Abstract
There are many kidney diseases that might be addressed by gene therapy. However, gene delivery to kidney cells is inefficient. This is due, in part, to the fact that the kidney excludes molecules above 50 kDa and that most gene delivery vectors are megaDaltons in mass. We compared the ability of adeno-associated virus (AAV), adenovirus (Ad), and lentiviral (LV) vectors to deliver genes to renal cells. When vectors were delivered by the intravenous (IV) route in mice, weak luciferase activity was observed in the kidney with substantially more in the liver. When gene delivery was observed in the kidney, expression was primarily in the glomerulus. To avoid these limitations, vectors were injected directly into the kidney by retrograde ureteral (RU) and subcapsular (SC) injections in mice. Small AAV vectors transduced the kidney, but also leaked from the organ and mediated higher levels of transduction in off-target tissues. Comparison of AAV2, 6.2, 8, and rh10 vectors by direct kidney injection demonstrated highest delivery by AAV6.2 and 8. Larger Ad and LV vectors transduced kidney cells and mediated less off-target tissue transduction. These data demonstrate the utility of direct kidney injections to circumvent the kidney size exclusion barrier. They also identify the effects of vector size on on-target and off-target transduction. This lays the foundation for the use of different vector platforms for gene therapy of diverse kidney diseases.
Keywords: kidney, retro-ureter, subcapsular, AAV, adenovirus, lentivirus, gene therapy
Introduction
Chronic kidney disease (CKD) affects 30 million individuals in the United States and ∼11–13% of humans worldwide.1 Some individuals may develop CKD due to lifestyle choices or due to kidney injuries, while others may develop CKD due to underlying genetic causes. At least 80 genes have been implicated as the causes of various genetic kidney diseases (reviewed in Hildebrandt2). These genetic kidney diseases can be grouped as cystic, glomerular basement membrane disorders, and tubulopathies, depending on how the disease affects normal physiology (reviewed in Leung3).
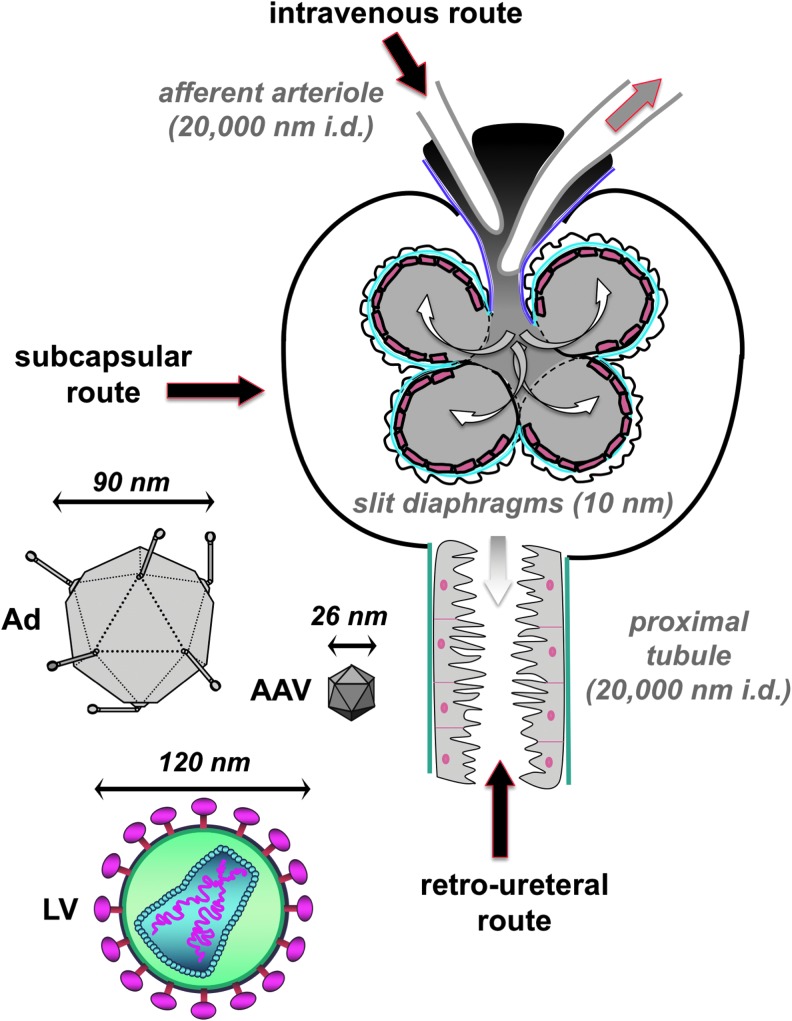
While gene therapy has made great strides over the past 30 years to treat diseases in other tissues, relatively little progress has been made in targeting kidney diseases. This is due, in part, to the stringent filtering functions intrinsic to the kidney. Viral and nonviral vectors can be 25 to 200 nm in diameter and have masses in megaDaltons. In contrast, the kidney actively excludes proteins above 50 kDa in size.4 In addition, podocytes within the glomerulus create slit diaphragms that are only 10 nm in diameter, which are well below the diameter of most gene therapy vectors, including popular adeno-associated virus (AAV) vectors (Fig. 1).
Figure 1.
Schematic of routes by which vectors transduce cells in the kidney. For IV injections, vectors would enter the kidney through the afferent arteriole, which has an estimated diameter of 10 nm. For RU injections, vectors would enter the kidney through the distal nephron. Shown is the PT lumen with an estimated diameter of 10 μm. Other downstream segments of the nephron such as the collecting duct have lumen as wide as 100 μm. The tubules of the nephron are generally large enough to accommodate the passage of AAV, Ad, and LV. AAV, adeno-associated virus; Ad, adenovirus; IV, intravenous; LV, lentiviral; PT, proximal tubule; RU, retrograde ureteral.
To better understand these pharmacologic barriers, we evaluated the ability of small 25 nm AAV vectors and larger 100 nm adenovirus (Ad) and 120 nm lentiviral vectors to transduce cells in the kidney. We tested three injection routes for kidney gene delivery: (1) intravenous by tail vein injection (IV), (2) retrograde infusion into the ureter or retro-ureteral (RU), and (3) subcapsular (SC) injection through the kidney capsule. We hypothesized that IV delivered vectors would suffer the filtering effects of the glomerulus and this would attenuate transduction in downstream tubule cells of the nephron. We hypothesized that delivery by the RU5–10 route could theoretically avoid these problems, but could be limited by running upstream against the natural flow of solutes from the nephrons. Similarly, SC delivery might avoid both problems, but may instead suffer from entrapment of vectors outside of tubules and limit their ability to reach other kidney cells.
Materials and Methods
Animal studies
Animals were housed in the Department of Comparative Medicine animal facilities at Mayo Clinic. All experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of the Institutional Animal Care and Use Committee at Mayo Clinic.
AAV, Ad, and lentiviral vector administration
AAV was administered at 1012 genome copies (GC) per mouse, except where otherwise noted. Ad was administered at 1011 viral particles (VP) per mouse. Lentiviral (LV) was administered at 6.4 × 106 transducing units (TU) per mouse. Vectors were administered either IV through standard tail vein injection, RU, or SC. Injection volumes ranged from 50 to 100 μL, contingent upon viral vector titer. For RU and SC injections, mice were anesthetized with ketamine/xylazine or isoflurane and laid in prone position. An incision of ∼4 mm was made in the right posterior so that the right kidney was accessible and could be gently lifted with tweezers and rested upon the wound. RU injections were made into the ureter adjacent to the renal pelvis. SC injections were performed by piercing the capsule of the kidney down to the bevel of the needle, then slowly injecting. One milliliter Sub-Q syringes (26G; BD, Franklin Lakes, NJ) were used for injections. The surgical wounds were then sutured, and mice were placed on a heating pad until they awoke and began to walk. All surgical procedures were performed with standard aseptic technique.
AAV vectors
AAV vectors were purchased from the University of Pennsylvania Vector Core.
Ad vectors
Replication-defective Ad vectors were produced in 293 cells and purified by double banding on CsCl gradients. Cre expression is driven by the CMV promoter.
LV vectors
Lentiviral vector LV-Luc2-P2A-Puro expressing luciferase driven by the Spleen focus-forming virus promoter was purchased from Imanis Life Sciences (Rochester, MN).
In vivo bioluminescence imaging
Mice were anesthetized with isoflurane and injected intraperitoneally with 150 μL of D-Luciferin (20 mg/mL; RR Labs, Inc., San Diego, CA). Images were taken using Xenogen IVIS 200 ten minutes after D-Luciferin administration, and luminescence was quantified using Living Image software. After extracting kidneys from euthanized mice, kidneys were injected with an additional 150 μL of D-Luciferin and imaged in a six-well plate.
Luciferase assay
Kidneys were removed from euthanized mice and completely homogenized using a standard glass homogenizer in 1 mL of Glo Lysis Buffer, 1X (Promega Corporation, Madison, WI). Varying amounts of the respective lysates were then mixed with Bright-Glo Luciferase Assay System to make a ½ dilution, and the samples were read for luminescence in a 96-well clear bottom black microplate (Corning, Inc., Corning, NY) using Beckman Coulter DTX 880 Multimode Detector.
Tissue sectioning and confocal microscopy
Tissues from mice with membrane-bound fluorescent proteins were fixed by overnight immersion in 4% paraformaldehyde (PFA)–phosphate-buffered saline (PBS) at 4°C and then cryoprotected overnight in 15% sucrose-PBS and 30% sucrose-PBS, successively, at 4°C. Trimmed tissues were then flash frozen by dry ice-cooled isopentane in optimal cutting temperature (OCT) medium (Sakura Finetek). Cryosections (18 μm thickness) were prepared with a Leica CM1860 UV cryostat (Leica Biosystems) and mounted on slides (SuperFrost Plus; Thermo Fisher Scientific, Waltham, MA) with VECTASHIELD with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and CytoSeal-60 coverslip sealant (Thermo Fisher Scientific). Confocal imaging was performed at the Microscopy and Cell Analysis Core facility at Mayo Clinic Rochester (Rochester, MN), using a Zeiss LSM780 laser confocal microscope (Carl Zeiss Jena, Jena, Germany). Lambda mode and linear unmixing were used to image Brainbow fluorophores.
To prevent leakage of cytoplasmic GFP from cells transduced by AAV8-CAG-EGFP, tissues were flash frozen in dry ice-cooled isopentane in OCT medium, cryosectioned at 6 μm thickness, and were then mounted on slides. The slides containing tissue sections were fixed with 4% PFA for 15 min, washed with PBS, treated with 5% normal goat serum (Abcam Catalog No. ab7481), and 0.5% IGEPAL® CA-630 (Sigma I8896) dissolved in PBS blocking buffer for 1 h at room temperature. The slides were then incubated with a 1:00 dilution of mouse GFP monoclonal antibody (Invitrogen Catalog No. A-11120) and a 1:100 dilution of biotinylated lotus tetragonolobus lectin (LTL) (Vector Laboratories Catalog No. B-1325) overnight at 4°C. The slides were washed and then incubated with a 1:250 dilution of goat anti-mouse IgG Alexa Fluor 488 polyclonal antibody (Invitrogen Catalog No. A-11001) and a 1:200 dilution of streptavidin-Alexa Fluor 594 (Invitrogen Catalog No. S11227) at room temperature for 1 h. The slides were washed, and coverslips were mounted using VECTASHIELD with DAPI.
Transgenic and wild-type mice
LSL-Luc mice (Stock No: 005125), mT/mG mice (Stock No: 007576), and Ksp1.3/Cre mice (Stock No: 012237) were originally purchased from The Jackson Laboratory. Outbred CD1 mice and Friend virus B strain (FVB) mice were purchased from Charles River.
Results
Double and triple reporter systems to analyze gene therapy vector pharmacology
We previously evaluated kidney transduction after IV injection with different AAV serotypes using Cre recombinase to track luciferase (Luc), GFP, and RFP in the same mouse.11 In this approach, AAV-Cre was injected into mice that are transgenic in the Rosa26 locus for different Cre-activated reporter genes.
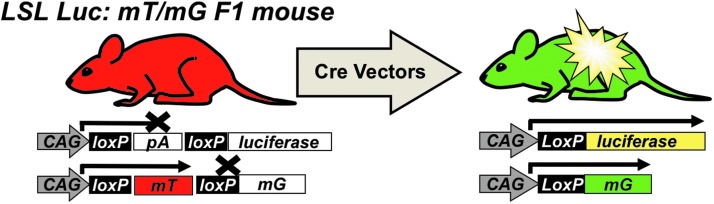
In LSL-luciferase mice, luciferase's expression is blocked by a LoxP-flanked (floxed) poly-adenylation (PolyA) cassette between the CAG promoter and Luc (Fig. 2). In the absence of Cre, no Luc is expressed. When Cre is delivered, it deletes the floxed PolyA to activate Luc.
Figure 2.
Hybrid triple reporter mouse model to assess transduction. Mice endogenously express the membrane targeted red fluorescent protein mTomato (mT). Any cell transduced by a Cre recombinase-expressing vector will stop expressing mTomato and begin to express mG and Luciferase. This hybrid mouse is useful for in vivo luminescent imaging and fluorescent microscopy to assess cell-by-cell transduction. mG, membrane targeted GFP.
In mT/mG mice, a floxed membrane-targeted red fluorescent protein mTomato (mT) is followed by membrane-targeted GFP (mG). In the absence of Cre, mT is expressed in all cells of the mouse and is membrane targeted. When Cre is delivered, mT is deleted and mG is expressed. At higher magnifications, these membrane-targeted reporter proteins provide substantial cell discrimination.11
By crossing LSL mice with mT/mG mice, hybrid mice have exactly one gene copy of Cre-activatable luciferase and exactly one gene copy of the mT/mG cassette. Therefore, these hybrid mice provide an “on/off” system to detect vector transduction and pharmacology. The presence of three reporter genes allows (1) in vivo imaging, (2) cell-specific transduction monitoring through mG expression, and (3) on/off confirmation of transduction by coordinated loss of mT with activation of mG.11 This system can also be augmented by tapping into the vast repertoire of mice engineered for tissue-specific Cre expression (Supplementary Fig. S1). In this previous work, we found that AAV9 and rh10 vectors mediated the highest levels of transduction in the kidney after IV injection compared to AAV1 and AAV811 (and unpublished observations).
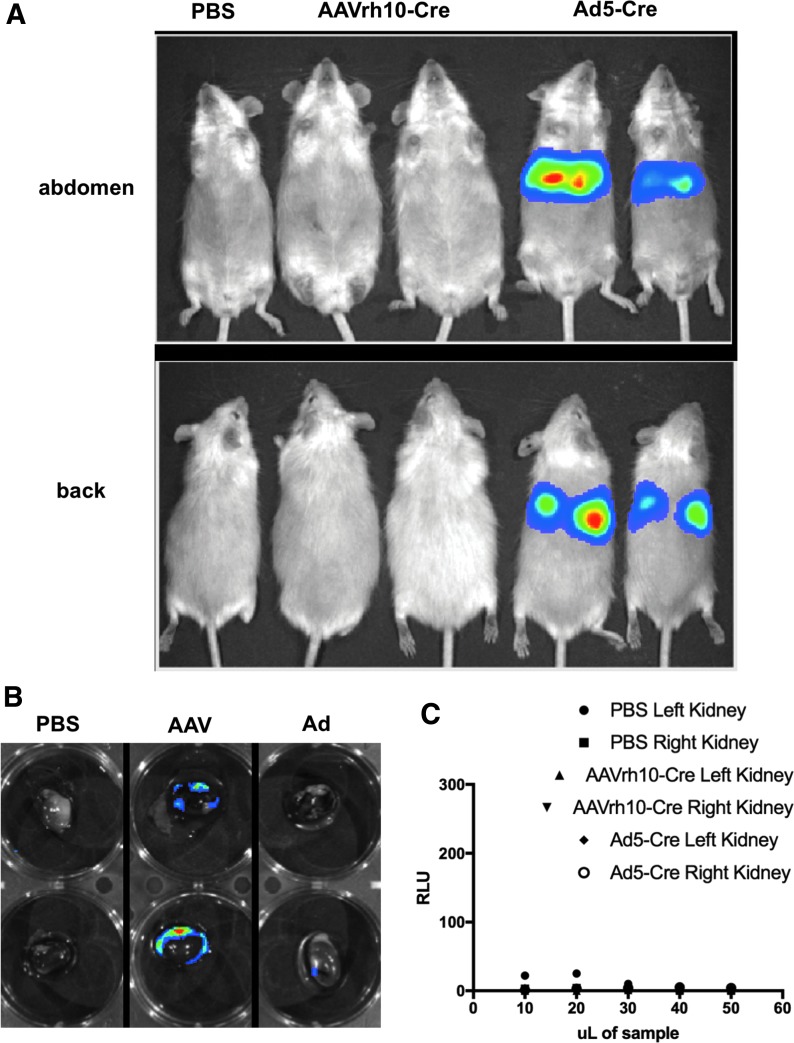
Given this prior art, in this study, we first compared AAVrh10-Cre transduction to that mediated by significantly larger Ad5-Cre by IV injection into LSL-Luc mice. LSL-Luc mice were injected with PBS, 1012 GC of AAVrh10-Cre, or 1011 VP of Ad5-Cre and were imaged for luciferase activity 3 days later (Fig. 3A). Under these conditions, luciferase activity was observed only in mice that were administered Ad5-Cre. This luminescence did not occur in the kidney, but instead arose from the liver of the mice. This observation is consistent with previous work that shows that the liver absorbs ∼98% of IV injected Ad.12,13 The kidneys from these mice were removed and analyzed ex vivo for luciferase activity. Imaging and enzymatic luciferase assays showed weak transduction in kidneys from mice injected IV with either AAVrh10-Cre or Ad5-Cre (Fig. 3B, C).
Figure 3.
IV injection mediates liver transduction. (A) Mice were administered IV injections of PBS (n = 1), 1e12 GC AAVrh10-Cre (n = 2), or 1e11 VP Ad5-Cre (n = 2) and imaged on day 3. Ad5-Cre mediated strong visible liver transduction. (B) Kidneys were removed from the euthanized mice and imaged. No transduction was observed except by AAVrh10-Cre in the capsule of the kidney. (C) Luciferase assay shows low levels of transduction in kidneys after IV injection. Kidneys from mice shown in Panel A were extracted and homogenized. The homogenate was used in a luciferase plate reader assay. No kidney homogenate showed higher levels than background (PBS). Luminescence is quantified in RLU. GC, genome copies; PBS, phosphate-buffered saline; RLU, relative light units.
RU and SC injections increase kidney transduction
This suggested that the glomerular barrier prevents large viral vectors from reaching the parenchyma of the kidney. We hypothesized that delivering the vectors from the reverse direction, (i.e., up the ureter), would avoid these size constraints and enable better gene delivery within the kidney. We also hypothesized that direct injection of vectors into the kidney parenchyma through the kidney capsule wall would also circumvent this barrier.
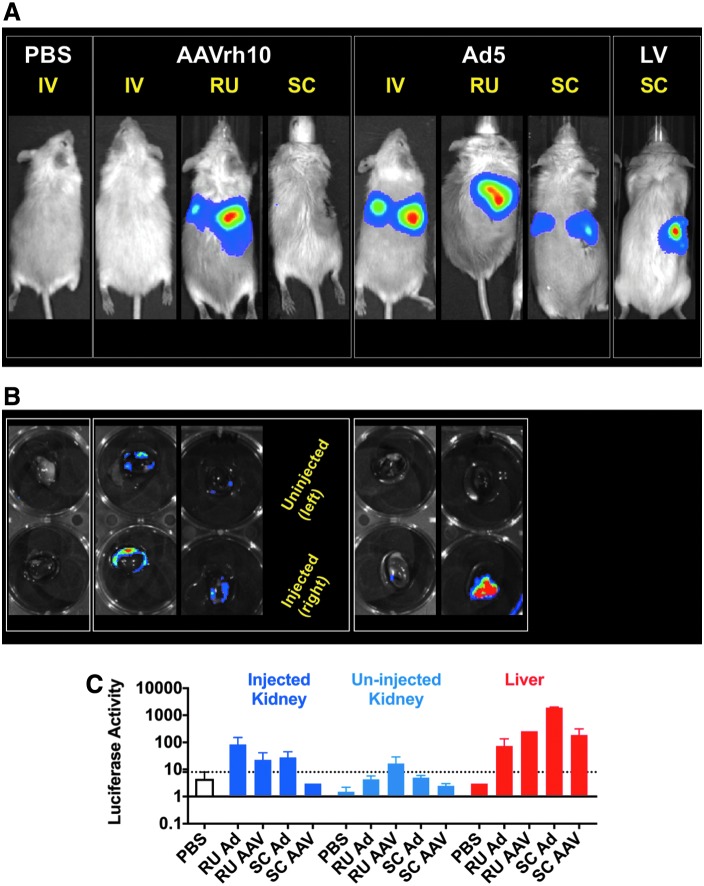
To test this, AAV-Cre and Ad-Cre were administered into the right kidneys in LSL-luciferase mice by RU and SC routes (Fig. 4A). RU-injected kidneys showed strong luciferase activity by imaging that appeared near the region of the kidney. Kidneys from the IV and RU injected mice were removed and imaged (Fig. 4B). The right kidney of the Ad5-Cre injected mouse showed markedly higher levels of transduction than the other samples. In addition, both the right and left kidney homogenates of the AAVrh10-Cre injected mouse showed detectable luciferase by imaging. When the RU injected and uninjected kidneys were homogenized and analyzed by enzyme activity, this showed highest activity in the Ad5 injected kidney with no activity in its contralateral kidney (Fig. 4C). In contrast, the AAV injected and uninjected kidneys both showed activity that was higher than in PBS control kidney extracts. This indicated that small AAV vector leaked from the injected site to transduce the contralateral kidney, whereas the large Ad vector did not. However, homogenate from the livers of these RU and SC injected mice showed even greater luciferase activity than the kidneys, indicating that both AAV and Ad leaked into the systematic compartment and transduced the liver.
Figure 4.
Enhanced levels of transduction in kidneys after RU and SC injections. (A) Mice were administered 1e12 GC AAVrh10-Cre, 1e11 VP Ad5-Cre, or 6.4e6 TU LV-Luc RU (n = 2 for AAVrh10 and n = 3 for Ad5) or SC (n = 2 for AAVrh10, n = 2 for Ad5, and n = 1 for LV). IV images are the same as those shown in Figure 3. (B) The extracted kidneys of the mice shown in Panel A were imaged. (C) Homogenates of these kidneys show high levels of transduction from Ad5 and for AAVrh10, weaker levels from both the injected (right) and uninjected (left) kidney. The liver homogenates of these mice also indicate that significant vector transduced the liver. Luminescence is quantified in RLU. SC, subcapsular; TU, transducing units.
Similar results were observed when LV expressing firefly luciferase was injected into nontransgenic mice by the SC route (Fig. 4A, right mouse). This resulted in a sustained kidney signal without off-target transduction indicating that the large LV vector likely integrated into kidney cells without leaking into the blood like the large Ad vector.
AAV-Brainbow vectors transduce tubule epithelial cells
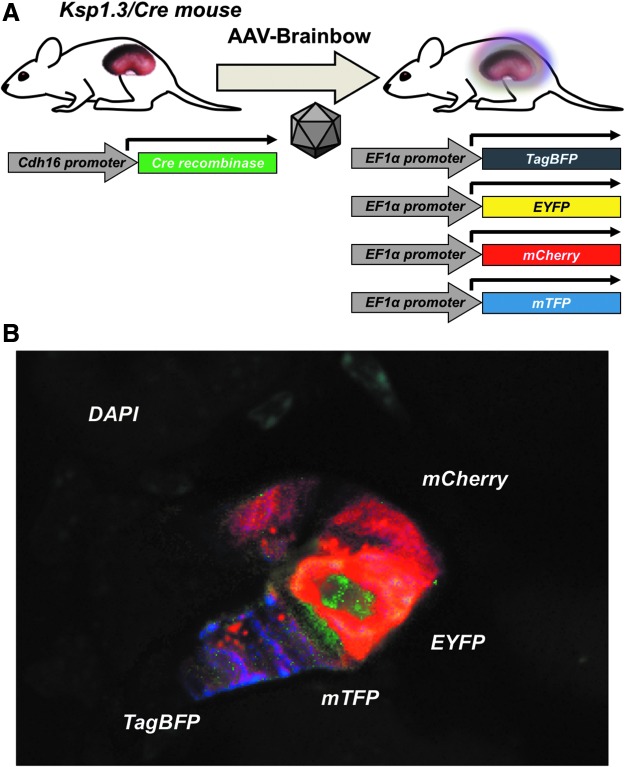
To further investigate the ability of AAV vectors to transduce renal tubule cells, we used AAV-Brainbow vectors14,15 in Cre-expressing mice. Like mT/mG mice, AAV-Brainbow vectors have inactivated fluorescent protein genes, but for four separate fluorescence proteins. In the presence of Cre, mCherry, mTFP, TagBFP, and EYFP genes are stochastically activated, generating cells that may express one or more of each of the fluorescent proteins.14,15
These vectors were injected into Ksp1.3/Cre mice that express Cre in renal tubule cells.16 Ksp1.3/Cre mice were injected with 5e11 GC of AAV9-TagBFP-EYFP and 5e11 GC of AAV9-mCherry-mTFP, by RU or SC injections (Fig. 5A). On day 24 after injection, the kidneys were harvested, sectioned, and examined by confocal microscopy. Under these conditions, stochastic transduction of cells was observed in sections (Fig. 5B). In most cases, a single fluorophore was expressed in tubule cells, although tubules could be found displaying all four of the Brainbow fluorophores in rare cases. The specificity of Ksp1.3/Cre in the kidney was confirmed by crossing these mice to mG/mT and LSL-Luc mice (Supplementary Fig. S1).
Figure 5.
Transduction of tubule cells by AAV9-Brainbow vectors in Ksp1.3/Cre mice. (A) Diagram of the reporter system. Ksp1.3/Cre mice express Cre driven by the Cadherin16 (Cdh16) promoter, which is active only in tubule epithelial cells of the kidney and the genitourinary tract. Cre flips AAV9-Brainbow vectors to the correct orientation to express TagBFP, EYFP, mCherry, or mTFP. (B) Mice were injected with a cocktail of 5e11 GC AAV9-TagBFP-EYFP and 5e11 GC AAV9-mCherry-mTFP RU (n = 2) or SC (n = 2). Shown is a confocal microscopy image of several cells in a tubule expressing the four Brainbow fluorophores. Magnification 40 × .
Comparison kidney transduction by different AAV serotypes
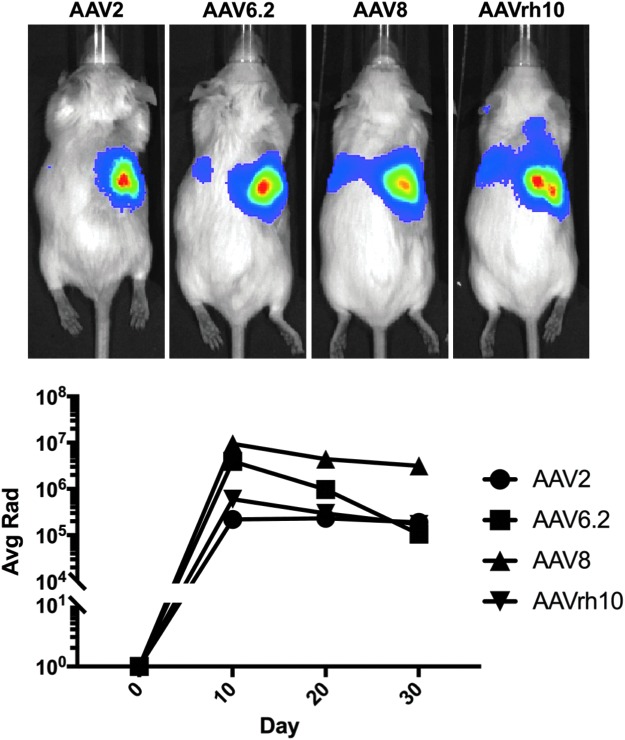
We found that AAV9 and rh10 were most robust by IV injection in previous work, but both primarily transduced cells within the glomerulus of the kidney. While these vectors are more robust than other AAV serotypes by the IV route17 (and reviewed in Saraiva et al.18), it was possible that they might not be best by direct kidney injection. In addition, the beneficial ability of AAV9 and rh10 to permeate into tissues after IV injection might actually be a liability for kidney injections by enabling them to leak out of the kidney and hit off-target tissues. Given this, we compared AAV2, AAV6.2, AAV8, and AAVrh10 vectors expressing Cre by SC injection into the kidneys of LSL-Luc-mT/mG mice (Fig. 6). Luciferase imaging over 30 days revealed that AAVrh10 surprisingly did not produce the strongest transduction. Instead, AAV8 and AAV6.2 mediated up to 10-fold higher expression than AAVrh10. AAV2 transduction was surprisingly similar to AAVrh10.
Figure 6.
AAV8 yields best kidney transduction determined by in vivo luminescence. Mice were given SC injections of 2e11 GC of AAV2-Cre, AAV6.2-Cre, AAV8-Cre, or AAVrh10-Cre (n = 1 for each serotype). Luminescent signals were measured at days 10, 20, and 30, and the signals typically declined from day 10 to 30. Luminescence is quantified in Average Radiance, p/sec/cm2/sr (Avg Rad).
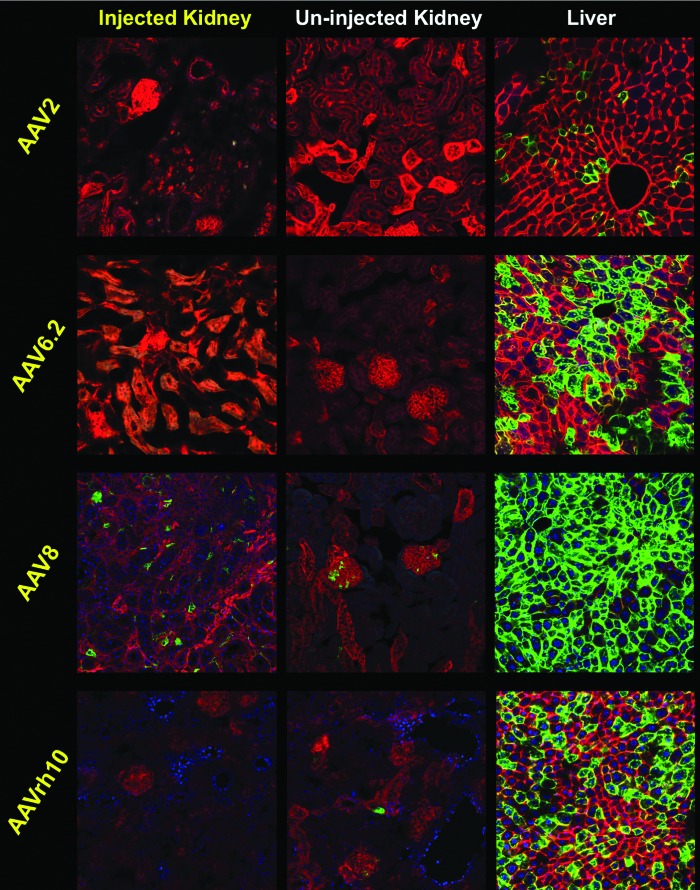
The mice were sacrificed and their kidneys were sectioned to observe transduction on a cell-by-cell basis. Under these conditions, the injected right kidney of the AAV8 mice yielded the highest number of transduced cells compared to the other AAV serotypes (Fig. 7). In parallel, the livers of these AAV8-injected mice also showed higher off-target transduction with nearly 100% of hepatocytes expressing mGFP. The livers of the mice injected with AAV6.2 and AAVrh10 also showed substantial levels of liver transduction. In contrast, AAV2 mediated as good of kidney transduction as AAVrh10 with relatively low off-target transduction of the liver. These data indicate that different AAV serotypes mediate different levels of transduction in the kidney after direct kidney injection. These data also indicate significant leakage of all of these AAV vectors from the organ that results in substantial off-target transduction of other tissues like the liver.
Figure 7.
Several AAV serotypes mediate varying levels of off-target liver transduction. 2e11 GC of AAV2-Cre, AAV6.2-Cre, AAV8-Cre, or AAVrh10-Cre were administered by SC injection to mice (n = 1 for each serotype; same group of mice shown in Fig. 6). For AAV2, no transduction of kidney cells was observed, while few liver cells were transduced. For AAV6.2, no transduced kidney cells were observed, while approximately half of liver cells were transduced. For AAV8, epithelial cells and some glomerular cells were stochastically transduced and nearly all liver cells were transduced. For AAVrh10, few kidneys cells were transduced, and approximately half of liver cells were transduced.
AAV8-Cre is a more sensitive reporter vector than AAV8-GFP
Our experiments thus far enabled us to determine which serotype of AAV is most effective at transducing kidney cells. However, the use of Cre to “fingerprint”11 or “footprint”19 transduced cells is a very sensitive system that can detect weakly or transiently transduced cells. This contrasts with standard episomal viral vector delivery of reporter genes whose expression depends on transgene copy numbers. The distinction between strong and weak transgene expression has important implications for viral vector-based genome editing applications, which may only require the latter.19
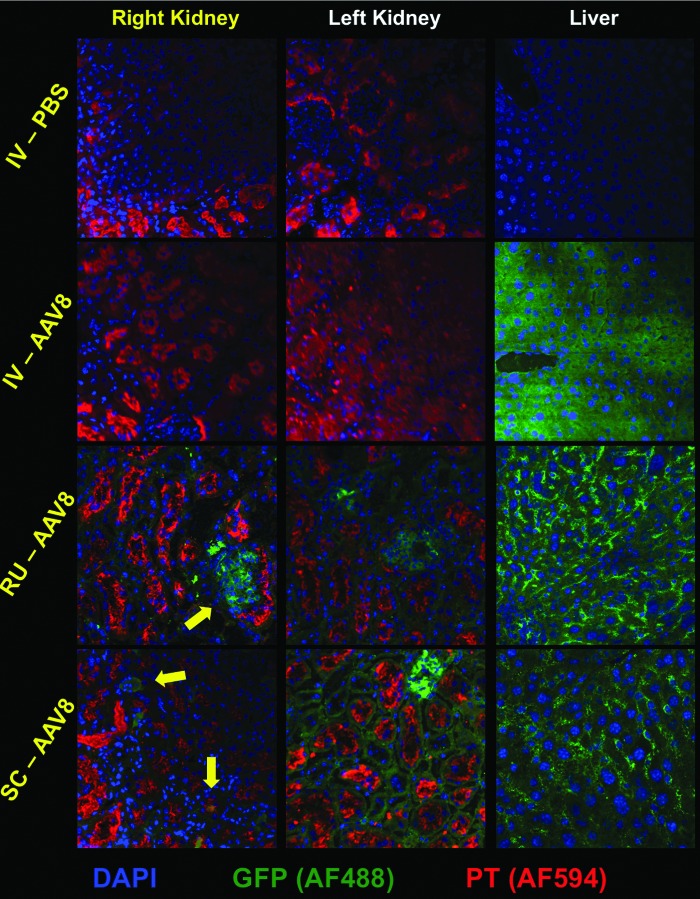
To evaluate kidney transduction with a more typical reporter gene, 2e11 GC of AAV8-GFP was injected into normal FVB mice by IV, RU, and SC routes. The mice were sacrificed 4 weeks later, and their kidneys and livers were analyzed by immunofluorescent staining for GFP (Fig. 8). IV injection of AAV8-GFP resulted in strong, nearly ubiquitous GFP expression in the liver, but no observable GFP expression in the kidney. Kidney tissues from RU and SC injections showed fewer GFP-positive cells than were observed in AAV-Cre injected mT/mG mice. Interestingly, the most abundant and consistent GFP expression mediated by RU and SC injections was in the glomeruli of injected and surprisingly also in uninjected kidneys. The SC-injected right kidneys also contained GFP-positive cells of tubular morphology, some of which colocalized with proximal tubule (PT) marker, lotus lectin (Fig. 8). The livers of the RU- and SC-injected mice also showed significant leakage of the AAV8-GFP vector to the liver, corroborating the previous data using the Cre reporter system.
Figure 8.
AAV8-CAG-GFP reporter vector mediates weaker expression than AAV8-Cre reporter vector. 2e11 GC of AAV8-CAG-GFP was administered by IV (n = 1), RU (n = 4), or SC (n = 4) injection to mice. PBS was injected as a control (n = 1 for each route). IV administration of AAV8-CAG-GFP mediated extremely robust GFP expression in the liver but none in either kidney. For both RU and SC injections, GFP expression was strongest and most abundant in the glomeruli (yellow arrow) of the injected (right) and uninjected (left) kidneys. The right kidney of the SC injected mouse also shows some transduction of tubule cells, some of which coincide with PT marker (lower yellow arrow) and some of which are likely distal tubule or collecting duct cells (upper yellow arrow). Liver sections from the RU and SC injected mice corroborate leakage of the vector to the liver. Tissue sections were stained using mouse anti-GFP primary plus Alexa Fluor 488 secondary and counterstained with lotus LTL plus Alexa Fluor 594 secondary to mark PT cells. LTL, tetragonolobus lectin.
Discussion
We previously examined the ability of several serotypes of AAV to transduce cells in various tissues in mice after IV injection with particular focus on kidney gene delivery.11 This study expands on this work to compare gene delivery by three different vector systems. This study also tested the utility of direct kidney injection of these vectors to avoid the natural ability of the glomerulus to exclude large molecules from downstream tubule epithelial cells of the nephron.
In our previous work, we compared selected AAV serotypes and determined that AAV1, AAV8, AAV9, and AAVrh10 all mediate varied levels of gene delivery in the kidney11 (and unpublished observations). However, this transduction was largely limited to cells within the glomeruli of the kidneys. While the ability to transduce glomerular cells may be relevant to treating inherited disorders such as Alport syndrome,20 the inability to transduce PT, distal tubule, and collecting duct cells obviates the ability to treat inherited tubulopathies and cystic diseases. We show here that it is indeed possible to transduce cells beyond the glomerulus, including key target cells in the tubule epithelium.
Delivery of reporter genes to the kidney by select viral vectors has been tested previously.5,21–24 Early work used AAV1, 2, 3, 4, and 5 serotypes to deliver β-galactosidase and GFP reporter genes. In more recent years, several groups have used RU or SC injection techniques for gene transfer similar to the one we use in this study.6,9,10,25 Injection through the renal artery or retrograde through the renal vein into the kidney has also had some success with various serotypes of AAV.23,26,27 These studies did not compare different vector systems for kidney gene delivery. They also generally did not look beyond the kidney to evaluate off-target gene delivery to other organs.
Our studies, as well as many others, indicate that vector delivery to the kidney through the systemic route is inefficient. This is in contrast to AAV vectors' ability to permeate into many tissues after IV injection. However, the kidney has evolved very specific permselectivity to control solute and protein entry into its parenchyma. This 50 kDa exclusion limit of the kidney is well below the megaDalton mass of even small AAV vectors, let alone larger vectors like Ad and LV. One can extrapolate these size effects beyond viral vectors to nonviral systems. Even nano-scale nonviral vectors like lipid nanoparticles will likely fail to pass the glomerular barrier unless they can be engineered to be below 50 kDa in mass. Considering that an average nucleotide is 325 Da in mass, this may mean that 150 base pairs of naked single-stranded DNA or RNA may have difficulties entering through the glomerulus. Small RNA or DNA like siRNAs may pass the barrier provided that their nonviral packaging does not bulk them up beyond the permselectivity of the glomerulus. This speculation assumes that vectors lack any special evolved or engineered mechanisms to enter the kidney by different means (e.g., natural retrograde infection, cell-targeting peptides, and so on).
Given the natural filtering function of the glomerulus, it is logical to avoid this barrier by direct or retrograde delivery into the kidney. There are two essential problems to address for efficient vector delivery to kidney cells by these direct kidney injection routes: the access problem and the tropism problem. The access problem regards whether or not vectors can actually make physical contact with the target cells. The tropism problem considers that if contact can be made whether vectors have the right cell-binding ligands for the receptors that are on the kidney target cells.
In the current study, we begin to address the access problem using novel injection routes that avoid the limitation of vectors' access to kidney cells from the bloodstream. We show the ability to stochastically transduce cells beyond the glomerulus, but are not yet able to saturate all cells that may be in need of therapy. We also begin to address the tropism problem by testing different vector systems and different AAV serotypes that target different receptors. The Ad5 vector we use can infect cells that express the coxsackie and Ad receptor and αv integrins (reviewed in Khare et al.28). Different AAV serotypes target a variety of receptors.18 One would expect these viral vectors that target different receptors to have different abilities to transduce kidney cells. In contrast, LV vectors that are pseudotyped with the glycoprotein from vesicular stomatitis virus (VSVg) (reviewed in Cronin et al.29) can infect nearly any cell provided they first satisfy the access problem. The repertoire of transduction will likely be expanded by the use of additional viral and nonviral vectors that target new receptors provided that they too can address the access problem.
Genetic and nongenetic kidney diseases affect many cells and structures in the organ.3 As mentioned, IV injections may be able to treat glomerulopathies, but neglect many cystic, glomerular basement membrane disorders, and tubulopathies. The ability to transduce renal tubular epithelial cells, as demonstrated with AAV9-Brainbow and Ksp1.3/Cre mice, has implications for treating several genetic diseases that impact these cells. Ksp1.3/Cre mice are characterized as having Cre expression in collecting ducts, thick ascending loop of Henle, and proximal and distal tubules.16 Targeting these cell types for transduction could pave the way for gene therapy approaches for diseases such as cystic kidney diseases, tubular diseases, and renal metabolic diseases (reviewed in Hildebrandt2). For example, delivering the SCNN1B or SCNN1G gene to epithelial cells of the collecting duct could perhaps treat the autosomal dominant metabolic disease Liddle Syndrome. Mouse models that recapitulate a variety of human monogenic renal disorders such as nephrotic syndrome, Alport syndrome, and Bartter's syndrome, among others, already exist (reviewed in Hofmeister et al.30). Therefore, the techniques and vectors used in this study may lay the groundwork for testing therapeutic interventions for these diseases in mouse models.
The kidney is an organ with complex vasculature (the human kidney filters ∼2,000 liters of blood per day). When livers of AAV-injected mice were analyzed, higher levels of liver transduction were observed than were observed even in the directly injected kidney. This indicates that a significant amount of these small vectors leak or permeate out of the kidney into the blood supply after direct kidney injection. This level of off-target transduction after kidney injection has not been reported previously in most cases simply because other organs were not examined in other studies. While Rocca et al. showed that AAV8-Luc and AAV9-Luc are expressed in the liver after left kidney injection,26 and Shen et al. showed that AAV9-GFP had some expression in the liver that did not affect organ function after transparenchymal kidney injection,25 the current study is the first to show the massive extent to which liver tissue is transduced after a direct kidney injection, with the most striking example being AAV8. This is important to note considering that off-target transduction and systemic effects of gene therapy vectors can cause dangerous side effects in animals and humans.31–39
AAV2 is generally thought to be a weak vector for IV gene therapy, whereas the newer AAVrh10 serotype is thought to be one of the most robust vectors for systemic therapy.40–43 While both were near equal in the injected kidney, it was interesting that the more robust AAVrh10 vector produced substantially higher off-target gene delivery in the liver than AAV2. We also observed a dose-dependent effect when using AAVrh10-Cre, with markedly more tubule cells being transduced after injecting 1e12 GC versus 2e11 GC (Supplementary Fig. S2). It is important to note that AAV serotypes that are deemed robust by virtue of their ability to permeate into tissues after IV injection may actually be a safety liability if one wants those vectors to stay local in a target organ like the kidney. Off-target transduction may not pose substantial safety issues unless expression of a kidney-specific protein provokes off-target cell dysfunctions or immune responses. In addition, vector transgene expression can be restricted through the use of tissue specific promoters, such as transthyretin for liver, muscle creatine kinase for muscle, and Ksp-cadherin for kidney.44–46 However, recent observations of severe toxicity after high dose IV injections in animals31–33,39 suggest that more targeted delivery to the kidney may be prudent when treating kidney-specific diseases.
While we have shown here that direct kidney injections are superior to IV injections for transduction of tubule epithelium, there is still much room for improvement. Another way to improve viral vectors for kidney gene delivery is to modify them to improve their tropism for kidney cells. For example, in vivo phage display can be used to select for peptides that can be added to AAV or Ad capsids for increased kidney cell tropism. A similar system called DARPins exist for LV.47 While this has been attempted through IV injection, it is yet to be attempted by RU or SC injections.48,49 AAV can also be put through “directed evolution” using DNA shuffling techniques to increase tropism for target tissue.50 In a similar manner, Ad proteins can be modified to target ligands that may be present on specific cell types.51 Modification of vector capsids to alter tropism holds promise not only for increasing specificity for the kidney but also for detargeting the liver. It is important to consider the relevance of smaller vectors such as AAV versus larger vectors such as Ad; while AAV is popular and less immunogenic, it would require at least two AAV vectors to carry Cas9, an accompanying single guide RNA, and a template for gene editing experiments, while Ad can easily carry all three of these components in addition to more cargo.
Lang et al. recently improved upon our original AAV-Cre “fingerprinting” studies comparing AAV-GFP-Cre and AAV-CRISPR to “footprint” cells that have been weakly or transiently transduced.19 This study showed that these principles also apply in transduction by direct kidney injection by testing AAV8-Cre versus AAV8-GFP, which has important implications in genome editing applications where only weak or transient transduction of CRISPR-Cas9 may be needed for a permanent genomic modification.
In conclusion, our studies demonstrate that genetic modifications of kidney cells can be increased by applying several different vector platforms by direct kidney injection. This work also reveals the previously unreported problem that massive amounts of the vectors that are injected into the kidney also appear to leak out of the organ to mediate rampant off-target tissue transduction. For some kidney diseases, this off-target transduction may not be a problem, but for others, this may increase the likelihood of significant side effects. Modifying vectors with kidney-specific promoters may help, but in reality just hide the fact that much of these injected doses are going elsewhere. Given this, next steps should include efforts to increase transduction of kidney cells and increase the retention of vectors within the organ.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Chris Chen for stimulating the need for these studies. We would like to thank Dr. Chris Chen and Dr. Matthew Hillestad for their technical input on kidney gene delivery and analyses. The authors thank Louisa Papke-Norton with help in tissue sectioning. The authors thank the Microscopy and Cell Analysis Core facility at Mayo Clinic Rochester for their assistance in confocal microscopy.
Author Disclosure
No competing financial interests exist.
Funding Information
This work was supported with reagents provided by the University of Pennsylvania Vector Core funded through the Gene Therapy Resource Program (GTRP) from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health. This work was supported by grants from Mayo Translational Polycystic Kidney Disease Center (P30-DK090728). This work was also supported by the Department of Molecular Medicine at Mayo Clinic (J.D.R.), National Institute of Diabetes Digestive and Kidney Diseases Grant Number 1F31DK123858–01 (J.D.R.), and the Department of Laboratory Medicine and Pathology at Mayo Clinic (M.A.B.).
Supplementary Material
References
- 1. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 2016;11:e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hildebrandt F. Genetic kidney diseases. Lancet 2010;375:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung JC. Inherited renal diseases. Curr Pediatr Rev 2014;10:95–100 [DOI] [PubMed] [Google Scholar]
- 4. Boron WF, Boulpaep EL. Glomerular filtration and renal blood flow. In: Medical Physiology. Philadelphia, PA: Elsevier, 2017:739.e732–753.e732 [Google Scholar]
- 5. Ito K, Chen J, Khodadadian JJ, et al. Adeno-associated viral vector transduction of green fluorescent protein in kidney: effect of unilateral ureteric obstruction. BJU Int 2008;101:376–381 [DOI] [PubMed] [Google Scholar]
- 6. Chung DC, Fogelgren B, Park KM, et al. Adeno-associated virus-mediated gene transfer to renal tubule cells via a retrograde ureteral approach. Nephron Extra 2011;1:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konkalmatt PR, Asico LD, Zhang Y, et al. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight 2016;1:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe S, Ogasawara T, Tamura Y, et al. Targeting gene expression to specific cells of kidney tubules in vivo, using adenoviral promoter fragments. PLoS One 2017;12:e0168638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodard LE, Cheng J, Welch RC, et al. Kidney-specific transposon-mediated gene transfer in vivo. Sci Rep 2017;7:44904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asico LD, Cuevas S, Ma X, et al. Nephron segment-specific gene expression using AAV vectors. Biochem Biophys Res Commun 2018;497:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hillestad ML, Guenzel AJ, Nath KA, et al. A vector-host system to fingerprint virus tropism. Hum Gene Ther 2012;23:1116–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lieber A, He CY, Meuse L, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol 1997;71:8798–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Worgall S, Wolff G, Falck-Pedersen E, et al. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997;8:37–44 [DOI] [PubMed] [Google Scholar]
- 14. Livet J, Weissman TA, Kang H, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007;450:56–62 [DOI] [PubMed] [Google Scholar]
- 15. Cai D, Cohen KB, Luo T, et al. Improved tools for the Brainbow toolbox. Nat Methods 2013;10:540–547 [PubMed] [Google Scholar]
- 16. Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 2002;13:1837–1846 [DOI] [PubMed] [Google Scholar]
- 17. Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006;14:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saraiva J, Nobre RJ, Pereira de Almeida L. Gene therapy for the CNS using AAVs: the impact of systemic delivery by AAV9. J Control Release 2016;241:94–109 [DOI] [PubMed] [Google Scholar]
- 19. Lang JF, Toulmin SA, Brida KL, et al. Standard screening methods underreport AAV-mediated transduction and gene editing. Nat Commun 2019;10:3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kruegel J, Rubel D, Gross O. Alport syndrome—insights from basic and clinical research. Nat Rev Nephrol 2013;9:170–178 [DOI] [PubMed] [Google Scholar]
- 21. Moullier P, Friedlander G, Calise D, et al. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int 1994;45:1220–1225 [DOI] [PubMed] [Google Scholar]
- 22. McDonald GA, Zhu G, Li Y, et al. Efficient adenoviral gene transfer to kidney cortical vasculature utilizing a fiber modified vector. J Gene Med 1999;1:103–110 [DOI] [PubMed] [Google Scholar]
- 23. Chen S, Agarwal A, Glushakova OY, et al. Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors. J Am Soc Nephrol 2003;14:947–958 [DOI] [PubMed] [Google Scholar]
- 24. Takeda S, Takahashi M, Mizukami H, et al. Successful gene transfer using adeno-associated virus vectors into the kidney: comparison among adeno-associated virus serotype 1–5 vectors in vitro and in vivo. Nephron Exp Nephrol 2004;96:e119–e126 [DOI] [PubMed] [Google Scholar]
- 25. Shen X, Xu Y, Bai Z, et al. Transparenchymal renal pelvis injection of recombinant adeno-associated virus serotype 9 vectors is a practical approach for gene delivery in the kidney. Hum Gene Ther Methods 2018;29:251–258 [DOI] [PubMed] [Google Scholar]
- 26. Rocca CJ, Ur SN, Harrison F, et al. rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther 2014;21:618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rocca CJ, Cherqui S. Gene transfer to mouse kidney in vivo. Methods Mol Biol 2019;1937:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khare R, Chen CY, Weaver EA, et al. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 2011;11:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 2005;5:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofmeister AF, Komhoff M, Weber S, et al. Disease modeling in genetic kidney diseases: mice. Cell Tissue Res 2017;369:159–170 [DOI] [PubMed] [Google Scholar]
- 31. Varnavski AN, Zhang Y, Schnell M, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol 2002;76:5711–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003;80:148–158 [DOI] [PubMed] [Google Scholar]
- 33. Varnavski AN, Calcedo R, Bove M, et al. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther 2005;12:427–436 [DOI] [PubMed] [Google Scholar]
- 34. Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007;317:477. [DOI] [PubMed] [Google Scholar]
- 35. Chandler RJ, LaFave MC, Varshney GK, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 2015;125:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russell DW, Grompe M. Adeno-associated virus finds its disease. Nat Genet 2015;47:1104–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nault JC, Datta S, Imbeaud S, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet 2015;47:1187–1193 [DOI] [PubMed] [Google Scholar]
- 38. Berns KI, Byrne BJ, Flotte TR, et al. Adeno-associated virus type 2 and hepatocellular carcinoma? Hum Gene Ther 2015;26:779–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hinderer C, Katz N, Buza EL, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 2018;29:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanguy Y, Biferi MG, Besse A, et al. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front Mol Neurosci 2015;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Bell P, Somanathan S, et al. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol Ther 2015;23:1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hocquemiller M, Giersch L, Audrain M, et al. Adeno-associated virus-based gene therapy for CNS diseases. Hum Gene Ther 2016;27:478–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perdomini M, Dos Santos C, Goumeaux C, et al. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21(-/-) mouse model. Gene Ther 2017;24:275–281 [DOI] [PubMed] [Google Scholar]
- 44. Greig JA, Nordin JML, White JW, et al. Optimized adeno-associated viral-mediated human factor VIII gene therapy in Cynomolgus macaques. Hum Gene Ther 2018. [Epub ahead of print]; DOI: 10.1089/hum.2018.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakamura-Takahashi A, Miyake K, Watanabe A, et al. Treatment of hypophosphatasia by muscle-directed expression of bone-targeted alkaline phosphatase via self-complementary AAV8 vector. Mol Ther Methods Clin Dev 2016;3:15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shao X, Johnson JE, Richardson JA, et al. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 2002;13:1824–1836 [DOI] [PubMed] [Google Scholar]
- 47. Munch RC, Muhlebach MD, Schaser T, et al. DARPins: an efficient targeting domain for lentiviral vectors. Mol Ther 2011;19:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Denby L, Work LM, Seggern DJ, et al. Development of renal-targeted vectors through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol Ther 2007;15:1647–1654 [DOI] [PubMed] [Google Scholar]
- 49. Diaconu I, Denby L, Pesonen S, et al. Serotype chimeric and fiber-mutated adenovirus Ad5/19p-HIT for targeting renal cancer and untargeting the liver. Hum Gene Ther 2009;20:611–620 [DOI] [PubMed] [Google Scholar]
- 50. Yang L, Li J, Xiao X. Directed evolution of adeno-associated virus (AAV) as vector for muscle gene therapy. Methods Mol Biol 2011;709:127–139 [DOI] [PubMed] [Google Scholar]
- 51. Shashkova EV, May SM, Doronin K, et al. Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol Ther 2009;17:2121–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.