Abstract
Aim
To provide reliable survival estimates for people with chronic heart failure and explain variation in survival by key factors including age at diagnosis, left ventricular ejection fraction, decade of diagnosis, and study setting.
Methods and results
We searched in relevant databases from inception to August 2018 for non‐interventional studies reporting survival rates for patients with chronic or stable heart failure in any ambulatory setting. Across the 60 included studies, there was survival data for 1.5 million people with heart failure. In our random effects meta‐analyses the pooled survival rates at 1 month, 1, 2, 5 and 10 years were 95.7% (95% confidence interval 94.3–96.9), 86.5% (85.4–87.6), 72.6% (67.0–76.6), 56.7% (54.0–59.4) and 34.9% (24.0–46.8), respectively. The 5‐year survival rates improved between 1970–1979 and 2000–2009 across healthcare settings, from 29.1% (25.5–32.7) to 59.7% (54.7–64.6). Increasing age at diagnosis was significantly associated with a reduced survival time. Mortality was lowest in studies conducted in secondary care, where there were higher reported prescribing rates of key heart failure medications. There was significant heterogeneity among the included studies in terms of heart failure diagnostic criteria, participant co‐morbidities, and treatment rates.
Conclusion
These results can inform health policy and individual patient advanced care planning. Mortality associated with chronic heart failure remains high despite steady improvements in survival. There remains significant scope to improve prognosis through greater implementation of evidence‐based treatments. Further research exploring the barriers and facilitators to treatment is recommended.
Keywords: Heart failure, Prognosis, Survival analysis, Systematic review, Meta‐analysis
Introduction
One to two in every 100 adults in the general population, and more than one in 10 people aged over 70 years are diagnosed with heart failure (HF).1, 2 The true prevalence is likely closer to 4%, as HF often goes unrecognised or misdiagnosed, particularly in older people.3, 4 Prevalence has risen by almost 25% since 2002 due to factors such as population ageing, improved survival following coronary events and an increase in the prevalence of HF risk factors, including hypertension and atrial fibrillation.5 HF is associated with significant morbidity and mortality equivalent to common forms of cancer.6
Much existing research on HF prognosis has focused on survival rates for people with 'acute' HF who have been admitted to hospital with a sudden deterioration in symptoms.7 An acute decompensation is itself a poor prognostic sign and therefore these survival estimates are not directly applicable to people with 'chronic' HF, who have had an extended period of symptom stability.7 Previous research suggests 1‐year survival in acute HF is between 55% and 65%,8, 9 compared to 80% to 90% in chronic HF.10, 11
The majority of patients have chronic HF and are treated in ambulatory settings.12 This chronic phase should be a time to discuss advanced care planning and anticipated disease progression with patients and their families. These conversations rely on healthcare professionals providing accurate prognostic information, yet survival estimates for chronic HF vary significantly across studies. The pattern of disease progression in HF is also unpredictable and varies considerably between individuals.13 Uncertainty over disease trajectory is one reason active HF treatment often persists into the terminal phases of illness, resulting in a large increase in resource use in the last 6 months of life.14 It also explains why some clinicians lack confidence in discussing HF prognosis and so avoid the subject.15, 16 Not all patients wish to know or discuss their prognosis, but for those who do, the ambiguity around their future can be distressing and many would welcome more information.17 Where patients are not informed of their expected prognosis, they tend to significantly overestimate their likely life expectancy.18
Reliable prognostic estimates can help to promote advanced care planning, improve shared understanding of treatment goals and facilitate integrated treatment with specialist services, including palliative care.16 The aim of this systematic review was to assimilate the existing evidence base to provide accurate survival estimates for people with chronic HF. We also aimed to identify key factors which explain the existing variation in prognostic estimates, including age at time of diagnosis, left ventricular ejection fraction (LVEF), decade of diagnosis, and study setting.
Methods
The protocol was published on PROSPERO (registration number CRD42017075680) and in Systematic Reviews.19 Reporting adheres to the 'Meta‐analysis Of Observational Studies in Epidemiology' (MOOSE) guidelines (online supplementary Methods S1 ).20
Search strategy
We conducted a systematic search of relevant databases from inception to August 2018, incorporating Medical Subject Heading Indexation (MESH) terms and integrated validated search filters from the Scottish Intercollegiate Guidelines Network21 (online supplementary Table S1 ). A hand search of the included papers' references and relevant review articles was completed to achieve literature saturation.
Eligibility criteria
Eligible studies reported survival time for adult patients with a diagnosis of HF in the 'chronic' or 'stable' phase.7 Survival times were calculated from diagnosis, or from point of study recruitment if this information was unavailable. Studies with under 1‐year follow‐up were excluded given the lack of information on long‐term prognosis. We included studies reporting outcomes for both acute and chronic HF where it was possible to extract survival rates for chronic HF. If the results were combined, we attempted to contact study authors. As our aim was to report survival time in the context of usual care, we excluded interventional studies, service evaluations and studies where participants had been recruited on the basis of another co‐morbidity. Conference abstracts were excluded as having insufficient detail for quality assessment.
Data analysis
Two authors (N.R.J., I.A.) independently completed two rounds of screening, the first based on titles and abstracts and the second a full text review. Foreign language papers were translated before assessment. Disagreements were checked with a third reviewer (C.J.T.). Two authors (N.R.J., I.A.) also completed independent duplicate data extraction.
Pooled survival rates were calculated at pre‐specified time points using a random effects model given the anticipated variability in study methods. We used the metaprop command in Stata 14, designed for meta‐analysis of binomial data.22 We calculated the study‐specific 95% confidence intervals using the score statistic via the cimethod(score) function and used the ftt command to perform the Freeman–Turkey double arcsine transformation and stabilise variance in our weighted pooled estimates.22 Heterogeneity and consistency were assessed using Chi‐squared and I2 statistics respectively. Sources of heterogeneity were explored using pre‐specified sensitivity and subgroup analyses.
We conducted subgroup analyses and meta‐regression for study date, setting, age and LVEF. To pool study dates, we categorised each included study or relevant subgroup by the decade of participant recruitment. Mean participant age was used to categorise results as either < 65, 65–74 or ≥ 75 years. Study setting was determined by point of recruitment and majority of management. Where there was evidence of significant input across both primary and secondary care, studies were classified as 'cross‐discipline'. HF was categorised as HF with preserved ejection fraction (HFpEF) if LVEF ≥ 50%, HF with mid‐range ejection fraction (HFmrEF) with LVEF in the range 40–49%, and HF with reduced ejection fraction (HFrEF) if LVEF < 40%. Some earlier studies did not include a mid‐range group and so categorised HFpEF as LVEF ≥ 40%. Studies reporting pooled outcomes for all three groups or not measuring LVEF were grouped as 'mixed' ejection fraction. Data were unavailable to allow all subgroups of interest to be included together as covariates in a meta‐regression analysis, therefore each covariate was considered separately in meta‐regression models of survival rates at 1 and 5 years.
Two authors (N.R.J., I.A.) independently completed a risk of bias assessment for each study using the Quality in Prognosis Studies (QUIPS) tool, recommended by the Cochrane Prognosis Methods Group.23 We conducted a sensitivity analysis excluding studies at moderate or high risk of bias. We report a Grading of Recommendations Assessment, Development and Evaluation (GRADE) score to provide an estimate of confidence in the cumulative outcomes (online supplementary Methods S2 ).24
Results
Study characteristics
We included 60 studies after screening, 5423 studies at the title and abstract stage and 97 full texts (online supplementary Figure S1 ). A number of studies reported survival rates from the same dataset. Where these provided relevant information for our pre‐specified subgroup analyses, we included both studies in the review but only one in any single meta‐analysis. Two studies met the inclusion criteria but reported survival rates at time points which could not be pooled; these are reported narratively.16, 25
The majority of included studies were conducted in Europe or North America and recruited participants from primary care (n = 23), cardiology outpatient clinics (n = 20), or both (n = 15). Over half were longitudinal cohort studies (n = 34) but many recent studies have analysed big databases of routinely collected patient information.9 HF diagnosis was most frequently captured using validated database codes (n = 19), though many studies also defined HF using Framingham (n = 12), or European Society of Cardiology (n = 10) criteria (Table 1).1, 10, 11, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 In eight studies the criteria for defining HF was unspecified or relied on a clinical diagnosis. There were insufficient data to conduct a meaningful analysis comparing outcomes by sex.
Table 1.
Summary of included studies
| First author | Year | Study dates | Country | Study setting | Study design | HF definition | Total participants | HF sample | Participants | QUIPS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Cleland26 | 1987 | Not stated | UK | Cardiology outpatient | Prospective cohort | Diagnosis based on clinical, radiological and echocardiogram findings | 152 | 152 | Symptomatically stable, NYHA class II–IV | High |
| Ho27 | 1993 | 1948–1988 | USA | Primary care | Prospective cohort | Framingham criteria | 9405 | 652 | Incident HF cases in Framingham and Framingham offspring studies | Moderate |
| Senni28 | 1998 | 1991 | USA | Cross‐discipline | Routinely collected data | 'Slight modification' of Framingham criteria | 216 | 216 | Incident HF cases in Rochester Epidemiology Project | Low |
| McAlister29 | 1999 | 1989–1995 | Canada | Cardiology outpatient | Prospective cohort | Framingham criteria | 566 | 566 | Consecutive, confirmed cases of HF at a specialist HF clinic | Moderate |
| Niebauer30 | 1999 | 1980–1993 | UK | Cardiology outpatient | Prospective cohort | Not defined | 99 | 99 | Patients from HF outpatient clinic with very low LVEF (≤20%) | High |
| Cicoira31 | 2001 | 1992–1998 | UK | Cardiology outpatient | Prospective cohort | Typical symptoms + radiological or clinical evidence of HF. | 188 | 188 | Consecutive patients aged >70 years from HF clinic | High |
| Mosterd10 | 2001 | 1990–1993, follow‐up to 1996 | Netherlands | Primary care | Prospective cohort | Two‐step process involving typical signs, evidence of cardiovascular disease and exclusion of COPD | 5255 | 181 | Incident HF cases in Rotterdam Study | Low |
| Chen32 | 2002 | 1996–1997 | USA | Cross‐discipline | Prospective cohort | Database code of HF, validated using Framingham criteria | 83 | 83 | Incident HF cases in Rochester Epidemiology Project, with LVEF >45% and no valve disease | Low |
| Levy33 | 2002 | 1950–1999 | USA | Primary care | Prospective cohort | Framingham criteria | 10 311 | 1075 | Incident HF cases in Framingham study | Moderate |
| Muntwyler34 | 2002 | 1999–2000 | Switzerland | Primary care | Prospective cohort | ESC and Framingham criteria | 411 | 411 | Incident HF cases (NYHA class II–IV) in 'Improvement of HF' primary care survey | Moderate |
| Ansari35 | 2003 | 1996 | USA | Cardiology outpatient | Retrospective cohort | ICD‐9 | 403 | 403 | Incident HF cases at Northern California Kaiser Medical Centre | Moderate |
| Koseki36 | 2003 | 2000–2001 | Japan | Secondary care (mixed) | Registry | LVEF >50%, LVDD >55 mm documented history of congestive HF | 721 | 702 | Chronic HF population within regional registry | High |
| MacCarthy37 | 2003 | 1993–1995 | UK | Cardiology outpatient | Prospective cohort | Typical symptoms and objective evidence of cardiac dysfunction | 522 | 522 | Incident, stable, symptomatic HF cases in UK HEART study | Moderate |
| Nielsen38 | 2004 | 1993–1996 | Denmark | Cross‐discipline | Prospective cohort | Typical symptoms or an abnormal chest X‐ray and current prescription for a loop diuretic | 2157 | 115 | Incident cases of HF from four general practices | Moderate |
| Bleumink1 | 2004 | 1989–1993 follow‐up to 2000 | Netherlands | Primary care | Prospective cohort | Validated score based on ESC criteria | 7734 | 725 | Incident HF cases in Rotterdam Study | Moderate |
| Raymond39 | 2004 | 1997–2000 | Denmark | Primary care | Prospective cohort | ESC criteria | 764 | 36 | Volunteer sample from select GPs screened for HF | Low |
| Roger40 | 2004 | 1979–2000 | USA | Primary care | Prospective cohort | ICD‐9‐CM, validated with Framingham criteria | 4537 | 4537 | Incident HF cases in Rochester Epidemiology Project | Low |
| Cacciatore41 | 2005 | 1992–2003 | Italy | Primary care | Prospective cohort | Medical note review and physical examination to confirm cases, categorised by NYHA status | 1259 | 120 | Random sample of elderly patients enrolled in the Southern Italy community cohort | Moderate |
| Senni42 | 2005 | 1995 and 1999 | Italy | Cardiology outpatient | Routinely collected data | Framingham criteria | 1315 | 1315 | The 'IN‐CHF' National Registry of elderly cardiology outpatients with HF | Low |
| Barker43 | 2006 | 1970–1974 and 1990–1994 | USA | Cross‐discipline | Routinely collected data | Framingham criteria | 40 671 | 1942 | Incident HF cases amongst Kaiser Northwest Region health‐plan members | Moderate |
| van Jaarsveld44 | 2006 | 1993–1998 | Netherlands | Primary care | Prospective cohort | International classification of primary care criteria | 5279 | 293 | Incident HF cases in Groningen Longitudinal Aging Study (GLAS) | Moderate |
| Tsutsui45 | 2007 | 2004–2005 | Japan | Cross‐discipline | Registry | Framingham criteria | 2685 | 2685 | Prospective multicentre JCARE‐GENERAL HF registry, including primary care and outpatient data | Low |
| Ammar46 | 2007 | 1997–2000 | USA | Cross‐discipline | Prospective cohort | American College of Cardiology, American Heart Association definitions | 2029 | 244 | Incident HF cases in Rochester Epidemiology Project | Moderate |
| Hobbs47 | 2007 | 1995–1999 follow‐up to 2004 | UK | Primary care | Prospective cohort | ESC criteria | 6162 | 449 | Randomly sampled from four discrete primary care populations and screened for LVSD and HF | Low |
| Huang48 | 2007 | 1991–1993 | Taiwan | Primary care | Prospective cohort | Framingham criteria | 2660 | 147 | Incident HF cases amongst volunteer community sample | Moderate |
| Curtis49 | 2008 | 1994–2003 | USA | Cross‐discipline | Routinely collected data | ICD‐9‐CM | 622 786 | 622 786 | Incident HF cases amongst Medicare patients | Moderate |
| Henkel50 | 2008 | 1979–2002 | USA | Cross‐discipline | Prospective cohort | ICD‐9 CM | 1063 | 1063 | Incident HF cases in Rochester Epidemiology Project | Low |
| Castillo51 | 2009 | 1999–2003 | Spain | Cardiology outpatient | Registry | Clinician decided. No stated diagnostic criteria | 4720 | 1416 | Patients with confirmed HFpEF within the BADAPIC registry | Low |
| Goda52 | 2009 | 2004–2005 | Japan | Secondary care (mixed) | Prospective cohort | Diagnosis based on clinical, radiological and echocardiogram findings. No stated diagnostic criteria | 4255 | 597 | Incident HF cases, NYHA class II–IV, at The Cardiovascular Institute Hospital, Tokyo | Moderate |
| Parashar53 | 2009 | 1989–1993 | USA | Primary care | Prospective cohort | Individual clinician diagnosis and on active HF treatment | 5888 | 1264 | Incident cases of HF within the Cardiovascular Health Study | Low |
| Jimenez‐Navarro54 | 2010 | 2000–2003 | Spain | Cardiology outpatient | Registry | ESC criteria | 4720 | 4720 | BADAPIC registry across 62 centres with HF specific unit | Moderate |
| Devroey55 | 2010 | 2005–2006 | Belgium | Primary care | Prospective cohort | Individual clinician diagnosis | 754 | 557 | Incident HF cases from 178 sentinel GPs | High |
| Pons56 | 2010 | 2001–2008 | Spain | Cardiology outpatient | Prospective cohort | Not stated | 960 | 960 | Consecutive referrals to specialist HF unit | High |
| Gomez‐Soto57 | 2011 | 2000–2007 | Spain | Cross‐discipline | Prospective cohort | Framingham criteria | 4793 | 4793 | Incident HF cases amongst all residents in region of Southern Spain | Low |
| Grundtvig58 | 2011 | 2000–2006 | Norway | Cardiology outpatient | Prospective cohort | Typical symptoms + radiological or clinical evidence of HF | 3632 | 3632 | Incident cases of HF from 24 outpatient clinics | Low |
| Yeung59 | 2012 | 1997–2007 | Canada | Cross‐discipline | Routinely collected data | ICD‐9/ICD‐10 code | 5 175 179 | 203 361 | Incident cases of HF within the Ontario Health Insurance Plan database | Low |
| Taylor60 | 2012 | 1995–1999 follow‐up to 2009 | UK | Primary care | Prospective cohort | ESC criteria | 6162 | 449 | Random sample from 16 socio‐economically diverse GPs screened for HF | Low |
| Fragasso61 | 2013 | 1992–2005 | Italy | Cardiology outpatient | Routinely collected data | ESC criteria | 372 | 372 | Consecutive HF outpatient clinic patients with LVEF <45% | Moderate |
| Frigola‐Capell62 | 2013 | 2005–2007 | Spain | Primary care | Retrospective cohort | ICD‐10‐GM | 13 008 | 5659 | Combined data from urban and rural primary care units in Catalonia, Spain | Low |
| Gupta63 | 2013 | 1993–1995 | USA | Primary care | Prospective cohort | Gothenburg criteria or ICD‐9 code | 1962 | 116 | Incident HF cases amongst middle‐aged African American people within ARIC study | Low |
| Maggioni64 | 2013 | 2009–2010 | 12 European countries | Cardiology outpatient | Prospective cohort | Clinical diagnosis by individual clinicians | 5118 | 4118 | Incident HF cases in EURObservational Programme | Moderate |
| Zarrinkoub65 | 2013 | 2006–2010 | Sweden | Cross‐discipline | Routinely collected data | ICD‐10 code | 88 038 | 88 038 | Incident HF cases within Stockholm Health Registry | Low |
| Singh25 | 2014 | 2002–2007 | UK | Cardiology outpatient | Retrospective cohort | Modified ESC criteria | 1041 | 513 | Consecutive patients referred to HF assessment clinic ‐ the Darlington Retrospective outpatient study (DROPSY) | Moderate |
| Stalhammar66 | 2014 | 2005–2006 | Sweden | Primary care | Retrospective cohort | ICD‐10 codes | 137 | 137 | Incident cases of HF with LVEF >50% in 31 primary care centres | Moderate |
| James67 | 2015 | 2002–2012 | Ireland | Cardiology outpatient | Routinely collected data | Typical symptoms, raised BNP and echocardiogram changes | 733 | 285 | Consecutive primary care referrals to Rapid Access Clinic for suspected HF (NYHA class II–III) | Moderate |
| Sarria‐Santamera68 | 2015 | 2006–2010 | Spain | Primary care | Retrospective cohort | ICD‐10 codes | 227 984 | 3061 | HF codes on primary care database | Low |
| Crespo‐Leiro69 | 2016 | 2011–2013 | 12 European countries | Cardiology outpatient | Registry | ESC criteria | 12 440 | 12 440 | Long‐term HF prospective registry across 21 European countries | Moderate |
| Akwo70 | 2017 | 2002–2010 | USA | Primary care | Prospective cohort | ICD‐9 codes | 27 078 | 4341 | Incident HF cases in Southern Community Cohort Study | Low |
| Al‐Khateeb71 | 2017 | 2000–2015 | Saudi Arabia | Cardiology outpatient | Retrospective cohort | Clinical diagnosis + LVEF <45% | 2298 | 2298 | Consecutive patients seen in HF clinic, with LVEF <45% | Moderate |
| Dokainish72 | 2017 | 2012–2014 | International | Cardiology outpatient | Prospective cohort | Clinical diagnosis by individual clinicians | 5823 | 5823 | Consecutive sample of outpatients and inpatients with HF across six regions | High |
| Farre73 | 2017 | 2012 | Spain | Cross‐discipline | Registry | ICD‐9‐CM | 88 195 | 88 195 | Longitudinal study of all prevalent cases of HF within Catalonian public health database | Low |
| Farre74 | 2017 | 2001–2015 | Spain | Cardiology outpatient | Prospective cohort | ESC criteria | 3580 | 3580 | Consecutive sample from four HF units | Low |
| Koudstaal75 | 2017 | 1997–2010 | UK | Primary care | Routinely‐collected data | ICD‐9 and10 | 2 130 000 | 89 554 | CALIBER linked data from CPRD, MINAP, HES & ONS to identify newly recorded HF cases from 674 GP surgeries | Moderate |
| Mamas76 | 2017 | 2002–2011 | UK | Primary care | Routinely collected data | Database HF code | 1 750 000 | 56 658 | Incident HF cases using Scottish Primary Care Clinical Informatics Unit data | Low |
| Pascual‐Figal77 | 2017 |
MUSIC 2003–2004 REDINSCOR 2007–2011 |
Spain | Cardiology outpatient | Prospective cohort | HF diagnostic criteria of local institutions | 3446 | 3446 | Data from MUSIC registry (8 specialist HF clinics with chronic symptomatic HF NYHA class II–III) and REDINSCOR registry (consecutive patients with HF NYHA class II–IV from 18 outpatient clinics) | Low |
| Taylor11 | 2017 | 1998–2012 | UK | Primary care | Routinely collected data | Database codes based on NHS Clinical Terminology Browser and QOF guidelines | 2 728 841 | 54 313 | Incident HF cases in UK primary care from The Health Improvement Network (THIN) | Low |
| Sahle78 | 2017 | 1995–2001 | Australia | Primary care | Prospective cohort | Defined as; 'significant dyspnoea with or without peripheral oedema together with definite physical signs of either left‐sided or congestive cardiac failure and/or the characteristic chest X‐ray appearance of left ventricular failure' | 6083 | 145 | Incident cases of HF within Australian National BP study – open‐label study of people with hypertension aged 65–84 years | Moderate |
| Stork79 | 2017 | 2009–2013 | Germany | Cross‐discipline | Routinely collected data | ICD‐10‐GM | 3 132 337 | 123 925 | Patients with two HF‐related diagnoses within the German Health Risk Institute database | Moderate |
| Avula80 | 2018 | 2005–2012 | USA | Cross‐discipline | Routinely collected data | ICD‐9 codes | 28 914 | 28 914 | Incident cases of HF among Kaiser Permanente Northern California healthcare members | Moderate |
| Eriksson81 | 2018 | 2001–2014 | Sweden | Cross‐discipline | Registry | Individual clinician diagnosis | 9654 | 9654 | Incident HF cases in Swedish HF Registry, with LVEF ≥40% | Low |
BNP, B‐type natriuretic peptide; BP, blood pressure; CPRD, Clinical Practice Research Datalink; COPD, chronic obstructive pulmonary disease; ESC, European Society of Cardiology; GP, general practice; HES, Hospital Episodes Statistics; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; ICD‐9/10, International Classification of Diseases 9/10 (CM, GM refer to version used); LVDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MINAP, Myocardial Ischaemia National Audit Project; NHS, National Health Service; NYHA, New York Heart Association; ONS, Office for National Statistics; QOF, Quality and Outcomes Framework; QUIPS, Quality in Prognosis Studies.
Demographic and baseline participant characteristics differed significantly between studies (online supplementary Table S2 ). Reporting of this information was inconsistent with ethnicity and deprivation indices only rarely included. However, co‐morbid cardiovascular disease was common, with hypertension the most frequent co‐morbidity, followed by diabetes and ischaemic heart disease. Treatment rates of key HF medications including angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, beta‐blockers and mineralocorticoid receptor antagonists improved over time. Some recent studies reported treatment rates close to 90%. Detailed prescribing information was lacking, meaning it was not possible to determine how many participants were treated with optimum dosage or the recommended combination of all three agents.
Summary survival rates and causes of death
The pooled survival rates at 1 month, and 1, 2, 5 and 10 years, respectively, were 95.7% (95% confidence interval 94.3–96.9), 86.5% (85.4–87.6), 72.6% (67.0–76.6), 56.7% (54.0–59.4) and 34.9% (24.0–46.8)(Figure 1; online supplementary Figures [Link] , [Link] , [Link] , [Link] , [Link] ). Only 19 studies reported data on cause of death, but in 14 of these a cardiovascular cause accounted for over 50% of the total deaths (Table 2).25, 26, 34, 45, 47, 50, 51, 52, 53, 56, 60, 61, 63, 64, 67, 69, 72, 73, 74, 77 HF tended to be the most frequent cause of death but there was significant variation in the reported proportion of deaths related directly to HF, ranging from 8% to 64%.
Figure 1.
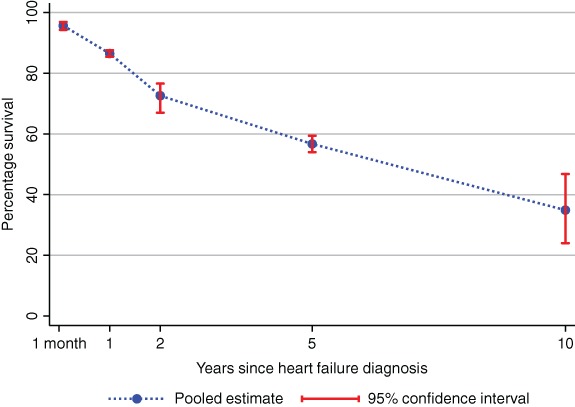
Combined survival rates for people with heart failure over time.
Table 2.
Causes of mortality reported in included studies
| First author | Year | Study subgroup | Cardiovascular mortality | Subgroups of cardiovascular mortality | Non‐cardiac mortality | Subgroups of non‐cardiovascular mortality | Unknown cause | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF | Stroke | Sudden cardiac death | Coronary heart disease (including MI) | Pulmonary disease | Cancer | GI or GU disease | Other | ||||||
| Cleland26 | 1987 | Overall | 8 | 1.3 | 75a | 8 | 1.3 | ||||||
| Tsutsui45 | 2007 | Overall | 36 | 32 | 32 | ||||||||
| Henkel50 | 2008 | Overall | 57 | 36 | 43 | 12 | 10.8 | 5.2 | 5.2 (CNS disease) | ||||
| HFpEF | 51 | 29 | 49 | 14.2 | 11.3 | 5.4 | 6.9 (CNS disease) | ||||||
| HFrEF | 64 | 43 | 36 | 10.1 | 9.7 | 5 | 3.6 (CNS disease) | ||||||
| Crespo‐Leiro69 | 2016 | Overall | 49.8 | 23.2 | 27 | ||||||||
| Dokainish72 | 2017 | Overall | 46 | 16 | 38 | ||||||||
| Gupta63 | 2013 | HFpEF | 56 | 44 | |||||||||
| HFrEF | 74 | 26 | |||||||||||
| Fragasso61 | 2013 | Overall | 63 | 24.6 | 7.6 | 15.8 | 13.9 | 37 | 16.3 | 5.6 | |||
| James67 | 2015 | Overall | 52.4 | 22.6 | Cardiovascular non‐HF 29.8 | 20.2 | 9.5 | 6 | 11.9 | ||||
| HFrEF | 58.5 | 26.8 | Cardiovascular non‐HF 31.7 | 17.1 | 12.2 | 2.4 | 9.8 | ||||||
| HFpEF | 46.5 | 18.6 | Cardiovascular non‐HF 27.9 | 23.3 | 7 | 9.3 | 14 | ||||||
| Maggioni64 | 2013 | Across regions | 54.5 | 22 | 16.3 | 29.2 | |||||||
| Pons56 | 2010 | Overall | 65.5 | 32.2 | 2.6 | 16 | 8.3 | 26.8 | 9.6 | 39.4 | 11.7 | 25.5 (sepsis) | 7.7 |
| Muntwyler34 | 2002 | Overall | 79 | ||||||||||
| Castillo51 | 2009 | Total | 95 | 64 | 24a | 7 | 5 | ||||||
| Goda52 | 2009 | Overall | 85 | 47.5 | 22.5 | 15 | 15 | ||||||
| Hobbs47 | 2007 | HF, no LVSD | 44.8 | 17.2 definite, 23 probable ± | 8 | 1.1a | 13.8 | 55.2 | 23 | 14.9 | 3.4 | 5.7 (renal) | |
| HF and LVSD | 74 | 38.5 definite, 12.5 probable ± | 7.7 | 3.8a | 25 | 26 | 10.6 | 6.7 | 1 | 1.9 (renal) | |||
| Taylor60 | 2012 | HF, LVSD | 72 | 32.1 definite ± | 22.6 | 28 | 13.7 | 7.1 | |||||
| HF, no LVSD | 48.4 | 19 definite ± | 12 | 51.6 | 21.2 | 13 | |||||||
| Parashar | 2009 | White women | 51.9 | ||||||||||
| African‐American women | 57.9 | ||||||||||||
| White men | 56 | ||||||||||||
| African‐American men | 45.4 | ||||||||||||
| Singh25 | 2014 | LVSD | 69 | 33.1 | 9.8 | 20.2 | 31 | 8.6 | 14.7 | ||||
| HFpEF | 43 | 15.3 | 13.6 | 13.6 | 57 | 13.6 | 21.2 | ||||||
| Farre73, 74 | 2017 | Overall | 46.2 | 27.1 | 7.5 | 29.6 | 24.2 | ||||||
| HFrEF | 48.1 | 26.3 | 9.9 | 25.9 | 25.9 | ||||||||
| HFmrEF | 45.2 | 26.2 | 5.9 | 32.6 | 22.2 | ||||||||
| HFpEF | 42.3 | 29.5 | 2.7 | 36.7 | 20.9 | ||||||||
| Pascual‐Figal77 | 2017 | HFrEF | 80 | 49.7 | 24.5 | 20 | |||||||
| HFmrEF | 72.7 | 42.2 | 22.7 | 27.3 | |||||||||
| HFpEF | 61.8 | 39.3 | 13.5 | 38.2 | |||||||||
Only studies reporting cause of mortality included. Blank cells indicate data were not reported in the original study. All figures refer to proportion of total mortality within the study. Selected subgroups of both cardiovascular and non‐cardiovascular mortality were reported in some studies, meaning in some cases the sum of the subgroup results are not equal to the combined mortality result.
CNS, central nervous system; GI, gastrointestinal; GU, genitourinary; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVSD, left ventricular systolic dysfunction; MI, myocardial infarction.
± HF cases recorded as either 'definite' or 'probable'. In Taylor, 'probable' HF mortality results not reported.
Not specified that all cases of sudden death attributable to cardiac causes.
Sensitivity analysis
The majority of studies were rated at low (n = 26) or moderate (n = 27) overall risk of bias (Table S3 ). Excluding the studies at moderate or high risk of bias in a sensitivity analysis did not alter the results. The pooled survival rate at 1 year across the remaining studies was 85.9% (84.1–87.7) and at 5 years 56.9% (52.1–61.7). The GRADE assessment suggests there is 'high' certainty in the summary findings (Table S4 ).
Subgroup analysis by age
Evidence from the forest plots and meta‐regression suggests survival rates decreased with increasing age at diagnosis (1‐year survival: R2 = 15.6%, P trend = 0.005; 5‐year survival: R2 = 42.6%, P trend < 0.001). Pooled survival rates at 1 year for people aged <65 years were 91.5% (88.2–94.3) compared to 83.3% (81.8–84.9) for people aged ≥ 75 years. By 5 years the respective survival rates were 78.8% (75.5–82.0) and 49.5% (46.3–52.7) (Figure 2; online supplementary Table S5 ).
Figure 2.
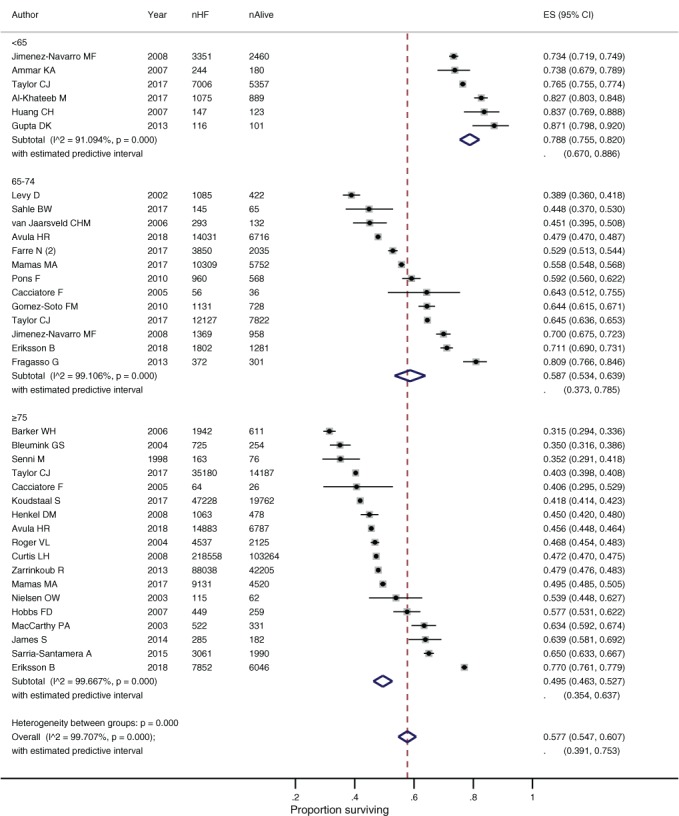
Survival of people with heart failure (HF) at 5 years by age at diagnosis. CI, confidence interval; ES, effect size.
The trend towards a worse prognosis in relation to age at diagnosis was also reported within individual studies.11, 41 In a recent analysis of survival rates within the UK THIN database, 5‐year survival rates were 50% amongst participants aged 75–84 years, compared to 81% amongst the youngest participants aged 45–54 years.11 In both cases, survival rates were significantly worse than for age‐matched participants of 72% and 98%, respectively.11
Subgroup analysis by study setting
The pooled 1‐ and 5‐year survival rates were significantly better for participants in secondary care studies compared to cross‐discipline studies (Figure 3). There was some evidence of improved survival in secondary care studies compared to primary care, with around 5% more participants alive at 1 year and 10% more at 5 years. The association between survival and setting was confirmed by meta‐regression (online supplementary Table S5 ). Individual secondary care studies with the poorest survival rates were those that purposively recruited either elderly frail participants, or those with a significant reduction in LVEF.30, 31 The primary care studies reporting the best survival rates used screening to detect incident HF cases.48, 63 Rates of key HF medication prescribing were consistently better in secondary care.
Figure 3.
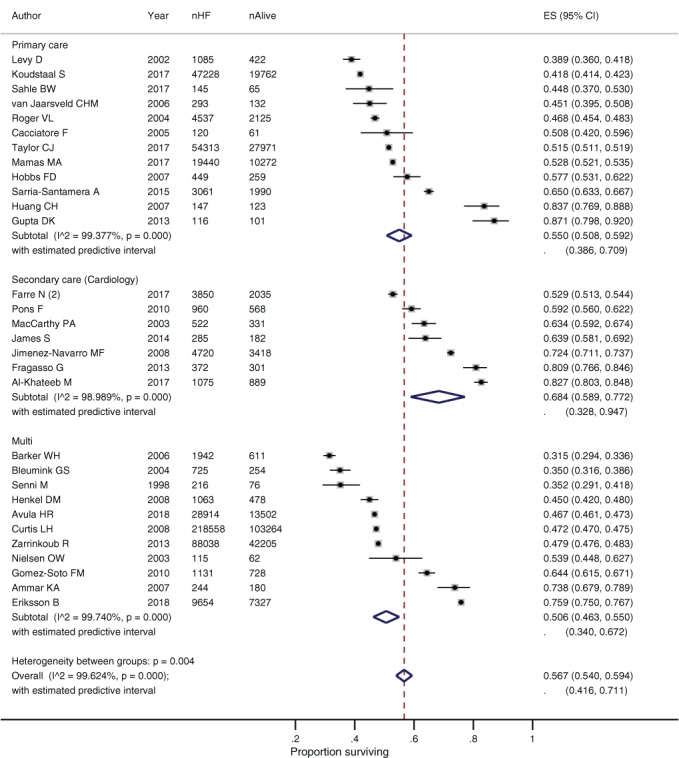
Survival of people with heart failure (HF) at 5 years by study setting. CI, confidence interval; ES, effect size.
The four studies36,45,48,52 conducted in South‐East Asia reported better survival rates compared to Europe and North America, despite recruiting participants of comparable age and co‐morbid disease burden. One of these studies48 used screening to detect incident cases and the proportion of participants prescribed HF medication was also relatively high, which may explain this survival difference.
Subgroup analysis by left ventricular ejection fraction
The pooled survival rate at 5 years was better for patients with HFrEF than mixed ejection fraction (Figure 4). There was no significant difference in the pooled survival rates for HFpEF compared to HFrEF at either 1 or 5 years (online supplementary Table S5 ). A number of studies compared the risk of death by LVEF in their individual populations and found a preserved ejection fraction was associated with improved survival. Survival analysis from a community‐based screened cohort found patients with a LVEF <40% compared to LVEF >50% had a 1.80 (1.55–2.10) times greater risk of death over the study period, when adjusted for key factors such as age and sex.60 Other studies found the risk of death to be even greater for those with HFrEF, with hazard ratio of 2.62 (1.45–4.75),50 and 3.72 (1.80–7.68) reported.39 In every study reporting cause of death data categorised by LVEF, the proportion of total mortality attributed to cardiovascular disease and HF‐related mortality was greater for people with HFrEF than HFpEF (Table 2).
Figure 4.
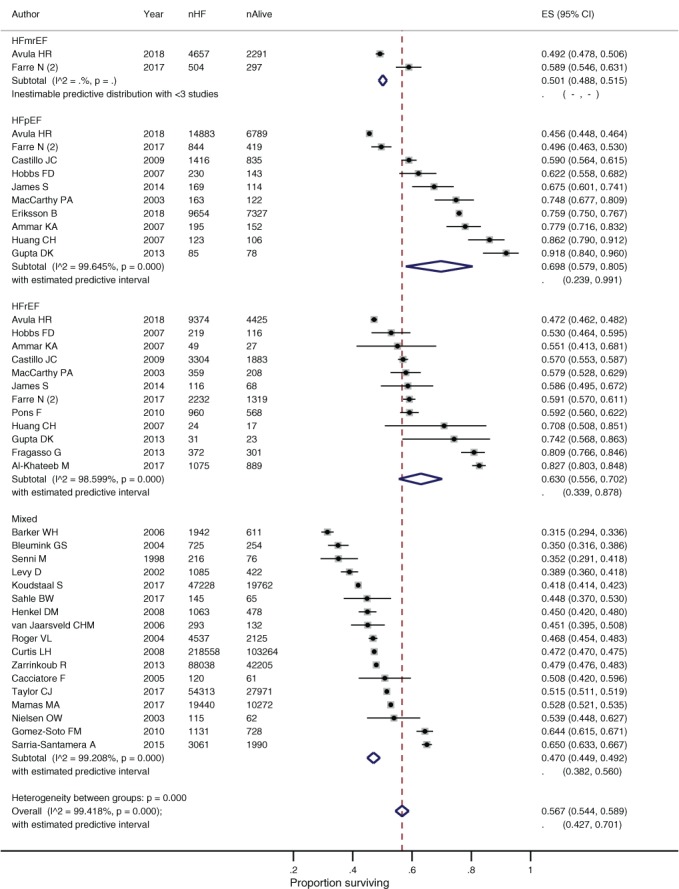
Survival of people with heart failure (HF) at 5 years by left ventricular ejection fraction. CI, confidence interval; ES, effect size.
Change in survival rates over time
Survival rates within each decade had high levels of heterogeneity (Figure 5), however over time and across the included studies there was a trend towards improvement in 1‐ and 5‐year survival rates (1‐year survival: R2 = 36.3%, P trend < 0.001; 5‐year survival: R2 = 23.2%, P trend = 0.013). Each decade since the 1970s has seen improving survival rates. The 1‐ and 5‐year pooled survival rates were 70.8% (64.7–76.3) and 35.2% (29.3–41.5) from the earliest reported time period, 1950–1969.33 By 2010–2019, 1‐ and 5‐year survival rates had reached 89.3% (84.3–93.4) and 59.7% (54.7–64.6).
Figure 5.
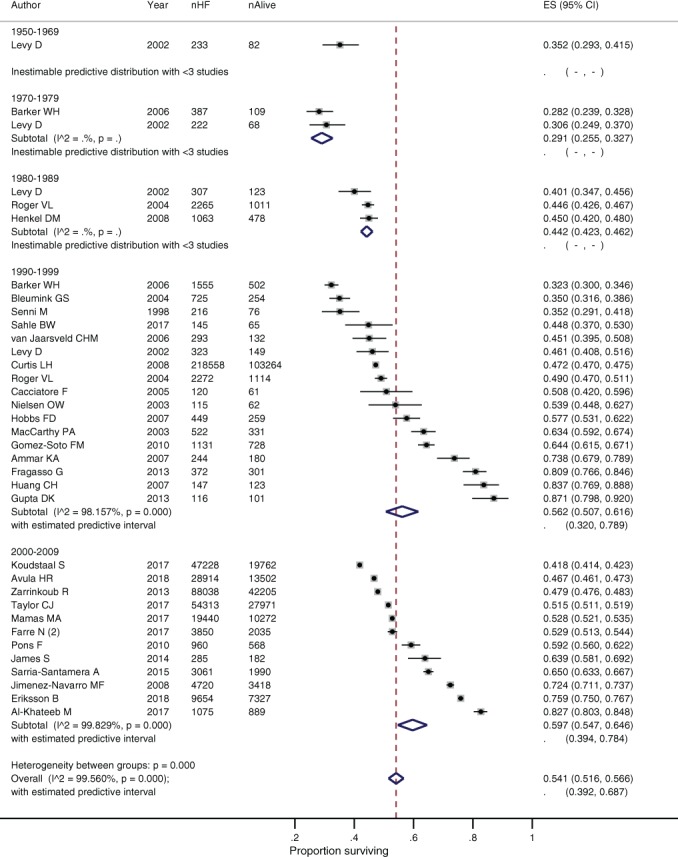
Survival of people with heart failure (HF) at 5 years by study decade. CI, confidence interval; ES, effect size.
Given the changes in treatment recommendations in the late 1990s, we conducted a subgroup analysis of pooled survival rates amongst all studies recruiting participants from the year 2000 onwards. The 1‐month, and 1‐, 2‐, and 5‐year survival rates for these groups were 95.2% (92.1–97.6), 89.3% (87.9–90.6), 78.9% (74.2–83.2) and 59.7% (54.7–64.6), respectively, slightly better than the overall pooled survival. Only one study reported 10‐year follow‐up data for participants post‐2000 with a survival rate of 29.5% (28.9–30.2).11
A number of studies have also demonstrated improving survival rates over time within their individual population. Framingham data show an improvement in 5‐year survival between 1950–1969 to 1990–1999 from 30% to 41% for men and from 43% to 55% for women.33 This trend is also seen in the Rochester Epidemiology Project.40 Recently, there have been more modest improvements in survival. A database study of over 400 000 people with HF in Ontario, found 1‐year mortality fell amongst outpatients with HF from 17.7% in 1997 to 16.2% in 2007.59 A study of 600 000 Medicare patients with incident HF reported a reduction in mortality from 67.5% to 64.9% for men and from 61.7% to 60.2% for women between 1994 and 2003.49
Discussion
This is the first systematic review of prognosis in chronic HF and provides contemporary survival estimates applicable across high income countries. The analyses draw on survival data from 1.5 million people with chronic HF across 60 studies.
Survival rates have improved over time and 20% more people survive at both 1‐ and 5‐year follow‐up today compared to between 1950 and 1969. Survival rates improved sharply from the 1970s to 1990s, but there has been only a modest reduction in mortality in the past two decades. Increasing age at diagnosis is one key factor associated with a poor prognosis. Survival rates amongst people aged ≤ 65 years were almost 10% better at 1 year and over 30% better at 5 years, when compared to people aged ≥ 75 years. Survival rates were higher in studies recruiting participants from cardiology outpatient settings compared to cross‐discipline or primary care.
There was no significant difference in survival between HFrEF and HFpEF in our pooled analysis, though individual studies reported improved survival rates and lower rates of hospital admission and cardiovascular mortality for people with HFpEF. Both survival rates and prescribing of HF medication were significantly lower for patients where LVEF was not reported or analysed. This may be due to older trials with worse survival rates not reporting LVEF. It may also reflect certain populations, such as nursing home residents or older patients, are less likely to have LVEF measured despite having a worse prognosis. Nevertheless, recognising that patients who are not categorised by LVEF have a poorer outlook may have important implications for future assessment and treatment pathways.
The search strategy and eligibility criteria were designed to be inclusive, drawing studies from a wide range of geographical and healthcare settings. Source data from developing countries were less abundant but landmark cross‐continental studies provide data for these healthcare settings. Internationally, the lowest mortality rates were in South‐East Asian studies.
Limitations
The diversity in study design and setting captured by the inclusive search strategy resulted in high levels of heterogeneity in each individual meta‐analysis. This included variations in participant characteristics that are likely to impact on prognosis. Screening was used to detect early HF in a small number of studies.82 Not all studies reported HF survival from time of diagnosis. Whilst primary care studies generally used routinely collected data sources to identify a first coded episode of HF, secondary care studies tended to calculate survival from first clinic visit, which may have been several years after diagnosis. Studies were categorised by setting to account for this potential time lag, though this was not apparent in our results. In practice, most patients with a confirmed diagnosis of HF will have input at some point from a cardiologist, except for some very frail patients who may be limited by cognitive or mobility issues. It is possible the differences seen in survival between settings reflect such variation in participant characteristics, though secondary care studies also reported higher rates of prescribing for key HF treatment. We plan to report more detail on prescribing rates in a separate paper. The definitions of cardiovascular and non‐cardiovascular death varied between studies as did the categories used in the cause of death subgroup analyses, making it difficult to compare these outcomes directly.
Outcome data are pooled from across a wide time period to capture changing survival rates over time. However, survival rates may not be directly comparable across these studies given there have been significant changes in HF management in the past 70 years, including the introduction of medications proven to improve prognosis for people with HFrEF. The statistical heterogeneity also reflects the large sample sizes of the included studies, which resulted in narrow confidence intervals. Even small differences in survival rates resulted in non‐overlapping confidence intervals and high I2 scores, a recognised limitation of this statistical measure in observational meta‐analysis.
The review included observational studies to present the real‐world outcomes for people with HF, outside of trial settings. Confounding is a recognised problem in these non‐randomised trials and reporting of important covariates was inconsistent. Missing data were a particular problem in earlier studies and those drawing on data from large primary care databases. Some meta‐regression results rely on data from a small number of studies, such as for HFmrEF and general secondary care clinics. However, similar results were observed when these small subgroups were combined with adjacent categories. Few studies reported echocardiogram findings or categorisation of HF by LVEF, despite the prognostic significance of this information. Accurate coding of HF is also a recognised limitation in routinely collected datasets.83, 84 However, this approach to epidemiological research is still felt to be valid and coding has been improving in line with performance payments and better access to diagnostic tests in primary care.85
Comparison with existing literature
A recent European secondary care study reported 1‐year mortality rates for people with acute and chronic HF of 23% and 6%, respectively, compared to 3% for matched controls.69 In our pooled analysis, 1‐year mortality in chronic HF was above 10%. This may be because some people with a very poor prognosis are never admitted to hospital or referred to secondary care. Categorisation of HF has changed over time to recognise the importance of LVEF when considering treatment options and prognosis. Survival rates are better for people with HFpEF compared to HFrEF, once adjusted for key covariates including age, sex, and aetiology of HF.86 However, people with HFpEF are more likely to be older and have significant co‐morbid disease, meaning the unadjusted HFrEF and HFpEF survival rates are similar. This may explain why there was no significant difference in survival in our subgroup analysis based on LVEF.
Research implications
Our results provide a reference source for clinicians, patients and policy makers, to inform population prognostic estimates. The subgroup analyses help to provide adjusted survival estimates based on key variables, such as age at time of diagnosis. Further work is needed to refine prognostic models for individuals with chronic HF. Existing tools, such as the Seattle Heart Failure Model and MAGGIC HF risk tool, lack specificity and sensitivity data that are applicable to clinical practice.86, 87 Reducing uncertainty and confusion about the outcomes in HF could lead to improvements in advanced care planning, treatment adherence and integration with wider healthcare teams such as palliative care.16, 88
Survival rates in HF remain poor despite modest improvements over time. Investment in healthcare infrastructure and public health initiatives for conditions with similar outcomes such as cancer and stroke have seen improvements in morbidity and mortality.89, 90 This review suggests that targeted allocation of resources towards improving early diagnosis, prescribing and treatment adherence and multi‐disciplinary models of care may lead to further reductions in mortality for people with HF.
Conclusion
There have been modest improvements in survival rates for people with chronic HF over the past 70 years. Despite this, the 5‐year survival rate is close to 50% and many people will die directly from HF or from related cardiovascular disease. Older populations are at the greatest risk of death, presenting a looming challenge to healthcare systems given changing global demographics. Our results draw from very heterogeneous data sources and when applying survival estimates to any individual, consideration should be given to factors such as their age, co‐morbid disease, treatment, and LVEF. Further research is needed to develop the evidence base around key prognostic indicators for patients with chronic HF that will enable population estimates to be refined for individuals. Greater understanding and awareness of chronic HF survival rates can facilitate better multi‐disciplinary team working and inform advanced care planning between patients and healthcare professionals.
Supporting information
Methods S1. MOOSE (Meta‐analyses Of Observational Studies in Epidemiology) checklist.
Methods S2. Risk of bias and quality assessment.
Table S1. Search strategy.
Table S2. Prevalence of co‐morbid disease, cardiovascular risk factors and heart failure medication across studies.
Table S3. Risk of bias assessment using the Quality in Prognosis Studies tool.
Table S4. GRADE risk of bias assessment across studies.
Table S5. Subgroup and meta‐regression analyses by age at diagnosis, setting, left ventricular ejection fraction, and date.
Figure S1. Survival of people with heart failure at 1 month.
Figure S2. Survival of people with heart failure at 1 year.
Figure S3. Survival of people with heart failure at 2 years.
Figure S4. Survival of people with heart failure at 5 years.
Figure S5. Survival of people with heart failure at 10 years.
Figure S6. PRISMA flow diagram of study selection.
Acknowledgements
Thanks to Nia Roberts and Jenny Hirst for their feedback on the search strategy. This work was conducted as part of N.R.J.'s MSc in Evidence Based Health Care at the University of Oxford. This work uses data provided by patients and collected by the NHS as part of their care and support and would not have been possible without access to these data. The National Institute for Health Research recognises and values the role of patient data, securely accessed and stored, both in underpinning and leading to improvements in research and care.
Funding
Nicholas R. Jones is supported by a Wellcome Trust Doctoral Research Fellowship [grant number 203921/Z/16/Z]. This project was completed during his time as a NIHR Academic Clinical Fellow. Andrea Roalfe is funded by the NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust. F.D. Richard Hobbs acknowledges his part‐funding from the NIHR School for Primary Care Research, the NIHR Collaboration for Leadership in Health Research Care (CLAHRC) Oxford, the NIHR Oxford Biomedical Research Centre (BRC), and the NIHR Oxford Medtech and In‐Vitro Diagnostics Co‐operative (MIC). The project was supported by the NIHR Oxford BRC and CLAHRC. Clare J. Taylor is a NIHR Academic Clinical Lecturer. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Conflict of interest: C.J.T reports speaker fees from Vifor and Novartis and non‐financial support from Roche outside the submitted work. F.D.R.H. reports personal fees and other from Novartis, Boehringer Ingelheim, and grants from Pfizer outside the submitted work. The other authors have nothing to disclose.
References
- 1. Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J 2004;25:1614–1619. [DOI] [PubMed] [Google Scholar]
- 2. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18:242–252. [DOI] [PubMed] [Google Scholar]
- 3. Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A; EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail 2002;4:531–539. [DOI] [PubMed] [Google Scholar]
- 4. Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005;26:1887–1894. [DOI] [PubMed] [Google Scholar]
- 5. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJ, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Katus HA, Debus J, Bischof M. Long‐term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 2010;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 8. Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long‐term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009;119:515–523. [DOI] [PubMed] [Google Scholar]
- 9. Ko DT, Alter DA, Austin PC, You JJ, Lee DS, Qiu F, Stukel TA, Tu JV. Life expectancy after an index hospitalization for patients with heart failure: a population‐based study. Am Heart J 2008;155:324–331. [DOI] [PubMed] [Google Scholar]
- 10. Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. The prognosis of heart failure in the general population: the Rotterdam study. Eur Heart J 2001;22:1318–1327. [DOI] [PubMed] [Google Scholar]
- 11. Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FD, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract 2017;34:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cleland JG, Cohen‐Solal A, Aguilar JC, Dietz R, Eastaugh J, Follath F, Freemantle N, Gavazzi A, van Gilst WH, Hobbs FD, Korewicki J, Madeira HC, Preda I, Swedberg K, Widimsky J; IMPROVEMENT of Heart Failure Programme Committees and Investigators. Improvement programme in evaluation and management; Study Group on Diagnosis of the Working Group on Heart Failure of The European Society of Cardiology. Management of heart failure in primary care (the IMPROVEMENT of Heart Failure Programme): an international survey. Lancet 2002;360:1631–1639. [DOI] [PubMed] [Google Scholar]
- 13. Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA; American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012;125:1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord 2018;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harding R, Selman L, Beynon T, Hodson F, Coady E, Read C, Walton M, Gibbs L, Higginson IJ. Meeting the communication and information needs of chronic heart failure patients. J Pain Symptom Manage 2008;36:149–156. [DOI] [PubMed] [Google Scholar]
- 16. McIlvennan CK, Allen LA. Palliative care in patients with heart failure. BMJ 2016;353:i1010. [DOI] [PubMed] [Google Scholar]
- 17. Barclay S, Momen N, Case‐Upton S, Kuhn I, Smith E. End‐of‐life care conversations with heart failure patients: a systematic literature review and narrative synthesis. Br J Gen Pract 2011;61:e49–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, Dodson GC, O'Connor CM, Felker GM. Discordance between patient‐predicted and model‐predicted life expectancy among ambulatory patients with heart failure. JAMA 2008;299:2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones NR, Roalfe AK, Adoki I, Richard Hobbs FD, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta‐analysis protocol. Syst Rev 2018;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 21.Scottish Intercollegiate Guidelines Network (SIGN). Search filters for observational studies. https://www.sign.ac.uk/search-filters.html [accessed 5 August 2019].
- 22. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh R. Long Term Outcomes in Patients with Heart Failure: The Darlington Retrospective OutPatient Study (DROPSY). Durham, UK: Durham University; 2014. http://etheses.dur.ac.uk/9491/ [accessed 5 August 2019]. [Google Scholar]
- 26. Cleland JG, Dargie HJ, Ford I. Mortality in heart failure: clinical variables of prognostic value. Br Heart J 1987;58:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 28. Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998;98:2282–2289. [DOI] [PubMed] [Google Scholar]
- 29. McAlister FA, Teo KK, Taher M, Montague TJ, Humen D, Cheung L, Kiaii M, Yim R, Armstrong PW. Insights into the contemporary epidemiology and outpatient management of congestive heart failure. Am Heart J 1999;138:87–94. [DOI] [PubMed] [Google Scholar]
- 30. Niebauer J, Clark AL, Anker SD, Coats AJ. Three year mortality in heart failure patients with very low left ventricular ejection fractions. Int J Cardiol 1999;70:245–247. [DOI] [PubMed] [Google Scholar]
- 31. Cicoira M, Davos CH, Florea V, Shamim W, Doehner W, Coats AJ, Anker SD. Chronic heart failure in the very elderly: clinical status, survival, and prognostic factors in 188 patients more than 70 years old. Am Heart J 2001;142:174–180. [DOI] [PubMed] [Google Scholar]
- 32. Chen HH, Lainchbury JG, Senni M, Bailey KR, Redfield MM. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail 2002;8:279–287. [DOI] [PubMed] [Google Scholar]
- 33. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 34. Muntwyler J, Abetel G, Gruner C, Follath F. One‐year mortality among unselected outpatients with heart failure. Eur Heart J 2002;23:1861–1866. [DOI] [PubMed] [Google Scholar]
- 35. Ansari M, Alexander M, Tutar A, Massie BM. Incident cases of heart failure in a community cohort: importance and outcomes of patients with preserved systolic function. Am Heart J 2003;146:115–120. [DOI] [PubMed] [Google Scholar]
- 36. Koseki Y, Watanabe J, Shinozaki T, Sakuma M, Komaru T, Fukuchi M, Miura M, Karibe A, Kon‐no Y, Numaguchi H, Ninomiya M, Kagaya Y, Shirato K, CHART Investigators. Characteristics and 1‐year prognosis of medically treated patients with chronic heart failure in Japan. Circ J 2003;67:431–436. [DOI] [PubMed] [Google Scholar]
- 37. MacCarthy PA, Kearney MT, Nolan J, Lee AJ, Prescott RJ, Shah AM, Brooksby WP, Fox KA. Prognosis in heart failure with preserved left ventricular systolic function: prospective cohort study. BMJ 2003;327:78–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen OW, McDonagh T, Cowburn P, Blue L, Robb SD, Dargie H. Patient differences related to management in general practice and the hospital: a cross‐sectional study of heart failure in the community. Eur Heart J 2004;25:1718–1725. [DOI] [PubMed] [Google Scholar]
- 39. Raymond I, Mehlsen J, Pedersen F, Dimsits J, Jacobsen J, Hildebrandt PR. The prognosis of impaired left ventricular systolic function and heart failure in a middle‐aged and elderly population in an urban population segment of Copenhagen. Eur J Heart Fail 2004;6:653–661. [DOI] [PubMed] [Google Scholar]
- 40. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jaco bsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 41. Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long‐term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005;35:723–730. [DOI] [PubMed] [Google Scholar]
- 42. Senni M, De Maria R, Gregori D, Gonzini L, Gorini M, Cacciatore G, Gavazzi A, Pulignano G, Porcu M, Maggioni AP. Temporal trends in survival and hospitalizations in outpatients with chronic systolic heart failure in 1995 and 1999. J Card Fail 2005;11:270–278. [DOI] [PubMed] [Google Scholar]
- 43. Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well‐defined older population, 1970‐1974 and 1990‐1994. Circulation 2006;113:799–805. [DOI] [PubMed] [Google Scholar]
- 44. van Jaarsveld CH, Ranchor AV, Kempen GI, Coyne JC, van Veldhuisen DJ, Sanderman R. Epidemiology of heart failure in a community‐based study of subjects aged ≥57 years: incidence and long‐term survival. Eur J Heart Fail 2006;8:23–30. [DOI] [PubMed] [Google Scholar]
- 45. Tsutsui H, Tsuchihashi‐Makaya M, Kinugawa S, Goto D, Takeshita A, JCARE‐GENERAL Investigators. Characteristics and outcomes of patients with heart failure in general practices and hospitals. Circ J 2007;71:449–454. [DOI] [PubMed] [Google Scholar]
- 46. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–1570. [DOI] [PubMed] [Google Scholar]
- 47. Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R; Midlands Research Practices Consortium (MidReC). Prognosis of all‐cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow‐up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 2007;28:1128–1134. [DOI] [PubMed] [Google Scholar]
- 48. Huang CH, Chien KL, Chen WJ, Sung FC, Hsu HC, Su TC, Lee YT. Impact of heart failure and left ventricular function on long‐term survival – report of a community‐based cohort study in Taiwan. Eur J Heart Fail 2007;9:587–593. [DOI] [PubMed] [Google Scholar]
- 49. Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med 2008;168:418–424. [DOI] [PubMed] [Google Scholar]
- 50. Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail 2008;1:91–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castillo JC, Anguita MP, Jimenez M; BADAPIC Group. Outcome of heart failure with preserved ejection fraction: a multicentre Spanish registry. Curr Cardiol Rev 2009;5:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goda A, Yamashita T, Suzuki S, Ohtsuka T, Uejima T, Oikawa Y, Yajima J, Koike A, Nagashima K, Kirigaya H, Sagara K, Ogasawara K, Isobe M, Sawada H, Aizawa T. Prevalence and prognosis of patients with heart failure in Tokyo: a prospective cohort of Shinken database 2004‐5. Int Heart J 2009;50:609–625. [DOI] [PubMed] [Google Scholar]
- 53. Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, Gottdiener JS. Race, gender, and mortality in adults ≥65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol 2009;103:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jimenez‐Navarro MF, Ramirez‐Marrero MA, Anguita‐Sanchez M, Castillo JC; BADAPIC Investigators. Influence of gender on long‐term prognosis of patients with chronic heart failure seen in heart failure clinics. Clin Cardiol 2010;33:E13–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Devroey D, Van Casteren V. The incidence and first‐year mortality of heart failure in Belgium: a 2‐year nationwide prospective registration. Int J Clin Pract 2010;64:330–335. [DOI] [PubMed] [Google Scholar]
- 56. Pons F, Lupon J, Urrutia A, González B, Crespo E, Díez C, Cano L, Cabanes R, Altimir S, Coll R, Pascual T, Valle V. Mortality and cause of death in patients with heart failure: findings at a specialist multidisciplinary heart failure unit. Rev Esp Cardiol 2010;63:303–314. [DOI] [PubMed] [Google Scholar]
- 57. Gomez‐Soto FM, Andrey JL, Garcia‐Egido AA, Escobar MA, Romero SP, Garcia‐Arjona R, Gutierrez J, Gomez F. Incidence and mortality of heart failure: a community‐based study. Int J Cardiol 2011;151:40–45. [DOI] [PubMed] [Google Scholar]
- 58. Grundtvig M, Gullestad L, Hole T, Flønæs B, Westheim A. Characteristics, implementation of evidence‐based management and outcome in patients with chronic heart failure: results from the Norwegian heart failure registry. Eur J Cardiovasc Nurs 2011;10:44–49. [DOI] [PubMed] [Google Scholar]
- 59. Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ 2012;184:E765–E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten‐year prognosis of heart failure in the community: follow‐up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail 2012;14:176–184. [DOI] [PubMed] [Google Scholar]
- 61. Fragasso G, Marinosci G, Calori G, Spoladore R, Arioli F, Bassanelli G, Salerno A, Cuko A, Puccetti P, Silipigni C, Palloshi A, Margonato A. Improved survival in patients with chronic mild/moderate systolic heart failure followed up in a specialist clinic. J Cardiovasc Med (Hagerstown) 2013;14:57–65. [DOI] [PubMed] [Google Scholar]
- 62. Frigola‐Capell E, Comin‐Colet J, Davins‐Miralles J, Gich‐Saladich IJ, Wensing M, Verdú‐Rotellar JM. Survival in Mediterranean ambulatory patients with chronic heart failure. A population‐based study. Rev Esp Cardiol (Engl Ed) 2013;66:539–544. [DOI] [PubMed] [Google Scholar]
- 63. Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart failure with preserved ejection fraction in African Americans: The ARIC (Atherosclerosis Risk In Communities) study. JACC Heart Fail 2013;1:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L; Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 65. Zarrinkoub R, Wettermark B, Wandell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013;15:995–1002. [DOI] [PubMed] [Google Scholar]
- 66. Stalhammar J, Stern L, Linder R, Sherman S, Parikh R, Ariely R, Deschaseaux C, Wikström G. The burden of preserved ejection fraction heart failure in a real‐world Swedish patient population. J Med Econ 2014;17:43–51. [DOI] [PubMed] [Google Scholar]
- 67. James S, Barton D, O'Connell E, Voon V, Murtagh G, Watson C, Murphy T, Prendiville B, Brennan D, Hensey M, O'Neill L, O'Hanlon R, Waterhouse D, Ledwidge M, Gallagher J, McDonald K. Life expectancy for community‐based patients with heart failure from time of diagnosis. Int J Cardiol 2015;178:268–274. [DOI] [PubMed] [Google Scholar]
- 68. Sarria‐Santamera A, Prado‐Galbarro FJ, Martin‐Martinez MA, Carmona R, Gamiño Arroyo AE, Sánchez‐Piedra C, Garrido Elustondo S, del Cura González I. Survival of patients with heart failure in primary care. Aten Primaria 2015;47:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A; Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 70. Akwo EA, Kabagambe EK, Wang TJ, Harrell FE Jr, Blot WJ, Mumma M, Gupta DK, Lipworth L. Heart failure incidence and mortality in the Southern Community Cohort Study. Circ Heart Fail 2017;10:e003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Al‐Khateeb M, Qureshi WT, Odeh R, Ahmed AM, Sakr S, Elshawi R, Bdeir MB, Al‐Mallah MH. The impact of digoxin on mortality in patients with chronic systolic heart failure: a propensity‐matched cohort study. Int J Cardiol 2017;228:214–218. [DOI] [PubMed] [Google Scholar]
- 72. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, Palileo‐Villaneuva L, Lopez‐Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley‐Cote E, Balasubramanian K, Islam S, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Bangdiwala SI, Yusuf S; INTER‐CHF Investigators. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER‐CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–e672. [DOI] [PubMed] [Google Scholar]
- 73. Farre N, Vela E, Cleries M, Bustins M, Cainzos‐Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdú‐Rotellar JM, Comín‐Colet J. Real world heart failure epidemiology and outcome: A population‐based analysis of 88,195 patients. PLoS One 2017;12:e0172745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Farre N, Lupon J, Roig E, Gonzalez‐Costello J, Vila J, Perez S, de Antonio M, Solé‐González E, Sánchez‐Enrique C, Moliner P, Ruiz S, Enjuanes C, Mirabet S, Bayés‐Genís A, Comin‐Colet J; GICCAT Investigators. Clinical characteristics, one‐year change in ejection fraction and long‐term outcomes in patients with heart failure with mid‐range ejection fraction: a multicentre prospective observational study in Catalonia (Spain). BMJ Open 2017;7:e018719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koudstaal S, Pujades‐Rodriguez M, Denaxas S, Gho JM, Shah AD, Yu N, Patel RS, Gale CP, Hoes AW, Cleland JG, Asselbergs FW, Hemingway H. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population‐based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail 2017;19:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail 2017;19:1095–1104. [DOI] [PubMed] [Google Scholar]
- 77. Pascual‐Figal DA, Ferrero‐Gregori A, Gomez‐Otero I, Vazquez R, Delgado‐Jimenez J, Alvarez‐Garcia J, Gimeno‐Blanes JR, Worner‐Diz F, Bardají A, Alonso‐Pulpon L, Gonzalez‐Juanatey JR, Cinca J; MUSIC and REDINSCOR I Research Groups. Mid‐range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–270. [DOI] [PubMed] [Google Scholar]
- 78. Sahle BW, Owen AJ, Wing LM, Beilin LJ, Krum H, Reid CM; Second Australian National Blood Pressure Study Management Committee. Long‐term survival following the development of heart failure in an elderly hypertensive population. Cardiovasc Ther 2017;35:e12303. [DOI] [PubMed] [Google Scholar]
- 79. Stork S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, Hupfer S, Klebs S. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 2017;106:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Avula HR, Leong TK, Lee KK, Sung SH, Go AS. Long‐term outcomes of adults with heart failure by left ventricular systolic function status. Am J Cardiol 2018;122:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Eriksson B, Wandell P, Dahlstrom U, Nasman P, Lund LH, Edner M. Comorbidities, risk factors and outcomes in patients with heart failure and an ejection fraction of more than or equal to 40% in primary care‐ and hospital care‐based outpatient clinics. Scand J Prim Health Care 2018;36:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 83. Deaton C, Benson J. Time for correct diagnosis and categorisation of heart failure in primary care. Br J Gen Pract 2016;66:554–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jordan K, Porcheret M, Croft P. Quality of morbidity coding in general practice computerized medical records: a systematic review. Fam Pract 2004;21:396–412. [DOI] [PubMed] [Google Scholar]
- 85. Bottle A, Kim D, Aylin P, Cowie MR, Majeed A, Hayhoe B. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104:600–605. [DOI] [PubMed] [Google Scholar]
- 86. Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 87. National Institute for Health and Care Excellence (NICE) . Chronic heart failure in adults: diagnosis and management. NICE guideline [NG106]. September 2018. https://www.nice.org.uk/guidance/ng106 [accessed 5 August 2019]. [PubMed]
- 88. Kavalieratos D, Mitchell EM, Carey TS, Dev S, Biddle AK, Reeve BB, Abernethy AP, Weinberger M. "Not the 'grim reaper service'": an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc 2014;3:e000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E, Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time‐trend analysis from the General Practice Research Database. BMJ Open 2011;1:e000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. MOOSE (Meta‐analyses Of Observational Studies in Epidemiology) checklist.
Methods S2. Risk of bias and quality assessment.
Table S1. Search strategy.
Table S2. Prevalence of co‐morbid disease, cardiovascular risk factors and heart failure medication across studies.
Table S3. Risk of bias assessment using the Quality in Prognosis Studies tool.
Table S4. GRADE risk of bias assessment across studies.
Table S5. Subgroup and meta‐regression analyses by age at diagnosis, setting, left ventricular ejection fraction, and date.
Figure S1. Survival of people with heart failure at 1 month.
Figure S2. Survival of people with heart failure at 1 year.
Figure S3. Survival of people with heart failure at 2 years.
Figure S4. Survival of people with heart failure at 5 years.
Figure S5. Survival of people with heart failure at 10 years.
Figure S6. PRISMA flow diagram of study selection.


