Abstract
Background:
It is known that once heart failure occurs in older patients with diabetes, the overall prognosis is extremely poor. We investigated whether early initiation of SGLT2 inhibitor therapy after admission was beneficial for diabetic patients requiring inpatient treatment for acute heart failure.
Methods:
We retrospectively assessed consecutive patients with comorbid diabetes who were admitted to the Department of Cardiology in Tosei General Hospital for treatment of acute heart failure. Patients were divided into two groups: those who initiated SGLT2 inhibitor therapy (SGLT2 inhibitor group; mean age: 73 ± 9 years) and those who did not receive the inhibitors during hospitalization (conventional treatment group; mean age: 75 ± 10 years).
Results:
No intergroup differences were observed in the distribution of either the severity or classes of heart failure on admission. Glycosylated hemoglobin levels were significantly higher in the SGLT2 inhibitor group (HbA1c: 8.1% ± 0.8%) than in the conventional treatment group (HbA1c: 7.1% ± 0.8%) (p = 0.003). After admission, patients in both groups recovered equally well, and in almost the same period of time, before discharge. The rate of diuretics use at the time of discharge in the SGLT2 inhibitor group (n = 8, 67%) was significantly lower than that in the conventional treatment group (n = 19, 100%) (p = 0.016). In particular, the dose of loop diuretics in the conventional treatment group was 34 ± 4 mg/day while that in the SGLT2 inhibitor group was significantly lower at 13 ± 5 mg/day (p = 0.008). During hospitalization, the incidence of acute kidney injury was significantly higher in the conventional treatment group (n = 11, 58%) than in the SGLT2 inhibitor group (n = 2, 16%) (p = 0.031).
Conclusions:
For the treatment and management of heart failure in patients with diabetes, early initiation of SGLT2 inhibitor therapy appears to be effective.
Keywords: acute kidney injury, diabetes mellitus, heart failure, SGLT2 inhibitor
Introduction
In developed countries, the number of patients with type 2 diabetes mellitus is steadily increasing. The progression of this pathological condition, accompanied by concomitant cardiovascular disease, markedly impairs patient prognoses. This is particularly true among individuals aged 65 years or older.1,2 Approximately 20–40% of aged individuals who have type 2 diabetes experience concomitant heart failure, and the presence of type 2 diabetes itself is a risk factor for the subsequent development of heart failure.2,3 Additionally, it is known that once heart failure occurs in older patients with diabetes, the overall prognosis is extremely poor.2 Thus, for older patients with diabetes, it is important to design therapeutic strategies with early reduction in the risk of heart failure in mind.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are recently developed antidiabetic agents that act on the proximal renal tubules to promote urinary glucose excretion and to lower blood glucose levels by inhibiting SGLT2s, which are responsible for the reabsorption of glucose from the urine.4,5 Because of this underlying mechanism, these inhibitors may affect blood glucose levels, body weight, blood pressure, and serum lipid levels as well as increase diuresis, and improve pancreatic and renal function. According to recent reports from the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) and the Canagliflozin Cardiovascular Assessment Study (CANVAS), SGLT2 inhibitors were found to be effective for medium- and long-term inhibition of major adverse cardiovascular events and the progression of renal dysfunction.6–9 In particular, the incidence of heart failure necessitating hospitalization during the follow-up period was reduced substantially by the administration of SGLT2 inhibitors. Thus, SGLT2 inhibitors are expected to act as antidiabetic agents that are also effective in the prevention of cardiovascular events including heart failure. However, because of the mechanism of action of SGLT2 inhibitors, patients being administered these drugs should be carefully monitored for urinary tract infection, dehydration, stroke, ketoacidosis, and other adverse health conditions.10,11 Caution is particularly required when these drugs are administered to older people, who are less likely to notice dehydration symptoms.12
We recently assessed the safety and efficacy of SGLT2 inhibitors in older Japanese patients (mean age, 73 years) with diabetes who also had underlying cardiovascular disease.13 Because no signs of dehydration were observed during 6 months of SGLT2 inhibitor administration, the results of that study confirmed that the inhibitors exerted a favorable hypoglycemic effect. In the present study, we investigated whether early initiation of SGLT2 inhibitor therapy after admission was beneficial for diabetic patients requiring inpatient treatment for acute heart failure.
Materials and methods
Study population
This is a retrospective study conducted at a single center, not a randomized trial. We retrospectively assessed consecutive patients with comorbid diabetes who were admitted to the Department of Cardiology in Tosei General Hospital for treatment of acute heart failure. The enrollment period for these patients was between January and December of 2017. All patients were diagnosed on the basis of the Framingham criteria.14 Patients were also classified according to the New York Heart Association (NYHA) Functional Class and Clinical scenario rubrics.15 The study included patients aged 18 years or more at the time of presentation to the emergency center with at least one symptom (respiratory discomfort or orthopnea) and one clinical sign (pedal edema, engorged jugular vein, or pulmonary congestion on radiographs) and required hospitalization. A medical history was obtained to document the etiology and severity of their heart failure, as well as additional symptoms, medications, and comorbid diseases. Routine laboratory results [e.g. for red blood cell count, hemoglobin (Hb), hematocrit (Hct), glycosylated hemoglobin (HbA1c), blood urea nitrogen (BUN), creatinine (Cre), glomerular filtration rate (GFR), and brain natriuretic peptide (BNP)] and concomitant cardiac medications were recorded. For assessment of loop diuretic doses, 30 mg of azosemide or 4 mg of torasemide was considered to be equivalent to 20 mg of furosemide. The following patients were excluded: those aged 90 years or older, those with clinical scenarios 4 and 5, those with end-stage renal failure who were also on dialysis, those with comorbid cancer, those who died from an infection or other cause during hospitalization, and those who received oral SGLT2 inhibitors before admission. Written informed consent was obtained from each patient, and the study was approved by the ethics committee of Tosei General Hospital (No 698: 2018/07/30).
Study groups and the length of follow up
On admission, the additional prescription of SGLT2 inhibitors was considered for patients with comorbid, poorly controlled diabetes. Specifically, administration of SGLT2 inhibitors was initiated in patients with an HbA1c level of 6.5 or higher who consented to the additional prescription of SGLT2 inhibitors within 24 h of admission. Namely, patients were divided into two groups: those who initiated SGLT2 inhibitor therapy (SGLT2 inhibitor group) and those who did not receive the inhibitors during hospitalization (conventional treatment group). The assignment to treatment groups was based on routine clinical care at the hospital. The hospital stay length was defined as the length of follow up, because this study aimed to assess the impact of the selection of drugs for acute-phase treatment on the incidence of acute kidney injury (AKI) and diuretic doses.
Biomarker analyses
Blood samples were obtained at each outpatient visit. Complete blood counts were performed using a Sysmex XE-5000 hematology analyzer (Sysmex, Kobe, Japan). Biochemical data were measured using a LABOSPECT 008 automatic analyzer (Hitachi Co., Tokyo, Japan). Estimated GFR (eGFR) levels were calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration).16
Echocardiography
Echocardiographic examination was performed by an experienced sonographer using Vivid E9 with XD clear (GE Healthcare, Tokyo, Japan). The images were recorded in Console and analyzed offline. The left ventricular ejection fraction (LVEF) was calculated using a modified version of Simpson’s rule.13 HFrEF (heart failure with reduced ejection fraction), HFpEF (heart failure with preserved ejection fraction), or HFmrEF (HF with mid-range ejection fraction) was defined according to the AHA and ESC guidelines.17,18
Definition of acute kidney injury
AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria as an increase in a serum Cre levels by 0.3 mg/dl or higher within 48 h, or to a level at least 0.5 times higher than reference levels within 7 days, or a recorded urinary output of 0.5 ml/kg/h or less for 6 or more hours.19
Statistical analyses
All analyses were performed using PASW Statistics 18 software (SPSS Inc., Chicago, IL, USA). The Fisher exact test was used to compare the two groups. Continuous variables were compared using a t test and presented as means ± standard deviation (SD). AKI free survival rate was determined by a Kaplan–Meier analysis with the log-rank test. In all analyses, p < 0.05 was considered statistically significant.
Results
Baseline characteristics
During the study period, 31 patients with acute heart failure complicated by type 2 diabetes were admitted. Administration of SGLT2 inhibitors was initiated in patients with an HbA1c level of 6.5 or higher on admission who also consented to the additional prescription of the inhibitors within 24 h of admission. There were 12 patients who initiated SGLT2 inhibitor therapy (SGLT2 inhibitor group) and 19 patients who did not receive an inhibitor during hospitalization (conventional treatment group) (Table 1). Oral administration of SGLT2 inhibitors was initiated on average 17 ± 15 h (median, 13 h) after admission. Nine patients (75%) received empagliflozin and three patients (25%) received canagliflozin. No patient had to suspend or discontinue use of SGLT2 inhibitors due to side effects during follow-up period.
Table 1.
Patient characteristics at baseline.
| Control (n = 19) | SGLT2-I (n = 12) | p value | |
|---|---|---|---|
| Age (years) | 75 ± 10 | 73 ± 9 | 0.623 |
| Male sex [n (%)] | 14 (73) | 9 (75) | 0.688 |
| Body weight (kg) | 64 ± 13 | 62 ± 12 | 0.320 |
| Body mass index (kg/m2) | 25 ± 5 | 24 ± 4 | 0.414 |
| NYHA classification | |||
| NYHA II | 2 (10) | 2 (17) | 0.507 |
| NYHA III | 7 (37) | 1 (8) | 0.086 |
| NYHA IV | 10 (53) | 9 (75) | 0.194 |
| Clinical scenario (CS) | |||
| CS1 | 12 (63) | 6 (50) | 0.470 |
| CS2 | 7 (37) | 5 (42) | 0.541 |
| CS3 | 0 (0) | 1 (8) | 0.387 |
| Previous history [n (%)] | |||
| Hypertension | 18 (95) | 12 (100) | 0.613 |
| Dyslipidemia | 17 (89) | 11 (92) | 0.648 |
| Chromic kidney disease | 17 (89) | 10 (83) | 0.507 |
| Acute myocardial infarction | 5 (26) | 4 (33) | 0.489 |
| Angina pectoris | 7 (37) | 6 (50) | 0.470 |
| Atrial fibrillation | 8 (42) | 4 (33) | 0.459 |
| Stroke | 3 (16) | 0 (0) | 0.216 |
| ADHF | 7 (37) | 5 (42) | 0.541 |
| Result of blood test | |||
| Hb (g/dl) | 12 ± 2 | 12 ± 2 | 0.672 |
| Hct (mg/dl) | 38 ± 6 | 38 ± 5 | 0.789 |
| Cre (mg/dl) | 1.3 ± 0.5 | 1.3 ± 0.3 | 0.961 |
| eGFR (ml/min/1.73 m2) | 48 ± 22 | 45 ± 17 | 0.666 |
| Na (mEq/|) | 140 ± 4 | 137 ± 4 | 0.113 |
| K (mEq/|) | 4.2 ± 0.6 | 4.2 ± 0.5 | 0.910 |
| Cl (mEq/|) | 106 ± 3 | 105 ± 5 | 0.534 |
| BNP (pg/ml) | 681 ± 624 | 881 ± 1270 | 0.586 |
| HbA1c (%) | 7.1 ± 0.8 | 8.1 ± 0.8 | 0.003 |
| Echocardiography | |||
| Ejection fraction (%) | 42 ± 18 | 53 ± 14 | 0.089 |
| Inferior vena cava (mm) | 17 ± 5 | 17 ± 4 | 0.826 |
| HFpEF | 8 (50) | 8 (50) | 0.183 |
| HFmrEF | 2 (10) | 1 (8) | 0.672 |
| HFrEF | 9 (47) | 3 (25) | 0.194 |
ADHF, acute decompensated heart failure; BNP, brain natriuretic peptide; Control, conventional treatment group; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; SGLT2-I, SGLT2 inhibitor group.
The mean age on admission was 75 ± 10 years in the conventional treatment group and 73 ± 9 years in the SGLT2 inhibitor group, with no significant difference between the two groups (p = 0.623). No significant differences were observed in the male-to-female ratio (p = 0.688), body weight (p = 0.320), or body mass index (p = 0.414) between the two groups. At the time of the initial visit, the severity of heart failure among individuals in the conventional treatment group were classified as: NYHA class II in two patients (10%), III in 7 (37%), and IV in 10 (53%). In the SGLT2 inhibitor group, severity was classified as NYHA class II in two patients (17%), III in 1 (8%), and IV in 9 (75%). According to the classification of clinical scenarios (CSs) in heart failure, the conventional treatment group included 12 patients with CS1 (63%) and 7 with CS2 (37%) while the SGLT2 inhibitor group included 6 with CS1 (50%), 5 with CS2 (42%), and 1 with CS3 (8%). Thus, on admission, no significant differences in distribution of the severity or CS classes of heart failure were observed between the two groups. In addition, no significant differences between the two groups were observed in terms of the prevalence of hypertension (p = 0.613), dyslipidemia (p = 0.648), chronic kidney disease (p = 0.507), myocardial infarction (p = 0.489), a history of revascularization for angina pectoris (p = 0.470), atrial fibrillation (p = 0.459), stroke (p = 0.216), or a history of hospitalization for acute decompensated heart failure (ADHF) (p = 0.541). Furthermore, no significant differences were observed between the two groups in levels of Hb, Hct, renal function indices (e.g. Cre, eGFR, and electrolytes), or BNP. Echocardiography further revealed no significant differences in LVEF (p = 0.089) or in the average diameter of the inferior vena cava (p = 0.826) between the two groups. The proportions of HFpEF, HFmrEF, and HFrEF patients did not differ between the two groups (p = 0.183, p = 0.672, p = 0.194, respectively). HbA1c levels, however, did significantly differ between the groups. In the conventional treatment group, this level was 7.1% ± 0.8%, whereas in the SGLT2 inhibitor group it was significantly higher at 8.1% ± 0.8% (p = 0.003).
Clinical course during hospitalization
The average hospital stay length was 20 ± 12 days in the conventional treatment group and 18 ± 11 days in the SGLT2 inhibitor group. These values were not significantly different (p = 0.512) (Table 2). At discharge, the severity of heart failure in the conventional treatment group was a NYHA class I in 17 patients (89%) and II in 2 (11%). In the SGLT2 inhibitor group, 10 patients were classified as NYHA class I (83%) and 2 as class II (17%). No significant differences were observed between the two groups in Hb or Hct levels, or renal function indices (e.g. Cre, eGFR and electrolytes) at the time of discharge. Moreover, no significant differences between the two groups were observed in the duration of oxygen therapy during hospitalization or in the number of patients who required intravenous injection of catecholamine or human atrial natriuretic peptide. Thus, patients in both groups recovered equally well from heart failure across the same period of time and were discharged to begin outpatient treatment in comparable numbers.
Table 2.
Patient characteristics at discharge.
| Control (n = 19) | SGLT2-I (n = 12) | p value | |
|---|---|---|---|
| Length of hospital stay (days) | 20 ± 12 | 18 ± 11 | 0.512 |
| NYHA classification | |||
| NYHA I | 17 (89) | 10 (83) | 0.672 |
| NYHA II | 2 (11) | 2 (17) | 0.672 |
| Hb (g/dl) | 12 ± 2 | 13 ± 3 | 0.280 |
| Hct (mg/dl) | 37 ± 5 | 39 ± 8 | 0.261 |
| Cre (mg/dl) | 1.3 ± 0.5 | 1.4 ± 0.4 | 0.921 |
| eGFR (ml/min/1.73 m2) | 45 ± 20 | 41 ± 16 | 0.634 |
| Na (mEq/|) | 139 ± 4 | 138 ± 4 | 0.192 |
| K (mEq/|) | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.082 |
| Cl (mEq/|) | 103 ± 4 | 105 ± 4 | 0.366 |
Control, conventional treatment group; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; SGLT2-I, SGLT2 inhibitor group.
Details of oral drugs used at the time of discharge
Next, the details of prescription drugs used at the time of discharge were compared and analyzed between the conventional treatment and SGLT2 inhibitor groups (Table 3). No significant difference in the use of RAS inhibitors (p = 0.226), beta-blockers (p = 0.281), or calcium channel blockers (p = 0.459) was found between the two groups. There were also no differences in the use of either statins or antidiabetic agents, other than SGLT2 inhibitors, between the two groups. In contrast, rates of diuretic and aldosterone blocker use were significantly lower in the SGLT2 inhibitor group than in the conventional treatment group (p = 0.016 and p = 0.032, respectively). There were no differences in the use of tolvaptan between the two groups. Prescribed doses of loop diuretics at the time of discharge were also noted. Diuretic dose in the conventional treatment group (34 ± 4 mg/d) was significantly greater than in the SGLT2 inhibitor group (13 ± 5 mg/d) (p = 0.008).
Table 3.
Concomitant medication at discharge.
| Control (n = 19) | SGLT2-I (n = 12) | p value | |
|---|---|---|---|
| RAS inhibitor [n (%)] | 14 (74) | 11 (92) | 0.226 |
| Beta-blocker [n (%)] | 17 (89) | 9 (75) | 0.281 |
| Calcium channel blockers [n (%)] | 8 (42) | 4 (33) | 0.459 |
| Statin [n (%)] | 13 (68) | 6 (50) | 0.258 |
| DPP-4 inhibitor [n (%)] | 15 (79) | 8 (67) | 0.362 |
| α-Glucosidase inhibitor [n (%)] | 9 (47) | 2 (17) | |
| Metformin [n (%)] | 4 (21) | 0 (0) | 0.123 |
| Sulfonylurea [n (%)] | 3 (16) | 0 (0) | 0.216 |
| Glinide [n (%)] | 4 (21) | 3 (25) | 0.086 |
| Pioglitazone [n (%)] | 0 (0) | 0 (0) | – |
| Insulin [n (%)] | 2 (11) | 1 (8) | 0.653 |
| Diuretics [n (%) ] | 19 (100) | 8 (67) | 0.016 |
| Aldosterone blockers [n (%)] | 11 (57) | 2 (17) | 0.032 |
| Tolvaptan [n (%)] | 9 (47) | 3 (25) | 0.194 |
| Dose of loop diuretics (Furosemide-equivalent dose, mg) | 33 ± 4 | 13 ± 5 | 0.008 |
Control, conventional treatment group; DDP: dipeptidyl peptidase; RAS: renin–angiotensin system; SGLT2-I, SGLT2 inhibitor group.
Events during hospitalization
Finally, clinical events during hospitalization were compared and analyzed between the conventional treatment and SGLT2 inhibitor groups. No cardiovascular events, including acute myocardial infarction or stroke, occurred in either group. Moreover, no incidence of hypoglycemic episodes or ketoacidosis, which are associated with antidiabetic treatment, occurred in either group. According to the KDIGO criteria, AKI was diagnosed in 11 patients in the conventional treatment group (58%) and two patients in the SGLT2 inhibitor group (16%). The incidence of AKI was significantly lower in the SGLT2 inhibitor group (p = 0.031). Furthermore, Kaplan-Meier analysis demonstrated that patients in the SGLT2 inhibitor group had significantly higher AKI free survival rate compared with those in the conventional treatment group (log-rank p < 0.047) (Figure 1).
Figure 1.
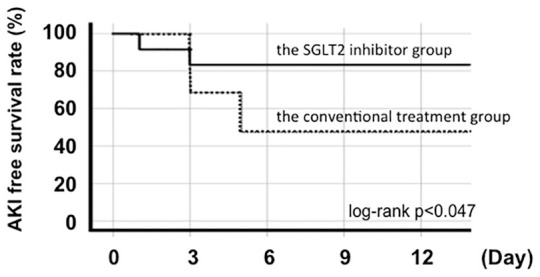
Kaplan–Meier estimate of the time to AKI free survival rate.
AKI, acute kidney injury.
Discussion
In the present study, we assessed whether early initiation of SGLT2 inhibitor therapy was effective for the treatment and management of heart failure in diabetic patients requiring inpatient treatment. The conventional treatment group included 19 patients with a mean age of 75 years, and the SGLT2 inhibitor group included 12 patients with a mean age of 73 years. Diabetic patients were compared and analyzed. Early initiation of SGLT2 inhibitor therapy after the onset of acute heart failure allowed for less diuretic use and reduced loop diuretic doses. Furthermore, the use of SGLT2 inhibitors contributed significantly to the prevention of AKI during treatment. According to recent reports from the EMPA-REG OUTCOME and CANVAS trials, administration of SGLT2 inhibitors prevents hospitalization for heart failure and the progression of renal dysfunction.6–9 Thus, the results of the present study are consistent with those of these other, larger-scale trials.
In the treatment of heart failure, a bolus injection of loop diuretics has been reported to be a potential factor for poor prognosis of heart failure.20–22 However, to control intractable heart failure, the use of loop diuretics and increases in their doses are necessary under present circumstances.23 In the present study, heart failure was well controlled with extremely low doses of loop diuretics in the SGLT2 inhibitor group. Upon recovery from heart failure and subsequent discharge, the mean doses of loop diuretics were 34 mg/day in the conventional treatment group and 13 mg/day in the SGLT2 inhibitor group. Because early, first-line use of SGLT2 inhibitor therapy for heart failure preserves the option of later treatment via diuretic dose increases, this therapy may be beneficial for the medium- and long-term clinical management of heart failure.
AKI has been known as a predictor of cardiovascular disease prognosis.24–26 It has also been reported that, despite recovery from transient pathological conditions, renal dysfunction gradually progresses and sometimes results in end-stage renal failure.27 In addition, AKI-complicating acute heart failure aggravates heart failure and further impairs circulation.24–26 In such cases, the prognosis is extremely poor. Because the incidence of AKI is very high among patients admitted for heart failure (approximately 40%), the prevention of AKI’s development is a critical issue in the treatment and management of acute heart failure.28
In terms of AKI prevention, the efficacy of loop diuretics has also been questioned.29 Because there are reports that the use of loop diuretics increases the incidence of AKI, many nations’ guidelines do not recommend the use of loop diuretics except for when they are used to correct fluid overload.19,30,31
In the EMPA-REG OUTCOME and CANVAS trials, SGLT2 inhibitors were found to be more effective at preventing the progression of diabetic nephropathy than were conventional treatments.7,9 Moreover, the results of a meta-analysis suggest that the administration of SGLT2 inhibitors may prevent the long-term development of AKI.32 Similarly, in the present study, the incidence of AKI during hospitalization for heart failure was significantly lower in the SGLT2 inhibitor group. Thus, early initiation of SGLT2 inhibitor therapy in the management of heart failure may be an extremely effective strategy for the prevention of AKI.
Tissue perfusion injury secondary to heart failure reduces blood flow in the renal arteries. It is further understood that, when the kidneys detect circulatory disturbances, reabsorption of sodium and water by the renal tubules is enhanced.33 In this circumstance, sodium-potassium-chloride-adenosine-triphosphatase and renal tubule sodium-chloride-adenosine-triphosphatase pump hyperactivity eventually aggravates the hypoxic environment surrounding the renal tubules.34 In addition to the hyperactivity of these pumps, the activity of the sodium-potassium-adenosine-triphosphatase pump around the proximal renal tubules is also enhanced in diabetic patients. As a consequence of this, the hypoxic state is aggravated and renal tubular injury is aggravated. SGLT2 inhibitors may ameliorate such proximal renal tubular injury and thus exert renoprotective effects.35 The prevention of AKI by using SGLT2 inhibitors, as seen in the present study, may be attributable to these inhibitors’ direct inhibitory effects on renal tubular injury and titration of loop diuretic doses.
There was no difference in the levels of eGFR between the two groups, although the rate of AKI occurrence was significantly different. As one of the reasons, it is suggested that we have only observed a short-term effect of SGLT2 inhibitor in diabetic patients with acute heart failure. SGLT2 inhibitors often induce an initial decrease in eGFR in patients with type2 diabetes. The acute GFR lowering effect of SGLT2 inhibitor relates to the contribution of SGLT2 to the primary tubular hyper-reabsorption in the diabetic kidney that secondarily causes glomerular hyperfiltration.36,37 On the other hand, AKI is observed in patients with hypovolemia treated with high dose of furosemide or other diuretics.36,37
Our study has several limitations. This is a retrospective study conducted at a single center, not a randomized trial. The sample size was relatively small. In the future, prospective studies are warranted to validate these findings in a larger population. Meanwhile, an analysis using the G*power software was performed to calculate the sample size required to yield a significant correlation in the incidence of AKI between the conventional treatment group and the SGLT2 inhibitor group. Further, the sample size of this study was confirmed to be appropriate (total sample size: 16, actual power: 0.95). In addition, few studies have reported an association between the use of SGLT2 inhibitors and doses of loop diuretics during treatment of acute heart failure. In this study, early initiation of SGLT2 inhibitor therapy after the onset of acute heart failure contributed to a reduction in the doses of loop diuretics to approximately one third. Furthermore, there are few reports on the association between the use of SGLT2 inhibitors and incidence of AKI during treatment of acute heart failure. In this study, the incidence of AKI was significantly lower in the SGLT2 inhibitor group than that in the conventional treatment group. Despite the small sample size, we consider this study to be significant because it provides new findings regarding acute-phase treatment for heart failure.
Conclusion
The present study demonstrated that the use of SGLT2 inhibitors was safe and effective in diabetic patients who required inpatient treatment for acute heart failure. Early initiation of SGLT2 inhibitor therapy after the onset of acute heart failure reduced the doses of loop diuretics that these patients required and contributed to greater prevention of AKI. For the treatment and management of heart failure in diabetic patients, early initiation of SGLT2 inhibitor therapy appears to be effective.
Footnotes
Author contributions: T.K., R.S., T.M. and M.A. designed and carried out the studies. T.K., H.O., H.A. and M.A. analyzed the data. R.S. wrote the paper.
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declared no conflict of interest.
ORCID iD: Rei Shibata  https://orcid.org/0000-0003-4179-8453
https://orcid.org/0000-0003-4179-8453
Data availability: The data used to support the findings of this study are available from the corresponding author upon request.
Contributor Information
Takahiro Kambara, Department of Cardiovascular Medicine, Tosei General Hospital, Seto, Japan.
Rei Shibata, Department of Advanced Cardiovascular Therapeutics, Nagoya University Graduate School of Medicine, 65 Tsurumai, Showa, Nagoya, 466-8550, Japan.
Hiroyuki Osanai, Department of Cardiovascular Medicine, Tosei General Hospital, Seto, Japan.
Yoshihito Nakashima, Department of Cardiovascular Medicine, Tosei General Hospital, Seto, Japan.
Hiroshi Asano, Department of Cardiovascular Medicine, Tosei General Hospital, Seto, Japan.
Toyoaki Murohara, Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Masayoshi Ajioka, Department of Cardiovascular Medicine, Tosei General Hospital, Seto, Japan.
References
- 1. Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol 2000; 35: 1628–1637. [DOI] [PubMed] [Google Scholar]
- 2. Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 3. Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; the Rotterdam study. Eur Heart J 1999; 20: 447–455. [PubMed] [Google Scholar]
- 4. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010; 27: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14: 5–14. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 8. Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin cardiovascular assessment study). Circulation 2018; 137: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Hu X, Liu X, et al. An overview of the effect of sodium glucose cotransporter 2 inhibitor monotherapy on glycemic and other clinical laboratory parameters in type 2 diabetes patients. Ther Clin Risk Manag 2016; 12: 1113–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li D, Wang T, Shen S, et al. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 2017; 19: 348–355. [DOI] [PubMed] [Google Scholar]
- 12. Terauchi Y, Yokote K, Nakamura I, et al. Safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): interim results of a post-marketing surveillance study. Expert Opin Pharmacother 2016; 17: 463–471. [DOI] [PubMed] [Google Scholar]
- 13. Kambara T, Shibata R, Osanai H, et al. Use of sodium-glucose cotransporter 2 inhibitors in older patients with type 2 diabetes mellitus. Geriatr Gerontol Int 2018; 18: 108–114. [DOI] [PubMed] [Google Scholar]
- 14. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 15. Mebazaa A, Gheorghiade M, Pina IL, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36: S129–S139. [DOI] [PubMed] [Google Scholar]
- 16. Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307: 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 19. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184. [DOI] [PubMed] [Google Scholar]
- 20. Hasselblad V, Gattis Stough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007; 9: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, et al. Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure: a report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). Circ J 2012; 76: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 22. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006; 97: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 23. Palazzuoli A, Ruocco G, Ronco C, et al. Loop diuretics in acute heart failure: beyond the decongestive relief for the kidney. Crit Care 2015; 19: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 2004; 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 25. Metra M, Nodari S, Parrinello G, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 2008; 10: 188–195. [DOI] [PubMed] [Google Scholar]
- 26. Belziti CA, Bagnati R, Ledesma P, et al. Worsening renal function in patients admitted with acute decompensated heart failure: incidence, risk factors and prognostic implications. Rev Esp Cardiol 2010; 63: 294–302. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein SL, Jaber BL, Faubel S, et al. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 2013; 8: 476–483. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014; 9: e114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 2006; 333: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hertzberg D, Ryden L, Pickering JW, et al. Acute kidney injury-an overview of diagnostic methods and clinical management. Clin Kidney J 2017; 10: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lassnigg A, Donner E, Grubhofer G, et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol 2000; 11: 97–104. [DOI] [PubMed] [Google Scholar]
- 32. Zhang XL, Zhu QQ, Chen YH, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc 2018; 7 pii: e007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shamseddin MK, Parfrey PS. Mechanisms of the cardiorenal syndromes. Nat Rev Nephrol 2009; 5: 641–649. [DOI] [PubMed] [Google Scholar]
- 34. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341: 577–585. [DOI] [PubMed] [Google Scholar]
- 35. Kamezaki M, Kusaba T, Komaki K, et al. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep 2018; 8: 4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 2018; 132: 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsimihodimos V, Filippatos TD, Elisaf MS. SGLT2 inhibitors and the kidney: effects and mechanisms. Diabetes Metab Syndr 2018; 12: 1117–1123. [DOI] [PubMed] [Google Scholar]


