Abstract
Kynurenic acid (KYNA), a glial-derived metabolite of tryptophan metabolism, is an antagonist of the alpha 7 nicotinic acetylcholine receptor and the glycine-binding site of N-methyl-d-aspartate (NMDA) receptors. Kynurenic acid levels are increased in both the brain and cerebrospinal fluid of several psychiatric disorders including bipolar disorder, schizophrenia, and Alzheimer disease. In addition, pro-inflammatory cytokines have been found to be elevated in the blood of schizophrenic patients suggesting inflammation may play a role in psychiatric illness. As both pro-inflammatory cytokines and KYNA can be elevated in the brain by peripheral lipopolysaccharide (LPS) injection, we therefore sought to characterize the role of neuroinflammation on learning and memory using a well-described dual-LPS injection model. Mice were injected with an initial injection (0.25 mg/kg LPS, 0.50 mg/kg, or saline) of LPS and then administrated a second injection 16 hours later. Our results indicate both 0.25 and 0.50 mg/kg dual-LPS treatment increased l-kynurenine and KYNA levels in the medial pre-frontal cortex (mPFC). Mice exhibited impaired acquisition of CS+ (conditioned stimulus) Pavlovian conditioning. Notably, mice showed impairment in reference memory while working memory was normal in an 8-arm maze. Taken together, our findings suggest that neuroinflammation induced by peripheral LPS administration contributes to cognitive dysfunction.
Keywords: Kynurenic-acid, reference memory, spatial memory, medial-prefrontal cortex, lipopolysaccharide
Introduction
Intensive research into the molecular pathophysiology of neuropsychiatric disorders has highlighted the importance of the neuroinflammation-induced activation of the kynurenine pathway (KP) of tryptophan catabolism in schizophrenia (SCZ), bipolar disorder (BP), and major depressive disorder (MDD). Kynurenic acid (KYNA) is an endogenous molecule derived from the metabolism of tryptophan (TRP) and released by astrocytes or microglia.1-4 Briefly, pro-inflammatory cytokines induce the rate-limiting enzymes, indoleamine 2,3 dioxygenase (IDO-1) and tryptophan 2,3, dioxygenase (TDO2), of the KP.5,6 The multistep chemical transformation of TRP into KYNA along the KP is known to regulate immune response and is hypothesized to play an integral role in the counter-regulatory mechanism pertaining to inflammation.7 Kynurenic acid, an endogenous molecule derived from this pathway, is synthesized and released by astrocytes.1-4 In high concentrations, KYNA is a competitive antagonist of the glycine-binding site of N-methyl-d-aspartate receptors (NMDARs).8,9 In addition, KYNA is known to modulate α7-nicotinic acetylcholine receptors (α7nAChRs).10 Neurotransmission through N-methyl-d-aspartate (NMDA) and nAChRs are crucial for a large number of cognitive functions. Interestingly, KYNA is found to be elevated in the cerebrospinal fluid (CSF) and postmortem brain tissue in patients diagnosed with SCZ and BP which has prompted the hypothesis that increased brain KYNA concentrations in the brain may underscore the glutamatergic and cholinergic alterations of cognitive function observed in individuals with SCZ and BP.11-18 Interestingly, increased synthesis of endogenous KYNA has been demonstrated to impair spatial and contextual fear memory in rats.19 Furthermore, brain-penetrant inhibitors of the enzyme kynurenine aminotransferase (KAT) II, which is responsible for neosynthesis of KYNA, have been shown to markedly reduce brain KYNA levels20-22 and to improve cognitive functions in rodents and non-human primates.20 Mice with targeted KAT II deletion exhibit decreased brain KYNA levels and enhanced performance in tests of cognitive paradigms.23 Overall, the existing literature stresses the importance of KYNA as a significant player in higher cognitive functions.
Compared to single-shot LPS (1×-LPS), 2×-LPS administration increase brain KYNA concentrations in CSF of mice.1 In our study, we investigate the effects of dual-injection of lipopolysaccharide (2×-LPS) on brain KYNA production and characterize the resulting behavior anomalies pertaining to cognitive function and memory. By employing this paradigm, we investigated KYNA concentrations from medial pre-frontal cortex (mPFC) whole tissue lysate of adult C57BL6/J male mice aged 12 weeks following 2×-LPS administration across 144 hours post LPS injection. In addition, we examined the behavioral effects of elevated KYNA in Pavlovian conditioning, the 8-arm radial maze, sensorimotor gating, and motor coordination and motor incoordination. Our experiments highlight that neuroinflammation stemming from peripheral 2×-LPS treatment induces dysregulation of the KP which may further serve as a useful model to study the subtle cognitive impairments associated with psychiatric disorder.
Materials and Methods
Animals
Male C57BL/6J mice used for this study were acquired from Jackson Laboratories (Bar Harbor, ME, USA). All mice used in this study were age matched at –3 months of age and were cared for as previously described.1,24 Food and water were available ad libitum, although food was restricted only in mice that underwent Pavlovian conditioning and 8-arm radial maze (8-ARM) testing. Animals were group housed with 5 mice per cage on a 12-hour lights on/off cycle (lights on at 06:00 hours). Temperature was maintained at 25°C and humidity between 40% and 60%. Experiments were approved by and performed in accordance with the guidelines of the Mayo Clinic Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Experimental design
Mice in this study were separated into 3 groups that received either consecutive dual LPS injections (2×-LPS; Escherichia coli serotype 0111:B4, Sigma Aldrich, St. Louis, MO, USA) at 0.25 mg/kg or 0.5 mg/kg (0.1 mL/10 g body weight i.p.) or saline vehicle (0.9%), freshly prepared prior to initial (T0) LPS injection. Vehicle or LPS were initially administered at T0 with a second injection commencing 16 hours later (T1; Figure 1A) as previously described.1 Mice used for this study were weighed daily to assess changes in body weight during Pavlovian conditioning and 8-ARM testing as well as to monitor health as a result of LPS administration. Given that LPS administration is known to decrease body weight,25 we matched food restriction weight with LPS-induced decreases in body weight for behavioral comparisons during Pavlovian conditioning and 8-ARM testing.
Figure 1.
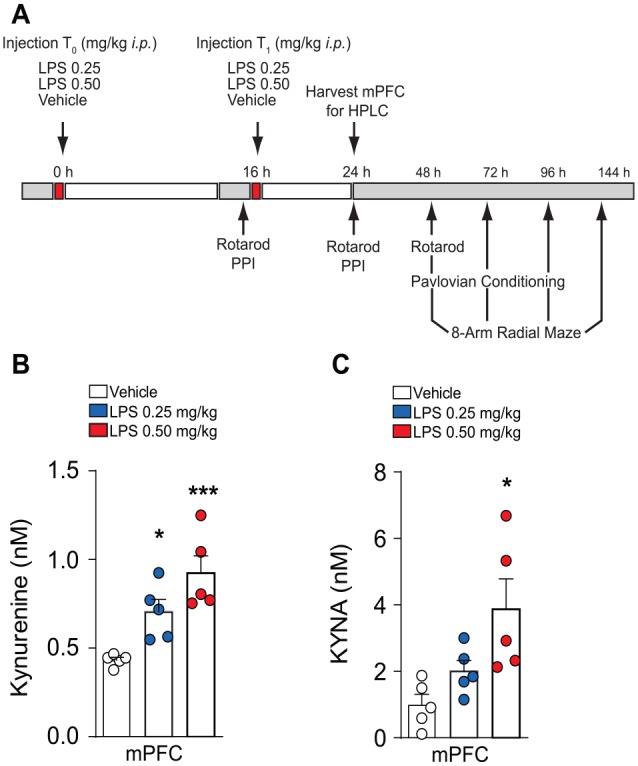
Double-LPS treatment activates KP pathway. (A) Experimental schedule used to assess the effects of dual-LPS treatment on cognitive behavior. (B) HPLC measurement of l-kynurenine 24 hours post initial LPS injection. n = 5/treatment. (C) HPLC measurement of kynurenic acid 24 hours post initial LPS injection. n = 5/treatment. Results are expressed as mean ± SEM. ANOVA indicates analysis of variance; HPLC, high-performance liquid chromatography; KP, kynurenine pathway; LPS, lipopolysaccharide; mPFC, medial pre-frontal cortex; SEM, standard error of the mean.
*P < .05, **P < .01, ***P < .001; 1-way ANOVA.
Tissue extraction
Mice were rapidly anesthetized using CO2, followed by decapitation. Whole brains were extracted and subsequently dissected to isolate mPFC. Tissue was immediately snap frozen in dry ice and stored in a −80°C freezer until used for biochemical assessment.
High-performance liquid chromatography analysis
Isolated mPFC tissue samples were mixed with a 1:5 (tissue to reagents) ratio of 0.4M perchloric acid (PCA), 0.1% sodium metabisulfite (Na2S2O5), and 0.05% EDTA. Each mPFC tissue sample was homogenized using 0.5 mm zirconium oxide beads in a Storm 24 magnetic Bullet Blender for 3 minutes with speed set to 4 (Next Adventure Inc., Averill Park, NY, USA). Next, the samples were centrifuged (21 000 × g) at 4°C for 5 minutes, and supernatant retained. The supernatant was mixed with 10% volume of 70% PCA and again centrifuged (21 000 × g) for an additional 5 minutes. The supernatant was then transferred to a new 1.5-mL Eppendorf tube and immediately stored at −20°C for later use.
To measure KYN and KYNA levels in the respective brain regions, an isocratic reverse-phase high-performance liquid chromatography (HPLC) system with a UV detector (Shimadzu SPD-10A, 360 nm) and fluorescence detector (FP-2020 Plus, Jasco Ltd., Hachioji, Japan; 344 nm excitation wavelength, 398 nm emission wavelength 18 nm bandwidth) was used. Samples (50 µL) were injected of mice treated with either vehicle (n = 5) or 2×-LPS (0.25 and 0.50 mg/kg, n = 5/treatment dose). The mobile phase consisting of 50 mM sodium acetate and 7% acetonitrile (pH 6.2) was pumped through a ReproSil-Pur C18 column (4 mm × 150 mm, Dr. Maisch GmbH, Ammerbuch, Germany). A second mobile phase consisting of 0.5M Zinc acetate in dH2O was delivered post-column, immediately preceding the fluorescence detector, by a Pharmacia P-500 (GE healthcare; Uppsala, Sweden) with a flow rate of 10 mL/h. Any signals received from the detectors were transferred to a computer equipped with Datalys Azur (Grenoble, France) for analysis. The retention time for KYN was 4 minutes, and the retention time for KYNA was 7 minutes.
Behavior Testing
Pavlovian conditioning
Mice were allowed 30 minutes of acclimation in the testing room prior to baseline measurement of general activity inside standard mouse operant chambers (MED-307A-B2, Med Associates Inc., St. Albans VT). For baseline measurement, mice were allowed to freely explore the operant chamber for 30 minutes with the house light (HL) and cubicle fan (CF) turned on. Assessment of baseline occurred before 2×-LPS or vehicle administration. Total session time, left and right nosepokes (inactive), and magazine head-entries (magazine-entries) were recorded (ENV-302D head entry detector, Med-Associated). Given that previous studies have shown deficits in conditioned stimulus (CS+) learning during LPS treatment,26 we questioned whether 2×-LPS administration would attenuate the acquisition of stimulus-reward learning during Pavlovian conditioning.27,28 To control for the decreased weight of the 2×-LPS-treated mice, vehicle-treated mice were food restricted and weights were closely monitored daily during conditioning. To establish a tone-reward contingency during conditioning, mice were given a random series of CS+ tone cues (65 dB and 0.25 seconds duration; ENV-323HAM, 4500 Hz Sonalert, Med-Associates), paired with delivery of a 10 µL sucrose reward.24,29 During conditioning, the CS+ was randomly spaced by intervals (2-60 seconds), such that on average, a reward was delivered every 15 seconds. The acquisition of Pavlovian learning was determined by measuring the latency between the CS+ and the subsequent CS+ potentiated magazine entry (CS+ reaction time) that resulted, thereby providing a measurement of stimulus-induced attentional processing. Sessions were terminated after reaching a designated number of CS+ presentations and indicated by HL and CF turning off. Average reaction time was determined as: Average Reaction Time = [(sum of latency from each trial/CS+ presentations)].
The effects of vehicle or 2×-LPS injection (0.25 and 0.50 mg/kg) on Pavlovian conditioning were examined in 3 groups of mice. For baseline assessment during Pavlovian conditioning (Experiment 1), we measured changes across testing sessions in reaction time latencies between CS+ presentations and the next immediate magazine entry to determine acquisition of conditioning. We also investigated the number of inactive hole magazine entries, as well as the number of nose-poke entries to determine how LPS affects general behavior during conditioning. Baseline was conducted prior to T0 vehicle or 2×-LPS administration. Pavlovian conditioning was assessed at 48, 72, and 96 hours after T0 administrations (Veh n = 13, LPS 0.25 mg/kg n = 10, LPS 0.50 mg/kg n = 5). Pavlovian conditioning sessions concluded after 65 CS+ presentations.
Accelerated rotarod
To probe the effects of 2×-LPS treatment on motor function, a mouse rotarod treadmill (UGO Basile, Verese, Italy) was used. A program was used to incrementally accelerate from 2 to 40 r/min over a time interval of 300 seconds. The time latency it took for a mouse to fall from the treadmill was the output measure of motor coordination. Each group of mice (vehicle n = 8, LPS 0.25 mg/kg n = 5, LPS 0.50 mg/kg n = 4) performed rotarod resting at 16 and 24 hours post T0 administration.
Radial 8-ARM maze
Mice undergoing 8-ARM testing were subjected to food restriction to 85% of their ad libitum feeding body weight. A typical 8-ARM (MED-RAM-U-1M, Med Associates, St. Albans, VT) was used to assess spatial learning and memory in mice after treatment with either vehicle or 2×-LPS. The maze was elevated 65 cm on a table and contained an octagonal central chamber equipped with 8 computer-controlled guillotine doors. Connected to the central chamber, there were 8 arms that extended from each side of the central chamber. The 8 extended arms were of equal length (35 cm long × 7.25 cm wide) and composed of transparent plastic to allow mice the use of external visual cues as a support for spatial navigation. At the distal end of each arm extending from the central chamber, there was a food trough equipped with sensors that would dispense 20 mg chocolate food pellets (Bio-Serv, Flemington NJ, USA) when activated.
During behavior testing, all mice were allowed 30 minutes to acclimation in the testing room prior to beginning the test. For habituation, mice were allowed to acclimate in center chamber for 2 minutes after which all guillotine doors opened allowing the mice to freely explore the maze for 10 minutes. During habituation, all 8 arms and magazine troughs located at the distal end of each arm were baited with a food pellet to encourage exploration. During subsequent habituation days, the same procedure was followed except only the magazine trough was baited, and the session time was decreased to 5 minutes. After completion of daily habituation, the mice were returned back to home cage. Upon successful habituation performance (consumption of all 8 baits within 5-minute session time), the mice were subjected to reference memory and working memory testing. Mice that failed in habituation performance were excluded from reference memory and working memory evaluation.
For reference and working memory testing, the session time was set to a maximum of 15 minutes at which point the session would be terminated automatically. To determine reference or working memory, arms 1, 3, and 7 were not baited, while arms 2, 4, 5, 6, and 8 were baited. Deficits in these parameters were displayed by mice that repeatedly entered non-baited arms to check whether there was a reward at the magazine trough. Mice with intact memory function decrease the frequency of entries into non-baited arms. Animals were tested for 8 consecutive days to determine whether 2×-LPS administration induced deficits in long term memory (LTM) or short-term memory (STM). The following ratio equations shown below were used to determine working memory and reference memory, as previously described30,31
| (1) |
For working memory, this ratio measures the percentage of all arm entries into the baited arms that resulted in food reinforcement. Therefore, errors were tabulated as excessive entries into arms where mice first retrieved a reward, in relation to the number of times animals revisited those arms. The most efficient performance is for animals to only visit baited arms and only visit them once
| (2) |
For reference memory, this ratio is expressed as the number of visits to the baited arms in relation to entries into all arms. Therefore, reference memory relies on remembering the location of arms that were baited throughout habituation and testing, while establishing cognitive distinction of the location of the arms that never contained a reward (unbaited arms). The most efficient performance is for animals to primarily visit baited arms and minimize unbaited arm entries; therefore, errors were tabulated as excessive entries into unbaited arms relative to baited arms.
Prepulse inhibition
To determine whether 2×-LPS treatment has any effects on sensorimotor gating, mice were (i.p.) administered initial injection of vehicle (n = 7) or LPS (0.50 mg/kg; n = 6). Prepulse inhibition (PPI) was measured 16 hours (ie, T1) after T0 immediately prior to the second LPS injection and then again tested for PPI at 24 hours following T0 LPS administration (Figure 1A). Prepulse inhibition testing was conducted as previously described.24,28 PPI was measured in sound-attenuating boxes (SR-Lab, San Diego Instruments, San Diego CA, USA) equipped with a loudspeaker and house light. Each chamber accommodated a cylindrical plexiglass animal enclosure which rested on a platform containing a piezoelectric accelerometer mounted beneath. The piezoelectric accelerometer was responsible for converting vibrations of the mouse enclosed within the cylindrical plexiglass to analog signals that were then stored on the computer. At the beginning of the startle stimulus, 65 readings were recorded at 1-ms interval to capture the maximum startle amplitude. Each session was started with a 5-minute acclimation period immediately preceded by 4 successive 120 dB stimulus alone trials. These 4 initial trials were excluded from analysis. Four different trial types were then presented at random: “no stimulus” (background, 65 dB), “Startle pulse alone” (120 dB; 40 ms), “prepulse alone” (4, 8, 16 dB above background; 20 ms), or “prepulse + startle pulse” (4, 8, or 16 dB prepulse given 100 ms before 120 dB startle pulse). The intertrial intervals were randomly varied ranging from 5 to 15 seconds, and all trials were presented 5 times except the “no stimulus” and “startle pulse alone” trials which were presented 10 times. The average maximum amplitude of vibrations from the “no stimulus” trial was subtracted from all startle response values which corrects for baseline movement in the chambers. The average percentage of acoustic PPI was calculated as: % PPI = 1 − [(prepulse + startle pulse)/(startle pulse alone)] × 100. For the startle response calculation, the maximum startle amplitude (v-max) within each PPI session was used to ascertain the magnitude of this reflex.
Statistical analysis
Measurements of KYN and KYNA concentrations in the mPFC were statistically analyzed with 1-way analysis of variance (ANOVA). We used repeated-measures 2-way ANOVA (RM 2-way ANOVA) for body weight measurement, accelerated rotarod, as well as CS+ reaction times, magazine entries, and inactive-hole entries during Pavlovian conditioning. For behavioral analysis of memory function during 8-ARM testing, we used RM 2-way ANOVA. For PPI, prepulse intensities were examined using RM 2-way ANOVA. For startle response, analysis was performed with unpaired 2-tailed Student t test. Statistical significances for all ANOVAs were followed by Tukey multiple comparisons, where appropriate. For all reported data, results were considered statistically significant when P ⩽ .05 and values represented are mean ± SEM (GraphPad Prism, La Jolla CA, USA).
Results
Double-intraperitoneal injection of LPS increases kynurenine and KYNA in mPFC
To determine whether peripheral low-dose LPS injection alters kynurenine metabolism, we intraperitoneally administered with 0.25 mg/kg or 0.5 mg/kg LPS, followed by administration of a second injection of 0.25 mg/kg or 0.5 mg/kg LPS 16 hours post initial injection (Figure 1A). Mice treated with 0.25 mg/kg displayed significant increases in KYN (1-way ANOVA, F2,12 = 12.61, P = .001; Tukey post hoc for vehicle vs LPS 0.25 mg/kg, P = .04, vehicle vs 0.5 mg/kg, P = .0008, LPS 0.25 mg/kg vs LPS 0.5 mg/kg, P = .01). In addition, our findings indicate significant increases in KYNA after double LPS administration (1-way ANOVA F2,12 = 6.31, P = .01; Tukey post hoc for vehicle vs LPS 0.25 mg/kg, P = .45, vehicle vs LPS 0.5 mg/kg, P = .01, LPS 0.25 mg/kg vs LPS 0.5 mg/kg, P = .997). These data suggest that peripheral administration of low-dose LPS is capable of modulating kynurenine metabolism, although these effects may be dose dependent.
LPS administration impairs Pavlovian conditioning
To investigate the effects of increased kynurenine metabolism induced by 2×-LPS administration on cognitive function, mice underwent conditioning to assess acquisition of Pavlovian learning. Testing was performed at testing 24, 48, and 72 hours post initial (T0) LPS injection (Figure 1A). Our results indicate a significant main effect of training as both vehicle and LPS injected mice displayed decreased reaction times to retrieve reward following conditioned stimulus (CS+) presentations across testing sessions (F2,50 = 19.21, P < .001) (Figure 2A). Notably, a main effect of treatment (F2,25 = 9.91, P = .0007) was detected as 0.25 mg/kg and 0.5 mg/kg 2×-LPS administration of significantly impaired reaction times (Figure 2A) when tested at 48, 72, and 96 hours post T0 LPS administration (all P < .05). There was no significant interaction detected. An examination of magazine entries (Figure 2B), an indicator of emotive reward seeking, detected a drug treatment effect (F2,25 = 9.43, P = .0009) as both 0.25 and 0.50 mg/kg 2×-LPS treatment displayed decreased frequency of magazine entries at 72 and 96 hours when compared to vehicle treatment (all P < .05). However, there was no effect of training across testing sessions (F2,50 = 2.68, P = .07), nor an interaction (F4,50 = 1.12, P = 0.35) (Figure 2B). Investigation of inactive-hole entries (Figure 2C), a measure or general activity, did not find differences between treatment groups which suggests that LPS treatment did not impair general exploratory activity and mice were properly Pavlovian conditioned because any responses into the inactive-hole did not deliver reinforcement.
Figure 2.
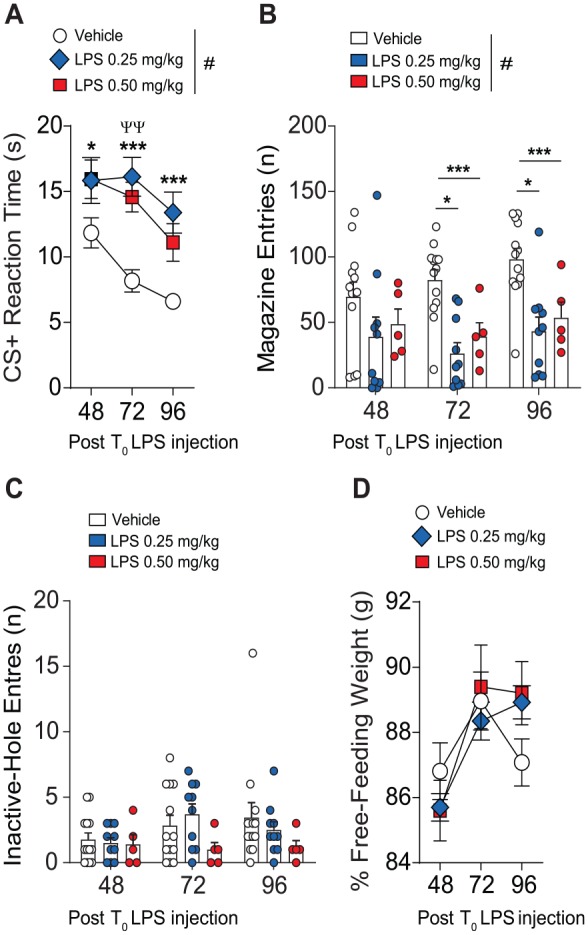
Effects of double-LPS treatment on Pavlovian conditioning. (A) Compared to vehicle-treated mice, mice undergoing dual-LPS treatment and tested for acquisition of Pavlovian behavior at 24, 48, and 72 hours post initial injection displayed slower reaction times to the CS+ stimulus. (B) In comparison to vehicle treatment, mice treated with 2×-LPS displayed significantly reduced magazine entries. (C) There was no difference detected in inactive-hole entries or a change in percentage free-feeding weight (D). Results are expressed as mean ± SEM. n = 5/vehicle, n = 10/LPS 0.25 mg/kg, n = 5/LPS 0.50 mg/kg. ANOVA indicates analysis of variance; CS+, conditioned stimulus; LPS, lipopolysaccharide; SEM, standard error of the mean.
#P < .05 by 2-way ANOVA. *P < .05 and **P < .01 by Tukey multiple comparisons relative to vehicle treatment.
Notably, all treatment groups had comparable body weights during Pavlovian conditioning, indicating that decreases in body weight at the doses tested is not a confounding variable affecting acquisition of conditioning in these experiments (Figure 2D).
Dual-LPS treatment impairs reference memory
To further investigate the effects of 2×-LPS administration on cognitive function, we assessed memory in mice utilizing the 8-arm radial maze (8-ARM). Our results show that 0.25 mg/kg LPS does not significantly affect short-term memory compared to vehicle treatment (F1,11 = 1.44, P = .25) (Figure 3A). Interestingly, 0.25 mg/kg LPS treatment induces deficits in reference memory (Figure 3B) compared to vehicle-treated counterparts (F1,11 = 7.98, P = .01); however, there was no effect of training across sessions (F4,44 = 1.12, P = .35) or an interaction (F4,44 = 2.15, P = .08). As denoted by significantly lower reference memory ratios in comparison to vehicle-treated mice, mice administered LPS committed a higher frequency of incorrect entries into arms that never contained a reward, in relation to entries into arms that previously contained a reward. This suggests that LPS-induced neuroinflammation can result in prolonged impairment of reference memory (long-term memory), as these deficits persisted up to 144 hours post T0 LPS administration.
Figure 3.
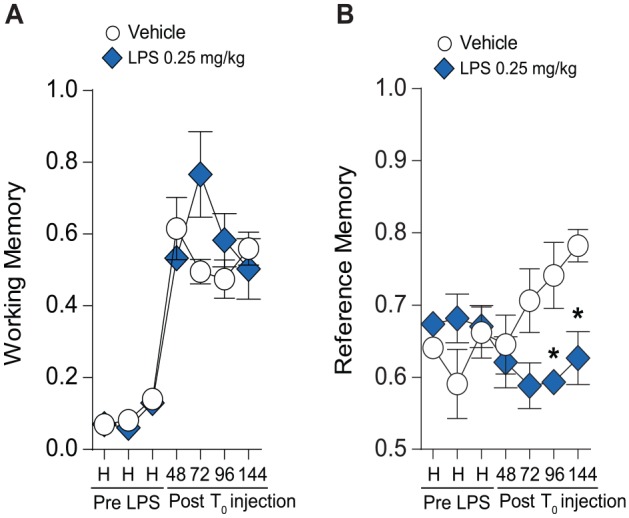
Effects of 2× LPS treatment on radial 8-arm maze. (A) Double treatment with LPS 0.25 mg/kg showed no effect on working memory compared to vehicle treatment; however, reference memory was impaired (B). ANOVA indicates analysis of variance; LPS, lipopolysaccharide.
#P < .05 by 2-way ANOVA. *P < .05 and **P < .01 by Tukey multiple comparisons relative to vehicle treatment.
Effects of LPS administration on motor skill learning
To interrogate whether 2×-LPS injection treatment had a significant effect on motor skill learning, we tested each group of mice on the accelerated rotarod. As depicted in Figure 4, we did not detect drug treatment effects on rotarod performance (F2,14 = 1.83, P = .19); however, there was an effect of rotarod training (F2,28 = 3.96, P = .03) as all treatment groups improved motor performance over time, suggesting that 2×-LPS treatment does not impair motor coordination or acquisition of motor skill learning.
Figure 4.
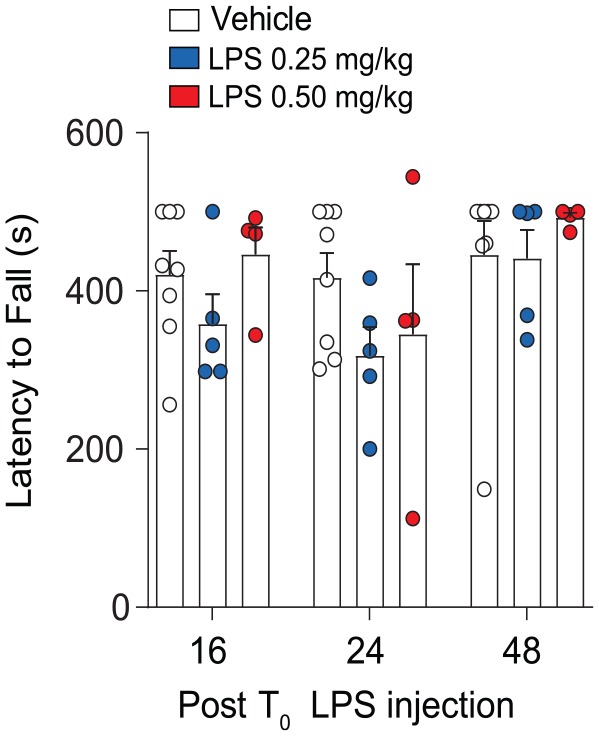
Effects of 2× LPS treatment on motor coordination. Compared to vehicle, mice treated with dual-LPS showed no deficits in motor skill learning on the accelerated rotarod. Results are expressed as mean ± SEM (n = 5/group). LPS indicates lipopolysaccharide; SEM, standard error of the mean.
Effect of LPS treatment on sensorimotor gating startle responses
Sensorimotor gating is the pre-attentive involuntary reduction of motor responses cued by a sensory event. Prepulse inhibition is the inhibition of reflexive motor response to a startling auditory pulse prior to presentation of a subthreshold prepulse. Due to deficits in sensorimotor gating reported in numerous neurocognitive disorders, we questioned whether mice treated with double-LPS injections would displayed reductions in PPI. Our results revealed that sensorimotor gating at 16 hours following T0 of 0.50 mg/kg LPS is intact compared to vehicle-treated mice (F1,33 = 1.98, P = .16), as PPI improved upon increased prepulse intensity (F2,33 = 23.31, P < .0001; Figure 5A). In addition, LPS treatment 16 hours post T0 injection did not significantly alter startle response (Figure 5B) compared to vehicle-treated controls (t11 = 0.88, P = .39). Analysis of PPI 24 hours post T0 LPS treatment (Figure 5C) revealed that PPI was unaffected as sensorimotor gating improved upon increased prepulse intensity (F2,33 = 9.07, P = .0007), and there was no treatment effect (F1,33 = 3.37, P = .06). Interestingly, mice treated with 0.50 mg/kg LPS displayed suppressed startle response compared to vehicle treated controls (t11 = 3.71, P = .003; Figure 5D).
Figure 5.
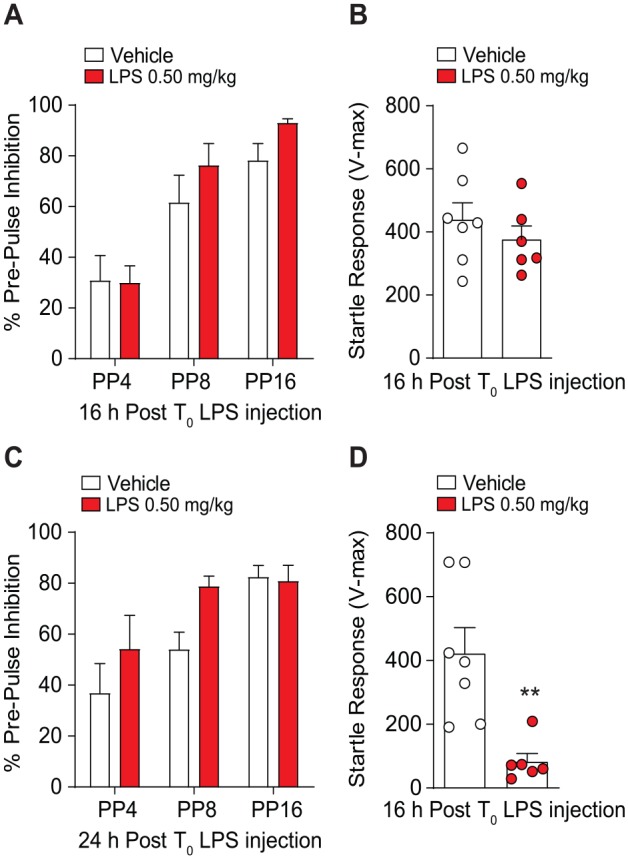
Prepulse inhibition (PPI) and startle response in mice treated with 2× LPS. (A) Sensorimotor gating tested 16 hours after initial injection LPS did not show significant difference in PPI compared to vehicle treatment at 4 dB (PP4), 8 dB (PP8), or 16 dB (PP16). (B) Mice tested 16 hours after initial LPS treatment showed no differences in startle response compared to vehicle treatment. (C) Mice tested for sensorimotor gating 24 hours after initial LPS treatment did not show any differences in PPI compared to vehicle; however, LPS treatment did result in decreased startle response relative to vehicle treatment at 24 hours (D). Results are expressed as mean ± SEM. HPLC indicates high-performance liquid chromatography; LPS, lipopolysaccharide; mPFC, medial pre-frontal cortex; SEM, standard error of the mean.
**P < .01, Student t test (n = 7/vehicle and n = 6/LPS 0.50 mg/kg).
Discussion
Neuroinflammatory responses have increasingly garnered investigative interest to determine its involvement in the pathogenesis of neuropsychiatric disease.32 Indeed, aberrant tryptophan metabolism resulting from inflammatory sequelae has been shown to play a potential role in the development of impairments of behavior.33 Therefore, the objective of this study was to investigate the role of neuroinflammation induced by consecutive peripheral LPS insults on cognitive function. Our data show that immune activation by repeated systemic LPS administration increased kynurenine and KYNA metabolism in the mPFC, in conjunction with deficits in acquisition of Pavlovian responding. These findings are in agreement with previous studies showing brain KP modulation following consecutive LPS administration albeit at a dose established to induce depressive like symptomatology34 and impaired reward motivated learning.1,24 Bacterial, viral, and parasitical infections are associated with activation of Toll-like receptors (TLRs) signaling, which can be activated experimentally by LPS. The induction of the KP via TLR activation provides a potential mechanism by which changes in kynurenine metabolism may affect brain development. For instance, studies have shown that rats administered perinatal influenza virus induces cerebral KP metabolism, thereby impairing sensorimotor function into adulthood.35 In line with this study, a recent publication shows that activation of TLR4 by LPS increase KYNA production in human peripheral monocytes.36
The assimilation of pooled sensory modalities allows us to adapt to various environments.37 PPI is the ability of a non-startling pulse to inhibit responding to a subsequent startling “pulse” or stimulus. Deficits in sensorimotor gating, as measured by PPI, are well described in patients diagnosed with SCZ and other psychiatric disorders.38,39 Inadequacies in sensorimotor gating are suspected to contribute to sensory overload, cognitive fragmentation, and interceptive stimuli which may result in psychotic symptoms and cognitive decencies. Interestingly, our current study supports our previous investigation demonstrating that consecutive LPS administration had no effects on PPI, although it significantly impairs startle responses.24 It is worth mentioning that Custódio et al40 reported decreased PPI performance 24 hours after a single i.p. injection of 0.5 mg/kg LPS, although this study was conducted using the SWR/J inbred strain mice. Investigations of inbred laboratory rat and mice strains revealed that PPI performance is a genetic trait and genetic background is an important contributor to PPI behavior.41 Thus, the discrepancy between the 2 studies is likely an effect of mouse strain and experimental methodologies. Given that the effects of LPS on brain function can be persistent well after the immediate sickness effects, it is possible that assessment of PPI at a later time point may reveal latent PPI deficits. Indeed, additional investigations are warranted to further delineate genetic mechanisms that may underlie sensorimotor gating.
Perhaps the most intriguing finding of our study was the reduction in reference memory capacity that persisted 144 hours after the initial LPS treatment during the 8-arm radial maze task. The important role of working memory for short-term information guidance and analysis of complex behavior has been well recognized.42 Of interest, neocortical brain regions such as the mPFC have been shown to be involved in working memory and reference spatial memory.43,44 The significant increase in KYNA within the mPFC shown in our study may support the hypothesis of LPS-induced neuroinflammation contributing to cognitive impairments, potentially via the antagonistic action of KYNA on nAChR receptors. In addition, our work provides evidence supporting the notion that working and reference memory are regulated by different brain systems as working memory was not altered by dual-LPS treatment. Considering the dynamic interactions between the mPFC and the hippocampus, further studies are necessary to interrogate the effects of double-LPS treatment on cognition.
Admittedly, there are some limitations to our study. Lipopolysaccharide activates a number of pathways including TLR-4 and elicits several pro-inflammatory responses. As such, LPS is not exclusive and activates many pathways involved in inflammation and is not specific toward the induction of the KP pathway. It is a limitation of our study that specific brain-penetrable KATII inhibitors were not used to decrease elevated KYNA levels resulting from 2×-LPS treatment. In addition, the use of an Alzet implantable osmotic pump could have been utilized to infuse l-kynurenine, thus increasing KYNA levels without the need for LPS which would have allowed us to better dissociate the effects of elevated KYNA on cognitive function apart from the inflammatory effects. Moving forward, a long-term study should be performed to determine if impairments in cognitive function resulting from 2×-LPS return to normal after longer periods of time. Clinically, sex difference in cognitive function have been reported in schizophrenic patients with studies indicating male schizophrenic patients are afflicted with more serious cognitive deficits compared to females.45 For this reason, we have decided to conduct our experiments using only male mice. However, future study should compare the possible difference between sexes.
In summary, intraperitoneal administration of dual-LPS injections strongly induce the KP metabolism resulting in increased production of KYNA. Furthermore, our findings provide support for the feasibility of LPS-induced neuroinflammatory process contributing to cognitive impediment.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Mayo-KI Collaborative Travel Award, Mayo Metabolomics Core Pilot Grant, the Samuel C. Johnson Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, the Swedish Medical Research Council (SE: 2017-00875), the Swedish Brain Foundation, Märta Lundqvists Stiftelse, Petrus och Augusta Hedlunds Stiftelse, Torsten Söderbergs Stiftelse, and NIH T32 Predoctoral Training Grant to L.P. (GM072474).
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.-S.C. is a scientific advisory board member to Peptron Inc. Peptron had no role in preparation, review, or approval of the manuscript. S.E. has served as speaker for Otsuka Pharmaceuticals and has received grants from AstraZeneca as co-PI. All the other authors declare no biomedical financial interests or potential conflicts of interest.
Author Contributions: AO, SE and D-SC designed the experiments. LP, AO, MT, LS, and PS performed the experiments and analyzed the data. LP, AO, GE, SE, and D-SC wrote the manuscript.
ORCID iDs: Sopie Erhardt  https://orcid.org/0000-0001-7359-5250
https://orcid.org/0000-0001-7359-5250
Doo-Sup Choi  https://orcid.org/0000-0002-6796-9938
https://orcid.org/0000-0002-6796-9938
References
- 1. Larsson MK, Faka A, Bhat M, et al. Repeated LPS injection induces distinct changes in the kynurenine pathway in mice. Neurochem Res. 2016;41:2243-2255. [DOI] [PubMed] [Google Scholar]
- 2. Parrott JM, Redus L, O’Connor JC. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation. 2016;13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wirthgen E, Hoeflich A, Rebl A, Günther J. Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. 2018;8:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wurfel BE, Drevets WC, Bliss SA, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7:e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sellgren CM, Kegel ME, Bergen SE, et al. A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol Psychiatry. 2015;21:1342-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197-209. [DOI] [PubMed] [Google Scholar]
- 8. Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309-379. [PubMed] [Google Scholar]
- 9. Stone TW. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci. 2007;25:2656-2665. [DOI] [PubMed] [Google Scholar]
- 10. Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463-7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96-98. [DOI] [PubMed] [Google Scholar]
- 13. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521-530. [DOI] [PubMed] [Google Scholar]
- 14. Lavebratt C, Olsson S, Backlund L, et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2013;19:334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2010;38:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315-322. [DOI] [PubMed] [Google Scholar]
- 17. Olsson SK, Sellgren C, Engberg G, Landén M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord. 2012;14:719-726. [DOI] [PubMed] [Google Scholar]
- 18. Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2010;37:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chess AC, Landers AM, Bucci DJ. L-Kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325-331. [DOI] [PubMed] [Google Scholar]
- 20. Kozak R, Campbell BM, Strick CA, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592-10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linderholm KR, Alm MT, Larsson MK, et al. Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology. 2016;102:42-47. [DOI] [PubMed] [Google Scholar]
- 22. Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Potter MC, Elmer GI, Bergeron R, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliveros A, Wininger K, Sens J, et al. LPS-induced cortical kynurenic acid and neurogranin-NFAT signaling is associated with deficits in stimulus processing during Pavlovian conditioning. J Neuroimmunol. 2017;313:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci. 2017;24:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sparkman NL, Kohman RA, Garcia AK, Boehm GW. Peripheral lipopolysaccharide administration impairs two-way active avoidance conditioning in C57BL/6J mice. Physiol Behav. 2005;85:278-288. [DOI] [PubMed] [Google Scholar]
- 27. Flagel SB, Robinson TE. Neurobiological basis of individual variation in stimulus-reward learning. Curr Opin Behav Sci. 2017;13:178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveros A, Heckman MG, Del Pilar Corena-McLeod M, Williams K, Boules M, Richelson E. Sensorimotor gating in NTS1 and NTS2 null mice: effects of d-amphetamine, dizocilpine, clozapine and NT69L. J Exp Biol. 2010;213:4232-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savage LM, Ramos RL. Reward expectation alters learning and memory: the impact of the amygdala on appetitive-driven behaviors. Behav Brain Res. 2009;198:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hritcu L, Clicinschi M, Nabeshima T. Brain serotonin depletion impairs short-term memory, but not long-term memory in rats. Physiol Behav. 2007;91:652-657. [DOI] [PubMed] [Google Scholar]
- 31. van der Staay FJ. Spatial working memory and reference memory of Brown Norway and WAG rats in a holeboard discrimination task. Neurobiol Learn Mem. 1999;71:113-125. [DOI] [PubMed] [Google Scholar]
- 32. Nutma E, Willison H, Martino G, Amor S. Neuroimmunology—the past, present and future. Clin Exp Immunol. 2019;197:278-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wonodi I, McMahon RP, Krishna N, et al. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr Res. 2014;160:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millett CE, Phillips BE, Saunders EFH. The sex-specific effects of LPS on depressive-like behavior and oxidative stress in the hippocampus of the mouse. Neuroscience. 2019;399:77-88. [DOI] [PubMed] [Google Scholar]
- 35. Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1-/- mice. Int J Neuropsychopharmacol. 2010;13:475-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orhan F, Bhat M, Sandberg K, et al. Tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor stimulation in peripheral monocytes. Scand J Immunol. 2016;84:262-271. [DOI] [PubMed] [Google Scholar]
- 37. Ishii D, Takeda K, Yamamoto S, et al. Effect of visuospatial attention on the sensorimotor gating system. Front Behav Neurosci. 2019;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mena A, Ruiz-Salas JC, Puentes A, Dorado I, Ruiz-Veguilla M, De la Casa LG. Reduced prepulse inhibition as a biomarker of schizophrenia. Front Behav Neurosci. 2016;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parwani A, Duncan EJ, Bartlett E, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662-669. [DOI] [PubMed] [Google Scholar]
- 40. Custódio CS, Mello BS, Cordeiro RC, et al. Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur J Pharmacol. 2013;713:31-38. [DOI] [PubMed] [Google Scholar]
- 41. Aubert L, Reiss D, Ouagazzal A-M. Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav. 2006;5:423-431. [DOI] [PubMed] [Google Scholar]
- 42. Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L. Neurocognitive architecture of working memory. Neuron. 2015;88:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith R, Lane RD, Alkozei A, et al. The role of medial prefrontal cortex in the working memory maintenance of one’s own emotional responses. Sci Rep. 2018;8:3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jo YS, Park EH, Kim IH, et al. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27:13567-13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li R, Ma X, Wang G, Yang J, Wang C. Why sex differences in schizophrenia? J Transl Neurosci. 2016;1:37-42. [PMC free article] [PubMed] [Google Scholar]


