Abstract
Background:
Post hip fracture surgery delirium (PHFD) is a significant clinical problem in older patients, but an adequate, simple risk prediction model for use in the preoperative period has not been developed.
Methods:
The 2016 American College of Surgeons National Quality Improvement Program Hip Fracture Procedure Targeted Participant Use Data File was used to obtain a cohort of patients aged 60 years and older who underwent hip fracture surgery (n=8871; randomly assigned to derivation [70%] or validation [30%] cohorts). A parsimonious prediction model for PHFD was developed in the derivation cohort using stepwise multivariable logistic regression with further removal of variables by evaluating changes in the area under the receiver operator characteristic curve (AUC). A risk score was developed from the final multivariable model.
Results:
Of 6210 patients in the derivation cohort, PHFD occurred in 1816 (29.2%). Of 32 candidate variables, nine were included in the final model: 1) preoperative delirium (adjusted odds ratio [aOR] 8.32 [95% confidence interval [CI] 6.78–10.21], 8 risk score points); 2) preoperative dementia (aOR 2.38 [95% CI 2.05–2.76], 3 points); 3) age (reference 60–69 years) (age 70–79: aOR 1.60 [95% CI 1.20–2.12], 2 point), (age 80–89: aOR 2.09 [95% CI 1.59–2.74], 3 points), (age ≥90: aOR 2.43 [95% CI 1.82–3.23], 3 points); 4) medical co-management (aOR 1.43 [95% CI 1.13–1.81], 1 point); 5) ASA Physical Status III-V (aOR 1.40 [95% CI 1.14–1.73], 1 point); 6) functional dependence (aOR 1.37 [95% CI 1.17–1.61], 1 point); 7) smoking (aOR 1.36 [95% CI 1.07–1.72], 1 point); 8) systemic inflammatory response syndrome/sepsis/septic shock (aOR 1.34 [95% CI 1.09–1.65], 1 point); and 9) preoperative use of mobility aid (aOR 1.32 [95% CI 1.14–1.52], 1 point), resulting in a risk score ranging from 0 to 20 points. The AUCs of the logistic regression and risk score models were 0.77 [95% CI 0.76–0.78] and 0.77 (95% CI 0.76–0.78), respectively, with similar results in the validation cohort.
Conclusions:
A risk score based on nine preoperative risk factors can predict PHFD in older adult patients with fairly good accuracy.
INTRODUCTION
Hip fractures are a major public health problem for older adults. In the United States, there are an estimated 250,000 cases of hip fracture annually among people aged 65 years and older, with most requiring surgery to restore their functional status.1 A common complication associated with hip fracture repair is delirium, with an estimated incidence of 16% to 65% in geriatric patients.2 Postoperative delirium is characterized by an acute fluctuation in consciousness, cognition, and attention,2 and is associated with significantly increased mortality, morbidity, functional and cognitive decline, and healthcare costs.2 There has been increased attention to the postoperative management of geriatric patients to improve cognitive outcomes and reducing postoperative delirium has been identified as a target for surgical quality improvement.3
Risk factors for postoperative delirium can be classified into predisposing factors (cognitive impairment, age, functional dependency, and multiple comorbidities) and precipitating factors (surgery, anesthesia, polypharmacy, infection, acute exacerbation of coexisting disease, dehydration, and laboratory abnormalities).4,5 Identifying high risk patients for delirium and early implementation of proactive multifactorial interventions for high risk patients may reduce the occurrence of delirium.5 Prediction models for postoperative delirium have been developed, such as in medical patients6 or postsurgical patients,7 but a risk model for delirium specific to hip fracture surgery patients may provide unique insights in this vulnerable population.
In this study, we developed, internally validated, and tested a risk score model for postoperative delirium in hip fracture patients using data from the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) Participant Use Data File (PUF) and Hip Fracture Procedure Targeted Participant Use Data File (HFPT-PUF), a large, multicenter national database.
METHODS
Data Source
The Columbia University Medical Center IRB determined that this study met the criteria for Not Human Subjects Research and was not subject to further review as it did not require access to protected health information. The ACS-NSQIP PUF was merged with the HFPT-PUF which contains additional data specific for hip fracture patients. The ACS-NSQIPa is a validated, prospectively collected national dataset aimed at improving surgical quality and outcomes.8 Detailed descriptions of case inclusion criteria, systematic sampling process, quality measures, and variable descriptions are available from the ACS-NSQIP website.9
Study Population and Data Collection
The 2016 HFPT-PUF, containing data on 9390 patients undergoing hip fracture surgery (Supplemental Figure 1) was used to derive and internally validate our predictive model. Delirium is associated with the older patients aged 60 years or older,10 so we excluded patients <60 years of age (N=519). Of the remaining 8871 patients, 6210 (70%) were randomly assigned to the derivation cohort and 2661 (30%) to the validation cohort. The 2017 HFPT-PUF, containing data on 10,506 patients, provided the test cohort for an initial form of external validation in which the derivation and test cohorts are separated by time. After excluding 623 patients <60 years of age, the test cohort contained 9883 patients.
Outcomes
The primary outcome was the presence of postoperative delirium within 30 days after hip fracture surgery. Delirium was determined from medical chart review, a method previously validated in medical patients11 and intensive care unit patients12 and applied to various medical and surgical patients for delirium detection.13
Specifically, post hip fracture surgery delirium (PHFD) was defined by the dataset as:
“Delirium is a common syndrome in hospitalized older adults and is associated with increased mortality, hospital costs, and long-term cognitive and functional impairment. Delirium is a serious disturbance in a person’s mental abilities that results in a decreased awareness of one’s environment and confused thinking. The onset of delirium is usually sudden, often within hours or a few days.
“Delirium may be recorded as: Delirium, confusion, sundowning, somnolent, crying out, inattentive, disorientation, incoherent, hallucinating, restlessness, or combative. It can also be described as “metabolic encephalopathy,” acute confusional state, acute organic mental disorder, acute organic brain syndrome.”
The ACS-NSQIP Geriatric Surgery Pilot study3 provided an investigational infrastructure for measuring delirium in a national multicenter database study and established the feasibility of chart extraction-based methods for detecting delirium. Trained clinical reviewers examined the entire medical record, including items such as progress notes, procedural notes, consultation notes, and nursing notes.
Variables
Candidate predictors of the primary outcome were obtained from the ACS-NSQIP dataset including demographic information, medical comorbidities, and surgical characteristics, including type of surgery. Laboratory values included preoperative hematocrit (%), white blood cell count (WBC; 103/μl), serum sodium (mEq/L), and serum creatinine (mg/dl). The relationships between continuous variables and PHFD were assessed by plotting the deciles of each variable by the log-odds of PHFD in univariable analyses. WBC and hematocrit were modeled as continuous variables. Serum sodium was categorized as (<143 and ≥143) and serum creatinine was categorized as (≤0.95 and >0.95). Other preoperative risk factors from the HFPT-PUF included preoperative delirium, preoperative dementia, use of mobility aid, pressure sore, medical co-management, standardized hip fracture care program, time from admission to operation, type/ location of hip fracture, and pathologic fracture.
Statistical analysis
The derivation cohort was used to develop a logistic regression model to determine the associations between risk factors and PHFD. Initial candidate variables were identified using clinical judgement and prior known delirium risk factors. Differences in predictor variables between patients with and without PHFD were compared using the chi-squared test or t-test as appropriate. Variable selection for the PHFD prediction model was performed in multiple steps. First, we fit a univariable logistic regression model for each candidate variable. Variables with P < 0.20 were entered into the multivariable analysis, initially consisting of a forward stepwise logistic regression model with an entry criterion of P<0.20 and a removal criterion of P>0.20. The remaining variables were sequentially removed, beginning with the variable with the smallest effect size (β coefficient), and were eliminated if there was no significant reduction in the area under the curve (AUC) of the receiver operator characteristic (ROC) curve after removal.14 Using the final logistic regression model, a risk score for PHFD was developed as previously described.15
Model discrimination was determined by assessing the AUC of the ROC curve and model calibration was assessed with calibration plots. Internal validity was assessed by comparing model discrimination and calibration between the derivation and validation cohorts. An initial analysis of external validity, evaluating cohorts separated by time, was performed by comparing the derivation and test cohorts. Missing data was observed in several variables (Supplemental Table 1). BMI had a high rate of missing data (14.3%) but did not remain in the final model in either complete case analysis or when coded with a missing category level. We did not adjust for missing data in the remaining variables due to the low rate of missing data, and complete case analysis was performed.
The α level was set at 0.05 for significance. Statistical analyses were performed using SPSS Software version 22.0.0 (SPSS, Inc., Chicago, IL) and SAS Software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
Of the 6210 patients in the derivation cohort, PHFD was observed in 1816 patients (29.2%). Baseline characteristics are presented in Table 1. PHFD was more likely to occur in patients who were older, had American Society of Anesthesiologists Physical Status (ASA PS) III–V, functional dependence, transferred from a chronic care facility, had systemic inflammatory response syndrome (SIRS)/sepsis/septic shock, bleeding disorder, preoperative delirium, preoperative dementia, pre-fracture bone protection medication, preoperative use of mobility aid, preoperative pressure sore, and medical co-management (Table 1).
Table 1.
Incidence of post hip fracture surgery delirium by patient characteristics, American College of Surgeons National Surgical Quality Improvement Program Hip Fracture Procedure Targeted Participant Use Data File, 2016.
| Characteristics | Total patientsa (n = 6210) n (%) |
Delirium (n = 1816) n (%) |
Incidence rate (29.2%) % |
|---|---|---|---|
| Age (y) | |||
| 60–69 | 710 (11.4%) | 89 (4.9%) | 12.5% |
| 70–79 | 1344 (21.6%) | 298 (16.4%) | 22.2% |
| 80–89 | 2608 (42.0%) | 833 (45.9%) | 31.9% |
| ≥ 90 | 1548 (24.9%) | 596 (32.8%) | 38.5% |
| Sex | |||
| Female | 4412 (71.0%) | 1278 (70.4%) | 29.0% |
| Male | 1798 (29.0%) | 538 (29.6%) | 29.9% |
| ASA physical status | |||
| I-II | 931 (15.0%) | 160 (8.8%) | 17.2% |
| III-V | 5268 (84.8%) | 1653 (91.0%) | 31.4% |
| Body mass index (kg/m2) | |||
| BMI ≤ 18.5 | 458 (7.4%) | 154 (8.5%) | 33.6% |
| BMI > 18.5 and ≤25 | 2594 (41.8%) | 789 (43.4%) | 30.4% |
| BMI >25 and ≤30 | 1456 (23.4%) | 387 (21.3%) | 26.6% |
| BMI >30 and ≤35 | 555 (8.9%) | 136 (7.5%) | 24.5% |
| BMI >35 | 259 (4.2%) | 59 (3.2%) | 22.8% |
| missing | 888 (14.3%) | 291 (16.0%) | 32.8% |
| Functional status | |||
| Independent | 4789 (77.1%) | 1141 (62.8%) | 23.8% |
| Partially/Totally dependent | 1359 (21.9%) | 648 (35.7%) | 47.7% |
| Transfer status | |||
| Admitted directly from home | 4646 (74.8%) | 1219 (67.1%) | 26.2% |
| Chronic care facility | 726 (11.7%) | 341 (18.8%) | 47.0% |
| Other | 838 (13.5%) | 256 (14.1%) | 30.5% |
| Smoking within one year | 597 (9.6%) | 146 (8.0%) | 24.5% |
| Diabetes | 1111 (17.9%) | 302 (16.6%) | 27.2% |
| Dyspnea | 480 (7.7%) | 161 (8.9%) | 33.5% |
| COPD | 668 (10.8%) | 213 (11.7%) | 31.9% |
| Pneumonia | 53 (0.9%) | 28 (1.5%) | 52.8% |
| Ascites | 20 (0.3%) | 8 (0.4%) | 40.0% |
| Congestive heart failure | 258 (4.2%) | 92 (5.1%) | 35.7% |
| Hypertension | 4211 (67.8%) | 1274 (70.2%) | 30.3% |
| Acute renal failure | 29 (0.5%) | 11 (0.6%) | 37.9% |
| Preoperative dialysis | 122 (2.0%) | 34 (1.9%) | 27.9% |
| Disseminated cancer | 165 (2.7%) | 31 (1.7%) | 18.8% |
| Wound infection | 238 (3.8%) | 88 (4.8%) | 37.0% |
| SIRS/Sepsis/Septic shock | 609 (9.8%) | 248 (13.7%) | 40.7% |
| Chronic steroid use | 353 (5.7%) | 86 (4.7%) | 24.4% |
| Unintentional weight loss | 81 (1.3%) | 31 (1.7%) | 38.3% |
| Bleeding disorder | 1123 (18.1%) | 377 (20.8%) | 33.6% |
| Preoperative transfusion | 314 (5.1%) | 98 (5.4%) | 31.2% |
| Preoperative laboratory data | |||
| Hematocrit (%), mean (SD) | 34.8 (5.4) | 34.7 (5.5) | |
| WBC count (109/L), mean (SD) | 10.0 (3.7) | 10.3 (3.7) | |
| Serum sodium level | |||
| Na ≥143 mEq/L | 597 (9.6%) | 230 (12.7%) | 38.5% |
| Creatinine > 0.95 mg/dl | 2467 (39.7%) | 809 (44.5%) | 32.8% |
| Preoperative delirium | 771 (12.4%) | 615 (33.9%) | 79.8% |
| Preoperative dementia | 1851 (29.8%) | 983 (54.1%) | 53.1% |
| Pre-fracture bone protection medication | 1791 (28.8%) | 598 (32.9%) | 33.4% |
| Preoperative use of mobility aid | 3368 (54.2%) | 1182 (65.1%) | 35.1% |
| Preoperative pressure sore | 211 (3.4%) | 87 (4.8%) | 41.2% |
| Medical co-management | 5577 (89.8%) | 1689 (93.0%) | 30.3% |
| Standardized hip fracture care program | 3406 (54.8%) | 1022 (56.3%) | 30.0% |
| Emergency | 2119 (34.1%) | 588 (32.4%) | 27.7% |
| Time from admission to operation | |||
| ≤ 1 day | 4711 (75.9%) | 1343 (74.0%) | 28.5% |
| > 1 day | 1279 (20.6%) | 413 (22.7%) | 32.3% |
| Type of surgery | |||
| Intramedullary Implant | 3117 (50.2%) | 900 (49.6%) | 28.9% |
| Extramedullary Implant | 871 (14.0%) | 244 (13.4%) | 28.0% |
| Internal Fixation or Prosthesis | 2222 (35.8%) | 672 (37.0%) | 30.2% |
| Type of hip fracture | |||
| Femoral neck fracture-undisplaced | 569 (9.2%) | 151 (8.3%) | 26.5% |
| Femoral neck fracture-displaced | 1734 (27.9%) | 538 (29.6%) | 31.0% |
| Intertrochanteric | 3353 (54.0%) | 993 (54.7%) | 29.6% |
| Subtrochanteric | 394 (6.3%) | 89 (4.9%) | 22.6% |
| Other/Cannot be determined | 160 (2.6%) | 45 (2.5%) | 28.1% |
| Pathologic fracture | |||
| None | 6038 (97.2%) | 1787 (98.4%) | 29.6% |
| Tumor | 89 (1.4%) | 16 (0.9%) | 18.0% |
| Atypical | 83 (1.3%) | 13 (0.7%) | 15.7% |
Totals within variables may vary because of missing data.
Continuous variables expressed as mean (SD). Categorical variables expressed as counts (%).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; SIRS, systemic inflammatory response syndrome; WBC, white blood cell; Na, sodium; BUN, blood urea nitrogen; Cr, creatinine.
Development of multivariable logistic regression model for post hip fracture surgery delirium
A total of 32 variables with P<0.20 in univariate logistic regression were entered in stepwise multivariable logistic regression (bolded variables in Supplemental Table 2), yielding 19 variables: preoperative delirium, pneumonia, preoperative dementia, advanced age, medical co-management, functional dependence, smoking, ASA PS, SIRS/sepsis/septic shock, preoperative use of mobility aid, bleeding disorder, preoperative pressure sore, dyspnea, serum sodium level, serum creatinine level, standardized hip fracture care program, hypertension, time from admission to operation and diabetes (Supplemental Table 2).
Beginning with these 19 variables, we sequentially removed variables, beginning with the variable with the smallest effect size, and evaluated changes in the AUC of the ROC curves (Supplemental Table 3). There was no significant decrease in AUC after removing diabetes, after removing time from admission to operation, after removing hypertension, after removing standardized hip fracture care program, after removing serum creatinine level, after removing serum sodium level, after removing dyspnea, after removing preoperative pressure sore, and after removing bleeding disorder. There was a significant decrease in AUC after removing preoperative use of mobility aid (AUC decrease 0.004 95% CI 0.001–0.007; P=0.008), and variable selection ended with 10 variables. In a logistic regression model with these 10 variables (Supplemental Table 4), pneumonia was not a significant predictor of PHFD (P=0.15), likely due to differences in the samples between the 19-variable logistic regression model and the 10-variable model; furthermore, removal of pneumonia did not significantly reduce the AUC of the model (P=0.40) and pneumonia was eliminated.
The final logistic regression model for PHFD included the following nine variables: preoperative delirium, preoperative dementia, age, medical co-management, ASA PS, functional dependence, smoking, SIRS/Sepsis/Septic shock, and preoperative use of mobility aid (Table 2). The model had an AUC of 0.77 (95% CI 0.76–0.78) (Figure 1A) and was well-calibrated (Figure 1B). We tested all combinations of 2-way interactions among these 9 variables and there were no interactions that significantly increased the AUC of the model (data not shown).
Table 2.
Multivariable logistic regression model for post hip fracture surgery delirium, American College of Surgeons National Surgical Quality Improvement Program Hip Fracture Procedure Targeted Participant Use Data File, 2016.
| Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Preoperative delirium | 8.32 (6.78–10.21) | <0.001 |
| Preoperative dementia | 2.38 (2.05–2.76) | <0.001 |
| Age (y) | <0.001 | |
| 60–69 | — | |
| 70–79 | 1.60 (1.20–2.12) | |
| 80–89 | 2.09 (1.59–2.74) | |
| ≥ 90 | 2.43 (1.82–3.23) | |
| Medical co-management | 1.43 (1.13–1.81) | <0.001 |
| ASA physical status | ||
| III-V vs I-II | 1.40 (1.14–1.73) | 0.002 |
| Functional dependence | 1.37 (1.17–1.61) | <0.001 |
| Smoking | 1.36 (1.07–1.72) | 0.011 |
| SIRS/Sepsis/Septic shock | 1.34 (1.09–1.65) | 0.006 |
| Preoperative use of mobility aid | 1.32 (1.14–1.52) | <0.001 |
Abbreviations: ASA, American Society of Anesthesiologists; SIRS, systemic inflammatory response syndrome.
Figure 1.
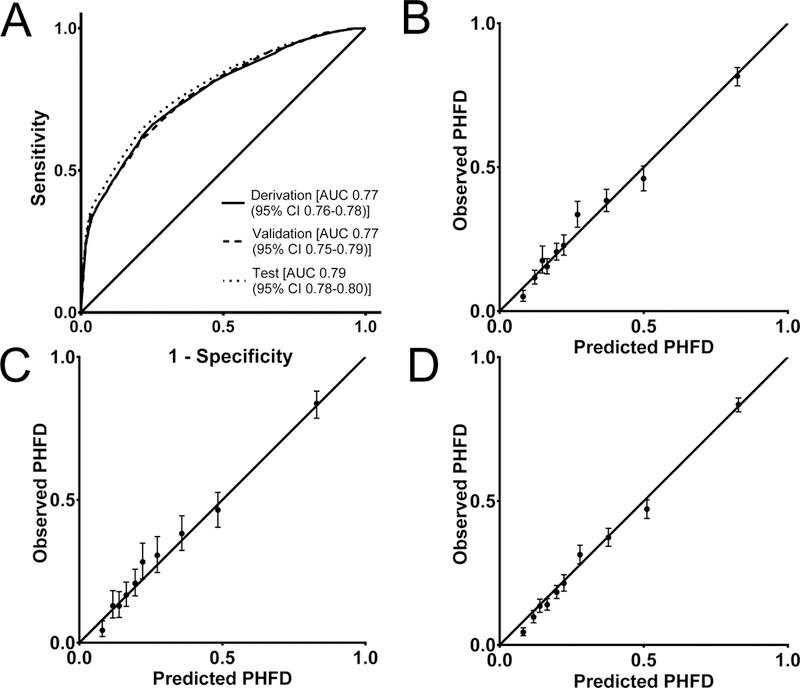
Receiver operator characteristic (ROC) curves of logistic regression model for post hip fracture delirium (PHFD) in derivation, validation, and test cohorts (A). Calibration plots showing observed and predicted risks across deciles of predicted risk for derivation (B), validation (C), and test cohorts (D).
Validation of the logistic regression model
There were no differences in the patient characteristics and PHFD rates between the derivation and validation cohorts (data not shown). The parameter estimates from the final logistic regression model in the derivation cohort were applied to the validation cohort, and the ROC curves in the derivation and validation cohorts were similar (Figure 1A). The AUC in the validation cohort was 0.77 (95% CI 0.75–0.79) with good calibration in the validation cohort (Figure 1C).
Development and validation of a risk score to predict post hip fracture surgery delirium
Based on the β coefficients of the final logistic regression model with 9 predictors, points were assigned to each predictor with a total score from 0 to 20 (Table 3). The predicted risk of PHFD ranged from 4.5% with a risk score of 0 to 92.0% with a risk score of 20 (Supplemental Table 5). The risk score model had an AUC 0.77 (95% CI 0.76–0.78). When applied to the validation cohort, the risk score model had an AUC of 0.77 (95% CI 0.75–0.79). The ROC curves of the risk score model using the derivation and validation cohorts were similar, and the models were well-calibrated in both cohorts (Supplemental Figure 2).
Table 3.
Risk score for post hip fracture surgery delirium, American College of Surgeons National Surgical Quality Improvement Program Hip Fracture Procedure Targeted Participant Use Data File, 2016.
| Variables | Points Assigned |
|---|---|
| Preoperative Delirium | 8 |
| Preoperative Dementia | 3 |
| Age | |
| 60–69 | 0 |
| 70–79 | 2 |
| ≥80 | 3 |
| Medical co-management | 1 |
| ASA physical status III-V | 1 |
| Functional dependence | 1 |
| Smoking | 1 |
| SIRS/Sepsis/Septic shock | 1 |
| Preoperative use of mobility aid | 1 |
Abbreviations: ASA, American Society of Anesthesiologists; SIRS, systemic inflammatory response syndrome.
Test cohort for model
The rate of PHFD in the test cohort was 28.4% (2803/9883), which was not different from the derivation cohort (P=0.23). There were no differences in the baseline characteristics between the derivation and test cohorts. When applied to the test cohort, the AUCs of the logistic regression and risk score models were 0.79 (95% CI 0.78–0.80) (Figure 1A) and 0.79 (95% CI 0.77–0.80) (Supplemental Figure 2A), respectively, and both models were well-calibrated (Figure 1D, Supplemental Figure 2D).
Sensitivity analysis: Patients without preoperative delirium
We performed a sensitivity analysis on the subset of patients without preoperative delirium. Of the 6210 patients in the derivation cohort, 5439 did not have preoperative delirium, and of these, 22% (N=1201) had PHFD. A stepwise forward logistic regression model was fit with 31 candidate variables (bolded variables in Supplemental Table 2, excluding preoperative delirium) using the same criteria as above. This model retained 14 variables (Supplemental Table 6) and the AUC was 0.69 (95% CI 0.68–0.71).
Discussion
We developed and internally validated a practical prediction model for PHFD consisting of nine clinical factors: preoperative delirium, preoperative dementia, age, medical co-management, ASA PS, functional dependence, smoking, SIRS/sepsis/septic shock, and preoperative use of mobility aid. This model can be applied by perioperative physicians at the time of surgery to assess the risk of PHFD and was developed using a large multicenter dataset of patients with data collected specifically for hip fracture surgery patients. The incidence of delirium after hip fracture surgery was ~29%, comparable to the incidence of 24–35% in prior studies of hip fracture patients16–18 and the predictors for PHFD identified by our study were also consistent with previous studies.4,7,16,19,20
Notably, our analysis included patients with preoperative delirium – affecting ~12% of our patients – and this was the strongest predictor of PHFD, accounting for 8 out of the total 20 points in the risk score. Preoperative delirium occurs in 13.5 to 53% of hip fracture patients21–23 but patients with preoperative delirium are typically excluded from studies6,19,24 and this risks excluding a significant proportion of patients at risk for PHFD.25 The inclusions of patients with preoperative delirium in our analysis are consistent with the real-world clinical population of patients presenting with hip fracture. In a sensitivity analysis, we found that when patients with preoperative delirium were excluded, the magnitude of the associations (ORs) of the remaining risk factors were similar to the original analysis, but model discrimination was worse, confirming the importance of preoperative delirium as a risk factor in our model.
Previous reviews of delirium prediction consistently identified advanced age, cognitive impairment, functional and sensory impairment, and comorbidity burden as leading risk factors.4,7,16,19,20 Among them, preoperative cognitive impairment is the most important risk factor for postoperative delirium.5 In our cohort, the incidence of preoperative dementia was 30% (1851/6210) and half of these patients (983/1851; 53%) developed PHFD, comparable to previous studies.20,26 A causal relationship between dementia and delirium has not yet been established4 but patients with dementia are at risk for preoperative delirium after a precipitating factor such as hip fracture.18 In our cohort, 72% of patients with preoperative delirium had preoperative dementia (554/771), comparable to previous studies.18,22 Delirium occurring in older patients with dementia has been associated with a dramatic deterioration of cognitive function and patients often fail to return to their baseline cognitive state.4
Frailty can be characterized as increased vulnerability to stress related to compromised homeostatic capacity in multiple physiologic systems and has many similarities with delirium.27 Advanced age, a central aspect of frailty,28 is a risk factor for PHFD, likely due to the relationship between aging and impaired physiologic compensatory function and the gradual accumulation of cerebral damage in the setting of surgical stress.20 Frail patients have a decline in their physical function, and several studies have demonstrated a significant association between functional dependence and PHFD,4,20,26 though a recent review did not find this relationship.19 This may be due to differences in how this variable is defined, and in our study, functional dependence was measured by impairments in the activities of daily living and was a significant predictor of PHFD. Impairments in the activities of daily living can be viewed as a symptom of frailty29 and dependence on mobility aids may represent frailty, especially in older adults.30
An increased comorbidity burden is a marker of frailty.31 Comorbidity burden, as assessed by the ASA PS or Charlson Comorbidity Index, is associated with PHFD.4,16,19,20,26 In addition to the ASA PS, our model identified medical co-management, defined as the need for a preoperative medical consultation and a likely indicator of advanced comorbidity burden, as a predictor of PHFD. We did not incorporate a frailty index in our model but frailty has been measured in ACS-NSQIP data32 and the utility of a frailty measure will require further investigation.
One of the leading mechanisms for delirium is neuroinflammation.4 In our cohort, the incidence of preoperative SIRS/sepsis/septic shock was 9.8 % (609/6210) and 40% (248/609) patients developed PHFD, comparable to previous studies.33 Smoking was a predictor of PHFD in our model. Although the exact link between smoking and delirium is unclear, acute nicotine withdrawal may increase delirium34 and others have identified smoking as a risk factor for postoperative delirium.35
Several prediction models for PHFD have been developed6,36,37 but these models have some limitations when applied to femur fracture patients. Kalisvaart et al.6 applied a medical risk model to hip-surgery patients but the sample consisted of elective hip replacement patients in addition to hip fracture repair patients, and it may be problematic to include both types of patients as the incidence of delirium was 4-fold greater in the hip fracture patients as compared to the elective patients. Moerman et al.37 included a significant number of healthy patients where 67% of patients had ASA PS I/II while in our study, 85% of patients had ASA PS III-V, and one of their factors (ability to draw a clock) is not something routinely assessed in clinical practice. Freter et al.36 applied an instrument developed in elective orthopedic surgery patients to older adults with femur fracture, but the study had a small sample size and only one variable in the instrument (cognitive impairment) was strongly associated with PHFD. A recent study, also using ACS NSQIP data, identified predictors of PHFD, but their analyses included postoperative variables and cannot be applied as a preoperative risk assessment tool.38 Patients identified as being high-risk for PHFD should be managed to minimize the risk for delirium such as proactive, multicomponent interventions and geriatrics consultation.5
Hospital Elder Life Program (HELP) focused on the intervention of modifiable delirium risk factors such as mobilization, orientation, nonpharmacological sleep enhancement, visual/hearing aids, and hydration.39 HELP effectively reduced the incidence of delirium and has been used internationally.5 The proactive geriatrics consultation, which is known as another effective nonpharmacologic strategy for delirium prevention, has a structured protocol to reduce opioid use (round-the-clock acetaminophen and local pain management) and discontinuing sleeping pills.5 Previous studies showed this method reduced the incidence of delirium in patients with hip fracture.5
The primary limitation of our analysis is the lack of a validated clinical screening or assessment tool for delirium, such as the Confusion Assessment Method and Delirium Rating Scale.40 The ACS-NSQIP is not an intervention per se, and cannot mandate the implementation of any specific clinical tests or measures, and the data are derived from multiple centers, so it may be difficult to apply a standardized screening tool across multiple hospitals. However, a chart-based method for the identification of delirium is a validated method with 74% sensitivity and 83% specificity, and overall agreement was 82% in older adults. A chart-based method can also detect delirium occurring during the night or on weekends, which may be missed by a screening or assessment tool based method.13 Other limitations are that some risk factors for postoperative delirium (e.g., substance abuse, sensory impairment, presence of depression, medications) are not available in the dataset. In addition, this study does not have data on the severity and duration of delirium. In future studies, a prediction model accounting for the severity of the delirium would be of great clinical benefit. Our risk score was derived, internally validated, and tested on ACS-NSQIP data. While the ACS-NSQIP HFPT-PUF contains data from 117 institutions, there is an over-representation of large, academic medical center, and external validation in different patient populations is necessary to determine the generalizability of our model.
In conclusion, we built a practical model with nine clinical factors to predict postoperative delirium for older adult patients with hip fracture. It will be necessary in future studies to externally validate our results to investigate how this risk score model affects the prevention of postoperative delirium in clinical practice.
Supplementary Material
Key Points.
Question: Is there a practical risk score that can predict the risk of postoperative delirium in older patients undergoing hip fracture repair?
Finding: The risk score for post hip fracture delirium included nine variables: preoperative delirium (8 points), preoperative dementia (3 points), age (0–3 points), medical co-management (1 point), ASA Physical Status III-V (1 point), functional dependence (1 point), smoking (1 point), systemic inflammatory response syndrome/sepsis/septic shock (1 point), and preoperative use of mobility aid (1 point), with an area under the curve of the receiver operator characteristic curve of 0.77 [95% confidence interval 0.76–0.78].
Meaning: Our prediction model can be applied by perioperative physicians at the time of hip fracture surgery to assess the risk of postoperative delirium.
Acknowledgments
Funding: This publication was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant Number KL2TR001874 (MK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This author was involved in study design, data analysis, and manuscript preparation. Dr. Li approved the final manuscript and attests to the integrity of the original data and analysis reported in this manuscript.
This author was involved in study design, conduct of study, data analysis, and manuscript preparation. Dr. M Kim approved the final manuscript and attests to the integrity of the original data and analysis reported in this manuscript. Dr. M Kim is the archival author.
This author was involved in study design, conduct of study, data analysis, and manuscript preparation. Dr. EM Kim approved the final manuscript and attests to the integrity of the original data and analysis reported in this manuscript. No conflict of interest exists.
Glossary of Terms
- ACS-NSQIP
American College of Surgeons National Quality Improvement Program
- PUF
Participant Use Data File
- HFPT-PUF
Hip Fracture Procedure Targeted Participant Use Data File
- PHFD
post hip fracture surgery delirium
- WBC
white blood cell count
- AUC
area under the curve
- ROC
receiver operator characteristic
- ASA PS
American Society of Anesthesiologists Physical Status
- SIRS
systemic inflammatory response syndrome
- HELP
Hospital Elder Life Program
Footnotes
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Reprints will not be available from the authors.
No conflict of interest exists.
Contributor Information
Eun Mi Kim, Department of Anesthesiology, Columbia University Medical Center, New York, NY; Department of Anesthesia and Pain Medicine, Kangnam Sacred Heart Hospital, Hallym University, Seoul, Korea.
Guohua Li, Department of Anesthesiology, Columbia University Medical Center, New York, NY; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY.
Minjae Kim, Department of Anesthesiology, Columbia University Medical Center, New York, NY; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY.
References
- 1.Endo A, Baer HJ, Nagao M, Weaver MJ. Prediction Model of In-Hospital Mortality After Hip Fracture Surgery. J Orthop Trauma. 2018;32:34–8. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berian JR, Zhou L, Russell MM et al. Postoperative Delirium as a Target for Surgical Quality Improvement. Ann Surg. 2018;268:93–9. [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377:1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalisvaart KJ, Vreeswijk R, de Jonghe JF et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc. 2006;54:817–22. [DOI] [PubMed] [Google Scholar]
- 7.Lindroth H, Bratzke L, Purvis S et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ open. 2018;8:e019223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink AS, Campbell DA Jr, Mentzer RM Jr. et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Surgeons. American College of Surgeons National Surgical Quality Improvement Program Participant Use File. [November 26, 2017]; Available from: https://www.facs.org/quality-programs/acs-nsqip/participant-use.
- 10.de Wit HA, Winkens B, Mestres Gonzalvo C et al. The development of an automated ward independent delirium risk prediction model. Int J Clin Pharm. 2016;38:915–23. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Leo-Summers L, Zhang Y et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–8. [DOI] [PubMed] [Google Scholar]
- 12.Pisani MA, Araujo KL, Van Ness PH et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saczynski JS, Kosar CM, Xu G et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 15.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–60. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Zhao X, Dong T et al. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2017;29:115–26. [DOI] [PubMed] [Google Scholar]
- 17.Bitsch M, Foss N, Kristensen B, Kehlet H. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthop Scand. 2004;75:378–89. [DOI] [PubMed] [Google Scholar]
- 18.Neerland BE, Krogseth M, Juliebo V et al. Perioperative hemodynamics and risk for delirium and new onset dementia in hip fracture patients; A prospective follow-up study. PloS one. 2017;12:e0180641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh ES, Li M, Fafowora TM et al. Preoperative risk factors for postoperative delirium following hip fracture repair: a systematic review. Int J Geriatr Psychiatry. 2015;30:900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan MM, Hawkes WG, Zimmerman SI et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M527–34. [DOI] [PubMed] [Google Scholar]
- 22.Juliebo V, Bjoro K, Krogseth M et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354–61. [DOI] [PubMed] [Google Scholar]
- 23.Adunsky A, Levy R, Heim M, Mizrahi E, Arad M. The unfavorable nature of preoperative delirium in elderly hip fractured patients. Arch Gerontol Geriatr. 2003;36:67–74. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Jia P, Zhang J et al. Prevalence and risk factors of postoperative delirium in elderly hip fracture patients. J Int Med Res. 2016;44:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurunathan U, Marudhachalam K, Reynolds K. Preoperative Delirium: Not to Be Overlooked. Anesth Analg. 2016;122:1727. [DOI] [PubMed] [Google Scholar]
- 26.Mosk CA, Mus M, Vroemen JP et al. Dementia and delirium, the outcomes in elderly hip fracture patients. Clin Interv Aging. 2017;12:421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinlan N, Marcantonio ER, Inouye SK et al. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59 Suppl 2:S262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juma S, Taabazuing MM, Montero-Odasso M. Clinical Frailty Scale in an Acute Medicine Unit: a Simple Tool That Predicts Length of Stay. Can Geriatr J 2016;19:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley JE, Haren MT, Rolland Y, Kim MJ. Frailty. Med Clin North Am. 2006;90:837–47. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7. [DOI] [PubMed] [Google Scholar]
- 32.Chimukangara M, Helm MC, Frelich MJ et al. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc. 2017;31:2509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morch SS, Tantholdt-Hansen S, Pedersen NE et al. The association between pre-operative sepsis and 30-day mortality in hip fracture patients-A cohort study. Acta Anaesthesiol Scand. 2018;62:1209–14. [DOI] [PubMed] [Google Scholar]
- 34.Lim TS, Lee JS, Yoon JH et al. Cigarette smoking is an independent risk factor for post-stroke delirium. BMC Neurol. 2017;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makiguchi T, Yokoo S, Kurihara J. Risk factors for postoperative delirium in patients undergoing free flap reconstruction for oral cancer. Int J Oral Maxillofac Surg. 2018;47:998–1002. [DOI] [PubMed] [Google Scholar]
- 36.Freter SH, George J, Dunbar MJ et al. Prediction of delirium in fractured neck of femur as part of routine preoperative nursing care. Age Ageing. 2005;34:387–8. [DOI] [PubMed] [Google Scholar]
- 37.Moerman S, Tuinebreijer WE, de Boo M et al. Validation of the Risk Model for Delirium in hip fracture patients. Gen Hosp Psychiatry. 2012;34:153–9. [DOI] [PubMed] [Google Scholar]
- 38.Malik AT, Quatman CE, Phieffer LS, Ly TV, Khan SN. Incidence, risk factors and clinical impact of postoperative delirium following open reduction and internal fixation (ORIF) for hip fractures: an analysis of 7859 patients from the ACS-NSQIP hip fracture procedure targeted database. Eur J Orthop Surg Traumatol. 2019;29:435–46. [DOI] [PubMed] [Google Scholar]
- 39.Inouye SK, Bogardus ST, Jr., Charpentier PA et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. [DOI] [PubMed] [Google Scholar]
- 40.De J, Wand AP. Delirium Screening: A Systematic Review of Delirium Screening Tools in Hospitalized Patients. Gerontologist. 2015;55:1079–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


