Short abstract
Huntington’s disease, like other neurodegenerative diseases, continues to lack an effective cure. Current treatments that address early symptoms ultimately fail Huntington’s disease patients and their families, with the disease typically being fatal within 10–15 years from onset. Huntington’s disease is an inherited disorder with motor and mental impairment, and is associated with the genetic expansion of a CAG codon repeat encoding a polyglutamine-segment-containing protein called huntingtin. These Huntington’s disease mutations cause misfolding and aggregation of fragments of the mutant huntingtin protein, thereby likely contributing to disease toxicity through a combination of gain-of-toxic-function for the misfolded aggregates and a loss of function from sequestration of huntingtin and other proteins. As with other amyloid diseases, the mutant protein forms non-native fibrillar structures, which in Huntington’s disease are found within patients’ neurons. The intracellular deposits are associated with dysregulation of vital processes, and inter-neuronal transport of aggregates may contribute to disease progression. However, a molecular understanding of these aggregates and their detrimental effects has been frustrated by insufficient structural data on the misfolded protein state. In this review, we examine recent developments in the structural biology of polyglutamine-expanded huntingtin fragments, and especially the contributions enabled by advances in solid-state nuclear magnetic resonance spectroscopy. We summarize and discuss our current structural understanding of the huntingtin deposits and how this information furthers our understanding of the misfolding mechanism and disease toxicity mechanisms.
Impact statement
Many incurable neurodegenerative disorders are associated with, and potentially caused by, the amyloidogenic misfolding and aggregation of proteins. Usually, complex genetic and behavioral factors dictate disease risk and age of onset. Due to its principally mono-genic origin, which strongly predicts the age-of-onset by the extent of CAG repeat expansion, Huntington’s disease (HD) presents a unique opportunity to dissect the underlying disease-causing processes in molecular detail. Yet, until recently, the mutant huntingtin protein with its expanded polyglutamine domain has resisted structural study at the atomic level. We present here a review of recent developments in HD structural biology, facilitated by breakthrough data from solid-state NMR spectroscopy, electron microscopy, and complementary methods. The misfolded structures of the fibrillar proteins inform our mechanistic understanding of the disease-causing molecular processes in HD, other CAG repeat expansion disorders, and, more generally, protein deposition disease.
Keywords: Neurodegeneration, structural biology, aggregation, proteins, biophysics, nuclear magnetic resonance
Introduction
Huntington’s disease (HD) is an inherited neurodegenerative disease (NDD) in which the mutated protein undergoes misfolding and aggregation in patients’ neuronal cells. As such, it is one example of an expanding class of protein misfolding and deposition diseases that include Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).1 The nature of the disease-causing mutation makes HD the most well-known example of a family of CAG repeat expansion disorders. In each of these disorders, the mutation affects a different gene with a naturally occurring CAG repeat, which, when expanded past a disease-specific threshold length, results in an age-dependent NDD (Figure 1(a)). On the protein level, this CAG repeat translates into a polyglutamine repeat, which in the wild-type proteins is typically between 10 and 35 glutamines long. Although the mutated proteins are involved in divergent functional roles, and include both soluble and membrane-bound proteins, the diseases share common features. Six CAG repeat expansion diseases are classified as spinocerebellar ataxias (SCAs) with cerebellum atrophy leading to symptoms such as poor hand or speech coordination, eye movement, or cognitive impairment. Dentatorubral-pallidoluysian atrophy (DRPLA) is characterized by dementia, ataxia, and choreoathetosis. Thus, a common phenotype seems independent of the functions of the mutated protein, which could be rationalized by commonalities in disease-mechanisms driven by protein-based gain-of-toxic-function in these and other protein misfolding diseases,1 rather than merely a loss of the native function. In these NDDs, amyloid formation is a common hallmark, reflecting protein aggregation accompanied by a conformational change of the affected protein to a characteristic β-sheet architecture.1 In AD and PD, our understanding of this conformational transition was recently boosted by high-resolution structures of protein filaments.2–4 Such structural information on the misfolded protein assemblies has proved more challenging to obtain in the case of HD, but important recent progress will be examined in this review.
Figure 1.
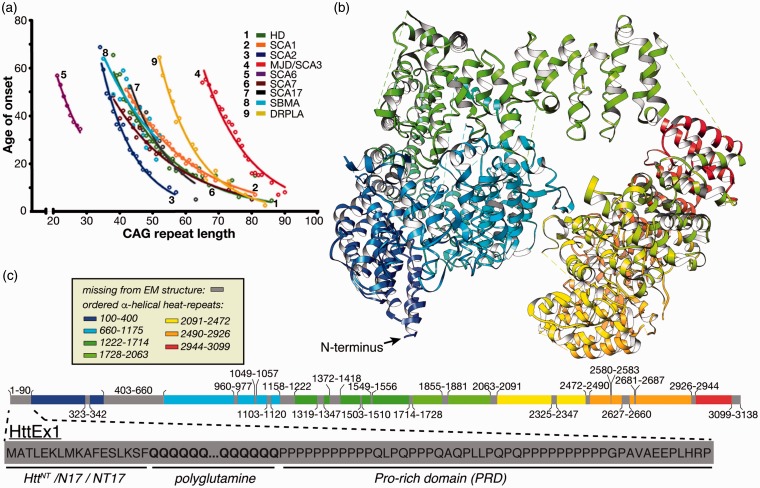
Genetic aspects of HD and other CAG repeat disorders. (a) Age of onset inversely correlates with the extent of expansion, for lengths beyond a disease-specific threshold. The figure was adapted from Kuiper et al.9 with permission. (b) Wild-type Htt structure solved by cryoEM (PDB 6EZ8; Guo et al.8). (c) Color-coded resolved and invisible domain segments from the Htt cryoEM structure, with the HttEx1 that contains the polyglutamine stretch enlarged below.
HD mutant protein
First documented by George Huntington in 1872, HD causes chorea and cognitive disruptions.5 It strikes adults and rarely adolescents, but the latter are afflicted with much harsher symptoms. The connection between HD and mutation of the huntingtin (Htt) protein was identified in the 1990s.6 The age of onset of HD and other polyglutamine expansion diseases is inversely dependent on the length of the CAG repeat (Figure 1(a)). Therefore, HD disease-risk is strongly predicted by its characteristic mutation, in contrast to the genetic and environmental complexities of AD and PD.
Wild-type Htt is a large multidomain protein with functions in various cellular processes.7 Structurally, Htt is prone to dynamic disorder with the most recognizable folded domains forming so-called HEAT repeats. HEAT repeats are α-helical domains found in a number of proteins, whose names explain the HEAT acronym: huntingtin, elongation factor 3, A subunit of protein phosphatase 2A and TOR1 signaling kinase. The large size of Htt and its inherent disorder presented obstacles to structural determination until 2018 when cryo-electron microscopy (cryoEM) was successfully applied to a complex of full-length wild-type Htt stabilized by huntingtin-associated protein 40 (HAP40),8 yielding the Htt structure shown in Figure 1(b). Nonetheless, segments constituting almost a quarter of Htt remain invisible in this structure due to high local flexibility, notably including the first exon of Htt (HttEx1) that features the polyglutamine segment mutated in HD (Figure 1(c)).
This inherent disorder of HttEx1 (and its polyglutamine segment) may be functionally relevant as the native role of polyglutamine segments likely depends on their tendency to be flexible and without well-defined secondary structure. Attempts at capturing a polyglutamine domain by X-ray crystallography have yielded structures in which the polyglutamine segment is either invisible or present in a variety of structural states.10–12 Accordingly, polyglutamine domains seem to act as semi-flexible linkers that connect other functionally relevant domains. Polyglutamine’s conformational ensemble may have unique properties necessary for proper positioning of the domains that flank it.13 Structural studies using fluorescence lifetime imaging microscopy detection of Förster resonance energy transfer (FLIM-FRET) indicate a hinge-like function of polyglutamine domains14,15 showing the importance of intramolecular proximity between N-terminal and proline-rich domains. Others propose an innate ability for interactions with specific protein partners based on a glutamine-rich composition or multi-protein coiled–coil formation.16,17
Aggregation by mutant Htt fragments
The flexible regions of Htt harbor a number of caspase cleavage sites with apparent relevance to HD.18 Caspase 3 cleavage products formed in vitro are consistent with Htt fragments in HD cerebellum, striatum and cortex, including fragments that map onto HttEx1.19 A notable, but as yet poorly understood aspect of the different-length Htt fragments is that they cause different levels of toxicity. For instance, in drosophila the HttEx1 fragments exert particularly high toxicity.20 In human neurons, N-terminal Htt fragments have been identified in neural intranuclear inclusions (NIIs) and dystrophic neurites (DNs) (Figure 2(a)), with their C-terminal counterparts in the cytoplasm.21,22 Remarkably, an HttEx1 fragment also forms via erroneous splicing of the mutant protein.23
Figure 2.
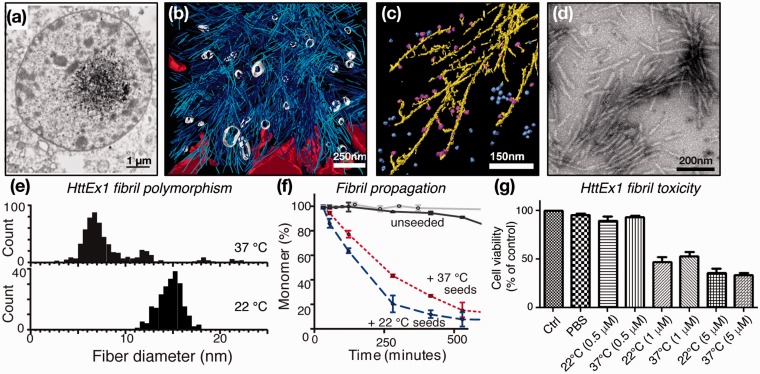
Mutant HttEx1 fibrils in vivo and in vitro. (a) Neuronal inclusions from HD patient; adapted from DiFiglia et al.21 with permission from AAAS. (b) 3D rendering of tomographic EM showing the interaction zone between an inclusion body and cellular membranes in a HttQ97-transfected HeLa cell. ER membranes (red), ER-bound ribosomes (green), HttQ97 fibrils (cyan), and vesicles inside the IB (white). Adapted from Bauerlein et al.27 with permission from Cell. (c) Annotated 3D EM tomogram of Q51-HttEx1 fibrils (yellow) in presence of TRiC chaperones (blue/magenta). Adapted with permission from Shahmoradian et al.64 (d) Negative-stain EM of Q44-HttEx1 fibrils used for ssNMR study. Adapted from Hoop et al.56 (e) Q44-HttEx1 forms temperature-dependent polymorphs with different widths, which seed polyglutamine protein aggregation (f) and cause neuronal toxicity (g). Panels (e–g) were adapted with permission from Lin et al.32
The findings described above led to many studies of HttEx1 in model animals and neuronal cells, which commonly observe HD-like symptoms, neuronal degeneration, and HttEx1 inclusions.24–26 A recent cryoEM tomography study provided a view of the structures formed by aggregated HttEx1 in a cellular context.27 A cluster of filamentous structures is observed (Figure 2(b)), with individual filaments resembling HttEx1 fibrils formed in vitro (Figure 2(c) and (d)). Of potential disease-mechanistic relevance, the fibrils interact with various subcellular organelles, leading, for example, to deformation of the endothelial reticulum (ER) membrane. Thus, μm-sized puncta seen in fluorescence studies likely contain numerous much smaller filaments. In isolation, these filaments are hard or impossible to detect unless super-resolution optical methods are applied.28,29 This distinction may in part underlie the apparent disconnect between observable aggregate load and neurotoxicity, given also that isolated fibrils (assembled in vitro) are toxic to cells but may be small enough to be missed in fluorescence assays (Figure 2(g)).28,30–32
Polyglutamine segments have long been known to undergo self-assembly in vitro. This aggregation propensity depends on the repeat length,33,34 both in polyglutamine peptides and in the context of HttEx1. Morphologically, both polyglutamine peptides and HttEx1 form filamentous assemblies (Figure 2), as seen by negative-stain transmission electron microscopy (TEM) or atomic force microscopy (AFM).27,33,35,36 Crucially, the kinetics of self-assembly are highly dependent on not only the polyglutamine length but also the segments flanking the polyglutamine (Figure 3(a)).37 The aggregation of HttEx1 is dramatically more efficient than that of the corresponding polyglutamine peptide,38,39 while the flanking regions also affect the aggregates’ morphology and toxicity.37,40,41
Figure 3.
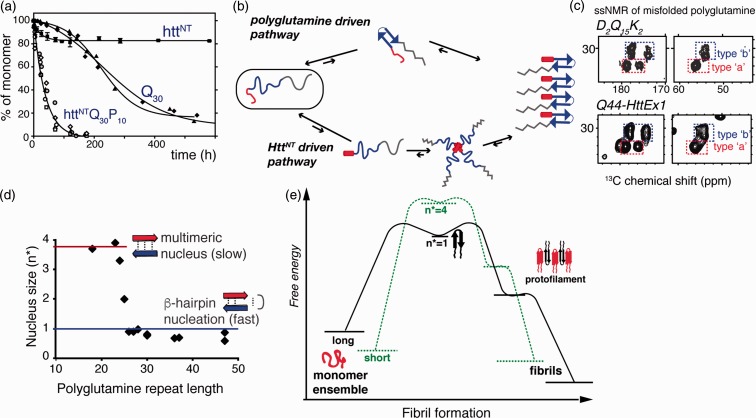
Mechanisms of aggregation. (a) Expanded polyglutamine without (Q30) and with (httNTQ30P10K2) Htt flanking regions attached show dramatically different aggregation kinetics. Adapted from Sivanandam et al.77 with permission from the American Chemical Society. (b) Intrinsically disordered HttEx1 can aggregate via both polyglutamine driven (top) and HttNT- driven mechanisms (bottom). (c) Both mechanisms yield products with the same ssNMR signals for the polyglutamine fibril core structure (see Figure 4): spectra of Q44-HttEx1 and polyglutamine peptide, D2Q15K2. Adapted from Hoop et al.56. (d) Length-dependent nucleus size data96–98 show that fast aggregation of long polyglutamine is facilitated by monomeric nucleation, likely involving β-hairpin formation. (e) Schematic free energy profile of fibril formation and growth, for short and long polyglutamine, based on published nucleation and fiber elongation energy values.96,97,99,100
The enhancement of aggregation has been traced to the N-terminal segment preceding the polyglutamine domain, commonly known as N17, NT17, or HttNT (Figure 1(c)).38,41,42 Note that in vivo the first Met is likely removed to yield a 16-residue HttNT starting with an acetylated Ala. HttNT is critical for the trafficking and localization of Htt and has a highly conserved primary sequence. In isolation, HttNT is in a concentration-dependent equilibrium between disordered monomers and α-helical multimers.38,43,44 Intermolecular interactions, including HttNT self-assembly, stabilize amphipathic α-helical structure in HttNT.10,45,46 The C-terminal proline-rich domain (PRD) of HttEx1 greatly reduces the propensity for aggregation,47,48 but its effect is outweighed by HttNT, when present (Figure 3(a)). PRD-binding proteins, such as FE65,49 often do so by recognizing the polyproline II (PPII) helical structure of the oligoproline motifs. This same PPII propensity is thought to underpin the aggregation inhibition.38,47,48
Clearly there is an important interplay in the unaggregated protein between the behavior of the polyglutamine domain and its respective flanking domains. Numerous studies, both experimental and computational, have probed this soluble structural ensemble50–52 but in the current review we will focus on structural studies of the misfolded aggregates.
Structural analysis of polyglutamine aggregates
Shortly after the discovery that expanded polyglutamine proteins result in the protein deposition of HD, models of the aggregate structures were proposed. Perutz et al.53 advocated a structure of pleated antiparallel β-sheets, stabilized by hydrogen bonds between the glutamine side chain and backbone. Early X-ray structural studies of polyglutamine aggregates revealed a cross-β diffraction pattern that is the hallmark of amyloids and amyloid-like structures.54 Similar data were obtained for both polyglutamine peptides and fibrillar HttEx1,54 a finding reproduced by later studies.55,56 The fiber diffraction data contain insufficient information to define a unique atomic-level structure but did lead to a new structural hypothesis. First, Perutz et al. 57 proposed a parallel β-sheet-based tubular fold, offering a potential rationale for the HD expansion threshold. However, a later report argued for an alternative explanation of the same data, favoring an antiparallel rather than parallel β-sheet structure, and featuring β-hairpin motifs.58 Note that the idea of β-hairpin formation during polyglutamine aggregation was invoked very early on by Perutz et al.54 The antiparallel β-sheet architecture was supported by an independent X-ray study, which reported subtle variations among different-length polyglutamine peptides and proposed distinct structures with kinked side chains.55 Thus, while X-ray studies provided unambiguous evidence of an amyloid-like architecture that was shared by aggregated polyglutamine and HttEx1, they were unable to resolve a unique atomic structure.
Various other techniques provided important clues regarding the structure of aggregated polyglutamine. Circular dichroism (CD), Fourier-transform infrared spectroscopy (FTIR), and UV resonance Raman (UVRR) spectroscopy indicated the formation of β-sheets, and specifically antiparallel β-sheets.59–63 Notably, the Zanni group combined multidimensional IR with isotopic labeling to observe β-hairpin structures in aggregated K2Q24K2W.61 As discussed below, β-hairpin motifs were subsequently also detected in the expanded polyglutamine segment of aggregated mutant HttEx1.32
Recent progress in HttEx1 fibril structure
The recent productive efforts toward a structural understanding of disease-relevant mutant HttEx1 aggregates combined a number of biophysical methods. In isolation, techniques like EM, nuclear magnetic resonance (NMR), and electron spin resonance (ESR) provide incomplete information, but together yield a compelling view of the fibrils’ structure and dynamics. We have already introduced various key contributions made by EM (Figure 2). Moreover, EM and AFM have both revealed other significant characteristics of HttEx1 fibrils, such as their propensity to display branch points that is attributed to surface-mediated secondary-nucleation events.35,64,65 However, unlike recent breakthrough studies of other protein filaments,3,4,66 EM thus far failed to resolve the atomic structure of HttEx1 or polyglutamine aggregates, seemingly due to the fibrils’ structural heterogeneity.27,64,67
NMR studies of fibrillar HttEx1
Liquid-state NMR studies have probed soluble ensembles of polyglutamine-containing peptides and proteins.68–74 This technique excels at providing structural and dynamic data on rapidly tumbling molecules in solution. However, as soon as expanded polyglutamine proteins self-assemble, they quickly form structures that no longer tumble fast enough to be tractable by solution NMR. This is due to the fact that immobilized or slowly tumbling molecules yield very broad spectra with low intensities, thus preventing effective characterization. In recent years, modern solid-state NMR (ssNMR) protocols and instrumentation have made it feasible to study large and immobilized protein assemblies, even in presence of dynamic and static disorder,75,76 resulting in ssNMR becoming an essential tool for amyloid protein research.2,75 Using magic angle spinning (MAS), the immobilized large protein assemblies are rapidly rotated in the magnetic field to generate high-resolution ssNMR spectra, independent of assembly size. The initial applications of ssNMR to polyglutamine-based aggregates made less than a decade ago71,77 immediately revealed a number of intriguing and important features. First, despite dramatic differences in aggregation kinetics or propensity, different labs consistently reported a highly characteristic “signature” for the self-assembled misfolded state of the polyglutamine stretch itself (Figures 3(c) and 4(a)). This ssNMR signature consistently combines highly atypical resonance frequencies with two equally populated sets of distinct NMR signals, in a unique combination that is only seen in polyglutamine aggregates and not in any other proteins studied by solution or solid-state NMR.78 The latter peak doubling arises even when a single residue is labeled, indicating a structural heterogeneity on the single-residue level.
Figure 4.
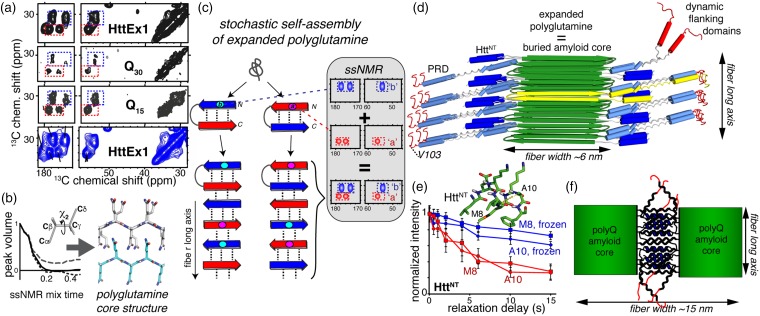
SSNMR structural studies of misfolded fibrils. (a) Fibrillar polyglutamine gives an identical ssNMR signature, whether in Q44-HttEx1 fibrils (top), Q46-HttEx1 fibrils (bottom), or polyglutamine peptides with 15 or 30 glutamines. Data from Lin et al.32 and Sivanandam et al.77 Data for Q46-HttEx1 were adapted with permission from Isas et al.79 copyright 2015 American Chemical Society. (b) Tailored ssNMR dihedral angle measurements probe the polyglutamine core structure, and reveal two differently structured β-strand types (adapted from Hoop et al.56). (c) A stochastic assembly process of the alternating β-strand structures explains the ssNMR spectral signature of polyglutamine amyloid. Adapted with permission from Hoop et al.56 (d) Architecture of mutant HttEx1 fibrils derived from ssNMR and EM constraints. (e) SSNMR relaxation measurements show dynamic changes in the solvent-exposed HttNT α-helical segment upon solvent freezing. Adapted from Hoop et al.,80 with permission from the American Chemical Society. (f) Schematic illustration of flanking-domain interactions enabling higher order assembly of the wider HttEx1 fibril polymorphs shown in Figure 2(e). Panels (d) and (f) are adapted with permission from Lin et al.32 (A color version of this figure is available in the online journal.)
Structural ssNMR measurements and mechanistic studies provide a compelling rationale for this surprising, but reproducible, doubled ssNMR signature (see below; Figures 3(c) and 4(a)). First, ssNMR measurements of backbone and side chain torsion angles confirmed that the two signals derive from two distinct conformations (Figure 4(b)).56 Inter-residue ssNMR correlations show that each of these conformers is present in surprisingly long, uninterrupted stretches.56,74,77–80 More specifically, they form equal amounts of two distinct types of uninterrupted β-strands. The two strands differ in their side chain torsion angles, but within each strand all residues have the same backbone and side chain geometry (Figure 4(b)). Both β-strands do share a 180° χ2 angle – implying an extended side chain structure56 akin to the steric zipper concept seen in other amyloids.81 A recent ssNMR study that employed dynamic nuclear polarization (DNP) allowed for the structural fingerprinting of unlabeled HttEx1 aggregates and provided further evidence for their antiparallel β-sheet assembly.56,82 In such an antiparallel arrangement, the two ssNMR-revealed β-strand types appear capable of inter-strand hydrogen bonding to each other (but not themselves). An assembled antiparallel β-sheet requires the presence of equal amounts of the two complementary β-strand geometries.83 Thereby, these findings provide a rationale for the polyglutamine amyloid ssNMR signature featuring equal amounts of the two corresponding sets of ssNMR signals. All ssNMR studies of polyglutamine-based aggregates with a single labeled residue show both signals for that labeled residue.56,77,78,80,84 This points to a stochastic assembly process in which any glutamine segment (or residue) has a 50/50 chance of adopting either of the two complementary β-strand motifs (Figure 4(c)56).
Polyglutamine length and the HD disease threshold
Polyglutamine retains solubility up to a length of approximately seven residues,44,85 but peptides of eight or more (well below the HD threshold) are routinely studied in their aggregated state. Interestingly, polyglutamine peptides with 15 to over 50 residues yield identical chemical shift patterns by ssNMR that are indistinguishable from those of HttEx1 fibrils’ amyloid core (Figures 3(c) and 4(a)).71,77,79,80,86 The ssNMR chemical shifts are very sensitive to changes in structure and typically show clear differences between amyloid polymorphs with different core structures.87 In ab initio calculations, the experimental ssNMR chemical shifts are inconsistent with the pre-ssNMR structural models of polyglutamine discussed above.56 Therefore, these ssNMR data are in apparent contradiction with reports arguing for dramatic changes in architecture between short and long polyglutamine aggregates.55,88,89
Does this imply there is no length-dependent transition in the misfolded structure or misfolding mechanism as a rationale for the disease threshold? One potential rationale has been proposed that integrates much of the available structural and mechanistic data. Mixed-isotope ssNMR experiments, reminiscent of the above-mentioned IR study,61 identified intramolecularly hydrogen-bonded β-hairpins in the amyloid core of Q44-HttEx1 fibrils (Figure 4(c) and (d)).56 The weak intensity from non-β-strand residues indicated that no more than a single turn was present. This implied 20-residue strands constitute the β-hairpin, which is reminiscent of the detection in a prior ssNMR study of surprisingly long strand segments in polyglutamine aggregates.71 Their study of a 15-residue polyglutamine peptide with isotopic labels mid-peptide did not reveal any turn structure signals. The Q15 peptides featured a single β-strand per peptide, while the β-hairpin Q44-HttEx1 fibrils contained two 20-residue strands (Figure 4(d)). Thus, the propensity for β-hairpin formation during polyglutamine (or HttEx1) aggregation would depend on the polyglutamine expansion length, with direct implications for the aggregation kinetics. In this model, β-hairpin formation may be required to achieve a sufficiently high aggregation propensity that cannot be suppressed by the cellular protein homeostasis machinery (under in vivo conditions). Interestingly, the currently available in vitro data (Figure 3(d)) point to the switch to monomeric nucleation occurring well below the typical disease thresholds. This could imply that β-hairpin-mediated aggregation is necessary but not sufficient for disease onset. Alternatively, there may be an as-yet unknown effect of environmental (cellular) factors modulating the polyglutamine aggregation process in vivo, dictating whether or not a particular polyglutamine length follows a β-hairpin-driven aggregation process or not.
HttEx1 polymorphism
Various studies have noted that a particular HttEx1 protein can adopt multiple types of misfolded or aggregated structures (e.g. Figure 2(e)).31,60 Such amyloid polymorphism is also common in other disease-associated protein aggregates.87 Early studies suggested that HttEx1 polymorphism stems from an architectural change of the polyglutamine stretch.60 However, ssNMR experiments consistently report identical signatures for the polyglutamine stretch, with polymorph-dependent differences localized to the non-amyloid flanking segments. This suggests that expanded polyglutamine forms a reproducible protofilament structure that can co-assemble into different supramolecular architectures, stabilized by variable flanking region interactions.32 Conceptually analogous “supramolecular polymorphs”3 have subsequently been described in several other disease-related amyloids, indicating that this may be a more general phenomenon.3,4,66,90,91
Both ssNMR and ESR studies have been instrumental in probing the fate of the flanking regions in HttEx1 post-aggregation.32,45,74,77,79,80,84,86 In contrast with the rigid and well-structured polyglutamine motif, both flanking segments are consistently found to display significant dynamics. The greatest flexibility is associated with the C-terminal end of HttEx1, which usually retains similar dynamics and accessibility before and after aggregation.32,79,92,93 Approaching the rigid polyglutamine amyloid core, the PRD becomes increasingly rigid, presumably due to intermolecular interactions.32,79,92,93 The HttNT flanking segment also has increased mobility and solvent accessibility (Figure 4(d) and (e)) relative to the polyglutamine fibril core.32,77,79,80 The reported dynamics and secondary structure of the HttNT varied among different studies, which may relate to a combination of fibril polymorphism, differences in protein constructs employed, and different fibril formation protocols. In our own work,32,77,84 ssNMR studies of HttNT in the fibrils consistently point to an α-helical conformation and a partial, though incomplete, immobilization (Figure 4(e)), likely facilitated by dense packing of the flanking segments on the fibril surface (Figure 4(d)).
When comparing different HttEx1 polymorphs (Figure 2(e)), ssNMR studies show clear differences in the dynamics and solvent accessibility of the flanking segments.32,93 Antibodies that recognize specific parts of the HttEx1 sequence also bind differently.32 The variable flanking segment exposure in HttEx1 polymorphs points to a mechanism for stabilizing the supramolecular polymorphs (Figure 4(f)), leading to the distinct fiber widths observed by EM (Figure 2(e)). These differences in burial or exposure of the flanking sequences should also modulate the biological (e.g. toxic) activity of the misfolded protein. For example, exposed and dynamic79,80,92 HttNT are implicated in membrane interactions, and their accessibility will affect the filaments’ ability to bind cellular membranes.27,94,95 The flanking segments’ varying ability to engage with biological membranes, chaperones, and other protein binding partners will have direct relevance for various pathogenic mechanisms.
Biological and mechanistic implications
The increased availability of structural information has greatly improved our understanding of the mechanisms by which polyglutamine proteins misfold, aggregate, and contribute to disease. Like other amyloidogenic processes, polyglutamine aggregation is nucleation driven. The nucleation event is the rate-limiting step with a positive free energy (ΔG) and very small equilibrium constant for nucleus formation (Kn*)96 (Figure 3(e)). Once a minimal nucleus is formed, the elongation process is thermodynamically favorable and spontaneous.99,101 For polyglutamine, Kn* increases with longer repeat lengths, leading to ever faster aggregation of proteins with longer polyglutamine repeats.96 Thus, Htt fragments with very long glutamine repeats nucleate more quickly than those with short repeats. Why does Kn* show this length dependence, and which conformation can lower the free energy of nuclei for longer repeat lengths? The size of the critical nucleus depends on polyglutamine length (Figure 3(d))100,101: although short polyglutamines aggregate, they require a multimeric nucleation event. The rate-limiting step for long polyglutamine manifests instead as a monomeric event.100,101 Various lines of evidence support the idea that monomeric nucleation reflects the formation of a β-hairpin within the expanded polyglutamine monomer. Structural studies detect β-hairpin structures in the end-product of aggregated polyglutamine and Q44-HttEx1.56,61 Moreover, β-hairpin-favoring mutations accelerate aggregation kinetics and increase fiber stabilities, without changing the characteristic polyglutamine ssNMR signature.78
Molecular dynamics (MD) simulation studies can examine transient events and dynamic processes that are hard to probe experimentally. Given the focus on this review, we refer readers to other recent articles.50,51 We do note an interesting set of MD studies that probed polyglutamine folds that may be compatible with the noted “monomeric” mechanism,100,102 since they favored β-hairpin motifs over less stable β-nanotube or β-pseudohelix conformations.
As noted above, all polyglutamine aggregate studied by ssNMR to date share a common characteristic ssNMR signature. One intriguing possibility is that this reflects a common core structure that may extend to all polyglutamine proteins associated with disease and that all the expanded-polyglutamine proteins aggregate via an analogous misfolding pathway. The different disease thresholds may relate to the impact of protein context (i.e. flanking domains) on the propensity for the polyglutamine region to adopt an elongation-capable β-hairpin. In HD, the flanking regions are dynamically disordered, allowing the polyglutamine segment termini to readily approach each other. Yet, in other polyglutamine proteins, combinations of steric interactions and/or electrostatic repulsion of the flanking domains may hinder β-hairpin formation. On the other hand, the protein fold of the α1 subunit of the neuronal P/Q-type voltage-gated calcium channel (associated with SCA6 disease103,104) may place its polyglutamine stretch in a conformation that encourages β-hairpin formation. This could rationalize the reduced threshold seen in SCA6, compared to the other polyglutamine expansion diseases104 (Figure 1(a)). Further structural and mechanistic studies are needed to test these proposals.
Oligomerization
Some reports claim that long polyglutamine peptides form small spherical oligomers (dimers and trimers) that coexist with larger ones in solution.89 However, other studies offer compelling evidence that polyglutamine de novo aggregation proceeds without formation of defined oligomers.96,97 There is more evidence for the formation of prefibrillar oligomeric assemblies for expanded HttEx1.105–108 In cells, wild-type HttEx1 can undergo a type of liquid–liquid phase separation, while polyglutamine-expanded HttEx1 assembles into more compact irreversible inclusions.109 When studied structurally, HttEx1 oligomers generally appear to be α-helical, rather than β-sheet-rich like Aβ oligomers.38,44,77,110 This helical structure is stabilized by bundling of α-helical HttNT, with the α-helical structure likely extending into the initial parts of the polyglutamine.44,77
The above differs notably from the extensively studied oligomers formed by the AD Aβ peptide. Aβ oligomers feature antiparallel β-sheet structures, which are distinct from the in-register parallel β-sheet fibrils, and which by many are proposed to reflect β-hairpins. An intriguing possibility is that β-hairpin formation may similarly occur during polyglutamine and Aβ aggregation, but that (unlike polyglutamine) the Aβ β-hairpin motif is not accommodated in the filamentous structure, instead becoming trapped in the semi-stable oligomeric intermediates.
The role of aggregates in disease
There is an ongoing debate whether Htt-related aggregates in the brain are cytotoxic and cause disease development, or the toxicity comes from other species, such as soluble oligomers or even misfolded monomers. Some studies showed no correlation between Htt aggregates and cell toxicity.111,112 On the other hand, many studies have shown toxic effects of HttEx1 or polyglutamine aggregates on neuronal cells.60,113–115 Various rationales have been proposed to unify these seemingly contradictory findings, and we will discuss some with an eye on the current knowledge of HttEx1 aggregate structure. First, as noted above, fluorescence-based assays commonly used in evaluating aggregate load may not reliably measure sub-diffraction sized protein deposits. As illustrated in Figure 2, individual filaments have widths on the nm-scale and are thus orders of magnitude smaller than visible inclusions. Thus, cells lacking μm-sized puncta may nonetheless contain significant amounts of protein filaments. Second, different polymorphs can exhibit substantially different levels of toxicity. The toxicity of polyglutamine proteins is dependent of the flanking sequences, protein–protein interactions, and fibril polymorphism.40,60 For example, in a mouse that expressed a truncated fragment of expanded Htt that is longer than HttEx1 (shortstop mouse), the existence of neural inclusions did not lead to neural abnormalities or degeneration.112
This also raises the fundamental question of the mechanism of toxicity. In our view, this issue remains largely unresolved. One mechanism specific to polyglutamine proteins is the recruitment or sequestration of other proteins featuring polyglutamine repeats by the aggregates, which similarly affects wild-type and expanded polyglutamine proteins. Thus, the cellular concentrations of essential proteins could be lowered to the point of dysfunction or toxicity. A study that attempted to test this mechanism using D-amino-acid polyglutamine aggregates found that even these fibrils induced toxicity in PC12 neuronal cells, but also offered a potential structural rationale by which observed recruitment could cross the chiral barrier.115 Aggregated Htt is also thought to impair intracellular trafficking into organelles such as the mitochondria and nucleus, potentially by interacting with the import/export protein machinery. Moreover, polyglutamine protein inclusions sequester many non-polyglutamine proteins, including components of the protein quality control machinery. Thus, fibrils are known to have various detrimental effects. There is also a growing interest in the finding that Htt fibers are able to propagate from one cell to another, enabling disease propagation via a prion-like process.116,117 This phenomenon requires the fibers to have conformations amenable to them crossing cellular membranes and seeding the self-assembly of other proteins in nearby cells. In other words, the polymorphism of Htt-derived aggregates, which we are only just beginning to understand, can likely dramatically alter their biological behavior and pathogenic effects in a way that is orthogonal to the apparent aggregate load.
Counteracting and controlling protein aggregation
Nature has deployed cellular protection mechanisms to counteract disease-causing protein misfolding and aggregation. Molecular chaperones refold misfolded proteins and prevent pathogenic protein aggregation.118–120 Chaperones such as TRiC, DNAJB6 and DNAJB8 have been shown to inhibit Htt aggregation.64,121,122 The proposed molecular mechanisms by which these chaperones act are mirrored in the abovementioned structural data for the aggregates. TRiC binds the α-helical HttNT that was detected by ssNMR in the HttEx1 fibrils, while the mentioned DNAJ co-chaperones appear to inhibit primary nucleation by recognizing β-hairpins. Thus, the fiber structures mirror the very structural features that can be targeted for the inhibition or modulation of aggregation. Along similar lines, post-translational modifications (PTMs) offer a mechanism for modulating disease risk and aggregation. Phosphorylation of HttNT is dependent on the glutamine repeat length123,9 and changes aggregation and toxicity.84,125 The clustering of the PTMs’ repulsive charges in the densely packed HttNT in misfolded Htt (Figure 4) leads to a destabilization that enhances the neurons’ ability to target and clear the Htt aggregates. Thus, insights into the structures of disease-associated protein deposits can direct efforts to design novel or enhanced treatment strategies. One critical component in such efforts will be the delineation of the structural variables (e.g. supramolecular architecture and/or exposure of flanking domains) that most strongly predict biological functions such as neuronal toxicity, membrane interactions, and inter-neuron propagation. We hope that progress made in understanding HD may also inform our understanding (and treatment) of more complex NDDs like AD, PD and ALS.
ACKNOWLEDGMENTS
We thank James Conway, Matt Lee, Mingyue Li, and Talia Piretra for their careful reading of the manuscript and their insightful suggestions.
Authors’ contributions
Both authors performed the literature review, preparation of figures, writing and reviewing of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge funding from NIH grants R01 GM112678 and AG019322 supporting our polyglutamine research.
References
- 1.Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 2017; 86:27–68 [DOI] [PubMed] [Google Scholar]
- 2.van der Wel P. Insights into protein misfolding and aggregation enabled by solid-state NMR spectroscopy. Solid State Nucl Magn Reson. 2017; 88:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres S. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017; 547:185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R, Britschgi M, Stahlberg H. Cryo-EM structure of alpha-synuclein fibrils. Elife 2018; 7:43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 2000; 403:544–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993; 72:971–83 [DOI] [PubMed] [Google Scholar]
- 7.Saudou F, Humbert S. The biology of Huntingtin. Neuron 2016; 89:910–26 [DOI] [PubMed] [Google Scholar]
- 8.Guo Q, Bin H, Cheng J, Seefelder M, Engler T, Pfeifer G, Oeckl P, Otto M, Moser F, Maurer M, Pautsch A, Baumeister W, Fernandez-Busnadiego R, Kochanek S. The cryo-electron microscopy structure of huntingtin. Nature 2018; 555:117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuiper EF, de Mattos EP, Jardim LB, Kampinga HH, Bergink S. Chaperones in polyglutamine aggregation: beyond the Q-stretch. Front Neurosci 2017; 11:145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambashivan S, Liu Y, Sawaya MR, Gingery M, Eisenberg D. Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature 2005; 437:266–9 [DOI] [PubMed] [Google Scholar]
- 11.Kim MW, Chelliah Y, Kim SW, Otwinowski Z, Bezprozvanny I. Secondary structure of Huntingtin amino-terminal region. Structure 2009; 17:1205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MW. Beta conformation of polyglutamine track revealed by a crystal structure of Huntingtin N-terminal region with insertion of three histidine residues. Prion 2013; 7:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgini F. A flexible polyglutamine hinge opens new doors for understanding Huntingtin function. Proc Natl Acad Sci U S A 2013; 110:14516–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caron NS, Desmond CR, Xia J, Truant R. Polyglutamine domain flexibility mediates the proximity between flanking sequences in huntingtin. Proc Natl Acad Sci U S A 2013; 110:14610–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truant R, Atwal RS, Desmond C, Munsie L, Tran T. Huntington’s disease: revisiting the aggregation hypothesis in polyglutamine neurodegenerative diseases. FEBS J 2008; 275:4252–62 [DOI] [PubMed] [Google Scholar]
- 16.Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and polyQ proteins. Cell 2010; 143:1121–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon MJ, Han MH, Bagley JA, Hyeon DY, Ko BS, Lee YM, Cha IJ, Kim SY, Kim DY, Kim HM, Hwang D, Lee SB, Jan YN. Coiled-coil structure-dependent interactions between polyQ proteins and Foxo lead to dendrite pathology and behavioral defects. Proc Natl Acad Sci U S A 2018; 18:E10748--E10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thornberry N, Pinsky L, Kakizuka A, Ross CA, Nicholson DW, Bredesen DE, Hayden MR. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem 1998; 273:9158–67 [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, Qin ZH, Aronin N, DiFiglia M. Caspase 3-cleaved N-terminal fragments of wild-type and mutant Huntingtin are present in normal and Huntington’s disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A 2001; 98:12784–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbaro BA, Lukacsovich T, Agrawal N, Burke J, Bornemann DJ, Purcell JM, Worthge SA, Caricasole A, Weiss A, Song W, Morozova OA, Colby DW, Marsh JL. Comparative study of naturally occurring Huntingtin fragments in Drosophila points to exon 1 as the most pathogenic species in Huntington‘s disease. Hum Mol Genet 2015; 24:913–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 1997; 277:1990–3 [DOI] [PubMed] [Google Scholar]
- 22.Jones AL. The localization and interactions of huntingtin. Philos Trans R Soc Lond B Biol Sci 1999; 354:1021–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathasivam K, Neueder A, Gipson TA, Landles C, Benjamin AC, Bondulich MK, Smith DL, Faull RLM, Roos RAC, Howland D, Detloff PJ, Housman DE, Bates GP. Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease. Proc Natl Acad Sci U S A 2013; 110:2366–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling G, Klevytska A, Tebbenkamp ATN, Juenemann K, Cooper J, Gonzales V, Slunt H, Poirer M, Ross CA, Borchelt DR. Characterization of huntingtin pathologic fragments in human Huntington disease, transgenic mice, and cell models. J Neuropathol Exp Neurol 2007; 66:313–20 [DOI] [PubMed] [Google Scholar]
- 25.Morton AJ, Howland DS. Large genetic animal models of Huntington’s disease. J Huntingtons Dis 2013; 2:3–19 [DOI] [PubMed] [Google Scholar]
- 26.Stricker-Shaver J, Novati A, Yu-Taeger L, Nguyen HP. Genetic rodent models of Huntington disease. Adv Exp Med Biol 2018; 1049:29–57 [DOI] [PubMed] [Google Scholar]
- 27.Bäuerlein FJB, Saha I, Mishra A, Kalemanov M, Martínez-Sánchez A, Klein R, Dudanova I, Hipp MS, Hartl FU, Baumeister W, Fernández-Busnadiego R. In situ architecture and cellular interactions of polyQ inclusions. Cell 2017; 171:179–87.e10 [DOI] [PubMed] [Google Scholar]
- 28.Sahl SJ, Weiss LE, Duim WC, Frydman J, Moerner WE. Cellular inclusion bodies of mutant huntingtin exon 1 obscure small fibrillar aggregate species. Sci Rep 2012; 2:895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duim WC, Jiang Y, Shen K, Frydman J, Moerner WE. Super-resolution fluorescence of huntingtin reveals growth of globular species into short fibers and coexistence of distinct aggregates. ACS Chem Biol 2014; 9:2767–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrasate M, Finkbeiner S. Protein aggregates in Huntington’s disease. Exp Neurol 2012; 238:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant Huntingtin and the risk of neuronal death. Nature 2004; 431:805–10 [DOI] [PubMed] [Google Scholar]
- 32.Lin HK, Boatz JC, Krabbendam IE, Kodali R, Hou Z, Wetzel R, Dolga AM, Poirier MA, van der Wel P. Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core. Nat Commun 2017; 8:15462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 1997; 90:549–58 [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Berthelier V, Yang W, Wetzel R. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol 2001; 311:173–82 [DOI] [PubMed] [Google Scholar]
- 35.Dahlgren PR, Karymov MA, Bankston J, Holden T, Thumfort P, Ingram VM, Lyubchenko YL. Atomic force microscopy analysis of the Huntington protein nanofibril formation. Nanomedicine 2005; 1:52–7 [DOI] [PubMed] [Google Scholar]
- 36.Legleiter J, Lotz GP, Miller J, Ko J, Ng C, Williams GL, Finkbeiner S, Patterson PH, Muchowski PJ. Monoclonal antibodies recognize distinct conformational epitopes formed by polyglutamine in a mutant Huntingtin fragment. J Biol Chem 2009; 284:21647–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nozaki K, Onodera O, Takano H, Tsuji S. Amino acid sequences flanking polyglutamine stretches influence their potential for aggregate formation. Neuroreport 2001; 12:3357–64 [DOI] [PubMed] [Google Scholar]
- 38.Thakur AK, Jayaraman M, Mishra R, Thakur M, Chellgren VM, Byeon IJL, Anjum DH, Kodali R, Creamer TP, Conway JF, Gronenborn AM, Wetzel R. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol 2009; 16:380–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, Frydman J. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol 2009; 16:1279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duennwald ML, Jagadish S, Muchowski PJ, Lindquist SL. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci U S A 2006; 103:11045–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum Mol Genet 2007; 16:61–77 [DOI] [PubMed] [Google Scholar]
- 42.Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet 2007; 16:2600–15 [DOI] [PubMed] [Google Scholar]
- 43.Kelley NW, Huang X, Tam S, Spiess C, Frydman J, Pande VS. The predicted structure of the headpiece of the huntingtin protein and its implications on huntingtin aggregation. J Mol Biol 2009; 388:919–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaraman M, Kodali R, Sahoo B, Thakur AK, Mayasundari A, Mishra R, Peterson CB, Wetzel R. Slow amyloid nucleation via α-helix-rich oligomeric intermediates in short polyglutamine-containing huntingtin fragments. J Mol Biol 2012; 415:881–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalek M, Salnikov ES, Bechinger B. Structure and topology of the huntingtin 1-17 membrane anchor by a combined solution and solid-state NMR approach. Biophys J 2013; 105:699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Genst E, Chirgadze DY, Klein FAC, Butler DC, Matak-Vinković D, Trottier Y, Huston JS, Messer A, Dobson CM. Structure of a single-chain Fv bound to the 17 N-terminal residues of huntingtin provides insights into pathogenic amyloid formation and suppression. J Mol Biol 2015; 427:2166–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharyya A, Thakur AK, Chellgren VM, Thiagarajan G, Williams AD, Chellgren BW, Creamer TP, Wetzel R. Oligoproline effects on polyglutamine conformation and aggregation. J Mol Biol 2006; 355:524–35 [DOI] [PubMed] [Google Scholar]
- 48.Darnell G, Orgel JP, Pahl R, Meredith SC. Flanking polyproline sequences inhibit beta-sheet structure in polyglutamine segments by inducing PPII-like helix structure. J Mol Biol 2007; 374:688–704 [DOI] [PubMed] [Google Scholar]
- 49.Chow WNV, Luk HW, Chan HYE, Lau K-F. Degradation of mutant huntingtin via the ubiquitin/proteasome system is modulated by FE65. Biochem J 2012; 443:681–9 [DOI] [PubMed] [Google Scholar]
- 50.Wetzel R. Physical chemistry of polyglutamine: intriguing tales of a monotonous sequence. J Mol Biol 2012; 425:466–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papaleo E, Invernizzi G. Conformational diseases: structural studies of aggregation of polyglutamine proteins. Curr Comput Aided Drug Des 2011; 7:23–43 [DOI] [PubMed] [Google Scholar]
- 52.Hoffner G, Djian P. Polyglutamine aggregation in Huntington disease: does structure determine toxicity? Mol Neurobiol 2015; 52:1297–314 [DOI] [PubMed] [Google Scholar]
- 53.Perutz MF, Staden R, Moens L, De Baere I. Polar zippers. Curr Biol 1993; 3:249–53 [DOI] [PubMed] [Google Scholar]
- 54.Perutz MF, Johnson T, Suzuki M, Finch JT. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A 1994; 91:5355–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma D, Shinchuk LM, Inouye H, Wetzel R, Kirschner DA. Polyglutamine homopolymers having 8-45 residues form slablike beta-crystallite assemblies. Proteins 2005; 61:398–411 [DOI] [PubMed] [Google Scholar]
- 56.Hoop CL, Lin HK, Kar K, Magyarfalvi G, Lamley JM, Boatz JC, Mandal A, Lewandowski JR, Wetzel R, van der Wel PC. Huntingtin exon 1 fibrils feature an interdigitated beta-hairpin-based polyglutamine core. Proc Natl Acad Sci U S A 2016; 113:1546–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perutz MF, Finch JT, Berriman J, Lesk A. Amyloid fibers are water-filled nanotubes. Proc Natl Acad Sci U S A 2002; 99:5591–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sikorski P, Atkins E. New model for crystalline polyglutamine assemblies and their connection with amyloid fibrils. Biomacromolecules 2005; 6:425–32 [DOI] [PubMed] [Google Scholar]
- 59.Sharma D, Sharma S, Pasha S, Brahmachari SK. Peptide models for inherited neurodegenerative disorders: conformation and aggregation properties of long polyglutamine peptides with and without interruptions. FEBS Lett 1999; 456:181–5 [DOI] [PubMed] [Google Scholar]
- 60.Nekooki-Machida Y, Kurosawa M, Nukina N, Ito K, Oda T, Tanaka M. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci U S A 2009; 106:9679–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchanan LE, Carr JK, Fluitt AM, Hoganson AJ, Moran SD, de Pablo JJ, Skinner JL, Zanni MT. Structural motif of polyglutamine amyloid fibrils discerned with mixed-isotope infrared spectroscopy. Proc Natl Acad Sci U S A 2014; 111:5796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong K, Punihaole D, Asher SA. UV resonance Raman spectroscopy monitors polyglutamine backbone and side chain hydrogen bonding and fibrillization. Biochemistry 2012; 51:5822–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monsellier E, Redeker V, Ruiz-Arlandis G, Bousset L, Melki R. Molecular interaction between the chaperone Hsc70 and the N-terminal flank of huntingtin exon 1 modulates aggregation. J Biol Chem 2015; 290:2560–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shahmoradian SH, Galaz-Montoya JG, Schmid MF, Cong Y, Ma B, Spiess C, Frydman J, Ludtke SJ, Chiu W. TRiC’s tricks inhibit huntingtin aggregation. Elife 2013; 2:e00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner AS, Politi AZ, Ast A, Bravo-Rodriguez K, Baum K, Buntru A, Strempel NU, Brusendorf L, Hänig C, Boeddrich A, Plassmann S, Klockmeier K, Ramirez-Anguita JM, Sanchez-Garcia E, Wolf J, Wanker EE. Self-assembly of mutant huntingtin exon-1 fragments into large complex fibrillar structures involves nucleated branching. J Mol Biol 2018; 430:1725–44 [DOI] [PubMed] [Google Scholar]
- 66.Iadanza MG, Silvers R, Boardman J, Smith HI, Karamanos TK, Debelouchina GT, Su Y, Griffin RG, Ranson NA, Radford SE. The structure of a β2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism. Nat Commun 2018; 9:4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen K, Calamini B, Fauerbach JA, Ma B, Shahmoradian SH, Serrano Lachapel IL, Chiu W, Lo DC, Frydman J. Control of the structural landscape and neuronal proteotoxicity of mutant huntingtin by domains flanking the polyQ tract. Elife 2016; 5:e18065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masino L, Kelly G, Leonard K, Trottier Y, Pastore A. Solution structure of polyglutamine tracts in GST-polyglutamine fusion proteins. FEBS Lett 2002; 513:267–72 [DOI] [PubMed] [Google Scholar]
- 69.Bennett MJ, Huey-Tubman KE, Herr AB, West AP, Ross SA, Bjorkman PJ. A linear lattice model for polyglutamine in CAG-expansion diseases. Proc Natl Acad Sci U S A 2002; 99:11634–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chellgren BW, Miller A-F, Creamer TP. Evidence for polyproline II helical structure in short polyglutamine tracts. J Mol Biol 2006; 361:362–71 [DOI] [PubMed] [Google Scholar]
- 71.Schneider R, Schumacher MC, Mueller H, Nand D, Klaukien V, Heise H, Riedel D, Wolf G, Behrmann E, Raunser S, Seidel R, Engelhard M, Baldus M. Structural characterization of polyglutamine fibrils by solid-state NMR spectroscopy. J Mol Biol 2011; 412:121–36 [DOI] [PubMed] [Google Scholar]
- 72.Eftekharzadeh B, Piai A, Chiesa G, Mungianu D, Garcia J, Pierattelli R, Felli IC, Salvatella X. Sequence context influences the structure and aggregation behavior of a polyQ tract. Biophys J 2016; 110:2361–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baias M, Smith PES, Shen K, Joachimiak LA, Żerko S, Koźmiński W, Frydman J, Frydman L. Structure and dynamics of the huntingtin exon-1 N-terminus: a solution NMR perspective. J Am Chem Soc 2017; 139:1168–76 [DOI] [PubMed] [Google Scholar]
- 74.Bravo-Arredondo JM, Kegulian NC, Schmidt T, Pandey NK, Situ AJ, Ulmer TS, Langen R. The folding equilibrium of huntingtin exon 1 monomer depends on its polyglutamine tract. J Biol Chem 2018; 293:19613–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Comellas G, Rienstra CM. Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys 2013; 42:515–36 [DOI] [PubMed] [Google Scholar]
- 76.van der Wel P. New applications of solid-state NMR in structural biology. Emerg Top Life Sci 2018; 2:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sivanandam VN, Jayaraman M, Hoop CL, Kodali R, Wetzel R, van der Wel PC. The aggregation-enhancing huntingtin N-terminus is helical in amyloid fibrils. J Am Chem Soc 2011; 133:4558–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kar K, Hoop CL, Drombosky KW, Baker MA, Kodali R, Arduini I, van der Wel PCA, Horne WS, Wetzel R. β-hairpin-mediated nucleation of polyglutamine amyloid formation. J Mol Biol 2013; 425:1183–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isas JM, Langen R, Siemer AB. Solid-state nuclear magnetic resonance on the static and dynamic domains of huntingtin exon-1 fibrils. Biochemistry 2015; 54:3942–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoop CL, Lin HK, Kar K, Hou Z, Poirier MA, Wetzel R, van der Wel PC. Polyglutamine amyloid core boundaries and flanking domain dynamics in huntingtin fragment fibrils determined by solid-state nuclear magnetic resonance. Biochemistry 2014; 53:6653–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature 2005; 435:773–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith AN, Märker K, Piretra T, Boatz JC, Matlahov I, Kodali R, Hediger S, van der Wel PCA, De PaëPe G. Structural fingerprinting of protein aggregates by dynamic nuclear polarization-enhanced solid-state NMR at natural isotopic abundance. J Am Chem Soc 2018; 140:14576–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nielsen JT, Bjerring M, Jeppesen MD, Pedersen RO, Pedersen JM, Hein KL, Vosegaard T, Skrydstrup T, Otzen DE, Nielsen NC. Unique identification of supramolecular structures in amyloid fibrils by solid-state NMR spectroscopy. Angew Chem Int Ed 2009; 48:2118–21 [DOI] [PubMed] [Google Scholar]
- 84.Mishra R, Hoop CL, Kodali R, Sahoo B, van der Wel PCA, Wetzel R. Serine phosphorylation suppresses huntingtin amyloid accumulation by altering protein aggregation properties. J Mol Biol 2012; 424:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ceccon A, Schmidt T, Tugarinov V, Kotler SA, Schwieters CD, Clore GM. Interaction of huntingtin exon-1 peptides with lipid-based micellar nanoparticles probed by solution NMR and Q-band pulsed EPR. J Am Chem Soc 2018; 140:6199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isas JM, Langen A, Isas MC, Pandey NK, Siemer AB. Formation and structure of wild type huntingtin exon-1 fibrils. Biochemistry 2017; 56:3579–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tycko R. Amyloid polymorphism: structural basis and neurobiological relevance. Neuron 2015; 86:632–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perevozchikova T, Stanley CB, McWilliams-Koeppen HP, Rowe EL, Berthelier V. Investigating the structural impact of the glutamine repeat in huntingtin assembly. Biophys J 2014; 107:411–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanley CB, Perevozchikova T, Berthelier V. Structural formation of huntingtin exon 1 aggregates probed by small-angle neutron scattering. Biophys J 2011; 100:2504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee V-Y, George JM, Rienstra CM. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol 2016; 23:409–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seuring C, Verasdonck J, Ringler P, Cadalbert R, Stahlberg H, Böckmann A, Meier BH, Riek R. Amyloid fibril polymorphism: almost identical on the atomic level, mesoscopically very different. J Phys Chem B 2017; 121:1783–92 [DOI] [PubMed] [Google Scholar]
- 92.Bugg CW, Isas JM, Fischer T, Patterson PH, Langen R. Structural features and domain organization of huntingtin fibrils. J Biol Chem 2012; 287:31739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caulkins BG, Cervantes SA, Isas JM, Siemer AB. Dynamics of the proline-rich C-terminus of huntingtin exon-1 fibrils. J Phys Chem B 2018; 122:9507–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monsellier E, Bousset L, Melki R. α-Synuclein and huntingtin exon 1 amyloid fibrils bind laterally to the cellular membrane. Sci Rep 2016; 6:19180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burke KA, Kauffman KJ, Umbaugh CS, Frey SL, Legleiter J. The interaction of polyglutamine peptides with lipid membranes is regulated by flanking sequences associated with huntingtin. J Biol Chem 2013; 288:14993–5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhattacharyya AM, Thakur AK, Wetzel R. polyglutamine aggregation nucleation: thermodynamics of a highly unfavorable protein folding reaction. Proc Natl Acad Sci U S A 2005; 102:15400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Landrum E, Wetzel R. Biophysical underpinnings of the repeat length dependence of polyglutamine amyloid formation. J Biol Chem 2014; 289:10254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen MC, Tsai MY, Zheng WH, Wolynes PG. The aggregation free energy landscapes of polyglutamine repeats. J Am Chem Soc 2016; 138:15197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kar K, Jayaraman M, Sahoo B, Kodali R, Wetzel R. Critical nucleus size for disease-related polyglutamine aggregation is repeat-length dependent. Nat Struct Mol Biol 2011; 18:328–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miettinen MS, Knecht V, Monticelli L, Ignatova Z. Assessing polyglutamine conformation in the nucleating event by molecular dynamics simulations. J Phys Chem B 2012; 116:10259–65 [DOI] [PubMed] [Google Scholar]
- 101.Miettinen MS, Monticelli L, Nedumpully-Govindan P, Knecht V, Ignatova Z. Stable polyglutamine dimers can contain β-hairpins with interdigitated side chains but not α-helices, α-nanotubes, β-pseudohelices, or steric zippers. Biophys J 2014; 106:1721–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 1997; 15:62–9 [DOI] [PubMed] [Google Scholar]
- 103.Du X, Gomez CM. Spinocerebellar ataxia type 6: molecular mechanisms and calcium channel genetics. Adv Exp Med Biol 2018; 1049:147–73 [DOI] [PubMed] [Google Scholar]
- 104.Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem 2002; 277:41032–7 [DOI] [PubMed] [Google Scholar]
- 105.Sanchez I, Mahlke C, Yuan J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 2003; 421:373–9 [DOI] [PubMed] [Google Scholar]
- 106.Nucifora LG, Burke KA, Feng X, Arbez N, Zhu S, Miller J, Yang G, Ratovitski T, Delannoy M, Muchowski PJ, Finkbeiner S, Legleiter J, Ross CA, Poirier MA. Identification of novel potentially toxic oligomers formed in vitro from mammalian-derived expanded huntingtin exon-1 protein. J Biol Chem 2012; 287:16017–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi Y, Okamoto Y, Popiel HA, Fujikake N, Toda T, Kinjo M, Nagai Y. Detection of polyglutamine protein oligomers in cells by fluorescence correlation spectroscopy. J Biol Chem 2007; 282:24039–48 [DOI] [PubMed] [Google Scholar]
- 108.Peskett TR, Rau F, O’Driscoll J, Patani R, Lowe AR, Saibil HR. A liquid to solid phase transition underlying pathological huntingtin exon1 aggregation. Mol Cell 2018; 17:588–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jayaraman M, Mishra R, Kodali R, Thakur AK, Koharudin LMI, Gronenborn AM, Wetzel R. Kinetically competing huntingtin aggregation pathways control amyloid polymorphism and properties. Biochemistry 2012; 51:2706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 1998; 95:55–66 [DOI] [PubMed] [Google Scholar]
- 111.Slow EJ, Graham RK, Osmand AP, Devon RS, Lu G, Deng Y, Pearson J, Vaid K, Bissada N, Wetzel R, Leavitt BR, Hayden MR. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci U S A 2005; 102:11402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet 2002; 11:2905–17 [DOI] [PubMed] [Google Scholar]
- 113.Pieri L, Madiona K, Bousset L, Melki R. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 2012; 102:2894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kar K, Arduini I, Drombosky KW, van der Wel PCA, Wetzel R. D-polyglutamine amyloid recruits L-polyglutamine monomers and kills cells. J Mol Biol 2014; 426:816–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ast A, Buntru A, Schindler F, Hasenkopf R, Schulz A, Brusendorf L, Klockmeier K, Grelle G, McMahon B, Niederlechner H, Jansen I, Diez L, Edel J, Boeddrich A, Franklin SA, Baldo B, Schnoegl S, Kunz S, Purfürst B, Gaertner A, Kampinga HH, Morton AJ, Petersén Å, Kirstein J, Bates GP, Wanker EE. mHTT seeding activity: a marker of disease progression and neurotoxicity in models of Huntington’s disease. Mol Cell 2018; 71:675–88.e6 [DOI] [PubMed] [Google Scholar]
- 116.Masnata M, Cicchetti F. The evidence for the spread and seeding capacities of the mutant huntingtin protein in in vitro systems and their therapeutic implications. Front Neurosci 2017; 11:2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakahira H, Breuer P, Hayer-Hartl MK, Hartl FU. Molecular chaperones as modulators of polyglutamine protein aggregation and toxicity. Proc Natl Acad Sci U S A 2002; 99:16412–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, Bottomley SP. Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proc Natl Acad Sci U S A 2010; 107:10424–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carmichael J, Chatellier J, Woolfson A, Milstein C, Fersht AR, Rubinsztein DC. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington’s disease. Proc Natl Acad Sci U S A 2000; 97:9701–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gillis J, Schipper-Krom S, Juenemann K, Gruber A, Coolen S, van den Nieuwendijk R, van Veen H, Overkleeft H, Goedhart J, Kampinga HH, Reits EA. The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine peptides. J Biol Chem 2013; 288:17225–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kakkar V, Mansson C, de Mattos EP, Bergink S, van der Zwaag M, van Waarde M, Kloosterhuis NJ, Melki R, van Cruchten RTP, Al-Karadaghi S, Arosio P, Dobson CM, Knowles TPJ, Bates GP, van Deursen JM, Linse S, van de Sluis B, Emanuelsson C, Kampinga HH. The S/T-rich motif in the DNAJB6 chaperone delays polyglutamine aggregation and the onset of disease in a mouse model. Mol Cell 2016; 62:272–83 [DOI] [PubMed] [Google Scholar]
- 122.Thompson LM, Aiken CT, Kaltenbach LS, Agrawal N, Illes K, Khoshnan A, Martinez-Vincente M, Arrasate M, O’Rourke JG, Khashwji H, Lukacsovich T, Zhu YZ, Lau AL, Massey A, Hayden MR, Zeitlin SO, Finkbeiner S, Green KN, LaFerla FM, Bates G, Huang L, Patterson PH, Lo DC, Cuervo AM, Marsh JL, Steffan JS. IKK phosphorylates huntingtin and targets it for degradation by the proteasome and lysosome. J Cell Biol 2009; 187:1083–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aiken CT, Steffan JS, Guerrero CM, Khashwji H, Lukacsovich T, Simmons D, Purcell JM, Menhaji K, Zhu YZ, Green K, Laferla F, Huang L, Thompson LM, Marsh JL. Phosphorylation of threonine 3: implications for huntingtin aggregation and neurotoxicity. J Biol Chem 2009; 284:29427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gu X, Greiner ER, Mishra R, Kodali RB, Osmand AP, Finkbeiner S, Steffan JS, Thompson LM, Wetzel R, Yang XW. Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron 2009; 64:828–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen S, Berthelier V, Hamilton JB, O’Nuallain B, Wetzel R. Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry 2002; 41:7391–9 [DOI] [PubMed] [Google Scholar]