Abstract
Background: The percentage of papillae is a crucial criterion in differentiating noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) from papillary thyroid carcinomas (PTCs) and in subclassifying PTC into classic and follicular variant. Since the description of NIFTP, three studies have shown that the presence of any papillae may be associated with nodal metastasis, which led to modification of the NIFTP criterion from <1% papillae to no true papillae allowed. We aim at providing clinical evidence-based data on the impact that papillary growth has on nodal spread and tumor genotype in tumors previously diagnosed as encapsulated unifocal PTC.
Methods: A meticulous histopathologic examination was performed on 235 cases previously diagnosed as unifocal encapsulated PTC (U-EPTC). One hundred of these cases were subjected to BRAFV600E and NRASQ61R immunohistochemistry.
Results: In our cohort, 27 patients (12%) had lymph node metastasis (N1) at the time of initial resection. Overall, 89% of the tumors in the N1 group contained ≥50% papillae, compared with 13% in the N0/Nx group. Nodal metastases were only present in tumors with ≥1% papillae. In noninvasive U-EPTC (n = 161), N1 disease was seen only in tumors with ≥10% papillae. A higher percentage of papillae within the tumor also correlated with an increased frequency of BRAFV600E and decreased rate of NRASQ61R. None of the 26 NRAS-positive cases had nodal disease, including the invasive tumors. Among 216 patients with follow-up (median: 5.2 years), 3 patients (1.5%) had distant metastases, all detected at the initial presentation. All three tumors displayed 100% follicular growth, and capsular or vascular invasion. There was no locoregional recurrence in the entire cohort.
Conclusion: In U-EPTC, there is a strong correlation between high percentage of papillary growth, presence of nodal metastasis, and BRAF+/RAS− genotype regardless of invasive status. Nodal metastases were not seen in tumors with <1% papillae irrespective of invasive status. These findings indicate that the initial criterion of <1% papillae is still valid for the diagnosis of NIFTP. Reinstituting this criterion will spare a carcinoma diagnosis and unnecessary therapy with its side effects on patients who have negligible clinical risk.
Keywords: encapsulated variant, papillary thyroid carcinoma, papillary, follicular
Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid cancer and it has shown a dramatic increase of incidence for the past several decades (1,2). For the past 40 years, the pathologic diagnosis of PTC has been based purely on its nuclear features: (i) enlarged elongated shape; (ii) chromatin clearing; and (iii) nuclear membrane irregularity (1,3,4). On the other hand, the subtyping of PTC factors depends on several parameters, including architectural pattern (e.g., follicular variant with follicular growth pattern and conventional [classic] PTC with well-formed papillae), cytomorphology (e.g., oncocytic variant, columnar variant, hobnail variant, and tall cell variant), encapsulation (e.g., encapsulated variant surrounded completely by a tumor capsule), and size (papillary microcarcinoma that measures 1 cm or less) (1,4). In particular, and pertinent to this study, follicular variants of PTC (FVPTC) are those PTCs with “exclusively or almost exclusively follicular growth pattern” (1,3) that have been traditionally translated into a cutoff of 1% papillae to separate FVPTC (<1% papillae) and conventional PTCs with a follicular predominant growth pattern (≥1% papillae) (4).
In 2014, a comprehensive genomic analysis by The Cancer Genome Atlas (TCGA) has shown that the follicular variant and conventional PTC have distinct molecular signatures. The FVPTC is associated with RAS mutations, an RAS-like molecular signature, and is highly differentiated with a high thyroid differentiation score (TDS). The classical PTC (CPTC) has a high frequency of BRAFV600E mutations, a BRAF-like molecular signature, and a low TDS (5). Clinically, FVPTC, especially the encapsulated and noninvasive form, tends to follow a highly indolent clinical course with negligible risk of recurrence and lymph node metastasis (3,6,7). Based on these compelling clinical and molecular evidence, a subset of noninvasive encapsulated FVPTC with (near) exclusive follicular growth pattern, <30% solid growth, and ≤1% papillae was renamed as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in 2016, to highlight the indolent nature of these lesions by eliminating the word “carcinoma” from the diagnosis, with the aim of avoiding overtreatment (3,8).
However, since the publication of the NIFTP consensus paper, three studies have reported that encapsulated follicular lesions with less than 1% of true papillae and PTC nuclei have a small risk (2% to 5.5%) of lymph node metastasis (9–11). As a result, commentaries were published subsequently on behalf of the NIFTP consensus group to revise the diagnostic criteria from less than 1% of true papillae to no true papillae allowed (8,12). This revision practically means that the finding of a single papilla in an otherwise noninvasive encapsulated follicular-patterned lesion with papillary-like nuclear features will result in a diagnosis of cancer.
In this retrospective cohort of 235 patients with unifocal encapsulated PTC (U-EPTC), we aimed at studying the correlation between architectural patterns, in particular the percentage of true papillae, with the risk of nodal metastasis and underlying BRAF/NRAS alterations. Our focus was to establish a clinical evidence-based cutoff for the percentage of papillae that can be used to define NIFTP and subclassify PTC. This, in turn, would lead to a better patient stratification, potentially sparing unnecessary therapy to individuals at a negligible clinical risk.
Materials and Methods
Characteristics of the study cohort
The study was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center (MSKCC). The institutional database was searched for all cases with a diagnosis of U-EPTC operated at MSKCC between 1980 and 2015. All cases with adequate material were examined microscopically by two head and neck pathologists with special interests in thyroid neoplasia (RG and BX). Cases with multifocal carcinoma or NIFTP, thyroid carcinomas with infiltrative growth pattern, and other types of thyroid carcinoma (e.g., follicular carcinoma, Hürthle cell carcinoma, and poorly differentiated thyroid carcinoma) were excluded from the study to minimize the impact of other pathologic confounding factors in predicting outcome.
A tumor was categorized as encapsulated if it was completely surrounded by a fibrous capsule, or if it had a well-demarcated border without a definite capsule. A total of 235 cases of unifocal EPTC were included in the study. The sampling of these tumors was as follows: the entire tumor sampled, including its capsule in 158 (67%) cases, entire tumor capsular interface sampled in 19 (8%), and representative sampling with a median of 9 sections sampled per tumor (range: 1–23) in 58 (25%) cases. Among tumors with <10% papillae, 113 (66%) were entirely sampled, including their capsule, 17 (10%) had tumor capsular interface entirely sampled; and 42 (24%) were representatively sampled with a median of 9 sections per tumor (range 2–21). One hundred and twelve cases fulfilled the original diagnostic criteria of NIFTP proposed in 2016 (3).
Pathologic and clinical review
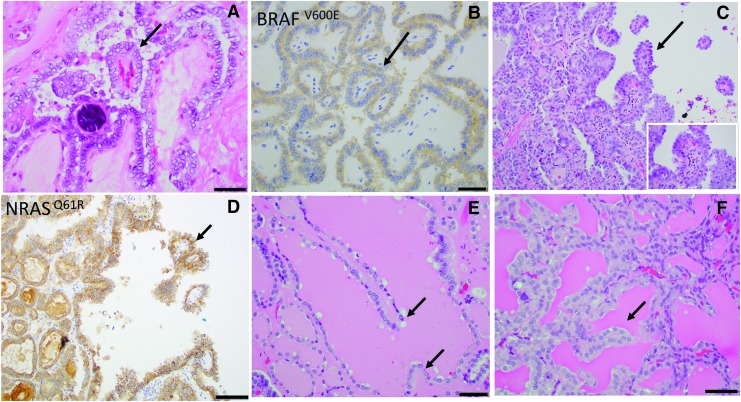
The architectural patterns and their respective percentage were collected for each tumor. The papillary pattern was defined by the presence of projections lined by lesional follicular epithelium and a well-defined fibrovascular core (“true” papillae) (Fig. 1A–D). Obliquely cut septa with fibrovascular cores and abortive papillae lacking a fibrovascular core were not included (Fig. 1E, F). It is our experience that in the day-to-day practice it may be difficult to differentiate “true” papillae from obliquely-cut septa with fibrovascular cores and abortive papillae. Further, it is not uncommon to find foci of papillary hyperplasia in follicular-patterned lesions lacking the PTC nuclear features. For the purpose of this study as stated earlier, only “true” papillae lined by lesional follicular cells and a fibrovascular core were considered (Fig. 1). For cases with 0.1% to 10% papillae, the presence and percentage of papillae were determined at consensus review sessions between BX and RG. Follicular pattern was characterized by follicles of any size (macrofollicles or microfollicles) with central colloid surrounded by follicular cells. A solid pattern consisted of areas of solid, trabecular, or insular growth.
FIG. 1.
Papillae in U-EPTC. (A, B) True papillae with fibrovascular cores (arrows) (A, H&E) in a classical PTC almost completely composed of papillae BRAFV600E-positive by immunohistochemistry (B). (C, D) An encapsulated PTC with follicular predominant growth pattern containing occasional (<1%) papillary structures (arrows). Inset shows typical PTC nuclei (C, H&E); neoplastic cells are positive for NRASQ61R by immunohistochemistry (D). (E) Pseudopapillae not fulfilling the definition of true papillae since they lack fibrovascular core (arrows) (F) Pseudopapilla not fulfilling the definition of true papillae since it lacks fibrovascular core and appears to represent an artefactually ruptured septa. H&E, hematoxylin and eosin; PTC, papillary thyroid carcinoma; U-EPTC, unifocal encapsulated PTC.
Tumor size was measured as the maximum diameter of the resected tumor specimen. Capsular invasion was defined as complete penetration of the capsule by tumor, and the number of these foci was recorded. Capsular invasion was recorded as “focal” when there were <4 foci of complete penetration of the tumor capsule and was recorded as “extensive” when the foci were ≥4. Vascular invasion (blood vessel invasion) was defined according to the criteria outlined by the Armed Forces Institute of Pathology (AFIP) fascicle (13) and the WHO classification (1). In brief, only when the invasive focus protruded into the lumen of the vessel in a polypoid manner covered by endothelial cells, or when it was attached to the vessel wall or associated with thrombus formation within an intra-capsular or extra-capsular vasculature, it was considered true vascular invasion. Vascular invasion was recorded as “focal” when there were <4 foci and was recorded as “extensive” when there were ≥4 foci.
The patient's charts were reviewed for age at diagnosis, sex, type of surgery, radioactive iodine therapy, and outcome. The primary outcome of the study was the presence (AJCC pN1) or absence (AJCC pNx or pN0) of lymph node metastases at the time of initial resection. The secondary outcomes were disease-free survival and disease-specific survival.
BRAFV600E and NRASQ61R immunohistochemistry and genotyping
Immunohistochemistry (IHC) staining for BRAFV600E and NRASQ61R was performed in a subset of 100 randomly selected cases with an anti-BRAFV600E monoclonal antibody (clone: VE1, dilution: 1:400; Abcam, Cambridge, MA), and an anti-NRASQ61R monoclonal antibody (clone: SP174, dilution: 1:25; Abcam) using the Leica Bond III system (Leica Biosystems, Inc., Buffalo Grove, IL) according to the manufacturer's recommendations. A tumor was considered as BRAFV600E or NRASQ61R positive when diffuse granular cytoplasmic stain was identified.
BRAF and RAS DNA mutation status was previously examined in 19 cases by using Thyroseq version 2 in preoperative fine needle aspiration (n = 1), Sequenom (n = 9), or MSK-IMPACT platforms (n = 9). The cases analyzed by Sequenom and MSK-IMPACT were included in previous publications from our group (14,15). Sequenom is a MassARRAY system based on matrix-assisted laser desorption/ionization time-to-flight mass spectrometry that was used to interrogate the presence of single nucleotide variation in 91 hot-spots of 8 oncogenes: EGFR, KRAS, BRAF, PIK3CA, AKT1, NRAS, MEK1, and ERBB2 (14). MSK-IMPACT is a targeted capture massive parallel sequencing platform detecting somatic genetic alterations in 410 cancer-related genes (15). The DNA mutation status was documented and correlated with the IHC results.
Statistics
All statistical analyses were performed by using the SPSS software 24.0 (IBM Corporation, New York, NY). Clinico-pathologic characteristics, in particular the percentage of each architectural pattern and the BRAF/RAS genotype determined by using IHC, were compared between cases with and without lymph node metastases by using appropriate statistical tests, that is, Chi-square test or Fisher's exact test. The prognostic significance of papillary architecture was calculated by using log rank test for disease-free survival. p-Values less than 0.05 were considered statistically significant.
Results
Results are summarized in Tables 1–4, and they are illustrated in Figures 1 and 2.
Table 1.
Clinico-Pathologic Characteristics and BRAF/NRAS Mutation in Unifocal Encapsulated Papillary Thyroid Carcinoma According to the Nodal Status at the Time of Initial Surgery
| All patients (n = 235) | N0/Nx (n = 208) | N1 (n = 27) | p-Values | |
|---|---|---|---|---|
| Architectural patterns | ||||
| Percentage of papillae | ||||
| 0% | 127 (54%) | 127 (61%) | 0 (0%) | <0.001 |
| 0.1–0.9% | 31 (13%) | 31 (15%) | 0 (0%) | |
| 1–9% | 14 (6%) | 13 (6%) | 1 (4%) | |
| 10–24% | 6 (3%) | 4 (2%) | 2 (7%) | |
| 25–49% | 5 (2%) | 5 (2%) | 0 (0%) | |
| ≥50% | 52 (22%) | 28 (13%) | 24 (89%) | |
| Percentage of follicles | ||||
| 0–24% | 52 (22%) | 31 (15%) | 21 (78%) | <0.001 |
| 25–49% | 6 (3%) | 3 (1%) | 3 (11%) | |
| ≥50% | 177 (75%) | 174 (84%) | 3 (11%) | |
| Percentage of solid growth | ||||
| 0–24% | 222 (94%) | 195 (94%) | 27 (100%) | 0.409 |
| 25–49% | 5 (2%) | 5 (2%) | 0 (0%) | |
| ≥50% | 8 (3%) | 8 (4%) | 0 (0%) | |
| BRAF and NRAS mutations (n = 100) | ||||
| BRAF/NRAS | ||||
| Positive BRAFV600E | 24 (24%) | 10 (12%) | 14 (74%) | <0.001 |
| Positive NRASQ61R | 26 (26%) | 26 (32%) | 0 (0%) | |
| Negative | 50 (50%) | 45 (56%) | 5 (26%) | |
| Other characteristics | ||||
| Sex | ||||
| Female | 156 (66%) | 141 (68%) | 15 (56%) | 0.206 |
| Male | 79 (34%) | 67 (32%) | 12 (44%) | |
| Age, median (range) | 46 (8–82) | 47 (8–82) | 36 (22–59) | <0.001 |
| Tumor size, cm, median (range) | 2.2 (0.1–7.5) | 2.3 (0.1–7.5) | 2.0 (0.3–4.7) | 0.088 |
| Type of surgery (n = 234) | ||||
| Lobectomy | 102 (44%) | 94 (45%) | 8 (30%) | 0.15 |
| Total thyroidectomy | 132 (56%) | 113 (55%) | 19 (70%) | |
| Capsular invasion | ||||
| Absent | 166 (71%) | 154 (74%) | 12 (44%) | <0.001 |
| Focal | 57 (24%) | 47 (23%) | 10 (37%) | |
| Extensive | 12 (5%) | 7 (3%) | 5 (19%) | |
| Vascular invasion | ||||
| Absent | 212 (90%) | 186 (89%) | 26 (96%) | 0.481 |
| Focal | 16 (7%) | 15 (7%) | 1 (4%) | |
| Extensive | 7 (3%) | 7 (3%) | 0 (0%) | |
| Extrathyroidal extension | ||||
| Absent | 231 (98%) | 207 (99.5%) | 24 (89%) | <0.001 |
| Present | 4 (2%) | 1 (0.5%) | 3 (11%) | |
| Margin status (n = 234) | ||||
| Negative | 234 (100%) | 207 (100%) | 27 (100%) | NA |
| Radioactive iodine (n = 222) | ||||
| No | 163 (74%) | 147 (76%) | 16 (62%) | 0.109 |
| Yes | 56 (26%) | 46 (24%) | 10 (38%) | |
| Clinical outcome (n = 219) | ||||
| Follow-up period, years, median (range) | 5.4 (0.1–28.1) | 5.4 (0.1–28.1) | 6.4 (0.1–26) | 0.796 |
| Distant metastasis | ||||
| Absent | 216 (99%) | 190 (98%) | 26 (100%) | 0.520 |
| Present | 3 (1%) | 3 (2%) | 0 (0%) | |
| Locoregional recurrence | ||||
| Absent | 219 (100%) | 193 (100%) | 26 (100%) | NA |
p-Values are obtained by using Fisher's exact test or Chi-square test for categorical variables, and log rank test for outcome (distant metastasis-free survival). Significant p-values are highlighted in bold.
The BRAF and NRAS mutational status was determined by immunohistochemistry using monoclonal antibodies for BRAFV600E and NRASQ61R in a subset of 100 randomly selected cases.
NA, not applicable.
Table 2.
Percentage of Papillae Within the Tumor, BRAF/NRAS Mutation, Nodal Metastasis, and Outcomes According to Invasion Status in Unifocal Encapsulated Papillary Thyroid Carcinoma
| Cases with invasion (capsular and/or vascular) | ||||
|---|---|---|---|---|
| All patients (n = 79) | N0/Nx (n = 64) | N1 (n = 15) | p-Values | |
| Percentage of papillae | ||||
| 0% | 27 (34%) | 27 (42%) | 0 (0%) | 0.001 |
| 0.1–0.9% | 11 (14%) | 11 (17%) | 0 (0%) | |
| 1–9% | 5 (6%) | 4 (6%) | 1 (7%) | |
| 10–24% | 3 (4%) | 2 (3%) | 1 (7%) | |
| 25–49% | 3 (4%) | 3 (5%) | 0 (0%) | |
| ≥50% | 30 (38%) | 17 (27%) | 13 (87%) | |
| BRAF/NRAS | ||||
| Positive BRAFV600E | 13 (46%) | 4 (24%) | 9 (82%) | 0.007 |
| Positive NRASQ61R | 6 (21%) | 6 (35%) | 0 (0%) | |
| Negative | 9 (32%) | 7 (41%) | 2 (18%) | |
| Follow-up period, years, median (range) | 6.7 (0.1–28.0) | 6.8 (0.1–28.0) | 5.5 (0.1–22.3) | 0.491 |
| Distant metastasis (n = 75) | ||||
| Absent | 72 (96%) | 58 (95%) | 14 (100%) | 0.451 |
| Present | 3 (4%) | 3 (5%) | 0 (0%) | |
| Noninvasive cases (capsular and/or vascular) | ||||
|---|---|---|---|---|
| All patients (n = 156) | N0/Nx (n = 144) | N1 (n = 12) | p-Values | |
| Percentage of papillae | ||||
| 0% |
100 (64%) |
100 (69%) |
0 (0%) |
<0.001 |
| 0.1–0.9% |
20 (13%) |
20 (14%) |
0 (0%) |
|
| 1–9% |
9 (6%) |
9 (6%) |
0 (0%) |
|
| 10–24% |
3 (2%) |
2 (1%) |
1 (8%) |
|
| 25–49% |
2 (1%) |
2 (1%) |
0 (0%) |
|
| 50% |
22 (14%) |
11 (8%) |
11 (92%) |
|
| BRAF/NRAS | ||||
| Positive BRAFV600E |
11 (15%) |
6 (9%) |
5 (62.5%) |
<0.001 |
| Positive NRASQ61R |
20 (28%) |
20 (31%) |
0 (0%) |
|
| Negative |
41 (57%) |
38 (59%) |
3 (37.5%) |
|
| Follow-up period, years, median (range) |
5.2 (0.1–28.1) |
5.1 (0.1–28.1) |
6.5 (0.1–26.0) |
0.340 |
| Distant metastasis (n = 144) | ||||
| Absent | 144 (100%) | 132 (100%) | 12 (100%) | NA |
The BRAF and NRAS mutational status was determined by immunohistochemistry using monoclonal antibodies for BRAFV600E and NRASQ61R in a subset of 100 randomly selected cases. Significant p-values are highlighted in bold.
Table 3.
Nodal Status and BRAF/NRAS Mutation According to the Percentage of Papillae Within the Tumor in Unifocal Encapsulated Papillary Thyroid Carcinoma
| Percentage of papillae |
||||||
|---|---|---|---|---|---|---|
| 0% | 0.1–0.9% | 1–9% | 10–24% | 25–49% | ≥50% | |
| All cases | ||||||
| pN stage | ||||||
| N0/NX | 127 (100%) | 31 (100%) | 13 (93%) | 4 (67%) | 5 (100%) | 28 (54%) |
| N1 | 0 (0%) | 0 (0%) | 1 (7%)a | 2 (33%) | 0 (0%) | 24 (46%) |
| RAS/BRAF | ||||||
| BRAFV600E | 1 (2.5%)b | 1 (4%)c | 0 (0%) | 1 (25%) | 3 (75%) | 18 (82%) |
| NRASQ61R | 15 (37.5%) | 10 (42%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Negative | 24 (60%) | 13 (54%) | 5 (83%) | 3 (75%) | 1 (25%) | 4 (18%) |
| Invasive cases | ||||||
| pN stage | ||||||
| N0/NX | 27 (100%) | 11 (100%) | 4 (80%) | 2 (67%) | 3 (100%) | 17 (57%) |
| N1 | 0 (0%) | 0 (0%) | 1 (20%)a | 1 (33%) | 0 (0%) | 13 (43%) |
| RAS/BRAF | ||||||
| BRAFV600E | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 2 (100%) | 10 (91%) |
| NRASQ61R | 1 (25%) | 5 (56%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Negative | 3 (75%) | 4 (44%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (9%) |
| Noninvasive cases | ||||||
| pN stage | ||||||
| N0/NX | 100 (100%) | 20 (100%) | 9 (100%) | 2 (67%) | 2 (100%) | 11 (50%) |
| N1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%)d | 0 (0%) | 11 (50%) |
| RAS/BRAF | ||||||
| BRAFV600E | 1 (3%)b | 1 (7%)c | 0 (0%) | 0 (0%) | 1 (50%) | 8 (73%) |
| NRASQ61R | 14 (39%) | 5 (33%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Negative | 21 (58%) | 9 (60%) | 4 (80%) | 3 (100%) | 1 (50%) | 3 (27%) |
The difference in BRAFV600E positivity and NRASQ61R positivity between cases with ≥10% papillae and those with <10% papillae is highly significant (Fisher's exact test, p < 0.001).
The percentage of papillae within this encapsulated tumor with capsular invasion was 5%; both the primary tumor and the nodal metastasis were negative for BRAFV600E and NRASQ61R.
A 0.2-cm noninvasive well-circumscribed tumor composed entirely of follicles. No true papillae were identified on multiple histologic levels examined.
A 1.5-cm noninvasive encapsulated lesion with 10% of total tumor volume showing tall cell cytomorphology.
The percentage of papillae within this noninvasive encapsulated tumor with lymph node metastasis was 10%.
Table 4.
Detailed Characteristics of Patients with Unifocal Encapsulated Papillary Thyroid Carcinoma and Distant Metastasis
| Age | Sex | Architecture | Sx | Size (cm) | CI | VI | pN | BRAF/NRAS | Molecular | FU (years) | Status at last FU | DM at presentation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 100% follicle | TT | 1.5 | Focal | Absent | N0 | NA | NA | 3.5 | DOD | Yes |
| 2 | 62 | F | 100% follicle | TT | 5.0 | Absent | Ext | Nx | NA | NA | 14.3 | AWD | Yes |
| 3 | 66 | M | 100% follicle | TT | 5.0 | Ext. | Ext | Nx | NRAS | NRASQ61R | 2.0 | DOD | Yes |
AWD, alive with disease; CI, capsular invasion; DOD, dead of disease; DM, distant metastasis; Ext, extensive; F, female; FU, follow up; M, male; NA, not available; Sx, surgery; TT, total thyroidectomy; VI, vascular invasion.
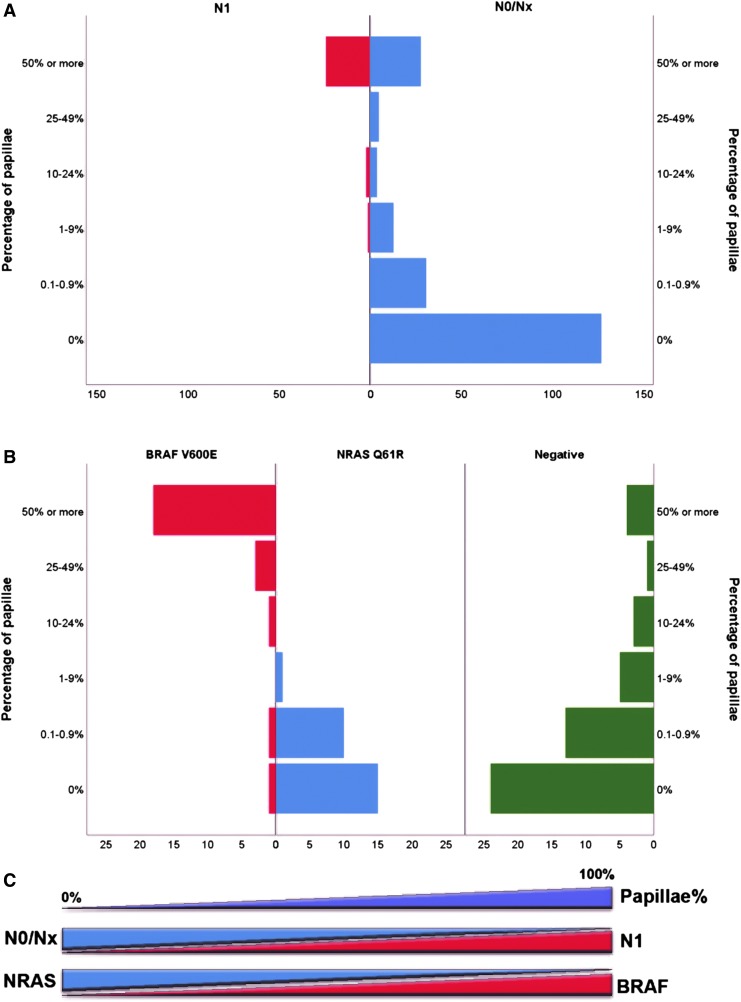
FIG. 2.
Percentage of papillae within the tumor, nodal metastasis, and BRAF/NRAS mutation. (A) Papillae within the tumor and risk of nodal metastasis. (B) Papillae within the tumor and BRAFV600E/NRASQ61R status subdivided to BRAFV600E positive, NRASQ61R positive and negative for both immunostains; on the y axis, the tumor groups are subdivided based on the percentage of papillae, and the x axis shows the number of tumors. (C) Diagram showing how an increasing percentage of papillae within the tumor is associated with a higher rate of N1 disease, BRAFV600E, and a lower frequency of NRASQ61R. The BRAF and NRAS mutational status was determined by immunohistochemistry using monoclonal antibodies for BRAFV600E and NRASQ61R in a subset of 100 randomly selected cases.
Clinico-pathologic characteristics of the study cohort
The clinico-pathologic features of the 235 patients are reported in Table 1. The female to male ratio was 2:1. The median age at diagnosis was 46 (range: 8–82). The median tumor size was 2.2 cm (range: 0.1–7.5 cm). One hundred and two patients (44%) were treated with lobectomy alone, while the remaining 132 (56%) underwent total thyroidectomy.
Seventy-nine tumors (34%) demonstrated evidence of invasion, being capsular invasion (56 out of 79, 71%), vascular invasion (10 out of 79, 13%), or both (13 out of 79, 16%). Seven tumors showed extensive vascular invasion (4 to 8 foci).
Correlation between papillary architecture and risk of lymph node metastasis at the time of initial surgery
Twenty-seven patients (12%) in our cohort had lymph node metastasis (AJCC pN1) at the time of initial resection. Compared with the patients without nodal metastases (AJCC pNx or pN0), those with nodal disease were associated with younger age (p < 0.001), had a higher prevalence of capsular invasion (66% in N1 group and 26% in N0/Nx group, p < 0.001), and of microscopic extrathyroidal extension into perithyroidal fibroadipose tissue (3 out of 77, 11% in N1 group and 1 out of 208, 0.5% in N0/Nx group, p < 0.001, Table 1). More importantly, the N1 and N0 groups were associated with different architectural patterns: 89% of the tumors in the N1 group had a predominance (≥50% of total tumor volume) of papillary architecture, whereas 75% of Nx/N0 tumors showed (near) exclusive follicular growth with 0% to <1% papillae (p < 0.001). A small percentage of tumors contained a significant amount of solid architecture: 5 cases (2%) with 25–49% and 8 (3%) with ≥50% of solid growth pattern. None of these 13 cases with significant solid growth had pN1 disease. The presence of any amount of solid pattern did not correlate with nodal status (N0/Nx vs. N1, p = 0.409).
All tumors were further classified into several categories according to the percentage of papillae within the tumor: 127 (54%) with no (0%) papillae, 31 (13%) with 0.1–0.9% of papillae, 14 (6%) had 1–9% papillae, 6 (3%) had 10–24% papillae, 5 (2%) had 25–49% papillae, and 52 (22%) had at least 50% papillae. The risk of lymph node metastasis according to the percentage of papillae within the tumor is illustrated in Table 3 and Figure 2A. None of the 158 patients with less than 1% papillae developed lymph node metastases, while 24 out of 52 tumors (46%) with at least 50% papillae had pN1 disease. The frequency of nodal metastasis in tumors with 1–9%, 10–24%, and 25–50% papillae was 7% (1 out of 13), 33% (2 out of 6), and 0% (0 out of 5), respectively.
When subdividing the tumors into those with invasion (capsular and/or vascular, n = 79) and without invasion (n = 156), there was a significant association between papillary architectural pattern and nodal metastasis regardless of the invasion status (p < 0.001, Table 2). Encapsulated PTC with ≥50% papillae had a 50% risk (11 out of 22 cases) of nodal metastasis even without invasion, whereas noninvasive encapsulated tumors with less than 10% of papillae (129 cases) did not metastasize to lymph nodes (Table 3).
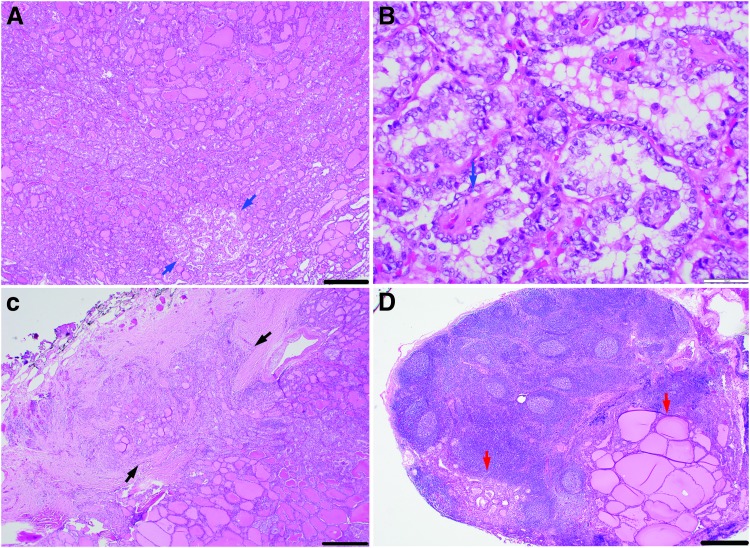
The case of U-EPTC with nodal metastasis and the lowest percentage of papillae (Fig. 3 and the case marked with superscript “C” in Table 3) had 5% papillae, had extensive capsular invasion, and measured 2.0 cm.
FIG. 3.
Microscopic pictures of a patient with a 2-cm encapsulated PTC harboring 5% papillae with capsular invasion and nodal metastases. (A) Low-power view of primary tumor with area of papillae formation (blue arrows). (B) High-power view of papillary area in A showing papillae with fibrovascular cores (blue arrow). (C) Primary tumor displaying mushrooming capsular invasion (black arrows). (D) Lymph node with metastatic deposits (red arrows). Scale bar: 500 μm in (A, C, and D), 50 μm in (B).
The cases of U-EPTC without invasion with the lowest percentage of papillae had 0.1% papillae: They included 6 tumors and measured from 2.5 to 5.0 cm.
Within each PTC group based on the percentage of papillae, the overall invasion status (capsular and/or vascular) did not have a significant impact on the risk of nodal metastasis (p ranged from 0.286 to 1.000 for each individual category, data not shown).
Phenotype-genotype correlation: increased percentage of papillae is associated with BRAFV600E
Table 3 and Figure 2B summarize the association between papillary percentage and BRAF/NRAS mutation status detected by using IHC (Fig. 1). Tumors with ≥10% papillae were predominantly BRAFV600E-related (22/30, 73%) and lack NRAS mutations (0/30, 0%), whereas PTC with <10% papillae are enriched in RAS mutations (26/70, 37%, p < 0.001). Tumors without any papillae had a high frequency (15/40, 37.5%) of NRASQ61R mutation and a low rate (1/40, 2.5%) of BRAFV600E mutation. The case positive for BRAFV600E IHC was a well-circumscribed 0.2-cm tumor without invasion that was composed entirely of follicles. No true papillae or tall cell cytomorphology were identified on multiple histologic levels examined. There was also a 1.5-cm U-EPTC without invasion showing predominant follicular growth, 0.1–0.9% papillae, and 10% area with tall cell cytomorphology that was positive for BRAFV600E IHC.
The frequency of BRAFV600E positivity increased with the increasing proportion of papillae within the tumor, being 4% in tumors with 0.1–0.9% papillae, 0% in tumors with 1–9% papillae, 25% in tumors with 10–24% papillae, 75% in tumors with 25–49% papillae, and 82% in those with ≥50% papillae. In contrast, the rate of NRASQ61R decreased. In our cohort, no tumor with ≥10% papillae was positive for NRASQ61R IHC. The difference in BRAFV600E positivity and NRASQ61R positivity between cases with ≥10% papillae and those with <10% papillae is highly significant (Fisher's exact test, p < 0.001). Tumors with ≥10% papillae are predominantly BRAFV600E-related (22/30, 73%) and lack NRAS mutations (0/30, 0%), whereas PTC with <10% papillae are enriched in RAS mutations (26/70, 37%).
BRAF and RAS genotypes were available in 19 cases that had been analyzed by mass spectrometry or next-generation sequencing before our study. All 19 cases were negative for BRAFV600E by IHC as well as by molecular testing with mass spectrometry or next-generation sequencing.
Nine of the 19 cases were positive for NRASQ61R by IHC. The molecular alterations detected in these 9 cases were NRASQ61R in four, KRASQ61R in two, and HRASQ61R in one; in two cases, no RAS mutations were detected by using the Sequenom mass spectrometry platform (14), an assay with relatively low sensitivity. Molecular testing by mass spectrometry or next-generation sequencing identified NRASQ61K, HRASQ61K, HRASG13C, and KRASG12D in four cases negative for NRASQ61R IHC.
Clinical outcome and adverse events
Follow-up data were available in 216 patients, with a median of 5.4 years (range: 0.1 to 28.1 years). There was no locoregional recurrence in the entire cohort. Three patients (1.4%) developed distant metastases and two of them died of their disease. Distant metastases were identified at the time of the initial diagnosis in all three patients (Table 4). The primary tumors were encapsulated FVPTC with exclusive follicular architecture devoid of any solid or papillary component. Two tumors had extensive vascular invasion (8 foci in each tumor), while one tumor had focal capsular invasion. One patient tested was positive for NRASQ61R mutation by IHC and by the Sequenom assay.
Discussion
FVPTC are those PTCs with an almost exclusive follicular growth pattern (1,3) that, when encapsulated, need to be distinguished from encapsulated forms of conventional PTC, which may also have a predominantly follicular growth pattern with a few papillae. Traditionally, a cutoff of 1% has been used to separate FVPTC (<1% papillae) and conventional PTCs with a follicular predominant growth pattern (≥1% papillae). The distinction is important since FVPTC has an RAS-like molecular signature and a tendency to metastasize to distant sites rather than to regional lymph nodes, whereas conventional PTC, even when encapsulated, is BRAF-like and typically metastasizes to lymph nodes first and only later to distant sites (4). The existence of full encapsulation is relatively uncommon but well documented in classic PTC, and it has been associated with a favorable clinical outcome compared with the far more common infiltrative forms of conventional PTC (16–18).
Surprisingly, however, less than a handful of studies included a systematic evaluation of the percentage of papillae formation in their study design. Liu et al. defined FVPTC as a tumor with <1% papillae, and reported an overall nodal metastasis rate of 17%, including a 5% rate of nodal metastasis in the encapsulated FVPTC, and 0% nodal metastasis in the noninvasive encapsulated forms (19). Zidan et al. (20) allowed up to 20% papillae to define FVPTC and reported a nodal metastasis rate of 22% in FVPTC.
How many papillae are there in encapsulated PTCs that behave like classic PTC that tend to develop lymph node metastasis? The ability to reliably answer this question has gained a new relevance since the introduction of the NIFTP terminology with the clinical implication of a negligible to absent risk for the patient carrying such a tumor to develop lymph node or distant metastases.
In the attempt to address the issue, Cho et al. have reported a nodal metastasis risk of 3% in noninvasive encapsulated FVPTC with rare (<1%) papillae, and 2% in those without any papillae (10). Similarly, Kim et al. (11) showed a 2% risk of lymph node metastasis in noninvasive encapsulated FVPTC with 0% papillae. However, these two studies included tumors with synchronous papillary microcarcinomas. As it is well-established that papillary microcarcinoma may give rise to nodal metastasis (21–24), the reported nodal metastasis risk in noninvasive encapsulated FVPTC may be exaggerated.
Parente et al. (9) reported a 5% risk of nodal metastasis and 1% risk of distant metastasis in unifocal noninvasive encapsulated FVPTC without any papillae. However, molecular analyses were not done in the positive cases on the primary tumor and the metastases to confirm that the metastatic deposit emanated from the identified primary tumor. Since metastatic nodal and even distant disease has been reported in total thyroidectomy specimens negative for carcinoma and entirely submitted for microscopic examination (25,26), one cannot exclude without molecular analysis that the metastatic deposits may have originated from separate infiltrative small carcinomas embedded in the paraffin block or which had undergone regression. Thus, in our opinion, these findings remain to be validated in other studies.
In contrast to the reports just cited, multiple studies, including the NIFTP consensus cohort, showed 0% risk of nodal metastasis in NIFTP, originally defined as a noninvasive encapsulated follicular-patterned lesion with <1% papillae and papillary-like nuclear features (3,6,15,27–32). Nevertheless, essentially based on the data from Cho et al. (10) and Parente et al. (9), a proposal has been made to revise the diagnostic criteria for NIFTP from <1% papillae to no true papillae (8,12).
All this recent work highlights the relevance of our study. We provide—to our knowledge for the first time—a detailed, clinical evidence-based analysis dissecting the relationship between papillary percentage, tumor behavior, and BRAF/NRAS status in encapsulated PTC.
In this study, we demonstrate a strong correlation between the percentage of papillae, risk of nodal metastasis, and underlying BRAF/RAS mutation status in encapsulated PTC. Tumors with ≥50% of papillae were associated with 46% risk of nodal metastasis and frequent (82%) BRAFV600E mutation, whereas tumors with (near) exclusive follicular growth pattern and very few (<1%) true papillae had 0% risk of nodal metastasis and were enriched with NRASQ61R (37.5%) mutations.
There may be a certain degree of subjectivity and ambiguity in defining one single true papilla and, consequently, diagnosing papillary carcinoma, and thus establishing a “cancer” diagnosis. We show that in any case—even when accounting for the occasional equivocal interpretation of histologic findings of papillae—tumors with rare (<1%) papillae are not uncommon (13% of the entire cohort) and are highly indolent with 0% risk of recurrence or lymph node metastasis. Therefore, the <1% papillae criterion as a cutoff for NIFTP appears sound for diagnostic purposes. The discrepant nodal metastatic rates between this study and the earlier mentioned three publications (9–11) could be due to two factors. First, we selected only unifocal carcinoma, while the study of Cho et al. and Kim et al. included tumors with synchronous papillary microcarcinomas. Further, a difference in the interpretation of the percentage of papillae could be an additional reason for these variable results.
We also demonstrate that there is an incremental risk of nodal metastasis according to the percentage of papillae, being 7% in those with 1–9% papillae and 46% in those with ≥50% papillae. In our cohort, invasion by itself did not predict the risk of nodal disease. Although capsular invasion is significantly more frequent in tumors with N1 disease, such an association is no longer significant when cases are substratified by the percentage of papillae within the tumor. Taken together, the percentage of papillae is an important histologic feature, and could be more relevant than invasive status in predicting the behavior of encapsulated PTC. As tumors with at least 1% papillae in an encapsulated follicular-predominant lesion are at risk of developing nodal metastasis, they are best classified as CPTC, with a follicular predominant growth pattern.
The link between papillae and BRAF/RAS signature has been previously implied by multiple studies (3,5,19,33,34). For example, the TCGA PTC study showed that tumors enriched with true papillae (e.g., classic and tall cell variants) have a high frequency (89% for tall cell and 67% for classic variant) of BRAFV600E mutation, whereas PTCs with (near) exclusive follicular growth pattern (i.e., FVPTC) are enriched with RAS mutations (38%) (5,35). The reported frequency of BRAFV600E mutations in a tumor fulfilling the original NIFTP diagnostic criteria (i.e., <1% papillae) ranges from 0% (15,30,36,37) to 8% (10,11). The 8% rate was reported by two Korean studies. The 0% rates were studies from North America and/or Europe. It is plausible to assume that variation in ethnic background may have an impact on the discrepancy. This study is the first to demonstrate a strong association between a measured percentage of papillae and BRAF/RAS alterations in encapsulated PTC.
Ten percent papillae in an encapsulated PTC appears to be associated with a genotype switch from RAS mutations to BRAFV600E mutations. Tumors with ≥10% papillae are predominantly BRAFV600E-related (73%) and lack NRAS mutations (0%), whereas PTC with <10% papillae are enriched in RAS mutations (37%). Two tumors with <1% papillae were positive for BRAFV600E, one of which contained 10% of cells showing tall cell cytomorphology, which would exclude this tumor from a diagnosis of NIFTP. As 89% to 95% of tall cell variant PTC harbor BRAFV600E mutations (5,38), the BRAFV600E immunopositivity in this tumor is explained by the presence of tall cell cytomorphology. In our cohort, among the 119 tumors meeting the original morphologic diagnostic criteria of NIFTP (i.e., <1% of papillae), one (0.8%) was positive for BRAFV600E. This tumor was a 0.2-cm well-circumscribed tumor completely devoid of papillae and tall cell cytomorphology on multiple hematoxylin and eosin levels examined. It is possible that papillae had not yet developed, given the very small size of the tumor. Whatever the reason, we fully agree with the statement by some of the authors of the NIFTP consensus group that BRAFV600E immunopositivity should lead to an exhaustive search for papillae and invasion (12). However, as stated by the same authors, it cannot be used solely to exclude NIFTP, which is a morphologic diagnosis (12).
BRAFV600E and NRASQ61R IHC have been shown to be highly sensitive and specific in detecting underlying mutations in thyroid tumors (39,40). An interesting finding of our study is that NRASQ61R IHC cross-reacts with HRASQ61R and KRASQ61R mutant proteins in thyroid carcinoma. A similar phenomenon has been previously described in medullary thyroid carcinoma (41) and melanoma (42). Regardless, we demonstrate a strong correlation between BRAF/NRAS IHC status and the route and risk of metastasis. While 14 of 24 (58%) of BRAFV600E-positive encapsulated PTC developed nodal metastasis, none of the 26 NRASQ61R IHC-positive tumors had nodal metastasis. Rather, one of the tumors with 100% follicular growth and distant metastasis at presentation was positive for NRASQ61R by IHC and genetic analysis.
The link between BRAFV600E-mutated tumors and nodal metastasis and between RAS-mutated carcinoma and distant metastasis have been previously reported by Fakhruddin et al. (43) in PTC and by Landa et al. (44) in poorly differentiated thyroid carcinoma. Given these results, BRAFV600E and NRASQ61R IHC may be utilized clinically as an additional tool to predict the route of metastasis and assist clinical decision regarding appropriate follow-up and management. In addition, these IHC stains may help in the diagnosis of tumors that are morphologically bordering on NIFTP. BRAFV600E immunopositivity, for example, should prompt extensive histopathologic examination for true papillae.
Our cohort also contains 13 tumors (6%) with a significant amount (≥25%) of solid architecture. Although none of these 13 tumors developed nodal metastases, the number is too small to draw any definite conclusion in regard to the impact of solid growth on nodal metastasis in encapsulated PTC.
The study has some limitation. First, it is a single institution study from a large tertiary cancer center and may not reflect the population at large. Second, the number of encapsulated PTC with 1% to 49% of papillae is relatively small as most of the tumors clustered at the two ends of the spectrum (<1% or ≥50% papillae).
In conclusion, in this study, we show that an increased percentage of papillae within an encapsulated PTC is associated with a high risk of nodal metastasis and BRAFV600E mutation, regardless of invasion status. U-EPTCs have an overall favorable behavior. The only patients who developed distant metastases harbored them at presentation, had 100% follicular growth, were positive for NRASQ61R, and had capsular/vascular invasion. Tumors with less than 1% papillae do not develop lymph node metastases irrespective of their invasive status. Nodal disease or recurrence are also absent in encapsulated PTC without invasion with <10% papillary growth. These findings indicate that the original criterion of <1% papillae within the tumor is still sound for a diagnosis of NIFTP. Reinstituting this criterion will spare a carcinoma diagnosis and unnecessary therapy with its side effects on patients who have a really negligible clinical risk.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
References
- 1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. 2017. WHO Classification of Tumours of Endocrine Organs. International Agency for Research on Cancer (IARC), Lyon [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. 2018. Cancer statistics, 2018. CA Cancer J Clin 68:7–30 [DOI] [PubMed] [Google Scholar]
- 3. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. 2016. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tallini G, Tuttle RM, Ghossein RA. 2017. The history of the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 102:15–22 [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson LD. 2016. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol 29:698–707 [DOI] [PubMed] [Google Scholar]
- 7. Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. 2009. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern). Thyroid 19:119–127 [DOI] [PubMed] [Google Scholar]
- 8. Lloyd RV, Asa SL, LiVolsi VA, Sadow PM, Tischler AS, Ghossein RA, Tuttle RM, Nikiforov YE. 2018. The evolving diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum Pathol 74:1–4 [DOI] [PubMed] [Google Scholar]
- 9. Parente DN, Kluijfhout WP, Bongers PJ, Verzijl R, Devon KM, Rotstein LE, Goldstein DP, Asa SL, Mete O, Pasternak JD. 2018. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: is NIFTP truly benign? World J Surg 42:321–326 [DOI] [PubMed] [Google Scholar]
- 10. Cho U, Mete O, Kim MH, Bae JS, Jung CK. 2017. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 30:810–825 [DOI] [PubMed] [Google Scholar]
- 11. Kim MJ, Won JK, Jung KC, Kim JH, Cho SW, Park DJ, Park YJ. 2018. Clinical characteristics of subtypes of follicular variant papillary thyroid carcinoma. Thyroid 28:311–318 [DOI] [PubMed] [Google Scholar]
- 12. Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, Wenig BM. 2018. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol 4:1125–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, Tallini G. 2015. Tumor of the Thyroid and Parathyroid Gland (AFIP Atlas of Tumor Pathology Series 4). American Registry of Pathology Press, Silver Spring, MD [Google Scholar]
- 14. Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. 2010. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 23:1191–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu B, Reznik E, Tuttle RM, Knauf J, Fagin JA, Katabi N, Dogan S, Aleynick N, Seshan V, Middha S, Enepekides D, Casadei GP, Solaroli E, Tallini G, Ghossein R, Ganly I. 2019. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine 64:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroder S, Bocker W, Dralle H, Kortmann KB, Stern C. 1984. The encapsulated papillary carcinoma of the thyroid. A morphologic subtype of the papillary thyroid carcinoma. Cancer 54:90–93 [DOI] [PubMed] [Google Scholar]
- 17. Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. 1985. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer 55:805–828 [DOI] [PubMed] [Google Scholar]
- 18. Evans HL. 1987. Encapsulated papillary neoplasms of the thyroid. A study of 14 cases followed for a minimum of 10 years. Am J Surg Pathol 11:592–597 [DOI] [PubMed] [Google Scholar]
- 19. Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. 2006. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer 107:1255–1264 [DOI] [PubMed] [Google Scholar]
- 20. Zidan J, Karen D, Stein M, Rosenblatt E, Basher W, Kuten A. 2003. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer 97:1181–1185 [DOI] [PubMed] [Google Scholar]
- 21. Gao X, Zhang X, Zhang Y, Hua W, Maimaiti Y, Gao Z. 2016. Is papillary thyroid microcarcinoma an indolent tumor?: a retrospective study on 280 cases treated with radioiodine. Medicine (Baltimore) 95:e5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, McCabe C, Boelaert K, Franklyn JA. 2014. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab 99:2834–2843 [DOI] [PubMed] [Google Scholar]
- 23. Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, Rosai J. 2013. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Hum Pathol 44:556–565 [DOI] [PubMed] [Google Scholar]
- 24. Tallini G, de Biase D, Durante C, Acquaviva G, Bisceglia M, Bruno R, Bacchi Reggiani ML, Casadei GP, Costante G, Cremonini N, Lamartina L, Meringolo D, Nardi F, Pession A, Rhoden KJ, Ronga G, Torlontano M, Verrienti A, Visani M, Filetti S. 2015. BRAF V600E and risk stratification of thyroid microcarcinoma: a multicenter pathological and clinical study. Mod Pathol 28:1343–1359 [DOI] [PubMed] [Google Scholar]
- 25. Xu B, Scognamiglio T, Cohen PR, Prasad ML, Hasanovic A, Tuttle RM, Katabi N, Ghossein RA. 2017. Metastatic thyroid carcinoma without identifiable primary tumor within the thyroid gland: a retrospective study of a rare phenomenon. Hum Pathol 65:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishikawa M, Toyoda N, Yonemoto T, Fujiyama A, Ogawa Y, Tokoro T, Sakaguchi N, Yoshimura M, Yoshikawa N, Tabata S, Kumazawa H, Yamashita T, Sakaida N, Okamura A, Kasagi K, Inada M. 1998. Occult papillary thyroid carcinoma in Hashimoto's thyroiditis presenting as a metastatic bone tumor. Endocr J 45:111–116 [DOI] [PubMed] [Google Scholar]
- 27. Xu B, Farhat N, Barletta JA, Hung YP, Biase D, Casadei GP, Onenerk AM, Tuttle RM, Roman BR, Katabi N, Nose V, Sadow P, Tallini G, Faquin WC, Ghossein R. 2018. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category? Endocrine 59:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA. 2017. Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 27:512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mariani RA, Kadakia R, Arva NC. 2018. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: should it also be reclassified in children? Pediatr Blood Cancer 65:e26966. [DOI] [PubMed] [Google Scholar]
- 30. Johnson DN, Furtado LV, Long BC, Zhen CJ, Wurst M, Mujacic I, Kadri S, Segal JP, Antic T, Cipriani NA. 2018. Noninvasive follicular thyroid neoplasms with papillary-like nuclear features are genetically and biologically similar to adenomatous nodules and distinct from papillary thyroid carcinomas with extensive follicular growth. Arch Pathol Lab Med 142:838–850 [DOI] [PubMed] [Google Scholar]
- 31. Shafique K, LiVolsi VA, Montone K, Baloch ZW. 2018. Papillary thyroid microcarcinoma: reclassification to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a retrospective clinicopathologic study. Endocr Pathol 29:339–345 [DOI] [PubMed] [Google Scholar]
- 32. Vivero M, Kraft S, Barletta JA. 2013. Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid 23:273–279 [DOI] [PubMed] [Google Scholar]
- 33. Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, Figge JJ, Mandel S, Haugen BR, Burman KD, Baloch ZW, Lloyd RV, Seethala RR, Gooding WE, Chiosea SI, Gomes-Lima C, Ferris RL, Folek JM, Khawaja RA, Kundra P, Loh KS, Marshall CB, Mayson S, McCoy KL, Nga ME, Ngiam KY, Nikiforova MN, Poehls JL, Ringel MD, Yang H, Yip L, Nikiforov YE. 2019. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol 5:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. 2006. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222 [DOI] [PubMed] [Google Scholar]
- 35. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson DN, Sadow PM. 2018. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum Pathol 82:32–38 [DOI] [PubMed] [Google Scholar]
- 37. Howitt BE, Chang S, Eszlinger M, Paschke R, Drage MG, Krane JF, Barletta JA. 2015. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 144:850–857 [DOI] [PubMed] [Google Scholar]
- 38. Ghossein R, Livolsi VA. 2008. Papillary thyroid carcinoma tall cell variant. Thyroid 18:1179–1181 [DOI] [PubMed] [Google Scholar]
- 39. Ghossein RA, Katabi N, Fagin JA. 2013. Immunohistochemical detection of mutated BRAF V600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. J Clin Endocrinol Metab 98:E1414–E1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oishi N, Kondo T, Vuong HG, Nakazawa T, Mochizuki K, Kasai K, Inoue T, Tahara I, Hirokawa M, Miyauchi A, Katoh R. 2016. Immunohistochemical detection of NRAS(Q61R) protein in follicular-patterned thyroid tumors. Hum Pathol 53:51–57 [DOI] [PubMed] [Google Scholar]
- 41. Reagh J, Bullock M, Andrici J, Turchini J, Sioson L, Clarkson A, Watson N, Sheen A, Lim G, Delbridge L, Sidhu S, Sywak M, Aniss A, Shepherd P, Ng D, Oei P, Field M, Learoyd D, Robinson BG, Clifton-Bligh RJ, Gill AJ. 2017. NRASQ61R mutation-specific immunohistochemistry also identifies the HRASQ61R mutation in medullary thyroid cancer and may have a role in triaging genetic testing for MEN2. Am J Surg Pathol 41:75–81 [DOI] [PubMed] [Google Scholar]
- 42. Felisiak-Golabek A, Inaguma S, Kowalik A, Wasag B, Wang ZF, Zieba S, Pieciak L, Rys J, Kopczynski J, Sarlomo-Rikala M, Gozdz S, Lasota J, Miettinen M. 2018. SP174 antibody lacks specificity for NRAS Q61R and cross-reacts with HRAS and KRAS Q61R mutant proteins in malignant melanoma. Appl Immunohistochem Mol Morphol 26:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fakhruddin N, Jabbour M, Novy M, Tamim H, Bahmad H, Farhat F, Zaatari G, Aridi T, Kriegshauser G, Oberkanins C, Mahfouz R. 2017. BRAF and NRAS mutations in papillary thyroid carcinoma and concordance in BRAF mutations between primary and corresponding lymph node metastases. Sci Rep 7:4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]