Abstract
Land models are often used to simulate terrestrial responses to future environmental changes, but these models are not commonly evaluated with data from experimental manipulations. Results from experimental manipulations can identify and evaluate model assumptions that are consistent with appropriate ecosystem responses to future environmental change. We conducted simulations using three coupled carbon‐nitrogen versions of the Community Land Model (CLM, versions 4, 4.5, and—the newly developed—5), and compared the simulated response to nitrogen (N) and atmospheric carbon dioxide (CO2) enrichment with meta‐analyses of observations from similar experimental manipulations. In control simulations, successive versions of CLM showed a poleward increase in gross primary productivity and an overall bias reduction, compared to FLUXNET‐MTE observations. Simulations with N and CO2 enrichment demonstrate that CLM transitioned from a model that exhibited strong nitrogen limitation of the terrestrial carbon cycle (CLM4) to a model that showed greater responsiveness to elevated concentrations of CO2 in the atmosphere (CLM5). Overall, CLM5 simulations showed better agreement with observed ecosystem responses to experimental N and CO2 enrichment than previous versions of the model. These simulations also exposed shortcomings in structural assumptions and parameterizations. Specifically, no version of CLM captures changes in plant physiology, allocation, and nutrient uptake that are likely important aspects of terrestrial ecosystems' responses to environmental change. These highlight priority areas that should be addressed in future model developments. Moving forward, incorporating results from experimental manipulations into model benchmarking tools that are used to evaluate model performance will help increase confidence in terrestrial carbon cycle projections.
Keywords: Community Land Model, nitrogen enrichment, elevated CO2, land model, biogeochemistry
Key Points
Experimental manipulations provide critical insights into ecosystem responses to environmental change that can evaluate land models
Parametric and structural changes to the Community Land Model version 5 improve the simulated response to environmental change
Model assumptions related to nutrient acquisition strategies and trade‐offs between carbon and nitrogen limitation deserve further attention
1. Introduction
Large uncertainties in terrestrial carbon (C) cycle projections presents significant barriers to developing greenhouse gas emissions that are compatible with particular climate change scenarios (Friedlingstein et al., 2014; Jones et al., 2013). In particular, the projected concentrations of atmospheric CO2 and the magnitude of carbon‐climate feedback shows persistently high uncertainty that is largely related to structural uncertainty among land models (Arora et al., 2013; Friedlingstein et al., 2006; Lovenduski & Bonan, 2017). Indeed, since the IPCC Fifth Assessment Report, significant efforts have gone into understanding model deficiencies, improving the representation of particular land processes, and developing the current generation of land models that will be used in upcoming climate change assessments (Eyring et al., 2016; Jones et al., 2016; Lawrence et al., 2016). Some of these advances include addition or modifications of land model simulations of the nitrogen (N) cycle and its constraints on terrestrial carbon balance (Thornton et al., 2007; Zaehle et al., 2010). As the science behind Earth system models matures, it creates a need to develop uniform metrics that can be used to evaluate model performance and to understand what model assumptions lead to appropriate, or likely, responses to environmental change.
Model benchmarking tools, like the International Land Model Benchmarking Project (ILAMB), offer powerful insights into the biogeophysical and biogeochemical representations of land models (Collier et al., 2018; Hoffman et al., 2017; Luo et al., 2012). By standardizing the observational data sets and scoring metrics by which models are evaluated, researchers gain understanding of model strengths and deficiencies (Lawrence et al., 2019). While matching historical trends and present‐day observations are an important prerequisite for credible land model simulations, they offer relatively little insight into the accuracy of future projections that such models are intended to make. Given that future environmental changes will likely exceed the range of historical conditions, improvements in benchmarking scores over the historical era do not necessarily improve confidence in future projections.
To gain a greater understanding of ecological processes that are represented in models we need to move beyond these static benchmarks. Evaluating terrestrial models against observed responses to experimental manipulations (e.g., atmospheric CO2 enrichment) helps to identify and evaluate assumptions that are important for simulating appropriate ecosystem responses to future environmental change (De Kauwe et al., 2014; Medlyn et al., 2015; Walker, Hanson, et al., 2014; Zaehle et al., 2014). When experimental manipulations are replicated in models, the outcomes provide additional insights into model strengths and weaknesses that will influence future projections and could be incorporated into benchmarking packages like ILAMB. Toward this end, we conducted simulations using three coupled carbon‐nitrogen (C‐N) versions of the Community Land Model (CLM), the land component of the Community Earth System Model, and compared the global response to nitrogen and CO2 enrichment simulated by the models to meta‐analyses from similar experimental manipulations.
The initial representations of terrestrial biogeochemical cycles in early climate models simulate leaf gas exchange and plant productivity based on Ball‐Berry stomatal conductance and Farquhar photosynthesis, respectively (Bonan, 2015). Although implementation and canopy scaling of these schemes among models remains uncertain (Rogers et al., 2017), they typically project a strong carbon‐concentration feedback and robust terrestrial carbon sink under elevated CO2 (Arora et al., 2013). These carbon‐only models potentially exaggerate the magnitude of the land carbon sink (Hungate et al., 2003; Wieder, Cleveland, Smith, & Todd‐Brown, 2015; Zaehle et al., 2015), thus motivating the representation of the terrestrial nitrogen cycle into land models, which typically reduces ecosystem sensitivity to elevated CO2 and decreases terrestrial carbon uptake in future scenarios (Thornton et al., 2007; Wang & Houlton, 2009; Zaehle et al., 2010). Alternatively, inclusion of coupled C‐N dynamics can also offset or even reverse the direction of the carbon‐climate feedback, when warming‐enhanced mineralization of N from soil organic matter stimulates more CO2 uptake by plants than is lost to warming‐enhanced decomposition (Sokolov et al., 2008). Although the inclusion of coupled C‐N dynamics into global‐scale models affords opportunities to integrate ecological and biogeochemical insights into historically geophysical models, it also creates significant uncertainties in model structure and parameterization that underscore our incomplete understanding of terrestrial ecosystem function and how to represent them at global scales. For example, the mechanisms by which nitrogen fundamentally limits terrestrial productivity remain uncertain (Meyerholt & Zaehle, 2015; Meyerholt & Zaehle, 2018; Thomas et al., 2015). Similarly, the magnitude and duration of ecosystem response to elevated CO2 remains poorly constrained (De Kauwe et al., 2014; Zaehle et al., 2014). This paper aims to quantify sensitivities to nitrogen enrichment and elevated CO2 in three coupled C‐N versions of CLM. Despite their structural similarities, over the course of model development each version of CLM makes different assumptions about plant and soil biogeochemical processes that lead to different responses to N enrichment and elevated CO2. Here we evaluate these assumptions with data from experimental manipulations that increase N and CO2 availability and suggest that similar activities afford opportunities to extend insights from future model intercomparison projects.
2. Methods
2.1. Model Overview
In the Coupled Model Intercomparison Project phase 5 (CMIP5) ensemble of models, the Community Land Model, version 4 (CLM4) was the only terrestrial model that included coupled carbon‐nitrogen biogeochemistry (Thornton et al., 2007). This version of the model showed strong nitrogen limitation (Bonan & Levis, 2010; Thomas, Zaehle, et al., 2013) and a weak response to elevated CO2 (Hoffman et al., 2014; Zaehle et al., 2014). The intermediate model version, CLM4.5 (Oleson et al., 2013), implemented modifications to canopy photosynthesis (Bonan et al., 2011; Bonan et al., 2012) and a vertically resolved representation of soil biogeochemistry, which resulted in a twentieth‐century land carbon sink that better matched observationally derived estimates (Koven et al., 2013), but still showed strong nitrogen limitation of the global carbon cycle (Wieder, Cleveland, Lawrence, & Bonan, 2015). Finally, CLM5 implemented extensive modifications to the representation of plant nitrogen dynamics (Fisher et al., 2019), snow and soil hydrology (Brunke et al., 2016; Swenson & Lawrence, 2014, 2015; van Kampenhout et al., 2017), plant hydraulic stress (Kennedy et al., 2019), and the capacity to simulate transient land use that includes a prognostic crop model (Lawrence et al., 2019). While extending the scientific capacity of CLM, these modifications also improved model performance relative to the ILAMB benchmarking metrics (Lawrence et al., 2019). Here we briefly summarize significant developments in the biogeochemical representations applied in the latest version of the CLM, with more details available in associated CLM5 papers (Bonan et al., 2019).
The representation of CLM's nitrogen cycle, particularly plant nitrogen dynamics, changed in several major ways as the model developed from versions 4.0 to 4.5 and 5.0. Most importantly, CLM4 and 4.5 calculated instantaneous (or potential) gross primary productivity (GPP), without consideration of soil nitrogen availability (Thornton et al., 2007). Subsequently, N availability and stoichiometric constraints downregulated potential GPP to provide actual GPP estimates. This approach decouples leaf exchange of carbon from associated water and energy fluxes (Zaehle et al., 2014). Further, CLM4 and 4.5 prescribed foliar nitrogen concentrations (leaf C:N ratios) and associated photosynthetic capacities that were static with regard to environmental conditions. Assumptions in CLM4 and 4.5 that related nitrogen availability to plant productivity were not consistent with observations, especially regarding how plants respond to environmental change. Modifications to CLM5 changed these assumptions in three important ways, with the addition of three new modules.
First, recognizing that leaf nitrogen content is dynamic, implementation of “FlexCN” functionality affords flexible plant stoichiometry and thus a single realized GPP calculation, as opposed to separating potential and actual fluxes (Ghimire et al., 2016). Second, recognizing that foliar photosynthetic capacity responds to environmental conditions and changes over space and time, the Leaf Utilization of Nitrogen for Assimilation (LUNA) module provides a prognostic optimization of maximum leaf carboxylation and electron transport rates (Ali et al., 2016; Xu et al., 2012; see also Ellsworth et al., 2004; Kattge et al., 2009; Walker, Beckerman, et al., 2014). Third, recognizing that changes in nitrogen supply or demand have consequences for plant productivity, the Fixation and Uptake of Nitrogen (FUN) module calculates the carbon costs of various nitrogen acquisition strategies and adjusts carbon expenditure on nitrogen uptake among biological fixation, active uptake, and retranslocation (Brzostek et al., 2014; J. B. Fisher, Sitch, et al., 2010; Shi et al., 2016; see also Rastetter et al., 2001). Note, as currently implemented the plant C costs calculated by FUN only serve as an additional source of autotrophic respiration, not as an actual belowground C flux to roots or mycorrhizae that interacts with the soil biogeochemistry simulated in CLM5. Collectively, FlexCN, LUNA, and FUN more mechanistically represent plant nitrogen dynamics in CLM5 and afford opportunities to understand, refine, and improve simulated C‐N biogeochemical dynamics. In addition to these modifications to plant nitrogen cycling, the dynamic plant carbon allocation scheme that was used in CLM4 and 4.5 produced biased plant productivity‐biomass relationships, at least in the tropics (Negrón‐Juárez et al., 2015); thus, a fixed allocation scheme, which assigns carbon in constant proportions to leaves, stems, and coarse and fine roots, was applied in CLM5. Modifications to plant allocation have indirect influences on nitrogen dynamics by altering the nitrogen requirements for a given level of net primary productivity (NPP).
2.2. Simulations and Analyses
The simulations presented here were initialized using the baseline (control) simulations presented in Lawrence et al. (2019). Briefly, simulations of CLM4, 4.5, and 5 were spun up to bring the carbon pools to equilibrium by cycling over the first 20 years of the GSWP3v1 climate forcing data set (http://hydro.iis.u-tokyo. ac.jp/GSWP3/). Subsequently, all versions of the model were run through the historical period (1850–2014) at ~1° horizontal spatial resolution using fully transient atmospheric CO2 concentrations, nitrogen deposition rates, and land use change data sets that are consistent with second‐generation land use harmonization and CMIP6 protocols (Lawrence et al., 2016; Lawrence et al., 2019). In these control simulations we focused on differences in the spatial distribution of GPP among different versions of the model (averaged over 2010–2014). We compare results with globally gridded GPP estimates from FLUXNET‐MTE (Beer et al., 2010; Jung et al., 2011), and calculated the bias from CLM simulations and FLUXNET‐MTE averaged over the periods 1995–2008. In general, changes to GPP tend to cascade through relevant ecosystem fluxes, like NPP, and pools, like vegetation carbon, and soil carbon stocks. Thus, we also calculated area weighted global sums of relevant ecosystem fluxes and pools.
For this study, we started two additional runs in 1995 where we initiated a global step increase of (1) nitrogen deposition—5 g N m−2 yr−1 above ambient (evenly distributed over the year through the N deposition stream) or (2) atmosphere CO2 concentration—200 ppm over ambient and ran the model forward until 2014. Although individual experimental manipulation used different amounts of N or CO2 enrichment, global values used in our simulations were chosen for N enrichment in order to remain on the low side of N‐addition experiments (Liu & Greaver, 2010) and the upper limit of projected N deposition rates over the twenty‐first century (Frey et al., 2014; Lamarque et al., 2013). Similarly, values for CO2 enrichment were chosen to approximate experimental manipulations for Free Air CO2 Enrichment (FACE) experiments (Ainsworth & Long, 2005).
To quantify model sensitivities to nitrogen enrichment and elevated CO2, we compared the effect size (treatment/control) averaged for each grid cell over the last five years of the simulation (2010–2014). Across grid cells, we report the mean effect size and the 50% prediction interval (calculated to provide an estimate of spatial variation) in each version of the model and compare simulated results with available observations from meta‐analyses of experimental manipulations (see below). To avoid biasing global results with grid cells having small initial carbon fluxes (and thus potentially very large effect sizes), we only calculated effect sizes where mean annual GPP was greater than 100 g C m−2 yr−1. Where appropriate, we repeated the analysis for each plant functional type (PFT) within grid cells and weighted mean results by the fractional area of each PFT. The broadleaf evergreen shrub PFT represents a small fraction of the vegetated land area in these simulations (<1,000 km2) and was excluded from PFT‐level analyses.
Several meta‐analyses quantify aspects of ecosystem responses to nitrogen enrichment. In this study, we compared simulated NPP responses to nitrogen enrichment to the aboveground net primary productivity response reported by LeBauer and Treseder (2008); simulated effect sizes for NPP and aboveground net primary productivity are nearly identical (Wieder unpublished). We compared changes in simulated vegetation carbon stocks with observed changes in total tree biomass in response to N enrichment reported by Janssens et al. (2010). We also compared changes in litter and soil carbon pools with observations reported by Lu et al. (2011) (see also Liu & Greaver, 2010, who report similar results, but with fewer sites). Finally, heterotrophic respiration rates simulated by CLM in response to nitrogen enrichment were compared with observations from Janssens et al. (2010).
Plant physiological and ecosystem responses to elevated CO2 simulated by CLM were compared with results synthesized in a meta‐analysis by Ainsworth and Long (2005). Some meta‐analyses from experimental manipulations report the natural log of the response ratio plus or minus the 95% confidence interval (e.g., Ainsworth & Long, 2005). For consistency we back transformed these values and report the observed mean effect size and 95% confidence interval. We recognize a temporal mismatch between the length of experimental manipulations (generally less than a decade) and their spatial bias (toward temperate ecosystems) with the multidecade and global‐scale simulations used in this analysis. We also acknowledge the mismatch in scales between leaf photosynthetic assimilation that can be measured in experimental manipulations and the upscaled canopy GPP that is simulated in the model. Despite these scale mismatches, we contend that the general trends in sign and magnitude of effect sizes that observed in experimental manipulations are still useful to compare to model results. Finally, we recognize that other syntheses of experimental manipulations have been published, and we use some of these studies to contextualize our main findings in the discussion of results.
3. Results
3.1. Control Simulations
The three different versions of CLM showed large differences in the spatial distribution and global sums of relevant biogeochemical fluxes and stocks (Table 1). For example, GPP—the gateway through which CO2 enters terrestrial ecosystems—simulated by CLM4 was very high in tropical forests and low across the Arctic, compared to later versions of the model (Figure 1). Reducing biases in GPP, relative to FLUXNET‐MTE observations (Figure 2a), was a major focus of subsequent model development (Bonan et al., 2011; Bonan et al., 2012; Koven et al., 2013; Lawrence et al., 2019). Successive versions of the model subsequently showed an overall reduction in global GPP (Table 1), with a shift in the spatial distribution of GPP from the tropics to higher‐latitude ecosystems (Figures 1b and 1c). This reduced the global GPP bias from +12.1, −4.6, to +1.0 Pg C yr‐1 in versions 4, 4.5, and 5 of the model, respectively (Figure 2). Significant biases in GPP remain in CLM5, notably where modeled GPP is too high across high latitudes (Figure 2c); but on balance, newer versions of the model appear to more realistically capture historical spatial patterns and the total amount of terrestrial productivity.
Table 1.
Global Sums of Ecosystem Fluxes and Stocks and Their Change Relative to the Control Run After Nitrogen and CO2 Enrichment Simulated by Successive Versions of the Community Land Model
| Model | Treatment | GPP (Pg C yr‐1) | NPP (Pg C yr‐1) | Veg C (Pg C) | Veg N (Pg N) | Soil C (Pg C) | Soil N (Pg N) | N fix (Tg N yr‐1) |
|---|---|---|---|---|---|---|---|---|
| Control | 132.9 | 45.6 | 472 | 3.0 | 513 | 50.3 | 109 | |
| 4 | +N | 41.4 | 17.2 | 67 | 0.9 | 44 | 4.1 | 24 |
| +CO2 | 10.1 | 2.9 | 28 | 0.2 | 1 | 0.1 | 3 | |
| Control | 116.4 | 48.2 | 469 | 3.2 | 1027 | 91.0 | 98 | |
| 4.5 | +N | 24.6 | 13.9 | 34 | 0.6 | 35 | 3.1 | 9 |
| +CO2 | 7.8 | 3.0 | 30 | 0.2 | 8 | 0.3 | 3 | |
| Control | 122.2 | 50.5 | 500 | 4.7 | 1100 | 97.6 | 99 | |
| 5 | +N | 12.1 | 11.3 | 22 | 0.5 | 35 | 3.0 | −35 |
| +CO2 | 22.6 | 9.2 | 65 | 0.4 | 14 | 1.0 | 53 |
All sums for gross primary productivity (GPP), net primary productivity (NPP), vegetation carbon and nitrogen stocks (Veg C and N), soil organic matter stocks (Soil C and N, which includes soil and litter stocks, 0–100 cm for CLM4.5 and CLM5), and nitrogen fixation (N fix, the sum of free‐living and symbiotic N fixation in CLM5) are averaged over the last five years of simulations (2010–2014) that were forced with GSWP3 climate reanalysis.
Figure 1.
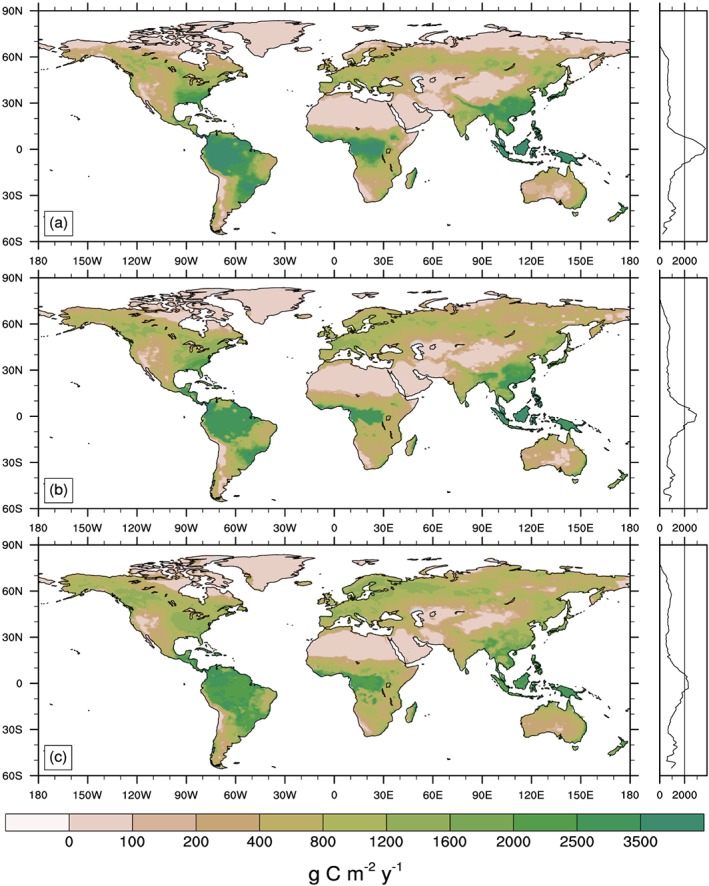
Spatial distribution and zonal mean plots of mean annual gross primary productivity (GPP) simulated in successive versions of the Community Land Model (a) CLM4, (b) CLM4.5, and (c) CLM5 under common atmospheric forcings from GSWP3 (control simulation). All units are g C m−2 yr−1 and averaged over the last five years of the control simulation (2010–2014).
Figure 2.
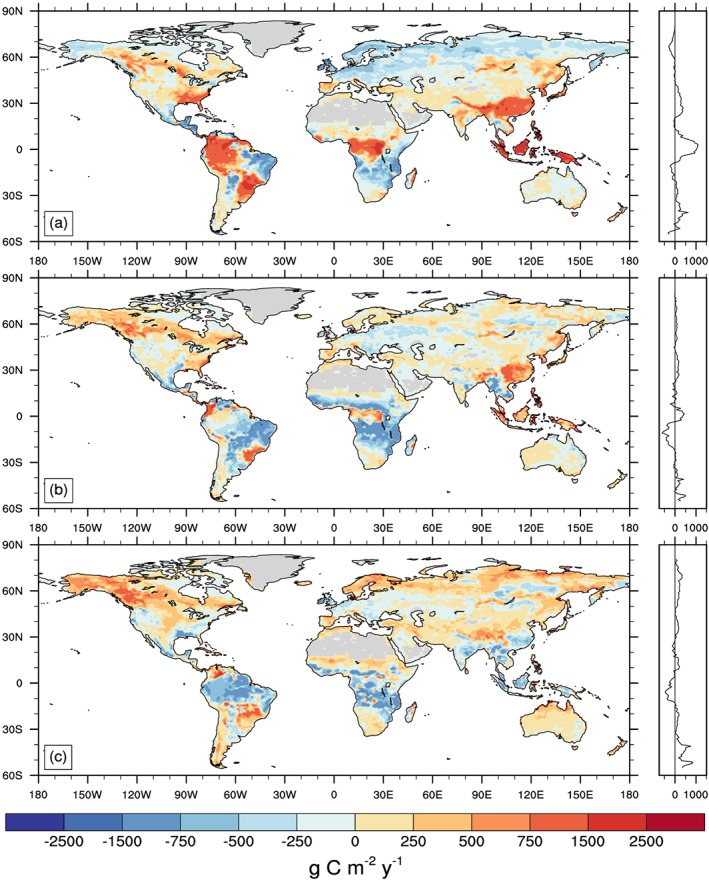
Spatial distribution and zonal mean plots showing biases in mean annual GPP simulated by successive versions of the Community Land Model (a) CLM4, (b) CLM4.5, and (c) CLM5 compared to observationally derived estimates from FLUXNET‐MTE. All units are g C m−2 yr−1 and averaged over the period 1995–2008.
Despite overall reductions in GPP between CLM4 and CLM5, net primary productivity (NPP), or the amount of carbon retained in ecosystems to build plant biomass, slightly increased with successive iterations of the model (Table 1). As with GPP, NPP increased at high latitudes and declined in the tropics in CLM4.5 and 5, relative to CLM4. Globally averaged ecosystem carbon use efficiency (calculated here as the ratio of NPP to GPP) increased from 0.34 in CLM4 to 0.41 in versions 4.5 and 5 of the model (Figure S1). For comparison, observations suggest that efficiencies range from 0.4 and 0.6 across multiple ecosystems, with higher values in managed and more fertile ecosystems (Campioli et al., 2015; DeLucia et al., 2007; Vicca et al., 2012). In tropical forests CLM5 estimates carbon use efficiency values between 0.3 and 0.45 (Figure S1), which also compare favorably with observational estimates between 0.32 and 0.49 by Malhi et al. (2009). Thus, CLM5 seems to simulate more realistic carbon use efficiency values across most ecosystems than previous versions of the model, perhaps with the exception of tropical savannas—although data from these ecosystems are sparse.
The allocation of NPP to vegetation carbon pools with different turnover times, and subsequent transfers to litter and soil C pools, resulted in variation in vegetation and soil C stocks. Global vegetation C stocks varied by 31 Pg C among models, roughly 6% (Table 1), but these totals mask important geographic and ecological variation among models. As with plant productivity, CLM4.5 and 5 both show a poleward shift in vegetation C stocks, relative to estimates in CLM4. Vegetation C stocks averaged over evergreen tropical forests decreased in successive versions of the model (from 25, 24, and 20 kg C m−2 in CLM4, 4.5, and 5, respectively), and increased in boreal needleleaf evergreen forests (from 3.9, 6.5, and 16 kg C m−2, respectively). Similar patterns are evident in soil C stocks, where CLM4 maintained notably low belowground C stocks (Table 1). Shifting to the CENTURY‐like representation of vertically resolved soil biogeochemistry in CLM4.5 (Koven et al., 2013), combined with higher productivity in high‐latitude ecosystems, more than doubled belowground C stocks relative to CLM4 (data for CLM4.5 and 5 are for top meter of soil). As noted earlier, the terrestrial C stocks and fluxes simulated by CLM5 are improved, relative to previous versions of the model and according to the ILAMB benchmarking package (Lawrence et al., 2019).
3.2. Nitrogen Fertilization
Nitrogen fertilization increased GPP in all models, but this effect was strongest in CLM4 where global GPP increased 31% relative to the control simulation, compared to a 21% increase in CLM4.5 and to a 10% increase with CLM5 (mean of 2010–2014 values; Table 1). Across biomes, the nitrogen effect size for GPP was also largest for CLM4 and smallest for CLM5 (Figure 3). Variation in the nitrogen effect size among PFTs with CLM5, however, was greater than the average differences across versions of CLM (Table S1). The dampening in N effect size with successive model versions also generally held for multiple carbon pools and fluxes downstream of gross primary productivity (Figure 4). At the grid scale level, CLM4 showed a 60% increase in NPP in response to N enrichment, almost double the 29% increase in aboveground NPP reported in observations (Figure 4a; LeBauer & Treseder, 2008). CLM4.5 and 5 better approximated the mean observed response, although considerable variation among individual PFTs remained (Table S1). By contrast, observed changes in vegetation C stocks (Janssens et al., 2010) were better approximated in CLM4, while CLM5 underestimates changes in vegetation C storage under N fertilization (Figure 4). We note that observed changes in vegetation C stocks shown in Figure 4a, however, mostly come from very young forested ecosystems (Janssens et al., 2010). Yet in the CLM5 simulations, woody PFTs generally showed more muted effect sizes for both NPP and vegetation C stocks compared to observations (Table S1), suggesting that additional modifications are necessary to represent N limitation of forest ecosystems and capture appropriate changes in productivity and allocation that occur in response to N enrichment in woody PFTs. Globally, CLM4.5 and 5 both appear to better approximate observed changes in litter and soil C stocks in response to N fertilization (Figure 4b). When combined with NPP effects, these findings suggest that later versions of the model may better simulate global C sensitivity to N enrichment.
Figure 3.
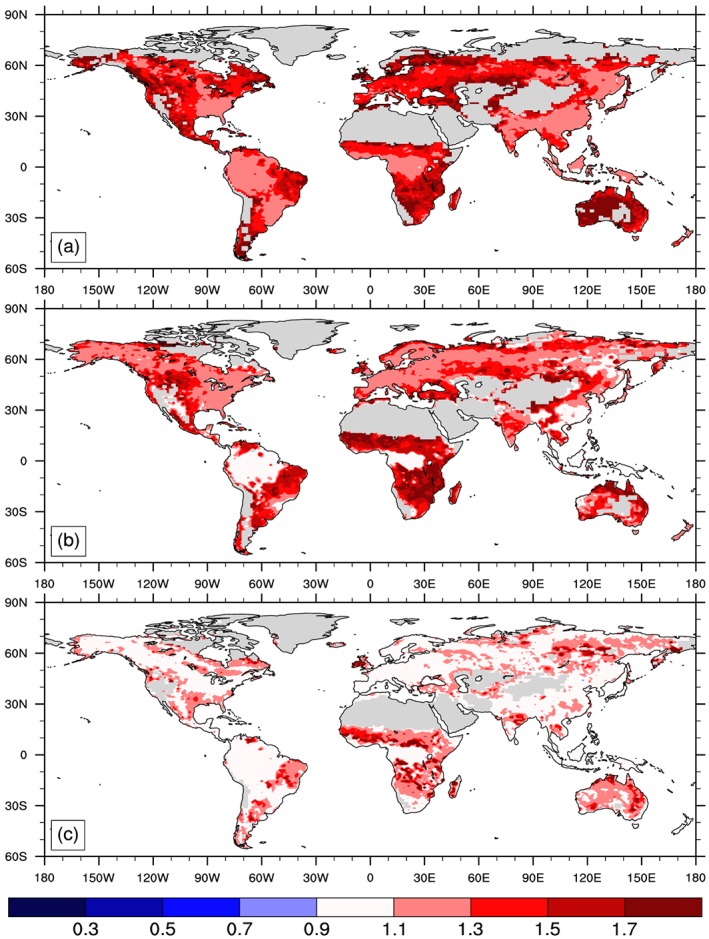
Spatial distribution and zonal mean plots showing the effect size of nitrogen enrichment on GPP simulated by successive versions of the Community Land Model (a) CLM4, (b) CLM4.5, and (c) CLM5. Effect sizes were calculated for each grid cell as the mean annual GPP of treatment divided control simulations over the last five years of the experiment (2010–2014) using cells with mean GPP > 100 g C m−2 yr−1.
Figure 4.
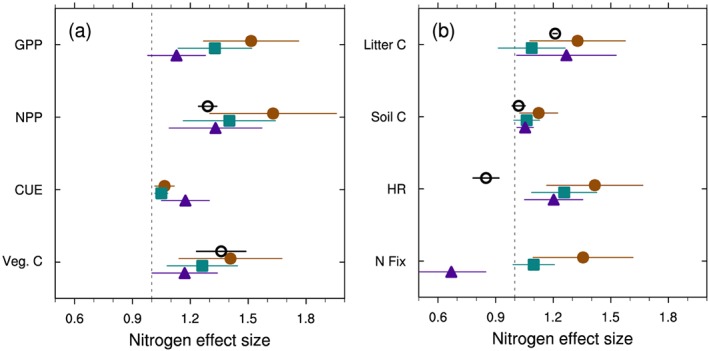
Observed (open circles) and simulated (solid shapes) effect size of nitrogen enrichment on select ecosystem fluxes and pools. Observations, where available, show the mean (±95% confidence interval) from various meta‐analyses (see section 2). Modeled responses show the global mean of grid cell effect sizes (±50% prediction interval) for version 4, 4.5, and 5 CLM (brown circles, turquoise squares, and purple triangles, respectively), calculated using cells with mean GPP > 100 g C m−2 yr−1. The vertical dashed line represents no effect. Variables listed include (a) gross and net primary productivity (GPP and NPP), carbon use efficiency (CUE), vegetation carbon pools (Veg C) and (b) litter and soil C pools, heterotrophic respiration (HR), and N fixation (N fix) rates.
We also present simulated and observed changes in heterotrophic respiration, as well as simulated rates of nitrogen fixation in response to N enrichment (Figure 4b). In the models, increases in productivity result in increased litterfall rates that lead to accumulation of carbon in litter and, ultimately, soil carbon pools (Figure 4). Thus, N fertilization necessarily increases heterotrophic respiration rates in models where decomposition CO2 fluxes are directly proportional to upstream C pool sizes (Figure 4b). Observations from experimental manipulations, however, suggest that under some circumstances N enrichment drives shifts in soil microbial community composition and often suppresses decomposition rates and heterotrophic respiration rates (Frey et al., 2014; Janssens et al., 2010; Knorr et al., 2005; Leff et al., 2015; Liu & Greaver, 2010; but see also Lu et al., 2011).
Finally, modeled responses of nitrogen fixation highlight uncertainties about how to represent ecosystem responses to N enrichment. Following N enrichment, N fixation rates increased dramatically with CLM4, but decreased in CLM5 (Figure 4b). This change in sign reflects differences in how N fixation was simulated. In CLM4 and 4.5, nitrogen fixation was estimated using an empirical relationship between N fixation rate and NPP (Cleveland et al., 1999; Wieder, Cleveland, Lawrence, & Bonan, 2015). As such, fertilization‐driven increases in modeled NPP subsequently drove increases in estimated N fixation rates. In CLM5, declines in N fixation with fertilization reflect decreases in plant N uptake costs calculated by FUN relative to the energetic cost for N fixation. In both natural and agricultural systems nitrogen enrichment reduces rates of biological N fixation (Batterman et al., 2013; McAuliffe et al., 1958), suggesting that the decreases in N fixation rates simulated by FUN in CLM5 are likely more appropriate. These contrasting model responses more broadly highlight uncertainties among models in how to appropriately represent ecological processes like fixation in global‐scale models (Meyerholt et al., 2016; Wieder, Cleveland, Lawrence, & Bonan, 2015). Overall, structural modifications to CLM5 muted the global carbon cycle response to nitrogen fertilization.
3.3. Elevated CO2
Increasing atmospheric CO2 concentrations by 200 ppm increased productivity in all three versions of the model, increasing GPP just over 7% in CLM4 and 4.5, but eliciting an 18% increase in GPP in CLM5 relative to the control simulation (Table 1). The low CO2 effects in CLM4 and 4.5 were equally distributed across the globe, although deciduous tropical forests and C3 nonarctic grasses showed the largest response in productivity and downstream C stocks (Figures 5a and 5b and Table S2). By contrast, nearly all PFTs showed significant increases in productivity and downstream ecosystem C stocks in CLM5, with the exception of C4 plants (Figure 5c and Table S2). Indeed, regions dominated by C4 grasses and C4 crops showed negligible effects to elevated CO2 in all versions of the model (Table S2), although this pattern is most evident in the CLM5 simulations (Figure 5c). This result aligns well with shorter‐term studies in grasslands and theoretical expectations of the response of the C4 photosynthetic pathway to elevated concentrations of CO2 (Ainsworth & Long, 2005; Ehleringer & Bjorkman, 1977); however, recent work by Reich et al. (2018) calls into question this established paradigm.
Figure 5.
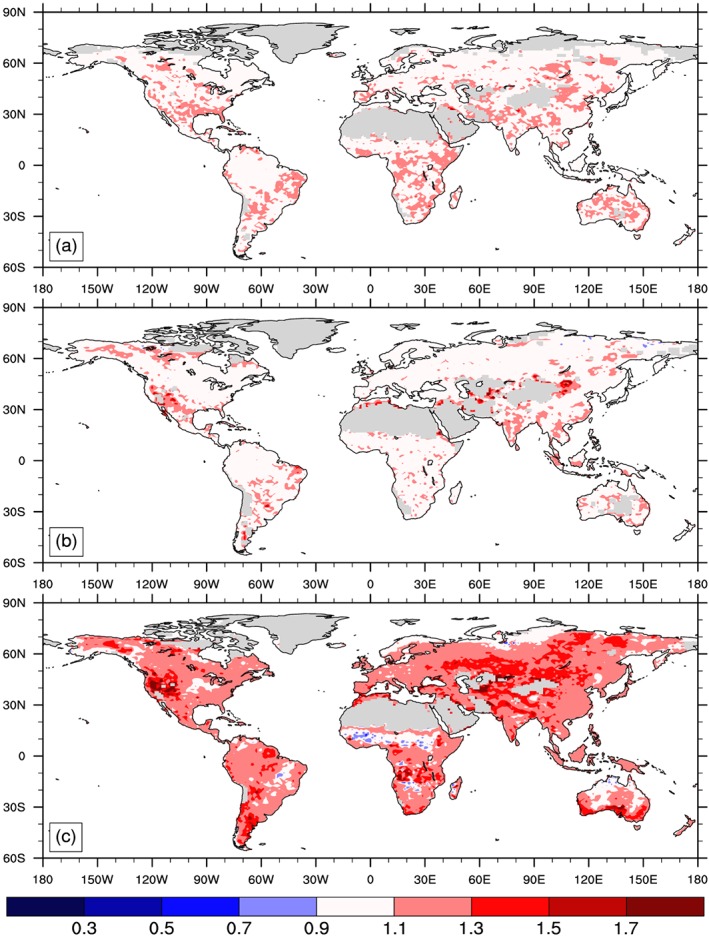
Spatial distribution and zonal mean plots showing the effect size of CO2 enrichment on GPP simulated by successive versions of the Community Land Model (a) CLM4, (b) CLM4.5, and (c) CLM5. Effect sizes were calculated for each grid cell as the mean annual GPP of treatment divided control simulations over the last five years of the experiment (2010–2014) using cells with mean GPP > 100 g C m−2 yr−1.
Canopy‐scale measurement of many relevant fluxes are not commonly made in FACE experiments, since the scale of CO2 enrichment plots is smaller than the typical footprint of an eddy covariance tower; therefore, we compared the response ratio of leaf measurements from FACE experiments with their closest canopy‐scale model analogue in Figure 6. For example, Ainsworth and Long (2005) report that diurnal photosynthetic assimilation (A′) increased 28% under elevated CO2, which compares well to the mean of daily maximum GPP rates simulated by CLM5, but not with previous versions of the model (Figure 6a). Similarly, observed dry matter production increased in the FACE experiments by 17% under elevated CO2 (with the largest increases in trees), results which again compare favorably to changes in NPP simulated by CLM5. Changes in NPP under elevated CO2 resulted in greater accumulations of vegetation C stocks, which increased 28, 30, and 65 Pg C in successive versions of the model (Table 1). However, the CO2 induced increases in productivity and vegetation C storage for CLM5 were potentially amplified by increases in leaf area index (LAI) that were greater than observed responses (Figure 6a).
Figure 6.
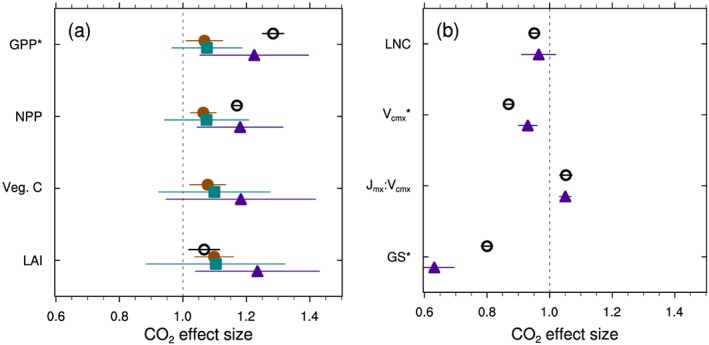
Observed (open circles) and simulated (solid shapes) effect size of CO2 enrichment on various ecosystem carbon and leaf traits. Observations, where available, show the mean (±95% confidence interval; Ainsworth & Long, 2005). Modeled responses show the global mean of grid cell effect sizes (±50% prediction interval) for versions 4, 4.5, and 5 of CLM (brown circles, turquoise squares, and purple triangles, respectively), calculated using cells with mean GPP > 100 g C m−2 yr−1. The vertical dashed line represents no effect. Variables listed include (a) gross and net primary productivity (GPP and NPP), vegetation carbon pools (Veg C), leaf area index (LAI) and (b) leaf N content (LNC), maximum carboxylation rates (Vcmax), the ratio of maximum electron transport rates (Jmax) to Vcmax, and stomatal conductance (GS). Asterisk denotes values for the mean of daily maximum rates.
Both CLM4 and 4.5 applied fixed foliar C:N ratios, but CLM5 includes the capacity for flexible tissue stoichiometry (Ghimire et al., 2016), a feature which predicts declines in leaf nitrogen content (LNC) under elevated CO2 of the appropriate sign and magnitude to observed values (Figure 6b). LNC was subsequently used to calculate the maximum rate of carboxylation (Vcmax) and the maximum rate of electron transport (Jmax), and subsequent rates of photosynthesis, using the LUNA module in CLM5 (Ali et al., 2016). Although simulated Vcmax declined under elevated CO2, the magnitude of this decrease is less than observations suggest, but changes in the ratio of Jmax to Vcmax were of an appropriate magnitude (Figure 6b). Finally, whereas changes in Vcmax simulated in CLM5 are not sensitive enough to elevated CO2, the changes in stomatal conductance (gs) show a strong sensitivity to CO2 enrichment (Figure 6b). This is likely caused by the Jmax:Vcmax ratio simulated by the LUNA model (Franks et al., 2017). Observations support reduced stomatal and canopy conductance under elevated CO2, but these responses are highly variable and likely less pronounced than the changes simulated by CLM5 (Ainsworth & Long, 2005; Norby & Zak, 2011; Walker, Beckerman, et al., 2014; Walker, Hanson, et al., 2014). Our global simulations indicate that accounting for changes in leaf physiology and stoichiometry in CLM5 increased the model's response to CO2 fertilization and better agreement with observations from experimental manipulations.
3.4. Trade‐Offs Between N and CO2 Limitation
Figure 7 illustrates broad differences in N and CO2 sensitivities among these three versions of the model. We focused on the effect size of NPP to experimental manipulations, as it represents one of the better quantified ecosystem responses and it also serves as a proximal control over the strength of the land C sink simulated in models. Our results illustrate the large sensitivity of terrestrial ecosystems in CLM4 to nitrogen enrichment and their relatively low sensitivity to elevated CO2 compared to CLM4.5 and CLM5 (Figure 7a). Adjustments to photosynthesis and soil biogeochemistry in CLM4.5 decreased the N sensitivity so it is closer to measured ranges, but this intermediate model still shows a muted response to elevated CO2. At a global scale, CLM5 represents responses to N and CO2 enrichment that are more in line with observations, relative to previous versions of the model.
Figure 7.
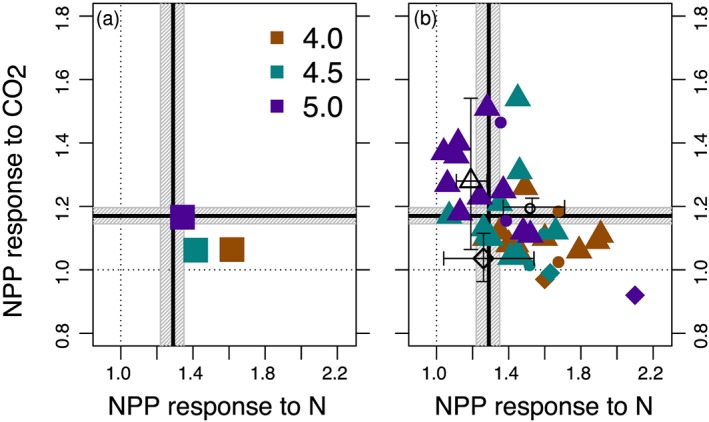
Simulated effect sizes of nitrogen versus CO2 enrichment on rates of net primary productivity (NPP) that was calculated (a) globally or (b) for each plant functional type in CLM4, 4.5, and 5 (brown, turquoise, and purple symbols, respectively). In both panels, observational constraints for the nitrogen response (aboveground NPP from LeBauer and Treseder (2008)) and CO2 response (dry matter production from Ainsworth and Long (2005)) are shown with the vertical and horizontal lines, respectively (mean ± 95% confidence interval). The right panel shows the observed (open symbols) and simulated (filled symbols) effect sizes of individual plant functional types for woody vegetation, C3 grasses, and C4 grasses (triangles, circles, and diamonds, respectively).
Breaking down these global mean effects into the mean responses of individual plant functional types illustrates the wide variation within and among models (Figure 7b). We highlight several patterns that seem more broadly relevant to the evaluation of terrestrial biogeochemical models. First, observations from experimental manipulations indicate that nutrient availability clearly modulates ecosystem responses to elevated CO2 (Norby et al., 2010; Reich et al., 2006; Reich & Hobbie, 2013). Similarly, CLM results suggest that in the model there appears to be a trade‐off between simulated N and CO2 responses. That is, a model or PFT that is strongly limited by N availability cannot have a very strong response to elevated CO2. Conversely, a model or PFT that shows a strong response to elevated CO2 experiences relatively low levels of N limitation. Second, some elements of variation among growth forms appear to be broadly captured by CLM. For example, lower CO2 fertilization effects of C4 plants are consistent with observations from FACE experiments and captured in the model (Figure 7b and Table S2; Ainsworth & Long, 2005), but contradicted by observations from Reich et al. (2018). The degree of N limitation simulated in C4 grasses, however, appears too strong—especially in CLM5, whereas observations suggest that annual C3 grasses show the largest response to N enrichment (LeBauer & Treseder, 2008). Woody vegetation shows a greater sensitivity to elevated CO2, albeit highly variable, and potentially a lower sensitivity to N (triangles in Figure 7b). Finally, all versions of the model project sustained increases in global‐scale productivity through the 20‐year simulation, a finding not supported by observations from some FACE experiments (Norby et al., 2010; Norby & Zak, 2011). Contradictions between simulated and observed results highlight opportunities to assess, refine, and revise the structural assumptions and particular parameterizations of land models.
3.5. Biogeophysical Feedback
Beyond the direct biogeochemical effects of N enrichment and elevated CO2, our simulations begin to shed light on potential future biogeophysical feedbacks to water and energy exchange between the land and atmosphere. A motivation for conducting FACE experiments was to assess if reductions in leaf stomatal conductance ultimately influence canopy conductance, evapotranspiration, and runoff—or if concurrent changes in LAI under elevated CO2 would attenuate whole plant and ecosystem changes in water use (Norby & Zak, 2011; Wullschleger et al., 2002). In our simulations N and CO2 enrichment both increased plant productivity and LAI in all versions of the model, but decreased stomatal conductance was only observed under elevated CO2 with CLM5.
The biophysical feedback on latent heat fluxes show similar magnitude but the opposite sign under +N compared to +CO2 enrichment. With N addition, average latent fluxes increased 3.6, 2.2, and 2.9% in CLM4, 4.5, and 5, respectively, and decreased global runoff by a similar magnitude. These global similarities, however, mask distinct regional differences among models. For example, CLM4 showed larger changes in latent heat fluxes with N fertilization than other versions of the model, especially in tropical forests (Figure S2). By contrast, nitrogen effects on latent heat fluxes in CLM4.5 and 5 were most prominent in tropical savannas. Under elevated CO2, globally averaged latent heat fluxes decreased in all models, by 3.9, 2.8, and 3.5% in CLM4, 4.5, and 5, respectively, which similarly increased global runoff. Again, decreases in latent fluxes under elevated CO2 were largest in forested regions with CLM4 and CLM4.5, albeit to a lesser extent, whereas CLM5 shows relatively larger effect in the tropics and in the high latitudes (Figure S3).
4. Discussion
Structural and parametric changes in successive versions of the Community Land Model resulted in shifts of terrestrial productivity and carbon storage from tropical forests toward temperate and high‐latitude regions (Figure 1). This reduced GPP bias, relative to estimates from FLUXNET‐MTE (Figure 2; Beer et al., 2010; Jung et al., 2011) and resulted in an overall improvement in model skill score across multiple metrics used to benchmark land models (Lawrence et al., 2019). A number of aspects in the model changed during CLM5 development, as detailed by a companion paper that is part of the CESM2 special issue (Lawrence et al., 2019). We have not isolated individual contributions from particular development activities, but other work begins to quantify sensitivities in parameter perturbation experiments (Fisher et al., 2019), explicit representation of crop management and phenology, and atmospheric forcing uncertainty (Bonan et al., 2019). In all of these efforts the ILAMB model benchmarking package provided critical insights into areas of model improvements and degradation (Collier et al., 2018). Moving forward, we contend that simultaneously assessing the representation of multiple ecological processes in land models will help reduce uncertainty and evaluate the likelihood of future projections (Medlyn et al., 2015). By comparing observed and simulated ecosystem responses to experimental manipulation of ecosystem N and CO2 availability we evaluated three versions of the Community Land Model. Over the course of model development, CLM transitioned from a model that showed strong sensitivity to nitrogen enrichment and little response to CO2 (in CLM4) to a model with stronger sensitivity to elevated CO2 (in CLM5; Figures 3 and 5). On balance, our results suggest that the globally integrated ecosystem responses to nitrogen and CO2 enrichment are better approximated with CLM5 than with previous versions of CLM (Figures 4 and 6). These findings are consistent with recent observations suggesting relatively weak N limitations on CO2 fertilization of plant productivity over the twentieth century (Campbell et al., 2017). Our results also highlight areas where further work, especially related to changes in plant allocation, is needed to align model assumptions with real‐world ecosystem responses to environmental change.
More broadly, our findings suggest a trade‐off between nitrogen and carbon limitation in land models. This trade‐off can be seen both among different versions of the CLM and between individual plant functional types represented in the model (Figure 7). Globally, averaged responses to nitrogen and CO2 enrichment suggest that successive versions of the model are better at approximating plant productivity responses to these experimental manipulations (Figure 7a). Individual PFT responses suggest that particular assumptions and parameterizations across versions of CLM tend to favor either N or C limitation of plant productivity (Figure 7b). Evaluating whether similar trade‐offs occur in natural ecosystems will help improve the representation of coupled biogeochemical cycles in land models. For example, recent studies suggest that plant nutrient economies largely determine ecosystem responses to elevated CO2, but that long‐term shifts in belowground function and nutrient cycling may be slow to manifest (Reich et al., 2018; Terrer et al., 2018). Our findings also suggest that greater attention should be given to model assumptions and ecosystem traits related to plant N acquisition strategies and the nature of plant‐soil feedback (Fisher et al., 2019; Rastetter et al., 1997). More widely testing these findings may be helpful in disentangling uncertainties in upcoming model intercomparison projects (e.g., C4MIP or LUMIP; Jones et al., 2016; Lawrence et al., 2016), with the aim of ultimately improving global‐scale terrestrial biogeochemical projections. Below we discuss the model assumptions that are responsible for simulated sensitivities to nitrogen and CO2 enrichment and highlight avenues for future research related to plant allocation and plant‐soil interactions.
4.1. Nitrogen Fertilization
Uncertainties in how to represent nitrogen effects on photosynthetic carbon uptake lead to divergent model responses to nitrogen enrichment (Meyerholt & Zaehle, 2015; Thomas, Bonan, & Goodale, 2013; Table 1 and Figure 3). The separate calculation of potential and nitrogen‐downregulated GPP in CLM4 and 4.5 generates the large GPP responses to N fertilization (Figures 3a and 3b; Thomas, Zaehle, et al., 2013). It is possible to reduce the N fertilization response within this approach by decreasing parameter values for the maximum photosynthetic rate. In particular, in CLM4.5, Bonan et al. (2011, 2012) decreased the potential photosynthetic rates of evergreen tropical trees to more realistic levels which accounts for the lower sensitivity of tropical forests to N enrichment, relative to CLM4 simulations (Figure 3). The FlexCN module in CLM5 removes the assumption of N unlimited potential GPP, instead calculating plant photosynthetic capacity as a function of time‐varying foliar N content (Fisher et al., 2019; Ghimire et al., 2016). Thus, changes in GPP with N fertilization are more dampened in CLM5 (Figure 3c), and attributable to measurable changes in ecosystem properties like leaf nitrogen content and LAI.
Nitrogen enrichment increased LNC in CLM5 by only 3% globally whereas LAI increased 25%. Observations, however, indicate that nitrogen enrichment typically drives relatively larger changes in LNC and more modest changes in leaf biomass (Xia & Wan, 2008). Additional experimental manipulations and meta‐analyses may be warranted here, as current work tends to bias sampling in late successional or closed canopy ecosystems where changes in LNC may be more apparent. Our results, however, indicate that additional model developments may be necessary to capture the correct sensitivities of both LNC and LAI to nitrogen availability. Such concerns are not trivial, as they involve reconciling assumptions and interactions between plant allocation, flexible stoichiometry represented by FlexCN, and C costs of N uptake that are calculated by FUN. Both FlexCN and FUN are new, independently developed contributions for CLM5 that address particular gaps in the model, but reconciling how these modules interact with each other as well as the plant allocation scheme and competition for soil N remains a challenge. Observations from experimental manipulations consistently show shifting patterns of plant C and N allocation (Janssens et al., 2010; Liu & Greaver, 2010) and provide a robust set of targets that models should try to replicate.
Our results highlight model assumptions that generate distinct ecosystem responses to nitrogen enrichment among versions of CLM. For example, CLM5 shows a stronger NPP response to N enrichment, relative to GPP, which increases plant carbon use efficiency (Figure 4a). Increasing carbon use efficiency in CLM5 results because the FUN module calculates lower carbon costs of nitrogen uptake following N enrichment (Fisher et al., 2019; Shi et al., 2016). This allows plants to build more biomass per unit of GPP. Further, the greater ecosystem N availability also decreases rates of symbiotic nitrogen fixation since active uptake of soil N is cheaper than N fixation, and thus preferred by plants (Table 1 and Figure 4). Indeed, observations do suggest that fertile ecosystems build biomass more efficiently, mainly through shifting allocation of C to aboveground biomass (Campioli et al., 2015; Vicca et al., 2012), and that nutrient enrichment reduces biological nitrogen fixation rates (Batterman et al., 2013; McAuliffe et al., 1958). Thus, assumptions in the FUN module that adjust plant C expenditure on nitrogen acquisition and sources of nitrogen uptake are supported by ecosystem observations. Other assumptions, however, related to heterotrophic respiration fluxes and shifts in plant C allocation (see section 4.3) are not well supported by data.
Heterotrophic respiration fluxes simulated in models that apply first‐order soil biogeochemical models are directly proportional to litter inputs and soil carbon stocks (see Gerber et al., 2010). Thus, increasing productivity in response to nitrogen enrichment necessarily increases heterotrophic respiration fluxes (Figure 4b). Meta‐analyses, however, show weakly positive (Lu et al., 2011) to strongly negative responses of heterotrophic respiration and litter decomposition to N enrichment (Janssens et al., 2010; Knorr et al., 2005; Liu & Greaver, 2010). Indeed, shifting plant allocation away from belowground investment for nutrient acquisition toward production of aboveground biomass may directly reduce root and heterotrophic respiration rates, especially in rhizosphere soils and in ecosystems where plants associate with ectomycorrhizal fungi (Phillips et al., 2013; Phillips & Fahey, 2007; Treseder, 2004). None of the three versions of CLM appropriately capture observed shifts in plant C allocation and belowground ecosystem function in response to nitrogen enrichment, in part because they do not yet consider plant allocation of carbon to microbial priming or mycorrhizae. These discrepancies highlight assumptions in the model that deserve further revisions, especially regarding how ecosystems respond to changes in resource availability.
4.2. Elevated CO2
The low sensitivity of CLM4 to elevated CO2 concentrations has been noted elsewhere (Koven et al., 2013; Zaehle et al., 2014), leading to biases in the atmospheric fraction of anthropogenic CO2 emissions in coupled simulations (Hoffman et al., 2014). Similar patterns are clearly evident in the global results presented here (Figure 5a). Given modifications to CLM4.5 that resulted in improvements in the terrestrial C balance over the twentieth century (Koven et al., 2013), we expected this intermediate version of the model to have a stronger sensitivity to elevated CO2. Our results, however, contradict this expectation, with effect sizes for GPP to elevated CO2 in CLM4.5 that largely mirror those from CLM4 (Figure 5b and Table 1). In contrast, CLM5 simulates larger increases in GPP with CO2 enrichment (Figure 5c), and the magnitude of change for both GPP and NPP simulated by CLM5 seem to better match observations (Figure 6a). We note that short‐term increases in leaf photosynthesis in response to CO2 enrichment have been reported elsewhere, but that concurrent changes in aboveground NPP are not universally observed—especially over longer time scales (Ellsworth et al., 2017; Norby et al., 2005; Norby et al., 2010). Thus, both CLM4.5 and CLM5 are able to match globally integrated estimates of the land carbon sink over the historical period, but by different means (Bonan et al., 2019; Koven et al., 2013; Lawrence et al., 2019).
Developments in CLM5 provide insights into the assumptions responsible for model responses to elevated CO2. For example, CLM5 applies a prognostic calculation of foliar nitrogen content and photosynthetic capacity, which were fixed parameters for each PFT in CLM4 and 4.5 (Ali et al., 2016; Fisher et al., 2019; Ghimire et al., 2016). Under elevated CO2, foliar nitrogen concentrations decrease by an appropriate magnitude, compared to observations from FACE experiments (Figure 6b; Ainsworth & Long, 2005). As expected, Vcmax calculated by the LUNA module and implemented in CLM5 decreased, but it still remained too high compared to observations from FACE experiments (Figure 6b; Ainsworth & Long, 2005). Indeed, the relative importance of electron transport, Rubisco, and triose phosphate utilization limited photosynthesis under elevated CO2 from observations remains uncertain (Franks et al., 2017; Lombardozzi et al., 2018; Medlyn et al., 2015). Thus, future efforts should consider the assumptions and parameterizations related to photosynthetic limitation and stomatal conductance in order to better match observations from FACE experiments. By introducing flexible leaf stoichiometry and prognostic photosynthetic capacity CLM5 more faithfully represents leaf physiology, but these advancements also introduce new uncertainties—especially related to leaf photosynthetic and stomatal responses to elevated CO2 and their canopy scaling.
Observed changes in vegetation and ecosystem C stocks under elevated CO2 remain uncertain, with comparisons among sites complicated by changes in plant C allocation, nutrient availability, and plant‐soil interactions (Norby & Zak, 2011; Reich et al., 2006; Reich et al., 2018; Reich & Hobbie, 2013; Terrer et al., 2018). Increased productivity and vegetation C storage for CLM5, however, were in part driven by increases in LAI that exceeded observed responses (Figure 6a; Ainsworth & Long, 2005). Some of the largest changes occurred in tropical forests (data not shown); and although free air CO2 enrichment studies in the Amazon are just beginning (Norby et al., 2015), meta‐analyses from temperate sites suggest that the response of LAI to elevated CO2 decreases with canopy closure (Norby & Zak, 2011). Instead, observations show that leaf mass per area (LMA) typically increases under elevated CO2, resulting in increased canopy biomass but not LAI (Ainsworth & Long, 2005; Kovenock & Swann, 2018; Medlyn et al., 2015). By contrast, models, including all three versions of CLM presented here, hold LMA at the top of the canopy constant while at the same time they assume that there is a negative LMA profile from the top to the base of the canopy (Thornton et al., 2007; see also Kovenock & Swann, 2018). This means that in the model, the additional increment of leaf biomass under elevated CO2 causes the canopy‐mean LMA to actually decrease slightly. It also means that the LAI increases slightly more than the leaf biomass. Thus, with high photosynthetic capacity and greater LAI, the increases in plant productivity and biomass simulated by CLM5 under elevated CO2 may be overestimated, especially over multidecadal time scales.
4.3. Allocation and Nutrient Uptake
Here we focus on the representation of plant allocation and the temporal dynamics of nutrient limitation, as these topics are relevant to both N and CO2 enrichment simulations presented here. The allocation of carbon into different vegetation components (leaves, wood, and fine roots) remains a significant source of uncertainty in land models (Litton et al., 2007; Malhi et al., 2011; Negrón‐Juárez et al., 2015). Meta‐analyses of experimental manipulations suggest that allocation changes, stoichiometric change, and acclimation are important aspects of terrestrial ecosystem responses to environmental change (Liu & Greaver, 2010; Luo et al., 2006; Reich et al., 2006). Our simulations, however, indicate that CLM has limited capacity to capture these responses—highlighting areas to be addressed in future model developments.
Observations demonstrate that plants change their allocation strategies in response to resource availability. For example, nitrogen enrichment tends to favor carbon allocation into woody biomass (at least in forests). It also causes a shift toward aboveground productivity, at the expense of belowground carbon investment (Aerts & Chapin, 2000; Janssens et al., 2010; Liu & Greaver, 2010). By contrast, CO2 enrichment tends to increase belowground carbon allocation as plants increase investment in nutrient, or nutrient and water, acquisition (Drake et al., 2011; Iversen et al., 2008), and occasionally an increase in allocation to woody biomass (De Kauwe et al., 2014). Indeed, understanding the nuances of changing belowground allocation in response to various environmental change scenarios is an active field of research (Giardina et al., 2005; Terrer et al., 2018; Treseder et al., 2018), and as such capturing these dynamics in models remains an outstanding challenge.
Both CLM4 and 4.5 applied a dynamic allocation scheme that increased allocation to wood with increases in NPP. This approach qualitatively captured the appropriate allocation response under N enrichment, but not elevated CO2 (Figures 4 and 6). This assumption also results in large increases in woody biomass, especially in tropical forests with CLM4 (Bonan & Levis, 2010), and causes an unrealistic productivity‐biomass relationship across tropical forests (Negrón‐Juárez et al., 2015). To rectify these shortcomings, CLM5 uses a fixed allocation scheme where the same fractions of carbon are assigned to leaves, stems, and coarse and fine roots irrespective of NPP (Lawrence et al., 2019). Accordingly, CLM5 cannot capture the changes in belowground allocation that have been observed with N or CO2 enrichment. Further, relative allocation to leaves is constant in CLM5, and so leaf allocation is not responsive to the economics of leaves in the lower layers of the canopy, which would in reality experience increasing light limitation. Reduction of resource allocation for leaves in negative carbon balance is a feature of the CLM (FATES) model (R. A. Fisher, McDowell, et al., 2010; Fisher et al., 2015). This allows LAI predictions to be responsive to leaf traits and their implications for the plant carbon economy, and will be incorporated into future releases of CLM.
Mounting evidence suggests that flexible allocation of carbon and nitrogen are necessary to capture observed ecosystem responses to both N and CO2 enrichment (De Kauwe et al., 2014; Meyerholt & Zaehle, 2015). The introduction of FlexCN in CLM5 provides appropriate changes in leaf nitrogen content with nitrogen fertilization (Xia & Wan, 2008) and CO2 enrichment (Ainsworth & Long, 2005; Figure 6b). Over the course of model development, however, only minor adjustments have been made to assumptions regarding plant N uptake and the nature of plant‐microbial N competition. For example, Koven et al. (2013) modified assumptions to decrease denitrification N losses and increase ecosystem nitrogen retention in CLM4.5 and 5 (see also Fan et al., 2015). Several studies, however, document unrealistically rapid plant N uptake in the CLM (Cheng et al., 2018; Thomas, Bonan, & Goodale, 2013). This could be addressed with alternative model structures that better resolve competitive interactions between plants and microbes (Zhu et al., 2017). Additionally, changes to the cost functions in the FUN module (Brzostek et al., 2014; Shi et al., 2016) in CLM5 could modify plant affinity for N and its change over time. More broadly, these findings highlight opportunities to better incorporate ecological understanding into representation of coupled biogeochemical cycles and their representation in global scale models.
Simulated responses to nitrogen enrichment and elevated CO2 also reflect different model assumptions related to plant nutrient acquisition strategies between versions of the CLM. Plants apply multiple strategies to acquire nitrogen, which may include symbiotic N2 fixation, as well as associations with different types of mycorrhizal fungi (Goodale, 2017; Nave et al., 2013; Phillips et al., 2013; Terrer et al., 2018; Treseder, 2004). In control simulations, the global sum (Table 1) and spatial distribution of contemporary symbiotic nitrogen fixation seems reasonable in CLM5, although these estimates may be high in temperate and boreal forests in all versions of the model (Houlton et al., 2008; Vitousek et al., 2013). Nitrogen fixation declines in CLM5 with nitrogen fertilization, as plants utilize less energetically expensive N uptake pathways (Figure 4). This response aligns with observations from agricultural systems (McAuliffe et al., 1958)—although fewer data are available for natural vegetation, especially in tropical forests and savannas (Batterman et al., 2013)—and suggests that the sign of the nitrogen fixation response to N fertilization seems more appropriate with CLM5 than previous versions of the model.
Under elevated CO2, both CLM4 and CLM4.5 have limited ability to increase N acquisition rates. These versions of the model increased stocks of nitrogen deployed in new growth by roughly 50 Tg N globally (~5%) at the end of the elevated CO2 experiment. In contrast, the FUN module of CLM5 affords ways for plants to acquire new nitrogen; thus, plant nitrogen accumulation in new growth increased 150 Tg N globally (~15%) with this version of the model. Much of this nitrogen was supplied by existing ecosystem nitrogen pools, although a significant fraction of “new” nitrogen was fixed via symbiotic nitrogen fixation with CLM5, which doubled under elevated CO2 (Table 1). Examples of nitrogen fixing strategies can be found in multiple ecosystems (Houlton et al., 2008; Menge et al., 2009), but we doubt their relative abundance (a parameter that determines the fraction of N fixing species in a PFT) and activity under elevated CO2 are as high as assumed in the current CLM5 configuration. Moreover, even where N2‐fixing strategies are common, FACE experiments do not support the large and sustained increases in N2‐fixation rates currently simulated by CLM5 (Hungate et al., 2004; Reich et al., 2006; van Groenigen et al., 2006), which likely avoids progressive N limitation in the model. As currently applied in CLM5, the carbon costs calculated by FUN are incurred as a plant respiration flux, but future modifications to link plant nutrient limitation with shifts in plant carbon allocation or ability to acquire soil nutrients may serve to correct assumptions in the model and its response to environmental change, especially in a CO2‐rich world.
5. Conclusion
Model benchmarking serves as a useful tool to objectively quantify areas of model improvements and deficiencies. Indeed, a myriad of parametric and structural changes throughout the family of CLM models evaluated here illustrates improvements in gross primary productivity and other carbon cycle metrics simulated in CLM5 (Lawrence et al., 2019). Moreover, using data from experimental manipulations to compare similarly perturbed model simulations affords additional insights into model assumptions. Our results suggest that ecosystem nitrogen limitation was greater than observed in CLM4 and 4.5, likely because of the assumptions related to nitrogen limitation that downregulated GPP for a higher potential state in these versions of the model. Given their strong N limitation, CLM4 and 4.5 show sensitivities to elevated CO2 that are lower than observations. Subsequent modifications to CLM5 afforded more appropriate sensitivities to both nitrogen and CO2 enrichment. Results from these global‐scale experimental manipulations suggest that new capabilities in CLM5 that improve plant nitrogen uptake and use—namely FlexCN, LUNA, and FUN—are largely responsible for these improvements.
Our results also suggest that the mechanisms by which CLM5 matches these observational targets deserve further refinement and integration, specifically related to shifts in plant allocation and nutrient acquisition strategies under environmental change. As such, additional research is needed to more completely understand, quantify, and simulate changes in plant physiology, allocation, and nutrient uptake that occur under environmental change. Long‐term experimental manipulations consistently show surprising ecosystem responses that are not expected from theoretical expectations or initial observations (Hungate et al., 2004; Melillo et al., 2017; Norby et al., 2010; Reich et al., 2018). Thus, synthesizing results across diverse ecosystems, continuing collection of long‐term data sets from experimental manipulations, and integrating these insights into models are critical to further refine and evaluate the representation of terrestrial ecosystems in land models that show increasingly sophisticated consideration of ecological processes. By comparing the effect size of modeled and observed responses to experimental manipulations, our approach affords opportunities to move beyond static benchmarks that can be used to understand and evaluate ecological processes that are represented in models.
Model Availability
CLM5.0 is publicly available through the Community Terrestrial System Model (CTSM) git repository (https://github.com/ESCOMP/ctsm).
6.
Author Contribution
W.R.W. conducted the simulations and wrote the manuscript with contributions from all other authors.
Supporting information
Supporting Information S1
Acknowledgments
This material is based upon work supported by the National Center for Atmospheric Research, which is a major facility sponsored by the National Science Foundation under cooperative agreement 1852977. Computing resources (doi:10.5065/D6RX99HX) were provided by the Climate Simulation Laboratory at NCAR's Computational and Information Systems Laboratory, sponsored by the National Science Foundation and other agencies. W.R.W. was supported by the U.S. Department of Agriculture NIFA award 2015‐67003‐23485; the Environmental Protection Agency's National Center for Environmental Assessment, through an Interagency Agreement with the National Science Foundation and the National Center for Atmospheric Research (DW‐49‐92447301‐0); and NASA Interdisciplinary Science Program award NNX17AK19G. This research was partially supported by the Reducing Uncertainties in Biogeochemical Interactions through Synthesis and Computation Scientific Focus Area (RUBISCO SFA), which is sponsored by the Regional and Global Model Analysis (RGMA) Program in the Climate and Environmental Sciences Division (CESD) of the Office of Biological and Environmental Research (BER) in the U.S. Department of Energy Office of Science.
Wieder, W. R. , Lawrence, D. M. , Fisher, R. A. , Bonan, G. B. , Cheng, S. J. , Goodale, C. L. , et al. (2019). Beyond static benchmarking: Using experimental manipulations to evaluate land model assumptions. Global Biogeochemical Cycles, 33, 1289–1309. 10.1029/2018GB006141
This article is a companion to Bonan et al. (2019), https://doi.org/10.1029/2019GB006175
Data Availability Statement
Data are publicly available at the UCAR/NCAR Climate Data Gateway, doi:10.5065/d6154fwh.
References
- Aerts, R. , & Chapin, F. S. I. (2000). The mineral nutrition of wild plants revisited: A re‐evaluation of processes and patterns. Advances in Ecological Research, 10, 402–407. 10.1016/S0065-2504(08)60016-1 [DOI] [Google Scholar]
- Ainsworth, E. A. , & Long, S. P. (2005). What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2 . New Phytologist, 165(2), 351–372. 10.1111/j.1469-8137.2004.01224.x [DOI] [PubMed] [Google Scholar]
- Ali, A. A. , Xu, C. , Rogers, A. , Fisher, R. A. , Wullschleger, S. D. , Massoud, E. C. , Vrugt, J. A. , Muss, J. D. , McDowell, N. G. , Fisher, J. B. , Reich, P. B. , & Wilson, C. J. (2016). A global scale mechanistic model of photosynthetic capacity (LUNA v1.0). Geoscientific Model Development, 9(2), 587–606. 10.5194/gmd-9-587-2016 [DOI] [Google Scholar]
- Arora, V. K. , Boer, G. J. , Friedlingstein, P. , Eby, M. , Jones, C. D. , Christian, J. R. , Bonan, G. , Bopp, L. , Brovkin, V. , Cadule, P. , Hajima, T. , Ilyina, T. , Lindsay, K. , Tjiputra, J. F. , & Wu, T. (2013). Carbon‐concentration and carbon‐climate feedbacks in CMIP5 Earth system models. Journal of Climate, 26(15), 5289–5314. 10.1175/jcli-d-12-00494.1 [DOI] [Google Scholar]
- Batterman, S. A. , Hedin, L. O. , van Breugel, M. , Ransijn, J. , Craven, D. J. , & Hall, J. S. (2013). Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature, 502(7470), 224–227. 10.1038/nature12525 [DOI] [PubMed] [Google Scholar]
- Beer, C. , Reichstein, M. , Tomelleri, E. , Ciais, P. , Jung, M. , Carvalhais, N. , Rodenbeck, C. , Arain, M. A. , Baldocchi, D. , Bonan, G. B. , Bondeau, A. , Cescatti, A. , Lasslop, G. , Lindroth, A. , Lomas, M. , Luyssaert, S. , Margolis, H. , Oleson, K. W. , Roupsard, O. , Veenendaal, E. , Viovy, N. , Williams, C. , Woodward, F. I. , & Papale, D. (2010). Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science, 329(5993), 834–838. 10.1126/science.1184984 [DOI] [PubMed] [Google Scholar]
- Bonan, G. B. (2015). Ecological climatology: Concepts and applications, (3rd ed.). Cambridge: Cambridge University Press. [Google Scholar]
- Bonan, G. B. , Lawrence, P. J. , Oleson, K. W. , Levis, S. , Jung, M. , Reichstein, M. , Lawrence, D. M. , & Swenson, S. C. (2011). Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. Journal of Geophysical Research, 116, G02014 10.1029/2010JG001593 [DOI] [Google Scholar]
- Bonan, G. B. , & Levis, S. (2010). Quantifying carbon‐nitrogen feedbacks in the Community Land Model (CLM4). Geophysical Research Letters, 37, L07401 10.1029/2010GL042430 [DOI] [Google Scholar]
- Bonan, G. B. , Oleson, K. W. , Fisher, R. A. , Lasslop, G. , & Reichstein, M. (2012). Reconciling leaf physiological traits and canopy flux data: Use of the TRY and FLUXNET databases in the Community Land Model version 4. Journal of Geophysical Research, 117, G02026 10.1029/2011JG001913 [DOI] [Google Scholar]
- Bonan, G. B. , Lombardozzi, D. L. , Wieder, W. R. , Oleson, K. W. , Lawrence, D. M. , Hoffman, F. M. , & Collier, N. (2019). Model Structure and Climate Data Uncertainty in Historical Simulations of the Terrestrial Carbon Cycle (1850–2014). Global Biogeochemical Cycles, 33 10.1029/2019GB006175 [DOI] [Google Scholar]
- Brunke, M. A. , Broxton, P. , Pelletier, J. , Gochis, D. , Hazenberg, P. , Lawrence, D. M. , Leung, L. R. , Niu, G. Y. , Troch, P. A. , & Zeng, X. (2016). Implementing and evaluating variable soil thickness in the Community Land Model, version 4.5 (CLM4.5). Journal of Climate, 29(9), 3441–3461. 10.1175/Jcli-D-15-0307.1 [DOI] [Google Scholar]
- Brzostek, E. R. , Fisher, J. B. , & Phillips, R. P. (2014). Modeling the carbon cost of plant nitrogen acquisition: Mycorrhizal trade‐offs and multipath resistance uptake improve predictions of retranslocation. Journal of Geophysical Research: Biogeosciences, 119, 1684–1697. 10.1002/2014JG002660 [DOI] [Google Scholar]
- Campbell, J. E. , Berry, J. A. , Seibt, U. , Smith, S. J. , Montzka, S. A. , Launois, T. , Belviso, S. , Bopp, L. , & Laine, M. (2017). Large historical growth in global terrestrial gross primary production. Nature, 544(7648), 84–87. 10.1038/nature22030 [DOI] [PubMed] [Google Scholar]
- Campioli, M. , Vicca, S. , Luyssaert, S. , Bilcke, J. , Ceschia, E. , Chapin Iii, F. S. , Ciais, P. , Fernández‐Martínez, M. , Malhi, Y. , Obersteiner, M. , & Olefeldt, D. (2015). Biomass production efficiency controlled by management in temperate and boreal ecosystems. Nature Geoscience, 8(11), 843–846. 10.1038/ngeo2553 [DOI] [Google Scholar]
- Cheng, C. , Hess, P. , Wieder, W. R. , Thomas, R. Q. , & Nadelhoffer, K. (2018). Decadal impacts of nitrogen additions on temperate forest carbon sinks: A data‐model comparison. Biogeosciences Discussions, 2018, 1–38. 10.5194/bg-2018-505 [DOI] [Google Scholar]
- Cleveland, C. C. , Townsend, A. R. , Schimel, D. S. , Fisher, H. , Howarth, R. W. , Hedin, L. O. , Perakis, S. S. , Latty, E. F. , von Fischer, J. C. , Elseroad, A. , & Wasson, M. F. (1999). Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochemical Cycles, 13(2), 623–645. 10.1029/1999GB900014 [DOI] [Google Scholar]
- Collier, N. , Hoffman, F. M. , Lawrence, D. M. , Keppel‐Aleks, G. , Koven, C. D. , Riley, W. J. , Mu, M. , & Randerson, J. T. (2018). The International Land Model Benchmarking (ILAMB) system: Design, theory, and implementation. Journal of Advances in Modeling Earth Systems, 10, 2731–2754. 10.1029/2018MS001354 [DOI] [Google Scholar]
- De Kauwe, M. G. , Medlyn, B. E. , Zaehle, S. , Walker, A. P. , Dietze, M. C. , Wang, Y. P. , Luo, Y. , Jain, A. K. , El‐Masri, B. , Hickler, T. , & Wårlind, D. (2014). Where does the carbon go? A model‐data intercomparison of vegetation carbon allocation and turnover processes at two temperate forest free‐air CO2 enrichment sites. New Phytologist, 203(3), 883–899. 10.1111/nph.12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia, E. H. , Drake, J. E. , Thomas, R. B. , & Gonzalez‐Meler, M. (2007). Forest carbon use efficiency: Is respiration a constant fraction of gross primary production? Global Change Biology, 13(6), 1157–1167. 10.1111/j.1365-2486.2007.01365.x [DOI] [Google Scholar]
- Drake, J. E. , Gallet‐Budynek, A. , Hofmockel, K. S. , Bernhardt, E. S. , Billings, S. A. , Jackson, R. B. , Johnsen, K. S. , Lichter, J. , McCarthy, H. R. , McCormack, M. L. , Moore, D. J. P. , Oren, R. , Palmroth, S. , Phillips, R. P. , Pippen, J. S. , Pritchard, S. G. , Treseder, K. K. , Schlesinger, W. H. , DeLucia, E. H. , & Finzi, A. C. (2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long‐term enhancement of forest productivity under elevated CO2 . Ecology Letters, 14(4), 349–357. 10.1111/j.1461-0248.2011.01593.x [DOI] [PubMed] [Google Scholar]
- Ehleringer, J. , & Bjorkman, O. (1977). Quantum yields for CO2 uptake in C3 and C4 plants: Dependence on temperature, CO2, and O2 concentration. Plant Physiology, 59(1), 86–90. 10.1104/pp.59.1.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth, D. S. , Anderson, I. C. , Crous, K. Y. , Cooke, J. , Drake, J. E. , Gherlenda, A. N. , Gimeno, T. E. , Macdonald, C. A. , Medlyn, B. E. , Powell, J. R. , Tjoelker, M. G. , & Reich, P. B. (2017). Elevated CO2 does not increase eucalypt forest productivity on a low‐phosphorus soil. Nature Climate Change, 7(4), 279–282. 10.1038/nclimate3235 [DOI] [Google Scholar]
- Ellsworth, D. S. , Reich, P. B. , Naumburg, E. S. , Koch, G. W. , Kubiske, M. E. , & Smith, S. D. (2004). Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free‐air CO2 enrichment experiments in forest, grassland and desert. Global Change Biology, 10(12), 2121–2138. 10.1111/j.1365-2486.2004.00867.x [DOI] [Google Scholar]
- Eyring, V. , Bony, S. , Meehl, G. A. , Senior, C. A. , Stevens, B. , Stouffer, R. J. , & Taylor, K. E. (2016). Overview of the Coupled Model Intercomparison Project phase 6 (CMIP6) experimental design and organization. Geoscientific Model Development, 9(5), 1937–1958. 10.5194/gmd-9-1937-2016 [DOI] [Google Scholar]
- Fan, Y. , Roupsard, O. , Bernoux, M. , Le Maire, G. , Panferov, O. , Kotowska, M. M. , & Knohl, A. (2015). A sub‐canopy structure for simulating oil palm in the Community Land Model (CLM‐Palm): Phenology, allocation and yield. Geoscientific Model Development, 8(11), 3785–3800. 10.5194/gmd-8-3785-2015 [DOI] [Google Scholar]
- Fisher, J. B. , Sitch, S. , Malhi, Y. , Fisher, R. A. , Huntingford, C. , & Tan, S. Y. (2010). Carbon cost of plant nitrogen acquisition: A mechanistic, globally applicable model of plant nitrogen uptake, retranslocation, and fixation. Global Biogeochemical Cycles, 24, GB1014 10.1029/2009GB003621 [DOI] [Google Scholar]
- Fisher, R. A. , McDowell, N. , Purves, D. , Moorcroft, P. , Sitch, S. , Cox, P. , Huntingford, C. , Meir, P. , & Woodward, F. I. (2010). Assessing uncertainties in a second‐generation dynamic vegetation model caused by ecological scale limitations. New Phytologist, 187(3), 666–681. 10.1111/j.1469-8137.2010.03340.x [DOI] [PubMed] [Google Scholar]
- Fisher, R. A. , Muszala, S. , Verteinstein, M. , Lawrence, P. , Xu, C. , McDowell, N. G. , Knox, R. G. , Koven, C. , Holm, J. , Rogers, B. M. , Spessa, A. , Lawrence, D. , & Bonan, G. (2015). Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4.5(ED). Geoscientific Model Development, 8(11), 3593–3619. 10.5194/gmd-8-3593-2015 [DOI] [Google Scholar]
- Fisher, R. A. , Wieder, W. R. , Sanderson, B. M. , Koven, C. D. , Oleson, K. W. , Xu, C. , Fisher, J. , Shi, M. , Walker, A. P. , & Lawrence, D. M. (2019). Parametric controls on vegetation responses to biogeochemical forcing in the CLM5. Journal of Advances in Modeling Earth Systems. 10.1029/2019MS001609 [DOI] [Google Scholar]
- Franks, P. J. , Berry, J. A. , Lombardozzi, D. L. , & Bonan, G. B. (2017). Stomatal function across temporal and spatial scales: Deep‐time trends, land‐atmosphere coupling and global models. Plant Physiology, 174(2), 583–602. 10.1104/pp.17.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, S. D. , Ollinger, S. , Nadelhoffer, K. , Bowden, R. , Brzostek, E. , Burton, A. , Caldwell, B. A. , Crow, S. , Goodale, C. L. , Grandy, A. S. , Finzi, A. , Kramer, M. G. , Lajtha, K. , LeMoine, J. , Martin, M. , McDowell, W. H. , Minocha, R. , Sadowsky, J. J. , Templer, P. H. , & Wickings, K. (2014). Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry, 121(2), 305–316. 10.1007/s10533-014-0004-0 [DOI] [Google Scholar]
- Friedlingstein, P. , Cox, P. , Betts, R. , Bopp, L. , von Bloh, W. , Brovkin, V. , Cadule, P. , Doney, S. , Eby, M. , Fung, I. , Bala, G. , John, J. , Jones, C. , Joos, F. , Kato, T. , Kawamiya, M. , Knorr, W. , Lindsay, K. , Matthews, H. D. , Raddatz, T. , Rayner, P. , Reick, C. , Roeckner, E. , Schnitzler, K. G. , Schnur, R. , Strassmann, K. , Weaver, A. J. , Yoshikawa, C. , & Zeng, N. (2006). Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. Journal of Climate, 19(14), 3337–3353. 10.1175/jcli3800.1 [DOI] [Google Scholar]
- Friedlingstein, P. , Meinshausen, M. , Arora, V. K. , Jones, C. D. , Anav, A. , Liddicoat, S. K. , & Knutti, R. (2014). Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. Journal of Climate, 27(2), 511–526. 10.1175/jcli-d-12-00579.1 [DOI] [Google Scholar]
- Gerber, S. , Hedin, L. O. , Oppenheimer, M. , Pacala, S. W. , & Shevliakova, E. (2010). Nitrogen cycling and feedbacks in a global dynamic land model. Global Biogeochemical Cycles, 24, GB1001 10.1029/2008GB003336 [DOI] [Google Scholar]
- Ghimire, B. , Riley, W. J. , Koven, C. D. , Mu, M. , & Randerson, J. T. (2016). Representing leaf and root physiological traits in CLM improves global carbon and nitrogen cycling predictions. Journal of Advances in Modeling Earth Systems, 8, 598–613. 10.1002/2015MS000538 [DOI] [Google Scholar]
- Giardina, C. P. , Coleman, M. D. , Hancock, J. E. , King, J. S. , Lilleskov, E. A. , Loya, W. M. , Pregitzer, K. S. , Ryan, M. G. , & Trettin, C. C. (2005). The response of belowground carbon allocation in forests to global change In Tree species effects on soils: implications for global change (pp. 119–154). Dordrecht: Springer. [Google Scholar]
- Goodale, C. L. (2017). Multiyear fate of a 15N tracer in a mixed deciduous forest: Retention, redistribution, and differences by mycorrhizal association. Global Change Biology, 23(2), 867–880. 10.1111/gcb.13483 [DOI] [PubMed] [Google Scholar]
- Hoffman, F. M. , Randerson, J. T. , Arora, V. K. , Bao, Q. , Cadule, P. , Ji, D. , Jones, C. D. , Kawamiya, M. , Khatiwala, S. , Lindsay, K. , Obata, A. , Shevliakova, E. , Six, K. D. , Tjiputra, J. F. , Volodin, E. M. , & Wu, T. (2014). Causes and implications of persistent atmospheric carbon dioxide biases in Earth system models. Journal of Geophysical Research: Biogeosciences, 119, 141–162. 10.1002/2013JG002381 [DOI] [Google Scholar]
- Hoffman, F. M. , Koven, C. D. , Keppel‐Aleks, G. , Lawrence, D. M. , Riley, W. J. , Randerson, J. T. , Ahlström, A. , Abramowitz,G. Baldocchi, D. D. , Best, M. J. , Bond‐Lamberty, B. , De Kauwe, M. G. , Denning, A. S. , Desai, A. , Eyring, V. , Fisher, J. B. , Fisher, R. A. , Gleckler, P. J. , Huang, M. , Hugelius, G. , Jain, A. K. , Kiang, N. Y. , Kim, H. , Koster, R. D. , Kumar, S. V. , Li, H. , Luo, Y. , Mao, J. , McDowell, N. G. , Mishra, U. , Moorcroft, P. R. , Pau, G. S. H. , Ricciuto, D. M. , Schaefer, K. , Schwalm, C. R. , Serbin, S. P. , Shevliakova, E. , Slater, A. G. , Tang, J. , Williams, M. , Xia, J. , Xu, C. , Joseph, R. , & Koch, D. (2017). International Land Model Benchmarking (ILAMB) 2016 Workshop Report,DOE/SC‐0186. Germantown,MD: U.S. Department of Energy, Office of Science; 10.2172/1330803 [DOI] [Google Scholar]
- Houlton, B. Z. , Wang, Y. P. , Vitousek, P. M. , & Field, C. B. (2008). A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature, 454(7202), 327–330. 10.1038/nature07028 [DOI] [PubMed] [Google Scholar]
- Hungate, B. A. , Dukes, J. S. , Shaw, R. , Luo, Y. , & Field, C. B. (2003). Nitrogen and climate change. Science, 302(5650), 1512–1513. 10.1126/science.1091390 [DOI] [PubMed] [Google Scholar]
- Hungate, B. A. , Stiling, P. D. , Dijkstra, P. , Johnson, D. W. , Ketterer, M. E. , Hymus, G. J. , Hinkle, C. R. , & Drake, B. G. (2004). CO2 elicits long‐term decline in nitrogen fixation. Science, 304(5675), 1291 10.1126/science.1095549 [DOI] [PubMed] [Google Scholar]
- Iversen, C. M. , Ledford, J. , & Norby, R. J. (2008). CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytologist, 179(3), 837–847. 10.1111/j.1469-8137.2008.02516.x [DOI] [PubMed] [Google Scholar]
- Janssens, I. A. , Dieleman, W. , Luyssaert, S. , Subke, J. A. , Reichstein, M. , Ceulemans, R. , Ciais, P. , Dolman, A. J. , Grace, J. , Matteucci, G. , Papale, D. , Piao, S. L. , Schulze, E. D. , Tang, J. , & Law, B. E. (2010). Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience, 3(5), 315–322. 10.1038/ngeo844 [DOI] [Google Scholar]
- Jones, C. , Arora, V. , Friedlingstein, P. , Bopp, L. , Brovkin, V. , Dunne, J. , Graven, H. , Hoffman, F. , Ilyina, T. , John, J. G. , & Jung, M. (2016). C4MIP‐The Coupled Climate‐Carbon Cycle Model Intercomparison Project: Experimental protocol for CMIP6. Geoscientific Model Development, 9(8), 2853–2880. 10.5194/gmd-9-2853-2016 [DOI] [Google Scholar]
- Jones, C. , Robertson, E. , Arora, V. , Friedlingstein, P. , Shevliakova, E. , Bopp, L. , Brovkin, V. , Hajima, T. , Kato, E. , Kawamiya, M. , Liddicoat, S. , Lindsay, K. , Reick, C. H. , Roelandt, C. , Segschneider, J. , & Tjiputra, J. (2013). Twenty‐first‐century compatible CO2 emissions and airborne fraction simulated by CMIP5 Earth system models under four Representative Concentration Pathways. Journal of Climate, 26(13), 4398–4413. 10.1175/Jcli-D-12-00554.1 [DOI] [Google Scholar]
- Jung, M. , Reichstein, M. , Margolis, H. A. , Cescatti, A. , Richardson, A. D. , Arain, M. A. , Arneth, A. , Bernhofer, C. , Bonal, D. , Chen, J. , Gianelle, D. , Gobron, N. , Kiely, G. , Kutsch, W. , Lasslop, G. , Law, B. E. , Lindroth, A. , Merbold, L. , Montagnani, L. , Moors, E. J. , Papale, D. , Sottocornola, M. , Vaccari, F. , & Williams, C. (2011). Global patterns of land‐atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. Journal of Geophysical Research, 116, G00J07 10.1029/2010JG001566 [DOI] [Google Scholar]
- Kattge, J. , Knorr, W. , Raddatz, T. , & Wirth, C. (2009). Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global‐scale terrestrial biosphere models. Global Change Biology, 15(4), 976–991. 10.1111/j.1365-2486.2008.01744.x [DOI] [Google Scholar]
- Kennedy, D. , Swenson, S. , Oleson, K. W. , Lawrence, D. M. , Fisher, R. , da Costa, A. C. L. , & Gentine, P. (2019). Implementing plant hydraulics in the Community Land Model, version 5. Journal of Advances in Modeling Earth Systems, 11, 485–513. 10.1029/2018MS001500 [DOI] [Google Scholar]
- Knorr, M. , Frey, S. D. , & Curtis, P. S. (2005). Nitrogen additions and litter decomposition: A meta‐analysis. Ecology, 86(12), 3252–3257. 10.1890/05-0150 [DOI] [Google Scholar]
- Koven, C. D. , Riley, W. J. , Subin, Z. M. , Tang, J. Y. , Torn, M. S. , Collins, W. D. , Bonan, G. B. , Lawrence, D. M. , & Swenson, S. C. (2013). The effect of vertically‐resolved soil biogeochemistry and alternate soil C and N models on C dynamics of CLM4. Biogeosciences, 10(11), 7109–7131. 10.5194/bg-10-7109-2013 [DOI] [Google Scholar]
- Kovenock, M. , & Swann, A. L. S. (2018). Leaf trait acclimation amplifies simulated climate warming in response to elevated carbon dioxide. Global Biogeochemical Cycles, 32, 1437–1448. 10.1029/2018GB005883 [DOI] [Google Scholar]
- Lamarque, J. F. , Dentener, F. , McConnell, J. , Ro, C. U. , Shaw, M. , Vet, R. , Bergmann, D. , Cameron‐Smith, P. , Dalsoren, S. , Doherty, R. , Faluvegi, G. , Ghan, S. J. , Josse, B. , Lee, Y. H. , MacKenzie, I. A. , Plummer, D. , Shindell, D. T. , Skeie, R. B. , Stevenson, D. S. , Strode, S. , Zeng, G. , Curran, M. , Dahl‐Jensen, D. , Das, S. , Fritzsche, D. , & Nolan, M. (2013). Multi‐model mean nitrogen and sulfur deposition from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP): Evaluation of historical and projected future changes. Atmospheric Chemistry and Physics, 13(16), 7997–8018. 10.5194/acp-13-7997-2013 [DOI] [Google Scholar]
- Lawrence, D. M. , Fisher, R. M. , Koven, C. D. , Oleson, K. W. , Swenson, S. C. , et al. (2019). The Community Land Model version 5: Description of new features, benchmarking, and impact of forcing uncertainty. Journal of Advances in Modeling Earth Systems. in review 10.1029/2018MS001583 [DOI] [Google Scholar]
- Lawrence, D. M. , Hurtt, G. C. , Arneth, A. , Brovkin, V. , Calvin, K. V. , Jones, A. D. , Jones, C. D. , Lawrence, P. J. , de Noblet‐Ducoudré, N. , Pongratz, J. , Seneviratne, S. I. , & Shevliakova, E. (2016). The Land Use Model Intercomparison Project (LUMIP) contribution to CMIP6: Rationale and experimental design. Geoscientific Model Development, 9(9), 2973–2998. 10.5194/gmd-9-2973-2016 [DOI] [Google Scholar]
- LeBauer, D. S. , & Treseder, K. K. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89(2), 371–379. 10.1890/06-2057.1 [DOI] [PubMed] [Google Scholar]
- Leff, J. W. , Jones, S. E. , Prober, S. M. , Barberán, A. , Borer, E. T. , Firn, J. L. , Harpole, W. S. , Hobbie, S. E. , Hofmockel, K. S. , Knops, J. M. H. , McCulley, R. L. , la Pierre, K. , Risch, A. C. , Seabloom, E. W. , Schütz, M. , Steenbock, C. , Stevens, C. J. , & Fierer, N. (2015). Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proceedings of the National Academy of Sciences., 112(35), 10,967–10,972. 10.1073/pnas.1508382112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litton, C. M. , Raich, J. W. , & Ryan, M. G. (2007). Carbon allocation in forest ecosystems. Global Change Biology, 13(10), 2089–2109. 10.1111/j.1365-2486.2007.01420.x [DOI] [Google Scholar]
- Liu, L. L. , & Greaver, T. L. (2010). A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecology Letters, 13(7), 819–828. 10.1111/j.1461-0248.2010.01482.x [DOI] [PubMed] [Google Scholar]
- Lombardozzi, D. L. , Lu, Y. , Lawrence, P. , Lawrence, D. M. , Swenson, S. C. , Oleson, K. W. , Wieder, W. R. , & Ainsworth, L. (2018). Simulating transient crop management in the Community Land Model version 5. Journal of Geophysical Research: Biogeosciences, in revision. [Google Scholar]
- Lombardozzi, D. L. , Smith, N. G. , Cheng, S. J. , Dukes, J. S. , Sharkey, T. D. , Rogers, A. , Fisher, R. , & Bonan, G. B. (2018). Triose phosphate limitation in photosynthesis models reduces leaf photosynthesis and global terrestrial carbon storage. Environmental Research Letters, 13(7), 074025 10.1088/1748-9326/aacf68 [DOI] [Google Scholar]
- Lovenduski, N. S. , & Bonan, G. B. (2017). Reducing uncertainty in projections of terrestrial carbon uptake. Environmental Research Letters, 12(4), 044020 10.1088/1748-9326/aa66b8 [DOI] [Google Scholar]
- Lu, M. , Zhou, X. H. , Luo, Y. Q. , Yang, Y. H. , Fang, C. M. , Chen, J. K. , & Li, B. (2011). Minor stimulation of soil carbon storage by nitrogen addition: A meta‐analysis. Agriculture Ecosystems & Environment, 140(1‐2), 234–244. 10.1016/J.Agee.2010.12.010 [DOI] [Google Scholar]
- Luo, Y. Q. , Hui, D. F. , & Zhang, D. Q. (2006). Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta‐analysis. Ecology, 87(1), 53–63. 10.1890/04-1724 [DOI] [PubMed] [Google Scholar]
- Luo, Y. Q. , Randerson, J. T. , Abramowitz, G. , Bacour, C. , Blyth, E. , Carvalhais, N. , Ciais, P. , Dalmonech, D. , Fisher, J. B. , Fisher, R. , Friedlingstein, P. , Hibbard, K. , Hoffman, F. , Huntzinger, D. , Jones, C. D. , Koven, C. , Lawrence, D. , Li, D. J. , Mahecha, M. , Niu, S. L. , Norby, R. , Piao, S. L. , Qi, X. , Peylin, P. , Prentice, I. C. , Riley, W. , Reichstein, M. , Schwalm, C. , Wang, Y. P. , Xia, J. Y. , Zaehle, S. , & Zhou, X. H. (2012). A framework for benchmarking land models. Biogeosciences, 9(10), 3857–3874. 10.5194/bg-9-3857-2012 [DOI] [Google Scholar]
- Malhi, Y. , Aragao, L. E. O. C. , Metcalfe, D. B. , Paiva, R. , Quesada, C. A. , Almeida, S. , Anderson, L. , Brando, P. , Chambers, J. Q. , Da Costa, A. C. , & Hutyra, L. R. (2009). Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Global Change Biology, 15(5), 1255–1274. 10.1111/j.1365-2486.2008.01780.x [DOI] [Google Scholar]
- Malhi, Y. , Doughty, C. , & Galbraith, D. (2011). The allocation of ecosystem net primary productivity in tropical forests. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1582), 3225–3245. 10.1098/rstb.2011.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe, C. , Chamblee, D. S. , Uribe‐Arango, H. , & Woodhouse, W. W. (1958). Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by 15N. Agronomy Journal, 50(6), 334–337. 10.2134/agronj1958.00021962005000060014x [DOI] [Google Scholar]
- Medlyn, B. E. , Zaehle, S. , De Kauwe, M. G. , Walker, A. P. , Dietze, M. C. , Hanson, P. J. , Hickler, T. , Jain, A. K. , Luo, Y. , Parton, W. , & Prentice, I. C. (2015). Using ecosystem experiments to improve vegetation models. Nature Climate Change, 5(6), 528–534. 10.1038/nclimate2621 [DOI] [Google Scholar]
- Melillo, J. M. , Frey, S. D. , DeAngelis, K. M. , Werner, W. J. , Bernard, M. J. , Bowles, F. P. , Pold, G. , Knorr, M. A. , & Grandy, A. S. (2017). Long‐term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science, 358(6359), 101–105. 10.1126/science.aan2874 [DOI] [PubMed] [Google Scholar]
- Menge, D. N. L. , Levin, S. A. , & Hedin, L. O. (2009). Facultative versus obligate nitrogen fixation strategies and their ecosystem consequences. American Naturalist, 174(4), 465–477. 10.1086/605377 [DOI] [PubMed] [Google Scholar]
- Meyerholt, J. , & Zaehle, S. (2015). The role of stoichiometric flexibility in modelling forest ecosystem responses to nitrogen fertilization. New Phytologist, 208(4), 1042–1055. 10.1111/nph.13547 [DOI] [PubMed] [Google Scholar]
- Meyerholt, J. , & Zaehle, S. (2018). Controls of terrestrial ecosystem nitrogen loss on simulated productivity responses to elevated CO2 . Biogeosciences, 15(18), 5677–5698. 10.5194/bg-15-5677-2018 [DOI] [Google Scholar]
- Meyerholt, J. , Zaehle, S. , & Smith, M. J. (2016). Variability of projected terrestrial biosphere responses to elevated levels of atmospheric CO2 due to uncertainty in biological nitrogen fixation. Biogeosciences, 13(5), 1491–1518. 10.5194/bg-13-1491-2016 [DOI] [Google Scholar]
- Nave, L. E. , Nadelhoffer, K. J. , Le Moine, J. M. , van Diepen, L. T. A. , Cooch, J. K. , & Van Dyke, N. J. (2013). Nitrogen uptake by trees and mycorrhizal fungi in a successional northern temperate forest: Insights from multiple isotopic methods. Ecosystems, 16(4), 590–603. 10.1007/s10021-012-9632-1 [DOI] [Google Scholar]
- Negrón‐Juárez, R. I. , Koven, C. D. , Riley, W. J. , Knox, R. G. , & Chambers, J. Q. (2015). Observed allocations of productivity and biomass, and turnover times in tropical forests are not accurately represented in CMIP5 Earth system models. Environmental Research Letters, 10(6), 064017 10.1088/1748-9326/10/6/064017 [DOI] [Google Scholar]
- Norby, R. J. , DeLucia, E. H. , Gielen, B. , Calfapietra, C. , Giardina, C. P. , King, J. S. , Ledford, J. , McCarthy, H. R. , Moore, D. J. P. , Ceulemans, R. , de Angelis, P. , Finzi, A. C. , Karnosky, D. F. , Kubiske, M. E. , Lukac, M. , Pregitzer, K. S. , Scarascia‐Mugnozza, G. E. , Schlesinger, W. H. , & Oren, R. (2005). Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings Of The National Academy Of Sciences Of The United States Of America, 102(50), 18,052–18,056. 10.1073/pnas.0509478102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby, R. J. , Kauwe, M. G. D. , Domingues, T. F. , Duursma, R. A. , Ellsworth, D. S. , Goll, D. S. , Lapola, D. M. , Luus, K. A. , MacKenzie, A. R. , Medlyn, B. E. , & Pavlick, R. (2015). Model–data synthesis for the next generation of forest Free‐Air CO2 Enrichment (FACE) experiments. New Phytologist, 209(1), 17–28. 10.1111/nph.13593 [DOI] [PubMed] [Google Scholar]
- Norby, R. J. , Warren, J. M. , Iversen, C. M. , Medlyn, B. E. , & McMurtrie, R. E. (2010). CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings Of The National Academy Of Sciences Of The United States Of America, 107(45), 19,368–19,373. 10.1073/pnas.1006463107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby, R. J. , & Zak, D. R. (2011). Ecological lessons from Free‐Air CO2 Enrichment (FACE) experiments. Annual Review of Ecology, Evolution, and Systematics, 42(1), 181–203. 10.1146/annurev-ecolsys-102209-144647 [DOI] [Google Scholar]
- Oleson, K. W. , Lawrence, D. M. , Bonan, G. B. , Drewniak, B. , Huang, M. , Koven, C. D. , Levis, S. , Li,F. Riley, W. J. , Subin, Z. M. , Swenson, S. C. , Thornton, P. E. , Bozbiyik, A. , Fisher, R. , Heald, C. L. , Kluzek, E. , Lamarque, J.‐E. , Lawrence, P. J. , Leung, L. R. , Lipscomb, W. , Muszala, S. , Ricciuto, D. M. , Sacks, D. M. , Sun, Y. , Tang, J. , & Yang, Z.‐L. (2013). Technical description of version 4.5 of the Community Land Model (CLM). NCAR Technical Note NCAR/TN‐503+STR. In (pp. 420). doi: 10.5065/D6RR1W7M [DOI]
- Phillips, R. P. , Brzostek, E. , & Midgley, M. G. (2013). The mycorrhizal‐associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytologist, 199(1), 41–51. 10.1111/nph.12221 [DOI] [PubMed] [Google Scholar]
- Phillips, R. P. , & Fahey, T. J. (2007). Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytologist, 176(3), 655–664. 10.1111/j.1469-8137.2007.02204.x [DOI] [PubMed] [Google Scholar]
- Rastetter, E. B. , Agren, G. I. , & Shaver, G. R. (1997). Responses of N‐limited ecosystems to increased CO2: A balanced‐nutrition, coupled‐element‐cycles model. Ecological Applications, 7(2), 444–460. 10.1890/1051-0761(1997)007[0444:RONLET]2.0.CO;2 [DOI] [Google Scholar]
- Rastetter, E. B. , Vitousek, P. M. , Field, C. , Shaver, G. R. , Herbert, D. , & Agren, G. I. (2001). Resource optimization and symbiotic nitrogen fixation. Ecosystems, 4(4), 369–388. 10.1007/s10021-001-0018-z [DOI] [Google Scholar]
- Reich, P. B. , & Hobbie, S. E. (2013). Decade‐long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nature Climate Change, 3(3), 278–282. 10.1038/Nclimate1694 [DOI] [Google Scholar]
- Reich, P. B. , Hobbie, S. E. , Lee, T. D. , & Pastore, M. A. (2018). Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20‐year field experiment. Science, 360(6386), 317–320. 10.1126/science.aas9313 [DOI] [PubMed] [Google Scholar]
- Reich, P. B. , Hungate, B. A. , & Luo, Y. Q. (2006). Carbon‐nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annual Review of Ecology Evolution and Systematics, 37(1), 611–636. 10.1146/annurev.ecolsys.37.091305.110039 [DOI] [Google Scholar]
- Rogers, A. , Medlyn, B. E. , Dukes, J. S. , Bonan, G. , von Caemmerer, S. , Dietze, M. C. , Kattge, J. , Leakey, A. D. B. , Mercado, L. M. , Niinemets, Ü. , Prentice, I. C. , Serbin, S. P. , Sitch, S. , Way, D. A. , & Zaehle, S. (2017). A roadmap for improving the representation of photosynthesis in Earth system models. New Phytologist, 213(1), 22–42. 10.1111/nph.14283 [DOI] [PubMed] [Google Scholar]
- Shi, M. , Fisher, J. B. , Brzostek, E. R. , & Phillips, R. P. (2016). Carbon cost of plant nitrogen acquisition: Global carbon cycle impact from an improved plant nitrogen cycle in the Community Land Model. Global Change Biology, 22(3), 1299–1314. 10.1111/gcb.13131 [DOI] [PubMed] [Google Scholar]
- Sokolov, A. P. , Kicklighter, D. W. , Melillo, J. M. , Felzer, B. S. , Schlosser, C. A. , & Cronin, T. W. (2008). Consequences of considering carbon‐nitrogen interactions on the feedbacks between climate and the terrestrial carbon cycle. Journal of Climate, 21(15), 3776–3796. 10.1175/2008jcli2038.1 [DOI] [Google Scholar]
- Swenson, S. C. , & Lawrence, D. M. (2014). Assessing a dry surface layer‐based soil resistance parameterization for the Community Land Model using GRACE and FLUXNET‐MTE data. Journal of Geophysical Research: Atmospheres, 119, 10,299–210,312. 10.1002/2014JD022314 [DOI] [Google Scholar]
- Swenson, S. C. , & Lawrence, D. M. (2015). A GRACE‐based assessment of interannual groundwater dynamics in the Community Land Model. Water Resources Research, 51, 8817–8833. 10.1002/2015WR017582 [DOI] [Google Scholar]
- Terrer, C. , Vicca, S. , Stocker, B. D. , Hungate, B. A. , Phillips, R. P. , Reich, P. B. , Finzi, A. C. , & Prentice, I. C. (2018). Ecosystem responses to elevated CO2 governed by plant‐soil interactions and the cost of nitrogen acquisition. New Phytologist, 217(2), 507–522. 10.1111/nph.14872 [DOI] [PubMed] [Google Scholar]
- Thomas, R. Q. , Bonan, G. B. , & Goodale, C. L. (2013). Insights into mechanisms governing forest carbon response to nitrogen deposition: A model‐data comparison using observed responses to nitrogen addition. Biogeosciences, 10(6), 3869–3887. 10.5194/bg-10-3869-2013 [DOI] [Google Scholar]
- Thomas, R. Q. , Brookshire, E. N. , & Gerber, S. (2015). Nitrogen limitation on land: How can it occur in Earth system models? Global Change Biology, 21(5), 1777–1793. 10.1111/gcb.12813 [DOI] [PubMed] [Google Scholar]
- Thomas, R. Q. , Zaehle, S. , Templer, P. H. , & Goodale, C. L. (2013). Global patterns of nitrogen limitation: Confronting two global biogeochemical models with observations. Global Change Biology, 19(10), 2986–2998. 10.1111/gcb.12281 [DOI] [PubMed] [Google Scholar]
- Thornton, P. E. , Lamarque, J.‐F. , Rosenbloom, N. A. , & Mahowald, N. M. (2007). Influence of carbon‐nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochemical Cycles, 21, GB4018 10.1029/2006GB002868 [DOI] [Google Scholar]
- Treseder, K. K. (2004). A meta‐analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytologist, 164(2), 347–355. 10.1111/j.1469-8137.2004.01159.x [DOI] [PubMed] [Google Scholar]
- Treseder, K. K. , Allen, E. B. , Egerton‐Warburton, L. M. , Hart, M. M. , Klironomos, J. N. , Maherali, H. , & Tedersoo, L. (2018). Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait‐based predictive framework. Journal Of Ecology, 106(2), 480–489. 10.1111/1365-2745.12919 [DOI] [Google Scholar]
- van Groenigen, K. J. , Six, J. , Hungate, B. A. , de Graaff, M. A. , van Breemen, N. , & van Kessel, C. (2006). Element interactions limit soil carbon storage. Proceedings of the National Academy of Sciences of the United States of America, 103(17), 6571–6574. 10.1073/pnas.0509038103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kampenhout, L. , Lenaerts, J. T. M. , Lipscomb, W. H. , Sacks, W. J. , Lawrence, D. M. , Slater, A. G. , & van den Broeke, M. R. (2017). Improving the representation of polar snow and firn in the Community Earth System Model. Journal of Advances in Modeling Earth Systems, 9, 2583–2600. 10.1002/2017MS000988 [DOI] [Google Scholar]
- Vicca, S. , Luyssaert, S. , Peñuelas, J. , Campioli, M. , Chapin, F. S. , Ciais, P. , Heinemeyer, A. , Högberg, P. , Kutsch, W. L. , Law, B. E. , & Malhi, Y. (2012). Fertile forests produce biomass more efficiently. Ecology Letters, 15(6), 520–526. 10.1111/j.1461-0248.2012.01775.x [DOI] [PubMed] [Google Scholar]
- Vitousek, P. M. , Menge, D. N. L. , Reed, S. C. , & Cleveland, C. C. (2013). Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1621). 10.1098/rstb.2013.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. P. , Beckerman, A. P. , Gu, L. , Kattge, J. , Cernusak, L. A. , Domingues, T. F. , Scales, J. C. , Wohlfahrt, G. , Wullschleger, S. D. , & Woodward, F. I. (2014). The relationship of leaf photosynthetic traits—Vcmax and Jmax—to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta‐analysis and modeling study. Ecology and Evolution, 4(16), 3218–3235. 10.1002/ece3.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. P. , Hanson, P. J. , De Kauwe, M. G. , Medlyn, B. E. , Zaehle, S. , Asao, S. , Dietze, M. , Hickler, T. , Huntingford, C. , Iversen, C. M. , & Jain, A. (2014). Comprehensive ecosystem model‐data synthesis using multiple data sets at two temperate forest free‐air CO2 enrichment experiments: Model performance at ambient CO2 concentration. Journal of Geophysical Research: Biogeosciences, 119, 937–964. 10.1002/2013JG002553 [DOI] [Google Scholar]
- Wang, Y.‐P. , & Houlton, B. Z. (2009). Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon‐climate feedback. Geophysical Research Letters, 36, L24403 10.1029/2009GL041009 [DOI] [Google Scholar]
- Wieder, W. R. , Cleveland, C. C. , Lawrence, D. M. , & Bonan, G. B. (2015). Effects of model structural uncertainty on carbon cycle projections: Biological nitrogen fixation as a case study. Environmental Research Letters, 10(4), 044016 10.1088/1748-9326/10/4/044016 [DOI] [Google Scholar]
- Wieder, W. R. , Cleveland, C. C. , Smith, W. K. , & Todd‐Brown, K. (2015). Future productivity and carbon storage limited by terrestrial nutrient availability. Nature Geoscience, 8(6), 441–444. 10.1038/ngeo2413 [DOI] [Google Scholar]
- Wullschleger, S. D. , Tschaplinski, T. J. , & Norby, R. J. (2002). Plant water relations at elevated CO2—Implications for water‐limited environments. Plant, Cell & Environment, 25(2), 319–331. 10.1046/j.1365-3040.2002.00796.x [DOI] [PubMed] [Google Scholar]
- Xia, J. , & Wan, S. (2008). Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist, 179(2), 428–439. 10.1111/j.1469-8137.2008.02488.x [DOI] [PubMed] [Google Scholar]
- Xu, C. , Fisher, R. , Wullschleger, S. D. , Wilson, C. J. , Cai, M. , & McDowell, N. G. (2012). Toward a mechanistic modeling of nitrogen limitation on vegetation dynamics. Plos One, 7(5), e37914 10.1371/journal.pone.0037914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle, S. , Friedlingstein, P. , & Friend, A. D. (2010). Terrestrial nitrogen feedbacks may accelerate future climate change. Geophysical Research Letters, 37, L01401 10.1029/2009GL041345 [DOI] [Google Scholar]
- Zaehle, S. , Jones, C. D. , Houlton, B. , Lamarque, J. F. , & Robertson, E. (2015). Nitrogen availability reduces CMIP5 projections of twenty‐first‐century land carbon uptake. Journal of Climate, 28(6), 2494–2511. 10.1175/Jcli-D-13-00776.1 [DOI] [Google Scholar]
- Zaehle, S. , Medlyn, B. E. , De Kauwe, M. G. , Walker, A. P. , Dietze, M. C. , Hickler, T. , Luo, Y. , Wang, Y. P. , El‐Masri, B. , Thornton, P. , & Jain, A. (2014). Evaluation of 11 terrestrial carbon–nitrogen cycle models against observations from two temperate Free‐Air CO2 Enrichment studies. New Phytologist, 202(3), 803–822. 10.1111/nph.12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q. , Riley, W. J. , & Tang, J. (2017). A new theory of plant–microbe nutrient competition resolves inconsistencies between observations and model predictions. Ecological Applications, 27(3), 875–886. 10.1002/eap.1490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data are publicly available at the UCAR/NCAR Climate Data Gateway, doi:10.5065/d6154fwh.


