Abstract
Although clinical studies identify traumatic brain injury (TBI) as a risk factor for the development of substance use disorder, much remains unknown about the possible underlying pathogenesis and age-specific effects. Thus, the aim of this study is to test the hypothesis that at an age of ongoing maturation, adolescent TBI alters elements of the reward pathway, resulting in increased sensitivity to the rewarding effects of a subthreshold dose of cocaine that does not induce significant behavioral changes in naïve, non-injured mice. Specifically, these results were derived from the combination of the controlled cortical impact model of TBI, performed on either adolescent (6 weeks) or young adult (8 weeks) mice, followed by the cocaine-induced conditioned place preference assay 2 weeks later. Using three-dimensional isosurface rendering and volumetric image analysis, TBI was found to induce neuromorphological changes such as decreased dendritic complexity and reduced spine density in brain regions essential for reward perception and processing of drug-induced euphoria. Further, we demonstrated that these neuronal changes may affect the differential expression of dopamine-associated genes. Our analysis also provided evidence for age-related differences in immune response and the distinct involvement of augmented microglial phagocytic activity in the remodeling of neuronal structures in the adolescent TBI brain. Our studies suggest that TBI during adolescence, a period associated with ongoing maturation of dopaminergic systems, may subsequently enhance the abuse liability of cocaine in adulthood.
Keywords: addiction, adolescent, behavior, neuroinflammation, traumatic brain injury
Introduction
According to the Centers for Disease Control and Prevention, more than 1 million traumatic brain injuries (TBIs) occur annually in the United States.1,2 Recent clinical advancements in the diagnosis and management of TBI have led to decreased numbers of TBI-related fatalities but increased numbers of TBI patients who survive and endure life-long consequences of their injuries. Current reports estimate that more than 5 million Americans live with disabilities and psychiatric complications associated with TBI.3,4 This number likely is an underestimate as many TBIs remain undiagnosed due to underreporting or misdiagnosis. Long-term negative consequences of TBI include sleep disturbances, difficulty concentrating, anxiety, depression, aggression, and deficits in decision making and impulsivity. Recent studies have begun to establish the links between TBI and comorbid psychiatric disorders, including substance use disorders (SUD).4 TBI remains a public health epidemic, which despite increased awareness and characterization of persistent TBI sequalae such as risk of SUD, the underlying pathology remains poorly understood.5–8
The population at highest risk for experiencing a TBI includes children and adolescents (ages 15-19 years old), a period during which primary neuronal networks that regulate reward behaviors remain under development.1,2 Notably, the rate of concussive brain injuries among children under the age of 19 more than doubled during the period of 2001-2012.9 By contrast, the age range of people most likely to experiment with drugs of abuse (20-24 years old) immediately follows the peak age at risk for having a TBI.10,11 The studies presented herein were designed to capture this specific chronology. Age of injury versus age of onset of drug use is highly relevant to risk for developing SUD because one of the most significant neural transformations that occurs during adolescence is the maturation of dopaminergic projections to the prefrontal cortex (PFC).12-14 These projections are a central component of the neuronal network comprised of both anatomical and functional connectivity between the PFC, nucleus accumbens (NAc), and ventral tegmental area (VTA), which have been implicated in addiction behavior.15 Dysregulation of dopaminergic signaling within this circuit, a known consequence of TBI, has consistently been linked to SUD.16,17 However, to date, mechanisms underlying how neurotrauma-induced molecular changes during development ongoing throughout adolescence affect brain structures essential for reward perception and processing of drug-induced euphoria, including PFC, NAc, and VTA, remain unknown.
Clinical data strongly support a link between having a history of TBI and subsequent SUD development. SUD is the third most frequent de novo neuropsychiatric disorder diagnosis among TBI patients.5 In fact, adolescent TBI recently was identified as a risk factor for increased vulnerability for problematic drug use.18,19 Pre-clinical studies have begun to explore the pathogenesis of addiction-like phenotypes following TBI. However, these investigations have mainly focused on the correlation between early-life TBI and alcohol use.20–23 Our previous studies were the first to extend these findings to an illicit drug of abuse and showed that male mice subjected to adolescent TBI displayed enhanced cocaine conditioned place preference (CPP) scores.24 Our results also highlighted that TBI induces neuroinflammation in regions that regulate motivation, stimulus saliency, and perception of reward, deficits commonly reported in TBI and SUD populations.24,25 In further support of this notion, a recent report provides evidence that TBI-induced neuroinflammation contributes to the increase in the acquisition of cocaine self-administration in rats.26 Overall, these studies demonstrate an increase in cocaine-seeking behavior following exposure to brain injury during adolescence, which is recognized as a developmental period associated with increased sensitivity to drugs of abuse. However, the question remains whether adolescent neurotrauma induces neurobiological changes augmenting age-dependent susceptibility to the rewarding effects of cocaine. Thus, the following study is the first pre-clinical investigation that addresses whether TBI exacerbates adolescent sensitivity by testing behavioral responses to a subthreshold dose of cocaine.
Here, we used the controlled cortical impact model of TBI (CCI-TBI), a prevailing model of TBI, in combination with the CPP assay as a model of behavior in response to cocaine reward in adult and adolescent male mice. We aimed to test the hypothesis that at an age of ongoing maturation, adolescent TBI alters elements of the reward pathway resulting in increased sensitivity to the rewarding effects of a subthreshold dose of cocaine that does not induce significant behavioral changes in naïve, non-injured mice. In parallel, we also examined whether the behavioral changes induced by TBI were selective for the rewarding effects of cocaine by assessing cocaine-induced locomotion. Microglia, the brain's resident immune cells that are centrally involved in neuroinflammation, also are actively involved in pruning of neuronal dendritic spines, a process that peaks during adolescence. We further hypothesized that in the adolescent brain, microglia actively participating in spine pruning offer a primed environment to exacerbate the immune response following brain injury. Therefore, we also evaluated TBI-induced immune cell infiltration and quantified differences in activated microglial phagocytosis of neuronal proteins and subsequent changes in neuronal dendritic arborization and spine density in the injured hemisphere, as well the brain region essential for reward-motivated behavior, the NAc. Finally, we examined how TBI affects the expression of dopamine-associated genes in brain regions critical for the perception of the rewarding effects of cocaine. These experiments were designed to advance our understanding of the pathophysiology underlying increased cocaine use disorder (CUD) vulnerability following adolescent TBI.
Methods
Subjects
For all experiments, 6- or 8-week-old male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Upon arrival, mice were group housed under a 12-h/12-h light/dark cycle with ad libitum access to food and water. Mice were given a 5- to 7-day period of acclimation to the University Laboratory Animal Research facility at the Lewis Katz School of Medicine at Temple University (Philadelphia, PA) prior to the administration of experimental traumatic brain injury. Only male mice were used in this study as sex as a biological variable is beyond the scope of this study and is considered in ongoing experiments. All experiments were approved by the Institutional Animal Care and Use Committee at Temple University and the NIDA-IRP Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865-23).
Controlled cortical impact
Following the acclimation period, experimental traumatic brain injury (TBI) of three severities (sham, mild, and moderate), was administered to different groups of mice using an Impact One™ Stereotaxic CCI Instrument (Leica Microsystems, Buffalo Grove, IL) outfitted with a piston (2-mm diameter) as previously described.24 The CCI model offers the advantage of reproducing the key pathological features that are seen in human TBI.27,28 For instance, the resulting neuropathology closely simulates the cortical tissue loss, acute subdural hematoma, neuronal (cell body and axonal) injury, inflammation (immune recruitment, astrocytic and microglial activation), blood–brain barrier (BBB) dysfunction, and concussion. Mice were anesthetized using vaporized isoflurane (5% induction, 2% maintenance) and placed in a Just for Mouse™ Stereotaxic Instrument (Stoelting Co., Wood Dale, IL). An Ideal Micro-Drill™ (CellPoint Scientific Inc., Gaithersburg, MD) with a 0.5-mm rounded burr was used to perform a craniectomy removing a 4-mm bone flap located to the right of the sagittal suture between lambda and bregma under a Zeiss Stemi 2000-C stereomicroscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) equipped with a SCHOTT EasyLED Ringlight (SCHOTT North America Inc., Elmsford, NY). The electro-magnetically–driven piston was placed at the surface of the exposed parenchyma and a single discharge (moderate: speed: 4.5 m/sec, dwell time: 0.5 sec, depth: 2 mm; mild: speed: 2.0 m/sec, dwell time: 0.5 sec, depth: 1 mm) produced mild- or moderate- (Mod) CCI-TBI in 6-week-old adolescent and 8-week-old young adult male mice. The craniectomy site was then covered by a sterile, 6-mm glass cover slip (ProSciTech Pty Ltd, Kirwan, Australia) adhered to the skull using Vetbond™ tissue adhesive (3M, St. Paul, MN). Post-operatively, mice were individually housed to ensure surgical recovery and weighed and monitored daily for 7 days. Sham controls (craniectomy only) underwent identical surgical procedures except impactor discharge. Naïve controls were individually housed at the same time as experimental mice.
Rotarod
Motor performance was assessed at 48 h and 7 days following induction of experimental TBI. A rotarod apparatus equipped with a 3 cm rotating rod and five lanes each with an automatic timer and falling sensor was used (40 cm width × 30 cm depth × 38 cm height; Ugo Basile, Mt. Laurel, NJ). Before the testing sessions, mice were habituated to stay on the rod at a constant speed of 4 rpm for two 3-min trials. During testing sessions, mice were placed on the rod with the speed set to accelerate from 4-40 rpm over a duration of 5 min. Each testing session consisted of three 5-min trials. Latency to fall from the rotating rod was recorded. Post-injury motor performance was compared with within-subject baseline performance recorded the day before injury to evaluate any motor deficits resulting from TBI. For each TBI severity, there were n = 8 mice per group.
Conditioned Place Preference
Two weeks following TBI, behavior was assessed using a biased CPP assay as previously described.24,25 Briefly, this assay consists of three phases that each take place in custom-designed Plexiglas two-compartment chambers (13.75 inches length × 5.25 inches width × 5.00 inches height; KB Acrylics Inc., Westville, NJ). One compartment was fitted with black walls and a coarse-textured floor, the other was fitted with white and black striped walls and a smooth-surfaced floor. Compartment bias was determined during the first phase (pre-test) when mice had access to both compartments through a door opening in the divider wall for 30 min and time spent in each compartment was recorded. The compartment with a lower residence time was assigned as the drug-paired, least-preferred compartment. During the second phase (conditioning), mice were randomly assigned to receive one of three doses of cocaine, administered in the morning by intraperitoneal (IP) injection (vehicle, 2.5 mg/kg or 10.0 mg/kg). In rodents, cocaine reaches a maximum concentration of 10.1 μM around 20-30 min post–IP injection and then gradually declines thereafter, especially from 120 min and beyond. Therefore, each mouse was immediately placed in its least-preferred compartment for 30 min following IP cocaine without access to the preferred compartment. After a minimum of a 4-h washout period, mice were injected with 0.9% saline and placed in the preferred compartment for 30 min.29-31 This schedule persisted for 6 days.
During the third phase (post-test), mice were placed in the chamber again for 30 min with free access to both compartments without any injections. The rewarding effects of cocaine were evaluated by a place preference shift, which was calculated by subtracting the time spent in the drug-paired compartment during the pre-test from the time spent in the drug-paired compartment during the post-test. When testing or conditioning occurred on the same day, mice assigned to various injury severity conditions were distributed equally to control for any influence of time of test. This assay was repeated in Naïve and Mod-CCI-TBI only using vehicle, 30 mg/kg cocaine, or 50 mg/kg cocaine to assess the descending curve of the typical inverted-U shaped dose response curve in adolescent mice. For each TBI severity and dose of cocaine tested, there were n = 8-14 mice per group.
Locomotor activity monitoring
Two weeks following TBI, a separate cohort of experimental mice was evaluated for the effect of TBI on cocaine-induced locomotor activity according to procedures described previously.24,32 Locomotor activity of individual mice was measured in open-field activity chambers (18 inches length × 14 inches width × 8 inches height) using the AccuScan Home Cage Activity System (Omnitech Electronics, Inc., Columbus, OH). Sixteen photobeams arranged along the horizontal axis of the activity chamber were used to automatically collect ambulatory activity (horizontal beam breaks) and stereotypy activity (repeated beam breaks) data from the sensor panels in 5-min intervals. Basal locomotor activity was measured for 60 min prior to the administration of either vehicle or 2.5 mg/kg or 10.0 mg/kg cocaine. Locomotor activity was recorded for an additional 60 min following injections. For each TBI severity and dose of cocaine tested, there were n = 8 mice per group.
Composite Neuroscore
At time-points ranging from 24 h to 3 weeks post-TBI, mice were assessed for neurological motor function on a 4-point scale known as the Composite Neuroscore. This battery uses a series of five neurobehavioral tests: 1) forelimb extension; 2) forelimb paw placement; 3) hindlimb flexion; 4) lateral pulsion; and 5) observation of abnormal twisting behavior. Animals received scores from +4 (uninjured) to 0 (nonfunctional) for both left and right forelimbs in the forelimb extension task and forelimb paw placement, the left and right hindlimbs in hindlimb flexion, and left and right sides for the lateral pulsion test. If no twisting was observed, the animal was scored as normal (+1), and if twisting was present the animal was scored as abnormal (0). The total possible score was 33. Total number of errors (total possible score of 33 – total actual score) is reported. Scores are reported as the average error values given by three different experimenters who were blind to animal condition. The maximum standard deviation of scores between experimenters was 0.57 errors. For each TBI severity, there were n = 8 mice per group.
Y-Maze
Additional experimental mice were evaluated for the effect of TBI on cognition 2 weeks post-TBI using the Y-Maze Spontaneous Alternation test. The maze was comprised of three arms at a 120° angle from each other (32 cm length × 610 cm width × 26 cm height; San Diego Instruments, San Diego, CA). Individual mice were placed in the center of the Y-shaped maze and allowed to freely explore the three arms for a duration of 5 min. The total number of arm entries and the sequential order of entries were recorded. Spontaneous alternations were defined as three consecutive entries into three different arms. Percent alternation was calculated as the total number of alternations divided by the total number of arm entries. For each TBI severity, there were n = 8 mice per group.
Flow cytometry analysis of immune infiltration and microglia
Brain tissue was harvested from a separate cohort of mice at either 2, 5, 14, or 28 days post–CCI-TBI and analyzed for both the impacted and non-impacted hemispheres. Tissue was placed into Hank's Balanced Salt Solution and held on ice until processing. The cerebellum was removed, and hemispheres were separated using a mouse brain matrix (Zivic Instruments, Pittsburgh, PA) and clean razor blades. Each hemisphere was homogenized in RPMI in a 5-mL ounce homogenizer (12-16 strokes) using a previous protocol.4 In short, homogenized brains were combined to a final 30% Percoll solution (9 mL Percoll, 1 mL 10 × phosphate-buffered saline (PBS) without calcium/magnesium, 10 mL RPMI), then centrifuged in a fixed angle rotor at 7800 g for 30 min at room temperature (RT). Floating myelin debris was removed, and the remaining cell solution was passed through a 40 μm cell strainer. The volume was brought up to 50 mL with RPMI and centrifuged at 1500 rpm for 5 min at RT. The cell pellet was resuspended in 1 mL FACS Buffer and transferred to 5 mL polystyrene flow cytometry tubes. A total of 1 mL of Ficoll-Paque PREMIUM was carefully layered under the cell solution, then spun at 2500 rpm for 25 min at RT without brake. The white layer at the interface (∼1 mL) was transferred to a new flow cytometry tube, washed with FACS buffer, and pelleted at 1500 rpm for 5 min. Fc receptors were blocked for 15 min, then cells labeled using the following antibodies/reagents purchased from eBioscience Inc./Thermo Fisher Scientific unless indicated otherwise: CD11b (Pacific Blue), CD45 (AmCyan), Ly6G (APC), fixability dye and lineage markers (FVD, CD3, CD9, Siglec F, NK1.1, APC-Cy7), Ly6C (PerCP-5.5), CX3CR-1 (Pe-Cy7, Biolegend). The intracellular protein post-synaptic density scaffolding protein 95 (PSD95; Abcam ab76115, PE secondary) was labeled after fixation and permeabilization. Samples were read on a BD FACS Canto II and data analysis was performing using FlowJo® software (FlowJo, LLC).
Our gating strategy involved gating on singlet populations on FSC-H versus FSC-A, then on live lineage negative populations. Then microglial and leukocytes were gated as CD11b+, CD45+. Neutrophils were excluded by Ly6G. Then gating on Ly6C vs. CD45, monocytes (CD45high) and microglia (CD45low) could be identified. Monocyte subtypes were identified by differential Ly6C expression (low, intermediate, high [Ly6Clow, Ly6Cint, Ly6Chigh]). Microglia were then gated for CX3CR-1low and PSD95+ cells. For each TBI severity, there were n = 3-4 mice per group.
Nucleus accumbens (NAc) punch-outs were processed using a modified version of the above protocol with reduced volumes. Brains were sectioned using the mouse matrix and tissue containing the NAc was excised using a brain microdissection tool 1.25 mm in diameter (Stoelting Co.). A total of 1.25 mm punchouts of the NAc from the right (impacted) hemisphere were homogenized using a pestle. Punchouts were resuspended in a 30% Percoll solution as above and centrifuged at 7800 g for 30 min RT. Myelin debris was removed, and cells were washed, centrifuged, and underlaid with Ficoll. The interface was collected after a spin at 2500 rpm for 25 min at RT without brake. Cells were then washed and stained as described above. For each TBI severity, there were n = 4-5 mice per group.
Golgi-Cox staining and imaging
Two weeks post-TBI, brains were harvested and placed in Golgi-Cox impregnation solution according to manufacturer's instructions (FD Rapid GolgiStain™ Kit; FD NeuroTechnologies Inc., Columbia, MD). After 24 h, the impregnation solution was refreshed, and brains were incubated for an additional 2 weeks at RT in the dark. Golgi-Cox impregnated brains were transferred to a 30% sucrose/30% ethylene glycol/1% PVP-40 cryoprotectant solution and allowed to equilibrate at 4°C. Brains were subsequently segmented in 2 mm increments and sectioned at a thickness of 200 μm using medium speed and frequency settings on the VT1000 S vibratome (Leica Biosystems). Sections were collected in cryoprotectant solution, mounted on gelatin-coated slides (FD NeuroTechnologies, Inc.; Cat. PO101), and allowed to dry for 48 h. Dried sections were rehydrated in water and stained via a 10-min dark immersion in freshly prepared 20% ammonia solution (Fisher, Cat A669-212), and a 10-min dark immersion in freshly prepared 1% sodium thiosulfate solution (Fisher, Cat S445-500). Stained sections were dehydrated through a graded ethanol series and cleared in xylene. Sections were mounted with Permount Mounting Medium (Fisher, Cat SP15-100) and high tolerance No. 1.5 cover glass (Marienfeld, Cat 0107242) and allowed to dry for 72 h prior to imaging.
Golgi-Cox stained sections were scanned with a long working distance water immersion 20 × objective APO lens (NA 0.95) under 1.5 × optimal zoom using a DS-Ri2 color camera outfitted on Nikon's A1R resonant scanning confocal system. Images were collected at a resolution of 4908 × 3264 pixels, with a pixel resolution of 0.24 μm, to allow for maximal discrimination of dendritic spines. Sections were scanned at multiple z levels and screened against the Mouse Brain Atlas (Paxinos and Franklin, 2nd edition) for coordinate determination. For all treatment groups, 100 μm multi-point z-stacks medial and ventral to the anterior commissure, with a step size of 0.6 μm, were collected from the rostral end of sections spanning approximately +1.78 bregma to +1.94 bregma. Resultant brightfield z-stack images were converted to grayscale, inverted and converted to a 16-bit format to create a pseudo-fluorescence image, and three dimensionally deconvolved via the Richardson-Lucy algorithm. All inversions and conversions were carried out on Nikon's NIS-Elements AR 5.11.00 software interface. Subsequent analyses of dendritic spine metrics within the NAc core and shell were performed using the FilamentTracer module within the Imaris v8.1.2 imaging software (Bitplane; Zurich, Switzerland).
Once the Z-stacks were loaded in Imaris, the surface module was used to render the images into three-dimensional (3D) volumes. The surface rendered neurons in the volume were then processed with the filament module for the identification and measurement of neuronal dendrite and dendritic spine characteristics. The measurements presented in the results are based on the following parameters. Dendrites starting point diameter = 12 microns, dendrite seed points = 0.5 microns, dendrite diameter threshold = 5.2 microns, and the dendrite diameter algorithm was set to distance map. Spine seed point diameter = 0.3 microns, spine maximum length = 10.0 microns, no branched spine detection was enabled, spine seed point threshold = 1153.46 microns, spine diameter threshold was set to automatic, and spine diameter algorithm was set to distance map. For each TBI severity, there were n = 3-4 mice per group.
Real-time polymerase chain reaction
The expression of genes associated with the dopamine system and synaptic plasticity was evaluated by real-time quantitative polymerase chain reaction (RT-qPCR) as previously described.24,25 Briefly, 2 weeks post-injury, brains were removed following transcardial perfusion with 20 mL 1 × PBS (Corning) and segmented in 2-mm segments using the mouse matrix. Segments were immediately placed into RNALater Solution (Thermo Fisher Scientific) and stored at 4°C overnight. A total of 1.25 mm punch-outs containing the NAc were excised as described above, processed to isolate RNA using TRIzol Reagent (Thermo Fisher Scientific), and quantified using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized using a High Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher Scientific) and an Eppendorf Mastercycler pro (Eppendorf AG, Hauppauge, NY). The prepared cDNA was then mixed with nuclease-free water, TaqMan Fast Universal PCR Master Mix (Thermo Fisher Scientific) and the following TaqMan probes for dopamine and plasticity genes: DAT, DR1, DR2, TH, VMAT, MAOA, and PSD-95. Data were analyzed using ExpressionSuite Software (Thermo Fisher Scientific) using the delta-delta threshold cycle (Ct) method (Relative Quantification). Data are expressed as the relative fold change compared with age-matched, sham controls. For each TBI severity, there were n = 4 mice per group.
Statistical analysis
Data were analyzed for statistical significance using Prism software (version 6.0h; GraphPad Software Inc., La Jolla, CA). Two-way analysis of variance (ANOVA) with Tukey's post hoc tests were performed to analyze the place preference shift data from our cocaine CPP assay and locomotor activity counts. Analysis of immune infiltration and microglia were analyzed by one-way ANOVA followed by Tukey's post hoc tests. Our gene expression data was analyzed by ΔΔCt, normalized to 18S, and are expressed as mean ± standard error of the mean (SEM) fold change, and statistical significance was determined by Student's t-tests, corrected for multiple comparisons using the Holm-Sidak method. For all tests, statistical significance was defined at p < 0.05. For any outlier analysis, the Q coefficient was set to 1% using the ROUT method in GraphPad Prism in order to generate strict thresholds for ruling out outliers.
Results
Adolescent TBI significantly increased sensitivity to the rewarding effects of a subthreshold dose of cocaine
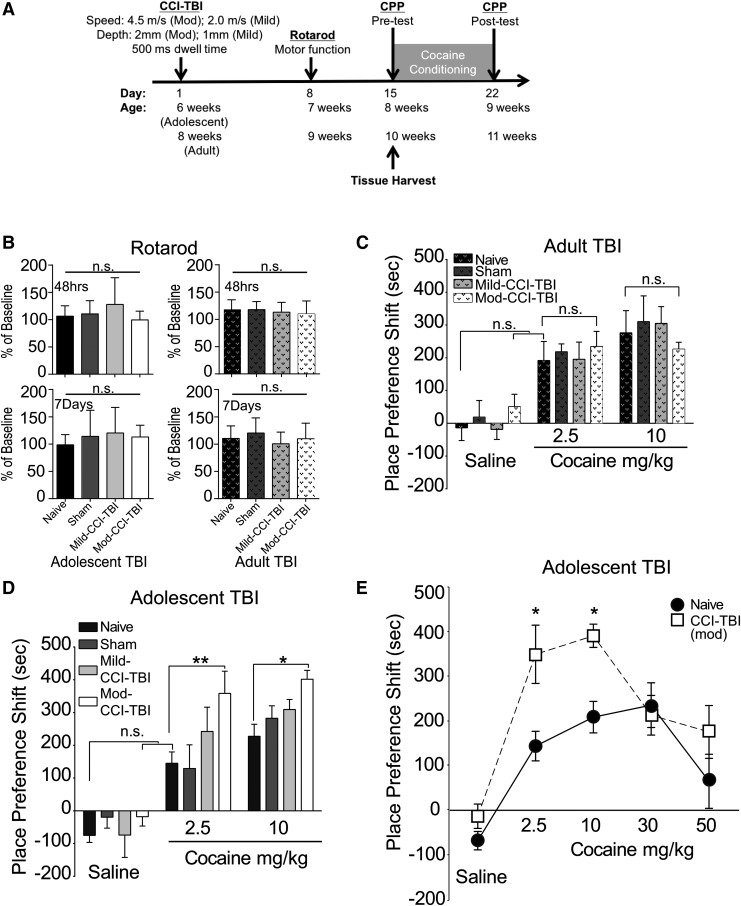
A schematic illustrating the staging of CCI-TBI with respect to the initiation of behavioral assays and tissue harvesting is shown in Figure 1A. We first used the rotarod performance test to assess mice for any motor deficits induced by TBI as a means of exclusionary criteria. Pre-injury baseline performance was used as a percentage standard to show progression of ambulatory function. Mice were considered impaired and excluded from the CPP assay if they fell below 1.5 times the standard deviation from the mean latency to fall of no craniectomy control mice. To this degree, a total of three mice (one adolescent sham, one adolescent Mod-CCI-TBI, and one adult Mod-CCI-TBI) were excluded from further testing following TBI. We found no differences in rotarod performance at either 48 h or 7 days post-injury in either adult or adolescent mice [Fig. 1B; one-way ANOVA, injury severity effect: F (3, 56) = 1.960, p > 0.05]. This finding suggests no change in motor function or coordination following CCI-TBI.
FIG. 1.
Cocaine conditioned place preference (CPP) shift is augmented in mice following adolescent controlled cortical impact model of traumatic brain injury (CCI-TBI). (A) Schematic representing timeline of experimental end-points. (B) Rotarod assay: TBI does not affect rotarod performance in adolescent or adult mice 48 h (top panels) or 7 days (bottom panels) after injury, justifying exclusion of subjects exhibiting rotarod deficits. Data are normalized to pre-injury performance. Injury severity did not affect motor performance. (C) CPP shift for cocaine in adult male mice tested 2 weeks following CCI-TBI. No dose-related differences were detected in CPP shift in adult mice. (D) Dose-related CPP shift for cocaine in adolescent male mice tested 2 weeks following CCI-TBI. Moderate (Mod)-CCI-TBI mice show significantly higher CPP shifts for subthreshold and behaviorally effective cocaine doses of 2.5 and 10.0 mg/kg cocaine, respectively. (E) Dose–effect curve for adolescent naïve (solid line) compared with adolescent Mod-CCI-TBI (dotted line), revealing a leftward shift and atypical responding to high doses of cocaine. n = 7-14 per condition, two-way analysis of variance with Tukey's post hoc tests; cocaine dose effect: F (2, 77) = 53.62, p < 0.01. **p < 0.01, *p < 0.05.
Two weeks following induction of CCI-TBI, a biased CPP assay was conducted to evaluate behavioral responses to the rewarding effects of cocaine in our adolescent and adult experimental mice. Cocaine was administered once daily at a dose of 10.0 or 2.5 mg/kg for 6 consecutive days. These doses were chosen to represent the optimal dose to induce the peak CPP response and the subthreshold dose that did not induce a significant CPP shift in naïve (non-injured) adolescent mice, respectively. Control mice received an equivalent volume of 0.9% saline. In mice injured during young adulthood, the CPP shift of mice that received either 2.5 or 10.0 mg/kg did not differ from naïve mice (Fig. 1C). By contrast, mice injured during adolescence exhibited a significant 10 mg/kg cocaine CPP shift after Mod-CCI-TBI compared with naïve controls [two-way ANOVA with Tukey's post hoc tests; cocaine dose effect: F (2, 77) = 53.62, p < 0.05; Fig. 1D], consistent with our previous findings.24,25 Notably, the subthreshold dose of 2.5 mg/kg cocaine was also identified with a significant CPP shift in adolescent Mod-CCI-TBI compared with naïve mice [two-way ANOVA with Tukey's post hoc tests; cocaine dose effect: F (2, 77) = 53.62, p < 0.01; Fig. 1D]. Further CPP testing of cocaine doses that lie on both the ascending-to-peak and descending portions of their respective inverted U-shape curves33,34 demonstrated a leftward shift of ascending doses following adolescent Mod-CCI-TBI [two-way ANOVA with Tukey's post hoc tests; effect of drug: F (4, 66) = 15.90, p < 0.05; Fig. 1E, dotted line]. While the CPP shift at the higher dose (50 mg/kg cocaine) in naïve adolescent mice is declining (Fig. 1E, solid line), adolescent Mod-CCI-TBI responded atypically to the rewarding effects of cocaine by maintaining an increased CPP shift for 50 mg/kg cocaine, but this effect did not reach statistical significance. These results suggest that TBI during adolescence may enhance the abuse liability of cocaine in adulthood and reduce the threshold for cocaine's rewarding effects.
Adolescent TBI does not alter other behaviors such as cocaine-induced locomotion
To examine whether the behavioral changes induced by TBI were specific to the rewarding effects of cocaine and did not affect additional pharmacodynamic properties of cocaine (e.g., cocaine-induced locomotion), we conducted parallel behavioral assays in an additional cohort of adolescent mice at the same time-point CPP assays were initiated. At 2 weeks post-injury, we report that cocaine dose-dependently increased total locomotor activity [Fig. 2A; cocaine dose effect: F (2,82) = 27.94, p < 0.001]. However, cocaine-induced locomotion was not significantly affected by severity of injury.
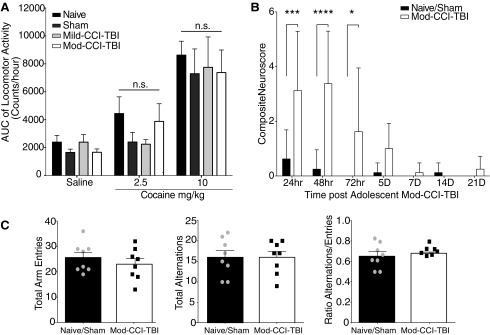
FIG. 2.
Traumatic brain injury (TBI) does not alter cocaine-induced locomotor behavior or long-term Neuroscore or Spontaneous Alternation Behavior. (A) Area under the curve (AUC) analysis of locomotor activity. Total locomotor activity recorded as a combination of ambulatory and stereotypy activity counts over 60 min following a single injection of either 0, 2.5, or 10.0 mg/kg cocaine 2 weeks following increasing degrees of adolescent controlled cortical impact model of traumatic brain injury (CCI-TBI). Data are presented as mean ± standard error of the mean (SEM). We report an effect of cocaine dose on induced locomotor activity [two-way analysis of variance (ANOVA), cocaine dose effect: F (2, 82) = 27.94, p < 0.001], but no effect of brain injury (two-way ANOVA, p > 0.05). (B) Composite Neuroscore includes a battery of neurological motor function tests including forelimb extension, lateral pulsion, forelimb paw placement, hindlimb flexion, and twisting. These were assessed up to 3 weeks following adolescent moderate (Mod)-CCI-TBI, where a maximum total score is 33. Data are presented as mean of errors (total possible score 33 – total actual score) ± SEM. Significant injury effects were observed for the Composite Neuroscore test between naïve and Mod-CCI-TBI mice at 24 [two-way ANOVA with Tukey's post hoc test; Interaction effect of Injury and Time: F (6, 98) = 5.252, p < 0.001], 48 h (DF = 98, p < 0.0001), and 72 h (DF = 98, p < 0.05) post-injury, (Fig. 2B). By 5 days and 1 week post-injury, no difference was observed, indicating Mod-CCI-TBI mice fully recovered from neurological motor deficits prior to the initiation of conditioned place preference (DF = 98, p > 0.05). (C) Spontaneous alternations were measured using the Y-Maze test at 2 weeks following adolescent Mod-CCI-TBI. Total number of arm entries and alternations were recorded for a total of 5 min. We report no differences in total number of arm entries, total number of alternations, or ratio of alternations/entries in adolescent naïve vs. Mod-CCI-TBI mice (unpaired t-tests, two-tailed, t = 0.0, DF = 14, p > 0.05). n = 8 mice per condition.
Analysis revealed no significant difference in ambulatory, stereotypical, or total activity between naïve, sham, Mild-CCI-TBI, or Mod-CCI-TBI mice [two-way ANOVA with Tukey's post hoc test; injury severity effect: F (3, 82) = 0.8169, p > 0.05]. Since, as expected, we observed no significant differences between naïve and sham mice, these experimental groups were combined from this point forward. Next, Naïve/Sham and Mod-CCI-TBI experimental mice were evaluated for neurological motor deficits for up to 3 weeks post-injury. Significant injury effects were observed for the Composite Neuroscore test between naïve and Mod-CCI-TBI mice at 24 h [two-way ANOVA with Tukey's post hoc test; Interaction effect of Injury and Time: F (6,98) = 5.252, p < 0.001], 48 h (DF = 98, p < 0.0001), and 72 h (DF = 98, p < 0.05) post-injury, (Fig. 2B). By 5 days and 1 week post-injury, no difference was observed indicating Mod-CCI-TBI mice fully recovered from neurological motor deficits prior to the initiation of CPP (DF = 98, p > 0.05). As shown in Figure 2C, compared with Naïve/Sham mice, Mod-CCI-TBI mice showed no difference in the number of Y-maze arm entries, number of alternations, or percentage of alternations as calculated as a ratio of number of alternations/entries (unpaired t-tests, two-tailed, t = 0.0, DF = 14, p > 0.05). Taken together, these results indicate that enhanced rewarding properties of cocaine following adolescent CCI-TBI are not likely due to the drug's alternate pharmacodynamic properties affecting locomotion and cognition or neurological impairment after TBI. Therefore, TBI during adolescence may enhance the abuse liability of cocaine and reduce the threshold for cocaine's rewarding effects.
TBI alters immune cell infiltration, microglial activation, and phagocytosis of neuronal proteins
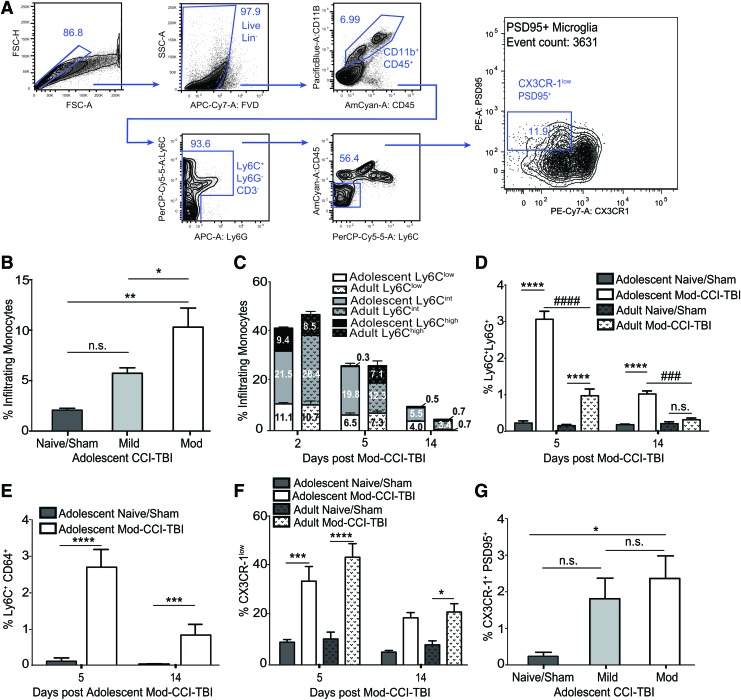
To further interrogate potential pathological mechanisms underlying the behavioral changes observed in CPP, we next investigated the immunological profile of the brain as a consequence of CCI-TBI. It is known that TBI induces a pervasive and robust inflammatory response, including glial activation and the infiltration of immune cells.24,25,35-37 Therefore, in effort to comprehensively characterize the profiles of these events, brains were homogenized and analyzed by flow cytometry at various time-points following either adolescent or adult CCI-TBI. Flow cytometry enables the quantitative characterization and distinction of resident immune cells (microglia) from infiltrating immune cells (monocytes/macrophages). To this end, Figure 3A shows the gating strategy that included identifying live, single cells that were CD11b+CD45+. Neutrophils were identified as CD11b+CD45+Ly6G+. The remaining cells were characterized as CD45low microglia and CD45high monocytes/macrophages. Microglia were then gated on CX3CR-1 and expression of PSD95, a protein involved in the structure and support of dendritic spines and not endogenously expressed by microglia, to evaluate the microglial phagocytosis of neuronal proteins important for synaptic plasticity (Fig. 3A).
FIG. 3.
Traumatic brain injury (TBI) induces immune infiltration and microglial phagocytosis of neuronal proteins. (A) Gating strategy for identification of immune cells and microglia. (B) The percentage of infiltrating monocytes identified as CD11b+ CD45+ Ly6G- Ly6C+ demonstrated a severity-dependent step-wise increase 2 weeks following adolescent controlled cortical impact model of traumatic brain injury (CCI-TBI). Moderate (Mod)-CCI-TBI resulted in a higher percentage of infiltrated monocytes compared with naïve, sham (DF = 12, p < 0.01), and Mild-CCI-TBI [DF = 12, p < 0.05; one-way analysis of variance (ANOVA) with Tukey's post hoc test: F (3, 12) = 13.23, p = 0.004]. (C) At various time-points post-adolescent Mod-CCI-TBI, infiltrating monocytes could be further separated by differential Ly6C expression into high, intermediate (int), and low. At 2 weeks post-injury, the profile of infiltrating monocytes comprised 5.5% Ly6Cint of a total of 10%. When compared with adult Mod-CCI-TBI (dotted), adolescent-injured animals had significantly higher percentages of Ly6Cint at both 5 (DF = 7, p = 0.021) and 14 days post–Mod-CCI-TBI (DF = 8, p = 0.018; Student's t-test, correcting for multiple t-tests using the Sidak-Bonferroni method). Adolescent-injured animals also had significantly higher percentage of persisting Ly6Clow monocytes at 14 days post-injury (DF = 7, Student's t-tests, p < 0.05). Further analysis of Ly6Cint monocytes revealed significant co-expression of Ly6G (D) and CD64 (E) [two-way analysis of variance (ANOVA) with Tukey's post hoc test; Interaction effect of Injury and Time: F (3,29) = 37.01, p < 0.0001: Age-matched Naïve/Sham vs. Mod-CCI-TBI p < 0.0001, p < 0.001: Adolescent Mod-CCI-TBI vs. Adult Mod-CCI-TBI]. (F) The population of microglia was identified as CD11b+ CD45+ Ly6G- Ly6C+. In both adolescent and adult Mod-CCI-TBI, microglia had a pronounced downregulation of CX3CR-1 in the impacted hemisphere at 5 days post-injury, which persisted and was still strongly present at 14 days post-TBI. (G) Two weeks following adolescent Mod-CCI-TBI, microglia that had downregulated CX3CR-1 also were positive for the neuronal marker post-synaptic density protein (PSD95). Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia compared with sham and naïve adolescent mice [one-way ANOVA: effect of injury: F (3,12) = 5.250 with Tukey's post hoc test; *p < 0.05]. n = 3-4 per condition. Color image is available online.
Two weeks after adolescent injury, the percent of infiltrating monocytes increased in a step-wise fashion as a function of injury severity, with Mod-CCI-TBI demonstrating significantly higher infiltration compared with Naïve/Sham (DF = 12; p < 0.01), and Mild-CCI-TBI [DF = 12, p < 0.05; one-way ANOVA with Tukey's post hoc test: F (3, 12) = 13.23, p = 0.004; Fig. 3B]. Further exploration of the characterization of the significantly increased infiltrating monocytes in Mod-CCI-TBI at 2 weeks post-injury revealed three distinct populations (Ly6Chigh, Ly6Cint, and Ly6Clow), which were quantified separately. Monocytes (Ly6Chigh/low) peaked at 2 days before decreasing over the time course (Fig. 3C, 3D). Ly6Cint monocytes were the most persistent following adolescent CCI-TBI compared with adult, with a significant difference peaking at 5 days (DF = 7; p = 0.021) and continuing 2 weeks post-injury (DF = 8; p = 0.018; Student's t-test, correcting for multiple t-tests using the Sidak-Bonferroni method; Fig. 3C). Further evaluation of the Ly6Cint monocyte population for the expression of CD64 and Ly6G revealed a significant increase in the percent of Ly6C+Ly6G+ at 5 days and 2 weeks following injury [two-way ANOVA with Tukey's post hoc test; Interaction effect of Injury and Time: F (3,29) = 37.01, p < 0.0001: Age-matched Naïve/Sham vs. Mod-CCI-TBI p < 0.0001, p < 0.001: Adolescent Mod-CCI-TBI vs. Adult Mod-CCI-TBI; Fig. 3D]. Similarly, Ly6Cint monocytes demonstrated a significant increase in the percent of Ly6C+CD64+ cells at both 5 days and 2 weeks following Adolescent Mod-CCI-TBI [two-way ANOVA with Tukey's post hoc test; Interaction effect of Injury and Time: F (1,12) = 69.17, p < 0.0001: Adolescent Naïve/Sham vs. Mod-CCI-TBI; Fig. 3E].
Microglia had a clear and pronounced downregulation of CX3CR-1 post-TBI. Approximately 40% of microglia could be quantified as CX3CR-1low following TBI, with no difference between age of injury, at 5 days post-injury, which persisted above 20% until 14 days post-injury [two-way ANOVA with Tukey's post hoc test; Interaction effect of Injury and Time: F (3,30) = 3.995, p < 0.001: Age-matched Naïve/Sham vs. Mod-CCI-TBI; Fig. 3F]. Two weeks following adolescent CCI-TBI, a significant percentage of CX3CR-1low microglia were also positive for PSD95. Specifically, we found a significant increase in CX3CR-1low/PSD95+ microglia in adolescent Mod-CCI-TBI hemispheres compared with adolescent Naïve/Sham controls [Fig. 3G; one-way ANOVA: effect of injury: F (3,12) = 5.250 with Tukey's post hoc test; p < 0.05]. These results suggest that inflammatory responses following TBI are age-specific as the activation and phagocytosis status of microglia and infiltration of monocytes persists in the adolescent injured brain compared with the faster resolution seen in adult injury.
TBI alters microglial activation and phagocytosis of neuronal proteins in the nucleus accumbens
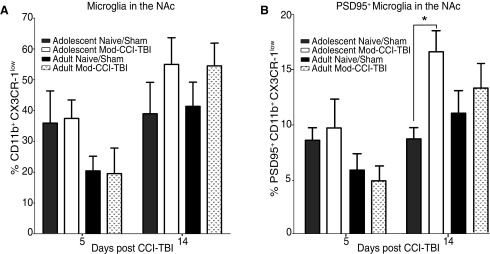
To observe whether a similar profile of microglial response was observed in key regions of the reward pathway that mediate reward-motivated behavior seen in CPP, analyses were then focused on the degree of microglial phagocytosis of neuronal proteins using punch-outs of the PFC and NAc. We report no differences in TBI-induced CX3CR-1low and PSD95+ microglia 2 weeks following adolescent or adult injury in the PFC (data not shown). While we report no difference in the total induction of CX3CR-1low microglia between adolescent and adult Mod-CCI-TBI at either 5 days or 2 weeks post-injury in punch-outs of the NAc (Fig. 4A), increased numbers of microglia with low CX3CR-1 and the co-expression of PSD95 were found at 2 weeks post-TBI in adolescent Mod-CCI-TBI mice only [two-way ANOVA with Sidak's multiple comparisons test: effect of time post-injury: F (1, 28) = 12.52, p < 0.05; Fig. 4B]. This data suggests that TBI affects microglial interaction with neurons in mesolimbic brain regions known to be ongoing during adolescence but not adulthood.
FIG. 4.
Adolescent moderate controlled cortical impact model of traumatic brain injury (Mod-CCI-TBI) increases microglial phagocytosis of neuronal proteins in the nucleus accumbens. At either 5 days or 2 weeks following adolescent Mod-CCI-TBI, the nucleus accumbens (NAc) was excised and processed for microglia that had downregulated CX3CR-1 and also were positive for the neuronal marker post-synaptic density protein (PSD95). (A) Mod-CCI-TBI increased CX3CR-1low microglia compared with sham in both adolescent and adult injuries, while no differences in total percentages of CX3CR-1low microglia in the NAc following either adult or adolescent Mod-CCI-TBI. (B) Only adolescent Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia in the NAc compared with sham mice at 2 weeks post-injury. [two-way ANOVA with Sidak's multiple comparisons test: effect of time post-injury: F (1, 28) = 12.52, *p < 0.05]. n = 4-6 per condition.
Adolescent TBI reduces synaptic arborization complexity and spine density in the nucleus accumbens
The increase in microglial phagocytosis of neuronal proteins may be indicative of structural changes in neuronal arborization and synaptic plasticity in the NAc after TBI. Therefore, we performed morphometric analyses of Golgi-Cox–stained medium spiny neurons in the NAc using reflective confocal microscopy and 3D volumetric rendering. Neuronal dendrites and spines were identified in adolescent Naïve/Sham, adolescent Mod-CCI-TBI (Fig. 5A, 5B), adult Naïve/Sham, and adult Mod-CCI-TBI. Apical dendritic branches projecting away from the cell body were evaluated for the number of branching points per neuron. Using two-way ANOVA with Tukey's post hoc test, we report an Interaction effect of injury severity and age and significant reduction in dendritic branching points in adolescent Mod-CC-TBI when compared with adolescent Naïve/Sham, Mild-CCI-TBI, or to adult mice in a step-wise increase of injury severity [two-way ANOVA, Interaction effect of injury severity and age; F (2, 12) = 6.509, adolescent Naïve/Sham/Mild-CCI-TBI: p < 0.01, adult Naïve/Sham: p < 0.01, adult Mild-CCI-TBI: p < 0.001, adult Mod-CCI-TBI: p < 0.0001; Fig. 5C]. Similarly, we report significant reduction in total dendritic length [μm; F (2, 12) = 20.51, adolescent Naïve/Sham: p < 0.05, adolescent Mild-CCI-TBI: p < 0.001, adult Naïve/Sham, Mild-, and Mod-CCI-TBI: p < 0.01; Fig. 5D] and spine density per 100 μm in NAc following only adolescent Mod-CCI-TBI [F (2, 12) = 6.656 adolescent Naïve/Sham/Mild-CCI-TBI: p < 0.01, adult Naïve/Sham, Mild- and Mod-CCI-TBI: p < 0.001; Fig. 5E]. Interestingly, analysis of spine length revealed the opposite pattern in that adolescent Mod-CCI-TBI NAc neurons demonstrated significantly increased average spine length compared with adolescent Naïve/Sham, Mild-CCI-TBI or adult mice of any injury severity [two-way ANOVA interaction effect of injury severity and age: F (2, 12) = 46.96: p < 0.001; Fig. 5F]. Collectively, these findings indicate that TBI during adolescence may result in aberrant synaptic and neuronal pruning that could lead to maladaptive remodeling of neuronal structures within reward substrates.
FIG. 5.
Adolescent moderate controlled cortical impact model of traumatic brain injury (Mod-CCI-TBI) alters neuronal dendrite and spine morphology in the nucleus accumbens. (A) Representative images of Golgi-Cox staining of 300-μm segment of cerebral tissue. Magnified area demonstrates spine morphology in the NAc of mice 2 weeks post-adolescent CCI-TBI. (B) Compiled Z stack dataset in high resolution examines morphological features of Golgi-Cox stained neurons from mice in three dimensionally rendered reconstructions using the Imaris software (scale bar = 30 μm). Magnified area shows a representative neuronal soma (black) and apical dendrites (red), with distinct elongated spine morphology (blue; scale bar = 10 μm). Quantification of differences between varied adult and adolescent TBI severities at 2 weeks post-injury reveal adolescent Mod-CCI-TBI resulted in decreased dendritic branching points (C), dendritic length (D), and spine density (E). Average spine length was increased following adolescent Mod-CCI-TBI (F). Two-way analysis of variance interaction effect of injury severity and age: F (2, 12) = 46.96, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n = 3-4 per condition. Color image is available online.
Moderate CCI-TBI alters expression of dopamine system–related genes
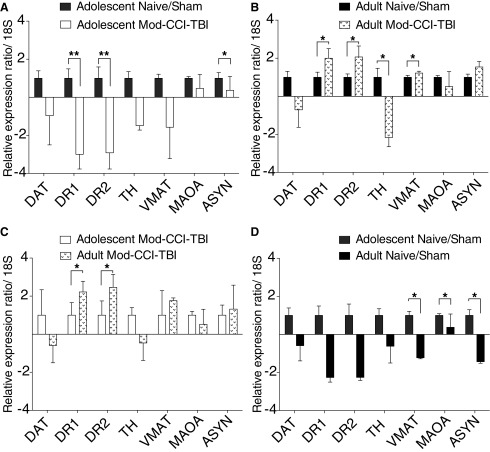
Given our findings that adolescent Mod-CCI-TBI results in changes to the reward circuitry at the structural level of neuronal morphology in the NAc, we next investigated how these structural modifications could affect functional dopamine (DA) neurotransmission by evaluating changes in DA-associated gene expression. Alterations in DA transmission have been observed following TBI.38-40 To determine whether the effect on cocaine sensitivity may be related to TBI-induced changes in expression of DA related genes, qRT-PCR was performed in the NAc 2 weeks following adolescent and adult CCI-TBI. Figure 6 demonstrates that while we found no effects of TBI on expression of the DAT, both DA receptor type 1 (DR1) and DR2 were significantly downregulated in the NAc following adolescent Mod-CCI-TBI (Fig. 6A) yet significantly upregulated in adult TBI compared with adult naïve/sham (Fig. 6B) or with adolescent Mod-CCI-TBI (Fig. 6C). In addition, changes in genes related to vesicular transport, vesicular monoamine transporter (VMAT) and synthesis of DA, tyrosine hydroxylase (TH), were seen only following adult TBI in the NAc (Fig. 6B).
FIG. 6.
Controlled cortical impact model of traumatic brain injury (CCI-TBI) alters the expression of dopamine-associated genes in the reward circuitry in age-specific patterns. The nucleus accumbens (NAc) was isolated 2 weeks following either sham or moderate (Mod)-CCI-TBI in adolescent and adult animals. Quantitative real-time polymerase chain reaction was used to profile changes in the following dopamine-associated genes: DAT, DR1, DR2, VMAT, TH, MAOA, and aSYN. Data were analyzed by ΔΔCt, normalized to 18S, and are expressed as mean ± standard error of the mean fold change. (A) Adolescent Naïve/sham vs. Adolescent Mod-CCI-TBI comparisons show significant downregulation of DR1, DR2, and aSYN following injury. (B) Adult Naïve vs. Adult Mod-CCI-TBI comparisons show significant downregulation of TH and upregulation of DR1, DR2, and VMAT following injury. (C) Adolescent Mod-CCI-TBI vs. Adult Mod-CCI-TBI comparisons show significant age-specific differences in DR1 and DR2 expression following injury. (D) Adolescent Naïve/Sham vs. Adult Naïve/Sham comparisons show age-dependent, basal differences in expression of VMAT, MAOA, and aSYN. Statistical significance was determined by Student's t-tests, corrected for multiple comparisons using the Holm-Sidak method. *p < 0.05, **p < 0.01. n = 4 per condition.
To evaluate whether the structural changes and phagocytosis of synaptic proteins coincide with changes in their gene expression, qRT-PCR analyses were also performed on alpha synuclein (aSYN), a pre-synaptic neuronal protein involved in DA release and transport. Figure 6 also reveals downregulation of aSYN in the NAc following adolescent CCI-TBI (Fig. 6A). Adolescent Naïve/Sham versus adult Naïve/Sham comparisons show age-dependent, basal differences in expression of VMAT, MAOA, and aSYN (Fig. 6D). While DA is not the only important neurochemical system important for the processing of rewarding stimuli in the NAc, additional analyses revealed no significant changes in genes associated with of alternate neurochemical systems (GABA, glutamate, serotonin) 2 weeks following adolescent Mod-CCI-TBI (Supplementary Fig. S1). Consequently, these analyses point to both age- and injury-mediated effects on the DA system within nuclei implicated in processing the rewarding effects of cocaine, as evident by changes in key dopaminergic gene expression.
Discussion
The results presented herein suggest that moderate TBI during adolescence results in augmented cocaine place conditioning cocaine compared with naïve, non-injured control mice.24,25 Importantly, a comprehensive analysis of cocaine doses along the ascending and descending portions of the inverted-U shaped CPP shift revealed that mice subjected to adolescent TBI also demonstrate significant preference for a subthreshold dose of cocaine that does not induce a significant CPP shift in naïve mice.33,34 These results suggest that TBI during adolescence increases sensitivity to the rewarding effects of cocaine and possibly, the abuse liability of psychostimulants in those who have a history of early-life TBI. Additional behavioral analyses suggest that TBI may be more selective for rewarding properties of cocaine, a process regulated by the transmission of dopamine in the NAc, with less impacts on other effects including cocaine-induced locomotion or PFC-dependent cognition. Notably, the increased sensitivity to a subthreshold dose of cocaine coincides with a delayed resolution of injury-induced immune cell infiltration and an increase of microglial phagocytosis of neuronal proteins within the NAc. Further examination of NAc medium spiny neurons revealed decreased dendritic arborization complexity, dendrite length, spine density, and an increase in average spine length following adolescent Mod-CCI-TBI. Real-time PCR was used to elucidate subsequent changes in the dopaminergic and synaptic plasticity signatures within the NAc 2 weeks following TBI. Collectively, these results support our hypothesis that TBI-induced neuroinflammation and subsequent aberrant microglial-neuronal interactions, particularly during adolescence when brain regions that mediate the processing of drug reinforcement are not fully mature, play a critical role in increasing susceptibility to SUD compared with non-injured mice.
Adolescent TBI and increased sensitivity to the reinforcing effects of a subthreshold dose of cocaine
The outcome observed by CPP extends our previous findings and supports clinical data suggesting that cocaine reward is enhanced when brain injury occurs during adolescence and when brain injury is severe.24,41 Moreover, these results are the first to examine the effect of adolescent TBI on adult sensitivity to a subthreshold dose of cocaine. Pre-clinical studies have previously established age-dependent sensitivity to the rewarding effects of subthreshold doses of cocaine, but not in the context of TBI. For example, Zakharova and colleagues found that adolescent male rats developed a significant CPP place preference shift at lower cocaine doses than adult rats.42 Badanich and colleagues also found that adolescent rats were more likely to establish CPP to subthreshold cocaine doses and that ontogenetic differences in dopamine release and reuptake, as measured by microdialysis, may account for age-specific sensitivities to the reinforcing properties of cocaine, and ultimately increased vulnerability to addiction during adolescence.43 Similar to our findings, in a neurochemical brain injury model induced by PFC administration of the dopaminergic neurotoxin 6-hydroxydopamine, Schenk and colleagues reported that lesioned rats reliably responded for the subthreshold intravenous doses of self-administered cocaine, suggestive of increased sensitivity to the rewarding effects of cocaine.34
However, two recent studies investigating the effects of adult TBI on cocaine self-administration reported conflicting results. Muelbl and colleagues found no difference in cocaine self-administration following adult exposure to mild blast TBI,44 consistent with our findings, while Vonder Haar and colleagues reported increased adult cocaine self-administration lever presses and infusions following mild or severe bilateral TBI to the medial PFC.26 Yet, this study administered bilateral TBI of greater severity than commonly seen among adolescent TBI patients, which could explain the increase in adult cocaine intake in their model. Despite study differences, the data in Vonder Haar and colleagues, Muelbl and colleagues, and our present and prior study24 support the notion that TBI increases cocaine preference and that different types and severities of injury at different ages could result in varying degrees of vulnerability to the development of CUD. The use of a biased CPP procedure with cocaine conditioning occurring in the least-preferred compartment introduces a limitation of this study as it cannot be entirely excluded that mice with a history of TBI may have experienced higher levels of stress or anxiety in the initially least-preferred side, thus adding to a potential anxiolytic effect of cocaine that may have amplified cocaine CPP in TBI mice. Similarly, although a minimum of 4 h wash-out period was implemented between cocaine and saline conditioning sessions, this experimental design limits the ability to differentiate between place preference from the rewarding effects of cocaine versus place avoidance resulting from potential acute, negative effects after cocaine metabolization that could be associated with the saline-paired, preferred compartment.
TBI does not affect cocaine-induced locomotion or other behaviors
We performed additional behavior assays to test for behavioral selectivity of adolescent TBI and for the potential confound of TBI-induced locomotor impairment. We report no effects of TBI in cocaine-induced locomotion, neurological motor deficits 2 weeks post-injury, or spontaneous alternation performance, a PFC-dependent cognitive task. We are the first to report no effect of TBI on cocaine-induced locomotor activity. However, Xu and colleagues demonstrated that the locomotor activity, a measure of total distance traveled, was reduced 2 weeks post-injury in CCI-TBI administered to CD-1 mice at 2-3 months of age.45 Additional reports examining behavioral deficits following TBI produced conflicting results. Doran and colleagues, reported motor and spontaneous alternation deficits following CCI-TBI in similarly aged mice; however, behaviors were assessed sooner after injury than in our study.46 Tomasevic and colleagues reported Composite Neuroscore deficits that persisted for up to 1 week post-injury but resolved by 3 weeks following CCI-TBI, similar to our results.47 Collectively, these findings indicate that behavioral deficits are dependent on injury severity and post-injury time of assessment. Thus, subject age at injury, mode and severity of injury, and recovery period, are important factors to consider when interpreting findings related to effects of TBI on behavior.
TBI-induced immune cell infiltration
Adolescent TBI severity affected infiltration of monocytes in step-wise manner and persisted for 14 and 28 days after moderate TBI compared with the immune cell infiltration profile in adult Mod-CCI-TBI mice. Interestingly, the total percentage of infiltrating monocytes did not differ following adolescent or adult Mod-CCI-TBI. However, the majority of persistent monocytes seen in adolescent TBI were classified as Ly6Cint, which are associated with a more destructive, prolonged inflammatory response compared with classical (proinflammatory) Ly6Chigh or non-classical (patrolling, pro-resolving) Ly6Clow infiltrating immune cells.48–50 Specifically, following adolescent Mod-CCI-TBI, our observed increase in Ly6Cint also positive for CD64 expression agrees with Li and colleagues, who reported finding Ly6Cint monocytes also positive for phagocytic markers such as CD64. Such expression was induced by and correlated with levels of interferon-1 in a systemic lupus model.49 In a stroke model, Jones and colleagues reported that prolonged immune cell infiltration may be associated with worse pathology, inflammation, and poorer outcome.48 Notably, our detection of higher levels of unresolved immune infiltration at 14 days post-adolescent Mod-CCI-TBI, the time-point at which mice were first exposed to the cocaine CPP assay, supports the possibility that inflammation within the reward circuitry increases sensitivity to cocaine and perhaps to other drugs of abuse. Collectively, these findings and our results suggest that inflammatory system responses are more complicated than supported by a binary (proinflammatory vs. pro-resolution) classification and suggest that additional studies are needed to better characterize how subset-specific functions alter brain function, brain development, and behavior.
Microglial activation by TBI and dendrite and spine morphological changes
Beyond classifying microglia status as either ramified when quiescent or ameboid when activated, several studies also have reported pruning-dependent phagocytosis of neuronal proteins by microglia, demonstrating a functional role of the brain's innate immune cells in the process of neuronal pruning.51–54 Although our findings of increased microglial phagocytosis of neuronal proteins in combination with decreased dendritic spine density in the NAc following adolescent TBI do not provide mechanistic causality, they suggest that there may be an age-specific susceptibility to activated microglia mediating ongoing synaptic pruning. We also report increased average spine length in the NAc of adolescent Mod-CCI-TBI mice, a known phenotype of filopodia or long, thin spines.55 Long thin spines also are associated with excitatory synaptic activity.56 Therefore, the changes in dendritic spine morphology as a potential consequence of TBI-induced microglial activation may account for increased sensitivity to the reinforcing properties of cocaine after adolescent brain injury.
Throughout adolescent cognitive development, the maturation of neural circuits remains incomplete due to two ongoing processes: pruning and myelination.57 Active myelination during adolescence is responsible for strengthening white matter tracts in a posterior-anterior direction wherein neural projections to the PFC are the last to mature, later in adulthood.58 Particularly in the PFC, cortical innervation of dopaminergic projections underlying sensitivity to rewarding stimuli, such as the reinforcing properties of cocaine, remains inchoate throughout adolescence.12-14 This myelination and strengthening of white matter tracts is associated with neural signaling that regulates executive cognitive functions that are underdeveloped during adolescence. Therefore, impairments in decision making, complex reasoning, and impulse control also may contribute to behavioral deficits observed in SUD populations.14,58–61 Further, neuronal synaptic pruning, the process underlying gray matter volume and cortical maturation, peaks during adolescence.62 Thus, the adolescent brain, already undergoing peaked pruning and underdeveloped myelination, may be a primed environment, wherein TBI-induced inflammation exacerbates microglial activation resulting in maladaptive pruning and compromised neuronal circuitry development. This altered neural circuitry could result in increased sensitivity to the rewarding effects of subthreshold doses of cocaine and perhaps other drugs of abuse, as indicated by our findings.
Chronically activated microglia and/or macrophages are considered a hallmark of unresolved inflammation.63 CX3CR-1 is expressed by neurons while the receptor for CX3CR-1 is expressed by microglia. Further, Paolicelli and colleagues showed that mice with microglia lacking the fractalkine receptor have higher densities of neuronal spines and functional synapses during early postnatal development.52 Their group also demonstrated that CX3CR-1KO mice exhibited a transient reduction of microglia and a consequential deficit in synaptic pruning.64 While microglial phagocytosis of dead or dying neurons is considered a beneficial and natural brain remodeling processes, there now is evidence that microglia also phagocytose viable neurons during certain conditions such as inflammation.65 For example, Wynne and colleagues demonstrated downregulation of CX3CR-1 expression in microglia following LPS-injection (4 and 24 h) with a simultaneous increase in IL-1β production (4 h). In addition, prolonged reduction of CX3CR-1 correlated with a delayed recovery from sickness behavior, prolonged IL production, and decreased TG expression.64 Our results demonstrate reduced CX3CR-1 in microglia following TBI, which in contrast to Paolicelli and colleagues, was correlated with a significant increase in the phagocytosis of neuronal proteins, an effect that may be suggestive of aberrant synaptic pruning as a result of adolescent TBI. Further, an established consequence of reactive microglia is an exaggerated neuroinflammatory cytokine response.
Dopamine neurotransmission is altered by TBI
The results of our qPCR data are consistent with previous evidence that DA neurotransmission is altered by addiction and by TBI.67–71 Clinically, the persistence of DA deficits in the striatum and NAc of TBI patients has been observed several months after injury.38 Pre-clinical studies expand upon these findings and demonstrate a biphasic DA response following experimental TBI. Huger and Patrick showed an early increase in DA levels in several brain regions, including cortex and striatum, within hours after TBI.72 In contrast, Chen and colleagues recently evaluated the effect of adolescent TBI on nicotine-reward behavior impairment and confirmed previous findings of suppressed release of DA in the NAc 2 weeks following TBI40 without nicotine on board, and reported an exacerbation of nicotine suppression of DA release by TBI.73 Similarly, the recent work by Vonder Haar and colleagues that reported significantly higher cocaine self-administration after TBI also identified changes in dopaminergic signaling 2 weeks post-TBI.26 Specifically, they found that mild and severe TBI resulted in lower DR1 levels in the striatum and that mild TBI increased DR1 levels in the NAc. Principal component analyses also revealed lower DR2 expression in the accumbens in combination with lower DAT in the striatum in severe TBI animals compared with sham controls.26 Subsequently, Wagner and colleagues reported significant deficits in DA release and reuptake via the DA transporter (DAT) 2 weeks following TBI. Their study also showed significant downregulation of DAT expression in the striatum of TBI animals.39 Further, Yan and colleagues report significant increases of tyrosine hydroxylase (TH), the rate limiting enzyme in DA synthesis, in the cortex, striatum, and substantia nigra up to 4 weeks following TBI.74,75 While our findings did not detect NAc increases in TH or TBI-induced differences in DAT levels in the NAc, we do report significant down regulation of DR1 and DR2 of our adolescent Mod-CCI-TBI animals. Although the cellular and neurochemical mechanisms underlying enhanced cocaine rewarding efficacy in adolescent TBI mice is unclear, dopamine D2 receptor downregulation may be a contributing factor. Indeed, a recent meta-analysis study investigating dopamine dysfunction found that human cocaine users displayed lower levels of dopamine D2/3 receptors compared with cocaine-naïve controls.76 Previous pre-clinical studies indicate that rhesus monkeys with lower dopamine D2 receptor availability are especially responsive to cocaine's reinforcing effects.77,78 Given that adolescent TBI mice in our study showed both an enhanced preference for cocaine and a decrease in D2 receptor gene expression in the mesolimbic circuit, it is possible that brain injury suffered during adolescence lowers the availability of D2 receptors, thereby enhancing vulnerability to CUD. Future experiments assessing protein levels of dopamine markers and extracellular levels of dopamine in mesolimbic substrates are planned to further probe dopaminergic dysfunction as a link between TBI and cocaine reward and dependence.
Conclusions
It is important to acknowledge that aspects of the experiments herein such as our CCI model of TBI, the inclusion of a biased CPP design, and behavioral components assessed are limitations and must be considered when interpreting our findings as it translates to the human condition. In our study, we found significant increases in sensitivity to the rewarding effects of cocaine in mice that experienced a moderate TBI, whereas the majority of TBI patients experience mild injuries.1,2 Further, while there is no correlate to the clinically used Glasgow Coma Scale for categorizing TBI in mice, the Composite Neuroscore has been widely used as a proxy for neurological performance following exposure to the CCI model.46,79,80 Our model of drug-seeking behavior has several caveats. For example, while the biased design accounts for inherent preference expressed by the mice, it introduces the potential for mice to develop the association of the saline-paired compartment with a less-rewarding context. Although cocaine's stimulant effects following systemic IP administration in rodents normally peak at 20-30 min, and then gradually decline 90-120 min post-administration, the inclusion of only a 4-h washout period between conditioning sessions yields the possibility that the effects of withdrawing from the physiological effects of cocaine could coincide with conditioning to the saline-paired compartment as well.81 Therefore, future experiments could incorporate longer washout intervals to control for the above.
This study is the first to describe increased sensitivity to the reinforcing properties of cocaine following adolescent TBI. Prolonged immune cell infiltration and microglial phagocytosis of neuronal proteins were identified as potential underlying mechanisms that distinguish behavioral disparities between mice that respond atypically to conditioned cocaine exposure. We postulate that TBI-induced inflammation and microglial activation contribute to maladaptive synaptic pruning and dopaminergic transmission, which may increase vulnerability to CUD development. Further studies are needed to better understand the effect of adolescent experimental TBI-induced augmented cocaine place preference behavior, by mechanistically exploring whether TBI impairs maturation within the reward circuitry; pre-clinical studies of adolescent TBI offer a useful model to examine developmental changes in sensitivity to the reinforcing properties of cocaine and perhaps other drugs of abuse.
Supplementary Material
Funding Statement
This work was supported by National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) P30 DA013429-16 (SHR, SMR), R01DA046833 01 (SHR), T32 DA007237 (LAC), NIH/National Institute of Neurological Disorders and Stroke (NINDS), R01 NS086570-01 (SHR), and the PA-CURE program (Pennsylvania Department of Health).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Faul M., Xu L, Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. National Center for Injury Prevention and Control; Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 2. Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 4. Vaughn M.G., Salas-Wright C.P., John R., Holzer K.J., Qian Z., and Veeh C. (2019). Traumatic brain injury and psychiatric Co-Morbidity in the United States. Psychiatr. Q. 90, 151–158 [DOI] [PubMed] [Google Scholar]
- 5. Whelan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 6. Corrigan J.D., Bogner J., and Holloman C. (2012). Lifetime history of traumatic brain injury among persons with substance use disorders. Brain Inj. 26, 139–150 [DOI] [PubMed] [Google Scholar]
- 7. Corrigan J.D. and Deutschle J.J. Jr (2008). The presence and impact of traumatic brain injury among clients in treatment for co-occurring mental illness and substance abuse. Brain Inj. 22, 223–231 [DOI] [PubMed] [Google Scholar]
- 8. Ponsford J., Draper K., and Schonberger M. (2008). Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J. Int. Neuropsychol. Soc. 14, 233–242 [DOI] [PubMed] [Google Scholar]
- 9. Coronado V.G., Haileyesus T., Cheng T.A., Bell J.M., Haarbauer-Krupa J., Lionbarger M.R., Flores-Herrera J., McGuire L.C., and Gilchrist J. (2015). Trends in sports- and recreation-related traumatic brain injuries treated in US emergency departments: the National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001-2012. J. Head Trauma Rehabil. 30, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Substance Abuse and Mental Health Services Administration. (2014). Results from the 2013 National Survey on Drug Use and Health: Summary of national findings. www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf (Last accessed July30, 2019) [PubMed]
- 11. United Nations Office on Crime and Drugs. (2018). World Drug Report 2018. www.unodc.org/wdr2018 (Last accessed July30, 2019)
- 12. Manitt C., Mimee A., Eng C., Pokinko M., Stroh T., Cooper H.M., Kolb B., and Flores C. (2011). The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J. Neurosci. 31, 8381–8394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naneix F., Marchand A.R., Di Scala G., Pape J.R., and Coutureau E. (2012). Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci. 32, 16223–16232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker D.M., Bell M.R., Flores C., Gulley J.M., Willing J., and Paul M.J. (2017). Adolescence and reward: making sense of neural and behavioral changes amid the chaos. J. Neurosci. 37, 10855–10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalivas P.W. and Volkow N.D. (2005). The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413 [DOI] [PubMed] [Google Scholar]
- 16. Merkel S.F., Cannella L. A., Razmpour R., Lutton E., Raghupathi R., Rawls S.M., and Ramirez S.H. (2017). Factors affecting increased risk for substance use disorders following traumatic brain injury: What we can learn from animal models. Neurosci. Biobehav. Rev. 77, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volkow N.D., Fowler J.S., Wang G.J., Telang F., Logan J., Jayne M., Ma Y., Pradhan K., Wong C., and Swanson J.M. (2010). Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage 49, 2536–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corrigan J.D., Bogner J., Mellick D., Bushnik T., Dams-O'Connor K., Hammond F.M., Hart T., and Kolakowsky-Hayner S. (2013). Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database. Arch. Phys. Med. Rehabil. 94, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ilie G., Mann R.E., Boak A., Adlaf E.M., Hamilton H., Asbridge M., Rehm J., and Cusimano M.D. (2016). Cross-sectional examination of the association of co-occurring alcohol misuse and traumatic brain injury on mental health and conduct problems in adolescents in Ontario, Canada. BMJ Open 6, e011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weil Z.M., Karelina K., Gaier K.R., Corrigan T.E., and Corrigan J.D. (2016). Juvenile traumatic brain injury increases alcohol consumption and reward in female mice. J. Neurotrauma 33, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim Y.W., Meyer N.P., Shah A.S., Budde M.D., Stemper B.D., and Olsen C.M. (2015). Voluntary alcohol intake following blast exposure in a rat model of mild traumatic brain injury. PloS One 10, e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowing J.L., Susick L.L., Caruso J.P., Provenzano A.M., Raghupathi R. and Conti A.C. (2014). Experimental traumatic brain injury alters ethanol consumption and sensitivity. J. Neurotrauma 31, 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayeux J.P., Teng S.X., Katz P.S., Gilpin N.W., and Molina P.E. (2015). Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav. Brain Res. 279, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merkel S.F., Razmpour R., Lutton E.M., Tallarida C.S., Heldt N.A., Cannella L.A., Persidsky Y., Rawls S.M., and Ramirez S.H. (2017). Adolescent traumatic brain injury induces chronic mesolimbic neuroinflammation with concurrent enhancement in the rewarding effects of cocaine in mice during adulthood. J. Neurotrauma 34, 165–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merkel S.F., Andrews A.M., Lutton E.M., Razmpour R., Cannella L.A., and Ramirez S.H. (2017). Dexamethasone attenuates the enhanced rewarding effects of cocaine following experimental traumatic brain injury. Cell Transplant. 26, 1178–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vonder Haar C., Ferland J.N., Kaur S., Riparip L.K., Rosi S., and Winstanley C.A. (2018). Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. Eur. J. Neurosci. 2018 Aug 17; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 28. Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mooney J. and Rawls S.M. (2017). KCNQ2/3 channel agonist flupirtine reduces cocaine place preference in rats. Behav. Pharmacol. 28, 405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J., Connelly K.L., Unterwald E.M., and Rawls S.M. (2017). Chemokines and cocaine: CXCR4 receptor antagonist AMD3100 attenuates cocaine place preference and locomotor stimulation in rats. Brain Behav. Immun. 62, 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tallarida C.S., Tallarida R.J., and Rawls S.M. (2015). Levamisole enhances the rewarding and locomotor-activating effects of cocaine in rats. Drug Alcohol Depend. 149, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hicks C., Gregg R.A., Nayak S.U., Cannella L.A., Schena G.J., Tallarida C.S., Reitz A.B., Smith G.R., and Rawls S.M. (2017). Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: a role for N-acetylaspartylglutamate (NAAG). Psychopharmacology (Berl) 234, 1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zakharova E., Leoni G., Kichko I., and Izenwasser S. (2009). Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav. Brain Res. 198, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schenk S., Horger B.A., Peltier R., and Shelton K. (1991). Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 543, 227–235 [DOI] [PubMed] [Google Scholar]
- 35. Loane D.J. and Byrnes K.R. (2010). Role of microglia in neurotrauma. Neurotherapeutics 7, 366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russo M.V. and McGavern D.B. (2016). Inflammatory neuroprotection following traumatic brain injury. Science 353, 783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beschorner R., Nguyen T.D., Gozalan F., Pedal I., Mattern R., Schluesener H.J., Meyermann R., and Schwab J.M. (2002). CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 103, 541–549 [DOI] [PubMed] [Google Scholar]
- 38. Donnemiller E., Brenneis C., Wissel J., Scherfler C., Poewe W., Riccabona G., and Wenning G.K. (2000). Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur. J. Nucl. Med. 27, 1410–1414 [DOI] [PubMed] [Google Scholar]
- 39. Wagner A.K., Sokoloski J.E., Ren D., Chen X., Khan A.S., Zafonte R.D., Michael A.C., and Dixon C.E. (2005). Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 95, 457–465 [DOI] [PubMed] [Google Scholar]
- 40. Chen Y.H., Huang E.Y., Kuo T.T., Hoffer B.J., Miller J., Chou Y.C. and Chiang Y.H. (2017). Dopamine release in the nucleus accumbens is altered following traumatic brain injury. Neuroscience 348, 180–190 [DOI] [PubMed] [Google Scholar]
- 41. Ramesh D., Keyser-Marcus L.A., Ma L., Schmitz J.M., Lane S.D., Marwitz J.H., Kreutzer J.S., and Moeller F.G. (2015). Prevalence of traumatic brain injury in cocaine-dependent research volunteers. Am. J. Addict. 24, 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zakharova E., Wade D., and Izenwasser S. (2009). Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 92, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Badanich K.A., Adler K.J., and Kirstein C.L. (2006). Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur. J. Pharmacol. 550, 95–106 [DOI] [PubMed] [Google Scholar]
- 44. Muelbl M.J., Slaker M.L., Shah A.S., Nawarawong N.N., Gerndt C.H., Budde M.D., Stemper B.D., and Olsen C.M. (2018). Effects of mild blast traumatic brain injury on cognitive- and addiction-related behaviors. Sci. Rep. 8, 9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu X., Cao S., Chao H., Liu Y., and Ji J. (2016). Sex-related differences in striatial dopaminergic systems after traumatic brain injury. Brain Res. Bull. 124, 214–221 [DOI] [PubMed] [Google Scholar]
- 46. Doran S.J., Ritzel R.M., Glaser E.P., Henry R.J., Faden A.I., and Loane D.J. (2019). Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. J. Neurotrauma 36, 1040–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomasevic G., Laurer H.L., Mattiasson G., van Steeg H., Wieloch T., and McIntosh T.K. (2012). Delayed neuromotor recovery and increased memory acquisition dysfunction following experimental brain trauma in mice lacking the DNA repair gene XPA. J. Neurosurg. 116, 1368–1378 [DOI] [PubMed] [Google Scholar]
- 48. Jones K.A., Maltby S., Plank M.W., Kluge M., Nilsson M., Foster P.S., and Walker F.R. (2018). Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav. Immun. 67, 299–307 [DOI] [PubMed] [Google Scholar]
- 49. Li Y., Lee P.Y., Kellner E.S., Paulus M., Switanek J., Xu Y., Zhuang H., Sobel E.S., Segal M.S., Satoh M., and Reeves W.H. (2010). Monocyte surface expression of Fcgamma receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res. Ther. 12, R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fischer M.A., Davies M.L., Reider I.E., Heipertz E.L., Epler M.R., Sei J.J., Ingersoll M.A., Rooijen N.V., Randolph G.J., and Norbury C.C. (2011). CD11b(+), Ly6G(+) cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection. PLoS Pathog. 7, e1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown G.C. and Neher J.J. (2014). Microglial phagocytosis of live neurons. Nat. Rev. Neurosci.15, 209–216 [DOI] [PubMed] [Google Scholar]
- 52. Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., and Gross C.T. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 [DOI] [PubMed] [Google Scholar]
- 53. Dissing-Olesen L., LeDue J.M., Rungta R.L., Hefendehl J.K., Choi H.B., and MacVicar B.A. (2014). Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J. Neurosci. 34, 10511–10527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wake H., Moorhouse A.J., Miyamoto A., and Nabekura J. (2013). Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 36, 209–217 [DOI] [PubMed] [Google Scholar]
- 55. Spiga S., Mulas G., Piras F., and Diana M. (2014). The “addicted” spine. Front. Neuroanat. 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matsuzaki M., Honkura N., Ellis-Davies G.C., and Kasai H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tau G.Z. and Peterson B.S. (2010). Normal development of brain circuits. Neuropsychopharmacology 35, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hartley C.A. and Somerville L.H. (2015). The neuroscience of adolescent decision-making. Curr. Opin. Behav. Sci. 5, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ryan N.P., Genc S., Beauchamp M.H., Yeates K.O., Hearps S., Catroppa C., Anderson V.A., and Silk T.J. (2018). White matter microstructure predicts longitudinal social cognitive outcomes after paediatric traumatic brain injury: a diffusion tensor imaging study. Psychol. Med. 48, 679–691 [DOI] [PubMed] [Google Scholar]
- 60. Yu F., Shukla D.K., Armstrong R.C., Marion C.M., Radomski K.L., Selwyn R.G., and Dardzinski B.J. (2017). Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J. Neurotrauma 34, 1364–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 62. Penzes P., Cahill M.E., Jones K.A., VanLeeuwen J.E., and Woolfrey K.M. (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McKee C.A. and Lukens J.R. (2016). Emerging roles for the immune system in traumatic brain injury. Front. Immunol. 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., and Gross C.T. (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 [DOI] [PubMed] [Google Scholar]
- 65. Neher J.J., Neniskyte U., and Brown G.C. (2012). Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front. Pharmacol. 3, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wynne A.M., Henry C.J., Huang Y., Cleland A., and Godbout J.P. (2010). Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 24, 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nestler E.J. (2005). Is there a common molecular pathway for addiction? Nat. Neurosci. 8, 1445–1449 [DOI] [PubMed] [Google Scholar]
- 68. Koob G.F. (2013). Negative reinforcement in drug addiction: the darkness within. Curr. Opin. Neurobiol. 23, 559–563 [DOI] [PubMed] [Google Scholar]
- 69. Wee S. and Koob G.F. (2010). The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl.) 210, 121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Di Chiara G. and Imperato A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 85, 5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McFarland K., Davidge S.B., Lapish C.C., and Kalivas P.W. (2004). Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 24, 1551–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huger F. and Patrick G. (1979). Effect of concussive head injury on central catecholamine levels and synthesis rates in rat brain regions. J. Neurochem. 33, 89–95 [DOI] [PubMed] [Google Scholar]
- 73. Chen Y.H., Kuo T.T., Yi-Kung Huang E., Chou Y.C., Chiang Y.H., Hoffer B.J., and Miller J. (2018). Effect of traumatic brain injury on nicotine-induced modulation of dopamine release in the striatum and nucleus accumbens shell. Oncotarget 9, 10016–10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan H.Q., Kline A.E., Ma X., Hooghe-Peters E.L., Marion D.W., and Dixon C.E. (2001). Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport 12, 2323–2327 [DOI] [PubMed] [Google Scholar]
- 75. Yan H.Q., Ma X., Chen X., Li Y., Shao L., and Dixon C.E. (2007). Delayed increase of tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 1134, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ashok A.H., Mizuno Y., Volkow N.D., and Howes O.D. (2017). Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nader M.A., Morgan D., Gage H.D., Nader S.H., Calhoun T.L., Buchheimer N., Ehrenkaufer R., and Mach R.H. (2006). PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 9, 1050–1056 [DOI] [PubMed] [Google Scholar]
- 78. Czoty P.W., Gage H.D., Nader S.H., Reboussin B.A., Bounds M., and Nader M.A. (2007). PET imaging of dopamine D2 receptor and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J. Addict. Med. 1, 33–39 [DOI] [PubMed] [Google Scholar]
- 79. Marklund N., Morales D., Clausen F., Hanell A., Kiwanuka O., Pitkanen A., Gimbel D.A., Philipson O., Lannfelt L., Hillered L., Strittmatter S.M., and McIntosh T.K. (2009). Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice. Neuroscience 163, 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marklund N., Bareyre F.M., Royo N.C., Thompson H.J., Mir A.K., Grady M.S., Schwab M.E., and McIntosh T.K. (2007). Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J. Neurosurg. 107, 844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nicolaysen L.C., Pan H.T., and Justice J.B. Jr (1988). Extracellular cocaine and dopamine concentrations are linearly related in rat striatum. Brain res. 456, 317–323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.