Abstract
Immune checkpoint therapy has revolutionized cancer treatment by blocking inhibitory pathways in T cells that limits the an effective anti-tumor immune response. Therapeutics targeting CTLA-4 and PD1/PDL1 have progressed to first line therapy in multiple tumor types with some patients exhibiting tumor regression or remission. However, the majority of patients do not benefit from checkpoint therapy emphasizing the need for alternative therapeutic options. Lymphocyte Activation Gene 3 (LAG3) or CD223 is expressed on multiple cell types including CD4+ and CD8+ T cells, and Tregs, and is required for optimal T cell regulation and homeostasis. Persistent antigen-stimulation in cancer or chronic infection leads to chronic LAG3 expression, promoting T cell exhaustion. Targeting LAG3 along with PD1 facilitates T cell reinvigoration. A substantial amount of pre-clinical data and mechanistic analysis has led to LAG3 being the third checkpoint to be targeted in the clinic with nearly a dozen therapeutics under investigation. In this review, we will discuss the structure, function and role of LAG3 in murine and human models of disease, including autoimmune and inflammatory diseases, chronic viral and parasitic infections, and cancer, emphasizing new advances in the development of LAG3-targeting immunotherapies for cancer that are currently in clinical trials.
Keywords: LAG3, immune checkpoint, inhibitory receptor, cancer immunotherapy, autoimmunity, chronic viral infection
1: Introduction
Inhibitory receptors (IRs) play a pivotal role in modulating the immune response and are mediators of T cell dysfunction in autoimmunity and chronic disease [1, 2]. In fact, targeting IRs such as Programmed Cell Death Protein 1 (PD1) and Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) in cancer patients is able to re-invigorate the anti-tumor immune response, which has been reported in several landmark clinical trials [3–5]. Despite tumor regression and remission in some patients, response to these treatments is still limited with >80% of patients not responding [6, 7]. In addition, some immune quiescent tumors, such as pancreatic and prostate cancer, are still resistant to these approaches [8], ultimately offering further incentive for new immunotherapeutic interventions. One of the most promising new IR targets currently in the clinic is Lymphocyte Activation Gene 3 (LAG3) or CD223, an immune checkpoint receptor expressed by both activated and exhausted CD4+ and CD8+ T cells as well as regulatory T cells (Tregs) [2, 9, 10]. LAG3 functions by delivering inhibitory signals that regulate immune cell homeostasis, T cell activation, proliferation, cytokine production, cytolytic activity and other functions [2]. When LAG3 regulated homeostasis is perturbed, such as in the setting of chronic autoimmunity, there is rapid, immune-mediated tissue damage [9, 11]. Further, persistent antigen-stimulation, such as in cancer and chronic viral infection, results in elevated levels of chronic LAG3 expression, which leads to T cell exhaustion and subsequent impairment of T cell function [12]. Numerous immunotherapies targeting LAG3 are in clinical trials in combination with antibodies against other IRs, such as PD1/PDL1, to treat cancer [13].
2: LAG3 structure, function and ligands
LAG3 was discovered in 1990 [14] as a transmembrane molecule that is expressed on CD4+ and CD8+ T cells, natural killer T (NKT) cells, natural killer (NK) cells, plasmacytoid dendritic cells (pDCs), and regulatory T cells (Tregs) [15, 16]. In most cell types, LAG3 expression is regulated via activation with the exception of pDCs and Tregs in which expression appears to be constitutive [17, 18]. The majority of studies on LAG3 outlined below have been described in murine models. However, there has been some analysis of LAG3 expression and function in humans with many more studies likely underway given the number of LAG3-targeting immunotherapies that are currently in clinic.
2.1: Structure and Ligands
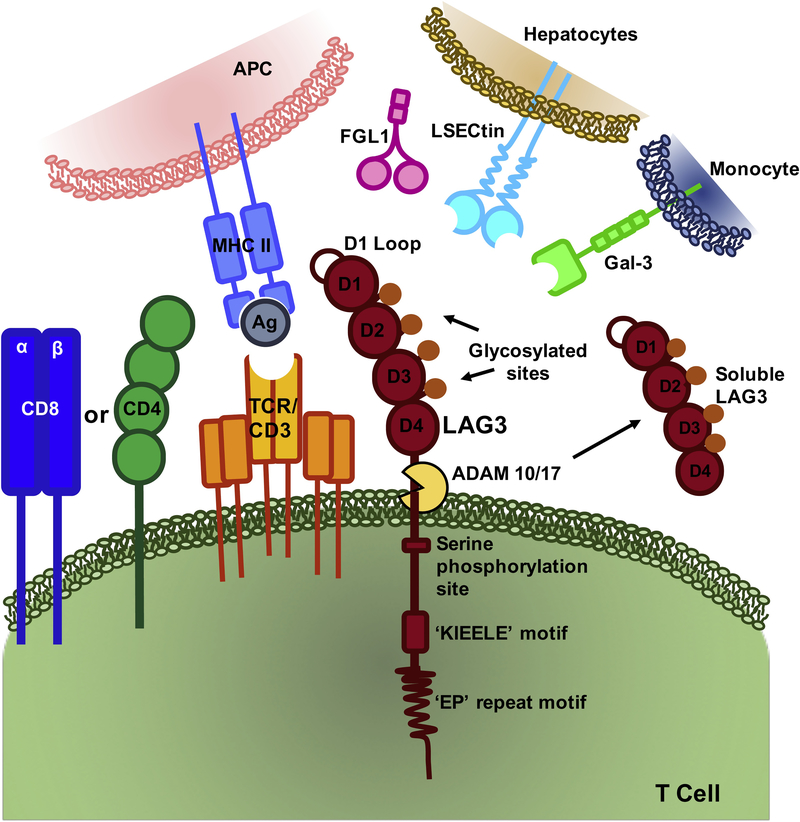
LAG3 resides on chromosome 12 (12p13.32) in humans and chromosome 6 in mice, encoding a 498-amino acid protein [19]. The LAG3 locus is adjacent to the gene encoding the CD4 co-receptor and has a similar intron/exon organization. Like CD4, LAG3 binds to MHC class II but with a much higher affinity (Fig 1). Combined, these observations suggesting that LAG3 may have evolved from a gene duplication event of the CD4 locus. Both CD4 and LAG3 consist of four extracellular immunoglobulin superfamily-like domains (D1-D4), although structure of LAG3 has yet to be solved. However, LAG3 does appear to possess two unique structural features. First, unlike CD4 the interaction between LAG3 and MHC class II is mediated through a unique, proline-rich, thirty amino acid loop within D1 [20]. Second, LAG3 has a longer connecting peptide between the fourth Ig domain and the transmembrane region rendering it more susceptible to cell surface shedding by a disintegrin and metalloproteinase domain-containing protein (ADAM) [20, 21].
Figure 1: LAG3 Structure and Ligands.
LAG3 is composed of four Ig-like domains and contains three highly conserved regions in the cytoplasmic tail. LAG3 binds MHC class II through a thirty amino acid loop in the D1 domain. Galectin-3, LSECtin and FGL1 have also be reported to bind to LAG3.
The cytoplasmic tail of LAG3 consists of three conserved motifs (Fig.1). However, the function and downstream signaling events remain unknown. The first motif contains a putative serine phosphorylation site, which contains two serine residues in humans and one in mice [Ser454]). Thus far, no function has been ascribed to this motif. The second motif is a highly unique and conserved six amino acid sequence (KIEELE) that has been shown to be required for LAG3 to downregulate T cell function [9, 22]. The third motif is a glutamic acid and proline dipeptide repeat (EP) that is phynologically conserved. It has been suggested that this motif binds LAG3-associated protein (LAP) which permits LAG3 co-localization with CD3, CD4, and/or CD8 molecules within lipid rafts [23]. However, mutations in the EP motifs maintain LAG3 activity and function, suggesting that it may not be essential [9] and no follow-up studies have been reported to date.
The primary, canonical ligand for LAG3 is MHC class II, which as noted above binds to a conserved, extended loop in the LAG3 D1 domain [24]. Once LAG3 is bound to MHC class II, it transmits inhibitory signals via its cytoplasmic domain, although the mechanism of signal transduction remains obscure [9]. Human melanoma cancer cells exhibit elevated expression of MHC class II which has been linked to poor patient prognosis [25]. LAG3:MHC class II binding contributes to tumor escape from apoptosis [26], and recruitment of tumor-specific CD4+ T cells, which subsequently leads to a reduction of the CD8+ T cell response [27].
However, over the past five years, other potential ligands have emerged. Galectin-3 (Gal-3), a 31 kDa galactose-binding lectin that regulates T cell activation, has been shown to bind to LAG3, which appears to be required for optimal inhibition of CD8+T cell cytotoxic function [28] (Fig 1). Gal-3 can be expressed on different cell types thus exerting its regulatory function on CD8+ T cells via multiple mechanisms [29]. LSECtin has also been proposed as LAG3 ligand. It binds to the four glycosylated sites on LAG3 and is a member of the DC-SIGN family of molecules. LSECtin is expressed in the liver and melanoma tumor cells, suggesting a mechanism by which LAG3 can regulate CD8+ T cells and NK cell function in these environments [30].
Fibrinogen-like protein 1 (FGL1) was recently described as a new ligand for LAG3 [31] (Fig 1). FGL1 is a member of the fibrinogen family, sharing a similar structure with fibrinogen beta and gamma, with no known role on platelets or in clot formation [32]. FGL1 is normally secreted by hepatocytes in the liver, however tumor cells can also express high levels of FGL1, which correlates with poor patient prognosis and resistance to immunotherapy [31]. Strikingly, blocking the interaction between LAG3 and FGL1 with a monoclonal antibody increases intratumoral T cell responses, which leads to decreased tumor size in murine models of melanoma, presenting a new mechanism for targeted immunotherapy. In summary, LAG3 appears to have multiple ligands but it remains to be determined if all are valid ligands, and when and where each are critical for LAG3 function.
2.2: Regulation of LAG3 expression
Cell surface expression of LAG3 is regulated by two mechanisms. First, LAG3 is stored in lysosomal compartments to facilitate rapid translocation to the cell surface following TCR stimulation to control T cell responses [33, 34]. In resting T cells, LAG3 is degraded in the lysosomal compartments. This degradation is the limiting step for the surface expression of LAG3, and inhibition of lysosomal activity increases its surface expression. Moreover, its translocation to the cell surface is mediated by protein kinase C signaling through the cytoplasmic domain [34].
Second, LAG3 cell surface expression is regulated by proteolytic cleavage, resulting in the shedding of a soluble form of LAG3 (sLAG3) [20]. LAG3 cleavage is mediated by a disintegrin and metalloproteinase domain-containing protein 10 and 17 (ADAM10 and ADAM17), whose activity can also induce the cleavage of other immune receptors such as TNFα, CD62L, TIM3 and VEGFR2 [35]. Cell surface expression of ADAM10 results in constitutive LAG3 cleavage while the activity of ADAM17 is controlled by serine phosphorylation in a TCR- and PKCθ-dependent manner [21]. Shedding occurs at the connecting peptide between the D4 domain and the transmembrane region. There does not appear to be a function for sLAG3 activity as it does not compete with surface LAG3 for binding to bind MHC II and it is rapidly degraded in vivo [20]. However, its cleavage is necessary for optimal T cell function as prevention of LAG3 shedding by non-cleavable LAG3 mutants reduced T cell function, as exhibited by decreased proliferation and attenuated IL-2 and IFN-γ production [21]. Overall, expression of LAG3 is tightly regulated through two separate mechanisms highlighting the importance of tightly controlling LAG3 expression and function to ensure optimal immune homeostasis.
3: Role of LAG3 in murine models and human disease
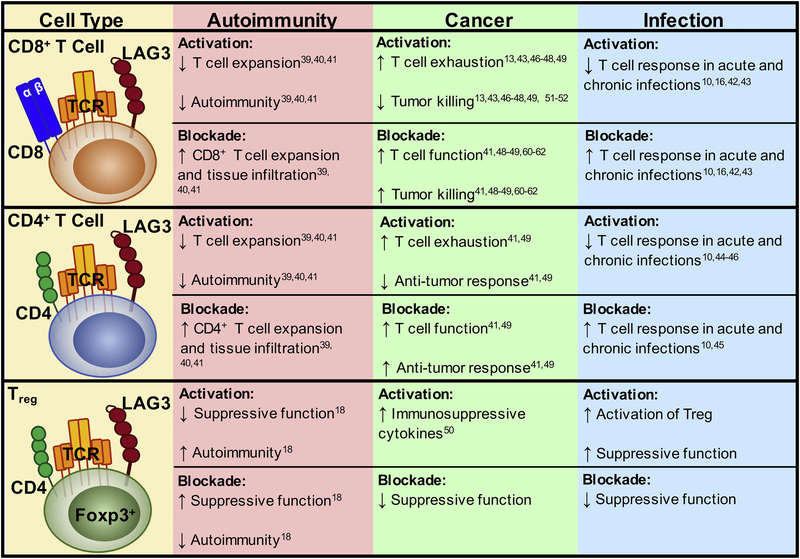
LAG3 is an IR expressed on activated and dysfunctional T cells. When engaged, it negatively regulates T cell function. Thus, LAG3 is an emerging target for modulating T cell responses in disease. Below, we will discuss studies that support the role of LAG3 as an important IR that impacts autoimmunity, chronic infection (viral and parasitic) and cancer. While LAG3 has been studied in multiple disease models, given the emergence and prominence of immunotherapy for cancer, the majority of studies to date in mice and humans have examined the role of LAG3 in cancer (Fig 2).
Figure 2:
Role of LAG3 on Different Cell Types in Multiple Diseases
3.1: Autoimmune and Inflammatory Diseases
IRs play a pivotal role in regulating autoimmune and inflammatory diseases [36]. In fact, symptoms of autoimmunity are often a side effect of checkpoint blockade for solid tumors due to loss of self-tolerance [37, 38]. Loss of LAG3 does not induce autoimmunity unless the mice are on a permissive genetic background [39, 40]. However, deletion of both LAG3 and PD1 leads to substantive, lethal autoimmunity [41]. On a non-obese diabetic (NOD) background, LAG3 is required for controlling T cell expansion in the islet. Antigen-specific Lag3−/− CD4+ and CD8+ T cells infiltrate the pancreas faster in younger mice, showing accelerated development of autoimmune diabetes compared to age-matched wild-type mice. Moreover, in wild-type NOD mice, administration of an anti-LAG3 blocking antibody also accelerates type 1 diabetes [39]. In contrast, Treg-specific deletion of LAG3 in NOD mice leads to a significant delay in the development of autoimmune diabetes [18]. This is due to an increase in Treg proliferation and survival via enhanced IL-2/STAT5 signaling and Eos (Ikzf4)-driven transcription, which ultimately improves Treg suppressive function. This suggests that the immunologic effect of LAG3 deletion or blockade on all T cells is more pronounced on enhancing the function of diabetogenic CD4+ effector T cells over Tregs. However, in autoimmune environments where chronic inflammation dominates, LAG3 may be constitutively expressed on Tregs, ultimately limiting their capacity to suppress auto-reactive effector T cells.
Targeting LAG3 has also generated interest in clinical applications for autoimmune disease in humans. An anti-LAG3 mAb (GSK2831781; GlaxoSmithKline [GSK]) has been developed, which is a humanized Antibody Dependent Cell Cytotoxicity (ADCC) enhanced monoclonal afucosylated antibody, that depletes LAG3-expressing immune cells in patients with autoimmune disease. This agent has completed a phase 1, first-in-human study in patients with plaque psoriasis () and will soon proceed to a phase 2 study. Another agonistic monoclonal antibody (mAb) targeting LAG3 (IMP761; Immutep) is currently in pre-clinical development for the treatment of autoimmune disease. These novel agents highlight that modulation of the immune response via enhanced LAG3 activity may have therapeutic potential in treating autoimmune and inflammatory diseases.
3.2: Chronic Viral and Parasitic Infections
IRs are highly expressed on dysfunctional and exhausted T cells in chronic viral and parasitic infections. The role of LAG3 in acute and chronic infections in vivo has been highlighted by multiple groups [16, 42, 43]. We initially demonstrated that LAG3 deficient CD4+ and CD8+ T cells displayed increased proliferation and IFN-γ production in acute (Sendai) and chronic (γ-herpervirus) viral infections, further underlining the role of LAG3 as a negative regulator of the immune system [10]. T cell exhaustion and chronic viral infection has also been robustly studied in the LCMV clone 13 model. In this model, the severity of the infection was directly proportional to LAG3 expression. Although LAG3 blockade alone only reduced viral load slightly [16, 42], co-blockade of LAG3 and PDL1 synergistically enhanced CD8+ T cell responses and significantly reduced viral load [16].
In a nonhuman primate model (rhesus macaques) of mycobacterium tuberculosis (MTB) infection, LAG3 is highly induced on CD4+ T cells and NK cells in the lungs and particularly in the granulomatous lesions of macaques. This coincided with high bacterial burden and changes in the host Th1 response [44]. Similarly, in malaria (Plasmodium falciparum) infection in mice, LAG3 is expressed by exhausted, parasite-specific CD4+ T cells [45]. In vivo LAG3 blockade in murine models has demonstrated synergy with PDL1 blockade to restore CD4+ T cell function, amplify the number of follicular helper T cells, germinal-center B cells, and plasmablasts, and enhance protective antibodies that led to rapid clearance of blood-stage malaria. Correlative studies done in humans have shown that infection with Plasmodium falciparum resulted in higher expression of LAG3 and PD1 associated with T cell dysfunction [45].
In summary, although there is limited efficacy in targeting LAG3 alone in treating chronic infections, targeting LAG3 for a synergistic effect with anti-PD1/PDL1 holds significant promise as an immuno-therapeutic approach for the treatment of chronic viral, bacterial or parasitic infections in humans. Further clinical studies in patients will be needed to define the feasibility, safety and efficacy of this approach.
3.3: Cancer
In cancer, T cells are constantly exposed to antigen, leading to a progressive loss of cytokine production and the capacity of CD8+ T cells to specifically kill tumor cells [46]. LAG3 expression is elevated on tumor-infiltrating lymphocytes (TILs) in many types of cancer [13, 43, 47]. In the context of vaccination, LAG3 blockade is specifically required for antigen-specific CD8+ T cell responses. Mice vaccinated with vaccinia virus–HA (VV-HA) in a tolerance model in combination with anti-LAG3 blocking antibody demonstrate accumulation of CD8+ T cells in areas of high antigen expression [48]. In several pre-clinical murine models of melanoma, colon adenocarcinoma and ovarian cancer, co-blockade of LAG3 and PD1, expressed on both CD4+ and CD8+ TILs, induced an increased anti-tumor response [41, 49]. LAG3 is also expressed on intratumoral Tregs, which can contribute to tumor immune escape. LAG3 expression on Tregs induces production of IL-10 and TGF-β1 [50]. Thus, blockade of LAG3 on Tregs may reduce their suppressive function, leading to reinvigoration of CD8+ TIL activity.
LAG3 reduces T cell proliferation and cytokine secretion, as demonstrated by enhanced T cell activation following addition of a blocking LAG3 antibody [22]. Chronic tumor antigen stimulation leads to persistent up-regulation of LAG3 and PD1 on CD8+ tumor antigen-specific T cells, which leads to their functional exhaustion [13, 46]. Expression of LAG3 on CD8+ T cells defines a more exhausted state of CD8+ T cells than PD1 expression alone. For example, tumor-specific (Melan-A/MART-1) CD8+ TIL isolated from melanoma metastases expressed higher levels of LAG3 and CTLA-4 when compared to the tumor-specific CD8+ T cells in the patient peripheral blood lymphocytes (PBL) [51]. They also failed to produce IFN-γ after peptide stimulation, compared to the tumor-specific CD8+ T cells in the PBL, which retained competent effector function. In ovarian cancer patients, the tumor antigen (NY-ESO-1)-specific CD8+TIL expressed higher levels of PD1, with some populations co-expressing PD1 and LAG3, as compared to the same NY-ESO-1-specific CD8+ T cells in the matched patient PBL [49]. Functionally, CD8+PD1+LAG3+ ovarian cancer TIL have significantly decreased production of IFN-γ, TNF-α and IL-2, compared to PD1+LAG3– or LAG3–PD1– subsets. Interestingly, the co-expression of LAG3 and PD1 on the CD8+ ovarian cancer TIL was driven by IL-6/IL-10 and tumor-derived APCs, known immunosuppressive components of the TME found in malignant ascites. Blockade of LAG3 in addition to PD1 was found to synergistically enhance proliferation and cytokine production of NY-ESO-1-specific CD8+ TIL when compared to PD1 blockade alone [49].
LAG3 expression in the TME is also correlated with increased tumor mutational burden. For instance, cancers with high microsatellite instability (MSIhi), such as a subset of colorectal cancer patients, exhibit higher somatic mutations and higher levels of immunogenic neoantigens. The tumor microenvironment of these MSIhi tumors are characterized by increased expression of multiple IRs including LAG3, PD1, PDL1, CTLA4 and IDO, as compared with microsatellite stabile (MSS) tumors [52]. These data may suggest a correlation between tumor mutational burden, anti-tumor immune response, and the co-expression of LAG3 and other IRs. In fact, the presence of LAG3 and other co-expressed IRs in the TME may explain why MSIhi tumors are not naturally eliminated despite a hostile immune microenvironment [52].
Studies to evaluate the predictive potential of LAG3 in the context of immunotherapy-treated patients is an area of active research. Currently, short-term expression of LAG3 after immunotherapy treatment can serve as a marker of T cell infiltration and general immune activation, and thus, better responsiveness to immune checkpoint blockade. In a study of longitudinal tissue biopsies from 46 metastatic melanoma patients initially treated with CTLA4 blockade followed by PD1 blockade at progression, the early on-treatment biopsies of responders displayed significantly increased expression of LAG3, as well as other immune biomarkers (PD1, PDL1, CD3, CD8, CD4), as compared to the same patients’ pre-treatment biopsies [53]. In contrast, significant expression of these immune biomarkers on pre-treatment or early post-treatment biopsies was not observed in non-responders. In a more recent study comparing global gene expression profiles of melanoma patient tumors pre- and post-anti-PD1 (nivolumab) therapy, it was found that many immune checkpoint genes, with LAG3 in particular, exhibited increased expression (LAG3, PD1, PDL1, CTLA-4, CD80, ICOS, 4–1BB) regardless of response to therapy [54]. Further analysis of responders vs. non-responder tumors identified a broader spectrum of differentially-expressed immune-related genes, including additional immune checkpoint molecules, as well as genes involved in lymphocyte activation, chemotaxis, cytokine signaling and immune cytolytic activities [54]. More in depth analyses must be performed to evaluate the role of LAG3 in the context of immunotherapy at the protein level, as all current studies focus on gene expression. Ultimately, the expression of LAG3, in concert with other IRs such as PD1, could serve as a prognostic immune biomarker for multiple solid tumors.
4: Clinical development of therapeutic agents to target LAG3 in the tumor microenvironment
There are currently ten experimental therapeutic agents targeting LAG3 that are being tested in clinical trials for various human cancers (Table 1). The initial first-in-class agent was IMP321 (Immutep), which is a 200 kDa soluble chimeric recombinant fusion protein of the extracellular portion of LAG3 and antibody Fc domain, which is proposed to activate antigen-presenting cells (APCs) via interaction with its canonical ligand, MHC class II. In pre-clinical studies, IMP321 has been found to activate APCs to promote the proliferation of dendritic cells (DCs), ameliorate Treg immunosuppression, and improve antigen cross-presentation to CD8+ T cells [55]. IMP321 has completed three clinical trials in renal cell carcinoma, metastatic breast carcinoma and melanoma, but has only exhibited limited success with small patient cohorts. However, in a completed phase 1 trial for metastatic breast cancer (), preliminary efficacy of an IMP321 plus chemotherapy (paclitaxel) combination exhibited a 50% overall response rate (ORR) compared to 25% with paclitaxel alone [56]. The efficacy of IMP321 was correlated with increased activation of multiple immune cell types in patients. There are ongoing clinical trials in other cancer types to further evaluate its therapeutic potential (Table 1).
Table 1:
Clinical trials for LAG-3-targeted immunotherapy in human cancer
| Trial number/Ref | Study population | Interventions | Phase | Status | Immune Outcome | Patient outcome |
|---|---|---|---|---|---|---|
| IMP321 (a soluble LAG-3 Ig) | ||||||
| [66] | Stage IV renal Cell Carcinoma | IMP321 | I | Completed 10/2008 | - Increased CD8+ percentage/activation at higher (>6 mg) doses | -Better PFS and tumor growth reduction at higher (>6 mg) doses |
| [67] | Metastatic breast carcinoma patients receiving first-line paclitaxel | IMP321 | I | Completed 1/2010 | -Increase in the number/activation of APCs (monocytes and DCs), NK, and CD8+ T cells. | -Objective tumor response rate:50% (vs. 25% with paclitaxel alone) -Clinical benefit in 90% of patients |
| [68] | Melanoma | Lympho-depletion, vaccine, IMP321 adjuvant | I | Completed 11/2011 | -Expansion of MART-1 specific CD8+ T cells in 83% of patients. -Reduced Tregs expansion |
- No confirmed responses per RECIST criteria |
| Solid tumors Peritoneal carcinomatosis |
IMP321 | I | Recruiting Estimated completion date: 2/2019 |
N/A | N/A | |
| Hormone receptor-positive metastatic breast cancer | Paclitaxel + IMP321/Placebo | II | Recruiting Estimated completion date: 12/2020 |
N/A | N/A | |
| (TACTI-mel) [69] | Unresectable/metastatic Stage III/IV Melanoma | IMP321 + Pembrolizumab (anti-PD-1) | I | Active, not recruiting Estimated completion date: 12/2018 |
N/A | - ORR: 33% (from the start of combination therapy). DCR: 66%. |
| Untreated or recurrent/refractory unresectable/metastatic NSCLC, HNSCC | IMP321+Pembrolizumab | II | Not yet recruiting Estimated completion date: 6/2021 |
N/A | N/A | |
| BMS-986016 (Relatlimab, anti-LAG3 mAb) | ||||||
| (CA224–020) [70–72] | Advanced solid tumors | Relatlimab ± Nivolumab (BMS-936558, anti-PD-1) | I/IIa | Recruiting Estimated completion date: 10/2019 |
-Increased proliferation/activation of NK and CD8+ T cells in the treated patients | -ORR: 11.5% (3-fold higher RR in melanoma patients with ≥ 1% LAG3) DCR: 49% |
| (CA224–034) | Advanced solid tumors | Relatlimab ± Nivolumab | I | Recruiting Estimated completion date: 7/2020 |
N/A | N/A |
| (CA224–047) | Previously untreated metastatic or unresectable melanoma | Nivolumab ± Relatlimab | II/III | Active, not recruiting Estimated completion date: 3/2022. |
N/A | N/A |
| (CA224–048) | Advanced malignant tumors | Relatlimab ± Nivolumab + BMS-986205 (anti-IDO1)/Ipilimumab (anti-CTLA4) | I/II | Recruiting Estimated completion date: 5/2022. |
N/A | N/A |
| Treatment-naïve metastatic melanoma | 1 cycle of lead-in phase (Nivolumab, Relatlimab, Nivolumab + Relatlimab) followed by Nivolumab + Relatlimab combination | II | Recruiting Estimated completion date: 10/2022. |
N/A | N/A | |
| Resectable stage IIIB or IV (oligometastatic) melanoma | Neoadjuvant Nivolumab ± Relatlimab/Ipilimumab | II | Recruiting Estimated completion date: 2/2020 |
N/A | N/A | |
| Advanced chordoma | Relatlimab + Nivolumab | II | Not yet recruiting Estimated completion date: 7/2022 |
N/A | N/A | |
| Virus associated solid tumors | Relatlimab ± nivolumab | I/II | Recruiting Estimated completion date: 12/2019 |
N/A | N/A | |
| Recurrent and metastatic microsatellite high (MSI-Hi) and microsatellite-stable (MSS) colon cancer | Relatlimab ± Nivolumab | II | Active, not recruiting Estimated completion date: 12/2019 |
N/A | N/A | |
| Metastatic/locally advanced microsatellite stable (MSS) colon cancer | Relatlimab + Nivolumab | II | Recruiting Estimated completion date: 10/2022 |
N/A | N/A | |
| Advanced mismatch repair deficient solid tumors resistant to prior PD-(L)1 inhibitor | Relatlimab + Nivolumab | II | Recruiting Estimated completion date: 10/2022 |
N/A | N/A | |
| Relapsed/refractory Hematologic Malignancies |
Relatlimab ± Nivolumab | I/IIa | Recruiting Estimated completion date: 1/2020 |
N/A | N/A | |
| Glioblastoma Gliosarcoma Recurrent brain neoplasm |
A1: Relatlimab A2: Urelumab (anti-CD137) B1: Relatlimab ± Nivolumab B2: Urelumab + Nivolumab |
I | Recruiting Estimated completion date: 12/2019 |
N/A | N/A | |
| Stage II/III resectable esophageal/GE junction cancer | Pre-operative nivolumab ± relatlimab combined with chemoradiation prior to surgical resection | Ib | Recruiting Estimated completion date: 2/2025 |
N/A | N/A | |
| Advanced gastric cancer | BMS-986016 + Nivolumab | II | Recruiting Estimated completion date: 11/2021 |
N/A | N/A | |
| Advanced non-small cell lung cancer | Nivolumab ± Relatlimab | II | Recruiting Estimated completion date: 4/2021 |
N/A | N/A | |
| Advanced renal cell carcinoma | Nivolumab + Relatlimab/BMS-986205 (anti-IDO1)/Ipilimumab | II | Recruiting Estimated completion date: 1/2022 |
N/A | N/A | |
| Advanced solid tumors | Adaptive matching design: A: Nivolumab + Lirilumab (anti-KIR) B: Nivolumab + Relatlimab C: Nivolumab + Cabiralizumab (anti-CSF1R) D: Nivolumab + Ipilimumab E: Nivolumab + BMS-986156 (anti-GITR) F: Nivolumab + BMS-986205 (anti-IDO1) G: Nivolumab + Radiation therapy |
I | Recruiting Estimated completion date: 4/2022 |
N/A | N/A | |
| LAG525 (anti-LAG-3 mAb) | ||||||
| [73] | Advanced solid and hematologic malignancies | LAG525 + PDR001 (spartalizumab, anti-PD-1) | II | Active, not recruiting Estimated completion date: 2/2021 |
N/A | N/A |
| Advanced solid tumors | LAG525 + Spartalizumab (anti-PD-1) | I/II | Active, not recruiting Estimated completion date: 4/2019 |
N/A | N/A | |
| Triple-negative breast cancer | A1: LAG525 + Spartalizumab A2: LAG525 + Spartalizumab + Carboplatin A3: LAG525 + Carboplatin |
II | Recruiting Estimated completion date: 2/2019 |
N/A | N/A | |
| Triple-negative breast cancer6 | LAG3 + Spartalizumab ± NIR-178 (anti-A2AR)/INC-280 (Capmatinib, anti-c-Met)/MCS110 (anti-M-CSF)/Canakinumab (anti-IL1β). | Ib | Recruiting Estimated completion date: 7/2020 |
N/A | N/A | |
| Previously treated unresectable or metastatic melanoma | A1: LAG525 + Spartalizumab A2: Capmatinib + Spartalizumab A3:Canakinumab + Spartalizumab |
II | Recruiting Estimated completion date: 3/2022 |
N/A | N/A | |
| MK-4280 (anti-LAG3 mAb) | ||||||
| Hodgkin’s and Non-Hodgkin’s B-cell lymphomas | MK-4280 ± Pembrolizumab | I/II | Recruiting Estimated completion date: 12/2025 |
N/A | N/A | |
| [74, 75] | Advanced solid tumors | MK-4280 ± Pembrolizumab | I | Recruiting Estimated completion date: 7/2021 - Preliminary results reported at 2018 SITC |
N/A | - MK-4280: ORR:6% DCR: 17% -Combination with Pembrolizumab: ORR: 27% DCR: 40% |
| Advanced NSCLC | Pembrolizumab + Relatlimab/Lenvatinib based on GEP and TMB | II | Recruiting Estimated completion date: 5/2022 |
N/A | N/A | |
| REGN3767 (anti-LAG-3 mAb) | ||||||
| [76] | Advanced solid/hematologic malignancies | REGN3767 ± REGN2810 (anti-PD-1) | I | Recruiting Estimated completion date: 10/2020 |
N/A | N/A |
| TSR-033 (anti-LAG-3 mAb) | ||||||
| [77] | Advanced solid tumors | TSR-033 ± anti-PD-1 | I | Recruiting Estimated completion date: 5/2021 |
-Increased proliferation and cytokine production of CD8+ and CD4+ T cells in vitro and in vivo -Reduced M2 TAMs in vivo |
N/A |
| Sym022 (anti-LAG-3 mAb) | ||||||
| [78] | Advanced solid tumors and lymphomas | Sym022 | I | Recruiting Estimated completion date: 8/2020 |
-Increased cytokine production by T-cells in vitro and tumor growth inhibition in vivo - Decreased total LAG3 surface levels via internalization/Shedding |
N/A |
| Advanced solid tumors and lymphomas | Sym021 (anti-PD-1) ± Sym022/Sym023 (anti-Tim3) | I | Recruiting Estimated completion date: 11/2020 |
N/A | N/A | |
| INCAGN02385 (anti-LAG-3 mAb) | ||||||
| [79] | Selected advanced solid tumors and lymphomas | INCAGN02385 | I | Recruiting Estimated completion date: 5/2020 |
-Enhanced T-cell responsiveness to TCR stimulation in vivo | N/A |
| MGD013 (a PD-1/LAG-3 bispecific DART®) protein | ||||||
| [80] | Advanced solid tumors, hematologic malignancies | MGD013 | I | Recruiting Estimated completion date: 8/2022 |
-Enhanced cytokine production upon antigenic re-challenge of prior superantigen-stimulated T cells in vitro. | N/A |
| Advanced that have progressed on or after prior PD-1/PD-L1 containing therapy | Recruiting date: 5/2020 |
CD8+ T cells in vitro. - Increased CD8:Tregs ratio in vivo. - Induced loss of LAG3 surface expression on CD4+ and CD8+ T cells in vivo. |
||||
Abbreviations: PD-1=Programmed death 1; PD-L1 = Programmed death ligand 1; CTLA-4=Cytotoxic T lymphocyte antigen 4; Treg: T-regulatory cells; NK: Natural Killer cells; TAM: Tumor-associated macrophages. M-CSF: Macrophage colony-stimulatory factor; IDO-1= Indoleamine 2,3-dioxygenase; mAb=monoclonal antibody; DART= Dual-Affinity Re-Targeting. PFS: Progression-free survival; ORR: Overall response rate; DCR: Disease-control rate.
LAG3-specific blocking mAbs have dominated the therapeutic modalities currently under investigation. There are now seven mAbs in development: Relatilimab (BMS-986016, Bristol-Myers Squibb; fully human IgG4 mAb), LAG525 (Novartis; humanized IgG4), MK-4280 (Merck; humanized IgG4), REGN3767 (Regeneron; human IgG4), TSR-033 (Tesaro; humanized IgG4), Sym022 (Symphogen; Fc-inert human mAb), and INCAGN02385 (InCyte; Fc-engineered IgG1κ) (Table 1). There is considerable interest in the development of mAbs targeting LAG3 for human cancer, particularly in combination with anti-PD1. This is based on preclinical findings that systemic PD1 and LAG3 expression is generally limited in mice and humans but is highly upregulated on intratumoral, dysfunctional TILs [41, 49, 57–59]. Therefore, co-blockade of both IRs may have a more restricted effect within the tumor microenvironment, limiting adverse events [2, 5, 13, 41, 60–62].
Two bispecific antibodies have also recently entered the clinic that target LAG3 and PD1 (MGD013; MacroGenics) or PDL1 (FS118; F-star) (Table 1). MGD013 consists of humanized high-affinity anti-PD1 and anti-LAG3 mAbs that are assembled into an Fc-bearing DART (Dual-affinity Re-targeting) protein. It targets PD1/PDL1, PD1/PDL2 and LAG3/MHC class II interactions. Further in vitro functional characterization has demonstrated enhanced cytokine secretion (i.e. in response to antigenic re-challenge of prior superantigen-stimulated T cells), as compared to the same T cells with either anti-PD1 or anti-LAG3 blocking alone. This agent also demonstrated a prolonged circulating half-life in cynomolgus macaques. FS118 was generated by incorporating an an engineered Fc region with anti-LAG3 antigen binding capability (Fcab) onto a PDL1-specific antibody. Upon administration, FS118 simultaneously targets LAG3 expressed on T cells in the TME and PDL1 on tumor cells or APCs. In vitro functional assays demonstrated increased activation of human CD8+ T cells in response to MHC class I restricted peptides. In vivo studies of this agent in MC38 tumor-bearing mice demonstrated significant anti-tumor activity equivalent to a combination of antibodies targeting LAG3 and PDL1. This correlated with loss of LAG3 surface expression on CD4+ and CD8+ T cells, and an increase in the CD8:Treg ratio. These novel bispecific agents are currently being tested in phase I clinical trials.
4.1: Clinical trials with anti-LAG3 blocking antibodies in cancer
Relatlimab (BMS-986016) is a fully human LAG3-specific antibody that was isolated following immunization of transgenic mice expressing human immunoglobulin (Ig) genes. It is expressed as an immunoglobulin G4 (IgG4) isotype antibody and includes a stabilizing hinge mutation (S228P). It is the first anti-LAG3 humanized mAb to be developed and, of all the anti-LAG3 mAbs, is the furthest along in clinical development. The initial phase I/IIa trial launched in 2013 was designed to evaluate the safety and efficacy of LAG3 blockade as a monotherapy or in combination with Nivolumab for patients with advanced solid malignancies (cervical, ovarian, bladder, colorectal, HPV-positive HNSCC, gastric, hepatocellular, RCC), who were not previously exposed to immunotherapy (). It is now being evaluated in 18 phase I and II/III trials in a variety of solid and hematological malignancies.
The combination of Relatlimab and Nivolumab (anti-PD1) demonstrated exciting preliminary efficacy in melanoma patients who were refractory to previous immunotherapy () [63]. In this phase I/IIa dose escalation and cohort expansion study, these pre-treated patients, all of whom had progressed on immunotherapy, and 47% of whom had failed 3 or more prior therapies, were treated with the combination of Nivolumab and Relatlimab. Among 48 evaluable patients, the objective response rate (ORR) was 12.5%. Of note, patients with LAG3 expression >1% on tumor-infiltrating lymphocytes (TIL) had a response rate of 20%, compared to 7.1% among patients with <1% LAG3 expression. Interestingly, the combination of Nivolumab and Relatlimab was well tolerated, with grade 3 or 4 treatment related immune adverse events seen in 9% of patients, which is similar to the frequency seen with Nivolumab alone. This suggest that an Nivolumab/Relatlimab combination may be safer that a Nivolumab/Ipilimumab (anti-CTLA4) combination. Among those patients with evaluable tumor specimens, the rate of LAG3 positivity, defined as the percentage of LAG3+ immune cells ≥ 1% of tissue, was approximately 60%. It is not known whether LAG3 positivity correlates with poorer response to Nivolumab or whether LAG3 expression increases at disease progression, as these were not tested in this trial. Based on these promising findings, there is now an ongoing phase II/III trial of Nivolumab + Relatlimab versus Nivolumab alone in the front-line setting against previously untreated unresectable/metastatic melanoma ().
Preliminary results were recently reported for the first-in-human phase I dose-finding study for the MK-4280 anti-LAG3 mAb (Merck) as monotherapy or in combination with Pembrolizumab for patients with advanced solid tumors ( [64]). MK-4280 monotherapy demonstrated 6% ORR, with 17% Disease Control Rate (DCR) in 18 patients, whereas MK-4280 + Pembrolizumab demonstrated 27% ORR, with 40% DCR in 15 patients. The majority of patients in both groups had received two or more lines of prior therapy. The MK-4280 monotherapy or in combination with Pembrolizumab were well-tolerated, with no dose-limiting toxicities.
Taken together, these early clinical trial findings are congruent with the findings in the murine studies and although anti-LAG3 blockade by itself does not currently demonstrate significant anti-tumor efficacy, there may be a synergistic anti-tumor immune response when combined with anti-PD1 blockade. In murine tumor models, as well as in some cancer patients who are refractory to anti-PD1 therapy, a combination of anti-LAG3 with anti-PD1 may help to overcome resistance to anti-PD1 immunotherapy. These are of significant interest for the ongoing exploration of LAG3 as an alternative immune checkpoint target, as well as a potential predictive biomarker of clinical response [65]. Randomized phase III clinical trials in the near future with rigorous comparison between the anti-LAG3 + anti-PD1 versus anti-PD1-treated groups, will be required to determine if there is clinical efficacy in multiple cancer types.
5: Conclusions and Future Directions
LAG3 is an IR that helps to maintain homeostasis in the immune system. We are just beginning to understand the complex biology of LAG3. Vital questions remain regarding how to apply and optimize the efficacy of LAG3-targeted immunotherapy, alone or in combination with anti-PD1/L1 and other immunotherapies, for autoimmune and inflammatory diseases, chronic infections, and cancer.
First, how LAG3 negatively regulates TCR signaling is essentially unknown. The unique intracellular cytoplasmic domain of LAG3 with the conserved ‘EP’ and ‘KEELE’ motifs, and absence of a tyrosine-based motif, distinguishes it from all other immune inhibitory receptors. We need to have a better understanding of how LAG3 works, as this will serve as an important conceptual advance and provide vital information for future immuno-therapeutic development.
Second, the discovery of fibrinogen-like protein (FGL1) as a novel ligand for LAG3 [31] also offers an exciting possibility of dual blockade of FGL1 and LAG3 to enhance cancer immunotherapy. It also remains to be determined how important LAG3:FGL1 interaction is in human cancer and if the current LAG3-targeting therapeutics block LAG3:FGL1 interaction.
Third, given that LAG3 is expressed on multiple immune cell types, understanding the difference and similarities in the function of LAG3 in these cell types and how they are impacted by anti-LAG3 blockade in the setting of autoimmunity, chronic infections and cancer is imperative. Of particular importance is understanding how anti-LAG3 would affect the function of CD4+ and CD8+ Teff versus Treg sub-populations, and the role of LAG3 on pDCs, NK cells and other cells types remains obscure.
Fourth, there are still important basic and translational questions that remain unanswered: (a) Can anti-LAG3 agonistic antibodies be generated and can they suppress autoimmune and inflammatory diseases, and if so, which immune cell population or subset mediates this effect? (b) What signaling pathway(s) underlie the complex synergistic interactions between LAG-3 and other immune checkpoints such as PD1? (c) How does anti-LAG3 alone or in combination with anti-PD1 alter the transcriptional program of exhausted T cells in the setting of chronic infections and cancer? (d) Can LAG3 expression and/or sLAG3 in plasma serve as a prognostic or predictive biomarker to inform combinatorial immunotherapy and would this be more important in certain tumor types or disease settings?
Elucidating these questions is critical for designing efficacious immunotherapeutic strategies that target LAG3. Based on the promising pre-clinical human and murine studies with LAG3-targeted therapies in combination with anti-PD1/PDL1, numerous clinical studies are ongoing to fully evaluate their safety and efficacy. It will also be intriguing to evaluate the results from ongoing clinical trials using LAG3 depleting or agonistic mAb for autoimmune and inflammatory disorders. Lastly, identifying approaches that combine anti-LAG3 with other therapeutic modalities, such as chemotherapy, radiation therapy and targeted therapy, may unleash further potential therapeutic approaches. As the third inhibitory receptor to be targeted in the clinic, behind CTLA4 and PD1, LAG3 remains an exciting target for immunotherapy in numerous diseases including chronic infection, autoimmunity, inflammatory disease, and of course cancer.
ACKNOWLEDGEMENTS
The authors wish to thank Lawrence Andrews for helpful comments. This work was supported by the National Institutes of Health [P01 AI108545 and R01 CA203689 to D.A.A.V, and R01 AI144422 to D.A.A.V. and C.J.W.]; NCI Comprehensive Cancer Center Support CORE grant [CA047904 to D.A.A.V.], HNSCC SPORE grant [P50 CA097190 to D.A.A.V.]; Associazione Italiana Per La Ricerca Sul Cancro (AIRC) postdoctoral fellowship [Project code: 22321 to E.R.]; and NCI Institutional National Research Service Award in Cancer Therapeutics [T32 CA193205 supporting R.W.; to E. Chu].
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors declare competing financial interests. D.A.A.V and C.J.W. have submitted patents covering LAG3 that are licensed or pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Brown KE, Freeman GJ, Wherry EJ, Sharpe AH, Role of PD-1 in regulating acute infections, Curr Opin Immunol 22(3) (2010) 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goldberg MV, Drake CG, LAG-3 in Cancer Immunotherapy, Curr Top Microbiol Immunol 344 (2011) 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gravitz L, Cancer immunotherapy, Nature 504(7480) (2013) S1. [DOI] [PubMed] [Google Scholar]
- [4].Couzin-Frankel J, Breakthrough of the year 2013. Cancer immunotherapy, Science 342(6165) (2013) 1432–3. [DOI] [PubMed] [Google Scholar]
- [5].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, N Engl J Med 366(26) (2012) 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M, Nivolumab plus ipilimumab in advanced melanoma, N Engl J Med 369(2) (2013) 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Postow MA, Callahan MK, Wolchok JD, Immune Checkpoint Blockade in Cancer Therapy, J Clin Oncol 33(17) (2015) 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, Juergens RA, Laurie SA, Nathan FE, Shen Y, Harbison CT, Hellmann MD, Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer, J Clin Oncol 34(25) (2016) 2980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Workman CJ, Dugger KJ, Vignali DA, Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3, J Immunol 169(10) (2002) 5392–5. [DOI] [PubMed] [Google Scholar]
- [10].Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA, Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo, J Immunol 172(9) (2004) 5450–5. [DOI] [PubMed] [Google Scholar]
- [11].Workman CJ, Vignali DA, Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223), J Immunol 174(2) (2005) 688–95. [DOI] [PubMed] [Google Scholar]
- [12].Wherry EJ, T cell exhaustion, Nat Immunol 12(6) (2011) 492–9. [DOI] [PubMed] [Google Scholar]
- [13].Andrews LP, Marciscano AE, Drake CG, Vignali DA, LAG3 (CD223) as a cancer immunotherapy target, Immunol Rev 276(1) (2017) 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T, LAG-3, a novel lymphocyte activation gene closely related to CD4, J Exp Med 171(5) (1990) 1393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA, Role of LAG-3 in regulatory T cells, Immunity 21(4) (2004) 503–13. [DOI] [PubMed] [Google Scholar]
- [16].Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ, Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection, Nat Immunol 10(1) (2009) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA, LAG-3 regulates plasmacytoid dendritic cell homeostasis, J Immunol 182(4) (2009) 1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Q, Chikina M, Szymczak-Workman AL, Horne W, Kolls JK, Vignali KM, Normolle D, Bettini M, Workman CJ, Vignali DAA, LAG3 limits regulatory T cell proliferation and function in autoimmune diabetes, Sci Immunol 2(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F, CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins, Eur J Immunol 25(9) (1995) 2718–21. [DOI] [PubMed] [Google Scholar]
- [20].Li N, Workman CJ, Martin SM, Vignali DA, Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223), J Immunol 173(11) (2004) 6806–12. [DOI] [PubMed] [Google Scholar]
- [21].Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP, Dempsey PJ, Workman CJ, Vignali DA, Metalloproteases regulate T-cell proliferation and effector function via LAG-3, EMBO J 26(2) (2007) 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Workman CJ, Vignali DA, The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells, Eur J Immunol 33(4) (2003) 970–9. [DOI] [PubMed] [Google Scholar]
- [23].Iouzalen N, Andreae S, Hannier S, Triebel F, LAP, a lymphocyte activation gene-3 (LAG-3)-associated protein that binds to a repeated EP motif in the intracellular region of LAG-3, may participate in the down-regulation of the CD3/TCR activation pathway, Eur J Immunol 31(10) (2001) 2885–91. [DOI] [PubMed] [Google Scholar]
- [24].Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, Maigret B, Dreano M, Triebel F, Characterization of the major histocompatibility complex class II binding site on LAG-3 protein, Proc Natl Acad Sci U S A 94(11) (1997) 5744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S, MHC antigens in human melanomas, Semin Cancer Biol 2(1) (1991) 35–45. [PubMed] [Google Scholar]
- [26].Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F, Al-Daccak R, Michel L, MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis, J Immunol 186(9) (2011) 5173–83. [DOI] [PubMed] [Google Scholar]
- [27].Donia M, Andersen R, Kjeldsen JW, Fagone P, Munir S, Nicoletti F, Andersen MH, Thor Straten P, Svane IM, Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T-Cells, Which Dampen CD8+ T-cell Antitumor Reactivity, Cancer Res 75(18) (2015) 3747–59. [DOI] [PubMed] [Google Scholar]
- [28].Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E, Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells, Cancer Immunol Res 3(4) (2015) 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dumic J, Dabelic S, Flogel M, Galectin-3: an open-ended story, Biochim Biophys Acta 1760(4) (2006) 616–35. [DOI] [PubMed] [Google Scholar]
- [30].Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F, LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses, Cancer Res 74(13) (2014) 3418–28. [DOI] [PubMed] [Google Scholar]
- [31].Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, Chen L, Chen Y, Zhu G, Yin W, Zheng L, Zhou T, Badri T, Yao S, Zhu S, Boto A, Sznol M, Melero I, Vignali DAA, Schalper K, Chen L, Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3, Cell 176(1–2) (2019) 334–347 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yamamoto T, Gotoh M, Sasaki H, Terada M, Kitajima M, Hirohashi S, Molecular cloning and initial characterization of a novel fibrinogen-related gene, HFREP-1, Biochem Biophys Res Commun 193(2) (1993) 681–7. [DOI] [PubMed] [Google Scholar]
- [33].Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, Drake CG, Vignali DA, Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4, Eur J Immunol 40(6) (2010) 1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bae J, Lee SJ, Park CG, Lee YS, Chun T, Trafficking of LAG-3 to the surface on activated T cells via its cytoplasmic domain and protein kinase C signaling, J Immunol 193(6) (2014) 3101–12. [DOI] [PubMed] [Google Scholar]
- [35].Moss ML, Minond D, Recent Advances in ADAM17 Research: A Promising Target for Cancer and Inflammation, Mediators Inflamm 2017 (2017) 9673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Q, Vignali DA, Co-stimulatory and Co-inhibitory Pathways in Autoimmunity, Immunity 44(5) (2016) 1034–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC, Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors, Diabetes 67(8) (2018) 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paluch C, Santos AM, Anzilotti C, Cornall RJ, Davis SJ, Immune Checkpoints as Therapeutic Targets in Autoimmunity, Front Immunol 9 (2018) 2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DA, Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3, J Immunol 187(7) (2011) 3493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee SY, Goverman JM, The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells, J Immunol 190(10) (2013) 4991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA, Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape, Cancer Res 72(4) (2012) 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Richter K, Agnellini P, Oxenius A, On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection, International immunology 22(1) (2010) 13–23. [DOI] [PubMed] [Google Scholar]
- [43].McLane LM, Abdel-Hakeem MS, Wherry EJ, CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer, Annu Rev Immunol (2019). [DOI] [PubMed] [Google Scholar]
- [44].Phillips BL, Mehra S, Ahsan MH, Selman M, Khader SA, Kaushal D, LAG3 expression in active Mycobacterium tuberculosis infections, Am J Pathol 185(3) (2015) 820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT, Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection, Nat Immunol 13(2) (2011) 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wherry EJ, Kurachi M, Molecular and cellular insights into T cell exhaustion, Nat Rev Immunol 15(8) (2015) 486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turnis ME, Andrews LP, Vignali DA, Inhibitory receptors as targets for cancer immunotherapy, Eur J Immunol 45(7) (2015) 1892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG, LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems, J Clin Invest 117(11) (2007) 3383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K, Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer, Proc Natl Acad Sci U S A 107(17) (2010) 7875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen J, Chen Z, The effect of immune microenvironment on the progression and prognosis of colorectal cancer, Med Oncol 31(8) (2014) 82. [DOI] [PubMed] [Google Scholar]
- [51].Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE, Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients, J Clin Invest 121(6) (2011) 2350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F, The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints, Cancer Discov 5(1) (2015) 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, De Macedo MP, Austin-Breneman JL, Jiang H, Chang Q, Reddy SM, Chen WS, Tetzlaff MT, Broaddus RJ, Davies MA, Gershenwald JE, Haydu L, Lazar AJ, Patel SP, Hwu P, Hwu WJ, Diab A, Glitza IC, Woodman SE, Vence LM, Wistuba II, Amaria RN, Kwong LN, Prieto V, Davis RE, Ma W, Overwijk WW, Sharpe AH, Hu J, Futreal PA, Blando J, Sharma P, Allison JP, Chin L, Wargo JA, Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade, Cancer Discov 6(8) (2016) 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL Jr., Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA, Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab, Cell 171(4) (2017) 934–949 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Abed Maillard S, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D, Michielin O, Romano E, Berthod G, Rimoldi D, Triebel F, Luescher I, Rufer N, Speiser DE, Vaccination with LAG-3Ig (IMP321) and Peptides Induces Specific CD4 and CD8 T-Cell Responses in Metastatic Melanoma Patients--Report of a Phase I/IIa Clinical Trial, Clin Cancer Res 22(6) (2016) 1330–40. [DOI] [PubMed] [Google Scholar]
- [56].Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C, Marcu M, Triebel F, First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity, J Transl Med 8 (2010) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, Hipkiss E, Vignali DA, Pardoll DM, Drake CG, Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells, Journal of Immunology 182(11) (2009) 6659–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Huard B, Gaulard P, Faure F, Hercend T, Triebel F, Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand, Immunogenetics 39(3) (1994) 213–217. [DOI] [PubMed] [Google Scholar]
- [59].Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA, Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3), Eur J Immunol 32(8) (2002) 2255–63. [DOI] [PubMed] [Google Scholar]
- [60].Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL, Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates, J.Clin.Oncol. 28(19) (2010) 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM, Safety and activity of anti-PD-L1 antibody in patients with advanced cancer, N Engl J Med 366(26) (2012) 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L, Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape, Sci.Transl.Med. 4(127) (2012) 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].PA M.I. Ascierto, Bhatia S, et al. , Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab in patients with melanoma previously treated with ant-PD-1/PD-L1 therapy, J Clin Oncol 35 (2017) suppl; abstr 9520. [Google Scholar]
- [64].Lakhani B, N., TM., Abraham AK, Luddy J, Palcza J, Chartash E, Healy J, Patnaik A, Abstract O26: The anti–LAG-3 antibody MK-4280 as monotherapy and in combination with pembrolizumab for advanced solid tumors: first-in-human phase 1 dose-finding study, Society for Immunotherapy of Cancer Annual Meeting, Washington, D.C., 2018. [Google Scholar]
- [65].Ascierto PA, McArthur GA, Checkpoint inhibitors in melanoma and early phase development in solid tumors: what’s the future?, J Transl Med 15(1) (2017) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brignone C, Escudier B, Grygar C, Marcu M, Triebel F, A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma, Clin Cancer Res 15(19) (2009) 6225–31. [DOI] [PubMed] [Google Scholar]
- [67].Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C, Marcu M, Triebel F, First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity, J Transl Med 8 (2010) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Romano E, Michielin O, Voelter V, Laurent J, Bichat H, Stravodimou A, Romero P, Speiser DE, Triebel F, Leyvraz S, Harari A, MART-1 peptide vaccination plus IMP321 (LAG-3Ig fusion protein) in patients receiving autologous PBMCs after lymphodepletion: results of a Phase I trial, J. Transl. Med 12 (2014) 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Triebel F, Mueller C, Brignone C, Atkinson V, Eastgate M, Amitesh Roy., Khattak A, Haydon A, Poster O28: Results from a Phase I dose escalation trial (TACTI-mel) with the soluble LAG-3 protein (IMP321, eftilagimod alpha) together with pembrolizumab in unresectable or metastatic melanoma, 2018 Society for Immunotherapy of Cancer Annual Meeting, Washington, D.C., 2018. [Google Scholar]
- [70].Ascierto MI, PA, Bhatia S, et al. , Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab in patients with melanoma previously treated with ant-PD-1/PD-L1 therapy, J Clin Oncol 35 (2017) suppl; abstr 9520. [Google Scholar]
- [71].Ascierto BP, PA, Bhatia S, Melero I, Nyakas M, Svane I, Larkin J, Gomez-Roca CA, Schadendorf D, Dummer R, Marabelle A, Hoeller C, Maurer M, et al. , Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma, ESMO Congress, Spain, Madrid, 2017. [Google Scholar]
- [72].Ascierto PA, McArthur GA, Checkpoint inhibitors in melanoma and early phase development in solid tumors: what’s the future?, J Transl Med 15(1) (2017) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Piha-Paul S, Amin A, Kovacs C, Magley A, Purkayastha DD., Zhuo Y, A phase 2, open-label study of the combination of spartalizumab (PDR001) and LAG525 for patients with advanced solid tumors and hematologic malignancies, American Society of Clinical Oncology (ASCO) Annual Meeting, Journal of Clinical Oncology Chicago, Illinois., 2018. [Google Scholar]
- [74].Haines BB, Javaid S, Cui L, Hirsch H, Cemerski S, McClanahan T, Sathe M, Zhang S, Rosenzweig M, Long B, Malefyt R.d.W., Abstract 4714: Blockade of LAG-3 amplifies immune activation signatures and augments curative antitumor responses to anti-PD-1 therapy in immune competent mouse models of cancer, Cancer research 77(13 Supplement) (2017) 4714–4714. [Google Scholar]
- [75].Lakhani B, N., TM., Abraham AK, Luddy J, Palcza J, Chartash E, Healy J, Patnaik A, Abstract O26: The anti–LAG-3 antibody MK-4280 as monotherapy and in combination with pembrolizumab for advanced solid tumors: first-in-human phase 1 dose-finding study, S ociety for Immunotherapy of Cancer Annual Meeting, Washington, D.C., 2018. [Google Scholar]
- [76].Papadopoulos KP, Lakhani NJ, Johnson ML, Park H, Wang D, Yap TA, Moore KN, Sims TN, Emeremni C, Karasarides M, Kroog GS, A study of REGN3767, an anti-LAG-3 antibody, alone and in combination with cemiplimab (REGN2810), an anti-PD1 antibody, in advanced cancers, Journal of Clinical Oncology 36(15_suppl) (2018) TPS3127–TPS3127. [Google Scholar]
- [77].Ghosh S, Sharma G, Travers J, Kumar S, Choi J, Jun HT, Kehry M, Ramaswamy S, Jenkins D, TSR-033, a novel therapeutic antibody targeting LAG-3 enhances T cell function and the activity of PD-1 blockade in vitro and in vivo, Mol Cancer Ther (2018). [DOI] [PubMed] [Google Scholar]
- [78].Grandal MM, Melander MC, Bhatia VK, Gjetting T, Lindsted T, Fröhlich C, Lantto J, Horak ID, Kragh M, Kofoed K, Pedersen MW, Abstract 5626: Preclinical characterization of Sym022, a novel anti-LAG3 antibody, Cancer research 78(13 Supplement) (2018) 5626–5626. [Google Scholar]
- [79].Savitsky D, Ward R, Riordan C, Mundt C, Jennings S, Connolly J, Findeis M, Sanicola M, Underwood D, Nastri H, Scherle P, Hollis G, Huber R, Stein R, Dijk M.v., Wilson NS, Abstract 3819: INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies, Cancer research 78(13 Supplement) (2018) 3819–3819. [Google Scholar]
- [80].LaMotte-Mohs R, Shah K, Smith D, Gorlatov S, Ciccarone V, Tamura J, Li H, Rillema J, Licea M, He L, Vasanwala F, Chen W, Yao X-T, Chen F, Brown J, Nordstrom J, Koenig S, Bonvini E, Johnson S, Moore P, Abstract 3217: MGD013, a bispecific PD-1 x LAG-3 Dual-Affinity Re-Targeting (DART®) protein with T-cell immunomodulatory activity for cancer treatment, Cancer research 76(14 Supplement) (2016) 3217–3217. [Google Scholar]
- [81].Kraman M, Fosh N, Kmiecik K, Everett K, Zimarino C, Faroudi M, Wydro M, Koers A, Young L, Gliddon D, Morrow M, Doody J, Tuna M, Brewis N, Abstract 2719: Dual blockade of PD-L1 and LAG-3 with FS118, a unique bispecific antibody, induces CD8+ T-cell activation and modulates the tumor microenvironment to promote antitumor immune responses, Cancer research 78(13 Supplement) (2018) 2719–2719. [Google Scholar]