live birth rate and gastrointestinal adverse effects. Secondary outcomes included other pregnancy outcomes and ovulation. We combined data to calculate pooled odds ratios (ORs) and 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I2 statistic and reported quality of the evidence for primary outcomes and reproductive outcomes using GRADE methodology.
Main results
We included 41 studies (4552 women). Evidence quality ranged from very low to moderate based on GRADE assessment. Limitations were risk of bias (poor reporting of methodology and incomplete outcome data), imprecision and inconsistency.
Metformin versus placebo or no treatment
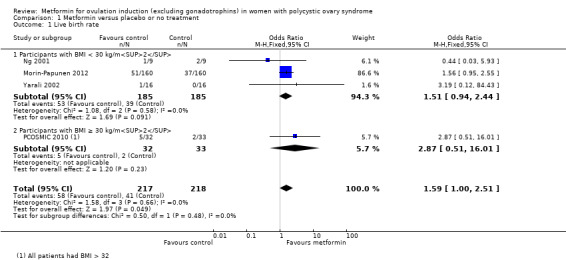
The evidence suggests that metformin may improve live birth rates compared with placebo (OR 1.59, 95% CI 1.00 to 2.51; I2 = 0%; 4 studies, 435 women; low‐quality evidence). For a live birth rate of 19% following placebo, the live birth rate following metformin would be between 19% and 37%. The metformin group probably experiences more gastrointestinal side effects (OR 4.00, 95% CI 2.63 to 6.09; I2 = 39%; 7 studies, 713 women; moderate‐quality evidence). With placebo, the risk of gastrointestinal side effects is 10% whereas with metformin this risk is between 22% and 40%. There are probably higher rates of clinical pregnancy (OR 1.98, 95% CI 1.47 to 2.65; I2 = 30%; 11 studies, 1213 women; moderate‐quality evidence). There may be higher rates of ovulation with metformin (OR 2.64, 95% CI 1.85 to 3.75; I2 = 61%; 13 studies, 684 women; low‐quality evidence). We are uncertain about the effect on miscarriage rates (OR 1.08, 95% CI 0.50 to 2.35; I2 = 0%; 4 studies, 748 women; low‐quality evidence).
Metformin plus CC versus CC alone
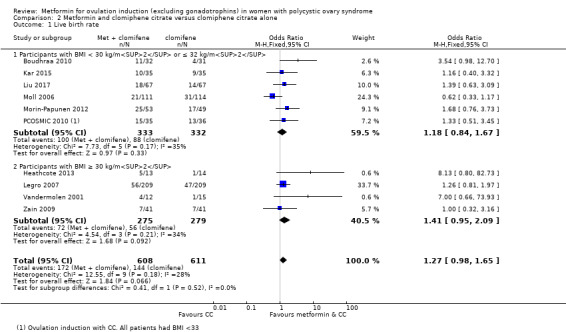
We are uncertain if metformin plus CC improves live birth rates compared to CC alone (OR 1.27, 95% CI 0.98 to 1.65; I2 = 28%; 10 studies, 1219 women; low‐quality evidence), but gastrointestinal side effects are probably more common with combined therapy (OR 4.26, 95% CI 2.83 to 6.40; I2 = 8%; 6 studies, 852 women; moderate quality evidence). The live birth rate with CC alone is 24%, which may change to between 23% to 34% with combined therapy. With CC alone, the risk of gastrointestinal side effects is 9%, which increases to between 21% to 37% with combined therapy. The combined therapy group probably has higher rates of clinical pregnancy (OR 1.62, 95% CI 1.32 to 1.99; I2 = 31%; 19 studies, 1790 women; moderate‐quality evidence). The combined group may have higher rates of ovulation (OR 1.65, 95% CI 1.35 to 2.03; I2 = 63%;21 studies, 1568 women; low‐quality evidence). There was no clear evidence of an effect on miscarriage (OR 1.35, 95% CI 0.91 to 2.00; I2 = 0%; 10 studies, 1206 women; low‐quality evidence).
Metformin versus CC
When all studies were combined, findings for live birth were inconclusive and inconsistent (OR 0.71, 95% CI 0.49 to 1.01; I2 = 86%; 5 studies, 741 women; very low‐quality evidence). In subgroup analysis by obesity status, obese women had a lower birth rate in the metformin group (OR 0.30, 95% CI 0.17 to 0.52; 2 studies, 500 women), while the non‐obese group showed a possible benefit from metformin, with high heterogeneity (OR 1.71, 95% CI 1.00 to 2.94; I2 = 78%, 3 studies, 241 women; very low‐quality evidence). However, due to the very low quality of the evidence we cannot draw any conclusions. Among obese women taking metformin there may be lower rates of clinical pregnancy (OR 0.34, 95% CI 0.21 to 0.55; I2 = 0%; 2 studies, 500 women; low‐quality evidence) and ovulation (OR 0.29, 95% CI 0.20 to 0.43; I2 = 0%; 2 studies, 500 women; low‐quality evidence) while among non‐obese women, the metformin group may have more pregnancies (OR 1.56, 95% CI 1.06 to 2.29; I2 = 26%; 6 studies, 530 women; low‐quality evidence) and no clear difference in ovulation rates (OR 0.80, 95% CI 0.52 to 1.25; I2 = 0%; 5 studies, 352 women; low‐quality evidence). We are uncertain whether there is a difference in miscarriage rates between the groups (overall: OR 0.92, 95% CI 0.51 to 1.66; I2 = 36%; 6 studies, 781 women; low‐quality evidence) and no studies reported gastrointestinal side effects.
Authors' conclusions
Our updated review suggests that metformin may be beneficial over placebo for live birth however, more women probably experience gastrointestinal side effects. We are uncertain if metformin plus CC improves live birth rates compared to CC alone, but gastrointestinal side effects are probably increased with combined therapy. When metformin was compared with CC, data for live birth were inconclusive, and the findings were limited by lack of evidence. Results differed by body mass index (BMI), emphasising the importance of stratifying results by BMI. No studies reported gastrointestinal side effects in this comparison. Due to the low quality of the evidence, we are uncertain of the effect of metformin on miscarriage in all three comparisons.
Keywords: Female; Humans; Pregnancy; Abortion, Spontaneous; Birth Rate; Body Mass Index; Clomiphene; Clomiphene/therapeutic use; Fertility Agents, Female; Fertility Agents, Female/therapeutic use; Infertility, Female; Infertility, Female/therapy; Metformin; Metformin/therapeutic use; Ovary; Ovary/surgery; Ovulation Induction; Ovulation Induction/methods; Polycystic Ovary Syndrome; Polycystic Ovary Syndrome/complications; Pregnancy Outcome; Pregnancy Rate; Randomized Controlled Trials as Topic
Plain language summary
Metformin for ovulation induction in women with a diagnosis of polycystic ovary syndrome and subfertility
Review question
Researchers reviewed the evidence about the effectiveness and safety of metformin compared with other ovulation induction agents, for inducing ovulation in women with polycystic ovary syndrome (PCOS). Of interest were live birth rate, gastrointestinal side effects and additional reproductive outcomes.
Background
Women with PCOS often have infrequent or no periods because they do not ovulate (release an egg), which can result in infertility. They may also develop problems such as obesity and diabetes. High levels of insulin, a hormone that allows the body to use sugar for energy, may be a cause of PCOS and levels are generally higher in obese women. Metformin helps the body use insulin more effectively and improves ovulation in women with PCOS. However, metformin may cause side effects such as nausea, diarrhoea or constipation (gastrointestinal side effects).
Study characteristics
We searched for studies in women with PCOS that compared metformin alone or with CC, letrozole or LOD, against CC, letrozole, LOD, placebo (sham treatment) or no treatment. This review updates the previous version of the review. We included 41 randomised controlled trials (where women were randomly allocated to a treatment) with 4552 women. 13 studies are new for this update. We combined results from the studies and assessed the quality of the studies to judge how confident we could be in their results. The evidence is current up to December 2018.
Key results
Metformin versus placebo/no treatment
Metformin may increase the chances of having a live birth compared with no treatment or placebo, however women taking metformin probably experience more gastrointestinal side effects. With placebo, the live birth rate is 19%, and it would be between 19% and 37% with metformin. The risk of gastrointestinal side effects is 10% with placebo, but higher with metformin, between 22% and 40%. Women taking metformin are probably more likely to get pregnant and may be more likely to ovulate. We are uncertain about the effect of metformin compared to placebo or no treatment on miscarriage.
Metformin plus CC versus CC alone
We are uncertain if metformin plus CC improves live birth rate compared to CC alone, but gastrointestinal side effects are probably more common. The live birth rate with CC alone is 24% which may change to between 23% to 34% with metformin and CC combined. With CC alone, the risk of gastrointestinal side effects is 9%, which increases to between 21% to 37% with metformin and CC combined. However, pregnancy rate is probably improved with metformin and CC. Ovulation rates may be improved with metformin and CC. There was no clear evidence of an effect on miscarriage.
Metformin versus CC
We combined all the studies and found that the quality of evidence was very low, results were inconsistent, and we could not confidently draw conclusions. Obese women had a lower birth rate with metformin, while non‐obese women showed a possible benefit from metformin. The live birth rate of non‐obese women with CC is 26%, which may increase to between 26% and 50% with metformin. However, in obese women, the live birth rate is 22% which may decrease to between 5% to 13% with metformin. Similarly, among obese women taking metformin there may be lower rates of clinical pregnancy and ovulation while, non‐obese women taking metformin may have more pregnancies; there was no clear difference in ovulation rates. We are uncertain whether there is a difference in miscarriage rates between women taking metformin or CC. No studies reported gastrointestinal side effects. It is possible that a woman's body mass index (a measure of healthy weight based on height and weight) affects which treatment she should take, although further research is required to establish this. The limited improvement in outcomes such as diabetes with metformin highlights the importance of weight loss and lifestyle adjustment, particularly in overweight women with PCOS.
Quality of the evidence
The quality of the evidence ranged from very low to moderate. The main problems were that the studies’ methods were poor or unclear, or they did not report all their results (risk of bias), or they were inaccurate and inconsistent.
Summary of findings
Summary of findings for the main comparison. Metformin compared with placebo or no treatment for women with polycystic ovary syndrome.
| Metformin compared with placebo or no treatment for women with polycystic ovary syndrome | ||||||
| Patient or population: women with polycystic ovary syndrome Settings: outpatient Intervention: metformin Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Metformin | |||||
| Live birth rate per woman | 188 per 1000 | 269 per 1000 (188 to 368) | OR 1.59 (1.00 to 2.51) | 435 (4 studies) | ⊕⊕⊝⊝ Lowa,b | |
| Adverse events (gastrointestinal) per woman | 97 per 1000 | 302 per 1000 (221 to 397) |
OR 4.00 (2.63 to 6.09) |
713 (7 studies) |
⊕⊕⊕⊝ Moderatea,c | I2 = 39% due to 1 study PCOSMIC 2010 |
| Clinical pregnancy rate per woman | 153 per 1000 | 263 per 1000 (210 to 323) |
OR 1.98 (1.47 to 2.65) |
1213 (11 studies) | ⊕⊕⊕⊝ Moderatea | |
| Ovulation rate per woman | 242 per 1000 |
457 per 1000 (371 to 545) |
OR 2.64 (1.85 to 3.75) |
684 (13 studies) | ⊕⊕⊝⊝ Lowa,d | I2 = 61% (82% in non‐obese group) |
| Miscarriage rate per woman | 35 per 1000 | 38 per 1000 (20 to 89) |
OR 1.08 (0.50 to 2.35) | 748 (4 studies) | ⊕⊕⊝⊝ Lowa,b | Miscarriage rate per pregnancy: OR 0.58, 95% CI 0.25 to 1.34; 200 pregnancies |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias related to failure to report methods of randomisation and/or serious risk of attrition bias in some of the studies. bDowngraded one level for serious imprecision as the event rate is low and findings are compatible with benefit in one or both groups or with no meaningful difference between the groups. cModerate inconsistency (I2 = 39%), but not downgraded, as all heterogeneity is attributable to a single small study and the direction of effect largely consistent. dDowngraded one level for serious inconsistency (I2 = 62%)
Summary of findings 2. Metformin combined with clomiphene citrate versus clomiphene citrate alone for women with polycystic ovary syndrome.
| Metformin combined with clomiphene citrate versus clomiphene citrate alone for women with polycystic ovary syndrome | ||||||
| Population: women with polycystic ovary syndrome Setting: outpatient Intervention: metformin combined with ovulation induction agent clomiphene citrate Comparison: Clomiphene citrate alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with CC alone | Risk with metformin combined with CC | |||||
| Live birth rate per woman | 236 per 1000 | 281 per 1000 (232 to 337) |
OR 1.27 (0.98 to 1.65) |
1219 (10 studies) | ⊕⊕⊝⊝ Lowa,b | |
| Adverse events (gastrointestinal) per woman | 85 per 1000 | 283 per 1000 (208 to 372) |
OR 4.26 (2.83 to 6.40) | 852 (6 studies) | ⊕⊕⊕⊝ Moderatea,c | |
| Clinical pregnancy rate per woman | 277 per 1000 | 383 per 1000 (336 to 432) |
OR 1.62 (1.32 to 1.99) | 1790 (19 studies) | ⊕⊕⊕⊝ Moderatea | |
| Ovulation rate per woman | 507 per 1000 | 629 per 1000 (581 to 676) |
OR 1.65 (1.35 to 2.03) |
1601 (22 studies) | ⊕⊕⊝⊝ Lowa,d,e | |
| Miscarriage rate per woman | 77 per 1000 | 101 per 1000 (70 to 142) |
OR 1.35 (0.91 to 2.00) | 1206 (10 studies) | ⊕⊕⊝⊝ Lowa,b | Miscarriage rate per pregnancy: OR 1.07 95% CI 0.69 to 1.66; 471 pregnancies |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CC: clomiphene citrate; CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias related to failure to describe study methods and/or serious risk of attrition bias in several of the studies. bDowngraded one level for serious imprecision as findings are compatible with benefit in one or both groups or with no meaningful difference between the group. cSome evidence of imprecision seen in obese group however only one study included therefore not downgraded, given clear effect seen in BMI < 30 kg/m2 group. dHigh heterogeneity (I2 = 63%), but not downgraded as direction of effect consistent and most inconsistency is due to a single small study. eDowngraded one level for evidence of publication bias seen with three studies outside the funnel plot and asymmetry around the line of effect
Summary of findings 3. Metformin compared with clomiphene citrate for women with polycystic ovary syndrome.
| Metformin compared with clomiphene citrate for women with polycystic ovary syndrome | ||||||
| Population: women with polycystic ovary syndrome Setting: outpatient Intervention: metformin Comparison: clomiphene citrate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with CC | Risk with metformin | |||||
|
Live birth rate per woman aParticipants with BMI < 30 kg/m2 or ≤ 32 kg/m2 |
256 per 1000 | 371 per 1000 (256 to 503) |
OR 1.71 (1.00 to 2.94) | 241 (3 studies) |
⊕⊝⊝⊝ very lowb,c,d |
High heterogeneity (I2 = 78%) 76 events |
|
Live birth rate per woman aParticipants with BMI ≥ 30 kg/m2 |
216 per 1000 | 76 per 1000 (45 to 125) |
OR 0.30 (0.17 to 0.52) |
500 (2 studies) |
⊕⊝⊝⊝ very lowb,c,d | 73 events |
|
Adverse events (gastrointestinal) |
Not reported by any of the included studies | |||||
| Clinical pregnancy rate per womanaParticipants with BMI < 30 kg/m2 or ≤ 32 kg/m2 | 258 per 1000 | 352 per 1000 (270 to 444) | OR 1.56 (1.06 to 2.29) | 530 (6 studies) | ⊕⊕⊝⊝ lowb,e | 160 events |
|
Clinical pregnancy rate per woman aParticipants with BMI ≥ 30 kg/m2 |
276 per 1000 | 115 per 1000 (74 to 173) | OR 0.34 (0.21 to 0.55) | 500 (2 studies) | ⊕⊕⊝⊝ lowb,c | 98 events |
|
Ovulation rate per woman fParticipants with BMI < 30 kg/m2 |
650 per 1000 | 597 per 1000 (491 to 699) | OR 0.80 (0.52 to 1.25) | 352 (5 studies) | ⊕⊕⊝⊝ lowb,c | 220 events |
|
Ovulation rate per woman fParticipants with BMI ≥ 30 kg/m2 |
516 per 1000 | 236 per 1,000 (176 to 314) | OR 0.29 (0.20 to 0.43) | 500 (2 studies) | ⊕⊕⊝⊝ lowb,c | 188 events |
|
Miscarriage rate per woman aParticipants with BMI < 30 kg/2 |
57 per 1000 | 83 per 1000 (36 to 182) | OR 1.51 (0.62 to 3.71) | 281 (4 studies) |
⊕⊕⊝⊝ lowb,c | 20 events Miscarriage rate per pregnancy: OR 1.02 (0.41 to 2.54) |
|
Miscarriage rate per woman aParticipants with BMI ≥ 30 kg/m2 |
64 per 1000 | 40 per 1000 (18 to 86) | OR 0.61 (0.27 to 1.38) | 500 (2 studies) | ⊕⊕⊝⊝ lowb,c | 26 events; only 1 study with events Miscarriage rate per pregnancy: OR 1.92 (0.72 to 5.12) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aData subgrouped by BMI, as pooling of BMI groups resulted in high heterogeneity (I2 > 85%) with differing directions of effect. bEvidence downgraded one level for risk of bias. cEvidence downgraded one level for serious imprecision: low event rate and/or wide confidence intervals. dEvidence downgraded for high heterogeneity. eEvidence downgraded for serious imprecision; many small studies with wide confidence intervals. fData subgrouped by BMI, as pooling of BMI groups resulted in high heterogeneity (I2 = 74%), though direction of effect was consistent.
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting at least 8% to 13% of women of reproductive age (Bozdag 2016; NHMRC 2018; Teede 2018). The disorder is heterogeneous, encompassing a broad spectrum of signs and symptoms of ovarian dysfunction. The classic presentation, as described by Stein and Leventhal (Stein 1935), with features of obesity, amenorrhoea and hirsutism is one end of the spectrum that, at the other end, includes women with normal menstrual cyclicity and yet with ultrasound, evidence of a polycystic ovarian appearance (Fauser 2012). Therefore, no single diagnostic criterion (such as hyperandrogenism or polycystic ovaries (PCO)) is sufficient for the clinical diagnosis. The 2003 Rotterdam Consensus' revised diagnostic criteria for a diagnosis of PCOS are as follows, with two of the following being required: 1. oligo or anovulation, or both, that is, menstrual disturbance; 2. clinical or biochemical signs, or both, of hyperandrogenism; 3. PCO on ultrasound; and exclusion of other aetiologies of menstrual disturbance and hyperandrogenism (such as congenital adrenal hyperplasia, androgen‐secreting tumours, Cushing's syndrome) (ESHRE/ASRM 2004). A recent update to guidelines in view of advancing ultrasound technology and resolution, state that the diagnostic criteria for ultrasound PCO morphology is either 20 or more follicles per ovary or increased ovarian volume, over 10 mL, when using a transvaginal ultrasound scan (NHMRC 2018).
Although PCOS is the commonest cause of anovulatory infertility (Balen 2014), up to 70% of women with PCOS remain undiagnosed (March 2010).
The expression of PCOS symptoms is multifaceted, and the reduced conception rates associated with PCOS may be related to hyperandrogenism, obesity and insulin resistance (Balen 2014). Over the last 20 years, the body of evidence indicating that increased insulin resistance and compensatory high insulin concentrations (hyperinsulinaemia) play a key role in the pathogenesis of PCOS has grown (Balen 2014; Rubin 2017). Insulin resistance is more common in overweight women but can also occur in non‐obese women with the disorder (Cassar 2016).
The insulin resistance associated with PCOS can worsen both women's symptom profile and their likelihood of achieving a live birth (Cassar 2016). Women with insulin resistance have a significantly higher level of testosterone and increased prevalence of hirsutism than women with non‐insulin‐resistant PCOS (Azziz 2016). Insulin‐resistant women with PCOS also have a lower ovulation rate and are more likely to develop resistance to ovulation induction with clomiphene citrate (CC) compared with women with non‐insulin resistant PCOS. Lifestyle modification including weight loss and exercise reduces central fat and improves insulin sensitivity, restoring ovulation in overweight, infertile women with PCOS (Azziz 2016).
The impaired glucose tolerance results can predispose women to the development of type 2 diabetes mellitus compared with the background population (Celik 2014). Celik 2014 conducted a prospective study of insulin resistance in 84 women with PCOS, with a mean follow‐up period of 2.6 years. Of those with normal glucose tolerance, 11.5% converted to insulin resistance (annual incidence rate 4.5%). This compares to 2.3% in the healthy control population (n = 45), with an annual progression of 0.9%. For women with impaired glucose tolerance at the outset, 33.3% developed diabetes (annual incidence rate 10.4%). The prevalence of insulin resistance in women with PCOS is influenced by body mass index (BMI) and at least 50% of women with PCOS are obese (Balen 2014; Cassar 2016). Correspondingly, a Mexican study found an increased prevalence of insulin resistance in obese women with PCOS compared to normal‐weight women with PCOS (78.2% and 19.3% respectively; Reyes‐Munoz 2016). Obesity, and particularly abdominal obesity as indicated by an increased waist to hip ratio, is correlated with reduced fecundity (Silvestris 2018). A small study demonstrated increased preterm birth and low birth‐weight infants in obese versus normal‐weight women with PCOS (De Frene 2014). Weight loss has been shown to improve the endocrine profile, menstrual cyclicity and the likelihood of ovulation (Silvestris 2018). Meta analyses have found that weight loss reduced testosterone and insulin resistance as well as improving reproductive outcomes (Moran 2011; Sim 2014). There is therefore considerable overlap between metabolic syndrome and the metabolic disturbances that feature in PCOS. Metabolic syndrome is a cluster of risk factors that confer an increased risk for cardiovascular disease and type II diabetes (Moran 2010). Women with metabolic syndrome may have a higher mortality from cardiovascular disease overall, coronary heart disease and stroke compared with women without the syndrome (Moran 2010). The prevalence of metabolic syndrome among women with PCOS was increased compared to the general population (OR 2.20, 95% CI 1.36 to 3.56 for BMI‐matched studies; Moran 2010). Women with PCOS are four times more likely to develop type 2 diabetes mellitus and be diagnosed four years earlier compared with non‐PCOS women (Rubin 2017). The prevalence also varies amongst different ethnic groups, which is likely to be influenced by the background prevalence of insulin resistance (Bozdag 2016). Furthermore, women with PCOS and metabolic syndrome tend to have a higher BMI, which has an increased risk of developing complications such as hypertension, insulin resistance, metabolic syndrome and endometrial hyperplasia (Sachdeva 2019). PCOS therefore affects reproductive outcomes and confers significant long‐term health risks to women. PCOS also has a significant psychological impact and is associated with low self‐esteem, anxiety and depression (Moran 2012). With the increasing prevalence of obesity in society, the prevalence of PCOS is likely to rise. There are therefore significant financial implications for the funding of PCOS management by healthcare providers. A 2005 study calculated approximately USD 4.36 billion are spent on managing reproductive‐age women with PCOS, of which USD 533 million is related to infertility (Azziz 2005; Azziz 2016).
Description of the intervention
Metformin is an antihyperglycaemic biguanide drug, widely used for the treatment of type 2 diabetes mellitus. However, the exact mechanism of action through which metformin has its glucose‐lowering effect is still being explored (Pernicova 2014). Metformin inhibits hepatic gluconeogenesis and reduces the action of glucagon, resulting in a reduction in circulating insulin and glucose. This is thought to occur via inhibition of mitochondrial complexes with downstream effects on cyclic adenosine monophosphate (AMP) and protein kinase signalling pathways. The effect on protein kinase may also modulate lipid synthesis. Metformin is known to exert its effect on several tissues affected by insulin resistance, including the liver, adipose tissue and the ovaries (Pernicova 2014).
We compared metformin with three alternative forms of ovulation induction: CC, letrozole and laparoscopic ovarian drilling (LOD).
CC is an anti‐oestrogen often used first line to induce ovulation (Balen 2017). CC is commenced on day two to five of the menstrual cycle, after pregnancy has been excluded, and given for five days. All women who are given CC are monitored by serial ultrasound assessments of follicular growth and if no menstruation by day 35, a withdrawal bleed is induced. Adverse effects of CC include luteinizing hormone (LH) hypersecretion, which reduces conception rates and increases miscarriage rates, possibly due to the anti‐oestrogen effects on the endometrium and cervical mucus (NHMRC 2018). CC can also lead to increased rates of multiple pregnancy and ovarian hyperstimulation syndrome (OHSS), and therefore close ultrasound surveillance is required.
Letrozole is an aromatase inhibitor, used for ovulation induction (Balen 2017). Letrozole inhibits the aromatisation of androgens to oestrogen and hence reduces the negative feedback otherwise induced by oestrogen on the hypothalamic‐pituitary axis. Rising levels of follicle‐stimulating hormone (FSH) leads to stimulation of follicle development, follicle maturation, and ovulation (NHMRC 2018). Improved pregnancy and live birth rates have been reported with letrozole, and reduced incidence of multiple pregnancy compared with CC (Franik 2014). However, concerns have risen regarding the possible association between letrozole use and congenital malformations (Biljan 2005). The World Health Organization (WHO) does support the use of letrozole as first‐line treatment for ovulation induction although many countries insist that more research on safety and efficacy is required (NHMRC 2018).
LOD is the surgical method of ovulation induction that has replaced the previous method of laparotomy and ovarian wedge resection (Balen 2017). LOD can be performed using monopolar, bipolar or laser diathermy to four separate points per ovary. This reduces LH and testosterone levels, leading to a resumption of regular menses. LOD provides an alternative treatment for women with CC resistance or for women who cannot be closely monitored for CC induction (NHMRC 2018). LOD may also be appropriate for women undergoing laparoscopic assessment of the pelvis for an alternative reason. A previous Cochrane Review compared the efficacy of LOD with combined metformin and CC and concluded that there was evidence of fewer live births in women with CC‐resistant PCOS undergoing LOD compared to metformin and CC (Farquhar 2012).
How the intervention might work
Increased insulin resistance, hyperandrogenism and obesity have a significant impact on menstrual cyclicity and reproductive health (Sachdeva 2019). Metformin may therefore have beneficial effects on anovulatory infertility in PCOS, with reduced hepatic glucose production, reduced levels of circulating insulin acting on the ovaries and restoration of ovarian function (Viollet 2012). Within the ovary itself, metformin may also have a direct impact on cells to reduce excessive steroidogenesis and follicular growth (Diamanti‐Kandarakis 2010). Metformin has been shown to reduce theca cell proliferation, reduce the number of small follicles and cysts, yet have higher percentages of antral follicles and corpora lutea, hence improving the chance of ovulation (Di Petro 2015).
As insulin resistance and resulting hyperinsulinaemia are key metabolic features in women with PCOS, their amelioration through metformin could improve PCOS‐associated symptoms and conception rates.
Why it is important to do this review
This is an updated Cochrane Review focusing on the impact of metformin on the reproductive outcomes in women with PCOS‐related subfertility, compared to or in combination with CC, letrozole and LOD. This follows on from previous reviews comparing the effects of metformin with thiazolidinediones including troglitazone, rosiglitazone and pioglitazone (first published in 2003 and most recently updated in 2017 (Lord 2002; Tang 2009; Tang 2012; Morley 2017). However, the most recent update in 2017 found insufficient evidence of benefit with thiazolidinediones and furthermore there has been a withdrawal of thiazolidinediones from the market due to adverse effects on liver function (FDA 2019). As a result we have excluded thiazolidinediones from this review.
The most recent 2017 update focused on live birth rate as the primary outcome. Metformin alone was found to be of benefit when compared with placebo, although the overall quality of evidence was low (Morley 2017). The live birth rate when comparing metformin versus CC was inconclusive. However, an improvement in clinical pregnancy and ovulation rates was observed with CC compared with metformin in obese women with PCOS. Results of this review differed by BMI and also by resistance to CC and maternal age. In addition, many older studies did not record live birth rate as an outcome. Anthropometric outcomes were included in the previous reviews, although these were documented inconsistently in the studies.
There is therefore scope for a Cochrane Review focusing on the reproductive outcomes in women being treated with metformin. We compared the efficacy of metformin versus alternative ovulation induction agents including CC, letrozole and LOD. A previous Cochrane Review looked specifically at gonadotrophins for ovulation induction in women with PCOS and therefore we excluded gonadotrophin therapy as a comparison from this review (Bordewijk 2017). The primary outcome of this review was the most important clinical end point, live birth rate. Subgroup analysis by BMI, maternal age and CC resistance, including high‐quality studies, will shed further light on the best management practice for anovulatory infertility.
Details of abbreviations used in this review and conversion factors of biochemical results can be found in Table 4 and Table 5, respectively.
1. Abbreviations used.
| Abbreviation | Definition |
| BMI | Body mass index |
| CC | Clomiphene citrate |
| CI | Confidence interval |
| CT | Computerised tomography scan |
| FSH | Follicle‐stimulating hormone |
| GTT | Glucose tolerance test |
| HbA1C | Glycosylated haemoglobin |
| LOD | Laparoscopic ovarian drilling |
| NIDDM | Non insulin dependent diabetes mellitus |
| PCO | Polycystic ovary |
| PCOS | Polycystic ovary syndrome |
| RCT | Randomised controlled trial |
| rFSH | Recombinant follicle‐stimulating hormone |
| SD | Standard deviation |
| SE | Standard error of the mean |
| vs | Versus |
| MD | Mean difference |
2. Conversion factors.
| Convert from | Convert to | Conversion factor | |
| Glucose | mg/dL | mmol/L | 0.056 |
| Testosterone | ng/dL | nmol/L | 0.03467 |
| Standard deviation | Standard error | Standard deviation | Sqrt n |
| Confidence intervals | Confidence intervals | Standard error | (upper limit ‐ lower limit)/3.92 |
Objectives
To evaluate the effectiveness and safety of metformin in combination with or in comparison to clomiphene citrate (CC), letrozole and laparoscopic ovarian drilling (LOD) in improving reproductive outcomes and associated gastrointestinal side effects for women with PCOS undergoing ovulation induction.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised and quasi‐randomised studies due to the high risk of bias. We included cross‐over studies but we only included data from the first phase of meta‐analyses.
Types of participants
We included women with oligo and anovulatory PCOS, based on the diagnostic criteria set by the Rotterdam Consensus (ESHRE/ASRM 2004), undergoing ovulation induction. We excluded women having in vitro fertilisation (IVF) or intracytoplasmic spermatic injection (ICSI), as this is covered in a separate Cochrane Review (Tso 2014).
Types of interventions
Metformin versus placebo or no treatment
Metformin and CC versus CC
Metformin versus CC
Metformin and letrozole versus letrozole
Metformin versus letrozole
Metformin and LOD versus LOD
Metformin versus LOD
We excluded thiazolidinediones (troglitazone, rosiglitazone and pioglitazone) because of the concerns about adverse effects such as hepatotoxicity, heart failure and bladder cancer leading to their subsequent withdrawal from the market (FDA 2019). The last update of this review found insufficient evidence of a benefit of thiazolidinediones for ovulation induction (Morley 2017).
Types of outcome measures
Primary outcomes
1. Live birth rate, as defined by included studies
2. Gastrointestinal side effects
Secondary outcomes
3. Clinical pregnancy rate, as defined by included studies (biochemical pregnancies were excluded)
4. Ovulation rate, as defined by included studies
5. Miscarriage rate
6. Multiple pregnancy rate
7. Anthropometric outcomes: BMI
8. Endocrine outcomes
a) Serum testosterone
b) Serum sex hormone‐binding globulin
9. Metabolic outcomes
a) Fasting blood glucose
b) Fasting insulin
Search methods for identification of studies
We searched for all published and unpublished RCTs without language restriction and in consultation with Cochrane Gynaecology and Fertility (CGF) Information Specialist. The original search was conducted in 2003, which included metformin and other insulin sensitisers compared with placebo or CC in PCOS. The first updated search was completed on 11 September 2008, the second update was completed on 3 October 2011, the third update was completed on 12 January 2017. The current search was completed on 13 December 2018 and included metformin only.
Electronic searches
We searched:
CGF Specialised Register of Controlled Trials, PROCITE platform (searched 13 December 2018; Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 12) via the Cochrane Register of Studies Online (CRSO) Web platform (Appendix 2);
MEDLINE Ovid (searched from 1946 to 13 December 2018; Appendix 3);
Embase Ovid (searched from 1980 to 13 December 2018; Appendix 4);
PsycINFO Ovid (searched form 1806 to 13 December 2018; Appendix 5); and
CINAHL EBSCO platform (searched from 1961 to 13 December 2018; Appendix 6).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). The Embase, PsycINFO and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) www.sign.ac.uk/search‐filters.html.
Other electronic sources of trials included:
-
trials registers for ongoing and registered trials;
ClinicalTrials.gov;
WHO International Clinical Trials Registry Platform (ICTRP);
2. PubMed and Google Scholar for recent trials not yet indexed in MEDLINE.
Searching other resources
We handsearched the reference sections of all studies obtained. In liaison with the CGF Information Specialist we searched relevant journal articles and conference abstracts that are not covered in the CGF register. We contacted study authors and experts in the field to identify additional studies.
Data collection and analysis
Selection of studies
The first review of this subject (Lord 2003), was undertaken by three review authors (JML, IHF and RJN), two of whom work in reproductive medicine (JML, RJN). Three review authors (TT, EY, AHB) updated the review (Tang 2009; Tang 2012). Three review authors (LCM, TT and AHB) performed the last update (Morley 2017). Five review authors (ANS, LCM, TT, RN and AHB) performed the current update. We employed the search strategy described previously to obtain titles and, where possible, relevant study abstracts. Two review authors (ANS and LCM) screened the titles and abstracts and then obtained copies of the relevant full‐text articles. Two review authors (ANS and LCM) independently assessed whether the studies met the inclusion criteria, with disagreements resolved by discussion with a third author (TT). For details of the screening and selection process see Figure 1.
1.
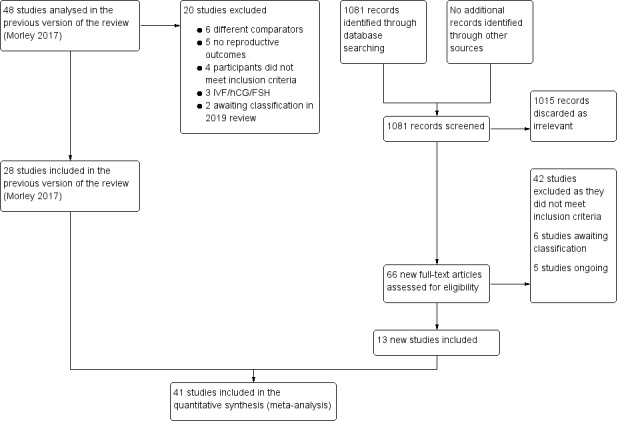
Study flow diagram 2019 update
Data extraction and management
Two review authors (ANS and LCM) independently extracted data from eligible studies onto a pre‐designed form, and resolved any disagreements by discussion with a third author (TT). Data extracted includes study characteristics and outcome data. We sought further information from the study authors where papers contained insufficient information.
Some studies were multi‐arm studies, and we excluded data from arms that did not meet the study criteria.
Assessment of risk of bias in included studies
Two review authors (ANS and LCM) independently assessed the risk of bias in accordance with the Cochrane 'Risk of bias' assessment tool (Higgins 2017).
We assessed selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias and summarised our judgements in the 'Risk of bias' tables, Figure 2 and Figure 3. We resolved disagreements by discussion. We incorporated the assessment of bias judgements into the interpretation of review findings by means of sensitivity analyses.
2.
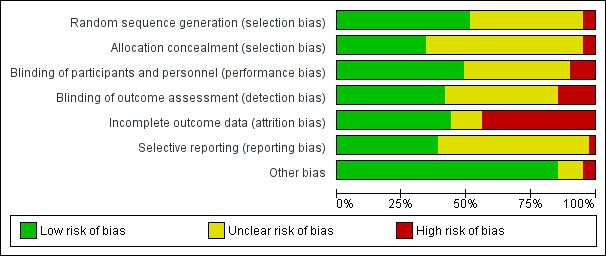
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
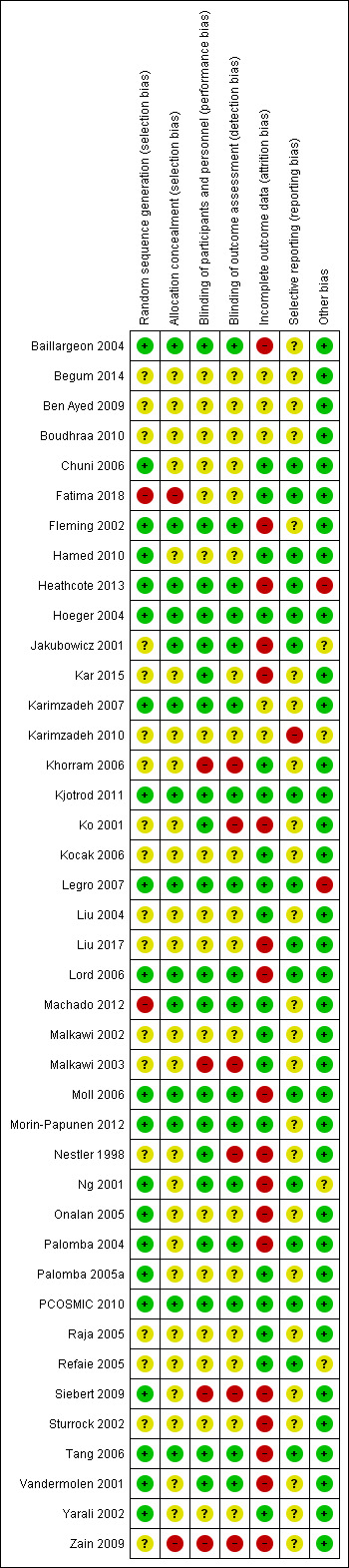
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Measures of treatment effect
We used odds ratio (OR) as the measure of effect for each dichotomous outcome and the mean difference (MD) for each continuous outcome. We have presented 95% confidence intervals (CI) for all outcomes.
Unit of analysis issues
The primary unit of analysis was each woman. For example, we calculated ovulation rate as rate of women in whom ovulation was confirmed. Where studies reported 'per‐cycle' data, we contacted the study authors to request 'per‐woman' data. When these data were not available, we did not pool the per‐cycle ovulation data but presented them in additional tables (Table 6; Table 7; Table 8; Table 9; Table 10). The exceptions to this were miscarriage and multiple pregnancy rates, which we analysed per woman, followed by a sensitivity analysis using per‐pregnancy data.
3. Metformin versus placebo: ovulation rate per cycle.
| Study ID | Metformin | Placebo | P value | ||
| Events | Cycles | Events | Cycles | ||
| BMI < 30 kg/m2 | |||||
| Baillargeon 2004 | 27 | 32 | 11 | 32 | P < 0.01 |
| Ng 2001 | 3 | 9 | 3 | 9 | P = 1.00 |
| Onalan 2005 | 17 | 153 | 20 | 150 | P = 0.81 |
| Yarali 2002 | 6 | 16 | 1 | 16 | P = 0.06 |
| BMI ≥ 30 kg/m2 | |||||
| Fleming 2002 | 37 | 45 | 30 | 47 | P = 0.05 |
| Hoeger 2004 | 3 | 9 | 6 | 11 | P = 0.35 |
| Hoeger 2004 | 4 | 9 | 3 | 9 | P = 0.63 |
| Jakubowicz 2001 | 8 | 28 | 0 | 28 | P = 0.03 |
| Lord 2006 | 9 | 22 | 9 | 22 | P = 1.00 |
| Nestler 1998 | 12 | 35 | 1 | 26 | P = 0.02 |
| Onalan 2005 | 5 | 63 | 5 | 51 | P = 0.73 |
| PCOSMIC 2010 | 17 | 32 | 13 | 33 | P = 0.27 |
| Sturrock 2002 | 0 | 12 | 1 | 14 | P = 0.54 |
| Vandermolen 2001 | 1 | 12 | 1 | 15 | P = 0.87 |
4. Metformin and clomiphene citrate versus clomiphene citrate alone: ovulation rate per cycle.
| Study ID | Metformin + clomiphene citrate | Clomiphene citrate alone | P value | ||
| Events | Cycles | Events | Cycles | ||
| BMI < 30 kg/m2 | |||||
| Ben Ayed 2009 | 10 | 16 | 6 | 16 | P = 0.16 |
| Boudhraa 2010 | 17 | 32 | 10 | 31 | P = 0.10 |
| Machado 2012 | 15 | 21 | 5 | 15 | P = 0.03 |
| Malkawi 2002 | 11 | 16 | 3 | 12 | P = 0.03 |
| Moll 2006 | 84 | 141 | 98 | 168 | P = 0.83 |
| Ng 2001 | 4 | 9 | 1 | 9 | P = 0.14 |
| PCOSMIC 2010 | 27 | 35 | 23 | 36 | P = 0.22 |
| BMI ≥ 30 kg/m2 | |||||
| Jakubowicz 2001 | 26 | 28 | 22 | 28 | P = 0.14 |
| Khorram 2006 | 7 | 16 | 1 | 15 | P = 0.04 |
| Legro 2007 | 582 | 964 | 462 | 942 | P < 0.01 |
| Nestler 1998 | 19 | 21 | 2 | 25 | P < 0.01 |
| Heathcote 2013 | 24 | 43 | 38 | 60 | P = 0.44 |
| Siebert 2009 | 34 | 52 | 36 | 55 | P = 0.99 |
| Sturrock 2002 | 5 | 12 | 4 | 14 | P = 0.49 |
| Vandermolen 2001 | 9 | 12 | 4 | 15 | P = 0.02 |
| Zain 2009 | 38 | 41 | 24 | 41 | P < 0.01 |
5. Metformin versus clomiphene citrate: ovulation rate per cycle.
| Metformin | Clomiphene citrate | ||||
| Study ID | Events | Cycles | Events | Cycles | P value |
| BMI < 30 kg/m2 | |||||
| Palomba 2005a | 129 | 205 | 148 | 221 | P = 0.38 |
| PCOSMIC 2010 | 23 | 35 | 23 | 36 | P = 0.87 |
| BMI ≥ 30 kg/m2 | |||||
| Legro 2007 | 296 | 1019 | 462 | 942 | P < 0.01 |
| Zain 2009 | 4 | 42 | 7 | 41 | P = 0.32 |
6. Metformin and letrozole vs letrozole: ovulation rate per cycle.
| Metformin and letrozole | Letrozole | ||||
| Study ID | Events | Cycles | Events | Cycles | P value |
| BMI < 30 kg/m2 | |||||
| Liu 2017 | 89 | 118 | 93 | 130 | P = 0.49 |
7. Metformin and laparoscopic ovarian drilling (LOD) vs LOD: ovulation rate per cycle.
| Metformin & LOD | LOD | ||||
| Study ID | Events | Cycles | Events | Cycles | P value |
| BMI ≥ 30 kg/m2 | |||||
| Kocak 2006 | 56 | 65 | 29 | 65 | P < 0.01 |
In order to reduce a carry‐over of treatment effect in cross‐over trials, we only used data from the first phase (such as before cross‐over) when the washout period was less than two months. The rationale is that oligo amenorrhoea is usually accepted as a menstrual cycle length over five to eight weeks. Therefore, the washout period of treatment effect on ovulation should ideally be more than eight weeks.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis where possible and sought any missing data from the study authors.
When this information was not available, we performed the analysis using the original number of women randomised.
Assessment of heterogeneity
Heterogeneity reflects any type of variability among the studies in a systematic review. A consistent treatment effect among the included studies suggests there is sufficient homogeneity for pooled analysis. We used the I2 statistic (Higgins 2003), to quantify the inconsistency among the studies. We regarded an I2 statistic of over 50% as indicative of substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise the potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we produced funnel plots for the primary outcome live birth, to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies; Sterne 2017).
Data synthesis
We performed statistical analyses according to the statistical guidelines for review authors developed by Cochrane and published in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We used Review Manager 5 (RevMan 5) to perform all the statistical analyses (Review Manager 2014).
We used OR, with 95% CI, as the measure of effect for each dichotomous outcome using the Mantel‐Haenszel method; whilst we presented continuous outcome differences between the two groups as MD with 95% CI. We employed a fixed‐effect model in the analysis, and have commented on significant heterogeneity where it occurred.
For clinical outcomes, we stratified comparisons by BMI, divided into obese and non‐obese groups, with an additional stratum for studies in which BMI was not reported. We defined 'obese' as BMI equal to or over 30 kg/m2.
Subgroup analysis and investigation of heterogeneity
As noted above, we subgrouped the primary analysis by BMI (obese or non‐obese), in order to assess any differences in effect within these subgroups.
We also conducted subgroup analyses by sensitivity to CC (sensitive or resistant), in relevant analyses (i.e. including CC group) where substantial heterogeneity was detected (I2 over 50%).
We also planned to explore other possible explanations where heterogeneity was substantial, by examining other clinical or methodological differences between the studies.
Sensitivity analysis
To determine that the conclusions of this review were robust, we performed sensitivity analyses after excluding studies with unclear or high risk of bias in sequence generation, allocation concealment or blinding method. We also performed a sensitivity analysis to compare the effect of reporting miscarriage and multiple pregnancy data 'per pregnancy'.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEpro GDT software (GRADEpro GDT). These tables evaluated the overall quality of the body of evidence for the main review outcomes (live birth, adverse events, clinical pregnancy, ovulation and miscarriage) with respect to the most clinically relevant comparisons (metformin versus placebo or no treatment, metformin and CC versus CC alone, metformin versus CC). Two review authors working independently evaluated the quality of the evidence using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified and documented our judgements about evidence quality (high, moderate, low or very low) and incorporated them into reporting of results for each outcome (Schünemann 2013; Schünemann 2017). We resolved any disagreements by consensus.
The previous update found a high heterogeneity when metformin was compared with CC for some outcomes, which was associated with BMI status. In this review, we have presented the data by BMI subgroup.
Details of abbreviations used in this review and conversion factors of biochemical results can be found in Table 4 and Table 5, respectively.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies for full details of the studies.
Results of the search
In this updated review there are 41 included studies and 42 excluded studies (Figure 1).
In this current update (fourth update, search period up to December 2018), we introduced changes to exclude thiazolidinediones and to include metformin compared with CC, letrozole and LOD. We included only studies that reported reproductive outcomes. We performed a new search up to December 2018. We considered the full texts of 94 articles (66 new studies and 28 studies from the previous review). Of these, we excluded 42 studies, five are ongoing clinical trials with no published results (NCT00005104; NCT00317928; NCT00558077; NCT01679574; NCT02562664), and six are awaiting classification (Ayaz 2013a; Beigi 2006; Jahan 2015; Robinson 2003; Singh 2001; Williams 2009) (see Figure 1). Of the 48 studies in the previous update, we have included 28 (Baillargeon 2004; Begum 2014; Ben Ayed 2009; Boudhraa 2010; Fleming 2002; Hoeger 2004; Jakubowicz 2001; Kar 2015; Karimzadeh 2007; Karimzadeh 2010; Khorram 2006; Legro 2007; Lord 2006; Machado 2012; Malkawi 2002; Moll 2006; Morin‐Papunen 2012; Nestler 1998; Ng 2001; Onalan 2005; Palomba 2005a; PCOSMIC 2010; Siebert 2009; Sturrock 2002; Tang 2006; Vandermolen 2001; Yarali 2002; Zain 2009). We have included 13 additional studies in this review (Chuni 2006; Fatima 2018; Hamed 2010; Heathcote 2013; Kjotrod 2011; Ko 2001; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2003; Palomba 2004; Raja 2005; Refaie 2005), one of which the Morley 2017 review excluded (Heathcote 2013) and have 41 studies in total.
Included studies
Study design and setting
The newly included studies for this current update (Chuni 2006; Fatima 2018; Hamed 2010; Heathcote 2013; Kjotrod 2011; Ko 2001; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2003; Palomba 2004; Raja 2005; Refaie 2005), all recorded reproductive outcomes following treatment.
Two compared metformin with placebo (Chuni 2006; Kjotrod 2011). Seven compared metformin and CC with CC alone (Fatima 2018; Heathcote 2013; Ko 2001; Liu 2004; Liu 2017; Raja 2005; Refaie 2005). One compared metformin and LOD with LOD (Kocak 2006). Three compared metformin with LOD (Hamed 2010; Malkawi 2003; Palomba 2004). One compared metformin and letrozole versus letrozole alone as well as metformin and CC versus CC alone (Liu 2017), and one compared metformin versus CC as well as metformin and CC (Liu 2004).
Twenty‐two of the included studies were documented as being double‐blind. Nine studies were not double‐blind (Boudhraa 2010; Khorram 2006; Ko 2001; Kocak 2006; Malkawi 2003; Nestler 1998; Raja 2005; Siebert 2009; Zain 2009), and the remainder were classified as unclear.
One of the studies was a cross‐over trial (Sturrock 2002). We only analysed the first phase from Sturrock 2002 as we considered the washout period to be short (four weeks).
The included studies originated from Australia, Bangladesh, Brazil, China, Denmark, Egypt, Finland, Hong Kong, India, Iran, Italy, Jordan, Malaysia, the Netherlands, Norway, New Zealand, Pakistan, South Africa, South Korea, Sweden, Tunisia, Turkey, UK, USA and Venezuela.
Participants
The number of women in the studies ranged from 18 to 626, with 4552 participants in total. The range of BMI in included participants was 20.96 to 38.9 kg/m2.
All the women had a diagnosis of PCOS based upon the Rotterdam Consensus criteria; two out of three of PCOS on ultrasound, oligo or anovulation, clinical or biochemical signs of hyperandrogenism (ESHRE/ASRM 2004). The age range of participants was 24.2 to 32.8 years with the range of fasting insulin concentrations between 6.3 and 54.7 mIU/L and testosterone levels of 1.5 to 11.4 nmol/L. However, several studies did not provide these data.
Interventions
In total, all 41 trials assessed the benefits of using metformin for women with PCOS. Eighteen trials compared metformin alone with placebo or no treatment (Baillargeon 2004; Chuni 2006; Fleming 2002; Hoeger 2004; Karimzadeh 2007; Karimzadeh 2010; Khorram 2006; Kjotrod 2011; Lord 2006; Morin‐Papunen 2012; Nestler 1998; Ng 2001; Onalan 2005; PCOSMIC 2010; Sturrock 2002; Tang 2006; Vandermolen 2001; Yarali 2002).
Eighteen studies investigated the benefits of using metformin combined with CC on reproductive outcomes (Ben Ayed 2009; Fatima 2018; Heathcote 2013; Jakubowicz 2001; Kar 2015; Karimzadeh 2010; Ko 2001; Legro 2007; Liu 2004; Liu 2017; Machado 2012; Malkawi 2002; Moll 2006; PCOSMIC 2010; Raja 2005; Refaie 2005; Siebert 2009; Zain 2009). Nine studies compared metformin versus CC (Begum 2014; Boudhraa 2010; Kar 2015; Karimzadeh 2010; Legro 2007; Liu 2004; Palomba 2005a; PCOSMIC 2010; Zain 2009).
One study compared metformin and letrozole to letrozole alone (Liu 2017). The same study also compared metformin and CC to CC.
One study compared metformin and LOD with LOD alone (Kocak 2006), and three studies compared metformin to LOD directly (Hamed 2010; Malkawi 2003; Palomba 2004).
Eleven studies included specific advice on lifestyle modification in the study protocol (Ben Ayed 2009; Boudhraa 2010; Heathcote 2013; Hoeger 2004; Karimzadeh 2010; Kjotrod 2011; Lord 2006; PCOSMIC 2010; Siebert 2009; Tang 2006; Zain 2009).
The duration of the studies ranged from 4 to 96 weeks with an average of 19.7 weeks. The median daily dose of metformin used in the studies was 1500 mg.
Outcomes
Most studies reported clinical pregnancy rate and ovulation rate but only 16 studies reported live birth rates (Boudhraa 2010; Heathcote 2013; Kar 2015; Kocak 2006; Legro 2007; Liu 2017; Malkawi 2003; Moll 2006; Morin‐Papunen 2012; Ng 2001; Palomba 2004; Palomba 2005a; PCOSMIC 2010; Vandermolen 2001; Yarali 2002; Zain 2009). The four largest studies reporting live birth rate were Legro 2007; Liu 2017; Moll 2006 and Morin‐Papunen 2012. Nineteen studies reported gastrointestinal side effects (Chuni 2006; Fleming 2002; Hamed 2010; Heathcote 2013; Hoeger 2004; Karimzadeh 2007; Kjotrod 2011; Legro 2007; Liu 2017; Malkawi 2003; Moll 2006; Morin‐Papunen 2012; Ng 2001; Onalan 2005; Palomba 2004; Palomba 2005a; PCOSMIC 2010; Raja 2005; Yarali 2002).
Excluded studies
In this fourth update, we excluded a total of 42 studies. Of these, we excluded eight because the comparators were not relevant to the meta‐analysis (Elgafor 2013; Fayed 2009; Hashim 2010; Hashim 2011; Melli 2010; Rezk 2018; Sohrabvand 2006; Weerakiet 2011), seven because there were no reproductive outcomes reported (Ashrafinia 2009; Aubuchon 2009; Chou 2003; Eisenhardt 2006; Maciel 2004; Moghetti 2000; Trolle 2007), four because they were review articles (Mayhew 2011; Palomba 2005c; Pinnow 2008; Wisniewski 2009), four because they were not RCTs (Kocak 2002; Neveu 2007; Palomba 2005b; Palomba 2007), two because they were quasi‐RCTs (Bonakdaran 2012; Chaudhury 2008), three because the participants underwent IVF or intrauterine insemination (Leanza 2014; Savic 2003; Ronsini 2006), 12 because they used human chorionic gonadotropin (hCG) or human menopausal gonadotropin in addition to ovulation agents to trigger ovulation (Abuelghar 2013; Ayaz 2013b; Aygen 2007; Gada 2000; Hwu 2005; Katica 2014; Kazerooni 2009; Maged 2015; Ramzy 2003; Sahin 2004; Santonocito 2009; Xiaolin 2014), one because the diagnosis of PCOS was made on ultrasound findings alone (Kore 2007), and one because we could not find the original abstract (Billa 2005).
A summary of studies included and excluded in this review can be found in Figure 1.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias.
We performed sensitivity analysis by including data only from studies with low risk of bias, as determined by sequence generation, allocation concealment and blinding methodology. Only 12 out of 41 studies met this criterion (Baillargeon 2004; Fleming 2002; Heathcote 2013; Hoeger 2004; Karimzadeh 2007; Kjotrod 2011; Legro 2007; Lord 2006; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010; Tang 2006). Two out of the 13 newly included studies met this criterion (Heathcote 2013; Kjotrod 2011).
Allocation
Sequence generation
Sequence generation was unclear in 18 studies (Begum 2014; Ben Ayed 2009; Boudhraa 2010; Jakubowicz 2001; Kar 2015; Karimzadeh 2010; Khorram 2006; Ko 2001; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2002; Malkawi 2003; Nestler 1998; Raja 2005; Refaie 2005; Sturrock 2002; Zain 2009). Two studies were high risk: Fatima 2018 included consecutive non‐probability sampling in their methods of randomisation; and in Machado 2012, participants' choice of pink or green bottle represented a sealed, opaque envelope. The remaining studies were low risk (Baillargeon 2004; Chuni 2006; Fleming 2002; Hamed 2010; Heathcote 2013; Hoeger 2004; Karimzadeh 2007; Kjotrod 2011; Legro 2007; Lord 2006; Moll 2006; Morin‐Papunen 2012; Ng 2001; Onalan 2005; Palomba 2004; Palomba 2005a; PCOSMIC 2010; Siebert 2009; Tang 2006; Vandermolen 2001; Yarali 2002), where they all used computer‐generated randomisation methods.
Allocation concealment
Allocation concealment was high risk in two studies: Fatima 2018 used consecutive sampling; and Zain 2009 used clearly labelled cards picked out of a box. Allocation concealment was low risk in 14 studies (Baillargeon 2004; Fleming 2002; Heathcote 2013; Hoeger 2004; Jakubowicz 2001; Karimzadeh 2007; Kjotrod 2011; Legro 2007; Lord 2006; Machado 2012; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010; Tang 2006). Allocation concealment was unclear in 25 studies (Begum 2014; Ben Ayed 2009; Boudhraa 2010; Chuni 2006; Hamed 2010; Kar 2015; Karimzadeh 2010; Khorram 2006; Ko 2001; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2002; Malkawi 2003; Nestler 1998; Ng 2001; Onalan 2005; Palomba 2004; Palomba 2005a; Raja 2005; Refaie 2005; Siebert 2009; Sturrock 2002; Vandermolen 2001; Yarali 2002).
Blinding
Performance bias was high risk in four studies (Khorram 2006; Malkawi 2003; Siebert 2009; Zain 2009), all of which did not blind participants. Malkawi 2003 compared LOD to metformin, therefore blinding was not feasible when comparing surgery to medications. We judged 20 studies at low risk of performance bias (Baillargeon 2004; Fleming 2002; Heathcote 2013; Hoeger 2004; Jakubowicz 2001; Kar 2015; Karimzadeh 2007; Kjotrod 2011; Ko 2001; Legro 2007; Lord 2006; Machado 2012; Moll 2006; Morin‐Papunen 2012; Nestler 1998; Ng 2001; Palomba 2004; PCOSMIC 2010; Tang 2006; Vandermolen 2001), where participants were blinded to the treatment, and we determined that 17 studies, where information was inadequate, were at unclear risk (Begum 2014; Ben Ayed 2009; Boudhraa 2010; Chuni 2006; Fatima 2018; Hamed 2010; Karimzadeh 2010; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2002; Onalan 2005; Palomba 2005a; Raja 2005; Refaie 2005; Sturrock 2002; Yarali 2002).
Detection bias was high risk in six studies (Khorram 2006; Ko 2001; Malkawi 2003; Nestler 1998; Siebert 2009; Zain 2009), where the investigators were not blinded to the treatment comparators; and low risk in 17 studies (Baillargeon 2004; Fleming 2002; Heathcote 2013; Hoeger 2004; Jakubowicz 2001; Karimzadeh 2007; Kjotrod 2011; Legro 2007; Lord 2006; Machado 2012; Moll 2006; Morin‐Papunen 2012; Ng 2001; Palomba 2004; PCOSMIC 2010; Tang 2006; Vandermolen 2001), where participants were blinded to the treatment. We judged 18 studies at unclear risk, where information was inadequate (Begum 2014; Ben Ayed 2009; Boudhraa 2010; Chuni 2006; Fatima 2018; Hamed 2010; Kar 2015; Karimzadeh 2010; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2002; Onalan 2005; Palomba 2005a; Raja 2005; Refaie 2005; Sturrock 2002; Yarali 2002).
Incomplete outcome data
Eighteen studies were at high risk of attrition bias due to high dropout rates, unequal dropouts between the groups, not providing missing data, not using intention‐to‐treat analysis or use of per‐protocol analysis (Baillargeon 2004; Fleming 2002; Heathcote 2013; Jakubowicz 2001; Kar 2015; Ko 2001; Liu 2017; Lord 2006; Moll 2006; Nestler 1998; Ng 2001; Onalan 2005; Palomba 2004; Siebert 2009; Sturrock 2002; Tang 2006; Vandermolen 2001; Zain 2009. Eighteen studies were at low risk of attrition bias (Chuni 2006; Fatima 2018; Hamed 2010; Hoeger 2004; Khorram 2006; Kjotrod 2011; Kocak 2006; Legro 2007; Liu 2004; Machado 2012; Malkawi 2002; Malkawi 2003; Morin‐Papunen 2012; Palomba 2005a; PCOSMIC 2010; Raja 2005; Refaie 2005; Yarali 2002). We classified the remaining studies as unclear risk because of insufficient information (Begum 2014; Ben Ayed 2009; Boudhraa 2010; Karimzadeh 2007; Karimzadeh 2010).
Selective reporting
We judged 16 studies to be at low risk of selective reporting (Chuni 2006; Fatima 2018; Hamed 2010; Heathcote 2013; Hoeger 2004; Jakubowicz 2001; Kjotrod 2011; Legro 2007; Liu 2017; Lord 2006; Moll 2006; Ng 2001; Palomba 2004; PCOSMIC 2010; Refaie 2005; Tang 2006), because they clearly reported all stated outcomes. One study (Karimzadeh 2010), did not report on all outcomes including endocrine and lipid profiles and we classified it as high risk. The remaining studies had insufficient information and we classified them as unclear risk (Baillargeon 2004; Begum 2014; Ben Ayed 2009; Boudhraa 2010; Fleming 2002; Kar 2015; Karimzadeh 2007; Khorram 2006; Ko 2001; Kocak 2006; Liu 2004; Machado 2012; Malkawi 2002; Malkawi 2003; Morin‐Papunen 2012; Nestler 1998; Onalan 2005; Palomba 2005a; Raja 2005; Siebert 2009; Sturrock 2002; Vandermolen 2001; Yarali 2002; Zain 2009).
Multi‐arm studies have an increased risk of reporting bias. There were five 3‐armed studies (Kar 2015; Karimzadeh 2010; Legro 2007; Liu 2004; PCOSMIC 2010), and two 4‐armed studies (Baillargeon 2004; Liu 2017), however, all studies clearly reported baseline characteristics and outcome data for each arm separately.
Other potential sources of bias
Two studies appeared to be at high risk of other sources of bias: Heathcote 2013 was not published, therefore had not undergone the peer review process; and Legro 2007 underwent an ad hoc change in sample size. We classified four studies as unclear risk. Jakubowicz 2001 reported a discrepant treatment period between groups. Karimzadeh 2010 may have duplicated some participants from a previous study with a crossover of recruitment periods, and there was no reply from the study author to clarify. Ng 2001 included participants who were anovulatory however, some of these participants did ovulate with no treatment. Refaie 2005 did not provide baseline characteristics between groups and hence there may be confounding factors present that affect the results. The majority of the studies were low risk with no evidence of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3
We have presented forest plots for the primary outcome live birth rate in Figure 4; Figure 5; Figure 6, for Analysis 1.1, Analysis 2.1 and Analysis 3.1, respectively.
4.
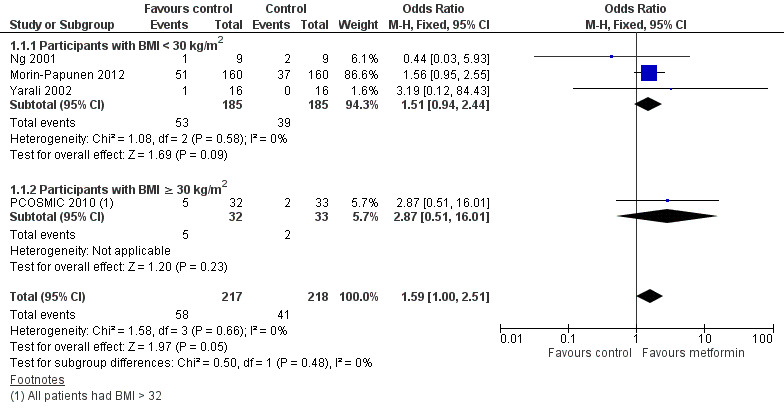
Forest plot of comparison 1. Metformin versus placebo or no treatment, outcome 1.1, live birth rate
5.
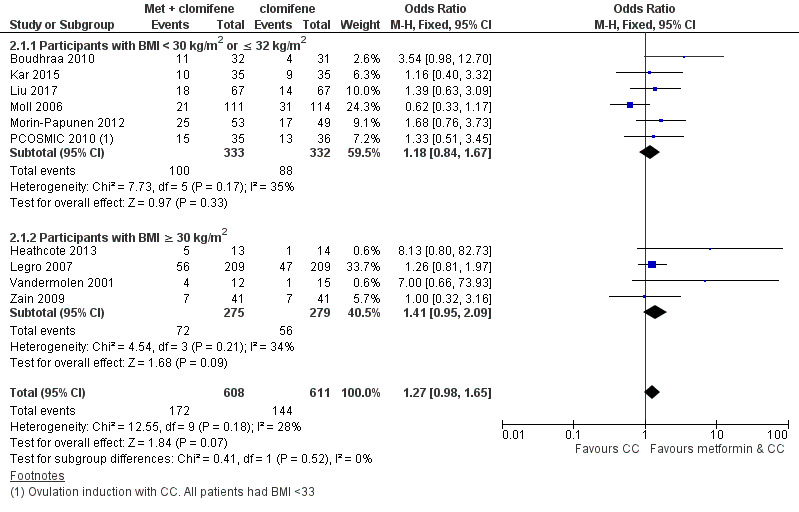
Forest plot of comparison 2. Metformin combined with clomiphene citrate versus clomiphene citrate alone, outcome: 2.1, live birth rate
6.
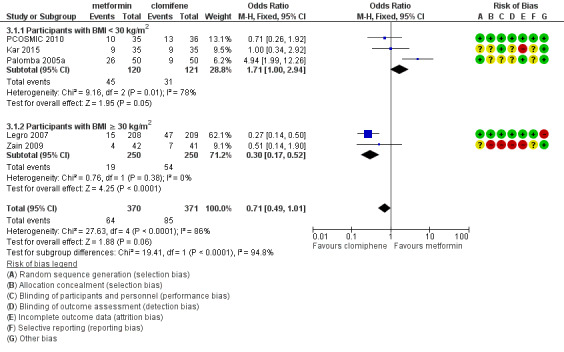
Forest plot of comparison 3. Metformin versus clomiphene citrate, outcome 3.1, live birth rate
1.1. Analysis.
Comparison 1 Metformin versus placebo or no treatment, Outcome 1 Live birth rate.
2.1. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 1 Live birth rate.
3.1. Analysis.
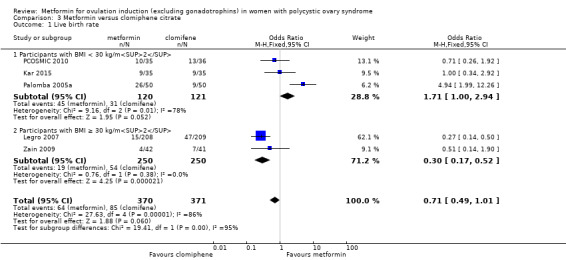
Comparison 3 Metformin versus clomiphene citrate, Outcome 1 Live birth rate.
1. Metformin versus placebo or no treatment
1.1 Live birth rate
When we compared metformin to placebo, only a limited number of studies reported live birth rate (Morin‐Papunen 2012; Ng 2001; PCOSMIC 2010; Yarali 2002). Pooled evidence from these four studies showed that live birth rate may improve slightly with metformin, with a number needed to treat for an additional beneficial outcome of 13 women (OR 1.59, 95% CI 1.01 to 2.50; I2 = 0%; 4 studies, 435 women; low‐quality evidence; Analysis 1.1). This suggests that for a live birth rate of 19% following placebo, the live birth rate following metformin would be between 19% and 37%. However, the wide‐ranging confidence intervals and low quality of the evidence make the advantage offered by metformin difficult to interpret clinically.
In the subgroup analysis by obesity status the test for differences showed no difference between obese and non‐obese women. There was no clear evidence of a difference in live birth rate in either subgroup (BMI of < 30 kg/m2: OR 1.51, 95% CI 0.94 to 2.44; I2 = 0%; 3 studies, 370 women; or BMI > 30 kg/ m2: OR 2.87, 95% CI 0.51 to 16.01; 1 study, 65 women). However, the broad confidence intervals due to reducing the number of combined studies for this analysis, render the results unclear. A sensitivity analysis, which excluded studies with unclear or high risk of bias left two studies remaining (OR 1.64, 95% CI 1.02 to 2.63; I2 = 0%; 2 studies, 385 women; Morin‐Papunen 2012; PCOSMIC 2010). The large and high‐quality study by Morin‐Papunen 2012 contributed 93.8% of the weight of the result (OR 0.95, 95% CI 0.95 to 2.55; 320 women). These results therefore suggest a potential benefit in live birth rate when using metformin compared with placebo, although the number of studies were small.
1.2 Adverse events (gastrointestinal side effects)
Women in the metformin group experienced a higher incidence of gastrointestinal side effects than the placebo group (OR 4.00, 95% CI 2.63 to 6.09; I2 = 39%; 7 studies, 713 women; moderate‐quality evidence; Analysis 1.2). This suggests that with placebo, the risk of adverse effects is 10% whereas with metformin the risk of adverse gastrointestinal side effects increases to between 22% and 40%. Despite the large confidence interval, the heterogeneity is moderate, which provides evidence that women are more likely to experience gastrointestinal side effects. The heterogeneity and wide confidence intervals could be explained by the subjective nature of gastrointestinal side effects and reliance on participant self‐reporting. Sensitivity analysis, which excluded studies with unclear or high risk of bias did not change the inference. In the subgroup analysis by BMI, the test for differences showed no evidence of a difference between obese and non‐obese women.
1.2. Analysis.
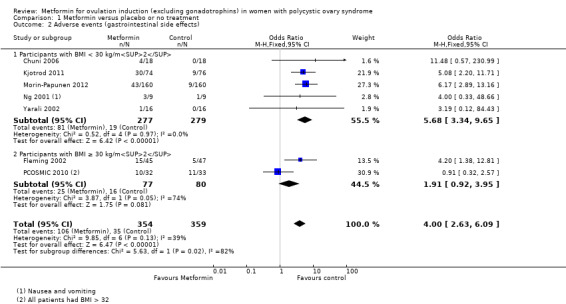
Comparison 1 Metformin versus placebo or no treatment, Outcome 2 Adverse events (gastrointestinal side effects).
1.3 Clinical pregnancy rate
Eleven trials reported clinical pregnancy rates (Chuni 2006; Fleming 2002; Karimzadeh 2007; Karimzadeh 2010; Kjotrod 2011; Lord 2006; Morin‐Papunen 2012; Ng 2001; PCOSMIC 2010; Tang 2006; Yarali 2002). Metformin probably improves pregnancy rates compared with placebo (OR 1.98, 95% CI 1.47 to 2.65; I2 = 30%; 11 studies, 1213 women; moderate‐quality evidence; Analysis 1.3). This suggests that the clinical pregnancy rate with placebo is 15%, which may increase to a range from 21% to 32% with metformin. In subgroup analysis by BMI the test for differences showed no evidence of a difference between obese and non‐obese women. In an attempt to improve heterogeneity we performed a sensitivity analysis, which excluded studies with unclear or high risk of bias, including the following studies: Fleming 2002; Karimzadeh 2007; Kjotrod 2011; Lord 2006; Morin‐Papunen 2012; PCOSMIC 2010; Tang 2006. However, this did not alter the inference or heterogeneity significantly.
1.3. Analysis.
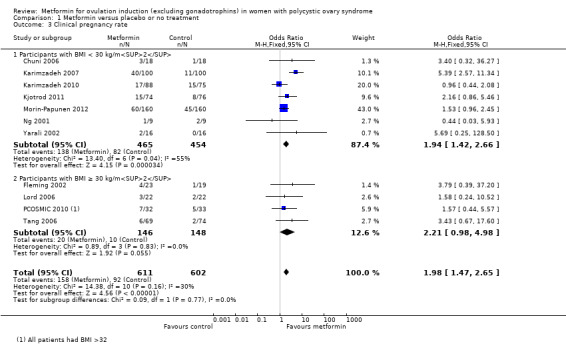
Comparison 1 Metformin versus placebo or no treatment, Outcome 3 Clinical pregnancy rate.
1.4 Ovulation rate
There was evidence that metformin may improve ovulation rate per woman (OR 2.64, 95% CI 1.85 to 3.75; I2 = 61%; 13 studies, 684 women; low‐quality evidence; Analysis 1.4). This suggests that the ovulation rate with placebo is 24%, which may increase to a range from 37% to 54% with metformin. We have presented ovulation rate per cycle in Table 6. Subgroup analysis by obesity status suggested no significant difference between women with a BMI of 30 kg/m2 or higher compared with women with a BMI of under 30 kg/m2 (test for subgroup differences: Chi² = 3.79, df = 1 (P = 0.05), I² = 73.6%. When we pooled both subgroups, heterogeneity was improved, after which included only five studies (Baillargeon 2004; Fleming 2002; Hoeger 2004; Lord 2006; PCOSMIC 2010), with an overall I2 statistic value of 76% . However, the overall inference remained unchanged.
1.4. Analysis.
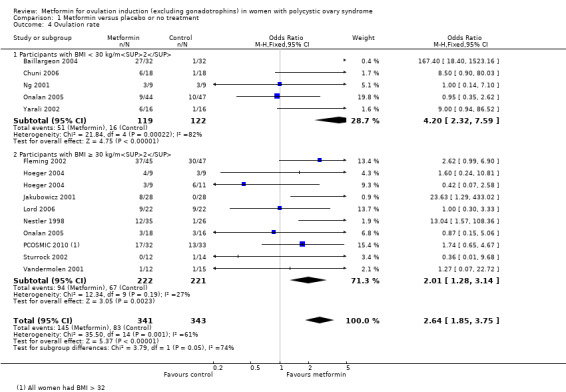
Comparison 1 Metformin versus placebo or no treatment, Outcome 4 Ovulation rate.
1.5 Miscarriage and 1.6 Miscarriage per pregnancy
There is no evidence that metformin compared with placebo increases miscarriage rate per woman (OR 1.08, 95% CI 0.50 to 2.35; I2 = 0%; 4 studies, 748 women; low‐quality evidence; Analysis 1.5). This suggests that a miscarriage rate of 4% with placebo may change to between 2% and 9% with metformin. A sensitivity analysis using per pregnancy rates was also inconclusive (OR 0.58, 95% CI 0.25 to 1.34; I2 = 0%; 4 studies, 200 pregnancies; low‐quality evidence; Analysis 1.6). A subgroup analysis by obesity status showed no evidence of a difference between the obese and non‐obese women. However, only one study was available with women with BMI more than 30 kg/m2 (PCOSMIC 2010).
1.5. Analysis.
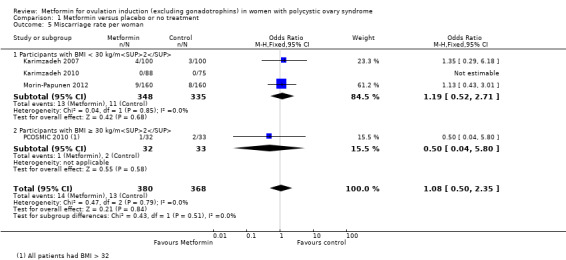
Comparison 1 Metformin versus placebo or no treatment, Outcome 5 Miscarriage rate per woman.
1.6. Analysis.
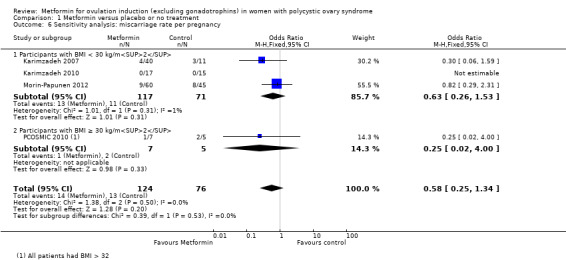
Comparison 1 Metformin versus placebo or no treatment, Outcome 6 Sensitivity analysis: miscarriage rate per pregnancy.
1.7 Multiple pregnancy and 1.8 Multiple pregnancy per pregnancy
Only one study reported multiple pregnancy rates (PCOSMIC 2010). We are uncertain of the effect of metformin compared with placebo on multiple pregnancy rates per woman (OR 0.33, 95% CI 0.01 to 8.49; 1 study, 65 women; Analysis 1.7). All women in this group were obese with BMI more than 32 kg/m2. A sensitivity analysis using per pregnancy rates was also inconclusive (OR 0.20, 95% CI 0.01 to 6.04; 1 study, 12 pregnancies; Analysis 1.8).
1.7. Analysis.
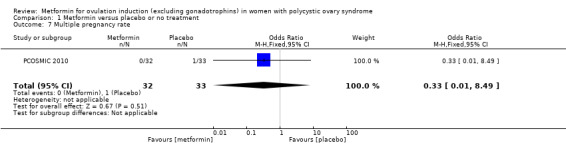
Comparison 1 Metformin versus placebo or no treatment, Outcome 7 Multiple pregnancy rate.
1.8. Analysis.
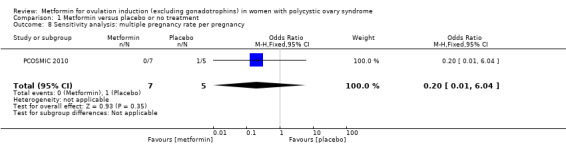
Comparison 1 Metformin versus placebo or no treatment, Outcome 8 Sensitivity analysis: multiple pregnancy rate per pregnancy.
Anthropometric outcomes
1.9 Body mass index
There is no evidence that metformin compared with placebo lowers BMI (MD −0.04, 95% CI −0.29 to 0.21; I2 = 0%; 10 studies, 589 women; Analysis 1.9) with an average duration of treatment of six months and average dose of 1500 mg. Baillargeon 2004 provided 79% of the weight of this analysis, which found no significant evidence of a difference in BMI (MD 0.00, 95% CI −0.28 to 0.28). The overall heterogeneity was low (I2 = 0%). Sensitivity analysis by study quality did not change the inference (Baillargeon 2004; Fleming 2002; Hoeger 2004; Morin‐Papunen 2012; Tang 2006).
1.9. Analysis.
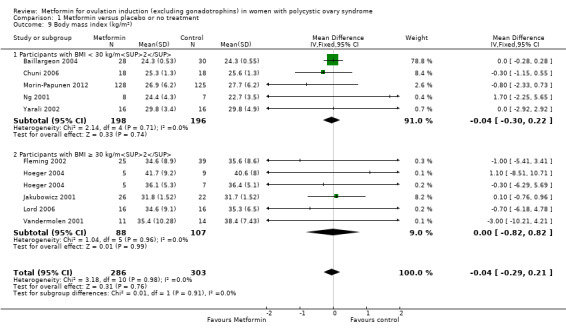
Comparison 1 Metformin versus placebo or no treatment, Outcome 9 Body mass index (kg/m2).
Endocrine outcomes
1.10 Serum testosterone
Evidence showed that metformin may reduce serum total testosterone levels with a MD of −0.41 nmol/L (95% CI −0.48 to −0.35; 11 studies, 707 women; Analysis 1.10). However, we observed high heterogeneity (I2 = 95%). In subgroup analysis by BMI, there was no evidence of a difference between obese and non‐obese women (test for subgroup differences: Chi² = 2.71, df = 1 (P = 0.10), I2 = 63.1%). Furthermore, different biochemical assays used in different studies could contribute to the heterogeneity. Sensitivity analysis by study quality did not improve the heterogeneity (I2 = 97%). However, removing the two extreme results (Baillargeon 2004; Jakubowicz 2001), improved heterogeneity (non‐obese group I2 = 0%; obese group I2 = 57%) without altering the inference. (MD ‐0.41, 95% CI ‐0.48 to ‐0.35; participants = 707; studies = 12; I2 = 95%)
1.10. Analysis.
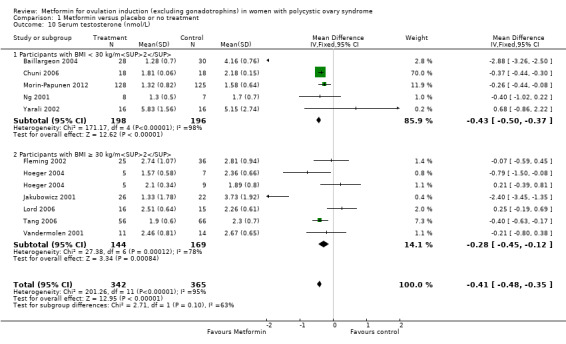
Comparison 1 Metformin versus placebo or no treatment, Outcome 10 Serum testosterone (nmol/L).
1.11 Serum sex hormone‐binding globulin
We are uncertain of the effect of metformin for serum sex hormone‐binding globulin levels (MD −1.70, 95% CI −4.77 to 1.36; I2 = 70%; 10 studies, 649 women; Analysis 1.11). Neither the subgroup analysis nor the sensitivity analysis by study quality changed the inference, yet removal of the studies with high or unclear risk of bias (Jakubowicz 2001; Nestler 1998; Ng 2001; Vandermolen 2001), did improve the heterogeneity (I2 = 6%).
1.11. Analysis.
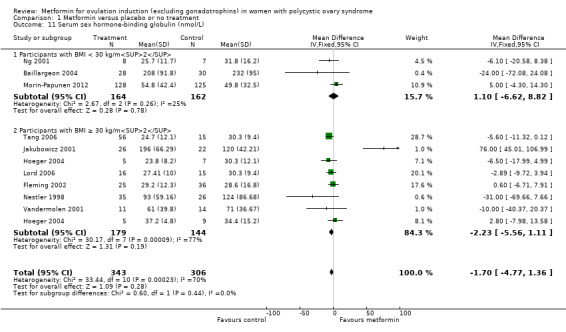
Comparison 1 Metformin versus placebo or no treatment, Outcome 11 Serum sex hormone‐binding globulin (nmol/L).
Metabolic outcomes
1.12 Fasting glucose
Metformin may reduce the fasting glucose levels compared with placebo (MD 0.01, 95% CI −0.04 to 0.06; I2 = 65%; 10 studies, 677 women; Analysis 1.12). Subgroup analysis only improved heterogeneity in the obese group (I2 = 49%) without changing the inference. Sensitivity analysis by study quality (Baillargeon 2004; Fleming 2002; Hoeger 2004; Morin‐Papunen 2012; Lord 2006; Tang 2006), improved overall heterogeneity (I2 = 20%) and the results indicated a minimal effect of metformin on fasting glucose concentrations (MD −0.09 mmol/L, 95% CI −0.17 to 0.00).
1.12. Analysis.
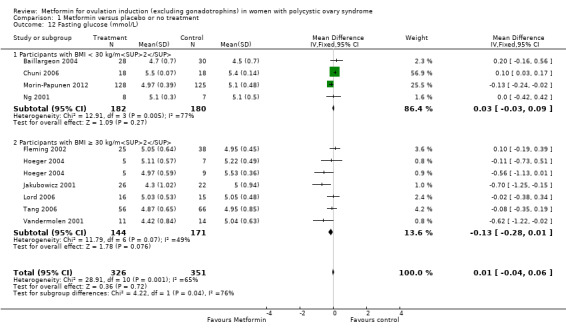
Comparison 1 Metformin versus placebo or no treatment, Outcome 12 Fasting glucose (mmol/L).
1.13 Fasting insulin
We are uncertain of an effect of metformin compared with placebo on fasting insulin levels with a MD −1.84 (95% CI −4.27 to 0.59; 8 studies, 361 women; Analysis 1.13) but with significant heterogeneity (I2 = 67%). In subgroup analysis by BMI the test for subgroup differences showed no evidence of a difference between obese and non‐obese women (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.97), I2 = 0%). Sensitivity analysis by study quality (Fleming 2002; Hoeger 2004; Lord 2006; Tang 2006), did not alter the inferences.
1.13. Analysis.
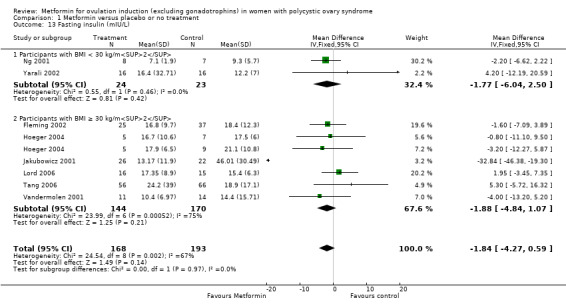
Comparison 1 Metformin versus placebo or no treatment, Outcome 13 Fasting insulin (mIU/L).
2. Metformin and CC versus CC alone
2.1 Live birth rate
We are uncertain of an effect of metformin and CC on live birth rates compared with CC alone (OR 1.27, 95% CI 0.98 to 1.65; I2 = 28%; 10 studies, 1219 women; low‐quality evidence; Analysis 2.1). The live birth rate with CC alone is 24%, which may change to between 23% to 34% with combined therapy.
In subgroup analysis, the test for subgroup differences showed no evidence of a difference between obese women (OR 1.41, 95% CI 0.95 to 2.09; 4 studies, 554 women) and non‐obese women (OR 1.18, 95% CI 0.84 to 1.67; 6 studies, 665 women) with a P value of 0.52. Sensitivity analysis by evidence quality (Heathcote 2013; Legro 2007; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010), with 843 women, also did not change the inference nor improve heterogeneity.
2.2 Adverse events
Metformin and CC probably increases the frequency of gastrointestinal side effects, including nausea and vomiting, compared with CC alone (OR 4.26, 95% CI 2.83 to 6.40; I2 = 8%; 6 studies, 852 women; moderate‐quality evidence; Analysis 2.2). With CC alone, the risk of gastrointestinal side effects is 9%, which increases to between 21% to 37% with combined therapy. The confidence interval is large, however, the heterogeneity is low and therefore suggests that women are probably more likely to experience gastrointestinal side effects compared with CC alone. Only one study included obese women (OR 2.36, 95% CI 0.19 to 29.71; 27 women; Heathcote 2013), and one study did not record BMI (OR 14.75, 95% CI 0.81 to 269.34; 100 women; Raja 2005). Sensitivity analysis by study quality (Heathcote 2013; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010), did not change our findings.
2.2. Analysis.
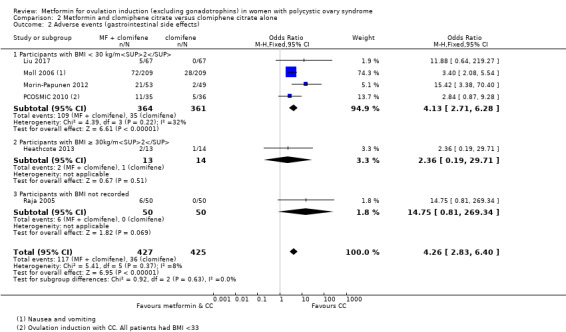
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 2 Adverse events (gastrointestinal side effects).
2.3 Clinical pregnancy rate
Metformin and CC probably improves pregnancy rate compared with CC alone (OR 1.62, 95% CI 1.32 to 1.99; I2 = 31%; 19 studies, 1790 women; moderate‐quality evidence; Analysis 2.3). This suggests that the clinical pregnancy rate with CC alone is 28% which may increase to between 34% and 43% with combination therapy.
2.3. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 3 Clinical pregnancy rate.
In subgroup analysis, the test for subgroup differences showed no evidence of a difference between the subgroups: the effect on pregnancy rates was seen in both analyses: obese group (OR 1.74, 95% CI 1.24 to 2.43; 8 studies, 666 women) and non‐obese group (OR 1.40, 95% CI 1.06 to 1.86; 9 studies, 896 women). Sensitivity analysis by study quality (Heathcote 2013; Legro 2007; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010), with 843 participants, did improve heterogeneity (I2 = 3%) but also altered the inference (OR 1.29, 95% CI 0.98 to 1.70).
2.4 Ovulation rate and 2.5 Ovulation rate by CC sensitivity and resistance
Metformin and CC may improve ovulation per woman compared with CC alone, (OR 1.65, 95% CI 1.35 to 2.03; I2 = 63%; 21 studies, 1568 women; low‐quality evidence; Analysis 2.4). This suggests that the ovulation rate with CC alone is 50% which may increase to between 58% and 68% with combination therapy. We have presented ovulation rate per cycle in Table 7. In subgroup analysis, the test for subgroup differences showed no evidence of a difference between obese and non‐obese women (P = 0.16). Heterogenity remained high (I2 = 70%) in the obese subgroup, but the direction of effect was consistent.
2.4. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 4 Ovulation rate.
Sensitivity analysis by study quality (Heathcote 2013; Legro 2007; Moll 2006; PCOSMIC 2010), did improve heterogeneity (I2 = 7%) however, it reduced the inference (OR 0.98, 95% CI 0.73 to 1.32; 5 studies, 777 women).
We conducted a subgroup analysis based on sensitivity to CC. Seven studies recorded CC‐resistance status. Six of these included women with CC resistance (Ko 2001; Machado 2012; Malkawi 2002; Ng 2001; Sturrock 2002; Vandermolen 2001). This analysis showed an improvement in ovulation rate with combined therapy (OR 4.97, 95% CI 2.46 to 10.03; I2 = 0%; 6 studies, 156 women; moderate‐quality evidence; Analysis 2.5). Only one small study of CC‐sensitive women was available, and we are unable to draw a conclusion from the result (OR 3.55, 95% CI 0.65 to 19.37; 56 women; Jakubowicz 2001).
2.5. Analysis.
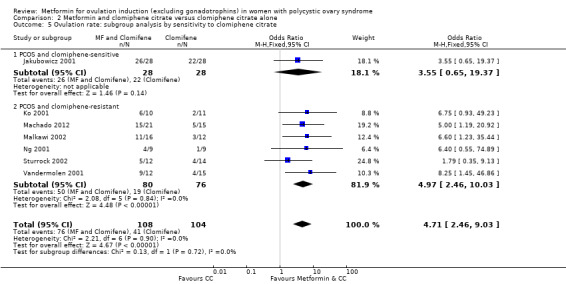
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 5 Ovulation rate: subgroup analysis by sensitivity to clomiphene citrate.
2.6 Miscarriage rate and 2.7 Miscarriage rate per pregnancy
When we pooled data from 10 studies, we found no effect of metformin and CC compared with CC alone on miscarriage (OR 1.35, 95% CI 0.91 to 2.00; I2 = 0%; 10 studies, 1206 women;low‐quality evidence; Analysis 2.6). This suggests that the miscarriage rate with CC alone is 8%, which may change to between 7% and 14% with combination therapy. When we analysed a subgroup by BMI, the test for subgroup differences showed no evidence of a difference between obese and non‐obese women (P = 0.72).
2.6. Analysis.
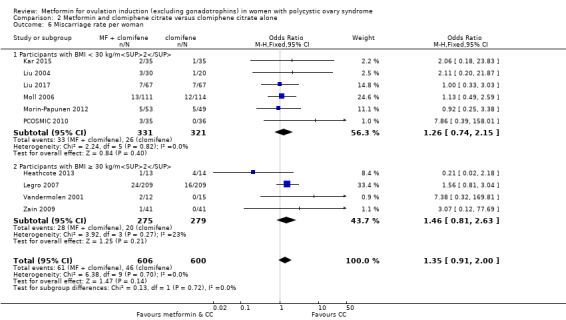
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 6 Miscarriage rate per woman.
When we performed an analysis of miscarriage rate per pregnancy, there was no clear evidence of a difference between the groups (OR 1.07, 95% CI 0.69 to 1.66; I2 = 0%; 10 studies, 471 pregnancies; Analysis 2.7), with no evidence of a difference between the BMI subgroups (P = 0.91). Sensitivity analysis by study quality (Heathcote 2013; Legro 2007; Moll 2006; Morin‐Papunen 2012; PCOSMIC 2010), also did not alter the inference. Any increase in miscarriage conferred by using CC therapy in isolation is therefore difficult to interpret and apply clinically.
2.7. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 7 Sensitivity analysis: miscarriage rate per pregnancy.
One study reported ectopic pregnancy rate (Liu 2017). There was one ectopic pregnancy in the metformin and CC group and one ectopic pregnancy in the CC‐alone group.
2.8 Multiple pregnancy rate and 2.9 Multiple pregnancy rate per pregnancy
We are uncertain of an effect of metformin and CC versus CC alone for multiple pregnancy rate (OR 0.56, 95% CI 0.18 to 1.68; I2 = 0%; 6 studies, 1003 women; Analysis 2.8). There was no evidence of a difference between BMI subgroups (P = 0.81). Sensitivity analysis using per pregnancy rates did not produce different findings (OR 0.46, 95% CI 0.15 to 1.42; I2 = 0%; 6 studies, 342 pregnancies; Analysis 2.9). Sensitivity analysis by study quality (Legro 2007; Moll 2006; PCOSMIC 2010), did not alter the inference either.
2.8. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 8 Multiple pregnancy rate per woman.
2.9. Analysis.
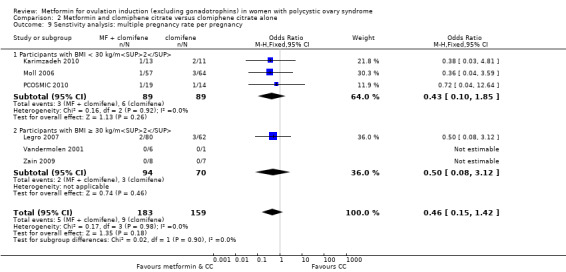
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 9 Senstivity analysis: multiple pregnancy rate per pregnancy.
Anthropometric outcomes
2.10 Body mass index
Metformin and CC probably reduce BMI compared with CC alone (MD −4.44, 95% CI −6.11 to −2.77; I2 = 0%; 3 studies, 105 women; Analysis 2.10), however, the number of participants is small. Only one study had non‐obese women (Liu 2004), however, there was no difference seen between subgroups of BMI (P = 0.50).
2.10. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 10 Body mass index (kg/m2).
Endocrine outcomes
2.11 Serum testosterone
We are uncertain of the effect of metformin and CC on reducing testosterone levels in women compared with CC alone (MD −0.37, 95% CI −0.60 to −0.13; I2 = 0%; 3 studies, 105 women; Analysis 2.11). There was no evidence of a difference between BMI subgroups (P = 0.80) however, there were only two studies with obese women (Ko 2001; Refaie 2005), and one study with non‐obese women (Liu 2004).
2.11. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 11 Serum testosterone (nmol/L).
Serum sex hormone‐binding globulin
Data were not available for this outcome.
Metabolic outcomes
2.12 Fasting glucose
We are uncertain of the effect of metformin and CC on reducing fasting glucose levels in women compared with CC alone (MD −0.21, 95% CI −0.29 to −0.12; I2 = 0%; 2 studies, 71 women; Analysis 2.12). There was no evidence of a difference between BMI subgroups (P = 0.58) however, there was only one study with obese women and one study with non‐obese women (Ko 2001 and Liu 2004 respectively).
2.12. Analysis.
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 12 Fasting glucose (mmol/L).
2.13 Fasting insulin
Metformin and CC may reduce insulin levels compared with CC alone (MD −6.57, 95% CI −7.84 to −5.29; I2 = 99%; 3 studies, 105 women; Analysis 2.13). Subgroup analysis of BMI suggested that non‐obese women responded better to combined metformin and CC alone (MD −15.20, 95% CI −18.33 to −12.07; 50 women) compared with obese women (MD −4.86, 95% CI −6.26 to −3.47; 55 women) however, there was only one study that included non‐obese women (Liu 2004).
2.13. Analysis.
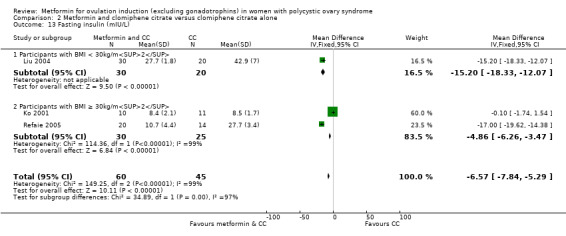
Comparison 2 Metformin and clomiphene citrate versus clomiphene citrate alone, Outcome 13 Fasting insulin (mIU/L).
3. Metformin versus CC
3.1 Live birth rate
When we combined the data from five studies (Kar 2015; Legro 2007; Palomba 2005a; PCOSMIC 2010; Zain 2009), we were uncertain of an effect of metformin compared with CC on live birth rate, with high heterogeneity (OR 0.70, 95% CI 0.48 to 1.01; I2 = 86%; 5 studies, 741 women; very low‐quality evidence; Analysis 3.1). However, in the subgroup analysis by obesity status, there was evidence of a difference between the obese and non‐obese women (test for subgroup differences: Chi2 = 19.41, df = 1, P < 0.0001, I2 = 94.8%). Among obese women, live birth rate was lower in the metformin group (OR 0.30, 95% CI 0.17 to 0.52; 2 studies, 500 women); 62% of the weight of this finding was provided by a single study (Legro 2007). In the non‐obese subgroup the direction of effect favoured metformin with high heterogeneity (OR 1.71, 95% CI 1.00 to 2.94; I2 = 78%; 3 studies, 241 women; very low‐quality evidence). This suggests that the live birth rate of non‐obese women with CC is 26%, which may increase to between 26% and 50% with metformin, whereas the live birth rate of obese women is 22%, which may decrease to between 5% to 13% with metformin.
Adverse events (gastrointestinal side effects)
Data were not available for this outcome.
3.2 Clinical pregnancy rate
The overall heterogeneity was high (I2 = 77%) and the data were not appropriate for pooling because the results were too discrepant between non‐obese and obese women. Subgroup analysis by obesity status showed evidence of a difference between the subgroups (test for subgroup differences: Chi² = 23.30, df = 1 (P < 0.00001, I2 = 95.7%; Analysis 3.2). In the obese group, higher pregnancy rates were seen amongst women taking CC compared with metformin (OR 0.34, 95% CI 0.21 to 0.55; I2 = 0%; 2 studies, 500 women; low‐quality evidence) whereas in the non‐obese group, metformin favoured higher pregnancy rates (OR 1.56, 95% CI 1.06 to 2.29; I2 = 26%; 6 studies, 530 women; low‐quality evidence). This suggests that the clinical pregnancy rate of non‐obese women with CC is 26%, which may increase to between 27% and 44% with metformin, whereas the clinical pregnancy rate of obese women is 28%, which may decrease to between 7% to 17% with metformin. Sensitivity analysis by study quality did not change the heterogeneity but did alter the overall inference (OR 0.42, 95% CI 0.27 to 0.65) however this was only based on two studies and 300 women (Legro 2007; PCOSMIC 2010).
3.2. Analysis.
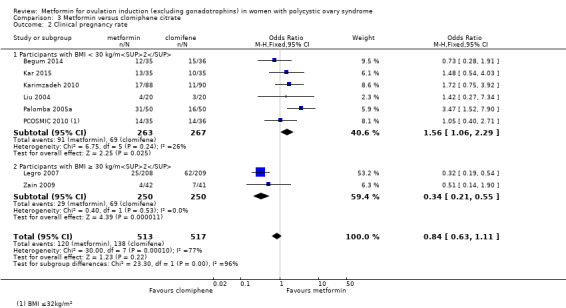
Comparison 3 Metformin versus clomiphene citrate, Outcome 2 Clinical pregnancy rate.
3.3 Ovulation rate
When we combined the data from seven studies (Begum 2014; Kar 2015; Liu 2004; Legro 2007; Palomba 2005a; PCOSMIC 2010; Zain 2009), we found that CC may improve ovulation rates slightly compared with metformin (OR 0.45, 95% CI 0.34 to 0.60; I2 = 53%; 7 studies, 852 women; Analysis 3.3).
3.3. Analysis.
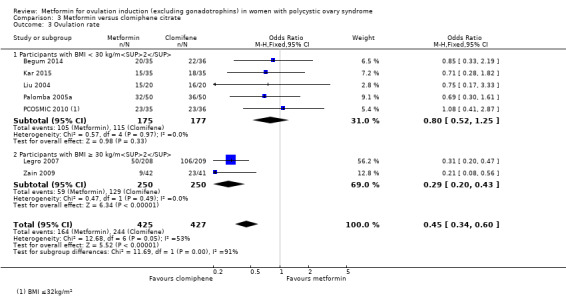
Comparison 3 Metformin versus clomiphene citrate, Outcome 3 Ovulation rate.
Subgroup analysis by obesity status again showed evidence of a difference between the obese and non‐obese women (test for subgroup differences: Chi² = 11.69, df = 1 (P = 0.0006), I² = 91.4%). In the obese group, combining the results from Legro 2007 and Zain 2009 found improved ovulation rates with CC therapy (OR 0.29, 95% CI 0.20 to 0.43; I2 = 0%; 2 studies, 500 women; low‐quality evidence). In the non‐obese group, the data were inconclusive (OR 0.80, 95% CI 0.52 to 1.25; I2 = 0%; 5 studies, 352 women; low‐quality evidence). This suggests that the ovulation rate of non‐obese women with CC is 65%, which may change to between 49% and 70% with metformin, whereas the ovulation rate of obese women is 52%, which may decrease to between 18% to 31% with metformin. Sensitivity analysis by study quality did not change the inference (OR 0.38, 95% CI 0.26 to 0.55; 2 studies, 488 women) but did increase the heterogeneity (I2 = 82%). We have presented ovulation rate per cycle in Table 8.
3.4 Miscarriage rate and 3.5 Miscarriage (sensitivity analysis)
We found no evidence that metformin compared with CC increased miscarriage rates across both BMI groups (OR 0.92, 95% CI 0.51 to 1.66; I2 = 36%; 6 studies, 781 women; low‐quality evidence; Analysis 3.4). On subgroup analysis of BMI, the test for subgroup differences showed no evidence of a difference between obese and non‐obese women (P = 0.14). This suggests that the miscarriage rate of non‐obese women with CC is 6%, which may change to between 4% and 18% with metformin, whereas the miscarriage rate of obese women is 6%, which may change to between 2% to 9% with metformin. Sensitivity analysis by study quality did not change the inference but did increase the heterogeneity (I2 = 71%).
3.4. Analysis.
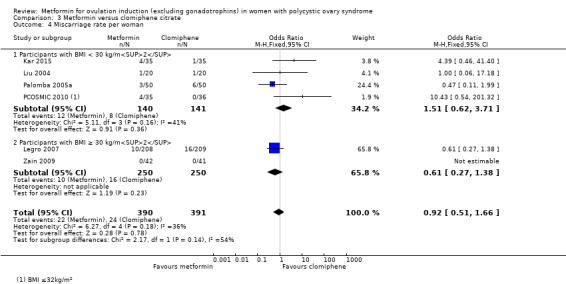
Comparison 3 Metformin versus clomiphene citrate, Outcome 4 Miscarriage rate per woman.
Analysis of miscarriage rate per pregnancy showed no clear evidence of a difference between the groups (OR 1.36, 95% CI 0.69 to 2.66; I2 = 60%; 6 studies, 203 pregnancies; Analysis 3.5), still with no evidence of a difference between the BMI subgroups (P = 0.36).
3.5. Analysis.
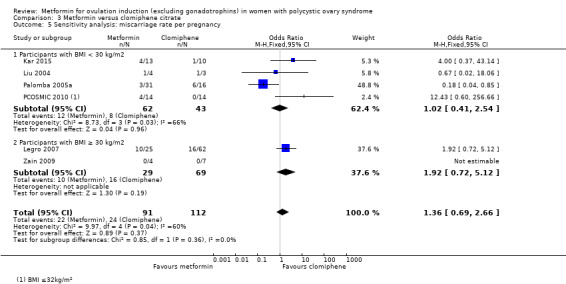
Comparison 3 Metformin versus clomiphene citrate, Outcome 5 Sensitivity analysis: miscarriage rate per pregnancy.
3.6 Multiple pregnancy rate and 3.7 Multiple pregnancy rate per pregnancy
We are uncertain of the effect of metformin compared with CC on multiple pregnancy rates (OR 0.29, 95% CI 0.06 to 1.43; I2 = 0%; 5 studies, 858 women; Analysis 3.6). In the subgroup analysis by obesity status, there was no evidence of a difference between the subgroups (P = 0.52). Sensitivity analysis by study quality did not change the inference or heterogeneity.
3.6. Analysis.
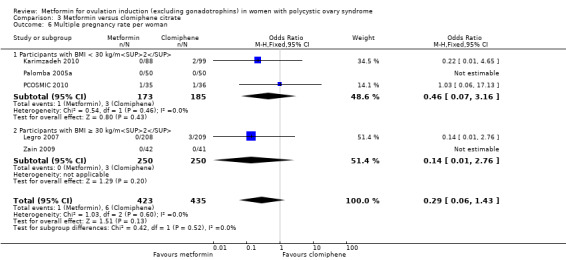
Comparison 3 Metformin versus clomiphene citrate, Outcome 6 Multiple pregnancy rate per woman.
Analysis of multiple pregnancy rate per pregnancy showed no clear evidence of a difference between metformin compared with CC (OR 0.33, 95% CI 0.06 to 1.68; I2 = 0%; 5 studies, 201 pregnancies; Analysis 3.7).
3.7. Analysis.
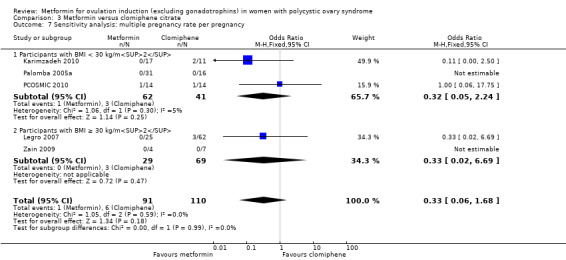
Comparison 3 Metformin versus clomiphene citrate, Outcome 7 Sensitivity analysis: multiple pregnancy rate per pregnancy.
Anthropometric outcomes
3.8 Body mass index
Only one study reported BMI (Liu 2004), which suggested that metformin did reduce BMI compared with CC (MD −5.10, 95% CI −9.40 to −0.80; 40 women; Analysis 3.8), however this is insufficient evidence to draw conclusions.
3.8. Analysis.
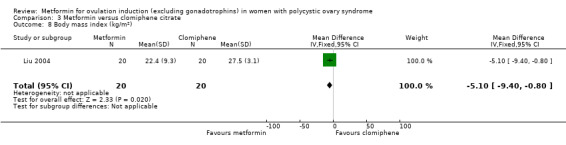
Comparison 3 Metformin versus clomiphene citrate, Outcome 8 Body mass index (kg/m2).
Endocrine outcomes
3.9 Serum testosterone
Only one study reported serum testosterone (Liu 2004), which found no effect of metformin on testosterone levels compared with CC (MD 0.30, 95% CI −0.82 to 1.42; 40 women; Analysis 3.9), however this is insufficient evidence to draw any conclusions.
3.9. Analysis.
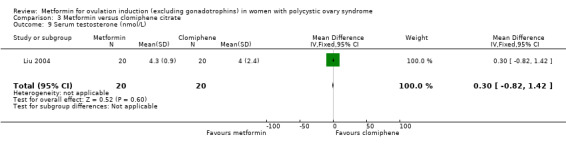
Comparison 3 Metformin versus clomiphene citrate, Outcome 9 Serum testosterone (nmol/L).
Serum sex hormone‐binding globulin
Data were not available for this outcome.
Metabolic outcomes
3.10 Fasting glucose
Only one study reported fasting blood glucose (Liu 2004), which found no effect of metformin on fasting blood glucose levels compared with CC (MD −0.20, 95% CI −0.79 to 0.39; 40 women; Analysis 3.10) however this is insufficient evidence to draw any conclusions.
3.10. Analysis.
Comparison 3 Metformin versus clomiphene citrate, Outcome 10 Fasting glucose (mmol/L).
3.11 Fasting insulin
Only one study reported fasting insulin levels (Liu 2004), which found a reduction in fasting insulin with metformin compared with CC (MD −13.00, 95% CI −16.96 to −9.04; 40 women; Analysis 3.11), although this is insufficient evidence to draw any conclusions.
3.11. Analysis.
Comparison 3 Metformin versus clomiphene citrate, Outcome 11 Fasting insulin (mIU/L).
4. Metformin and letrozole versus letrozole
4.1 Live birth rate
Only one study reported live birth rate (Liu 2017), and there was an identical number of live births in each group (OR 1.00, 95% CI 0.48 to 2.08; 134 women; Analysis 4.1), however this is insufficient evidence to draw any conclusions.
4.1. Analysis.
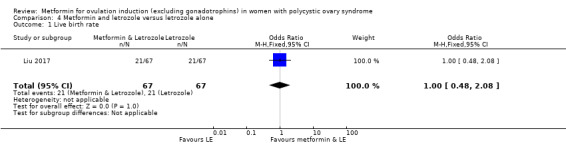
Comparison 4 Metformin and letrozole versus letrozole alone, Outcome 1 Live birth rate.
4.2 Adverse events (gastrointestinal side effects)
Only one study reported gastrointestinal adverse events (Liu 2017), and found no clear evidence that women taking combined metformin and letrozole had more side effects than with letrozole alone (OR 16.74, 95% CI 0.94 to 299.23; 134 women; Analysis 4.2). Seven of 67 women suffered gastrointestinal side effects with metformin and letrozole compared with 0 of 67 women with letrozole alone.
4.2. Analysis.
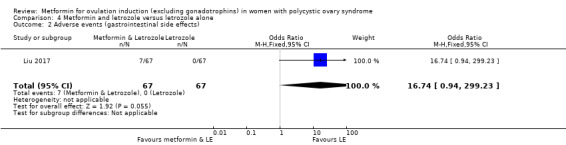
Comparison 4 Metformin and letrozole versus letrozole alone, Outcome 2 Adverse events (gastrointestinal side effects).
4.3 Clinical pregnancy rate
Only one study reported clinical pregnancy rate (Liu 2017), which found no clear evidence that women who had both metformin and letrozole had an improved clinical pregnancy rate compared with letrozole alone (OR 1.27, 95% CI 0.64 to 2.51; 134 women; Analysis 4.3), however the data are insufficient to allow us to draw conclusions.
4.3. Analysis.
Comparison 4 Metformin and letrozole versus letrozole alone, Outcome 3 Clinical pregnancy rate.
Ovulation rate
Data were not available for this outcome. Data for ovulation per cycle are available and presented in Table 9.
4.4 Miscarriage rate and 4.5 Miscarriage rate per pregnancy
Only one study reported miscarriage rate (Liu 2017), which found no difference in miscarriage rate between the two treatment groups (OR 1.61, 95% CI 0.61 to 4.23; 134 women; Analysis 4.4) however this is insufficient evidence to draw conclusions.
4.4. Analysis.
Comparison 4 Metformin and letrozole versus letrozole alone, Outcome 4 Miscarriage rate per woman.
Sensitivity analysis of miscarriage per pregnancy did not show clear evidence of a difference between metformin and letrozole compared with letrozole alone (OR 1.50, 95% CI 0.51 to 4.42; 62 pregnancies; Analysis 4.5).
4.5. Analysis.
Comparison 4 Metformin and letrozole versus letrozole alone, Outcome 5 Sensitivity analysis: miscarriage rate per pregnancy.
Multiple pregnancy rate
Data were not available for this outcome.
Anthropometric outcomes
Body mass index
Data were not available for this outcome.
Endocrine outcomes
Serum testosterone
Data were not available for this outcome.
Serum sex hormone‐binding globulin
Data were not available for this outcome
Metabolic outcomes
Fasting glucose
Data were not available for this outcome.
Fasting insulin
Data were not available for this outcome.
Metformin versus letrozole
We did not identify any suitable studies for this comparison.
5 Metformin and LOD versus LOD
5.1 Live birth rate
Only one study reported live birth rate (Kocak 2006), which found no clear evidence of a difference between metformin and LOD compared with LOD alone (OR 2.13, 95% CI 0.51 to 8.77; 42 women; Analysis 5.1), although the data are insufficient to allow us to draw conclusions.
5.1. Analysis.
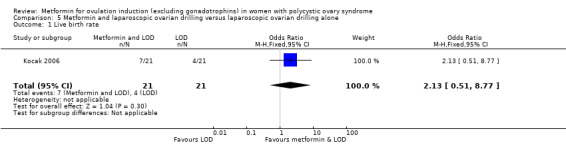
Comparison 5 Metformin and laparoscopic ovarian drilling versus laparoscopic ovarian drilling alone, Outcome 1 Live birth rate.
Adverse events (gastrointestinal side effects)
Data were not available for this outcome.
5.2 Clinical pregnancy rate
Only one study reported clinical pregnancy rate (Kocak 2006), which found no clear evidence of a difference between metformin and LOD compared with LOD alone (OR 3.19, 95% CI 0.79 to 12.80; 42 women; Analysis 5.2), although the data are insufficient to allow us to draw conclusions.
5.2. Analysis.
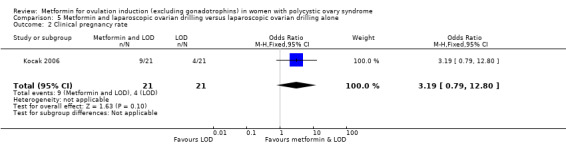
Comparison 5 Metformin and laparoscopic ovarian drilling versus laparoscopic ovarian drilling alone, Outcome 2 Clinical pregnancy rate.
Ovulation rate
Data were not available for this outcome. We have presented data for ovulation per cycle in Table 10.
5.3 Miscarriage rate and 5.4 Miscarriage rate per pregnancy
Only one study reported miscarriage rate (Kocak 2006), which found no clear evidence of a difference between metformin and LOD compared with LOD alone (OR 5.51, 95% CI 0.25 to 122.08; Analysis 5.3), although the data are insufficient to allow us to draw conclusions.
5.3. Analysis.
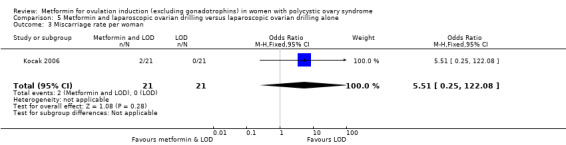
Comparison 5 Metformin and laparoscopic ovarian drilling versus laparoscopic ovarian drilling alone, Outcome 3 Miscarriage rate per woman.
Sensitvity analysis of miscarriage per pregnancy found no clear evidence of a difference between metformin and LOD compared with LOD alone (OR 3.00, 95% CI 0.12 to 77.64; 13 pregnancies; Analysis 5.4).
5.4. Analysis.
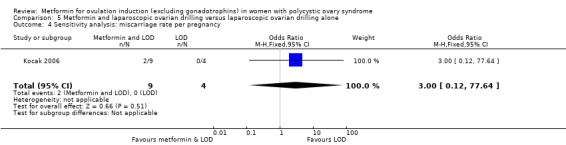
Comparison 5 Metformin and laparoscopic ovarian drilling versus laparoscopic ovarian drilling alone, Outcome 4 Sensitivity analysis: miscarriage rate per pregnancy.
Multiple pregnancy rate
Data were not available for this outcome
Anthropometric outcomes
Body mass index
Data were not available for this outcome.
Endocrine outcomes
Serum testosterone
Data were not available for this outcome.
Serum sex hormone‐binding globulin
Data were not available for this outcome.
Metabolic outcomes
Fasting glucose
Data were not available for this outcome.
Fasting insulin
Data were not available for this outcome.
6. Metformin versus LOD
6.1 Live birth rate
Only one study reported live birth rate (Palomba 2004), which found live birth rate to be improved in the metformin group compared with LOD (OR 2.29, 95% CI 1.09 to 4.78; 120 women; Analysis 6.1).
6.1. Analysis.
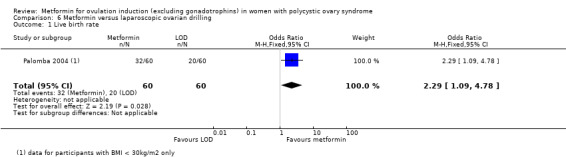
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 1 Live birth rate.
6.2 Adverse events (gastrointestinal side effects)
Two studies reported gastrointestinal events (Hamed 2010; Palomba 2004), and found that the LOD group had fewer adverse side effects compared with metformin (OR 7.77, 95% CI 2.43 to 24.89; 230 women; Analysis 6.2). Palomba 2004 included non‐obese women and Hamed 2010 included obese women, however there was no difference between the groups (test for subgroup differences: Chi² = 1.10, df = 1 (P = 0.30), I² = 8.8%).
6.2. Analysis.
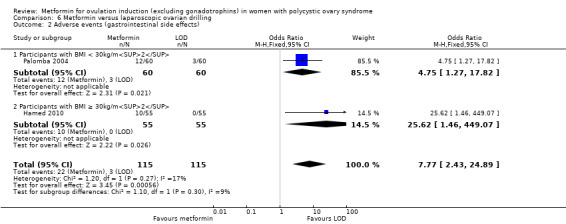
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 2 Adverse events (gastrointestinal side effects).
6.3 Clinical pregnancy rate
Two studies reported clinical pregnancy rate (Hamed 2010; Palomba 2004), and we are uncertain of the effect of metformin compared with LOD on clinical pregnancy rate (OR 0.93, 95% CI 0.54 to 1.59; 2 studies, 230 women; Analysis 6.3).
6.3. Analysis.
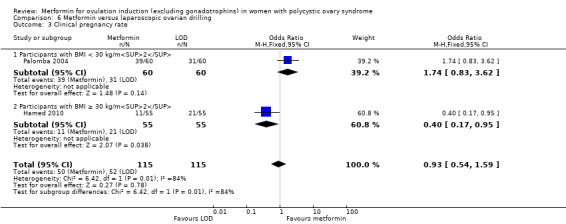
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 3 Clinical pregnancy rate.
Subgroup analysis showed a significant difference between the obese and the non‐obese group (test for subgroup difference: Chi² = 6.42, df = 1 (P = 0.01), I² = 84.4%) where obese women favoured LOD (OR 0.40, 95% CI 0.17 to 0.95; 1 study, 110 women).
6.4 Ovulation rate
Only one study reported ovulation per woman (Malkawi 2003), and we are uncertain of the effect of metformin compared with LOD on ovulation rate (OR 0.51, 95% CI 0.26 to 1.01; 145 women; Analysis 6.4).
6.4. Analysis.
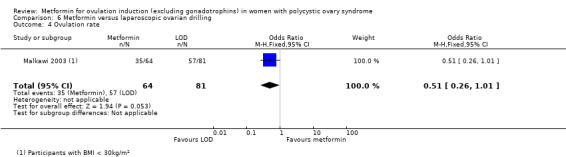
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 4 Ovulation rate.
Two studies reported data for ovulation per cycle, which we have presented in Table 11.
8. Metformin vs laparoscopic ovarian drilling (LOD): ovulation rate per cycle.
| Metformin | LOD | ||||
| Study ID | Events | Cycles | Events | Cycles | P value |
| BMI < 30 kg/m2 | |||||
| Palomba 2004 | 115 | 210 | 123 | 231 | P = 0.75 |
| BMI ≥ 30 kg/m2 | |||||
| Hamed 2010 | 94 | 281 | 131 | 258 | P < 0.01 |
6.5 Miscarriage rate and 6.6 Miscarriage per pregnancy
Two studies reported miscarriage rate (Hamed 2010; Palomba 2004), and found no evidence of an effect of metformin compared with LOD for miscarriage rate (OR 0.58, 95% CI 0.23 to 1.47; 2 studies, 230 women; Analysis 6.5). Subgroup analysis found no clear evidence of a difference between obese and non‐obese women (test for subgroup differences: Chi² = 0.07, df = 1 (P = 0.80), I² = 0%).
6.5. Analysis.
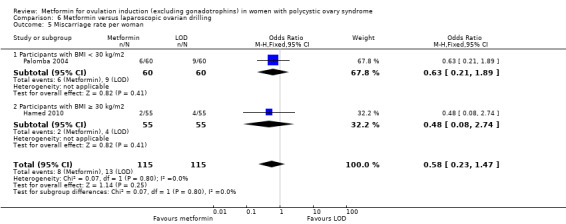
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 5 Miscarriage rate per woman.
Sensitvity analysis of miscarriage per pregnancy found no clear evidence of a difference between metformin and LOD (OR 0.55, 95% CI 0.20 to 1.48; 2 studies, 102 pregnancies; Analysis 6.6).
6.6. Analysis.
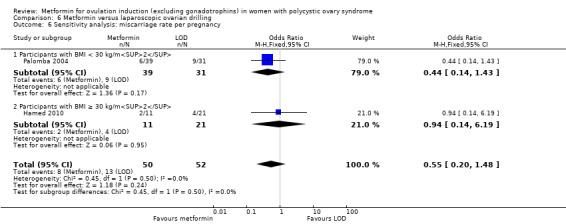
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 6 Sensitivity analysis: miscarriage rate per pregnancy.
Multiple pregnancy rate
Data were not available for this outcome.
Anthropometric outcomes
6.7 Body mass index
One study reported BMI (Hamed 2010), and we are uncertain of the effect of metformin versus LOD on BMI (MD −3.60, 95% CI −13.48 to 6.28; 110 women; Analysis 6.7).
6.7. Analysis.
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 7 Body mass index (kg/m2).
6.8 Endocrine outcomes
Serum testosterone
One study reported serum testosterone (Hamed 2010), and we are uncertain of the effect of metformin versus LOD on serum testosterone (MD −0.16, 95% CI −1.09 to 0.77; 110 women; Analysis 6.8).
6.8. Analysis.
Comparison 6 Metformin versus laparoscopic ovarian drilling, Outcome 8 Serum testosterone (nmol/L).
Serum sex hormone‐binding globulin
Data were not available for this outcome.
Metabolic outcomes
Fasting glucose
Data were not available for this outcome.
Fasting insulin
Data were not available for this outcome.
Publication bias
We assessed publication bias using a funnel plot (Figure 7; Table 1; Table 2; Table 3).
7.
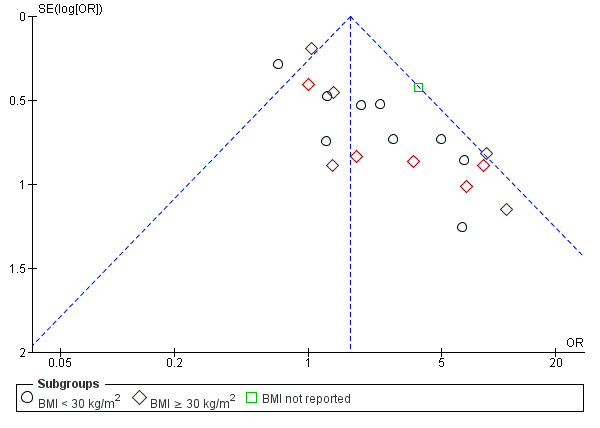
Funnel plot of comparison: 2 Metformin and clomiphene citrate versus clomiphene citrate alone, outcome: 2.4 Ovulation rate.
Discussion
Summary of main results
Metformin is associated with a beneficial effect on ovulation and clinical pregnancy rates, regardless of BMI, when compared with placebo. This update did not yield any further studies comparing metformin to placebo for live birth rate and therefore more high‐quality studies that report live birth as a primary outcome are still required. When comparing outcomes following the use of metformin or CC, higher ovulation rates suggest that CC is beneficial over metformin, in particular in women with a BMI of 30 kg/m2 or higher. However, there was no clear evidence to suggest that either treatment would increase the likelihood of a live birth or clinical pregnancy rate over the other.
Women who are known to be resistant to CC therapy may benefit from improved ovulation with the addition of metformin to CC. However, data were not available to determine if this would improve clinical pregnancy or live birth rates in this group of women. Women taking metformin should be advised that there does not appear to be an effect on miscarriage with treatment, but the likelihood of gastrointestinal side effects is higher than with placebo or CC. More studies that compare the effects of ovulation‐induction agents with CC‐resistant versus CC‐sensitive women are required.
There was insufficient evidence to determine a beneficial effect with metformin compared with or in combination with letrozole for live birth rate, clinical pregnancy or ovulation. There is some evidence that metformin is beneficial over LOD for improving live birth rate however this was based on one study and the findings did not correlate for clinical pregnancy and ovulation rate.
Reproductive outcomes
When compared with placebo, the results suggest a possible benefit of using metformin in improving live birth rates (Analysis 1.1). One high‐quality study included in this updated review contributed the majority of the weight (89.2%) to this finding (Morin‐Papunen 2012). However, the wide‐ranging confidence intervals and lower‐quality evidence when the Morin‐Papunen 2012 results were combined with other included studies, makes the advantage offered by metformin difficult to interpret clinically. However, clinical pregnancy rates were higher with the use of metformin for ovulation induction (Analysis 1.3). Ovulation also appeared to be improved with metformin versus placebo, which persisted following a subgroup analysis by BMI (Analysis 1.4).
There was no conclusive evidence that adding metformin in combination with CC, increased live birth compared with CC monotherapy (Analysis 2.1). However, clinical pregnancy and ovulation rates were improved with combination treatment in both BMI groups (Analysis 2.3; Analysis 2.4). We attempted to analyse data depending on whether women were known to be sensitive or resistant to CC. Unfortunately, these data were only available for ovulation rate. In women who are CC‐resistant, improved ovulation rate was seen with adding metformin to CC compared to women who were CC‐sensitive (Analysis 2.5).
When metformin was compared with CC, findings were complicated by a difference based on the obesity status of the participants. Here, women in the non‐obese group were more likely to achieve a live birth with metformin, whilst the obese women appeared to benefit from CC therapy (Analysis 3.1). This pattern was also evident for clinical pregnancy (Analysis 3.2), however, the studies in this review failed to show the same pattern with ovulation rate. There was evidence to suggest that CC increased ovulation rate compared with metformin for both obese and non‐obese groups, although the evidence in non‐obese groups was less clear (Analysis 3.3).
We did not find any eligible studies that compared metformin with letrozole directly. When metformin and letrozole in combination were compared with letrozole alone, there was insufficient evidence to suggest that adding metformin improved live birth or clinical pregnancy rates (Analysis 4.1; Analysis 4.3). We did not find any data on ovulation rate for these comparisons.
When adding metformin to LOD compared with LOD alone, there was no evidence to suggest an improvement in live birth rate or clinical pregnancy rate (Analysis 5.1; Analysis 5.2). We did not find any data on ovulation rate for these comparisons. When comparing metformin directly with LOD, there was evidence to suggest benefit with metformin compared with LOD for live birth rate however this was based on only one study (Palomba 2004; Analysis 6.1). There was insufficient evidence to suggest a benefit with metformin compared with LOD for clinical pregnancy rate (Analysis 6.2).
Miscarriage was not commonly reported as an outcome and when it was reported, the event rate was low (4.8%, 223 miscarriages of 4552 women). There was no evidence of an effect with metformin compared with placebo, with combined metformin and CC compared with CC alone or with metformin versus CC directly (Analysis 1.5; Analysis 2.6; Analysis 3.4). The previous review suggested an increase in miscarriage when CC combined with metformin was compared with CC alone. However, we did not see this effect in the current review with the addition of three new studies (Liu 2004; Liu 2017; Heathcote 2013). The results were inconclusive for miscarriage rates for metformin and letrozole versus letrozole, metformin and LOD versus LOD, or metformin versus LOD (Analysis 4.4; Analysis 5.3; Analysis 6.5).
For the multiple pregnancy outcome, there was only one study that reported multiple pregnancy rates for metformin compared with placebo and no available data regarding metformin and letrozole versus letrozole, metformin and LOD versus LOD or metformin versus LOD. The results were inconclusive for combination therapy versus CC monotherapy, and for the comparison between metformin and CC (Analysis 2.8; Analysis 3.6).
Adverse effects
Metformin was associated with higher rates of gastrointestinal side effects compared with placebo and LOD (Analysis 1.2; Analysis 6.2). Combination treatment of metformin and CC also had increased rates of gastrointestinal side effects compared with CC alone (Analysis 2.2). There were insufficient data available for metformin compared with CC or letrozole directly, and for when metformin was used in combination with letrozole and LOD.
Metabolic and anthropometric outcomes
This review included studies that specifically reported reproductive outcomes where women had taken metformin in an attempt to induce ovulation and conceive. We excluded studies that compared metformin to placebo to improve BMI or other metabolic outcomes only, without attempting to induce ovulation. Therefore we cannot provide a robust analysis of the effect of metformin compared to placebo on metabolic and anthropometric outcomes. Nonetheless, we did analyse the metabolic and anthropometric outcomes within these included studies for all comparisons that were relevant in view of reducing the risk of metabolic syndrome and associated complications of cardiovascular risk and type 2 diabetes mellitus, which can increase maternal and fetal morbidity and mortality. These outcomes include BMI, serum testosterone, serum sex hormone‐binding globulin, fasting insulin and fasting glucose. There was no evidence that metformin reduced BMI when compared with placebo (Analysis 1.9), and there was no significant difference between BMI subgroups. However, there was some evidence to suggest that metformin might reduce BMI when added to CC compared with CC alone as well as when compared with metformin directly (Analysis 2.10; Analysis 3.8). There was no evidence to suggest a beneficial effect when metformin was compared with LOD directly (Analysis 6.7), and there were no data available for metformin and letrozole compared with letrozole alone, nor metformin and LOD compared with LOD alone. The insufficient data across many of these comparators is likely due to the restrictions of only including studies with reproductive outcomes. The Australian National Health and Medical Research Council (NHRMC) reported that the use of metformin, not specifically for ovulation induction, improves metabolic and anthropometric outcomes including lowering BMI, testosterone, fasting insulin and cholesterol levels (NHMRC 2018).
With regards to endocrine outcomes, there was evidence to suggest a reduction in serum testosterone levels with metformin compared with placebo (Analysis 1.10), however, we did not see the same effect when we compared metformin and CC with CC alone, or metformin with CC or LOD (Analysis 2.11; Analysis 3.9; Analysis 6.8). Serum sex hormone‐binding globulin was only measured in the metformin versus placebo group and there was no evidence to suggest a benefit of using metformin compared with placebo (Analysis 1.11).
There was no conclusive evidence of an effect of metformin on serum glucose levels (Analysis 1.12; Analysis 2.12; Analysis 3.10). There was no conclusive evidence of an effect of metformin when compared with placebo for reducing serum insulin levels (Analysis 1.13) however, there was some evidence of an effect of metformin on reducing serum insulin levels when added to CC therapy compared with CC alone and when compared directly to CC (Analysis 2.13; Analysis 3.11).
It is therefore unclear whether these metabolic and endocrine effects would be of clinical benefit in reducing the risk of metabolic syndrome in women with PCOS, especially given that data on these outcomes were associated with high heterogeneity and some of the effects were created from single small studies. Furthermore, 11 studies included specific advice on lifestyle modification in the study protocol (Ben Ayed 2009; Boudhraa 2010; Heathcote 2013; Hoeger 2004; Karimzadeh 2010; Kjotrod 2011; Lord 2006; PCOSMIC 2010; Siebert 2009; Tang 2006; Zain 2009). Obesity has a significant detrimental impact on both maternal and fetal outcomes in pregnancy as well as longer‐term cardiovascular health (Cedergren 2004). As such, women with PCOS should still be advised to undergo lifestyle interventions before any fertility treatment (ESHRE/ASRM 2008).
Limitations
See Quality of the evidence and Potential biases in the review process.
Overall completeness and applicability of evidence
This review includes a large number of women, all meeting the Rotterdam diagnostic criteria for PCOS (ESHRE/ASRM 2004). However, we still observed significant heterogeneity in many of the analyses. This was particularly evident in the biochemical outcomes, even after adjustment for BMI, dosage of metformin and duration of treatment. Heterogeneity remained unchanged after sensitivity analysis by study quality. However, the prevalence and magnitude of insulin resistance are influenced by ethnicity (Kakoly 2018), therefore, combining trials from different study populations would introduce heterogeneity despite all meeting the diagnostic criteria of PCOS. Another factor is the range of biochemical assays used in different studies, which may introduce some heterogeneity. The efficacy of metformin in PCOS was first described by Velazquez 1997. A number of small, and often short‐duration, observational studies followed, which showed variable outcomes. Indeed, in a systematic review by Costello 2003 nine out of the 12 published studies on the effects of metformin alone on the menstrual cycle in women with PCOS had a sample size of fewer than 30 women. The first Cochrane Review by Lord 2002 included nearly 1000 women from 15 RCTs. However, most of the studies had relatively small sample sizes with the largest one containing 94 women (Fleming 2002). In this fourth updated review, we included 41 RCTs (4552 women), with the two largest studies of high quality being by Morin‐Papunen 2012 and Legro 2007, with sample sizes of 320 and 626 women, respectively.
Reproductive outcomes
This review focused on the effect of metformin on reproductive outcomes including ovulation rate, clinical pregnancy rate and live birth rate.
This review confirmed the findings from the previous review that there is a potential benefit of using metformin when compared with placebo to improve live birth rate, with a number needed to treat for an additional beneficial outcome of 13. This positive finding was consistent in both obese and non‐obese women. Similar findings were seen with ovulation rate and clinical pregnancy rate, which further strengthens any recommendation to use metformin compared with placebo for ovulation induction in PCOS women with subfertility. However, the impact on live birth rate was based on four studies, the majority with low‐quality evidence and we were unable to obtain any new studies to strengthen the recommendation. We included two additional studies featuring data on ovulation and clinical pregnancy rate (Chuni 2006; Kjotrod 2011). Obese women with PCOS have higher levels of insulin resistance and higher serum insulin concentrations and hence metformin may have a limited effect on reducing these high serum insulin concentrations in the obese group compared with non‐obese women (Tang 2006). Further studies comparing metformin with placebo must stratify by BMI in order to guide the use of metformin appropriately.
Traditionally, CC is the first‐line therapy for ovulation induction in PCOS women. In this review, we did not find any additional studies with data on live birth rates when directly comparing metformin and CC. Of the five studies included, the results differed with BMI. CC increased live births in the obese group, with a large weighting from the study by Legro 2007. In the non‐obese group however, metformin appeared to increase live births yet this analysis included only small studies of low quality. The same inference was seen with clinical pregnancy rate. More high‐quality studies are required with larger numbers of participants to assess metformin versus CC for live birth rate.
The addition of two new studies (Heathcote 2013; Liu 2017), did not change the inference that there was no clear evidence of improvement in live birth rate with combination metformin and CC compared with CC alone. However, there was evidence to suggest improvement in clinical pregnancy and ovulation rates. Similarly to the previous review, a larger effect was seen in the CC‐resistant group compared with the CC‐sensitive group, with low heterogeneity (I2 = 0%) in the CC‐resistant group. However, the CC‐sensitive group consisted of one study only (Jakubowicz 2001). Future studies to determine the effect of metformin compared with CC on live birth rate should specify CC sensitivity. Within the BMI subgroups, heterogeneity was high for both groups and there was no difference in effect between obese and non‐obese women (P = 0.16).
In this review, there was no evidence of an effect with miscarriage or multiple pregnancy rates attributable to metformin. However, women should be counselled on the increased gastrointestinal side effects associated with metformin use.
We did not find any eligible studies that directly compared metformin with aromatase inhibitors. One study compared combined metformin and letrozole with letrozole alone, although there was insufficient evidence of a beneficial effect on live birth rate, clinical pregnancy, miscarriage or adverse effects (Liu 2017). The study had an unclear risk of bias and larger studies of higher‐quality are required to determine the effect of metformin compared with letrozole.
We found three studies that directly compared metformin with LOD. There was evidence to suggest that metformin had a beneficial effect on live birth rate compared with LOD. However, only one small study reported this outcome (Palomba 2004), and hence more studies that report live birth rate are required. Furthermore, the findings do not correlate with other reproductive outcomes where there was insufficient evidence to suggest a beneficial effect with metformin compared with LOD for clinical pregnancy rate and ovulation rate. Of the three studies, only one study (Palomba 2004), showed low risk of bias and hence more, larger, high‐quality studies are required to clarify the uncertainty that these pooled results reveal on the effect of metformin compared with LOD. One study analysed the cost effectiveness of LOD compared with metformin (Palomba 2004), who compared the costs of the day‐surgery fee, the surgeon's fee, the anaesthetist's fee, assistant's fee as well as the equipment required for LOD with the cost of six cycles of metformin given at 1700 mg daily. The cost of LOD was significantly more expensive (EUR 1050) compared with the six‐month course of metformin (EUR 50; P < 0.05). This paper was published 15 years prior to this review and hence now we could expect an even greater discrepancy between medical versus surgical management. Hence, it is important to consider the cost effectiveness of medical versus surgical treatment especially in low‐income countries where access to surgery may be less easy.
Metabolic and anthropometric outcomes
There is still no long‐term data available on the use of metformin for women with PCOS in reducing the risk of developing diabetes or metabolic syndrome. This review found no evidence of an effect of metformin on reducing BMI when compared with placebo. Some reduction of BMI was seen when metformin was added to CC therapy and when compared with CC, although these results were based on small studies with unclear risks of bias. Testosterone levels were reduced with metformin therapy compared with placebo, although heterogeneity was high (I2 = 94%), which makes this finding difficult to interpret clinically. Other comparisons did not show an effect on testosterone levels nor serum insulin or serum glucose because only small numbers of low‐quality studies were available for analysis.
Quality of the evidence
Overall, we graded only 12 out of the 41 included studies as having a low risk of bias to sequence generation, allocation concealment and blinding. The main limitation of the comparisons in this review is therefore the risk of bias and imprecision within the included studies, as discussed in 'Table 1; Table 2; Table 3 and Figure 2 and Figure 3. However, sensitivity analysis on the studies with adequate sequence generation, allocation concealment and blinding method did not alter the clinical findings. We classified the overall quality of evidence for metformin versus placebo as low for live birth rate, ovulation rate and miscarriage rate, and moderate for clinical pregnancy rate and adverse effects (Table 1). This was due to a moderate risk of bias, marginal effect size and statistical imprecision. Despite many comparison studies demonstrating high heterogeneity, we did not downgrade if the direction of effect was consistent, nor if the heterogeneity was attributable to a single study. We regarded both the evidence for metformin and letrozole compared with letrozole, as well as metformin combined with LOD versus LOD alone as low quality, based on a single study only. The overall quality of evidence for metformin versus LOD was also low.
Potential biases in the review process
We conducted a thorough search, used sound methodology and are not aware of any biases in the review process.
There are some papers awaiting classification (Ayaz 2013a; Beigi 2006; Jahan 2015; Robinson 2003; Singh 2001; Williams 2009), and ongoing clinical trials (NCT00005104; NCT00317928; NCT00558077; NCT01679574; NCT02562664), that are likely to provide further useful information.
Agreements and disagreements with other studies or reviews
Reproductive outcomes
A 2016 systematic review investigated the efficacy of metformin in women with anovulatory infertility for the improvement of reproductive outcomes (Abu Hashim 2016). More recently, international guidelines have been released (NHMRC 2018), quoting results from the last Cochrane Review (Morley 2017), and with the addition of one more RCT, which has been included in this most current update (Kjotrod 2011). The general consensus from these guidelines, which is in accordance with this update, is that metformin improves live birth rate, clinical pregnancy rate, ovulation rate, yet increases gastrointestinal side effects compared with placebo (NHMRC 2018). Another recent meta‐analysis (Wang 2019), found that CC and metformin may improve clinical pregnancy rate compared to CC alone (RR 1.18, 95% CI 1.00 to 1.39, 8 studies, 1039 women) however, there was insufficient evidence of a difference on live birth (RR 1.08, 95% CI 0.87 to 1.35, 5 studies, 907 women) These results are similar to this updated review however, Wang 2019 included fewer studies. Furthermore some of these studies involve intrauterine insemination and the use of hCG as an ovulation trigger, which we excluded from our review (Leanza 2014;Sahin 2004).
Comparison of metformin with CC for ovulation induction has been determined in three meta‐analyses as well as the recent international guidelines (NHMRC 2018; Palomba 2009; Siebert 2012; Wang 2017). In accordance with our findings, they found improved ovulation rates with CC rather than metformin. There was no conclusive benefit of either treatment on clinical pregnancy or live birth rate, with wide confidence intervals noted. Palomba 2009 and Siebert 2012 therefore conclude that CC remains the “gold standard first‐line pharmacological treatment for ovulation induction in anovulatory infertile women with PCOS”. An analysis of four studies that compared metformin with CC in non‐obese women found no significant difference in reproductive outcomes (Misso 2013). The conclusions drawn by Abu Hashim 2016 echo the ESHRE consensus, which documented that the first‐line treatment for anovulatory infertility is CC, whilst obese women should be advised to undergo lifestyle modifications (ESHRE/ASRM 2008).
When evaluating the Palomba 2009 and Siebert 2012 meta‐analyses, Abu Hashim 2016 found no evidence of an improvement in live birth when metformin was used in combination with CC. Our review also found no conclusive evidence of a difference in live birth rate, although clinical pregnancy and ovulation were improved with co‐therapy. These results are similar to those found in a systematic review (Wang 2017), where improvements in clinical pregnancy and ovulation were seen with combination therapy however, this did not reflect in live birth rate. Wang 2017 reported clinical pregnancy rate as their primary outcome therefore only a small number of studies reported the outcome live birth rate, which hinders the statistical power and explains the lack of significance. Given the increased side‐effect profile with metformin, as found in our review (Morley 2017), Abu Hashim 2016 do not recommend adding in metformin to CC therapy. However, their results are not stratified by BMI. The recent international guidelines that quoted our most recent Cochrane Review as well as one RCT did stratify by BMI and similarly found no difference in live birth rate. We excluded this additional RCT from our recent update because it used hCG injection to trigger ovulation.
A recent meta‐analysis in 2018 reported that letrozole should be used first line for ovulation induction given that "the likelihood of live birth is increased 40‐60% with letrozole compared with CC", with reduced rates of failure to ovulate, multiple pregnancy rate and reduced side effects such as hot flushes (NHMRC 2018). Concerns arise that letrozole is associated with an increase in potential teratogenic effects however, these findings are yet to be confirmed, with multiple case reviews and a systematic review and meta‐analysis failing to determine any significant association (Diamond 2015; Wang 2017). It was beyond the scope of this Cochrane update to directly compare letrozole with CC however, we were unable to find any studies that directly compared metformin with letrozole. Letrozole is considered first line in some countries because it has been shown to improve ovulation, pregnancy, live birth and reduce the multiple pregnancy rate (Wang 2017).
The 2018 meta‐analysis compared LOD to metformin directly using two of the three studies included in this review (NHMRC 2018). They concluded, in accordance with our findings, that there were insufficient data on whether LOD improves live birth rate, clinical pregnancy or ovulation rates. As a result, they recommend LOD as second‐line treatment in women who are CC‐resistant or first‐line if laparoscopy is indicated for an alternative reason (NHMRC 2018). Similarly Wang 2017 found insufficient evidence of benefit with metformin compared with LOD. They emphasise that women should be counselled carefully about the risks of surgery, including periadnexal adhesion formation, risk of reduced ovarian reserve or loss of ovarian function and increased intra‐operative and post‐operative risks especially in obese women.
Metabolic and anthropometric outcomes
This update focused on women with subfertility with a desire to conceive, therefore we excluded studies if reproductive outcomes were not the aim of treatment. Nonetheless, an improvement in some metabolic and anthropometric outcomes may improve the success of reproductive outcomes and subsequent health.
Our review found mixed evidence of an effect of metformin on metabolic outcomes, which is of unclear clinical significance for the prevention of diabetes in the long term. These findings are supported by a Diabetes Prevention Program Research group study of over 3000 obese women (mean BMI 34 kg/m2) with an average follow‐up period of 2.8 years (Knowler 2002). They reported that both metformin and lifestyle‐intervention groups (7.8 and 4.8 cases per 100 person years respectively) had a lower incidence of diabetes compared with placebo (11 per 100 person years). However, the lifestyle‐intervention group achieved a significantly better weight reduction compared with the metformin group (58% versus 31%). Furthermore, the initial modest weight loss in the metformin group was not sustainable after three years of follow‐up. In contrast, in the lifestyle group, an average of 4% weight loss was still maintained after four years. Likewise, the Finnish Diabetes Prevention Study demonstrated that weight loss improved insulin sensitivity, waist circumference and serum triglyceride levels compared with controls in 150 obese women with impaired glucose tolerance (Uusitupa 2000). A 2007 meta‐analysis also concluded that lifestyle interventions are more effective than metformin in reducing the rate of progression to type 2 diabetes in obese women with impaired glucose tolerance (Gillies 2007).
Authors' conclusions
Implications for practice.
Our updated review suggests that metformin may be beneficial over placebo for live birth however, more women probably experience gastrointestinal side effects. Compared to placebo, metformin probably increases pregnancy rates and may increase ovulation rates. We are uncertain if metformin plus clomiphene citrate (CC) improves live birth rates compared to CC alone, but gastrointestinal side effects are probably increased with combined therapy. The combined therapy group probably has higher rates of clinical pregnancy and may have higher rates of ovulation. When metformin was compared with CC, data for live birth were inconclusive, and the findings were limited by lack of evidence. Results differed by body mass index (BMI), emphasising the importance of stratifying results by BMI. Improved clinical pregnancy and ovulation rates with metformin and CC versus CC alone suggests that combined therapy may be useful although we do not know whether this translates into increased live births. No studies reported gastrointestinal side effects in this comparison. Due to the low quality of the evidence, we are uncertain of the effect of metformin on miscarriage in all three comparisons.
Implications for research.
More high‐quality studies are required with adequate power that stratify BMI and CC sensitivity status. Only few studies compared metformin directly with, or in combination with, letrozole and laparoscopic ovarian drilling (LOD), therefore further studies are required to determine the effect these comparisons have on reproductive outcomes.
Possible future strategies for insulin‐sensitising drugs include glucagon‐like peptide 1 (GLP‐1) analogues, which have been studied recently in women with PCOS (Jensterle 2014). These agents include exenatide and liraglutide and are currently only licensed for the treatment of type 2 diabetes mellitus. One study reported improved pregnancy rates after combined liraglutide and metformin prior to in vitro fertilisation (Janez 2018). Future updates of this review may include comparative studies between metformin and these newer agents. Mitochondrial mutations have been associated with insulin resistance and PCOS (Ding 2017). The development of mitochondrial inhibitors may present an additional new therapeutic strategy for managing PCOS (Colca 2013; Zhang 2012).
Future studies of metformin should include live birth rate as the primary outcome. Studies should subdivide data on reproductive outcomes by resistance to CC and BMI (accounting for women having bariatric surgery). The magnitude of insulin resistance is also influenced by ethnicity (Bozdag 2016). Trials should therefore perform subgroup analyses according to the ethnic origin of participants. These subgroups may reduce the heterogeneity in meta‐analyses. It may be prudent to investigate the efficacy of early intervention in young women or adolescents, or both, with a diagnosis of PCOS. Further data in this area may improve patient selection when determining the appropriate therapeutic strategy. Studies should also focus on the long‐term impact of lifestyle changes and the use of insulin‐sensitising drugs to modulate the risk of developing metabolic syndrome.
Good‐quality studies of adequate power are required to investigate the efficacy and safety of any new insulin‐sensitising agents. Although there is no current evidence that metformin is teratogenic (Cassina 2014), if it is used widely to treat anovulation then it is possible that rare effects may be unmasked. Metformin therapy therefore needs to be kept under continuing surveillance with more stringent reporting of adverse outcomes including gastrointestinal side effects.
What's new
| Date | Event | Description |
|---|---|---|
| 14 August 2019 | New citation required but conclusions have not changed | When metformin is compared with placebo and CC treatment, the conclusions have not changed. There was insufficient evidence to draw conclusions about letrozole and LOD. |
| 13 December 2018 | New search has been performed | An update to the review, however, a change in protocol to include studies that compared metformin with placebo and/or CC, letrozole and LOD monotherapy or combination therapy and exclude studies on rosiglitazone, pioglitazone and D‐chiro‐inositol due to lack of reporting of reproductive outcomes and concerns about safety of these medications in pregnancy. 13 new studies added (Fatima 2018; Hamed 2010; Heathcote 2013; Kjotrod 2011; Ko 2001; Kocak 2006; Liu 2004; Liu 2017; Malkawi 2003; Chuni 2006; Palomba 2004; Raja 2005; Refaie 2005). |
History
Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 15 September 2017 | New search has been performed | Five new studies added (Ayaz 2013; Begum 2014; Kar 2015; Machado 2012; Morin‐Papunen 2012). Six studies reclassified as excluded (Chaudhry 2016; Chaudhury 2008; Constantino 2009; Farzadi 2006; Ladson 2011; Refaie 2005). The review now includes 48 studies. |
| 15 September 2017 | New citation required and conclusions have changed | The Inclusion and exclusion of studies at this update has led to a modification in the conclusions of this review. |
| 19 April 2012 | New citation required but conclusions have not changed | New studies added but no change to conclusions |
| 2 October 2011 | New search has been performed | New studies added: Ben Ayed 2009; Boudhraa 2010; Brettenthaler 2004; Carmina 2004; Karimzadeh 2010; Khorram 2006; Ladson 2011; Lam 2011; Otta 2010; Pasquali 2000; Romualdi 2010; Sahin 2004; Siebert 2009; Williams 2009 Re‐classified publications Rautio 2006a; Rautio 2006b into a single study Rautio 2006 Protocol changes: removed secondary outcomes of hirsutism, waist circumference and HDL cholesterol; Removed Kelly 2002, Re‐classification of risk of bias in included studies according to the CRG recommendations |
| 6 December 2010 | New search has been performed | New Studies added: PCOSMIC 2010 |
| 1 March 2010 | Amended | Error in abstract corrected |
| 12 June 2008 | New citation required and conclusions have changed | Converted to new review format. Twenty‐one new RCTs were added to the review: Baillargeon 2004, Chou 2003, Eisenhardt 2006, Gerli 2003, Glintborg 2005, Hoeger 2004 and b, Karimzadeh 2007, Legro 2007, Lord 2006, Maciel 2004 and b, Moll 2006 Onalan 2005 and b, Palomba 2005a, Rautio 2006, Rautio 2006b, Tang 2006, Trolle 2007 and Zain 2009. Some changes to the methodology were made in accordance with Revman 5 and one new comparison was added (Metformin versus CC). Studies using troglitazone were removed as this drug has been removed from the market because of safety concerns. |
| 7 December 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
A previous Cochrane Review compared the effects of LOD with other ovulation induction agents including metformin (Farquhar 2012). However, no studies were found that directly compared these two comparators. Two studies compared metformin and CC with LOD however, these comparisons were out of the scope of this review (Palomba 2004; Palomba 2010).
Acknowledgements
The authors wish to thank Cochrane Gynaecology and Fertility for their assistance with developing this systematic review. We also thank Dr Machado and Professor Morin‐Papunen for answering email queries regarding their studies.
We would also like to thank the corresponding authors of the following studies who took time to respond to our requests for further data, some of whom took the trouble to perform repeat analyses for us.
Chou 2003; Fleming 2002; Hoeger 2004; Heathcote 2013; Hwu 2005; Jakubowicz 2001; ; Legro 2007; Lord 2006; Malkawi 2002; Moghetti 2000; Morin‐Papunen 2012; Nestler 1998Ng 2001; Rautio 2006a; Sturrock 2002; Trolle 2007; Vandermolen 2001; Yarali 2002.
We would also like to thank the managing editor, Elena Kostova for her help and support throughout the review process. We would like to thank Marian Showell for conducting the database searches and assistance with retrieval of studies. We would also like to thank the peer reviewers; Katie Stocking, Andy Watson, Rebecca Gormley and the Co‐ordinating Editor Madelon van Wely. We would like to thank Ashley Ng for providing translations for foreign language papers.
The authors of the 2019 update wish to thank Dr Jonathan Lord and Dr E Yasmin for their contributions to all previous versions of this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
Searched 13 December 2018
PROCITE platform
Keywords CONTAINS "polycystic ovary syndrome" or "PCOS" or "ovarian failure" or "polycystic ovary morphology" or "hyperandrogenemia" or "hyperandrogenism" or "hyperinsulinaemia" or "hyperandrogenicity" or Title CONTAINS "polycystic ovary syndrome" or "polycystic ovary syndrome" or "PCOS" or "ovarian failure" or "polycystic ovary morphology" or "hyperandrogenemia" or "hyperandrogenism" or "hyperinsulinaemia" or "hyperandrogenicity"
AND
Keywords CONTAINS "metformin" or "glucophage" or Title CONTAINS "metformin" or "glucophage"
(442 hits)
Appendix 2. Cochrane Central Register of Studies Online (CRSO) search strategy
Searched 13 December 2018
Web platform
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 1267 #2 (PCOS or PCOD):TI,AB,KY 1964 #3 (polycystic ovar*):TI,AB,KY 2383 #4 #1 OR #2 OR #3 2592 #5 MESH DESCRIPTOR Metformin EXPLODE ALL TREES 3288 #6 Metformin:TI,AB,KY7 163 #7 (dimethylbiguanid* or dimethylguanylguanidine or glucophage or glucovance):TI,AB,KY 109 #8 #5 OR #6 OR #7 7167 #9 #4 AND #8 824
Appendix 3. MEDLINE search strategy
Searched from 1946 to 13 December 2018
OVID platform
1 Polycystic Ovary Syndrome/ (13144) 2 PCOS.ti,ab,sh. (9665) 3 polycystic ovar$.ti,ab,sh. (17186) 4 PCOD.ti,ab,sh. (283) 5 (stein‐leventhal or leventhal).tw. (718) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (89) 7 or/1‐6 (17765) 8 Metformin/ (11517) 9 metformin.ti,ab,sh. (18593) 10 (dimethylbiguanid$ or dimethylguanylguanidine or glucophage or glucovance).tw. (250) 11 or/8‐10 (18643) 12 7 and 11 (1556) 13 randomized controlled trial.pt. (472057) 14 controlled clinical trial.pt. (92771) 15 randomized.ab. (428370) 16 randomised.ab. (85530) 17 placebo.tw. (198968) 18 clinical trials as topic.sh. (185394) 19 randomly.ab. (301433) 20 trial.ti. (190942) 21 (crossover or cross‐over or cross over).tw. (78495) 22 or/13‐21 (1244547) 23 exp animals/ not humans.sh. (4519948) 24 22 not 23 (1145169) 25 12 and 24 (588)
Appendix 4. Embase search strategy
Searched from 1980 to 13 December 2018
OVID platform
1 exp ovary polycystic disease/ (24241) 2 PCOS.tw. (15248) 3 polycystic ovar$.tw. (20966) 4 PCOD.tw. (388) 5 (stein‐leventhal or leventhal).tw. (297) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (83) 7 or/1‐6 (28153) 8 Metformin/ (55132) 9 metformin.tw. (29780) 10 (dimethylbiguanid$ or dimethylguanylguanidine or glucophage or glucovance).tw. (1798) 11 or/8‐10 (57045) 12 7 and 11 (3875) 13 Clinical Trial/ (942899) 14 Randomized Controlled Trial/ (523265) 15 exp randomization/ (80398) 16 Single Blind Procedure/ (33297) 17 Double Blind Procedure/ (153077) 18 Crossover Procedure/ (57463) 19 Placebo/ (313868) 20 Randomi?ed controlled trial$.tw. (192316) 21 Rct.tw. (30475) 22 random allocation.tw. (1837) 23 randomly.tw. (391618) 24 randomly allocated.tw. (31084) 25 allocated randomly.tw. (2377) 26 (allocated adj2 random).tw. (797) 27 Single blind$.tw. (21733) 28 Double blind$.tw. (186112) 29 ((treble or triple) adj blind$).tw. (857) 30 placebo$.tw. (276040) 31 prospective study/ (488511) 32 or/13‐31 (2173583) 33 case study/ (57987) 34 case report.tw. (357892) 35 abstract report/ or letter/ (1039805) 36 or/33‐35 (1446525) 37 32 not 36 (2123511) 38 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5582439) 39 37 not 38 (1976585) 40 12 and 39 (1536)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 13 December 2018
OVID platform
1 exp Endocrine Sexual Disorders/ (1152) 2 PCOS.tw. (252) 3 polycystic ovar$.tw. (385) 4 PCOD.tw. (6) 5 (stein‐leventhal or leventhal).tw. (291) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (0) 7 or/1‐6 (1699) 8 metformin.tw. (418) 9 (dimethylbiguanid$ or dimethylguanylguanidine or glucophage or glucovance).tw. (2) 10 or/8‐9 (418) 11 7 and 10 (16)
Appendix 6. CINAHL search strategy
Searched from 1961 to 13 December 2018
EBSCO platform
S22 S9 AND S21 191 S21 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 1,289,129 S20 TX allocat* random* 9,584 S19 (MH "Quantitative Studies") 21,468 S18 (MH "Placebos") 11,083 S17 TX placebo* 54,535 S16 TX random* allocat* 9,584 S15 (MH "Random Assignment") 52,378 S14 TX randomi* control* trial* 162,277 S13 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) 990,611 S12 TX clinic* n1 trial* 237,099 S11 PT Clinical trial 86,802 S10 (MH "Clinical Trials+") 253,415 S9 S4 AND S8 469 S8 S5 OR S6 OR S7 6,457 S7 TX (dimethylbiguanid* or dimethylguanylguanidine or glucophage or glucovance) 47 S6 TX Metformin 6,451 S5 (MM "Metformin") 2,449 S4 S1 OR S2 OR S3 4,343 S3 TX polycystic ovar* 3,774 S2 TX PCOS or TX PCOD 2,309 S1 (MM "Polycystic Ovary Syndrome") 2,339
Data and analyses
Comparison 1. Metformin versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 4 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.00, 2.51] |
| 1.1 Participants with BMI < 30 kg/m2 | 3 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.94, 2.44] |
| 1.2 Participants with BMI ≥ 30 kg/m2 | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.51, 16.01] |
| 2 Adverse events (gastrointestinal side effects) | 7 | 713 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.00 [2.63, 6.09] |
| 2.1 Participants with BMI < 30 kg/m2 | 5 | 556 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.68 [3.34, 9.65] |
| 2.2 Participants with BMI ≥ 30 kg/m2 | 2 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.92, 3.95] |
| 3 Clinical pregnancy rate | 11 | 1213 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.47, 2.65] |
| 3.1 Participants with BMI < 30 kg/m2 | 7 | 919 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.42, 2.66] |
| 3.2 Participants with BMI ≥ 30 kg/m2 | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.98, 4.98] |
| 4 Ovulation rate | 13 | 684 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.85, 3.75] |
| 4.1 Participants with BMI < 30 kg/m2 | 5 | 241 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.20 [2.32, 7.59] |
| 4.2 Participants with BMI ≥ 30 kg/m2 | 9 | 443 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.28, 3.14] |
| 5 Miscarriage rate per woman | 4 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.50, 2.35] |
| 5.1 Participants with BMI < 30 kg/m2 | 3 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.52, 2.71] |
| 5.2 Participants with BMI ≥ 30 kg/m2 | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.80] |
| 6 Sensitivity analysis: miscarriage rate per pregnancy | 4 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.34] |
| 6.1 Participants with BMI < 30 kg/m2 | 3 | 188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.53] |
| 6.2 Participants with BMI ≥ 30 kg/m2 | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.02, 4.00] |
| 7 Multiple pregnancy rate | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.49] |
| 8 Sensitivity analysis: multiple pregnancy rate per pregnancy | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 6.04] |
| 9 Body mass index (kg/m2) | 10 | 589 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 9.1 Participants with BMI < 30 kg/m2 | 5 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| 9.2 Participants with BMI ≥ 30 kg/m2 | 5 | 195 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.82, 0.82] |
| 10 Serum testosterone (nmol/L) | 11 | 707 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.48, ‐0.35] |
| 10.1 Participants with BMI < 30 kg/m2 | 5 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.50, ‐0.37] |
| 10.2 Participants with BMI ≥ 30 kg/m2 | 6 | 313 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.45, ‐0.12] |
| 11 Serum sex hormone‐binding globulin (nmol/L) | 10 | 649 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐4.77, 1.36] |
| 11.1 Participants with BMI < 30 kg/m2 | 3 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐6.62, 8.82] |
| 11.2 Participants with BMI ≥ 30 kg/m2 | 7 | 323 | Mean Difference (IV, Fixed, 95% CI) | ‐2.23 [‐5.56, 1.11] |
| 12 Fasting glucose (mmol/L) | 10 | 677 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 12.1 Participants with BMI < 30 kg/m2 | 4 | 362 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.03, 0.09] |
| 12.2 Participants with BMI ≥ 30 kg/m2 | 6 | 315 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.28, 0.01] |
| 13 Fasting insulin (mIU/L) | 8 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐4.27, 0.59] |
| 13.1 Participants with BMI < 30 kg/m2 | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐6.04, 2.50] |
| 13.2 Participants with BMI ≥ 30 kg/m2 | 6 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐4.84, 1.07] |
Comparison 2. Metformin and clomiphene citrate versus clomiphene citrate alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 10 | 1219 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.98, 1.65] |
| 1.1 Participants with BMI < 30 kg/m2 or ≤ 32 kg/m2 | 6 | 665 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.67] |
| 1.2 Participants with BMI ≥ 30 kg/m2 | 4 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.95, 2.09] |
| 2 Adverse events (gastrointestinal side effects) | 6 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.26 [2.83, 6.40] |
| 2.1 Participants with BMI < 30 kg/m2 | 4 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.13 [2.71, 6.28] |
| 2.2 Participants with BMI ≥ 30kg/m2 | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.19, 29.71] |
| 2.3 Participants with BMI not recorded | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.75 [0.81, 269.34] |
| 3 Clinical pregnancy rate | 19 | 1790 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.32, 1.99] |
| 3.1 Participants with BMI < 30 kg/m2 | 9 | 896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.06, 1.86] |
| 3.2 Participants with BMI ≥ 30 kg/m2 | 8 | 666 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.24, 2.43] |
| 3.3 Participants with BMI not recorded | 2 | 228 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.39, 5.87] |
| 4 Ovulation rate | 21 | 1568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.35, 2.03] |
| 4.1 BMI < 30 kg/m2 | 9 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.03, 2.03] |
| 4.2 BMI ≥ 30 kg/m2 | 11 | 875 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.26, 2.16] |
| 4.3 BMI not reported | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.78 [1.65, 8.65] |
| 5 Ovulation rate: subgroup analysis by sensitivity to clomiphene citrate | 7 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.71 [2.46, 9.03] |
| 5.1 PCOS and clomiphene‐sensitive | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.65, 19.37] |
| 5.2 PCOS and clomiphene‐resistant | 6 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.97 [2.46, 10.03] |
| 6 Miscarriage rate per woman | 10 | 1206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.00] |
| 6.1 Participants with BMI < 30 kg/m2 | 6 | 652 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.74, 2.15] |
| 6.2 Participants with BMI ≥ 30 kg/m2 | 4 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.81, 2.63] |
| 7 Sensitivity analysis: miscarriage rate per pregnancy | 10 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| 7.1 Participants with BMI < 30 kg/m2 | 6 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.96] |
| 7.2 Participants with BMI ≥ 30 kg/m2 | 4 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.54, 2.02] |
| 8 Multiple pregnancy rate per woman | 6 | 1003 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.18, 1.68] |
| 8.1 Participants with BMI < 30 kg/m2 | 3 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.12, 2.04] |
| 8.2 Participants with BMI ≥ 30kg/m2 | 3 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.01] |
| 9 Senstivity analysis: multiple pregnancy rate per pregnancy | 6 | 342 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.15, 1.42] |
| 9.1 Participants with BMI < 30 kg/m2 | 3 | 178 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.10, 1.85] |
| 9.2 Participants with BMI ≥ 30 kg/m2 | 3 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.08, 3.12] |
| 10 Body mass index (kg/m2) | 3 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐4.44 [‐6.11, ‐2.77] |
| 10.1 Participants with BMI < 30kg/m2 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.20, ‐1.60] |
| 10.2 Participants with BMI ≥ 30kg/m2 | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐7.47, ‐2.61] |
| 11 Serum testosterone (nmol/L) | 3 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.60, ‐0.13] |
| 11.1 Participants with BMI ≥ 30kg/m2 | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.61, ‐0.13] |
| 11.2 Participants with BMI < 30kg/m2 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.47, 1.07] |
| 12 Fasting glucose (mmol/L) | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.29, ‐0.12] |
| 12.1 Participants with BMI < 30kg/m2 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.64, 0.04] |
| 12.2 Participants with BMI ≥ 30kg/m2 | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.29, ‐0.11] |
| 13 Fasting insulin (mIU/L) | 3 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐6.57 [‐7.84, ‐5.29] |
| 13.1 Participants with BMI < 30kg/m2 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐15.20 [‐18.33, ‐12.07] |
| 13.2 Participants with BMI ≥ 30kg/m2 | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐4.86 [‐6.26, ‐3.47] |
Comparison 3. Metformin versus clomiphene citrate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 5 | 741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.01] |
| 1.1 Participants with BMI < 30 kg/m2 | 3 | 241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.00, 2.94] |
| 1.2 Participants with BMI ≥ 30 kg/m2 | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.52] |
| 2 Clinical pregnancy rate | 8 | 1030 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.11] |
| 2.1 Participants with BMI < 30 kg/m2 | 6 | 530 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.06, 2.29] |
| 2.2 Participants with BMI ≥ 30 kg/m2 | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.55] |
| 3 Ovulation rate | 7 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.34, 0.60] |
| 3.1 Participants with BMI < 30 kg/m2 | 5 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.25] |
| 3.2 Participants with BMI ≥ 30 kg/m2 | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.20, 0.43] |
| 4 Miscarriage rate per woman | 6 | 781 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.51, 1.66] |
| 4.1 Participants with BMI < 30 kg/m2 | 4 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.62, 3.71] |
| 4.2 Participants with BMI ≥ 30 kg/m2 | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.27, 1.38] |
| 5 Sensitivity analysis: miscarriage rate per pregnancy | 6 | 203 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.66] |
| 5.1 Participants with BMI < 30 kg/m2 | 4 | 105 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.41, 2.54] |
| 5.2 Participants with BMI ≥ 30 kg/m2 | 2 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.72, 5.12] |
| 6 Multiple pregnancy rate per woman | 5 | 858 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.43] |
| 6.1 Participants with BMI < 30 kg/m2 | 3 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.07, 3.16] |
| 6.2 Participants with BMI ≥ 30 kg/m2 | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.76] |
| 7 Sensitivity analysis: multiple pregnancy rate per pregnancy | 5 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.68] |
| 7.1 Participants with BMI < 30 kg/m2 | 3 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.05, 2.24] |
| 7.2 Participants with BMI ≥ 30 kg/m2 | 2 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.69] |
| 8 Body mass index (kg/m2) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.40, ‐0.80] |
| 9 Serum testosterone (nmol/L) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.82, 1.42] |
| 10 Fasting glucose (mmol/L) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.79, 0.39] |
| 11 Fasting insulin (mIU/L) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐16.96, ‐9.04] |
Comparison 4. Metformin and letrozole versus letrozole alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.48, 2.08] |
| 2 Adverse events (gastrointestinal side effects) | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.74 [0.94, 299.23] |
| 3 Clinical pregnancy rate | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.64, 2.51] |
| 4 Miscarriage rate per woman | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.61, 4.23] |
| 5 Sensitivity analysis: miscarriage rate per pregnancy | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.51, 4.42] |
Comparison 5. Metformin and laparoscopic ovarian drilling versus laparoscopic ovarian drilling alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.51, 8.77] |
| 2 Clinical pregnancy rate | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.79, 12.80] |
| 3 Miscarriage rate per woman | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.51 [0.25, 122.08] |
| 4 Sensitivity analysis: miscarriage rate per pregnancy | 1 | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 77.64] |
Comparison 6. Metformin versus laparoscopic ovarian drilling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.09, 4.78] |
| 2 Adverse events (gastrointestinal side effects) | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.77 [2.43, 24.89] |
| 2.1 Participants with BMI < 30kg/m2 | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.75 [1.27, 17.82] |
| 2.2 Participants with BMI ≥ 30kg/m2 | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.62 [1.46, 449.07] |
| 3 Clinical pregnancy rate | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.54, 1.59] |
| 3.1 Participants with BMI < 30 kg/m2 | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.83, 3.62] |
| 3.2 Participants with BMI ≥ 30 kg/m2 | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.17, 0.95] |
| 4 Ovulation rate | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.01] |
| 5 Miscarriage rate per woman | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.47] |
| 5.1 Participants with BMI < 30 kg/m2 | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.89] |
| 5.2 Participants with BMI ≥ 30 kg/m2 | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.08, 2.74] |
| 6 Sensitivity analysis: miscarriage rate per pregnancy | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.48] |
| 6.1 Participants with BMI < 30 kg/m2 | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.43] |
| 6.2 Participants with BMI ≥ 30 kg/m2 | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.14, 6.19] |
| 7 Body mass index (kg/m2) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐13.48, 6.28] |
| 8 Serum testosterone (nmol/L) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.09, 0.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baillargeon 2004.
| Methods | RCT Setting: Venezuela Method of randomisation: fixed block‐of‐8 randomisation which was performed by the investigational pharmacist. Blinding: double Number randomised: 58 |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS women (BMI ≤ 27 kg/m2) Inclusion criteria: PCOS (oligomenorrhoea < 8 periods/year, hyperandrogenism total testosterone > 2.43 nmol/L Normal prolactin and TFT, fasting insulin < 15 μIU/mL and fasting glucose to insulin ratio > 4.5 Normal OGTT Hormonal contraceptives were not used before the study. Exclusion criteria: late onset adrenal hyperplasia, hypertension. Previous insulin‐sensitiser users Baseline characteristics of each group: metformin (n = 28) vs placebo (n = 30)
Dropouts: 4 (12.5%) in the metformin arm and 2 (6.3%) in the placebo group |
|
| Interventions | Main intervention: metformin 850 mg or placebo tablets twice daily Duration: 6 months Co‐interventions: none |
|
| Outcomes | Primary: none Secondary: menstrual frequency, ovulation: weekly progesterone measurement with a level > 4 ng/mL, BMI, testosterone, fasting glucose, fasting insulin |
|
| Notes | This study randomised 128 women into 4 groups (metformin alone, rosiglitazone alone, combined metformin and rosiglitazone, placebo alone). We included the metformin‐alone and placebo group for analysis. Women were predominantly white European emigrants to Venezuela | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fixed block‐of‐8 randomisation, which was performed by the investigational pharmacist |
| Allocation concealment (selection bias) | Low risk | Study drugs packed in coded boxes allocated by the research nurse. Study drugs were similar in appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 4 (12.5%) in the metformin arm and 2 (6.3%) in the placebo group. Details not provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study. 4‐arm study but only 2 arms included in this review. All outcomes for each arm clearly reported |
| Other bias | Low risk | No evidence of other bias |
Begum 2014.
| Methods | RCT Setting: Bangladesh (Infertility Department of women and children's hospital) Method of randomisation: envelopes used, but no other information Blinding: unclear Number randomised: 71 |
|
| Participants | Summary: metformin vs CC Inclusion criteria: subfertile women aged 20‐35 years with a diagnosis of PCOS according to Rotterdam criteria Exclusion criteria: age > 35 years, hypo‐ or hyperthyroidism, hyperprolactinaemia, diabetes mellitus and male factor infertility Baseline characteristics of each group: metformin (n = 35) vs CC (n = 36)
Dropouts: none stated |
|
| Interventions | Main intervention: Group 1: metformin 1500 mg/d. Group 2: CC 100 mg/d for 5 d Duration: 6 months Co‐interventions: none |
|
| Outcomes | Primary: none Secondary: pregnancy rate (urine pregnancy test), ovulation rate: serum progesterone on D21 >5 ng/mL |
|
| Notes | We have contacted study authors for further information regarding methodology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random sequence for distribution in envelopes is not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation to each group revealed in envelopes but not stated if opaque and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Ben Ayed 2009.
| Methods | RCT Setting: Tunisia Method of randomisation: not stated Blinding: not stated Number randomised: 32 |
|
| Participants | Summary: metformin and CC vs CC and placebo Inclusion criteria: Rotterdam criteria Exclusion criteria: late onset adrenal hyperplasia, Cushing's Syndrome, abnormal TFT, hyperprolactinaemia, androgen‐secreting tumour Baseline characteristics of each group: metformin and CC (n = 16) vs CC and placebo (n = 16)
Dropouts: none stated |
|
| Interventions | Main intervention: CC 100 mg from day 3 to day 7 of the cycle and Metformn 1700 mg/d or placebo Duration: up to 3 cycles Co‐interventions: lifestyle advice given to obese participants |
|
| Outcomes | Primary: none Secondary: ovulation; USS follicular tracking with follicular size > 16 mm |
|
| Notes | Inadequate information in the protocol to assess the quality of the study No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Boudhraa 2010.
| Methods | RCT Setting: Tunisia Method of randomisation: not stated* Blinding: unblinded Number randomised: 63 |
|
| Participants | Summary: metformin vs CC in PCOS non‐obese women Inclusion criteria: unclear. ?diagnostic criteria of PCOS used Exclusion criteria: male factor infertility, tubal disease Baseline characteristics of each group: metformin vs CC
Dropouts: none |
|
| Interventions | Main intervention: metformin 850 mg/d, CC 100 mg for 5 days Duration: not stated Co‐interventions: recommendations on healthy diet |
|
| Outcomes | Primary: live birth rate Secondary: clinical pregnancy, ovulation: method to confirm ovulation not stated, BMI |
|
| Notes | *Study protocol is too brief. Inadequate information to assess the quality of the study. No reply from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information |
| Selective reporting (reporting bias) | Unclear risk | Inadequate information |
| Other bias | Low risk | No evidence of other bias however, limited reported methodology |
Chuni 2006.
| Methods | RCT Setting: India Method of randomisation: computer‐generated tables Blinding: unclear Number randomised: 36 |
|
| Participants | Summary: metformin vs placebo
Inclusion criteria: PCOS diagnosed as oligomenorrhoea and clinical or biochemical features of hyperandrogenism, either raised LH:FSH ratio or raised LH or US features of PCO; normal serum prolactin concentrations, normal TFT Exclusion criteria: diabetes mellitus Baseline characteristics of each group: metformin (n = 18) vs placebo (n = 18)
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d or placebo 3/d Duration: 3 months Co‐interventions: CC 50 mg on day 3‐7 for 5 days (increased to a maximum of 200 mg/d) added in women who had not ovulated after 3 months |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, menstrual frequency, ovulation (progesterone > 8 ng/dL), BMI, fasting blood glucose, fasting insulin, serum testosterone, gastrointestinal side effects |
|
| Notes | No information on blinding. No results reported on menstrual frequency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly reported |
| Other bias | Low risk | No evidence of other bias |
Fatima 2018.
| Methods | RCT Setting: Pakistan Method of randomisation: consecutive non‐probability sampling Blinding: unclear Number randomised: 128 |
|
| Participants | Summary: metformin and CC versus CC alone Inclusion criteria: PCOS ‐ unclear how diagnosed; duration of fertility ≤ 3 years, age 20‐35 years Exclusion criteria: use of oral contraceptives, comorbid medical conditions, those not living with their husband Baseline characteristics of each group: metformin and CC (n = 64) vs CC (n = 64)
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 3 cycles Co‐interventions: CC 50 mg from day 2 until day 6 of cycle, increased to 100 mg then 150 mg for 3 consecutive cycles |
|
| Outcomes | Primary: none Secondary: clinical pregnancy: urine pregnancy test and confirmed on US |
|
| Notes | Endocrine and metabolic factors not measured, including BMI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive non‐probability sampling |
| Allocation concealment (selection bias) | High risk | Consecutive sampling |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | The primary outcome, clinical pregnancy, was clearly reported. |
| Other bias | Low risk | No evidence of other bias |
Fleming 2002.
| Methods | RCT Setting: UK Method of randomisation: computer‐generated randomisation by pharmacy in blocks of 4 Blinding: double‐blind Number randomised: 94 |
|
| Participants | Summary: metformin vs placebo in obese PCOS women Inclusion criteria: PCOS (oligomenorrhoea < 8 cycles/year, exclusion of other endocrinopathy, US finding of PCOS) Age < 35 years Exclusion criteria: diabetes mellitus, adrenal hyperplasia, thyroid dysfunction, hyperprolactinaemia, medication likely to influence hormonal profiles Baseline characteristics of each group metformin (n = 39) vs placebo (n = 26):
Dropouts: 30 (32%), with 22 in the treatment arm and 8 in the placebo, mainly due to gastrointestinal side effects in metformin group. Overall, 58% of the metformin arm completing the trial and 83% of the placebo arm. Included in ITT analysis |
|
| Interventions | Main intervention: 1 of metformin 850 mg 2/d, placebo Duration: 12‐16 weeks Co‐interventions: 1st week of treatment at 850 mg 1/d |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: clinical pregnancy, ovulation: by twice‐weekly serum oestradiol. Where oestradiol > 300 pmol/L, LH and progesterone (> 8 nmol/L in ≥ 2 successive samples defined ovulation*) were determined, BMI, testosterone, fasting glucose, fasting insulin |
|
| Notes | Diagnostic criteria different to other trials ‐ using US not hyperandrogaenemia (although 90% did have raised androgens, and mean entry‐FAI 10 with 5% CI 8.6). Subgroup analysis showed that those who ovulated in response to metformin had significantly lower androgens. High rate of background ovulation (64% on placebo ovulated at some stage) *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation by pharmacy in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Remote allocation. Identical metformin and placebo tablets. Randomisation code kept in the pharmacy department until the end of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was described as double‐blind although the details of this were not explained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was described as double‐blind although the details of this were not explained |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 30 (32%), with 22 in the treatment arm and 8 in the placebo, mainly due to gastrointestinal side effects in metformin group. Overall, 58% of the metformin arm completed the trial and 83% of the placebo arm. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Hamed 2010.
| Methods | RCT Setting: Egypt Method of randomisation: computer‐generated random number tables Blinding: unclear Number randomised: 110 |
|
| Participants | Summary: diagnostic laparoscopy and metformin versus LOD Inclusion criteria: PCOS (Rotterdam criteria), CC resistance, women aged 20‐35 years, insulin resistance fasting blood glucose to insulin ratio (G/l) < 4.5 Exclusion criteria: women on gonadotrophin or oral contraceptives 3 months prior to the study, women with hyperprolactinaemia or other endocrine disorders, hepatic or renal disorders, organic pelvic mass, previous abdominal surgery suggesting pelvic factor infertility Baseline characteristics of each group: metformin (n = 55) and diagnostic laparoscopy vs LOD (n = 55)
Dropouts: none |
|
| Interventions | Main intervention: Group 1: metformin 850 mg twice daily, Group 2: LOD (4‐8 punctures, each for 4 s with insulated needle and monopolar diathermy adjusted at 40‐60 watts) Duration: 6 cycles or 30 weeks, depending on which occurred first Co‐interventions: diagnostic laparoscopy was performed in group 1 |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: clinical pregnancy rate, menstrual frequency, ovulation: follicle tracking on transvaginal US or day 21 progesterone (≥ 5 ng/mL), BMI, fasting glucose to insulin ratio, serum testosterone, miscarriage rate |
|
| Notes | Allocation concealment using serially numbered opaque envelopes. Blinding unclear however no placebo drug in group 2 Ovulation rate per cycle available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes used however, does not state that the envelopes were sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available. Group 1 had diagnostic laparoscopy and metformin, group 2 had LOD. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available. Group 1 had diagnostic laparoscopy and metformin, group 2 had LOD. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were clearly reported. |
| Other bias | Low risk | No evidence of other bias |
Heathcote 2013.
| Methods | RCT Setting: Australia Method of randomisation: computer‐generated random number sequence performed by the hospital pharmacy department to ensure allocation concealment. Blinding: double blinding Number randomised: 27 |
|
| Participants | Summary: metformin and CC vs CC and placebo Inclusion criteria: PCOS (Rotterdam criteria) Exclusion criteria: other causes of anovulation (hyperprolactinaemia, pituitary disease, hypothyroidism, congenital adrenal hyperplasia), diabetes mellitus, male factor subfertility Baseline characteristics of each group metformin and CC (n = 11) vs CC and placebo (n = 12):
Dropouts: 4 (2 from metformin group and 2 from placebo group) |
|
| Interventions | Main intervention: Group 1: metformin 500 mg 3/d vs matched placebo Duration: 6 cycles Co‐interventions: CC 50 mg/d from day 3‐7. Women with BMI > 30 kg/m2 were referred to dietician |
|
| Outcomes | Primary: live birth rate Secondary: ovulation rate: serum progesterone > 10.6 nmol/L, miscarriage, gastrointestinal side effects |
|
| Notes | Unpublished paper with permission granted from the study authors (23 April 2019) to use the data for the review. The paper was supplied by the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled allocation and dispensing |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts stated with incomplete reasoning. 1 withdrew and 4 did not complete 6 cycles from the CC and placebo group, 2 withdrew and 4 did not complete 6 cycles from CC and metformin group |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were clearly reported |
| Other bias | High risk | Not an ITT analysis; paper has not been peer reviewed |
Hoeger 2004.
| Methods | RCT Setting: USA Method of randomisation: computer‐generated random number, randomisation and allocation conducted by the pharmacy department* Blinding: double Number randomised: 18 |
|
| Participants | Summary: metformin vs placebo in overweight or obese PCOS women Inclusion criteria: PCOS (oligomenorrhoea with < 6 menses/year and evidence of hyperandrogenism), BMI > 25, normal TSH, prolactin and FSH concentrations No hormonal treatment within 2 months before the study commenced Exclusion criteria: adrenal disease Baseline characteristics of each group: metformin (n = 9) vs placebo (n = 9)
Dropouts: 3 (33.3%) in the metformin arm and 2 (22.2%)in the placebo arm at 24 months of the trial |
|
| Interventions | Main intervention: metformin 850 mg 2/d or placebo Duration: 24 months Co‐interventions: none |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: ovulation: urinary pregnanediol glucuronide, BMI (weight), total testosterone, fasting glucose, fasting insulin*, menstrual pattern* |
|
| Notes | This study included 4 treatment arms: metformin alone vs placebo vs metformin and lifestyle intervention vs lifestyle intervention and placebo. For this review we have only included the metformin vs placebo groups. *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number |
| Allocation concealment (selection bias) | Low risk | Conducted by the pharmacy department |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. Drug and placebo packaged and labelled identically according to participant number by the pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 3 (33.3%) in the metformin arm and 2 (22.2%)in the placebo arm at 24 months of the trial. Further 4 (44.4%) in the metformin/lifestyle arm and 2 (18.2%) in the placebo/lifestyle arm at 24 months of the trial. Baseline characteristics between the participants completed and the dropouts were similar |
| Selective reporting (reporting bias) | Low risk | Study protocol available. Prespecified outcome measures (ovulation and testosterone levels) were reported |
| Other bias | Low risk | No evidence of other bias |
Jakubowicz 2001.
| Methods | RCT Setting: Venezuela (63% white, 31% Hispanic, 4% Arabic, 2% South American Indian) Method of randomisation: sequentially numbered, identical containers of identical drugs* Blinding: double‐blind Number randomised: 48 |
|
| Participants | Summary: metformin and CC vs placebo and CC in obese PCOS women, CC‐sensitive Inclusion criteria: PCOS (oligomenorrhoea ≤ 8 cycles/year, elevated free testosterone, exclusion of other endocrinopathy, US finding of PCOS), ovulation with CC 150 mg (demonstrated by serum progesterone > 12.7 pmol/L and US) Exclusion criteria: adrenal hyperplasia, thyroid dysfunction, hyperprolactinaemia, diabetes mellitus, failure to ovulate with CC as described above, medication that could affect insulin sensitivity* Baseline characteristics of each group: metformin (n = 26) vs placebo (n = 22)
Dropouts: after randomisation, 8 (14%), 2 in metformin arm and 6 in placebo. Not included in analysis |
|
| Interventions | Main intervention: 1 of metformin 500 mg 3/d, placebo Duration: 4‐5 weeks prior to CC, then for a further 19 d after commencing CC Co‐interventions: CC 150 mg for 5 d |
|
| Outcomes | Primary: none Secondary: menstrual frequency, ovulation: by serum progesterone > 12.7 pmol/L and US (ovulation checked on 2 occasions on day 23: once after metformin/placebo cycle and once after subsequent metformin/placebo with CC), BMI, fasting glucose, fasting insulin, total testosterone |
|
| Notes | Women that were given metformin and ovulated received an extra week’s course of treatment when compared with the placebo group High dropout rate between recruitment and randomisation (24%) as only those who ovulated with CC prior to randomisation were included The primary outcome measures are not relevant to this review, but the other parameters reported are It is assumed that the units quoted for testosterone are mmol/dL and not mmol/L *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical containers of identical drugs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: after randomisation, 8 (14%), 2 in metformin arm and 6 in placebo. Not included in analysis. Missing data not reported. High dropout rate between recruitment and randomisation (24%) as only those who ovulated with CC prior to randomisation were included |
| Selective reporting (reporting bias) | Low risk | The primary outcome measures are not relevant to this review, but the other parameters such as ovulation reported are |
| Other bias | Unclear risk | Women that were given metformin and ovulated received an extra week’s course of treatment when compared with the placebo group |
Kar 2015.
| Methods | RCT Setting: India (private hospital) Method of randomisation: envelopes prepared by a nurse "naive to this study" Blinding: double‐blind Number randomised: 105 |
|
| Participants | Summary: metformin and CC vs CC vs metformin in Asian Indian women with "treatment naive" PCOS
Inclusion criteria: history of infertility and oligomenorrhoea, meeting the Rotterdam criteria for PCOS Normal male factor, at least 1 patent tube by hysterosalpingography, treatment naive Exclusion criteria: any major systemic illness Baseline characteristics of each group: metformin and CC (n = 24) vs metformin (n = 24) vs CC (n = 32)
Dropouts: 25 (3 in the CC group, 11 in metformin group, 11 in combined group) |
|
| Interventions | Main intervention: 3 equal groups. Group 1: CC 50‐150 mg/d. Group 2: metformin 1700 mg/d. Group 3: CC plus metformin, doses as above) Duration: 6 months, or until pregnant, or until resistant to CC Co‐interventions: not applicable |
|
| Outcomes | Primary: live birth rate Secondary: ovulation: follicle tracking on US, clinical pregnancy rate, miscarriage |
|
| Notes | We have contacted the study authors for further information regarding methodology No units provided for fasting insulin and fasting glucose levels |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random sequence for distribution in envelopes not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation revealed in envelopes but not clear if opaque or sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of investigators unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22.9% dropout rate, without reasons given Data analysis not performed as ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study. 3‐arm study however data presented for all 3 arms clearly |
| Other bias | Low risk | No evidence of other bias |
Karimzadeh 2007.
| Methods | RCT Setting: Iran Method of randomisation: computer‐generated sequences that was sealed in envelopes Blinding: double Number randomised: 200 |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS Inclusion criteria: Rotterdam criteria 2003 Exclusion criteria: hyperprolactinaemia, CSH, thyroid disease, Cushings syndrome, androgen‐secreting tumour Baseline characteristics of each group:
Dropouts: not mentioned |
|
| Interventions | Main intervention: metformin 500 mg 3/d, placebo Duration: 3 months Co‐interventions: nil |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: clinical pregnancy rate, ovulation: progesterone > 10 ng/mL, BMI, fasting insulin, miscarriage |
|
| Notes | Women were recruited from a single centre. The primary objective of this study was to investigate the effect of metformin on lipid profile. The duration of the trial was relatively short. Therefore, it was difficult to ascertain the reliability on both of the ovulation rates and the improvement in menstrual patterns. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequences that were sealed in envelopes |
| Allocation concealment (selection bias) | Low risk | Sequences sealed in opaque envelopes and code kept in the pharmacy department |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Karimzadeh 2010.
| Methods | RCT Setting: Iran Method of randomisation: not stated Blinding: not stated Number randomised: 343 |
|
| Participants | Summary: metformin alone with placebo or no treatment; metformin and CC vs CC vs metformin in non‐obese PCOS Inclusion criteria: Rotterdam criteria 2003. Aged 19‐35, BMI 25‐29, primary infertility, normal prolactin levels, TFT, liver and renal functions Exclusion criteria: male factor infertility Baseline characteristics of each group: metformin and CC vs CC vs metformin
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 3‐6 months Co‐interventions: CC 100 mg day 3‐7; lifestyle group were advised to increase daily exercise for 30 min along with high carbohydrate diet |
|
| Outcomes | Primary: none Secondary: ovulation: USS follicular tracking, clinical pregnancy, fasting insulin, multiple pregnancy |
|
| Notes | This study compared the effect of CC, metformin, combined CC and metformin, and lifestyle modification on subfertile women with PCOS. Very little information can be extracted from the study protocol. A large sample size without any dropouts Some of the women may have been included in the previous trial Karimzadeh 2007. No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information |
| Selective reporting (reporting bias) | High risk | Not all the primary outcome measures (endocrine parameters, lipid profile) data available. 3‐arm study however data presented for all 3 arms clearly |
| Other bias | Unclear risk | Some of the women may have been included in the previous trial Karimzadeh 2007. No reply from study author |
Khorram 2006.
| Methods | RCT Setting: USA Method of randomisation: picking a card out of a box Blinding: none Number randomised: 31 |
|
| Participants | Summary: metformin vs placebo in obese PCOS Inclusion criteria: oligomenorrhoea (< 8 cycles/year), PCO on USS, clinical (acne, hirsutism, alopecia) or biochemical hyperandrogenism (elevated testosterone level) BMI > 29 Exclusion criteria: pregnancy, hepatic or renal disease, heart disease, alcoholism, pulmonary disorder, abnormal TFT, hyperprolactinaemia, CAH or androgen‐secreting tumour Baseline characteristics of each group:
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d. Placebo was not used Duration: 2 weeks from the start of the menstrual cycle. 1 trial cycle only Co‐interventions: CC 100 mg for 5 d from day 5 of the cycle |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, ovulation: method to detect ovulation was not stated, fasting blood glucose, fasting insulin, free and total testosterone |
|
| Notes | This study was designed to evaluate the effect of a short course of metformin treatment on the outcomes of CC ovulation induction therapy. All participants were Hispanic except 1 African American in the CC‐only group and 1 white woman in the combined group. None of the participants had taken CC before. The trial was unblinded. The method of randomisation and concealment were inadequate. Therefore, potential bias may have been introduced. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Picking a card out of a box |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias, however, limited methodology |
Kjotrod 2011.
| Methods | RCT Setting: 8 infertility centres in Denmark, Finland, Norway and Sweden Method of randomisation: hospital pharmacy used a computer‐generated list Blinding: double Number randomised: 150 |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS women Inclusion criteria: PCOS (Rotterdam criteria), aged < 38 years, BMI < 28 kg/m2, scheduled to undergo fertility treatment, minimum of 1 year infertility Exclusion criteria: contraindicated for rFSH, FSH > 10 IU/L, liver or kidney disease, diabetes, fasting blood glucose ≥ 7.0 mmol/L, alcoholism, drug abuse, hyperprolactinaemia, abnormal thyroid function tests, congenital adrenal hyperplasia, androgen‐secreting tumours, Cushing's syndrome; had received oral steroid hormones, cimetidine, anticoagulants, erythromycin or other macrolides. A 1‐month washout if woman previously had metformin. Baseline characteristics of each group: metformin (n = 74) vs placebo (n = 76)
Dropouts: 1 person withdrew from placebo group |
|
| Interventions | Main intervention: metformin 500 mg/d gradually increased to 2000 mg/d within first 2 weeks of treatment vs placebo Duration: 12 weeks Co‐interventions: diet and lifestyle advice were given to all patients |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: clinical pregnancy rate |
|
| Notes | Did not exclude alternative causes of infertility such as male factor, tubal disease, endometriosis The study continued with fertility treatment for those women who did not conceive spontaneously with metformin or placebo. Endocrine parameters measured but results not recorded Women who did not conceive spontaneously after metformin vs placebo, went on to have IVF. We have included up to the IVF stage for analysis in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Low risk | Pharmacy generated number and packaging of medications was identical |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated with reasoning where one participant withdrew consent after randomisation |
| Selective reporting (reporting bias) | Low risk | The primary outcome relevant to this review, spontaneous pregnancy, was clearly reported. |
| Other bias | Low risk | No evidence of other bias |
Ko 2001.
| Methods | RCT Setting: South Korea Method of randomisation: unclear Blinding: single Number randomised: 21 |
|
| Participants | Summary: metformin and CC vs CC alone Inclusion criteria: PCOS (diagnosis unclear), CC resistance, women aged 18‐35 years, weight 75‐98 kg, Exclusion criteria: alternative cause of infertility, chronic diseases such as diabetes and other endocrine disorders Baseline characteristics of each group: metformin and CC (n = 10) vs CC alone (n = 11)
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 7 weeks prior to CC Co‐interventions: CC 50 mg from day 2 until day 6 of cycle, increased to 100 mg then 150 mg until ovulation occurred |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, ovulation: progesterone level > 4 ng/mL, BMI, fasting glucose, fasting insulin, free testosterone |
|
| Notes | Women who ovulated after metformin alone (before CC treatment started) were excluded from analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method for randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No dropouts. Not an ITT analysis as participants were excluded if ovulated following metformin alone |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Kocak 2006.
| Methods | RCT Setting: Turkey Method of randomisation: unclear Blinding: none Number randomised: 42 |
|
| Participants | Summary: metformin and LOD vs LOD alone Inclusion criteria: PCOS (≥ 3 of the following criteria: oligomenorrhoea/amenorrhoea, infertility, hirsutism, obesity (BMI > 25 kg/m2), hyperandrogenism, chronic anovulation, ovarian cortical multiple follicles (≥ 10, 2‐10 mm diameter)), primary infertility, CC resistance (all women used CC for 3 cycles prior to the study), normal renal and liver function tests Exclusion criteria: male factor and tubal‐uterine factor infertility, other endocrine diseases, no medications within 12 weeks prior to the study known to affect pituitary‐gonadal function or carbohydrate metabolism Baseline characteristics of each group: metformin (n = 21) and diagnostic laparoscopy vs LOD (n = 21)
Dropouts: none |
|
| Interventions | Main intervention: Group 1: metformin 850 mg twice daily Duration: metformin 6 months Co‐interventions: Group 1 and 2 both had LOD (punctures of 8 mm depth, each for 2‐4 s with insulated needle and monopolar diathermy adjusted at 30‐40 watts) |
|
| Outcomes | Primary: live birth rate Secondary: clinical pregnancy, ovulation: follicle tracking on transvaginal US or day 21 progesterone (≥ 5 ng/mL), BMI, fasting blood glucose, fasting insulin, free and total testosterone, miscarriage, adverse effect: preterm delivery |
|
| Notes | Does not state number of punctures per ovary Only has metabolic and endocrine parameters (BMI, testosterone, fasting glucose, fasting insulin) for group1 after treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias, however, reported methodology limited |
Legro 2007.
| Methods | RCT Setting: USA Method of randomisation: a large multi‐centre, randomised, placebo‐controlled study (see Legro 2006a for detail) Blinding: double Number randomised: 626 |
|
| Participants | Summary: metformin and CC vs CC vs metformin in obese PCOS women Inclusion criteria: oligomenorrhoea (< 8 periods/year), biochemical hyperandrogenism (elevated testosterone level documented within the previous year on the basis of local laboratory results) Women should have at least 1 proven patent fallopian tube. Normal uterine cavity. Normal semen analysis (sperm concentration > 20 million/mL) Exclusion criteria: hyperprolactinaemia, CSH, thyroid disease, Cushings's syndrome, androgen‐secreting tumour Baseline characteristics of each group: metformin and CC vs CC vs metformin
Dropouts: 49 (23.7%) in the metformin and CC group, 55 (26.3%) in the placebo and CC group, 72 (34.6%) in the metformin group. The differences were not significant. |
|
| Interventions | Main intervention: 2 extended‐release metformin 500 mg or 2 placebo tablets twice daily Duration: up to 6 cycles or 30 weeks Co‐interventions: CC 50 mg or second matching placebo tablet was commenced concurrently from day 3‐7 of the cycle. When women had no or poor response, the dose was increased by 50 mg or 1 additional placebo tablet with the maximum dose of 150 mg or 3 placebo tablets |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, ovulation: progesterone > 5 ng/mL, BMI, fasting glucose, fasting insulin, serum testosterone, miscarriage, multiple pregnancy, other adverse events |
|
| Notes | Based on the initial sample size calculation, 678 was needed to detect a 15% absolute difference in live birth rates with a power of 80% and a type I error of 0.05. Due to limitations in the supplying metformin and the matching placebo tablets, the number of required women was reduced to 626. This was approved after the assessment by the data safety and monitoring board. Because the observed live birth rate was lower than projected, the number of recruited participants (626) was sufficient to detect a 15% difference with the same magnitude of power and type I error. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated; participants were randomised by means of an interactive voice system and stratified based on study site and previous exposure to study drugs |
| Allocation concealment (selection bias) | Low risk | Each participant received a medication package on a monthly basis that consisted of a bottle M (metformin or placebo) and a bottle C (CC or placebo). Data co‐ordinating centre at the clinical research institute Legro 2006a |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 49 (23.7%) in the metformin and CC group, 55 (26.3%) in the placebo and CC group, 72 (34.6%) in the metformin group. A much higher dropout rate in the metformin‐only group. The differences were not significant. Reasons for dropout given. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures reported. 3‐arm study however outcome data presented for all 3 arms clearly. |
| Other bias | High risk | The original sample size was 678 to detect a 15% absolute difference in live birth rates. However, due to drug supply logistics, the sample size later reduced to 626 after the data safety and monitoring board review. |
Liu 2004.
| Methods | RCT Setting: China Method of randomisation: Blinding: Number randomised: 70 |
|
| Participants | Summary: metformin and CC vs CC vs metformin in PCOS with insulin resistance Inclusion criteria: Exclusion criteria: Baseline characteristics of each group: metformin and CC vs CC vs metformin
Dropouts: |
|
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 3 months Co‐interventions: CC 50 mg 1/d from day 5‐9 |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, BMI, fasting blood glucose, fasting insulin, serum testosterone |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information in the study |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study. 3‐arm study however, outcome data presented for all 3 arms clearly |
| Other bias | Low risk | No evidence of other bias, however, reported methodology limited |
Liu 2017.
| Methods | RCT Setting: China Method of randomisation: computer‐generated random number Blinding: unclear Number randomised: 268 |
|
| Participants | Summary: CC and metformin vs CC alone vs letrozole and metformin vs letrozole alone Inclusion criteria: PCOS (Rotterdam criteria), minimum unilateral tubal patency, normal semen analysis Exclusion criteria: women with gynaecological tumours or genital tract malformations, severe systemic disease or acute/chronic urogenital tract infections, other endocrine disease (thyroid and adrenal disease), BMI > 30, age > 35 years or < 20 years Baseline characteristics of each group: CC and metformin (n = 67) vs CC alone (n = 67) vs letrozole and metformin (n = 67) vs letrozole alone (n = 67)
Dropouts: 28 |
|
| Interventions | Main intervention: metformin 1000‐1500 mg/d; CC 50 mg on day 3‐5 of cycle for 5 d and increased to 100 mg then 150 mg each cycle if no ovulation; letrozole 5 mg on day 3‐5 of cycle for 5 d; Duration: 3 cycles Co‐interventions: none |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, ovulation: follicle tracking on transvaginal US or basal body temperature, miscarriage, adverse effect: ectopic pregnancy |
|
| Notes | This review looked at 4 interventions; CC and metformin, CC alone, letrozole and metformin, letrozole alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer random‐number generator but participants numbered and randomly divided into groups according to the order of inclusion |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information in the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts stated but incomplete reasoning. 4 women in the CC group, 9 women in the metformin and CC group, 5 women in the letrozole group and 10 women in the letrozole and metformin group. Unclear if it is an ITT analysis because not clear whether dropouts were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were clearly reported. 4‐arm study, however outcome data presented for all 4 arms clearly |
| Other bias | Low risk | No evidence of other bias |
Lord 2006.
| Methods | RCT Setting: UK Method of randomisation: randomisation was conducted centrally by computer at the hospital pharmacy department using a block with sequential numbers. The code was kept sealed until the trial was completed.* Blinding: double Number randomised: 44 |
|
| Participants | Summary: metformin vs placebo in obese PCOS Inclusion criteria: oligomenorrhoea (< 6 periods/year), biochemical hyperandrogenism (FAI > 5.0) Age 18‐40 years Exclusion criteria: diabetes, thyroid disease, hyperprolactinaemia, CAH, the use of ovulation‐induction agents or drugs that could affect insulin metabolism within 2 months before the start of the trial Baseline characteristics of each group:
Dropouts: 3 women in the metformin group and 1 in the placebo were excluded after they were assigned to the group (did not meet the inclusion criteria). Furthermore, 3 (2 due to pregnancy and 1 lost to follow‐up) in the metformin arm and 5 (3 due to pregnancy and 2 lost to follow‐up) in the placebo arm did not complete the study. Overall, 6 (27.2%) in the metformin group and 6 (27.2%) in the placebo group withdrew from the study after they had been randomised. |
|
| Interventions | Main intervention: metformin 500 mg 3/d or placebo tablet 3/d Duration: 12 weeks Co‐interventions: general advice on diet and exercise |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, menstrual pattern, ovulation: progesterone > 30 nmol/L, BMI, fasting blood glucose, fasting insulin, testosterone |
|
| Notes | This study was to ascertain the effects of metformin on metabolic parameters, visceral and subcutaneous fat distribution in women with PCOS. The fat distribution was measured with areal planimetry (CT scan). There were no significant changes in any of the measures of fat distribution between the metformin and the placebo groups. Although, metformin significantly reduced serum cholesterol concentrations, treatment effects on androgens, insulin, triglycerides, ovulation and pregnancy were not observed. *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted centrally by computer at the hospital pharmacy department using a block with sequential numbers. |
| Allocation concealment (selection bias) | Low risk | The code was kept sealed until the study was completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 6 (27.2%) in the metformin group and 6 (27.2%) in the placebo group withdrew from the study after they had been randomised. Details of dropouts were not provided |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No evidence of other bias |
Machado 2012.
| Methods | RCT Setting: Brazil Method of randomisation: numbered, sealed, opaque envelopes Blinding: double Number randomised: 36 |
|
| Participants | Summary: metformin and CC vs CC and placebo in CC‐resistant PCOS Inclusion criteria: oligomenorrhoea or amenorrhoea, Rotterdam criteria for PCOS, lack of response to previous ovulation induction with CC Exclusion criteria: male factor and tubal infertility, endocrinology and chronic health conditions, the use of hormonal treatments within 60 days of the trial commencing Baseline characteristics of each group: placebo, metformin
Dropouts*: 67 women were initially included in the study. 21 women did not respond to CC alone and 13 became pregnant. 36 women were then randomised to receive metformin or placebo. All 36 women completed the study, with no women dropping out |
|
| Interventions | Main intervention: metformin 850 mg 2/d or placebo tablet 2/d Duration: 60 days Co‐interventions: CC 100 mg day 5‐9 with concurrent use of metformin or placebo |
|
| Outcomes | Primary: none Secondary: clinical pregnancy rate, ovulation: follicle tracking on US, progesterone > 3000 pg/mL on day 21, BMI, fasting insulin, fasting glucose |
|
| Notes | This study aimed to evaluate the efficacy of metformin with CC on ovulation in women previously resistant to CC alone. We did not perform a subgroup analysis by BMI in our analysis due to the small number of women in the study. *Additional information was provided by the study author on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Envelopes representing green or pink bottles where woman chose which envelope |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study author has confirmed in private correspondence that women and healthcare providers were blinded for the duration of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study author has confirmed in private correspondence that women and healthcare providers were blinded for the duration of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for all 36 women who participated in the study. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Malkawi 2002.
| Methods | RCT Setting: Jordan Method of randomisation: centralised randomisation process with women receiving a sequential number* Blinding: double‐blind* Number randomised: 28 |
|
| Participants | Summary: metformin and CC vs CC in non‐obese PCOS, CC‐resistant women Inclusion criteria: US findings of polycystic ovaries together with 3 of: oligomenorrhoea < 6 cycles in preceding year, Ferriman‐Gallwey score > 7, hyperandrogaenemia (free testosterone, androstenedione, DHEAS), elevated LH or LH:FSH > 2 CC resistance defined as failure to ovulate with 150 mg day 5‐9 for 3 months. Normal uterine cavity and patent tubes on hysterosalpingography. Normal semen analysis Exclusion criteria: raised prolactin, adrenal hyperplasia, thyroid dysfunction, Cushing's syndrome. Baseline characteristics of each group: metformin and CC vs CC
Dropouts: none |
|
| Interventions | Main intervention: 1 of metformin 850 mg 2/d, placebo Duration: 6 months Co‐interventions: CC 50 mg day 5‐9 in the first cycle, increasing by 50 mg up to 200 mg in each subsequent cycle until ovulation achieved |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, ovulation: serum progesterone on day 21 and 28 > 15.9 nmol/L, |
|
| Notes | Units of testosterone assumed to be ng/mL *Information kindly provided by the study author that was not in the original paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centralised randomisation process with women receiving a sequential number |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias however limited reported methodology |
Malkawi 2003.
| Methods | RCT Setting: Jordan Method of randomisation: unclear Blinding: none Number randomised: 161 |
|
| Participants | Summary: metformin vs LOD in CC‐resistant PCOS women Inclusion criteria: PCOS, diagnosed if polycystic ovaries on US and ≥ 3 of oligomenorrhoea, hirsutism, hyperandrogenism, elevated LH, LH:FSH ratio > 2, CC resistance (failure to ovulate or conceive after CC treatment up to a daily dose of 150 mg in at least 3 consecutive cycles), normal uterine cavity, normal tubal patency, normal semen parameters Exclusion criteria: congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinaemia, thyroid disease Baseline characteristics of each group: metformin (n = 64) vs LOD (n = 97)
Dropouts: none |
|
| Interventions | Main intervention: metformin 850 mg twice daily; LOD (8‐10 punctures per ovary, each for 2‐3 s with insulated needle adjusted at 40 watts, ovaries then washed with crystalloid solution) Duration: 3 months then if no ovulation, CC was added to both groups Co‐interventions: CC 50 mg/d starting on days 5‐9 of cycle. If no ovulation, dose increased to 100 mg/d then 150 mg/d in each consecutive cycle |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, menstrual frequency, ovulation: serum progesterone > 10 ng/mL, BMI, fasting blood glucose, fasting insulin, serum testosterone, miscarriage, multiple pregnancy, other adverse effects: ectopic pregnancy |
|
| Notes | No information on method of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information however the study compares LOD vs metformin therefore blinding not achievable |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information however the study compares LOD vs metformin therefore blinding not achievable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias however limited reported methodology |
Moll 2006.
| Methods | Multicentre RCT Setting: the Netherlands Method of randomisation: computer‐generated blocks of 4 Blinding: double‐blind Number randomised: 225 |
|
| Participants | Summary: metformin and CC vs CC and placebo in non‐obese women with PCOS Inclusion criteria: PCOS (according to Rotterdam consensus), normal FSH concentrations Exclusion criteria: age > 40 years, abnormal liver function tests or creatinine levels > 95 μmol/L, history of heart disease, history of male factor infertility with total motile sperm count < 10 x 106 Baseline characteristics of each group metformin and CC (n = 111) vs CC (n = 114):
Dropouts: no significant difference in the dropout rates, 28 (25%) in the metformin arm, 21 (18%) in the placebo arm |
|
| Interventions | Main intervention: metformin 2000 mg/d (increased from 500 mg to 2000 mg over a period of 7 d in order to limit the side effects) or placebo Duration: all women received metformin or placebo for 1 month before starting CC treatment (a maximum of 6 cycles for those who ovulated with CC) Co‐interventions: CC 50 mg from day 3 (spontaneous menstruation) or day 5 (progestogen‐induced menstruation) for a period of 5 days. If ovulation did not occur with this dose, CC was increased with steps of 50 mg with a maximum of 150 mg/d in the next cycles |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, ovulation: progesterone > 14 nmol/L in the second half of menstrual cycle, follicle tracking on US, miscarriage, multiple pregnancy, other adverse effects including OHSS, pregnancy complications |
|
| Notes | A large, multicentre RCT. The sample size calculation was based on the ovulation rate. In total, 228 women were initially screened and 3 were subsequently excluded. 111 women were randomised to receive metformin and CC; whilst 114 received placebo and CC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Allocation carried out in the co‐ordinating centre (Amsterdam) and the list was kept until inclusion was completed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: no significant difference in the dropout rates, 28 (25%) in the metformin arm, 21 (18%) in the placebo arm. Details of the dropout participants not mentioned; although number of dropouts in each group were similar |
| Selective reporting (reporting bias) | Low risk | Primary outcome (ovulation) and secondary outcome (pregnancy, miscarriage rates) measures reported |
| Other bias | Low risk | No evidence of other bias |
Morin‐Papunen 2012.
| Methods | Multicentre RCT (parallel‐group study) Setting: Finland Method of randomisation: randomisation codes remained concealed. Metformin and placebo identically packaged and consecutively numbered Blinding: double Number randomised: 320 |
|
| Participants | Summary: metformin vs placebo Inclusion criteria: PCOS diagnosed by Rotterdam criteria, anovulatory infertility for at least 6 months and 3 months since the last infertility treatment. Age range 18‐39 years Exclusion criteria: type 1 diabetes mellitus, liver, cardiac or renal disease, hormone medication, alcohol use, regular smoking Baseline characteristics of each group metformin vs placebo
Dropouts: 61 women were lost to follow‐up or discontinued but their data were included in the ITT analysis |
|
| Interventions | Main intervention: metformin 500 mg 1/d for 1 week, then increased weekly by 1 extra tablet/d to 1.5 g/d in non‐obese and 2 g/d in obese women versus placebo Duration: 3‐9 months Co‐interventions: if pregnancy had not occurred by 3 months, ovulation induction was started with CC. If unsuccessful after 4‐6 cycles, gonadotrophins or aromatase inhibitors were used |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy rate, BMI, miscarriage rate |
|
| Notes | This study was to ascertain the effects of metformin on pregnancy and live birth rates. Endocrine/metabolic outcomes not measured. Additional information sought from the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by hospital pharmacy with 1:1 allocation in random blocks of 10 using computer‐generated lists |
| Allocation concealment (selection bias) | Low risk | Metformin and placebo identically packaged and consecutively numbered. Randomisation codes remained blinded until database lock had taken place. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61 women were lost to follow‐up or discontinued but their data were included in the ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Nestler 1998.
| Methods | Multicentre RCT Setting: USA (3 participants), Venezuela (54 participants), Italy (4 participants)* Method of randomisation: centralised randomisation process* Blinding: single‐blind, participants blinded Number randomised: 61 |
|
| Participants | Summary: metformin vs placebo Inclusion criteria: PCOS (oligomenorrhoea < 6 cycles/year, hyperandrogaenemia (elevated free testosterone), exclusion of other endocrinopathy, US finding of PCO), BMI > 28 Exclusion criteria: diabetes mellitus, adrenal hyperplasia, thyroid dysfunction, hyperprolactinaemia, taking any medication for previous 2 months Baseline characteristics of each group:
Dropouts: none |
|
| Interventions | Main intervention: 1 of metformin 500 mg 3/d, placebo Duration: 34 d, then those who did not ovulate continued for a further 19 d Co‐interventions: those that did not ovulate after 34 days had CC 50 mg for 5 d and continued metformin/placebo for a total of 53 d |
|
| Outcomes | Primary: none Secondary: ovulation: by serum progesterone (≥ 25.6 nmol/L) measured on days 14, 28, 35 (and 44 and 53 in those that went on to receive CC), BMI, fasting glucose, fasting insulin, total and free testosterone |
|
| Notes | 89% of participants were recruited in Venezuela Most of the outcome measures were only reported for those that failed to ovulate during the metformin vs placebo phase of the trial. These have not been included in the analysis as a further analysis to include all participants was not possible. *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centralised randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinded (participant only) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No dropouts. Most of the outcome measures were only reported for those that failed to ovulate during the metformin vs placebo phase of the trial. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Ng 2001.
| Methods | RCT Setting: Hong Kong (Chinese women) Method of randomisation: computer‐generated list in sealed envelopes Blinding: double‐blind Number randomised: 20 |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS, CC resistance Inclusion criteria: PCOS (irregular cycles of ≤ 21 days or ≥ 35 days and cycle‐to‐cycle variation of > 4 days*, anovulation with mid‐luteal progesterone < 16 nmol/L whilst taking CC 100 mg for 5 d over 3 cycles, exclusion of other endocrinopathy (raised prolactin, thyroid disorder*), US findings of PCO, age < 40, day 2 FSH < 10, bilateral patent tubes demonstrated by laparoscopy, normal semen parameters Exclusion criteria: taking any sex hormones in previous 3 months, smokers, renal impairment Baseline characteristics of each group*:
Dropouts: 5 (25%), 3 in placebo arm, 2 in metformin. Analysis on ITT |
|
| Interventions | Main intervention: 1 of metformin 500 mg 3/d, placebo Duration: 3 months. Those who did not ovulate continued for a further cycle Co‐interventions: CC 100 mg for 5 d was given after 3 months if there was no ovulation |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, ovulation: by serum progesterone (> 16 nmol/L) weekly, BMI, fasting blood glucose, fasting insulin, testosterone |
|
| Notes | The BMI was lower than in other trials In spite of the fact that anovulation and CC resistance was an inclusion criteria, 7 out of 9 women taking placebo ovulated (3 with placebo alone, and 4 out of the 6 remaining in the trial who had CC and placebo) *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | In sealed envelopes however, does not state whether the envelopes were opaque. Double, identical appearance and packed by the hospital pharmacy. Code kept in the pharmacy department until the end of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 5 (25%), 3 in placebo arm, 2 in metformin. Analysis on ITT. Details not provided |
| Selective reporting (reporting bias) | Low risk | All primary outcome measures reported |
| Other bias | Unclear risk | In spite of the fact that anovulation and CC resistance was an inclusion criteria, 7 out of 9 women taking placebo ovulated (3 with placebo alone, and 4 out of the 6 remaining in the trial who had CC and placebo) |
Onalan 2005.
| Methods | RCT Setting: Turkey Method of randomisation: computer‐generated randomisation in blocks of 4 Blinding: double* Number randomised: 139 were randomised into 6 main groups according to the fasting glucose/insulin ratio (with a level < 4.5 classified as hyperinsulinaemia) and BMI (< 25, 25‐29.9 and > 30) |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS vs obese PCOS Inclusion criteria: oligomenorrhoea (< 6 periods/year), clinical hyperandrogenism (Ferriman‐Gallwey score > 7) and/or biochemical hyperandrogenism (free testosterone > 4 ng/dL) Exclusion criteria: other causes of hyperandrogenism, Cushing’s syndrome, CAH, hyperprolactinaemia, thyroid dysfunction Baseline characteristics of each group: non‐obese: metformin (n = 51) vs placebo (n = 50)
Baseline characteristics of each group: obese: metformin (n = 21) vs placebo (n = 17)
Dropouts: 15 in total, mainly due to gastrointestinal side effects. Further 8 women were excluded in the analysis because of pregnancy* |
|
| Interventions | Main intervention: metformin 850 mg or placebo tablet twice daily Duration: 6 months Co‐interventions: none |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: menstrual frequency, ovulation: progesterone > 5 ng/mL, BMI, fasting blood glucose, fasting insulin, free testosterone |
|
| Notes | The objective of this study was to investigate the effects of hyperinsulinaemia (fasting glucose/insulin ratio < 4.5 mg/10‐4 U and obesity (BMI > 30) on the responses to metformin treatment in women with PCOS. There were 6 subgroups, normoinsulinaemic lean (BMI < 25), overweight (BMI 25‐29.9) and obese (BMI >30); hyperinsulinaemic lean (BMI < 25), overweight (BMI 25‐29.9) and obese (BMI > 30). The results of the non‐obese subgroups were entered separately from the obese subgroup in the meta‐analysis. We have written to the study author regarding the details of randomisation and concealment. Additionally, we also asked the study author to provide further information of the anthropometric, hormonal and metabolic results at the end of the trial period. *No reply from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks‐of‐4 randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 15 in total (11%), mainly due to gastrointestinal side effects. Missing outcomes not addressed. Imbalance in missing data between the intervention and placebo groups |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome measures not stated. Inadequate study protocol reporting |
| Other bias | Low risk | No evidence of other bias |
Palomba 2004.
| Methods | RCT Setting: Italy Method of randomisation: computer‐generated random allocation sequence in double block Blinding: double Number randomised: 120 |
|
| Participants | Summary: diagnostic laparoscopy and metformin vs LOD and multivitamins Inclusion criteria: PCOS (NIH criteria), CC resistance (failure to ovulate during total of 3 consecutive cycles using CC 150 mg/d for 5 days from day 3‐7), overweight (BMI 25‐30) Exclusion criteria: age < 22 years or > 34 years, hypothyroidism, hyperprolactinaemia, Cushing's syndrome, nonclassical congenital adrenal hyperplasia, current or previous (within 6 months) use of oral contraceptives, glucocorticoids, antiandrogens, ovulation induction agents, antidiabetic or antiobesity drugs, other hormonal drugs. Comorbid conditions including neoplastic, metabolic, hepatic and cardiovascular disease. Diabetes, renal disease, malabsorptive disorders. Glucose intolerance, special diet or physical activity programme. Organic pelvic disease, previous pelvis surgery, suspected peritoneal factor infertility, tubal or male factor infertility. Smokers. Alcohol Baseline characteristics of each group: metformin (n = 60) vs LOD (n = 60)
Dropouts: 11 |
|
| Interventions | Main intervention: metformin 850 mg twice daily; LOD (3‐6 punctures per ovary, each for 2‐3 seconds with insulated needle adjusted at 40 watts, ovaries then washed with crystalloid solution, injured areas covered with hyaluronic acid gel) Duration: 6 months then CC added 150 mg/d from day 3‐6 Co‐interventions: diagnostic laparoscopy (group 1); multivitamins 2 tablets/d (group 2) |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy, menstrual frequency, ovulation: follicle tracking on US, miscarriage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation sequence concealed until the interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts stated with reasoning. 6 women in the diagnostic laparoscopy and metformin group, 5 women in the LOD group. Not an ITT analysis because dropouts were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes clearly reported |
| Other bias | Low risk | No evidence of other bias |
Palomba 2005a.
| Methods | RCT Setting: Italy Method of randomisation: computer‐generated random allocation sequence in double block Blinding: double Number randomised: 100 |
|
| Participants | Summary: metformin vs CC in non‐obese PCOS Inclusion criteria: National Institutes of Health criteria, age 20‐34 years, BMI < 30 kg/m2, tubal patency confirmed by HSG:, normal semen analysis Exclusion criteria: metabolic disorders, hepatic or renal dysfunction, thyroid disease, hyperprolactinaemia, Cushing's syndrome, CAH, hormonal drugs, pelvic diseases, previous pelvic surgery Baseline characteristics of each group: metformin (n = 45) vs CC (n = 47)
Dropouts: 5 in the metformin group and 3 in the metformin + CC group |
|
| Interventions | Main intervention: metformin 850 mg twice daily and placebo vs CC 150 mg on day 3‐7 of the cycle and placebo Duration: 6 months Co‐interventions: none |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy rate, menstrual frequency, ovulation: USS follicular tracking, miscarriage, multiple pregnancy, other adverse effects: various pregnancy complications |
|
| Notes | This study was designed to compare the effectiveness of metformin and CC treatment as a first‐line therapy in non‐obese anovulatory women with PCOS. The primary end point measure was the pregnancy rate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence in double block |
| Allocation concealment (selection bias) | Unclear risk | Allocation sequence concealed until the interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 5 in the metformin group and 3 in the metformin + CC group with reasoning |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
PCOSMIC 2010.
| Methods | Multicentre RCT Setting: New Zealand Randomisation: double‐blind Number randomised: 171 |
|
| Participants | Summary: metformin vs placebo in obese women, metformin and CC vs CC vs metformin in non‐obese women Inclusion criteria: women with PCOS according to Rotterdam consensus criteria Exclusion criteria: couples had undergone previous fertility treatment involving > 5 months' treatment with CC or metformin; tubal factor (at least 1 tube blocked); severe male factor (< 15 mil/mL); important medical disorders Obese women (BMI > 32 kg/m2): baseline characteristics: metformin (n = 32) vs placebo (n = 33)
Dropout: 7 (5 in placebo, 2 in metformin group) Non‐obese women (BMI: ≤ 32 kg/m2): baseline characteristics: metformin and CC (n = 35) vs metformin (n = 35) vs CC (n = 36)
Dropout: 9 (2 in metformin and CC, 3 in metformin and 4 in CC groups) |
|
| Interventions | Obese women were randomised to receive either metformin 500 mg 3/d (increasing dose over 2 weeks) or matching placebo Non‐obese women were randomised to receive either metformin 500 mg 3/d, CC 50 mg from day 2‐6 (increasing up to 150 mg over 3 months if no evidence of ovulation) or metformin 500 mg 3/d combined with CC 50 mg day 2‐6 (increasing up to 150 mg over 3 months if no evidence of ovulation) Duration: up to 6 months All study drugs were stopped once the participant was pregnant |
|
| Outcomes | Primary: live birth rate, gastrointestinal side effects Secondary: clinical pregnancy rate, ovulation: serum progesterone ≥ 25 nmol/L, miscarriage, multiple pregnancy, adverse effects: various pregnancy complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation (blocks of 10) |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation concealment was strictly maintained by a telephone call from the recruiting nurse to pharmacy, ...dispensing pre‐prepared drugs in a true third party randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis planned and protocol breach and losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | Protocol published and all outcomes reported. 3‐arm study, however data presented for all 3 arms clearly |
| Other bias | Low risk | No evidence of other bias |
Raja 2005.
| Methods | RCT Setting: Pakistan Method of randomisation: unclear Blinding: single‐blinded (ultrasonographers were blinded) Number randomised: 100 |
|
| Participants | Summary: metformin and CC vs CC alone Inclusion criteria: PCOS (diagnosed by presence of PCO on ultrasound and ≥ 2 of oligomenorrhoea, hirsutism, hyperandrogenism, elevated LH or LH:FSH ratio). Tubal patency and normal semen analysis Exclusion criteria: other endocrine disorders including congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinaemia and thyroid disease Baseline characteristics of each group: metformin and CC vs CC alone
Dropouts: none |
|
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 6 cycles Co‐interventions: CC 50 mg from day 2 until day 6 of cycle |
|
| Outcomes | Primary: gastrointestinal side effects Secondary: clinical pregnancy rate, ovulation: follicle tracking on transvaginal US and day 21 progesterone > 8 mg/mL, adverse effects: teratogenic effects |
|
| Notes | Old paper ‐ unable to read baseline testosterone levels No information on method of randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias, however, reporting of methodology limited |
Refaie 2005.
| Methods | RCT Setting: Egypt Method of randomisation: unclear Blinding: unclear Number randomised: 55 total (34 in group 1 randomised to metformin and CC vs CC alone) |
|
| Participants | Summary: Group 1 (insulin‐resistant): metformin and CC versus CC alone; Group 2 (non‐insulin‐resistant): CC alone Inclusion criteria: PCOS (Rotterdam criteria) Exclusion criteria: male factor infertility, tubal and peritoneal factors Baseline characteristics of each group: Group 1 (insulin‐resistant) vs group 2 (non‐insulin‐resistant)
Dropouts: none |
|
| Interventions | Main intervention: metformin 1500 mg/d Duration: 6 months or until pregnancy occurred Co‐interventions: CC 50 mg from day 2 until day 6 of cycle, increased up to 150 mg/d if no evidence of ovulation |
|
| Outcomes | Primary: none Secondary: clinical pregnancy rate, ovulation: midluteal progesterone > 10 ng/mL, BMI, fasting insulin, testosterone |
|
| Notes | Unable to distinguish baseline characteristics within group 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Group 1 was randomised to receive metformin and CC vs CC but there are no baseline characteristics available within these randomised groups |
Siebert 2009.
| Methods | RCT Setting: South Africa Method of randomisation: computer‐generated random numbers Blinding: unblinded Number randomised: 107 |
|
| Participants | Summary: metformin and CC vs CC in obese PCOS women Inclusion criteria: PCOS (according to Rotterdam consensus 2003), confirmed tubal patency Exclusion criteria: male factor subfertility Baseline characteristics of each group: metformin and CC (n = 42) vs CC (n = 48)
Dropouts: 17, 10 in metformin + CC group and 7 in CC‐only group |
|
| Interventions | Main intervention: metformin 850 mg twice daily Duration: 6 weeks before and throughout ovulation induction with CC Co‐interventions: CC 50‐150 mg day 4‐8 for 4 cycles |
|
| Outcomes | Primary: none Secondary: ovulation: day‐21 progesterone level (level not stated) |
|
| Notes | A single‐centre RCT investigated the benefit of using metformin in CC ovulation induction treatment. ITT was used in our analysis. Participant lost to follow‐up classified as non‐responder; whilst pregnant participants did not attend follow‐up visit (1 in each arm) were classified as responder No units for insulin, glucose and testosterone in the paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: no significant difference in the dropout rates, 10 in metformin + CC group and 7 in CC‐only group; no reason for dropout |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Sturrock 2002.
| Methods | Cross‐over RCT Setting: UK Method of randomisation: performed by pharmacy* Blinding: double‐blind Number randomised: 19 |
|
| Participants | Summary: metformin vs placebo in obese PCOS with CC resistance Inclusion criteria: oligomenorrhoea cycle > 40 d for 6 months, anovulation demonstrated by day 20‐22 progesterone ≤ 10 nmol/L, lack of response to CC 100 mg for 5 d with US showing endometrial thickness ≤ 5 mm and no ovarian follicle ≥ 14 mm. Age 18‐40 years Exclusion criteria: raised prolactin, adrenal hyperplasia, thyroid dysfunction, medication known to affect insulin action* Baseline characteristics of each group*:
Dropouts: 4 (40%) from metformin arm and 4 (44%) from placebo arm*. Not included in analysis |
|
| Interventions | Main intervention: 1 of metformin 500 mg 3/d, placebo Duration: 6 months Co‐interventions: 1st week of treatment at 500 mg 1/d, 2nd at 500 mg 2/d and 3rd at 500 mg 3/d Those that did not ovulate after 3 months had CC 50 mg days 2‐6, increased to 100 mg for a total of 3 cycles |
|
| Outcomes | Primay: none Secondary: clinical pregnancy, menstrual frequency, ovulation: by monthly serum progesterone (> 10 nmol/L) and presence of follicle ≥ 14 mm on ovarian US*, BMI, testosterone, fasting glucose, fasting insulin |
|
| Notes | This was designed as a cross‐over trial, with 6 months in the treatment/placebo arm followed by a 1‐month washout and then a 3‐month cross‐over. In this review, we only considered the first phase. The inclusion criteria were simply for CC‐resistant anovulation and not specifically PCOS. However only 2 women did not have US criteria of PCOS, and 75% had a raised FAI* In this review, only those participants who had a raised FAI were included in the analysis* *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Performed by pharmacy |
| Allocation concealment (selection bias) | Unclear risk | Performed by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 4 (40%) from metformin arm and 4 (44%) from placebo arm.* Not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias. See notes above |
Tang 2006.
| Methods | Multicentre RCT Setting: UK Method of randomisation: randomisation was performed by the research pharmacy department centrally. Using a random table, a block‐of‐4 randomisation technique was employed in the study. Medications were supplied centrally from the research pharmacy department. The code was kept in the pharmacy department until the end of the trial period. Blinding: double Number randomised: 143 |
|
| Participants | Summary: metformin vs placebo in obese PCOS Inclusion criteria: PCO on USS (> 10 cysts 2‐8 mm in diameter), oligomenorrhoea (cycle length > 35 d) or amenorrhoea (no period in 6 months) Age between 18‐39 years BMI > 30 normal semen analysis and the participant should have at least 1 proven patent fallopian tube Exclusion criteria: concurrent hormone therapy within previous 6 weeks, metabolic or chronic disease, renal or liver disease, diabetes, CAH, androgen‐secreting tumour Baseline characteristics of each group: metformin (n = 69) vs placebo (n = 74)
Dropouts: 11 (15.9%) in the metformin arm, 6 (8.1%). The difference was not significant |
|
| Interventions | Main intervention: metformin 850 mg or 1 placebo tablet twice daily Duration: 6 months Co‐interventions: lifestyle modification (combination of diet and exercise) aiming to reduce 500 kcal/d |
|
| Outcomes | Primary: none Secondary: clinical pregnancy, menstrual frequency, BMI, fasting blood glucose, fasting insulin, testosterone |
|
| Notes | A large multicentre randomised placebo‐controlled study was conducted to investigate the combined effects of the lifestyle modification and the use of metformin in obese women with PCOS (BMI > 30). A total of 8 centres in UK took part in the recruitment. All the participants were recruited from the infertility clinics. The ethnic origin of the participants was not recorded. Both the metformin and the placebo groups experienced improvement in weight loss and in menstrual pattern. However, the differences between the 2 groups were not significant. Participants in the metformin arm showed a greater reduction in total testosterone levels compared with women in the placebo arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed by the research pharmacy department centrally. Using a random table, a block‐of‐4 randomisation technique was employed in the study. |
| Allocation concealment (selection bias) | Low risk | Medications were supplied centrally from the research pharmacy department. The code was kept in the pharmacy department until the end of the trial period. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 11 (15.9%) in the metformin arm, 6 (8.1%). The difference was not significant. Details of the dropout participants were not mentioned. |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure (menstrual frequency) and secondary outcome measures (metabolic parameters) were reported. |
| Other bias | Low risk | No evidence of other bias |
Vandermolen 2001.
| Methods | Multicentre RCT Setting: USA Method of randomisation: computer generation in blocks of 6 Blinding: double‐blind Number randomised: 27 |
|
| Participants | Summary: metformin vs placebo in obese PCOS with CC resistance Inclusion criteria: PCOS (oligomenorrhoea < 6 cycles/year, anovulation with CC 150 mg for 5 d confirmed by progesterone < 4 ng/mL or amenorrhoea by day 35, hyperandrogaenemia (elevated androstenedione, free testosterone or total testosterone)* or hirsutism, exclusion of other endocrinopathy, US findings of PCO; age 18‐35; normal semen analysis; tubal patency if previous pelvic surgery or infection Exclusion criteria: diabetes mellitus, adrenal hyperplasia, thyroid dysfunction, hyperprolactinaemia, abnormal renal or liver function, medication known to affect insulin action* Baseline characteristics of each group:
Dropouts: 1 from each arm (7%); 1 in the placebo arm ovulated in response to CC but was excluded owing to non‐compliance. Not included in analysis |
|
| Interventions | Main intervention: 1 of metformin 500 mg 3/d, placebo Duration: 7 weeks initially, then those who did not ovulate continued for a further 6 cycles Co‐interventions: those that did not ovulate after 7 weeks had CC 50 mg for 5 d. If ovulation did not occur the dose was increased to 100 mg then 150 mg for a total of 6 cycles No change in usual eating habits, physical activity or lifestyle |
|
| Outcomes | Primary: live birth rate Secondary: clinical pregnancy, ovulation: serum progesterone ≥ 12.7 nmol/L on days 10, 20, 30 and 40 (and days 21 and 28 of subsequent cycles if received CC), BMI, fasting glucose, fasting insulin, total and free testosterone |
|
| Notes | Although obesity was not an inclusion criteria, the mean BMI was high in this study although similar in both arms. *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation in blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 1 from each arm (7%); 1 in the placebo arm ovulated in response to CC but was excluded owing to non‐compliance. Not included in analysis. Details not provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Yarali 2002.
| Methods | RCT Setting: Turkey Method of randomisation: computer‐generated numbers. Centralised randomisation process* Blinding: double‐blind Number randomised: 32 |
|
| Participants | Summary: metformin vs placebo in non‐obese PCOS, CC resistance Inclusion criteria: PCOS (oligomenorrhoea < 6 cycles/year, anovulation confirmed with progesterone < 5 ng/mL, testosterone > 2.4 nmol/L, exclusion of other endocrinopathy, US findings of PCO, CC resistance to 250 mg for 5 d for up to 6 months, normal semen analysis, normal HSG or laparoscopy within 6 months Exclusion criteria: diabetes mellitus, adrenal hyperplasia, Cushing’s syndrome, thyroid dysfunction, hyperprolactinaemia, medication known to alter insulin action, previous gonadotrophin treatment, infertility other than that caused by PCOS, previous pelvic surgery Baseline characteristics of each group: metformin (n = 16) vs placebo (n = 16)
Dropouts: 2 (6%) from the metformin/placebo part of the study owing to pregnancy. They were excluded from analysis |
|
| Interventions | Main intervention: 1 of metformin 850 mg 2/d, placebo Duration: 6 weeks initially, then those who did not ovulate continued for 1 cycle Co‐interventions: those that did not ovulate after 6 weeks had recombinant FSH in a low‐dose, step‐up protocol No change in usual eating habits |
|
| Outcomes | Primary: none Secondary: live birth rate, gastrointestinal side effects, pregnancy rate, ovulation: serum progesterone > 15.9 nmol/L weekly, BMI, fasting glucose, fasting insulin, total and free testosterone |
|
| Notes | Free testosterone was significantly higher in the metformin group. Fasting insulin was non‐significantly higher with a wide SD compared with placebo *Information not in the original paper kindly provided by the study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers. Centralised randomisation process* |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 2 (6%) from the metformin/placebo part of the study owing to pregnancy. They were excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Inadequate information |
| Other bias | Low risk | No evidence of other bias |
Zain 2009.
| Methods | RCT Setting: Malaysia Method of randomisation: picking a card out of a box Blinding: unblinded Number randomised: 124 |
|
| Participants | Summary: metformin and CC vs CC vs metformin in obese PCOS Inclusion criteria: newly diagnosed with PCOS (Rotterdam criteria), age < 40 years Exclusion criteria: diabetes, hepatic or renal dysfunction, heart disease, abnormal semen analysis (WHO criteria) Baseline characteristics of each group: metformin and CC vs CC vs metformin
Dropouts: 4 (9.5%) in the metformin group, 2 (4.9%) in the CC group and 3 (7.3%) in the combined metformin and CC group |
|
| Interventions | Main intervention: metformin 1500 mg/d Duration: 6 months Co‐interventions: CC 50 mg from day 2‐6 of the cycle. If women did not respond to the treatment, the dose increased by 50 mg to a maximum dose of 200 mg All the women were offered dietary advice. |
|
| Outcomes | Primary: live birth rate Secondary: clinical pregnancy, ovulation: USS follicular tracking, testosterone, miscarriage, multiple pregnancy |
|
| Notes | This study was designed to compare the live birth rates in women who received CC, metformin and combined CC and metformin treatments. Placebo tablets were not used in this unblinded RCT. Therefore, potential bias may be introduced. Most women were Malay (about 90%) Analysis was based on analysis per protocol, not ITT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Picking a card out of a box labelled A, B or C for metformin, CC and metformin and CC respectively |
| Allocation concealment (selection bias) | High risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 4 (9.5%) in the metformin group, 2 (4.9%) in the CC group and 3 (7.3%) in the combined metformin and CC group. Details not reported. Analysis was based on analysis per protocol, not ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the study |
| Other bias | Low risk | No evidence of other bias |
Baseline characteristics given in order of main intervention (drug, placebo).
Where the trial protocol included a statement such as, "all patients had ultrasound features of PCOS" then this has been included as an inclusion criteria (unless the study authors specifically state that it was not in which case it is recorded under notes).
Abbreviations Table 4:
BMI: body mass index; CAH: congenital adrenal hyperplasia; CC: clomiphene citrate; CI: confidence interval; CSH: chorionic somatomammotropin hormone; CT: computerised tomography scan; DHEAS: dehydroepiandrosterone sulphate; FAI: Free Androgen Index; FSH: follicle‐stimulating hormone; HSG: hysterosalpingogram; IQR: interquartile range; ITT: intention‐to‐treat; IVF: in vitro fertilisation; LH: luteinizing hormone; LOD: laparoscopic ovarian drilling; OGTT: Oral glucose tolerance test; OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial; rFSH: recombinant follicle‐stimulating hormone; PCO(S): polycystic ovary (syndrome); SD: standard deviation SE(M): standard error of the mean; TFT: thyroid function test; TSH: thyroid‐stimulating hormone; US(S): ultrasound (scan); WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abuelghar 2013 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. Reasons for losses to follow‐up not given. Not ITT analysis |
| Ashrafinia 2009 | This study compared metformin with LOD. The interventions were not blinded and the only reproductive outcome was menstrual frequency. |
| Aubuchon 2009 | A cross‐over study including 8 participants who were analysed to metformin vs placebo. Participants were asked to use barrier contraception during the entire study period. The study was mechanistic and was not for ITT. |
| Ayaz 2013b | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. The study was very similar to one we currently assigned to 'awaiting classification" (Ayaz 2013a), therefore we have contacted the authors to ask for confirmation as to whether HCG was used. |
| Aygen 2007 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Billa 2005 | Article not found |
| Bonakdaran 2012 | Quasi randomisation based on day referred to clinic |
| Chaudhury 2008 | Quasi‐randomised ‐ alternation used for randomisation |
| Chou 2003 | Participants were asked to use barrier contraception during the entire study period and the only reproductive outcome was menstrual frequency. |
| Eisenhardt 2006 | The study determined effect of metformin vs placebo on insulin resistance. The only reproductive outcome was menstrual frequency and participants that conceived dropped out of the study. |
| Elgafor 2013 | This study compared metformin and letrozole with LOD. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Fayed 2009 | This study compared metformin and CC vs rosiglitazone and CC. There were no relevant comparisons or placebo. |
| Gada 2000 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Hashim 2010 | This study compared metformin and CC vs letrozole. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Hashim 2011 | This study compared metformin and CC vs LOD. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Hwu 2005 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Katica 2014 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Kazerooni 2009 | This study evaluated the effect of short‐course pretreatment with metformin on hyperandrogenism, insulin resistance, cervical scores and pregnancy rates in women with CC‐resistant PCOS HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Kocak 2002 | Quasi‐randomised trial comparing combined CC and metformin with CC on ovulation in CC‐resistant women with PCOS. Inadequate randomisation and sequence generation (sequential by order of admission). Admission determined by day of menses. Allocation performed by nurse blinded to the study. Odd numbers allocated metformin, even numbers allocated placebo. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Kore 2007 | The diagnosis of PCOS was based on US features alone. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Leanza 2014 | Participants underwent intrauterine insemination and assisted reproduction is an exclusion criteria for this review. Aspects of the methodology are missing from the article. |
| Maciel 2004 | This study compared metformin with placebo however, there are no reproductive outcomes reported. |
| Maged 2015 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Mayhew 2011 | This study is a review. |
| Melli 2010 | This study compared metformin and CC with metformin and CC and fluoxetine. There are no relevant comparisons or placebo. |
| Moghetti 2000 | This study had 2 protocols. Firstly, metformin was compared with placebo however the only reproductive outcome was menstrual frequency. Secondly, long‐term effects of metformin on ovulation were assessed however, there was no placebo/control. |
| Neveu 2007 | This is not a RCT as women could choose which treatment: metformin and CC, CC alone or metformin alone |
| Palomba 2005b | This is a follow‐on study from Palomba 2005a where all participants who did not ovulate following 6 months' treatment of metformin or LOD/placebo, were given CC. |
| Palomba 2005c | This is a commentary of the previous paper Palomba 2005a. |
| Palomba 2007 | This is a non‐RCT comparing metformin vs CC. |
| Pinnow 2008 | This study is a review. |
| Ramzy 2003 | An open‐labelled, randomised trial comparing metformin 500 mg 3/d with placebo 6 weeks prior to CC treatment. In addition, randomisation was performed using alternate numbers. These factors introduced significant bias. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Rezk 2018 | This study compared metformin and CC with letrozole. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Ronsini 2006 | Participants in this study underwent intrauterine insemination and assisted reproduction is an exclusion criteria for this review. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Sahin 2004 | HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Santonocito 2009 | The objective of this study was to compare CC with metformin on ovulation rates. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Savic 2003 | Participants in this study underwent assisted reproduction, which is an exclusion criteria for this review. |
| Sohrabvand 2006 | This study compared metformin and CC with metformin and letrozole. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Trolle 2007 | The participants were asked to use barrier contraception during the entire study period and the only reproductive outcome was menstrual frequency. |
| Weerakiet 2011 | This study compared different doses of metformin (100 mg/d and 1700 mg/d) and added CC if no evidence of ovulation. There was no placebo/control. HCG hormone was used as an ovulation trigger, which may have added additional heterogeneity to the results. |
| Wisniewski 2009 | This study is a review. |
| Xiaolin 2014 | Human menopausal gonadotrophin hormone was used to stimulate the ovaries. Participants undergoing assisted reproduction is an exclusion criteria for this review. |
CC: clomiphene citrate; FSH: follicle‐stimulating hormone; hCG: human chorionic gonadotrophin; IVF: in vitro fertilisation; ITT: intention‐to‐treat; PCOS: polycystic ovary syndrome; RCT: randomised controlled trial; US(S): ultrasound (scan)
Characteristics of studies awaiting assessment [ordered by study ID]
Ayaz 2013a.
| Methods | RCT Setting: Saudi Arabia Method of randomisation: unclear Blinding: double Number randomised: 42 |
| Participants | Summary: metformin and CC vs CC alone Inclusion criteria: PCOS (Rotterdam criteria) Exclusion criteria: other endocrine disorders, male factor infertility, recent PID, tubal infertility Baseline characteristics of each group: metformin and CC vs CC alone Mean age (SD) 32 (3.5), 31.3 (2.9) BMI > 25 14 (56.7)), 15 (71.4) Mean TSH mIU/L (SD) 4.6 (1.3), 3.9 (1.7) Free thyroxin nmol/L (SD) 4.81 (1.6), 5.2 (1.8) Mean total testosterone: mmol/L (SD) 2.60 (0.78), 2.74 (0.65) Sex hormone‐binding globulin: nmol/L (SD) 21.7 (3.7), 18.9 (4.3) Dropouts: none |
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 6 months until 8 weeks of a confirmed pregnancy Co‐interventions: CC 50 mg from day 2 until day 6 of cycle |
| Outcomes | Ovulation: follicle tracking on transvaginal US Others: menstrual pattern, pregnancy rate, multiple pregnancy rate |
| Notes | Endocrine and metabolic outcomes not recorded Need to confirm whether hCG was used ‐ HTML link paper says hCG was used, www.ncbi.nlm.nih.gov/pmc/articles/PMC3713569/ Email sent to drus76@yahoo.com on 22.4.19 |
Beigi 2006.
| Methods | RCT Setting: Iran Method of randomisation: unclear Blinding: unclear Number randomised: 70 |
| Participants | Summary: metformin vs CC alone Inclusion criteria: PCOS based on a history of hyperandrogenism, anovulation, oligomenorrhoea or amenorrhoea, diagnostic US and laboratory findings Exclusion criteria: unclear Baseline characteristics of each group: metformin (n = 35) vs CC (n = 35) unclear Dropouts: unclear |
| Interventions | Main intervention: metformin 500 mg 3/d Duration: 6 months Co‐interventions: CC 50 mg 2/d from day 5‐9 of cycle |
| Outcomes | Ovulation: unclear how measured Others: live birth rate, miscarriage, clinical pregnancy, menstrual frequency |
| Notes | Only abstract found, contacted study authors to provide further information of methodology and results |
Jahan 2015.
| Methods | Prospective trial Setting: unclear Method of randomisation: unclear Blinding: unclear Number randomised: 460 |
| Participants | Summary: metformin vs CC vs letrozole Inclusion criteria: PCOS (diagnostic criteria unclear) Exclusion criteria: unclear Baseline characteristics of each group: metformin (n = 152) vs CC (n = 156) vs letrozole (n = 152) unclear Dropouts: unclear |
| Interventions | Main intervention: metformin 500 mg 3/d vs CC 100 mg/d from day 2‐6 vs letrozole 2.5 mg 2/d from day 2‐6 Duration: unclear Co‐interventions: none |
| Outcomes | Ovulation: follicle tracking on US and serum progesterone Others: live birth rate, miscarriage, clinical pregnancy, multiple pregnancy |
| Notes | Only abstract found, contacted study authors to provide further information of methodology and results |
Robinson 2003.
| Methods | RCT Setting: unclear Method of randomisation: unclear Blinding: double Number randomised: 48 |
| Participants | Summary: metformin and CC vs CC and placebo Inclusion criteria: PCOS (hyperandrogenic oligo‐ovulatory or anovulatory cycles) and 1‐year history infertility Exclusion criteria: other causes of infertility Baseline characteristics of each group: metformin and CC (n = 23) vs CC and placebo (n = 25) unclear Dropouts: unclear |
| Interventions | Main intervention: metformin 500 mg 3/d, CC 50 mg/d from day 5‐9 (increased up to maximum 250 mg/d in stepwise fashion) Duration: until ovulation confirmed and continued for 6 ovulatory cycles or until conception Co‐interventions: placebo |
| Outcomes | Ovulation: ovulation prediction kit, progesterone level Others: miscarriage, clinical pregnancy |
| Notes | Only abstract found, contacted study authors to provide further information of methodology and results |
Singh 2001.
| Methods | RCT Setting: India Method of randomisation: unclear Blinding: unclear Number randomised: 100 |
| Participants | Summary: metformin and CC vs CC Inclusion criteria: PCOS (oligomenorrhoea and/or anovulation, US appearance and reversed LH/FSH ratio > 2), non‐obese (BMI < 25), aged 18‐35 years Exclusion criteria: unclear Baseline characteristics of each group: metformin and CC (n = 53) vs CC (n = 47)
Dropouts: unclear |
| Interventions | Main intervention: metformin 1000 mg/d, CC 50 mg/d from day 3‐7 Duration: at least 4 months Co‐interventions: none |
| Outcomes | Others: clinical pregnancy |
| Notes | Only abstract found, contacted study authors to provide further information of methodology and results |
Williams 2009.
| Methods | RCT Setting: USA Method of randomisation: unclear Blinding: triple Number randomised: 55 |
| Participants | Summary: metformin and CC vs CC and placebo Inclusion criteria: PCOS (diagnostic criteria unclear) Exclusion criteria: unclear Baseline characteristics of each group: metformin and CC (n = 29) vs CC and placebo (n = 26) unclear Dropouts: unclear |
| Interventions | Main intervention: metformin 500 mg 3/d, CC 50 mg/d from day 5‐9 (increased up to maximum 200 mg/d in stepwise fashion) Duration: at least 4 months Co‐interventions: placebo |
| Outcomes | Ovulation: serum progesterone ≥ 5 ng/mL Others: clinical pregnancy |
| Notes | Only abstract found, contacted study authors to provide further information of methodology and results Participants who did not respond to 200 mg of CC on cycle days 5‐9 were unblinded and those in the placebo group were crossed over to metformin and CC group. |
CC: clomiphene citrate; FSH: follicle‐stimulating hormone; hCG: human chorionic gonadotrophin; LH: luteinizing hormone; PCOS: polycystic ovary syndrome; PID: pelvic inflammatory disease; RCT: randomised controlled trial; TSH: thyroid‐stimulating hormone; US: ultrasound
Characteristics of ongoing studies [ordered by study ID]
NCT00005104.
| Trial name or title | Randomised study of decreased hyperinsulinaemia on the ovulatory response to clomiphene citrate in women with polycystic ovary syndrome |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | Women with chronic anovulation to PCOS, whose treatment with CC failed |
| Interventions | Oral metformin vs oral placebo, with addition of CC if remain anovulatory after 49 days |
| Outcomes | Ovulation |
| Starting date | April 2000 |
| Contact information | University of Virginia |
| Notes |
NCT00317928.
| Trial name or title | Efficacy of metformin in PCOS: metabolic and hormonal factors |
| Methods | Randomised, double‐blind, cross‐over trial |
| Participants | Women with PCOS |
| Interventions | Metformin vs placebo |
| Outcomes | Ovulation |
| Starting date | April 2006 |
| Contact information | Aarhus University Hospital, Skejby |
| Notes |
NCT00558077.
| Trial name or title | Second‐line treatments for anovulatory infertility in PCOS patients |
| Methods | RCT |
| Participants | Infertile, anovulatory PCOS patients |
| Interventions | Diagnostic laparoscopy and metformin and CC vs LOD |
| Outcomes | Live birth rate, clinical pregnancy rate, miscarriage rate |
| Starting date | November 2007 |
| Contact information | Stefano Palomba |
| Notes |
NCT01679574.
| Trial name or title | Letrozole or combined clomiphene citrate and metformin as a first line treatment in women with polycystic ovarian syndrome (PCOS) |
| Methods | RCT |
| Participants | Women with PCOS |
| Interventions | Letrozole vs metformin and CC |
| Outcomes | Ovulation, pregnancy and miscarriage |
| Starting date | September 2012 |
| Contact information | Assiut university, Egypt |
| Notes |
NCT02562664.
| Trial name or title | Metformin improves clinical pregnancy rates in polycystic ovarian syndrome patients |
| Methods | Double‐blinded, RCT |
| Participants | Women with PCOS |
| Interventions | Metformin and CC vs CC and placebo |
| Outcomes | Pregnancy rate, fasting glucose, fasting insulin |
| Starting date | September 2015 |
| Contact information | Assiut University, Egypt |
| Notes |
CC: clomiphene citrate; LOD: laparoscopic ovarian drilling; PCOS: polycystic ovary syndrome; RCT: randomised controlled trial;
Differences between protocol and review
Changes in 2009 update
In the 2009 update of this review, the title was changed from 'Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for polycystic ovary syndrome' to 'Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility'.
The outcome measures were restructured. One new comparison was added (metformin versus CC).
Studies using troglitazone were excluded.
Changes in 2017 update
Unit of analysis
We added a note to the Methods section to clarify that miscarriage and multiple pregnancy data were analysed 'per woman' and added a sensitivity analysis to check the effect of analysing these outcomes 'per pregnancy'. In addition we restricted analysis of ovulation rates to per‐woman data and reported per‐cycle data in an additional table.
'Summary of findings' table
We added more detail in the Methods section to state which comparisons and outcomes would be included in the 'Summary of findings' table. We decided to include only the three most important clinical comparisons. For one comparison (metformin versus CC), there was high heterogeneity for some outcomes, which was associated with BMI status, so for this comparison we decided as a post hoc measure to present the data by BMI subgroup.
Changes in 2019 update
Inclusion criteria
We included randomised control trials only involving women with polycystic ovary syndrome (PCOS) that met the Rotterdam diagnostic criteria. We included papers only if reproductive outcomes were reported, at the least ovulation, but ideally also clinical pregnancy and live birth rate. We did not include menstrual frequency in this review because regular menstruation is a marker of ovulation and we had already included ovulation rates defined as a raised serum progesterone level in the luteal phase or follicle tracking on ultrasound. We excluded studies that only reported anthropometric, metabolic or endocrine outcomes and also studies where participants were asked to use barrier contraception or were not trying to conceive. We also excluded studies that used human chorionic gonadotropin injections to trigger ovulation because the aim was to directly compare prespecified ovulation induction agents: metformin, clomiphene citrate, letrozole, and laparoscopic ovarian drilling. Studies that assessed the effect of metformin in PCOS patients undergoing artificial reproductive techniques or induction with gonadotrophin therapy were excluded because these are the subject of different Cochrane Reviews.
Contributions of authors
ANS: literature search, assessment of studies, data collection, revising and preparing the review (2019 version) LCM: literature search, assessment of studies, data collection, revising and preparing the review (2019 and 2017 version) TT: checking the literature search, secondary assessment of studies and data analysis in the updated review (May 2008 to January 2019). Preparation of the previous reviews (2009 and 2012 versions) RN: read, commented on and approved the draft review (2009, 2012, 2017 and 2019 versions) AB: secondary assessment of studies and quality analysis. Revising and finalising the review (2009, 2012, 2017 and 2019 versions)
Sources of support
Internal sources
Peninsula Medical School, UK.
University of Adelaide, Australia.
Leeds Centre of Reproductive Medicine, Leeds, UK.
External sources
No sources of support supplied
Declarations of interest
ANS: none known LCM: none known TT: received consultancy fee from Finox Biotech for advisory board meeting in 2016; Finox do not manufacture insulin sensitisers. RN: received consultancy fee from Ferring for advisory board meeting; Ferring do not manufacture insulin sensitisers. AB: NHS Consultant in Reproductive Medicine and clinical lead for the Leeds Centre for Reproductive Medicine, which performs all fertility treatments funded by the NHS; partner in Genesis LLP, the private arm on the Leeds Centre for Reproductive Medicine, which performs all self‐funded fertility treatments using identical protocols to the NHS; Chair, Clinical Board, IVI, UK; Chair, British Fertility Society; Chair, NHS England IVF Pricing Development Expert Advisory Group; Chair, World Health Organization Expert Working Group on Global Infertility Guidelines, Management of PCOS; consultant for ad hoc advisory boards for Ferring Pharmaceuticals, Astra Zeneca, Merck Serono, IBSA, Clear Blue, Gideon Richter, Uteron Pharma & former member of ethics committee for OvaScience. Merck manufacture some products containing metformin.
New
References
References to studies included in this review
Baillargeon 2004 {published data only}
- Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertility and Sterility 2004;82:893‐902. [DOI] [PubMed] [Google Scholar]
Begum 2014 {published data only}
- Begum H, Chowdhury TA, Begum F, Begum SN, Kabir N, Haque MD. Effect of metformin and clomiphene citrate in improving fertility in subfertility women with polycystic ovary syndrome: a randomised controlled trial. Bangladesh Journal of Obstetrics and Gynaecology 2014;29:15‐20. [Google Scholar]
Ben Ayed 2009 {published data only}
- Ben Ayed B, Dammak Dit Mlik S, Ben Arab H, Trabelssi H, Chahtour H, Mathlouthi N. Metformin effects on clomifene‐induced ovulation in the polycystic ovary syndrome. Tunisie Medicale 2009;87(1):43‐8. [PubMed] [Google Scholar]
Boudhraa 2010 {published data only}
- Boudhraa K, Jellouli MA, Amri M, Farhat M, Torkhani F, Gara MF. Indication of metformin in the management of hormonal dysfunction secondary to polycystic ovarian syndrome: prospective comparative study about 63 cases. La Tunisie Medicale 2010;88(5):335‐40. [PubMed] [Google Scholar]
Chuni 2006 {published data only}
- Chuni N. Efficacy of sequential treatment of metformin and clomiphene citrate in clomiphene resistant women with polycystic ovary syndrome. Journal of Obstetrics and Gynecology of India 2007;57(1):69‐72. [Google Scholar]
Fatima 2018 {published data only}
- Fatima A, Khan SA, Saifuddin Z, Aslam R. Comparison of efficacy of clomiphene citrate alone and with metformin for treatment of infertility in polycystic ovarian syndrome. Rawal Medical Journal 2018;43(2):285‐8. [Google Scholar]
Fleming 2002 {published data only}
- Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism 2002;87(2):569‐74. [DOI] [PubMed] [Google Scholar]
- Fleming R, Hopkinson ZE, Wallace E, Greer IA, Sattar N. Metformin treatment improves ovulation frequency in women with oligomenorrhoea: a randomized, double blind placebo trial. Human Fertility 2000; Vol. 3, issue 4:389‐90.
Hamed 2010 {published data only}
- Hamed HO, Hasan AF, Ahmed OG, Ahmend MA. Metformin versus laparoscopic ovarian drilling in clomiphene‐ and insulin‐resistant women with polycystic ovary syndrome. International Journal of Gynecology and Obstetrics 2010;108:143‐7. [DOI] [PubMed] [Google Scholar]
Heathcote 2013 {unpublished data only}
- Heathcote G, Forbes K, Luscombe G, Boothroyd C. Live birth rate after metformin and clomiphene vs clomiphene alone in polycystic ovary syndrome (PCOS): a randomized, double‐blind placebo‐controlled trial. Unpublished data.
Hoeger 2004 {published data only}
- Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48‐week, placebo‐controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertility and Sterility 2004;82:421‐9. [DOI] [PubMed] [Google Scholar]
Jakubowicz 2001 {published data only}
- Jakubowicz DJ, Seppala M, Jakubowicz S, Rodriguez‐Armas O, Rivas‐Santiago A, Koistinen H, et al. Insulin reduction with metformin increases luteal phase serum glycodelin and insulin‐like growth factor‐binding protein 1 concentrations and enhances uterine vascularity and blood flow in the polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2001;86(3):1126‐33. [DOI] [PubMed] [Google Scholar]
Kar 2015 {published data only}
- Kar S. Metabolic risks of the lean PCOS woman. Fertility and Sterility ASRM abstracts 2013;100(S359):P‐730. [Google Scholar]
- Kar S, Sanchita S. Clomiphene citrate, metformin or a combination of both as the first line ovulation induction drug for Asian Indian women with polycystic ovarian syndrome: a randomized controlled trial. Journal of Human Reproductive Science 2015;8:197‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karimzadeh 2007 {published data only}
- Karimzadeh M, Tayebi N, Eftekhar M, Sakhavat L, Taheripanah R, Zare F. The effect of administration of metformin on lipid profile changes and insulin resistance in patients with polycystic ovary syndrome. Middle East Fertility Society Journal 2007;12(3):174‐8. [Google Scholar]
Karimzadeh 2010 {published data only}
- Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate‐metformin in patients with polycystic ovary syndrome. Fertility and Sterility 2010;94(1):216‐20. [DOI] [PubMed] [Google Scholar]
Khorram 2006 {published data only}
- Khorram O, Helliwell JP, Katz S, Bonpane CM, Jaramillo L, Khorram O, et al. Two weeks of metformin improves clomiphene citrate‐induced ovulation and metabolic profiles in women with polycystic ovary syndrome. Fertility and Sterility 2006;85:1448‐51. [DOI] [PubMed] [Google Scholar]
Kjotrod 2011 {published data only}
- Kjotrod SB, Carlsen SM, Rasmussen PE, Holst‐Larsen T, Mellembakken J, Thurin‐Kjellberg A, et al. Use of metformin before and during assisted reproductive technology in non‐obese young infertile women with polycystic ovary syndrome: a prospective, randomized, double‐blind, multi‐centre study. Human Reproduction 2011;26(8):2045‐53. [DOI] [PubMed] [Google Scholar]
Ko 2001 {published data only}
- Ko SH, Lee SH. The effect of metformin therapy on clomiphene citrate‐resistant polycystic ovarian syndrome women. Korean Journal of Fertility and Sterility 2001;28(4):255‐64. [Google Scholar]
Kocak 2006 {published data only}
- Kocak I, Ustin C. Effects of metformin on insulin resistance, androgen concentration, ovulation and pregnancy rates in women with polycystic ovary syndrome following laparoscopic ovarian drilling. Japan Society of Obstetrics and Gynecology January 2006;32(3):292‐8. [DOI] [PubMed] [Google Scholar]
Legro 2007 {published data only}
- Cataldo N, Barnhart H, Legro R, Myers E, Schlaff W, Carr B, et al. Extended‐release metformin does not reduce the clomiphene citrate dose required to induce ovulation in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3147‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. New England Journal of Medicine 2007;356:551‐66. [original article] [DOI] [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu Z, Xue, Y, Chen L, Cai Q, Chen H, Zhang J, et al. Clinical study on treating insulin resistance and promoting ovulation in polycystic ovary syndrome. Chinese Journal of Obstetrics and Gynaecology 2004;39(9):586‐90. [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu C, Feng G, Huang W, Wang Q, Yang S, Tan J, et al. Comparison of clomiphene citrate and letrozole for ovulation induction in women with polycystic ovary syndrome: a prospective randomized trial. Gynecological Endocrinology 2017;33(11):872‐6. [DOI] [PubMed] [Google Scholar]
Lord 2006 {published data only}
- Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome‐‐a randomised, double‐blind, placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 2006;113:817‐24. [DOI] [PubMed] [Google Scholar]
Machado 2012 {published data only}
- Machado RC, Machado NA, Geber S. Evaluation of the use of metformin for ovulation in patients with PCOS resistant to isolated use of clomiphene citrate. Brazilian Journal Assisted Reproduction 2012;16(1):27‐31. [Google Scholar]
Malkawi 2002 {published data only}
- Malkawi HY, Qublan HS. The effect of metformin plus clomiphene citrate on ovulation and pregnancy rates in clomiphene‐resistant women with polycystic ovary syndrome. Saudi Medical Journal 2002;23(6):663‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Malkawi 2003 {published data only}
- Malkawi HY, Qublan HS, Hamaideh AH. Medical vs. surgical treatment for clomiphene citrate‐resistant women with polycystic ovary syndrome. Journal of Obstetrics and Gynaecology 2003;23(3):289‐93. [DOI] [PubMed] [Google Scholar]
Moll 2006 {published data only}
- Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ 2006;332:1485. [original article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll E, Korevaar JC, Bossuyt PM, Veen F. Does adding metformin to clomifene citrate lead to higher pregnancy rates in a subset of women with polycystic ovary syndrome?. Human Reproduction 2008;23(8):1830‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll E, Wely M, Lambalk CB, Bossuyt PM, Veen F. Health‐related quality of life in women with newly diagnosed polycystic ovary syndrome randomized between clomifene citrate plus metformin or clomifene citrate plus placebo. Human Reproduction 2012;27:3273‐8. [DOI] [PubMed] [Google Scholar]
Morin‐Papunen 2012 {published data only}
- Morin‐Papunen L, Rantala AS, Unkila‐Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, et al. Metformin improves pregnancy and live‐birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double‐blind, placebo‐controlled randomized trial. Journal of Clinical Endocrinology and Metabolism 2012;97:1492‐500. [DOI] [PubMed] [Google Scholar]
Nestler 1998 {published data only}
- Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene‐induced ovulation in the polycystic ovary syndrome. New England Journal of Medicine 1998;338(26):1876‐80. [DOI] [PubMed] [Google Scholar]
Ng 2001 {published data only}
- Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene‐resistant polycystic ovaries: a randomized, double‐blinded placebo‐controlled trial. Human Reproduction 2001;16(8):1625‐31. [DOI] [PubMed] [Google Scholar]
Onalan 2005 {published data only}
- Onalan G, Goktolga U, Ceyhan T, Bagis T, Onalan R, Pabuccu R. Predictive value of glucose‐insulin ratio in PCOS and profile of women who will benefit from metformin therapy: obese, lean, hyper or normoinsulinemic?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123:204‐11. [DOI] [PubMed] [Google Scholar]
Palomba 2004 {published data only}
- Palomba S, Orio F, Nardo LG, Falbo A, Russo T, Corea D, et al. Metformin administration versus laparoscopic ovarian diathermy in clomiphene citrate‐resistant women with polycystic ovary syndrome: a prospective parallel randomized double‐blind placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism 2004;89(10):4801‐9. [DOI] [PubMed] [Google Scholar]
Palomba 2005a {published data only}
- Palomba S, Orio F Jr, Falbo A, Manguso F, Russo T, Cascella T, et al. Prospective parallel randomized, double‐blind, double‐dummy controlled clinical trial comparing clomiphene citrate and metformin as the first‐line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2005;90(7):4068‐74. [original article] [DOI] [PubMed] [Google Scholar]
- Palomba S, Orio F Jr, Falbo A, Russo T, Tolino A, Zullo F. Effects of metformin and clomiphene citrate on ovarian vascularity in patients with polycystic ovary syndrome. Fertility and Sterility 2006;86:1694‐701. [DOI] [PubMed] [Google Scholar]
PCOSMIC 2010 {published data only}
- Johnson NP, Bontekoe S, Stewart AW. Analysis of factors predicting success of metformin and clomiphene treatment for women with infertility owing to PCOS‐related ovulation dysfunction in a randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 2011;51:252‐6. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Stewart AW, Falkiner J, Farquhar CM, Milsom S, Singh V‐P, et al. on behalf of REACT‐NZ (REproduction And Collaborative Trials in New Zealand) a multi‐centre fertility trials group. PCOSMIC: a multi‐centre randomized trial in women with polycystic ovary syndrome evaluating metformin for infertility with clomiphene. Human Reproduction 2010;25(7):1675‐83. [MEDLINE: 10.1093/humrep/deq100] [DOI] [PubMed] [Google Scholar]
Raja 2005 {published data only}
- Raja A, Hashmi SN, Sultana N, Rashid H. Presentation of polycystic ovary syndrome and its management with clomiphene alone and in combination with metformin. Journal of Ayub Medical College, Abbottabad 2005;17(2):50‐3. [PubMed] [Google Scholar]
Refaie 2005 {published data only}
- Refaie AM, Ibrahim GA, Al Oash S. Characteristics of polycystic ovary syndrome with and without insulin resistance and the role of insulin sensitizing drug (metformin) in its management. Middle East Fertility Society Journal 2005;10(2):142‐9. [Google Scholar]
Siebert 2009 {published data only}
- Siebert T, Kruger T, Lombard C. Evaluating the equivalence of clomiphene citrate with and without metformin in ovulation induction in PCOS patients. Journal of Assisted Reproduction and Genetics 2009;26(4):165‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturrock 2002 {published data only}
- Sturrock ND, Lannon B, Fay TN. Metformin does not enhance ovulation induction in clomiphene resistant polycystic ovary syndrome in clinical practice. British Journal of Clinical Pharmacology 2002;53(5):469‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo‐controlled, double‐blind multicentre study. Human Reproduction 2006;21:80‐9. [DOI] [PubMed] [Google Scholar]
Vandermolen 2001 {published data only}
- Vandermolen DT, Ratts VS, Evans WS, Stovall DW, Kauma SW, Nestler JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertility and Sterility 2001;75:310‐5. [DOI] [PubMed] [Google Scholar]
Yarali 2002 {published data only}
- Yarali H, Yildiz BO, Demirol A, Zeyneloglu HB, Yigit N, Bukulmez O, et al. Co‐administration of metformin during rFSH treatment in patients with clomiphene citrate‐resistant polycystic ovarian syndrome: a prospective randomized trial. Human Reproduction 2002;17(2):289‐94. [DOI] [PubMed] [Google Scholar]
Zain 2009 {published data only}
- Zain MM, Jamaluddin R, Ibrahim A, Norman RJ. Comparison of clomiphene citrate, metformin, or the combination of both for first‐line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fertility and Sterility 2009;91(2):514‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abuelghar 2013 {published data only}
- Abuelghar WM, Elkady OS, Khamees AA. Clomiphene citrate alone, in combination with metformin or in combination with pioglitazone as first line therapy in induction of ovulation in infertile women with polycystic ovary syndrome, a randomised controlled trial. Middle East Fertility Society Journal 2013;18:135‐41. [Google Scholar]
Ashrafinia 2009 {published data only}
- Ashrafinia M, Hosseini R, Moini A, Eslami B, Asgari Z. Comparison of metformin treatment and laparoscopic ovarian diathermy in patients with polycystic ovary syndrome. International Journal of Gynecology and Obstetrics 2009;107:236‐9. [DOI] [PubMed] [Google Scholar]
Aubuchon 2009 {published data only}
- Abuchon M, Lieman H, Stein D, Cohen HW, Isaac B, Adel G, et al. Metformin does not improve the reproductive or metabolic profile in women with polycystic ovary syndrome (PCOS). Reproductive Sciences 2009;16(10):938‐46. [DOI] [PubMed] [Google Scholar]
Ayaz 2013b {published data only}
- Ayaz A, Alwan Y, Farooq MU. Metformin‐clomiphene citrate vs clomiphene citrate alone: Polycystic ovarian syndrome. Journal of Human Reproductive Sciences January‐March 2013;6(1):15‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aygen 2007 {published data only}
- Aygen EM, Guzel Z, Ozgun T, Atakul T, Sahin Y. The use of letrozole for ovulation induction in infertile women with polycystic ovarian syndrome. Erciyes Medical Journal 2007;29(3):195‐200. [Google Scholar]
Billa 2005 {published data only}
- Billa N, Kapolla N, Nicopoulou S, Koukkou E, Venaki E, Adamopoulos DA, et al. Effects of metformin on reproductive axis and metabolism in women with polycystic ovary syndrome (abstract). European Journal of Clinical Investigation 2005;35(Suppl 2). [Google Scholar]
Bonakdaran 2012 {published data only}
- Bonakdaran S, Khorasani ZM, Davachi B, Khorasani M. The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: a randomized placebo‐controlled clinical trial. Iran Journal of Reproductive Medicine 2012;10(5):465‐72. [PMC free article] [PubMed] [Google Scholar]
Chaudhury 2008 {published data only}
- Chaudhury K, Chaudhury S, Chowdhury S. Does metformin augment the ovulation inducing effects of clomiphene in non‐obese women with polycystic ovary syndrome?. Journal of Indian Medicine Association 2008;106(10):643‐8. [PubMed] [Google Scholar]
Chou 2003 {published data only}
- Chou KH, Eye Corleta H, Capp E, Spritzer PM. Clinical, metabolic and endocrine parameters in response to metformin in obese women with polycystic ovary syndrome: a randomized, double‐blind and placebo‐controlled trial. Hormone and Metabolic Research 2003;35:86‐91. [DOI] [PubMed] [Google Scholar]
Eisenhardt 2006 {published data only}
- Eisenhardt S, Schwarzmann N, Henschel V, Germeyer A, Wolff M, Hamann A, et al. Early effects of metformin in women with polycystic ovary syndrome: a prospective randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism 2006;91(3):946‐52. [DOI] [PubMed] [Google Scholar]
Elgafor 2013 {published data only}
- Elgafor IA. Efficacy of combined metformin‐letrozole in comparison with bilateral ovarian drilling in clomiphene‐resistant infertile women with polycystic ovarian syndrome. Archives of Gynecology and Obstetrics 2013;288:119‐23. [DOI] [PubMed] [Google Scholar]
Fayed 2009 {published data only}
- Fayed MR, Hamed T. Ovulation induction with rosiglitazone/clomiphene citrate versus metformin/clomiphene citrate in women with clomiphene citrate‐resistant polycystic ovary syndrome. Middle East Fertility Society Journal 2009;14(4):257‐64. [Google Scholar]
Gada 2000 {published data only}
- Gada NJ, Purandare CN, Lopez JA, Salunkhe R. Role of metformin in PCOD: a comparative study. XVI FIGO World Congress of O & G, 2000 Sep 3‐8; Washington DC. 2000; Vol. 2:107‐9.
Hashim 2010 {published data only}
- Hashim HA, Shokeir T, Badawy A. Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene‐resistant women with polycystic ovary syndrome: a randomized controlled trial. Fertility and Sterility 2010;94(4):1405‐9. [DOI] [PubMed] [Google Scholar]
Hashim 2011 {published data only}
- Hashum HA, Lakany NE, Sherief L. Combined metformin and clomiphene citrate versus laparoscopic ovarian diathermy for ovulation induction in clomiphene‐resistant women with polycystic ovary syndrome: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(3):169‐77. [DOI] [PubMed] [Google Scholar]
Hwu 2005 {published data only}
- Hwu YM, Lin SY, Huang WY, Lin MH, Lee RK. Ultra‐short metformin pretreatment for clomiphene citrate‐resistant polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics 2005;90:39‐43. [DOI] [PubMed] [Google Scholar]
Katica 2014 {published data only}
- Katica V, Rama A, Abadzic N. Efficacy of ovulation induction with clomiphene citrate and metformin in women with polycystic ovary syndrome ‐ pilot study. Folia Medica 2014;49(1):37‐42. [Google Scholar]
Kazerooni 2009 {published data only}
- Kazerooni T, Ghaffarpasand F, Kazerooni Y, Kazerooni M, Setoodeh S. Short‐term metformin treatment for clomiphene citrate‐resistant women with polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics 2009;107(1):50‐3. [DOI] [PubMed] [Google Scholar]
Kocak 2002 {published data only}
- Kocak M, Caliskan E, Simsir C, Haberal A. Metformin therapy improves ovulatory rates, cervical scores, and pregnancy rates in clomiphene citrate‐resistant women with polycystic ovary syndrome. Fertility and Sterility 2002;77(1):101‐6. [DOI] [PubMed] [Google Scholar]
Kore 2007 {published data only}
- Kore SJ, Lakhotia S, Parikh M, Ambiye VR. Polycystic ovarian disease: impact of metformin therapy. Bombay Hospital Journal 2007;49(2):1‐6. [Google Scholar]
Leanza 2014 {published data only}
- Leanza V, Coco L, Grasso F, Leanza G, Zarbo G, Palumbo M. Ovulation induction with clomiphene citrate and metformin in women with polycystic ovary syndrome. Minerva Ginecologica 2014;66(3):299‐301. [PubMed] [Google Scholar]
Maciel 2004 {published data only}
- Maciel GA, Soares Junior JM, Alves da Motta EL, Abi Haidar M, Lima GR, Baracat EC. Nonobese women with polycystic ovary syndrome respond better than obese women to treatment with metformin. Fertility and Sterility 2004;81(2):355‐60. [DOI] [PubMed] [Google Scholar]
Maged 2015 {published data only}
- Maged AM, Elsawah H, Abdelhafez A, Bakry A, Al Mostafa W. The adjuvant effect of metformin and N‐acetylcysteine to clomiphene citrate in induction of ovulation in patients with polycystic ovary syndrome. Gynecological Endocrinology 2015;31(8):635‐8. [DOI] [PubMed] [Google Scholar]
Mayhew 2011 {published data only}
- Mayhew MS. Treatment for polycystic ovary syndrome. Journal for Nurse Practitioners 2011;7(6):517‐18. [Google Scholar]
Melli 2010 {published data only}
- Melli MS, Kazemi‐Shishvani M. The effectiveness of clomiphene citrate (CC) + metformin vs CC + metformin +fluoxetine in PCOS CC‐resistant patients [P‐447]. Human Reproduction 2010;25 Suppl 1(6):[Abstract]. [Google Scholar]
Moghetti 2000 {published data only}
- Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomised, double‐blind, placebo‐controlled 6‐month trial, followed by open, long‐term clinical evaluation. Journal of Clinical Endocrinology and Metabolism 2000;85(1):139‐46. [DOI] [PubMed] [Google Scholar]
Neveu 2007 {published data only}
- Neveu N, Granger L, St‐Michel P, Lavoie HB. Comparison of clomiphene citrate metformin, or the combination of both for first‐line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertility and Sterility 2007;87(1):113‐20. [DOI] [PubMed] [Google Scholar]
Palomba 2005b {published data only}
- Palomba S, Orio Jr F, Falbo A, Russo T, Caterina G, Manguso F, et al. Metformin administration and laparoscopic ovarian drilling improve ovarian response to clomiphene citrate (CC) in oligo‐anovulatory CC‐resistant women wtih polycystic ovary syndrome. Clinical Endocrinology 2005;63:631‐5. [DOI] [PubMed] [Google Scholar]
Palomba 2005c {published data only}
- Palomba S, Orio Jr F, Nardo LG, Falbo A, Russo T, Corea D, et al. Live birth rate was higher with metformin treatment than after laparoscopic ovarian diathermy in women with PCOS. Reproductive Endocrinology and Infertility 2005;7:145‐6. [Google Scholar]
Palomba 2007 {published data only}
- Palomba S, Orio Jr F, Falbo A, Russo T, Tolino A, Zullo F. Clomiphene citrate versus metformin as first‐line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2007;92(9):3498‐503. [DOI] [PubMed] [Google Scholar]
Pinnow 2008 {published data only}
- Pinnow S, Kraus C. What is the most effective treatment for polycystic ovary syndrome for patients who cannot tolerate metformin?. Evidence‐based Practice September 2008;11:7‐8. [Google Scholar]
Ramzy 2003 {published data only}
- Ramzy A, El‐Kateb S, Al‐Inany H, Aboulmaaty Z, Badie M. The use of metformin in overweight and lean infertile patients with polycystic ovarian syndrome: a randomised controlled trial. Middle East Fertility Society Journal 2003;8:143‐9. [Google Scholar]
Rezk 2018 {published data only}
- Rezk M, Shaheen A, El‐Nasr IS. Clomiphene citrate combined with metformin versus letrozole for induction of ovulation in clomiphene‐resistant polycystic ovary syndrome: a randomized clinical trial. Gynecological Endocrinology 2018;34(4):298‐300. [DOI] [PubMed] [Google Scholar]
Ronsini 2006 {published data only}
- Ronsini S, Filippo MA, Grieco N, Cappeillo F, D'Aniello G. Ovulation induction in insulin‐resistant infertility. Human Reproduction 2006;21(Suppl):[Abstract]. [Google Scholar]
Sahin 2004 {published data only}
- Sahin Y, Yirmibes U, Kelestimur F, Aygen E. The effects of metformin on insulin resistance, clomiphene‐induced ovulation and pregnancy rates in women with polycystic ovary syndrome. European Journal of Obstetrics and Gynecology and Reproductive Biology 2004;113:214‐20. [DOI] [PubMed] [Google Scholar]
Santonocito 2009 {published data only}
- Santonocito V, Rapisarda V, Abruzzo SR, Pollicino R, Coco L, Zarbo G. Comparison between clomiphene citrate and metformin for induction of ovulatory cycles in infertile nonobese women with polycystic ovary syndrome. Giornale Italiano di Ostetricia e Ginecologia 2009;31(11‐12):455‐60. [Google Scholar]
Savic 2003 {published data only}
- Savic B, Milacic D, Zarkovic A. Insulin sensitising agents in PCOS treatment. International Journal of Obstetrics & Gynecology 2003;83(Suppl 3):[Abstract]. [Google Scholar]
Sohrabvand 2006 {published data only}
- Sohrabvand F, Ansari Sh, Bagheri M. Efficacy of combined metformin–letrozole in comparison with metformin–clomiphene citrate in clomiphene‐resistant infertile women with polycystic ovarian disease. Human Reproduction 2006;21(6):1432‐5. [DOI] [PubMed] [Google Scholar]
Trolle 2007 {published data only}
- Trolle B, Flyvbjerg A, Kesmodel U, Lauszus FF. Efficacy of metformin in obese and non‐obese women with polycystic ovary syndrome: a randomized, double‐blinded, placebo‐controlled cross‐over trial. Human Reproduction 2007;22(11):2967‐73. [DOI] [PubMed] [Google Scholar]
Weerakiet 2011 {published data only}
- Weerakiet S, Sophonsritsuk A, Lertvikool S, Satirapot C, Leelaphiwat S, Jultanmas R. Randomized controlled trial of different doses of metformin for ovulation induction in infertile women with polycystic ovary syndrome. Journal of Obstetrics and Gynaecology Research 2011;37(9):1229‐37. [DOI] [PubMed] [Google Scholar]
Wisniewski 2009 {published data only}
- Wisniewski M, Peterson M. Is metformin beneficial for the treatment of infertility in women with PCOS?. JAAPA 2009;22(4):49‐50. [DOI] [PubMed] [Google Scholar]
Xiaolin 2014 {published data only}
- Xiaolin C, Jiwei G, Jing X. Association between levels of serum leptin and insulin resistance in patients with polycystic ovary syndrome. Chinese Journal of Epidemiology December 2014;35(12):1389‐91. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ayaz 2013a {published data only}
- Ayaz A, Alwan Y, Farooq MU. Efficacy of combined metformin–clomiphene citrate in comparison with clomiphene citrate alone in infertile women with polycystic ovarian syndrome (PCOS). Journal of Medicine and Life 2013;6(2):198‐201. [PMC free article] [PubMed] [Google Scholar]
Beigi 2006 {published data only}
- Beigi A. Randomized trial comparing clomiphene citrate and metformin as the first‐line treatment for ovulation induction in polycystic ovary syndrome. Human Reproduction 2006;21(i129):[Abstract]. [Google Scholar]
Jahan 2015 {published data only}
- Jahan S. Comparative study of efficacy among metformin, clomiphene citrate and aromatase inhibitor (letrozole) as the first‐line medication for ovulation induction, achievement of pregnancy and live birth in Asian women with polycystic ovarian syndrome: a prospective trial.. International Journal of Gynecology and Obstetrics 2015;131(E503):[Abstract]. [Google Scholar]
Robinson 2003 {published data only}
- Robinson RD, Swezey M, Propst A, Bates GW. Metformin added to clomiphene citrate does not improve pregnancy rates in hyperandrogenic, chronic anovulatory women: a randomized trial. Fertility and Sterility 2003;80(3):S273‐S274, P‐464 [Abstract]. [Google Scholar]
Singh 2001 {published data only}
- Singh I, Bedaiwy MA, Hatwal A, Kumar A, Agarwal A. Increased pregnancy rates with metformin and clomiphene citrate in non‐obese patients with polycystic ovary syndrome: prospective randomized study. Fertility and Sterility 2001;76(3):Suppl 1, SP‐S94 [Abstract]. [Google Scholar]
Williams 2009 {published data only}
- Williams CD, Pastore LM, Shelly WB, Bailey AP, Baras DC, Bateman BG. A randomized, placebo‐controlled study of the influence of instant‐release metformin on response to clomiphene citrate and time to conception in polycystic ovary syndrome. Fertility and Sterility 2009;92(3):S105 [Abstract]. [Google Scholar]
References to ongoing studies
NCT00005104 {published data only}
- NCT00005104. Randomized study of decreased hyperinsulinemia on the ovulatory response to clomiphene citrate in women with polycystic ovary syndrome. clinicaltrials.gov/ct2/show/NCT00005104 (first posted April 2000). [Google Scholar]
NCT00317928 {published data only}
- NCT00317928. Efficacy of metformin in PCOS: metabolic and hormonal factors. clinicaltrials.gov/ct2/show/NCT00317928 (first received April 2006). [Google Scholar]
NCT00558077 {published data only}
- NCT00558077. Second‐line treatments for anovulatory infertility in PCOS patients. clinicaltrials.gov/ct2/show/NCT00558077 (first posted November 2007). [Google Scholar]
NCT01679574 {published data only}
- NCT01679574. Letrozole or combined clomiphene citrate metformin as a first line treatment in women with polycystic ovarian syndrome (PCO). clinicaltrials.gov/ct2/show/NCT01679574 (first posted September 2012). [Google Scholar]
NCT02562664 {published data only}
- NCT02562664. Metformin improves clinical pregnancy rate in polycystic ovarian syndrome patients. clinicaltrials.gov/ct2/show/NCT02562664 (first posted September 2015). [Google Scholar]
Additional references
Abu Hashim 2016
- Abu Hashim H. Twenty years of ovulation induction with metformin for PCOS; what is the best available evidence?. Reproductive BioMedicine Online 2016;32:44‐53. [DOI] [PubMed] [Google Scholar]
Azziz 2005
- Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care‐related economic burden of the polycystic ovary syndrome during the reproductive life span. Journal of Clinical Endocrinology and Metabolism 2005;90:4650‐8. [DOI] [PubMed] [Google Scholar]
Azziz 2016
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro R, et al. Polycystic ovary syndrome. Nature 2016;2:1‐18. [DOI] [PubMed] [Google Scholar]
Balen 2014
- Balen AH. Infertility in Practice. 4th Edition. Boca Raton: CRC Press, 2014. [13:978‐1‐84184‐5] [Google Scholar]
Balen 2017
- Balen AH. Polycystic ovary syndrome (PCOS). Obstetrician & Gynaecologist 2017;19:119‐29. [Google Scholar]
Biljan 2005
- Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertility and Sterility 2005;84(Supp 1):S95. [Google Scholar]
Bordewijk 2017
- Bordewijk EM, Nahuis M, Costello MF, Veen F, Tso LO, Mol BW, et al. Metformin during ovulation induction with gonadotrophins followed by timed intercourse or intrauterine insemination for subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD009090.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bozdag 2016
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Human Reproduction 2016;31(12):2841‐55. [DOI] [PubMed] [Google Scholar]
Cassar 2016
- Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta‐analysis of euglycaemic‐hyperinsulinaemic clamp studies. Human Reproduction 2016;31(11):2619‐31. [DOI] [PubMed] [Google Scholar]
Cassina 2014
- Cassina M, Donà M, Gianantonio E, Litta P, Clementi M. First‐trimester exposure to metformin and risk of birth defects: a systematic review and meta‐analysis. Human Reproduction Update 2014;20:656‐69. [DOI] [PubMed] [Google Scholar]
Cedergren 2004
- Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics and Gynecology 2004;103:219‐24. [DOI] [PubMed] [Google Scholar]
Celik 2014
- Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M. Progression to impaired glucose tolerance or type 2 diabetes mellitus in polycystic ovary syndrome: a controlled follow‐up study. Fertility and Sterility 2014;101:1123‐8. [DOI] [PubMed] [Google Scholar]
Colca 2013
- Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell‐Conrad AS, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)‐‐relationship to newly identified mitochondrial pyruvate carrier proteins. PloS One 2013 [Epub ahead of print]; Vol. 8. [DOI] [PMC free article] [PubMed]
Costello 2003
- Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertility and Sterility 2003;79:1‐13. [DOI] [PubMed] [Google Scholar]
De Frene 2014
- Frène V, Vansteelandt S, T'Sjoen G, Gerris J, Somers S, Vercruysse L, et al. A retrospective study of the pregnancy, delivery and neonatal outcome in overweight versus normal weight women with polycystic ovary syndrome. Human Reproduction 2014;29:2333‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Di Petro 2015
- Petro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, et al. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015;156(4):1453‐63. [DOI] [PubMed] [Google Scholar]
Diamanti‐Kandarakis 2010
- Diamanti‐Kandarakis E, Christakou CD, Kandarakis E, Economou F. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. European Journal of Endocrinology 2010;162:193‐212. [DOI] [PubMed] [Google Scholar]
Diamond 2015
- Diamond MP. Letrozole, gonadotropin, or clomiphene for unexplained infertility. New England Journal of Medicine 2015;373(13):1230‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2017
- Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mutations in mitochondrial tRNA genes may be related to insulin resistance in women with polycystic ovary syndrome. American Journal of Translational Research 2017;9(6):2984‐96. [PMC free article] [PubMed] [Google Scholar]
ESHRE/ASRM 2004
- The Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction 2004;19(1):41. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM 2008
- The Thessaloniki ESHRE/ASRM‐sponsored PCOS consensus workshop group. Consensus on infertility treatment related to polycystic ovary syndrome. Human Reproduction 2008;23:462‐77. [DOI] [PubMed] [Google Scholar]
Farquhar 2012
- Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2012, (6):1‐73. [DOI] [PubMed] [Google Scholar]
Fauser 2012
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM‐sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility 2012;97:28‐38. [PUBMED: 22153789] [DOI] [PubMed] [Google Scholar]
FDA 2019
- FDA (Federal Drug Administration). www.fda.gov Accessed 12 November 2019.
Franik 2014
- Franik S, Kremer JA, Nelen W, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010287.pub2] [DOI] [PubMed] [Google Scholar]
Gillies 2007
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta‐analysis. BMJ 2007;334:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 2015‐2017. Hamilton (ON): McMaster University (developed by Evidence Prime).
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Janez 2018
- Janez A, Jensterle M. GLP‐1 receptor agonist liraglutide increased IVF pregnancy rates in obese women with PCOS and previous poor response to first‐line reproductive treatments. Diabetes 2018;67 Suppl 1:1465‐P. [DOI] [PubMed] [Google Scholar]
Jensterle 2014
- Jensterle M, Kocjan T, Pfeifer M, Janez A. Short‐term intervention with liraglutide improved eating behaviour in obese women with polycystic ovary syndrome. Endocrine Research 2014;40:133‐8. [DOI] [PubMed] [Google Scholar]
Kakoly 2018
- Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta‐regression. Human Reproduction Update 2018;24:455‐67. [DOI] [PubMed] [Google Scholar]
Knowler 2002
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Legro 2006a
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, et al. and Reproductive Medicine Network. The Pregnancy in Polycystic Ovary Syndrome Study: baseline characteristics of the randomized cohort including racial effects. Fertility and Sterility 2006;86(4):914‐33. [DOI] [PubMed] [Google Scholar]
Lord 2002
- Lord J, Wilkin T. Polycystic ovary syndrome and fat distribution: the central issue?. Human Fertility (Cambridge) 2002;5:67‐71. [DOI] [PubMed] [Google Scholar]
March 2010
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human Reproduction 2010;25:544‐51. [DOI] [PubMed] [Google Scholar]
Misso 2013
- Misso ML, Costello MF, Garrubba M, Wong J, Hart R, Rombauts L, et al. Metformin versus clomiphene citrate for infertility in non‐obese women with polycystic ovary syndrome: a systematic review and meta‐analysis. Human Reproduction Update 2013;19:2‐11. [DOI] [PubMed] [Google Scholar]
Moran 2010
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta‐analysis. Human Reproduction 2010;16:347‐63. [DOI] [PubMed] [Google Scholar]
Moran 2011
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007506.pub3] [DOI] [PubMed] [Google Scholar]
Moran 2012
- Moran LJ, Deeks AA, Gibson‐Helm ME, Teede HJ. Psychological parameters in the reproductive phenotypes of polycystic ovary syndrome. Human Reproduction 2012;27:2082‐8. [DOI] [PubMed] [Google Scholar]
NHMRC 2018
- NHRMC. International evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Monash University, Melbourne Australia 2018;1(https://www.monash.edu/medicine/sphpm/mchri/pcos/guideline):1‐199. [Google Scholar]
Palomba 2009
- Palomba S, Pasquali R, Orio Jr F, Nestler JE. Clomiphene citrate, metformin or both as first‐step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head‐to‐head randomized controlled studies and meta‐analysis. Clinical Endocrinology 2009;70:311‐21. [DOI] [PubMed] [Google Scholar]
Palomba 2010
- Palomba S, Falbo A, Battista L, Russo T, Venturella R, Tolino A, et al. Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second‐line strategy for infertile anovulatory patients with polycystic ovary syndrome: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(6):577.el‐8. [DOI] [PubMed] [Google Scholar]
Pernicova 2014
- Pernicova I, Korbonits M. Metformin‐‐mode of action and clinical implications for diabetes and cancer. Nature Reviews. Endocrinology 2014;10:143‐56. [DOI: 10.1038/nrendo.2013.256] [DOI] [PubMed] [Google Scholar]
Rautio 2006a
- Rautio K, Tapanainen JS, Ruokonen A, Morin‐Papunen LC. Endocrine and metabolic effects of rosiglitazone in overweight women with PCOS: a randomized placebo‐controlled study. Human Reproduction 2006;21:1400‐7. [DOI] [PubMed] [Google Scholar]
Rautio 2006b
- Rautio K, Tapanainen JS, Ruokonen A, Morin‐Papunen LC. Rosiglitazone treatment alleviates inflammation and improves liver function in overweight women with polycystic ovary syndrome: a randomized placebo‐controlled study. Fertility and Sterility 2007;87(1):202‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes‐Munoz 2016
- Reyes‐Munoz E, Ortega‐Gonzalez C, Martinez‐Cruz N, Arce‐Sanchez L, Estrada‐Gutierrez G, Moran C, et al. Association of obesity and overweight with the prevalence of insulin resistance, pre‐diabetes and clinical–biochemical characteristics among infertile Mexican women with polycystic ovary syndrome: a cross‐sectional study. BMJ Open 2016;6:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 2017
- Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2017;102(10):3848‐57. [DOI] [PubMed] [Google Scholar]
Sachdeva 2019
- Sachdeva G, Gainder S, Suri V, Sachdeva N, Chopra S. Obese and non‐obese polycystic ovarian response: comparison of clinical, metabolic, hormonal parameters, and their differential response to clomiphene. Indian Journal of Endocrinology and Metabolism 2019;23(2):257‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Siebert 2012
- Siebert TI, Viola MI, Steyn DW, Kruger TF. Is metformin indicated as primary ovulation induction agent in women with PCOS? A systematic review and meta‐analysis. Gynecologic and Obstetric Investigation 2012;73:304‐13. [DOI] [PubMed] [Google Scholar]
Silvestris 2018
- Silvestris E, Pergola G, Rosania R, Loverro G. Obesity as a disruptor of the female fertility. Reproductive Biology and Endocrinology 2018;16:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sim 2014
- Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obesity Reviews 2014;15:839‐50. [DOI] [PubMed] [Google Scholar]
Stein 1935
- Stein IF. Amenorrhea associated with bilateral polycystic ovaries. American Journal of Obstetrics and Gynecology 1935;29:181‐91. [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Teede 2018
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. International PCOS Network. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Fertility and Sterility 2018;110(3):364‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tso 2014
- Tso L, Costello MF, Albuquerque LE, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD006105.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uusitupa 2000
- Uusitupa M, Louheranta A, Lindstrom J, Valle T, Sundvall J, Eriksson J, et al. The Finnish Diabetes Prevention Study. British Journal of Nutrition 2000;83 Suppl 1:137‐42. [DOI] [PubMed] [Google Scholar]
Velazquez 1997
- Velazquez E, Acosta A, Mendoza SG. Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstetrics and Gynecology 1997;90:392‐5. [DOI] [PubMed] [Google Scholar]
Viollet 2012
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical Science (London, England : 1979) 2012;122(6):253‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017
- Wang R, Kim BV, Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta‐analysis. BMJ 2017;356:j:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019
- Wang R, Li W, Bordeqijk EM, Legro RS, Zhang H, Wu X, et al. First‐line ovulation induction for polycystic ovary syndrome: an individual participant data meta‐analysis. Human Reproduction Update 2019;25:717‐32. [DOI] [PubMed] [Google Scholar]
Zhang 2012
- Zhang Y, Ye J. Mitochondrial inhibitor as a new class of insulin sensitizer. Acta Pharmacologica Sinica 2012;2:341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lord 2003
- Lord JM, Flight IH, Norman RJ. Insulin‐sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D‐chiro‐inositol) for polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003053] [DOI] [PubMed] [Google Scholar]
Morley 2017
- Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003053.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2009
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003053.pub4; PUBMED: 20091537] [DOI] [PubMed] [Google Scholar]
Tang 2012
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003053.pub5] [DOI] [PubMed] [Google Scholar]


