Abstract
Background
Social anxiety disorder (SAD) is an incapacitating disorder running in families. Previous work associated social fearfulness with a failure to habituate, but the habituation response to neutral faces has, as of yet, not been investigated in patients with SAD and their family members concurrently. Here, we examined whether impaired habituation to neutral faces is a putative neurobiological endophenotype of SAD by using data from the multiplex and multigenerational Leiden Family Lab study on SAD.
Methods
Participants (n = 110; age, 9.2 – 61.5 years) performed a habituation paradigm involving neutral faces, as these are strong social stimuli with an ambiguous meaning. We used functional magnetic resonance imaging data to investigate whether brain activation related to habituation was associated with the level of social anxiety within the families. Furthermore, the heritability of the neural habituation response was estimated.
Results
Our data revealed a relationship between impaired habituation to neutral faces and social anxiety in the right hippocampus and right amygdala. In addition, our data indicated that this habituation response displayed moderate ‐ to‐moderately high heritability in the right hippocampus.
Conclusion
The present results provide support for altered habituation as a candidate SAD endophenotype; impaired neural habitation cosegregrated with the disorder within families and was heritable. These findings shed light on the genetic susceptibility to SAD.
Keywords: amygdala, endophenotypes, family research, FSL (RRID:SCR_002823), functional neuroimaging, hippocampus, phobia, social
1. INTRODUCTION
Social anxiety disorder (SAD) is a highly prevalent and incapacitating disorder with a genetic background (Isomura et al., 2015; Stein & Stein, 2008; Stein et al., 2017). The underlying neurobiology is still not fully elucidated, hampering progress in prevention and therapies. A potential neurobiological marker for SAD is the reactivity of the brain to novel stimuli, and, more specific, the change in this response over time called habituation. Habituation, which can be reliably established using functional magnetic resonance imaging (fMRI; Plichta et al., 2014), is the adaptive decrease in the automatic response to a novel stimulus presented multiple times without meaningful consequences (Ramaswami, 2014; Rankin et al., 2009). Several lines of evidence implicate impaired habituation in social anxiety (SA): a prolonged habituation response, for example, in the amygdala, has been linked to inhibited temperament (Blackford, Allen, Cowan, & Avery, 2013; Blackford, Avery, Cowan, Shelton, & Zald, 2011; Schwartz, 2003; Schwartz et al., 2012), a stable trait which is considered to be a risk factor for SAD (Clauss, Avery, & Blackford, 2015; Clauss & Blackford, 2012); furthermore, a study in a community sample of young adults revealed slower neural habituation of neutral faces in individuals with higher levels of social fearfulness (Avery & Blackford, 2016). These findings are further supported by work in nonhuman primates with an anxious temperament (cf., Fox & Kalin, 2014), and a recent study demonstrating that a sustained amygdala response to neutral stimuli predicts a worse response to attention bias modification treatment in transdiagnostic clinical anxiety (Woody et al., 2019). Together, these observations support the link between impaired habituation and the vulnerability for SA. Furthermore, they provide an initial evidence for the neural habituation response to neutral faces, which could be considered as strong social stimuli with an ambiguous meaning in social situations and as such as ecologically relevant in the context of SA, as a SA endophenotype. Endophenotypes are measurable heritable characteristics that constitute a causal connection between a certain genotype and a phenotype, and shed light on genetically based disease mechanisms (Bas‐Hoogendam et al., 2016; Gottesman & Gould, 2003). Importantly, not all disease‐related traits are endophenotypes; by definition, endophenotypes should be associated with the disorder (Criterion 1), state‐independent and already present in a preclinical state (Criterion 2), and heritable (Criterion 3). Furthermore, an endophenotype should cosegregate with the disorder within families of probands, with nonaffected family members showing altered levels of the endophenotype in comparison to the general population (Criterion 4; Glahn, Thompson, & Blangero, 2007; Lenzenweger, 2013; Puls & Gallinat, 2008). Nevertheless, as the neural habituation response has, as of yet, not been investigated in patients with SAD and their family members simultaneously, evidence with respect to the endophenotype criteria of cosegregation within families and heritability is currently lacking. Investigating these criteria is, however, of importance, given the genetic susceptibility to SAD and the typical onset of SAD during adolescence (Knappe, Beesdo‐Baum, & Wittchen, 2010).
In this study, we investigated neural habituation in two generations of families genetically enriched for SAD; these families were a part of the Leiden Family Lab study on SAD (LFLSAD), a unique neuroimaging study with a multiplex and multigenerational design which was especially designed to delineate putative endophenotype of SA (Bas‐Hoogendam, Harrewijn et al., 2018). Here, we examined whether impaired habituation cosegregated with SA within families (first element of Criterion 4); furthermore, the family data enabled establishing the heritability of the neural habituation response (Criterion 3). Based on the evidence summarized above, we predicted an association between SA and impaired neural habituation; furthermore, as genetic influences on the neural habituation response have been demonstrated (Lonsdorf et al., 2011; Perez‐Rodriguez et al., 2017; Piel et al., 2018; Wiggins, Swartz, Martin, Lord, & Monk, 2014), we expected the habituation response to be at least moderately (h 2 ≥ 0.20) heritable.
2. MATERIALS AND METHODS
2.1. Participants
Participants (n = 110; eight families) originated from the LFLSAD (Figure 1a), families within the LFLSAD were invited based on the combination of a primary diagnosis of SAD in a parent (age 25–55 years; “proband”) and a child who met criteria for clinical or subclinical SAD (age 8–21 years and living at home with the proband; “proband's SA‐child”; Figure 1a). Together with these two SAD cases, first‐ and second‐degree family members of two generations were invited to participate, being the proband's partner and other children of the nuclear family (age ≥8 years; no upper age limit), as well as the proband's sibling(s) with their partners and children (age ≥8 years; no upper age limit). A detailed description of the study design, the exclusion criteria, the recruitment procedure, and an a priori power calculation are provided elsewhere (Bas‐Hoogendam, Harrewijn et al., 2018) and described in the Supporting Information; furthermore, a preregistration is publicly available (Bas‐Hoogendam et al., 2014a, 2014b). The study was approved by the Medical Ethical Committee of the Leiden University Medical Center and participants provided informed consent according to the Declaration of Helsinki. All participants completed a number of measurements, such as a diagnostic interview, self‐report questionnaires, and a magnetic resonance imaging (MRI) scan (Bas‐Hoogendam, Harrewijn et al., 2018).
Figure 1.
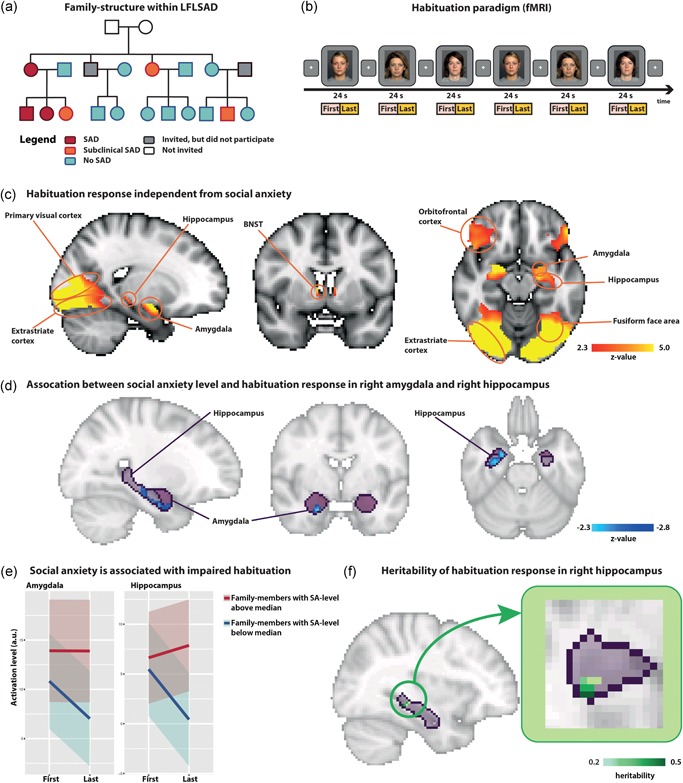
Failure to habituate within families genetically enriched for social anxiety disorder. (a) The LFLSAD sample comprises families who were invited to participate based on the combination of a primary diagnosis of SAD in a parent (age 25–55 years old; “proband”; depicted in red) and a proband's child with SAD (red) or subclinical SAD (orange). Furthermore, family members of two generations were invited (age ≥8 years), independent from the presence of SAD within these family members (no SAD: light blue; did not participate: gray). Grandparents (white) were not invited to participate. Squares and circles represent men and women, respectively. This figure is a modified reprint of Figure 1 of Bas‐Hoogendam, Harrewijn et al. (2018). (b) The habituation paradigm during functional (f)MRI scanning. (c) Significant habituation responses (brain activation “first half > last half”) in the bilateral amygdala, BNST, hippocampus, primary visual cortex, fusiform face area, extrastriate cortex, and orbitofrontal cortex (n = 105). Coordinates of displayed slices (MNI, x, y, z): 26, 2, −26 (left and right images); 24, −2, −26 (middle image). Images are displayed according to radiological convention: right in the image is left in the brain. (d) Negative association between SA‐level and habituation in the right amygdala and right hippocampus. Coordinates of displayed slices (MNI, x, y, z): 26, −10, −26 (left and right images); 26, −2, −20 (middle image). (e) SA‐level was positively related with brain activation levels during the presentation of the faces in the last half of the blocks, while there was no correlation between SA and activation during the first half of the presentation blocks. (f) Heritability of brain activation in the right hippocampus. Coordinates of displayed slices (MNI, x, y, z): 34, − 34, − 8. BNST, bed nucleus of stria terminalis; fMRI, functional magnetic resonance imaging; LFLSAD, Leiden Family Lab study on SAD; MNI, montreal neurological institute; MRI, magnetic resonance imaging; SA, social anxiety; SAD, social anxiety disorder
2.2. Data acquisition and analyses
2.2.1. Phenotyping
Experienced clinicians confirmed the presence of clinical SAD, subclinical SAD (hereafter, the term “(sub)clinical SAD” will be used to refer to both clinical and subclinical SAD), and other diagnostic and statistical manual of mental disorders (DSM‐IV) diagnoses using the Mini‐International Neuropsychiatric Interview (M.I.N.I.)‐Plus or the M.I.N.I.‐Kid interview. Clinical SAD was established using the DSM‐IV‐TR criteria for the generalized subtype of SAD, while the interviewer verified whether the DSM‐5 criteria for SAD were also met. A diagnosis of subclinical SAD was established when participants met the criteria for SAD as described in the DSM‐5, but did not show impairing limitations in important areas of functioning.
Furthermore, participants filled out age‐matched questionnaires on SA symptoms, being the Liebowitz SA scale (participants ≧18 years of age) or the SA scale for adolescents (participants <18 years of age; Fresco et al., 2001; La Greca & Lopez, 1998). To use these scores over the whole sample, z‐scores were computed as described elsewhere (Bas‐Hoogendam, Harrewijn et al., 2018). We refer the reader to the Supporting Information for an extended characterization of the LFLSAD sample.
2.2.2. Data analysis: Demographics and clinical characteristics
Incidental missing values on the questionnaires were replaced by the average value of the completed items. Participants with and without (sub)clinical SAD were compared by fitting regression models in R (R Core Team, 2016), with (sub)clinical SAD as the independent variable and the level of self‐reported SA (z‐score) as dependent variable. Gender and age were included as covariates; genetic correlations between family members were modeled by including random effects.
2.2.3. Habituation paradigm during fMRI
The habituation paradigm was a part of a larger scan protocol (total duration of MRI protocol: 54 min 47 s) consisting of structural scans (Bas‐Hoogendam, van Steenbergen et al., 2018) and functional task paradigms (Bas‐Hoogendam, van Steenbergen, Kreuk, van der Wee, &Westenberg, 2017; Bas‐Hoogendam, van Steenbergen, Tissier, van der Wee, & Westenberg, 2019). Details on the MRI experiment (3.0‐T Philips MRI scan) are provided in the Supporting Information Methods.
During the habituation paradigm, three neutral faces from the FACES database (Ebner, Riediger, & Lindenberger, 2010) were repeatedly presented (Figure 1b); see Supporting Information Methods for the selected faces. We chose neutral faces as they have an ambiguous meaning in a social context, leading to amygdala activation in both people with and without social fear (Whalen, 2007); thereby, these faces offer the best starting point for studying differential habituation patterns.
The habituation paradigm started with the presentation of a fixation cross (24 s) followed by the presentation of the neutral faces. The faces were presented in blocks of 24 s; within each block, a neutral face was repeatedly presented (48 times) for 200 ms with a 300 ms interstimulus interval. There were six face blocks (two blocks for each face) to resemble the design described previously (Wedig, Rauch, Albert, & Wright, 2005), and face blocks were separated by the presentation of a fixation cross (duration 12 s). An additional 12 s fixation cross was presented at the end of the paradigm. Gender‐matched faces were presented in pseudorandom order, and participants were instructed to keep looking at the faces and the fixation crosses. Before the acquisition of the fMRI data, participants rated the three faces on likeability and arousal (Supporting Information Methods). Association analyses on the likeability ratings indicated that they were not significantly related to SA‐level, ruling out that potential differences in habituation would be (partly) due to differences in threat valence assigned to the stimuli (cf., Yoon & Zinbarg, 2008).
2.2.4. fMRI data: Habituation response
fMRI data were prepreprocessed following standard procedures using FSL (RRID:SCR_002823) described in the Supporting Information Methods. Event‐related statistical analyses were performed in native space using FMRIB's improved linear model (FILM) with local autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001); in the general linear model, we included regressors modeling the presentation of the faces during the first half and last (second) half of the blocks (Figure 1b). Regressors were convolved with a canonical double‐gamma hemodynamic response function; furthermore, their temporal derivatives were included. We investigated the habituation by using the contrast “first half > last half” and applied a hypothesis‐driven region‐of‐interest (ROI) approach focusing on the regions described by Avery & Blackford (2016), being the amygdala, hippocampus, ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex, fusiform face area (FFA), primary visual cortex (V1), and extrastriate visual cortex; we added the bed nucleus of stria terminalis (BNST) given its role in anxiety (Avery, Clauss, & Blackford, 2015; Clauss, Avery, Benningfield, & Blackford, 2019; Figel et al., 2019). Specifics on these ROIs are available in the Supporting Information Online. We established in which ROIs habituation was present at the group level (cluster threshold, z > 2.3 and extent threshold, p < .05) and used these ROIs for the subsequent endophenotype analysis.
2.2.5. fMRI data: Endophenotype analysis
We examined the “cosegregation of the habituation response with the disorder within families” within the ROIs showing significant habituation‐related activation. We used voxelwise multivariable regression models in R; that is, for each voxel, we estimated the strength of the association between self‐reported SA‐level (z‐score, centered; predictor) and brain activation related to the contrast “first half > last half” (outcome variable); within these models, correlations between family members were modeled by including random effects using the R‐function lmekin, which created a kinship matrix representing the family structure (package coxme) and age and gender were included as covariates. Results (z‐scores) were transformed into a Niftin image with the same dimensions of the MNI T1‐template brain. Clusters within these images, mirroring the relation between SA and brain activation, were corrected for multiple comparisons within each bilateral ROI mask using the FSL‐tool easythresh (cluster threshold, z > 2.3, corresponding to p < .01, and extent threshold, p < .05; Woo, Krishnan, & Wager, 2014; Worsley, 2001). Given the fact that the ROIs were a priori defined and are functionally related as they are all part of a network of brain areas involved in social information processing (Avery & Blackford, 2016) and the innovative nature of the present study (to the best of our knowledge, this is the first comprehensive family study on SA), we report findings uncorrected for the number of ROIs. Results on a whole‐brain analysis are reported in the Supporting Information Results.
Next, we determined the heritability of brain activation for voxels in the significant clusters. Voxelwise heritability estimates were obtained using the statistical model developed by Tissier, Tsonaka, Mooijaart, Slagboom, and Houwing‐Duistermaat (2017). This method uses a multivariate‐mixed probit model in which the ascertainment of the families (based on SAD in the proband and (sub)clinical SAD in the proband's SA‐child) and the familial relationship are taken into account by jointly modeling SAD status in these participants and brain activation. To adjust for age and gender, these variables were included as covariates (both centered) in the marginal regression models. Variance of the random effects was determined using maximum‐likelihood estimates; subsequently, heritability was computed (Tissier et al., 2017). For reasons of completeness, we also performed analyses with (sub)clinical SAD as a discrete predictor, as well as sensitivity analyses on the effect of (comorbid) psychopathology other than SAD and the influence of depressive symptoms, and a sensitivity analysis excluding participants taking psychotropic medication (Supporting Information Methods and Results).
3. RESULTS
3.1. Demographics and clinical characteristics
Sample characteristics (n = 105 after quality control; see Supporting Information Results) are summarized in Table 1. Family members with (sub)clinical SAD were more often diagnosed with depression (past) and dysthymia (present), but these differences were only significant at an uncorrected significance level (cf., Bas‐Hoogendam, van Steenbergen et al., 2019). For a detailed phenotyping of the LFLSAD sample, we refer the reader to Supporting Information Results.
Table 1.
Characteristics of participants within the LFLSAD
| (Sub)clinical SAD (n = 37) a | No SAD (n = 61) | Statistical analysis | |
|---|---|---|---|
| Demographics | |||
| Male/female (n) | 18/19 | 31/30 | χ 2(1) = 0.04, p = 0.84 b |
| Generation 1/Generation 2 (n) | 19/18 | 27/34 | χ 2(1) = 0.47, p = 0.50 b |
| Age in years (mean ± SD, range) | 31.3 ± 15.2, 9.2–59.6 | 31.6 ± 15.2, 9.4–61.5 | β ± SE = −0.3 ± 3.1, p = 0.93 c |
| Estimated IQ (mean ± SD) | 103.8 ± 12.0 | 105.5 ± 10.5 | β ± SE = −2.0 ± 2.2, p = 0.36 c |
| Diagnostic information (n) | |||
| Clinical SAD | 17 | 0 | χ 2(1) = 33.9, p < .001 b |
| Depressive episode present | 1 | 1 | χ 2(1) = 0.2, p = .69 b |
| Depressive episode past | 12 | 9 | χ 2(1) = 4.9, p = .03 b |
| Dysthymia present | 3 | 0 | χ 2(1) = 5.4, p = .02 b |
| Dysthymia past | 1 | 1 | χ 2(1) = 0.2, p = .65 b |
| Panic disorder lifetime | 5 | 2 | χ 2(1) = 4.0, p = .05 b |
| Agoraphobia present | 3 | 2 | χ 2(1) = 1.3, p = .26 b |
| Agoraphobia past | 0 | 2 | χ 2(1) = 1.2, p = .28 b |
| Separation anxiety | 0 | 1 | χ 2(1) = 0.8, p = .38 b |
| Specific phobia | 2 | 3 | χ 2(1) = 0.02, p = .89 b |
| Generalized anxiety disorder | 1 | 0 | χ 2(1) = 1.8, p = .19 b |
| Obsessive‐compulsive disorder | 1 | 0 | χ 2(1) = 1.8, p = .19 b |
| Attention deficit hyperactivity disorder | 3 | 1 | χ 2(1) = 2.5, p = .11 b |
| Alcohol dependency present | 1 | 1 | χ 2(1) = 0.2, p = .70 b |
| Alcohol dependency lifetime | 1 | 3 | χ 2(1) = 0.2, p = .62 b |
| Present psychotropic medication | 4 | 3 | χ 2(1) = 1.1, p = .30 b |
| Antidepressants | 3 | 0 | |
| ADHD medication (methylphenidate) | 1 | 3 | |
| Self‐report measures | |||
| Social anxiety symptoms (z‐score; mean ± SD) | 2.9 ± 3.3 | 0.6 ± 1.5 | β ± SE = 2.5 ± 0.5, p < .001 c |
Abbreviations: ADHD, attention deficit/hyperactivity disorder; LFLSAD, Leiden Family Lab study on SAD; SA, social anxiety; SAD, social anxiety disorder; SD, standard deviation; SE, standard error.
Due to technical reasons, data on the presence of subclinical SAD were lost for seven family members. Data from these participants were, however, included in the endophenotype analyses using SA‐level (z‐score) as a predictor (n = 105).
χ 2 Tests in SPSS (version 25).
Regression models in R (https://www.r-project.org), in which genetic correlations between family members were modeled by including random effects.
3.2. fMRI analyses
3.2.1. Habituation response
Analyses over the whole sample revealed significant habituation responses (brain activation related to the contrast “first half > last half”) within most of the ROIs including the bilateral amygdala, BNST, hippocampus, V1, FFA, extrastriate cortex, and orbitofrontal cortex (Figure 1c; Table 2), confirming the effectiveness of the paradigm for studying the neural correlates of the habituation response (see Figure S1 for an illustration of activation levels over time). No significant habituation was present in the vmPFC.
Table 2.
Neural habituation in regions of interest (ROIs) at the group level
| Peak coordinates (MNI space) | ||||||
|---|---|---|---|---|---|---|
| ROI | Left/right | z‐score | x | y | z | Cluster size |
| Amygdala | Left | 5.26 | −20 | −12 | −12 | 191 |
| Right | 5.88 | 20 | −4 | −12 | 225 | |
| BNST | Left | 3.53 | −8 | 4 | 6 | 5 |
| Right | 4.5 | 8 | 4 | 6 | 9 | |
| Extrastriate cortex | Left/right | 9.49 | −30 | −86 | −14 | 8,736 |
| FFA | Left | 8.99 | −26 | −84 | −18 | 964 |
| Right | 8.67 | 30 | −78 | −2 | 1,200 | |
| Hippocampus | Left | 5.26 | −20 | −12 | −12 | 244 |
| Right | 5.88 | 20 | −4 | −12 | 233 | |
| Orbitofrontal cortex | Left | 4.76 | −52 | 36 | −12 | 952 |
| Right | 5.44 | 34 | 26 | −26 | 1,205 | |
| V1 | Left/right | 9.42 | 6 | −90 | 0 | 2,880 |
| vmPFC | No significant clusters | |||||
Abbreviations: BNST, bed nucleus of stria terminalis; FFA, fusiform face area; MNI, montreal neurological institute, V1, primary visual cortex; vmPFC, ventromedial prefrontal cortex.
3.2.2. Endophenotype analyses
Voxelwise regression analyses revealed that SA‐level was associated with reduced neural habituation in the right amygdala (cluster characteristics: 27 voxels, p = .013; max z‐value = 2.82) and right hippocampus (cluster characteristics: 136 voxels, p = .04; max z‐value = 3.13; Figure 1d). Follow‐up analyses (details included in Supporting Information Results) indicated that family members with higher SA‐levels showed a failed habituation response, and not a heightened novelty response (Figure 1e). To specify, SA‐level was positively related with brain activation levels during the presentation of the faces in the last half of the blocks (amygdala: β ± standard error [SE] = 1.23 ± 0.67, p = .07, and standardized effect size = 0.07; hippocampus: β ± SE = 1.53 ± 0.57, p = .007, and standardized effect size = 0.1), while there was no relation between SA and activation during the presentation of the faces in the first half of the blocks (amygdala: β ± SE = −0.39 ± 0.72, p = 0.59, and standardized effect size = −0.02; hippocampus: β ± SE = −0.25 ± 0.72, p = 0.73, and standardized effect size = −0.01; regression analyses corrected for age, gender, and family structure; see Supporting Information Results for a more detailed analysis on brain activation separately for the first and second blocks of face presentation). There were no SA‐related alterations in neural habituation in the other ROIs, nor did we find an association between (sub)clinical SAD (discrete predictor) and the habituation response (Supporting Information Results).
Voxelwise heritability analyses within the clusters showing an association with SA‐level revealed that the neural habituation response within the right hippocampus was heritable with 13 voxels showing at least moderate heritability (h 2 > 0.20; Figure 1f). In other ROIs, no association with SA was present at the predefined significance level.
4. DISCUSSION
The present findings provide evidence that altered habituation is an endophenotype of SAD. First, we showed that impaired habituation to neutral faces in neural structures supporting threat (amygdala) and memory‐related processes (hippocampus) is associated with SA within families genetically enriched for SAD, supporting the endophenotype criterion of cosegregration within families (Criterion 4, first element). Next, our data indicated that the habituation response to neutral faces in the hippocampus is partly heritable (endophenotype Criterion 3). Thereby, these results from the multiplex multigenerational LFLSAD add substantially to prior work indicating an association between impaired habituation and SA (endophenotype Criterion 1) and studies on the trait stability of the habituation response (endophenotype Criterion 2; Avery & Blackford, 2016; Blackford et al., 2013, 2011; cf., Bas‐Hoogendam et al., 2016), and shed light on the genetic pathways leading to SAD.
4.1. Impaired habituation in families genetically enriched for SAD
As habituation is an adaptive process, reflecting a basic nonassociative learning mechanism that acts like “an intelligent firewall” that filters out irrelevant sensory information (Poon & Young, 2006), the failure to habituate likely contributes to the feelings of uncertainty that characterize individuals with high SA‐levels: at the neurobiological level, these individuals keep considering neutral social stimuli as being alarming, which makes them feel uncomfortable in social situations and contributes to aberrant social behavior. Although previous neuroimaging studies on habituation in patients with SAD yielded divergent results (Campbell et al., 2007; Sladky et al., 2012), potentially due to differences in task characteristics (cf., the extended discussion in Bas‐Hoogendam et al. (2016)), our findings are in line with work on participants with high levels of behavioral inhibition (Blackford et al., 2013; Schwartz, 2003; Schwartz et al., 2012), as well as with the results of a study in individuals with high levels of social fearfulness (Avery & Blackford, 2016). Interestingly, impairments in neural habituation have also been reported in other neuropsychiatric disorders in which social behavior is altered, like autism and schizophrenia (Blackford, Williams, & Heckers, 2015; Kleinhans et al., 2009; Williams, Blackford, Luksik, Gauthier, & Heckers, 2013; and review by McDiarmid, Bernardos, & Rankin, 2017), indicating that impaired habituation is not specifically related to SA. However, as argued more extensively in Bas‐Hoogendam et al. (2016), specificity is not a prerequisite for an endophenotype, as endophenotypes that are related to more than one disorder could advance transdiagnostic research on the shared genetic background of these disorders (Bearden & Freimer, 2006).
4.2. Habituation response in hippocampus, but not the amygdala, is heritable
The dissociation in heritability of the habituation response between the amygdala and hippocampus was unexpected, as previous studies indicated genetic influences on both hippocampus activation (Kauppi, Nilsson, Persson, & Nyberg, 2014) and amygdala reactivity (Lonsdorf et al., 2011; Munafò, Brown, & Hariri, 2008; Murphy et al., 2013). However, the results presented here are in line with findings from a multigenerational family study in rhesus monkeys, revealing the significant heritability of metabolic activity predictive of anxious temperament in hippocampal regions, but not in the amygdala (Oler et al., 2010). Together, these findings suggest that the impaired habituation response in the amygdala, although associated with SA, does not meet the endophenotype criterion of heritability, illustrating the distinction between disease‐related neurobiological traits (biomarkers) and endophenotypes with the latter having a genetic link with the disorder (cf., Lenzenweger, 2013) and underscoring the value of studies using a family design (Glahn et al., 2018). Furthermore, these findings support the notion that both genes and environment play a role in the development of SAD (Bas‐Hoogendam, Roelofs, Westenberg, & van der Wee, 2019) and indicate that research on the interaction between these factors is important.
4.3. Habituation in other ROIs
Although the brain response to neutral faces habituated in several ROIs besides the amygdala and hippocampus, namely in the BNST, extrastriate cortex, FFA, orbitofrontal cortex, and V1, we did not find an association with SA within these regions. Thereby, we could not replicate previous work demonstrating a relationship between social fearfulness and a slower habituation response to neutral faces in the orbitofrontal cortex, FFA, extrastriate cortex, and V1 (Avery & Blackford, 2016). It should, however, be noted that this study employed a task paradigm in which the neutral faces were presented one, three, five, or seven times; as a result, their design allowed for the investigation of habituation within specific repetition windows, for example, from first‐to‐third presentation, third‐to‐fifth presentation, and fifth‐to‐seventh presentation (Avery & Blackford, 2016). Interestingly, the effect of social fearfulness on habituation in the hippocampus was present over the whole paradigm in line with our findings. The effects in the other ROIs were, however, only present in specific time windows (first‐to‐third and third‐to‐fifth presentations) which, arguably, could explain why we did not find associations with SA within these regions in the present study. Future studies, using the same task parameters and analysis methods as described by Avery and Blackford (2016), are needed to explore whether the associations between social fearfulness and neural habituation at specific timing intervals are also present in families genetically enriched for SAD.
4.4. Clinical implications
In addition to providing insight into the genetic susceptibility to SAD, our results might have potential clinical relevance, for example, when considering the effect of exposure therapy. Exposure therapy, targeted at diminishing anxiety levels by repeated confrontations with the feared stimulus (i.e., a social situation), is often applied in SAD as a part of cognitive‐behavioral therapy, with placebo‐controlled trials typically showing only moderate effects (Carpenter et al., 2018; Klumpp & Fitzgerald, 2018). The effect of exposure therapy is thought to rely (at least partly) on habituation responses, but it is important to note that fear extinction, defined as the decrease in fear during repeated exposure to a previously conditioned stimulus, which is now presented in the absence of an unconditioned stimulus, also plays an essential role during exposure therapy (Craske, 2015; Myers & Davis, 2006; Pittig, van den Berg, & Vervliet, 2016). In this study, we did not include active conditioning and fear extinction of the neutral faces; however, a recent research paper on adults with speaking anxiety indicated that less amygdala activation during extinction learning predicted greater reduction in SA symptoms 2 weeks after a session of exposure (Ball, Knapp, Paulus, & Stein, 2017), while another study in patients with SAD indicated that a decrease in regional cerebral blood flow in the amygdala was associated with anxiety reduction following repeated stress exposure (Åhs, Gingnell, Furmark, & Fredrikson, 2017). Furthermore, a meta‐analysis showed a positive association between both within‐session as well as between‐session habituations on the one hand, and treatment outcome on the other hand (Rupp, Doebler, Ehring, & Vossbeck‐Elsebusch, 2017). These results suggest that impaired habituation might have a negative consequence on the outcome of exposure therapy, but more research is needed to test this hypothesis. In this light, the role of inhibitory learning is also relevant: inhibitory learning, involving the amygdala, hippocampus, as well as the prefrontal cortex, and aimed at inhibiting the original feared association by a newly formed association representing safety, has been proposed as an alternative mechanism underlying exposure therapy (Craske, Liao, Brown, & Vervliet, 2012; Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). We hypothesize that a focus on inhibitory learning in exposure therapy might yield better outcomes in patients with anxiety with impaired habituation responses, but as the current study did not involve explicit learning of the meaning of the faces, future studies, in which patients with SAD are trained to interpret neutral faces in a more positive way, are needed to test this hypothesis.
4.5. Limitations and future studies
As the LFLSAD had a cross‐sectional design and was intended to investigate the endophenotype criteria with respect to cosegregation and heritability (Bas‐Hoogendam, Harrewijn et al., 2018), we were not able to establish the trait stability of the candidate endophenotype (endophenotype Criterion 2), nor could we examine the difference in neural habituation between nonaffected family members within the sample and participants from the general population (endophenotype Criterion 4, second element). To investigate whether neural habituation meets these criteria, longitudinal studies, including families enriched for SAD, as well as control families from the general population, are necessary. Furthermore, case‐control studies on participants with and without clinical SAD are essential to provide further support for the endophenotype criterion of “association with the disorder.”
In addition, it should be noted that we employed a hypothesis‐driven ROI approach, which showed an association between SA‐level and neural habituation in the amygdala and hippocampus ROI at a relatively lenient, but fairly common statistical threshold. These results did, however, not survive thresholding at the whole‐brain level (cf., Supporting Information Results) and had small effect sizes, emphasizing the need for replication studies (Blackford, 2017). Despite these limitations, we want to stress the unique character of the LFLSAD, as the family design enabled us to extend previous studies on habituation in unrelated individuals (Avery & Blackford, 2016; Blackford et al., 2013; Schwartz et al., 2012) by providing evidence for two important endophenotype criteria (cosegregation and heritability). Thereby, the present findings indicate that impaired neural habituation is not just a biomarker (i.e., associated with the disorder, but not necessarily involved in the mechanistic pathway from genotype to phenotype Lenzenweger (2013)), but could be seen as a promising candidate endophenotype, which provides information about the genetic vulnerability to develop SAD (Bas‐Hoogendam et al., 2016). In this light, the present promising results paved the way for future analyses on the genetics underlying neural habituation: We did collect genetic data on the LFLSAD sample (Bas‐Hoogendam, Harrewijn et al., 2018), but we have not yet investigated whether specific genetic variations or epigenetic changes (cf., Alisch et al., 2014; Dannlowski et al., 2011; Domschke et al., 2012; Ziegler et al., 2015) are associated with the impaired neural habituation response. Such an investigation would be the following stage in disentangling the genetic vulnerability to SAD. In addition, the present study employed only neutral faces, in line with previous work (Avery & Blackford, 2016; Schwartz et al., 2003; Wedig et al., 2005), and although there was no association between SA‐level and ratings of likeability before the start of the habituation paradigm, it is possible that the altered SA‐related habituation response is specific for faces with a neutral expression. Future studies could evaluate faces with different emotional valences to explore this possibility. Furthermore, it should be noted that our definition of habituation (first half > last half), although frequently used in neuroimaging research (see e.g., the work of Fischer et al. (2003); Kleinhans et al. (2009); Swartz, Wiggins, Carrasco, Lord, & Monk (2013); Wright et al. (2001)), did not allow for a very precise investigation of the neural response over time; such an investigation has been provided by cellular work on mammals and invertebrates (Ramaswami, 2014). We recommend future studies to thoroughly test habituation, using more elaborate designs involving multiple blocks, to examine the temporal dynamics of the neural response in more detail, and to enable testing whether dishabituation is present (i.e., after presentation of a new stimulus, an increase in the habituated response to the original stimulus can be measured; McDiarmid et al. (2017)).
Finally, given work reporting changes in functional and structural connectivity of the amygdala in SAD (Brühl, Delsignore, Komossa, & Weidt, 2014), as well as studies reporting on age‐related differences in habituation (Wedig et al., 2005), future studies should explore whether these aberrant connectivity patterns meet criteria for being candidate SAD endophenotypes and how the SA‐related alterations in the neural habituation response are influenced by age.
5. CONCLUSION
The findings reported here support the hypothesis that impaired neural habituation to neutral faces is a promising neural candidate endophenotype of SAD, as our data revealed that impaired habituation to neutral faces, expressed as a prolonged response to these faces in the right hippocampus and right amygdala, cosegregated with SA within families of probands. Next, our data indicated that brain activation related to habituation displayed moderate‐to‐moderately high heritability in the right hippocampus providing support for the endophenotype criterion of heritability. Thereby, the present results offer novel insights in the neurobiological pathways leading to SAD.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The LFLSAD and J. M. B.‐H. were funded by Leiden University Research Profile “Health, Prevention and the Human Life Cycle” and the Institute of Psychology of Leiden University. These funding sources had no involvement in writing this paper nor in the decision to submit this study for publication. J. M. B.‐H. had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Bas‐Hoogendam JM, van Steenbergen H, Blackford JU, Tissier RL, van der Wee NJ, Westenberg PM. Impaired neural habituation to neutral faces in families genetically enriched for social anxiety disorder. Depress Anxiety. 2019;36:1143–1153. 10.1002/da.22962
Present address Renaud L. M. Tissier, Department of Epidemiology and Biostatistics, Vrije Universiteit Medical Center, Amsterdam, The Netherlands.
References
REFERENCES
- Åhs, F. , Gingnell, M. , Furmark, T. , & Fredrikson, M. (2017). Within‐session effect of repeated stress exposure on extinction circuitry function in social anxiety disorder. Psychiatry Research: Neuroimaging, 261, 85–90. 10.1016/j.pscychresns.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Alisch, R. S. , Chopra, P. , Fox, A. S. , Chen, K. , White, A. T. J. , Roseboom, P. H. , … Kalin, N. H. (2014). Differentially methylated plasticity genes in the amygdala of young primates are linked to anxious temperament, an at risk phenotype for anxiety and depressive disorders. The Journal of Neuroscience, 34(47), 15548–15556. 10.1523/JNEUROSCI.3338-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , & Blackford, J. U. (2016). Slow to warm up: The role of habituation in social fear. Social Cognitive and Affective Neuroscience, 11(11), 1832–1840. 10.1093/scan/nsw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , & Blackford, J. U. (2015). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41(1), 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, T. M. , Knapp, S. E. , Paulus, M. P. , & Stein, M. B. (2017). Brain activation during fear extinction predicts exposure success. Depression and Anxiety, 34(3), 257–266. 10.1002/da.22583 [DOI] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , Blackford, J. U. , Brühl, A. B. , Blair, K. S. , van der Wee, N. J. A. , & Westenberg, P. M. (2016). Neurobiological candidate endophenotypes of social anxiety disorder. Neuroscience & Biobehavioral Reviews, 71, 362–378. 10.1016/j.neubiorev.2016.08.040 [DOI] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , Harrewijn, A. , van der Molen, M. J. W. , van Steenbergen, H. , van Vliet, I. , Houwing‐Duistermaat, J. , & Westenberg, P. M. (2014a). Preregistration: General background and key question of project, part of “profiling endophenotypes in social anxiety disorder: A neurocognitive approach” Retreived from 10.17605/OSF.IO/E368H [DOI]
- Bas‐Hoogendam, J. M. , Harrewijn, A. , van der Molen, M. J. W. , van Steenbergen, H. , van Vliet, I. , Houwing‐Duistermaat, J. , & Westenberg, P. M. (2014b). Preregistration: Methods: Study design and sample. Part of “profiling endophenotypes in social anxiety disorder: A neurocognitive approach” Retreived from 10.17605/OSF.IO/AQ3SV [DOI]
- Bas‐Hoogendam, J. M. , Harrewijn, A. , Tissier, R. L. M. , van der Molen, M. J. W. , van Steenbergen, H. , van Vliet, I. M. , … Westenberg, P. M. (2018). The Leiden Family Lab study on social anxiety disorder: A multiplex, multigenerational family study on neurocognitive endophenotypes. International Journal of Methods in Psychiatric Research, 27(2), e1616 10.1002/mpr.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , Roelofs, E. F. , Westenberg, P. M. , & van der Wee, N. J. A. (2019). Pathogenesis of SAD In Simon N., Hollander E., Rothbaum B. O., & Stein D. J. (Eds.), The American Psychiatric Association textbook of anxiety, trauma and OCD related disorders. Washington DC: American Psychiatric Association Publishing. [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Kreuk, T. , van der Wee, N. J. A. , & Westenberg, P. M. (2017). How embarrassing! The behavioral and neural correlates of processing social norm violations. PLoS One, 12(4), e0176326 10.1371/journal.pone.0176326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Tissier, R. L. M. , Houwing‐Duistermaat, J. J. , Westenberg, P. M. , & van der Wee, N. J. A. (2018). Subcortical brain volumes, cortical thickness and cortical surface area in families genetically enriched for social anxiety disorder—A multiplex multigenerational neuroimaging study. EBioMedicine, 36, 410–428. 10.1016/j.ebiom.2018.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Tissier, R. , van der Wee, N. , & Westenberg, P. M. (2019). Altered neurobiological processing of unintentional social norm violations: A multiplex, multigenerational functional magnetic resonance imaging study on social anxiety endophenotypes. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 10.1016/j.bpsc.2019.03.003. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Bearden, C. , & Freimer, N. (2006). Endophenotypes for psychiatric disorders: Ready for primetime? Trends in Genetics, 22(6), 306–313. 10.1016/j.tig.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Blackford, J. U. (2017). Leveraging statistical methods to improve validity and reproducibility of research findings. JAMA Psychiatry, 74(2), 119–120. 10.1001/jamapsychiatry.2016.3730 [DOI] [PubMed] [Google Scholar]
- Blackford, J. U. , Allen, A. H. , Cowan, R. L. , & Avery, S. N. (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8(2), 143–150. 10.1093/scan/nsr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford, J. U. , Avery, S. N. , Cowan, R. L. , Shelton, R. C. , & Zald, D. H. (2011). Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience, 6(5), 621–629. 10.1093/scan/nsq073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford, J. U. , Williams, L. E. , & Heckers, S. (2015). Neural correlates of out‐group bias predict social impairment in patients with schizophrenia. Schizophrenia Research, 164(1–3), 203–209. 10.1016/j.schres.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl, A. B. , Delsignore, A. , Komossa, K. , & Weidt, S. (2014). Neuroimaging in social anxiety disorder—A meta‐analytic review resulting in a new neurofunctional model. Neuroscience and Biobehavioral Reviews, 47, 260–280. 10.1016/j.neubiorev.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Campbell, D. W. , Sareen, J. , Paulus, M. P. , Goldin, P. R. , Stein, M. B. , & Reiss, J. P. (2007). Time‐varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry, 62(5), 455–463. 10.1016/j.biopsych.2006.09.017 [DOI] [PubMed] [Google Scholar]
- Carpenter, J. K. , Andrews, L. A. , Witcraft, S. M. , Powers, M. B. , Smits, J. A. J. , & Hofmann, S. G. (2018). Cognitive behavioral therapy for anxiety and related disorders: A meta‐analysis of randomized placebo‐controlled trials. Depression and Anxiety, 35(6), 502–514. 10.1002/da.22728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss, J. A. , Avery, S. N. , Benningfield, M. M. , & Blackford, J. U. (2019). Social anxiety is associated with BNST response to unpredictability. Depression and Anxiety, 36, 666–675. 10.1002/da.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss, J. A. , Avery, S. N. , & Blackford, J. U. (2015). The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta‐analysis. Progress in Neurobiology, 127–128, 23–45. 10.1016/j.pneurobio.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss, J. A. , & Blackford, J. U. (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta‐analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066.e1–1075.e1. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske, M. (2015). Optimizing exposure therapy for anxiety disorders: An inhibitory learning and inhibitory regulation approach. Verhaltenstherapie, 25(2), 134–143. 10.1159/000381574 [DOI] [Google Scholar]
- Craske, M. G. , Liao, B. , Brown, L. , & Vervliet, B. (2012). Role of inhibition in exposure therapy. Journal of Experimental Psychopathology, 3(3), 322–345. 10.5127/jep.026511 [DOI] [Google Scholar]
- Craske, M. G. , Treanor, M. , Conway, C. C. , Zbozinek, T. , & Vervliet, B. (2014). Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy, 58, 10–23. 10.1016/j.brat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski, U. , Kugel, H. , Franke, F. , Stuhrmann, A. , Hohoff, C. , Zwanzger, P. , … Domschke, K. (2011). Neuropeptide‐S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology, 36(9), 1879–1885. 10.1038/npp.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke, K. , Baune, B. T. , Havlik, L. , Stuhrmann, A. , Suslow, T. , Kugel, H. , … Dannlowski, U. (2012). Catechol‐O‐methyltransferase gene variation: Impact on amygdala response to aversive stimuli. NeuroImage, 60(4), 2222–2229. 10.1016/j.neuroimage.2012.02.039 [DOI] [PubMed] [Google Scholar]
- Ebner, N. C. , Riediger, M. , & Lindenberger, U. (2010). FACES—A database of facial expressions in young, middle‐aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362. 10.3758/BRM.42.1.351 [DOI] [PubMed] [Google Scholar]
- Figel, B. , Brinkmann, L. , Buff, C. , Heitmann, C. Y. , Hofmann, D. , Bruchmann, M. , … Straube, T. (2019). Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. NeuroImage: Clinical, 22, 101735 10.1016/j.nicl.2019.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, H. , Wright, C. I. , Whalen, P. J. , McInerney, S. C. , Shin, L. M. , & Rauch, S. L. (2003). Brain habituation during repeated exposure to fearful and neutral faces: A functional MRI study. Brain Research Bulletin, 59(5), 387–392. [DOI] [PubMed] [Google Scholar]
- Fox, A. S. , & Kalin, N. H. (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171(11), 1162–1173. 10.1176/appi.ajp.2014.14040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco, D. M. , Coles, M. E. , Heimberg, R. G. , Liebowitz, M. R. , Hami, S. , Stein, M. B. , & Goetz, D. (2001). The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self‐report and clinician‐administered formats. Psychological Medicine, 31(6), 1025–1035. 10.1017/S0033291701004056 [DOI] [PubMed] [Google Scholar]
- Glahn, D. C. , Nimgaonkar, V. L. , Raventós, H. , Contreras, J. , McIntosh, A. M. , Thomson, P. A. , … Blangero, J. (2018). Rediscovering the value of families for psychiatric genetics research. Molecular Psychiatry, 24, 523–535. 10.1038/s41380-018-0073-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn, D. C. , Thompson, P. M. , & Blangero, J. (2007). Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Human Brain Mapping, 28(6), 488–501. 10.1002/hbm.20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, I. I. , & Gould, T. D. (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry, 160(4), 636–645. 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Isomura, K. , Boman, M. , Rück, C. , Serlachius, E. , Larsson, H. , Lichtenstein, P. , & Mataix‐Cols, D. (2015). Population‐based, multi‐generational family clustering study of social anxiety disorder and avoidant personality disorder. Psychological Medicine, 45(8), 1581–1589. 10.1017/S0033291714002116 [DOI] [PubMed] [Google Scholar]
- Kauppi, K. , Nilsson, L.‐G. , Persson, J. , & Nyberg, L. (2014). Additive genetic effect of APOE and BDNF on hippocampus activity. NeuroImage, 89, 306–313. 10.1016/j.neuroimage.2013.11.049 [DOI] [PubMed] [Google Scholar]
- Kleinhans, N. M. , Johnson, L. C. , Richards, T. , Mahurin, R. , Greenson, J. , Dawson, G. , & Aylward, E. (2009). Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. The American Journal of Psychiatry, 166(4), 467–475. 10.1176/appi.ajp.2008.07101681 [DOI] [PubMed] [Google Scholar]
- Klumpp, H. , & Fitzgerald, J. M. (2018). Neuroimaging predictors and mechanisms of treatment response in social anxiety disorder: An overview of the amygdala. Current Psychiatry Reports, 20(10), 89 10.1007/s11920-018-0948-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe, S. , Beesdo‐Baum, K. , & Wittchen, H.‐U. (2010). Familial risk factors in social anxiety disorder: Calling for a family‐oriented approach for targeted prevention and early intervention. European Child & Adolescent Psychiatry, 19(12), 857–871. 10.1007/s00787-010-0138-0 [DOI] [PubMed] [Google Scholar]
- La Greca, A. M. , & Lopez, N. (1998). Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology, 26(2), 83–94. 10.1023/A:1022684520514 [DOI] [PubMed] [Google Scholar]
- Lenzenweger, M. F. (2013). Endophenotype, intermediate phenotype, biomarker: Definitions, concept comparisons, clarifications. Depression and Anxiety, 30(3), 185–189. 10.1002/da.22042 [DOI] [PubMed] [Google Scholar]
- Lonsdorf, T. B. , Golkar, A. , Lindstöm, K. M. , Fransson, P. , Schalling, M. , Öhman, A. , & Ingvar, M. (2011). 5‐HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biological Psychology, 87(1), 106–112. 10.1016/j.biopsycho.2011.02.014 [DOI] [PubMed] [Google Scholar]
- McDiarmid, T. A. , Bernardos, A. C. , & Rankin, C. H. (2017). Habituation is altered in neuropsychiatric disorders—A comprehensive review with recommendations for experimental design and analysis. Neuroscience and Biobehavioral Reviews, 80, 286–305. 10.1016/j.neubiorev.2017.05.028 [DOI] [PubMed] [Google Scholar]
- Munafò, M. R. , Brown, S. M. , & Hariri, A. R. (2008). Serotonin transporter (5‐HTTLPR) genotype and amygdala activation: A meta‐analysis. Biological Psychiatry, 63(9), 852–857. 10.1016/j.biopsych.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S. E. , Norbury, R. , Godlewska, B. R. , Cowen, P. J. , Mannie, Z. M. , Harmer, C. J. , & Munafò, M. R. (2013). The effect of the serotonin transporter polymorphism (5‐HTTLPR) on amygdala function: A meta‐analysis. Molecular Psychiatry, 18(4), 512–520. 10.1038/mp.2012.19 [DOI] [PubMed] [Google Scholar]
- Myers, K. M. , & Davis, M. (2006). Mechanisms of fear extinction. Molecular Psychiatry, 12, 120–150. 10.1038/sj.mp.4001939 [DOI] [PubMed] [Google Scholar]
- Oler, J. A. , Fox, A. S. , Shelton, S. E. , Rogers, J. , Dyer, T. D. , Davidson, R. J. , … Kalin, N. H. (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466(7308), 864–868. 10.1038/nature09282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Rodriguez, M. M. , New, A. S. , Goldstein, K. E. , Rosell, D. , Yuan, Q. , Zhou, Z. , … Hazlett, E. A. (2017). Brain‐derived neurotrophic factor Val66Met genotype modulates amygdala habituation. Psychiatry Research: Neuroimaging, 263, 85–92. 10.1016/j.pscychresns.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, J. H. , Lett, T. A. , Wackerhagen, C. , Plichta, M. M. , Mohnke, S. , Grimm, O. , … Erk, S. (2018). The effect of 5‐HTTLPR and a serotonergic multi‐marker score on amygdala, prefrontal and anterior cingulate cortex reactivity and habituation in a large, healthy fMRI cohort. European Neuropsychopharmacology, 28(3), 415–427. 10.1016/j.euroneuro.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Pittig, A. , van den Berg, L. , & Vervliet, B. (2016). The key role of extinction learning in anxiety disorders: Behavioral strategies to enhance exposure‐based treatments. Current Opinion in Psychiatry, 29, 39–47. 10.1097/YCO.0000000000000220 [DOI] [PubMed] [Google Scholar]
- Plichta, M. M. , Grimm, O. , Morgen, K. , Mier, D. , Sauer, C. , Haddad, L. , … Meyer‐Lindenberg, A. (2014). Amygdala habituation: A reliable fMRI phenotype. NeuroImage, 103, 383–390. 10.1016/j.neuroimage.2014.09.059 [DOI] [PubMed] [Google Scholar]
- Poon, C.‐S. , & Young, D. L. (2006). Nonassociative learning as gated neural integrator and differentiator in stimulus‐response pathways. Behavioral and Brain Functions, 2(1), 29 10.1186/1744-9081-2-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls, I. , & Gallinat, J. (2008). The concept of endophenotypes in psychiatric diseases meeting the expectations? Pharmacopsychiatry, 41(Suppl 1), S37–S43. 10.1055/s-2008-1081462 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Retrieved from https://www.r-project.org
- Ramaswami, M. (2014). Network plasticity in adaptive filtering and behavioral habituation. Neuron, 82(6), 1216–1229. 10.1016/j.neuron.2014.04.035 [DOI] [PubMed] [Google Scholar]
- Rankin, C. H. , Abrams, T. , Barry, R. J. , Bhatnagar, S. , Clayton, D. F. , Colombo, J. , … Thompson, R. F. (2009). Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory, 92(2), 135–138. 10.1016/j.nlm.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, C. , Doebler, P. , Ehring, T. , & Vossbeck‐Elsebusch, A. N. (2017). Emotional processing theory put to test: A meta‐analysis on the association between process and outcome measures in exposure therapy. Clinical Psychology & Psychotherapy, 24(3), 697–711. 10.1002/cpp.2039 [DOI] [PubMed] [Google Scholar]
- Schwartz, C. E. (2003). Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science, 300(5627), 1952–1953. 10.1126/science.1083703 [DOI] [PubMed] [Google Scholar]
- Schwartz, C. E. , Kunwar, P. S. , Greve, D. N. , Kagan, J. , Snidman, N. C. , & Bloch, R. B. (2012). A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry, 17(10), 1042–1050. 10.1038/mp.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, C. E. , Wright, C. I. , Shin, L. M. , Kagan, J. , Whalen, P. J. , McMullin, K. G. , & Rauch, S. L. (2003). Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biological Psychiatry, 53(10), 854–862. 10.1016/S0006-3223(02)01906-6 [DOI] [PubMed] [Google Scholar]
- Sladky, R. , Höflich, A. , Atanelov, J. , Kraus, C. , Baldinger, P. , Moser, E. , … Windischberger, C. (2012). Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by FMRI. PLoS One, 7(11), e50050 10.1371/journal.pone.0050050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. B. , Chen, C.‐Y. , Jain, S. , Jensen, K. P. , He, F. , Heeringa, S. G. , … Gelernter, J. (2017). Genetic risk variants for social anxiety. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 174(2), 120–131. 10.1002/ajmg.b.32520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. B. , & Stein, D. J. (2008). Social anxiety disorder. The Lancet, 371(9618), 1115–1125. 10.1016/S0140-6736(08)60488-2 [DOI] [PubMed] [Google Scholar]
- Swartz, J. R. , Wiggins, J. L. , Carrasco, M. , Lord, C. , & Monk, C. S. (2013). Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 52(1), 84–93. 10.1016/j.jaac.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier, R. , Tsonaka, R. , Mooijaart, S. P. , Slagboom, E. , & Houwing‐Duistermaat, J. J. (2017). Secondary phenotype analysis in ascertained family designs: Application to the Leiden longevity study. Statistics in Medicine, 36(14), 2288–2301. 10.1002/sim.7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedig, M. M. , Rauch, S. L. , Albert, M. S. , & Wright, C. I. (2005). Differential amygdala habituation to neutral faces in young and elderly adults. Neuroscience Letters, 385(2), 114–119. 10.1016/j.neulet.2005.05.039 [DOI] [PubMed] [Google Scholar]
- Whalen, P. J. (2007). The uncertainty of it all. Trends in Cognitive Sciences, 11(12), 499–500. 10.1016/j.tics.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Wiggins, J. L. , Swartz, J. R. , Martin, D. M. , Lord, C. , & Monk, C. S. (2014). Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Social Cognitive and Affective Neuroscience, 9(6), 832–838. 10.1093/scan/nst039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L. E. , Blackford, J. U. , Luksik, A. , Gauthier, I. , & Heckers, S. (2013). Reduced habituation in patients with schizophrenia. Schizophrenia Research, 151(1), 124–132. 10.1016/j.schres.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C.‐W. , Krishnan, A. , & Wager, T. D. (2014). Cluster‐extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody, M. L. , Yang, J. O. , Cummings, L. , Gilchrist, D. , Graur, S. , Siegle, G. J. , & Price, R. B. (2019). Protracted amygdalar response predicts efficacy of a computer‐based intervention targeting attentional patterns in transdiagnostic clinical anxiety. Translational Psychiatry, 9(1), 121 10.1038/s41398-019-0458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich, M. W. , Ripley, B. D. , Brady, M. , & Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage, 14(6), 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Worsley, K. J. (2001). Statistical analysis of activation images In Jezzard P., Matthews P. M., & Smith S. M. (Eds.), Functional magnetic resonance imaging: An introduction to methods (pp. 1–23). Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780192630711.003.0014 [DOI] [Google Scholar]
- Wright, C. I. , Fischer, H. , Whalen, P. J. , McInerney, S. C. , Shin, L. M. , & Rauch, S. L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12(2), 379–383. [DOI] [PubMed] [Google Scholar]
- Yoon, K. L. , & Zinbarg, R. E. (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117(3), 680–685. 10.1037/0021-843X.117.3.680 [DOI] [PubMed] [Google Scholar]
- Ziegler, C. , Dannlowski, U. , Bräuer, D. , Stevens, S. , Laeger, I. , Wittmann, H. , … Omschke, K. (2015). Oxytocin receptor gene methylation: Converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology, 40(6), 1528–1538. 10.1038/npp.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


