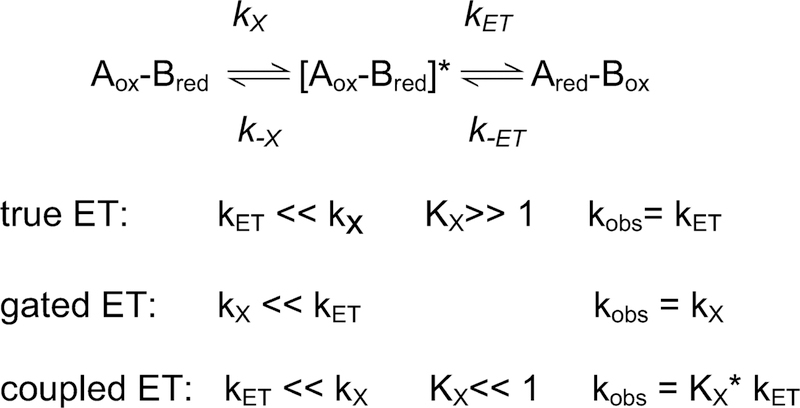Summary
Cupredoxins are small proteins that contain type I copper centers, which are ubiquitous in nature. They function as electron transfer shuttles between proteins. This review of the structure and properties of native cupredoxins, and those modified by site-directed mutagenesis, illustrates how these proteins may have evolved to specifically bind copper, develop recognition sites for specific redox partners, tune redox potential for a particular function, and allow for efficient electron transfer through the protein matrix. This is relevant to the general understanding of the roles of metals in energy metabolism, respiration and photosynthesis.
I. Copper in nature
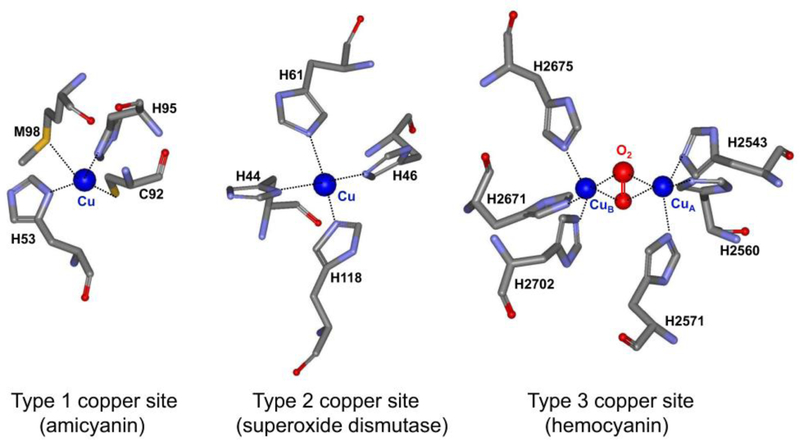
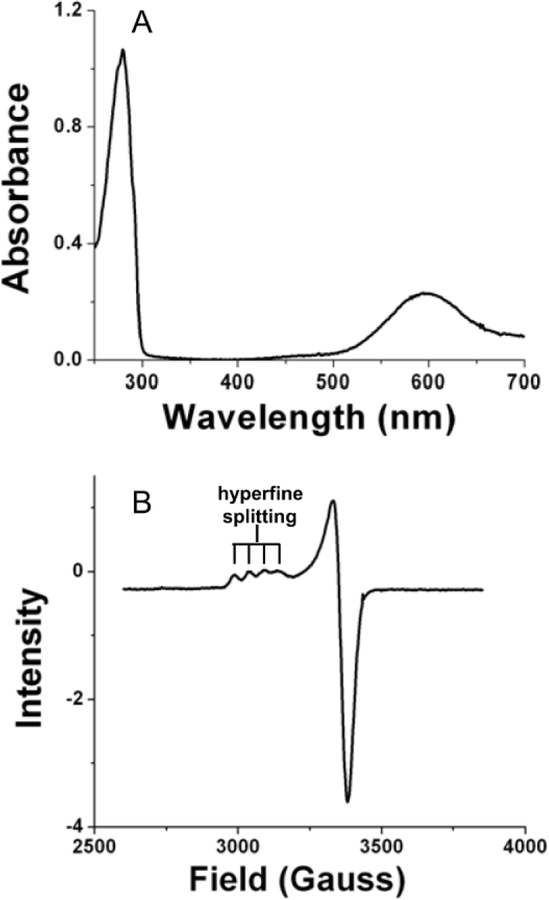
While present in trace amounts, copper is a critical component of biological systems. Very little free copper is found in living systems; rather it is bound to proteins and enzymes. The functional copper-binding sites in proteins are classified as type 1, 2 or 3 (Figure 1).1, 2 This designation depends primarily the spectroscopic properties of the protein-bound copper which is a consequence of the number of coppers bound and the copper ligation geometry. Type I copper centers bind a single copper atom with distorted tetrahedral ligation geometry. The copper is coordinated by two nitrogens from two His residues, a sulfur from a Cys residue and typically a sulfur from a Met residue. Relatively small proteins with a single type 1 copper center and no other metal or cofactor are commonly called blue copper proteins or cupredoxins. In the oxidized state, cupredoxins exhibit an intense visible absorption centered near 600 nm and an EPR spectrum characterized by very narrow hyperfine splitting in the direction parallel to the magnetic field (All) (Figure 2).3 Type 1 copper sites function primarily as mediators of electron transfer. Type 2 copper centers bind copper in a square planar coordination with nitrogen ligands typically provided by His and oxygen ligands provided by Asp or Tyr residues. Type 2 copper proteins exhibit a weak visible absorption around 700 nm, and an axial electron paramagnetic resonance (EPR) spectrum with copper hyperfine splitting in the parallel region similar to that observed in inorganic copper coordination compounds. The type 2 copper centers occur in enzymes, where they assist in biological oxidations. Type 3 copper centers are binuclear in that they bind two copper atoms, each coordinated by three nitrogens from three His residues. Type 3 copper proteins exhibit no EPR signal due to strong antiferromagnetic coupling metal ions. The type 3 copper centers occur in enzymes which are oxidases and oxygenases, and in oxygen-transporting proteins. In addition, there are enzymes that have been characterized that use multiple type 1, 2 and 3 copper sites, sometimes in combinations of different types to catalyze reactions.4 As seen in Table 1, the functions of copper-containing enzymes and proteins are diverse and their functions are critical for respiration, photosynthesis and proper metabolism.
Figure 1.
Examples of the three major classes of copper-binding sites in proteins. The structures were drawn using the structure coordinates from PDB files for the type 1 copper center of amicyanin from Paracoccus denitrificans (PDB code 2OV0), the type 2 copper center of Cu,Zn-superoxide dismutase from human bovine erythrocytes (PDB code 2SOD) and the type 3 copper center of hemocyanin from Octopus dofleini (PDB code 1JS8). The structures are presented as sticks colored gray for carbon, red for oxygen, blue for nitrogen and yellow for sulfur. The copper is displayed as a dark blue sphere.
Figure 2.
Spectroscopic properties of amicyanin which are characteristic of cupredoxins. (A) The visible absorption spectrum of oxidized (Cu2+) amicyanin. (B) X-band EPR spectrum of oxidized (Cu2+) amicyanin.
Table 1.
Examples of members of different types of copper proteins
| Examples (Source) | |
|---|---|
| Type 1 copper proteins | Amicyanin (bacteria) Azruin (bacteria) Plastocyanin (plants, algae) Pseudoazurin (bacteria) Stellacyanin (plants) Rusticyanin (bacteria) |
| Type 2 copper proteins | Galactose oxidase (fungi) Cu-Zn Superoxide dismutase (yeast, mammals) |
| Type 3 copper proteins | Hemocyanin (arthropods, mollusks) Tyrosinase (fungi, mammals) |
| Multicopper oxidases | Ceruloplasmin (mammals) Laccase (fungi, plants, insects) Ascorbate oxidase (plants) |
II. Type 1 copper proteins (cupredoxins)
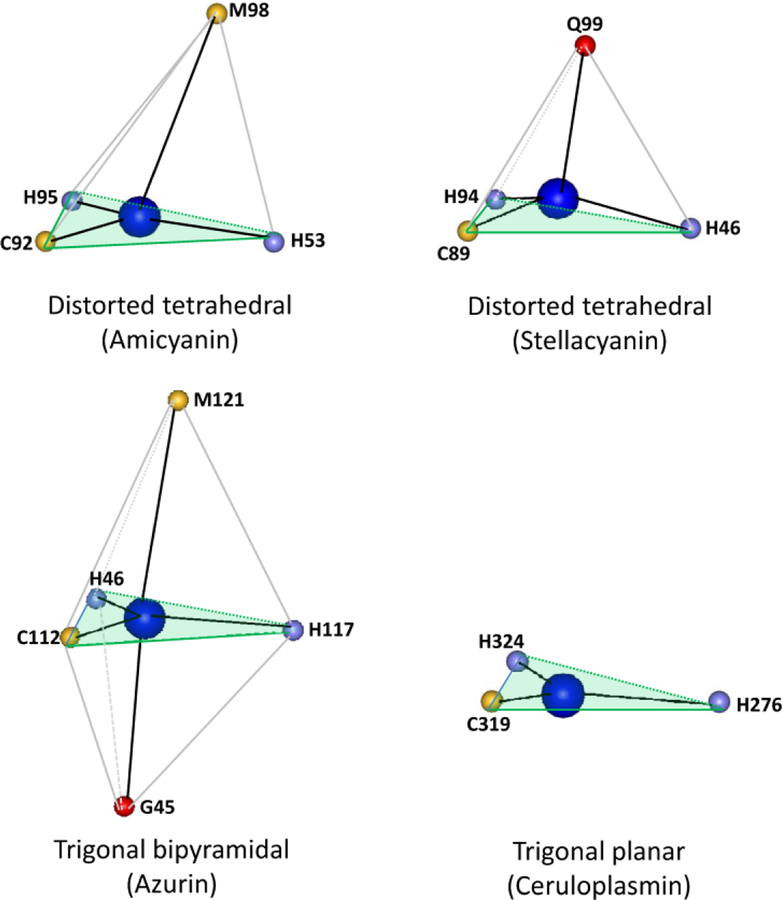
Type 1 copper sites are ubiquitous in nature and typically function as mediators of electron transfer through and between proteins. Many cupredoxins have been structurally and spectroscopically characterized.5 The type 1 copper site always involves strong coordination from a Cys and two His residues in an approximately trigonal planar arrangement with a weaker axial ligand, usually provided by a sulfur of a Met residue, which displaces the Cu2+ from the trigonal plane. The strong interaction between the Cu2+ and the sulfur of the Cys ligand is responsible for the intense blue color that arises from a band at approximately 600 nm due to a S(Cys)π→Cu(II)dx2-y2 ligand-to-metal charge transfer transition.3 This distorted tetrahedral geometry of the type 1 copper site of amicyanin, which is typical of most cupredoxins, is shown in Figure 3. An example of a type 1 copper site in which Met does not provide the axial ligand is stellacyanin where the axial ligand is a side-chain oxygen of a Gln residue.6 This stronger axial ligation, as evidenced by the shorter bond length further displaces the Cu2+ from the trigonal plane yielding a slightly more rhombic geometry. A variation of the typical type 1 geometry is seen in azurin, where a second weak axial interaction with the copper is provided by a backbone carbonyl oxygen atom.7 Another variation in the type 1 geometry is seen in the type 1 copper site of ceruloplasmin, which is not a small cupredoxin but a multicopper eukaryotic protein that is comprised of six cupredoxin-like domains and contains multiple types of copper sites. In one of the type 1sites, the position typically occupied by the axial ligand is a Leu residue which cannot provide a ligand, and the Cu2+ possesses only three ligands in a trigonal planar geometry,8 yet retains the spectroscopic features of a type 1 copper site.
Figure 3.
Variations in the ligation geometry of type 1 copper sites. The structures were drawn using the structure coordinates from PDB files for amicyanin from Paracoccus denitrificans (PDB code 2OV0), stellacyanin from Cucumis sativus (PDB code 1JER), azurin from Pseudomonas aeruginosa (PDB code 4AZU) and ceruloplasmin from human serum (PDB code 1KCW). Only the atom providing the copper ligand is shown with the one-letter amino acid code and residue number indicated. The plane described by the two His and one Cys ligand in each is shaded light green.
While a general function for cupredoxins as electron transfer mediators has been well established, the specific physiological redox partner proteins for specific cupredoxins have only been identified in a few cases. This review will focus on studies performed on amicyanin from Paracoccus denitrificans,9 a representative cupredoxin. Its physiological electron transfer donor protein, methylamine dehydrogenase (MADH),10 and electron transfer acceptor protein, cytochrome c-551i,11 have been identified, and the structure of the electron transfer complex of these proteins has been determined (Figure 4).12 The binding and rate constants for the reactions between these proteins have characterized,13–15 and extensive site-directed mutagenesis studies have elucidated a variety of structure function relationships. The results of these studies and those with other cupredoxins will be used to discuss four specific areas of cupredoxin structure, function and evolution. How did cupredoxin structure evolve to specifically bind copper? How did the relatively simple and highly conserved overall structure of cupredoxins evolve to react with a wide range of structurally diverse redox partners? How did cupredoxin structure evolve to tune the electronic properties of the highly structurally conserved copper site to span a wide range of oxidation-reduction midpoint potential (Em) values, thus expanding the range of roles it may play as a biological electron transfer mediator? Considering that different redox partner proteins of cupredoxins can bind to different sites on the surface of the protein, how did cupredoxins evolve to efficiently mediate electron transfer via different pathways through this conserved structure? The answers to these questions are relevant to understanding the roles of copper, and metals in general, in energy metabolism, respiration and photosynthesis.
Figure 4.
The methylamine dehydrogenase-amicyanin-cytochrome c-551i complex. One half of the symmetrical complex of the crystal structure (PDB code 2MTA) is shown with the cytochrome colored tan, amicyanin colored blue, the MADH α subunit colored olive green, and the MADH β subunit colored light green. The TTQ in MADH, copper in amicyanin and heme in the cytochrome are colored black.
III. How did metalloproteins arise?
It has been estimated that approximately one third of all enzymes contain metals. The choice of the metal depends on its ionic radius, on the reaction to be catalyzed, on the Em value needed, on the type of ligands available and on the biological availability of the metal. How and why metalloproteins came to be are fundamental questions. It seems unlikely that a protein evolved de novo to bind a specific metal for a specific function. More likely a non-metal-containing protein, which evolved and persisted for some other function, fortuitously was suited to bind a metal. Binding of this metal endowed the protein with enhanced stability or a new function, or both. This initiated new lines of evolution which fine-tuned this metalloprotein for a variety of new functions.
Type 1 copper proteins are a straightforward system with which to consider these questions of metalloprotein evolution and metal-binding specificity. Cupredoxins are relatively small proteins of 10–20 kDa and possess relatively simple structures comprised primarily of beta sheets and turns (Figure 5).2 The bacterial cupredoxins function in the periplasmic space of gram negative bacteria rather than in the cytoplasm, and there is no evidence for involvement of chaperones in cupredoxin assembly. One clear advantage for cupredoxins to bind a metal is to enhance their stability. Such an effect on thermal stability was demonstrated with P. denitrificans amicyanin. The folded apoamicyanin was prepared from the holoprotein by reduction with sodium dithionite followed by dialysis against 0.1 M KCN.16 The crystal structure of this apoamicyanin was identical to that of the holoprotein except for the absence of copper.17 Differential scanning calorimetry revealed that the midpoint temperature for thermal transitions (Tm value) for apoamicyanin was significantly lower for apoamicyanin than for the copper-containing holoprotein.18 This indicated significant structural de-stabilization in the absence of bound type 1 copper. It follows that during evolution a random mutation which allowed a pre-existing protein to bind a metal which enhanced the protein’s stability could provide a strong selective advantage. If binding of a metal also endowed the protein with a new beneficial function, then this would be an even greater selective advantage. This new function need not be the one which exists today. For example, free copper in solution is potentially toxic as it can generate reactive oxygen species under certain circumstances. If a bacterial periplasmic protein acquired the ability to tightly bind copper, then this could conceivably serve as a defense mechanism against copper toxicity. The periplasmic location of cupredoxins could also provide an opportunity for a redox-active metalloprotein to acquire roles in electron transfer and respiration. The periplasmic space in bacteria is analogous to the mitochondrial intermembrane space where cytochrome c functions as an electron transfer mediator during respiration. Cupredoxins are known to interact with components of the bacterial membrane-bound respiratory chains and mediate the entry of electrons into the respiratory chain from periplasmic dehydrogenases. It seems reasonable to believe that a stable redox-active metalloprotein could have adapted to perform these tasks and gain new physiological roles over time.
Figure 5.
Structures of representative cupredoxins. The secondary structures are highlighted with beta sheets colored light blue, alpha helices colored red, and unstructured loops and turns colored gray. The structures were drawn using the structure coordinates from PDB files for amicyanin from Paracoccus denitrificans (PDB code 2OV0), plastocyanin from Ulva pertusa (PDB code 1IUZ), azurin from Pseudomonas aeruginosa (PDB code 4AZU) and stellacyanin from Cucumis sativus (PDB code 1JER).
IV. What dictates the metal specificity for the type 1 copper site of proteins?
There is ample evidence that the type 1 center of cupredoxins can accommodate metals other than copper. It is possible to reversibly remove the copper to generate the metal-free apoprotein and then reconstitute the apoprotein with copper or some other metals. In particular cobalt- and nickel-substituted cupredoxins have been generated and studied.19–23 Furthermore, recombinant cupredoxins that are expressed in the cytoplasm of E. coli are sometimes isolated with zinc rather than copper occupying the site.20 These zinc-containing proteins are inert but stable. Given the very low concentrations of free soluble copper in biological systems, and the ability of the type 1 site to accommodate other metals, one wonders what factors dictate the specificity for copper insertion into the type 1 site in vivo.
It was shown for amicyanin that the nature of the axial ligand exerts a strong influence over the specificity for metal incorporation. Recombinant P. denitrificans amicyanin which was expressed in the periplasm of E. coli24 was isolated with full occupancy of the type 1 site by copper. Mutation of the axial Met98 ligand of this amicyanin to Gln, Ala, or Leu resulted in the synthesis and isolation of amicyanin which contained primarily zinc rather copper bound to the type 1 site.25–27 The Zn2+ could be removed by unfolding the protein in the presence of EDTA. Then refolding the protein in the presence of Cu2+ generated the copper-containing mutant amicyanins whose properties were altered by the mutations but which did react with the physiological redox partner proteins.26–28 The influence of the nature of the axial ligand on the specificity of metal binding was studied by comparing the relative affinities of native and M98Q apoamicyanins for Zn2+ versus Cu2+ in the folded, partially folded and unfolded states.25 Surprisingly, the influence of the axial ligand of the type 1 copper site on metal specificity was the strongest prior to the completion of protein folding and adoption of the final type 1 site geometry. Thus, at the point during the biosynthetic process when the type 1 copper site is not yet formed, the residue which ultimately provides the weakest ligand is a strong determinant of the specificity of the metal for incorporation. This also suggested that metal incorporation in vivo most likely occurs during protein folding in the periplasm and not to a preformed type 1 site.25 It should be noted that not all type 1 copper proteins possess a Met axial ligand, and stellacyanin naturally has an axial Gln ligand yet contains copper (see Figure 3).6 So while the presence of methionine as an axial ligand is not the sole and absolute requirement for copper specificity, there is evidence that it is a very strong determinant.
V. What dictates the specificity for the redox partner proteins that interact with different cupredoxins?
Many biological processes involve highly specific interactions between proteins which either strongly or weakly associate. “Static” complexes are characterized by a strong binding affinity with slow dissociation (e.g., antibody-antigen interactions and signal transduction protein complexes). “Transient” complexes have weaker binding affinity with a shorter lifetime. Electron transfer between soluble proteins typically involves formation of transient complexes. Readily reversible protein interactions allow continuous electron flow between redox partners. Specificity is also critical to ensure that electrons are not transferred to the wrong molecule which would waste energy and potentially lead to formation of damaging free radicals and reactive oxygen species. Since cupredoxins are electron transfer mediators, they must interact with two different proteins, one that donates electrons and one that accepts electrons. While few complexes of cupredoxins with their physiological redox partner protein have been structurally characterized, those that have provide insight into how the specificity of these interactions is achieved. Four examples are discussed below.
A. Amicyanin
Amicyanin9 serves as a mediator of electron transfer from methylamine dehydrogenase (MADH)10 to cytochrome c-551i.11 These three proteins are isolated from P. denitrificans as individual soluble proteins but they have to form a ternary complex to catalyze methylamine-dependent cytochrome c-551i reduction.14, 29 The complex of MADH, amicyanin, and cytochrome c-551i is one of the best characterized physiological protein electron transfer systems (Figure 4).30 The proteins have been structurally characterized by x-ray crystallography as the binary complex of MADH and amicyanin,31 and the ternary protein complex12 which includes cytochrome c-551i. The protein complexes were shown to be catalytically active and able to perform electron transfer in the crystalline state.32, 33 Electron transfer from MADH to the cytochrome is thermodynamically favorable but does not occur because the proteins are unable to interact with other. The Em value of amicyanin (+294 mV) is more positive than that of cytochrome c-551i (+190 mV).34 This means that when amicyanin is free in solution and interacts with the cytochrome, the direction of electron transfer would be from the cytochrome to amicyanin, the reverse of the physiological direction.29, 35 On complex formation with MADH, the Em value of amicyanin becomes more negative which allows the electron transfer in the physiological direction to become more favorable. This is because a redox state-dependent conformational change that is linked to the protonation of His95 of amicyanin is sterically constrained in the complex with MADH relative to free amicyanin.36 Thus, formation of the ternary protein complex is critical for electron transfer from MADH to cytochrome c-551i.
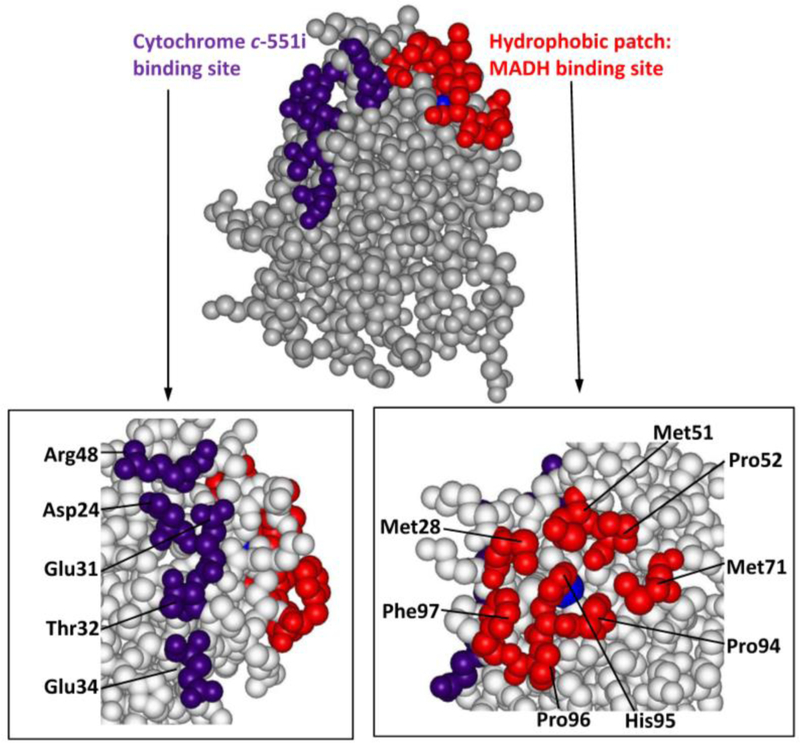
The structure of the protein complex indicated that the interface between MADH and amicyanin is stabilized largely by hydrophobic interactions (Figure 6), and possibly one or two intermolecular salt bridges at the periphery of this hydrophobic interface.31 The specific contributions of these hydrophobic and electrostatic interactions were investigated using site-directed mutagenesis.24 Conversion of Phe97 to a more polar Glu residue, increased the Kd for complex formation with MADH by two orders of magnitude, consistent with the importance of this hydrophobic patch. Arg99 and Lys68 of amicyanin were inferred from the structure to possibly form salt bridges with residues on MADH. While a K68A mutation did not affect binding, R99D and R99L mutations did significantly increase the Kd for complex formation and altered the ionic strength dependence of binding. These results demonstrate that a combination of specific hydrophobic and ionic interactions is required to stabilize complex formation between MADH and amicyanin, and that individual amino acid residues on the protein surface are able to dictate very specific interactions between soluble redox proteins. Interestingly the structure of the ternary protein complex reveals that the interaction of amicyanin with cytochrome c-551i is stabilized a different set of interactions. That protein interface involves multiple van der Waals and hydrogen bonding interactions including some with bridging water molecules.12
Figure 6.
Space filling model of the structure of amicyanin from Paracoccus denitrificans (PDB code 2OV0). The MADH binding site (red) seen in the structure of the protein complex is comprised of residues Met28, Met51, Pro52, Met71, Pro94, Pro96 and Phe97. The cytochrome c-551i binding site (purple) is comprised of Asp24, Glu31, Glu32, Thr34 and Arg48. The copper is displayed as a blue sphere.
B. Plastocyanin
Plastocyanin is critical for photosynthesis in plants and bacteria as it functions as a soluble mobile mediator of electron transfer from the membrane-bound cytochrome f of the cytochrome bf complex to the membrane-bound photosystem I.37 The crystal structures of several plastocyanins from plants and algae revealed two potential molecular recognition sites for its redox partners (Figure 7).38, 39 One is a hydrophobic patch; similar to what is seen in amicyanin (Figure 6), which is a region of nonpolar residues around the solvent accessible, exposed His ligand of copper. The other, which is unique to plastocyanin is an acidic patch which was thought to interact with a basic Lys-rich region on the surface of cytochrome f.38, 40–42 No crystal structure of a complex of plastocyanin with a redox partner protein is available but the solution structure of the complex between plastocyanin and cytochrome f was determined by NMR.43 This study confirmed the interaction between residues in the lysine-rich domain on cytochrome f with the acidic patch of plastocyanin, and interactions between the hydrophobic patch of plastocyanin and hydrophobic residues around the heme of cytochrome f. Given the close proximity of the copper and heme redox sites of the two proteins at the interface of the hydrophobic patches, this interaction is thought to be directly related to mediation of interprotein electron transfer. This has been confirmed by site-directed mutagenesis studies and the acidic patch appears to provide a docking site for the initial binding with cytochrome f.
Figure 7.
Redox partner binding sites of plastocyanins. Space filling models of the structure were prepared using the coordinates from the PDB files of plastocyanins from a higher plant (Silene PDB code 1BYO), from a green alga (Ulva pertusa PDB code 1IUZ) and from a cyanobacteria (Synechococuss sp. PDB code 1BXU). The hydrophobic patch which is the site of interaction with cytochrome f and is present in all three is red. The acidic patch which also interacts with cytochrome f in the plastocyanins of plants and algae is purple. The additional acid patch found only in plastocyanin from plants is green. Copper is indicated as a blue sphere.
The presence of the acidic patch is not common to all plastocyanins (Figure 7). Plastocyanin from plants actual have two acidic patches, one near the hydrophobic patch and the other one further away. Plastocyanin from algae has the one acidic patch, corresponding to the one that is further from the hydrophobic site. In addition to the eukaryotic plants and algae, plastocyanin is found in the prokaryotic cyanobacteria.44 Interestingly, the acidic patch is absent in prokaryotic plastocyanin which only exhibit the hydrophobic patch.45 Thus, plastocyanin provides an example of how residues on the surface of this protein, whose overall tertiary structure is highly conserved, have adapted during evolution to apparently enhance specificity for its redox protein partner by going from one to two to three distinct recognition sites for protein-protein interaction.
C. Azurin
Azurins are present in several bacteria and are located in the periplasmic space. Expression of azurin is typically constitutive and it is believed to serve a general function in mediation of electron transfer. A crystal structure is available for one complex of an azurin with its physiological redox partner, the complex of aromatic amine dehydrogenase (AADH) and azurin from Alcaligenes faecalis.46 The structure reveals that the AADH-azurin interface is largely hydrophobic with one direct hydrogen bond.
It is of particular interest to compare the protein interactions in this complex with those in the MADH-amicyanin complex described earlier. AADH from and MADH are structurally highly conserved proteins which each possessing tryptophan tryptophylquinone (TTQ)47 as its redox cofactor.46, 48 While the kinetic and structural properties of AADH and MADH are very similar,49, 50 the electron acceptor specificity is different; azurin rather than amicyanin.51 Neither copper protein can substitute for the other as an effective electron acceptor.51 The regions of MADH and AADH that bind their respective copper proteins are essentially the same. As does amicyanin, azurin also interacts with AADH via the hydrophobic patch near the copper site. However, the orientation of azurin with AADH is largely different from that of amicyanin with MADH being rotated by 90° degree with respect to azurin.46 Also, the fraction of hydrophobic residues in AADH-azurin complex is larger than that in MADH-amicyanin.46 This fact is consistent with solution studies which showed that the AADH-azurin complex was stabilized at high ionic strength,51 which is the opposite of what was observed for the MADH-amicyanin complex. Thus, subtle changes at the periphery of the hydrophobic patch, a hydrogen bond with azurin versus a salt bridge with amicyanin, allows these two cupredoxins to discriminate between two structural similar electron donor proteins.
D. Rusticyanin
Rusticyanin is localized in the periplasm of acidophilic bacteria and is involved in mediation of electron transfer from Fe2+ ions in the extracellular environment to the membrane-bound respiratory chain.52 The best studied rusticyanin is that from Thiobacillus ferrooxidans. Rusticyanin forms a complex with cytochrome c4 to mediate electron transfer.53 Rusticyanin is distinct from other cupredoxins in that it contains an N-terminal extension of 35 amino acids that protects the β-barrel core from its interaction with the solvent, maintaining a high degree of hydrophobicity of the protein.54, 55 No crystal or NMR structure of the rusticyanin-cytochrome c4 complex is available; however, modeling studies revealed possible hydrogen-bond mediated recognition sites that included several water molecules that could stabilize this complex.56, 57
VI. How do cupredoxins tune the redox potential of type 1 copper site?
Despite their highly conserved structures, type 1 copper sites in proteins exhibit Em values that range from 184 mV for stellacyanin58 to >1000 mV for type1 copper center in human ceruloplasmin.59 A common feature of cupredoxins is that the Em values are all more positive than that of the Cu2+/Cu+ aqua couple. This is likely due to structural constraints which are imposed upon the copper by the protein in the type 1 site. As discussed in the rack-induced concept of copper binding in cupredoxins, the type 1 copper protein forces the copper into a configuration intermediate between those preferred by Cu2+ and Cu+.60 This could minimize the difference in relative stabilities of Cu2+ and Cu+ in the type 1 copper site relative to those in solution which would account for the more positive Em values of cupredoxins than aqueous copper complexes. The Cu+-axial ligand bond distance in the reduced cupredoxin also influences the Em value.61 A normal Cu+-Smet bond length is about 2.3 Å, whereas in cupredoxins the observed bond length is about 2.9 Å. This increased distance would reduce the charge donation of the ligand to the copper ion, which would increase the Em value. Further variation in Em value is also to some extent due the nature of the axial ligand; however, most cupredoxins possess a Met ligand so clearly other factors are in play. These factors include desolvation or hydrophobic effects, hydrogen bonding pattern around the copper site, and intraprotein electrostatic interactions.62
A hydrophobic environment raises the Em value of the Cu2+/Cu+ redox couple by preferential stabilization of the less charged Cu+ oxidation state. All of the structurally characterized cupredoxins have their type 1 copper centers embedded in a hydrophobic region of nonpolar side chains. The highly positive Em value of T. ferrooxidants rusticyanin (680 mV) has been attributed in part to its unusually high hydrophobicity due to an N-terminal extension of 35 amino acids that contributes to the rigid hydrophobic core.54, 63, 64 Numerous site-directed mutagenesis studies with different cupredoxins have demonstrated the relationship between Em value and hydrophobicity of metal-binding site. When the residue providing the axial copper ligand is replaced with a hydrophobic amino acid such as Leu, the Em value typically becomes ~130mV more positive (Table 2). For example, an M98L amicyanin mutation increases Em value by 127 mV,27 an M148L rusticyanin mutation increases Em value by 131 mV,65 and an M121L azurin mutation increases Em value by 135 mV.66 A clear correlation between the Em value and amino acid side chain hydrophobicity of the axial ligands was also obtained using recombinant azurin in which the axial Met was replaced with unnatural amino acids which are iso-structural analogues of Met.66–68
Table 2.
Influence of the axial copper ligand on redox potential.
Site-directed mutagenesis studies of cupredoxins have clearly demonstrated that the hydrogen bonding pattern around the copper site, especially hydrogen bonds involving the thiolate of the Cys ligand, influences the Em value (Table 3). In P. denitrificans amicyanin mutation of Pro94 to Phe or Ala increased the Em value by 120 mV and 150 mV, respectively.69 High resolution x-ray crystal structures of the mutants revealed that in each an electron-withdrawing hydrogen bond to the copper-coordinating thiolate sulfur of Cys92 is introduced by movement of the amide nitrogens of Phe94 and Ala94 much closer to the thiolate than the amide N of Pro94, which in native amicyanin is too far from the thiolate to form a hydrogen bond.70 Similarly, a P80I mutation in A. faecalis pseudoazurin introduced an additional hydrogen bond from the protein backbone to the thiolate of the Cys ligand resulting in a 180 mV increase in Em value.71 Conversely in azurin, a F114P mutation which resulted in loss of a hydrogen bond decreased the Em value by 94 mV. 6, 67
Table 3.
Influence of Hydrogen bonding to the cysteine copper ligand on redox potential
Certain cupredoxins also exhibit a pH-dependent Em value. The Em value of amicyanin increases by approximately 60 mV per pH unit. This is because in reduced P. denitrificans amicyanin, a pH-dependent conformational change occurs on protonation of the His95 copper ligand with an associated pKa value of 7.7.36 When protonated, His95 rotates 180° about its Cβ—Cγ bond effectively removing it from the copper coordination sphere. A similar “histidine flip” is observed with plastocyanin,42 but it exhibits a more acidic pKa value of 4.9 for spinach plastocyanin.72 It should be noted that these different pKa values correlate approximately with the physiological pH of the periplasmic space and thylakoid space, respectively, where amicyanin and plastocyanin are localized and function.
VII. How do cupredoxins optimize rates of interprotein electron transfer reactions?
Cupredoxins have been extensively studied as model systems for protein electron transfer reactions. Azurin and plastocyanin, which were labeled at various sites on the protein surface with redox-active ruthenium complexes, have been used to study the distance-dependence of the rate of interprotein electron transfer to and from the copper.73–75 Using NMR it has also been possible to study electron transfer self-exchange reactions of cupredoxins.76, 77 These two approaches have yielded substantial information that provides insight into general mechanisms of protein electron transfer reactions. However, these excellent model systems do not describe physiological interprotein electron transfer reactions.
In physiological interprotein electron transfer reactions, the redox centers which serve as electron donor and acceptor are not in direct contact. They are not only separated by relatively long distances but also the gap between the two proteins. Furthermore, interprotein electron transfer is a bimolecular reaction which at a minimum requires a binding step to precede electron transfer and may also include additional non-electron transfer reactions steps such as protein rearrangements to optimize the orientation of the initial complex for the electron transfer reaction.78 The physiological electron transfer protein complex is that of MADH, amicyanin and cytochrome c-551i from P. denitrificans has been extensively studied.12, 30 The rates of the individual electron transfer reactions that occur within the complex have been determined by monitoring characteristic changes in the absorption spectra of the proteins that occur during the redox reactions. Site-directed mutagenesis studies of amicyanin have identified specific amino acid residues that influence electron transfer parameters for the reactions that occur within the complex (Table 4). It was also possible to generate mutations of amicyanin that alter the kinetic mechanisms of electron transfer reactions within the complex by converting true electron transfer reactions to ones that are gated or coupled.30 These studies showed that electron transfer rates may be significantly altered by subtle changes in protein structure by a variety of mechanisms.
Table 4.
Alteration of rates of true electron transfer rates by site-directed mutagenesis
| Electron transfer reaction | Reaction rate at 30° C (s−1) | ΔG° (kJ/mol) | λ (eV) | HAB (cm−1) | Reference |
|---|---|---|---|---|---|
| MADH to amicyanin | 9.8 | −3.2 | 2.3 ± 1 | 12 ± 7 | 93 |
| MADH to M98Q amicyanin | 0.2 | −3.3 | 2.7 ± 1 | 12 ± 4 | 28 |
| MADH to F97E amicyanin | 0.2 | −3.2 | 2.3 ± 1 | 3 ± 1 | 83 |
| MADH to P94F amicyanin | 53 | −21.7 | 2.3 ± 1 | 5 ± 1 | 94 |
| Amicyanin to cytochrome c-551i | 87 | +3.2 | 1.2 ± 1 | 0.3 ± 0.1 | 14 |
| P94F amicyanin to cytochrome c-551i | 0.6 | +21.7 | 1.3 ± 1 | 0.3 ± 0.1 | 94 |
The reactions rates which were altered and the parameters which were altered that caused the change in rate are highlighted in red.
Before giving specific examples of site-directed mutagenesis studies which have elucidated features of cupredoxin structure that influence electron transfer rates, a brief discussion of electron transfer theory is presented to provide a framework to understand how individual protein residues can influence rates of electron transfer. According to Marcus theory (eq 1)79 which is the prevalent form of electron transfer theory, the rate of electron transfer (kET) depends on these factors; driving force (ΔG°), reorganization energy (λ) and the donor-acceptor electronic coupling (HAB).
| (1) |
A. Activation energy
The activation energy for an electron transfer reaction is determined by both ΔG° and λ. The ΔG° for electron transfer reaction depends on the ΔEm for the electron donor and acceptor of redox centers. The mechanisms by which cupredoxins tune their Em values were discussed earlier. λ is the energy difference between the reactant and product states at the equilibrium nuclear configuration of the reactant, and it is the energy required to bring the reactant and product states to this common intermediate in which electron transfer occurs. The type 1 copper site seems to have evolved to minimize λ, thus maximizing kET. Redox reactions of copper in aqueous solution can exhibit high λ values < 2.4 eV80 due to the different ligation geometries preferred by Cu+ and Cu2+ and influence of solvent. However, in cupredoxins copper is shielded from solvent and the copper site is constrained by the protein into a geometry intermediate between that preferred for Cu+ and Cu2+. This minimizes changes in copper site geometry and solvent effects during the redox reaction which would otherwise increase λ.60
The importance of the geometry of the type 1 site in controlling λ was demonstrated with P. denitrificans amicyanin. It was shown that conversion of the axial Met ligand to Gln by site-directed mutagenesis decreased kET for the reaction between MADH and amicyanin by 45-fold due to a 0.4 eV increase in λ28 (Table 4). Structural studies indicated that this mutation increased the distance of the copper from the equatorial plane of the type 1 site and that this rhombic distortion of the type 1 geometry was responsible for the increase in λ.26, 28 This demonstrated that even subtle perturbation of the type 1 site geometry can significantly affect electron transfer rates by modulating λ.
B. Electronic coupling.
The rate of electron transfer also depends upon HAB, which depends on the electron transfer distance and the nature of the intervening medium.75, 81 It is generally accepted that electron transfer through a vacuum is much less efficient than electron transfer through a covalent bond.82 It follows that efficient electron transfer through proteins would occur primarily through bonds rather than through empty space. An F97E mutation of amicyanin decreased kET for the reaction between MADH and amicyanin by 24-fold83 (Table 4). The ΔG° and λ associated with the electron transfer reaction were unaffected by the mutation and the decrease in kET was due solely to a decrease in HAB. Phe97 is located at the MADH-amicyanin interface and had previously been shown to perturb the protein-protein interaction.24 From structural and computational analysis it was concluded that the F97E mutation caused an increase in the interprotein distance of 0.9 Å which accounts for the observed decreases in HAB and consequently kET. This illustrates the importance of the identity of surface residues at the protein interface and demonstrates that small (sub-angstrom) changes in the length of through-space segments of electron transfer pathways, particularly interprotein gaps, can significantly alter HAB.
C. Kinetic regulation of observed electron transfer rates.
For interprotein electron transfer reactions the observed rate of the redox reaction is not always a true electron transfer rate constant (kET). Given the complexity of the biological electron transfer reactions,84 the possibility exists that some non-electron transfer process (e.g., a protein conformation change or reconfiguration of proteins after initial complex formation) is influencing the observed rate. This may give rise to what have been described as gated and coupled electron transfer reactions (Scheme 1).30 Gated electron transfer occurs when the non-electron transfer reaction step (kx) is slower than the true electron transfer reaction. If the rates of these two sequential reactions cannot be kinetically distinguished, then the observed rate constant (kobs) for the redox reaction will be the rate constant for the preceding slower non-electron transfer reaction step rather than kET. Coupled electron transfer occurs when a true electron transfer reaction is preceded by a more rapid, but thermodynamically unfavorable non-electron transfer reaction (described by Kx=kx/k-x), which limits the availability of the optimized state. In this case the observed rate constant (kobs) for the redox reaction is actually equal to the product of kET and the equilibrium constant for the preceding reaction step (kobs=Kx*kET).85 Two examples in which site-directed mutagenesis of amicyanin has been used to convert true ET reactions within the MADH-amicyanin-cytochrome c-551i complex to coupled and gated reactions (Table 5) are described below.
Scheme 1.
Kinetic mechanisms of electron transfer reactions
Table 5.
Alteration of kinetic mechanism of electron transfer by site-directed mutagenesis
| Electron transfer reaction | Reaction rate at 30° C (s−1) | λ (eV) | HAB (cm−1) | Kinetic mechanism | Reference |
|---|---|---|---|---|---|
| MADH to amicyanin | 9.8 | 2.3 ± 1 | 12 ± 7 | True | 93 |
| MADH to M51A amicyanin | 1.3 | 3.1 ± 1 | 142 ± 20 | Gated | 87 |
| Amicyanin to cytochrome c-551i | 87 | 1.2 ± 1 | 0.3 ± 0.1 | True | 14 |
| P94A amicyanin to cytochrome c-551i | 0.4 | 2.3 ± 1 | 8.3 ± 5.5 | Coupled | 86 |
The reactions rates and the parameters which were altered by the change in kinetic mechanism are highlighted in red.
Pro94 which lies within the hydrophobic patch and the ligand loop of amicyanin was converted to Ala. The crystal structure of oxidized (Cu2+) P94A amicyanin was relatively unaffected by the mutation but the structure of reduced (Cu+) P94A amicyanin exhibited two alternate conformations with the positions of the copper 1.4 Å apart.70 One was similar to that of the native protein. In the other a water replaced Met98 as the copper ligand and the electron transfer distance to the heme of the cytochrome was increased by 1.4 Å, which would make electron transfer from this conformation much less favorable. Analysis of the electron transfer reaction from amicyanin to cytochrome c-551i suggested that this true electron transfer reaction had been converted to a coupled electron transfer reaction.86 A kinetic mechanism was proposed to explain these data in which after the reduction of Cu2+ by MADH, electron transfer from the favored conformation of Cu+ is coupled to an unfavorable equilibrium with the unfavorable conformation, thus limiting the availability of the optimized state and decreasing kET.
In another study, mutation of Met51 which lies within the hydrophobic patch of amicyanin was converted to Ala.87 Analysis of the electron transfer reaction from MADH to amicyanin revealed that the rate of the redox reaction had become much slower because the electron transfer reaction was now gated. Structural analysis revealed that intermolecular contacts between Met51 of amicyanin and MADH were lost as a consequence of the M51A mutation. The Kd for complex formation was not affected and it was concluded that the mutation slowed the rate of normally rapid conformational rearrangement that precedes electron transfer resulting in conversion of the true electron transfer reaction to a gated electron transfer reaction. It was subsequently shown using molecular dynamics simulations that the interactions between Met51 and MADH comprised a “molecular breakwater” that optimized the position of water molecules at the protein interface, and that the M51A mutation disrupted this breakwater resulting in a decreased availability of the optimum configuration for electron transfer.88 These results highlight the fact that specific individual residues at the surface of redox proteins not only dictate specificity for initial binding to their redox protein partners, but also are critical to optimize the configuration of the redox centers and intervening media within the protein complex for the electron transfer event.
VIII. Perspective
Copper, as an element, has captured our imagination and found a variety of practical uses over the years. The alchemist’s name for copper was Venus, the Roman goddess of love and beauty. Copper and its alloy, bronze, have been used by humans since ancient times. Today its electrical properties are exploited for use in electrical wiring and electromagnets, and its thermal conduction is exploited for use in heat exchangers. Cupredoxins have also intrigued and fascinated chemists and biochemists for many years due to their intense blue color. Cupredoxins were one of the first classes of metalloproteins to be studied in detail and at least in part contributed to the development of the field of bioinorganic chemistry. The roles of cupredoxins in nature are reasonably well-established. Perhaps because they are such well-defined proteins one is beginning to see reports of potential applications that employ cupredoxins in biotechnology and medicine. For example, cupredoxins may prove useful as components of bio-electronic devices,89, 90 and surprisingly the cupredoxin azurin has been shown to preferentially enter cancer cells and cause apoptosis.91, 92 Understanding how cupredoxins have evolved and adapted to perform their natural functions will hopefully provide insight into how protein engineering may be used to further adapt this class of metalloproteins for new applications.
Acknowledgements
Work from the author’s laboratory was supported by NIH grant GM41574.
Abbreviations
- AADH
Aromatic amine dehydrogenase
- EPR
Electron paramagnetic resonance
- Em
oxidation-reduction midpoint potential
- MADH
Methylamine dehydrogenase
- NMR
Nuclear magnetic resonance
- TTQ
Tryptophan tryptophylquinone
References
- 1.Solomon EI, Sundaram UM and Machonkin TE, Chem. Rev, 1996, 96, 2563–2606. [DOI] [PubMed] [Google Scholar]
- 2.Adman ET, Adv. Protein Chem, 1991, 42, 145–197. [DOI] [PubMed] [Google Scholar]
- 3.Solomon EI, Szilagyi RK, DeBeer George S and Basumallick L, Chem. Rev, 2004, 104, 419–458. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura K and Go N, Cell Mol. Life Sci, 2005, 62, 2050–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennison C, Coord. Chem. Rev, 2005, 249, 3025–3054. [Google Scholar]
- 6.Hart PJ, Nersissian AM, Herrmann RG, Nalbandyan RM, Valentine JS and Eisenberg D, Protein Sci, 1996, 5, 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nar H, Messerschmidt A, Huber R, van de Kamp M and Canters GW, J. Mol. Biol, 1991, 221, 765–772. [DOI] [PubMed] [Google Scholar]
- 8.Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A and Lindley P, J. Biol. Inorg. Chem, 1996, 1, 15–23. [Google Scholar]
- 9.Husain M and Davidson VL, J. Biol. Chem, 1985, 260, 14626–14629. [PubMed] [Google Scholar]
- 10.Davidson VL, Adv. Protein Chem, 2001, 58, 95–140. [DOI] [PubMed] [Google Scholar]
- 11.Husain M and Davidson VL, J. Biol. Chem, 1986, 261, 8577–8580. [PubMed] [Google Scholar]
- 12.Chen L, Durley RC, Mathews FS and Davidson VL, Science, 1994, 264, 86–90. [DOI] [PubMed] [Google Scholar]
- 13.Davidson VL and Jones LH, Anal. Chim. Acta, 1991, 249, 235–240. [Google Scholar]
- 14.Davidson VL and Jones LH, Biochemistry, 1996, 35, 8120–8125. [DOI] [PubMed] [Google Scholar]
- 15.Brooks HB and Davidson VL, Biochemistry, 1994, 33, 5696–5701. [DOI] [PubMed] [Google Scholar]
- 16.Husain M, Davidson VL and Smith AJ, Biochemistry, 1986, 25, 2431–2436. [DOI] [PubMed] [Google Scholar]
- 17.Durley R, Chen L, Lim LW, Mathews FS and Davidson VL, Protein Sci, 1993, 2, 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma JK, Bishop GR and Davidson VL, Arch. Biochem. Biophys, 2005, 444, 27–33. [DOI] [PubMed] [Google Scholar]
- 19.Carrell CJ, Wang X, Jones L, Jarrett WL, Davidson VL and Mathews FS, Biochemistry, 2004, 43, 9381–9389. [DOI] [PubMed] [Google Scholar]
- 20.Nar H, Huber R, Messerschmidt A, Filippou AC, Barth M, Jaquinod M, van de Kamp M and Canters GW, Eur. J. Biochem, 1992, 205, 1123–1129. [DOI] [PubMed] [Google Scholar]
- 21.Blackwell KA, Andersen BF and Baker EN, Acta Crystallogr. D Biol. Crystallogr, 1994, 50, 263–270. [DOI] [PubMed] [Google Scholar]
- 22.Moratal JM, Romero A, Salgado J, Perales-Alarcon A and Jimenez HR, Eur. J. Biochem, 1995, 228, 653–657. [DOI] [PubMed] [Google Scholar]
- 23.McMillin DR, Holwerda RA and Gray HB, Proc. Natl. Acad. Sci. USA, 1974, 71, 1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson VL, Jones LH, Graichen ME, Mathews FS and Hosler JP, Biochemistry, 1997, 36, 12733–12738. [DOI] [PubMed] [Google Scholar]
- 25.Ma JK, Lee S, Choi M, Bishop GR, Hosler JP and Davidson VL, J. Inorg. Biochem, 2008, 102, 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrell CJ, Ma JK, Antholine WE, Hosler JP, Mathews FS and Davidson VL, Biochemistry, 2007, 46, 1900–1912. [DOI] [PubMed] [Google Scholar]
- 27.Choi M, Sukumar N, Liu A and Davidson VL, Biochemistry, 2009, 48, 9174–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma JK, Mathews FS and Davidson VL, Biochemistry, 2007, 46, 8561–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson VL and Jones LH, J. Biol. Chem, 1995, 270, 23941–23943. [DOI] [PubMed] [Google Scholar]
- 30.Davidson VL, Acc. Chem. Res, 2008, 41, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Durley R, Poliks BJ, Hamada K, Chen Z, Mathews FS, Davidson VL, Satow Y, Huizinga E, Vellieux FM and et al. , Biochemistry, 1992, 31, 4959–4964. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari D, Di Valentin M, Carbonera D, Merli A, Chen Z-W, Mathews FS, Davidson VL and Rossi G-L, J. Biol. Inorg. Chem, 2004, 9, 231–237. [DOI] [PubMed] [Google Scholar]
- 33.Merli A, Brodersen DE, Morini B, Chen Z, Durley RC, Mathews FS, Davidson VL and Rossi GL, J. Biol. Chem, 1996, 271, 9177–9180. [DOI] [PubMed] [Google Scholar]
- 34.Gray KA, Knaff DB, Husain M and Davidson VL, FEBS Letters, 1986, 207, 239–242. [DOI] [PubMed] [Google Scholar]
- 35.Gray KA, Davidson VL and Knaff DB, J. Biol. Chem, 1988, 263, 13987–13990. [PubMed] [Google Scholar]
- 36.Zhu Z, Cunane LM, Chen Z, Durley RC, Mathews FS and Davidson VL, Biochemistry, 1998, 37, 17128–17136. [DOI] [PubMed] [Google Scholar]
- 37.Hope AB, Biochim. Biophys. Acta, 2000, 1456, 5–26. [DOI] [PubMed] [Google Scholar]
- 38.Colman PM, Freeman HC, Guss JM, Murata M, Norris VA, Ramshaw JAM, Venkatappa MP, Nature, 1978, 272, 319–324. [Google Scholar]
- 39.Shibata N, Inoue T, Nagano C, Nishio N, Kohzuma T, Onodera K, Yoshizaki F, Sugimura Y and Kai Y, J. Biol. Chem, 1999, 274, 4225–4230. [DOI] [PubMed] [Google Scholar]
- 40.Gewirth DT and Moore PB, Nuc. Acids Res, 1988, 16, 10717–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guss JM and Freeman HC, J. Mol. Biol, 1983, 169, 521–563. [DOI] [PubMed] [Google Scholar]
- 42.Guss JM, Harrowell PR, Murata M, Norris VA and Freeman HC, J. Mol. Biol, 1986, 192, 361–387. [DOI] [PubMed] [Google Scholar]
- 43.Ubbink M, Ejdeback M, Karlsson BG and Bendall DS, Structure, 1998, 6, 323–335. [DOI] [PubMed] [Google Scholar]
- 44.Bertini I, Ciurli S, Dikiy A, Fernandez CO, Luchinat C, Safarov N, Shumilin S and Vila AJ, J. Am. Chem. Soc, 2001, 123, 2405–2413. [DOI] [PubMed] [Google Scholar]
- 45.Crowley PB, Otting G, Schlarb-Ridley BG, Canters GW and Ubbink M, J. Am. Chem. Soc, 2001, 123, 10444–10453. [DOI] [PubMed] [Google Scholar]
- 46.Sukumar N, Chen ZW, Ferrari D, Merli A, Rossi GL, Bellamy HD, Chistoserdov A, Davidson VL and Mathews FS, Biochemistry, 2006, 45, 13500–13510. [DOI] [PubMed] [Google Scholar]
- 47.McIntire WS, Wemmer DE, Chistoserdov A and Lidstrom ME, Science, 1991, 252, 817–824. [DOI] [PubMed] [Google Scholar]
- 48.Govindaraj S, Eisenstein E, Jones LH, Sanders-Loehr J, Chistoserdov AY, Davidson VL and Edwards SL, J. Bacteriol, 1994, 176, 2922–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards SL, Davidson VL, Hyun YL and Wingfield PT, J. Biol. Chem, 1995, 270, 4293–4298. [DOI] [PubMed] [Google Scholar]
- 50.Hyun YL and Davidson VL, Biochemistry, 1995, 34, 12249–12254. [DOI] [PubMed] [Google Scholar]
- 51.Hyun YL and Davidson VL, Biochemistry, 1995, 34, 816–823. [DOI] [PubMed] [Google Scholar]
- 52.Blake RC 2nd and Shute EA, J. Biol. Chem, 1987, 262, 14983–14989. [PubMed] [Google Scholar]
- 53.Giudici-Orticoni MT, Guerlesquin F, Bruschi M and Nitschke W, J. Biol. Chem, 1999, 274, 30365–30369. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez B, Piccioli M, Moratal JM and Donaire A, Biochemistry, 2003, 42, 10396–10405. [DOI] [PubMed] [Google Scholar]
- 55.Grossmann JG, Hall JF, Kanbi LD and Hasnain SS, Biochemistry, 2002, 41, 3613–3619. [DOI] [PubMed] [Google Scholar]
- 56.Mukhopadhyay BP, Ghosh B, Bairagya HR, Bera AK, Nandi TK and Das SB, J. Biomol. Struct. Dyn, 2007, 25, 157–164. [DOI] [PubMed] [Google Scholar]
- 57.Mukhopadhyay BP, Ghosh B, Bairagya HR, Nandi TK, Chakrabarti B and Bera AK, J. Biomol. Struct. Dyn, 2008, 25, 543–551. [DOI] [PubMed] [Google Scholar]
- 58.Nersissian AM, Immoos C, Hill MG, Hart PJ, Williams G, Herrmann RG and Valentine JS, Protein Sci, 1998, 7, 1915–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machonkin TE, Zhang HH, Hedman B, Hodgson KO and Solomon EI, Biochemistry, 1998, 37, 9570–9578. [DOI] [PubMed] [Google Scholar]
- 60.Malmstrom BG, Eur. J. Biochem, 1994, 223, 711–718. [DOI] [PubMed] [Google Scholar]
- 61.Guckert JA, Lowery MD and Solomon EI, J. Am. Chem. Soc, 1995, 117, 2817–2844. [Google Scholar]
- 62.Li H, Webb SP, Ivanic J and Jensen JH, J. Am. Chem. Soc, 2004, 126, 8010–8019. [DOI] [PubMed] [Google Scholar]
- 63.Donaire A, Jimenez B, Moratal J, Hall JF and Hasnain SS, Biochemistry, 2001, 40, 837–846. [DOI] [PubMed] [Google Scholar]
- 64.Donaire A, Jimenez B, Fernandez CO, Pierattelli R, Niizeki T, Moratal JM, Hall JF, Kohzuma T, Hasnain SS and Vila AJ, J. Am. Chem. Soc, 2002, 124, 13698–13708. [DOI] [PubMed] [Google Scholar]
- 65.Hall JF, Kanbi LD, Strange RW and Hasnain SS, Biochemistry, 1999, 38, 12675–12680. [DOI] [PubMed] [Google Scholar]
- 66.Berry SM, Ralle M, Low DW, Blackburn NJ and Lu Y, J. Am. Chem. Soc, 2003, 125, 8760–8768. [DOI] [PubMed] [Google Scholar]
- 67.Marshall NM, Garner DK, Wilson TD, Gao YG, Robinson H, Nilges MJ and Lu Y, Nature, 2009, 462, 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garner DK, Vaughan MD, Hwang HJ, Savelieff MG, Berry SM, Honek JF and Lu Y, J. Am. Chem. Soc, 2006, 128, 15608–15617. [DOI] [PubMed] [Google Scholar]
- 69.Machczynski MC, Gray HB and Richards JH, J. Inorg. Biochem, 2002, 88, 375–380. [DOI] [PubMed] [Google Scholar]
- 70.Carrell CJ, Sun D, Jiang S, Davidson VL and Mathews FS, Biochemistry, 2004, 43, 9372–9380. [DOI] [PubMed] [Google Scholar]
- 71.Libeu CA, Kukimoto M, Nishiyama M, Horinouchi S and Adman ET, Biochemistry, 1997, 36, 13160–13179. [DOI] [PubMed] [Google Scholar]
- 72.Sinclair-Day JD, Sisley MJ, Sykes AG, King GC and Wright PE, J. Chem. Soc. Chem. Commun, 1985, 1985, 505–507. [Google Scholar]
- 73.Gray HB and Winkler JR, Annu. Rev. Biochem, 1996, 65, 537–561. [DOI] [PubMed] [Google Scholar]
- 74.Bjerrum MJ, Casimiro DR, Chang IJ, Di Bilio AJ, Gray HB, Hill MG, Langen R, Mines GA, Skov LK, Winkler JR and et al. , J. Bioenerg. Biomembr, 1995, 27, 295–302. [DOI] [PubMed] [Google Scholar]
- 75.Onuchic JN, Beratan DN, Winkler JR and Gray HB, Ann. Rev. Biophys. Biomol. Struct, 1992, 21, 349–377. [DOI] [PubMed] [Google Scholar]
- 76.Ma L, Philipp E and Led JJ, J Biomol NMR, 2001, 19, 199–208. [DOI] [PubMed] [Google Scholar]
- 77.Jensen MR, Hansen DF and Led JJ, J. Am. Chem Soc, 2002, 124, 4093–4096. [DOI] [PubMed] [Google Scholar]
- 78.Davidson VL, Acc. Chem. Res, 2000, 33, 87–93. [DOI] [PubMed] [Google Scholar]
- 79.Marcus R. A. a. N. S., Biochim. Biophys. Acta, 1985, 811, 265–322. [Google Scholar]
- 80.Winkler JR, Wittung-Stafshede P, Leckner J, Malmstrom BG and Gray HB, Proc. Natl. Acad. Sci. USA, 1997, 94, 4246–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Page CC, Moser CC, Chen X and Dutton PL, Nature, 1999, 402, 47–52. [DOI] [PubMed] [Google Scholar]
- 82.Regan JJ, Risser SM, Beratan DN and Onuchic JN, J. Phys. Chem, 1993, 97, 13083–13088. [Google Scholar]
- 83.Davidson VL, Jones LH and Zhu Z, Biochemistry, 1998, 37, 7371–7377. [DOI] [PubMed] [Google Scholar]
- 84.Davidson VL, Biochemistry, 1996, 35, 14035–14039. [DOI] [PubMed] [Google Scholar]
- 85.Davidson VL, Biochemistry, 2000, 39, 4924–4928. [DOI] [PubMed] [Google Scholar]
- 86.Sun D, Li X, Mathews FS and Davidson VL, Biochemistry, 2005, 44, 7200–7206. [DOI] [PubMed] [Google Scholar]
- 87.Ma JK, Wang Y, Carrell CJ, Mathews FS and Davidson VL, Biochemistry, 2007, 46, 11137–11146. [DOI] [PubMed] [Google Scholar]
- 88.de la Lande A, Babcock NS, Rezac J, Sanders BC and Salahub DR, Proc. Natl. Acad. Sci. USA, 2010, 107, 11799–11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shleev S, Tkac J, Christenson A, Ruzgas T, Yaropolov AI, Whittaker JW and Gorton L, Biosens. Bioelectron, 2005, 20, 2517–2554. [DOI] [PubMed] [Google Scholar]
- 90.Andolfi L, Bruce D, Cannistraro S, Canters GW, Davis JJ, Hill HAO, Crozier J, Verbeet MP, Wrathmell CL and Astier Y, J. Electroanal. Chem, 2004, 565, 21–28. [Google Scholar]
- 91.Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, Yamada T, Constantinou AI, Christov K, White B, Li G, Majumdar D, Chakrabarty AM and Das Gupta TK, Oncogene, 2004, 23, 2367–2378. [DOI] [PubMed] [Google Scholar]
- 92.Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, Hashimoto W, Schlarb-Ridley B, Cho W, Das Gupta TK and Chakrabarty AM, Biochemistry, 2007, 46, 1799–1810. [DOI] [PubMed] [Google Scholar]
- 93.Brooks HB and Davidson VL, J. Am. Chem. Soc, 1994, 116, 11201–11202. [Google Scholar]
- 94.Sun D and Davidson VL, Biochemistry, 2003, 42, 1772–1776. [DOI] [PubMed] [Google Scholar]