Abstract
The mu opioid receptor (MOR) is a diversely regulated target for the alleviation of pain in the clinical setting. However, untoward side effects such as tolerance, dependence, respiratory suppression, constipation, and abuse liability detract from their usefulness. Studies in genetically modified rodent models suggest that activating G protein signaling pathways while avoiding phosphorylation of the receptor or recruitment of βarrestin scaffolding proteins could preserve the analgesic properties of MOR agonists while avoiding certain side effects. With the development of novel MOR “biased” agonists, which lead to preferential activation of G protein pathways over receptor phosphorylation, internalization or interaction with other effectors, this hypothesis can be tested in a native, physiological setting. Overall, it is clear that the MOR is not a simple on and off switch and that the diverse means by which the receptor can be regulated may present an opportunity to refine therapeutics for the treatment of pain.
Keywords: Biased agonism, GPCR (G protein-coupled receptor), Arrestin, tolerance, dependence, antinociception, genetic mouse models
Introduction
Agonists of the mu opioid receptor (MOR) are clinically indispensable for their pain relieving properties but their deleterious effects including tolerance to the pain blocking effects, dependence, constipation, respiratory suppression, and abuse liability often give rise to serious medical complications, including death (1). From 1999 to 2017, there has been a six-fold increase in opioid-related deaths up to nearly 50,000 per year (2). Additionally, the economic burden associated with complications associated with opioid medications has been estimated to be over $78 billion (3). Given the severity of the complications and the ensuing epidemic, much of the MOR research has focused on reducing the aforementioned side effects. One promising mechanism by which this might be possible is via the concept of functional selectivity, also known as ligand bias.
The MOR is a seven transmembrane spanning, G-protein coupled receptor (GPCR) that signals via Gαi/o to suppress cyclic adenosine monophosphate (cAMP) formation by adenylyl cyclase (AC). The MOR also interacts with other cellular effectors including GPCR kinases (GRKs) that phosphorylate the receptor in a manner that facilitates binding of βarrestin proteins. GPCR-βarrestin interactions can lead to desensitization of receptor signaling through G proteins, as there may be steric hindrance for further coupling when the βarrestin is bound (4, 5) (Figure 1, top). However, as the multifunctional scaffolding proteins, βarrestin interactions can lead to other favorable interactions with signaling effectors, including G proteins and other regulators of receptor function (6, 7). Given that the mu opioid receptor resides in different neuronal populations and at different sites throughout the body, it will have opportunities to interact with different effectors upon activation. For example, a receptor expressed in a synaptic bouton will see different scaffolding partners than a receptor expressed in a dendritic spine. It is this concept that can be daunting for our attempts to understand receptor function in vivo; but simultaneously, it offers the opportunity to harness receptor signaling in a functionally selective manner (see Figure 1, bottom, for examples of how GPCRs signaling and regulation can be affected by where they are expressed). This concept is called “biased agonism” or “functional selectivity” of receptor signaling, and it refers to the ability to drive preferred signaling pathways and avoid adverse signaling pathways in a ligand-dependent manner. The challenge arises in the identification of which signaling pathways will be preserved in vivo and which should be avoided. In this review we will discuss some of the evidence supporting potential physiological pathways that may be harnessed to improve opioid analgesia and those that may be avoided to improve the side effect profile.
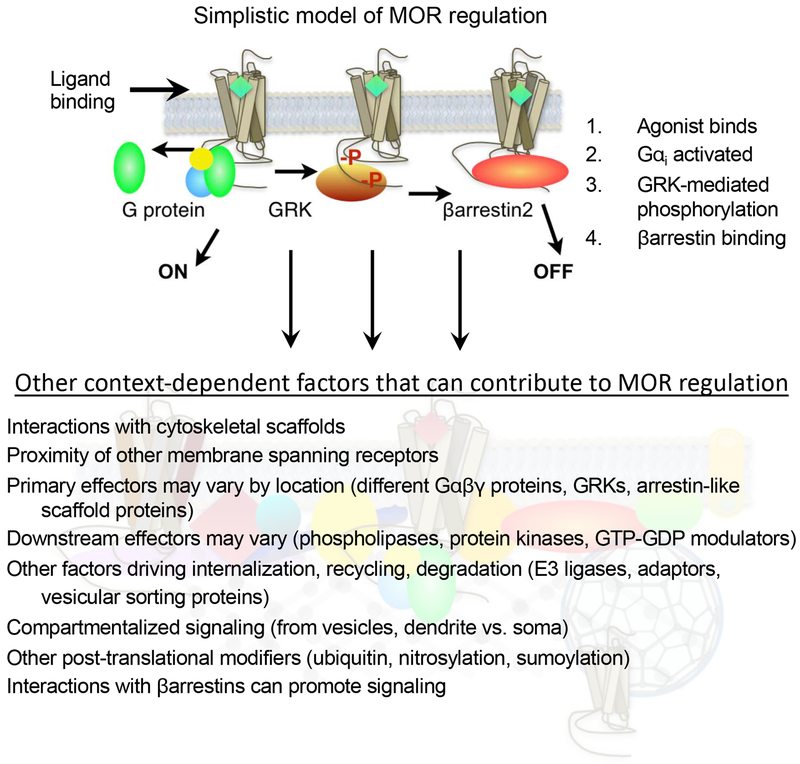
Figure 1.
Models of MOR signaling and regulation. The top model presents a simplistic linear progression of events wherein the agonist binds to the receptor with induces the activation of the heterotrimeric G protein and promotes dissociation of the G α protein from the βγ subunits. The receptor is then phosphorylated by a GPCR kinase (GRK) which then leads to the interactions with βarrestin proteins which prevents further interactions with the G protein. While these events can happen in this order, the simple model does not account for the interplay of variables that may differ based on where the receptor is expressed. The bottom figure represents the degree of complexity that GPCR activation may entail. All of these signaling events do not happen for all receptors in all locations, however, the diagram is meant emphasize the complexity of the system and the potential contributions that multiple effectors and regulators may have on the system. A few examples, but not comprehensive list of GPCR effectors have been included as examples. Each of these scenarios have the potential to influence the outcome of ligand-receptor-effector interactions and signaling events.
Genetically modified mouse models as indicators of physiological MOR regulation
Antinociception
The idea of pursuing biased signaling at the MOR has been inspired by work using genetically modified mouse models that lack βarrestin2. Constitutive deletion of βarrestin2 resulted in viable mice on a S129/C57BL6 background, and initial experiments with morphine revealed both enhanced potency and extended duration of action in the hot plate assay, a measure of supraspinally-mediated antinociception (8–10) as well as in the warm water tail immersion assay, a measure of spinal reflex to nociceptive stimuli (11, 12). These behavioral results have also been observed in mice treated intracerebroventricularly with via antigene RNA inhibition of βarrestin2 (13). Mice injected with siRNA to βarr2 but not βarr1 into the PAG showed enhanced and prolonged antinociception while overexpression of βarr2, but not βarr1, inhibited morphine-induced antinociception in the hot plate test (14). In a study using rats intrathecal infusion of siRNA silencing βarrestin2 expression acutely showed enhanced and prolonged antinociception in the tail flick assay (15).
βArrestins will bind to GPCRs upon phosphorylation by GRKs and it is postulated that the differential phosphorylation patterns, particularly in the C-terminus, may predict subsequent receptor fate (desensitization, down regulation, signaling, etc.) (16, 17). Unlike βarrestin2 knockout models, deletion of GRK3, GRK4, GRK5, and GRK6 did not display enhanced morphine antinociception (18–20). In the study by Glück et al. (19), GRK5-KO mice displayed less antinociception in response to morphine. Recently, generation of a mice expressing phosphorylation deficient mutations in the C-terminal of MOR revealed enhanced morphine and fentanyl antinociception in the hot plate test (21). A comprehensive study examining the role of the C-terminus (potential sites for phosphorylation and βarrestin interactions) using mice expressing MOR variants with truncated C-termini, revealed no enhancement of morphine antinociception overall (22).
Antinociceptive Tolerance:
Upon repeated dosing, βarr2-KO mice developed less tolerance to the antinociceptive effects of morphine in the hot plate assay (9) (23). However, when tested in the warm water tail withdrawal assay, a measure of spinally-mediated reflex to nociception, morphine produced tolerance in both genotypes of mice although the onset of tolerance in the βarr2-KO mice was delayed (11). Moreover, a nonselective protein kinase C (PKC) inhibitor, chelerythrine, injected systemically, restored morphine sensitivity in the tolerant cohort of βarr2-KO mice but had no significant impact on the WT mice. Both studies suggest that βarrestin2 regulates MOR sensitivity to morphine in the spinal cord, although it is clear that other regulatory proteins can also impact receptor function. Knockdown of siRNA for βarrestin2 but not βarrestin1 prevented morphine tolerance in mice in the hot plate assay (14).
In the hot plate test, GRK6-KO mice displayed equivalent tolerance as compared to WT mice when tested for tolerance development to morphine (20), as were GRK5-KO mice (19). GRK3-KO mice showed no improvement of tolerance upon chronic daily morphine administration, although fentanyl-treated mice exhibited significantly less tolerance relative to wild type controls in the hot plate test (24). In separate study, GRK3-KO mice were equivalently tolerant to morphine, but less tolerant to etonitazene (19), suggesting potential roles of efficacy and/or potency of agonists as a determinant of MOR-mediated tolerance in mice lacking GRK3.
A mouse line expressing MOR with a mutation at Ser 375 to Ala exhibited diminished tolerance to fentanyl and etonitazine but not morphine, indicating agonist specific regulation of MOR tolerance (25). In the C-termini truncation of exon7 deletion MOR mice, morphine tolerance was significantly attenuated in a radiant heat tail flick assay (22). The MOR phosphorylation deficient mutants (S375A, 10 or 11 Ser/Thr residues mutated to Ala) developed less tolerance to fentanyl, but only the multiple site mutants displayed less tolerance to morphine (21). Less morphine tolerance in the tail withdrawal assay was observed in rats treated with siRNA to βarrestin2 (22).
Physical dependence and withdrawal
Following implantation of a morphine pellet (75 mg pellet, 3 days), WT and βarr2-KO mice displayed the same extent of withdrawal in response to naloxone (9). Continuous infusion of lower concentration of morphine (24 mg/kg/day, subcutaneous minipump infusion over 6 days) resulted in fewer signs of naloxone-precipitated withdrawal observed in βarr2-KO mice relative to the WT mice (23). GRK6-KO mice displayed equivalent morphine dependence as WT mice (20); while GRK5-KO mice displayed less signs of withdrawal although these mice also responded less to morphine in pain assays (19). Phosphorylation deficient mutant MOR mice still displayed naloxone-precipitated morphine withdrawal signs (21). The deletion of exon4-encoded C-terminus of MOR attenuated morphine dependence, while the exon7 C-terminal deletion did not (the opposite of what was found for tolerance) (22).
It is important to note that the benefits observed in the βarrestin2-KO mice were somewhat uniquely observed for morphine as the opioid agonist (Bohn et al., 2004; Raehal and Bohn, 2011). Other opioids including fentanyl, oxycodone, methadone, and etorphine did not produce the separation in potency between the βarrestin2 genotypes for both supraspinal and spinal antinociception (11) (23). Moreover, no differences in the degree of tolerance or dependence developed between βarrestin2 null and wild type mice for oxycodone, methadone, and fentanyl were observed (23), suggesting differential regulation of morphine induced MOR signaling relative to the other tested opioids. Subsequent studies in cell based signaling assays suggest that morphine is better at recruiting βarrestin2 over βarrestin1 (10, 26, 27) and it was proposed that the elimination of βarrestin2 would therefore have the greatest impact on morphine-mediated events. In mouse studies of mice lacking βarrestin1 morphine-induced antinociception did not differ from WT mice (18). It is also possible that the impact of the removal of βarrestin2 is most revealed for agonists, such as morphine, which produce very little βarrestin2 recruitment and little receptor internalization (28, 29).
Constipation:
Morphine causes constipation by directly activating MOR in the enteric nervous system and indeed, the development of peripherally restricted antagonists have proven useful for reversing morphine-induced constipation (30). Within the gastrointestinal system, the MOR is expressed in enteric neurons located in both the myenteric and submucosal plexi and within different intestinal sections, thus there is potential for differential regulation (31, 32). The βarr2-KO mice displayed less delay in colonic bead expulsion and overall fecal accumulation in response to morphine; but ileum transport of a charcoal gavage was the same between the genotypes (33). In additional studies, both βarrestin2 and MOR were shown to be co-localized in neurons dissociated from the myenteric plexus of ileum and colon (34), and that βarrestin2 may play differential roles in morphine-sensitive neurons from the ileum versus the colon (35). In contrast, the phosphorylation site mutant mice displayed no protection from morphine-induced constipation (21), while mice lacking the exon4 (but not exon7) C-terminus of MOR were less responsive to morphine as well (22). GRK6-KO mice also displayed less constipation than WT mice while other GRK-KO mice have not been tested (20).
Respiratory suppression:
In response to morphine, mice display decreases in breathing frequency and subsequent decreases in arterial oxygen saturation (%O2). The βarr2-KO mice displayed less morphine-induced respiratory suppression than WT mice (33). No benefit was seen in the MOR phosphorylation-site mutants (21). Respiration studies have not been reported in the other mutant strains at this time.
Running behaviors and reward:
Although βarr2-KO mice showed smaller increases in locomotor stimulation relative to WT mice, dopamine release in striatum was similar to WT mice (36, 37). Mice lacking βarrestin1 responded to morphine similar to WT mice in locomotor activity (37). The βarr2-KO mice also displayed robust CPP in response to morphine that was slightly enhanced relative to WT mice (36). Mice lacking GRK5, however, did not develop CPP in response to morphine than their WT littermates while GRK3-KO and S375A mutant mice did (19). The exon7 C-terminal truncation MOR mice also showed decreased running behaviors in response to morphine however, these animals also displayed less morphine-induced conditioned place preference (CPP) (22). Morphine-stimulated locomotor activity was not affected in the C-terminal phosphorylation sites mutant mice (21). Further evaluations of abuse potential was not pursued in the global βarr2-KO mice as most GPCRs, including dopamine receptors, utilize these proteins (37). Extensive studies have been done to evaluate dopaminergic and serotonergic signaling (38) (39, 40) and more refined models and chemical probes will be necessary to understand the impact of these signaling modalities to opioid abuse potential.
Summary of the animal models:
The studies in the genetically modified mouse models point to an opportunity to avoid βarrestin (or βarrestin-associated) pathways as a means to improve the therapeutic outcome of opioid pain therapies. A summary of the models discussed in this section are presented in Supplemental Table 1. Importantly, removal of a particular GRK did not always recapitulate the removal of a βarrestin, and deletion of individual phosphorylation sites could also have disparate outcomes. This emphasizes that these signaling events may not be linearly exclusive. Phosphorylation at a particular site may have other biochemical and physiological consequences apart from βarrestin recruitment. Moreover, the GRK family of proteins may affect functionality in addition to phosphorylating receptors (41). This view is further complicated by the realization that these interactions are likely present in some cells where they can be important for some receptor-mediated physiologies but not others. Altogether, it evident that MOR is differentially regulated in a region-dependent and agonist-dependent manner. Cellular model systems have been essential for understanding the basics of MOR signaling and regulation; however, these models are often only providing a limited snapshot of signaling potential. As studies continue, we become more aware that signal transduction is product of the local environment. While it was once thought that receptor internalization was synonymous with turning receptors off, it is now known that internalization can lead to down regulation, recycling or even permit persistent receptor signaling (42). The question remains as to how the MOR signals in the different cells that control the different physiological responses opioid analgesics. Overall, there is an opportunity to attempt to capitalize on these differences as genetic model evidence suggests that avoiding the initial interaction with βarrestins might be a means to avoid certain adverse effects. However, it must be recognized that mouse models may not recapitulate the same signaling paradigms that are present across species. Therefore, the development of ligands that can promote or exclude the events suggested by the genetic models, will provide the opportunity to assess how divergence in receptor signaling can impact diverse physiological systems across species, and ultimately in humans. The following section will describe recent efforts towards the development of opioid analgesics with these properties.
Development of agonists that promote G protein signaling over βarrestin recruitment (G protein biased agonists)
The first published MOR agonist that appeared to have functional selectivity for recruiting G-proteins over βarrestin2 was herkinorin (43)(Figure 2). Herkinorin has limited bioavailability and is predicted to have a very short half-life and brain penetrance based on its close structural similarity to the kappa opioid receptor agonist, salvinorin A. While a local administration of herkinorin to paw pads produced potent antinociception in rats with limited tolerance upon repeated dosing in the formalin test, it is difficult to know whether this dosing strategy is sufficient to lead to receptor desensitization (44). Cellular immunoprecipitation studies demonstrated reduced phosphorylation at serine 375 of MOR and less internalization (by confocal microscopy and cell surface biotinylation studies) and βarrestin recruitment as determined by confocal microscopy. In these same studies, ERK1/2 posphorylation was still induced by herkinorin. Herkinorin was later shown to be 10X less potent than DAMGO in GTPγS binding assays although overexpression of GRK2 was insufficient to promote βarrestin2 recruitment (45). These early studies applied no mathematical modeling to compare relative potencies and efficacies and relied primarily on the presence or absence of an effect. Later studies utilizing an enzyme complementation assay to assess βarrestin2 recruitment show that the compound can induce recruitment (46), but no calculation of bias was presented.
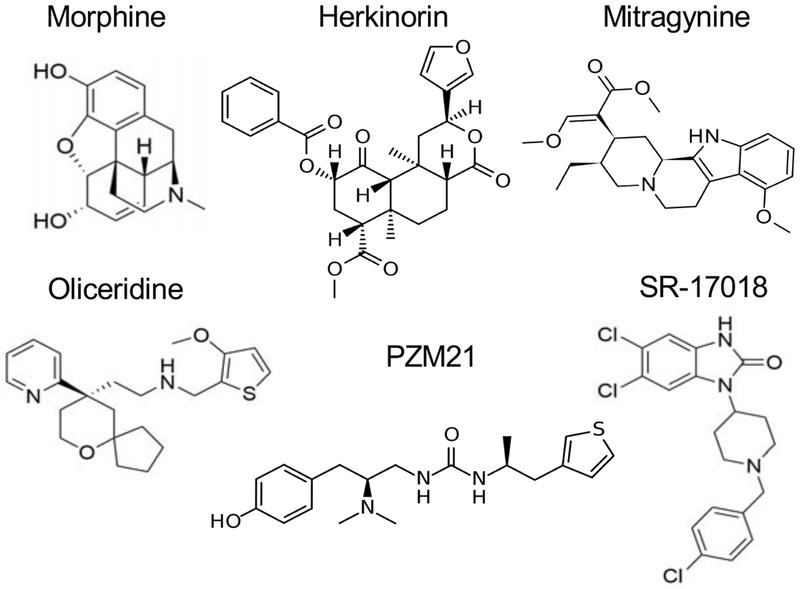
Figure 2.
Chemical structures opioid agonists of MOR discussed in this review.
These early studies highlight the importance of what we term as “biased” and what cellular assays we consider to be a surrogate for detecting relevant signaling differences. As new compounds are developed, it is increasingly apparent that cellular contexts can greatly impact on how ligands induce MOR signaling and will influence the perception of bias. Ultimately it will be important to understand which signaling profiles in which cellular assays will correlate with physiological responses. Herein we will discuss some of the more-studied compounds that have been reported to produce preference for G protein signaling over βarrestin2 recruitment. It should be recognized that while these compounds have been called “biased agonists” the criteria for calculating “bias” and the assay systems used vary between the studies. A summary of compounds discussed here are presented in Supplemental Tables 2 (in vitro studies) and 3 (in vivo studies).
Oliceridine (or TRV-130) was the first clinically pursued biased MOR agonist (47). Preclinical results suggested a modest selectivity (approximately 3 fold) for cAMP inhibition over βarrestin2 recruitment (48), Figure 2. MOR internalization was also markedly reduced relative to the full agonist DAMGO, in alignment with loss of βarrestin2 recruitment. In mouse and rat models oliceridine maintained the typical mu opioid attenuation of pain-like responses while demonstrating reductions in gastrointestinal and respiratory side effects (48). Other studies suggested that oliceridine could produce constipation at higher doses (49). In abuse liability assays, oliceridine was self-administered by rats (50) and potentiated intracranial self-stimulation responding (49), consistent with the expected abuse potential of MOR agonists. Oliceridine also evoked tolerance in an assay of spinal antinociception as well as somatic signs of withdrawal, although to a lesser degree than morphine treated mice (51). In clinical trials for post-operative pain, oliceridine exhibited some tangible benefit in safety profile in regard to respiration as compared to morphine (52–56) although this benefit was lost with higher doses of oliceridine. In trials, it produced opioid-like subjective effects in humans (52), suggesting a potential for abuse liability. These results taken together suggest that the development of compounds that have lower efficacy for recruiting βarrestin2 in vitro may be a means to separate pain relief from some but not all adverse events.
Another novel biased MOR agonist, PZM21, was designed utilizing the crystal structure of the MOR, specifically to find novel molecules that would mimic the binding pose of oliceridine docked in the inactive state MOR (46) (Figure 2). When cAMP accumulation versus βarrestin2 recruitment enzyme fragment complementation (EFC) assays were conducted, only a weak activation of βarrestin2 was observed, while PZM21 was potent and efficacious for activating G protein-mediated inhibition of cAMP accumulation. The dose response curve for PZM21 overlays that of oliceridine presented in the manuscript (46); in prior studies, the potency of oliceridine (TRV130) in the βarrestin2 EFC assays were reported as 80 nM (48). Within the supplemental data of the Manglik et al., manuscript, PZM21 is shown to activate βarrestin2 in the TANGO assay (~900 nM potency), but not in a βarrestin2 bioluminescence resonance energy transfer (BRET) assay. However, no calculations were made to determine bias factors for this compound (46).
In mouse models, PZM21 suppressed hot plate affective responses but not reflexive paw removal, which is unusual for a MOR agonist (46). It had no effect in the warm water tail immersion test but did produce efficacy in both phases of the formalin paw test. Initial measures of tolerance and abuse liability seemed promising for PZM21 where the compound produced no CPP or locomotor stimulation (46). When another assay was used to study the compound in vitro, PZM21 was a low efficacy agonist for stimulating [35S]GTPγS binding assay; in this study, where bias was calculated relative to DAMGO and compared to βarrestin2 recruitment (enzyme fragment complementation), no bias was observed (57). This group showed that in two strains of mice, PZM21 induced antinociception in the hot plate test (paw withdrawal) however they also observed respiratory suppression. Following 5 days of daily dosing, PZM21 produced tolerance in the hot plate test but not for respiratory suppression (57). The compound, and the many analogues produced in the initial report describing PZM21 (46), may provide important tools to understand MOR regulation of diverse pain pathways. However, its unusual signaling and behavioral features should be considered (e.g. lack of tail flick response, potency or efficacy in βarrestin2 assays detected differently in different assays) should also be considered along with its perceived signaling bias.
Mitragynine, the main component of kratom, has been described as a biased agonist at MOR (Figure 2). However, mitragynine also acts at other receptors, including kappa and delta opioid receptors (58), therefore its effects in vivo are difficult to completely attribute to its pharmacological profile at MOR alone. However, the compound produces antinociception with less apparent tolerance and spontaneous withdrawal. Additionally, experiments utilizing single doses of mitragynine derivatives appear to produce less constipation, respiratory suppression, and CPP, relative to morphine (58). However, establishing dose-response relationships for these endpoints are needed to determine whether these apparent benefits will be maintained across a wide dose range. More selective derivatives with more complete assessment of in vivo dose ranges may prove useful for probing MOR pharmacology.
Recently, our lab reported on a series of biased MOR agonists surveying many degrees of bias from 0.4- to 100-fold preference as measured by either GTPγS binding or cAMP accumulation versus either βarrestin2 recruitment PathHunter® enzymatic complementation or recruitment of GFP-conjugated βarrestin2 (59, 60). The compounds are N-benzyl piperidine 4-benzimidazolones and an example of SR-17018 is shown in Figure 2. They are collectively referred to as the Scripps Research (SR) series of compounds in this review. Unlike oliceridine and PZM21 in the EFC assay, the SR series of compounds produce rightward shifted βarrestin2 potencies relative to the enkephalin analog (DAMGO) reference. For characterization, a method of analysis described by the operational model of pharmacological agonism (61) was used to compare the performance of the compound across multiple signaling assays at both the mouse and human MOR. Dose response studies were then conducted in mice where efficacy and potency were demonstrated in the hot plate and tail flick pain assays, as well as in respiratory suppression measures. Potency ratios between the different pain assays and respiratory studies were generated to determine a therapeutic index which was then compared to the degree of bias observed in vitro. The high degree of correlation suggests that the greater the separation between G protein signaling and βarrestin recruitment in cells could widen the therapeutic window.
Ongoing studies are evaluating the effect of chronic administration although limitations of solubility have presented challenges. In mice, SR-17018 chronic oral administration did not lead to hot plate antinociceptive tolerance or morphine cross tolerance. Upon cessation of treatment, withdrawal signs were present, but they dissipated after one day in contrast to morphine withdrawal which persisted for 72 hours. Furthermore, when morphine-tolerant mice were switched to SR-17018 daily dosing, morphine-withdrawal could be prevented as is typical for opioid agonist substitution in a dependent animal. However, the daily dosing with SR-17018 restored morphine antinociceptive sensitivity, unlike buprenorphine substitution which could suppress withdrawal but also preserved morphine tolerance (62). In studies assessing drug discrimination and rat tail flick antinociception, SR-14968 and oliceridine produced fentanyl-like discriminative stimulus effects but showed an improved potency ratio (drug discrimination potency/ tail flick potency) compared to morphine and methadone (63). This paper also showed that SR-14968 has efficacy in a non-human primate model for antinociception. It is not clear however, how pharmacokinetics will play into the reinforcing properties of the compound, as SR-14968 has a long duration of action relative to oliceridine, morphine and methadone in the rodent models (60). Ongoing studies are needed determine if biased MOR agonists will have any improvement over conventional, clinically utilized opioids with regards to abuse liability and addiction.
Concluding Remarks
The use of genetically modified mice continues to be very valuable to the study of how GPCRs function in vivo. However, important caveats, such as developmental and environmental adaptations, strain differences (which implies different protein expression) and endogenous ligand tone, will likely impact on the display of receptor function. Indeed, many of the initial results with βarr2-KO mice have been recapitulated with novel biased agonists. Biased agonism is a useful approach to investigate receptor signaling potentials, but it is important to realize that the separation between two pathways observed in vitro may not reflect receptor signaling in the endogenous setting. For example, studies with KOR agonists that display bias between inhibition of cAMP and GTPγS binding in CHO cells do not display this bias in striatal neurons (64). For the MOR, which is expressed throughout many tissues and neuronal types throughout the body, it is likely that signaling and regulation mechanisms will differ according to changes in context. The best that we can hope of is that the cell-based signaling assays can be useful models as readouts of receptor-effector potentials and at best, can provide a glimpse of the signaling events that ensues at the receptor in the physiological setting. The growing collection of tool compounds with diverse pharmacological signatures should prove useful for gaining a greater understanding of how a receptor functions in a relevant cell to determine the biological response- whether desired (analgesia) or avoided (side effects). Introducing pathway selective signaling may present a novel means to separate physiologies, but only if distinct pathways control distinct physiologies. For the MOR, which mediates many distinct physiological responses in both mouse and human, the opportunity for refinement is promising.
Supplementary Material
Acknowledgements:
Work on the mu opioid receptor biased agonists in the Bohn laboratory is funded by NIH grants from NIDA: DA038964, DA033073, and DA047039.
Financial Disclosures: Dr. Bohn has served as a consultant for Axovant Biosciences and Goldfinch Bio, Inc. on non-opioid research in the past two years. She has received research funding on an unrelated project from Axovant Biosciences. Dr. Bohn has filed for a patent on the Scripps Research compounds described herein. Dr. Grim and Dr. Acevedo-Canabal report no biomedical financial interests or potential conflicts of interest to disclose.
Abbreviations
- AC
adenylyl cyclase
- BRET
bioluminescence resonance energy transfer
- cAMP
cyclic adenosine monophosphate
- CHO
Chinese hamster ovary
- CPP
conditioned place preference
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- EFC
enzyme fragment complementation
- GPCR
G-protein coupled receptor
- GRK
G-protein coupled receptor kinase
- GTPγS
guanosine 5’-O-[gamma-thio]triphosphate)
- KO
knockout
- MOR
mu opioid receptor
- PKC
protein kinase C
- RNA
ribonucleic acid
- siRNA
short interfering RNA
- WT
wild type
Reference:
- 1.Pasternak GW (2018): Mu Opioid Pharmacology: 40 Years to the Promised Land. Adv Pharmacol. 82:261–291. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Collins FS (2017): The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 377:391–394. [DOI] [PubMed] [Google Scholar]
- 3.Florence CS, Zhou C, Luo F, Xu L (2016): The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med Care. 54:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilger D, Masureel M, Kobilka BK (2018): Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol. 25:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, et al. (2014): Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 512:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurevich VV, Gurevich EV (2019): The structural basis of the arrestin binding to GPCRs. Mol Cell Endocrinol. 484:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomsen ARB, Plouffe B, Cahill TJ 3rd, Shukla AK, Tarrasch JT, Dosey AM, et al. (2016): GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell. 166:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999): Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- 9.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000): Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 408:720–723. [DOI] [PubMed] [Google Scholar]
- 10.Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS (2004): Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 66:106–112. [DOI] [PubMed] [Google Scholar]
- 11.Bohn LM, Lefkowitz RJ, Caron MG (2002): Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 22:10494–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal N, Tan M, Egbuta O, Desai N, Crawford C, Xie CW, et al. (2012): Evidence that behavioral phenotypes of morphine in beta-arr2−/− mice are due to the unmasking of JNK signaling. Neuropsychopharmacology. 37:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bu H, Liu X, Tian X, Yang H, Gao F (2015): Enhancement of morphine analgesia and prevention of morphine tolerance by downregulation of beta-arrestin 2 with antigene RNAs in mice. Int J Neurosci. 125:56–65. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Liu X, Liu C, Kang J, Yang J, Pei G, et al. (2009): Improvement of morphine-mediated analgesia by inhibition of beta-arrestin2 expression in mice periaqueductal gray matter. Int J Mol Sci. 10:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CH, Huang HW, Chen KH, Chen YS, Sheen-Chen SM, Lin CR (2011): Antinociceptive potentiation and attenuation of tolerance by intrathecal beta-arrestin 2 small interfering RNA in rats. Br J Anaesth. 107:774–781. [DOI] [PubMed] [Google Scholar]
- 16.Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, et al. (2011): Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 286:11506–11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer D, Damberger FF, Samarasimhareddy M, Feldmueller M, Vuckovic Z, Flock T, et al. (2019): Distinct G protein-coupled receptor phosphorylation motifs modulate arrestin affinity and activation and global conformation. Nat Commun. 10:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004): Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 27:107–144. [DOI] [PubMed] [Google Scholar]
- 19.Gluck L, Loktev A, Mouledous L, Mollereau C, Law PY, Schulz S (2014): Loss of morphine reward and dependence in mice lacking G protein-coupled receptor kinase 5. Biol Psychiatry. 76:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raehal KM, Schmid CL, Medvedev IO, Gainetdinov RR, Premont RT, Bohn LM (2009): Morphine-induced physiological and behavioral responses in mice lacking G protein-coupled receptor kinase 6. Drug Alcohol Depend. 104:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, et al. (2019): Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Lu Z, Narayan A, Le Rouzic VP, Xu M, Hunkele A, et al. (2017): Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Invest. 127:1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raehal KM, Bohn LM (2011): The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 60:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, et al. (2004): G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol. 141:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grecksch G, Just S, Pierstorff C, Imhof AK, Gluck L, Doll C, et al. (2011): Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A mu-opioid receptor knock-in mice. J Neurosci. 31:13890–13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groer CE, Schmid CL, Jaeger AM, Bohn LM (2011): Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 286:31731–31741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M (2015): Biased Agonism of Endogenous Opioid Peptides at the mu-Opioid Receptor. Mol Pharmacol. 88:335–346. [DOI] [PubMed] [Google Scholar]
- 28.Whistler JL, von Zastrow M (1998): Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci U S A. 95:9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, et al. (1998): Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 95:7157–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood JD, Galligan JJ (2004): Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 16 Suppl 2:17–28. [DOI] [PubMed] [Google Scholar]
- 31.Briggs SL, Sawyer DC, Rech RH, Galligan JJ (1995): Oxymorphone-induced analgesia and colonic motility measured in colorectal distension. Pharmacol Biochem Behav. 52:561–563. [DOI] [PubMed] [Google Scholar]
- 32.Burks TF, Galligan JJ, Hirning LD, Porreca F (1987): Brain, spinal cord and peripheral sites of action of enkephalins and other endogenous opioids on gastrointestinal motility. Gastroenterol Clin Biol. 11:44B–51B. [PubMed] [Google Scholar]
- 33.Raehal KM, Walker JK, Bohn LM (2005): Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 314:1195–1201. [DOI] [PubMed] [Google Scholar]
- 34.Maguma HT, Datta De D, Bhave S, Dewey WL, Akbarali HI (2014): Specific localization of beta-Arrestin2 in myenteric plexus of mouse gastrointestinal tract. PLoS One. 9:e103894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith TH, Grider JR, Dewey WL, Akbarali HI (2012): Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS One. 7:e45251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, et al. (2003):Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 23:10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urs NM, Daigle TL, Caron MG (2011): A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 36:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005): An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 122:261–273. [DOI] [PubMed] [Google Scholar]
- 39.Schmid CL, Bohn LM (2010): Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 30:13513–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, et al. (2008): Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 105:13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watari K, Nakaya M, Kurose H (2014): Multiple functions of G protein-coupled receptor kinases. J Mol Signal. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoeber M, Jullie D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, et al. (2018): A Genetically Encoded Biosensor Reveals Location Bias of Opioid Drug Action. Neuron. 98:963–976 e965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, et al. (2007): An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol. 71:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE (2012): Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: new concepts in mu opioid receptor pharmacology: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 121:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, et al. (2008): Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem. 51:2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. (2016): Structure-based discovery of opioid analgesics with reduced side effects. Nature. 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, et al. (2013): Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 56:8019–8031. [DOI] [PubMed] [Google Scholar]
- 48.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, et al. (2013): A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 344:708–717. [DOI] [PubMed] [Google Scholar]
- 49.Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS (2017): Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 31:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Austin Zamarripa C, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB (2018): The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend. 192:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang DY, Li WW, Nwaneshiudu C, Irvine KA, Clark JD (2018): Pharmacological Characters of Oliceridine, a mu-Opioid Receptor G-Protein[FIGURE DASH]Biased Ligand in Mice. Anesth Analg. [DOI] [PubMed] [Google Scholar]
- 52.Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. (2014): Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 155:1829–1835. [DOI] [PubMed] [Google Scholar]
- 53.Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, et al. (2014): First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 54:351–357. [DOI] [PubMed] [Google Scholar]
- 54.Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, et al. (2017): A randomized, Phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res. 10:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singla NK, Skobieranda F, Soergel DG, Salamea M, Burt DA, Demitrack MA, et al. (2019): APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein-Biased Ligand at the mu-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty. Pain Pract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N (2019): APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the micro-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res. 12:927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, et al. (2018): The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol. 175:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, et al. (2016): Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit beta-Arrestin-2. J Med Chem. 59:8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, et al. (2018): Optimization of a Series of Mu Opioid Receptor (MOR) Agonists with High G Protein Signaling Bias. J Med Chem. 61:8895–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, et al. (2017): Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell. 171:1165–1175 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Black JW, Leff P (1983): Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 220:141–162. [DOI] [PubMed] [Google Scholar]
- 62.Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho JH, Acevedo-Canabal A, et al. (2019): A G protein signaling-biased agonist at the mu-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, et al. (2019): Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology. 150:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho JH, Stahl EL, Schmid CL, Scarry SM, Aube J, Bohn LM (2018): G protein signaling-biased agonism at the kappa-opioid receptor is maintained in striatal neurons. Sci Signal. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.