ABSTRACT
Long intergenic non-coding RNAs (lincRNAs) have been implicated in gene regulation, but their requirement for development needs empirical interrogation. We computationally identified nine murine lincRNAs that have developmentally regulated transcriptional and epigenomic profiles specific to early heart differentiation. Six of the nine lincRNAs had in vivo expression patterns supporting a potential function in heart development, including a transcript downstream of the cardiac transcription factor Hand2, which we named Handlr (Hand2-associated lincRNA), Rubie and Atcayos. We genetically ablated these six lincRNAs in mouse, which suggested genomic regulatory roles for four of the cohort. However, none of the lincRNA deletions led to severe cardiac phenotypes. Thus, we stressed the hearts of adult Handlr and Atcayos mutant mice by transverse aortic banding and found that absence of these lincRNAs did not affect cardiac hypertrophy or left ventricular function post-stress. Our results support roles for lincRNA transcripts and/or transcription in the regulation of topologically associated genes. However, the individual importance of developmentally specific lincRNAs is yet to be established. Their status as either gene-like entities or epigenetic components of the nucleus should be further considered.
KEY WORDS: Heart development, Long non-coding RNA, Gene regulation
Highlighted Article: Although cardiac-specific long intergenic noncoding RNAs display striking expression patterns in the embryo, they appear largely dispensable for proper heart development.
INTRODUCTION
A substantial proportion of the mammalian genome is transcribed throughout development, although only a small fraction of this yields functional protein (ENCODE Project Consortium, 2012; Hon et al., 2017; Wong et al., 2001). The remaining noncoding RNA is arbitrarily classified into long (lncRNA) and short transcripts based upon length greater or less than 200 nt. A few lncRNAs have been implicated to be important for cardiac development (Anderson et al., 2016; Grote et al., 2013; Han et al., 2014; Kurian et al., 2015). However, these RNA molecules are often products of the pervasive bidirectional transcription taking place at most genes (Katayama et al., 2005), which makes independently dissecting their function difficult. On the other hand, thousands of putative intergenic lncRNAs (lincRNAs) with little protein-coding potential exist as stand-alone units (Carninci et al., 2005). They can exhibit characteristics indicative of epigenetic control, such as histone H3 trimethylation at lysine 4 (H3K4me3) and acetylation of lysine 27 (H3K27Ac) at promoters, and trimethylation at lysine 36 (H3K36me3) throughout their gene body, splicing, 5′ m7G capping and polyadenylation (Derrien et al., 2012; Quinn and Chang, 2016; Sati et al., 2012). LincRNAs also can display considerable sequence conservation and are dynamically expressed in specific tissues at developmentally discrete times (Diederichs, 2014; Mattioli et al., 2019; Perry and Ulitsky, 2016). For example, the lincRNAs braveheart (Bvht), meteor (ThemisM4Btlr) and carmen (Carmn) seem to play significant roles in precardiac mesodermal differentiation, at least in cellular differentiation systems in vitro (Alexanian et al., 2017; Hou et al., 2017; Klattenhoff et al., 2013; Ounzain et al., 2015). However, meteor knockout in vivo resulted in milder phenotypes than observed in vitro (Guo et al., 2018), while braveheart and carmen have not been tested in embryos. Thus, few lincRNAs have been shown to be required for cardiac development in vivo. Given the great number of lincRNAs expressed within the cell, the energy investment into the processing and maintenance of these transcripts suggests their putative importance. Therefore, efforts must be taken to interrogate specific lincRNA requirements for proper embryogenesis.
We were most interested in lincRNAs that might act to influence the early commitment of nascent mesoderm into the cardiac lineage. We hypothesized that as yet unstudied transcripts were important for this most fundamental stage of cardiac development. Therefore, we screened for the expression of candidates during mouse embryonic stem cell (mESC) in vitro differentiation into cardiomyocytes (CM) through nascent mesoderm (MES), cardiac mesoderm (cMES) and cardiac progenitor (CP) intermediates. Of more than 114,000 long noncoding RNA annotations, we identified a small cohort of lincRNAs with epigenetic regulation, clear splice structure and cardiac progenitor specificity, which we then validated in vivo in the early embryo. Ablation of these noncoding genes revealed regulatory roles within their topologically associated domains (TADs) but very mild or undetectable phenotypes in heart development or postnatal function.
RESULTS
LincRNAs with cardiac-specific expression and epigenetic regulation in vitro
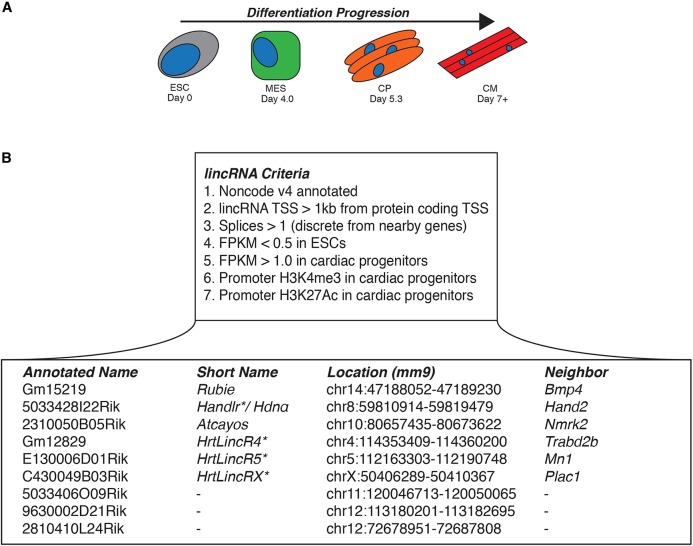
We hypothesized that, like many canonical genes, a subset of lincRNAs would be specifically expressed in the cardiac lineage. We also predicted that those most crucial for heart formation would function early in its development. To find candidate lincRNAs, we re-mapped stranded raw RNA-seq reads from differentiations of mouse ESCs into cardiomyocytes (Fig. 1A,B) (Devine et al., 2014; Wamstad et al., 2012) against Noncode version 4.0-annotated transcripts (Xie et al., 2014). Additionally, we integrated parallel histone modification ChIP-seq data (Wamstad et al., 2012) to discriminate loci under epigenetic regulation. To screen for cardiac developmental specificity, we chose to focus on elements that were lowly expressed in ESCs (FPKM<0.5), while strongly upregulated in cardiac mesoderm or cardiac progenitors (FPKM>1.0). The majority of protein coding genes display antisense transcription from their promoters (Carninci et al., 2005), including numerous studied lncRNAs (Anderson et al., 2016; Daneshvar et al., 2016; Gore-Panter et al., 2016; Grote et al., 2013; Han et al., 2014; Kino et al., 2010; Kurian et al., 2015; Ramos et al., 2015; Xu et al., 2016). We focused instead on genomic elements that could be altered independently from their nearby protein-coding genes. Therefore, we filtered for RNA annotations whose transcriptional start site (TSS) began more than 1 kilobase (kb) from the TSS of known protein-coding genes. To avoid spurious transcripts, we required candidates be spliced and then further refined the list to those displaying histone H3 lysine-4 trimethylation (H3K4me3) and H3 lysine-27 acetylation (H3K27Ac) at their promoters. After removing annotated transcripts that splice into nearby protein coding genes (i.e. A930006K02Rik into Ifnar1), we found that these criteria narrowed candidates to only nine total lincRNAs out of 114,104 considered transcripts (Fig. 1B).
Fig. 1.
Epigenetically regulated cardiac lincRNAs. (A) Differentiation progression of mESCs into cardiomyocytes used for lincRNA candidate selection. ESC, embryonic stem cell; MES, mesoderm; CP, cardiac progenitor; CM, cardiomyocyte. (B) Criteria for lincRNA identification and the resulting nine candidates. Asterisks indicate names assigned by the Bruneau lab.
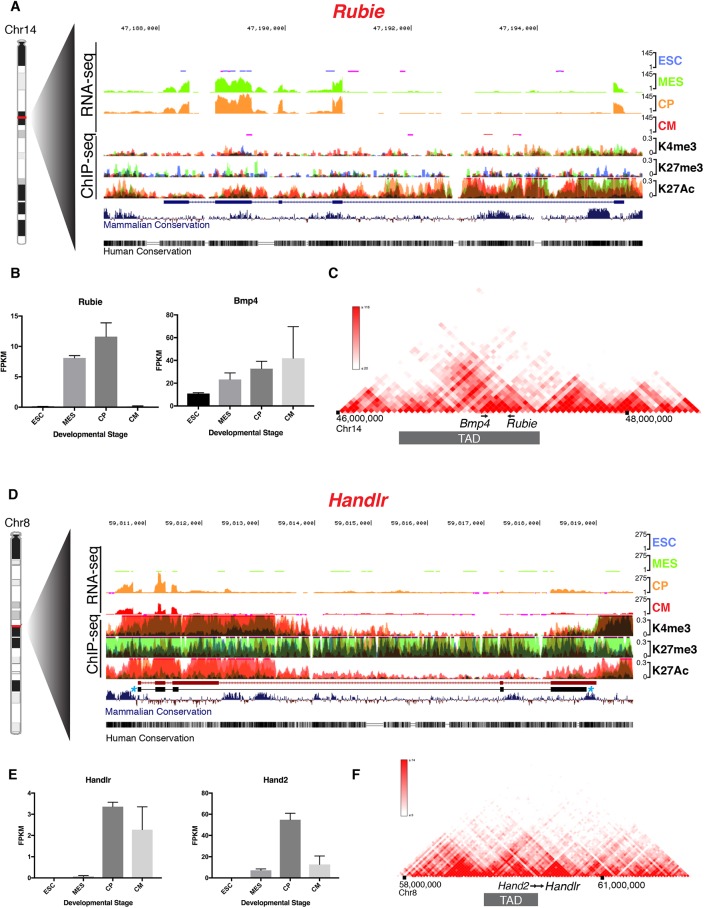
The lincRNA Rubie (RNA upstream Bmp4 in the inner ear, Gm15219) is known to co-express with the TGFβ signaling protein Bmp4 (Wozney et al., 1988) after E15.0 in the mouse inner ear, and its perturbed splicing has previously been implicated in ear vestibule malformation and consequent circling behavior (Roberts et al., 2012). However, our candidate screen revealed it to be expressed much earlier in the developing cardiac mesoderm (Fig. 2A). As in the inner ear, its expression in vitro overlapped Bmp4, and these genes, which are separated by ∼176 kb, co-occupied a strongly interacting region within the same topologically associated domain (TAD; Fig. 2B,C) (Dixon et al., 2012; Nora et al., 2012).
Fig. 2.
Genomic characterization of Rubie and Handlr in vitro. (A) UCSC Genome Browser tracks of Rubie RNA-seq and overlaid histone H3 ChIP-seq at ESC, MES, CP and CM stages of in vitro differentiation; RefSeq annotation in blue. (B) Quantified expression of Rubie and Bmp4 at each differentiation stage. (C) 3D Genome Browser Hi-C heatmap of chromosome interactions around Bmp4 and Rubie loci. (D) UCSC Genome Browser tracks of Handlr RNA-seq and overlaid histone H3 ChIP-seq at ESC, MES, CP and CM stages of in vitro differentiation. Ensembl annotation in red; observed exon structure of predominant Handlr transcript in black with blue asterisks. (E) Quantified expression of Handlr and Hand2 at each differentiation stage. (F) 3D Genome Browser Hi-C heatmap of chromosome interactions around Handlr and Hand2 loci. ESC, embryonic stem cell; MES, mesoderm; CP, cardiac progenitor; CM, cardiomyocyte; blue, ESC; green, MES; orange, CP; red, CM; K4me3, histone H3 lysine 4 trimethylation; K27me3, histone H3 lysine 27 trimethylation; K27Ac, histone H3 lysine 27 acetylation; TAD, topologically associated domain. Data are mean±s.e.m. in B and E.
Hand2, a transcription factor that is crucial for heart development (Srivastava et al., 1997), has previously been shown to be regulated by antisense transcription of the noncoding RNA upperhand (Uph; Hand2os1) locus 5′ from its promoter (Anderson et al., 2016). Our search identified 5033428l22Rik as a candidate lincRNA ∼8 kb downstream of Hand2, which we named Handlr (Hand2-associated lincRNA). During the course of our study, others also studied this locus, which they named handsdown (Hdn) (Ritter et al., 2019), in the cardiac lineage. This region displayed numerous transcribed splice forms, but 3′ rapid amplification of cDNA ends (RACE) of E9.5 cDNA revealed a single predominant 5-exon, polyadenylated isoform that varied from its Ensembl- and RefSeq-annotated structures (Fig. 1D, Fig. S1A,B). The expression of Handlr overlapped that of Hand2 in vitro (Fig. 2E), but these genes sat near a TAD border (Fig. 2F) and were separated by a CTCF insulation site (Martin et al., 2011), suggesting a potential topological division between the two.
Seven additional annotated lincRNAs met the criteria for subsequent analyses. We discovered that Atcayos (2310050B05Rik) transcription spanned the important cardiomyocyte metabolic regulator Nmrk2 (Diguet et al., 2018) and preceded its expression in differentiating cardiac progenitors and cardiomyocytes (Fig. S2A,B). Gm12829, named HrtLincR4 (heart lincRNA of chromosome 4) was correlatively expressed within a genomic domain in frequent proximity to Trabd2b, a Wnt protein-binding metalloprotease (Zhang et al., 2012b). In addition, its expression was only transiently detected within an 18 h window at the cardiac mesoderm (cMES) stage of differentiation (Fig. S2C-E). E130006D01Rik, named HrtLincR5 (heart lincRNA of chromosome 5), was expressed within a Mn1-interacting DNA domain ∼275 kb downstream of this transcriptional co-activator (van Wely et al., 2003). This transcript displayed highly stereotypic splicing and was only detected at the cardiac progenitor stage of differentiation (Fig. S3A-C). C430049B03Rik, named HrtLincRX (heart lincRNA of X chromosome) was highly expressed early in our differentiation model and contained a miRNA cluster in its 3′ tail that had previously been shown to drive cardiomyocyte specification (Shen et al., 2016). This lincRNA also lies ∼12.5 kb downstream of – and overlapped expression with – the essential placental gene Plac1 (Jackman et al., 2012), which is not normally expressed in most somatic tissues (Fant et al., 2010) (Fig. S2D-F). Finally, 5033406O09Rik, 9630002D21Rik and 2810410L24Rik also fulfilled the criteria of our screen (Fig. S4A-C).
All nine lincRNAs contained regions with highly homologous sequence to human and/or mammalian genomes (Fig. 2, Figs S2, S3 and S4). To assess the protein-coding potential of these candidates, we employed multiple tests. First, we evaluated PhyloCSF (Lin et al., 2011) codon scores in all three frames for each transcript. Whereas this algorithm readily detected stretches with coding potential in known genes such as Bmp4 and the micropeptide-containing Apela/Toddler (Pauli et al., 2014) and Dworf (Strit1) (Nelson et al., 2016), we found no evidence for protein-coding potential in our lincRNA cohort, with one exception. A 28 amino acid reading frame in the second exon of HrtLincR4 was predicted to represent a possible conserved coding region (Fig. S5A), although HrtLincR4, as well as each additional member of the cohort displayed negative coding-non-coding indices (CNCI, Fig. S5B) (Sun et al., 2013) similar to the known paraspeckle-associated lincRNA Neat1 (Hutchinson et al., 2007). However, CPAT (Wang et al., 2013) calculation of hexamer usage bias (Wang et al., 2013) and Ficket nucleotide composition and codon usage bias (Fickett, 1982) could not differentiate our lincRNA group from Apela or Dworf (Fig. S5C). We next tested Rubie, Handlr, Atcayos, HrtLincR4, HrtLincR5 and HrtLincRX localization in fractionated cardiac progenitor cells. These lincRNAs were biased to the nucleus, where Rubie and Handlr were enriched even more so than Neat1 (Fig. S5D). Additionally, these six lincRNAs could generate cDNA using oligo dT primers at least as efficiently as Actb and Neat1, which are known to be polyadenylated (Fig. S5E, Table S1) (Sasaki et al., 2009). Taken together, we classified these lincRNAs as nuclear enriched, polyadenylated, transcripts with little translational capacity. However, we could not rule out the coding potential of the minority HrtLincR4 fraction that reaches the cytoplasm.
A cohort of screened cardiac lincRNAs display dynamic expression in vivo in the developing mouse heart
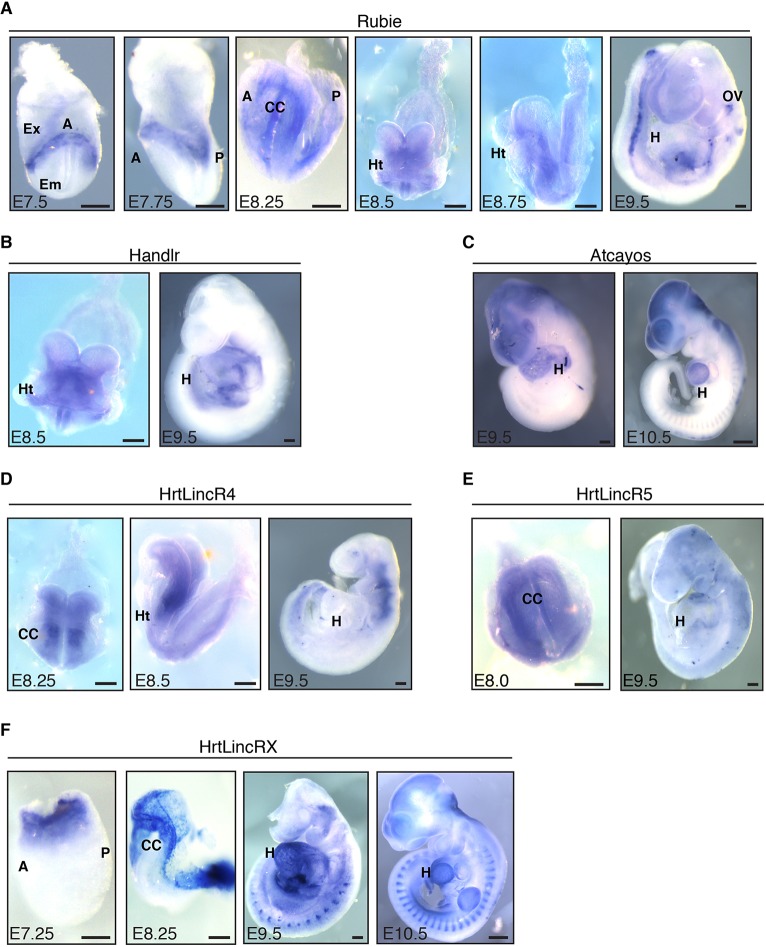
We examined the spatiotemporal expression patterns in the developing embryo for each of the nine candidate lincRNAs by whole-mount in situ hybridization from E7.25 through E10.5 using transcript-specific probes (Table S2). The expression patterns observed in vitro were largely predictive of those observed in vivo. Rubie was first observed in the E7.75 embryo, where, similar to Bmp4 (Perea-Gomez et al., 1999), it strongly demarcated the extra-embryonic boundary and flanked the eventual heart field. From E8.0 to E8.5, its expression became less focused, spreading throughout the developing cardiac crescent and heart tube, respectively. Rubie transcription at E8.75 was largely non-cardiac, and by E9.5, it was strongly localized to posterior mesoderm and the otic vesicle (Fig. 3A). This patterning overlapped a somewhat refined subset of what had previously been established for Bmp4 (Danesh et al., 2009).
Fig. 3.
lincRNA expression patterns in vivo. (A) In situ hybridization staining for Rubie from E7.5 to E9.5. (B) In situ hybridization staining for Handlr at E8.5 and E9.5. (C) In situ hybridization staining for Atcayos at E9.5 and E10.5. (D) In situ hybridization staining for HrtLincR4 at E8.25, E8.5 and E9.5. (E) In situ hybridization staining for HrtLincR5 at E8.0 and E9.5. (F) In situ hybridization staining for HrtLincRX from E7.25 through E10.5. A, anterior; P, posterior; Em, embryonic region; Ex, extra-embryonic region; CC, cardiac crescent; Ht, heart tube; H, heart; OV, otic vesicle. Scale bars: 100 µm; 500 µm for E10.5 in C and F. All images are white balanced for clarity.
From E8.5 through E9.5, Handlr was transcribed in the developing heart tube, posterior cardiac progenitors, branchial arches and lateral plate mesoderm (Fig. 3B). These patterns overlapped what was also shown for Hand2 at this developmental stage (Charite et al., 2000), suggesting common regulation between Hand2 and Handlr. Atcayos, as predicted by in vitro expression patterns, was weakly expressed during early stages of heart tube formation, while it was dramatically upregulated after E9.5 in the developing ventricles, as well as cranial structures and somitic mesenchyme (Fig. 3C).
From E8.25 through E9.5, HrtLincR4 displayed strong expression in developing pharyngeal mesoderm, immediately dorsal to the developing cardiac crescent (Fig. 3D). Given its highly transient expression within differentiating cardiac mesoderm in vitro, these data suggested that HrtLincR4 was quickly specified to the secondary heart field and/or adjacent tissues during the onset of cardiac lineage commitment. HrtLincR5 was broadly expressed throughout the mesoderm, including the nascent cardiac crescent, at E8.25. As expected by its short-lived in vitro expression pattern, HrtLincR5 was predominantly lost in vivo by E9.5 (Fig. 3E).
HrtLincRX was strongly expressed by E7.5 during cardiac lineage formation in anterior mesoderm at the extra-embryonic boarder, as well as in extra-embryonic tissues. At E8.25, it was strongly expressed in the cardiac crescent, amniotic membranes and the developing allantois. Although expression of the adjacent miR322/503 cluster was previously shown to be cardiac specific (Shen et al., 2016), this lincRNA was widely expressed throughout the heart, forelimb and somitic mesoderm at E9.5 and E10.5 (Fig. 3F). This suggested divergent regulation and/or compounding roles for HrtLincRX versus its miRNA components. We could not effectively validate the expression of 5033406O09Rik, 9630002D21Rik or 2810410L24Rik beyond diffuse low levels in the developing mouse embryo (Fig. S6A-C). These experiments established the striking expression patterns of numerous tissue-specific lincRNAs identified from our screen of in vitro cardiac differentiation. Therefore, we aimed to test the developmental importance of Rubie, Handlr, Atcayos, HrtLincR4, HrtLincR5 and HrtLincRX expression during embryonic development.
Cas9 ablation of lincRNA promoter regions in vivo identifies local gene regulatory roles
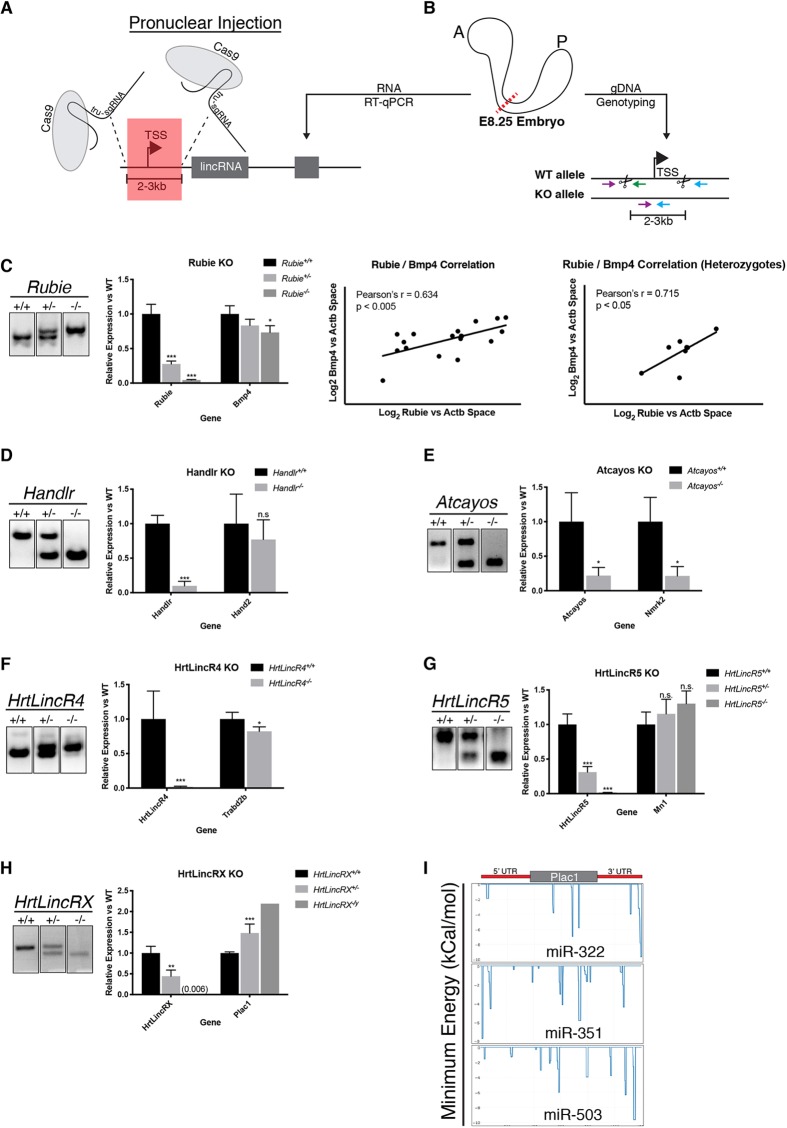
To determine the requirement for the six lincRNAs that displayed compelling in vivo expression, we generated knockout mouse lines through pronuclear Cas9 mRNA and tru-sgRNA (Fu et al., 2014) injections. For each knockout, paired tru-sgRNAs were co-injected to induce 2-3 kb deletions flanking the respective lincRNA transcriptional start site (TSS) (Fig. 4A, Table S3), which successfully generated heritable alleles for all six target regions. After substantial outbreeding (more than three backcrosses into C57Bl/6j background), we crossed heterozygotes and harvested the anterior half of E8.25 embryos for RT-qPCR (Fig. 4B). We found that these deletions ablated downstream transcription of each lincRNA (Fig. 4C-H). As these lincRNAs were nuclear enriched, we hypothesized they might be involved in transcriptional regulation within their local genomic environments. To test this, we measured expression of neighboring protein-coding genes sharing the same respective TADs (Table S1). While Rubie was previously associated with BMP4 signaling in the inner ear (Roberts et al., 2012), its requirement for Bmp4 expression had not been established. We found that loss of Rubie resulted in significant reduction of Bmp4 expression during cardiac specification. Furthermore, the amount of transcribed Rubie was directly correlated with Bmp4 levels in this region at the same time point. This effect was maintained even within equivalent underlying genotypes, whereby Rubie and Bmp4 transcript levels were still significantly correlated among Rubie+/− offspring only (Fig. 4C). These data strongly suggested that either the act of Rubie transcription and/or its physical RNA molecule were responsible for quantitative regulation of Bmp4 expression.
Fig. 4.
Cas9 ablation of cardiac lincRNAs in vivo and effects on local gene expression. (A) lincRNA TSS/promoter ablation strategy. TSS, transcriptional start site; tru-sgRNA, truncated single guide RNA. (B) Schematic for RT-qPCR on the anterior half of an E8.25 embryo and gDNA genotyping on the posterior half of the embryo. A, anterior; P, posterior; dotted red line indicates a bisection point; colored arrows indicate the orientation of genotyping primers; scissors, Cas9 cut sites. (C) Left: electrophoresed gDNA PCR genotyping products of Rubie alleles and quantification of the resulting Rubie and Bmp4 expression in anterior E8.25 embryos. Right: correlation between Rubie expression and Bmp4 expression for all genotypes or Rubie+/− only. (D) Electrophoresed gDNA PCR genotyping products of Handlr alleles and quantification of the resulting Handlr and Hand2 expression in anterior E8.25 embryos. (E) Electrophoresed gDNA PCR genotyping products of Atcayos alleles and quantification of the resulting Atcayos and Nmrk2 expression in anterior E8.25 embryo. (F) Electrophoresed gDNA PCR genotyping products of HrtLincR4 alleles and quantification of the resulting HrtLincR5 and Mn1 expression in anterior E8.25 embryos. (G) Electrophoresed gDNA PCR genotyping products of HrtLincR5 alleles and quantification of the resulting HrtLincR4 and Trabd2b expression in anterior E8.25 embryos. (H) Left: electrophoresed gDNA PCR genotyping products of HrtLincRX alleles and quantification of the resulting HrtLincRX and Plac1 expression in anterior E8.25 embryos. (I) IntaRNA 2.0 binding prediction between HrtLincRX 3′ miRNAs and Plac1. *P<0.05; **P<0.01; ***P<0.005; n.s., not significant; Student's two-tailed t-test. Data are mean±s.e.m. in C-H.
Despite proximity to and co-expression with Handlr, Hand2 activation was not dependent on Handlr lincRNA (or its underlying promoter DNA sequence, Fig. 4D). We speculated that the TAD architecture and CTCF boundary between these genes may introduce complex dynamics within the region. We also could not find a correlation between the expression of Mn1 and HrtLincR5 (Fig. 4E). In contrast, Nmrk2 and Trabd2b expression was dependent on Atcayos (Fig. 4F) and HrtLincR4 (Fig. 4G), respectively. Furthermore, Plac1 transcription was significantly and inversely correlated to HrtLincRX levels, whereby loss of HrtLincRX resulted in an approximately twofold increase in expression of Plac1 (Fig. 4H). However, using IntaRNA (Mann et al., 2017) analysis, we calculated stable RNA-RNA interactions between all three miRNAs constituents of its 3′ tail (miRNA-322, miRNA-351 and miRNA-503) and the 5′- and 3′-untranslated regions (UTRs) of Plac1 (Fig. 4I). Therefore, this relationship could likely be explained by the loss of inhibitory miRNA binding to Plac1 primary transcript. Nonetheless, these data indicate a potential role for HrtLincRX and/or its miRNAs in demarcating embryonic from extra-embryonic mesoderm during gastrulation and early cardiogenesis.
Cardiac lincRNAs are not required for viable mouse development
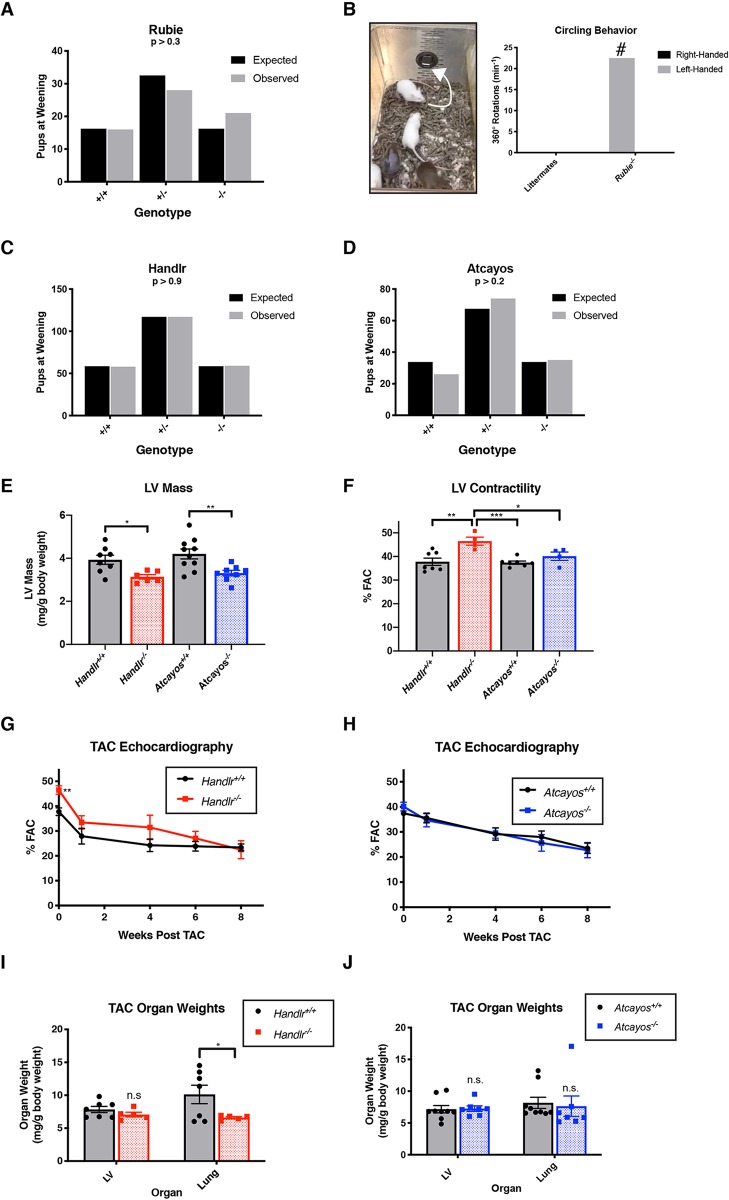
To determine the requirement of our lincRNA cohort for viable embryonic development in vivo, we bred heterozygotes for each gene and examined ratios of expected offspring that survived to weaning (Table S4). We could not establish any reduction in viability within null progeny for any tested lincRNA (Fig. 5A,C,D, Fig. S7A-C). Furthermore, homozygous offspring for these loci lived to adulthood and were fertile. However, the Rubie-null genotype did sporadically recapitulate the circling behavior described by Roberts et al., which they observed as a result of aberrant Rubie splicing in the SWR/J genetic background (Roberts et al., 2012). Although rarely observed, circling was only present in Rubie−/− mice after more than 2 years of colony breeding (Fig. 5B). Nonetheless, despite their clear expression within the developing heart, we concluded that none of the lincRNAs were individually required for viable development or fertility in the FVBn; C57BL/6j mixed background.
Fig. 5.
Viability and phenotypic effects after lincRNA knockout. (A) Offspring recovered at weaning from Rubie+/−×Rubie+/− cross versus expected Mendelian ratios. (B) Representative sporadic circling behavior in Rubie−/− offspring; #, only observed in Rubie−/− genotype over 2+ years of observation (left-handedness not exclusive in all observed cases). (C) Offspring recovered at weaning from Handlr+/−×Handlr+/− cross versus expected Mendelian ratios. (D) Offspring recovered at weaning from Atcayos+/−×Atcayos+/− cross versus expected Mendelian ratios; P-values in A, C and D derived from χ2 test. (E) LV mass calculated by echocardiographic measurements in Handlr wild-type versus null and in Atcayos wild-type versus null litter-matched adult males. (F) LV contractility measured by echocardiography in Handlr wild-type versus null and in Atcayos wild-type versus null litter-matched adult males. (G) Time course of cardiac contractility after TAC in Handlr wild-type versus null litter-matched adult males. (H) Time course of cardiac contractility after TAC in Atcayos wild-type versus null litter-matched adult males. (I) Week 8 post-TAC LV and lung weights in Handlr wild-type versus null litter-matched adult males. (J) Week 8 post-TAC LV and lung weights in Atcayos wild-type versus null litter-matched adult males. *P<0.05; **P<0.01; ***P<0.005; Student's two-tailed t-test, except I, Z-test. LV, left ventricle; FAC, fractional area shortening; TAC, transverse aortic constriction. Phenotypic data are mean±s.e.m. n=4-9 for all measurements.
Neither Handlr nor Atcayos play important roles in the cardiac stress response
Gene knockout models often require external stressors to materialize overt phenotypes. Handlr and Atcayos were the only cohort lincRNAs expressed in adult hearts, and the very high expression of Atcayos was reduced ∼50% after transverse aortic constriction (TAC)-induced cardiac hypertrophy (Fig. S7D,E) (Duan et al., 2017). Therefore, we performed TAC experiments on Handlr- and Atcayos-null mice, and compared their responses to wild-type littermates. At baseline, calculated left ventricular (LV) masses (from echocardiographic measurements) were modestly reduced in Handlr−/− and Atcayos−/− adults (−18.4%, P<0.05; −22.4%, P<0.01, respectively; Fig. 5E; Tables S5 and S6). Additionally, Handlr-null adults had slightly but significantly increased fractional area contractility (FAC) over Handlr+/+, Atcayos+/+ and Atcayos−/− genotypes (46.5% versus 37.8%, P<0.01; 37.4%, P<0.005; and 40.1%, P<0.05, respectively; Fig. 5F, Tables S5 and S6). However, neither loss of Handlr nor Atcayos could invoke a significant alteration to the LV mass increase or to the LV fractional shortening decrease after TAC-mediated stress. Of note, the severity of the TAC-response in Handlr-related experiments was greater than that for Atcayos due to genetic background and/or surgical differences for each cohort. This resulted in a sharper fractional shortening decrease and a longer duration under cardiac failure (%FAC<30%) for these mice. Consequently, Handlr+/+ males displayed greater incidence of progression into heart failure-induced lung hypertrophy versus Handlr−/− individuals (which began the experiment with greater contractile function), while less frequent lung hypertrophy arose in the Atcayos groups (Fig. 5G-J). This result was not interpreted to indicate an altered cardiac hypertrophic response in Handlr-null individuals but further supports their increased LV FAC measured at baseline. We also could not detect any noticeable changes versus wild-type in the expression of canonical hypertrophic response genes Nppa, Nppb or Acta1 due to Handlr or Atcayos knockout (data not shown). Therefore, despite strong Atcayos expression in the adult heart and the known hypertrophic involvement of Hand2 (the neighbor of Handlr) loss of these transcripts did not induce an altered response to LV pressure overload. Therefore, we concluded that neither of these lincRNAs played important roles in the physiological response to heart failure.
Compound heterozygosity reveals a genetic interaction between Rubie and Bmp4 but not between Handlr and Hand2
Despite the lack of overt lethality in lincRNA-deficient offspring, we carefully examined morphological heart development in Handlr- and Rubie-null embryos, the only conditions that produced noticeable physiological effects. We harvested E15.5 hearts and examined transverse histological sections to establish any change to chamber septation, myocardial trabeculation and/or compaction, or to ventricular outflow tract (OFT) development. Handlr−/− adults exhibited increased left ventricular fractional shortening over wild-type controls, but we could not associate this functionality with overt changes in cardiac anatomy (Fig. S8A). Owing to overlapping expression patterns between Hand2 and Handlr in the developing heart, we next tested embryos from Hand2+/−×Handlr+/− crosses to eliminate one allele of either Hand2 or Handlr per chromosome. However, neither Hand2 heterozygosity nor Hand2+/−; Handlr+/− compound heterozygosity resulted in any clear effects on heart morphogenesis (Fig. S8B,C). In addition, we did not notice any elevated lethality in Hand2+/−; Handlr+/− offspring (data not shown, n=63).
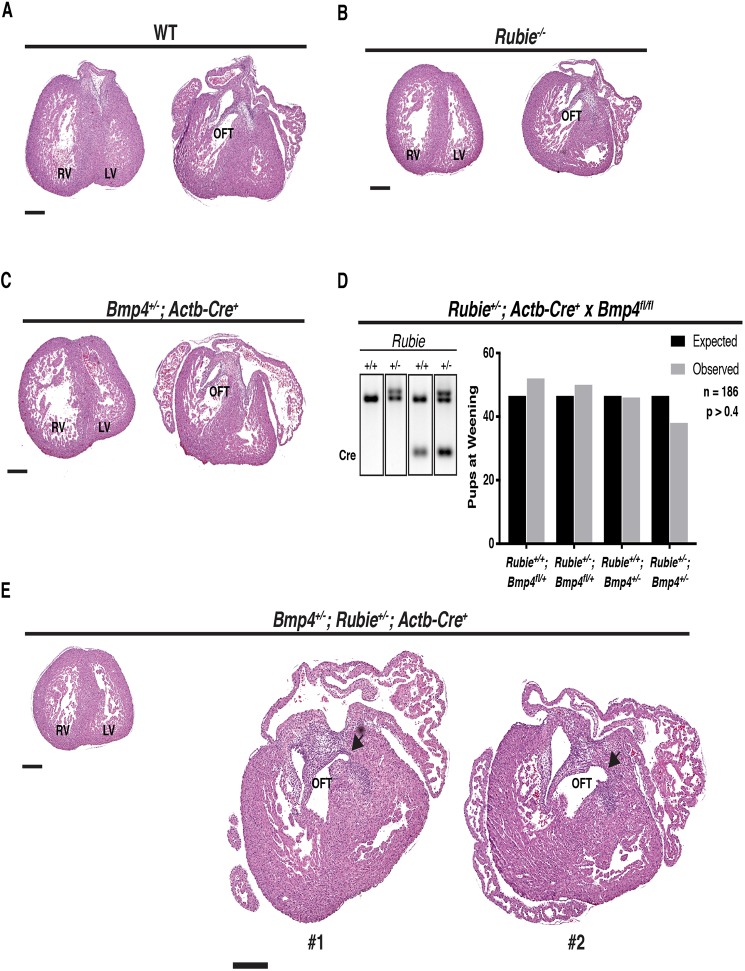
Bmp4 expression in the embryo was correlated to the amount of Rubie transcript. Numerous studies have established the requirement of the correct BMP4 dose for normal septation of the atria, ventricles and outflow tract (OFT), as well as for viable embryo development (Dunn et al., 1997; Goldman et al., 2009; Jiao et al., 2003). Therefore, we tested the hypothesis that compound haploidy of Bmp4 and Rubie together would result in an exacerbated onset of resulting phenotypes. For this, we bred Bmp4fl/fl×Rubie+/−; Actb-Cre+ (single transgene integration) in the FVB/n; C57BL/6j mixed genetic background. When we examined E15.5 hearts, neither the Rubie−/− background nor loss of a single Bmp4 allele could induce an abnormal cardiac phenotype. We also recovered these offspring in expected ratios at weaning (Fig. 6A-D). However, we did find a sustained ∼20% reduction in recovered pups carrying the Bmp4+/−/Rubie+/− compound genotype (Fig. 6D; n=186), although dramatically more breeding rounds would be required to deem this result statistically significant (n>585 needed for P<0.05 at observed lethality rate). In addition, these offspring exhibited incidences of OFT distortion out of the right ventricle beyond its typical boundary. In these cases, the origins of the pulmonary artery skewed toward the left ventricular OFT and aortic valve (Fig. 6E). Although we were unable to clearly establish communication between the pulmonary and aortic outflow systems in these instances, the data point toward a modest genetic interaction between Rubie and Bmp4 in cardiac morphogenesis.
Fig. 6.
Effect of Rubie ablation on heart development at E15.5. (A-C,E) Oblique transverse Hematoxylin and Eosin histological sections of cardiac ventricular and OFT morphogenesis at E15.5. (A) Representative wild-type morphology. (B) Representative Rubie−/− morphology. (C) Representative Bmp4+/− (Actb-Cre+) morphology. (D) Left: electrophoresed gDNA PCR genotyping products of Rubie and Actb-Cre transgene alleles. Right: offspring recovered at weaning from Rubie+/−; Actb-Cre+×Bmp4fl/fl mating versus expected Mendelian ratios. (E) Representative Bmp4+/−; Rubie+/− (Actb-Cre+) morphology in two separate individuals. RV, right ventricle; LV, left ventricle; OFT, outflow tract; WT, wild type. Scale bars: 300 µm. Arrows indicate distorted OFT orientation. P-values were obtained using a χ2 test.
DISCUSSION
With thousands of uncharacterized noncoding transcriptional elements expressed throughout the genome, efforts must be taken to better understand the functional relevance of unstudied lincRNAs. To achieve this, these experiments intended to identify and test the requirement for cardiac progenitor-specific lincRNAs in the developing embryo. Out of numerous considered annotated lncRNAs that we initially surveyed, our selection criteria led to a highly restricted set that contained epigenetically regulated promoter signatures and cardiac-specific expression in vivo. Ablation of these transcripts in the developing mouse revealed modulatory roles of Rubie, Atcayos, HrtLincR4 and HrtLincRX within their local genomic environments. In particular, we found a requirement for the Rubie locus for normal Bmp4 dose. Furthermore, loss of Handlr/Hdn and Atcayos led to minor reduction in LV mass in adult mice, along with modestly increased contractility among Handlr−/− individuals. Loss of either of these two transcripts did not influence the hypertrophic or LV contractile response to pressure overload. Most importantly, despite clear transcription in the developing heart, none of the tested lincRNAs alone were required for embryo viability. However, when we generated compound heterozygotes for Rubie and Bmp4, we observed a slight yet consistent reduction in recovered offspring and a modest perturbation of right ventricular outflow tract orientation. Of course, this cohort of lincRNAs that we ablated does not include the entirety of all relevant cardiac transcripts. Nonetheless, we argue this set of transcripts represents a sufficient consideration of the early cardiac lincRNA landscape to predict modest individual roles for most noncoding transcripts of this type.
Similar to our observations, an independent study (Ritter et al., 2019) reported that the Handlr/Hdn locus produced multiple transcripts. Namely, Hdn was described as originating from the same TSS but splicing ∼15 kb 3′ beyond Handlr, along with Hdna, which shares TSS and splice patterning (albeit one less exon) with Handlr. Hdn/Hdna was described as localized to the cytoplasm near the nuclear envelope. Our results clearly supported Handlr enrichment in the nucleus, though we did not interrogate to which subnuclear component. Most importantly, they showed that complete deletion of the >20 kb Hdn locus produced embryonic-lethal cardiac and extra-embryonic phenotypes. This accompanied perturbation of a cardiac gene expression program, including hyper-expression of Hand2. They attributed the act of transcription from the Hdn/Hdna TSS as an important regulatory component of this phenotype, whereas Hdna-specific RNA molecules were dispensable. In our work, ablation of the Handlr/Hdn/Hdna TSS, which terminated downstream transcription, resulted in no lethality, dramatic cardiac phenotype or altered Hand2 expression. Therefore, both sets of experiments agree on the lack of requirement for Handlr/Hdna as a functional lincRNA during cardiogenesis, but disagree on a link between Handlr/Hdn/Hdna transcription and Hand2 expression. Importantly, a clear regulatory mechanism/effect for transcription per se from the Handlr/Hdn/Hdna TSS has yet to be established. We predict the large genomic deletion reported by Ritter et al. (2019) affects crucial cardiac enhancer elements within the Hdn locus. Therefore, future work must more definitively assign a mechanistic role for RNA synthesis, splicing and/or molecular function at this locus before attributing them to the mild or severe phenotypes observed by either study.
The subtle effects created by ablation of our collection of cardiac lincRNAs are consistent with the results most often obtained by the efforts of others to knockout developmentally-specific lincRNAs (Amândio et al., 2016; Goff et al., 2015; Goudarzi et al., 2019; Lai et al., 2015; Nakagawa et al., 2012, 2011; Sauvageau et al., 2013; Zhang et al., 2012a). In most instances where strong phenotypes have been observed, conflicting regulatory mechanisms, in vivo versus in vitro effects, lack of in vivo reproducibility, and/or conflated DNA-/RNA-/transcription-/splicing-based mechanisms prevent clean parsing of the underlying importance of lincRNA molecules. However, several lincRNAs in our cohort did seem to function within the nucleus to impact gene expression in their local environments, including the influence of Rubie on Bmp4. Consequently, future experiments are needed to dissect the physical mechanisms that underlie these effects. We speculate that the vast majority of singular lncRNAs fit as cogs into the multifaceted regulatory architecture of the nucleus, which individually can be compensated for during organogenesis in vivo. More so we hypothesize that overt phenotypic impacts in most lincRNA-centric experiments will be observed only after additional contextual molecular components are also manipulated. It may prove more beneficial to interpret most nuclear lincRNAs as collective epigenomic components analogous to histone/DNA modifications – with potential capabilities to alter the physical properties and orientation of the genome, and recruit regulatory machinery, more so than as individual gene-like elements.
MATERIALS AND METHODS
Informatic search for cardiac lincRNAs
Raw stranded, total RNA-seq reads from ESC (day 0), MES (day 4), CP (day 5.3) and CM (day 10) stages (Wamstad et al., 2012), and cMES (day 4.75) stage (Devine et al., 2014) of mESC in vitro differentiation into cardiomyocytes were mapped to the mouse genome (mm9) and aligned to Noncode v4 (Xie et al., 2014) annotated lncRNAs using the Cufflinks suite of software (Trapnell et al., 2010). ChIP-seq domains positive for trimethylation of histone 3 lysine 4 (H3K4me3), acetylation of histone 3 lysine 27 (H3K27Ac) and trimethylation of histone 3 lysine 27 (H3K27me3) for ESC, MES, CP and CM stages were obtained from Wamstad et al. (2012). The following criteria were used to generate a candidate list of lincRNAs: (1) fewer than 0.5 fragments per kilobase per million reads (FPKM) in mESCs; (2) greater than 1.0 FPKM at the CP or cMES stage of differentiation (expression at other time points was not factored into selection); (3) positive H3K4me3 ChIP-seq signal at TSS during CP stage; (4) positive H3K27Ac at TSS during CP stage; (5) at least one exon splice in transcript; (6) no splice events into neighboring protein coding genes; and (7) TSS at least 1 kb from nearest protein-coding gene TSS. Screened candidates tracks were then visually inspected via the UCSC Genome Browser (Kent, 2002) to filter for lincRNAs with expression patterns that matched the assigned lincRNA structure and not simply spurious reads at the general locus.
Analysis of lincRNA-coding potential
PhyloCSF (Lin et al., 2011) browser tracks were uploaded to the UCSC Genome Browser for interrogation. Ficket and hexamer scores were calculated with CPAT software (Wang et al., 2013) and visualized with the ‘ggplot2’ package (Wickham, 2016) in R version 3.4.0 (R Core Team, 2017). CNCI scores were obtained from NONCODEv4 annotations (Xie et al., 2014).
mESC differentiation and cardiac progenitor cell fractionation
Directed cardiomyocyte differentiations were performed as previously described (Wamstad et al., 2012) using the Smarcd3-F6nlsEGFP mESC line (Devine et al., 2014) with minor modifications to improve differentiation efficiency. Briefly, 3 days before differentiation induction (day −3), mESCs were split into 2i+LIF media on gelatin. The following day (day −2), 2i+LIF was replaced with 15% FBS (HiClone) in DMEM, 1× non-essential amino acids, 1× sodium pyruvate 1× GlutaMAX, 1× β-mercaptoethanol, 1× penicillin/streptomycin+1000 U/ml LIF (ESGRO, EMD). The following day (day −1), cells were fed again with the same 15% FBS-LIF media to complete their conversion to epiblast-like stem cells. One day later (day 0), cardiac differentiation was initiated as per Wamstad et al. (Wamstad et al., 2012). On day 5.3, CP cells were dissociated with TrypLE (Gibco) and quenched in DMEM:F12 (Gibco)+10% FBS (HiClone). After washing in D-PBS, nuclei were isolated using the Nuclei EZ Prep kit (Sigma Aldrich), pelleted and supernatant was collected as a cytoplasmic fraction. Nuclei were washed again, and the pellet was harvested with Trizol (Invitrogen) as a nuclear fraction in subsequent qPCR quantification experiments. Cytoplasmic fractions were processed equivalently.
Whole-mount in situ hybridization
Primers were designed to amplify in situ probe templates between 440 bp and 1.5 kb for each candidate lincRNA off cDNA from the CP stage of in vitro differentiation (Table S2). Templates were electrophoresed in 1.0% agarose gel and purified using QIAquick gel extraction kit (Qiagen). These templates were then TOPO TA cloned into pCR4-TOPO using the TOPO TA cloning kit (Invitrogen) and Sanger sequenced to validate orientation in plasmid and proper composition. Linearized vector for each lincRNA template (2 µg) was then input into digoxigenin (DIG) RNA synthesis kit reactions (Roche) in a 40 µl total volume using either T7 or T3 primers, depending on template orientation. Transcription was carried out for 2 h at 37°C. Afterwards, 8 U DNase I (NEB) were added to each reaction and incubated for 15 min at 37°C to degrade DNA. DNase reactions were quenched with 1.5 µl EDTA, and DIG-RNA probes were cleaned and concentrated with RNeasy Mini Columns (Qiagen), ethanol precipitated and washed and resuspended in 20 µl H2O. DIG probes were then diluted to 100 µg/ml in HYB buffer [50% formamide, 5× SSC (pH 4.5), 50 µg/ml yeast tRNA, 75 µg/ml heparin, 0.2% Tween-20, 0.5% CHAPS, 5 mM EDTA]. E7.5 through E12.5 mouse embryos were liberated from the uterus and dissected from extra-embryonic tissues and membranes. Embryos were washed with D-PBS and fixed overnight in 4% paraformaldehyde and then washed three times in in PBT (PBS+0.1% Tween-20) on ice. Embryos were dehydrated in a methanol series (25%, 50%, 75% and twice in 100% for 5 min each). Then, samples were rehydrated by reversing this series, including two extra PBT washes. Embryos were bleached in 6% H2O2 in PBT for 15 min at room temperature with rocking. Embryos were washed for 3×5 min in PBT and treated with 10 μg/ml proteinase K for 5 min (E7.5), 10 min (E8.5), 20 min (E9.5) or 30 min (E10.5+) with rocking at room temperature and then quenched twice with 2 mg/ml glycine in PBT followed by 3×5 min washes in PBTw. Embryos were re-fixed in 4% paraformaldehyde and 0.2% glutaraldehyde for 20 min with rocking, and washed for an additional 5×5 min with PBT. Embryos were then rinsed twice in 65°C HYB buffer and incubated in HYB buffer for 3 h at 65°C. LincRNA-specific probes (in HYB) were the added to final concentration of 1 µg/ml and hybridized overnight at 65°C. Embryos were rinsed for 3×5 min at 65°C in WASH1 buffer [50% formamide, 5×SSC (pH 4.5), 1% SDS] and then incubated again for 2×30 min at 65°C in WASH1 buffer. Next, embryos were washed for 2×30 min at 65°C in WASH2 buffer [50% formamide, 2×SSC (pH 4.5), 0.1% Tween-20], followed by 3×5 min washes at room temperature in TTBS [25 mM Tris HCl (pH 7.4), 135 mM NaCl, 2.5 mM KCl, 0.1% Tween-20]. Embryos were then blocked in TTBS containing 20% sheep serum for 3 h at room temperature and stained overnight with alkaline phosphatase (AP)-conjugated anti-DIG Fab fragments in TTBS+1% sheep serum (1:5000, Roche). Embryos were then rinsed for 3×5 min in RT TTBS, followed by 6×1 h TTBS washes at room temperature. A final TTBS wash was then performed overnight at 4°C. Embryos were then washed for 2×30 min in AP buffer [100 mM Tris (pH 9.5), 50 mM MgCl2, 100 mM NaCl, 0.1% Tween-20] at room temperature. Boehringer Purple AP substrate was then added to embryos to initiate staining reactions. Reactions were allowed to progress in the dark until suitable contrast was observed. AP reactions were quenched with three PBT washes containing 1 mM EDTA, followed by multiple PBT (pH 5.5) washes. A final fixation was then performed overnight in 4% paraformaldehyde and 0.1% glutaraldehyde at 4°C. Finally, embryos were dehydrated again in methanol series and stored in 100% methanol at −20°C. Embryos were imaged on an upright microscope, and images were white balanced using Adobe Photoshop.
Cas9 lincRNA knockout, mouse husbandry and genotyping
All mouse experiments were carried out in accordance with IACUC protocols and cared for by the UCSF LARC. For each lincRNA, two cut sites were targeted to induce a 2-3 kb deletion flanking the TSS/promoter. Two sequence-specific truncated single guide RNA (tru-gRNA, Table S3 (Fu et al., 2014)) regions were separately cloned into pX330 (Addgene). After generating T7 promoter-containing sgRNA templates by PCR using Phusion TAC polymerase (NEB), tru-sgRNAs were transcribed using the Hiscribe T7 High Yield RNA Synthesis Kit (NEB). Tru-sgRNA was extracted using Trizol reagent (Invitrogen) and dual chloroform purifications before immunoprecipitating with isopropanol. Each tru-gRNA pair was then resuspended in sterile 5 mM Tris-HCl before pronuclear injection by the Gladstone transgenic mouse core. Injections were carried out as previously described (Yang et al., 2014). To increase efficiency of obtaining deletions for each target site, all pairs were co-injected into each of 70 Mus musculus FVB/n pronuclei. All genotyping was performed on tail clips stored at −20°C. To extract gDNA, tail clips were suspended in 100 µl 50 mM NaOH in H2O and incubated at 95°C for 40 min. Tubes were agitated to break up tissue, and remaining solids were allowed to settle before use. pH was normalized by addition of 7.0 µl of 1 M Tris HCl pH 7.4. 1.5 µl was then input into PCR reactions using Q5 2X master mix (NEB) and 3 gene-specific primers for simultaneous WT and KO allele amplification (Table S4). Thus, any KO allele products larger than WT reflect primer positioning and not inserted DNA sequence. Reactions were carried out according to manufacturer-specified recommendations. F0 founders for single lincRNA deletions were first identified and bred into Mus musculus C57BL/6j to establish germline transmission (F1). Separate F1 heterozygotes for each individual lincRNA deletion were then outbred into the C57BL/6j background for multiple generations to reduce off-target effects. Handlr- and Atcayos-null alleles were generated in collaboration with the Jackson Laboratory using the same targeting strategy but in a homogenous C57BL/6j background. Statistical significance of deviation from expected Mendelian ratios in offspring was analyzed using a χ2 test.
Transverse aortic constriction cardiac hypertrophy models
Surgery was performed under IACUC protocols and monitored by the UCSF Laboratory Animal Resource Center. Experiments were performed as described previously (Duan et al., 2017). For transverse aortic constriction (TAC), 12- to 20-week-old male mice were anaesthetized with ketamine/xylazine and mechanically ventilated. After thoracotomy, TAC was executed between the left common carotid and the brachiocephalic arteries using a 7-0 silk suture and 27-gauge needle. After surgery, pressure overload was confirmed by Doppler probe measurement of flow velocity at the carotid artery. Echocardiography was performed at baseline, 1 week, 4 weeks, 6 weeks and 8 weeks after the operation to measure left ventricle (LV) fractional area change (%FAC). LV areas were obtained from two-dimensional measurements at the end-diastole and end-systole. At baseline, non-biological echocardiography variability required outlier removal. Week 0 (pre-TAC) mice measured to be in cardiac failure (%FAC<30.0%) with increased % FAC 1 week after TAC were deemed failed measurements. One or two instances of this were observed in all groups. From remaining data, ‘1.5× interquartile range rule’ was used to eliminate outliers. The LV mass was estimated by M-mode measurements and the equation
from Marwick et al. (2015), where MLV is the left ventricular mass, IVSD is the diastolic interventricular septum width, LVIDD is the diastolic left ventricular internal diameter and LVPWD is diastolic left ventricular posterior wall thickness.
At 8 weeks post-surgery, mice were sacrificed for analysis. First, left ventricle, lung and body weights were measured. Subsequently, a 10-20 mg concentric short axis slice of the left ventricle was collected and preserved in RNAlater reagent (ThermoFisher). Heart sections were disrupted in PureZOL (Bio-Rad) on a TissueLyser II (Qiagen). RNA was then purified with Aurum purification kit (BioRad). qRT-PCR was performed using TaqMan chemistry, including FastStart Universal Probe Master (Roche), labeled probes from the Universal Probe Library (Roche) and gene-specific oligonucleotide primers run on a 7900HT (ThermoFisher) cycler with absolute quantification. Gene expression levels were normalized to cycloB and Actb internal controls using the ΔCt method. Statistical significance was determined by Student's two-tailed t-test (P<0.05), except for week 8 organ weights due to bimodal hypertrophy progression (P values calculated using a Z-test).
E8.25 RNA isolation and RT-qPCR analysis
At E8.25, embryos were removed from the uterus and dissected from extra-embryonic tissues and membranes. Only embryos displaying late cardiac crescent formation before heart tube expansion and cavitation were kept and deemed to be at E8.25. The anterior half of each embryo was washed twice in ice-cold PBS and transferred into Trizol (Invitrogen), while the posterior half was washed in PBS and stored at −20°C for genotyping. RNA from Trizol samples was precipitated using standard protocols and further purified/condensed using Qiagen RNeasy MinElute columns. RNA (250 ng) was reverse transcribed using the AffinityScript Reverse Transcription kit (Agilent) with 200 ng random hexamer and/or 100 ng dT20 primers, where appropriate. RT-qPCR was subsequently performed with 5.0 ng cDNA and 500 nM gene-specific primers (Table S1) in PowerUP SYBR Green master mix (Thermo Fisher). Reactions were run on a 7900HT (Thermo Fisher) cycler with absolute quantification. Gene expression levels were normalized to Actb internal controls using the ΔCt method. Statistical significance was determined by Student's two-tailed t-test (P<0.05), except for correlation analysis, which was calculated using Pearson's r statistic and N-2 degrees of freedom.
E15.5 histology
At E15.5, embryos were liberated from the uterus and dissected from extra-embryonic tissues and membranes. Whole hearts were removed, rinsed twice in D-PBS and fixed overnight in 4% paraformaldehyde. Each heart was then embedded in paraffin wax and sectioned at an oblique transverse plane for four chamber visualization. Hematoxylin and Eosin staining and imaging were performed by the Gladstone Histology Core (UCSF).
Supplementary Material
Acknowledgements
We thank Junli Zhang (Gladstone Transgenic Core) for pronuclear injection, Hazel Salunga for help with echocardiography, and the Gladstone Histology and Microscopy Core for embryo heart sectioning and histology. We are also grateful to Judy Morgan, Leslie Goodwin and Laura Reinholdt (JAX) for mouse production.
Footnotes
Competing interests
B.G.B. is a co-founder of and owns equity in Tenaya Therapeutics. M.R.G. is an employee of and owns equity in Vascugen. S.M.H. is an executive and shareholder of Amgen and a co-founder with equity stake in Tenaya Therapeutics. These interests are not related to the work described here.
Author contributions
Project design and direction: B.G.B. and M.R.G. All experiments: M.R.G. (except mouse echocardiography and surgery: Q.D. and Y.H. under the supervision of S.M.H.). QRT-PCR work: A.N. Interpretation of cardiac anatomy: I.S.K and K.R. Manuscript writing: M.R.G. and B.G.B with contribution from all authors.
Funding
This work was supported by grants from the National Institutes of Health National Heart, Lung and Blood Institute (R01HL114948 and Bench to Bassinet Program UM1HL098179) and The Younger Family Fund to B.G.B.; by the National Institutes of Health National Heart, Lung, and Blood Institute (HL127240 to S.M.H.); by the American Heart Association and a Lawrence J. and Florence A. DeGeorge Charitable Trust Predoctoral Fellowship (15PRE24470159 to M.R.G.); by grants from the Society of Pediatric Anesthesia, the UCSF Research Allocation Program, The Hellman Family Fund and the Department of Anesthesia at UCSF to I.S.K; and by the National Institutes of Health National Center for Research Resources (C06 RR018928 to the J. David Gladstone Institutes). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.185314.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.185314.reviewer-comments.pdf
References
- Alexanian M., Maric D., Jenkinson S. P., Mina M., Friedman C. E., Ting C.-C., Micheletti R., Plaisance I., Nemir M., Maison D. et al. (2017). A transcribed enhancer dictates mesendoderm specification in pluripotency. Nat. Commun. 8, 1806 10.1038/s41467-017-01804-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amândio A. R., Necsulea A., Joye E., Mascrez B. and Duboule D. (2016). Hotair is dispensible for mouse development. PLoS Genet. 12, e1006232 10.1371/journal.pgen.1006232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. M., Anderson D. M., McAnally J. R., Shelton J. M., Bassel-Duby R. and Olson E. N. (2016). Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433-436. 10.1038/nature20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carninci P., Kasukawa T., Katayama S., Gough J., Frith M. C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C. et al. (2005). The transcriptional landscape of the mammalian genome. Science 309, 1559-1563. 10.1126/science.1112014 [DOI] [PubMed] [Google Scholar]
- Charite J., McFadden D. G. and Olson E. N. (2000). The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development 127, 2461-2470. [DOI] [PubMed] [Google Scholar]
- Danesh S. M., Villasenor A., Chong D., Soukup C. and Cleaver O. (2009). BMP and BMP receptor expression during murine organogenesis. Gene Expr. Patterns 9, 255-265. 10.1016/j.gep.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar K., Pondick J. V., Kim B.-M., Zhou C., York S. R., Macklin J. A., Abualteen A., Tan B., Sigova A. A., Marcho C. et al. (2016). DIGIT is a conserved long noncoding RNA that regulates GSC expression to control definitive endoderm differentiation of embryonic stem cells. Cell Rep. 17, 353-365. 10.1016/j.celrep.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D. G. et al. (2012). The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22, 1775-1789. 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine W. P., Wythe J. D., George M., Koshiba-Takeuchi K. and Bruneau B. G. (2014). Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3, e03848 10.7554/eLife.03848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S. (2014). The four dimensions of noncoding RNA conservation. Trends Genet. 30, 121-123. 10.1016/j.tig.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Diguet N., Trammell S. A. J., Tannous C., Deloux R., Piquereau J., Mougenot N., Gouge A., Gressette M., Manoury B., Blanc J. et al. (2018). Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256-2273. 10.1161/CIRCULATIONAHA.116.026099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J. S. and Ren B. (2012). Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376-380. 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., McMahon S., Anand P., Shah H., Thomas S., Salunga H. T., Huang Y., Zhang R., Sahadevan A., Lemieux M. E. et al. (2017). BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci. Transl. Med. 9, eaah5084 10.1126/scitranslmed.aah5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. R., Winnier G. E., Hargett L. K., Schrick J. J., Fogo A. B. and Hogan B. L. M. (1997). Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev. Biol. 188, 235-247. 10.1006/dbio.1997.8664 [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57-74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant M., Farina A., Nagaraja R. and Schlessinger D. (2010). PLAC1 (Placenta-specific 1): a novel, X-linked gene with roles in reproductive and cancer biology. Prenat. Diagn. 30, 497-502. 10.1002/pd.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickett J. W. (1982). Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 10, 5303-5318. 10.1093/nar/10.17.5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Sander J. D., Reyon D., Cascio V. M. and Joung J. K. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279-284. 10.1038/nbt.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff L. A., Groff A. F., Sauvageau M., Trayes-Gibson Z., Sanchez-Gomez D. B., Morse M., Martin R. D., Elcavage L. E., Liapis S. C., Gonzalez-Celeiro M. et al. (2015). Spatiotemporal expression and transcriptional perturbations by long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA 112, 6855-6862. 10.1073/pnas.1411263112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. C., Donley N. and Christian J. L. (2009). Genetic interaction between Bmp2 and Bmp4 reveals shared functions during multiple aspects of mouse organogenesis. Mech. Dev. 126, 117-127. 10.1016/j.mod.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore-Panter S. R., Hsu J., Barnard J., Moravec C. S., Van Wagoner D. R., Chung M. K. and Smith J. D. (2016). PANCR, the PITX2 adjacent noncoding RNA, is expressed in human left atria and regulates PITX2c expression. Circulation Arrhythm. Electrophysiol. 9, e003197 10.1161/CIRCEP.115.003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M., Berg K., Pieper L. M. and Schier A. F. (2019). Individual long non-coding RNAs have no overt functions in zebrafish embryogenesis, viability and fertility. eLife 8, e40815 10.7554/eLife.40815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote P., Wittler L., Hendrix D., Koch F., Währisch S., Beisaw A., Macura K., Bläss G., Kellis M., Werber M. et al. (2013). The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24, 206-214. 10.1016/j.devcel.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Xu Y., Wang Z., Wu Y., Chen J., Wang G., Lu C., Jia W., Xi J., Zhu S. et al. (2018). A Linc1405/Eomes complex promotes cardiac mesoderm specification and cardiogenesis. Cell Stem Cell 22, 893-908.e6. 10.1016/j.stem.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Han P., Li W., Lin C.-H., Yang J., Shang C., Nurnberg S. T., Jin K. K., Xu W., Lin C.-Y., Lin C.-J. et al. (2014). A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514, 102-106. 10.1038/nature13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon C.-C., Ramilowski J. A., Harshbarger J., Bertin N., Rackham O. J. L., Gough J., Denisenko E., Schmeier S., Poulsen T. M., Severin J. et al. (2017). An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 543, 199-204. 10.1038/nature21374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Long H., Zhou C., Zheng S., Wu H., Guo T., Wu Q., Zhong T. and Wang T. (2017). Long noncoding RNA Braveheart promotes cardiogenic differentiation of mesenchymal stem cells in vitro. Stem Cell Res. Ther. 8, 4 10.1186/s13287-016-0454-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J. N., Ensminger A. W., Clemson C. M., Lynch C. R., Lawrence J. B. and Chess A. (2007). A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8, 39 10.1186/1471-2164-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S. M., Kong X. and Fant M. E. (2012). Plac1 (placenta-specific 1) is essential for normal placental and embryonic development. Mol. Reprod. Dev. 79, 564-572. 10.1002/mrd.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K., Kulessa H., Tompkins K., Zhou Y., Batts L., Baldwin H. S. and Hogan B. L. M. (2003). An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17, 2362-2367. 10.1101/gad.1124803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama S., Tomaru Y., Kasukawa T., Waki K., Nakanishi M., Nakamura M., Nishida H., Yap C. C., Suzuki M., Kawai J. et al. (2005). Antisense transcription in the mammalian transcriptome. Science 309, 1564-1566. 10.1126/science.1112009 [DOI] [PubMed] [Google Scholar]
- Kent W. J. (2002). BLAT--the BLAST-like alignment tool. Genome Res. 12, 656-664. 10.1101/gr.229202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T., Hurt D. E., Ichijo T., Nader N. and Chrousos G. P. (2010). Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3, ra8 10.1126/scisignal.2000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff C. A., Scheuermann J. C., Surface L. E., Bradley R. K., Fields P. A., Steinhauser M. L., Ding H., Butty V. L., Torrey L., Haas S. et al. (2013). Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152, 570-583. 10.1016/j.cell.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian L., Aguirre A., Sancho-Martinez I., Benner C., Hishida T., Nguyen T. B., Reddy P., Nivet E., Krause M. N., Nelles D. A. et al. (2015). Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 131, 1278-1290. 10.1161/CIRCULATIONAHA.114.013303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.-M. V., Gong G., Atanasio A., Rojas J., Quispe J., Posca J., White D., Huang M., Fedorova D., Grant C. et al. (2015). Diverse phenotypes and specific transcription patterns in twenty mouse lines with ablated LincRNAs. PLoS ONE 10, e0125522 10.1371/journal.pone.0125522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. F., Jungreis I. and Kellis M. (2011). PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 27, i275-i282. 10.1093/bioinformatics/btr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M., Wright P. R. and Backofen R. (2017). IntaRNA 2.0: enhanced and customizable prediction of RNA-RNA interactions. Nucleic Acids Res. 45, W435-W439. 10.1093/nar/gkx279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Pantoja C., Fernandez Miñán A., Valdes-Quezada C., Moltó E., Matesanz F., Bogdanović O., de la Calle-Mustienes E., Domínguez O., Taher L. et al. (2011). Genome-wide CTCF distribution in vertebrates defines equivalent sites that aid the identification of disease-associated genes. Nat. Struct. Mol. Biol. 18, 708-714. 10.1038/nsmb.2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick T. H., Gillebert T. C., Aurigemma G., Chirinos J., Derumeaux G., Galderisi M., Gottdiener J., Haluska B., Ofili E., Segers P. et al. (2015). Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur. Heart J. Cardiovasc. Imaging 16, 577-605. 10.1093/ehjci/jev076 [DOI] [PubMed] [Google Scholar]
- Mattioli K., Volders P.-J., Gerhardinger C., Lee J. C., Maass P. G., Melé M. and Rinn J. L. (2019). High-throughput functional analysis of lncRNA core promoters elucidates rules governing tissue specificity. Genome Res. 29, 344-355. 10.1101/gr.242222.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Naganuma T., Shioi G. and Hirose T. (2011). Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J. Cell Biol. 193, 31-39. 10.1083/jcb.201011110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Ip J. Y., Shioi G., Tripathi V., Zong X., Hirose T. and Prasanth K. V. (2012). Malat1 is not an essential component of nuclear speckles in mice. RNA 18, 1487-1499. 10.1261/rna.033217.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. R., Makarewich C. A., Anderson D. M., Winders B. R., Troupes C. D., Wu F., Reese A. L., McAnally J. R., Chen X., Kavalali E. T. et al. (2016). A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271-275. 10.1126/science.aad4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora E. P., Lajoie B. R., Schulz E. G., Giorgetti L., Okamoto I., Servant N., Piolot T., van Berkum N. L., Meisig J., Sedat J. et al. (2012). Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381-385. 10.1038/nature11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounzain S., Micheletti R., Arnan C., Plaisance I., Cecchi D., Schroen B., Reverter F., Alexanian M., Gonzales C., Ng S. Y. et al. (2015). CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J. Mol. Cell. Cardiol. 89, 98-112. 10.1016/j.yjmcc.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Pauli A., Norris M. L., Valen E., Chew G.-L., Gagnon J. A., Zimmerman S., Mitchell A., Ma J., Dubrulle J., Reyon D. et al. (2014). Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636-1248636 10.1126/science.1248636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A., Shawlot W., Sasaki H., Behringer R. R. and Ang S. (1999). HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development 126, 4499-4511. [DOI] [PubMed] [Google Scholar]
- Perry R. B.-T. and Ulitsky I. (2016). The functions of long noncoding RNAs in development and stem cells. Development 143, 3882-3894. 10.1242/dev.140962 [DOI] [PubMed] [Google Scholar]
- Quinn J. J. and Chang H. Y. (2016). Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47-62. 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramos A. D., Andersen R. E., Liu S. J., Nowakowski T. J., Hong S. J., Gertz C., Salinas R. D., Zarabi H., Kriegstein A. R. and Lim D. A. (2015). The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell 16, 439-447. 10.1016/j.stem.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter N., Ali T., Kopitchinski N., Schuster P., Beisaw A., Hendrix D. A., Schulz M. H., Müller-McNicoll M., Dimmeler S. and Grote P. (2019). The lncRNA locus handsdown regulates cardiac gene programs and is essential for early mouse development. Dev. Cell 50, 644-657.e8. 10.1016/j.devcel.2019.07.013 [DOI] [PubMed] [Google Scholar]
- Roberts K. A., Abraira V. E., Tucker A. F., Goodrich L. V. and Andrews N. C. (2012). Mutation of Rubie, a novel long non-coding RNA located upstream of Bmp4, causes vestibular malformation in mice. PloS one 7, e29495 10.1371/journal.pone.0029495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y. T. F., Ideue T., Sano M., Mituyama T. and Hirose T. (2009). MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc. Natl. Acad. Sci. USA 106, 2525-2530. 10.1073/pnas.0807899106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati S., Ghosh S., Jain V., Scaria V. and Sengupta S. (2012). Genome-wide analysis reveals distinct patterns of epigenetic features in long non-coding RNA loci. Nucleic Acids Res. 40, 10018-10031. 10.1093/nar/gks776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M., Goff L. A., Lodato S., Bonev B., Groff A. F., Gerhardinger C., Sanchez-Gomez D. B., Hacisuleyman E., Li E., Spence M. et al. (2013). Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife 2, e01749 10.7554/eLife.01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Soibam B., Benham A., Xu X., Chopra M., Peng X., Yu W., Bao W., Liang R., Azares A. et al. (2016). miR-322/-503 cluster is expressed in the earliest cardiac progenitor cells and drives cardiomyocyte specification. Proc. Natl Acad. Sci. USA 113, 9551-9556. 10.1073/pnas.1608256113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D., Thomas T., Lin Q., Kirby M. L., Brown D. and Olson E. N. (1997). Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat. Genet. 16, 154-160. 10.1038/ng0697-154 [DOI] [PubMed] [Google Scholar]
- Sun L., Luo H., Bu D., Zhao G., Yu K., Zhang C., Liu Y., Chen R. and Zhao Y. (2013). Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 41, e166-e166 10.1093/nar/gkt646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., Salzberg S. L., Wold B. J. and Pachter L. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511-515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wely K. H. M., Molijn A. C., Buijs A., Meester-Smoor M. A., Aarnoudse A. J., Hellemons A., den Besten P., Grosveld G. C. and Zwarthoff E. C. (2003). The MN1 oncoprotein synergizes with coactivators RAC3 and p300 in RAR-RXR-mediated transcription. Oncogene 22, 699-709. 10.1038/sj.onc.1206124 [DOI] [PubMed] [Google Scholar]
- Wamstad J. A., Alexander J. M., Truty R. M., Shrikumar A., Li F., Eilertson K. E., Ding H., Wylie J. N., Pico A. R., Capra J. A. et al. (2012). Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151, 206-220. 10.1016/j.cell.2012.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Park H. J., Dasari S., Wang S., Kocher J.-P. and Li W. (2013). CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 41, e74 10.1093/nar/gkt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Wong G. K.-S., Passey D. A. and Yu J. (2001). Most of the human genome is transcribed. Genome Res. 11, 1975-1977. 10.1101/gr.202401 [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M. and Wang E. A. (1988). Novel regulators of bone formation: molecular clones and activities. Science 242, 1528-1534. 10.1126/science.3201241 [DOI] [PubMed] [Google Scholar]
- Xie C., Yuan J., Li H., Li M., Zhao G., Bu D., Zhu W., Wu W., Chen R. and Zhao Y. (2014). NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res. 42, D98-D103. 10.1093/nar/gkt1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhang Y., Wang Q., Xu Z., Jiang J., Gao Y., Gao M., Kang J., Wu M., Xiong J. et al. (2016). Long non-coding RNA GAS5 controls human embryonic stem cell self-renewal by maintaining NODAL signalling. Nat. Commun. 7, 13287 10.1038/ncomms13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H. and Jaenisch R. (2014). Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956-1968. 10.1038/nprot.2014.134 [DOI] [PubMed] [Google Scholar]
- Zhang B., Arun G., Mao Y. S., Lazar Z., Hung G., Bhattacharjee G., Xiao X., Booth C. J., Wu J., Zhang C. et al. (2012a). The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2, 111-123. 10.1016/j.celrep.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Abreu J. G., Yokota C., MacDonald B. T., Singh S., Coburn K. L. A., Cheong S.-M., Zhang M. M., Ye Q.-Z., Hang H. C. et al. (2012b). Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 149, 1565-1577. 10.1016/j.cell.2012.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.