Abstract
Lung resident memory CD8 T cells (TRM) are critical for protection against respiratory viruses, but the cellular interactions required for their development are poorly understood. Herein we describe the necessity of classical monocytes for the establishment of lung TRM following influenza infection. We find that, during the initial appearance of lung TRM, monocytes and dendritic cells are the most numerous influenza antigen-bearing APCs in the lung. Surprisingly, depletion of DCs after initial T cell priming did not impact lung TRM development or maintenance. In contrast, a monocyte deficient pulmonary environment in CCR2−/− mice results in significantly less lung TRM following influenza infection, despite no defect in the antiviral effector response or in the peripheral memory pool. Imaging shows direct interaction of antigen-specific T cells with antigen-bearing monocytes in the lung, and pulmonary classical monocytes from the lungs of influenza infected mice are sufficient to drive differentiation of T cells in vitro. These data describe a novel role for pulmonary monocytes in mediating lung TRM development through direct interaction with T cells in the lung.
INTRODUCTION
During respiratory virus infections, effector CD8 T cells are primed in the lung-draining lymph nodes by antigen presenting cells (APCs) that have migrated from the infected lung 1, 2, 3. Following a program of proliferation and differentiation that is regulated by antigen encounter, co-stimulation receptor engagement, and the local cytokine environment, virus-specific effector CD8 T cells then traffic back to the infected lung to mediate their effector functions 4, 5. Upon entry into the lung tissue, virus-specific effector CD8 T cells may re-encounter antigen presented by local APCs, including monocytes, macrophages, dendritic cells, and infected epithelial cells. In addition to antigen re-encounter, cues from the local microenvironment can further influence the differentiation of virus-specific CD8 T cells into short-lived effector or long-lived memory cells, ultimately directing cell fate 6, 7, 8. Despite the significance of these cell fate decisions for pathogen clearance and the establishment of immune memory, the importance of the local microenvironment on T cell differentiation in the tissue and the roles of individual tissue-resident APC subsets that provide these signals for the development of T cell memory are not well understood.
Following respiratory virus clearance, subsets of the CD8 memory precursor cells in the lung will differentiate into tissue-resident memory T cells (TRM). Lung TRM have been shown to be critical for protective cellular immunity against secondary heterosubtypic respiratory infections, enabling the rapid detection of the invading pathogen and thereby limiting pathogen replication and immunopathology 9, 10, 11. The programming of CD8 TRM has been extensively investigated in recent years, and key cytokines such as TGF-β and IL-15 have been shown to be important for their development 12, 13, 14. Although antigen stimulation is required to initiate the effector T cell response in the lymph nodes, its role in TRM development in the tissue had been less well characterized. Recently, it has been demonstrated that differentiation of lung TRM during influenza virus infection requires virus-specific T cells to re-encounter antigen in the lung tissue 15, 16. However, it is not clear if this requires interaction between effector T cells and specific APC subsets in the lung. Previous reports have shown that targeting vaccines to pulmonary CD103+ dendritic cells or alveolar macrophages promotes the establishment of lung TRM, but it is unclear whether this was due to the ability of these APC subsets to promote TRM programming during initial priming in the lymph node, or whether these APC subsets regulate lung TRM establishment through antigen re-encounter in the lung itself 17, 18. Given the importance of TRM for protective cellular immunity in the lung, it is critical to define the cellular and molecular requirements for their establishment and identify new approaches for optimizing vaccines against respiratory pathogens.
To better define the factors that promote TRM in the lung, we investigated the role of different lung-resident APC subsets in virus-specific CD8 TRM development. Although essential for initial virus-specific CD8 T cell activation, depletion of CD11c+ dendritic cells during the peak of the effector T cell response did not impact the number of virus-specific lung CD8 TRM following influenza infection. Surprisingly, analysis of pulmonary APC subsets around the time of viral clearance showed that monocytes were among the most numerous lung APC subsets harboring influenza antigens, and this correlated with the initial appearance of virus-specific lung CD8 TRM. While monocytes have been investigated for their pro-inflammatory roles in innate immunity, their ability to influence T cell responses through direct interactions with virus-specific CD8 T cells has not been as extensively investigated 19, 20, 21. Using CCR2-deficient mice, which are defective in monocyte trafficking to the lung during influenza infection 22, we observed a significant decrease in the number of virus-specific lung CD8 TRM in both the parenchyma and airways, but there was no effect on the number of circulating virus-specific memory CD8 T cells in the spleen. Notably, there were no differences in the number of virus-specific effector CD8 T cells or virus-specific memory CD8 precursor cells generated in the lung at the time of viral clearance when comparing wild-type and CCR2−/− mice, demonstrating the role for monocytes in TRM development was restricted to antigen re-encounter in the tissue and not initial T cell priming. In support of this, imaging of the lung revealed a close interaction between virus-specific CD8 T cells and monocytes. Furthermore, pulmonary monocytes sorted from infected lungs were sufficient to activate naive antigen specific T cells, as well as induce their expression of CD103 on a subset of highly-divided cells in vitro. Together, these data define a novel role for lung tissue-resident monocytes as critical mediators in the establishment of lung CD8 TRM but not circulating T cell memory following respiratory infection, through presentation of viral antigens to T cells in the infected lung.
RESULTS
Lung CD8 TRM develop immediately following viral clearance
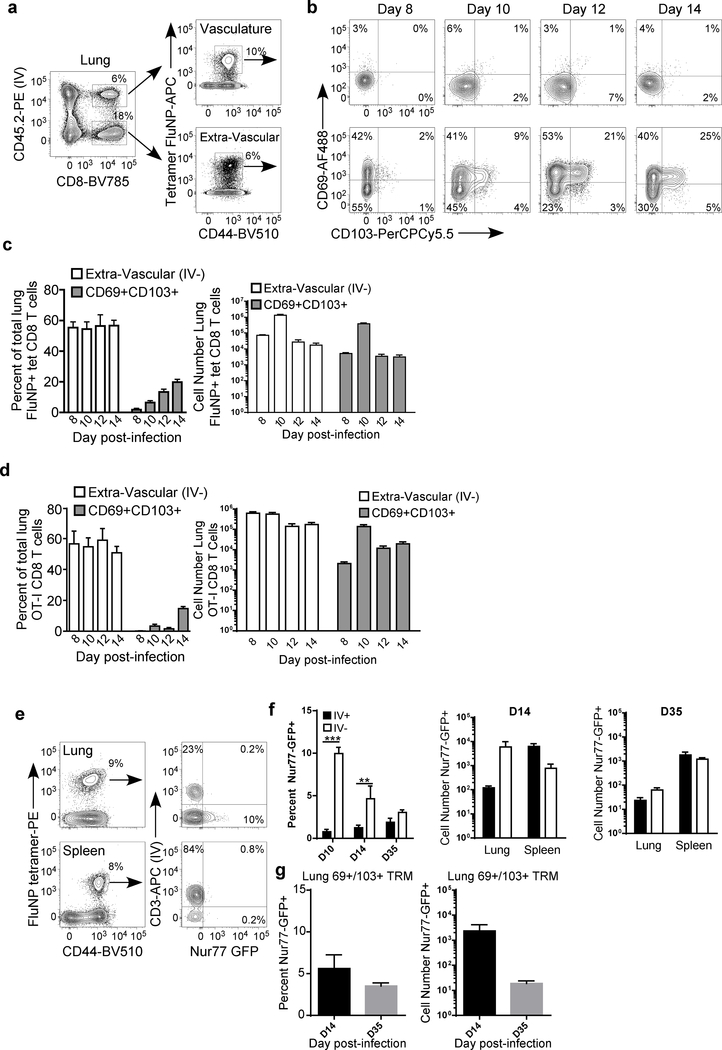
While there is growing appreciation for the role of tissue resident memory T cells in protection against viral challenges at mucosal surfaces, less is known about their ontogeny. To determine the cellular interactions critical for lung TRM development, we first sought to define the kinetics of the appearance of CD8 TRM in the lung following influenza infection. Flu-specific CD8 T cells were identified in both the lung vasculature and lung parenchyma following intravital labeling. The appearance of cells with a tissue-resident phenotype (CD69+ CD103+) was observed in the lung parenchyma beginning on day 8 post infection (Fig. 1A and 1B), just preceding the peak of the effector CD8 T cell response at day 10 post infection, as well as influenza viral clearance 23, 24. Over the course of the effector T cell response in the lung, the frequency of vascular versus tissue-resident flu-specific CD8 T cells remains relatively constant. However, the frequency of CD69+ CD103+ flu-specific CD8 T cells steadily increases within the resident population from day 8–14 post-infection (Fig. 1C). We see a similar pattern in the number of lung TRM, with a peak at D10 and a decline following viral clearance that mimics the kinetics of the total effector response (Fig. 1c). Similar to polyclonal flu-specific T cells, transgenic OT-I T cells resident in the lung show an increase in the frequency of CD69+ CD103+ cells from days 8–14 following infection with influenza x31-OVA (Fig. 1D), and numbers of lung OT-I TRM matched the kinetics of the overall effector T cell response. Coincident with the expansion of CD69+ CD103+ cells we observed continued antigen stimulation, as measured by Nur77-GFP expression, in flu-specific CD8 T cells resident in the lung, but not in the lung vasculature or the spleen, on day 10 post-infection (Fig. 1E and 1F). Increased Nur77-GFP expression in lung flu-specific CD8 T cells continued through day 14 post-infection, but was largely absent by day 30 post-infection. In addition, there were increased numbers of flu-specific CD69+ CD103+ cells in the lung at day 14 post-infection (Figure 1G). Together, these data show the seeding of the lung-resident T cell pool occurs rapidly following the resolution of influenza infection and is associated with continued antigen recognition by flu-specific T cells in the lung tissue.
Figure 1. Rapid appearance of lung resident CD8 T cells following influenza infection.
(A) Gating strategy and representative flow plots for the intravital labeling of FluNP-specific CD8 T cells in the lung. (B) Representative flow plots for CD69 and CD103 staining on FluNP-specific CD8 T cells in the lung vasculature (top row) or lung extra-vascular compartments (bottom row). (C) Frequency and number of total extra-vascular FluNP-specific CD8 T cells and CD69+ CD103+ resident FluNP-specific CD8 T cells among total lung FluNP-specific CD8 T cells over time. (D) Frequency and number of total extra-vascular OT-I CD8 T cells and CD69+ CD103+ resident OT-I CD8 T cells among total lung OT-I CD8 T cells over time. (E) Representative staining of Nur77-GFP expression in FluNP-specific CD8 T cells. (F) Frequency and number of Nur77-GFP+ FluNP-specific CD8 T cells that are circulating (IV+) or extra-vascular (IV−) in the lung and spleen on days 10, 14, and 35 post-infection. (G) Frequency and number of Nur77-GFP+ FluNP-specific tissue-resident CD8 T cells expressing both CD69 and CD103 in the lung extra-vascular population. *** p<0.001 (two-tailed Student’s t-test) All graphs error bars are S.E.M. Data are representative of 3 independent experiments with 5 mice per time point (A-C and G) or 2 independent experiments with 4 mice each (E and F).
Depletion of lung dendritic cells after initial T cell priming does not alter lung CD8 TRM establishment
Previous studies have shown that the establishment of lung-resident T cell memory requires antigen re-encounter in the lung 15, and flu-specific T cells continue to receive antigen stimulation in the lung following viral clearance 25, 26. Dendritic cells, specifically, are appreciated as efficient mediators of T cell activation and differentiation. As well, the importance of dendritic cells in the initiation of a T cell response against influenza infection has been well documented 4, 22, 27, 28, 29, but the role pulmonary DCs may be playing in the differentiation of flu-specific TRM in the tissue after initial priming is less well understood. To address this question, we investigated the establishment of lung TRM following depletion of DCs after initial T cell priming (Fig. 2A). Treatment of CD11c-DTR (Itgax-DTR) chimeras with diphtheria toxin (DTx) beginning on day 5 post-infection was sufficient to deplete the majority of CD11c+ cells in the lung, which include both DCs and alveolar macrophages, while leaving the CD11b+ cells intact (Fig. 2B, 2C, and S1). Surprisingly, depletion of CD11c+ cells before TRM generation but after initial T cell activation had no effect on the phenotype or numbers of flu-specific lung TRM in the airways or parenchyma on day 14 post-infection (Fig. S1D and S1E), or at memory (Fig. 2D and 2E). Therefore, despite their importance for the initial priming of naïve CD8 T cells, interactions between DCs and virus-specific CD8 T cells in the tissue are not required for the establishment of lung TRM.
Figure 2. Depletion of DCs after initial priming does not affect establishment of lung TRM.
(A) Experimental diagram of DC depletion using CD11c-DTR (Itgax-DTR) chimeras. (B) Representative images from the lung on day 11 post-infection (day 3 post-depletion) demonstrating the specific depletion of CD11c+ cells. (C) Frequency and number of total CD11c expressing cells in the lung and BAL at D10 post infection after DTX or PBS treatment. * p<0.05 (two-tailed Student’s t-test) (D) Representative staining of FluNP-specific CD8 T cells 30 days post-infection in control or DTx-treated mice. BAL and lung plots are gated on extra-vascular (IV−) cells. (E) Number of FluNP-specific CD8 T cells in BAL, lung extra-vascular (LEV), lung vasculature (LV), and spleen 30 days post-infection in control or DTx-treated mice. Data are representative of three independent experiments with 3–4 mice per group. All graphs error bars are S.E.M.
Lung-resident dendritic cells and monocytes are among the most numerous cell types presenting influenza antigens during the initial appearance of lung TRM
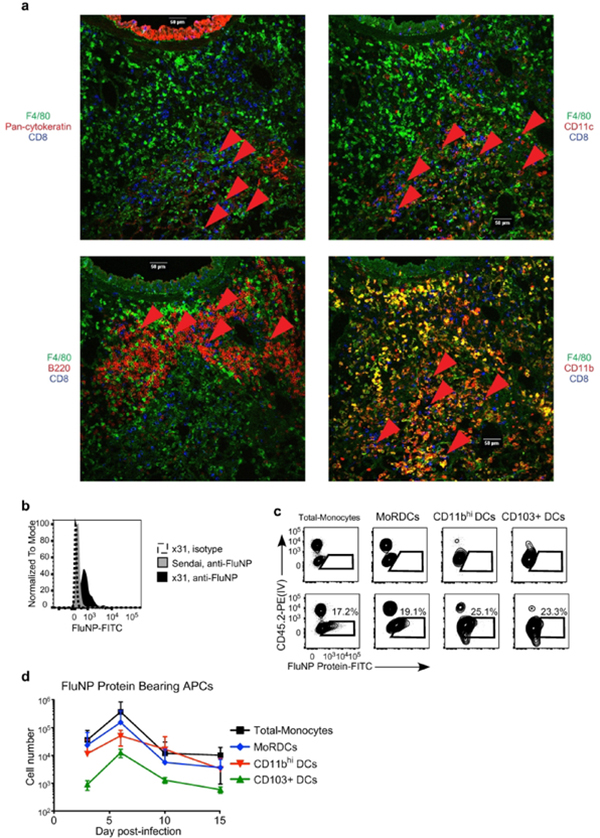
This finding led us to broaden our approach to identify additional APC subsets potentially involved in lung TRM differentiation. We investigated which APC subsets in the lung were displaying antigen to flu-specific T cells, particularly at the conclusion of the effector T cell response, when resident memory is established. CD8 T cells in the lung make direct contact with many different APC subsets, including dendritic cells, macrophages, and monocytes (Fig. 3A). To determine which APC subsets were capable of presenting influenza antigens in the lung, we performed intravital labeling to identify different lung extra-vascular APC subsets, (Fig. S2) and assessed intracellular influenza nucleoprotein (FluNP) content 30. This staining approach was specific for the influenza nucleoprotein, as isotype staining, as well as staining of lung APCs following Sendai virus infection, failed to give a positive signal (Fig. 3B). Intracellular FluNP staining was detected in pulmonary monocytes, monocytic respiratory dendritic cells (MoRDCs), CD11bhi DCs, and CD103+ DCs, and this staining was restricted to the lung-resident (CD45.2−) population of each subset (Fig. 3C). The number of cells containing FluNP in each subset was highest at day 6 post-infection, and substantial numbers of pulmonary APCs containing FluNP were detected through day 15 post-infection (Fig. 3D) 30. Although, as expected, substantial numbers of FluNP+ DCs were present in the lung, it was surprising to observe that pulmonary monocytes were also among the most numerous FluNP+ APC subset from days 10–15 post-infection. Thus, we began to assess what role these monocytes may play in the differentiation of lung TRM, as well as any differences in the roles of the classical and non-classical monocyte subsets.
Figure 3. Large numbers of pulmonary DCs and monocytes contain influenza antigens following viral clearance and during the initial appearance of lung TRM.
(A) Representative confocal microscopy images showing the localization of CD8 T cells and various APC subsets in the lung of influenza infected mice. Red arrows highlight areas of T cell and APC co-localization. (B) Representative staining showing the specificity of intracellular FluNP staining in pulmonary monocytes following infection with influenza x31 (black histogram) or Sendai virus (gray histogram). Isotype control staining is shown by the dashed line. (C) Representative staining of intracellular FluNP in extra-vascular (CD45.2 IV−) lung APC subsets on day 7 post-infection. (D) The number of FluNP-containing total monocytes (black), MoRDCs (blue), CD11bhi DCs (red), and CD103+ DCs (green) in the lung over the course of influenza infection. Data are representative of 3 independent experiments with 5 mice per time point. All graphs error bars are S.E.M.
Inhibiting monocyte recruitment to the lung significantly decreases lung CD8 TRM development
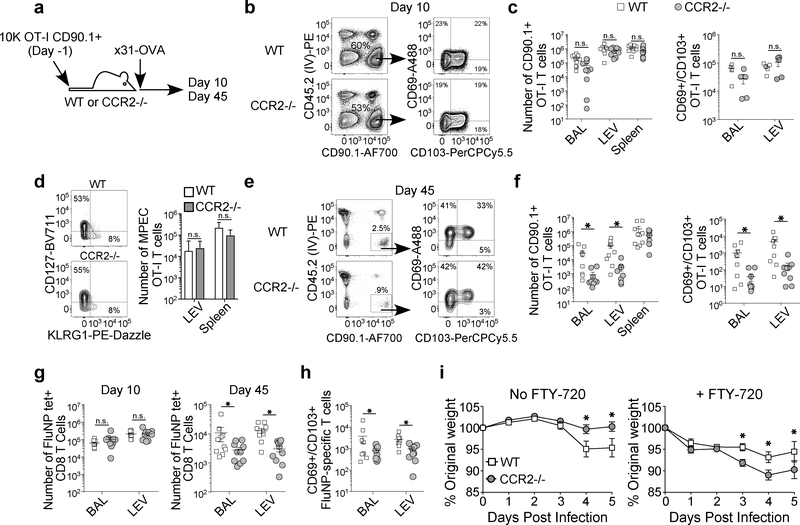
To investigate the role pulmonary monocytes may be playing in the development of lung TRM in vivo, we used mice deficient in C-C chemokine receptor type 2, or CCR2, which lack the ability to efficiently traffic monocytes from the circulation into sites of mucosal inflammation. Previous studies characterizing influenza infection in CCR2−/− mice observed no defect in the flu-specific effector CD8 T cell response or viral clearance 31, 32, but the mice do show decreased monocyte-driven immunopathology 22. To test this, we seeded WT and CCR2−/− mice with naïve OT-I T cells, infected the mice with x31-OVA, and tracked the OVA-specific as well as endogenous fluNP-specific T cell response (Fig. 4A). As expected, we observed a significant decrease in the number of monocytes recruited to the lung in CCR2−/− mice following influenza infection, but no difference in the numbers of other lung APC subsets, including MoRDC, CD103+, and CD11bhi DC subsets (Fig. S3). Similar to previous reports, at day 10 post-infection there were no differences in the number of OT-I effector T cells in the BAL, lung interstitium (lung extra-vascular, LEV), or spleen between WT and CCR2−/− mice (Fig. 4B and 4C) 31. In addition, there was no difference in the number of CD69+ CD103+ lung-resident OT-I cells at this peak of the acute CD8 T cell response (Fig. 4C). To determine whether CCR2−/− mice showed a defect in overall memory T cell development, we assessed the number of memory precursor cells (MPECs) in the lung and spleen (Fig. 4D). Similar to our observations of the overall effector T cell pool, there was no difference in the number of CD127hi KLRG1lo MPECs in the lung or spleen. Thus, CCR2−/− mice showed no defect in the flu-specific effector CD8 T cell response, even within the lung tissue and airways (BAL).
Figure 4. Inhibiting monocyte recruitment to the lung significantly reduces the number virus-specific lung extra-vascular and lung TRM following influenza infection.
(A) Experimental design for investigating the role of pulmonary monocytes in lung TRM establishment. (B) Representative staining and (C) numbers of total and CD69+ CD103+ OT-I CD8 T cells in the airways (BAL), lung extra-vascular (LEV), and spleen on day 10 post-infection in WT and CCR2−/− mice. (D)Representative staining and numbers of CD127+ KLRG1− MPEC OT-I T cells in the lung and spleen on day 10 post-infection. (E) Representative staining and (F) numbers of total and CD69+ CD103+ OT-I CD8 T cells in the airways (BAL), lung extra-vascular (LEV), and spleen on day 45 post-infection in WT and CCR2−/− mice. (G) Number of FluNP-specific CD8 T cells in the airways (BAL) and extra-vascular in the lung (LEV) on days 10 and 45 post-infection in WT and CCR2−/− mice. (H) Number of CD69+ CD103+ FluNP-specific CD8 T cells resident in the airways (BAL) and lung (LEV) on days 10 and 45 post-infection in WT and CCR2−/− mice. (I) Weight loss of WT and CCR2−/− influenza x31-OVA-immune mice challenged with PR8-OVA in the presence (right graph) or absence (left graph) of FTY-720. Data represent 3 independent experiments with 5 mice per group (B-H), or 3 independent experiments with 6 mice per group (I). All graphs error bars are S.E.M. * p<0.05(two-tailed Student’s t-test).
In contrast to the effector T cell response, CCR2−/− mice showed a significant decrease in the number of OT-I lung TRM in both the airway (BAL) and interstitium (LEV) at memory (Fig. 4E and 4F). Importantly, there was no difference in the number of OT-I memory T cells in the spleen, indicating that the role of pulmonary monocytes in the generation of CD8 T cell memory was restricted to the lung-resident pool. In addition to a significant decrease in the total number of lung extra-vascular T cells, there was a significant decrease in the number of CD69+ CD103+ TRM OT-I cells in both the airways and interstitium in CCR2−/− mice (Fig. 4F). Although there is some overlap between the number of lung TRM observed in WT and CCR2−/− mice, the compiled data show a significant defect in the average number of flu-specific lung TRM in CCR2-deficient mice.
To differentiate between the potential role classical and non-classical monocytes may be playing in the CCR2−/− model, we generated CX3CR1-DTR bone marrow chimeras to allow for the selective depletion of CX3CR1+ non-classical monocytes (Figure S4A) 32. This model showed no difference at memory between the PBS and DTx treated mice, with similar numbers of memory OT-Is being generated in the lung extra-vascular compartment, the airways, and the spleen (Fig S4B and S4C). This indicated that the classical monocytes, but not non-classical monocytes, had a prominent role in driving the differentiation of lung TRM following influenza infection.
To confirm the results of the transferred OT-I cells in the CCR2−/− model, we also investigated the endogenous polyclonal flu-specific T cell response. Similar to the OT-I response, there was no difference in the number of effector fluNP-specific CD8 T cells in the airways and lung on day 10 post-infection, but a significant decrease in the number of fluNP-specific lung extra-vascular T cells in the airways and interstitium in CCR2−/− mice at memory (Fig. 4G). Furthermore, in CCR2−/− mice, the overall decrease in fluNP-specific lung extra-vascular T cells was paralleled by a significant decrease in the number of CD69+ CD103+ TRM in the airway and interstitium (Fig. 4H).
Assessing the impact of reduced numbers of lung TRM on cellular immune protection in CCR2−/− mice is complicated due to the reduced monocyte recruitment, which results in decreased immunopathology and weight loss 22, 33. To overcome this issue, we treated (H3N2) influenza x31-immune WT and CCR2−/− mice with FTY-720 to sequester circulating immune cells in secondary lymphoid organs and limit the response to the virus to lung TRM and challenged the mice with heterologous (H1N1) influenza PR8 to assess the protective efficacy of the lung TRM population. Similar to previous reports, mock FTY-720-treated CCR2−/− mice showed decreased weight loss compared to WT mice following PR8 challenge (Fig 4I). In contrast, treatment with FTY-720 resulted in significantly greater weight loss in CCR2−/− mice compared to WT mice, indicating that the decreased numbers of lung TRM in CCR2−/− mice are associated with impaired immune protection.
Pulmonary monocytes interact with virus-specific CD8 T cells in vivo and are sufficient to drive CD8 T cell activation and differentiation in vitro
The effect of defective monocyte trafficking on lung TRM establishment suggested two possibilities: that the inflammatory milieu of the lung was altered in a manner detrimental to TRM differentiation, or that TRM development is driven by direct interactions between antigen-specific T cells and monocytes in the lung tissue. Given that antigen re-encounter in the tissue is necessary to establish lung TRM following an influenza infection 15, we focused on the potential role of pulmonary monocytes presenting antigens directly to virus-specific CD8 T cells. The lung is a large organ, making the precise localization of individual cells crucial if they are to directly engage one another. As monocytes are among the most abundant APC subsets containing influenza antigens when lung TRM first appear, we examined whether different fluorescently-marked monocyte subsets were interacting with virus-specific CD8 T cells in the lung (Fig. 5A). Influenza antigen-bearing monocytes were observed in close contact with virus-specific CD8 T cells in the lung on day 12 post-infection (Fig. 5B, red arrows). To ensure that the monocytes not only contained but were presenting antigen on MHC-I as described by Jakubzick et al. 34, we stained lung monocytes for H-2Kb bound to SIINFEKL, finding that both classical and non-classical monocytes were capable of presenting virus-derived peptide on MHC-I (Fig. 5C). Furthermore, we found that a subset of these H-2Kb-SIINFEKL positive cells also contained intracellular FluNP protein, thus demonstrating both flu antigen uptake as well as presentation on MHC-I (Fig 5D). Though the cells were in contact, it was still possible they did not contribute to T cell activation and differentiation. To test this, we sorted classical and non-classical monocytes from the lungs of mice infected with x31, pulsed the monocytes with SIINFEKL peptide, and cultured the monocytes with naïve OT-I T cells. Both monocytes subsets were able to induce substantial proliferation of OT-I cells as measured by CTV dilution (Fig. 5D). However, in highly divided cells, only classical monocytes were able to generate a population of OT-I T cells that co-expressed the TRM markers CD69 and CD103 (Fig. 5E and 5F). Surprisingly, dendritic cell subsets, while able to induce CD103 upregulation, did so to a lesser degree than classical monocytes (Fig. 5H). Both subsets of monocytes were able to induce the expression of CD127 on highly divided cells, indicating that they were capable of generating OT-I cells with the potential to become memory T cells (Fig. S5). Together, these data indicate that lung monocytes, particularly classical monocytes, are present in the lung micro-environment, co-localize with CD8 T cells, and are sufficient to drive T cell differentiation, including the expression of CD103 on a subset of highly-divided cells. Overall, these data demonstrate a novel role for antigen presentation by pulmonary monocytes in the establishment of virus-specific lung TRM, but not systemic T cell memory, following influenza infection.
Figure 5. Pulmonary monocytes interact with T cells in the lung during infection, present influenza-derived antigen, and are sufficient to drive the activation and differentiation of TRM-like CD8 T cells in vitro.
(A) Experimental design for using CX3CR1+/GFP CCR2+/RFP dual reporter mice infected with x31-OVA for microscopy and monocyte isolation for in vitro culture. (B) Representative fluorescent microscopy images from the lung at day 12 post-infection. OT-I T cells interacting with FluNP-containing monocytes are indicated with red arrows. Scale bar is 20 μm. (C) Representative co-staining of uninfected and x31-OVA-I infected mice with surface H-2Kb-OVA and intracellular FluNP protein, showing specificity of staining (left plot) as well as monocytes with both surface H-2Kb-OVA and intracellular FluNP protein (right plot). (D) Number of lung extra-vascular monocytes co-expressing FluNP protein and H-2Kb-OVA staining day 8 post infection. (E) Cell trace violet dilution of OT-I T cells cultured in the presence or absence of classical or non-classical monocytes for three days. (F) Representative staining of CD69 and CD103 on OT-I CD8 T cells with 5+ cell divisions as indicated by CTV dilution. (G) Frequency of CD103+ OT-I CD8 T cells stimulated by classical or non-classical monocytes with more than 5 cell divisions. **** p<0.0001 (two-tailed Student’s t-test) (H) Frequency of CD103+ OT-I CD8 T cells stimulated by CD103+ DC, MoRDC, or CD11bhi DC subsets with more than 5 cell divisions. *p<.05 (two-tailed Student’s t-test) Data are representative of 3 independent experiments, with each in vitro culture (D-I) run in triplicate. All graphs error bars are S.E.M.
DISCUSSION
Many studies have shown the importance of dendritic cells for the initiation of antiviral T cell responses following influenza infection, with particular subsets such as CD8α+ and CD103+ DCs playing specific roles in naïve T cell activation and differentiation 4, 35, 36, 37. Given the requirement for antigen re-encounter in the tissue for establishing lung TRM, it was surprising that depletion of CD11c+ cells after initial T cell activation showed that DCs were dispensable for lung TRM formation. In contrast, inhibiting monocyte recruitment to the lung had a dramatic impact on the establishment of lung TRM, despite having no effect on the magnitude of the effector T cell response. Thus, the ability of monocytes to promote T cell responses against influenza is not through the initial priming and expansion of antiviral T cells, but through their ability to present viral antigens to effector T cells in the infected lung tissue and drive T cell differentiation.
Classical monocytes have been characterized as innate inflammatory mediators that produce large amounts of IL-1, IL-6, and TNFα, and promote tissue damage 38, but their ability to drive adaptive immune responses through antigen presentation has been understudied 39. Monocytes have been shown to promote TH1 responses during viral infection through direct priming of naïve T cells in the lymph node 4, 40, 41. However, as we observed no defect in the flu-specific effector CD8 T cell response or the systemic flu-specific memory CD8 T cell pool in CCR2−/− mice, it is unlikely that the decreased numbers of lung TRM in these mice are due to a defect in the initial priming of the flu-specific T cell response. Rather, our data show that antigen presentation by pulmonary monocytes to effector CD8 T cells in the lung tissue is important for lung TRM establishment. Consistent with these findings, Ly6C+ inflammatory monocytes are efficient at cross-presentation to CD8 T cells in the presence of TLR agonists, especially TLR7 34, 42, 43, 44, 45. Together, these findings support a model where monocytes in the infected lung are activated by viral TLR agonists, promoting efferocytosis of dying cells and enhancing cross-presentation of influenza antigens to flu-specific CD8 T cells, which ultimately drives the establishment of lung TRM.
Several studies have identified roles for specific APC subsets in the establishment or maintenance of TRM. Cross-presentation by DNGR-1+ dendritic cells was shown to be required for optimal generation of TRM, but not circulating memory T cells, in a model of cutaneous Vaccinia virus infection46. In the lung, targeting vaccines to respiratory dendritic cells or alveolar macrophages was shown to induce local TRM that could protect mice against respiratory challenge17, 47. Recently, it was reported that inflammatory monocytes were important for the maintenance of both lung TRM and circulating memory T cell subsets following Vaccinia virus infection, but the mechanism by which monocytes were promoting memory maintenance was unknown31. We have extended these findings to demonstrate a critical role for antigen presentation by monocytes in establishing lung TRM. Although we did not observe a defect in the circulating memory T cell pool in our study, this discrepancy is likely due to the differences in tissue tropism between influenza and Vaccinia viruses. As influenza replication and inflammation is localized to the respiratory tract, the impact of monocyte antigen-presentation on memory T cell development during a respiratory virus infection would be limited to the lung.
Although our data support a direct role for antigen presentation by pulmonary monocytes in driving lung TRM establishment, there may be additional monocyte-derived factors contributing to this process, such as cytokines or the propagation of tissue repair. For example, monocytes can produce IL-15, which has been implicated in the initial lodgment of TRM48. Monocytes are also prevalent in areas of tissue repair following viral clearance in the lung, and these sites have been identified as anatomical niches, termed repair-associated memory depots, that promote the maintenance of lung TRM49. Future studies investigating antigen-independent functions of monocytes in TRM differentiation and maintenance, and the interplay between monocytes and other APCs in these processes, will be required to fully understand the contributions of monocytes for the development of resident T cell memory.
Monocytes and effector CD8 T cells are recruited to discrete sites of inflammation in the lung through CCR2- and CXCR3-dependent mechanisms, respectively3, 19. Given the large size of the lung, having a mechanism to recruit both APCs and virus-specific CD8 T cells to the same inflamed local microenvironment where virus is present is an efficient means to ensure T cells have access to antigen and accessory signals such as co-stimulation and cytokines that will promote their differentiation. The high number of antigen-bearing monocytes in the lung, their co-localization with antigen specific T cells, and their sufficiency to drive T cell differentiation in vitro all point to monocytes being critical mediators of TRM differentiation.
It should be noted that monocytes did not induce robust expression of TRM markers such as CD103 on all OT-I cells in our in vitro culture system, and we did not examine the transcriptional profile of these cells to assess the complete TRM program50, 51. As the in vitro culture system cannot recapitulate all the complex interactions that guide CD8 T cell differentiation in vivo, we believe it is unlikely that monocytes alone are sufficient to program lung TRM development following an influenza inflection. It seems likely that multiple T cell – APC interactions, separated by time (initial T cell priming and antigen re-encounter) and location (lymph node and infected lung) are required. One potential developmental pathway suggested by our data is that dendritic cells may induce the initial TRM program, and that pulmonary monocytes may provide additional signals that lead to further progression or enforcement of this program. We are currently investigating these possibilities.
While significant technical hurdles remain, if strategies can be devised by which vaccine-derived antigens are presented by monocytes to activated T cells in the lung, this may enhance vaccine efficacy through the establishment of greater numbers of lung TRM. These findings also provide a rationale for antibody-targeted mucosal vaccines, such as used by Villadangos et al18, to directly target vaccine antigens to lung monocytes in order to produce more robust TRM responses. Combined with the previously demonstrated efficacy of dendritic cell targeting vaccines, this could offer an avenue towards a combination vaccine capable of generating robust cellular immunity in the lung. In summary, we have identified a novel role for antigen presentation by pulmonary monocytes in driving the establishment of lung TRM following influenza virus infection. Further exploration of the mechanisms by which monocytes promote TRM differentiation may aid in the development of new strategies for vaccination against respiratory pathogens.
MATERIALS AND METHODS
Mice and infections
C57BL/6J (WT), B6.PL-Thy1a/CyJ (CD90.1), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1), B6.129S4-Ccr2tm1Ifc/J (CCR2−/−), B6.129P-CX3CR1tm1Litt/J (CX3CR1-GFP), B6.129(Cg)-CCR2tm2.1Ifc/J (CCR2-RFP), C57BL/6-Tg(Nr4a1-EGFP/cre)820Khog/J (Nur77-GFP), B6N.129P2-Cx3cr1tm3(DTR)Litt/J (CX3CR1-DTR), and B6.FVB-1700016L21RikTg(Itgax-DTR/EGFP)57Lan/J (CD11c-DTR) mice from The Jackson Laboratory were housed under SPF conditions at Emory University and Kindai University. B6.129P-CX3CR1tm1Litt/J and B6.129(Cg)-CCR2tm2.1Ifc/J mice were crossed to generate F1 dual reporter mice (CX3CR1+/GFP CCR2+/RFP) for imaging. Intranasal infection with influenza A/HKx31 (H3N2) at 30,000 50% egg infectious doses (EID50), A/HKx31-OVAI expressing SIINFEKL peptide at 30,000 EID50, and influenza A/PR8-OVAI expressing SIINFEKL peptide (H1N1) at 6,000 EID50 were performed as previously described 52. In some experiments, 104 CD90.1+ naïve OT-I CD8 T cells were injected i.v. into recipient mice one day prior to infection. In protection experiments, mice were injected daily i.p. with 150 μg FTY720 (Cayman Chemical) suspended in PBS. All experiments were completed in accordance with the Institutional Animal Care and Use Committee guidelines of Emory University.
Generation of CD11c-DTR and CX3CR1-DTR chimeras
Recipient CD45.1 mice were injected i.p. with 600 μg of busulfan (Otsuka Pharmaceutical). The next day and 5 × 106 BM cells isolated from CD11c-DTR (Itgax-DTR) or CX3CR1-DTR mice were injected intravenously. Chimeras were rested for 6 weeks for reconstitution, and were bled to confirm the presence of the donor CD45.2+ cells prior to virus infection. Some groups of mice were injected i.n. with 60 μg of Diphtheria Toxin (DTx) (Sigma-Aldrich) or PBS following infection with influenza x31 or x31-OVA.
Tissue collection and flow cytometry
Intravital staining was performed immediately before mouse euthanasia and tissue harvest as previously described 53. Briefly, to identify T cells resident in various tissues, including the lung parenchyma, 1.5μg of fluorophore-conjugated α-CD45.2 antibody in 200λ 1× PBS was intravenously injected into the tail vein of mice; five minutes post-injection, mice were euthanized with Avertin (2,2,2-Tribromoethanol, Sigma) and exsanguinated prior to harvest of BAL and other tissues. Cells in the lung airways were recovered by lavage with 5 × 1 ml R10 media. Lung tissues were digested by collagenase D (Roche) for 30 min at 37°C and enriched by centrifugation in 40/80% Percoll gradient. Splenocytes were obtained by straining through nylon mesh, followed by RBC lysis in buffered ammonium chloride. Cells were blocked first with mAbs to FcRγIII/II and then stained with APC-conjugated influenza NP366–374/Db tetramer. Tetramer-labeled cells were washed and stained with fluorophore-conjugated reagents purchased from BD Biosciences (CD103, CD11c, Ly6C, Siglec-F), BioLegend (CD103, CD11b, CD127, CD69, CD8a, CD90.1, KLRG-1, Ly6G, I-A/I-E, CD45.2), eBiosciences (CD4, CD44, H2KB-OVA), anti influenza NP (Abcam) and R&D (CCR2). Intracellular staining was performed using the Cytofix/Cytoperm Kit according to manufacturer protocol (BD Biosciences). Tetramers were generated by the NIH Tetramer Core at Emory University. Samples were run on LSRFortessa and LSR-II flow cytometers (BD), and data were analyzed using FlowJo software (Tree Star). Cell sorting was performed on an SH800 (Sony) or FACSAria III cell sorter (BD Biosciences).
Fluorescence and confocal microscopy
Mouse lungs were inflated by intratracheal administration of optimal cutting temperature (OCT) media to preserve lung morphology followed by snap freezing in liquid nitrogen. Six or Seven - μm-thick cryosections were fixed for 2 min with acetone / ethanol, and blocked with combined rat serum, donkey serum, mouse serum and FcBlock (anti CD16/32 2.4G2) or Blocking One reagent (Nacalai Tesque) followed by blocking with endogenous avidin and biotin blocking system (Abcam). Sections were then stained with antibodies purchased from Biolegend (CD90.1, anti-GFP, donkey anti rabbit IgG, CD8a, CD11b, CD11c, B220), F4/80 (Bay Bioscience), pan-Cytokeratin (Bioss Antibodies), Abcam (fluNP), Lifetech (streptavidin Alexa Fluor 405), Invitrogen (a-RFP rabbit polyclonal) and mounted with ProLong™ Diamond Antifade (Thermo Fisher). Images were acquired on an AxioObserver.Z1 (Zeiss) using a 100x oil objective at room temperature or a C2si confocal microscope (Nikon). Images were processed using Zen 2.3 blue edition software.
Monocyte and T Cell in vitro co-culture
Classical and non-classical monocytes were sorted from lungs of day 10 ×31 influenza infected mice (CD45+, CD11b+, MHC-II−, Ly6g−, Ly6c+, CCR2+ classical monocytes; CD45+, CD11b+, MHC-II−, Ly6g−, Ly6c−, CCR2− non-classical monocytes). Sorted monocytes were pulsed for 2 hours with 1μM OVA peptide (SIINFEKL) in round bottom plates at 37°C. and cultured with naive CD8 OT-I T cells isolated from spleens using the EasySep™ Mouse CD8+ T Cell Isolation Kit (Stem Cell Technologies) and stained with Cell Trace Violet (ThermoFisher) Co-cultures were performed at a 1:2 monocyte to T cell ratio for 3 days prior to analysis 54.
Statistical analysis
Statistical analysis was performed using Prism 5 (GraphPad Software), and significance was determined by an unpaired two-tailed Student’s t test unless otherwise noted in the figure legend. P-values less than 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC I tetramers and the Emory Flow Cytometry Core for cell sorting. This work is supported by National Institutes of Health grants HL122559, HL138508, and Centers of Excellence in Influenza Research and Surveillance Contract HHSN272201400004C (to J.E.K.), and Grant-in-Aid for Scientific Research (C) 16K08850 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from Life Science Foundation of Japan, the Japan Foundation for Pediatric Research (16-007), the Naito Foundation, and SENSHIN Medical Research Foundation (to S.T.). P.R.D. is supported by National Institutes of Health Grant F31 AI124611 and S.L.H. is supported by National Institutes of Health Grant F31 HL136101.
REFERENCES
- 1.Villadangos JA & Heath WR Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol 17, 262–272 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J & Steinman RM Dendritic cells and the control of immunity. Nature 392, 245–252 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Henri S et al. The dendritic cell populations of mouse lymph nodes. J Immunol 167, 741–748 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Kim TS & Braciale TJ Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One 4, e4204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohlmeier JE et al. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol 183, 4378–4384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui G et al. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell 161, 750–761 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laidlaw BJ et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol 16, 871–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poholek AC et al. IL-10 induces a STAT3-dependent autoregulatory loop in TH2 cells that promotes Blimp-1 restriction of cell expansion via antagonism of STAT5 target genes. Sci Immunol 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KG & Masopust D Editorial: Pulmonary resident memory CD8 T cells: here today, gone tomorrow. J Leukoc Biol 95, 199–201 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Schenkel JM, Fraser KA & Masopust D Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192, 2961–2964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMaster SR, Wilson JJ, Wang H & Kohlmeier JE Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 195, 203–209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandau MM, Kohlmeier JE, Woodland DL & Jameson SC IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol 184, 35–44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YW, Kim HG, Perry CJ & Kaech SM CCR7 expression alters memory CD8 T-cell homeostasis by regulating occupancy in IL-7- and IL-15-dependent niches. Proc Natl Acad Sci U S A 113, 8278–8283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed J et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat Immunol 17, 414–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMaster SR et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzolla A et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Vega-Ramos J & Villadangos JA Consequences of direct and indirect activation of dendritic cells on antigen presentation: functional implications and clinical considerations. Mol Immunol 55, 175–178 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Wakim, LM, Smith, J, Caminschi I, Lahoud MH & Villadangos JA Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8, 1060–1071 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Tsou CL et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117, 902–909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G & Brandtzaeg P Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104, 3021–3027 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Astiz M, Saha D, Lustbader D, Lin R & Rackow E Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med 128, 594–600 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Aldridge JR Jr. et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A 106, 5306–5311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohlmeier JE & Woodland DL Immunity to respiratory viruses. Annu Rev Immunol 27, 61–82 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Li W, Moltedo B & Moran TM Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 86, 12304–12312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TS, Hufford MM, Sun J, Fu YX & Braciale TJ Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med 207, 1161–1172 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JR et al. Club cells surviving influenza A virus infection induce temporary nonspecific antiviral immunity. Proc Natl Acad Sci U S A 113, 3861–3866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikhak Z, Strassner JP & Luster AD Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med 210, 1855–1869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richert LE et al. Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat Res Biol 11, 196–202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J, Waithman J, Xiao K, Oveissi S & Chen W Optimal conditions required for influenza A infection-enhanced cross-priming of CD8(+) T cells specific to cell-associated antigens. Immunol Cell Biol 91, 576–582 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Zaynagetdinov R Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 49, 180–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai P, Tahiliani V, Stanfield J, Abboud G & Salek-Ardakani S Inflammatory monocytes contribute to the persistence of CXCR3(hi) CX3CR1(lo) circulating and lung-resident memory CD8(+) T cells following respiratory virus infection. Immunol Cell Biol 96, 370–378 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehl GE et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494, 116–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin KL, Suzuki Y, Nakano H, Ramsburg E & Gunn MD CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180, 2562–2572 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Larson SR et al. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ 23, 997–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infusini G et al. Respiratory DC Use IFITM3 to Avoid Direct Viral Infection and Safeguard Virus-Specific CD8+ T Cell Priming. PLoS One 10, e0143539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao X, Kim TS & Braciale TJ Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol 82, 4908–4919 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TS, Gorski SA, Hahn S, Murphy KM & Braciale TJ Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity 40, 400–413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leeuwen-Kerkhoff N et al. Transcriptional profiling reveals functional dichotomy between human slan(+) non-classical monocytes and myeloid dendritic cells. J Leukoc Biol 102, 1055–1068 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Jakubzick CV, Randolph GJ & Henson PM Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 17, 349–362 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Nakano H et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 10, 394–402 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zigmond E et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Desch AN et al. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun 5, 4674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kastenmuller K et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest 121, 1782–1796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh JZ, Kurche JS, Burchill MA & Kedl RM TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 118, 3028–3038 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu C, Nguyen VA, Merad M & Randolph GJ MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol 182, 3650–3659 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iborra S et al. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1(+) Dendritic Cells. Immunity 45, 847–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macdonald DC et al. Harnessing alveolar macrophages for sustained mucosal T-cell recall confers long-term protection to mice against lethal influenza challenge without clinical disease. Mucosal Immunol 7, 89–100 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Mackay LK et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Takamura S et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213, 3057–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar BV et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20, 2921–2934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackay LK et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC & Woodland DL Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson KG et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189, 2702–2706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis MD et al. A reproducible method for the expansion of mouse CD8+ T lymphocytes. J Immunol Methods 417, 134–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.