Abstract
Aims:
Special AT-rich sequence-binding protein 2 (SATB2) is a transcriptional regulator with critical roles in brain, craniofacial, and skeletal development. It has emerged as a key marker of lower gastrointestinal (GI) tract columnar epithelial and osteoblastic differentiation. Transcription factor immunohistochemistry is useful in assigning site of origin in well-differentiated neuroendocrine tumors (NETs) and has had a limited role in poorly differentiated neuroendocrine carcinomas (NECs).
Methods and results:
Tissue microarrays were constructed from the following: 317 NETs (37 thyroid, 46 lung, 12 duodenum, 70 pancreas, 106 jejunoileum, 24 appendix, 5 rectosigmoid), 44 pheochromocytomas/paragangliomas, and 79 NECs (29 Merkel cell, 30 lung, 20 extrapulmonary visceral); 9 appendiceal and 19 rectal NETs were examined in whole sections. SATB2 immunohistochemistry was scored for extent (%) and intensity (0–3+) with an H-score calculated. SATB2 was expressed by 96% of rectosigmoid, 79% of appendiceal, and only 7% of other well-differentiated neoplasms (p<0.0001). Expression in lower GI tract NETs (median H-score=255) was stronger than in other positive tumors (median H-score=7) (p<0.0001). Any SATB2 expression was 86% sensitive/93% specific for lower GI tract origin. SATB2 was expressed by 79% of Merkel cell (median H-score=300), 33% of lung (median H-score=23), and 60% of extrapulmonary visceral (median H-score=110) NECs, with stronger expression in Merkel cell carcinoma (p<0.001). At an H-score cutoff of ≥150, SATB2 was 69% sensitive/90% specific for Merkel cell carcinoma.
Conclusions:
SATB2 is frequently, strongly expressed by lower GI tract NETs; we have adopted it as our rectal NET marker. Relatively frequent, strong expression in Merkel cell carcinoma may have value in assigning NEC site of origin.
Keywords: SATB2, immunohistochemistry, transcription factor, neuroendocrine, Merkel cell carcinoma, differential diagnosis, site of origin
Introduction:
Well-differentiated neuroendocrine tumors (NETs) frequently (10–20%) present as metastases of occult origin.(1)Determination of primary site has prognostic and therapeutic significance. For example, pancreatic NETs are more likely to present with distant metastases and are associated with inferior median survival relative to jejunoileal NETs; NET primaries are often resected to militate against local complications, even in the face of distant metastasis; and different biologic and cytotoxic therapies are approved based on primary site.(2–4) Our group frequently employs a panel of immunohistochemical stains in this diagnostic setting, including CDX2 (midgut); PAX6, Islet 1 (pancreas); and OTP (lung).(5, 6) In the past, we had employed prostatic acid phosphatase (PrAP) as a rectal NET marker, though when we formally studied it, we also found frequent expression by midgut NETs (poster presentation at USCAP 2014). Rectal NETs often co-express the pancreatic NET markers PAX6 and Islet 1, a diagnostic pitfall.(1)
Aside from TTF-1 and CK20 for visceral and cutaneous origin, respectively, and perhaps neurofilament for Merkel cell carcinoma and CM2B4 to identify Merkel cell polyomavirus-associated tumors, immunohistochemistry has had a limited role in assigning site of origin in poorly differentiated neuroendocrine carcinomas (NECs).(1) Historically, platinum/etoposide (i.e., “small cell lung cancer chemotherapy”) has been applied in the metastatic setting, regardless of primary site.(7) This distinction is no longer of merely academic interest. Checkpoint inhibitor therapy (specifically anti-PD-L1 therapy) has proven remarkably effective in Merkel cell carcinoma (and only modestly so in visceral NECs) and has rapidly emerged as first-line therapy in the metastatic setting.(8–10) Unlike in adenocarcinomas, transcription factors are frequently expressed in NECs independent of site of origin—a phenotype referred to as “transcription factor lineage infidelity.”(11) There is precedent, though, for the value of a transcription factor in this diagnostic context (i.e., TTF-1).
Special AT-rich sequence-binding protein 2 (SATB2) is a transcriptional regulator that plays critical roles in neocortical and craniofacial development. It was simultaneously discovered as the gene disrupted in two cleft palate patients with balanced translocations involving chromosome 2q32 and in a screen of murine and human databases for sequences with 1) homology to known nuclear matrix attachment (MAR)-binding proteins and 2) expression in pre-B cells.(12, 13) It was subsequently independently discovered by two laboratories investigating transcriptional regulation of neocortical development.(14, 15) SATB2 was introduced to the diagnostic pathology community in the context of the immunohistochemistry-based protein expression profiling of the Human Protein Atlas, which uncovered relatively restricted expression in colonic epithelium and colon cancer.(16) SATB2 has subsequently gained significant traction as a lower gastrointestinal (GI) tract-specific adenocarcinoma marker.(17)
In a follow up to the initial Human Protein Atlas study, Dragomir and colleagues reported an 18-month prospective study in which SATB2 was performed on all tumors (n=840) in which CK20 was ordered in the conduct of routine clinical care.(18) I was intrigued by the finding (included in two tables but not specifically mentioned in the text) that 40% of “carcinoid” tumors expressed SATB2 and that, among the positives, 35% were extensively so. In that same study, the authors reported SATB2 expression by 3 Merkel cell carcinomas. Given our group’s experience with transcription factor expression in NETs, I hypothesized that SATB2 might be differentially expressed based on neuroendocrine cell lineage or anatomic site and that SATB2 expression in Merkel cell carcinoma likely reflected transcription factor lineage infidelity.
SATB2 expression was examined in a large cohort of well-differentiated neuroendocrine neoplasms (i.e., NETs and pheochromocytoma/paraganglioma) and NECs. SATB2 expression is sensitive and specific for a lower GI origin among NETs and strong SATB2 expression, though only moderately sensitive, is fairly specific for Merkel cell carcinoma among NECs. Based on these results, our group has adopted SATB2 as our primary rectal NET marker. We would consider using SATB2 as a second-line NEC site of origin marker in ambiguous diagnostic settings.
Materials and methods:
NETs, NECs, pheochromocytomas and paragangliomas were identified from the surgical pathology archives of the University of Iowa Hospitals and Clinics. Original glass slides were reviewed, the diagnosis was confirmed, and a “best tumor block” was identified. Tissue microarrays (TMAs) were constructed using the Manual Tissue Arrayer MTA-1 (Beecher Instruments; Sun Prairie, WI), with the following tumors arrayed as triplicate 1 mm cores: 317 NETs [37 thyroid, 46 lung (40 typical carcinoid, 6 atypical carcinoid), 12 duodenum, 70 pancreas, 106 jejunoileum, 24 appendix, 1 sigmoid, 4 rectum), 79 NECs (29 Merkel cell, 30 lung, 20 extrapulmonary visceral), 22 pheochromocytomas, and 22 paragangliomas. An additional 9 appendiceal and 19 rectal NETs were examined in whole sections.
SATB2 immunohistochemistry was performed on 4-μm-thick NET TMA tissue sections after deparaffinization, rehydration, and PT Link (Agilent Dako; Santa Clara, CA) heat-induced epitope retrieval in HpH Target Retrieval Solution (Agilent Dako; pH 9) on an Autostainer Link 48 (Agilent Dako) using a rabbit monoclonal antibody (clone EPNCIR130A; Abcam; Cambridge, MA; 1:500 dilution; 15 minute incubation) and the polymer-based EnVision+ detection system (Agilent Dako; 15 minute incubation). Four of five NEC TMA sections had been stained previously as part of a study examining transcription factor lineage infidelity in NEC. In that protocol, immunohistochemistry was performed manually after Decloaking Chamber (Biocare Medical; Pacheco, CA) pressure cooker heat-induced epitope retrieval using a rabbit polyclonal antibody (product number HPA001042; Sigma Aldrich; St. Louis, MO; 1:250 dilution; 2 hour incubation). The same pretreatment solution and detection chemistry were used as above. The whole sections of appendiceal and rectal NETs were also stained with this prior protocol. A subsequently constructed NEC TMA, including 10 pulmonary and 1 extrapulmonary visceral neoplasms, was stained with the same protocol applied to the NET TMAs. A colon cancer served as the positive control, while a multi-tissue block including normal tonsil, placenta, lung, and myometrial tissue served as the negative control.
SATB2 expression was evaluated for extent (0–100%) and intensity (0–3+) in each TMA core or whole section slide and an overall H-score (mean extent*intensity) was calculated for each tumor. Any non-zero H-score was considered “positive” for expression. Frequency of expression and sensitivity and specificity were examined at various H-score cutoffs. Two-sided 1) Fisher’s exact and 2) Mann Whitney or Kruskal-Wallis tests (the latter with Dunn post tests) were used to analyze categorical and continuous data, respectively, with p<0.05 considered significant. For Merkel cell carcinomas, results of CK20 immunohistochemistry (previously performed) were reviewed, and for “aberrant” SATB2-high expressing visceral NECs, results of TTF-1, CK20, p53, and total Rb immunohistochemistry (recently performed in the context of a separate project) were reviewed. TTF-1 and CK20 had been evaluated with an H-score. p53 had been scored as missense mutation-pattern (diffuse, strong staining obscuring nuclear detail), null pattern (complete absence of tumor staining with wild-type background in internal control), and wild type-pattern (patchy weak to moderate staining allowing for scattered strongly staining nuclei) and total Rb had been scored as null pattern (complete absence of tumor staining with wild-type background in internal control) or wild type-pattern (any nuclear staining).(11, 19) Missense mutation and null patterns were taken as evidence of biallelic inactivation of the implicated tumor suppressor.
For the 4 CK20-negative Merkel cell carcinomas, the diagnosis was supported by the results of clinical examination (2 patients had palpable regional lymph node involvement at presentation) and imaging studies (CT and PET); CM2B4 immunohistochemistry was subsequently performed and was negative (as tends to be the case in CK20-negative Merkel cell carcinoma). This research was conducted with University of Iowa Institutional Review Board approval.
Results:
SATB2 Expression in Well-Differentiated Neuroendocrine Neoplasms:
SATB2 expression was detected in 96% of 25 rectosigmoid, 79% of 33 appendiceal, and only 7% of the remaining well-differentiated neuroendocrine neoplasms (p<0.0001 for the combined appendiceal/rectal vs remaining groups). Among positive cases, the combined appendiceal/rectal tumor group tended to show diffuse, strong staining (mean/median H-score=212/255), while the other positive tumors showed rare to focal staining, which tended to be weak (mean/median H-score=13/7) (p<0.0001). SATB2-positive rectal NETs demonstrated higher H-scores (mean/median=253/285) than appendiceal tumors (mean/median=174/168) (p=0.01), though the overall frequency of positivity was not statistically significant between these two groups (p=0.12). SATB2 expression data in the well-differentiated neoplasms stratified by site of origin are presented in Table 1, and representative photomicrographs are shown in Figures 1–3.
Table 1:
SATB2 Expression in Well-Differentiated Neuroendocrine Tumors and Pheochromocytoma/Paraganglioma
| Site of Origin | n | % positive (any non-zero H-score) | % at least moderate positive (H-score ≥100) | % high positive (H-score ≥200) | Mean (median) H-score, if positive | Range of positive H-scores |
|---|---|---|---|---|---|---|
| Lung | 46 | 13% | 0% | 0% | 23 (15) | 3–70 |
| Thyroid (Medullary) | 37 | 14% | 0% | 0% | 4 (2) | 1–7 |
| Stomach | 16 | 0% | 0% | 0% | NA | NA |
| Duodenum | 12 | 0% | 0% | 0% | NA | NA |
| Pancreas | 70 | 0% | 0% | 0% | NA | NA |
| Jejunoileum | 106 | 7% | 0% | 0% | 15 (8) | 3–43 |
| Appendix | 33 | 79% | 55% | 36% | 174 (168) | 15–300 |
| Rectosigmoid | 25 | 96% | 92% | 80% | 253 (285) | 57–300 |
| Pheochromocytoma | 22 | 0% | 0% | 0% | NA | NA |
| Paraganglioma | 22 | 14% | 0% | 0% | 5 (5) | 3–7 |
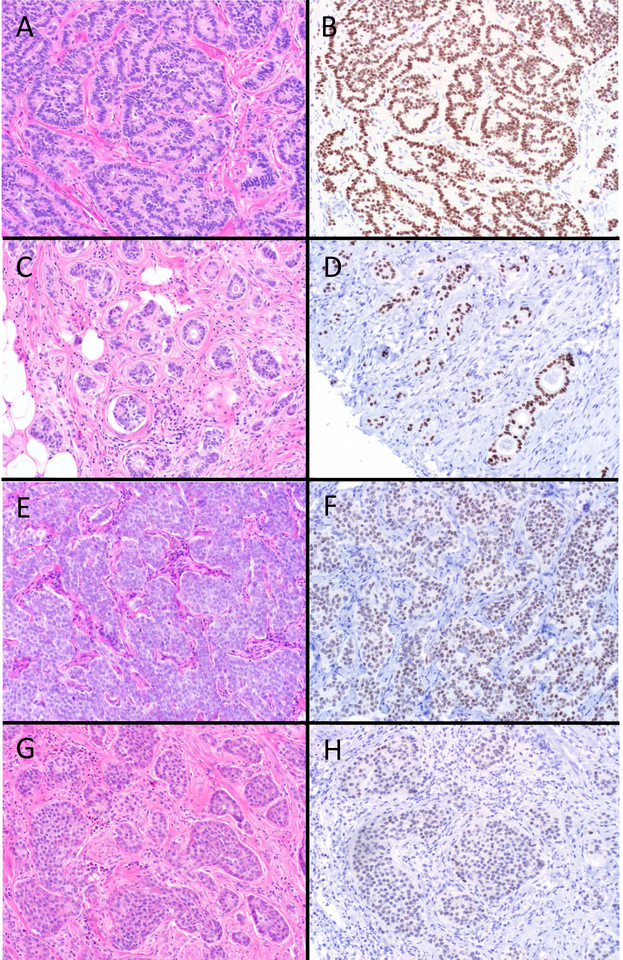
Figure 1.
SATB2 Expression in Rectal and Appendiceal Neuroendocrine Tumors (NETs). The vast majority of rectal NETs (A) demonstrated diffuse, strong SATB2 expression (B). While most appendiceal NETs were also SATB2-positive, they showed a greater range of expression. This tubular carcinoid (C) displays diffuse, strong staining (D), this EC-cell tumor (E) shows only slightly weaker staining (F), while this second EC-cell tumor (G) demonstrates more modest staining (H) (original magnification of each image 200x).
Figure 3.
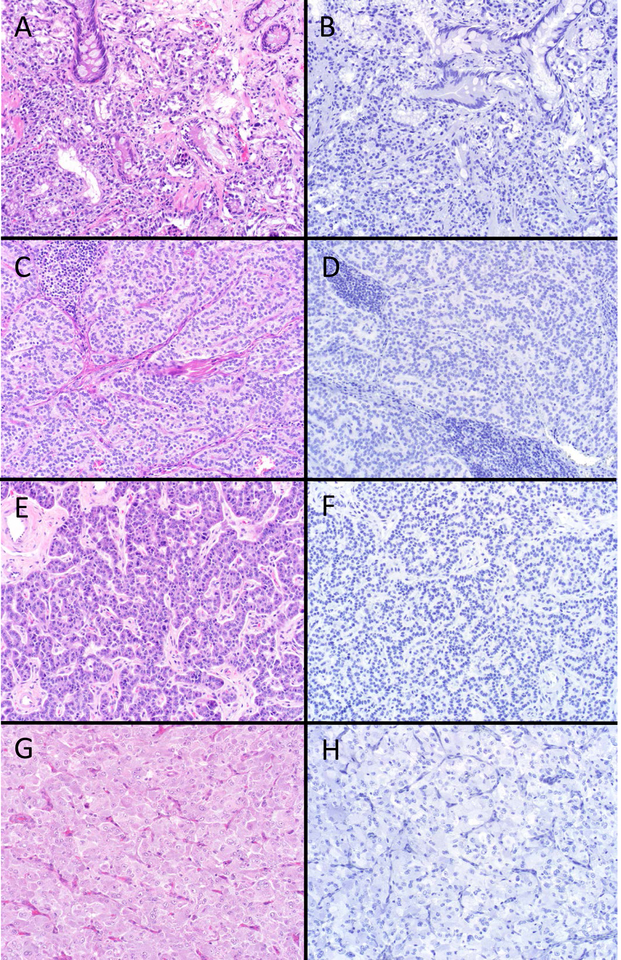
SATB2 “Rarely Positive” Well-Differentiated Neuroendocrine Neoplasms. Around 10% of bronchopulmonary, thyroid and jejunoileal NETs and paragangliomas were SATB2-positive, with H-scores typically in the single digits to few dozen. Several of the strongest “aberrant” expressors are depicted here. Atypical carcinoid tumors of lung origin (A; note focus of punctate necrosis at the lower left) were more likely to be SATB2-positive (B) than typical carcinoid tumor. A medullary thyroid carcinoma (C) demonstrating rare cells staining (D). Ileal NET (E) demonstrating moderately strong staining (F); of note, this tumor was encountered clinically subsequent to the completion of the study and represents the strongest SATB2-positivity I have seen in a NET outside of the appendix and rectum. Paraganglioma (G) displaying rare cells staining (H) (original magnification of each image 200x).
Although only 13% of lung NETs were SATB2-positive, atypical carcinoid tumors (50%; 3/6) were more likely to the positive than typical carcinoid tumors (8%;3/40) (p=0.02). The positive atypical carcinoid tumors had H-scores of 20, 30, and 70; while the positive typical carcinoid tumors had H-scores of 3.3, 3.3, and 10. This apparent difference in H-score did not achieve statistical significance (p=0.1), likely given the small sample size.
For positivity defined as any non-zero H-score, SATB2 was 86% sensitive and 93% specific for an appendiceal or rectal origin among well-differentiated neuroendocrine neoplasms. At an H-score cutoff of >70, SATB2-positivity was 74% sensitive and 100% specific.
SATB2 Expression in Poorly Differentiated Neuroendocrine Carcinomas:
SATB2 expression was detected in 79% of 29 Merkel cell, 33% of 30 lung, and 60% of 20 extrapulmonary visceral NECs (p=0.0017 for the overall Fisher’s exact test). Merkel cell carcinomas were more likely to be SATB2-positive than lung tumors (p=0.0006) but not extrapulmonary visceral tumors (p=0.2). There was a trend toward more frequent expression in extrapulmonary visceral than lung NECs (p=0.08). There was a difference in the median H-scores between the three groups (p<0.001) with SATB2-expressing Merkel cell carcinomas demonstrating typically diffuse, strong staining (mean/median H-score=237/300), which was significantly greater than the positive H-scores in the lung (mean/median H-score=58/23; p<0.001) and extrapulmonary visceral (mean/median H-score 124/110; p<0.001) groups. SATB2 expression data in the poorly differentiated neuroendocrine carcinomas stratified by site of origin are presented in Table 2, and representative photomicrographs are shown in Figure 4.
Table 2:
SATB2 Expression in Poorly Differentiated Neuroendocrine Carcinomas
| Site of origin | n | % positive (any non-zero H-score) | % at least moderate positive (H-score ≥100) | % high positive (H-score ≥200) | Mean (median) H-score, if positive | Range of positive H-scores |
|---|---|---|---|---|---|---|
| Skin (Merkel cell carcinoma) | 29 | 79% | 72% | 55% | 237 (300) | 1–300 |
| Lung | 30 | 33% | 10% | 0% | 58 (23) | 8–195 |
| Extrapulmonary viscera | 20 | 60% | 35% | 15% | 124 (110) | 3–280 |
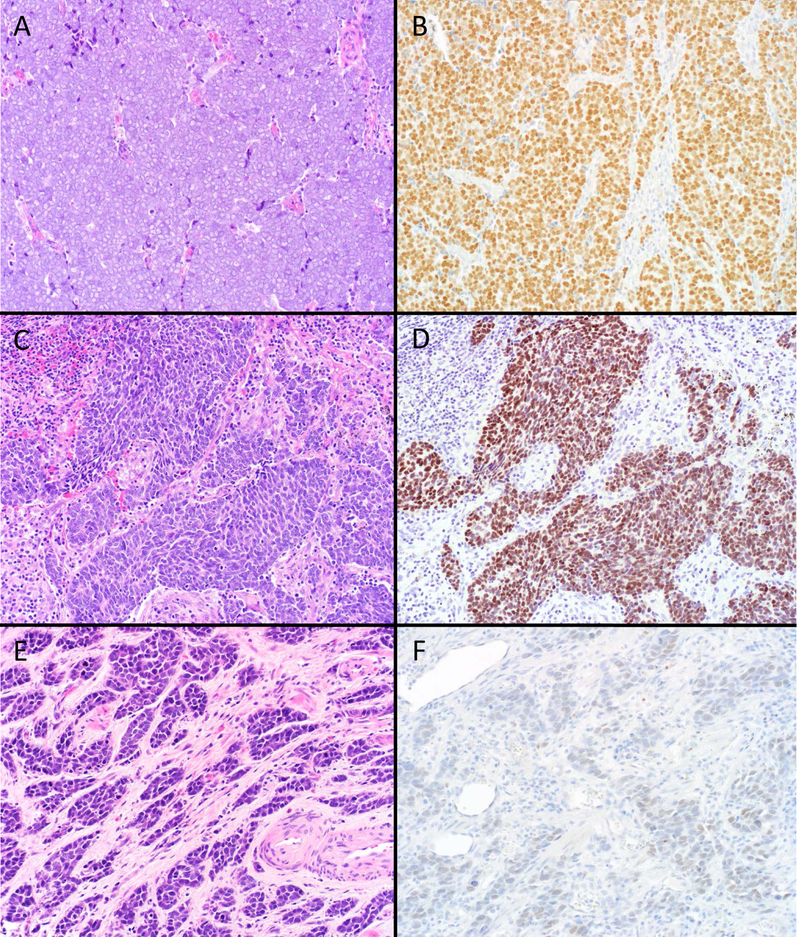
Figure 4.
SATB2 Expression in Neuroendocrine Carcinomas (NECs). Around two-thirds of Merkel cell carcinomas (A) demonstrated diffuse, strong SATB2 expression (B), which was 90% specific among NECs for a cutaneous origin. This small cell lung cancer (C) was among the minority of visceral NECs to demonstrate similarly strong staining (D). This small cell carcinoma from the bladder (E) displays weak staining (F). Weak, patchy SATB2 staining was seen in a third of visceral NECs, likely a manifestation of transcription factor lineage infidelity (original magnification of each image 200x).
For positivity defined as any non-zero H-score, SATB2 was 79% sensitive and only 56% specific for Merkel cell carcinoma among NECs. Visual inspection of the data revealed a cutpoint between “low” and “high” expressors at an H-score ≥150. At this threshold, SATB2 was 69% sensitive and 90% specific for Merkel cell carcinoma. Any SATB2 expression was seen in 80% (20/25) of CK20-positive and 75% (3/4) of CK20-negative tumors (p=1), while SATB2 high expression was seen in 76% (19/25) of CK20-positive but only 25% of CK20-negative Merkel cell carcinomas (0.076).
SATB2 high-expressing visceral NECs included primaries from the following sites (1 each): lung, parotid, uterine cervix, vagina, and pancreas. Results of TTF-1/CK20/p53/total Rb immunostains for these cases are presented in Table 3. Of note, the extrapulmonary visceral group included 3 rectal NECs, which demonstrated H-scores of 0, 3.3, and 12.
Table 3:
Additional Immunohistochemical Results in “Aberrant” SATB2-High Expressing Visceral Poorly Differentiated Neuroendocrine Carcinomas
| Primary Site | TTF-1 | CK20 | p53 | Total Rb |
|---|---|---|---|---|
| Lung | (+) H-score=300 | (−) | Missense mutation-pattern | Null pattern |
| Parotid | (−) | (−) | Null pattern | Null pattern |
| Uterine cervix | (−) | (−) | Null pattern | Wild type-pattern |
| Vagina | (−) | (+)H-score=83 | Null pattern | No residual tumor |
| Pancreas | (−) | (+) H-score=300 | Wild type-pattern | Wild type-pattern |
Discussion:
SATB2 was strongly expressed by nearly all rectal (96%) and the vast majority of appendiceal (79%) NETs—with only rare to focal expression in a minority (7%) of well-differentiated neoplasms from other anatomic sites. SATB2 expression was identified in only 7% of jejunoileal (median H-score 8; highest H-score 43) and in 0% of 70 pancreatic NETs. This finding is of note because metastatic NETs of occult origin most commonly arise from these two sites.(1, 20) Any SATB2 expression was 86% sensitive and 93% specific for an appendiceal or rectal origin. Because expression in lower GI tract NETs was typically strong and “aberrant” expression by NETs at other sites tended to be weak, applying an H-score threshold of 70 resulted in a sensitivity of 74% and a specificity of 100% for lower GI tract origin.
These results are similar to those from the one prior study that systematically examined SATB2 expression in NETs. Li and colleagues reported SATB2-positivity in 90% of hindgut (52/58), 12% of midgut (3/25), and 17% (14/84) of foregut tumors.(21) This comparator study utilized the same monoclonal antibody applied to the NET TMAs here and scored SATB2-positivity as follows: 0 (no staining), 1+ (1–25% tumor cells staining), 2+ (26–50%), 3+ (51–75%), 4+ (76–100%). Among positive hindgut NETs, 79% were 4+; the 3 positive midgut tumors were all 1 or 2+; and while 8 of the positive foregut tumors were 1 or 2+, 4 were 3 or 4+. Compared to this prior study, the current study includes a larger number of cases (389 well-differentiated neoplasms vs 167 NETs) and tumor types not previously represented (i.e., medullary thyroid carcinoma, pheochromocytoma, paraganglioma), provides more granular H-score data, and also formally examines NECs. Li et al’s study included more rectosigmoid tumors (58 vs 25) but only included 4 appendiceal (vs 33) and 16 jejunoileal (vs 106) NETs.
Otherwise, there are scant published data regarding SATB2 expression in NETs. As discussed in the introduction, Dragomir and colleagues reported SATB2-positivity in 40% of “carcinoids,” not further specified.(18) This same group reported no staining in 10 “carcinoids” in a subsequent study focused on SATB2 expression in NECs.(22) Lin and colleagues studied SATB2 expression in a large cohort of nearly 2000 neoplasms. They formally reported no SATB2-positivity in 12 medullary thyroid carcinomas and 13 pheochromocytomas.(23) In their discussion they shared an unpublished observation that SATB2 expression is seen in a “majority of NETs of the colon, rectum, and appendix but rarely in NETs from the stomach, duodenum, small intestine, pancreas, and lung.” Moh and colleagues, in a study focused on the distinction of metastatic lower GI tract adenocarcinomas to the ovary from mucinous ovarian or endometrioid primaries, reported SATB2-negativity in 1 “small intestinal carcinoid” metastatic to the ovary.(24) Finally, in a study focused on identifying sensitive and specific colon cancer diagnostic makers, Li and colleagues reported SATB2 expression in 6% of 32 (31 pancreatic; 1 small intestinal) “neuroendocrine carcinomas.”(25) Given the rarity of NEC at these anatomic sites, these almost certainly represented NETs. Table 4 summarizes the results of previous studies reporting on SATB2 expression in neuroendocrine neoplasms. Of note, these studies employed 6 different SATB2 monoclonal antibodies (1 study using 3 different antibodies), and the current study employed 1 of these 6, as well as a polyclonal antibody; unlike some other immunohistochemical markers (e.g., TTF-1 8G7G3/1 vs. SPT24; GATA-3 L50–823 vs. HG3–31) no consistent clone-based performance differences have been noted to date.
Table 4:
Results of Previous Studies Addressing SATB2 Expression in Neuroendocrine Neoplasms
| Dragomir et al (2014)(18) | Lin et al (2014)(23) | Li et al (2015)(21) | Fukuhara et al (2016)(22) | Kervarrec et al (2018)(26) | |
|---|---|---|---|---|---|
| Clone | CL0276 | SATBA4B10 and EP281/EPNCIR130B | EPNCIR130A | CL0320 | Not specified |
| NET | “Carcinoid” (n=54): 0–1%: 59% 2–25%: 22% 26–75%: 2% >75%: 13% |
|
|
“Carcinoid” (n=10): 0% | Not examined |
| Pheochromocytoma/paraganglioma | Not reported | Pheochromocytoma (n=13): 0% | Not examined | Not examined | Not examined |
| NEC | 3 Merkel cell carcinomas showed staining in >75% of nuclei | Not examined | Unpublished observation that SATB2 is expressed by “one third of PDNECs of various organs” |
|
|
Dragomir and colleagues’ observation of extensive (>75% cells staining) SATB2-positivity in 3 Merkel cell carcinomas led to a follow up study in which the group, utilizing a 10% cutoff, found SATB2-positivity in 15 of 20 (75%) Merkel cell carcinomas and 0 of 4 (0%) small cell lung cancers. I was initially dismissive of the potential diagnostic importance of this result, given our group’s experience with transcription factor lineage infidelity in NECs and the very small number of visceral NECs examined in this study. In a previous study, we found SATB2 to be among a group of 15 transcription factors (36 examined in total) to be expressed by at least 20% of Merkel cell, lung, and/or extrapulmonary visceral NECs (platform presentation at USCAP 2014). In fact, it was one of the most frequently expressed transcription factors across tumor types (57% overall), trailing only SOX2 (96%), FLI1 (87%), ISL1 (85%), and PLAG1 (67%). Among the 15 transcription factors, it was one of 6 to be significantly differentially expressed across the 3 tumor types, but because it was not significantly differentially expressed between Merkel cell carcinoma and extrapulmonary visceral NEC and because it was still expressed by a significant minority of lung NECs, SATB2 did not garner much of our attention. Of note, this previous analysis had not taken H-score into account.
While I was preparing a manuscript limited to a description of SATB2 expression in NETs, Kervarrec and colleagues reported a detailed evaluation of a set of 10 immunohistochemical markers that had previously shown promise in assigning NEC site of origin. When positive was thresholded as “diffuse, strong, and homogeneous staining identified by low magnification (x5),” SATB2 was one of the best performing markers [along with neurofilament, Merkel cell polyomavirus (by PCR), and CK20], with a sensitivity of 64%, specificity of 98%, and positive likelihood ratio of 36.6 for Merkel cell carcinoma.(26) These results caused me to re-examine SATB2 expression in NECs using H-scores. At an H-score threshold of ≥150 (analogous to the qualitative threshold used by Kervarrec et al.), SATB2 was 69% sensitive and 90% specific for Merkel cell carcinoma. We both appeared to identify more frequent SATB2-high expressors among CK20-positive (76% current study, 61% prior study) than CK20-negative (25% and 38%) tumors, though this result did not achieve significance in either study.
In the current study, the 5 outlier SATB2-high expressing visceral NECs arose in the lung, parotid, uterine cervix, vagina, and pancreas. The lung tumor demonstrated diffuse strong TTF-1 expression, did not express CK20, demonstrated missense mutation-pattern p53 staining, and null pattern total Rb staining—the prototypical staining pattern for a small cell (neuroendocrine) carcinoma of lung origin. The parotid tumor failed to express TTF-1 or CK20 and demonstrated null pattern staining for p53 and total Rb. As with the lung tumor, the results of p53 and total Rb immunostains suggest biallelic inactivation of these tumor suppressors, which is the genetic hallmark of small cell lung cancer and is frequently encountered in other visceral NECs but is not typical of Merkel cell carcinoma.(27, 28) The uterine cervical tumor was again TTF-1 and CK20-negative and demonstrated null pattern p53 and wild-type pattern total Rb. This pattern would be typical of an adenocarcinoma, in which p53 inactivation is frequent and Rb inactivation is uncommon. A subset of NECs, including many large cell NECs of lung origin and extrapulmonary visceral NECs, bear genetic signatures similar to the non-neuroendocrine carcinomas that arise at that site.(28, 29) The vaginal tumor was TTF-1-negative, CK20 modestly positive (H-score 83), and p53 null pattern. Tissue was depleted on total Rb staining. NECs of the gynecological tract are among the most likely non-Merkel cell NECs to express CK20 (with primary parotid tumors the most likely), but the weaker staining here would be unusual for Merkel cell carcinoma.(1) Of all the outlier NECs, the pancreatic tumor immunophenotypically most resembles a Merkel cell carcinoma. It demonstrated diffuse, strong CK20 expression, did not express TTF-1, and was wild type-pattern for p53 and total Rb. This 68-year-old woman presented with obstructive jaundice and underwent a Whipple procedure after imaging demonstrated a 7 cm pancreatic tumor and no evidence of metastatic disease. She subsequently underwent adjuvant carboplatin/etoposide and radiation therapy. A total skin examination revealed only actinic keratoses and a basal cell carcinoma of the right cheek. She was feeling well at 3-years follow up.
As mentioned in the introduction, SATB2 was introduced to the diagnostic pathology community as a potential colon cancer diagnostic marker.(16) Subsequent work has established its role as a lower GI tract-specific columnar marker, which is especially useful in the distinction of colon cancer from variably CDX2-expressing esophageal, gastric, small intestinal, and pancreatic adenocarcinomas and in the distinction of disseminated low-grade appendiceal mucinous neoplasm from primary mucinous ovarian tumor.(18, 23–25, 30–33) Although data is limited to one study, it has also been shown to be more sensitive than CK20 and CDX2 in medullary (undifferentiated) colon cancer, with Lin and colleagues reporting any and >50% cells staining in 89% and 75% of 18 cases.(23)
This study highlights two additional diagnostic applications of SATB2 immunohistochemistry—namely assigning a lower GI tract origin in a NET and a cutaneous origin in a NEC. Although rectal NETs only rarely present as metastases of occult origin, our group sorely needed a reliable rectal NET marker for a couple reasons. First, while 10–20% of NETs present as metastases of occult origin, given a referral bias to our NET Center of Excellence, the number approaches 40% of 200+ new clinic patients a year. Outside pathology is reviewed before patients are scheduled for a clinic appointment, with the pathologist typically armed with only the prior pathology report, in which an attempt to assign site of origin has rarely been made. Second, our primary NET site of origin immunopanel includes the “pancreas NET markers” PAX6 and ISL1, which are often expressed by rectal NETs. Rectal NETs metastatic to the liver are thus apt to be misclassified as pancreatic in origin. In addition, we are occasionally consulted by colleagues who have applied our site or origin algorithm to typical small rectal primaries, alarmed by the results. For those who have unnecessarily “opened this can of worms,” a positive SATB2 immunostain allays their concerns (see Figure 5). SATB2 would also be useful in distinguishing appendiceal and ileal NETs, an uncommon clinical scenario in which S-100 has been historically applied.(1)
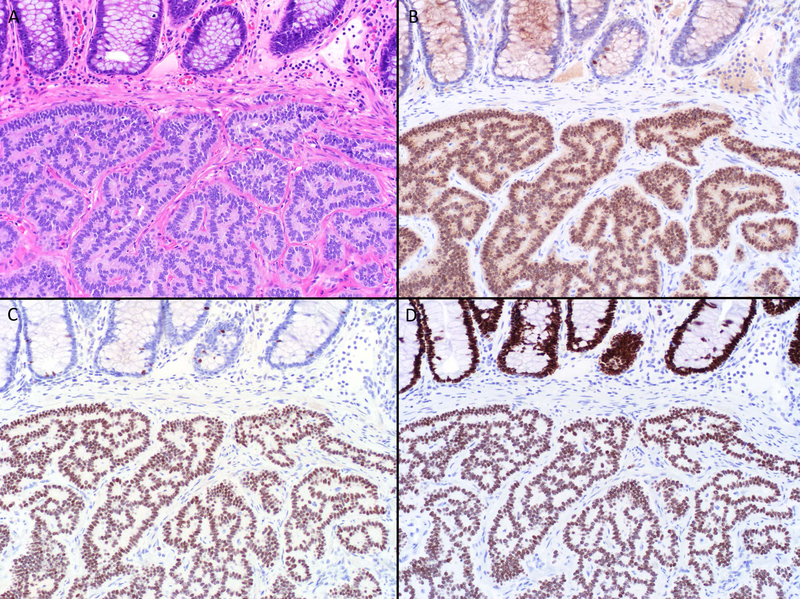
Figure 5.
Rectal Neuroendocrine Tumor Transcription Factor Immunohistochemistry. This rectal tumor (A) was found to express PAX6 (B) and Islet 1 (C), initially raising concern for metastasis from the pancreas. SATB2 immunostain demonstrates diffuse, strong staining in the tumor and overlying crypt epithelium, confirming a rectal origin (D). Note scattered enteroendocrine cell staining in B and (especially evident in) C. Rectal NETs recapitulate enteroglucagon-producing L cells, with are phenotypically similar to pancreatic α cells (original magnification of each image 200x).
The nature of SATB2 expression in NETs, which appears to be organ-specific, is fundamentally different than that of CDX2 (EC cells) and PAX6/ISL1 (islet cells in the pancreas, enteroglucagon-producing L cells in the rectum), which are enteroendocrine cell lineage-specific. SATB2 is expressed by every epithelial cell type (stem cells, endocrine cells, Paneth cells, goblet cells, enterocytes) in appendiceal and colonic crypts and thus marks both appendiceal and rectal NETs, despite the fact that appendiceal EC-cell NETs are otherwise much more similar to jejunoileal tumors, while rectal NETs are much more similar to pancreatic ones. Unlike the situation in the brain, face, and skeleton, the basic science of SATB2 in the tubal gut is essentially unexplored. There is no described lower GI tract phenotype in patients with germline balanced translocations, mutations, or deletions that lead to SATB2 haploinsufficiency, and papers describing Satb2 knockout mouse models did not mention expression in the lower GI tract or describe a lower GI tract phenotype.(12, 34–39) In a recent description of the development of pluripotent stem cell-derived human colonic organoids, the authors used SATB2 as the “definitive marker of the presumptive large intestinal epithelium,” but it was identified by mining several publically available expression databases, including the Human Protein Atlas, rather than being arrived at experimentally.(40) The authors of this study did, though, identify SATB2 expression in mouse posterior intestine.
The ever-changing nomenclature of neuroendocrine neoplasms has at various times chosen to refer to these tumors as simply “endocrine,” rather than “neuroendocrine.” The “neuro-” character of neuroendocrine neoplasms is reflected in their secretory organelles, which are highly analogous to those seen in neurons. The “general neuroendocrine marker” synaptophysin is intrinsic to subsets of these organelles, and, in fact, in neurobiology research synaptophysin expression is often utilized to quantify synaptic density. Merkel cells are cutaneous mechanoreceptors; they synapse onto somatosensory afferent nerve fibers. Neurofilaments are intermediate filament proteins forming part of the neuronal cytoskeleton. There is a small body of literature suggesting that neurofilament expression is relatively sensitive and highly specific for Merkel cell carcinoma in the differential diagnosis with small cell lung cancer, with limited available data in extrapulmonary visceral NECs.(1) Most recently, Kervarrec and colleagues reported neurofilament (clone 2F11) expression in 75% of 97 Merkel cell carcinomas and only 5% of 59 visceral NECs.(26) Given that SATB2 is expressed in the brain and spinal cord, its (along with neurofilament’s) preferential expression in Merkel cell carcinoma may reflect a neuronal phenotype. Supporting the conclusion that SATB2 is functioning as a neuronal marker in this context, SATB2 expression has also been described in sudomotor cholinergic neurons and our group has observed it in ganglion cells of the enteric nervous system (the latter an unpublished observation).(41) In this light, the descriptor “neuroendocrine” seems appropriate, with Merkel cell carcinoma perhaps representing the most neuronal of all NECs.
Conclusion:
Strong SATB2 expression is restricted to NETs of lower GI tract origin and among NECs is reasonably sensitive and fairly specific for a cutaneous origin (i.e., Merkel cell carcinoma). In the former instance SATB2 appears to be functioning as a pan-lower GI tract epithelial marker, while in the latter it may specifically reflect a component of neuronal differentiation. SATB2 is an exemplar next-generation immunohistochemical marker, its role as a potential colon cancer marker having been suggested by the results of expression profiling and its further role as an osteoblast lineage marker supported by the developmental biology literature. SATB2 has the distinct advantage of being an oligospecific transcription factor, with laboratories clinically deploying it being able to utilize it in multiple unique diagnostic contexts.
Figure 2.
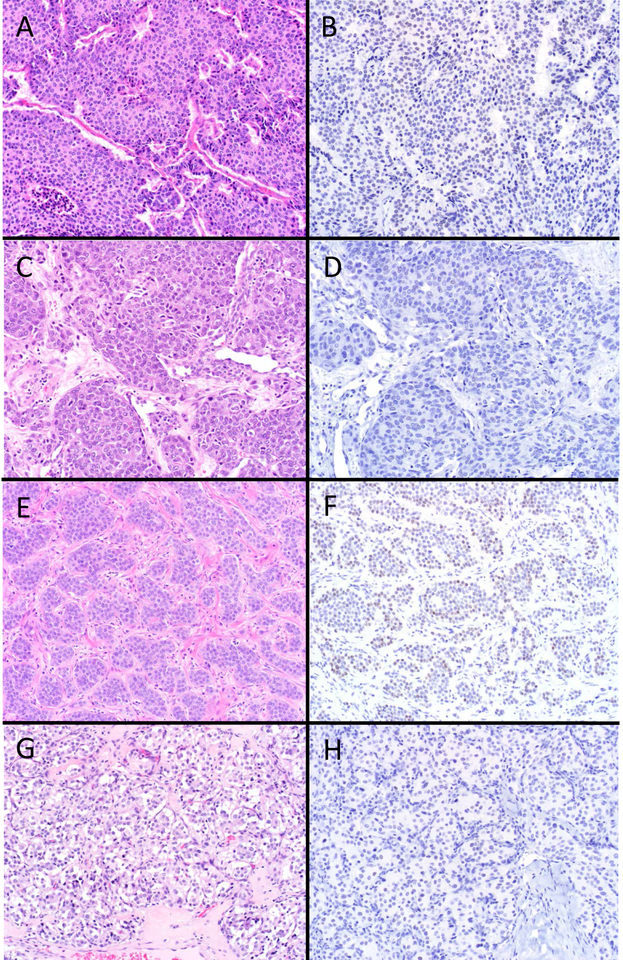
SATB2 “Never Positive” Well-Differentiated Neuroendocrine Neoplasms. Examined NETs from the stomach (A), duodenum (C), and pancreas (E), as well as pheochromocytomas (G) were never SATB2-positive (B, D, F, and H, respectively) (original magnification of each image 200x).
Acknowledgements:
This work was supported by NIH grant P50 CA174521–01A1 (AMB).
Footnotes
Nothing To Disclose – The author has indicated that he has no conflicts of interest that relate to the content of this manuscript.
References:
- 1.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Advances in anatomic pathology. 2013;20(5):285–314. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(18):3063–72. [DOI] [PubMed] [Google Scholar]
- 3.Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39(6):735–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas. 2017;46(6):707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156(6):1359–65; discussion 65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeczok TW, Stashek KM, Maxwell JE, O’Dorisio TM, Howe JR, Hornick JL, et al. Clusterin in Neuroendocrine Epithelial Neoplasms: Absence of Expression in a Well-differentiated Tumor Suggests a Jejunoileal Origin. Applied immunohistochemistry & molecular morphology : AIMM. 2018;26(2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Angelo SP, Russell J, Lebbe C, Chmielowski B, Gambichler T, Grob JJ, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA oncology. 2018;4(9):e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakkala S, Owonikoko TK. Immune checkpoint inhibitors in small cell lung cancer. Journal of thoracic disease. 2018;10(Suppl 3):S460–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. The New England journal of medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Bellizzi AM. Immunohistochemistry in Gastroenterohepatopancreatobiliary Epithelial Neoplasia: Practical Applications, Pitfalls, and Emerging Markers. Surgical pathology clinics. 2013;6(3):567–609. [DOI] [PubMed] [Google Scholar]
- 12.FitzPatrick DR, Carr IM, McLaren L, Leek JP, Wightman P, Williamson K, et al. Identification of SATB2 as the cleft palate gene on 2q32-q33. Human molecular genetics. 2003;12(19):2491–501. [DOI] [PubMed] [Google Scholar]
- 13.Dobreva G, Dambacher J, Grosschedl R. SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression. Genes & development. 2003;17(24):3048–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britanova O, Akopov S, Lukyanov S, Gruss P, Tarabykin V. Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. The European journal of neuroscience. 2005;21(3):658–68. [DOI] [PubMed] [Google Scholar]
- 15.Szemes M, Gyorgy A, Paweletz C, Dobi A, Agoston DV. Isolation and characterization of SATB2, a novel AT-rich DNA binding protein expressed in development- and cell-specific manner in the rat brain. Neurochemical research. 2006;31(2):237–46. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. The American journal of surgical pathology. 2011;35(7):937–48. [DOI] [PubMed] [Google Scholar]
- 17.Ordonez NG. SATB2 is a novel marker of osteoblastic differentiation and colorectal adenocarcinoma. Advances in anatomic pathology. 2014;21(1):63–7. [DOI] [PubMed] [Google Scholar]
- 18.Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: Results of a pathology-based clinical prospective study. American journal of clinical pathology. 2014;141(5):630–8. [DOI] [PubMed] [Google Scholar]
- 19.Chen BJ, Marino-Enriquez A, Fletcher CD, Hornick JL. Loss of retinoblastoma protein expression in spindle cell/pleomorphic lipomas and cytogenetically related tumors: an immunohistochemical study with diagnostic implications. The American journal of surgical pathology. 2012;36(8):1119–28. [DOI] [PubMed] [Google Scholar]
- 20.Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Archives of surgery. 2010;145(3):276–80. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Yuan J, Wei L, Zhou L, Mei K, Yue J, et al. SATB2 is a sensitive marker for lower gastrointestinal well-differentiated neuroendocrine tumors. International journal of clinical and experimental pathology. 2015;8(6):7072–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuhara M, Agnarsdottir M, Edqvist PH, Coter A, Ponten F. SATB2 is expressed in Merkel cell carcinoma. Archives of dermatological research. 2016;308(6):449–54. [DOI] [PubMed] [Google Scholar]
- 23.Lin F, Shi J, Zhu S, Chen Z, Li A, Chen T, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Archives of pathology & laboratory medicine. 2014;138(8):1015–26. [DOI] [PubMed] [Google Scholar]
- 24.Moh M, Krings G, Ates D, Aysal A, Kim GE, Rabban JT. SATB2 Expression Distinguishes Ovarian Metastases of Colorectal and Appendiceal Origin From Primary Ovarian Tumors of Mucinous or Endometrioid Type. The American journal of surgical pathology. 2016;40(3):419–32. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Rock JB, Roth R, Lehman A, Marsh WL, Suarez A, et al. Dual Stain With SATB2 and CK20/Villin Is Useful to Distinguish Colorectal Carcinomas From Other Tumors. American journal of clinical pathology. 2018;149(3):241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kervarrec T, Tallet A, Miquelestorena-Standley E, Houben R, Schrama D, Gambichler T, et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018. [DOI] [PubMed] [Google Scholar]
- 27.George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31(12):1770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(14):3618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez Montiel D, Arispe Angulo K, Cantu-de Leon D, Bornstein Quevedo L, Chanona Vilchis J, Herrera Montalvo L. The value of SATB2 in the differential diagnosis of intestinal-type mucinous tumors of the ovary: primary vs metastatic. Annals of diagnostic pathology. 2015;19(4):249–52. [DOI] [PubMed] [Google Scholar]
- 31.Ramos BD, Brettfeld S, Berry RS, Routh JK, Martin DR, Hanson JA. A Comprehensive Evaluation of Special AT-rich Sequence-binding Protein 2 (SATB2) Immunohistochemical Staining in Mucinous Tumors From Gastrointestinal and Nongastrointestinal Sites. Applied immunohistochemistry & molecular morphology : AIMM. 2017. [DOI] [PubMed] [Google Scholar]
- 32.Ma C, Lowenthal BM, Pai RK. SATB2 Is Superior to CDX2 in Distinguishing Signet Ring Cell Carcinoma of the Upper Gastrointestinal Tract and Lower Gastrointestinal Tract. The American journal of surgical pathology. 2018;42(12):1715–22. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Sun L, Zhang L, Zhou L, Zhao M, Peng Y, et al. Diagnostic Utility of SATB2 in Metastatic Krukenberg Tumors of the Ovary: An Immunohistochemical Study of 70 Cases With Comparison to CDX2, CK7, CK20, Chromogranin, and Synaptophysin. The American journal of surgical pathology. 2018;42(2):160–71. [DOI] [PubMed] [Google Scholar]
- 34.Leoyklang P, Suphapeetiporn K, Siriwan P, Desudchit T, Chaowanapanja P, Gahl WA, et al. Heterozygous nonsense mutation SATB2 associated with cleft palate, osteoporosis, and cognitive defects. Human mutation. 2007;28(7):732–8. [DOI] [PubMed] [Google Scholar]
- 35.Urquhart J, Black GC, Clayton-Smith J. 4.5 Mb microdeletion in chromosome band 2q33.1 associated with learning disability and cleft palate. European journal of medical genetics. 2009;52(6):454–7. [DOI] [PubMed] [Google Scholar]
- 36.Bengani H, Handley M, Alvi M, Ibitoye R, Lees M, Lynch SA, et al. Clinical and molecular consequences of disease-associated de novo mutations in SATB2. Genetics in medicine : official journal of the American College of Medical Genetics. 2017;19(8):900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125(5):971–86. [DOI] [PubMed] [Google Scholar]
- 38.Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, et al. Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. American journal of human genetics. 2006;79(4):668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–92. [DOI] [PubMed] [Google Scholar]
- 40.Munera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, et al. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell stem cell. 2017;21(1):51–64 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apostolova G, Loy B, Dorn R, Dechant G. The sympathetic neurotransmitter switch depends on the nuclear matrix protein Satb2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(48):16356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]