Abstract
Background
Geriatricians are often confronted with unexpected health outcomes in older adults with complex multimorbidity. Aging researchers have recently called for a focus on physical resilience as a new approach to explaining such outcomes. Physical resilience, defined as the ability to resist functional decline or recover health following a stressor, is an emerging construct.
Methods
Based on an outline of the state‐of‐the‐art in research on the measurement of physical resilience, this article describes what tests to predict resilience can already be used in clinical practice and which innovations are to be expected soon.
Results
An older adult's recovery potential is currently predicted by static tests of physiological reserves. Although geriatric medicine typically adopts a multidisciplinary view of the patient and implicitly performs resilience management to a certain extent, clinical management of older adults can benefit from explicitly applying the dynamical concept of resilience. Two crucial leads for advancing our capacity to measure and manage the resilience of individual patients are advocated: first, performing multiple repeated measurements around a stressor can provide insight about the patient's dynamic responses to stressors; and, second, linking psychological and physiological subsystems, as proposed by network studies on resilience, can provide insight into dynamic interactions involved in a resilient response.
Conclusion
A big challenge still lies ahead in translating the dynamical concept of resilience into clinical tools and guidelines. As a first step in bridging this gap, this article outlines what opportunities clinicians and researchers can already exploit to improve prediction, understanding, and management of resilience of older adults. J Am Geriatr Soc 67:2650–2657, 2019
Keywords: adaptive capacity, complex dynamical system, personalized medicine, resistance, time series analysis
Dealing with uncertain outcomes in older adults is inherent in the work of geriatricians. When older persons face a stressor, geriatricians often observe health outcomes they could not predict, nor fully understand: a surprising restoration of functioning in a patient with multimorbidity or an unforeseen worsening in an older person who was not judged to be frail. In current clinical reasoning, we are inclined to explain what we observe in terms of linear cause‐effect relations (stressor → functional decline) or simple additive burden of disease (multiple/larger stressors → worse outcome). However, frequently outcomes are not proportional to stressor burden. While managing clinical uncertainty is an integral part of “the art of medicine”,1 we do not need to accept all uncertainty as inevitable. Much can be gained if we target not just the disorder(s) a patient is confronted with but also the person's capacity to recover from disease, which is called physical resilience. An individual's potential for recovery after a health stressor can only be defined or measured in the presence of a stressor that elicits a complex, dynamic process of recovery.2, 3, 4, 5, 6, 7, 8 If the spectrum from robustness to frailty reflects the physiological potential one has to recover from stressors, resilience refers to the actualization of that potential.8
The emerging construct of physical resilience was characterized in a systematic review by Whitson and colleagues, published in 2015.9 This article put forward the notion that a better understanding of a person's ability to recover health after a health stressor has significant clinical implications. Finding ways to measure a person's physical resilience was termed as one of the research priorities by the National Institute of Aging.4 This article is based on an updated review of the literature on physical resilience, presented in Supplementary Materials. Building on the current state of evidence, it outlines opportunities for researchers and clinicians to apply the dynamical concept of resilience, which can give rise to new tools for quantitative, personalized prediction of recovery potential in older persons. It is beneficial to involve clinicians in the emerging dialogues on physical resilience for two reasons. First, clinicians can already apply recent theoretical insights in their clinical management of older persons. Second, the clinical perspective offers valuable knowledge about the recovery potential of older adults that is complementary to the research perspective that has dominated the debate on physical resilience so far.
STATE OF THE ART: STATIC TESTS OF PHYSIOLOGICAL RESERVES
To start with, this article will briefly describe the current state of knowledge. To this end, a literature search was performed, specifically addressing predictors of recovery or resilience in (frail) older adults. Since this is an update to the 2015 systematic review,9 the search was limited to articles published within the last 5 years. “Recovery” was added as a search term because studies may address aspects of physical resilience without explicitly using the term. Methods and results of this literature review are detailed in the Supplementary Information. Table 1 summarizes the most important insights gained from the literature.
Table 1.
Summary of current literature on prediction of recovery potential
|
The 27 selected studies reflect an important amount of work on improving the assessment of the recovery potential of older adults. From the literature review, it can be concluded that most well‐studied clinical predictors of recovery are static tests of physiological reserves over multiple dimensions of functioning (eg, physical, psychological, and social). This is in line with resilience being a whole‐person capacity. However, while comparing two measurements—one before (T0) and one directly after the stressor (T1)—with an outcome in the future (T2) (Figure 1A) is more informative with regard to tracking the recovery process, most studies did not include the T1 measurement. Moreover, if three measurements (T0, T1, and T2) around a stressor are performed, the resulting trajectory still does not capture the variably fluctuating physiologic response of an individual (Figure 1B). In addition, the various stressors eliciting the functional decline were—if specified in the first place—not quantified. Last, the outcomes studied were often single and dichotomous (ie, recovery or no recovery) while multidimensional and quantified outcomes are much more relevant.10, 11
Figure 1.
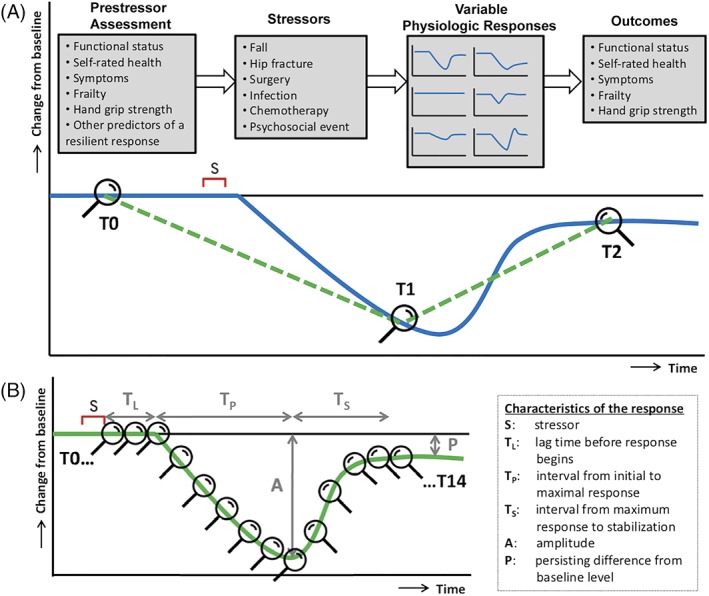
A, The recovery paradigm, as currently applied by most studies on predicting recovery potential. The measurements before the stressor (T0), after the stressor (T1), and in the future (T2) enable us to draw the green dashed line, which is an improvement over two time points (T0 and T2). However, the green line still does not capture the variable “real‐world” physiological responses of individuals, of which one example is represented by the blue solid line. B, The dynamical resilience paradigm allows the construction of more detailed trajectories of recovery from a stressor that provide insight in individual dynamic responses. This trajectory can be drawn if multiple repeated measurements (eg, T0‐T14) are performed. In this case, different characteristics of the response to a stressor can be quantified and used as measures of resilience. Figure 1B adapted from Hadley et al.4
Overlooking patients' variable physiological responses over time by performing too few measurements is a common pitfall of biomedical research and hinders progress in the development of personalized medicine.12 Many of the selected articles directly or indirectly referred to the difficulty of studying real‐world functional recovery in a geriatric population with great clinical complexity. This underscores the need for the development of new study designs and analysis approaches. Building on the findings from literature review, the remainder of this article will describe how the dynamical concept of resilience can provide opportunities to get a better grip on the recovery potential of older adults.
ADDED VALUE OF THE RESILIENCE CONCEPT
Although medicine has traditionally focused on managing disease, in geriatric medicine, resilience management is already implicitly performed to a certain extent, albeit not explicitly defined as such.13, 14 In geriatric evaluation and management units (GEMUs), older adults' recovery potential is routinely estimated by performing a comprehensive geriatric assessment (CGA). This holistic approach provides information regarding the expected rehabilitation time and possibility to return home.15 The successful implementation of CGA and GEMUs and availability of multidisciplinary geriatric teams to deliver personalized, multidisciplinary care has been shown to improve the outcomes of (frail) older adults admitted to the hospital16 and those living at home.17 However, we are still far from a wide, systematic, and standardized implementation of CGA in the evaluation of older persons. Building a robust CGA‐based network within the healthcare system would greatly facilitate the assessment of resilience. In addition to CGA, research on frailty has enabled us to assess the reserves accounting for the likelihood of recovery. However, although frailty is considered a dynamic process, it has been operationalized as a static measure that by definition cannot reflect the body's complex dynamic interactions in response to a stressor that are key to the recovery process.3, 8, 18
Building further on the important foundation of CGA and frailty, adding a dynamical dimension through resilience measurements may offer a way forward in personalizing the prediction and management of recovery.19 Quantitative tools to dynamically measure resilience can complement CGA‐based clinical intuitions and hence boost clinical resilience management.4, 9 In this review, two crucial leads for developing such tools and expanding our capacity to manage the resilience of individual patients are advocated: first, performing multiple repeated measurements around a stressor can provide insight about the patient's dynamic responses to stressors; and, second, linking psychological and physiological subsystems can provide insight into dynamic interactions involved in a resilient response. We describe both lines with regard to what opportunities clinicians and researchers can already exploit and what knowledge and tools still need to be developed.
DYNAMICAL RESILIENCE MEASUREMENTS
The first and simplest strategy to add dynamical measurements to recovery research is to increase the number of measurements around a stressor. This allows us to draw and compare detailed recovery trajectories (Figure 1B) and acknowledge the large heterogeneity in the recovery response between persons.
Resilience Trajectories
Essential to constructing resilience trajectories prospectively is synchronization at the time of the stressor. Unless the stressor is planned (eg, elective surgery), the prestressor assessment has to be performed retrospectively, which may introduce subjectivity and recall bias, especially in the setting of cognitive impairment. An alternative study design is performing repeated measurements in a large cohort and waiting for stressors to occur. Longitudinal studies with multiple repeated measurements over a prolonged period that also include details about the period around the stressor (eg, through “measurement‐burst” study designs20) are scarce but valuable. The five studies from the literature review that included monthly,21 weekly,22 or daily23, 24, 25 measurements after hospitalization or surgery showed that mapping recovery of functioning with higher temporal resolution can delineate distinct recovery patterns for individual patients. Moreover, recording recovery across multiple domains (ie, activities of daily living, Geriatric Depression Scale, and Mini‐Mental State Examination) revealed that the course of recovery varies between organ subsystems of the body.22 In addition to questionnaires, physical tests can be performed repeatedly. This could also be done at home, as community‐dwelling older adults appeared able and willing to self‐assess maximum step length and gait speed weekly for a 6‐month period.26 Furthermore, step counters could be used to monitor patients at home and allow healthcare professionals to intervene early.27 Also, nonwearable technology is increasingly available, such as infrared sensors placed in older adults' homes that measure walking speed, total daily activity, and time out of home.28 Incorporating such measurements in routine clinical practice is timely and could provide a rich resource for constructing resilience trajectories sensitively and objectively.29
Stimulus‐Response Tests
Another dynamical measurement is a stimulus‐response test that involves standardized probing of a physiological function with an experimental stressor and monitoring the response.3 Well‐known examples are monitoring heart rate around an exercise stress test or blood pressure around an orthostatic challenge. Heart rate recovery after treadmill testing is an independent predictor of mortality in older adults who are able to exercise.30 Impaired systolic blood pressure recovery in the first minute after standing is associated with frailty and with mortality in older adults.31, 32 Also, longer recovery times of glucose levels after a high‐glucose challenge are related to frailty.33 In clinical care, stimulus‐response tests could potentially be used to support the identification of older adults at risk of functional decline after major treatments (eg, surgery or chemotherapy) or after being admitted to the emergency department for an acute illness, where they may help expedite subsequent treatment and appropriate disposition.
However, one challenge is to develop stimulus‐response tests that are reasonably safe and practically feasible for frail patients. In addition, important questions about stimulus‐response tests remain unanswered. Does the resilience of a specific physiological subsystem stressed by a certain stimulus reflect resilience of the whole person or only the resilience of the subsystem itself? And, if so, which subsystem(s) should be probed, and in what context, to most reliably estimate whole‐person resilience? Is an older person's response to an experimental stressor that is considered to be safe and exerted under controlled conditions indicative of resilience under real‐life circumstances? The “holy grail” measure of whole‐person resilience to any stressor may not exist; instead, several measures may be needed to fully capture a person's systemic resilience.
Microrecoveries in Response to Natural Perturbations
There might be a way to circumvent some of the drawbacks of stimulus‐response tests. Instead of artificially perturbing the body, one could also use the fact that a human being is constantly subject to natural perturbations from the environment and must respond to these tiny challenges to maintain homeostasis. When continuously monitoring system parameters, the system's dynamic responses to such everyday challenges can be captured. Although most natural perturbations may be small, zooming in on the “microrecoveries” of system parameters may give an impression of the system's resilience. In time series with sufficiently high frequency and length, dynamical indicators of resilience (DIORs), such as variance and temporal autocorrelation, can be calculated. DIORs have been developed as predictors in other complex dynamical systems, such as ecosystems and the climate,34 and have been hypothesized as a means to quantify resilience of humans as well.35, 36
DIORs were tested in a previous study that monitored self‐rated health in a small group of older adults.37 These older persons rated their own physical, mental, and social health daily for 100 consecutive days. It was hypothesized that during this period, a frail older adult would have more ups and downs (resulting in increased variance) and would recover more slowly from perturbations, such as a fall, an infection, or an emotional stressor (resulting in increased temporal autocorrelation) than a nonfrail older adult. By showing that these two DIORs were related to frailty scores, preliminary evidence for DIORs as measures of resilience and the empirical link between the concepts of frailty and resilience were provided. In another study, DIORs were tested on time series of postural balance and showed that these were related to successful aging of high‐functioning older adults.38 A third study measured DIORs in time series of mood (eg, rated 10 times a day during 5‐6 consecutive days) and found them to mark the risk of a major depression later in life,39 also within one person.40 DIORs may provide complementary insights to other time series metrics, such as the complexity of the fast dynamics of physiological parameters that may be lost with aging and disease.7, 41
Applicability in Clinical Care
The discussed dynamical resilience measurements (trajectories, stimulus‐response tests, and microrecoveries) are not yet sufficiently robust, validated, and technologically embedded in clinical workflow to be translated to guideline‐driven resilience measurements in routine clinical care. However, they are ready to be used and enhance clinicians' understanding of the resilience of the whole patient and/or subsystems. For example, recovery of systolic blood pressure after change of posture to less than 80% of baseline after 60 seconds in beat‐to‐beat blood pressure measurements may be used as an easily available marker for decreased cardiovascular resilience and increased mortality risk.32 Similarly, recovery trajectories after a recent disease (eg, influenza, cardiac decompensation, or chronic obstructive pulmonary disease exacerbation) or intervention (eg, hip replacement) may be one of the best available individual predictors of the upcoming recovery trajectory after a highly similar stressor. In a recent systematic review on risk factors for the development of postoperative delirium, history of delirium proved to have the highest odds ratio.42 While the reliance on past recovery patterns to predict future outcomes still needs to be validated for many stressor‐outcome scenarios, this concept has high face validity. For widespread use, however, it would be crucial that repeated measures of function after a health stressor are carefully documented in clinical care, which is not common practice. For example, step counters, tracking patients' recovery after surgery, seem promising in predictive value,25, 27 but they were only used in a research setting, which does not (yet) allow real‐time feedback to be sent to the clinicians in charge of the patient. If future studies prove that such dynamical measurements have added value, efforts toward technological embedding in health records could be envisioned in the near future.
The 2015 systematic review provided a table with a summary of key research questions and directions concerning the measurement of physical resilience.9 To stimulate translation of future research efforts to clinical care, this article makes specific recommendations to researchers as well as clinicians (Table 2).
Table 2.
Recommendations to advance the measurement of physical resilience of older adults
|
Goal for Researchers: to develop valid dynamical resilience measurements that can inform clinicians' intuitions about the resilience of their patients.
How:
|
|
Goal for Clinicians: to improve their clinical management of older persons by applying recent research insights about physical resilience.
How:
|
Abbreviation: SHERPA, Sharing Evidence Routine for a Person‐Centered Plan for Action.
Characterization of the Stressor
Differences between individual responses to perturbations not only depend on the person's resilience but also on the type of stressor. Therefore, a response always needs to be indexed with reference to the stressor.3, 4, 6, 7, 43 For example, elective hip replacement surgery, as it is well planned and less injurious, may generally be considered a smaller stressor as compared to a trauma resulting in hip fracture requiring surgery. As a result, the accompanying recovery trajectories will differ. Stressors can include (non)elective surgery, hospitalization, chemotherapy, periods of (in)activity, and numerous acute pathophysiologic events (eg, infections, ischemic cardiovascular events, and fall‐related physical complaints), as well as psychosocial stressors (eg, death of a spouse and moving house). Acute stressors (perturbations) are contrasted with chronic exposures (eg, chronic mental stress) that slowly drive the system toward a less resilient state.
Efforts directed toward characterizing stressors should be included in future longitudinal data collections, beginning with carefully describing the type, intensity, frequency, and timing of the stressor(s). Identification and quantification of stressors have remained elusive due to their unpredictability and the highly variable responses elicited by them. The clear‐cut, smooth response to a known stressor that is shown in Figure 1 is rather artificial—in reality, multiple stressors may act at the same time, with different strengths and in different directions (positive/negative). Furthermore, stressors occurring simultaneously may produce unforeseeable, disproportional effects in the individual. Recognizing the real‐world complexity of the geriatric patient is important but does not preclude the advancement of our understanding and assessment of resilience.
LINKING INTERACTIONS BETWEEN SUBSYSTEMS
The understanding and assessment of resilience could be increased further by not only looking at a single global outcome (ie, the level of overall functioning), but also monitoring the functioning of multiple physiological subsystems over time. Two emerging fields—network medicine and network physiology—are poised to provide new insights into the interactions among organ systems driving resilience44 and to advance personalization of healthcare.45 In the field of psychopathology, the network theory of mental disorders has received considerable attention and recognition in recent years.46 It challenges the traditional way of thinking based on the “disease paradigm,” which assumes that symptoms are caused by a distinct underlying medical condition (eg, depressed mood, insomnia, and fatigue are caused by a depression). Instead, the mental state of an individual is conceptualized as a network of symptoms and factors (eg, physical activity), and mental disorders arise from the interplay between these symptoms and factors.6 A similar shift in thinking has been suggested with regard to geriatric syndromes, which were conceptualized as not having a single underlying pathophysiology but emerge from the complex interactions between multiple vulnerabilities of an individual and environmental challenges.47, 48 The same interconnections also allow for the unique and spontaneous recovery of some patients.49 The following paragraphs outline how a focus on dynamic interactions among physiological subsystems can be adopted in research and clinical practice.
Resilience and Organ Cross‐Correlations
Linking the dynamic functioning of multiple organs will provide insight about the degree to which they rely on each other. Aging is characterized by a gradual decrease in reserves of physiological systems, rendering them less resilient on their own (Figure 2A) and becoming more mutually dependent (Figure 2B). Hence, a disturbance in the functioning of one organ is more readily reflected in another organ. For example, in a frail older adult, a “simple” bladder infection may not only provide a challenge to the urinary tract and the immune system but also elicit delirium and functional decline. When synchronously monitoring multiple physiological parameters over time, preliminary data show that the resulting time series become more correlated.50 This has also been shown in models of complex nonhuman biological subsystems.51
Figure 2.
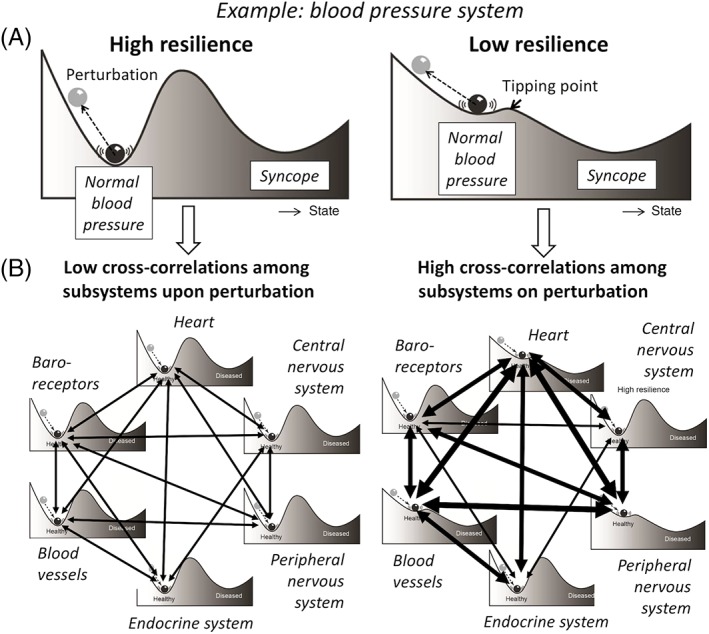
A, Each bodily system has its own level of resilience, depicted by the resilience landscape. When the ball (eg, the blood pressure system) lies in a deep well (has a high resilience), even a large perturbation will not push it over the tipping point to a different state (eg, syncope). While for a ball lying in a shallow well (low resilience), a small stressor (eg, orthostasis) is already sufficient to start the ball rolling. B, Each bodily system is, in turn, a network of subsystems with different levels of resilience. Since subsystems with low resilience are theorized to recover more slowly, they will also become more mutually dependent on each other, here illustrated by the on average stronger hypothetical links between blood pressure regulation subsystems. Hence, perturbations will spread more readily throughout the network, reflected by higher cross‐correlations between the dynamic fluctuations of physiological subsystems. This process diminishes the recovery potential of the person as a whole. Figure adapted from Scheffer et al.35
This idea was further explored in self‐rated health time series. It was hypothesized that, at the time of experiencing a physical dip, a frail older adult is more likely to feel also mentally and socially less well compared to a person with higher resilience. Indeed, frailty scores were associated with increased cross‐correlations among the physical, mental, and social time series.37 Such cross‐correlations among subsystems can be studied as a third DIOR when time series data capturing synchronous fluctuations of multiple bodily functions are collected.
Adopting a Network Approach in Research
Advancing this field will require an empirical foundation for the links among bodily subsystems. Adopting the network approach means defining and analyzing relationships between the patient's clinical signs, without assuming a priori that such relationships arise from a disease as a single common cause.52 Several empirical studies that applied the network approach to aging and frailty have already been published.53, 54, 55 A study of network models of frailty deficits demonstrated that deficits that have the most connections to other deficits are major contributors to the risk of death.53 This suggests that targeting highly connected deficits with therapy may be an effective strategy to improve resilience. Moreover, a study of networks of comorbidities tracking their structural changes over the life course revealed that patients predominantly develop diseases that are in close network proximity to disorders that they already have (eg, hypertension and chronic ischemic heart disease).54 Gaining insight into the relationships among diseases and disabilities allows patients, families, and clinicians to set priorities in the care plan and prevent new disabilities.
Extending Clinical Reasoning by Addressing Interconnections
All healthcare providers working with older adults can increase their understanding of resilience and recovery by explicitly taking note of the observed interactions between the patient's signs and symptoms in daily clinical communication. This exploration may help increase awareness and make sense of the multisystem connections at all levels that contribute to the patient's varying course of recovery or decline and may offer unique opportunities for resilience management. A step‐by‐step plan for such innovative clinical reasoning was recently proposed in the Sharing Evidence Routine for a Person‐Centered Plan for Action (SHERPA) framework.56 The SHERPA step plan results in a network of problems written on paper that can facilitate shared decision making with patients and colleagues. Rather than giving a snapshot of a patient's condition, the recovery trajectories of multiple subsystems (including the mental and social domains) are followed over time, beginning before the stressor and continuing throughout the clinical encounter, treatment, and the recovery or further decline.57
Sharing resilience narrative storylines of observed multisystem dynamics in real patients will already increase our understanding of how resilience comes about. Experienced geriatricians will find it more intuitive to extend their clinical reasoning in this way. They can mentor novice physicians to recognize the interactions between the patient's signs, symptoms, test results, and subsequent consequences. Since nurses traditionally have a holistic view on patient care, involving them (and other healthcare professionals) in this line of thinking is a natural step.58 Together, we can develop tools for describing what is actually happening with the patient and foster the advancement of multidisciplinary clinical resilience management.
CONCLUSION
Physical resilience as a paradigm may offer a next step to take geriatric medicine to a higher level. Resilience cannot be grasped in its entirety in one study or measurement. However, by performing dynamical, multisystem measurements and comparing these with clinical data, clinicians and researchers together could aim to assess the signatures of successful clinical intervention in (frail) older adults. Researchers need to work on the design of future longitudinal studies that capture the dynamic responses to stressors by including repeated or continuous measurements in the period directly before and after health stressors. In addition, ways to quantify the stressor need to be tested to be able to compare recovery trajectories of individual patients. Clinicians need to extend their clinical reasoning by addressing links between multiple subsystems over time. Importantly, any tool to objectively measure physical resilience will always serve to inform—not replace—clinical intuitions about the recovery potential of the patient receiving care. Although there are already opportunities at hand to benefit from the physical resilience concept in clinical care, a big challenge lies ahead in its translation into clinical tools, evidence, and guidelines. An important first step for clinicians is to introduce resilience‐related terminology into clinical reasoning and explicitly consider the question: “How resilient is this patient?”
Supporting information
Supplementary Appendix S1. Supplementary Information.
ACKNOWLEDGMENTS
The authors acknowledge the intellectual contributions to Figure 1A of Chhanda Dutta and Basil Eldadah (Division of Geriatrics and Clinical Gerontology, National Institute on Aging) that resulted from personal communication. We are grateful to Thea Heil and Julian Lieverse (Department of Geriatrics, Radboud University Medical Center, Nijmegen, The Netherlands) for critical reading of the manuscript and providing feedback.
Financial Disclosure
Dr. Whitson's contributions are supported by the Duke Claude D. Pepper Older American Independence Center (P30AG028716), the Physical Resilience Indicators and Mechanisms in the Elderly Collaborative (UH2AG056925), the Advancing Geriatrics Infrastructure and Network Growth Initiative (R33AG057806), and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002553).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
S.M.W.G.: drafting of the manuscript. R.J.F.M. and M.G.M.O.R.: critical revision of multiple versions of the manuscript for important intellectual content. H.E.W., I.A.L., M.S., D.A., and J.L.R.: critical revision of the manuscript. All authors: final approval for submission.
Sponsor's Role
None.
REFERENCES
- 1. Simpkin AL, Schwartzstein RM. Tolerating uncertainty: the next medical revolution? N Engl J Med. 2016;375:1713‐1715. [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. [DOI] [PubMed] [Google Scholar]
- 3. Varadhan R, Seplaki CL, Xue QL, Bandeen‐Roche K, Fried LP. Stimulus‐response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129:666‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hadley EC, Kuchel GA, Newman AB. Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalisch R, Baker DG, Basten U, et al. The resilience framework as a strategy to combat stress‐related disorders. Nat Hum Behav. 2017;1:784‐790. [DOI] [PubMed] [Google Scholar]
- 6. Nelson B, McGorry PD, Wichers M, Wigman JTW, Hartmann JA. Moving from static to dynamic models of the onset of mental disorder: a review. JAMA Psychiat. 2017;74:528‐534. [DOI] [PubMed] [Google Scholar]
- 7. Varadhan R, Walston JD, Bandeen‐Roche K. Can a link be found between physical resilience and frailty in older adults by studying dynamical systems? J Am Geriatr Soc. 2018;66:1455‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitson HE, Cohen HJ, Schmader KE, Kuchel GA, Colon‐Emeric CS. Physical resilience: not simply the opposite of frailty. J Am Geriatr Soc. 2018;66:1459‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitson HE, Duan‐Porter WD, Schmader KE, Morey MC, Cohen HJ, Colon‐Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2015;71:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colón‐Emeric C, Pieper CF, Schmader K, et al. Two approaches to classifying and quantifying physical resilience in longitudinal data. J Gerontol A Biol Sci Med Sci. 2019; https://www.ncbi.nlm.nih.gov/pubmed/30993327 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cosco TD, Kaushal A, Hardy R, Richards M, Kuh D, Stafford M. Operationalising resilience in longitudinal studies: a systematic review of methodological approaches. J Epidemiol Community Health. 2017;71:98‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563:619‐621. [DOI] [PubMed] [Google Scholar]
- 13. Van Craen K, Braes T, Wellens N, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta‐analysis. J Am Geriatr Soc. 2010;58:83‐92. [DOI] [PubMed] [Google Scholar]
- 14. Colón‐Emeric CS, Whitson HE, Pavon J, Hoenig H. Functional decline in older adults. Am Fam Physician. 2013;88:388‐394. [PMC free article] [PubMed] [Google Scholar]
- 15. Abrahamsen JF, Haugland C, Ranhoff AH. Assessment of recovery in older patients hospitalized with different diagnoses and functional levels, evaluated with and without geriatric assessment. Eur Rev Aging Phys Act. 2016;13:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stuck AE, Minder CE, Peter‐Wüest I, et al. A randomized trial of in‐home visits for disability prevention in community‐dwelling older people at low and high risk for nursing home admission. Arch Intern Med. 2010;160:977‐986. [DOI] [PubMed] [Google Scholar]
- 18. Kuchel GA. Aging and homeostatic regulation In: Halter JB, Ouslander JG, Studenski S, et al., eds. Hazzard's Geriatric Medicine and Gerontology. 7th ed New York, NY: McGraw‐Hill; 2017:681‐690. [Google Scholar]
- 19. Corazza GR, Formagnana P, Lenti MV. Bringing complexity into clinical practice: an internistic approach. Eur J Intern Med. 2018;61:9‐14. [DOI] [PubMed] [Google Scholar]
- 20. Ram N, Gerstorf D. Time‐structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychol Aging. 2009;24:778‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maxwell CA, Mion LC, Mukherjee K, et al. Preinjury physical frailty and cognitive impairment among geriatric trauma patients determine postinjury functional recovery and survival. J Trauma Acute Care Surg. 2016;80:195‐203. [DOI] [PubMed] [Google Scholar]
- 22. Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199:762‐772. [DOI] [PubMed] [Google Scholar]
- 23. Hatheway OL, Mitnitski A, Rockwood K. Frailty affects the initial treatment response and time to recovery of mobility in acutely ill older adults admitted to hospital. Age Ageing. 2017;46:920‐925. [DOI] [PubMed] [Google Scholar]
- 24. Hubbard RE, Eeles EMP, Rockwood MRH, et al. Assessing balance and mobility to track illness and recovery in older inpatients. J Gen Intern Med. 2011;26:1471‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg. 2013;96:1057‐1061. [DOI] [PubMed] [Google Scholar]
- 26. Bongers KTJ, Schoon Y, Olde Rikkert MGM. Feasibility of repeated self‐measurements of maximum step length and gait speed by community‐dwelling older persons. BMJ Open. 2016;6:e011538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghomrawi HMK, Baumann LM, Kwon S, et al. Using accelerometers to characterize recovery after surgery in children. J Pediatr Surg. 2018;53:1600‐1605. [DOI] [PubMed] [Google Scholar]
- 28. Kaye JA, Maxwell SA, Mattek N, et al. Intelligent systems for assessing aging changes: home‐based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011;66:i180‐i190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeBrasseur NK. Physical resilience: opportunities and challenges in translation. J Gerontol A Biol Sci Med Sci. 2017;72:978‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Messinger‐Rapport B, Pothier Snader CE, Blackstone EH, Yu D, Lauer MS. Value of exercise capacity and heart rate recovery in older people. J Am Geriatr Soc. 2003;51:63‐68. [DOI] [PubMed] [Google Scholar]
- 31. O'Connell MDL, Savva GM, Finucane C, Romero‐Ortuno R, Fan CW, Kenny RA. Impairments in hemodynamic responses to orthostasis associated with frailty: results from The Irish Longitudinal Study on Ageing (TILDA). J Am Geriatr Soc. 2018;66:1475‐1483. [DOI] [PubMed] [Google Scholar]
- 32. Lagro J, Schoon Y, Heerts I, et al. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69:471‐478. [DOI] [PubMed] [Google Scholar]
- 33. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose‐insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67A:1300‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheffer M, Bascompte J, Brock WA, et al. Early‐warning signals for critical transitions. Nature. 2009;461:53‐59. [DOI] [PubMed] [Google Scholar]
- 35. Scheffer M, Bolhuis JE, Borsboom D, et al. Quantifying resilience of humans and other animals. Proc Natl Acad Sci U S A. 2018;115:11883‐11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olde Rikkert MGM, Dakos V, Buchman TG, et al. Slowing down of recovery as generic risk marker for acute severity transitions in chronic diseases. Crit Care Med. 2016;44:601‐606. [DOI] [PubMed] [Google Scholar]
- 37. Gijzel SMW, Van de Leemput IA, Scheffer M, Roppolo M, Olde Rikkert MGM, Melis RJF. Dynamical resilience indicators in time series of self‐rated health correspond to frailty levels in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:991‐996. [DOI] [PubMed] [Google Scholar]
- 38. Gijzel SMW, van de Leemput IA, Scheffer M, et al. Dynamical indicators of resilience in postural balance time series are related to successful aging in high‐functioning older adults. J Gerontol A Biol Sci Med Sci. 2019;74:1119‐1126. [DOI] [PubMed] [Google Scholar]
- 39. van de Leemput IA, Wichers M, Cramer AO, et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci U S A. 2014;111:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wichers M, Groot PC, Psychosystems, ESM Group, EWS Group . Critical slowing down as a personalized early warning signal for depression. Psychother Psychosom. 2016;85:114‐116. [DOI] [PubMed] [Google Scholar]
- 41. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57A:115‐125. [DOI] [PubMed] [Google Scholar]
- 42. Watt J, Tricco AC, Talbot‐Hamon C, et al. Identifying older adults at risk of delirium following elective surgery: a systematic review and meta‐analysis. J Gen Intern Med. 2018;33:500‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller JD, Halter J, Austad SN, et al. Frameworks for proof‐of‐concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci. 2016;71:1415‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ivanov PC, Liu KKL, Bartsch RP. Focus on the emerging new fields of network physiology and network medicine. New J Phys. 2016;18:100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greene JA, Loscalzo J. Putting the patient Back together — social medicine, network medicine, and the limits of reductionism. N Engl J Med. 2017;377:2493‐2499. [DOI] [PubMed] [Google Scholar]
- 46. Fried EI, Boschloo L, Borkulo CD, Cramer AOJ, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol. 2017;52:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olde Rikkert MGM. Conceptualizing geriatric syndromes In: Michel J, Beattie BL, Martin FC, Walston JD, eds. Oxford Textbook of Geriatric Medicine. 3rd ed Oxford, UK: Oxford University Press; 2017:355‐361. [Google Scholar]
- 49. Cesari M, Araujo de Carvalho I, Jotheeswaran AT, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci. 2018;73:1653‐1660. [DOI] [PubMed] [Google Scholar]
- 50. Chen L, Liu R, Liu ZP, Li M, Aihara K. Detecting early‐warning signals for sudden deterioration of complex diseases by dynamical network biomarkers. Sci Rep. 2012;2:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dakos V, Van Nes EH, Donangelo R, Fort H, Scheffer M. Spatial correlation as leading indicator of catastrophic shifts. Theor Ecol. 2010;3:163‐174. [Google Scholar]
- 52. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rutenberg AD, Mitnitski AB, Farrella SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol. 2018;107:126‐129. [DOI] [PubMed] [Google Scholar]
- 54. Chmiel A, Klimek P, Turner S. Spreading of diseases through comorbidity networks across life and gender. New J Phys. 2014;16:115013. [Google Scholar]
- 55. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2015;56:52‐61. [DOI] [PubMed] [Google Scholar]
- 56. Jack E, Maskrey N, Byng R. SHERPA: a new model for clinical decision making in patients with multimorbidity. Lancet. 2018;392:1397‐1399. [DOI] [PubMed] [Google Scholar]
- 57. Greenhalgh T, Hurwitz B. Why study narrative? Br Med J. 1999;318:48‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mann‐Salinas EA, Engebretson J, Batchinsky AI. A complex systems view of sepsis: implications for nursing. Dimens Crit Care Nurs. 2013;32:12‐17. [DOI] [PubMed] [Google Scholar]
- 59. Wojtusiak J, Levy CR, Alemi F. Predicting functional decline and recovery for residents in Veterans Affairs nursing homes. Gerontologist. 2015;56:42‐51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1. Supplementary Information.


