Abstract
Objective
Mindfulness‐based interventions (MBIs) are increasingly used within psycho‐oncology. Since the publication of the most recent comprehensive meta‐analysis on MBIs in cancer in 2012, the number of published trials has more than doubled. We therefore conducted a systematic review and meta‐analysis of randomized controlled trials (RCTs), testing the efficacy of MBIs on measures of psychological distress (primary outcome) and other health outcomes in cancer patients and survivors.
Methods
Two authors conducted independent literature searches in electronic databases from first available date to 10 October 2018, selected eligible studies, extracted data for meta‐analysis, and evaluated risk of bias.
Results
Twenty‐nine independent RCTs (reported in 38 papers) with 3274 participants were included. Small and statistically significant pooled effects of MBIs on combined measures of psychological distress were found at post‐intervention (Hedges's g = 0.32; 95%CI: 0.22‐0.41; P < .001) and follow‐up (g = 0.19; 95%CI: 0.07‐0.30; P < .002). Statistically significant effects were also found at either post‐intervention or follow‐up for a range of self‐reported secondary outcomes, including anxiety, depression, fear of cancer recurrence, fatigue, sleep disturbances, and pain (g: 0.20 to 0.51; p: <.001 to.047). Larger effects of MBIs on psychological distress were found in studies (a) adhering to the original MBI manuals, (b) with younger patients, (c) with passive control conditions, and (d) shorter time to follow‐up. Improvements in mindfulness skills were associated with greater reductions in psychological distress at post‐intervention.
Conclusions
MBIs appear efficacious in reducing psychological distress and other symptoms in cancer patients and survivors. However, many of the effects were of small magnitude, suggesting a need for intervention optimization research.
Keywords: cancer, meta‐analysis, mindfulness, mindfulness‐based cognitive therapy, mindfulness‐based stress reduction, oncology, systematic review
1. BACKGROUND
Approximately 38% of US citizens will be diagnosed with cancer at some point in their lives, and the number of cancer survivors increased from 10 million in 2002 to 14 million in 2012.1, 2 Furthermore, cancer mortality has steadily declined since the late 1980s, eg, in the EU with reductions of 1.6% per year in men and 1% per year in women.3 Increased survival rates bring new rehabilitation challenges as more than one in three cancer patients and survivors experience significant levels of psychological distress.4 The National Comprehensive Cancer Network defines distress as a multi‐determined unpleasant emotional experience.5 Significant psychological distress impairs quality of life (QoL)6 and requires psychological treatment,4 underscoring the need for evidence‐based rehabilitation programs.7 In the last two decades, mindfulness‐based interventions (MBIs) have increasingly been used to reduce psychological distress in patients during as well as after cancer treatment.
A meta‐analysis from 2012 of the nine RCTs available at the time found that MBIs reduced anxiety and depression with effects corresponding to small effect sizes (ESs; Hedges's g: 0.37 and 0.44).8 These findings have since been supported in a number of more recent meta‐analyses focusing on depression and anxiety in cancer patients,9 primarily patients with breast cancer.10, 11, 12 In addition, these meta‐analyses have found positive effects of MBIs on a range of other cancer‐related outcomes.10, 12
However, although previous meta‐analyses on MBIs for cancer patients and survivors have contributed to our current knowledge, some issues remain. First, previous meta‐analyses have been restricted in their scope by being either relatively narrow, eg, focusing only on effects of mindfulness‐based stress reduction (MBSR) in breast cancer survivors,13 or very broad, eg, focusing on “mind‐body approaches.”14 Second, the majority of previous meta‐analyses have only included a small number of psychological outcomes,9, 11, 15 although psychological and physical consequences of cancer can be multifaceted.16 Third, none of the previous meta‐analyses have explored the possible moderating role of between‐study differences in patient, cancer, and intervention characteristics, eg, mindfulness‐based cognitive therapy (MBCT) versus MBSR or adapted versions like mindfulness‐based cancer recovery (MBCR).17 Knowing how between‐study differences in such characteristics may influence the efficacy of MBIs may inform clinical practice as to “what works for whom.” Fourth, information that points to the working mechanisms of MBIs may be an important step toward optimization of MBIs,18 and associations between changes in possible mediators and effects of MBIs in cancer patients and survivors have not previously been explored in meta‐analyses. Finally, the possible associations between the quality of the MBIs and their effects in cancer patients and survivors have not yet been studied. As the number of RCTs of MBIs in cancer patients and survivors has more than doubled since the first comprehensive meta‐analysis,8 an update is desirable.
On this background, we conducted an updated and comprehensive systematic review and meta‐analysis of the immediate and longer‐term effects of MBIs in cancer patients and survivors on the primary outcome of psychological distress. Psychological distress included various individual measures as well as combinations of anxiety, depression, and distress, representing central aspects of the psychological symptom cluster identified in this patient group.19 Furthermore, we explored effects on a number of secondary outcomes, namely, cancer‐related QoL and a range of individual psychological and physical symptoms commonly experienced by cancer patients and survivors, including anxiety, depression, post‐traumatic stress symptoms, fear of cancer recurrence, pain, fatigue, and sleep disturbances. In addition, we examined the possible moderating role of a number of patient, cancer, and intervention characteristics. Finally, we explored the associations between changes in putative MBI mechanisms, including mindfulness skills, self‐compassion, and rumination, and effects of MBIs on psychological distress.
2. METHODS
The present review was preregistered with PROSPERO (registration number: CRD42018096911)20 and conducted and reported in accordance with the PRISMA guidelines.21, 22
2.1. Search strategy
Electronic databases (PubMed, Web of Science, PsychINFO, and CINAHL) were independently searched by two authors (LC and MJ) for publications from first available date to 10 October 2018 with prespecified search terms to identify RCTs of MBIs for cancer patients and survivors. The final search strategy was based on the PICO approach,23 combining the following search terms: Population (cancer OR neoplasm) AND Intervention (mindful* OR meditation OR MBCT OR MBSR OR MBCR) AND Outcome (anxiety OR depression OR depressive OR symptom OR fear OR adaptation OR “mental health” OR “psychological distress” OR distress OR reaction; full search string provided in the Supplementary material). No search term for Comparison was included as this proved too restrictive. In addition, a backward search (snowballing) was conducted of reference lists from identified reports and earlier systematic reviews together with a forward search (citation tracking) until no additional relevant reports were found.
2.2. Selection procedure and data extraction
Two authors (LC and MJ) independently performed title and abstract screening, followed by full‐text screening. Inter‐rater reliability for full‐text screening was adequate (agreement = 92.6%, kappa = .70). Disagreements were discussed with a third author (AS or RZ) and a final decision negotiated.
Study eligibility was assessed using the PICO approach.23 Population: Adult (≥18 years) cancer patients or survivors (any type and stage). Intervention: MBIs with mindfulness as the main component, as opposed to being a subcomponent of a program (eg, acceptance and commitment therapy) and including formal meditation homework. Comparison: RCTs with at least one non‐MBI control arm. Outcomes: One or more measures of distress, including perceived stress, anxiety, depression, and combined measures of distress, eg, the Hospital Anxiety and Depression Scale (HADS) total score.24 If results of the same trial were reported in more than one publication, additional publications reporting secondary outcomes could be included. Only original research reported in English was included.
Data were extracted by LC and included (a) study characteristics (publication year, study design, type of control group [passive, active, competing], intent‐to‐treat [ITT] analyses [yes, no], and assessment time points); (b) patient characteristics (gender, age, cancer type, cancer stage, time since diagnosis, symptom levels as study inclusion criteria); (c) MBI characteristics (number and length of sessions [hours], intervention based on MBSR or MBCT, MBI changes/adaptations [minor, major], actual home practice [minutes]); and (d) self‐reported outcomes (psychological distress, PTSD symptoms, fear of cancer recurrence, physical symptoms, and cancer‐related QoL) and hypothesized mediators (mindfulness skills, self‐compassion, and rumination). Unadjusted means, standard deviations, and number of participants based on the ITT sample were extracted. If the data reported were insufficient for meta‐analysis, authors were contacted and asked to provide these data. As our aim was to evaluate the overall efficacy, in case of more than two trial arms (K = 5), we included the most passive control condition. For example, if a nutrition intervention and a waitlist were the control conditions, the waitlist condition was included as comparison.
2.3. Risk of bias assessment
Risk of bias was assessed by LC and MJ using the Cochrane Risk of Bias Tool.25 This tool evaluates risk of bias regarding (a) random sequence generation, (b) allocation concealment, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) incomplete outcome data, and (f) selective reporting. Risk of bias was rated as low, unclear or high.
2.4. MBI quality assessment
Two authors (LC and MJ) evaluated MBI quality using five criteria inspired by Shaw and colleagues.26 The criteria included (a) clear description of structure and themes of the intervention (1 point) and possible adaptations (1 point; if the study did not involve any adaptations, full points were given); (b) relevant profession of mindfulness instructor (mental health specialist: 1 point); (c) adequate experience and training/education of mindfulness instructor (clinical experience: 1 point; mindfulness education: 1 point); (d) adherence to intervention protocol (assessed adherence: 1 point; adherence reported in the results: 1 point); and (e) assessment of teacher competence (assessed competence: 1 point; competence reported in the results: 1 point). The total MBI quality score ranged from 0 to 9. Inter‐rater reliability was good (agreement 87.6%, kappa = 0.81).
2.5. Quality of evidence assessment
The GRADE system27 was used to rate the overall quality of evidence of the meta‐analytic results as high, moderate, low, or very low. GRADE assessment goes beyond risk of bias, which addresses internal validity of the included studies, as the GRADE assessment reflects the general confidence in the overall ES. GRADE uses a baseline rating of high for RCTs. This rating can be downgraded based on five assessment criteria: risk of bias, inconsistency of the results, indirectness, imprecision, and publication bias. The ratings were conducted and negotiated by all authors.
2.6. Computing effect sizes
All analyses were performed with Comprehensive Meta‐Analysis.28 The primary outcome was overall psychological distress, which consisted of measures of perceived stress, anxiety, depression, and combined measures of distress, eg, the HADS total score.24 We chose a broad outcome to represent the psychological symptom cluster,19 which, in addition, enabled us to include more studies and improve the statistical power of moderation analyses. To address possible differences between psychological symptoms, we also examined anxiety and depression separately as secondary outcomes. Additional secondary outcomes were PTSD symptoms, fear of cancer recurrence, fatigue, pain, sleep disturbance, and QoL. Hedges's g, a variation of Cohen's d,29 correcting for possible bias due to small sample sizes,30 was used as the standardized ES. Hedges's g can be characterized as small (0.2), medium (0.5), or large (0.8).29 ESs were calculated for pre‐ to post‐treatment and for pre‐treatment to the last follow‐up. Whenever possible, ESs were computed using reported means and standard deviations. If these data were unavailable, authors were contacted. If authors did not respond or were unable to provide the data, ESs were based on reported ESs or calculated based on N and other reported statistics, eg, P values and F‐values. Pooled ESs were weighted by the inverse standard error, taking into account the precision of each study, with positive values chosen to indicate effects in the hypothesized direction. When multiple outcomes from one study were included in the same analysis, the average ES was calculated and weighted by the precision of the individual ESs. As differences in ESs can only very rarely be assumed to be purely attributed to sampling error, a random effects model was chosen a priori for all analyses.
2.7. Publication bias
The possibility of publication bias was evaluated with funnel plots and Egger's tests.31, 32 If the results were suggestive of publication bias, an adjusted ES was calculated using the Duval and Tweedie trim‐and‐fill method,33 which imputes “missing” studies and recalculates the ES accordingly. In case of statistically significant results (P < .05), we calculated the failsafe N, ie, the number of unpublished studies with null findings that would reduce the results to statistical non‐significance (P > .05). A failsafe N exceeding 5K+10 (K = number of studies) has been suggested to be sufficiently robust in the face of possible publication bias.34
2.8. Analytical strategy
Pooled ESs were calculated for the primary outcome, ie, psychological distress, as various combinations of anxiety, depression, and stress measures, as well as for all secondary psychological and physical outcomes (see Table S1 in the Supporting Information for included outcomes per study). To satisfy the assumption of independence,35 effects were averaged within and across outcomes, so that any given study in any given analysis was only represented once in each analysis. The influence of possible outliers was explored with sensitivity analyses omitting ESs above or below two standard deviations from the pooled ES.36 In addition, the influence of studies with online MBIs was examined with sensitivity analyses omitting these studies.
When available for at least eight studies per parameter in the analysis, possible moderators of the effect on the primary outcome were explored with meta‐regression. Categorical and continuous moderators related to study design included type of control group (passive control, active control, and competing intervention), time to post‐intervention (weeks) and follow‐up (months), attrition rates (percentage), risk of bias score, and publication year (year). Patient‐related moderators included sample mean age (years), gender (percent women), and symptom inclusion criteria (yes, no). Cancer‐related moderators included cancer type (breast, mixed), cancer stage (non‐metastatic, mixed), and time since diagnosis (months). Intervention‐related moderators included MBI type (MBSR, MBCT), intervention dose (hours), adaptations from the original protocol (minor, major), and MBI quality score. Moderation analyses were based on random‐effects models and the Maximum Likelihood method.
3. RESULTS
In total, 38 research papers describing results of 29 independent RCTs were included. The study selection process is shown in Figure 1.
Figure 1.
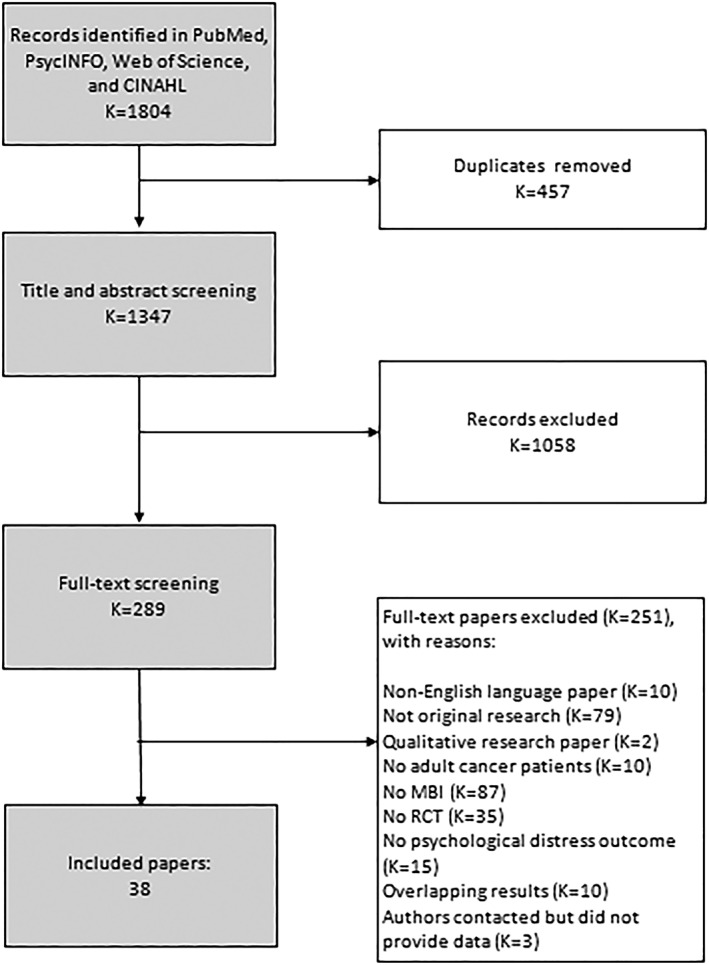
Flowchart of study selection
3.1. Study characteristics
The main characteristics of the included studies are presented in Table 1 (for further details, see Table S2 ). The 29 RCTs included a total of 3274 participants with an average study sample size of 117 (range: 16‐245). The majority (86%) of participants were women, 70% had breast cancer, and sample mean age was 55 years (range: 46‐71). The most common type of MBI was MBSR (K = 13, 45%). Interventions involved on average 16.6 contact hours (range: 4.5‐28.5 hours). Mean follow‐up time was 6.6 months (range 3‐24 months) in the 19 studies, which included follow‐up assessment. The outcomes included in each study are shown in Table S1.
Table 1.
Main characteristics of included studies
| Study (authors + year) | Participant characteristics: type of cancer, stage of cancer, time since diagnosis, severity inclusion criteria | Total n in analyses | Intervention and control groups (n a) |
|---|---|---|---|
| Blaes et al. 201637 | Mixed, 69% breast; within 6 mo of completion of chemotherapy/radiation | 41 | MBCR (28) |
| WL (14) | |||
| Bower et al. 201538; Boyle et al. 201739 | Breast; stage 0 to III; 4 y since diagnosis | 71 | MAPS (39) |
| WL (32) | |||
| Bränström et al. 201040; Bränström et al. 201241 | Mixed,76% breast | 71 | MBSR (32) |
| WL (39) | |||
| Bruggeman‐Everts et al. 201742 | Mixed, 47% breast; with severe fatigue | 84 | Online eMBCT (55) |
| Ambulant activity feedback (62)b | |||
| PE emails (50) | |||
| Carlson et al. 201343; Schellekens et al.201744 | Breast; stage 0 to IV; 26 mo since diagnosis; with distress | 134 | MBCR (113) |
| SET (104)b | |||
| SMS (54) | |||
| Chambers et al. 201745 | Prostate; advanced; 6 y since diagnosis | 189 | MBCT in teleconference groups (94) |
| Enhanced UC (95) | |||
| Compen et al. 201846 | Mixed, 62% breast; 4 y since diagnosis; with at least mild psychological distress | 245 | MBCTc (77) |
| Online eMBCTc (90) | |||
| UC (78) | |||
| Foley et al. 201047 | Mixed, 42% breast; stage I to IV; 2.1 y since diagnosis | 115 | MBCT (55) |
| WL (60) | |||
| Garland et al. 201448; Garland et al. 201549 | Mixed, 48% breast; non‐metastatic; with insomnia | 110 | MBSR (64) |
| CBT (47) | |||
| Henderson et al. 201250 | Breast; stage I or II | 114 | MBSR (56) |
| NEP (52)b | |||
| UC (58) | |||
| Hoffman et al. 201251 | Breast; 0 to III; 18 mo since diagnosis | 214 | MBSR (114) |
| WL (115) | |||
| Jang et al. 201652 | Breast; 0 to III; treatment less than 2 y ago | 24 | MBAT (12) |
| UC WL (12) | |||
| Johannsen et al. 201653; Johannsen et al. 201854 | Breast; non‐metastatic; 42 mo since surgery; with post‐treatment pain | 128 | MBCT (67) |
| WL (62) | |||
| Johns et al. 201555 | Mixed, 86% breast; stage I to IV, with cancer‐related fatigue | 35 | MBSR‐CRF (18) |
| WL (17) | |||
| Johns, Brown, et al. 201656; Johns, Von Ah, et al. 201657 | Breast (85%) and colorectal; 2.4 y since cancer treatment completion; with cancer‐related fatigue | 71 | Tailored MBSR (35) |
| PE support group (36) | |||
| Kenne Sarenmalm et al. 201758 | Breast | 114 | MBSR (62) |
| MBSR‐self‐instruct (52)b | |||
| No intervention (52) | |||
| Kingston et al. 201559 | Mixed, 38% breast; with mild to moderate symptoms of depression/anxiety | 13 | MBCT (8) |
| WL UC (8) | |||
| Lengacher et al. 201660; Reich et al. 201719 | Breast; 0 to III; 33 wk since treatment | 332 | MBSR (BC) (167) |
| UC (155) | |||
| Lengacher et al. 200961 | Breast; stage 0 to III; 19 wk since treatment | 82 | MBSR (BC) (41) |
| WL UC (43) | |||
| Lerman et al. 201262 | Mixed, 71% breast; 3.8 y since diagnosis | 68 | MBSR (53) |
| WL (24) | |||
| Monti et al. 200663 | Mixed, 46% breast; no terminal patients; beyond four months and within 2 y of an original diagnosis (or recurrence) | 111 | MBAT (56) |
| WL (55) | |||
| Monti et al. 201364 | Breast; all stages; 34 mo since diagnosis | 191 | MBAT (126) |
| Support group (125) | |||
| Nakamura et al. (2013)65 | Mixed, 54% breast; 42 mo since diagnosis; with clinically significant sleep disturbance | 38 | Mindfulness meditation (20) |
| PE sleephygiene (18) | |||
| Mind‐body bridging program (19)d | |||
| Reynolds et al. 201766 | Mixed, 40% breast; non‐metastatic; 3 mo since diagnosis | 68 | Brief MBT (32) |
| Relaxation (36) | |||
| Schellekens et al. 201767 | Lung; stage I to IV; 7 mo since diagnosis | 45 | MBSR (31) |
| UC (32) | |||
| Speca et al. 200068 | Mixed, 41% breast; stage I to IV; | 90 | MBCR (53) |
| WL (37) | |||
| Würtzen et al. 201369; Würtzen et al. 201570; Andersen et al. 201371 | Breast; stage I to III; 8 mo since diagnosis | 336 | MBSR (168) |
| UC (168) | |||
| Zernicke et al. 201472 | Mixed, 34% breast; stage I to IV; completed primary cancer treatment in last 3 y; with at least moderate distress | 62 | Online MBCR (30) |
| WL‐UC (32) | |||
| Zhang et al. 201773 | Breast; Stage I to III; within 2‐6 mo after surgery | 58 | MBSR (BC) (28) |
| WL UC (30) |
Abbreviations: MAPS, mindfulness awareness practices; MBAT, mindfulness‐based art therapy; MBCR, mindfulness‐based cancer recovery; MBCT, mindfulness‐based cognitive therapy; MBSR, mindfulness‐based stress reduction; MBT, mindfulness‐based training; na, not available; NEP, nutrition education program; PE, psycho‐education; SMS, stress management seminar; UC, usual care; WL, waitlist control group
Sample size represents number of participants randomized to each group.
Not included in the meta‐analysis, due to primary focus on efficacy.
MBCT and eMBCT were combined for analyses.
Not included in meta‐analysis due to overlap with mindfulness‐based interventions.
3.2. Pooled effects at post‐treatment
A forest plot of ESs for psychological distress is shown in Figure 2. As seen in Table 2, MBIs had a statistically significant effect on psychological distress at post‐treatment corresponding to a small ES (Hedges's g = 0.32). In addition, MBIs were found to have statistically significant effects on self‐reported symptoms of anxiety, depression, fear of cancer recurrence, and fatigue with ESs ranging from small to medium (Hedges's g: 0.29‐0.51; Table 2). With the exception of fear of cancer recurrence, the majority of the statistically significant results were robust with failsafe Ns exceeding the criterion.
Figure 2.
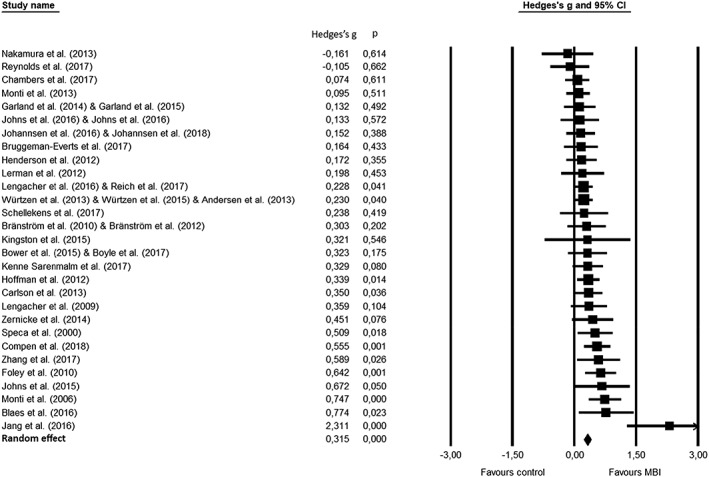
Forest plot of effect sizes for combined psychological distress outcomes at post‐intervention
Table 2.
Pooled post‐intervention and follow‐up effects of mindfulness‐based interventions on psychological and physical health outcomes and proposed mediators in cancer patients and survivors
| Sample size | Heterogeneityb | Global effect sizes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Post‐intervention | K | N | Q | df | P | I2 | Hedges's g c | 95% CI | P | Failsafe Nd | Criterione |
| Psychological distressa | 29 | 3274 | 45.1 | 28 | .022 | 37.9 | 0.32 | 0.22‐0.41 | <.001 | 536 | 155 |
| Adj. for publication bias f | (31) | 0.29 | 0.19‐0.40 | ‐ | |||||||
| Anxiety | 13 | 1396 | 18.4 | 12 | .104 | 34.8 | 0.36 | 0.22‐0.51 | <.001 | 138 | 75 |
| Adj. for publication bias f | (15) | 0.33 | 0.15‐0.50 | ‐ | |||||||
| Depression | 13 | 1406 | 34.7 | 12 | .001 | 65.4 | 0.38 | 0.18‐0.58 | <.001 | 126 | 75 |
| PTSD symptoms | 6 | 482 | 4.4 | 5 | .488 | 0.0 | 0.09 | −0.08‐0.27 | .299 | ‐ | ‐ |
| Fear of cancer recurrence | 4 | 838 | 3.7 | 3 | .291 | 19.8 | 0.29 | 0.12‐0.45 | .001 | 13 | 30 |
| Pain | 4 | 587 | 5.7 | 3 | .125 | 47.7 | 0.18 | −0.07‐0.43 | .152 | ‐ | ‐ |
| Sleep disturbance | 8 | 1021 | 28.0 | 7 | <.001 | 75.0 | 0.22 | −0.07‐0.50 | .137 | ‐ | ‐ |
| Fatigue | 6 | 626 | 11.9 | 5 | <.036 | 58.1 | 0.51 | 0.22‐0.79 | .001 | 43 | 40 |
| Quality of life | 9 | 987 | 34.2 | 8 | <.001 | 76.6 | 0.26 | −0.02‐0.55 | .066 | ‐ | ‐ |
| Mindfulness skills | 17 | 2138 | 28.9 | 16 | .025 | 44.5 | 0.23 | 0.11‐0.36 | <.001 | 93 | 95 |
| Self‐compassion | 6 | 335 | 2.4 | 5 | .796 | 0.0 | 0.42 | 0.21‐0.64 | <.001 | 20 | 40 |
| Rumination | 3 | 361 | 2.7 | 2 | .258 | 26.3 | 0.43 | 0.14‐0.72 | .003 | 7 | 25 |
| Follow‐up | |||||||||||
| Psychological distressa | 18 | 2207 | 28.6 | 17 | .038 | 40.6 | 0.19 | 0.07‐0.30 | 002 | 76 | 100 |
| Anxiety | 8 | 1048 | 15.7 | 7 | .028 | 55.3 | 0.36 | 0.15‐0.56 | .001 | 51 | 50 |
| Depression | 8 | 1058 | 13.9 | 7 | .053 | 49.7 | 0.20 | 0.01‐0.40 | .040 | 14 | 50 |
| PTSD symptoms | 6 | 482 | 5.2 | 5 | .386 | 4.7 | 0.17 | −0.02‐0.35 | .072 | ‐ | ‐ |
| Pain | 4 | 587 | 1.4 | 3 | .698 | 0.0 | 0.20 | 0.04‐0.36 | .016 | 1 | 30 |
| Sleep disturbance | 8 | 1021 | 17.8 | 7 | .013 | 60.7 | 0.23 | 0.00‐0.45 | .047 | 15 | 50 |
| Fatigue | 6 | 645 | 25.8 | 5 | .001 | 80.6 | 0.40 | −0.01‐0.80 | .057 | ‐ | ‐ |
| Quality of life | 5 | 615 | 7.9 | 4 | .096 | 49.2 | 0.09 | −0.15‐0.32 | .477 | ‐ | ‐ |
| Mindfulness skills | 11 | 1450 | 11.7 | 10 | .309 | 14.2 | 0.20 | 0.08‐0.32 | .001 | 31 | 65 |
| Self‐compassion | 5 | 332 | 1.9 | 4 | .758 | 0.0 | 0.32 | 0.10‐0.54 | .005 | 7 | 35 |
Abbreviation: PTSD, post‐traumatic stress disorder.
Bold values indicate statistical significance.
Primary outcome variable—psychological distress, anxiety, and depression.
Q‐statistic: P values < .1 taken to suggest heterogeneity. I2 statistic: 0% (no heterogeneity), 25% (low heterogeneity), 50% (moderate heterogeneity), and 75% (high heterogeneity).
ES = Hedges's g. A positive value indicates an effect size in the hypothesized direction. All ESs were combined using a random effects model. Conventions: small (0.2); medium (0.5); and large (0.8).29
Failsafe N = number of non‐significant studies that would bring the P value to non‐significant (P > .05).
A Failsafe N exceeding the criterion (5x+10) indicates a robust result.34
If analyses indicated possible publication bias, missing studies were imputed and an adjusted ESR calculated (italics).33 K indicates number of published studies + number of imputed studies.
[Correction added on 28 October 2019, after first online publication: In Table 2, under follow‐up, in the psychological distress row, the P is missing a point before the number and it has been corrected from ‘038’ to ‘.038’ and the I2 in the same row has been corrected from ‘40.’ to ‘40.6’. The alignment of columns has also been fixed in this current version.]
Indications of possible publication bias were found for both the primary outcome of combined psychological distress and the secondary outcome of anxiety. When adjusting for publication bias, ESs remained statistically significant and were only slightly smaller. A sensitivity analysis excluding studies of online MBIs did not substantially change the pooled ES for psychological distress. Likewise, omitting the outlier ES of one study52 (Hedges's g = 2.31) did not substantially change the results (Hedges's g = 0.29). Heterogeneity was relatively low (38%) for psychological distress, and omitting the outlier52 further reduced heterogeneity to 10% (data not shown). As seen in Table 2, heterogeneity of the remaining outcomes varied from none (PTSD symptoms) to high (QoL).
As seen in Table 3, only two out of the 17 possible moderators, mean sample age, and type of control group reached statistical significance at post‐intervention. Larger ESs were found in studies with younger participants (β = −0.02) and with passive (g = 0.40) compared with active control groups (g = 0.15; β = 0.23; for detailed subgroup analyses, see Table S3).
Table 3.
Exploring moderators of effects on psychological distressa at post‐intervention and follow‐up: results of meta‐regression
| Moderator (post‐intervention)b | Kc | Betad | 95%CI | P (two‐tailed) |
|---|---|---|---|---|
| Cancer type: breast (referent: mixed) | 27 | −0.10 | −0.25‐0.05 | .174 |
| Cancer stage: non‐metastatic (referent: mixed) | 24 | −0.08 | −0.24‐0.09 | .349 |
| Distress level inclusion criterion (referent: no) | 27 | −0.02 | −0.19‐0.15 | .818 |
| Control: passive (referent: active) | 25 | 0.23 | 0.03‐0.43 | .027 |
| Intervention adaptation: major (referent: minor) | 28 | 0.00 | −0.15‐0.15 | .991 |
| Intention to treat: yes (referent: no) | 29 | −0.06 | −0.29‐0.17 | .632 |
| Gender(% women) | 29 | 0.00 | 0.00‐0.01 | .137 |
| Mean sample age (years) | 29 | −0.02 | −0.03‐0.00 | .017 |
| Time since cancer diagnosis | 12 | 0.00 | −0.01‐0.00 | .698 |
| Time to post‐intervention (weeks) | 29 | 0.00 | −0.04‐0.03 | .794 |
| Intervention dose (total hours intervention) | 26 | 0.01 | −0.00‐0.02 | .122 |
| Home practice/day (minutes) | 11 | 0.01 | 0.00‐0.03 | .082 |
| Attrition rate at post‐intervention (%) | 28 | −0.00 | −0.01‐0.01 | .549 |
| Publication year | 29 | −0.02 | −0.04‐0.00 | .062 |
| Mindfulness‐based intervention quality (range:0‐9) | 28 | −0.04 | −0.10‐0.02 | .193 |
| Moderator (follow‐up)a | K | Betab | 95%CI | P (two‐tailed) |
|---|---|---|---|---|
| Cancer type: breast (referent: mixed) | 16 | −0.05 | −0.25‐0.16 | .670 |
| Cancer stage: non‐metastatic (referent: mixed) | 16 | 0.12 | −0.09‐0.32 | .273 |
| Distress level inclusion criterion (referent: no) | 16 | 0.00 | −0.22‐0.23 | .985 |
| Control: passive (referent: active) | 14 | 0.29 | 0.06‐0.52 | .014 |
| Intervention adaptation: major (referent: minor) | 18 | −0.21 | −0.39‐−0.03 | .024 |
| Intention to treat: yes (referent: no) | 18 | −0.70 | −1.12‐−0.28 | .001 |
| Gender(% women) | 18 | 0.00 | 0.00‐0.01 | .078 |
| Mean sample age (Years) | 18 | −0.02 | −0.03‐0.00 | .028 |
| Time since cancer diagnosis (months) | 8 | −0.00 | −0.01‐0.00 | .062 |
| Time to follow‐up (weeks) | 18 | −0.02 | −0.04‐0.00 | .046 |
| Intervention dose (total hours intervention) | 15 | 0.00 | −0.01‐0.02 | .662 |
| Attrition rate at follow‐up (%) | 16 | −0.01 | −0.02‐0.00 | .124 |
| Publication year | 18 | 0.01 | −0.04‐0.05 | .778 |
| Mindfulness‐based intervention quality (range: 0‐9) | 18 | −0.05 | −0.14‐0.04 | .283 |
Bold values indicate statistical significance.
Primary outcome variable—psychological distress, anxiety, and depression.
Analyses conducted when > 8 studies available for the analysis.
Number of included studies.
Maximum likelihood.
3.3. Pooled effects at follow‐up
As shown in Table 2, statistical significant pooled effects of MBIs were found for both psychological distress (g = 0.19) and for the secondary outcomes of depressive symptoms, sleep disturbance, pain, and symptoms of anxiety (g: 0.20‐0.36). Heterogeneity varied from none (pain) to high (fatigue). With the exception of anxiety, the failsafe Ns did not exceed the criteria, suggesting less robust findings, but there were no indications of publication bias.
As shown in Table 3, five out of the 16 analyzed moderators reached statistical significance at follow‐up. The significant moderators were type of control group, time to follow‐up, ITT analysis, mean sample age, and adaptation of MBI, with larger ESs found for studies with passive (g = 0.30) versus active control groups (g = 0.01; β = 0.29), for studies with shorter time to follow‐up (β = −0.02), for studies with no ITT analysis (g = 0.84) versus studies with ITT analysis (g = 0.14; β = −0.70), for studies with younger samples (β = −0.02), and for studies including none or minor (g = 0.25) versus major adaptations of MBI (g = 0.05; β = −0.21; for results of subgroup analyses, see Table S3).
3.4. Exploring possible MBI mechanisms
As seen in Table 2, statistically significant changes were found for all analyzed possible mechanisms of change at both post‐intervention and follow‐up. As indicated by the failsafe Ns, the results were less robust. A statistically significant association was found between improvements from pre‐ to post‐intervention in mindfulness skills and psychological distress (K = 17, β = 0.49, 95%CI: 0.10‐0.89; P = .015). The association did not reach statistical significance at follow‐up (K = 11, β = 0.20, 95%CI: −0.35‐0.75; P = .472). The studies were too few (K < 8) to explore the associations of changes in self‐compassion and rumination with improvements in psychological distress.
3.5. Risk of bias and MBI quality
As shown in Table S4, most included RCTs were categorized as being at low risk regarding the domains of randomization sequence generation and incomplete outcome data (K = 18, 62% and K = 22, 76%, respectively). The risk of bias was high or unclear for blinding of participants/personnel and outcome assessment in a majority of studies (K = 27, 93% and K = 20, 67%, respectively). Likewise, allocation concealment often went unreported (K = 16, 55%), and twelve studies (41%) were evaluated as being at high risk of bias with respect to selective reporting.
Concerning MBI study quality, most interventions were well‐described (K = 28, 97%), and, in the majority of studies, mindfulness instructors were reported to be mental health professionals (K = 21, 72%; see Table S4). A full description of both education and experience of the mindfulness instructors was only found in 11 RCTs (40%), and only two studies (7%) provided a full description of adherence to the intervention protocol and teacher competency. As shown in Table 3, MBI quality scores were unassociated with effects on psychological distress at both post‐intervention and follow‐up.
3.6. Overall quality of the evidence
Using GRADE,27 the overall quality of the evidence was rated as moderate, suggesting a moderate level of confidence in the effect estimate. The level of evidence for RCTs was downgraded from high to moderate due to serious concerns regarding inconsistency, ie, considerable heterogeneity and inability to identify the reasons for the heterogeneity. Overall, no serious concerns were found for risk of bias, indirectness, imprecision, or publication bias.
4. DISCUSSION
The present comprehensive meta‐analysis provides updated ES estimates for the efficacy of MBIs in cancer patients and survivors and is the first to address moderators and putative working mechanisms in this group. Our results showed small but robust effects of MBIs in cancer patients and survivors on psychological distress combined as well as individual symptoms of anxiety and depression at post‐intervention. The effect on anxiety at follow‐up was robust and of similar magnitude, whereas the effects on overall psychological distress and depression were smaller and less robust. The results in our updated meta‐analysis are generally similar to those reported in previous meta‐analyses of fewer studies for anxiety8, 10, 12 and depression8, 12 but smaller than those reported in two previous meta‐analyses of seven RCTs with usual care or no‐intervention control groups (respectively, SMD = −0.75[anxiety],9 −0.90[depression],9 and −1.13[depression]10).
In addition, MBIs were found to reduce self‐reported fear of cancer recurrence and fatigue at post‐treatment and pain and sleep disturbance at follow‐up, but no effects were found for measures of cancer‐related QoL or post‐traumatic stress symptoms. A previous meta‐analysis of MBSR and MBCT for breast cancer patients did find small but significant effects of these interventions on health‐related QoL in general.12 Possibly, MBIs target general health‐related QoL and not specifically cancer‐related QoL. Studies with passive control conditions (compared with active/competing conditions) and studies with younger participants reported greater reductions in psychological distress at post‐intervention. At follow‐up, larger effects were found for MBIs adhering to the original protocols74, 75 and studies with shorter follow‐up periods. Changes in mindfulness were related to reductions in psychological distress at post‐treatment but not at follow‐up.
4.1. Clinical implications
Our estimates of effects were generally of small magnitude. Although ESs cannot be directly interpreted in terms of clinical relevance, the combined ESs in the literature do not appear to reach the threshold of a minimal clinically important effect.76 It should be noted here that the studies included not only passive control conditions but also active controls and competing interventions, which were found associated with smaller effects and should be considered when interpreting effects.77 Furthermore, despite modest effects, MBIs have previously been found to be cost‐effective,78 easily accessible, and non‐pathologizing.79 It should also be noted that the theoretical underpinnings of mindfulness suggest that, in addition to symptom reduction, MBIs can lead to beneficial effects across various domains, eg, personal growth and healthy lifestyle changes.80 Finally, ESs of MBIs are similar to those found for cognitive behavioral therapy (CBT) with breast cancer patients, with CBT being the currently most commonly used psychosocial intervention for psychological distress in cancer.81 Taken together, MBIs may be relevant treatment options for cancer patients and survivors, although direct comparisons of MBIs with, eg, CBTs are needed for this patient group. This is an important area for future research.
Our results indicate that time since diagnosis was unrelated to intervention gain and that patients with different types and at different stages of cancer may benefit from MBIs, which is accordance with previous qualitative research.82 Younger patients, on the other hand, appeared to benefit more from MBIs than older patients. Considering that participants were relatively young (mean sample age: 55 years) compared with the general population of cancer patients (median age 66 years at diagnosis),2 clinical oncologists are advised that older cancer patients may benefit less from MBIs.
The guidelines of the National Comprehensive Cancer Network suggest that treatment only be offered when distress symptoms are significant.5 These guidelines are inconsistent with our results showing that studies that included patients based on high baseline symptomatology did not find larger effects of MBIs compared with studies that did not. This could, on the other hand, also suggest a possible bias, namely, that patients willing to participate in RCTs are also those who are likely to benefit, even when baseline symptoms are minimal, especially when self‐referral sampling strategies are used.83 In terms of clinical practice, this could indicate that patients with a preference for MBIs are also those who should be referred to MBIs, which is in accordance with research suggesting that patients benefit more when they receive their preferred treatment.84
We did not find differences in efficacy between MBSR and MBCT‐based interventions, suggesting that different types of MBIs may be equally beneficial for cancer patients. However, our results also indicate that it may be important to use MBIs that adhere strongly to the original MBSR or MBCT protocols,74, 75 as these interventions showed larger effects compared with MBIs with major adaptations in duration and content. The number of contact hours during the intervention did not fully explain the difference in efficacy of MBIs with major versus minor adaptations. Exploring MBI quality showed that adherence to the intervention protocol was frequently not reported, and monitoring protocol adherence and trainer quality85 is recommended for clinical practice and future research.
4.2. Research implications
GRADE evaluation showed a moderate level of confidence in our effect estimates due to some level of heterogeneity, with patient, cancer, and intervention characteristics unable to fully explain this heterogeneity. Other type of moderators, eg, psychological traits, could be relevant to explore in future research aiming to optimize intervention gain. The risk of bias assessment also indicated that reporting of allocation concealment and blinding of outcome assessors can be improved. In addition, most studies failed to assess adverse effects, an issue to be addressed in future of MBIs with cancer patients and survivors.86
Changes in mindfulness skills were associated with post‐treatment effects on psychological distress, which is in concordance with findings of a meta‐analysis focusing on mediating mechanisms in a broader sample of participants.87 However, we found no associations at follow‐up, thus impeding an unambiguous interpretation of mindfulness skills as a working mechanism.
4.3. Study limitations
Among the strengths of the present meta‐analysis are its comprehensiveness, including the relatively large number of RCTs (K = 29) reporting on a broad range of cancer‐related symptoms and late effects and focusing on both immediate and longer‐term effects. In addition, to our knowledge, the present meta‐analysis is the first to explore moderators and putative working mechanisms of MBI in this patient group. The primary outcome of psychological distress was broad and included several aspects of psychological distress. This can be viewed as a strength, as it enabled the inclusion of a large number of studies and represents a common psychological symptom cluster in cancer patients and survivors.19 It could, nevertheless, also be a potential cause of heterogeneity challenging the interpretability of results. The heterogeneity of the results was, however, only medium and was mainly due to one outlier study, and we also analyzed all individual distress outcomes separately. Another possible limitation relates to the large number of moderator analyses increasing the risk of type‐1 error. Although we did not adjust for multiple comparisons, as this could increase the risk of type‐2 error, it is recommended that results are interpreted not only according to their statistical significance but also their ES.
In conclusion, MBIs appear efficacious in reducing psychological distress and a number of other psychological and physical symptoms in cancer patients and survivors, while noting that the effects are generally of small magnitude. Patients with different types of cancer at different stages may benefit, and MBIs adhering closely to the original protocols (MBSR and MBCT) appear to have larger effects. Future research could focus on non‐inferiority trials comparing MBI with other psychosocial interventions as well as on working mechanisms of MBIs with the aim of optimizing treatment effects. Moderation studies are needed to identify for whom MBIs may work best, supporting the development of individualized healthcare.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose.
Supporting information
Table S1: Included outcome measures
Table S2: Characteristics of included studies
Table S3: Subgroup analyses
Table S4: Risk of bias and quality of the mindfulness‐based intervention
Cillessen L, Johannsen M, Speckens AEM, Zachariae R. Mindfulness‐based interventions for psychological and physical health outcomes in cancer patients and survivors: A systematic review and meta‐analysis of randomized controlled trials. Psycho‐Oncology. 2019;28:2257–2269. 10.1002/pon.5214
Pre‐registration number PROSPERO: CRD42018096911
[Correction added on 28 October 2019, after first online publication: the affiliations of Anne E.M. Speckens were missing from the author byline and have now been tagged to this version.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. De Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Prevent Biomarkers. 2013;22(4):561‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975‐2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 3. Bosetti C, Bertuccio P, Malvezzi M, et al. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann Oncol. 2013;24(10):2657‐2671. [DOI] [PubMed] [Google Scholar]
- 4. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90(12):2297‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . Distress management. Clinical practice guidelines. Version 2.2018. NCCN Guidelines. 2018.
- 6. Nayak MG, George A, Vidyasagar MS, et al. Quality of life among cancer patients. Indian J Palliat Care. 2017;23(4):445‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weis J. Psychosocial care for cancer patients. Breast Care. 2015;10(2):84‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piet J, Wurtzen H, Zachariae R. The effect of mindfulness‐based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta‐analysis. J Consult Clin Psychol. 2012;80(6):1007‐1020. [DOI] [PubMed] [Google Scholar]
- 9. Zhang M‐F, Wen Y‐S, Liu W‐Y, Peng L‐F, Wu X‐D, Liu Q‐W. Effectiveness of mindfulness‐based therapy for reducing anxiety and depression in patients with cancer: A meta‐analysis. Medicine. 2015;94(45):e0897‐e0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Xu R, Wang B, Wang J. Effects of mindfulness‐based therapy for patients with breast cancer: A systematic review and meta‐analysis. Complement Ther Med. 2016;26:1‐10. [DOI] [PubMed] [Google Scholar]
- 11. Cramer H, Lauche R, Paul A, Dobos G. Mindfulness‐based stress reduction for breast cancer—A systematic review and meta‐analysis. Current Oncology. 2012;19(5):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haller H, Winkler MM, Klose P, Dobos G, Kümmel S, Cramer H. Mindfulness‐based interventions for women with breast cancer: An updated systematic review and meta‐analysis. Acta Oncol. 2017;56(12):1665‐1676. [DOI] [PubMed] [Google Scholar]
- 13. Huang H‐p, He M, Wang H‐y, Zhou M. A meta‐analysis of the benefits of mindfulness‐based stress reduction (MBSR) on psychological function among breast cancer (BC) survivors. Breast Cancer. 2016;23(4):568‐576. [DOI] [PubMed] [Google Scholar]
- 14. Hall DL, Luberto CM, Philpotts LL, Song R, Park ER, Yeh GY. Mind‐body interventions for fear of cancer recurrence: A systematic review and meta‐analysis. Psychooncology. 2018;27(11):2546‐2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness‐based stress reduction on mental health of breast cancer patients: A meta‐analysis. Psychooncology. 2013;22(7):1457‐1465. [DOI] [PubMed] [Google Scholar]
- 16. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long‐term and late effects of cancer. Cancer. 2008;112(S11):2577‐2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlson LE, Speca M, Segal Z. Mindfulness‐based cancer recovery. Oakland, CA: New Harbinger; 2010. [Google Scholar]
- 18. Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3(1):1‐27. [DOI] [PubMed] [Google Scholar]
- 19. Reich RR, Lengacher CA, Alinat CB, et al. Mindfulness‐based stress reduction in post‐treatment breast cancer patients: Immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage. 2017;53(1):85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst Rev. 2012;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sackett DL. Evidence‐based medicine: How to practice and teach EBM: WB Saunders Company; 1997.
- 24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaw JM, Sekelja N, Frasca D, Dhillon HM, Price MA. Being mindful of mindfulness interventions in cancer: A systematic review of intervention reporting and study methodology. Psychooncology. 2018;27(4):1162‐1171. [DOI] [PubMed] [Google Scholar]
- 27. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed). 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biostat inc . Comprehensive meta‐analysis. version 3 2018.
- 29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: erlbaum; 1988. [Google Scholar]
- 30. Hedges L, Olkin I. Statistical methods for meta‐analysis. New York: Academic Press; 1985. [Google Scholar]
- 31. Copas J, Shi JQ. Meta‐analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247‐262. [DOI] [PubMed] [Google Scholar]
- 32. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882‐893. [DOI] [PubMed] [Google Scholar]
- 33. Duval S, Tweedie R. Trim and fill: A simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638‐641. [Google Scholar]
- 35. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta‐analysis. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- 36. Lipsey MW, Wilson DB. Practical meta‐analysis. Thousand Oaks, CA, US: Sage Publications, Inc; 2001. [Google Scholar]
- 37. Blaes AH, Fenner D, Bachanova V, et al. Mindfulness‐based cancer recovery in survivors recovering from chemotherapy and radiation. J Commun Supportive Oncol. 2016;14(8):351‐358. [Google Scholar]
- 38. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2015;121(8):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyle CC, Stanton AL, Ganz PA, Crespi CM, Bower JE. Improvements in emotion regulation following mindfulness meditation: Effects on depressive symptoms and perceived stress in younger breast cancer survivors. J Consult Clin Psychol. 2017;85(4):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bränström R, Kvillemo P, Brandberg Y, Moskowitz JT. Self‐report mindfulness as a mediator of psychological well‐being in a stress reduction intervention for cancer patients—A randomized study. Ann Behav Med. 2010;39(2):151‐161. [DOI] [PubMed] [Google Scholar]
- 41. Bränström R, Kvillemo P, Moskowitz JT. A randomized study of the effects of mindfulness training on psychological well‐being and symptoms of stress in patients treated for cancer at 6‐month follow‐up. Int J Behav Med. 2012;19(4):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bruggeman‐Everts FZ, Wolvers MD, van de Schoot R, Vollenbroek‐Hutten MM, Van der Lee ML. Effectiveness of two web‐based interventions for chronic cancer‐related fatigue compared to an active control condition: Results of the “Fitter na kanker” randomized controlled trial. J Med Internet Res. 2017;19(10):e336 10.2196/jmir.7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of mindfulness‐based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31(25):3119‐3126. [DOI] [PubMed] [Google Scholar]
- 44. Schellekens MP, Tamagawa R, Labelle LE, et al. Mindfulness‐Based Cancer Recovery (MBCR) versus Supportive Expressive Group Therapy (SET) for distressed breast cancer survivors: Evaluating mindfulness and social support as mediators. J Behav Med. 2017;40(3):414‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chambers SK, Occhipinti S, Foley E, et al. Mindfulness‐based cognitive therapy in advanced prostate cancer: A randomized controlled trial. J Clin Oncol. 2017;35(3):291‐297. [DOI] [PubMed] [Google Scholar]
- 46. Compen F, Bisseling E, Schellekens M, et al. Face‐to‐face and internet‐based mindfulness‐based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: A multicenter randomized controlled trial. J Clin Oncol. 2018;36(23):2413‐2421. [DOI] [PubMed] [Google Scholar]
- 47. Foley E, Baillie A, Huxter M, Price M, Sinclair E. Mindfulness‐based cognitive therapy for individuals whose lives have been affected by cancer: A randomized controlled trial. J Consult Clin Psychol. 2010;78(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 48. Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness‐based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449‐457. [DOI] [PubMed] [Google Scholar]
- 49. Garland SN, Rouleau CR, Campbell T, Samuels C, Carlson LE. The comparative impact of mindfulness‐based cancer recovery (MBCR) and cognitive behavior therapy for insomnia (CBT‐I) on sleep and mindfulness in cancer patients. Explore: J Sci Healing. 2015;11(6):445‐454. [DOI] [PubMed] [Google Scholar]
- 50. Henderson VP, Clemow L, Massion AO, Hurley TG, Druker S, Hébert JR. The effects of mindfulness‐based stress reduction on psychosocial outcomes and quality of life in early‐stage breast cancer patients: A randomized trial. Breast Cancer Res Treat. 2012;131(1):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness‐based stress reduction in mood, breast‐and endocrine‐related quality of life, and well‐being in stage 0 to III breast cancer: A randomized, controlled trial. J Clin Oncol. 2012;30(12):1335‐1342. [DOI] [PubMed] [Google Scholar]
- 52. Jang S‐H, Kang S‐Y, Lee H‐J, Lee S‐Y. Beneficial effect of mindfulness‐based art therapy in patients with breast cancer—A randomized controlled trial. Explore: J Sci Healing. 2016;12(5):333‐340. [DOI] [PubMed] [Google Scholar]
- 53. Johannsen M, O'Connor M, O'Toole MS, Jensen AB, Højris I, Zachariae R. Efficacy of mindfulness‐based cognitive therapy on late post‐treatment pain in women treated for primary breast cancer: A randomized controlled trial. J Clin Oncol. 2016;34(28):3390‐3399. [DOI] [PubMed] [Google Scholar]
- 54. Johannsen M, O'Connor M, O'Toole MS, Jensen AB, Zachariae R. Mindfulness‐based cognitive therapy and persistent pain in women treated for primary breast cancer: Exploring possible statistical mediators results from a randomized controlled trial. Clin J Pain. 2018;34(1):59‐67. [DOI] [PubMed] [Google Scholar]
- 55. Johns SA, Brown LF, Beck‐Coon K, Monahan PO, Tong Y, Kroenke K. Randomized controlled pilot study of mindfulness‐based stress reduction for persistently fatigued cancer survivors. Psychooncology. 2015;24(8):885‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johns SA, Brown LF, Beck‐Coon K, et al. Randomized controlled pilot trial of mindfulness‐based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support Care Cancer. 2016;24(10):4085‐4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johns SA, Von Ah D, Brown LF, et al. Randomized controlled pilot trial of mindfulness‐based stress reduction for breast and colorectal cancer survivors: Effects on cancer‐related cognitive impairment. J Cancer Surviv. 2016;10(3):437‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kenne Sarenmalm E, Mårtensson LB, Andersson BA, Karlsson P, Bergh I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017;6(5):1108‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kingston T, Collier S, Hevey D, et al. Mindfulness‐based cognitive therapy for psycho‐oncology patients: An exploratory study. Irish J Psychol Med. 2015;32(3):265‐274. [DOI] [PubMed] [Google Scholar]
- 60. Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness‐based stress reduction in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2016;34(24):2827‐2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lengacher CA, Johnson‐Mallard V, Post‐White J, et al. Randomized controlled trial of mindfulness‐based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261‐1272. [DOI] [PubMed] [Google Scholar]
- 62. Lerman R, Jarski R, Rea H, Gellish R, Vicini F. Improving symptoms and quality of life of female cancer survivors: A randomized controlled study. Ann Surg Oncol. 2012;19(2):373‐378. [DOI] [PubMed] [Google Scholar]
- 63. Monti DA, Peterson C, Kunkel EJS, et al. A randomized, controlled trial of mindfulness‐based art therapy (MBAT) for women with cancer. Psycho‐Oncol: J Psychol, Social Behav Dimensions Cancer. 2006;15(5):363‐373. [DOI] [PubMed] [Google Scholar]
- 64. Monti DA, Kash KM, Kunkel EJ, et al. Psychosocial benefits of a novel mindfulness intervention versus standard support in distressed women with breast cancer. Psychooncology. 2013;22(11):2565‐2575. [DOI] [PubMed] [Google Scholar]
- 65. Nakamura Y, Lipschitz DL, Kuhn R, Kinney AY, Donaldson GW. Investigating efficacy of two brief mind–body intervention programs for managing sleep disturbance in cancer survivors: A pilot randomized controlled trial. J Cancer Surviv. 2013;7(2):165‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reynolds LM, Bissett IP, Porter D, Consedine NS. A brief mindfulness intervention is associated with negative outcomes in a randomised controlled trial among chemotherapy patients. Mind. 2017;8(5):1291‐1303. [Google Scholar]
- 67. Schellekens MPJ, Hurk DGM, Prins JB, et al. Mindfulness‐based stress reduction added to care as usual for lung cancer patients and/or their partners: A multicentre randomized controlled trial. Psychooncology. 2017;26(12):2118‐2126. [DOI] [PubMed] [Google Scholar]
- 68. Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait‐list controlled clinical trial: The effect of a mindfulness meditation‐based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62(5):613‐622. [DOI] [PubMed] [Google Scholar]
- 69. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self‐reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for stage I–III breast cancer. Eur J Cancer. 2013;49(6):1365‐1373. [DOI] [PubMed] [Google Scholar]
- 70. Würtzen H, Dalton SO, Christensen J, et al. Effect of mindfulness‐based stress reduction on somatic symptoms, distress, mindfulness and spiritual wellbeing in women with breast cancer: Results of a randomized controlled trial. Acta Oncol. 2015;54(5):712‐719. [DOI] [PubMed] [Google Scholar]
- 71. Andersen SR, Würtzen H, Steding‐Jessen M, et al. Effect of mindfulness‐based stress reduction on sleep quality: Results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52(2):336‐344. [DOI] [PubMed] [Google Scholar]
- 72. Zernicke KA, Campbell TS, Speca M, McCabe‐Ruff K, Flowers S, Carlson LE. A randomized wait‐list controlled trial of feasibility and efficacy of an online mindfulness‐based cancer recovery program: The eTherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76(4):257‐267. [DOI] [PubMed] [Google Scholar]
- 73. Zhang J‐Y, Zhou Y‐Q, Feng Z‐W, Fan Y‐N, Zeng G‐C, Wei L. Randomized controlled trial of mindfulness‐based stress reduction (MBSR) on posttraumatic growth of Chinese breast cancer survivors. Psychol Health Med. 2017;22(1):94‐109. [DOI] [PubMed] [Google Scholar]
- 74. Kabat‐Zinn J, Hanh TN. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Delta; 2009. [Google Scholar]
- 75. Segal ZV, Williams JMG, Teasdale JD. Mindfulness‐based cognitive therapy for depression. New York: Guilford Press; 2013. [Google Scholar]
- 76. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582‐592. [DOI] [PubMed] [Google Scholar]
- 77. Rosenkranz MA, Dunne JD, Davidson RJ. The next generation of mindfulness‐based intervention research: What have we learned and where are we headed? Current Opinion in Psychology. 2019. [DOI] [PMC free article] [PubMed]
- 78. Compen FR, Adang EMM, Bisseling EM, Van der Lee ML, Speckens AEM. Cost‐effectiveness of individual internet‐based and face‐to‐face mindfulness‐based cognitive therapy compared to treatment as usual in reducing psychological distress in cancer patients. Submitted [DOI] [PMC free article] [PubMed]
- 79. Monteiro LM, Compson JF, Musten F. Practitioner's guide to ethics and mindfulness‐based interventions. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 80. Ludwig DS, Kabat‐Zinn J. Mindfulness in medicine. JAMA. 2008;v300(11):1350‐1352. [DOI] [PubMed] [Google Scholar]
- 81. Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: A meta‐analysis. J Behav Med. 2006;29(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 82. Bisseling EM, Schellekens MP, Jansen ET, van Laarhoven HW, Prins JB, Speckens AE. Mindfulness‐based stress reduction for breast cancer patients: A mixed method study on what patients experience as a suitable stage to participate. Support Care Cancer. 2017;25(10):3067‐3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thewes B, Rietjens JA, van den Berg SW, et al. One way or another: The opportunities and pitfalls of self‐referral and consecutive sampling as recruitment strategies for psycho‐oncology intervention trials. Psychooncology. 2018;27(8):2056‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carlson LE, Tamagawa R, Stephen J, et al. Tailoring mind‐body therapies to individual needs: Patients' program preference and psychological traits as moderators of the effects of mindfulness‐based cancer recovery and supportive‐expressive therapy in distressed breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014(50):308‐314. [DOI] [PubMed] [Google Scholar]
- 85. Crane RS, Eames C, Kuyken W, et al. Development and validation of the mindfulness‐based interventions–teaching assessment criteria (MBI: TAC). Assessment. 2013;20(6):681‐688. [DOI] [PubMed] [Google Scholar]
- 86. Baer R, Crane C, Miller E, Kuyken W. Doing no harm in mindfulness‐based programs: Conceptual issues and empirical findings. Clin Psychol Rev. 2019;71:101‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness‐based cognitive therapy and mindfulness‐based stress reduction improve mental health and wellbeing? A systematic review and meta‐analysis of mediation studies. Clin Psychol Rev. 2015;37:1‐12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Included outcome measures
Table S2: Characteristics of included studies
Table S3: Subgroup analyses
Table S4: Risk of bias and quality of the mindfulness‐based intervention
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


