Abstract
Birds are the most abundant terrestrial vertebrates and their diversity is greatly shaped by the feathers. How avian evolution is linked to feather evolution has long been a fascinating question. Numerous excellent studies have shed light on this complex relationship by investigating feather diversity and its underlying molecular mechanisms. However, most have focused on adult domestic birds, and the contribution of feather diversity to environmental adaptation has not been well-studied. In this review, we described bird diversity using the traditional concept of the altricial-precocial spectrum in bird hatchlings. We combined the spectrum with a recently published avian phylogeny to profile the spectrum evolution. We then focused on the discrete diagnostic character of the spectrum, the natal down, and propose a hypothesis for the precocial-to-altricial evolution. For the underlying molecular mechanisms in feather diversity and bird evolution, we reviewed the literature and constructed the known mechanisms for feather tract definition and natal down development. Finally, we suggested some future directions for research on altricial-precocial divergence, which may expand our understanding of the relationship between natal down diversity and bird evolution.
Keywords: Avian evolution, Feather, Altricial, Precocial, Development
INTRODUCTION
Avian specialty and the precocial-altricial spectrum
Birds, with more than 10,000 known species, are the most abundant terrestrial vertebrates. They are highly diverse in body size, shape, and color (Wright 2006). Bird evolutionary innovations include feathers, toothless beaked jaws, hard-shelled eggs, a higher metabolic rate, and a light but strong skeleton, enabling them to occupy different ecological niches. Thus, birds are an excellent model to study animal evolution and environmental adaptations.
The feathers show the highest degree of complexity and diversity among the evolutionary novelties in birds (Chen et al. 2015, Prum 2005, Prum and Brush 2002, Strasser et al. 2015). For example, down feathers composed of barbs and barbules can keep the body warm, while contour feathers possessing rachis provide physical protection and attraction. Feather diversity has likely contributed to avian diversity.
Avian hatchlings display variation in apparent maturity, which is called the altricial-precocial spectrum (Table 1). The hatchlings of altricial birds, such as Psittaciformes and Passeriformes songbirds, are close to the embryonic state, with almost naked skin and closed eyes. On the other hand, precocial hatchlings, such as Galliformes and Anseriformes, are close to the adult state, with open eyes and feathers (Starck and Ricklefs 1998). The functional maturity of the hatchlings determines the care they need from their parents and the environment to which they are going to adapt (Starck and Ricklefs 1998, Vleck and Vleck 1987).
Table 1. Diagnostic features of developmental modes in bird hatchlings.
| Developmental modea | Plumage | Eyes | Nest attendance | Parental care |
| P1 | Contour feather | Open | Leave | None |
| P2 | Down | Open | Leave | Brooding |
| P3 | Down | Open | Leave | Food showing |
| P4 | Down | Open | Leave | Parental feeding |
| SemiP | Down | Open | Nest area | Parental feeding |
| SemiA1 | Down | Open | Stay | Parental feeding |
| SemiA2 | Down | Closed | Stay | Parental feeding |
| A | None | Closed | Stay | Parental feeding |
aP1: precocial 1; P2: precocial 2; P3: precocial 3; P4: precocial 4; SemiP: semiprecocial; SemiA1: semialtricial 1; SemiA2: semialtricial 2; A: altricial.
Plumage is a main diagnostic feature in defining the altricial-precocial spectrum. However, the definition is not clear-cut, and the underlying molecular mechanism is not well characterized. In this review, we first re-analyzed the evolutionary pattern of the altricial- precocial spectrum using a recently published avian phylogeny. Second, we then surveyed the literature and hypothesized a scenario for the evolution feathers. Third, we reviewed the known molecular mechanisms responsible for the natal down divergence and discussed unresolved questions. Finally, we suggested possible research directions to uncover the mystery of feather evolution in modern birds.
Definitions of precocial and altricial birds and their evolution
Altricial and precocial hatchling divergence has been thought to be associated with environmental adaptation. However, how to define the divergence is still debated (Starck and Ricklefs 1998). In habitat selection, most altricial birds tend to nest above ground, and their chicks need to spend more time growing before they can leave the nest on their own (Bicudo 2010). In contrast, most precocial birds tend to be ground nesting, and their chicks can walk away from the nest soon after hatching. Several morphological and behavioral characters in hatchlings have been used as diagnostic features for the spectrum (Table 1), such as downy plumage, motor activity, locomotor activity, parental care, food search and feed alone, staying in the nest, eyes closed at hatching, without external feathers at hatching, no interaction with parents, and contour feathers in hatchlings (Bicudo 2010, Nice 1962, Skutch 1976, Starck and Ricklefs 1998). A comprehensive analysis reorganizing and summarizing the spectrum is shown in table 1 (Starck and Ricklefs 1998).
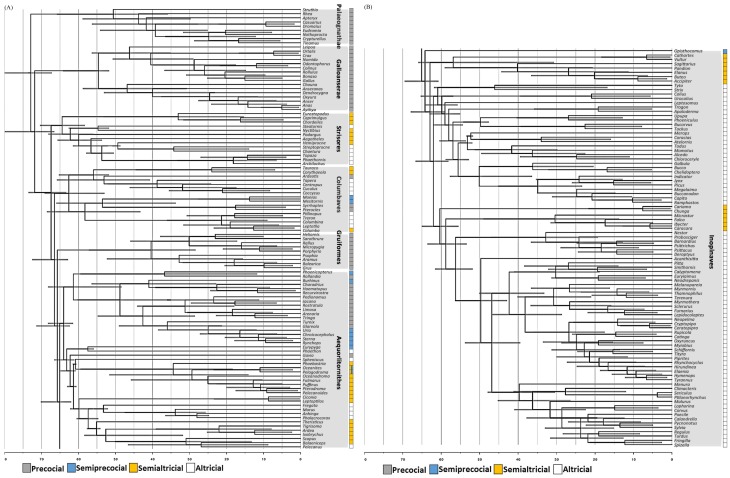
We then plotted the updated altricial-precocial spectrum to a recently published avian phylogeny that covers by far the most bird families (Fig. 1) (Hart et al. 2017, Prum et al. 2015, Starck and Ricklefs 1998). Due to limited data availability, we only mapped the avian species to four modes: precocial, semiprecocial, semialtricial, and altricial modes. Several patterns consistent with previous findings were revealed. First, the trend from precocial toward altricial avian evolution is supported by studies in both fossils and extant birds (Charvet and Striedter 2011, Starck and Ricklefs 1998, Xing et al. 2017, Zhou and Zhang 2004). The same trend is also seen in the phylogeny (Fig. 1). Second, the precocial toward altricial evolution occurred multiple times (Charvet and Striedter 2011, Chen et al. 2016, Starck and Ricklefs 1998). Some details are described below.
Fig. 1.
Fig. 1. The modified time calibrated Bayesian tree and a plot of four major avian developmental modes (Prum et al. 2015). The complete tree is divided into parts A and B. Scale in the Y-axis: millions of years ago.
Palaeognathae and Galloanserae are two oldest avian lineages and all of their members belong to the precocial developmental mode (Fig. 1). Neoaves include most of the living bird lineages (Strisores, Columbaves, Gruiformes, Aequorlitornithes, and Inopinaces) (Fig. 1). The most derived lineage, Inopinaves, belong to either the semialtricial or the altricial mode. Opisthocomus hoazin is the only exception. It belongs to the semiprecocial mode and is the most primitive Inopinaves (Fain and Houde 2004, Hackett et al. 2008, Jarvis et al. 2014). Therefore, our plotting implies that an altricial evolution event occurred after the emergence of Inopinaves.
In Neoaves, Strisores and Columbaves are two basal lineages and have terrestrial life. Strisores is peculiar in that, despite its early emergence, all its members belong to either the semialtricial or altricial mode, implying that the earliest altricial mode evolution event occurred in this lineage. Columbaves encompasses all four developmental modes. Among Columbaves, two distinct clades (Cuculiformes and Columbiformes) belong to the altricial mode, suggesting that at least two altricial mode evolution events occurred within this lineage.
The remaining two lineages, Gruiformes and Aequorlitornithes, are mostly water birds, or land birds without good flying ability. According to the phylogeny, Gruiformes could be basal to Aequorlitornithes. All Gruiformes are water birds and belong to the precocial groups, whereas Aequorlitornithes are highly diverse in their developmental mode. Interestingly, within Aequorlitornithes, the developmental mode evolved from precocial toward altricial, resembling the whole avian evolutionary trend. At least one altricial evolution event occurred in this lineage.
In summary, the plotting suggests that at least five independent altricial mode evolutionary events occurred during modern avian evolution.
Trends of natal down evolution during avian evolution
Natal down is a diagnostic feature of the altricial-precocial spectrum. Natal down, evolved from environmental adaptation, provides insulation to keep hatchlings warm (Pap et al. 2017). However, the description of the downy plumage in bird hatchlings is still scarce, not to mention the underlying molecular mechanism. Here we reviewed published data to construct a possible scenario of natal down evolution during avian evolution.
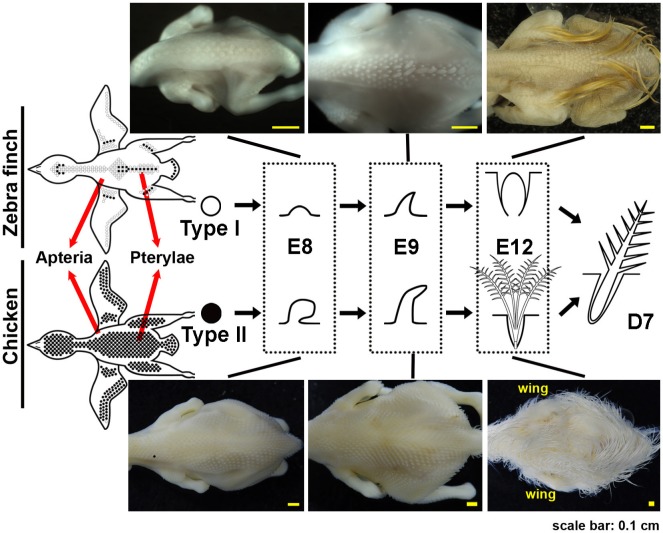
According to previous studies, the plumage differences between altricial and precocial bird hatchlings could be due to macropattern and micropattern changes in feather development (Chen et al. 2016). Feather macropattern, or pterylosis, is the feather tract distribution of a bird (Fliniaux et al. 2004, Ho et al. 2019, Olivera-Martinez et al. 2004). In feather tracts, the follicles of contour feathers are concentrated in dense tracts called pterylae and are separated by bare areas called apteria (Fig. 2). The feather tract area is smaller in altricial birds than precocial birds, but the reason for the divergence is still not clear. Three explanations have been proposed: (1) The apterium may be an adaptation to reduce the total feather weight. (2) The movement of the body and the feathers could be better accommodated by an increase in apterium. (3) The apterium may aid birds in thermoregulation during flight or brooding (Chen et al. 2016, Stettenheim 2015). In other words, flying ability contributes to the feather tract divergence and altricial birds are usually better in flight. Our previous data showed that feather tracts cover almost the entire body surface in chicken hatchlings, whereas in zebra finch hatchlings they only cover part of the body (Fig. 2) (Chen et al. 2016). This phenomenon implies a relationship between feather macropatterning and avian evolution.
Fig. 2.
Fig. 2. Schematic diagram of feather tract and types of natal down formation in zebra finch and chicken. Zebra finch embryos show two types of feather formation: Type I feather formation (open circles), in which the feather buds do not develop into downy feather; Type II feather formation (black circles), in which the feather buds develop into downy feathers, which are later replaced by contour feathers. Chicken embryos exhibit only the Type II feather formation. E8, E9, and E12: embryo day 8, 9 and 12, respectively. D7: 7 days post-hatch. Scale bar = 0.1 cm. The figure was modified from our previous study (Chen et al. 2017).
Feather micropatterning is the development of individual feather buds. Feather diversity appears in different body regions in adult birds and at developmental stages of a bird. For example, flight feathers enable the bird to fly, body contour feathers provide physical protection and shape the body outline, and down feathers keep the body warm. Our previous studies revealed regulatory differences among different body feathers or among different parts of a feather in chickens (Ng et al. 2014 2015, Wu et al. 2015). However, most bird hatchlings only show natal down and we found differences in natal down growth patterns between the precocial chicken and altricial zebra finch, implying that feather micropatterning contributed to avian evolution.
To address the contribution of micropatterning to avian evolution, we reviewed the literature in natal down development. We considered four major developmental modes: precocial, semiprecocial, semialtricial, and altricial hatchlings. However, we found that the staging of semiprecocial bird embryos has never been characterized. Therefore, we focused on natal down development of a basal precocial bird (emu), a semialtricial bird (pigeon), and an altricial sister clade of finch (parrot).
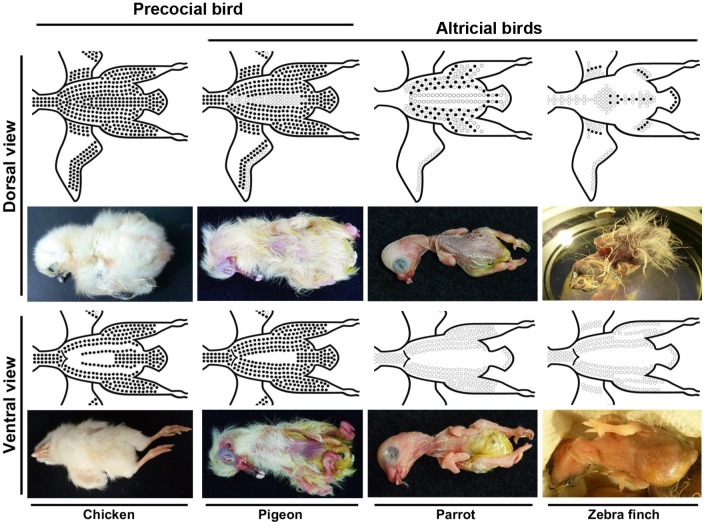
Emu, Dromaius novaehollandiae, is a member of Paleognathae, which also includes other ground living birds: ostrich, rhea, tinamou, kiwi, and cassowary (Nagai et al. 2011). Paleognathae is the basal lineage of modern birds and all the members belong to the precocial mode (Fig. 1). Compared to chicken hatchlings, emu hatchlings come from larger eggs, have a larger body size, require a longer incubation time, and develop peculiar limb types (Nagai et al. 2011). However, like chicken hatchlings, emu hatchlings are covered with natal down throughout the body surface and can feed by themselves soon after hatching (Nagai et al. 2011). These observations imply that precocial birds share similar body natal down distribution and feeding behaviors. Strisores and Columbaves include the basal altricial and semialtricial lineages. Pigeon, Columba livia, belongs to Columbaves, but its developmental mode is debated. Some studies assign it as an altricial bird, while others classify it as a semialtricial bird (Dyke and Kaiser 2010, Łukasiewicz and Boruc 2014, Olea and Sandoval 2012, Starck and Ricklefs 1998). Although pigeon hatchlings demand much parental care, in the literature and our own breeding study, we found that natal down covers most of the body surface, except the posterior ventral region (Fig. 3). Therefore, by using natal down as the indicator, we classify the pigeon to the semialtricial mode.
Fig. 3.
Fig. 3. Schematic diagram of bird hatchlings. Dorsal (upper row) and ventral (lower row) views of chicken (precocial), pigeon (semialtricial), parrot (altricial) and zebra finch.
Zebra finch, Taeniopygia guttata, belongs to the most derived and diverse bird lineage, Passerineform. All passerines are classified as altricial birds. Their hatchlings show no or sparse natal down on the body surface and demand much parental care. We found that zebra finch hatchlings have two types of feather formation. In the pterylae region, Type I feather buds grow contour feather directly from the follicles without going through the natal down; that is, the natal down follicle forms, but feather filament does not. Type II feather buds, like the feather buds in precocial birds, develop into natal down feather filament and later grow contour feathers after replacing the natal down in the same follicles (Chen et al. 2016). The contour feathers grow from both types of feather buds at similar stages (D7, Fig. 2).
Parrot (Myiopsitta monachus), a sister clade to finch, is an altricial bird (Carril and Tambussi 2015, Prum et al. 2015). However, its natal down development is different from that of zebra finch. According to previous studies and our observation, the hatchlings of both species show no natal down on ventral skin; on dorsal skin, zebra finch hatchlings show mature natal down, while parrot hatchlings show growing natal down, starting from naked-like hatchlings in newborns to downy feathers on dorsal skin in later stages (Fig. 3). We can only find the Type I feather formation in the ventral region, and immature natal down forms in the dorsal region of parrot hatchlings. Thus, the natal down growth pattern may differ between parrot and zebra finch.
By comparing the hatchlings of the above four avian species, we propose an evolutionary scenario of natal down plumage evolution during avian evolution: natal down initially covered the entire body in precocial hatchlings. After precocial birds occupied most of the land niches, semiprecocial and semialtricial birds with better flight ability evolved and extended their habitat to waters, oceans, or higher places. Semialtricial birds can build nests in hidden places. Therefore, their hatchlings can snuggle in the nest and their ventral natal downs are no longer necessary. Finally, altricial birds evolved because they have higher intelligence and can build sophisticated nests, so the hatchlings needed less natal down and can re-allocate their saved energy to other organs, such as digestive organs (Blom and Lilja 2005).
In summary, although natal down plumage is a diagnostic character to distinguish between developmental modes, it is not a simple diagnostic character because of the diversity in semiprecocial, semialtricical and altricical bird lineages. Further study in natal down plumage classifications is needed.
Molecular mechanisms of feather development
The source tissues for feather tract development are embryonic somatopleure and somite (Fliniaux et al. 2004). The proximal somatopleural mesoderm forms a feather-forming dermis at E2 (2 days of incubation) (Fliniaux et al. 2004). At the molecular level, like HOX gene clusters that define the animal body plan, feather macropatterns are defined by regional identity, and some regulators have been identified (Fliniaux et al. 2004, Ho et al. 2019, Houghton et al. 2005). The members of the ectodysplasin pathway, Ectodysplasin A (EDA), Ectodysplasin A receptor (EDAR) and ectodysplasin receptor associated death domain (EDARADD) are known to be involved in the patterning and could be directed by β-catenin signaling and/or BMP2 (Bone morphogenetic proteins 2) (Ho et al. 2019, Houghton et al. 2005). During chicken early skin development (E6.5), the expanding expression of EDA imposes the travelling wave of feather formation. The EDA wave spreads across a mesenchymal cell density gradient and lowers the threshold of mesenchymal cells required to begin the feather bud formation, further triggering the pattern formation. Interestingly, such waves and the precise arrangement of feather primordia are lost in the flightless emu and ostrich (Ho et al. 2019). In zebra finch, the expression pattern of EDA is unclear. We observed that the regular arrangement of feather primordia remains, but how the reduced feather tract formed is still unclear (Chen et al. 2017).
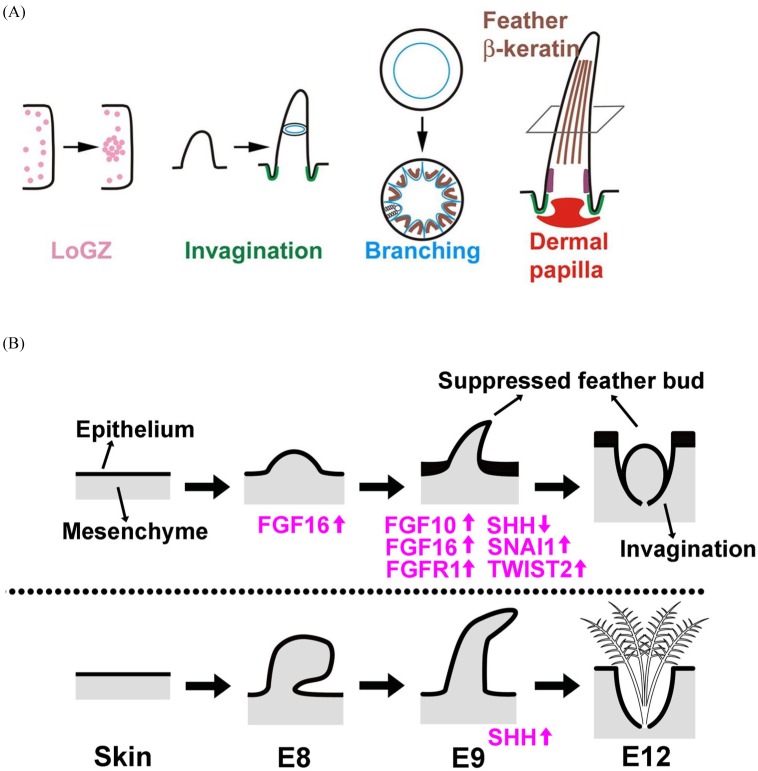
Once feather tracts are established, the feather micropatterning is initiated. Five properties, localized growth zone (LoGZ), invagination, branching, feather β-keratin, and dermal papilla (stem cell), have been characterized in feathers (Fig. 4A) (Wu et al. 2018). To localize the growth zone, the spatial arrangement and regular outgrowth of feathers are regulated by the epithelio-mesenchymal molecular interactions between the dermis and the overlying epidermis (Chen et al. 2015, Ho et al. 2019, Meinhardt and Gierer 2000, Mou et al. 2011, Wells et al. 2012). Many molecules that act with the process have been identified. For example, WNT/b-catenin signaling and TWIST2 are promoters at the early stages of skin patterning, not only for feather tract establishment but also for feather growth initiation (Hornik et al. 2005, Noramly et al. 1999, Widelitz et al. 2000). Some FGFs (fibroblast growth factors) and SHH (sonic hedgehog) are promoters or activators, while some BMPs (bone morphogenetic proteins) are inhibitors of feather placode formation (Jung et al. 1998, Mandler and Neubuser 2004, McKinnell et al. 2004, Song et al. 2004). Branching formation within a feather filament is formed by invagination of the multilayered filament epithelium surrounding the mesenchyme. This event takes place in the ramogenic zone, and NOTCH, FGF, GDF10 and GREM1 that modulate the BMP signaling were reported to regulate the periodic-branching process (Cheng et al. 2018, Li et al. 2017). Feather rachis is formed by the fusion of barb ridges at the anterior end of the feather, and BMP, NOG, SPRY, and FGF are known to regulate the periodic invagination that forms barb and rachis (Chen et al. 2015, Chuong et al. 2014, Ng and Li 2018).
Fig. 4.
Fig. 4. Five developmental stages of feathers and the regulators for natal down growth suppression in zebra finch. (A) Schematic diagram shows the five developmental stages of feathers: LoGZ, invagination, branching, feather β-keratin, and dermal papilla (Wu et al. 2018). (B) A summary of the mRNAs identified in Type I and Type II feather formations in zebra finch (Chen et al. 2016).
Although the feather developmental process and the underlying regulators are largely identified, all the researches related to it only have been done in domestic chicken. The regulatory mechanisms in diverse natal down growth in altricial birds are less studied. The genes underlying some chicken featherless mutants have been characterized. The regulatory differences in BMPs cause the naked neck phenotype in chicken, and a nonsense mutation in FGF20 is associated with the featherless trait (Mou et al. 2011, Wells et al. 2012). However, both chicken variants show no established feather buds on the naked region, unlike the Type I feather formation process we observed in zebra finch and parrot. By using comparative transcriptomics, we found that FGF16 and the mitogen-activated protein kinase (MAPK) signaling pathway are involved in natal down growth suppression in zebra finch (Fig. 4B) (Chen et al. 2016). However, how the regulation is achieved and whether it has been conserved in altricial birds, or natal down suppressed birds, need further study.
CONCLUSIONS AND PERSPECTIVE
The altricial-precocial spectrum traditionally describes the developmental modes of mammals and birds at birth (Augustine et al. 2019). The developmental modes are highly associated with parental care, habitat selection, and environmental adaptation. Natal down plumage is one of the indicators for the altricial-precocial spectrum and is strongly related to avian evolution. However, previous studies rarely described the natal down plumage precisely when analyzing the spectrum. In this review, we first plotted the altricial-precocial spectrum to the recently published avian phylogeny to explore the evolution of the developmental modes during avian evolution. We then focused on the natal down plumage differences in representative precocial, semialtricial, and altricial birds to construct the probable evolutionary steps for natal down plumage degeneration. Finally, we reviewed the molecular mechanism for feather development and described the possible mechanisms involved in natal down plumage diversification among birds.
The phylogeny of birds has been difficult to construct because of the rapid species radiation from the Cretaceous to Paleogene (K-Pg) (Jarvis et al. 2014). Jarvis et al. (2014) recently published a whole-genome- based avian phylogeny. However, species phylogeny construction relies not only on the sequencing coverage of the genome, but also on the nodes of the species. The avian phylogeny including all major avian lineages with lower genome sequencing coverage was constructed (Prum et al. 2015). Although the two phylogenetic trees show some differences (for example, the position of birds of prey), both trees fit our model. Here we adopt a phylogeny with a lower genome coverage but a larger node number as our analysis backbone (Prum et al. 2015). However, as large scale bird genome sequencing is progressing rapidly, the phylogeny may soon be revised. A recent large scale genomic study in passerines revealed their precise phylogeny and an interesting evolutionary trajectory, and a similar study in semiprecocial and semialtricial species will largely improve the study of evolution of the altricial- precocial spectrum (Oliveros et al. 2019). Moreover, the accumulation of the morphological data can also alter the phylogeny. Most morphological data are from investigations and records from birds in the wild, so it is difficult to know whether the bird hatchlings were recorded at birth or several days after birth. We have observed that, at least in parrots, natal down grows vigorously after birth, so the spectrum should be characterized at birth.
Feather development involves the interaction of numerous molecules. The major regulators at different developmental stages were basically identified as mentioned above. However, how those regulators are regulated in feather development is still unclear. The phenotypic changes could have resulted from coding and/or non-coding sequence changes. Coding sequence variation is known to be a key factor in domestic chicken variation (Mou et al. 2011, Ng and Li 2018, Ng et al. 2012, Wells et al. 2012). However, such cases have rarely been identified in wild birds, and non- coding sequence variation contributes more to the intra- species variation (Küpper et al. 2016, Lamichhaney et al. 2015 2016). Indeed, despite the abundance of bird species, their chromosomal structures have been surprisingly well conserved over evolutionary time (Frankl-Vilches et al. 2015, Skinner and Griffin 2012). Also, coding sequence evolution is relatively slower in birds than other vertebrates (Nam et al. 2010, Weber et al. 2014). Therefore, coding sequence variation should not be the major evolutionary effector for bird diversity. Non-coding sequences can act as gene regulatory regions, such as promoters and enhancers, or non- coding transcripts, such as non-coding RNAs. In mouse, regional specificity regulation has been characterized at the epigenetic level (Ezhkova et al. 2011, Fabre et al. 2017, Rodriguez-Carballo et al. 2017) and in non- coding RNA (Caley et al. 2010). Similar mechanisms may act in the avian genome, but further studies will be needed.
Research on feather development and diversity expands our view of how complex structures can evolve to increase an organism’s survival and persistence. However, the importance of the “natal down diversity”, in terms of their types and topological distribution, could have long been underestimated. The evolution from precocial to altricial modes has been thought to be environmental adaptation. However, most of the diagnostic characters are difficult to qualify or quantify, so the spectrum evolution has been debated for a long time. Natal down is the only discrete character for the diagnosis, and our view in that altricial-precocial spectrum evolution is based on our characterization of different types and patterns of the natal down. Furthermore, different developmental stages of bird exhibit different types of feather. Juvenile birds are ready to leave the nest and most natal downs are replaced by contour feathers (Podulka et al. 2004), which enable the birds to fly. After moulting several times, more functional feathers are derived from the juvenile feather follicles to achieve specific functions in adult birds (Terres 1991, National Audubon Society), including feathers used in camouflage, migration, overwintering, or courtship (Dunn et al. 2011). The hatchling (natal down) to juvenile (contour feather) plumage transition happens only once in most birds, but the mechanism has never been characterized. A continuous investigation of hatchling to juvenile plumage change will further help us explore the mystery of evolution from precocial to altricial modes in birds.
Acknowledgments
Acknowledgment: We thank Drs Ping Wu and Cheng Ming Chuong for their suggestions and for permission to use the feather development model. We thank Dr. Richard Prum for his permission to use of the phylogenetic tree. This study was supported by MOST, Taiwan (MOST 107-2311-B-001-016-MY3).
Footnotes
Authors’ contributions: C.-C.K. and W.-H.L. designed the research; C.-C.K., S.-M.W., and H.-F.C. performed the research and analyzed the data; and C.-C. K. and W.-H.L. wrote the paper.
Competing interests: The authors declare no competing interests.
Availability of data and materials: The datasets used and analyzed during the current study are available from the first or corresponding author on request.
Ethics approval consent to participate: All the data were downloaded from the internet.
References
- Augustine S, Lika K, Kooijman Salm. Altricial-precocial spectra in animal kingdom. J Sea Res. 2019;143:27–34. [Google Scholar]
- Bicudo Jepw. Ecological and environmental physiology of birds. Oxford; New York: Oxford University Press; 2010. [Google Scholar]
- Blom J, Lilja C. A comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology. 2005;108:81–95. doi: 10.1016/j.zool.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Caley D P, Pink R C, Trujillano D, Carter D R. Long noncoding RNAs, chromatin, and development. Sci World J. 2010;10:90–102. doi: 10.1100/tsw.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carril J, Tambussi C P. Development of the superaltricial monk parakeet (Aves, Psittaciformes): embryo staging, growth, and heterochronies. Anat Rec. 2015;298:1836–1847. doi: 10.1002/ar.23256. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental modes and developmental mechanisms can channel brain evolution. Front Neuroanat. 2011;5:4. doi: 10.3389/fnana.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C F, Foley J, Tang P C, Li A, Jiang T X, Wu P, Widelitz R B, Chuong C M. Development, regeneration, and evolution of feathers. Annu Rev Anim Biosci. 2015;3:169–195. doi: 10.1146/annurev-animal-022513-114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C K, Ng C S, Wu S M, Chen J J, Cheng P L, Wu P, Lu M Y, Chen D R, Chuong C M, Cheng H C, Ting C T, Li W H. Regulatory differences in natal down development between altricial zebra finch and precocial chicken. Mol Biol Evol. 2016;33:2030–2043. doi: 10.1093/molbev/msw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C K, Yu C P, Li S C, Wu S M, Lu M J, Chen Y H, Chen D R, Ng C S, Ting C T, Li W H. Identification and evolutionary analysis of long non-coding RNAs in zebra finch. BMC Genomics. 2017;18 doi: 10.1186/s12864-017-3506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Yan X, Qiu G, Zhang J, Wang H, Feng T, Tian Y, Xu H, Wang M, He W, Wu P, Widelitz R B, Chuong C M, Yue Z. Contraction of basal filopodia controls periodic feather branching via Notch and FGF signaling. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C M, Bhat R, Widelitz R B, Bissell M J. Snapshot: Branching morphogenesis. Cell. 2014;158:1212–1212. doi: 10.1016/j.cell.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J L, Alderfer J K. National geographic field guide to the birds of north america. Washington, D.C.: National Geographic Society; 2011. [Google Scholar]
- Dyke G J, Kaiser G W. Cracking a developmental constraint: Egg size and bird evolution. Proceedings of the VII International Meeting of the Society of Avian Paleontology and Evolution. 2010;62:207–216. [Google Scholar]
- Ezhkova E, Lien W H, Stokes N, Pasolli H A, Silva J M, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre P J, Leleu M, Mormann B H, Lopez-Delisle L, Noordermeer D, Beccari L, Duboule D. Large scale genomic reorganization of topological domains at the HoxD locus. Genome Biol. 2017;18 doi: 10.1186/s13059-017-1278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain M G, Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Viallet J P, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–3966. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- Frankl-Vilches C, Kuhl H, Werber M, Klages S, Kerick M, Bakker A, Oliveira E H, Reusch C, Capuano F, Vowinckel J, Leitner S, Ralser M, Timmermann B, Gahr M. Using the canary genome to decipher the evolution of hormone-sensitive gene regulation in seasonal singing birds. Genome Biol. 2015;16 doi: 10.1186/s13059-014-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett S J, Kimball R T, Reddy S, Bowie R C, Braun E L, Braun M J, Chojnowski J L, Cox W A, Han K L, Harshman J, Huddleston C J, Marks B D, Miglia K J, Moore W S, Sheldon F H, Steadman D W, Witt C C. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- Hart L A, Downs C T, Brown M. Keeping it regular: Development of thermoregulation in four tropical seabird species. J Therm Biol. 2017;64:19–25. doi: 10.1016/j.jtherbio.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Ho Wkw, Freem L, Zhao D, Painter K J, Woolley T E, Gaffney E A, Mcgrew M J, Tzika A, Milinkovitch M C, Schneider P, Drusko A, Matthäus F, Glover J D, Wells K L, Johansson J A, Davey M G, Sang H M, Clinton M, Headon D J. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik C, Krishan K, Yusuf F, Scaal M, Brand-Saberi B. cDermo-1 misexpression induces dense dermis, feathers, and scales. Dev Biol. 2005;277:42–50. doi: 10.1016/j.ydbio.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Houghton L, Lindon C, Morgan B A. The ectodysplasin pathway in feather tract development. Development. 2005;132:863–872. doi: 10.1242/dev.01651. [DOI] [PubMed] [Google Scholar]
- Jarvis E D, Mirarab S, Aberer A J, Li B, Houde P, Li C. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346:1320–1331. doi: 10.1126/science.1253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H S, Francis-West P H, Widelitz R B, Jiang T X, Ting-Berreth S, Tickle C, Wolpert L, Chuong C M. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Küpper C, Stocks M, Risse J E, Remedios Dos, Farrell N, Mcrae L L, Morgan S B, Karlionova T C, Pinchuk N, Verkuil P, Kitaysky Y I, Wingfield A S, Piersma J C, Zeng T, Slate K, Blaxter J, Lank M, Burke D B. A supergene determines highly divergent male reproductive morphs in the ruff. Nat Genet. 2016;48:79–83. doi: 10.1038/ng.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhaney S, Berglund J, Almén M S, Maqbool K, Grabherr M, Martinez-Barrio A, Promerová M, Rubin C J, Wang C, Zamani N, Grant B R, Grant P R, Webster M T, Andersson L. Evolution of darwin's finches and their beaks revealed by genome sequencing. Nature. 2015;518:371–375. doi: 10.1038/nature14181. [DOI] [PubMed] [Google Scholar]
- Lamichhaney S, Fan G, Widemo F, Gunnarsson U, Thalmann D S, Hoeppner M P, Kerje S, Gustafson U, Shi C, Zhang H, Chen W, Liang X, Huang L, Wang J, Liang E, Wu Q, Lee S M, Xu X, Höglund J, Liu X, Andersson L. Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax) Nat Genet. 2016;48:84–88. doi: 10.1038/ng.3430. [DOI] [PubMed] [Google Scholar]
- Li A, Figueroa S, Jiang T X, Wu P, Widelitz R, Nie Q, Chuong C M. Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme. Nat Commun. 2017;8 doi: 10.1038/ncomms14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasiewicz M, Boruc K. Biology of embryo development in pigeon Columba livia domesticus in conditions of artificial incubation. Adv Anim Vet Sci. 2014;2:401–406. [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–3343. doi: 10.1242/dev.01203. [DOI] [PubMed] [Google Scholar]
- Mckinnell I W, Turmaine M, Patel K. Sonic Hedgehog functions by localizing the region of proliferation in early developing feather buds. Dev Biol. 2004;272:76–88. doi: 10.1016/j.ydbio.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. BioEssays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mou C, Yu L, Burt D W, Bed'hom B, Tixier-Boichard M, Painter K J, Headon D J. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Mak S S, Weng W, Nakaya Y, Ladher R, Sheng G. Embryonic development of the emu, Dromaius novaehollandiae. Dev Dyn. 2011;240:162–175. doi: 10.1002/dvdy.22520. [DOI] [PubMed] [Google Scholar]
- Nam K, Mugal C, Nabholz B, Schielzeth H, Wolf J B, Backström N, Künstner A, Balakrishnan C N, Heger A, Ponting C P, Clayton D F, Ellegren H. Molecular evolution of genes in avian genomes. Genome Biol. 2010;11 doi: 10.1186/gb-2010-11-6-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C S, Chen C K, Fan W L, Wu P, Wu S M, Chen J J, Lai Y T, Mao C T, Lu Myj, Chen D R, Lin Z S, Yang K J, Sha Y A, Tu T C, Chen C F, Chuong C M, Li W H. Transcriptomic analyses of regenerating adult feathers in chicken. BMC Genomics. 2015;16 doi: 10.1186/s12864-015-1966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C S. Genetic and molecular basis of feather diversity in birds. Genome Biol Evol. 2018;10:2572–2586. doi: 10.1093/gbe/evy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C S, Wu P, Fan W L, Yan J, Chen C K, Lai Y T, Wu S M, Mao C T, Chen J J, Lu M Y, Ho M R, Widelitz R B, Chen C F, Chuong C M, Li W H. Genomic organization, transcriptomic analysis, and functional characterization of avian α-and β-keratins in diverse feather forms. Genome Biol Evol. 2014;6:2258–2273. doi: 10.1093/gbe/evu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C S, Wu P, Foley J, Foley A, Mcdonald M L, Juan W T, Huang C J, Lai Y T, Lo W S, Chen C F, Leal S M, Zhang H, Widelitz R B, Patel P I, Li W H, Chuong C M. The chicken frizzle feather is due to an α-keratin (KRT75) mutation that causes a defective rachis. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice MM. Development of behavior in precocial birds. New York: [Linnaean Society. 1962.
- Noramly S, Freeman A, Morgan B A. β-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Olea G B, Sandoval M T. Embryonic development of Columba livia (Aves: Columbiformes) from an altricial-precocial perspective. Revista Colombiana De Ciencias Pecuarias. 2012;25:3–13. [Google Scholar]
- Olivera-Martinez I, Viallet J P, Michon F, Pearton D J, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–115. doi: 10.1387/ijdb.15272376. [DOI] [PubMed] [Google Scholar]
- Oliveros C H, Field D J, Ksepka D T, Barker F K, Aleixo A, Andersen M J. Earth history and the passerine superradiation. Proc Natl Acad Sci. 2019;116:7916–7925. doi: 10.1073/pnas.1813206116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap P L, Vincze O, Wekerle B, Daubner T, Vágási C I, Nudds R L. A phylogenetic comparative analysis reveals correlations between body feather structure and habitat. Funct Ecol. 2017;31:1241–1251. [Google Scholar]
- Podulka S, Rohrbaugh R W, Bonney R. Handbook of bird biology. 2nd ed. Ithaca, NY: Cornell Lab of Ornithology in association with Princeton University Press; 2004. [Google Scholar]
- Prum R O. Evolution of the morphological innovations of feathers. J Exp Zool B Mol Dev Evol. 2005;304:570–579. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- Prum R O, Berv J S, Dornburg A, Field D J, Townsend J P, Lemmon E M, Lemmon A R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 2015;526:569–573. doi: 10.1038/nature15697. [DOI] [PubMed] [Google Scholar]
- Prum R O, Brush A H. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Carballo E, Lopez-Delisle L, Zhan Y, Fabre P J, Beccari L, El-Idrissi I, Huynh Thn, Ozadam H, Dekker J, Duboule D. The HoxD cluster is a dynamic and resilient TAD boundary controlling the segregation of antagonistic regulatory landscapes. Genes Dev. 2017;31:2264–2281. doi: 10.1101/gad.307769.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B M, Griffin D K. Intrachromosomal rearrangements in avian genome evolution: evidence for regions prone to breakpoints. Heredity. 2012;108:37–41. doi: 10.1038/hdy.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutch A F. Parent birds and their young. Austin: University of Texas Press; 1976. [Google Scholar]
- Song H K, Lee S H, Goetinck P F. FGF-2 signaling is sufficient to induce dermal condensations during feather development°. Dev Dyn. 2004;231:741–749. doi: 10.1002/dvdy.20243. [DOI] [PubMed] [Google Scholar]
- Starck J M, Ricklefs R E. Avian growth and development: Evolution within the altricial-precocial spectrum. New York: Oxford University Press; 1998. [Google Scholar]
- Stettenheim P R. The integumentary morphology of modern birds-an overview 1. American Zoologist. 2015;40:461–477. [Google Scholar]
- Strasser B, Mlitz V, Hermann M, Tschachler E, Eckhart L. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol Biol. 2015;15 doi: 10.1186/s12862-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terres J K. The audubon society encyclopedia of north American birds. New York: Wings Books: Distributed by Outlet Book Co; 1991. [Google Scholar]
- Vleck C M, Vleck D. Metabolism and energetics of avian embryos. J Exp Zool Suppl. 1987;1:111–125. [PubMed] [Google Scholar]
- Weber C C, Nabholz B, Romiguier J, Ellegren H. Kr/Kc but not dN/dScorrelates positively with body mass in birds, raising implications for inferring lineage-specific selection. Genome Biol. 2014;15 doi: 10.1186/s13059-014-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K L, Hadad Y, Ben-Avraham D, Hillel J, Cahaner A, Headon D J. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz R B, Jiang T X, Lu J, Chuong C M. β-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated β-Catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Wright M T. Birds of the world: Recommended English names. London: Christopher Helm; 2006. [Google Scholar]
- Wu P, Ng C S, Yan J, Lai Y C, Chen C K, Lai Y T, Wu S M, Chen J J, Luo W, Widelitz R B, Li W H, Chuong C M. Topographical mapping of α-and β-keratins on developing chicken skin integuments: Functional interaction and evolutionary perspectives. Proc Natl Acad Sci. 2015;112:6770–6779. doi: 10.1073/pnas.1520566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Yan J, Lai Y C, Ng C S, Li A, Jiang X, Elsey R M, Widelitz R, Bajpai R, Li W H, Chuong C M. Multiple regulatory modules are required for scale-to-feather conversion. Mol Biol Evol. 2018;35:417–430. doi: 10.1093/molbev/msx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Connor O', Mckellar J K, Chiappe R C, Tseng L M, Li K, Bai G. A mid-Cretaceous enantiornithine (Aves) hatchling preserved in Burmese amber with unusual plumage. Gondwana Research. 2017;49:264–277. [Google Scholar]
- Zhou Z, Zhang F. A precocial avian embryo from the Lower Cretaceous of China. Science. 2004;306 doi: 10.1126/science.1100000. [DOI] [PubMed] [Google Scholar]