Abstract
Objective
Bevacizumab maintenance following platinum-based chemotherapy is an effective treatment for epithelial ovarian cancer (EOC), both in primary and recurrent disease. Our aim was to identify criteria to select elderly patients who can safely benefit from bevacizumab addition.
Methods
This is a case-control study on patients with primary or recurrent EOC who received platinum-based chemotherapy plus bevacizumab, between January 2015 and December 2016. Patient characteristics, treatment details and adverse events were reviewed and analyzed in 2 settings: younger (<65 years, group 1) and elderly (≥65 years, group 2). A binary logistic model was applied to correlate clinical variables and severe (grade ≥3) toxicity risk.
Results
Overall, 283 patients with EOC were included, with 72 (25.4%) older patients compared with 211 (74.6%) younger women. Bevacizumab had been administered to 234 patients (82.7%) as first-line treatment and in 49 (17.3%) with recurrent disease. At diagnosis, elderly patients presented with at least one comorbidity and were taking at least 1 medication in 84.7% and 80.6% of the cases respectively, compared with correspondingly 47.4% and 37.4% in group 1 (p<0.001). Nonetheless, the occurrence of serious (grade ≥3) adverse events did not increase among the older group. Creatinine serum levels >1.1 g/dL, estimated glomerular filtration rate (eGFR) ≤60 mL/min, ≥3 comorbidities were independently associated with a higher severe toxicity.
Conclusions
Elderly patients with EOC can safely be treated with bevacizumab; factors other than age, as higher creatinine serum levels, eGFR and number of comorbidities should be considered to better estimate bevacizumab-related toxicity risk.
Keywords: Ovarian Cancer, Bevacizumab, Toxicity, Elderly, Chemotherapy
INTRODUCTION
The incidence of cancer has been estimated to increase dramatically in the elderly population, from 1.6 million in 2010 to 2.3 million in 2030 in the United States [1]. Due to the lengthening of life expectancy, 70% of all cancer diagnoses will occur in elderly patients and, despite the substantial improvements made in treatment and prevention strategies, the number of deaths from cancer has expected to rise further [1,2,3].
Older age is a poor prognostic factor in ovarian cancer (OC), and the management of a disease that predominantly affects an “elderly” population is a challenge. At present, the standard of care of OC was defined by the results of 2 randomized trials (ICON-7 and GOG218) that established the activity of bevacizumab in combination with 3-weekly paclitaxel and carboplatin for the first line treatment [4,5]. The safety of bevacizumab was investigated in subgroup analyses from 3 international studies (AURELIA, OTILIA and ROSiA), but data on elderly population were scant, mainly because these patients are under-represented in clinical trials [6,7,8]. The studies concluded that older age should not preclude the use of antiangiogenetic drugs, although a geriatric assessment is required in order to improve the appropriate selection of patients.
The application of a geriatric assessment in oncology is becoming crucial because it is valued as an indicator of risk stratification in older cancer patients. In this scenario, clinical criteria to adequately select elderly patients who can benefit from the efficacy of bevacizumab under safe conditions are needed. The aims of this single institution retrospective study were 1) to assess the tolerability of bevacizumab; 2) to identify predictors of developing toxicity from bevacizumab administration in elderly patients affected by OC.
MATERIALS AND METHODS
To address the study purposes, we identified from our electronic database a consecutive series of patients affected by primary advanced or recurrent OC, who received a bevacizumab-based treatment, admitted at the Gynaecologic Oncology Unit, Fondazione Policlinico A. Gemelli IRCCS in Rome, between January 2015 and December 2016. This case-control study was approved by the Institutional Review Board (n° prot. approval. IST CICOG-31-10-18\123).
All patients included in the analysis should have received primary debulking surgery; no case of bevacizumab given in the neoadjuvant setting has been included. As first-line chemotherapy we administered carboplatin (area under the curve [AUC] 5) plus paclitaxel (175 mg/mq) and bevacizumab (15 mg/kg) for 6 cycles followed by 16 cycles of maintenance therapy with bevacizumab (15 mg/kg) q21, while for platinum-sensitive OC relapse we administered carboplatin (AUC 4) plus gemcitabine 800 mg/mq or paclitaxel 175 mg/mq and bevacizumab (15 mg/kg) followed by bevacizumab maintenance therapy until disease progression. Patients receiving other bevacizumab combinations in the recurrent setting were excluded.
Clinical charts were retrospectively reviewed and patient characteristics at initial diagnosis (demographics, comorbidities, Eastern Cooperative Oncology Group [ECOG] performance status [PS], tumor stage, histological type, tumor grade), surgical procedures, perioperative complications, chemotherapy treatment, and follow-up status were reported for each patient. Items for chemotherapy included the administered drugs, number of cycles, hematological and nonhematological toxicities, dose intensity, dose reductions, treatment delays, treatment discontinuation, PS according to ECOG at the end of chemotherapy, and number of chemotherapy lines administered.
In order to identify potential predictors of toxicity from bevacizumab, we considered factors generally recognized to hinder bevacizumab use, such as advanced age (≥65 years) [9], and kidney failure (creatinine serum levels >1.1 mg/dL) [10]. Other factors of interest in the field of geriatric oncologic assessment associated to worse outcomes were included such as a high comorbidity burden (≥3 comorbidities), polypharmacy, defined as the concomitant use of ≥5 drugs [11] and ECOG ≥1 [12]. The estimated glomerular filtration rate (eGFR) according to Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) [13], recently suggested to accurately predict renal impairment in the elderly with cancer [14], was also included.
Patients were divided into 2 groups according to age category: <65 years (group 1) and ≥65 years (group 2) [9] and surgical and medical treatments with the related complications and toxicities were analyzed according to patient age cut-off value. Chi-square or Fisher's exact test were used for comparison of categorical variables. A binary logistic model was applied to determine the effect of independent variables as previously defined on the risk of toxicity. Regarding survival analysis, progression free survival (PFS) was defined as the time elapsed between the date of diagnosis (staging laparoscopy) and recurrence. Medians and life tables were computed using the product limit estimate by the Kaplan-Meier method, and the log-rank test was used to assess statistical significance.
In addition to standard statistics, a decision tree methodology was applied to define significant clinical predictors of severe toxicity rate. The following predictor variables were used: ECOG, age, high grade serous OC, grade, comorbidities, BMI and creatinine. The dependent variable referred to severe toxicity (no or yes). An algorithmic rule — that splits group into 2 groups that are internally as similar as possible — was used to decide which variable to split and which splitting value to take at each step of the tree's construction [15]. To create the decision-tree the part package was used and the minimum error rule (size producing the minimum cross-validation error) was applied to determine the optimal tree size.
All statistical calculations were carried out using SPSS 20.0 for Mac (SPSS Inc., Chicago, IL, USA) and RStudio-0.98.1091 software.
RESULTS
The study population included 283 women, receiving a bevacizumab containing treatment for primary advanced or recurrent epithelial OC. Mean age of the overall population was 55 years (±11.1, standard deviation), with 211 patients (74.6%) younger than 65 years (group 1) and 72 older patients (group 2). The majority of patients presented with FIGO stage IIIC at diagnosis (183; 68%) and with a serous histotype (248; 92.5%). Stage and histotype distribution were homogeneous among older and younger patients (Table 1).
Table 1. Clinical characteristic of the overall population and according to age.
| Variable | All cases | Group 1 (age <65 yr) | Group 2 (age ≥65 yr) | p-value | |
|---|---|---|---|---|---|
| Patients | 283 | 211 (74.6) | 72 (25.4) | - | |
| Mean age (yr) | 55.6±11.1 | 51±8.6 | 70±3.0 | 0.001 | |
| ECOG PS | 0.001 | ||||
| 0 | 259 (91.5) | 203 (96.2) | 56 (77.8) | ||
| 1 | 24 (8.5) | 8 (3.8) | 16 (22.2) | ||
| Comorbidities* | 0.001 | ||||
| 0 | 122 (43.1) | 111 (52.6) | 11 (15.3) | ||
| 1 | 88 (31.1) | 69 (32.7) | 19 (26.4) | ||
| 2 | 45 (15.9) | 21 (10.0) | 24 (33.3) | ||
| 3+ | 28 (9.9) | 10 (4.7) | 18 (25.0) | ||
| Comorbidities type | |||||
| Hypertension | 83 (29.3) | 44 (20.9) | 39 (54.2) | 0.001 | |
| Diabetes | 10 (3.5) | 4 (1.9) | 6 (8.3) | 0.020 | |
| Obesity | 5 (1.8) | 4 (1.9) | 1 (1.4) | 0.623 | |
| CV disease | 38 (13.4) | 20 (9.5) | 18 (25.0) | 0.001 | |
| GI disease | 18 (6.4) | 10 (4.7) | 8 (11.1) | 0.056 | |
| Pulmonary | 9 (3.2) | 2 (0.9) | 7 (9.7) | 0.001 | |
| Neurological | 25 (8.8) | 12 (5.7) | 13 (18.1) | 0.001 | |
| Other cancer | 0 | 0 | 0 | - | |
| Mean CR serum levels | 0.75±0.16 | 0.73±0.15 | 0.82±0.18 | 0.010 | |
| Mean eGFR CDK-EPI | 88.68±18.01 | 93.15±16.83 | 75.58±14.70 | 0.001 | |
| Mean of assumed drugs | 1.10±1.65 | 0.70±1.18 | 2.28±2.18 | 0.001 | |
| Histotype† | 0.591 | ||||
| HGS | 248 (92.5) | 186 (91.6) | 62 (95.4) | ||
| Endometrioid | 12 (4.5) | 10 (4.9) | 2 (3.1) | ||
| Other | 8 (3.0) | 7 (3.5) | 1 (1.5) | ||
| Tumor grade | 0.243 | ||||
| G1 | 7 (2.6) | 7 (3.4) | 0 | ||
| G2 | 7 (2.6) | 6 (3) | 1 (1.5) | ||
| G3 | 254 (94.8) | 190 (93.6) | 64 (98.5) | ||
| FIGO stage‡ | 0.267 | ||||
| I–II | 11 (3) | 9 (4.4) | 2 (3) | ||
| III–IV | 264 (93) | 198 (95.6) | 66 (97) | ||
| Surgical approach at primary surgery | 0.001 | ||||
| PDS | 191 (68) | 156 (74) | 35 (51) | ||
| NACT+IDS | 92 (32) | 55 (26) | 37 (49) | ||
| Perioperative complications§ | |||||
| Abscess | 1 (0.3) | 0 | 1 (1.5) | 0.082 | |
| Dehiscence | 2 (0.7) | 1 (0.4) | 1 (1.5) | 0.426 | |
| Sub-occlusion | 3 (1) | 3 (1.4) | 0 | 0.305 | |
| Sepsis | 2 (0.7) | 1 (0.4) | 1 (1.5) | 0.426 | |
| Bleeding | 1 (0.3) | 0 | 1 (1.5) | 0.082 | |
| Temporary/permanent stoma | 0.067 | ||||
| No | 242 (85) | 185 (88) | 57 (79) | ||
| Yes | 41 (15) | 26 (12) | 15 (21) | ||
| Chemotherapy regimen | 0.035 | ||||
| CBDCA-PTX-BEV (1st line) | 234 (82.7) | 168 (79.6) | 66 (91.7) | ||
| CBDCA-PTX-BEV (2nd line) | 12 (4.2) | 12 (5.7) | 0 (0.0) | ||
| CBDCA-GEM-BEV (2nd line) | 37 (13.1) | 31 (14.7) | 6 (8.3) | ||
Values are presented as number (%) or mean±standard deviation.
BEV, bevacizumab; CBDCA, carboplatin; CR, caloric restriction; CV, cardiovascular; ECOG PS, Eastern Cooperative Oncology Group performance status; eGFR CDK-EPI, estimated glomerular filtration rate according to Chronic Kidney Disease Epidemiology Collaboration equation [13]; FIGO, International Federation of Gynecology and Obstetrics; GEM, gemcitabine; GI, gastrointestinal; HGS, high-grade serous; NACT+IDS, neoadjuvant chemotherapy followed by interval debulking surgery; PDS, primary debulking surgery; PTX, paclitaxel.
*Patients might have experiences more than 1 comorbidity; †Data of 15 patients were missing; ‡Data of 8 patients were missing; §Within 30 days after surgery.
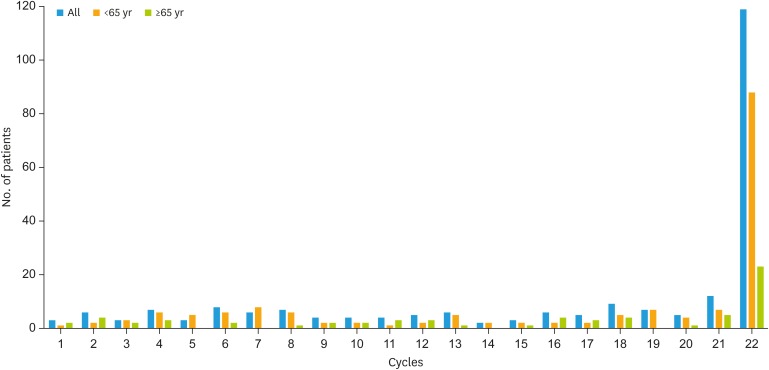
The majority of patients (234, 82.7%) received carboplatin plus paclitaxel plus bevacizumab for 6 cycles, followed by maintenance therapy as first-line chemotherapy; treatment schedules are reported in Table 1. Among 49 patients who received bevacizumab at recurrence, 12 (5.4%) had antiangiogenic treatment both at first and second line; they were all younger than 65 years of age. Among those receiving bevacizumab in the first-line setting, median duration of therapy was 16 cycles (range 1–22), without differences age-related (17 cycles in the younger group compared with 15 cycles in the older, p=0.09) (Fig. 1). Similarly, no difference was found in the recurrent setting, with a median of 20 cycles received in group 1, compared with 22 in group 2 (p=0.57).
Fig. 1. Summary of treatment exposure to bevacizumab maintenance in the first-line setting, in the all population and according to age.
Overall, 161 of 283 (56.9%) patients had at least one comorbidity (range 1–6), and 126 (51.2%) did not assume any drug. Older patients presented with at least 1 comorbidity (61; 84.7%) and 18 (25%) had 3 or more comorbidities, compared with 10 (4.7%) in the younger population. Hypertension was the most reported comorbidity followed by cardiovascular disease among the older patients (54.2% and 25% of patients, respectively) (Table 1).
Accordingly, the number of assumed drugs was higher among the elderly patients, with 58 (80.6%) of them taking at least 1 medication and 28 (38.9%) taking 3 or more drugs, compared with 79 (37.4%) of the younger patients who received at least 1 medication (p<0.001).
Creatinine serum levels and eGFR values were significantly higher and lower in the elderly group than in the younger population (p<0.01 and p<0.001, respectively, Table 1). Although most of the patients had ECOG PS equal to zero, a significantly higher number of the elderly had grade 1 compared to younger patients (16; 22.2% vs. 8; 3.8%; p<0.001) (Table 1).
Twenty-seven (9.5%) patients reported grade 3 or 4 (G3/G4) toxicity in the whole population (Table 2), with 8 patients having more than one adverse event of grade 3 or worse. The most frequent adverse event reported was proteinuria (8, 2.8%), followed by gastrointestinal toxicity (7, 2.4%), venous thromboembolic event (6, 2.1%) and hypertension (6, 2.1%). No significant difference regarding type and number of toxicities were found between different age groups. Among the overall population and the age groups, 10 (4.7%, group 1) and 7 (9.7%, group 2) of women discontinued due to treatment related adverse events (p=0.355) (Table 2). None of patients in both groups discontinued due to worsening of pre-existing comorbidities.
Table 2. Adverse events G3/G4 among patients and according to age.
| Variable | All cases | Group 1 (age <65 yr) | Group 2 (age ≥65 yr) | p-value | |
|---|---|---|---|---|---|
| Patients | 283 | 211 (74.6) | 72 (25.4) | - | |
| Patients with G3/G4 AEs | 27 (9.5) | 19 (9.0) | 8 (11.1) | 0.599 | |
| BEV related G3/G4 AEs* | |||||
| Hematologic | 5 (1.97) | 4 (1.9) | 1 (1.4) | 0.631 | |
| Gastrointestinal (bowel perforation, subocclusion, volvulus) | 7 (2.4) | 6 (2.8) | 1 (1.4) | 0.684 | |
| Venous thromboembolic events | 6 (2.1) | 4 (1.9) | 2 (2.8) | 0.643 | |
| Arterial thromboembolic events/stroke/ictus | 1 (0.3) | 1 (0.5) | 0 | 0.748 | |
| Hypertension | 6 (2.1) | 5 (2.3) | 1 (1.4) | 0.535 | |
| Proteinuria | 8 (2.8) | 4 (1.9) | 4 (5.6) | 0.112 | |
| Chronic kidney failure† | 8 (2.8) | 3 (1.4) | 5 (7) | 0.627 | |
| Bleeding | 1 (0.3) | 1 (0.4) | 0 | 0.554 | |
| Wound healing complication | 3 (1) | 2 (0.9) | 1 (1.4) | 0.753 | |
| Fistula/abscess | 1 (0.3) | 0 | 1 (1.4) | 0.082 | |
| Congestive heart failure | 0 | 0 | 0 | NA | |
| Posterior reversible encephalopathy syndrome | 0 | 0 | 0 | NA | |
| Arthralgia | 1 (0.3) | 0 | 1 (1.4) | 0.082 | |
| Liver (high liver enzymes) | 1 (0.3) | 1 (0.4) | 0 | 0.554 | |
| Main reason for BEV discontinuation | |||||
| Disease progression | 96 (34) | 70 (33.1) | 26 (36.1) | 0.488 | |
| Treatment related AEs | 17 (6) | 10 (4.7) | 7 (9.7) | 0.355 | |
| Worsening comorbidity | 0 | 0 | 0 | NA | |
| Other | 1 (0.3) | 1 (0.5) | 0 | 0.956 | |
Values are presented as number (%).
BEV, bevacizumab; AE, adverse event; eGFR CDK-EPI, estimated glomerular filtration rate according to Chronic Kidney Disease Epidemiology Collaboration equation.
*Patient might have more than 1 AE; †Expressed as eGFR CDK-EPI <60 mL/min.
Among patients receiving carboplatin plus paclitaxel plus bevacizumab, 12 of them had previously received bevacizumab; these patients were younger than 65 years of age and none of them developed G3/G4 toxicity.
A logistic regression taking into account variables showing a statistically significant difference between young and elderly patients, was performed, to evaluate their association with the development of G3/G4 toxicity (Table 3). Interestingly, creatinine serum levels higher than 1.1 mg/dL (odds ratio [OR]=12.68; 95% confidence interval [CI]=2.92–55.07), eGFR <60 mL/min (CKD-EPI) (OR=10.35; 95% CI=3.66–29.31), presence of 3 or more comorbidities (OR=6.60; 95% CI=1.87–23.36) were independently associated with a higher risk of developing G3/G4 toxicity. Conversely, neither being older than 65 years of age nor being on polypharmacy regimen were associated to toxicity.
Table 3. Logistic regression of factors predictive of G3/G4 adverse events.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age ≥65 yr | 0.52 | 0.16–1.68 | 0.278 |
| ECOG PS ≥1 | 2.18 | 0.50–9.47 | 0.298 |
| Comorbidies number ≥3 | 6.60 | 1.87–23.36 | 0.003 |
| Creatinine serum levels ≥1.1 mg/dL | 12.68 | 2.92–55.07 | 0.001 |
| eGFR CDK-EPI <60 mL/min | 10.35 | 3.66–29.31 | 0.001 |
| Polypharmacy | 1.57 | 0.25–8.76 | 0.799 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; eGFR CDK-EPI: estimated glomerular filtration rate according to Chronic Kidney Disease Epidemiology Collaboration equation; OR, odds ratio.
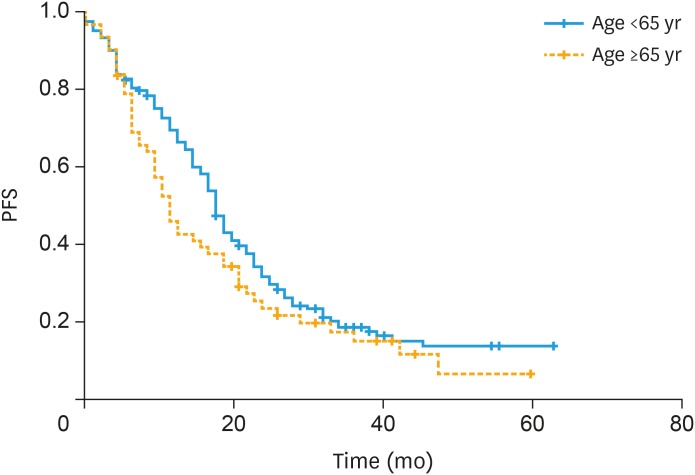
Among those patients receiving bevacizumab maintenance in the first-line setting, median PFS was 17 vs. 11 months in patients younger than 65 years vs. older, respectively (p=0.09; Fig. 2). Similarly, median PFS among those receiving bevacizumab in the recurrent setting was similar among groups (12 vs. 14 months, in patients younger than 65 years vs. older, respectively, p=0.87).
Fig. 2. PFS in patients receiving bevacizumab maintenance in the first-line setting: median PFS in group 1 (<65 years) 17 months vs. 11 months in group 2 (≥65 years) (p=0.09).
PFS, progression free survival.
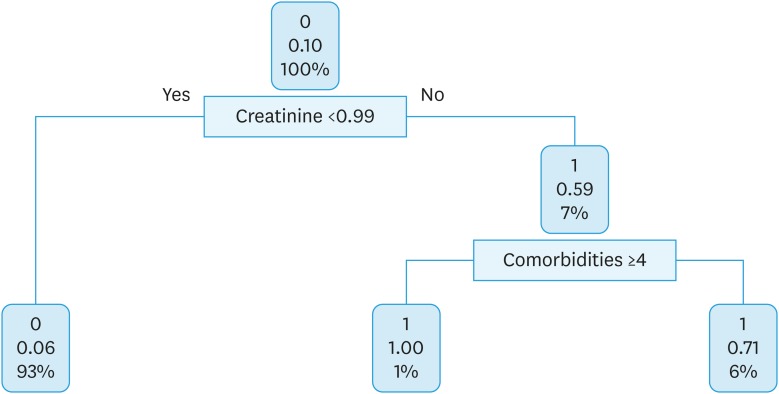
At this point, in order to produce a more useful classification, a decision tree algorithm has been proposed for the entire population. Creatinine level and comorbidities were of relative importance to predict severe toxicity in OC patients who received platinum-based chemotherapy plus bevacizumab. The split at the top of the tree resulted in 2 large branches: the left-hand branch included patients with creatinine level <0.99 (93% of the overall sample, with 6% probability of severe toxicity); the right-hand branch corresponded to creatinine level ≥0.99 (7% of the overall sample, with 59% probability of severe toxicity). The right branch is further subdivided, in one step, by comorbidities (<4 vs. ≥4). Details are shown in Fig. 3.
Fig. 3. Classification tree for ovarian cancer patients who received platinum-based chemotherapy plus bevacizumab.
DISCUSSION
In the present study, we found that bevacizumab is not associated with an increase in G3/G4 toxicity among the elderly, suggesting that age per se is not a predictive factor of adverse events for those receiving bevacizumab.
Data about the use of bevacizumab in the specific setting of old patients, are available from the ROSiA study [7], which evaluated an extended duration of bevacizumab (up to 24 months) as frontline therapy for OC, and the single-arm OTILIA trial [8] assessing the safety and efficacy of carboplatin-paclitaxel plus bevacizumab in routine oncology practice. Although both agreed bevacizumab can be safely considered in the frontline treatment irrespective of age, our real-life study shows that its use is rarely considered in patients older than 65 years old.
In order to avoid exclusion of older patients from bevacizumab therapy, we then investigated some specific and simple clinical elements that can be considered when choosing chemotherapy schedule for OC patients. Indeed, in our study population, even if the number of comorbidities, number of assumed drugs, ECOG PS or serum creatinine level were reasonably higher among patients older than 65 years, G3/G4 toxicity were similar between the groups and the main reason for bevacizumab interruption was disease progression.
Results suggest that presence of renal dysfunction (as defined by creatinine serum levels ≥1.1 mg/dL or eGFR <60 mL/min according to CKD-EPI equation) and a higher comorbidity burden (≥3) are significantly associated with G3/G4 toxicity in women receiving bevacizumab in maintenance (Table 3). In particular, the CKD-EPI equation, which was shown to give a more accurate GFR estimation of renal function, without additional laboratory costs, compared with other GFR measures [13] is one of the strongest predictive factors of developing G3/G4 toxicity. Interestingly, none of the 12 patients receiving 2 chemotherapy lines with bevacizumab complained of G3/G4 toxicity at second line treatment, confirming that it is a reasonable choice even as a rechallenge [16].
Given the retrospective observational nature of our study, several limitations should be noted. Nonetheless, we have also tried to overcome this issue by a decision tree analysis which further confirmed the limited value of age in the decision-process of elderly patients suitable for bevacizumab. Of course, results should be confirmed in a controlled prospective trial and further machine learning algorithms should be developed in order to identify predictive features of side effects in this setting of patients.
Furthermore, because patients with poorer ECOG PS might, in general, be less likely to receive bevacizumab in first-line treatment and more likely to experience adverse events, and as we were unable to control for factors that might have influenced treatment decision-making, our findings could have underestimated the relative risk of bevacizumab use.
For the same reason, other geriatric parameters such as physical performance or cognitive impairment have not been assessed. To verify if older patients are suitable for a specific chemotherapy, a comprehensive assessment with validated tools should be mandatory, in order to identify vulnerable patients with higher risk of toxicities [17]. Of course, clinical and laboratoristic parameters should be evaluated but, in the era of personalized cancer care, throughout the use of validated measures (Comprehensive Geriatric Assessment), the oncologist should become able to perform specific tests to determine a personalized antineoplastic treatment for elderly patients [18,19].
In conclusion, the use of bevacizumab is extremely common in OC treatment for both first- and second-line treatment and our findings suggest that its use in the elderly population should be considered as safe and manageable. Some parameters such as higher creatinine serum levels, lower eGFR according to CKD-EPI equation and number of comorbidities might be of help when establishing the risk of bevacizumab-related toxicities. We believe that these simple rules will be particularly useful in the era of combination of different target treatments, where toxicity profile can be unexpected especially in older women. Further studies including a more in-depth assessment of the geriatric population are awaited to further personalized OC treatment in the elderly.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: F.A., A.G., F.D., C.G.
- Data curation: A.G., B.C., D.S.M., S.F.
- Formal analysis: M.C., V.E.R., F.A.
- Investigation: M.C., V.E.R., F.A.
- Supervision: F.A., S.G.
- Writing - original draft: A.G., M.C., F.A.
- Writing - review & editing: F.A., M.C., F.D.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures, 2017 [Internet] Atlanta, GA: American Cancer Society; 2017. [cited 2019 Mar 28]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Google Scholar]
- 3.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 5.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 7.Selle F, Colombo N, Korach J, Mendiola C, Cardona A, Ghazi Y, et al. Safety and efficacy of extended bevacizumab therapy in elderly (≥70 Years) versus younger patients treated for newly diagnosed ovarian cancer in the international ROSiA study. Int J Gynecol Cancer. 2018;28:729–737. doi: 10.1097/IGC.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 8.Mustea A, Wimberger P, Oskay-Oezcelik G, Jungberg P, Meinerz W, Reichert D, et al. Impact of age on the safety and efficacy of bevacizumab (BEV)-containing therapy in patients(pts) with primary ovarian cancer (OC): analyses of the OTILIA German non-interventional study on behalf of the North-Eastern German Society of Gynaecological Oncology Ovarian Cancer Working Group. Ann Oncol. 2016;27:vi296–vi312. [Google Scholar]
- 9.Kim J, Chang Y, Kim TJ, Lee JW, Kim BG, Bae DS, et al. Optimal cutoff age for predicting prognosis associated with serous epithelial ovarian cancer: what is the best age cutoff? J Gynecol Oncol. 2019;30:e11. doi: 10.3802/jgo.2019.30.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin R, Grunkemeier GL, Brown JR, Furnary AP. Estimated glomerular filtration rate and renal function. Ann Thorac Surg. 2008;86:1–3. doi: 10.1016/j.athoracsur.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7:346–353. doi: 10.1016/j.jgo.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusco D, Allocca E, Rocco Villani E, Franza L, Laudisio A, Colloca G. An update in breast cancer management for elderly patients. Transl Cancer Res. 2018;7:S319–28. [Google Scholar]
- 13.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SY, Lee KW. Renal assessment using CKD-EPI equation is useful as an early predictor of contrast- induced nephropathy in elderly patients with cancer. J Geriatr Oncol. 2017;8:44–49. doi: 10.1016/j.jgo.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Efron B, Hastie T. Computer age statistical inference: algorithms, evidence, and data science. New York: Cambridge University Press; 2016. Chapter 8.5: notes and details; pp. 125–128. [Google Scholar]
- 16.Pignata S, Lorusso D, Florence J, Gallo C, Colombo N, Sessa C, et al. Chemotherapy plus or minus bevacizumab for platinum-sensitive ovarian cancer patients recurring after a bevacizumab containing first line treatment: The randomized phase 3 trial MITO16B-MaNGO OV2B-ENGOT OV17. J Clin Oncol. 2018;36 suppl:5506. [Google Scholar]
- 17.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 18.Tortorella L, Vizzielli G, Fusco D, Cho WC, Bernabei R, Scambia G, et al. Ovarian cancer management in the oldest old: improving outcomes and tailoring treatments. Aging Dis. 2017;8:677–684. doi: 10.14336/AD.2017.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. 2017;9:CD006211. doi: 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]