Abstract
A species’ distribution provides fundamental information on: climatic niche, biogeography, and conservation status. Species distribution models often use occurrence records from biodiversity databases, subject to spatial and taxonomic biases. Deficiencies in occurrence data can lead to incomplete species distribution estimates. We can incorporate other data sources to supplement occurrence datasets. The general public is creating (via GPS-enabled cameras to photograph wildlife) incidental occurrence records that may present an opportunity to improve species distribution models. We investigated (1) occurrence data of a cryptic group of animals: non-marine snakes, in a biodiversity database (Global Biodiversity Information Facility (GBIF)) and determined (2) whether incidental occurrence records extracted from geo-tagged social media images (Flickr) could improve distribution models for 18 tropical snake species. We provide R code to search for and extract data from images using Flickr’s API. We show the biodiversity database’s 302,386 records disproportionately originate from North America, Europe and Oceania (250,063, 82.7%), with substantial gaps in tropical areas that host the highest snake diversity. North America, Europe and Oceania averaged several hundred records per species; whereas Asia, Africa and South America averaged less than 35 per species. Occurrence density showed similar patterns; Asia, Africa and South America have roughly ten-fold fewer records per 100 km2than other regions. Social media provided 44,687 potential records. However, including them in distribution models only marginally impacted niche estimations; niche overlap indices were consistently over 0.9. Similarly, we show negligible differences in Maxent model performance between models trained using GBIF-only and Flickr-supplemented datasets. Model performance appeared dependent on species, rather than number of occurrences or training dataset. We suggest that for tropical snakes, accessible social media currently fails to deliver appreciable benefits for estimating species distributions; but due to the variation between species and the rapid growth in social media data, may still be worth considering in future contexts.
Keywords: Snake, Social media, Species distribution models, Tropical, Occurrence, GBIF, Flickr, Low detectability
Introduction
Species distribution models can yield insight into a species’ niche and habitat (Santos et al., 2006). Information on a species’ niche provides some ability to predict species’ responses to environmental change (Penman et al., 2010; Yousefi et al., 2015; Ahmadi et al., 2019). Predictions from species distribution models can also inform protected area allocation (Tulloch et al., 2016), support conservation status assessments (Solano & Feria, 2007; Fourcade et al., 2013), invasion potential (Pearson, 2015; Mutascio et al., 2018; although with complications Phillips, Chipperfield & Kearney, 2008) and identify potential human-wildlife conflicts (Yañez-Arenas et al., 2014).
The utility of a species distribution model is dependent on the underlying species occurrence data used in constructing the model. Gaps and incomplete data can lead to misidentifying the target species’ niche (Monsarrat et al., 2019). Underestimating species distributions can further mask the impacts of human activity on distributions, contributing to shifting baseline syndrome where progressively eroded species distributions or populations are accepted as normal or healthy (Cromsigt, Kerley & Kowalczyk, 2012). Ways to mitigate data gaps need thorough investigation.
Technological advances, global survey effort and digitisation of museum records have developed large biodiversity databases, pulling together disparate data sources to make global occurrence records more accessible and comprehensive. However, considerable gaps in biodiversity databases exist because of: detection difficulties, inconsistent surveying, and inadequate (or sometimes inaccurate) locality data for museum specimens (Yesson et al., 2007; Beck et al., 2013; Troudet et al., 2017).
Novel supplementary data sources could help fill biodiversity database gaps (Toivonen et al., 2019). With the proliferation of GPS enabled devices, the public is generating huge datasets on Web 2.0 platforms that can describe and/or predict a variety of phenomena including: protests (Alanyali, Preis & Moat, 2016), land-use (Antoniou et al., 2016), tourism (García-Palomares, Gutiérrez & Mínguez, 2015; Chua et al., 2016), hurricane damage (Preis et al., 2013) and protected area use (Orsi & Geneletti, 2013; Hausmann et al., 2018). Some Web 2.0 data, in the form of geo-tagged images, can communicate the identity and location of species (Barve, 2014). The geo-tagged images in a searchable social media platform may be a source for incidentally collected records of species (Allain, 2019; Barve, 2014; ElQadi et al., 2017; Jiménez-Valverde et al., 2019).
Incidental biodiversity records have already improved data availability for butterflies, snowy owls (Barve, 2014), spiders (Jiménez-Valverde et al., 2019), bees, flowers (ElQadi et al., 2017) and turtles (Allain, 2019). However, we do not know whether social media can provide similar benefits for (mostly) unpopular and low-detectability species.
Snakes present a model to explore the utility of social media data. Because snakes have been historically overlooked in research (Shine & Bonnet, 2000; De Miranda, 2017) and are difficult to detect (Steen, 2010; Durso & Seigel, 2015) they are likely suffering from a lack of primary biodiversity data. The need to generate more primary biodiversity data is underscored by: snakes’ important regulatory and keystone roles in ecosystem functioning (Willson & Winne, 2016; De Miranda, 2017); major gaps in reptile conservation assessments (Bland & Böhm, 2016; Tingley, Meiri & Chapple, 2016; Hughes, 2017); and frequent involvement in human-wildlife conflicts (Whitaker & Shine, 2000; Akani et al., 2002; Meek, 2012; Miranda, Ribeiro & Strüssmann, 2016; Marshall et al., 2018).
We describe the current state of snake occurrence records in the Global Biodiversity Information Facility (GBIF) database, highlighting gaps in surveying. We then explore the potential utility of a supplementary data source, a photography sharing platform Flickr, in the modelling of tropical snake distributions.
Materials & Methods
We completed all data analyses in R v.3.5.3 (R Core Team, 2019) and R Studio v.1.2.1335 (R Studio Team, 2019). For data visualisation we used ggplot2 (Wickham, 2016), ggrepel (Slowikowski, 2019) and scico (Pedersen & Crameri, 2018) packages. We have included a directory of scripts, packages (using packrat Ushey et al., 2018), and data used in analyses at: doi: 10.5281/zenodo.3243983.
Data retrieval
GBIF
Before acquiring data from the GBIF database (GBIF.org, 2019) we generated a comprehensive list of snake species. We used the taxize package (Chamberlain et al., 2018) to access GBIF and National Center for Biotechnology Information (NCBI; Benson et al., 2008; Sayers et al., 2009) records for all squamates families, filtering for those mentioning Serpentes in downstream classification. For each family within the suborder Serpentes we then queried the GBIF database and downloaded occurrence records, on a per genus basis, using the dismo (Hijmans et al., 2017) and rgbif packages (Chamberlain et al., 2019). Once we had downloaded all records, we queried the GBIF database a second time to ensure that downloaded files were complete and included all available occurrences. All GBIF downloads and metadata (including a list of data sources) are available at doi: 10.5281/zenodo.3243983.
After downloading, we compiled the resulting genus occurrence files, filtering out marine snake families (data manipulation performed with the dplyr (Wickham et al., 2017), data.table (Dowle & Srinivasan, 2019) and reshape2 (Wickham, 2007) packages). Due to the size of the dataset, we automated cleaning. We opted to use the CoordinateCleaner package to clean each species individually (Zizka et al., 2019). Following the process outlined in Zizka et al. (2019), we removed records with locations as NAs, zeros, identical values, near GBIF headquarters, near biodiversity institutions, within oceans, and that were extreme outliers for that species (using interquartile range outlier detection). Species with over 15,000 records (e.g., Thamnophis spp., Natrix spp. and Vipera berus) failed or produced erroneous results so we examined these species manually, removing outlying occurrences (those occurring on incorrect continents).
Flickr
To acquire data from Flickr we generated a list of species names, both common and binomial as search terms. We used the taxize package (Chamberlain et al., 2018) to query the GBIF and NCBI databases for all species downstream of the Serpentes families. We then compiled results from both databases into a single list, removing duplicates. Common name queries of GBIF and NCBI databases were inadequate or failed for many species. Therefore, we created a query system that accessed The Reptile Database (Uetz, Freed & Hošek, 2019) (using XML (Lang & CRAN Team, 2019a), xml2 (Wickham, Hester & Ooms, 2018) and rvest (Wickham, 2019)). For each species we retrieved all common names the Reptile Database listed. We parsed each set of common names to separate them to generate a list of search terms for each species, attempting to anticipate as many notation styles as possible used by Reptile Database. We relied on the stringr package (Wickham, 2018) to handle recurring character patterns.
We then accessed Flickr’s API (Flickr Development Team, 2019), via R packages XML (Lang & CRAN Team, 2019a), RCurl (Lang & CRAN Team, 2019b) and httr (Wickham, 2017), using each species’ search terms to retrieve search results for images. Photos had to be tagged as snake and geo-tagged so that a location was evident. During this process we saved the URL and year (extracted with the lubridate package Grolemund & Wickham, 2011) for each photo to later manually verify species identification. We then manually reviewed each image for the 18 selected tropical species (1166 images total), removing records of non-target species or images judged to have been taken within captive settings (captive settings were inferred by the presence of artificial substrate, white-balances associated with artificial lighting and geographic proximity to zoos, i.e., occurred within a raster cell and a suburban/urban area contiguous with a Google Maps labelled zoo).
Raster layers
We used the raster package (Hijmans, 2019) to retrieve climatic raster data from WorldClim (Fick & Hijmans, 2017). To guard against over-parameterisation and over-fitting during species distribution modelling (Fourcade, Besnard & Secondi, 2018), we discarded 14 of WorldClim’s bioclimatic layers. We discarded layers until between-layer correlations with an R value >0.6 were removed (Merow, Smith & Silander, 2013; Castellanos et al., 2019). We explored different combinations that reduced the correlation, and opted for a set of five variables covering a variety of climatic aspects likely important to snake range delimitation (Kearney, Shine & Porter, 2009; Fourcade, Besnard & Secondi, 2018). The remaining layers were: BIO1 (annual mean temperature), BIO2 (mean diurnal temperature range), BIO7 (temperature annual range), BIO12 (annual precipitation), and BIO15 (precipitation seasonality).
We limited the remaining WorldClim data to three regions of interest: Tropical Asia (longitude: 50°E, 150°E; latitude: −25°N, 50°N), Africa (longitude: −40°E, 40°E; latitude: −25°N, 75°N) and South America (longitude: −120°E, −25°E; latitude: −60°N, 25°N). We also downloaded global elevation data (Danielson & Gesch, 2011; U.S. Geological Survey, 2016) and human footprint index (Venter et al., 2016a; Venter et al., 2016b). Then we downscaled and reprojected elevation and footprint layers using projectRaster (Hijmans, 2019) with the default bilinear method to match the regional WorldClim layers’ projection, extent and resolution. We have included the resulting raster layers used in analysis in the supplementary data.
Point Process Analysis
We examined the distribution of GBIF occurrences via several point process analyses. We set the data within a landmass polygon (to account for water bodies during calculations) downloaded from natural earth using the rnaturalearth package (South, 2017). Then using the spatstat package (Baddeley & Turner, 2005) we tested for spatial conformity. We performed four types of spatial tests. We ran quadrat tests (with quadrats roughly equivalent to 10 degrees squared) to examine the spatial randomness of points. We calculated nearest neighbour distance functions (G) with Kaplan–Meier, border, and hazard corrections to examine the distribution of distances from points to their nearest neighbour. We estimated the empty space function (F) with Kaplan–Meier, border, and Chiu-Stoyan correction to examine how empty space was distributed between points. Finally, we estimated Ripley’s reduced second moment function (K), with no correction applied due to the prohibitively large dataset, for further examination of spatial non-randomness.
We estimated continental area and occurrence density using the rnaturalearth landmass. To estimate continental area, we projected the landmass for each continent using the closest Albers equal area conic projection (specifications obtained from https://epsg.io) with the rgeos (Bivand & Rundel, 2018) and sp packages (Pebesma et al., 2019). For standard error calculations we used the pracma package (Borchers, 2019).
Modelling
Species selection
We selected 18 species to investigate: nine selected manually and nine selected randomly. Our manual selection was based on relative taxonomic stability, their charismatic appearance and ease of photographic identification: Bitis arietans MERREM, 1820; Bothriechis schlegelii (BERTHOLD, 1846); Bungarus fasciatus (SCHNEIDER, 1801); Calloselasma rhodostoma (KUHL, 1824); Coelognathus radiatus (BOIE, 1827); Dendroaspis polylepis GÜNTHER, 1864; Eunectes murinus (LINNAEUS, 1758); Malayopython reticulatus (SCHNEIDER, 1801); and Ophiophagus hannah (CANTOR, 1836). Our manual selection represented all three tropical regions (Tropical Asia: 5, Africa: 2, South America: 2).
In addition to the nine manually selected species, we used the sample_n function in dplyr (Wickham et al., 2017) to randomly select nine more species that fitted the following criteria: occurs entirely within one of the three tropical regions, and be considered taxonomically stable. We defined the second criteria using the names listed on Reptile Database. Any species with a single binomial name listed since 2000, we considered stable. Once we had filtered the list of species by those criteria, we randomly selected nine species from 25 species with the most Flickr results. We had to repeat the random selection to avoid species with too few occurrences to model or an insufficiently sized distribution to be estimated with the resolution of raster layers. Porthidium spp . also had to be excluded because of the difficulties verifying species identity in images. The final nine randomly selected species were: Aplopeltura boa (BOIE, 1828); Atheris nitschei TORNIER, 1902; Boiga cynodon (BOIE, 1827); Boiga kraepelini STEJNEGER, 1902; Chironius carinatus (LINNAEUS, 1758); Echis coloratus GÜNTHER, 1878; Enhydris enhydris (SCHNEIDER, 1799); Hydrodynastes gigas (DUMÉRIL, BIBRON & DUMÉRIL, 1854); and Sinonatrix percarinata (BOULENGER, 1899) (Tropical Asia: 5, Africa: 2, South America: 2).
Model settings
We created four training datasets per species. First, we used SPthin (Aiello-Lammens et al., 2014) with a grid size equal to the raster cell to thin the data, ensuring only a single occurrence per cell. We split the GBIF occurrences into five randomly assigned groups in geographic space, limiting non-independence in environmental space (Roberts et al., 2017; Castellanos et al., 2019). We used the BlockCV package (Valavi et al., 2018) with the recommended block size based on the climatic and elevation raster layers (using 100,000 samples, group assignment was optimised across 500 iterations). Where the recommended block size failed to assign at least one occurrence to every group, we decreased the block size by 5% and re-ran the assignment until all groups were represented. Once groups had been successfully assigned, we set aside the median sized group of points from testing. We used the remaining points to train the geo-independent model. We generated a second GBIF data-only training set with a random subset of the original data removed. We removed this subset with no space weighting (to replicate random k-folds frequently used in the modelling literature), and the size was equal to the subset removed for the geo-independent model training dataset. We refer to the second model as the GBIF random model. The final models used the two GBIF training datasets described above supplemented by the Flickr data collected for that species.
We generated an array of 10,000 background points for each species, the array was consistent between model runs and training datasets. We bounded background point generation with a minimum convex polygon around all species occurrence records (Castellanos et al., 2019), plus a buffer equal to half the mean distance between occurrences. Whereas studies usually choose a fixed buffer to create the bounding area, the disparity in our 18 species distributions required us to use species-specific buffers based on relative occurrence record spread. Relying on a compromised fixed buffer for all species could underestimate AUC scores for species with large distributions, while inflating AUC scores for species with small distributions (Anderson & Raza, 2010). Because survey effort is undocumented and unequal (Tulloch et al., 2013), we weighted background point distribution using a bias layer to areas that are likely to have had increased survey effort (Phillips et al., 2009; Merow, Smith & Silander, 2013). We chose human footprint as proxy for survey likelihood, under the assumption that increased access and human presence leads to greater occurrence records.
We used the ENMeval package (Muscarella et al., 2014) to run Maxent models across varying model settings. We chose Maxent because of its flexibility and performance relative to other methods (Elith et al., 2006). We used combinations of linear and quadratic feature classes and ran models using a sequence of regularization values from 1 to 8 to reduce the chances of overfitting (Shcheglovitova & Anderson, 2013; Merow, Smith & Silander, 2013; Radosavljevic & Anderson, 2014) and set internal cross validation to user groups defined with BlockCV (Valavi et al., 2018).
Model evaluation
The metrics used to assess species distribution model performance are debated. Due to their reliance on pseudo-absences some of the ways of evaluating models are unhelpful. We chose to follow Castellanos et al.’s, (2019) advice and use multiple metrics. We selected receiver operating characteristic AUC (ROC AUC) because of its wide use and ability to compare models based on different datasets. Use of ROC AUC has drawbacks (Lobo, Jiménez-valverde & Real, 2008): it is sensitive to background area (Anderson & Raza, 2010), and is liable to overestimate model performance (Fernandes, Scherrer & Guisan, 2018). To supplement ROC AUC evaluation, we use precision-recall values (PRRC)—recently recommended as a metric insensitive to background area and species rarity because it ignores correctly predicted absences (Sofaer, Hoeting & Jarnevich, 2019). For every model created by the four training datasets, we calculated ROC AUC and PRRC values for all three test datasets with the PRROC package (Grau, Grosse & Keilwagen, 2015).
As an additional measure of the Flickr data’s contribution to models, we examined the niche overlap between models trained on only GBIF records and those trained on datasets supplemented with Flickr occurrences. We estimated niche overlap using Schoener’s D measure with the ENMeval package (Muscarella et al., 2014).
We explored Maxent model performance using GLM and GLMMs with the lme4 package (Bates et al., 2015). We created models using combinations of number of occurrences, species, and training dataset as predictors of PRRC and ROC AUC values. The full list of models tested can be found in Table S1. We used Spearman’s rank test to explore the relationships between area and occurrence count, after testing for normality with qqplots (from the car package Fox & Weisberg, 2011) and Shapiro–Wilk tests.
Results
Data summary
Our assessments of GBIF snake occurrences reveal strong spatial bias in the 302,386 unique locations of non-marine snakes. Flickr data searches produced only 44,689 images tagged with snakes and location information; Flickr data was also spatially nonuniform.
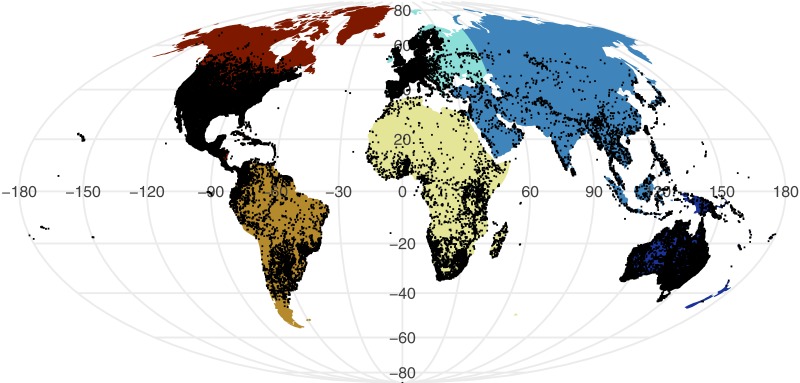
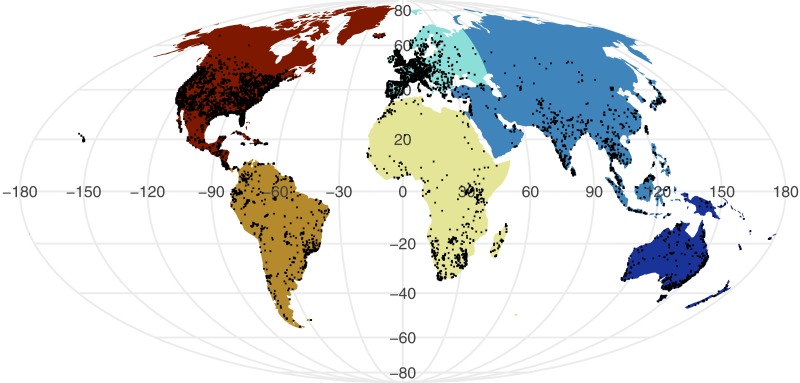
All point process analysis showed that the distribution of GBIF and Flickr points are not randomly distributed: multiple metrics suggest spatial clustering (GBIF data Quadrat test: X2 = 2425600, df = 288, p-value <2.2e−16; Flickr: Quadrat test: X2 = 426820, df = 288, p-value <2.2e−16; G-function: Fig. S1; F-function: Fig. S2; K-function: Fig. S3). The clustering is apparent in Figs. 1 and 2, illustrating points concentrated in North America, Europe and Australia –both GBIF and Flickr appear to follow similar distributions.
Figure 1. Global distribution of all GBIF non-marine snake records displayed against continental divisions.
Figure 2. Global distribution of geo-tagged Flickr photos that appeared across all searches.
Examining the GBIF results per continent reveals the scale of spatial bias (Table 1). The number of occurrence records are considerably lower in Africa, Asia, and South America, despite their large area and diversity of snake species. This pattern is particularly apparent in the density of occurrence records that are approximately ten-fold lower.
Table 1. Summary information of GBIF snake records.
| Continent | # Species | # Occurrences | Mean occurrences per species | SE | Area (million km2) | Occurrences per 100 km2 |
|---|---|---|---|---|---|---|
| Africa | 513 | 17,108 | 33.35 | 3.38 | 29.89 | 0.006 |
| Asia | 576 | 19,187 | 33.31 | 5.69 | 44.67 | 0.004 |
| Europe | 99 | 42,892 | 433.25 | 169.78 | 8.97 | 0.048 |
| North America | 680 | 157,923 | 232.24 | 33.62 | 24.64 | 0.064 |
| Oceania | 236 | 49,247 | 208.67 | 32.56 | 8.92 | 0.055 |
| South America | 633 | 16,029 | 25.32 | 2.21 | 17.91 | 0.009 |
Notes.
- # Species
- Number of species appearing in GBIF data, not the actual number of species known to exist across the continent
- # Occurrences
- Number of occurrence records in GBIF downloads
- Mean occurrences per species
- Total number of occurrences records in a continent divided by number of species in GBIF data
- SE
- Standard error associated with the mean occurrences per species
- Area
- Area in millions of km2 estimated using Albers equal area conic projection
- Occurrences per 100 km2
- Total number of occurrence records divided by the estimated continental area
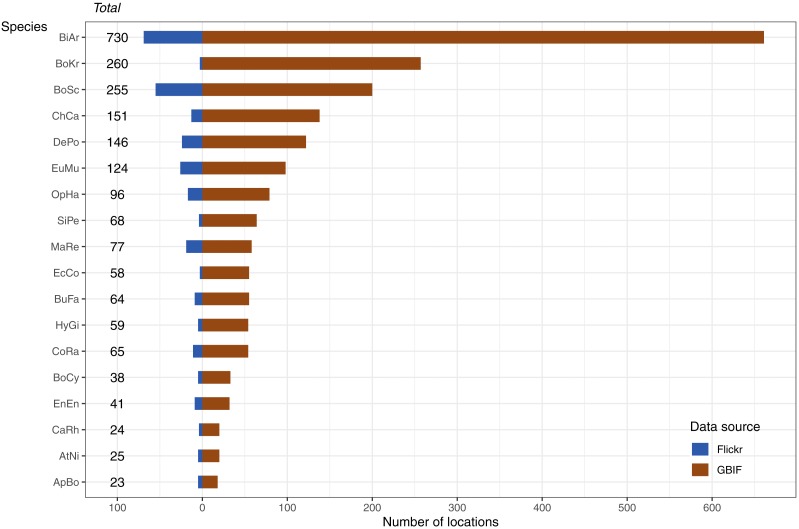
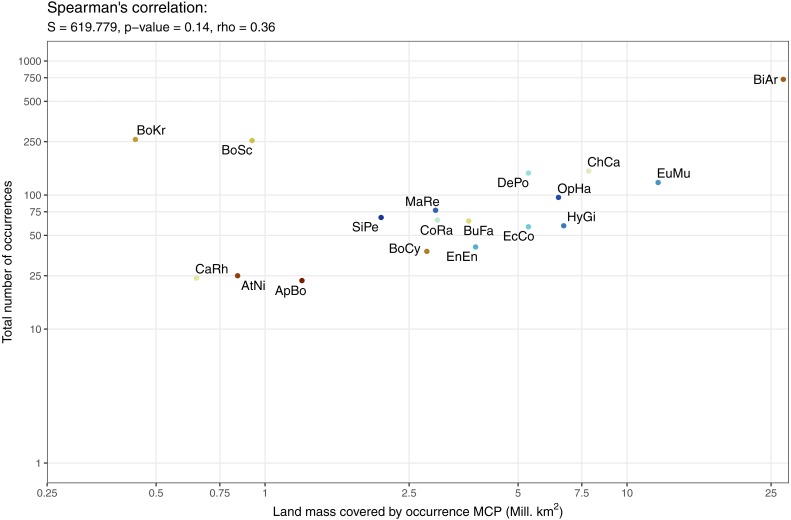
The data available for our 18 selected snake species varied dramatically (Fig. 3), and appeared to only be weakly positively associated with the size of the minimum convex polygon (MCP) of occurrence points (Fig. 4). Overall, we manually reviewed 1166 Flickr images, discarding 11.22 ± 5.68 non-snake or captive image locations per species (range = 0–92; percentage of images discarded per species 11.06 ± 4.86%, range = 0–77.97%).
Figure 3. Number and source of selected species occurrence records.
Species codes in order of appearance: BiAr = Bitis arietans, BoKr = Boiga kraepelini, BoSc = Bothriechis schlegelii, ChCa = Chironius carinatus, DePo = Dendroaspis polylepis, EuMu = Eunectes murinus, OpHa = Ophiophagus hannah, SiPe = Sinonatrix percarinata, MaRe = Malayopython reticulatus, EcCo = Echis coloratus, BuFa = Bungarus fasciatus, HyGi = Hydrodynastes gigas, CoRa = Coelognathus radiatus, BoCy = Boiga cynodon, EnEn = Enhydris enhydris, CaRh = Calloselasma rhodostoma, AtNi = Atheris nitschei, ApBo = Aplopeltura boa.
Figure 4. Relationship between number of occurrences and the minimum convex polygon (MCP) area cover by occurrence points.
Minimum convex polygon are clipped to exclude oceans. Both scales are presented as logs to make individual species visible. Species codes from left to right: BoKr = Boiga kraepelini, CaRh = Calloselasma rhodostoma, AtNi = Atheris nitschei, BoSc = Bothriechis schlegelii, ApBo = Aplopeltura boa, SiPe = Sinonatrix percarinata, BoCy = Boiga cynodon, MaRe = Malayopython reticulatus, CoRa = Coelognathus radiatus, BuFa = Bungarus fasciatus, EnEn = Enhydris enhydris, DePo = Dendroaspis polylepis, EcCo = Echis coloratus, OpHa = Ophiophagus hannah, HyGi = Hydrodynastes gigas, ChCa = Chironius carinatus, EuMu = Eunectes murinus, BiAr = Bitis arietans.
Modelling results
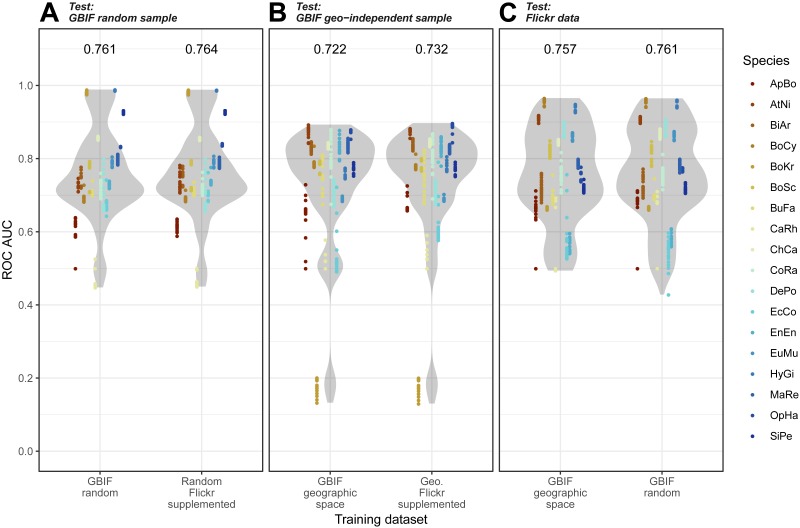
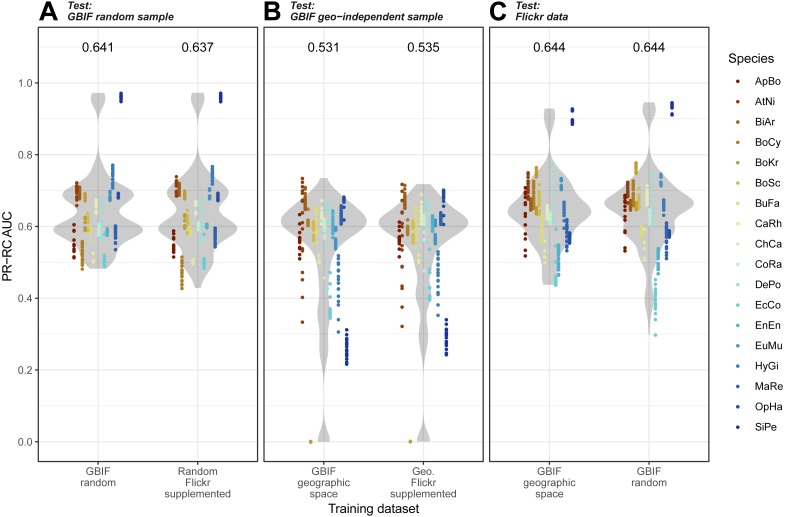
Overall, we found that models trained on GBIF supplemented with Flickr results were marginally better at predicting both randomly selected and geographically selected GBIF records when assessed using ROC AUC (Fig. 5). Precision-Recall values only saw the Flickr supplemented models perform better when predicting the geographically independent sample of GBIF records (Fig. 6).
Figure 5. Receiver Operating Characteristic results for the three models when tested against the three independent test datasets.
(A) GBIF random sample. (B) GBIF geo-independent sample. (C) Flickr data.Species codes in alphabetical order: ApBo = Aplopeltura boa, AtNi = Atheris nitschei, BiAr = Bitis arietans, BoCy = Boiga cynodon, BoKr = Boiga kraepelini, BoSc = Bothriechis schlegelii, BuFa = Bungarus fasciatus, CaRh = Calloselasma rhodostoma, ChCa = Chironius carinatus, CoRa = Coelognathus radiatus, DePo = Dendroaspis polylepis, EcCo = Echis coloratus, EnEn = Enhydris enhydris, EuMu = Eunectes murinus, HyGi = Hydrodynastes gigas, MaRe = Malayopython reticulatus, OpHa = Ophiophagus hannah, SiPe = Sinonatrix percarinata.
Figure 6. Precision-Recall results for the three models when tested against the three independent test datasets.
(A) GBIF random sample. (B) GBIF geo-independent sample. (C) Flickr data.Species codes in alphabetical order: ApBo = Aplopeltura boa, AtNi = Atheris nitschei, BiAr = Bitis arietans, BoCy = Boiga cynodon, BoKr = Boiga kraepelini, BoSc = Bothriechis schlegelii, BuFa = Bungarus fasciatus, CaRh = Calloselasma rhodostoma, ChCa = Chironius carinatus, CoRa = Coelognathus radiatus, DePo = Dendroaspis polylepis, EcCo = Echis coloratus, EnEn = Enhydris enhydris, EuMu = Eunectes murinus, HyGi = Hydrodynastes gigas, MaRe = Malayopython reticulatus, OpHa = Ophiophagus hannah, SiPe = Sinonatrix percarinata.
Models trained on randomly and geographically independent GBIF data performed similarly when tested against the Flickr data. The randomly subset GBIF models showed more variable results both for ROC AUC and PRRC. The respectable ability to predict Flickr results from only GBIF records suggest that Flickr results have little in the way of new climatic information.
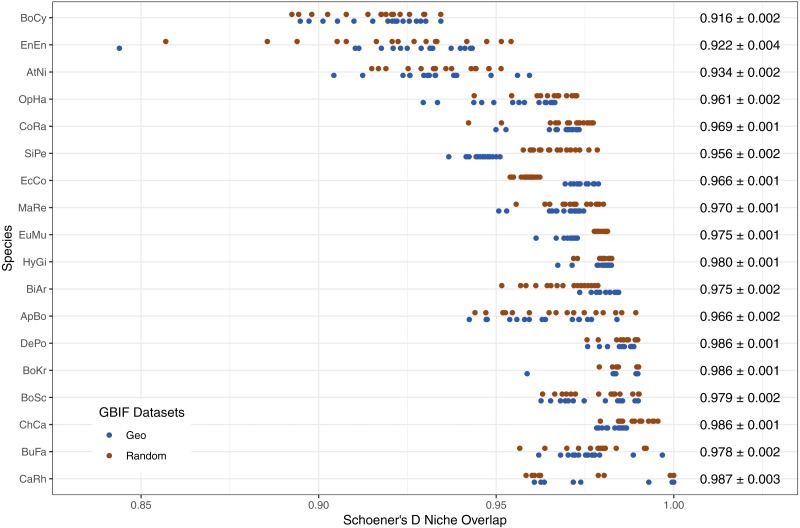
The limited new information provided by Flickr datasets is further supported by the high levels of niche overlap between models trained on GBIF-only and Flickr-supplemented datasets, albeit with variation between species (Fig. 7).
Figure 7. Schoener’s D measure of niche overlap for models trained on GBIF-only and Flickr supplemented datasets.
Right-hand side values show the overall niche overlap mean per species and the standard error. Species codes top to bottom: BoCy = Boiga cynodon, EnEn = Enhydris enhydris, AtNi = Atheris nitschei, OpHa = Ophiophagus hannah, CoRa = Coelognathus radiatus, SiPe = Sinonatrix percarinata, EcCo = Echis coloratus, MaRe = Malayopython reticulatus, EuMu = Eunectes murinus, HyGi = Hydrodynastes gigas, BiAr = Bitis arietans, ApBo = Aplopeltura boa, DePo = Dendroaspis polylepis, BoKr = Boiga kraepelini, BoSc = Bothriechis schlegelii, ChCa = Chironius carinatus, BuFa = Bungarus fasciatus, CaRh = Calloselasma rhodostoma.
When we investigated which variable predicts model performance, the mixed-models using the training dataset and species as random predictors were superior based on AIC. The resulting model agrees with Figs. 1 and 2 indicating variability between species and a weak trend driven by the training dataset. While the model investigations seem to support species as the driver behind Maxent model performance, the residuals from the models remain highly structured and non-normal (Sharpiro-Wilk test: PRRC as response, W = 0.7526, p-value <2.2e−16; ROC AUC as response, W = 0.94194, p-value <2.2e−16). Our models exploring change in model evaluation metrics suggested that the difference in sample size played a very small role (negative relationship with PRRC values: −0.0095 ±0.0039, p = 0.015; positive relationship with ROC AUC values: 0.0260 ± 0.0042, p < 0.001) and the changes were largely dependent on the species (model specification and AIC values can be found in Table S1).
Discussion
Spatial bias
Our results show a strong spatial bias in GBIF’s occurrence records for non-marine snakes. The lack of records in the critical snake hotspots mirrors investigations into other taxonomic groups (Yesson et al., 2007; Amano, Lamming & Sutherland, 2016; Roll et al., 2017). The identified gaps in GBIF records support efforts to make use of more diverse data sources: by filling gaps in GBIF coverage and boosting sample sizes, supplementary data sources could reduce the chances of underestimating species distributions and ecological niches (Beck et al., 2013; Monsarrat et al., 2019). However, while our efforts to retrieve occurrence records from social media were successful, the quantity of records was insufficient to make significant impacts on distribution models. The gaps in GBIF records (and similar gaps in Flickr derived data) are likely not the results of lack of knowledge in these locations (Tantipisanuh & Gale, 2018), but barriers limiting submissions to global biodiversity databases (Amano & Sutherland, 2013).
Other studies had highlighted the potential of social media photographs to supplement existing occurrence records (Allain, 2019; Barve, 2014; ElQadi et al., 2017), but stopped short of exploring how the records would impact distribution modelling and model predictive power. Studies that explored the impact on models’ predictive power targeted more readily photographed species in a region with greater interaction with biodiversity recording (Jiménez-Valverde et al., 2019). Tropical snakes provide a harsher assessment of the utility of community generated geo-tagged images. Our findings suggest that while there is a growing potential for social media to supplement biodiversity databases, the benefits are currently minimal for species with low-detectability and vary dramatically between species.
There are several reasons for researchers to consider using social media despite the marginal impacts shown here. First, is the low cost of initially screening for potential records. Flickr’s map user interface (https://www.flickr.com/map) can be used to gauge the number of potential records before undertaking the task of extracting (and reviewing) the records. Second, are the benefits of increased sample sizes that analyses of real-world data find difficult to quantify. Larger samples are less sensitive to false-positives/negatives and locational error (Wisz et al., 2008; Mitchell, Monk & Laurenson, 2017; Fernandes, Scherrer & Guisan, 2018). When working with species with fewer than 20-30 records, model performance is more likely to be improved by any additional records (Stockwell & Peterson, 2002); only three of our tested species had fewer than 30 records. Third, species distribution modelling techniques can vary in their sensitivity to changes in sample size (Thibaud et al., 2014; Fernandes, Scherrer & Guisan, 2018); Maxent tends to be a less sensitive technique (Thibaud et al., 2014).
Supplementary data sources limitations and potential
We highlight three limitations to implementing social media occurrence into species distribution efforts.
First is the number of geo-tagged images for low detectability species. The species with the most photographs relative to GBIF records tended to be more striking, either in size or colouration (e.g., Eunectes murinus, Malayopython reticulatus and Bothriechis schlegelii); a pattern reflected in GBIF records overall (Troudet et al., 2017). Public interest in reptiles has also been linked to whether a species is venomous, endangered, or widely distributed (Roll et al., 2016). It may be the case that traits associated with people’s interest in a species are mediated by traits that control how likely a species is to be photographed, such as its rarity or natural history (e.g., generalist species may be more photographed than purely cryptozoic species). Limitations associated with the quantity of photos will lessen over time as GPS enabled cameras become more common and the growth in geo-tagged images continues to increase (Fig. S4). Accessing other social media platforms containing geo-tagged images could additionally bolster occurrence datasets. However, current terms and conditions on several potential platforms prohibit data mining or have significant barriers to data access (Toivonen et al., 2019). Reliance on manual curation of occurrence records may be feasible when focusing on a single species but will become prohibitively time-consuming when assessing a wider clade.
The second limitation is the need to verify the identity of species depicted. While community science projects can have good identification rates for non-professional participants (Austen et al., 2016; Kosmala et al., 2016), species distribution modelling can be sensitive to false-positives (Fernandes, Scherrer & Guisan, 2018). Eliminating false-positives currently requires manual verification by the researchers, but there is significant progress being made in automated species identification (Botella et al., 2018; Wäldchen & Mäder, 2018; Toivonen et al., 2019). For snakes, a reliable system may be difficult to perfect given their crypsis and current taxonomic fluidity. Even if automated photographic verification can become reasonably reliable, it would be prudent to explicitly integrate the confidence of species identification into the distribution models, a practice that has already been demonstrated to improve predictions (Louvrier et al., 2018; Johnston et al., 2018).
Finally, researchers must consider the drivers behind different data sources distributions (Li, Goodchild & Xu, 2013). The use of bias layers in presence only modelling is the primary way to mitigate the impacts of an unknowable survey effort (Phillips et al., 2009; Merow, Smith & Silander, 2013). However, bias layers derived from the spatial patterns of one dataset may be inappropriate for another. This is why we opted for a bias layer, human footprint, that is likely connected to the overall distribution of wildlife observations. With larger datasets from more sources there may be a need to account for sampling bias on a per-dataset basis. Alternatively, social media derived datasets could be used only in model validation, proving a “semi-independent” dataset to supplement cross-validation (Gregr et al., 2019).
Conservation implications
Numerous reptiles lack proper conservation assessment due to data deficiency (Bland & Böhm, 2016). Discovering ways to fill data gaps (e.g., Callaghan et al., 2019) without having to fund additional surveying efforts would be valuable at a time when natural history investigations are under appreciated but macro-ecological questions are popular (Ríos-Saldaña, Delibes-Mateos & Ferreira, 2018; McCallen et al., 2019). Overcoming data deficiencies should be prioritised; delays could result in occurrence data derived from distributions defined by human activity (realised niche), rather than the climatic or absolute niche of a species (Monsarrat et al., 2019). Improvements in occurrence data may help identify current distributions, but unstructured occurrence data cannot help quantify population trends sorely needed for many reptile species (Bland & Böhm, 2016; Bayraktarov et al., 2019).
The quantity and accessibility of social media species occurrence records is open for abuse. In herpetology, there have been several cases of species being negatively affected by the scientific publication of location data (Stuart et al., 2006; Lindenmayer & Scheele, 2017) even though journals allow masked or partial publication (Lowe et al., 2017). While there is understandable fear in publishing the locations of new and desirable species in scientific literature, how long does it take for that information to enter the public sphere via geo-tagged photography? With the rapid growth geo-tagged images, being able to keep a desirable species protected by secrecy or gate-keeping may become increasingly difficult.
Conclusion
We have highlighted that there is considerable spatial bias in the GBIF records for non-marine snakes, with gaps in tropical regions that house exceptionally high snake diversity (Roll et al., 2017). While we encourage the investigation of supplementary data sources to help fill gaps in biodiversity databases, currently accessible social media occurrence records only improve species distribution models marginally. The data availability for tropical snakes is highly variable between species and emphasises the difficulties researchers face when studying low detectability species. Both GBIF and social media data sources are growing exponentially (although not uniformly across taxa Amano, Lamming & Sutherland, 2016); tapping the full potential of these resources may be best realised with integration of image recognition and identification confidence.
Supplemental Information
Red triangles show the per year number of GBIF records. Blue circles show the per year number of Flickr photographs, containing location data and tagged with the word snake.
Acknowledgments
We thank the Suranaree University of Technology for providing the resources required to undertake this research. We thank Inês Silva and Matt Crane for enduring long discussions on model evaluation metrics. We thank the Flickr team for creating an API that is accessible and searchable. We thank countless photographers across the globe for their enthusiasm for wildlife. We would also like to thank the editor, Assoc. Prof. Alastair Culham, and three anonymous reviewers for their comments and insight in improving this manuscript.
Funding Statement
This research was supported by the Suranaree University of Technology, Insitute of Research and Development. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Benjamin M. Marshall, Email: benjaminmichaelmarshall@gmail.com.
Colin T. Strine, Email: strine.conservation@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Benjamin M. Marshall conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Colin T. Strine conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
We have uploaded all data and code used in this study to Zenodo at DOI: 10.5281/zenodo.3243983.
The code does not include API keys to access data services. A Flickr API key can be acquired from https://www.flickr.com/services/apps/create/ after creating a Flickr account and submitting a request. There is documentation on obtaining and using the API at https://www.flickr.com/services/api/. A NCBI API key can be acquired from the accounts page https://www.ncbi.nlm.nih.gov/account/settings/ after creating an account with the NCBI. The API keys are only required for data curation code segments, we have included the datasets we curated during the study to allow the analysis code segments to run independently.
References
- Ahmadi et al. (2019).Ahmadi M, Hemami M-R, Kaboli M, Malekian M, Zimmermann NE. Extinction risks of a Mediterranean neo-endemism complex of mountain vipers triggered by climate change. Scientific Reports. 2019;9:6332. doi: 10.1038/s41598-019-42792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello-Lammens et al. (2014).Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP. spThin: functions for spatial thinning of species occurrence records for use in ecological models. v.0.1.0.1. Ecography. 2014;38:541–545. doi: 10.1111/ecog.01132. [DOI] [Google Scholar]
- Akani et al. (2002).Akani GC, Eyo E, Odegbune E, Eniang EA, Luiselli L. Ecological patterns of anthropogenic mortality of suburban snakes in an African tropical region. Israel Journal of Zoology. 2002;48:1–11. doi: 10.1092/NL55-UK13-XXQ9-NCYE. [DOI] [Google Scholar]
- Alanyali, Preis & Moat (2016).Alanyali M, Preis T, Moat HS. Tracking protests using geotagged flickr photographs. PLOS ONE. 2016;11:e0150466. doi: 10.1371/journal.pone.0150466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain (2019).Allain S. Mining Flickr: a method for expanding the known distribution of invasive species. Herpetological Bulletin. 2019;148:11–14. doi: 10.33256/hb148.1114. [DOI] [Google Scholar]
- Amano, Lamming & Sutherland (2016).Amano T, Lamming JDL, Sutherland WJ. Spatial gaps in global biodiversity information and the role of citizen science. BioScience. 2016;66:393–400. doi: 10.1093/biosci/biw022. [DOI] [Google Scholar]
- Amano & Sutherland (2013).Amano T, Sutherland WJ. Four barriers to the global understanding of biodiversity conservation: wealth, language, geographical location and security. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1756):20122649. doi: 10.1098/rspb.2012.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson & Raza (2010).Anderson RP, Raza A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. Journal of Biogeography. 2010;37:1378–1393. doi: 10.1111/j.1365-2699.2010.02290.x. [DOI] [Google Scholar]
- Antoniou et al. (2016).Antoniou V, Fonte C, See L, Estima J, Arsanjani J, Lupia F, Minghini M, Foody G, Fritz S. Investigating the feasibility of geo-tagged photographs as sources of land cover input data. ISPRS International Journal of Geo-Information. 2016;5(64):1–20. doi: 10.3390/ijgi5050064. [DOI] [Google Scholar]
- Austen et al. (2016).Austen GE, Bindemann M, Griffiths RA, Roberts DL. Species identification by experts and non-experts: comparing images from field guides. Scientific Reports. 2016;6:33634. doi: 10.1038/srep33634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley & Turner (2005).Baddeley A, Turner R. spatstat: an R package for analyzing spatial point patterns. v.1.59.0. Journal of Statistical Software. 2005;12:1–42. [Google Scholar]
- Barve (2014).Barve V. Discovering and developing primary biodiversity data from social networking sites: a novel approach. Ecological Informatics. 2014;24:194–199. doi: 10.1016/j.ecoinf.2014.08.008. [DOI] [Google Scholar]
- Bates et al. (2015).Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. v.1.1.21. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bayraktarov et al. (2019).Bayraktarov E, Ehmke G, O’Connor J, Burns EL, Nguyen HA, McRae L, Possingham HP, Lindenmayer DB. Do big unstructured biodiversity data mean more knowledge? Frontiers in Ecology and Evolution. 2019;6:239. doi: 10.3389/fevo.2018.00239. [DOI] [Google Scholar]
- Beck et al. (2013).Beck J, Ballesteros-Mejia L, Nagel P, Kitching IJ. Online solutions and the ‘Wallacean shortfall’: what does GBIF contribute to our knowledge of species’ ranges? Diversity and Distributions. 2013;19:1043–1050. doi: 10.1111/ddi.12083. [DOI] [Google Scholar]
- Benson et al. (2008).Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank Nucleic Acids Res. 2008. Jan, 1, 33. https://www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/ [DOI] [PMC free article] [PubMed]
- Bivand & Rundel (2018).Bivand R, Rundel C. rgeos: interface to geometry engine—open source (’GEOS’). v.0.4-2. https://CRAN.R-project.org/package=rgeos 2018
- Bland & Böhm (2016).Bland LM, Böhm M. Overcoming data deficiency in reptiles. Biological Conservation. 2016;204:16–22. doi: 10.1016/j.biocon.2016.05.018. [DOI] [Google Scholar]
- Borchers (2019).Borchers HW. pracma: practical numerical math functions. v.2.2.5. https://CRAN.R-project.org/package=pracma 2019
- Botella et al. (2018).Botella C, Joly A, Bonnet P, Monestiez P, Munoz F. Species distribution modeling based on the automated identification of citizen observations. Applications in Plant Sciences. 2018;6:e1029. doi: 10.1002/aps3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan et al. (2019).Callaghan CT, Rowley JJL, Cornwell WK, Poore AGB, Major RE. Improving big citizen science data: moving beyond haphazard sampling. PLOS Biology. 2019;17:e3000357. doi: 10.1371/journal.pbio.3000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos et al. (2019).Castellanos AA, Huntley JW, Voelker G, Lawing AM. Environmental filtering improves ecological niche models across multiple scales. Methods in Ecology and Evolution. 2019;10:481–492. doi: 10.1111/2041-210X.13142. [DOI] [Google Scholar]
- Chamberlain et al. (2019).Chamberlain S, Barve V, Mcglinn D, Oldoni D, Desmet P, Geffert L, Ram K. rgbif: interface to the global biodiversity information facility API. v.1.2.0. 2019. https://CRAN.R-project.org/package=rgbif https://CRAN.R-project.org/package=rgbif
- Chamberlain et al. (2018).Chamberlain S, Szoecs E, Foster Z, Arendsee Z, Boettiger C, Ram K, Bartomeus I, Baumgartner J, O’Donnell J, Oksanen J, Tzovaras BG, Marchand P, Tran V. taxize: taxonomic information from around the web. v.0.9.8.9140. 2018. https://github.com/ropensci/taxize https://github.com/ropensci/taxize
- Chua et al. (2016).Chua A, Servillo L, Marcheggiani E, Moere AV. Mapping Cilento: using geotagged social media data to characterize tourist flows in southern Italy. Tourism Management. 2016;57:295–310. doi: 10.1016/j.tourman.2016.06.013. [DOI] [Google Scholar]
- Cromsigt, Kerley & Kowalczyk (2012).Cromsigt JPGM, Kerley GIH, Kowalczyk R. The difficulty of using species distribution modelling for the conservation of refugee species—the example of European bison. Diversity and Distributions. 2012;18:1253–1257. doi: 10.1111/j.1472-4642.2012.00927.x. [DOI] [Google Scholar]
- Danielson & Gesch (2011).Danielson JJ, Gesch DB. Global multi-resolution terrain elevation data 2010 (GMTED2010): US Geological survey open-file report 2011-1073. http://pubs.usgs.gov/of/2011/1073/pdf/of2011-1073.pdf 2011
- De Miranda (2017).De Miranda EBP. The plight of reptiles as ecological actors in the tropics. Frontiers in Ecology and Evolution. 2017;5:159. doi: 10.3389/fevo.2017.00159. [DOI] [Google Scholar]
- Dowle & Srinivasan (2019).Dowle M, Srinivasan A. data.table: extension of ‘data.frame’. v.1.12.2. https://CRAN.R-project.org/package=data.table 2019
- Durso & Seigel (2015).Durso AM, Seigel RA. A snake in the hand is worth 10, 000 in the Bush. Journal of Herpetology. 2015;49:503–506. doi: 10.1670/15-49-04.1. [DOI] [Google Scholar]
- Elith et al. (2006).Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JMcCM, Townsend Peterson A, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- ElQadi et al. (2017).ElQadi MM, Dorin A, Dyer A, Burd M, Bukovac Z, Shrestha M. Mapping species distributions with social media geo-tagged images: case studies of bees and flowering plants in Australia. Ecological Informatics. 2017;39:23–31. doi: 10.1016/j.ecoinf.2017.02.006. [DOI] [Google Scholar]
- Fernandes, Scherrer & Guisan (2018).Fernandes RF, Scherrer D, Guisan A. Effects of simulated observation errors on the performance of species distribution models. Diversity and Distributions. 2018;25(3):400–413. doi: 10.1111/ddi.12868. [DOI] [Google Scholar]
- Fick & Hijmans (2017).Fick SE, Hijmans RJ. Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- Flickr Development Team (2019).Flickr Development Team Flickr API. https://www.flickr.com/services/api/ 2019
- Fourcade, Besnard & Secondi (2018).Fourcade Y, Besnard AG, Secondi J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Global Ecology and Biogeography. 2018;27:245–256. doi: 10.1111/geb.12684. [DOI] [Google Scholar]
- Fourcade et al. (2013).Fourcade Y, Engler JO, Besnard AG, Rödder D, Secondi J. Confronting expert-based and modelled distributions for species with uncertain conservation status: a case study from the corncrake (Crex crex) Biological Conservation. 2013;167:161–171. doi: 10.1016/j.biocon.2013.08.009. [DOI] [Google Scholar]
- Fox & Weisberg (2011).Fox J, Weisberg S. Sage; Thousand Oaks: 2011. [Google Scholar]
- García-Palomares, Gutiérrez & Mínguez (2015).García-Palomares JC, Gutiérrez J, Mínguez C. Identification of tourist hot spots based on social networks: a comparative analysis of European metropolises using photo-sharing services and GIS. Applied Geography. 2015;63:408–417. doi: 10.1016/j.apgeog.2015.08.002. [DOI] [Google Scholar]
- GBIF.org (2019).GBIF.org Global biodiversity information facility website. https://www.gbif.org. [05 August 2019];2019
- Grau, Grosse & Keilwagen (2015).Grau J, Grosse I, Keilwagen J. PRROC: computing and visualizing precision—recall and receiver operating characteristic curves in R. Bioinformatics. 2015;31:2595–2597. doi: 10.1093/bioinformatics/btv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregr et al. (2019).Gregr EJ, Palacios DM, Thompson A, Chan KMA. Why less complexity produces better forecasts: an independent data evaluation of kelp habitat models. Ecography. 2019;42:428–443. doi: 10.1111/ecog.03470. [DOI] [Google Scholar]
- Grolemund & Wickham (2011).Grolemund G, Wickham H. Dates and times made easy with lubridate. Journal of Statistical Software. 2011;40:1–25. [Google Scholar]
- Hausmann et al. (2018).Hausmann A, Toivonen T, Slotow R, Tenkanen H, Moilanen A, Heikinheimo V, Di Minin E. Social media data can be used to understand tourists’ preferences for nature-based experiences in protected areas: social media data in protected areas. Conservation Letters. 2018;11:e12343. doi: 10.1111/conl.12343. [DOI] [Google Scholar]
- Hijmans (2019).Hijmans RJ. raster: geographic data analysis and modeling. v.2.8-19. https://CRAN.R-project.org/package=raster 2019
- Hijmans et al. (2017).Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: species distribution modeling. v.1.1-4. 2017. https://CRAN.R-project.org/package=dismo https://CRAN.R-project.org/package=dismo
- Hughes (2017).Hughes AC. Mapping priorities for conservation in Southeast Asia. Biological Conservation. 2017;209:395–405. doi: 10.1016/j.biocon.2017.03.007. [DOI] [Google Scholar]
- Jiménez-Valverde et al. (2019).Jiménez-Valverde A, Peña Aguilera P, Barve V, Burguillo-Madrid L. Photo-sharing platforms key for characterising niche and distribution in poorly studied taxa. Insect Conservation and Diversity. 2019;12(5):389–403. doi: 10.1111/icad.12351. [DOI] [Google Scholar]
- Johnston et al. (2018).Johnston A, Fink D, Hochachka WM, Kelling S. Estimates of observer expertise improve species distributions from citizen science data. Methods in Ecology and Evolution. 2018;9:88–97. doi: 10.1111/2041-210X.12838. [DOI] [Google Scholar]
- Kearney, Shine & Porter (2009).Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer cold-blooded animals against climate warming. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmala et al. (2016).Kosmala M, Wiggins A, Swanson A, Simmons B. Assessing data quality in citizen science. Frontiers in Ecology and the Environment. 2016;14:551–560. doi: 10.1002/fee.1436. [DOI] [Google Scholar]
- Lang & CRAN Team (2019a).Lang DT, CRAN Team XML: tools for parsing and generating XML within R and S-Plus. v.3.98-1.20. https://CRAN.R-project.org/package=XML 2019a
- Lang & CRAN Team (2019b).Lang DT, CRAN Team RCurl: general network (HTTP/FTP/...) client interface for R. v.1.95-4.12. https://CRAN.R-project.org/package=RCurl 2019b
- Li, Goodchild & Xu (2013).Li L, Goodchild MF, Xu B. Spatial, temporal, and socioeconomic patterns in the use of Twitter and Flickr. Cartography and Geographic Information Science. 2013;40:61–77. doi: 10.1080/15230406.2013.777139. [DOI] [Google Scholar]
- Lindenmayer & Scheele (2017).Lindenmayer D, Scheele B. Do not publish. Science. 2017;356:800–801. doi: 10.1126/science.aan1362. [DOI] [PubMed] [Google Scholar]
- Lobo, Jiménez-valverde & Real (2008).Lobo JM, Jiménez-valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2008;17:145–151. doi: 10.1111/j.1466-8238.2007.00358.x. [DOI] [Google Scholar]
- Louvrier et al. (2018).Louvrier J, Molinari-Jobin A, Kéry M, Chambert T, Miller D, Zimmermann F, Marboutin E, Molinari P, Müeller O, Černe R, Gimenez O. Use of ambiguous detections to improve estimates from species distribution models. Conservation Biology. 2018;33:185–195. doi: 10.1111/cobi.13191. [DOI] [PubMed] [Google Scholar]
- Lowe et al. (2017).Lowe AJ, Smyth AK, Atkins K, Avery R, Belbin L, Brown N, Budden AE, Gioia P, Guru S, Hardie M, Hirsch T, Hobern D, La Salle J, Loarie SR, Miles M, Milne D, Nicholls M, Rossetto M, Smits J, Sparrow B, Terrill G, Turner D, Wardle GM. Publish openly but responsibly. Science. 2017;357:141–141. doi: 10.1126/science.aao0054. [DOI] [PubMed] [Google Scholar]
- Marshall et al. (2018).Marshall BM, Strine CT, Jones MD, Theodorou A, Amber E, Waengsothorn S, Suwanwaree P, Goode M. Hits close to home: repeated persecution of King Cobras (Ophiophagus hannah) in Northeastern Thailand. Tropical Conservation Science. 2018;11:194008291881840. doi: 10.1177/1940082918818401. [DOI] [Google Scholar]
- McCallen et al. (2019).McCallen E, Knott J, Nunez-Mir G, Taylor B, Jo I, Fei S. Trends in ecology: shifts in ecological research themes over the past four decades. Frontiers in Ecology and the Environment. 2019;17(2):109–116. doi: 10.1002/fee.1993. [DOI] [Google Scholar]
- Meek (2012).Meek R. Anthropogenic sources of mortality in the western whip snake, Hierophis viridiflavus, in a fragmented landscape in Western France. Herpetological Bulletin. 2012;120:4–8. [Google Scholar]
- Merow, Smith & Silander (2013).Merow C, Smith MJ, Silander JA. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- Miranda, Ribeiro & Strüssmann (2016).Miranda EBP, Ribeiro RP, Strüssmann C. The ecology of human-anaconda conflict: a study using internet videos. Tropical Conservation Science. 2016;9:43–77. doi: 10.1177/194008291600900105. [DOI] [Google Scholar]
- Mitchell, Monk & Laurenson (2017).Mitchell PJ, Monk J, Laurenson L. Sensitivity of fine-scale species distribution models to locational uncertainty in occurrence data across multiple sample sizes. Methods in Ecology and Evolution. 2017;8:12–21. doi: 10.1111/2041-210X.12645. [DOI] [Google Scholar]
- Monsarrat et al. (2019).Monsarrat S, Novellie P, Rushworth I, Kerley GIH. Shifted distribution baselines: neglecting long-term biodiversity records risks overlooking potentially suitable habitat for conservation management. bioRxiv. 2019 doi: 10.1101/565929. [DOI] [PMC free article] [PubMed]
- Muscarella et al. (2014).Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP. ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution. 2014;5:1198–1205. doi: 10.1111/2041-210X.12261. [DOI] [Google Scholar]
- Mutascio et al. (2018).Mutascio HE, Pittman SE, Zollner PA, D’Acunto LE. Modeling relative habitat suitability of southern Florida for invasive Burmese pythons (Python molurus bivittatus) Landscape Ecology. 2018;33:257–274. doi: 10.1007/s10980-017-0597-5. [DOI] [Google Scholar]
- Orsi & Geneletti (2013).Orsi F, Geneletti D. Using geotagged photographs and GIS analysis to estimate visitor flows in natural areas. Journal for Nature Conservation. 2013;21:359–368. doi: 10.1016/j.jnc.2013.03.001. [DOI] [Google Scholar]
- Pearson (2015).Pearson RG. Asian common toads in Madagascar: an urgent effort to inform surveys and eradication efforts. Global Change Biology. 2015;21:9–9. doi: 10.1111/gcb.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebesma et al. (2019).Pebesma E, Bivand R, Rowlingson B, Gomez-Rubio V, Hijmans R, Sumner M, MacQueen D, Lemon J, O’Brien J. Package ‘sp’. v.1.3.1. https://cran.r-project.org/web/packages/sp/index.html 2019
- Pedersen & Crameri (2018).Pedersen TL, Crameri F. scico: colour palettes based on the scientific colour-maps. v.1.1.0. 2018. https://CRAN.R-project.org/package=scico https://CRAN.R-project.org/package=scico
- Penman et al. (2010).Penman TD, Pike DA, Webb JK, Shine R. Predicting the impact of climate change on Australia’s most endangered snake, Hoplocephalus bungaroides: impact of climate change on an endangered snake. Diversity and Distributions. 2010;16:109–118. doi: 10.1111/j.1472-4642.2009.00619.x. [DOI] [Google Scholar]
- Phillips, Chipperfield & Kearney (2008).Phillips BL, Chipperfield JD, Kearney MR. The toad ahead: challenges of modelling the range and spread of an invasive species. Wildlife Research. 2008;35:222–234. doi: 10.1071/WR07101. [DOI] [Google Scholar]
- Phillips et al. (2009).Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, Leathwick J, Ferrier S. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecological Applications. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- Preis et al. (2013).Preis T, Moat HS, Bishop SR, Treleaven P, Stanley HE. Quantifying the digital traces of hurricane sandy on flickr. Scientific Reports. 2013;3:3141. doi: 10.1038/srep03141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019).R Core Team . R Foundation for Statistical Computing; Vienna: 2019. [Google Scholar]
- R Studio Team (2019).R Studio Team . RStudio, Inc; Boston: 2019. [Google Scholar]
- Radosavljevic & Anderson (2014).Radosavljevic A, Anderson RP. Making better Maxent models of species distributions: complexity, overfitting and evaluation. Journal of Biogeography. 2014;41:629–643. doi: 10.1111/jbi.12227. [DOI] [Google Scholar]
- Ríos-Saldaña, Delibes-Mateos & Ferreira (2018).Ríos-Saldaña CA, Delibes-Mateos M, Ferreira C. Are fieldwork studies being relegated to second place in conservation science? Global Ecology and Conservation. 2018;14:e00389. doi: 10.1016/j.gecco.2018.e00389. [DOI] [Google Scholar]
- Roberts et al. (2017).Roberts DR, Bahn V, Ciuti S, Boyce MS, Elith J, Guillera-Arroita G, Hauenstein S, Lahoz-Monfort JJ, Schröder B, Thuiller W, Warton DI, Wintle BA, Hartig F, Dormann CF. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography. 2017;40:913–929. doi: 10.1111/ecog.02881. [DOI] [Google Scholar]
- Roll et al. (2017).Roll U, Feldman A, Novosolov M, Allison A, Bauer AM, Bernard R, Böhm M, Castro-Herrera F, Chirio L, Collen B, Colli GR, Dabool L, Das I, Doan TM, Grismer LL, Hoogmoed M, Itescu Y, Kraus F, LeBreton M, Lewin A, Martins M, Maza E, Meirte D, Nagy ZT, De C Nogueira C, Pauwels OSG, Pincheira-Donoso D, Powney GD, Sindaco R, Tallowin OJS, Torres-Carvajal O, Trape J-F, Vidan E, Uetz P, Wagner P, Wang Y, Orme CDL, Grenyer R, Meiri S. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nature Ecology & Evolution. 2017;1:1677–1682. doi: 10.1038/s41559-017-0332-2. [DOI] [PubMed] [Google Scholar]
- Roll et al. (2016).Roll U, Mittermeier JC, Diaz GI, Novosolov M, Feldman A, Itescu Y, Meiri S, Grenyer R. Using Wikipedia page views to explore the cultural importance of global reptiles. Biological Conservation. 2016;204:42–50. doi: 10.1016/j.biocon.2016.03.037. [DOI] [Google Scholar]
- Santos et al. (2006).Santos X, Brito JC, Sillero N, Pleguezuelos JM, Llorente GA, Fahd S, Parellada X. Inferring habitat-suitability areas with ecological modelling techniques and GIS: a contribution to assess the conservation status of Vipera latastei. Biological Conservation. 2006;130:416–425. doi: 10.1016/j.biocon.2006.01.003. [DOI] [Google Scholar]
- Sayers et al. (2009).Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Mizrachi I, Ostell J, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Yaschenko E, Ye J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2009;37(suppl 1):D5–D15. doi: 10.1093/nar/gkn741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheglovitova & Anderson (2013).Shcheglovitova M, Anderson RP. Estimating optimal complexity for ecological niche models: a jackknife approach for species with small sample sizes. Ecological Modelling. 2013;269:9–17. doi: 10.1016/j.ecolmodel.2013.08.011. [DOI] [Google Scholar]
- Shine & Bonnet (2000).Shine R, Bonnet X. Snakes: a new model organism in ecological research? Trends in Ecology and Evolution. 2000;15:221–222. doi: 10.1016/S0169-5347(00)01853-X. [DOI] [PubMed] [Google Scholar]
- Slowikowski (2019).Slowikowski K. ggrepel: automatically position non-overlapping text labels with ggplot2. v.0.8.1. https://CRAN.R-project.org/package=ggrepel 2019
- Sofaer, Hoeting & Jarnevich (2019).Sofaer HR, Hoeting JA, Jarnevich CS. The area under the precision—recall curve as a performance metric for rare binary events. Methods in Ecology and Evolution. 2019;10:565–577. doi: 10.1111/2041-210X.13140. [DOI] [Google Scholar]
- Solano & Feria (2007).Solano E, Feria TP. Ecological niche modeling and geographic distribution of the genus Polianthes L. (Agavaceae) in Mexico: using niche modeling to improve assessments of risk status. Biodiversity and Conservation. 2007;16:1885–1900. doi: 10.1007/s10531-006-9091-0. [DOI] [Google Scholar]
- South (2017).South A. rnaturalearth: world map data from natural earth. v.0.1.0. 2017. https://CRAN.R-project.org/package=rnaturalearth https://CRAN.R-project.org/package=rnaturalearth
- Steen (2010).Steen DA. Snakes in the grass: secretive natural histories defy both conventional and progressive statistics. Herpetological Conservation and Biology. 2010;5:183–188. [Google Scholar]
- Stockwell & Peterson (2002).Stockwell DRB, Peterson AT. Effects of sample size on accuracy of species distribution models. Ecological Modelling. 2002;148:1–13. doi: 10.1016/S0304-3800(01)00388-X. [DOI] [Google Scholar]
- Stuart et al. (2006).Stuart BL, Rhodin AGJ, Grismer LL, Hansel T. Scientific description can imperil species. Science. 2006;312:1137. doi: 10.1126/science.312.5777.1137b. [DOI] [PubMed] [Google Scholar]
- Tantipisanuh & Gale (2018).Tantipisanuh N, Gale GA. Identification of biodiversity hotspot in national level—importance of unpublished data. Global Ecology and Conservation. 2018;13:e00377. doi: 10.1016/j.gecco.2018.e00377. [DOI] [Google Scholar]
- Thibaud et al. (2014).Thibaud E, Petitpierre B, Broennimann O, Davison AC, Guisan A. Measuring the relative effect of factors affecting species distribution model predictions. Methods in Ecology and Evolution. 2014;5:947–955. doi: 10.1111/2041-210X.12203. [DOI] [Google Scholar]
- Tingley, Meiri & Chapple (2016).Tingley R, Meiri S, Chapple DG. Addressing knowledge gaps in reptile conservation. Biological Conservation. 2016;204:1–5. doi: 10.1016/j.biocon.2016.07.021. [DOI] [Google Scholar]
- Toivonen et al. (2019).Toivonen T, Heikinheimo V, Fink C, Hausmann A, Hiippala T, Järv O, Tenkanen H, Di Minin E. Social media data for conservation science: a methodological overview. Biological Conservation. 2019;233:298–315. doi: 10.1016/j.biocon.2019.01.023. [DOI] [Google Scholar]
- Troudet et al. (2017).Troudet J, Grandcolas P, Blin A, Vignes-Lebbe R, Legendre F. Taxonomic bias in biodiversity data and societal preferences. Scientific Reports. 2017;7:9132. doi: 10.1038/s41598-017-09084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch et al. (2013).Tulloch AIT, Mustin K, Possingham HP, Szabo JK, Wilson KA. To boldly go where no volunteer has gone before: predicting volunteer activity to prioritize surveys at the landscape scale. Diversity and Distributions. 2013;19:465–480. doi: 10.1111/j.1472-4642.2012.00947.x. [DOI] [Google Scholar]
- Tulloch et al. (2016).Tulloch AIT, Sutcliffe P, Naujokaitis-Lewis I, Tingley R, Brotons L, Ferraz KMPMB, Possingham H, Guisan A, Rhodes JR. Conservation planners tend to ignore improved accuracy of modelled species distributions to focus on multiple threats and ecological processes. Biological Conservation. 2016;199:157–171. doi: 10.1016/j.biocon.2016.04.023. [DOI] [Google Scholar]
- Uetz, Freed & Hošek (2019).Uetz P, Freed P, Hošek J, editors. The reptile database. http://www.reptile-database.org. [17 April 2019];2019
- U.S. Geological Survey (2016).U.S. Geological Survey TopoTools. https://topotools.cr.usgs.gov/GMTED_viewer/gmted2010_global_grids.php. [01 December 2018];2016
- Ushey et al. (2018).Ushey K, McPherson J, Cheng J, Atkins A, Allaire JJ. packrat: a dependency management system for projects and their r package dependencies. v.0.5.0. https://CRAN.R-project.org/package=packrat 2018
- Valavi et al. (2018).Valavi R, Elith J, Lahoz-Monfort J, Guillera-Arroita G. blockCV: spatial and environmental blocking for k-fold cross-validation. v.1.0.0. 2018. https://github.com/rvalavi/blockCV https://github.com/rvalavi/blockCV
- Venter et al. (2016a).Venter O, Sanderson EW, Magrach A, Allan JR, Beher J, Jones KR, Possingham HP, Laurance WF, Wood P, Fekete BM, Levy MA, Watson JE. Global terrestrial Human Footprint maps for 1993 and 2009. Scientific Data. 2016a;3:160067. doi: 10.1038/sdata.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter et al. (2016b).Venter O, Sanderson EW, Magrach A, Allan JR, Beher J, Jones KR, Possingham HP, Laurance WF, Wood P, Fekete BM, Levy MA, Watson JEM. Data from: global terrestrial human footprint maps for 1993 and 2009. Dryad Digital Repository. https://doi.org/105061/dryad.052q52. 2016b doi: 10.1038/sdata.2016.67. [DOI] [PMC free article] [PubMed]
- Wäldchen & Mäder (2018).Wäldchen J, Mäder P. Machine learning for image based species identification. Methods in Ecology and Evolution. 2018;9:2216–2225. doi: 10.1111/2041-210X.13075. [DOI] [Google Scholar]
- Whitaker & Shine (2000).Whitaker PB, Shine R. Sources of mortality of large elapid snakes in an agricultural landscape. Journal of Herpetology. 2000;34:121–128. doi: 10.2307/1565247. [DOI] [Google Scholar]
- Wickham (2007).Wickham H. Reshaping data with the reshape package. Journal of Statistical Software. 2007;21:1–20. [Google Scholar]
- Wickham (2016).Wickham H. Springer-Verlag; New York: 2016. [Google Scholar]
- Wickham (2017).Wickham H. httr: tools for working with URLs and HTTP. v.1.4.1. https://CRAN.R-project.org/package=httr 2017
- Wickham (2018).Wickham H. stringr: simple, consistent wrappers for common string operations. v.1.4.0. https://CRAN.R-project.org/package=stringr 2018
- Wickham (2019).Wickham H. rvest: easily Harvest (Scrape) Web Pages. v.0.3.5. https://CRAN.R-project.org/package=rvest 2019
- Wickham et al. (2017).Wickham H, François R, Henry L, Müller K. dplyr: a grammar of data manipulation. v.0.8.3. https://CRAN.R-project.org/package=dplyr 2017
- Wickham, Hester & Ooms (2018).Wickham H, Hester J, Ooms J. xml2: parse XML. v.1.2.2. https://CRAN.R-project.org/package=xml2 2018
- Willson & Winne (2016).Willson JD, Winne CT. Evaluating the functional importance of secretive species: a case study of aquatic snake predators in isolated wetlands. Journal of Zoology. 2016;298:266–273. doi: 10.1111/jzo.12311. [DOI] [Google Scholar]
- Wisz et al. (2008).Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, Guisan A, NCEAS Predicting Species Distributions Working Group † Effects of sample size on the performance of species distribution models. Diversity and Distributions. 2008;14:763–773. doi: 10.1111/j.1472-4642.2008.00482.x. [DOI] [Google Scholar]
- Yañez-Arenas et al. (2014).Yañez-Arenas C, Peterson AT, Mokondoko P, Rojas-Soto O, Martínez-Meyer E. The use of ecological niche modeling to infer potential risk areas of snakebite in the Mexican State of Veracruz. PLOS ONE. 2014;9(6):e100957. doi: 10.1371/journal.pone.0100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesson et al. (2007).Yesson C, Brewer PW, Sutton T, Caithness N, Pahwa JS, Burgess M, Gray WA, White RJ, Jones AC, Bisby FA, Culham A. How global is the global biodiversity information facility? PLOS ONE. 2007;2:e1124. doi: 10.1371/journal.pone.0001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi et al. (2015).Yousefi M, Ahmadi M, Nourani E, Behrooz R, Rajabizadeh M, Geniez P, Kaboli M. Upward altitudinal shifts in habitat suitability of mountain vipers since the last glacial maximum. PLOS ONE. 2015;10:e0138087. doi: 10.1371/journal.pone.0138087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizka et al. (2019).Zizka A, Silvestro D, Andermann T, Azevedo J, Duarte Ritter C, Edler D, Farooq H, Herdean A, Ariza M, Scharn R, Svanteson S, Wengström N, Zizka V, Antonelli A. CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods in Ecology and Evolution. 2019;10(5):744–751. doi: 10.1111/2041-210X.13152. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red triangles show the per year number of GBIF records. Blue circles show the per year number of Flickr photographs, containing location data and tagged with the word snake.
Data Availability Statement
The following information was supplied regarding data availability:
We have uploaded all data and code used in this study to Zenodo at DOI: 10.5281/zenodo.3243983.
The code does not include API keys to access data services. A Flickr API key can be acquired from https://www.flickr.com/services/apps/create/ after creating a Flickr account and submitting a request. There is documentation on obtaining and using the API at https://www.flickr.com/services/api/. A NCBI API key can be acquired from the accounts page https://www.ncbi.nlm.nih.gov/account/settings/ after creating an account with the NCBI. The API keys are only required for data curation code segments, we have included the datasets we curated during the study to allow the analysis code segments to run independently.