Abstract
Counterconditioning (CC) is a form of retroactive interference that inhibits expression of learned behavior. But similar to extinction, CC can be a fairly weak and impermanent form of interference, and the original behavior is prone to relapse. Research on CC is limited, especially in humans, but prior studies suggest it is more effective than extinction at modifying some behaviors (e.g., preference or valence ratings) than others (e.g., physiological arousal). Here, we used a within-subjects design to compare the effects of aversive-to-appetitive CC versus standard extinction on two separate tests of long-term memory in human adults: implicit physiological arousal and explicit episodic memory. Participants underwent Pavlovian fear conditioning to two semantic categories (animals, tools) paired with an electric shock. Conditioned stimuli (i.e., category exemplars) from one category were then extinguished, while stimuli from the other category were paired with a positive outcome. Participants returned 24-h later for a test of skin conductance responses (SCR) to the conditioned exemplars, as well as a surprise recognition memory test for stimuli encoded the previous day. Results showed reduced SCRs at a test for unique stimuli from a category that had undergone CC, relative to stimuli from a category that had undergone standard extinction. Additionally, participants selectively remembered more stimuli encoded during CC than extinction. These results provide new evidence that aversive-to-appetitive CC, as compared to extinction, strengthens memory for items directly associated with a positive outcome, which may provide stronger retrieval competition against a fear memory at test to help diminish fear relapse.
The ability to learn and retain associations between neutral cues and threatening events is adaptive. But maintaining these associations when threat is no longer imminent can present a burden. Research on how to prevent maladaptive behavior tends to utilize Pavlovian extinction, wherein negative expectations are violated by the omission of threat. Yet, extinction is widely considered temporary and fragile, and the original behavior is prone to relapse (Bouton 2002; Vervliet et al. 2013). Consequently, there is interest in approaches besides standard extinction to persistently reduce maladaptive behavior (Craske et al. 2014; Dunsmoor et al. 2015b). One approach is to replace negative associations with a positive rewarding association, a technique known as counterconditioning (CC). Counterconditioning forms the basis for behavior modification strategies like systematic desensitization (Wolpe 1954). But laboratory research on CC is limited, particularly as compared to intensifying interest in standard extinction. In humans especially, it is unclear whether CC is any more or less effective than standard extinction in preventing fear relapse over time. It is also unknown how CC affects explicit memory processes, which play an important role in the etiology and maintenance of fear and anxiety (Mathews and MacLeod 2005; Rubin et al. 2008; Zlomuzica et al. 2014). Here, we compared the effects of standard extinction to appetitive CC on tests of implicit memory, measured through physiological arousal, and explicit episodic memory.
Counterconditioning refers to the technique and the process of using reinforcement of the opposite valence to modify a previously learned behavior (Bouton 1993). For example, in aversive-to-appetitive CC, a subject might first learn that a conditioned stimulus (CS; e.g., a tone) predicts a shock unconditioned stimulus (US), and therefore exhibits a defensive response to the CS. Next, the subject learns to associate the CS with a new positive US (e.g., food). Counterconditioning has long been thought to produce inhibitory interactions between negative and positive valence systems (Dickinson and Pearce 1977). Research on the long-term effects of CC on suppressing the originally learned behavior are mixed. The earliest research in humans dates to the “Little Peter” experiment (Jones 1924), in which a 3-yr old boy overcame his fear of rabbits by eating candy during gradual exposure to a rabbit. Influential work by Bouton and colleagues in rats, however, showed that CC is temporally (Bouton and Peck 1992) and contextually (Peck and Bouton 1990) limited, and behavior will eventually revert back to the original conditioned response.
The small number of contemporary aversive-to-appetitive CC studies in humans have produced mixed results (Raes and De Raedt 2012; Meulders et al. 2015; Kang et al. 2018; van Dis et al. 2019). Much of this research focuses on two separate forms of learning invoked in conditioning: expectancy-learning and evaluative learning (Baeyens and De Houwer 1995; Hermans et al. 2002). Expectancy-learning refers to learning the contingency between the CS and US, such that presentations of the CS evoke an expectation that the US will occur. In fear conditioning, expectancy-learning is often measured by changes in self-reported US expectancy ratings and anticipatory physiological arousal assessed via skin conductance responses (SCR). Some work shows that CC is equally as effective as standard extinction on modifying behaviors associated with expectancy-learning (Raes and De Raedt 2012; van Dis et al. 2019). In contrast, evaluative conditioning involves changes in preferences or in the valence of the CS regardless of whether or not the US is anticipated. Unlike extinction, CC can be effective at changing preferences or valence ratings of the CS (Baeyens et al. 1989; Kerkhof et al. 2011; Meulders et al. 2015; Newall et al. 2017; van Dis et al. 2019). Accordingly, CC might have different effects given the type of behavior under investigation.
Conditioning and extinction can also affect learning systems involved in forming long-term emotional episodic memories (Dunsmoor and Kroes 2019). For instance, Pavlovian conditioning selectively increases recognition memory for category exemplars (e.g., a semantic category of animals or tools) associated with a US (Dunsmoor et al. 2012, 2015a; Kroes et al. 2017; Patil et al. 2017; Starita et al. 2019). But when extinction immediately follows Pavlovian fear conditioning on the same day, CS exemplars encoded during the extinction phase are remembered at a lower rate than CSs from the same category encoded during the fear conditioning phase (Dunsmoor et al. 2018). Dunsmoor et al. (2018) proposed that a subtle break separating the conditioning and extinction phase can serve as an event boundary to segment competing memory traces of conditioning and extinction (cf. Totty et al. 2019). A consequence of weaker episodic memory representations for extinction, relative to the first experience of fear conditioning, could be that memories directly associated with fear conditioning are more easily retrieved and expressed, as commonly observed in standard Pavlovian conditioning (Bouton 1993, 2002; Rescorla 2004). As it regards episodic memory per se, memories of a past emotional learning experience (e.g., “these stimuli are signals of danger”) are more likely to persist, while memory representations of countervailing information incompatible with the emotional experience (e.g., “these conceptually related stimuli are not signals of danger”) are weaker and more prone to forgetting.
Whether CC has a unique effect on episodic memory as compared to standard extinction is unknown. One possibility is that CC produces stronger memories for items associated with a new positive outcome, as compared to memories for items associated with the mere absence of a negative outcome (i.e., standard extinction). Here, we investigated whether appetitive CC, as compared to standard extinction, diminishes the physiological relapse of fear after 24-h. We also examined whether CC affects episodic memory representations associated with a positive outcome, in comparison to standard extinction.
In a within-subjects design, subjects underwent fear conditioning to two semantic categories both paired with a shock (CS+’s; pictures of animals and tools) and one category never paired with shock (CS−; pictures of food). In contrast to repeated presentations of the same CS (e.g., a face or a colored square), the use of semantic categories as CSs effectively tags each CS presentation as an individual learning episode, thereby providing the opportunity to estimate memory as a function of when (i.e., during which experimental phase) each CS was encoded (see Dunsmoor and Kroes 2019). During fear conditioning on Day 1, one object category was then extinguished by omitting the shock, while the other object category was counterconditioned by omitting and replacing the shock with a positive picture. We then tested two forms of memory retrieval 24-h later: autonomic physiological fear responses (assessed by SCR) and episodic memory (assessed by a surprise recognition memory test). We predicted that appetitive CC, as compared to standard extinction, would diminish 24-h physiological arousal, while selectively enhancing long-term episodic memory representations.
Results
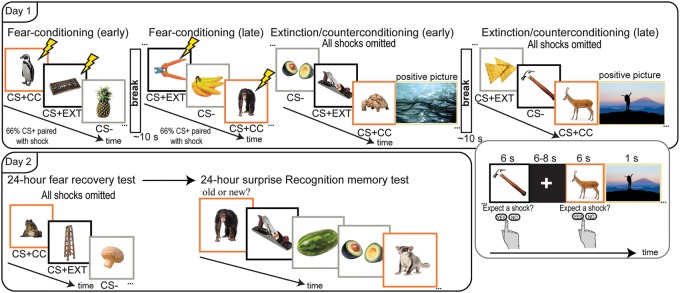
This study was a 2-d within-subjects Pavlovian category-conditioning task that measured both physiological arousal (SCRs) and 24-h episodic (recognition) memory. Additional methodological details are below in the Materials and Methods section. Day 1 involved a collection of SCRs while subjects underwent fear conditioning followed by extinction/CC. During conditioning, two object categories (CS+, animals, and tools) coterminated with an unpleasant electrical shock to the wrist, while pictures of food always served as a within-subjects shock unpaired control category (CS−). Following conditioning, items from one CS+ category (animals or tools, counterbalanced) were presented unpaired with shock (standard extinction, hereafter referred to as CS + EXT), while items from the other CS+ category (tools or animals, respectively) were paired with a positively valenced picture in place of the coterminating shock (CC, hereafter referred to as CS + CC). Subjects returned 24-h later for a test of physiological arousal (SCR) to novel images from the CS + EXT, CS + CC, and CS− categories, followed by a surprise recognition memory test for CSs from each object category encoded the previous day. The recognition memory test included an equal number of novel pictures of animals, tools, and food to control for false alarms (Fig. 1).
Figure 1.
Experimental design. Participants viewed 144 unique pictures of animals, tools, and food during fear conditioning, extinction/CC, and 24-h fear recovery test while they rated shock expectancy. During conditioning, CS+ items (animals and tools) were paired with a shock 66% of the time. During extinction/CC, CS + EXT items (animals or tools, counterbalanced) were never paired with a shock, while CS + CC items (tools or animals, respectively) were always paired with a positive picture of a scene. CS− items (food) were unpaired throughout conditioning and extinction/CC. An explicit ∼10 sec break separated the first and second half of fear conditioning and extinction/CC. The next day, participants viewed 30 novel basic level exemplars of the same CS categories (animals, tools, and food). This was followed by a surprise recognition memory test. Lightning bolts denote shock and colored borders are for illustrative purposes only.
Skin conductance responses
Fear conditioning
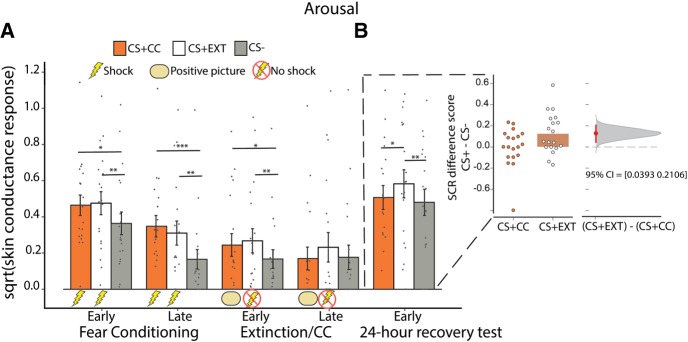
SCR results are presented in Figure 2. One subject was excluded from SCR analysis due to equipment malfunction. Analysis of mean SCRs from fear conditioning on Day 1 were separated into the first and second half of trials. Repeated-measures ANOVA showed a main effect of CS type (F(2,36) = 12.840, P < 0.001, ηp2 = 0.416) and half (F(1,18) = 25.190, P < 0.001, ηp2 = 0.583), but no interaction. Post-hoc paired samples t-tests showed successful conditioning to CS + CC versus CS−, and CS + EXT versus CS−, during the first and second half of fear conditioning (all P’s < 0.013). There was no difference between CS + CC and CS + EXT during either the first (P = 0.72) or second half (P = 0.29) of fear conditioning on Day 1. Thus, subjects exhibited similar levels of conditioned SCRs to the two CS+ categories, and SCRs were significantly elevated to both CS+ categories as compared to the CS− category.
Figure 2.
Skin conductance responses. (A) Results from fear conditioning indicated successful and equivalent acquisition of conditioned SCRs to both CS+ categories (CS + CC and CS + EXT), as mean SCRs were elevated relative to the unpaired CS− category. During late extinction, SCRs successfully diminished for the extinguished (CS + EXT) and counterconditioned (CS + CC) categories. (B) (Left) The following day, mean SCRs were relatively higher to new images from the CS + EXT category as compared to the CS + CC and CS− category. (B) (Right). A Gardner–Altman plot (Ho et al. 2019) shows the nonparametric effect size in the difference in means between CS + CC–CS− and CS + EXT–CS− SCR recovery scores. Five thousand bootstrap samples were drawn to build the distribution curve. The red dot depicts the mean difference of the two scores (CS + CC–CS− & CS + EXT–CS−) and the horizontal red line indicates the 95% confidence interval of the bootstrap differences. Error bars represent SEM (***) P < 0.001; (**) P < 0.01; (*) P < 0.05.
Extinction/counterconditioning
Analysis of mean SCRs from extinction/CC on Day 1 were separated into early (first half) and late (last three of each CS type) trials. Early extinction included the first half of trials. Late extinction focused on the mean of the last three trials in order to estimate whether SCRs had successfully diminished for each CS+ category relative to the CS− category by the end of Day 1. Repeated-measures ANOVA showed an effect of CS type (F(2,36) = 4.662, P = 0.016, ηp2 = 0.206), but no effect of early/late trials, nor an interaction. Post-hoc paired samples t-tests on the late CS trials showed no significant difference between CS + EXT versus CS− (P = 0.193) or CS + CC versus CS− (P = 0.855) during late extinction/CC on Day 1, indicating successful diminishment of conditioned SCRs via the absence of shock by the end of the session (Fig. 2A, extinction/CC).
Counterconditioning diminished 24-h fear recovery as compared to standard extinction
Twenty-four hours later, subjects returned for a test of physiological arousal to new images (animals, tools, food) from each CS category (CS + CC, CS + EXT, CS−). Shock electrodes were reattached and subjects were not given any new instructions from the previous day. Analysis of SCRs focused on the early trials (first 3). Focusing on the early trials of a long-term test of physiological arousal is consistent with extant work on testing extinction recall/retention in human research (Milad et al. 2009; Schiller et al. 2010; Kroes et al. 2017; Sevinc et al. 2019). These early trials capture the moment when the possibility for threat is most ambiguous. Including later trials in SCR analysis can obscure results, as these later trials likely represent reextinction through repeated presentations of the CS without the US. Note: the CS + CC trials were presented alone on Day 2, without the positive picture. Repeated-measures ANOVA showed a main effect of CS type(F(2,36) = 5.595, P = 0.008, ηp2 = 0.237). Post-hoc paired samples t-tests showed greater mean SCRs to CS + EXT versus CS− (t18 = 2.899, P = 0.010, 95% CI: 0.034–0.218) and CS + EXT versus CS + CC (t18 = 2.807, P = 0.012, 95% CI: 0.032–0.223), but no difference between CS + CC versus CS− (t18 = 0.026, P = 0.980, 95% CI: −0.088–0.090). Thus, participants expressed heightened physiological arousal to items from the object category that had undergone standard extinction the previous day (CS + EXT) as compared to items that had undergone CC (CS + CC) (Fig. 2B, 24-h recovery test). This same pattern of results held when what defined “early trials” was extended to include either the first four or five trials as well.
Some human fear extinction research has quantified postextinction return of fear by correcting for behavior during the end of extinction (Lonsdorf et al. 2017). Here, a subtraction of SCRs to each CS type at the test from SCRs at the end of extinction did not yield a significant difference between “recovery index” of CS + CC versus CS + EXT (P = 0.18). However, interpreting performance at the postextinction test by controlling for behavior at extinction is considered problematic for a variety of reasons. Crucially, within-session extinction performance is shown to poorly predict between-session performance (Craske et al. 2008; Plendl and Wotjak 2010; Lonsdorf et al. 2017).
Trial-by-trial shock expectancy ratings
On each trial subjects indicated whether or not they expected a shock using a two-alternative forced choice button press (Yes or No). During all phases of the experiment shock expectancy was higher on CS + CC versus CS− and CS + EXT versus CS− (all P’s < 0.005). Mean shock expectancy ratings during fear conditioning confirmed successful acquisition (mean ± SEM: CS + CC: 0.66 ± 0.02; CS + EXT: 0.55 ± 0.02; CS−: 0.06 ± 0.01). Mean expectancy during late (last 3) extinction trials showed diminished expectancy for CS+ categories via absence of the shock (mean ± SEM: CS + CC: 0.18 ± 0.05; CS + EXT: 0.13 ± 0.04; CS−: 0 ± 0). The next day there was recovery of shock expectancy to both CS+ categories (mean ± SEM CS + CC: 0.60 ± 0.06; CS + EXT: 0.50 ± 0.07) as compared to the (CS−: 0.13 ± 0.04). However, there was no difference in shock expectancy during early recovery test trials on Day 2 between CS + CC and CS + EXT, P = 0.24. Given the limited sensitivity of a two-alternative forced choice shock expectancy rating, this null finding was not surprising.
Episodic memory
Enhanced 24-h recognition memory for CS+ exemplars encoded during conditioning
Following the test of physiological arousal, subjects underwent a surprise recognition memory test comprised of CSs encoded the previous day during fear conditioning and extinction/CC. Memory results are presented in Figures 3 and 4. Similar to our past work (Dunsmoor et al. 2015a, 2018; Kroes et al. 2017), the use of separate object categories allowed us to test memory for CS+ and CS− items within-subjects, and as a function of when each item was encoded (i.e., during conditioning or CC/extinction). That is, we could assess memory as a time-ordered function of when each CS item was encoded, and which category the CS item was from (CS + CC, CS + EXT, CS−). This provides the opportunity to examine whether CC, as compared to standard extinction, uniquely affects what items are selectively remembered the next day and at what time point those items were encoded or forgotten.
Figure 3.
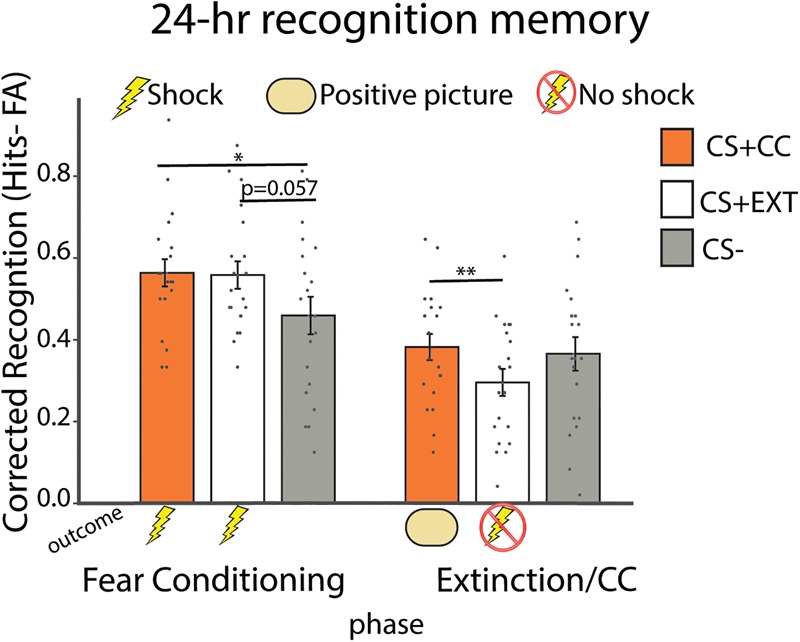
Twenty-four hours recognition memory results. Trial-unique (nonrepeating) exemplars from three different superordinate categories serves as conditioned stimuli (CS) and were incidentally encoded during fear conditioning and extinction/CC. Participants underwent a surprise recognition memory for these exemplars the next day. CS items encoded during fear conditioning that were from a category predictive of an electric shock (animals and tools) were recognized at a higher rate than unpaired CS− items (food) that were never paired to a shock. Different exemplars from these categories were also extinguished (CS + EXT) or paired with a positive picture (CS + CC). Participants recognized relatively more items paired with reward (CS + CC) than items that were extinguished (CS + EXT) via the mere absence of shock at the 24-h recognition memory test. Error bars represent SEM. (**) P < 0.01; (*) P < 0.05.
Figure 4.
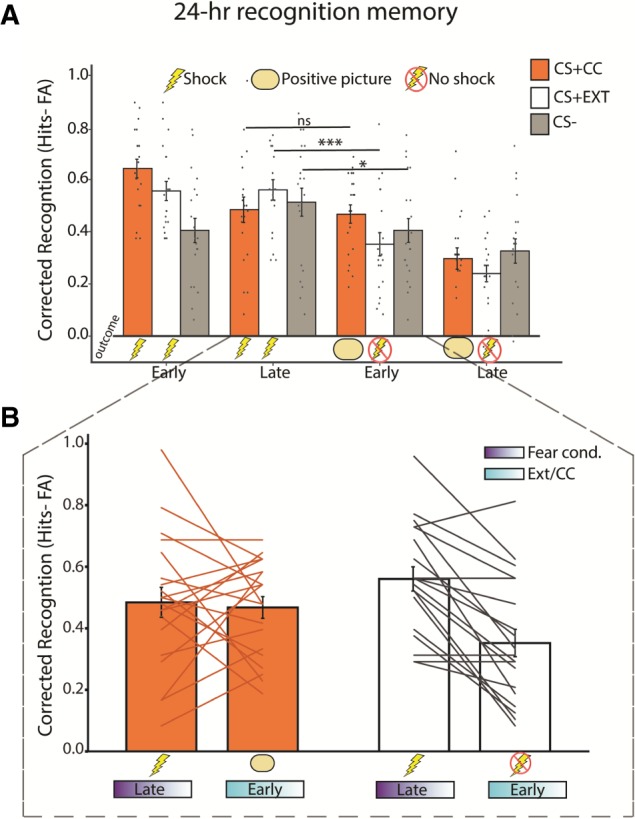
Counterconditioning prevents the immediate drop in episodic memory observed with standard extinction. (A) Recognition memory for conditioned stimuli (CSs) plotted as a function of when these items were encoded, broken into bins of early and late conditioning and extinction/CC. There is an immediate drop in recognition memory for items from the category that underwent standard extinction (CS + EXT) across the transition from late conditioning to early extinction. However, participants recognize an equivalent amount of items from the category that underwent CC (CS + CC) across the transition from late conditioning to early CC. (B) Corrected recognition performance for late conditioning and early extinction/CC is plotted for each subject. Error bars represent SEM. (***) P < 0.001; (*) P < 0.05. ns = not significant.
We analyzed mean corrected recognition memory performance (hits minus false alarms) for items from each CS category encoded during conditioning and extinction/CC (Fig. 3). The false alarm rate was low overall, and there was no main effect of CS type on false alarms (P = 0.621). Repeated-measures ANOVA of corrected recognition showed a main effect of Phase (F(1,19) = 46.232, P < 0.001, ηp2 = 0.709) and a Phase by CS type interaction (F(2,38) = 6.951, P = 0.003, ηp2 = 0.268). There was no main effect of CS type (P = 0.18). Post-hoc paired samples t-tests showed better corrected recognition memory for exemplars encoded during fear conditioning on Day 1 from the CS + CC versus CS− category (t19 = 2.594, P = 0.018, 95% CI: 0.020–0.188), and marginally better memory for CS + EXT versus CS− category (t19 = 2.027, P = 0.057, 95% CI: −0.003–0.201). There was no difference in corrected recognition memory for items from the CS + CC versus CS + EXT items encoded during fear conditioning (P = 0.903). Thus, subjects correctly recognized more items encoded during fear conditioning from the two categories associated with an electrical shock as compared to items from a shock unpaired control category, replicating prior results (Fig. 3, fear conditioning) (Dunsmoor et al. 2012, 2015a, 2018; Starita et al. 2019).
Enhanced memory for counterconditioned exemplars during extinction in comparison to standard extinction
Post-hoc paired samples t-tests for items that were encoded during CC/extinction on Day 1 showed that subjects correctly recognized significantly more items from the CS + CC versus CS + EXT category (t19 = 3.566, P = 0.002, 95% CI: −0.035–0.137) (Fig. 3, extinction/CC). Memory for CS + CC was no greater than for CS− (P = 0.70) or for CS + EXT versus CS− (P = 0.082). Thus, memory for CS+ items from a category associated with a positive outcome during CC were remembered at a higher rate than CS+ items from a category associated with the mere absence of shock.
We have previously reported a dramatic drop in 24-h recognition memory for CS+ items from the same category (e.g., animals) encoded during early extinction relative to late fear conditioning (Dunsmoor et al. 2018). Put another way, recognition memory for CS+ items encoded during the extinction phase is substantially weaker than for CS+ items from the same semantic category that were encoded just moments prior, during the fear conditioning phase. To investigate whether items that undergo CC are likewise sensitive to the same immediate drop in memory as shown for extinguished items (Dunsmoor et al. 2018), we compared memory for CSs encoded during the second half of conditioning to CSs from the same object category encoded during the first half of extinction/CC (Fig. 4). There was a significant drop in memory for items from the CS + EXT category across the transition from late conditioning to early extinction (t19 = 5.784, P < 0.001, 95% CI: 0.132–0.284), as previously shown (Dunsmoor et al. 2018). This drop in memory was also present for items from the CS− category (t19 = 2.436, P = 0.025, 95% CI: 0.015–0.201). However, recognition memory for items from the CS + CC category remained stable across the transition from late conditioning into early CC (t19 = 0.317, P = 0.755, 95% CI: −0.009–0.126). Thus, while the transition between fear conditioning and extinction is associated with a decrease in what subjects later remembered, memory is maintained across the transition to CC (Fig. 4B). Notably, 24-h recognition memory for CS + CC items encoded in the late half of CC diminished to a level equivalent to the other two CS categories (Fig 4A, far right).
Additionally, we used a change point analysis (see Materials and Methods) to detect where, in time, recognition memory for conditioned category exemplars significantly declined (Fig. 5). Here, a significant change in the slope of time-ordered data indicates a significant decline in recognition memory. In line with previous findings (Dunsmoor et al. 2018), memory for CS + EXT stimuli, which underwent standard extinction, significantly declined during early extinction, specifically when conditioning ended and extinction began. In contrast, subjects later recognized CS + CC items encoded during the early phase of CC, and memory declined for CS + CC items encoded during late CC. This analysis reveals that experience of reward during extinction prevented the rapid decrease in later memory obtained with standard extinction.
Figure 5.
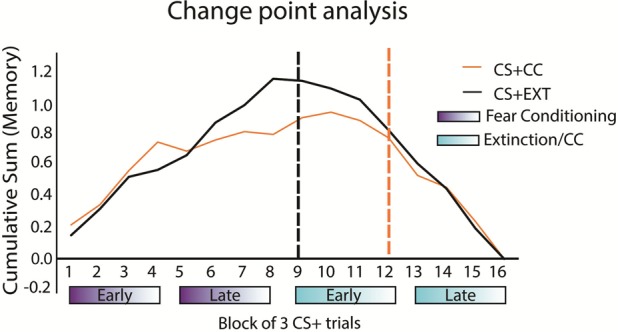
Counterconditioning prevents the sharp drop in memory at the boundary of fear conditioning and CC. A change-point analysis revealed that the decrease in CS + EXT memory (black dotted line) occurred for items encoded immediately following the transition separating conditioning from extinction. But this decline memory did not occur until the end of early CC (orange dotted line) for items that had undergone CC during encoding.
Discussion
Despite historical and clinical interest in CC as both a procedure and a process, this area has received negligible research attention in the past several decades. Direct comparisons between CC and extinction across species and across different sets of behavior have produced mixed results (e.g., Raes and De Raedt 2012; Thomas et al. 2012; Meulders et al. 2015; Holmes et al. 2016; van Dis et al. 2019). Using a within-subjects Pavlovian conditioning design and testing implicit and explicit forms of long-term memory, we found that appetitive CC, as compared to standard extinction, was associated with reduced physiological arousal and heightened recognition memory. These findings provide new evidence that CC has unique effects on long-term memory expression in humans.
A longstanding question in the animal learning literature concerns whether CC is a distinct phenomenon or merely another form of extinction (Lomont 1965; Nelson 1966; Wilson 1973; Dickinson and Pearce 1977; Peck and Bouton 1990). Nearly all associative learning theories of extinction view it as a form of new learning that generates a secondary memory trace, which then competes with the original conditioning memory for expression. Counterconditioning is likewise viewed as a form of retroactive interference that inhibits expression of the originally learned behavior (Bouton 1993, 2002). Importantly, both extinction and CC are sensitive to relapse to the original conditioned response, suggesting the impermanent nature of both learning processes. But there is evidence that CC is more effective than extinction at modifying certain forms of behavior (Kerkhof et al. 2011; Engelhard et al. 2014; Kang et al. 2018; van Dis et al. 2019), which suggests the two phenomena are at least partly distinct. One possible mechanism by which CC exerts a stronger influence than extinction concerns memory competition between the first and second learned association (Miller and Laborda 2011). That is, standard extinction relies on the omission of an expected US to generate a new memory, whereas CC relies on a new perceptible, salient outcome to generate a new memory. Simply put, the new memory formed by CC might just be stronger than the memory formed by extinction. A stronger memory representation for secondary learning might then facilitate retrieval of this competing memory at a future test.
The episodic memory results support the idea that CC produces a stronger memory that is more easily retrieved in the future. Subjects correctly recognized more CS+ items encoded during CC than extinction the next day, whereas memory for CS+ items encoded during fear conditioning were equivalent between the two categories. This ability to remember specific instances of safety could help diminish fear in the future. Indeed, broadly speaking, a goal in many forms of therapy for fear and anxiety disorders is to enhance memory for new associations to override maladaptive thoughts or associations (Craske et al. 2017). In systematic desensitization specifically, the goal is to generate associations that are directly incompatible with, and therefore inhibit, expression of fear and anxiety (Wolpe 1954). Approaches to improve the long-term episodic memory for therapeutic experiences, rather than focusing predominately on diminishing memories of fear, could be one target for optimizing treatment for some psychiatric disorders that warrants further exploration.
We have previously found that an explicit break between conditioning and extinction might serve as an event boundary segmenting competing emotional learning states (Dunsmoor et al. 2018). The transition from conditioning to CC would in principle serve as a strong event boundary to signal the change between fear and safety, which might presumably lead to a segmentation between memories formed during the competing phases of learning. Yet, the presence of a rewarding outcome on CC trials appears to maintain memories of items that would otherwise be more prone to forgetting during standard extinction, suggesting that the memory trace for fear conditioning and CC are both fairly well preserved.
One unexpected result from the SCR analysis was that the overall level of physiological arousal on Day 2 was substantially elevated to all three CS types relative to the end of extinction/CC on Day 1 (Fig. 2B, left). This global increase in SCRs between days is difficult to explain, particularly because the increase encompassed the CS−, which was never paired with shock at any point in the experiment. Thus, it cannot be said that CC prevented or alleviated the recovery of conditioned SCRs altogether, given the level of physiological arousal between days to all CSs. Nevertheless, the global increase in SCRs from Day 1 to Day 2 notwithstanding, it appears that CC was more effective at reducing SCRs relative to standard extinction. One procedural difference from a traditional two-day human fear extinction task worth considering is that each trial on Day 2 was an entirely unique exemplar from each CS category that was not shown the previous day. This may have contributed to a general rise in arousal to all stimuli above what is normally observed when the same CS (e.g., a face or a colored square) is repeated throughout all phases of the experiment.
Some limitations of the present study are worth considering. First, the use of a positive picture for CC is not necessarily a particularly salient appetitive reinforcement on par with the types of outcomes used in animal research. Animal CC research tend to incorporate food, social encounters with the opposite sex, or pleasurable drugs as appetitive stimuli, which serve as strong primary reinforcers. Human CC research has incorporated a number of different types of reinforcers that are far less salient to human subjects, such as positive pictures (Kang et al. 2018), small amounts of money (Meulders et al. 2015), sounds (Raes and De Raedt 2012), or positive film clips (van Dis et al. 2019). It is likely that some forms of reinforcement are more effective than others, but it is questionable whether any of these positive outcomes are as salient to human volunteers as the types of reinforcement received by rodents. Second, the task design used here was a trial-unique category-conditioning design, and thus the CS exemplars presented throughout the conditioning task were never repeated. Thus, built into the design is the idea that subjects learn and generalize the CS contingencies within, and not between, each semantic category. In this regard, it is noteworthy that subjects evinced a typical pattern of acquisition, extinction, and recovery despite the fact that a specific CS exemplar is never repeated. The use of trial-unique exemplars from a CS+ category is an innovative approach to assess generalized fear recovery that merits further attention. However, a limitation to this design is that we did not directly test physiological arousal to the exact same CS+ exemplars used during CC or extinction. Thus, we do not have a measure of 24-h recovery of physiological arousal to the precise CSs first associated with shock and then associated with either a positive outcome or no shock. Third, it is unclear what effect methodological considerations like the reinforcement rate during conditioning and CC could have on the results, but these procedural details warrant further consideration for future research. Finally, it is worth considering whether the presence of the positive outcome influenced how the CS + EXT was processed or perceived by the subjects. That is, it is possible that the CS + EXT attracted less attention by virtue of the positive outcome on CS + CC trials, or that the absence of positive outcome lowered the value of the CS + EXT. However, it is worth noting that the results from the CS + EXT condition were remarkably similar to those obtained in prior work without a CC condition (Dunsmoor et al. 2015a, 2018; Kroes et al. 2017), both in terms of the SCR and episodic memory results, suggesting that the positive outcome did not have a selectively deleterious effect on extinction.
In conclusion, these results provide new evidence that aversive-to-appetitive CC, as compared to standard extinction, can diminish physiological arousal and increase episodic memory strength. The limited attention to CC in the Pavlovian conditioning literature is surprising, given that it may be a more powerful approach to override fear expression and thus has important clinical implications. Further work in this area is warranted, including investigating whether the neurocircuitry supporting CC is distinct from that supporting standard extinction (Giustino and Maren 2015). Indeed, there is some limited work in rats showing that rewarded extinction might be more effective than standard extinction by engaging a medial prefrontal cortex-amygdala-striatal circuit (Correia et al. 2016). Additionally, emerging research suggests that dopamine plays a role in standard extinction processes (Kalisch et al. 2019). It is possible that conditioning might simply facilitate standard extinction processes by boosting dopamine system function through the unexpected presentation of a reward, rather than the mere omission of the expected US. This model might also fit with recent functional neuroimaging showing that simply replacing shocks with a tone engages the ventromedial prefrontal cortex and diminishes the return of fear in humans (Dunsmoor et al. 2019).
Materials and Methods
Participants
A sample size of 20 healthy adults was a priori based on extensive behavioral research of multiday fear conditioning in humans (Lonsdorf et al. 2017), as well as recent experiments combining Pavlovian conditioning with 24-h episodic memory tests (Dunsmoor et al. 2012, 2015a; Kroes et al. 2017; Patil et al. 2017; Starita et al. 2019). A total of 20 participants from the University of Texas at Austin and local Austin community completed the study (17 females; mean age = 22.7; range 18 to 39). One subject was excluded from the SCR analysis due to equipment malfunction, but this subject's memory data was included in the recognition memory analysis. All participants provided written informed consent and compensated $40 for completing the 2-d study. Procedures were in compliance with the Institutional Review Board of UT Austin (IRB # 2017-02-0094)
Conditioning task
This was a within-subjects 2-d experiment separated by ∼24 h. Conditioned stimuli were pictures of animals, tools, and food on a white background obtained from the website www.lifeonwhite.com or publicly available sources on the internet. The US was a 6 msec electrical shock delivered to the right wrist from the BIOPAC (Goleta, CA) STM200 and calibrated before the session to a level deemed “highly annoying but not painful.” Trial order was pseudorandomized such that no more than three pictures of the same category appeared in a row. The task was presented using E-Prime 2.0 and consisted of a trial-unique category conditioning design, meaning that each trial was a different basic-level exemplar with a unique name. For example, there were no two different pictures of a monkey. Pictures of common phobic stimuli (e.g., spiders, snakes, weapons) or highly appealing food images were not used in the CS set. CSs were on the screen for 6 sec, followed by a 6–8 sec waiting period with a fixation cross on a white background. On each trial, subjects rated expectancy for the shock using a two-alternative forced choice rating scale. Subjects were not instructed about the CS–US contingencies, but were told that if they paid attention then they might learn an association between the pictures and the shock.
Fear conditioning
Day 1 involved a total of 144 trials across fear conditioning and extinction/CC. During conditioning, pictures from two object categories (CS+, animals, and tools) coterminated with an electrical shock on 16 out of 24 trials, while 24 pictures of food always served as a within-subjects shock unpaired control category (CS−). There was a short (∼10 sec) explicit break midway through fear conditioning.
Counterconditioning/extinction
There was no explicit break between fear conditioning and the start of CC/extinction. 24 CS exemplars from each category (CS + EXT, CS + CC, CS−) were presented during extinction/CC, and there was a short (∼10 sec) explicit break midway throughout this phase. Items that underwent standard extinction (referred to as CS + EXT), (animals or tools, counterbalanced) was presented without shock while images from the other category (tools or animals, respectively) were paired with a unique image of a positive valence picture (referred to as CS + CC), depicting serene scenes or accomplishment. The positive picture appeared at the end of the trial, at the moment when the shock should be expected, for a duration of 1 sec. The positive pictures used for CC were pilot rated by a separate group of 19 subjects to confirm high valence and low arousal.
Twenty-four hour test of physiological arousal
The next day, subjects returned and were reattached to SCR and shock electrodes, but the shock was not recalibrated to avoid fear reinstatement. Subjects were not provided any new instructions from the previous day. A test of 24-h physiological fear recovery included 10 trials each of unique (i.e., not shown the previous day) animals, tools, and food. There were no shocks or positive pictures presented on Day 2. The first trial on Day 2 was always a CS− trial that was used to capture the initial orienting response and discarded from analysis (Schiller et al. 2013; Kroes et al. 2017).
Surprise recognition memory test
The episodic memory test was based on prior studies (e.g., Dunsmoor et al. 2012). Following the spontaneous recovery test, shock and SCR electrodes were removed and subjects were told that they would now be tested for their memory for the pictures they saw the day before. Subsequently, subjects were asked to rate whether or not they expected to receive a memory test. Out of 20 subjects, only one participant expected with confidence to receive a memory test. The memory test included a total of 288 pictures: 72 CS pictures shown during fear conditioning, 72 CS pictures shown during CC/extinction, and 144 new pictures of animals, tools, and food (novel foils, 48 each) to control for false alarms within the category. The recognition memory test was self-paced and subjects responded on each trial whether the picture was old or new and their level of confidence (“definitely old,” “maybe old,” “maybe new,” “definitely new”). Corrected recognition (hits minus false alarms) focused on high confidence responses as prior research shows emotion has a more substantive impact on items recalled with high confidence or a sense of recollection than items remembered with low confidence or a mere sense of familiarity (Talarico et al. 2004; Dolcos et al. 2005; Phelps and Sharot 2008).
Skin conductance responses
SCRs were recorded from the hypothenar eminence of the left palmar surface using pregelled snap electrodes (BIOPAC EL509) connected to the BIOPAC MP-160 System (Goleta, CA). SCR data were considered valid to CS presentation if the trough-to-peak deflection occurred between 0.5 to 6 sec following CS onset, was greater than 0.02 µS, and lasted for a maximum 5 sec. Trials that did not meet these criteria were scored as zero. SCRs were processed using an automated analysis script implemented in Matlab (Green et al. 2013), and were visually inspected by research assistants blind to the experimental conditions. SCR data were square-root transformed prior to statistical analysis to normalize the distributions.
Statistical analysis
The primary dependent variables were SCRs and corrected recognition memory data (hits minus false alarms). Shock expectancy ratings were also analyzed; but given the limited two-alternative forced choice option, no strong predictions were made regarding differences in US expectancy between the CS+ categories. Repeated measures analysis of variance (ANOVA) included factors for CS type (CS + CC, CS + EXT, CS-) and time (e.g., early, late phase) where appropriate. The main effects or interactions were followed by post-hoc paired t-tests, considered significant at P < 0.05.
Change point analysis
To conduct a change point analysis for the recognition memory, we binned CS+ items encoded during fear conditioning and extinction/CC into blocks of three trials, for a total of 16 bins per CS+. To visualize the data, we plotted the time-ordered cumulative sum of the mean of each bin (blocks of three trials) subtracted by the total memory average (computed separately by CS+ type, CS + CC and CS + EXT). A sudden change in the slope of the cumulative sum indicates a change in the running average of the trials. The Change-Point Analyzer (Taylor 2000) was used to compute a significant change in the time-ordered memory data, using 1000 bootstrap iterations with a confidence level of 95%.
Acknowledgments
We thank Alejandro Rey-Hipolito in assistance with data analysis and Mason McClay and Augustin Hennings in assistance with the generation of figures. This study was funded in part from National Institutes of Health NIH R00MH106719 to J.E.D. N.E.K. is supported by NIH R00MH106719-04S.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.050740.119.
References
- Baeyens F, De Houwer J. 1995. Evaluative conditioning is a qualitatively distinct form of classical conditioning: a reply to Davey (1994). Behav Res Ther 33: 825–831. 10.1016/0005-7967(95)00021-O [DOI] [PubMed] [Google Scholar]
- Baeyens F, Eelen P, Van den Bergh O, Crombez G. 1989. Acquired affective-evaluative value: conservative but not unchangeable. Behav Res Ther 27: 279–287. 10.1016/0005-7967(89)90047-8 [DOI] [PubMed] [Google Scholar]
- Bouton ME. 1993. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull 114: 80–99. 10.1037/0033-2909.114.1.80 [DOI] [PubMed] [Google Scholar]
- Bouton ME. 2002. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52: 976–986. 10.1016/S0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. 1992. Spontaneous recovery in cross-motivational transfer (counterconditioning). Anim Learn Behav 20: 313–321. 10.3758/BF03197954 [DOI] [Google Scholar]
- Correia SS, McGrath AG, Lee A, Graybiel AM, Goosens KA. 2016. Amygdala-ventral striatum circuit activation decreases long-term fear. Elife 5: e12669 10.7554/eLife.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. 2008. Optimizing inhibitory learning during exposure therapy. Behav Res Ther 46: 5–27. 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. 2014. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 58C: 10–23. 10.1016/j.brat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU. 2017. Anxiety disorders. Nat Rev Dis Primers 3: 17024 10.1038/nrdp.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Pearce JM. 1977. Inhibitory interactions between appetitive and aversive stimuli. Psychol Bull 84: 690 10.1037/0033-2909.84.4.690 [DOI] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. 2005. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci 102: 2626–2631. 10.1073/pnas.0409848102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MC. 2019. Episodic memory and Pavlovian conditioning: ships passing in the night. Curr Opin Behav Sci 26: 32–39. 10.1016/j.cobeha.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Martin A, LaBar KS. 2012. Role of conceptual knowledge in learning and retention of conditioned fear. Biol Psychol 89: 300–305. 10.1016/j.biopsycho.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Murty VP, Davachi L, Phelps EA. 2015a. Emotional learning selectively and retroactively strengthens memories for related events. Nature 520: 345–348. 10.1038/nature14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Niv Y, Daw ND, Phelps EA. 2015b. Rethinking extinction. Neuron 88: 47–63. 10.1016/j.neuron.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MC, Moscatelli CM, Evans MD, Davachi L, Phelps EA. 2018. Event segmentation protects emotional memories from competing experiences encoded close in time. Nat Hum Behav 2: 291 10.1038/s41562-018-0317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MC, Li J, Daw ND, Simpson HB, Phelps EA. 2019. Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. J Neurosci 39: 3264–3276. 10.1523/JNEUROSCI.2713-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard IM, Leer A, Lange E, Olatunji BO. 2014. Shaking that icky feeling: effects of extinction and counterconditioning on disgust-related evaluative learning. Behav Ther 45: 708–719. 10.1016/j.beth.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. 2015. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci 9: 298 10.3389/fnbeh.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SR, Kragel PA, Fecteau ME, LaBar KS. 2013. Development and validation of an unsupervised scoring system (Autonomate) for skin conductance response analysis. Int J Psychophysiol 91: 186–193. 10.1016/j.ijpsycho.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Vansteenwegen D, Crombez G, Baeyens F, Eelen P. 2002. Expectancy-learning and evaluative learning in human classical conditioning: affective priming as an indirect and unobtrusive measure of conditioned stimulus valence. Behav Res Ther 40: 217–234. 10.1016/S0005-7967(01)00006-7 [DOI] [PubMed] [Google Scholar]
- Ho J. 2019. Moving beyond P values: data analysis with estimation graphics. Nat Methods 7: 565–566. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Leung HT, Westbrook RF. 2016. Counterconditioned fear responses exhibit greater renewal than extinguished fear responses. Learn Mem 23: 141–150. 10.1101/lm.040659.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MC. 1924. A laboratory study of fear: the case of Peter. J Genet Psychol 31: 308–315. 10.1080/00221325.1991.9914707 [DOI] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AM, Duvarci S. 2019. A dopaminergic basis for fear extinction. Trends Cogn Sci 23: 274–277. 10.1016/j.tics.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Kang S, Vervliet B, Engelhard IM, van Dis EA, Hagenaars MA. 2018. Reduced return of threat expectancy after counterconditioning versus extinction. Behav Res Ther 108: 78–84. 10.1016/j.brat.2018.06.009 [DOI] [PubMed] [Google Scholar]
- Kerkhof I, Vansteenwegen D, Baeyens F, Hermans D. 2011. Counterconditioning: an effective technique for changing conditioned preferences. Exp Psychol 58: 31–38. 10.1027/1618-3169/a000063 [DOI] [PubMed] [Google Scholar]
- Kroes MC, Dunsmoor JE, Lin Q, Evans M, Phelps EA. 2017. A reminder before extinction strengthens episodic memory via reconsolidation but fails to disrupt generalized threat responses. Sci Rep 7: 10858 10.1038/s41598-017-10682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomont JF. 1965. Reciprocal inhibition or extinction? Behav Res Ther 3: 209–219. 10.1016/0005-7967(65)90029-X [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O. 2017. Don't fear ‘fear conditioning’: methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev 77: 247–285. 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. 2005. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 1: 167–195. 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- Meulders A, Karsdorp PA, Claes N, Vlaeyen JW. 2015. Comparing counterconditioning and extinction as methods to reduce fear of movement-related pain. J Pain 16: 1353–1365. 10.1016/j.jpain.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Laborda MA. 2011. Preventing recovery from extinction and relapse: a product of current retrieval cues and memory strengths. Curr Dir Psychol Sci 20: 325–329. 10.1177/0963721411418466 [DOI] [Google Scholar]
- Nelson F. 1966. Effects of two counterconditioning procedures on the extinction of fear. J Comp Physiol Psychol 62: 208 10.1037/h0023672 [DOI] [PubMed] [Google Scholar]
- Newall C, Watson T, Grant K-A, Richardson R. 2017. The relative effectiveness of extinction and counter-conditioning in diminishing children's fear. Behav Res Ther 95: 42–49. 10.1016/j.brat.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Patil A, Murty VP, Dunsmoor JE, Phelps EA, Davachi L. 2017. Reward retroactively enhances memory consolidation for related items. Learn Mem 24: 65–69. 10.1101/lm.042978.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CA, Bouton ME. 1990. Context and performance in aversive-to-appetitive and appetitive-to-aversive transfer. Learn Motiv 21: 1–31. 10.1016/0023-9690(90)90002-6 [DOI] [Google Scholar]
- Phelps EA, Sharot T. 2008. How (and why) emotion enhances the subjective sense of recollection. Curr Dir Psychol Sci 17: 147–152. 10.1111/j.1467-8721.2008.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. 2010. Dissociation of within and between-session extinction of conditioned fear. J Neurosci 30: 4990–4998. 10.1523/JNEUROSCI.6038-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes AK, De Raedt R. 2012. The effect of counterconditioning on evaluative responses and harm expectancy in a fear conditioning paradigm. Behav Ther 43: 757–767. 10.1016/j.beth.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Rescorla RA. 2004. Spontaneous recovery. Learn Mem 11: 501–509. 10.1101/lm.77504 [DOI] [PubMed] [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. 2008. Memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychol Rev 115: 985–1011. 10.1037/a0013397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463: 49–53. 10.1038/nature08637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. 2013. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci 110: 20040–20045. 10.1073/pnas.1320322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevinc G, Hölzel BK, Greenberg J, Gard T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, Lazar SW. 2019. Strengthened hippocampal circuits underlie enhanced retrieval of extinguished fear memories following mindfulness training. Biol Psychiatry 86: 693–702. 10.1016/j.biopsych.2019.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starita F, Kroes MCW, Davachi L, Phelps EA, Dunsmoor JE. 2019. Threat learning promotes generalization of episodic memory. J Exp Psychol Gen 148: 1426–1434. 10.1037/xge0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico JM, LaBar KS, Rubin DC. 2004. Emotional intensity predicts autobiographical memory experience. Mem Cognit 32: 1118–1132. 10.3758/BF03196886 [DOI] [PubMed] [Google Scholar]
- Taylor WA. 2000. Change-point analysis: a powerful new tool for detecting changes. (ed. Deerfield IBHC).
- Thomas BL, Cutler M, Novak C. 2012. A modified counterconditioning procedure prevents the renewal of conditioned fear in rats. Learn Motiv 43: 24–34. 10.1016/j.lmot.2012.01.001 [DOI] [Google Scholar]
- Totty MS, Payne MR, Maren S. 2019. Event boundaries do not cause the immediate extinction deficit after Pavlovian fear conditioning in rats. Sci Rep 9: 9459 10.1038/s41598-019-46010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dis EA, Hagenaars MA, Bockting CL, Engelhard IM. 2019. Reducing negative stimulus valence does not attenuate the return of fear: two counterconditioning experiments. Behav Res Ther 120: 103416 10.1016/j.brat.2019.103416 [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. 2013. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol 9: 215–248. 10.1146/annurev-clinpsy-050212-185542 [DOI] [PubMed] [Google Scholar]
- Wilson GT. 1973. Counterconditioning versus forced exposure in extinction of avoidance responding and conditioned fear in rats. J Comp Physiol Psychol 82: 105 10.1037/h0033819 [DOI] [PubMed] [Google Scholar]
- Wolpe J. 1954. Reciprocal inhibition as the main basis of psychotherapeutic effects. AMA Arch Neurol Psychiatry 72: 205–226. 10.1001/archneurpsyc.1954.02330020073007 [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Dere D, Machulska A, Adolph D, Dere E, Margraf J. 2014. Episodic memories in anxiety disorders: clinical implications. Front Behav Neurosci 8: 131 10.3389/fnbeh.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]