Abstract
Spinal cord injury (SCI) is a serious problem that primarily affects younger and middle-aged adults at its onset. To date, no effective regenerative treatment has been developed. Over the last decade, researchers have made significant advances in stem cell technology, biomaterials, nanotechnology, and immune engineering, which may be applied as regenerative therapies for the spinal cord. Although the results of clinical trials using specific cell-based therapies have proven safe, their efficacy has not yet been demonstrated. The pathophysiology of SCI is multifaceted, complex and yet to be fully understood. Thus, combinatorial therapies that simultaneously leverage multiple approaches will likely be required to achieve satisfactory outcomes. Although combinations of biomaterials with pharmacologic agents or cells have been explored, few studies have combined these modalities in a systematic way. For most strategies, clinical translation will be facilitated by the use of minimally invasive therapies, which are the focus of this review. In addition, this review discusses previously explored therapies designed to promote neuroregeneration and neuroprotection after SCI, while highlighting present challenges and future directions.
Impact Statement
To date there are no effective treatments that can regenerate the spinal cord after injury. Although there have been significant preclinical advances in bioengineering and regenerative medicine over the last decade, these have not translated into effective clinical therapies for spinal cord injury. This review focuses on minimally invasive therapies, providing extensive background as well as updates on recent technological developments and current clinical trials. This review is a comprehensive resource for researchers working towards regenerative therapies for spinal cord injury that will help guide future innovation.
Keywords: biomaterials, cell therapy, minimally invasive, regeneration, spinal cord injury
Introduction
Spinal cord injury (SCI) results from direct trauma to the tissue and is associated with loss of motor, sensory, and autonomic functions caudal to the site of injury.1 According to the World Health Organization (WHO),2 between 250,000 and 500,000 people around the world sustain an SCI each year. In the United States alone, there are around 11,000–20,000 new cases each year.3 However, the annual incidence of SCI varies in different countries; for example, it is 9.2 patients per million in Denmark and 40.1 per million in the United States.4 Around the world, the incidence ranges from 3.6 to 195.4 patients per million.5
In the United States, there are around 250,000 patients who are at present living with SCI.3 Over a lifetime, direct costs of SCI can reach 1.1–4.6 million USD per patient.6 Most often SCI occurs in young and middle-aged adults7 and those with SCI are two to five times more likely to die prematurely.2 SCI affects not only a patient's physical and psychological health but also one's family and the broader community and economy.
Due to the lack of plasticity and limited regenerative capacity of the central nervous system (CNS), recovery of neural function after SCI is rare. After injury, the spinal cord undergoes several changes on the cellular and molecular level that interfere with axonal regeneration. These complex events are poorly understood, hindering the development of treatments that can lead to complete recovery.8 Present SCI treatment is mostly conservative relying on stabilization of the patient, prevention of complications, and physical rehabilitation.
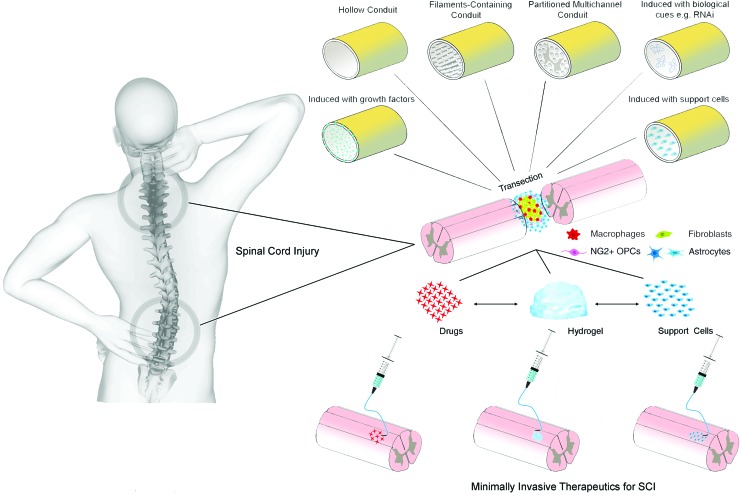
Extensive research into regenerative strategies for SCI has been performed over the last couple of decades. Improvements in our understanding of the pathophysiology of SCI coupled with advances in cell-based therapies, biomaterials, and biomolecules have enabled us to develop new therapeutic approaches for neuroregeneration. Promising results from in vivo experiments evaluating therapies based on biomaterials, cells, or biomolecules are being continuously reported.9–13 Because of the complexity of SCI pathophysiology, the use of combinatorial therapies may be more effective and lead to better regeneration.14,15 Although various procedures can be used to administer therapy after SCI (Fig. 1), the use of minimally invasive strategies, such as injection, is needed to reduce risk of complications, including introducing additional injury, and thus spare neuronal circuitry.16
FIG. 1.
A schematic illustration showing different methods that can be used for the treatment of SCI. SCI, spinal cord injury. Adapted from Führmann et al.182 Color images are available online.
This review focuses on minimally invasive SCI therapeutics in clinical trials,1,3,6,17 as well as those in preclinical9–13 and in vitro phases of development.18–21 In addition, major challenges are highlighted. Future successful approaches for the treatment of SCI will likely include the integration of several recent advances in various fields as combinatorial therapies in minimally invasive formats. This review summarizes accumulating knowledge, examines evidence and development, and highlights potential paths forward.
Pathophysiology
SCI is characterized by sequential primary, secondary, and chronic phases. The primary injury to the spinal cord is the result of initial trauma. The primary mechanical insult may occur from compression, shearing, laceration, stretch, distraction, hemorrhage, or vasospasm. Bone or tissue fragments from the primary injury can exacerbate swelling of the spinal cord and add to tissue damage.
Secondary injury follows in a progressive way, as a result of ischemia, inflammation, and development of a cytotoxic microenvironment, leading to death of functional cells and damage to the tissue microenvironment. The chronic stage of SCI is characterized by formation of astroglial and fibrous scar tissue around cystic cavitations. As a result, regeneration is thought to be inhibited, at least partially, by the extracellular matrix (ECM) and soluble factors secreted by inflammatory cells within the scar tissues (Fig. 2).6
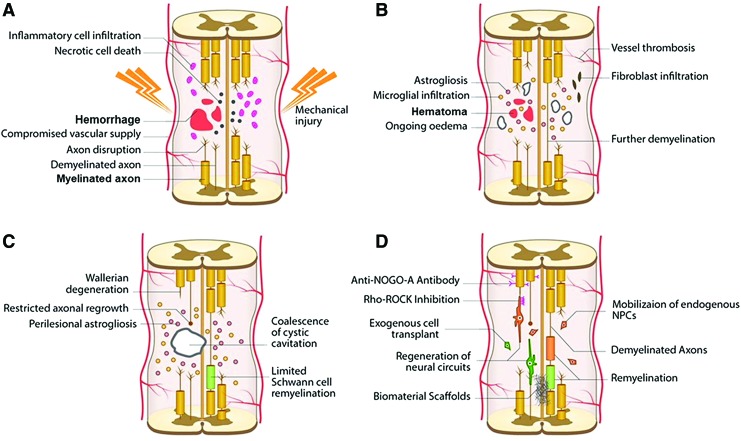
FIG. 2.
An illustration showing the development of pathophysiological changes following SCI. The acute phase takes 0–48 h and it involves hemorrhage, edema, and proapoptotic factors (A). This leads to further loss of function, more than that resulting from the initial insult occurs due to injury to neurons and oligodendrocytes. Astrocyte infiltration and release of additional proinflammatory factors are seen while demyelinated and injured axons begin to die back. In the late subacute (B) and intermediate (C) stages, microcystic cavities follow cell death. These cavities then coalesce forming barriers to regeneration in the chronic stage (>6 months). The final chronic stage scar, which is composed of a network of astrocytic processes and a dense fibrous deposit, acts as a physical and biochemical barrier to neurite outgrowth and cell migration. (D) A schematic illustration showing demyelination and axonal loss that follow SCI and various regenerative therapeutics that can be used including the use of biomaterials, cells, molecules, such as an anti-NOGO-A antibody treatment and Rho-ROCK inhibition, or agents to mobilize endogenous cells such as metformin. ROCK, Rho-associated protein kinase. Adapted from Ahuja et al.6 Color images are available online.
Any therapeutic intervention should consider and address the dynamic pathophysiological events occurring in SCI. For example, combating measures against increased extracellular amino acids should be applied early on, in minutes, while measures against inflammatory mediators in hours and measures against myelin-associated inhibitors within weeks after injury.22 Because inflammation continues and may persist for years, the administration of therapeutic measures should be considered to continue accordingly.22
Given practical limitations of treating acute SCI in a clinical setting, many therapeutic interventions in development aim to target either the secondary or chronic stages of injury. For example, accumulation of myelin debris during secondary injury is associated with inhibition of regeneration. Myelin-associated proteins lead to inhibition of neurite outgrowth, thus named NOGO inhibitors, which act through NOGO receptors leading to activation of GTPase Rho A. Its effector, Rho-associated protein kinase (ROCK), leads ultimately to apoptosis, axonal collapse, and neurite retraction.6 While a detailed discussion of the mechanisms of myelin-associated inhibition of axonal outgrowth is outside the scope of this review, we refer the reader to a review by Zhang et al. for more information.23
There is also an accumulation of extracellular amino acids at the site of injury, whose abundance relates to severity of SCI and neurotoxicity.22 These molecules are attractive targets for pharmacological intervention aimed at enhancing neuroprotection and neuroregeneration post-SCI and are discussed more in the following sections. During secondary injury, damage occurs on the cellular level through several mechanisms, including mitochondrial dysfunction leading to high calcium levels, increased production of oxygen free radicals, and oxidative injury.24
In chronic injury, scar formation is a problem and its disruption is a common strategy for promoting regeneration. For example, a recent study found that the protein tyrosine phosphatase σ (PTPσ) contributes to a transition of regenerating growth cones into dystrophic bulbs through interactions with chondroitin sulfate proteoglycans (CSPGs), which are abundant in the glial scar and many forms of which have been widely reported to inhibit axonal outgrowth.25 Experimental inhibition of PTPσ was found to promote regeneration in a rodent model of SCI by enabling axons to overcome CSPG-mediated growth inhibition and, consequently, serotonergic fiber navigation across the SCI lesion leading to some functional recovery.25
While the CSPG-rich glial scar was previously thought to be completely inhibitory to axonal growth, more recent studies have found that some aspects of scar ECM, which is secreted by astrocytes, are permissive and may even promote regeneration.26 Furthermore, prevention, attenuation, or ablation of chronic astrocytic scars increased lesion size and reduced axonal ingrowth.27 Recent studies found also that the ECM secreted by protoplasmic, but not fibrous, astrocytes can promote axonal elongation when injected into the rats' spinal cord after SCI.26 Taken together, these findings suggest that while some aspects of the astrocytic scar may be detrimental, other components may be required for regeneration.
Therapeutic Approaches
Standard treatments
The management of SCI represents a formidable challenge, and there is no treatment at present available that can effectively restore lost tissue function. Several factors are responsible for the failure of healing after SCI, including chronic local inflammation and the release of antiregenerative factors. Present treatment methods include the use of neuroprotective and neuroregenerative strategies. The first line of management for SCI patients includes resuscitation, stabilization, and critical care in specialized centers with early determination and localization of specific injuries. Stabilization may include removal of bone fragments or foreign objects, physical stabilization of the vertebral column, and spinal decompression.
Nonpharmacological methods to reduce inflammation, including hypothermia28 and cerebrospinal fluid drainage,29 have also been in phase I/II clinical trials with promising early results. Early surgical decompression (within 24 h), administration of anti-inflammatory drugs, and augmentation of blood pressure lead to reduced acute complications and hospital stay.6 Timely and early intervention is important and can be achieved by using neuroprotective measures (ideally in the acute stage).
The management of SCI also includes prevention and treatment of complications, and physical rehabilitation. Without these, there is very limited recovery of neurological function.1 Besides failure of regeneration, SCI is often complicated by other problems such as infection and pressure sores, which can be serious and ultimately lead to death, such as in the case of the actor Christopher Reeve following his SCI.30 Development of regenerative therapies to treat neurological deficit represents an active area of research and is a rapidly growing field.6 Improved clinical outcomes will emerge by harnessing the potential of recent advances in different fields and integrating them to create innovative therapeutic strategies.
Neuroregenerative and neuroprotective therapies
Despite active research and clinical studies, there is no effective and globally accepted standard treatment for SCI.8 Various minimally invasive, neuroprotective, and neuroregenerative strategies using cells, biomaterials, or pharmacological agents are in various stages of preclinical research and clinical trials around the world (Table 1 and Fig. 3). Clinical trials have evaluated agents that can neutralize axonal growth inhibitors, such as the Rho-ROCK inhibitor, Cethrin/VX-210, and anti-NOGO monoclonal antibody.6,31 However, no pharmacological agent for neuroregeneration (restoration of neurological function) has been approved by the U.S. Food and Drug Administration (FDA) for treatment of SCI.32
Table 1.
Biomaterials Studied for Treatment of Spinal Cord Injury
| Materials used | Cells or factors included | Injury model and delivery method | Notable findings | References |
|---|---|---|---|---|
| Phase-separated poly (2-hydroxyethyl methacrylate) (pHEMA) | N/A | Spinal lesion, injection | Reduced accumulation of GFAP and Neurocan; however, scaffold did not integrate into tissue | 134 |
| Alginate | EGF, bFGF | Clip-compression, intrathecal injection | Improved functional recovery; enhanced outgrowth of corticospinal tracts, and angiogenesis | 135 |
| HPMA copolymers | Bivalirudin peptides | Clip-compression, intrathecal injection | Decreased cellular proliferation, inflammation, and astrogliosis | 144 |
| Hyaluronic acid | IKVAV, BDNF | Clip-compression, intrathecal injection | BDNF-hydrogels showed greatest improvement in locomotor functional recovery | 152,155 |
| Agarose/carbomer hydrogels | RGD, ECM deposition | Clip-compression, intrathecal injection | Increased MP M2 population | 43 |
| Human mesenchymal cells | ||||
| BD PuraMatrix synthetic peptide | Human Schwann cells | Clip-compression, intrathecal injection | Reduced astrogliosis and promoted infiltration of endogenous S100-positive cells | 56 |
| Collagen | Laminin | Clip-compression, intrathecal injection | Enhanced long-term cell survival, vascularization, axonal regrowth, and infiltration | 11 |
| Schwann cells |
ECM, extracellular matrix.
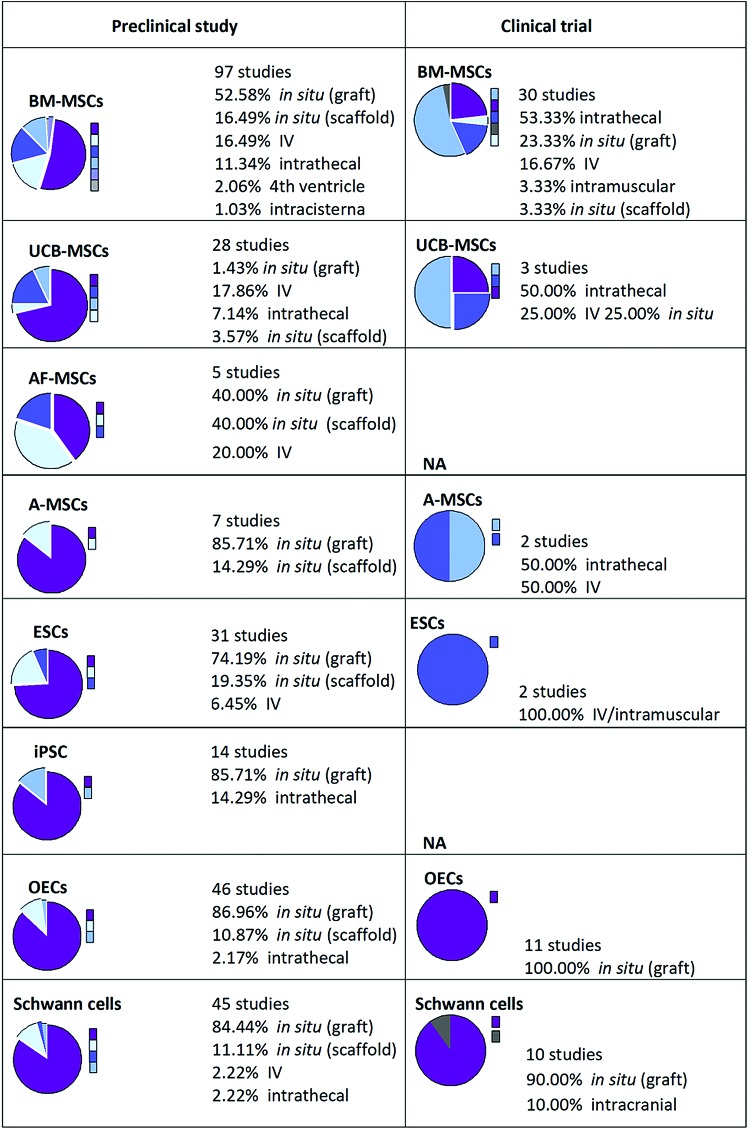
FIG. 3.
An illustration showing the global trends of clinical and preclinical studies in which cell transplantation is involved. Number of studies, type of cells (ESCs, BM-MSCs, A-MSCs, AF-MSCs, UCB-MSCs, or OECs), and routes of administration (IV, intrathecal, fourth ventricle, or intracisterna) are shown. When no data available, it was indicated as NA. AF-MSCs, amniotic fetal MSCs; A-MSCs, adipose tissue-derived mesenchymal stem cells; BM-MSCs, bone marrow-derived mesenchymal stem cells; ESCs, embryonic stem cells; IV, intravenous; OECs, olfactory ensheathing cells; UCB-MSCs, umbilical cord blood MSCs. Reproduced with permission from Vismara et al.70 Color images are available online.
Cell-based therapies may provide multiple benefits, including neuroprotection, angiogenesis, immune modulation, and tissue regeneration. Cell types evaluated have included stem cells (embryonic [ESCs], neural [NSPCs], or mesenchymal [MSCs]) and glial cells (Schwann cells, olfactory ensheathing cells [OECs], or oligodendrocyte precursor cells [OPCs]).1,6 In addition, electrical field stimulation of the injured spinal cord has been used to enhance neuronal plasticity and direct rewiring of circuitry into functional relays.33 As in wound healing of other tissues, angiogenesis within the injured spinal cord tissue has been correlated with positive outcomes, particularly in studies delivering biomaterial, cell, or angiogenic soluble factors.13,34,35
Biomaterials have also been investigated for their inherent regenerative potential as well as for the delivery of therapeutic agents, including cells,9,16,36–44 drugs,36,45 growth factors,16,37,38,46 or genes (Fig. 4).47 Combinations of these are also being investigated.16,36–38,48–51 Nevertheless, only one biomaterial has reached clinical trials,52 while most remain in preclinical animal43,53–56 or in vitro stages of investigation.57,58 Even developed therapies with potential for clinical translation still require extensive testing to prove their safety and efficacy in patients. Further complicating results is the fact that there are no comprehensive studies to compare different strategies under identical conditions. Research groups can vary greatly in the choice of animal or injury model and are understandably restricted in scope. To date, none of these neuroregenerative therapies is used routinely in clinical practice.
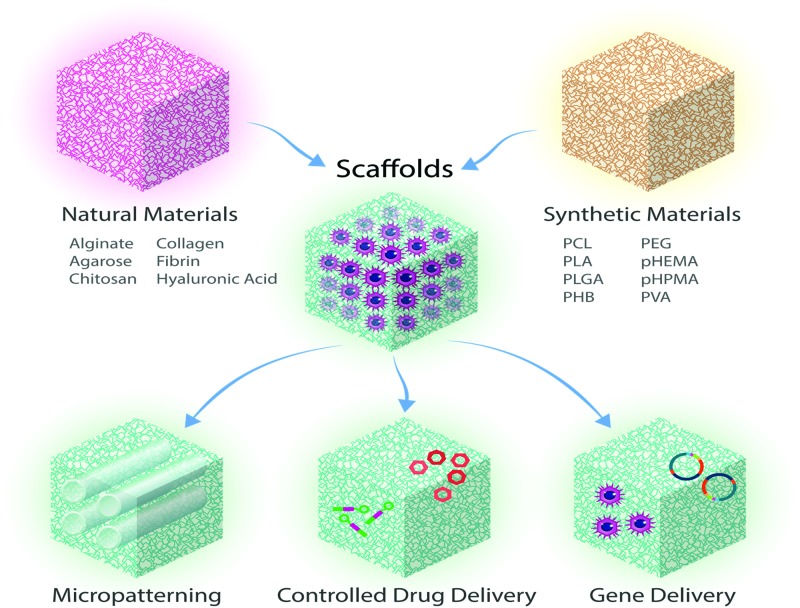
FIG. 4.
Illustration showing that scaffolds (made of synthetic or natural biomaterials) can be used for the treatment of SCI, as micropatterned devices or as matrices combined with drug delivery, or gene delivery to enable guided tissue regeneration. Adapted from Iyer et al.201 Color images are available online.
Cell-based therapies
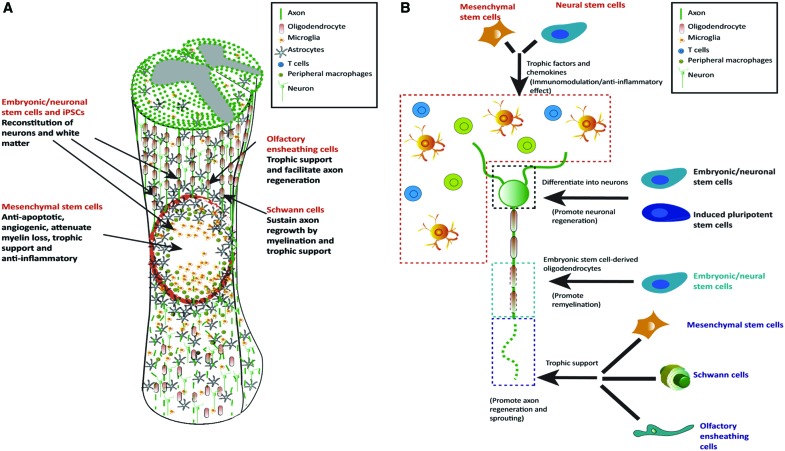
Cell-based therapy may be suitable for the treatment of discrete lesions in SCI (Figs. 3 and 5) to provide the following: (1) trophic support,59 (2) immunomodulation,60,61 (3) angiogenesis,35 (4) and generation of new CNS cells,1,6 which may form functional relays (Fig. 6).15,16,48 In addition, paracrine factors released by administered cells may aid in the activation of resident progenitors.62 While stem cell therapies have shown promising results in clinical trials,63,64 translation faces substantial challenges, including timing and method of administration,1 and risks that have to be carefully evaluated, including (1) poor survival of transplanted cells,46,63 (2) migration away from the delivery site,65,66 (3) formation of ectopic stem cell colonies or tumors,67 (4) excessive proliferation,37 (4) differentiation into unwanted, nonregenerative cell types,68 and (5) aberrant axonal growth leading to allodynia.69 Controlling stem cell differentiation or production of specific regenerative factors remains as additional key objectives.70,71
FIG. 5.
Schematic illustration showing the main cellular targets of cell therapy in SCI and how this works (A), with particular focus on the mechanism by which stem cells act to achieve immunomodulatory action, differentiation into neurons and oligodendrocytes, or providing trophic factors that can support axonal regeneration (B). Reproduced with permission from Vismara et al.70 Color images are available online.
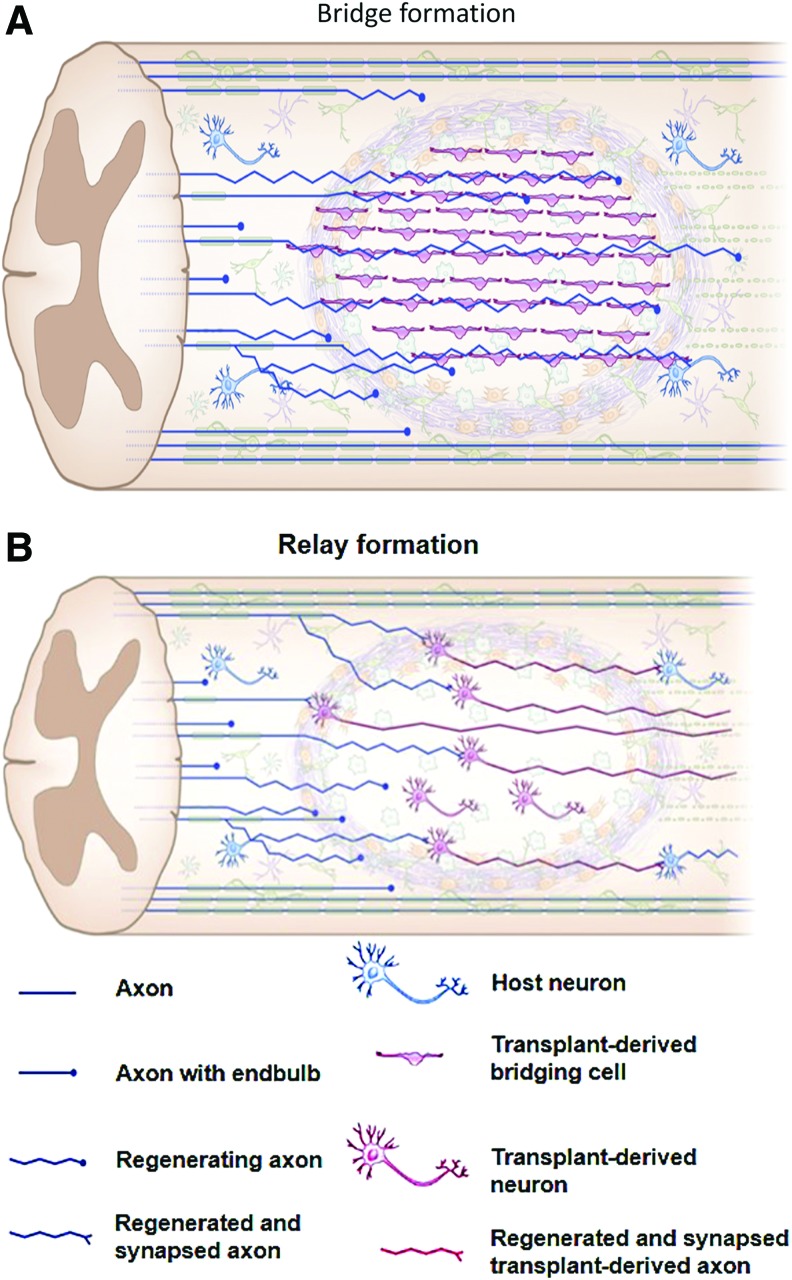
FIG. 6.
Schematic illustration showing bridge (A) or relay (B) formation during the repair process after the transplantation of cells for the treatment of SCI leading to neuronal connectivity. Reproduced with permission from Assinck et al.17 Color images are available online.
While various cell therapies are being evaluated in clinical trials, none of these trials has progressed past phase I/IIa.3 The absence of controls in these trials has limited their value, and the efficacy and long-term outcomes after treatment of SCI are yet to be demonstrated.17 Financial costs of cell-based therapies are another major hurdle. Of the more successful clinical trials, one was terminated due to limited long-term effects,72 while another was terminated for financial reasons.73 Questions relating to the type of cells used, number, and safety remain to be addressed.74 Stem cell therapy remains experimental and it cannot ethically be offered to patients as a proven form of treatment.
Despite these limitations, clinical and preclinical studies evaluating effects of transplanting several different cell types, including NSPCs, MSCs, Schwann cells, OPCs, and OECs, have shown promising results to date.1,6,17 NSPCs have been derived from human fetal spinal cord,63,75 human fetal brain,44,72,76 or pluripotent or ESCs64 and can differentiate into the glial and neuronal cells, which normally make up the spinal cord. MSCs, including those derived from adipose tissue (A-MSCs),77 umbilical cord blood,78 and Wharton's jelly,79 human OPCs derived from human ESC (hESC) lines,64 and human NSPCs derived from fetal spinal cord tissues63 have been investigated in clinical trials.
While not in clinical trials, there has also been recent interest in using human dental pulp cells (hDPCs) because they contain neural crest-derived ecto-MSCs. These cells were found to be more effective than bone marrow-derived MSCs in inducing functional recovery in rodents following SCI.80 Another promising cell source yet to be used in clinical trials is the human inducible pluripotent stem cell (iPSC), which may help overcome issues with immune rejection of allogenic transplants.
These types of cells vary greatly in their tissue source and processing methods, often making it difficult to directly compare results obtained from different studies. For example, there is strong evidence that NSPCs or OPCs derived from hESCs using different protocols or obtained from varying sources may have different regenerative capacities.46 For example, some preclinical studies reported robust results with the use of NSPCs, and it was found that axonal regeneration can be obtained even with the use of NSPCs obtained from a different species, for example, with the use of human NSCs in immunodeficient rats.16 In contrast, other reports indicated that CNS-derived stem cells were not efficient in the treatment of cervical spine contusion injury in mice and may even have negative effects.46
Recently, questions regarding the translation of preclinical data describing cell therapies to the clinic have been raised.46,81,82 It was found that there are differences in therapeutic effects between cell lots, such as that observed with the cell lots used for research and those of clinical grade of an NSPC product (human central nervous system stem cell [HuCNS-SC]) cell in preclinical studies by Anderson et al.46 Although the use of a research-grade cell lot was successful in rat and mouse thoracic SCI models, transplantation of the corresponding clinical-grade cell lot into a cervical SCI mouse model provided no benefits. Moreover, some data suggested negative effects.46
One possible reason for the difference is the heterogenicity of the fetal tissue from which these cells were derived. Donor genetic makeup, the location from which cells were taken, and the developmental stage at which the cells were isolated may all vary. In addition, protocols and reagents used to maintain and differentiate therapeutic cells may all lead to differences among study outcomes.81 These difficulties may have contributed to the termination of clinical trials using HuCNS-SC transplantation for the treatment of SCI, which were terminated based on business decisions unrelated to any safety concerns.72,83 Differences between research-grade and clinical-grade cells will undoubtedly be a critical factor in the translational capacity of stem cell therapies.
Timing of the administration of cell-based therapy after SCI is very important. Early delivery after an acute injury may not be appropriate because the hyperinflammatory environment may lead to death of transplanted cells. On the contrary, delayed administration may result in no neuroregenerative effects because of the loss of cell plasticity at the site of injury and extensive scar and cyst formation.74 Studies have suggested that cell transplantation during the subacute stage is more effective than that during acute or chronic stages.1 Hence, in most previous studies, cells were administered during the subacute stage.84 It was also found from a clinical study that cell application within the therapeutic window of 3–4 weeks post-SCI will have an important role in clinical settings to balance the feasibility of transplantation with optimal therapeutic efficacy.85
Soluble bioactive molecule-based therapies
Soluble bioactive molecules, such as growth factors and drugs, have been explored as SCI therapies for their neuroprotective, neuroregenerative, angiogenic, and immunomodulatory effects. As discussed in detail later in this review, biomolecules are often delivered in combination with biomaterials.16,86,87 The use of carriers88 such as hydrogels, and nanoparticles (NPs) can improve therapeutic drug delivery and efficacy.89 Promising neuroprotective therapies for use in the acute stage of SCI that are being investigated for translation to the clinic include the use of various pharmacological agents such as riluzole,90 magnesium,91 minocycline,92 hepatocyte growth factor (HGF),93 and granulocyte-colony stimulating factor (G-CSF).6,94
In preclinical studies, improved axonal sprouting has been demonstrated in experimental SCI in rats to which antibodies against NOGO were delivered by cells placed into the parietal cortex.95 Inhibition of phosphatase and tensin homologue (PTEN), a downstream target of NOGO-RhoA-ROCK in the sensorimotor cortex, has also been shown to promote corticospinal tract regeneration in rodents.96,97 Another recent study reported that the administration of connective tissue growth factor (CTGF) in zebrafish, after SCI leads to healing of SCI in these fish within weeks.98 Recently, pregabalin, an established drug treatment for several neurological disorders, was also found to block the axonal regeneration inhibitor in mice,99 and it is worth exploring its use further in SCI studies.
Biomolecules modulating the immune response are also candidates for SCI treatment. For example, blocking of the interleukin-7 (IL-7) receptor favors the generation of M2 phenotype macrophages and thus improves functional recovery after experimental SCI in mice.100 Delivery of IL-4 and IL-10 chemokines to promote macrophages to adopt an M2-like phenotype has also been shown to improve recovery after SCI in a mouse model.101,102 The use of fractalkine, a chemokine that preferentially recruits reparative monocytes, was found to dramatically increase the regeneration of experimental nerve defects in rats103 and thus may have benefits if used as a treatment for SCI.
Factors released by stem cells may also be useful in the treatment of SCI.70 Intrathecal injection of conditioned medium of MSCs into experimental SCI in rats was found to increase axonal regeneration and improve locomotor recovery 1–4 weeks after the treatment.59 Stem cell-conditioned media contain several potentially regenerative factors, including insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGF-β1), and HGF. It has been reported that the use of such media are more effective than using individual factors or their combinations in enhancing the survival of neurons and growth of neurites.104
Factors secreted by stem cells can also have an important role in regulating immune cell phenotypes, that is, immunomodulatory action. Such secreted factors may influence neutrophils, macrophages, dendritic cells, NK cells, T cells, and B cells.71 Furthermore, stem cell-derived exosomes can be used to modulate the recruitment and function of macrophages.105 It is important to have a deep understanding of stem cell-derived exosomes, and their effect, to enable the development of novel therapeutic methods.71 The use of these media or exosomes provides many future possibilities for their application in minimally invasive therapy of SCI.
Therapeutic biomolecules can also be used to stimulate resident NSPCs, various types of which are present throughout the spinal cord.106 It has been suggested that ependymal progenitor cells, present in the central canal of the spinal cord, may enable regeneration in some animals even after severe injury.107 Furthermore, ependymal progenitor cells in the glial scar can be beneficial. They can limit secondary injury by maintaining tissue integrity and releasing bioactive factors that promote neuronal survival.108,109 Thus, manipulation of the local ependymal stem cell niche107 or mobilization of other resident progenitors106 to stimulate self-repair through these cells may be a useful approach.110 For example, delivery of factors known to promote oligodendrocyte-lineage differentiation during development in a mouse model of acute SCI, including sonic hedgehog, platelet-derived growth factor-AA (PDGF-AA), and noggin, has been reported to increase the numbers of myelinated, regenerating axons and functional outcomes.111,112
It is worth highlighting some recent outcomes of clinical trials investigating the use of antiregeneration inhibitors (Rho-ROCK inhibitors113 and G-CSF114) for the treatment of SCI. Rho-ROCK inhibitors are thought to have neuroprotective and neuroregenerative effects.115 In a phase I/IIa clinical trial that involved 48 patients who had acute and complete cervical (C4-T1) or thoracic (T2-T12) SCI, the application of Rho inhibitor to the epidural space in a fibrin glue resulted in no increased adverse effects. Improvement in long-term motor function was best seen in cervical SCI patients who had the drug administered during 7.8–146 h after injury.115
Despite these promising results, a phase IIb trial for treatment of acute SCI with Cethrin/VX-120 was halted in late 2018 due to lack of efficacy.116 G-CSF is also being investigated for the treatment of SCI.114 Two phase I/IIa trials94,117 and a small-scale double-blind, randomized clinical trial118 have reported improved outcomes. However, future clinical trials that are larger in scale and randomized will be required to demonstrate the efficacy of the treatment with G-CSF.
While the majority of previous clinical studies have delivered potentially therapeutic agents either intravenously or orally, intrathecal delivery may provide better access to the injury and better approximates the design of many preclinical experimental studies with positive results. Thus, despite its more invasive nature, it has been argued that this route of administration will have better clinical success.119 A recent phase I clinical trial reported the safety and tolerability of intrathecal infusion of the human anti-NOGO antibody ATI355 in patients after acute SCI.31 In addition, a phase I/II clinical trial in which the HGF was intrathecally injected at the lumbar level in patients with cervical SCI was completed in 2018 in Japan, and results are expected in the near future.119
Biomaterial-based therapies
So far, no biomaterial-based regenerative therapy of SCI is in routine clinical practice. However, a biomaterial technology, the Neuro-Spinal Scaffold, has recently reached clinical trials.52,120,121 Biomaterials used for the treatment of SCI can be in the form of conduits,101,112,122 sheets,123 scaffolds,121,124 fibers,125 particles, or hydrogels (Table 1).126–130 Biomaterials are used to mechanically stabilize the injury site and provide an environment for interactions with host cells, physically fill SCI-associated cavities, reconstituting ECM, and bridging the injury to guide axonal growth across the gap.70,101,112,121,131–135 To guide axonal regeneration, several biomaterial architectures have been investigated, including channels,86,136–138 fibers,139 scaffolds, and magnetic microgels.58
Biomaterials for the treatment of SCI can also be used for the delivery of therapeutic agents and cells (Fig. 4).6,132 In addition, they can support cell survival of delivered cells in situ,44,50 as discussed later in this review, and recruit migrating endogenous cells, such as Schwann cells121 and stem cells,111,112,140 that can support regeneration. To avoid additional complexities associated with the use of cells, the use of acellular biomaterials for supporting spinal cord regeneration and remodeling after injury has been important to investigate.121,136,141,142
It is preferable that biomaterials be biodegradable and injectable for delivery through minimally invasive means.70 In the acute phase of SCI, the use of a hydrogel is preferred as it may be used to seal the dura and release anti-inflammatory drugs. At later stages the use of fibers and conduits may be better for achieving guided neural regeneration.132 However, it would be ideal to design a system that could be implanted acutely and remain for months to both guide regeneration and prevent chronic inflammation.
To achieve appropriate tissue repair, the implanted biomaterial should degrade as the host tissue regenerates. While biomaterials such as poly(lactide-co-glycolide) (PLGA) degrade hydrolytically,142,143 naturally derived materials such as fibrin are degraded by cell-produced enzymes15,48 and thus may have better potential for coupling degradation to native matrix formation, which is a challenging objective. Hybrids of synthetic and natural biomaterials can combine desirable properties of both types of materials. For example, synthetic biomaterials with controlled properties and tailored degradation profiles can be functionalized with peptides, which are susceptible to degradation by cell-produced enzymes,138,144 or provide sites for cell adhesion.121,125
Several different types of scaffolds have been investigated for the treatment of SCI. A scaffold made of a block copolymer of PLGA and poly-l-lysine (PLL) with a highly interconnected porous structure (approximately 250–500-μm-diameter pores) was shown to lead to improved functional recovery after SCI in rats.145 Later, the safety and efficacy of these scaffolds were evaluated in partial and complete hemisection models of thoracic SCI in Old-World primates (Chlorocebus sabaeus) and significant recovery of locomotion was observed.141 A significant increase in remodeled tissue with neural sprouting was seen 12 weeks after injury.
Results from preclinical studies, where these PLGA-PLL devices known as “Neuro-Spinal Scaffold” were implanted into a thoracic-level contusion in rats and minipigs after internal decompression was performed, reported reduced inflammation and lesion cavitation volumes and increased tissue sparing and deposition of new, endogenous laminin-rich tissue.121 However, no functional benefits were observed. The Neuro-Spinal Scaffold has recently been evaluated in small-scale clinical trials for treatment of thoracic-level SCI and has shown promising results.52,120
While porous polymeric scaffolds, such as those based on PLGA, have shown promise, hydrogel materials can be fabricated to better match hydration and mechanical properties of native spinal cord tissue, both of which are thought to reduce inflammation and scarring. In addition, hydrogels can be readily formulated to be injected and formed in situ in the spinal cord. In addition, microgels can be used for the delivery of cells,146,147 drugs, and/or biomolecules,70,89,148 and they can be interesting formulations to use in regenerative therapy of the spinal cord. For detailed reviews of hydrogels as minimally invasive therapies for spinal cord repair, the authors refer the readers to Perale et al.,127 Macaya and Spector,126 Pakulska et al.148 and Khaing et al.149
The use of polyethylene glycol (PEG), an FDA-approved biomaterial used for many applications, is considered promising for the treatment of SCI because it can support stem cell growth, migration, proliferation, and differentiation. PEG hydrogels can also help to reduce local glial scar invasion, and promote and guide axonal regeneration.138,150 While PEG is essentially biologically inert, hydrogels containing bioactive polymers provide the opportunity to more directly affect cells in the spinal cord, and thus, the repair process. In particular, polymers derived from the native ECM, including hyaluronic acid (HA),26,44,50,151–155 fibrin,37,42,48 and laminin,125,156,157 have shown success in preclinical studies.
In contrast to including individual ECM components, cell-secreted ECM compositions have also been shown to aide in regeneration.43,125,158 Alternatively, spinal cord tissue, decellularized to preserve not only the ECM composition but also the structure, has been found to enhance axonal regeneration in the spinal cord of rats.159
A number of studies have found that provision of a physical structure to guide regenerating axons along the longitudinal axis to their targets in the form of conduits or channels enhances functional recovery after SCI. For example, collagen-based neural conduits were functionalized with the neurotrophin-3 (NT-3) gene and used for the treatment of completely transected spinal cord in rats.47 Increased NT-3 levels seen in surrounding tissues and axonal regeneration, observed 1 month postoperatively, were significantly higher than in controls.
PLGA scaffolds with multiple guidance channels implanted in mice were found to be sufficient for promoting corticospinal tract axonal regeneration into the channels, through the injury site, and out of the conduits, where they have the opportunity to connect with synaptic targets caudal to the lesion.160 In this study, the extent of axonal regeneration was found to correlate with functional recovery. Hydrogels made from multiple base materials and containing guidance channels have been reported to promote regeneration in animal models of SCI.9,138,161,162 A recent report by Koffler et al. described the use of a microscale continuous projection printing method to create hydrogel scaffolds with guidance architectures that conform to contusion lesions in unique animals.161
While conduits and channels provide macroscale guidance structures, nanofibers that mimic the nanostructure of the native ECM have been developed for regenerative biomaterials and studied in vitro and in vivo.163,164 Nanofibers have been shown to guide the orientation of regenerating neurites parallel to their orientation.165 In addition, the use of electrospun nanofibers has been associated with ESC differentiation toward neural lineages.166 Recently, it was found that peptide nanofibers containing long laminin motif can influence neurogenesis both in vitro and in vivo.157 Synthetic poly(ɛ-caprolactone) (PCL) nanofibers were also investigated and found to successfully create electrically active human three-dimensional (3D) neuronal networks with synapses using brain neural progenitors.167
Engineered NPs have been explored for their use for the regeneration of the spinal cord. For example, PLGA NPs were used for local delivery of flavopiridol (a cyclin-dependent kinase inhibitor that inhibits astrocyte growth and inflammatory agent synthesis), and they resulted in recovery following SCI in rats.168 Using a biological ligand, gold NPs were bonded to neurons for optical excitation and they exhibited potential benefits over optogenetics.169 Pulsed infrared light and plasmonic gold nanorods were also used to enhance infrared neural stimulation by increasing neural responsivity.170
Intravenously injected NPs made of ferulic acid-modified glycol chitosan were shown to be effective in achieving functional restoration after SCI in rats.171 Moreover, magnetic NPs coupled to HA-based hydrogels can be used to modulate the activation of mechanosensitive ion channels in dorsal root ganglia neurons as it was demonstrated in vitro.20 Nanofibers can be combined with therapeutic agents and NPs164 to enhance their function. For example, gold NPs were incorporated into electrospun PCL/gelatin nanofiber-based scaffolds. Neuronal cells were subsequently seeded onto these scaffolds and resulted in axonal elongation forming 3D networks in vitro.18 In another example, zero valent zinc NPs included in a PCL matrix using electrospinning were found to promote neuroglial cell proliferation in vitro.19
In addition, electroconductive (e.g., graphene) or magnetic (e.g., iron oxide NPs) elements can be incorporated into scaffolds. The use of electroconductive materials and applied electromagnetic fields may help to influence cell migration, adhesion, proliferation, and differentiation.172 For example, magnetic NPs can be used to guide neurite growth173 and enable the control of cell organization and differentiation (Fig. 7).174 It was found that when polypyrrole-coated poly (l-lactide-co-ɛ-caprolactone) nanofiber nerve conduits were combined with electric stimulation and used in the treatment of experimental sciatic nerve defects in rats, results were similar to those obtained with the use of autografts.175 Cell elongation was found to occur in a direction parallel to that of the nanofibers.176
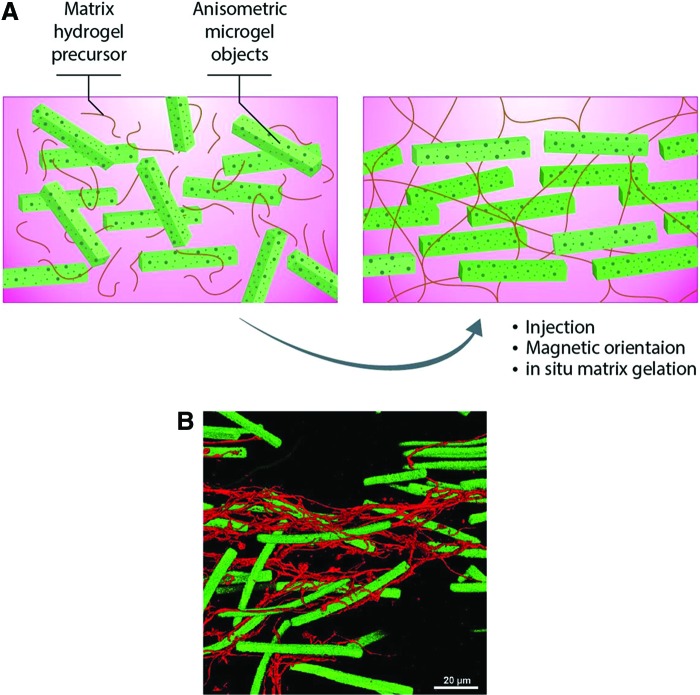
FIG. 7.
The use of magnetic rods in fibrin hydrogel to help guide regeneration that can be aligned upon exposure to magnetic field. (A). An illustration showing the method of preparation of injectable hybrid hydrogel. A unidirectional structure made of aligned rod-shaped, magnetoceptive microgels within the fibrin hydrogel is generated in situ by exposure to magnetic field. Following this, the surrounding liquid prehydrogel is crosslinked, so that microgel orientation is fixed to guide aligned cell ingrowth. Adapted from Rose et al.58 (B) Premixed fibroblasts can be seen to extend along the microgel axis (green), as visualized by stretched F-actin filaments (stained in red). Reproduced with permission Rose et al.58 Color images are available online.
Combined with electrical stimulation, conductive carbon nanofiber-based scaffolds were found to enhance the regenerative function of NSPCs.177 Moreover, core-shell-structured, piezoelectric polyvinylidene fluoride (PVDF) nanofibers that were chemically wrapped by graphene oxide lamellae showed a desirable out-of-plane piezoelectric constant higher than that of the conventional pure PVDF nanofibers.178 To produce graphene-containing silk, a recent method reported feeding of graphene to silk-producing worms to create silk with new properties,179 adding to the available fabrication methods.
The concept of biomaterials that can be injected and form a defined guidance architecture in situ is attractive; nevertheless, development of such biomaterials has posed a formidable challenge. Promising strategies to overcome this challenge include the use of scaffolds that are based on self-assembling fibers56,95,124,180 or that are embedded with magnetically responsive microgels.58 In an interesting study, poly(ethylene oxide-stat-propylene oxide) gels containing supermagnetic iron oxide nanorods were used to help the orientation of nerve extension parallel to the rods that were aligned by using an external magnetic field.58,181 This offers a great opportunity to develop minimally invasive and regenerative therapies for SCI.
Combinatorial therapeutic approaches
Because of the complex nature of pathophysiological changes that occur following SCI, combinatorial approaches that address different aspects of the problem are expected to be more effective. In such an approach, biomaterials may be combined with cells and soluble molecules and administered locally to an injury. It is evident that biomaterials can provide sustained drug delivery, enhance survival, growth, host tissue integration, and even differentiation of delivered cells (Fig. 4).182 Attention should be paid to proper biomaterial selection, tailored drug release, source and processing of therapeutic cells, and timing of intervention. Despite recent advances in these therapeutics, there is still a likely long way to go to achieve a therapy that can lead to significant recovery after SCI.183
Biomaterials and soluble biomolecules
In one approach for the treatment of SCI, biomaterials and bioactive molecules can be combined (Table 1). There are many examples of procedures using soluble molecule delivery, for example, growth factors,86,87,184,185 drugs,115 or other therapeutic agents,54,186–189 for the treatment of SCI. Combining biomaterials with diffusible factors, and even gene therapies, can maximize therapeutic effects by targeting more than one aspect involved in SCI.
Biomaterials can also maintain appropriate local therapeutic concentrations at or near the injury site over longer periods of time than bolus injection and hence better support regeneration.70 For example, hydrogels fabricated from a hydrolytically degradable macromer [poly(l-lactide)(PLA)-b-PEG-b-PLA] were used for sustained release of NT-3 into the injured spinal cords of rats over the course of 2 weeks, where this combinatorial therapy induced more axonal regrowth than either individual therapy.188 Such controlled release systems help to overcome the need for multiple injections or implantation events. Anderson et al. recently demonstrated that injectable biomaterials could be used as reservoirs for localized delivery of a combination of three growth factors (FGF2, EGF, and GDNF) whose synergistic actions were required to enhance recovery after SCI in a rodent model.185
Biomaterials and cells
The use of biomaterials to deliver cells after SCI has been reported by several studies to improve treatment efficacy (Table 1).190 Biomaterials may physically protect loaded cells from the hostile environment of SCI, retain administered cells near the injury site, and provide an adhesive matrix for cells and other cues to improve their survival.43,191 By minimizing the loss of delivered cells, the number of cells required for achieving a therapeutic effect can also be reduced.43 Promising results with the use of different biomaterial/cell combinations have been observed in experimental SCI models. These include neurotrophic factor expression,13 improved postimplantation cell survival,11,16,44,50,138 neuronal differentiation,10 and directed axonal regeneration.9
Although various forms of biomaterials can be used, hydrogels represent an attractive form as they can be easily used as minimally invasive therapies. A study by Kadoya et al. demonstrated some potential advantages of delivering therapeutic cells via methods that are minimally invasive, specifically those that maintain an intact dura.16 The study observed formation of ectopic stem cell colonies in rats that were administered a cell-laden fibrin matrix, which also contained a cocktail of growth factors, after opening the dura. However, no ectopic colonies were observed in closed dura delivery, where no growth factor cocktail was used.16 In addition, injection delivery of cells within hydrogel can help to reduce cell death induced by mechanical stresses.192
Several combinations of biomaterials with cells have previously reported varying outcomes of axonal regeneration and functional recovery in experimental models of SCI (Table 1). These have included HA hydrogels with NSPCs,44 self-assembling peptides with NSPCs,12 PEG hydrogels with human iPSC-derived NSPCs,10 polyurethane-based gel with MSCs,193 and Matrigel with Schwann cells.194 A fibronectin-mimetic, peptide-grafted gellan gum hydrogel was combined with A-MSCs and OECs and evaluated for the treatment of experimental SCI in rats. It was found that hydrogel encapsulation of both A-MSCs and OECs led to increased axonal regeneration and significant improvement in motor function.195 Delivery of OECs via PQLA/PLGA scaffolds improved cell survival and proregenerative efficacy in the rat SCI model.13 Delivery of mouse E14 spinal progenitors, loaded onto multichannel PLGA bridges providing a guidance architecture, showed marked improvement of axonal and functional regeneration in a mouse model of thoracic SCI.196 However, another study found that collagen microfibers combined with neural progenitor cells from an alternative source failed to form a bridge across the injury site, or recovery of the motor function in a mouse SCI model.39
It has been proposed that the combination of human MSCs and biomimetic hydrogels could modulate the immune cell microenvironment in SCI, leading to increased proregenerative M2 macrophage population.43 The use of an MSC-seeded alginate hydrogel was found to promote linear axonal regeneration in an experimental rat C5 hemisection SCI model.9
In a separate study, MSCs combined with a collagen sponge were found to facilitate neurite elongation in a rat model of SCI.197 In another study, injectable scaffolds made of self-assembling peptide nanofibers containing bone marrow homing, bioactive peptide motifs were described. When combined with human endometrium-derived stromal cells (hEnSCs), these materials were explored for the treatment of experimental chronic SCI in rats. The nanofibers were found to support neural differentiation of hEnSCs. In addition, higher axonal regeneration and myelination were also demonstrated.181 MSCs are thought to promote neuronal regeneration through neurotrophic protection and immunomodulation.60
Cells and soluble biomolecules
Although there are some studies that have combined cells or growth factors with biomaterials for SCI treatment,16,36–38 relatively few have used combinations of cells and growth factors alone. In some studies, cells themselves were applied as delivery vehicles for specific factors. For example, FGF2 was used to prime hDPCs that were subsequently injected into a complete transection model of SCI in rats. Some axonal regeneration and locomotor recovery were observed.198 In vitro, astrocytes were transfected to overexpress nerve growth factor (NGF) and encapsulated into a collagen scaffold. They were then added to rat dorsal root ganglion culture and found to significantly enhance axonal growth.21 Cells were conjugated with adjuvant drug-loaded NPs and were found to enable pseudoautocrine stimulation of transplanted cells.199 Finally, ESC-derived OPCs were combined with the ciliary neurotrophic factor (CNTF) and used to treat experimental SCI in rats leading to significant improvement in hind limb locomotor function.200
Biomaterials, soluble biomolecules, and cells
Combining biomaterials, stem cells, and biomolecules represents a critical201 and promising14 approach that may improve recovery after SCI.36 Exciting recent work has demonstrated that delivery of human NSPCs within a simple fibrin matrix containing a growth factor cocktail after SCI supports extensive engraftment with host neuronal circuits leading to functional recovery in both rodent15 and nonhuman primate models.48 Another recent study used a combinatorial therapy comprising a scaffold containing Schwann cells modified to overexpress NT-3 and adult stem cells modified to overexpress the NT-3 receptor TrkC.202 In each of these studies, results suggest that NSPCs can integrate with the host tissue and act as neuronal relays across SCI leading to functional gains.
A separate study incorporated NT-3 within silk fibroin coatings on poly(ɛ-caprolactone)-block-poly(l-lactide-co-ɛ-caprolactone) (PCLA) conduits, with enabled sustained release of the growth factor,203 which may provide advantages over delivery from a bolus or cell source. When combined with rat E14 NSPCs and transplanted into the rat thoracic spinal cavity after complete transection, incorporation of NT-3 increased NSPC survival, axonal regeneration, and functional improvement. Others have developed biomaterials as affinity-based delivery platforms (e.g., based on heparin-binding domains) for growth factors, including NT-3 and PDGF-AA, in combination with NSPCs and have found similar benefits in rodent models of SCI.37,50
Beyond growth factors, a hydrogel delivery platform based on SH3 affinity has been used to deliver chondroitinase ABC in combination with human iPSCs, resulting in better survival of both host and transplanted cells.51 Liu et al. have described an approach where alginate biomaterials with longitudinally aligned channels to provide guidance were seeded with Schwann cells and implanted into cervical lesions in rats.49 Adenovirus encoding BDNF was delivered caudally to the lesion to encourage axons to regenerate along the rostral/caudal axis and allow axons to exit biomaterial implants and re-enter the caudal spinal cord.
Other therapeutic approaches
Because of the complexity of the healing process of the spinal cord after SCI, taking advantage of non tissue engineering approaches may further support restoration of function. For example, small-scale clinical studies have demonstrated that the use of epidural electrical stimulation, in particular in conjunction with dynamic, task-specific training, can restore voluntary, coordinated motor activity in previously paralyzed individuals following SCI.204–207 It is envisioned that combining proregenerative drugs, biomolecules, and/or cells with electrical stimulation and/or rehabilitative training will lead to better results. This type of approach would be expected to create a proregenerative environment supportive of axonal growth and plasticity, which would then be guided to form and maintain relays for different bodily functions via direct neuromuscular stimulation.33 For example, Chen et al. found that treatment of rats with anti-NOGO-A antibodies after incomplete thoracic SCI followed by treadmill locomotive training improved motor recovery.208
Challenges and Future Perspectives
Many obstacles to the development of successful treatments for SCI remain, including reproducibility of the therapeutic paradigm (in particular with combinatorial or multimodal therapies), variations in the location and severity of SCI across patients, and clinical variability and recognizing the appropriate timing for treatment intervention, remain to be addressed. Cell-based therapies will face challenges concerning both preclinical processing and clinical administration, such as identifying the optimal number of cells for transplantation that balances efficacy and safety, improving survival of transplanted cells, and directing cell differentiation and functional integration with the host circuitry.
Important challenges also include issues related to interspecies variation and preclinical experimental models. For example, while rat SCI is more pathologically similar to the human case than mouse SCI,209 mice have been used more frequently in preclinical studies. Even the use of rat models is not ideal as these have fundamental differences from humans, and thus, results cannot always be translated to humans. For example, the efficacy of successful cell therapy observed in animal studies has not been reproduced in human clinical trials.17 In addition, while cervical SCI is the most common injury in human patients, it has been less often investigated in preclinical studies.70 Instead, many preclinical studies have used lumbar and thoracic models that are technically easier to perform.210 Therefore, more clinically representative models should be considered and developed in the future.
Given these considerations, preclinical results should be interpreted cautiously. For example, axonal regeneration does not always mean functional recovery. Efforts should be made to identify specific populations of axons responsible for functional recovery. The reversible neuronal silencing technique is a possible method for this purpose.211 However, regeneration of large numbers of axons, which can exit spinal cord lesions caudally, remains a significant challenge.17 There are also differences between animals and humans in biological cues, and hence, the extrapolation of results can be difficult.
Although there have been several reports of cell-based therapies for the treatment of SCI, including clinical trials, treatment efficacy is yet to be demonstrated in phase I-II trials.3 Recently, questions regarding the translation of preclinical data to the clinic have been raised.46,81,82 It is difficult to define potency in cell products and current good manufacturing practice (cGMP) requires testing for the markers that are essential to identify desired cells and contaminants and as indicators of potency. In addition, the FDA does not require a definitive potency assay for early clinical studies. While this policy may serve to speed up translation of some therapies, it leaves room for variations in final products leading to a risk of failure in clinical trials.81 Thus, researchers must balance the need for extensive product characterization with that for helping patients sooner. It should be noted that no preclinical model is ideal, and thus, the ultimate testing for efficacy lies in the human clinical setting.212
In the future, the use of iPSC-derived cells may help to avoid hurdles associated with possible immune rejection of allogenic cells. They may also mitigate the need to address issues with variability across lots, as each batch of therapeutic cells would be tailored for each patient. However, iPSCs will require thorough investigation into the safety of their clinical use, as possible genetic and epigenetic abnormalities may occur during their induction and tumorigenicity after their transplantation.84 Tools, including the use of clustered regularly interspaced short palindromic repeats (CRISPR)-mediated gene editing, have helped to identify key players involved in the cell response to injury, and may aid in developing methods to enhance the ability of stem cell-based therapies to restore function after SCI.213
Real-time, in vivo imaging, data communication and control can be integrated in future therapeutic strategies.214,215 For example, the next wave of innovations would likely include smart, multifunctional materials that can sense, respond, actuate, and report with remote control.215–217 The use of dynamically bioresponsive materials216,217 for developing a new generation of therapeutic constructs may also be useful.218 Such materials can be designed to provide graded, temporally and spatially controlled release of various agents,219 and even stimuli-responsive behavior than can be triggered by external or internal means. Biomaterials may also have electroconductive elements and electroresponsive properties. In addition, including in situ organizable structures (e.g., with the use of magnetic rods58) may promote organized guidance of regenerating axons/neurites. For example, the incorporation of superparamagnetic iron oxide NPs would help targetability under the influence of external magnetic field. This type of approach has the potential to yield new nonsurgical treatment options for SCI.220
Organ-on-a-chip technologies have been developed and used to build various models for studying normal physiology and pathological conditions.221–223 Such models may be used in the future to study the various stages of pathophysiology of SCI, and to develop and test new therapeutics. Organ-on-a-chip models can be more representative to native tissues than conventional two-dimensional cell culture and can possibly recapitulate human cell behavior better than animal studies.223–225 It would be a great advantage to have SCI-on-a-chip models that may help to overcome some of the present challenges, especially those related to the development of effective therapy for a difficult condition.
Commercialization of newly developed therapeutics will depend on the feasibility of scaling up for mass cGMP production, demonstration of clinical safety and efficacy, and obtaining regulatory approvals. When developing therapies integrating diverse technologies (e.g., devices, drugs, and cells), regulatory approval presents additional challenges, such as how these combination therapies affect the body differently than might be expected for each component alone. Thus, new combination products will require extensive preclinical characterization. Targeting more specific SCI pathologies when designing trials, and the use of new imaging technologies and biomarkers6 also promise to improve evaluation of outcomes.
There is a need to build upon already obtained clinical results to refine therapies and, in particular, cell-based therapies that are relatively new and for which the optimal potency, cell types, and dosing for therapeutic benefits are crucial to characterize. In addition, successful clinical translation will require development of safe methods for cell transplantation. Complications and risks related to the use of stem cells, such as the formation of ectopic colonies, need to be carefully assessed and resolved. More studies are needed to optimize minimally invasive delivery methods that may lead to reduced risk of cell migration.16
Moreover, a multidisciplinary approach involving clinicians, researchers, pharmacologists, materials scientists, and bioengineers will be indispensable to achieve successful treatment of patients with SCI, a very challenging, yet important, clinical goal. Development of a global consensus and standardization for cell-based therapies should be encouraged to curb the misuse of these interventions as curative means and maximize the ability of scientific studies to advance innovative treatments for SCI.74
Conclusions
SCI is a devastating condition with a complex pathophysiology for which no present therapies can restore lost neurological functions. The last decade has produced increasingly intensive research that has led to the development of a few cell-based therapies that have proceeded to clinical trials. Unfortunately, despite successes in preclinical animal studies, efficacy has not been reproduced in humans. To date, the most successful results have been obtained using a combinatorial therapy that used biomaterials, cells, and soluble molecules. It is anticipated that addition of electrical stimulation of the spinal cord to these therapies will achieve a better outcome. At present, functional recovery after SCI is a long process that may take years and it is unlikely that a full recovery will ever be achieved. The development of minimally invasive approaches is desired to reduce complications and avoid additional injury to the spinal cord. Nevertheless, several barriers remain to be overcome through a multidisciplinary approach and consensus based on integrated evidence and recruitment of advances made in different fields of science and technology.
Acknowledgment
Thanks to Mohammed Xohdy for help with drawing figures.
Funding Information
The authors also acknowledge funding from the National Institutes of Health (EB021857, AR066193, AR057837, CA214411, HL137193, EB024403, EB023052, EB022403, and EB021857), Air Force Office of Sponsored Research under award No. FA9550-15-1-0273, and a National Science Foundation Career Award (S.K.S., 1653730).
Disclosure Statement
The authors acknowledge that they have no competing interests.
References
- 1. O'Shea T.M., Burda J.E., and Sofroniew M.V.. Cell biology of spinal cord injury and repair. J Clin Invest 127, 3259, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization; The International Spinal Cord Society. International Perspectives on Spinal Cord Injury. Geneva, Switzerland: World Health Organization, 2013 [Google Scholar]
- 3. Cox C.S., Jr. Cellular therapy for traumatic neurological injury. Pediatr Res 83, 325, 2018 [DOI] [PubMed] [Google Scholar]
- 4. Singh A., Tetreault L., Kalsi-Ryan S., Nouri A., and Fehlings M.G.. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6, 309, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jazayeri S.B., Beygi S., Shokraneh F., Hagen E.M., and Rahimi-Movaghar V.. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J 24, 905, 2015 [DOI] [PubMed] [Google Scholar]
- 6. Ahuja C.S., Nori S., Tetreault L., et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 80, S9, 2017 [DOI] [PubMed] [Google Scholar]
- 7. Guilcher S.J., Munce S.E., Couris C.M., et al. Health care utilization in non-traumatic and traumatic spinal cord injury: a population-based study. Spinal Cord 48, 45, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Zeb H., Khan I.N., Munir I., et al. Updates on therapeutics in clinical trials for spinal cord injuries: key translational applications of human embryonic stem cells-derived neural progenitors. CNS Neurol Disord Drug Targets 15, 1266, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Gunther M.I., Weidner N., Muller R., and Blesch A.. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater 27, 140, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Mosley M.C., Lim H.J., Chen J., et al. Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young's Modulus gradient. J Biomed Mater Res A 105, 824, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Patel V., Joseph G., Patel A., et al. Suspension matrices for improved Schwann-cell survival after implantation into the injured rat spinal cord. J Neurotrauma 27, 789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zweckberger K., Ahuja C.S., Liu Y., Wang J., and Fehlings M.G.. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater 42, 77, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Blumenthal J., Cohen-Matsliah S.I., and Levenberg S.. Olfactory bulb-derived cells seeded on 3D scaffolds exhibit neurotrophic factor expression and pro-angiogenic properties. Tissue Eng Part A 19, 2284, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Shrestha B., Coykendall K., Li Y., Moon A., Priyadarshani P., and Yao L.. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res Ther 5, 91, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu P., Wang Y., Graham L., et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadoya K., Lu P., Nguyen K., et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med 22, 479, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assinck P., Duncan G.J., Hilton B.J., Plemel J.R., and Tetzlaff W.. Cell transplantation therapy for spinal cord injury. Nat Neurosci 20, 637, 2017 [DOI] [PubMed] [Google Scholar]
- 18. Baranes K., Shevach M., Shefi O., and Dvir T.. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett 16, 2916, 2016 [DOI] [PubMed] [Google Scholar]
- 19. Aydemir Sezer U., Ozturk K., Aru B., Yanikkaya Demirel G., Sezer S., and Bozkurt M.R.. Zero valent zinc nanoparticles promote neuroglial cell proliferation: a biodegradable and conductive filler candidate for nerve regeneration. J Mater Sci Mater Med 28, 19, 2017 [DOI] [PubMed] [Google Scholar]
- 20. Tay A., Sohrabi A., Poole K., Seidlits S., and Di Carlo D.. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv Mater 30, e1800927, 2018 [DOI] [PubMed] [Google Scholar]
- 21. Berndt M., Li Y., Seyedhassantehrani N., and Yao L.. Fabrication and characterization of microspheres encapsulating astrocytes for neural regeneration. ACS Biomater Sci Eng 3, 1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saghazadeh A., and Rezaei N.. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother 92, 128, 2017 [DOI] [PubMed] [Google Scholar]
- 23. Zhang X., Chen J., and Wang S.. Serum amyloid A induces a vascular smooth muscle cell phenotype switch through the p38 MAPK signaling pathway. Biomed Res Int 2017, 4941379, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Springer J.E., Visavadiya N.P., Sullivan P.G., and Hall E.D.. Post-injury treatment with NIM811 promotes recovery of function in adult female rats after spinal cord contusion: a dose-response study. J Neurotrauma 35, 492, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang B.T., Cregg J.M., DePaul M.A., et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 518, 404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson R.E., Pardieck J., Smith L., et al. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 162, 208, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson M.A., Burda J.E., Ren Y., et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tracy B., Armola R., and Micham J.. The “cold cord”: a review of therapeutic hypothermia for traumatic spinal cord injuries. Am J Crit Care 24, 540, 2015 [DOI] [PubMed] [Google Scholar]
- 29. Kwon B.K., Curt A., Belanger L.M. et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine 10, 181, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Sen C.K., Gordillo G.M., Roy S., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17, 763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kucher K., Johns D., Maier D., et al. First-in-man intrathecal application of neurite growth-promoting anti-Nogo-A antibodies in acute spinal cord injury. Neurorehabil Neural Repair 32, 578, 2018 [DOI] [PubMed] [Google Scholar]
- 32. Ross C.L., Syed I., Smith T.L., and Harrison B.S.. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med 36, 74, 2017 [DOI] [PubMed] [Google Scholar]
- 33. Hamid S., and Hayek R.. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur Spine J 17, 1256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rocha L.A., Sousa R.A., Learmonth D.A., and Salgado A.J.. The role of biomaterials as angiogenic modulators of spinal cord injury: mimetics of the spinal cord, cell and angiogenic factor delivery agents. Front Pharmacol 9, 164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z., Guo G.H., Wang G.S., Guan C.X., and Yue L.. Influence of neural stem cell transplantation on angiogenesis in rats with spinal cord injury. Genet Mol Res 13, 6083, 2014 [DOI] [PubMed] [Google Scholar]
- 36. Wilems T.S., Pardieck J., Iyer N., and Sakiyama-Elbert S.E.. Combination therapy of stem cell derived neural progenitors and drug delivery of anti-inhibitory molecules for spinal cord injury. Acta Biomater 28, 23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson P.J., Tatara A., McCreedy D.A., Shiu A., and Sakiyama-Elbert S.E.. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter 6, 5127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson P.J., Tatara A., Shiu A., and Sakiyama-Elbert S.E.. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant 19, 89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugai K., Nishimura S., Kato-Negishi M., et al. Neural stem/progenitor cell-laden microfibers promote transplant survival in a mouse transected spinal cord injury model. J Neurosci Res 93, 1826, 2015 [DOI] [PubMed] [Google Scholar]
- 40. Hatami M., Mehrjardi N.Z., Kiani S., et al. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy 11, 618, 2009 [DOI] [PubMed] [Google Scholar]
- 41. McCreedy D.A., Wilems T.S., Xu H., et al. Survival, differentiation, and migration of high-purity mouse embryonic stem cell-derived progenitor motor neurons in fibrin scaffolds after sub-acute spinal cord injury. Biomater Sci 2, 1672, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Itosaka H., Kuroda S., Shichinohe H., et al. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology 29, 248, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Caron I., Rossi F., Papa S., et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 75, 135, 2016 [DOI] [PubMed] [Google Scholar]
- 44. Mothe A.J., Tam R.Y., Zahir T., Tator C.H., and Shoichet M.S.. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 34, 3775, 2013 [DOI] [PubMed] [Google Scholar]
- 45. Wilems T.S., and Sakiyama-Elbert S.E.. Sustained dual drug delivery of anti-inhibitory molecules for treatment of spinal cord injury. J Control Release 213, 103, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson A.J., Piltti K.M., Hooshmand M.J., Nishi R.A., and Cummings B.J.. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Reports 8, 249, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao L., Daly W., Newland B., et al. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther 20, 1149, 2013 [DOI] [PubMed] [Google Scholar]
- 48. Rosenzweig E.S., Brock J.H., Lu P., et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 24, 484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu S., Sandner B., Schackel T., et al. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater 60, 167, 2017 [DOI] [PubMed] [Google Scholar]
- 50. Fuhrmann T., Tam R.Y., Ballarin B., et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83, 23, 2016 [DOI] [PubMed] [Google Scholar]
- 51. Fuhrmann T., Anandakumaran P.N., Payne S.L., et al. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed Mater 13, 024103, 2018 [DOI] [PubMed] [Google Scholar]
- 52. Theodore N., Hlubek R., Danielson J., et al. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery 79, E305, 2016 [DOI] [PubMed] [Google Scholar]
- 53. Hakim J.S., Esmaeili Rad M., Grahn P.J., et al. Positively charged oligo[poly(ethylene glycol) fumarate] scaffold implantation results in a permissive lesion environment after spinal cord injury in rat. Tissue Eng Part A 21, 2099, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen B.K., Madigan N.N., Hakim J.S., et al. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J Tissue Eng Regen Med 12, e398, 2018 [DOI] [PubMed] [Google Scholar]
- 55. Ruzicka J., Romanyuk N., Hejcl A., et al. Treating spinal cord injury in rats with a combination of human fetal neural stem cells and hydrogels modified with serotonin. Acta Neurobiol Exp (Wars) 73, 102, 2013 [DOI] [PubMed] [Google Scholar]
- 56. Moradi F., Bahktiari M., Joghataei M.T., et al. BD PuraMatrix peptide hydrogel as a culture system for human fetal Schwann cells in spinal cord regeneration. J Neurosci Res 90, 2335, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Yang Y.H., Khan Z., Ma C., Lim H.J., and Smith Callahan L.A.. Optimization of adhesive conditions for neural differentiation of murine embryonic stem cells using hydrogels functionalized with continuous Ile-Lys-Val-Ala-Val concentration gradients. Acta Biomater 21, 55, 2015 [DOI] [PubMed] [Google Scholar]
- 58. Rose J.C., Camara-Torres M., Rahimi K., Kohler J., Moller M., and De Laporte L.. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett 17, 3782, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kanekiyo K., Wakabayashi T., Nakano N., et al. Effects of intrathecal injection of the conditioned medium from bone marrow stromal cells on spinal cord injury in rats. J Neurotrauma 35, 521, 2018 [DOI] [PubMed] [Google Scholar]
- 60. Han S., Wang B., Li X., et al. Bone marrow-derived mesenchymal stem cells in three-dimensional culture promote neuronal regeneration by neurotrophic protection and immunomodulation. J Biomed Mater Res A 104, 1759, 2016 [DOI] [PubMed] [Google Scholar]
- 61. Torres-Espin A., Hernandez J., and Navarro X.. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS One 8, e76141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kobolak J., Dinnyes A., Memic A., Khademhosseini A., and Mobasheri A.. Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 99, 62, 2016 [DOI] [PubMed] [Google Scholar]
- 63. Curtis E., Martin J.R., Gabel B., et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell 22, 941, 2018 [DOI] [PubMed] [Google Scholar]
- 64. Asterias Biotherapeutics. Dose escalation study of AST-OPC1 in spinal cord injury. 2018. Available at: https://ClinicalTrials.gov/show/NCT02302157 (accessed July3, 2019)
- 65. Tuszynski M.H., Wang Y., Graham L., et al. Neural stem cell dissemination after grafting to CNS injury sites. Cell 156, 388, 2014 [DOI] [PubMed] [Google Scholar]
- 66. Steward O., Sharp K.G., and Matsudaira Yee K.. Long-distance migration and colonization of transplanted neural stem cells. Cell 156, 385, 2014 [DOI] [PubMed] [Google Scholar]
- 67. Steward O., Sharp K.G., Yee K.M., Hatch M.N., and Bonner J.F.. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J Neurosci 34, 14013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nguyen H.X., Hooshmand M.J., Saiwai H., et al. Systemic neutrophil depletion modulates the migration and fate of transplanted human neural stem cells to rescue functional repair. J Neurosci 37, 9269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hofstetter C.P., Holmstrom N.A., Lilja J.A., et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci 8, 346, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Vismara I., Papa S., Rossi F., Forloni G., and Veglianese P.. Current options for cell therapy in spinal cord injury. Trends Mol Med 23, 831, 2017 [DOI] [PubMed] [Google Scholar]
- 71. Lin L., and Du L.. The role of secreted factors in stem cells-mediated immune regulation. Cell Immunol 326, 24, 2018 [DOI] [PubMed] [Google Scholar]
- 72. StemCells, Inc. Study of human central nervous system (CNS) stem cell transplantation in cervical spinal cord injury. 2016. Available at: https://ClinicalTrials.gov/show/NCT02163876 (accessed July3, 2019)
- 73. Scott C.T., and Magnus D.. Wrongful termination: lessons from the Geron clinical trial. Stem Cells Transl Med 3, 1398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chhabra H.S., and Sarda K.. Clinical translation of stem cell based interventions for spinal cord injury—are we there yet? Adv Drug Deliv Rev 120, 41, 2017 [DOI] [PubMed] [Google Scholar]
- 75. Neuralstem, Inc. Safety study of human spinal cord-derived neural stem cell transplantation for the treatment of chronic SCI. 2017. Available at: https://ClinicalTrials.gov/show/NCT01772810 (accessed July3, 2019)
- 76. Ghobrial G.M., Anderson K.D., Dididze M., et al. Human neural stem cell transplantation in chronic cervical spinal cord injury: functional outcomes at 12 months in a phase II clinical trial. Neurosurgery 64, 87, 2017 [DOI] [PubMed] [Google Scholar]
- 77. Ferrer Internacional S.A. Safety and preliminary efficacy of FAB117-HC in patients with acute traumatic spinal cord injury (SPINE). 2018. Available at: https://ClinicalTrials.gov/show/NCT02917291 (accessed July3, 2019)
- 78. Rong L. Study of human central nervous system (CNS) stem cell transplantation in cervical spinal cord injury. Available at: https://ClinicalTrials.gov/show/NCT02163876 (accessed July3, 2019)
- 79. Banc de Sang i Teixits. Intrathecal administration of ex-panded Wharton's jelly mesenchymal stem cells in chronic traumatic spinal cord injury. 2018. Available at: https://ClinicalTrials.gov/show/NCT03003364 (accessed July3, 2019)
- 80. Sakai K., Yamamoto A., Matsubara K., et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122, 80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Temple S., and Studer L.. Lessons learned from pioneering neural stem cell studies. Stem Cell Reports 8, 191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Monuki E.S., Anderson A.J., Blurton-Jones M., and Cummings B.J.. Response to StemCells Inc. Stem Cell Reports 8, 195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. StemCells, Inc. Long-term follow-up of transplanted hu-man central nervous system stem cells (HuCNS-SC) in spinal cord trauma subjects. 2016. Available at: https://ClinicalTrials.gov/show/NCT01725880 (accessed July3, 2019)
- 84. Nagoshi N., and Okano H.. iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci 75, 989, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sykova E., Homola A., Mazanec R., et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 15, 675, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Tsai E.C., Dalton P.D., Shoichet M.S., and Tator C.H.. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 27, 519, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Nomura H., Katayama Y., Shoichet M.S., and Tator C.H.. Complete spinal cord transection treated by implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery 59, 183, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Ashammakhi N. Drug release: proper control to help clinical application. J Craniofac Surg 29, 124, 2018 [DOI] [PubMed] [Google Scholar]
- 89. Rossi F., Perale G., Papa S., Forloni G., and Veglianese P.. Current options for drug delivery to the spinal cord. Expert Opin Drug Deliv 10, 385, 2013 [DOI] [PubMed] [Google Scholar]
- 90. AOSpine North America Research Network. Riluzole in spinal cord injury study (RISCIS). 2019. Available at: https://ClinicalTrials.gov/show/NCT01597518 (accessed July3, 2019)
- 91. Acorda Therapeutics. AC105 in patients with acute trau-matic spinal cord injury (AC105). 2018. Available at: https://ClinicalTrials.gov/show/NCT01750684 (accessed July3, 2019)
- 92. Rick Hansen Institute. Minocycline in acute spinal cord injury (MASC). 2014. Available at: https://ClinicalTrials.gov/show/NCT01828203 (accessed July3, 2019)
- 93. Kringle Pharma, Inc. Phase I/II study of KP-100IT in acute spinal cord injury. 2019. Available at: https://ClinicalTrials.gov/show/NCT02193334 (accessed July3, 2019)
- 94. Takahashi H., Yamazaki M., Okawa A., et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J 21, 2580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bregman B.S., Kunkel-Bagden E., Schnell L., Dai H.N., Gao D., and Schwab M.E.. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378, 498, 1995 [DOI] [PubMed] [Google Scholar]
- 96. Danilov C.A., and Steward O.. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol 266, 147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lewandowski G., and Steward O.. AAVshRNA-mediated suppression of PTEN in adult rats in combination with salmon fibrin administration enables regenerative growth of corticospinal axons and enhances recovery of voluntary motor function after cervical spinal cord injury. J Neurosci 34, 9951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mokalled M.H., Patra C., Dickson A.L., Endo T., Stainier D.Y., and Poss K.D.. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354, 630, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tedeschi A., Dupraz S., Laskowski C.J., et al. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92, 419, 2016 [DOI] [PubMed] [Google Scholar]
- 100. Bao C., Wang B., Yang F., and Chen L.. Blockade of interleukin-7 receptor shapes macrophage alternative activation and promotes functional recovery after spinal cord injury. Neuroscience 371, 518, 2018 [DOI] [PubMed] [Google Scholar]
- 101. Park J., Decker J.T., Smith D.R., Cummings B.J., Anderson A.J., and Shea L.D.. Reducing inflammation through delivery of lentivirus encoding for anti-inflammatory cytokines attenuates neuropathic pain after spinal cord injury. J Control Release 290, 88, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Margul D.J., Park J., Boehler R.M., et al. Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioeng Transl Med 1, 136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mokarram N., Dymanus K., Srinivasan A., et al. Immunoengineering nerve repair. Proc Natl Acad Sci U S A 114, E5077, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nakano N., Nakai Y., Seo T.B., et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett 483, 57, 2010 [DOI] [PubMed] [Google Scholar]
- 105. Shen B., Liu J., Zhang F., et al. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int 2016, 1240301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Barnabe-Heider F., Goritz C., Sabelstrom H., et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470, 2010 [DOI] [PubMed] [Google Scholar]
- 107. Marichal N., Reali C., Rehermann M.I., Trujillo-Cenoz O., and Russo R.E.. Progenitors in the ependyma of the spinal cord: a potential resource for self-repair after injury. Adv Exp Med Biol 1015, 241, 2017 [DOI] [PubMed] [Google Scholar]
- 108. Sabelstrom H., Stenudd M., Reu P., et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 342, 637, 2013 [DOI] [PubMed] [Google Scholar]
- 109. Sabelstrom H., Stenudd M., and Frisen J.. Neural stem cells in the adult spinal cord. Exp Neurol 260, 44, 2014 [DOI] [PubMed] [Google Scholar]
- 110. Horner P.J., and Gage F.H.. Regenerating the damaged central nervous system. Nature 407, 963, 2000 [DOI] [PubMed] [Google Scholar]
- 111. Smith D.R., Margul D.J., Dumont C.M., et al. Combinatorial lentiviral gene delivery of pro-oligodendrogenic factors for improving myelination of regenerating axons after spinal cord injury. Biotechnol Bioeng 116, 155, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thomas A.M., Seidlits S.K., Goodman A.G., et al. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb) 6, 694, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Forgione N., and Fehlings M.G.. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg 82, e535, 2014 [DOI] [PubMed] [Google Scholar]
- 114. Khorasanizadeh M., Eskian M., Vaccaro A.R., and Rahimi-Movaghar V.. Granulocyte colony-stimulating factor (G-CSF) for the treatment of spinal cord injury. CNS Drugs 31, 911, 2017 [DOI] [PubMed] [Google Scholar]
- 115. Fehlings M.G., Theodore N., Harrop J., et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28, 787, 2011 [DOI] [PubMed] [Google Scholar]
- 116. Vertex Pharmaceuticals, Incorporated. Study to assess the efficacy and safety of VX-210 in subjects with acute traumatic cervical spinal cord injury. 2018. Available at: https://ClinicalTrials.gov/show/NCT02669849 (accessed July3, 2019)
- 117. Kamiya K., Koda M., Furuya T., et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J 24, 963, 2015 [DOI] [PubMed] [Google Scholar]
- 118. Derakhshanrad N., Saberi H., Yekaninejad M.S., Joghataei M.T., and Sheikhrezaei A.. Granulocyte-colony stimulating factor administration for neurological improvement in patients with postrehabilitation chronic incomplete traumatic spinal cord injuries: a double-blind randomized controlled clinical trial. J Neurosurg Spine 29, 97, 2018 [DOI] [PubMed] [Google Scholar]
- 119. Kitamura K., Nagoshi N., Tsuji O., Matsumoto M., Okano H., and Nakamura M.. Application of hepatocyte growth factor for acute spinal cord injury: the road from basic studies to human treatment. Int J Mol Sci 20, 1054, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. InVivo Therapeutics. The INSPIRE study: probable benefit of the neuro-spinal scaffold for treatment of AIS A thoracic acute spinal cord injury. 2018. Available at: https://ClinicalTrials.gov/show/NCT02138110 (accessed July3, 2019)
- 121. Guest J.D., Moore S.W., Aimetti A.A., et al. Internal decompression of the acutely contused spinal cord: differential effects of irrigation only versus biodegradable scaffold implantation. Biomaterials 185, 284, 2018 [DOI] [PubMed] [Google Scholar]
- 122. He L., Zhang Y., Zeng C., et al. Manufacture of PLGA multiple-channel conduits with precise hierarchical pore architectures and in vitro/vivo evaluation for spinal cord injury. Tissue Eng Part C Methods 15, 243, 2009 [DOI] [PubMed] [Google Scholar]
- 123. Kim H., Tator C.H., and Shoichet M.S.. Chitosan implants in the rat spinal cord: biocompatibility and biodegradation. J Biomed Mater Res A 97, 395, 2011 [DOI] [PubMed] [Google Scholar]
- 124. Gelain F., Panseri S., Antonini S., et al. Transplantation of nanostructured composite scaffolds results in the regeneration of chronically injured spinal cords. ACS Nano 5, 227, 2011 [DOI] [PubMed] [Google Scholar]
- 125. Tysseling-Mattiace V.M., Sahni V., Niece K.L., et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28, 3814, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Macaya D., and Spector M.. Injectable hydrogel materials for spinal cord regeneration: a review. Biomed Mater 7, 012001, 2012 [DOI] [PubMed] [Google Scholar]
- 127. Perale G., Rossi F., Sundstrom E., et al. Hydrogels in spinal cord injury repair strategies. ACS Chem Neurosci 2, 336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Woerly S., Petrov P., Sykova E., Roitbak T., Simonova Z., and Harvey A.R.. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng 5, 467, 1999 [DOI] [PubMed] [Google Scholar]
- 129. Hejcl A., Lesny P., Pradny M., et al. Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 6: 3D hydrogels with positive and negative surface charges and polyelectrolyte complexes in spinal cord injury repair. J Mater Sci Mater Med 20, 1571, 2009 [DOI] [PubMed] [Google Scholar]
- 130. Kataoka K., Suzuki Y., Kitada M., et al. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng 10, 493, 2004 [DOI] [PubMed] [Google Scholar]
- 131. Perale G., Rossi F., Santoro M., et al. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J Control Release 159, 271, 2012 [DOI] [PubMed] [Google Scholar]
- 132. Ziemba A.M., and Gilbert R.J.. Biomaterials for local, controlled drug delivery to the injured spinal cord. Front Pharmacol 8, 245, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fukunaga K., Tsutsumi H., and Mihara H.. Cell differentiation on disk- and string-shaped hydrogels fabricated from Ca(2+)-responsive self-assembling peptides. Biopolymers 106, 476, 2016 [DOI] [PubMed] [Google Scholar]
- 134. Li H.Y., Fuhrmann T., Zhou Y., and Dalton P.D.. Host reaction to poly(2-hydroxyethyl methacrylate) scaffolds in a small spinal cord injury model. J Mater Sci Mater Med 24, 2001, 2013 [DOI] [PubMed] [Google Scholar]
- 135. Grulova I., Slovinska L., Blasko J., et al. Delivery of alginate scaffold releasing two trophic factors for spinal cord injury repair. Sci Rep 5, 13702, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yang Y., De Laporte L., Zelivyanskaya M.L., et al. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A 15, 3283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Olson H.E., Rooney G.E., Gross L., et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A 15, 1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dumont C.M., Carlson M.A., Munsell M.K., et al. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater 86, 312, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li X., Yang C., Li L., et al. A therapeutic strategy for spinal cord defect: human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res Int 2015, 197183, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Espinosa-Jeffrey A., Oregel K., Wiggins L., et al. Strategies for endogenous spinal cord repair: HPMA hydrogel to recruit migrating endogenous stem cells. Adv Exp Med Biol 760, 25, 2012 [DOI] [PubMed] [Google Scholar]
- 141. Slotkin J.R., Pritchard C.D., Luque B., et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials 123, 63, 2017 [DOI] [PubMed] [Google Scholar]
- 142. Tuinstra H.M., Margul D.J., Goodman A.G., et al. Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration. Tissue Eng Part A 20, 1027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Makadia H.K., and Siegel S.J.. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 3, 1377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chu D.S., Sellers D.L., Bocek M.J., Fischedick A.E., Horner P.J., and Pun S.H.. MMP9-sensitive polymers mediate environmentally-responsive bivalirudin release and thrombin inhibition. Biomater Sci 3, 41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Teng Y.D., Lavik E.B., Qu X., et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 99, 3024, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kamperman T., Henke S., van den Berg A., et al. Single cell microgel based modular bioinks for uncoupled cellular micro- and macroenvironments. Adv Healthc Mater 6, 2017 [DOI] [PubMed] [Google Scholar]
- 147. Lienemann P.S., Rossow T., Mao A.S., Vallmajo-Martin Q., Ehrbar M., and Mooney D.J.. Single cell-laden protease-sensitive microniches for long-term culture in 3D. Lab Chip 17, 727, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pakulska M.M., Ballios B.G., and Shoichet M.S.. Injectable hydrogels for central nervous system therapy. Biomed Mater 7, 024101, 2012 [DOI] [PubMed] [Google Scholar]
- 149. Khaing Z.Z., Ehsanipour A., Hofstetter C.P., and Seidlits S.K.. Injectable hydrogels for spinal cord repair: a focus on swelling and intraspinal pressure. Cells Tissues Organs 202, 67, 2016 [DOI] [PubMed] [Google Scholar]
- 150. Kong X.B., Tang Q.Y., Chen X.Y., Tu Y., Sun S.Z., and Sun Z.L.. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen Res 12, 1003, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Horn E.M., Beaumont M., Shu X.Z., et al. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J Neurosurg Spine 6, 133, 2007 [DOI] [PubMed] [Google Scholar]
- 152. Park J., Lim E., Back S., Na H., Park Y., and Sun K.. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A 93, 1091, 2010 [DOI] [PubMed] [Google Scholar]
- 153. Khaing Z.Z., Milman B.D., Vanscoy J.E., Seidlits S.K., Grill R.J., and Schmidt C.E.. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng 8, 046033, 2011 [DOI] [PubMed] [Google Scholar]
- 154. Gupta D., Tator C.H., and Shoichet M.S.. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 27, 2370, 2006 [DOI] [PubMed] [Google Scholar]
- 155. Fuhrmann T., Obermeyer J., Tator C.H., and Shoichet M.S.. Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods 84, 60, 2015 [DOI] [PubMed] [Google Scholar]
- 156. Kubinova S., Horak D., Hejcl A., et al. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J Tissue Eng Regen Med 9, 1298, 2015 [DOI] [PubMed] [Google Scholar]
- 157. Tavakol S., Mousavi S.M.M., Tavakol B., Hoveizi E., Ai J., and Sorkhabadi S.M.R.. Mechano-transduction signals derived from self-assembling peptide nanofibers containing long motif of laminin influence neurogenesis in in-vitro and in-vivo. Mol Neurobiol 54, 2483, 2017 [DOI] [PubMed] [Google Scholar]
- 158. Tukmachev D., Forostyak S., Koci Z., et al. Injectable extracellular matrix hydrogels as scaffolds for spinal cord injury repair. Tissue Eng Part A 22, 306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhu J., Lu Y., Yu F., et al. Effect of decellularized spinal scaffolds on spinal axon regeneration in rats. J Biomed Mater Res A 106, 698, 2018 [DOI] [PubMed] [Google Scholar]
- 160. Pawar K., Cummings B.J., Thomas A., et al. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: association with recovery of forelimb function. Biomaterials 65, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Koffler J., Zhu W., Qu X., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 25, 263, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Liu S., and Blesch A.. Targeted tissue engineering: hydrogels with linear capillary channels for axonal regeneration after spinal cord injury. Neural Regen Res 13, 641, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ashammakhi N., Ndreu A., Yang Y., Ylikauppila H., Nikkola L., and Hasirci V.. Tissue engineering: a new take-off using nanofiber-based scaffolds. J Craniofac Surg 18, 3, 2007 [DOI] [PubMed] [Google Scholar]
- 164. Ashammakhi N., Ndreu A., Piras A.M., et al. Biodegradable nanomats produced by electrospinning: expanding multifunctionality and potential for tissue engineering. J Nanosci Nanotechnol 7, 862, 2007 [DOI] [PubMed] [Google Scholar]
- 165. Schnell E., Klinkhammer K., Balzer S., et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28, 3012, 2007 [DOI] [PubMed] [Google Scholar]
- 166. Xie J., Willerth S.M., Li X., et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30, 354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jakobsson A., Ottosson M., Zalis M.C., O'Carroll D., Johansson U.E., and Johansson F.. Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds. Nanomedicine 13, 1563, 2017 [DOI] [PubMed] [Google Scholar]
- 168. Ren H., Han M., Zhou J., et al. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 35, 6585, 2014 [DOI] [PubMed] [Google Scholar]
- 169. Carvalho-de-Souza J.L., Treger J.S., Dang B., Kent S.B., Pepperberg D.R., and Bezanilla F.. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Eom K., Kim J., Choi J.M., et al. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 10, 3853, 2014 [DOI] [PubMed] [Google Scholar]
- 171. Wu W., Lee S.Y., Wu X., et al. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 35, 2355, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ross C.L. The use of electric, magnetic, and electromagnetic field for directed cell migration and adhesion in regenerative medicine. Biotechnol Prog 33, 5, 2017 [DOI] [PubMed] [Google Scholar]
- 173. Omidinia-Anarkoli A., Boesveld S., Tuvshindorj U., Rose J.C., Haraszti T., and De Laporte L.. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small 13, 1702207, 2017 [DOI] [PubMed] [Google Scholar]
- 174. Du V., Luciani N., Richard S., et al. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat Commun 8, 400, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Song J., Sun B., Liu S., et al. Polymerizing pyrrole coated poly (l-lactic acid-co-epsilon-caprolactone) (PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front Mol Neurosci 9, 117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou J.F., Wang Y.G., Cheng L., Wu Z., Sun X.D., and Peng J.. Preparation of polypyrrole-embedded electrospun poly(lactic acid) nanofibrous scaffolds for nerve tissue engineering. Neural Regen Res 11, 1644, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhu W., Ye T., Lee S.J., et al. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomedicine 14, 2485, 2018 [DOI] [PubMed] [Google Scholar]
- 178. Liu X., Ma J., Wu X., Lin L., and Wang X.. Polymeric nanofibers with ultrahigh piezoelectricity via self-orientation of nanocrystals. ACS Nano 11, 1901, 2017 [DOI] [PubMed] [Google Scholar]
- 179. Wang Q., Wang C., Zhang M., Jian M., and Zhang Y.. Feeding single-walled carbon nanotubes or graphene to silkworms for reinforced silk fibers. Nano Lett 16, 6695, 2016 [DOI] [PubMed] [Google Scholar]
- 180. Tavakol S., Saber R., Hoveizi E., Aligholi H., Ai J., and Rezayat S.M.. Chimeric self-assembling nanofiber containing bone marrow homing peptide's motif induces motor neuron recovery in animal model of chronic spinal cord injury; an in vitro and in vivo investigation. Mol Neurobiol 53, 3298, 2016 [DOI] [PubMed] [Google Scholar]
- 181. Ashammakhi N., Kaarela O., and Ferretti P.. Pulling and pushing stem cells to control their differentiation. J Craniofac Surg 29, 804, 2018 [DOI] [PubMed] [Google Scholar]
- 182. Führmann T., Anandakumaran P.N., and Shoichet M.S.. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater 6, 1601130, 2017 [DOI] [PubMed] [Google Scholar]
- 183. Lin X.Y., Lai B.Q., Zeng X., et al. Cell transplantation and neuroengineering approach for spinal cord injury treatment: a summary of current laboratory findings and review of literature. Cell Transplant 25, 1425, 2016 [DOI] [PubMed] [Google Scholar]
- 184. Han Q., Jin W., Xiao Z., et al. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials 31, 9212, 2010 [DOI] [PubMed] [Google Scholar]
- 185. Anderson M.A., O'Shea T.M., Burda J.E., et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bakshi A., Fisher O., Dagci T., Himes B.T., Fischer I., and Lowman A.. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine 1, 322, 2004 [DOI] [PubMed] [Google Scholar]
- 187. Lee H., McKeon R.J., and Bellamkonda R.V.. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A 107, 3340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Piantino J., Burdick J.A., Goldberg D., Langer R., and Benowitz L.I.. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol 201, 359, 2006 [DOI] [PubMed] [Google Scholar]
- 189. Stokols S., and Tuszynski M.H.. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27, 443, 2006 [DOI] [PubMed] [Google Scholar]
- 190. Agbay A., Edgar J.M., Robinson M., et al. Biomaterial strategies for delivering stem cells as a treatment for spinal cord injury. Cells Tissues Organs 202, 42, 2016 [DOI] [PubMed] [Google Scholar]
- 191. Liu J., Chen J., Liu B., et al. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci 325, 127, 2013 [DOI] [PubMed] [Google Scholar]
- 192. Aguado B.A., Mulyasasmita W., Su J., Lampe K.J., and Heilshorn S.C.. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18, 806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ritfeld G.J., Rauck B.M., Novosat T.L., et al. The effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repair. Biomaterials 35, 1924, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Williams R.R., Henao M., Pearse D.D., and Bunge M.B.. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant 24, 115, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Gomes E.D., Mendes S.S., Leite-Almeida H., et al. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials 105, 38, 2016 [DOI] [PubMed] [Google Scholar]
- 196. Dumont C.M., Munsell M.K., Carlson M.A., Cummings B.J., Anderson A.J., and Shea L.D.. Spinal progenitor-laden bridges support earlier axon regeneration following spinal cord injury. Tissue Eng Part A 24, 1588, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Onuma-Ukegawa M., Bhatt K., Hirai T., et al. Bone marrow stromal cells combined with a honeycomb collagen sponge facilitate neurite elongation in vitro and neural restoration in the hemisected rat spinal cord. Cell Transplant 24, 1283, 2015 [DOI] [PubMed] [Google Scholar]
- 198. Nagashima K., Miwa T., Soumiya H., et al. Priming with FGF2 stimulates human dental pulp cells to promote axonal regeneration and locomotor function recovery after spinal cord injury. Sci Rep 7, 13500, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Stephan M.T., Moon J.J., Um S.H., Bershteyn A., and Irvine D.J.. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16, 1035, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cao Q., He Q., Wang Y., et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci 30, 2989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Iyer N.R., Wilems T.S., and Sakiyama-Elbert S.E.. Stem cells for spinal cord injury: strategies to inform differentiation and transplantation. Biotechnol Bioeng 114, 245, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhang X., Zeng Y., Zhang W., Wang J., Wu J., and Li J.. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. J Neurotrauma 24, 1863, 2007 [DOI] [PubMed] [Google Scholar]
- 203. Tang S., Liao X., Shi B., et al. The effects of controlled release of neurotrophin-3 from PCLA scaffolds on the survival and neuronal differentiation of transplanted neural stem cells in a rat spinal cord injury model. PLoS One 9, e107517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Angeli C.A., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J.. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Calvert J.S., Grahn P.J., Strommen J.A., et al. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J Neurotrauma 36, 1451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gad P.N., Kreydin E., Zhong H., Latack K., and Edgerton V.R.. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci 12, 432, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Gill M.L., Grahn P.J., Calvert J.S., et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24, 1677, 2018 [DOI] [PubMed] [Google Scholar]
- 208. Chen K., Marsh B.C., Cowan M., et al. Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol 292, 135, 2017 [DOI] [PubMed] [Google Scholar]
- 209. Bilgen M., and Al-Hafez B.. Comparison of spinal vasculature in mouse and rat: investigations using MR angiography. Neuroanatomy 5, 12, 2006 [Google Scholar]
- 210. Sekhon L.H., and Fehlings M.G.. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 26, S2, 2001 [DOI] [PubMed] [Google Scholar]
- 211. Hilton B.J., Anenberg E., Harrison T.C., Boyd J.D., Murphy T.H., and Tetzlaff W.. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci 36, 4080, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. StemCells Inc. former management. Reaction from StemCells, Inc. to two papers in stem cell reports on the efficacy of human NSCs in mouse models of Alzheimer's disease and spinal cord injury. Stem Cell Reports 8, 194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Tazaki A., Tanaka E.M., and Fei J.F.. Salamander spinal cord regeneration: the ultimate positive control in vertebrate spinal cord regeneration. Dev Biol 432, 63, 2017 [DOI] [PubMed] [Google Scholar]
- 214. Mostafalu P., Lenk W., Dokmeci M.R., Ziaie B., Khademhosseini A., and Sonkusale S.R.. Wireless flexible smart bandage for continuous monitoring of wound oxygenation. IEEE Trans Biomed Circuits Syst 9, 670, 2015 [DOI] [PubMed] [Google Scholar]
- 215. Ashammakhi N., Ahadian S., Darabi M.A., et al. Minimally invasive and regenerative therapeutics. Adv Mater 31, e1804041, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Ashammakhi N., and Kaarela O.. Stimuli-responsive biomaterials: next wave. J Craniofac Surg 28, 1647, 2017 [DOI] [PubMed] [Google Scholar]
- 217. Lu Y., Aimetti A.A., Langer R., and Gu Z.. Bioresponsive materials. Nat Rev Mater 2, 16075, 2017 [Google Scholar]
- 218. Ashammakhi N., Ahadian S., Zengjie F., et al. Advances and future perspectives in 4D bioprinting. Biotechnol J 13, e1800148, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Nikkola L., Viitanen P., and Ashammakhi N.. Temporal control of drug release from biodegradable polymer: multicomponent diclofenac sodium releasing PLGA 80/20 rod. J Biomed Mater Res B Appl Biomater 89, 518, 2009 [DOI] [PubMed] [Google Scholar]
- 220. Sivaraman B., Swaminathan G., Moore L., et al. Magnetically-responsive, multifunctional drug delivery nanoparticles for elastic matrix regenerative repair. Acta Biomater 52, 171, 2017 [DOI] [PubMed] [Google Scholar]
- 221. Marx U., Walles H., Hoffmann S., et al. “Human-on-a-chip” developments: a translational cutting-edge alternative to systemic safety assessment and efficiency evaluation of substances in laboratory animals and man? Altern Lab Anim 40, 235, 2012 [DOI] [PubMed] [Google Scholar]
- 222. Ahadian S., Civitarese R., Bannerman D., et al. Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater 7, 1700506, 2018 [DOI] [PubMed] [Google Scholar]
- 223. Ashammakhi N.A., and Elzagheid A.. Organ-on-a-chip: new tool for personalized medicine. J Craniofac Surg 29, 823, 2018 [DOI] [PubMed] [Google Scholar]
- 224. McLean I.C., Schwerdtfeger L.A., Tobet S.A., and Henry C.S.. Powering ex vivo tissue models in microfluidic systems. Lab Chip 18, 1399, 2018 [DOI] [PubMed] [Google Scholar]
- 225. Wang Y., Ma J., Li N., et al. Microfluidic engineering of neural stem cell niches for fate determination. Biomicrofluidics 11, 014106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]