Abstract
Abundant data are now available to evaluate relationships between seafood consumption in pregnancy and childhood and neurocognitive development. We conducted two systematic reviews utilizing methodologies detailed by the Dietary Guidelines for Americans Scientific Advisory Committee 2020–2025. After reviewing 44 publications on 102,944 mother-offspring pairs and 25,031 children, our technical expert committee developed two conclusion statements that included the following:
“Moderate and consistent evidence indicates that consumption of a wide range of amounts and types of commercially available seafood during pregnancy is associated with improved neurocognitive development of offspring as compared to eating no seafood. Overall, benefits to neurocognitive development began at the lowest amounts of seafood consumed (~4 oz/wk) and continued through the highest amounts, above 12 oz/wk, some range up to >100 oz/wk.”, “This evidence does not meet the criteria for “strong evidence” only due to a paucity of randomized controlled trials that may not be ethical or feasible to conduct for pregnancy” and “Moderate and consistent evidence indicates that consumption of >4 oz/wk and likely >12 oz/wk of seafood during childhood has beneficial associations with neurocognitive outcomes.”
No net adverse neurocognitive outcomes were reported among offspring at the highest ranges of seafood intakes despite associated increases in mercury exposures. Data are insufficient for conclusive statements regarding lactation, optimal amounts, categories or specific species characterized by mercury content and neurocognitive development; although there is some evidence that dark/oily seafood may be more beneficial. Research was conducted in healthy women and children and is generalizable to US populations. Assessment of seafood as a whole food integrates inherently integrates any adverse effects from neurotoxicants, if any, and benefits to neurocognition from omega-3 fats, as well as other nutrients critical to optimal neurological development.
Introduction
Maternal prenatal nutrition and nutrition during childhood are crucial factors in a child’s neurodevelopment, and failure to provide adequate amounts of key nutrients at critical periods may result in lifelong impairment in cognitive development and mental health that cannot be corrected by subsequent repletion of nutrients. Seafood is a rich source of key nutrients that are biologically essential for optimal fetal and child neurodevelopment including iodine, vitamin B12, iron, vitamin D, zinc, manganese and highly unsaturated omega-3 and omega-6 fatty acids [1]. Women are more likely to the achieve optimal intakes of these nutrients when consuming seafood in pregnancy [2]. Public health agencies in the United States [3, 4] Canada [5], and Europe [6, 7]reviewed evidence available through 2014 and concluded that seafood consumed by pregnant women is likely to benefit the neurocognitive development of their children as described in the accompanying article in this journal [8]. The evidence today is greater with at least 29 published studies evaluating seafood consumption during pregnancy (prenatal exposure) for 201,944 mother-child pairs, and 15 studies of 25,031 children who ate seafood (postnatal exposure).
Thus, it is timely and appropriate that the 2020 Dietary Guidelines Advisory Committee is conducting systematic reviews to examine the following questions identified by US Departments of Agriculture (USDA) and Health and Human Services (HHS): Question #40 “What is the relationship between seafood consumption during pregnancy and lactation and the neurocognitive development of the infant?” and Question #41 “What is the relationship between seafood consumption during childhood and adolescence (up to 18 years of age) and neurocognitive development?” https://www.dietaryguidelines.gov/work-under-way/review-science/topics-and-questions-under-review. The Dietary Guidelines for Americans (DGA) systematic review process detailed by the USDA’s Nutrition Evidence Systematic Review (NESR) team (https://nesr.usda.gov) is designed to be rigorous and transparent, such that it can be replicated by qualified professionals [https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews]. Our goals were to conduct systematic reviews for these two questions by adhering to the NESR methodology and to contribute the perspectives of an independent technical expert committee with extensive experience on these topics.
Critically, the 2020 Dietary Guidelines Advisory Committee uses the term “seafood” in these questions to define the independent or causal variable in these questions as opposed to any single nutritional or chemical component within seafood, whether naturally-occurring or an environmental contaminant. This approach allows development of guidance for the public regarding consumption of seafood as a whole food (e.g., whether to eat seafood during pregnancy and feed seafood to children to benefit their neurocognition) rather than focusing on harms or benefits of individual seafood components. Thus, we systematically reviewed studies that assessed relationships between seafood consumption as a net or whole package because data from these studies more directly and reliably address relationships to neurocognition than studies of individual constituents of seafoods. We also used the 2010 and 2015–2020 DGA definition of seafood as follows: “Seafood is a large category of marine animals that live in the sea and in freshwater lakes and rivers. Seafood includes fish, such as salmon, tuna, trout, and tilapia, and shellfish, such as shrimp, crab and oysters.” [3, 4]. Marine mammals (e.g. porpoises and whales, including pilot whales, which are not commonly consumed by Americans) and sea plants (seaweeds and algae) are not considered to be seafood in this definition.
We also considered the importance of the term “relationship” in of these questions. We evaluated the overall strength of evidence for whether seafood consumed in pregnancy or childhood is likely to benefit neurocognition, and if so, for the magnitude of those benefits, and whether they are clinically meaningful, lasting and consistent with the stages of development during which they were examined. Important secondary questions included a) determination of the lowest and highest amounts of consumption providing benefit, b) whether there is an optimum beneficial amount, c) whether some types of seafood are more beneficial than others (e.g. oily or fatty vs. lean or white fish) and d) whether differentiation by species (e.g. fresh vs. salt water) is merited.
Mercury, a neurotoxicant to which the fetus is susceptible, is present at some level in essentially all seafood [9, 10,]so an important question is whether and under what circumstances exposure to mercury from seafood affects neurocognitive outcomes. Nutritional status and mercury exposure could simultaneously influence developmental outcomes in opposite directions. Thus, the examination of seafood as the independent variable simultaneously evaluates the magnitude of adverse effects from exposure to mercury and beneficial effects from nutrients on cognitive development. Considering this, we also sought to determine if any reported levels of seafood consumption resulted in net harms to neurocognition in pregnancy and in childhood. As to be expected, all of the studies that attempted to measure exposure to mercury in addition to maternal seafood consumption found it in maternal blood or hair or in cord blood. In describing these findings, hereafter we use the term “mercury” rather than “methylmercury” for consistency because most studies tested for total mercury, which includes methylmercury.
Methods
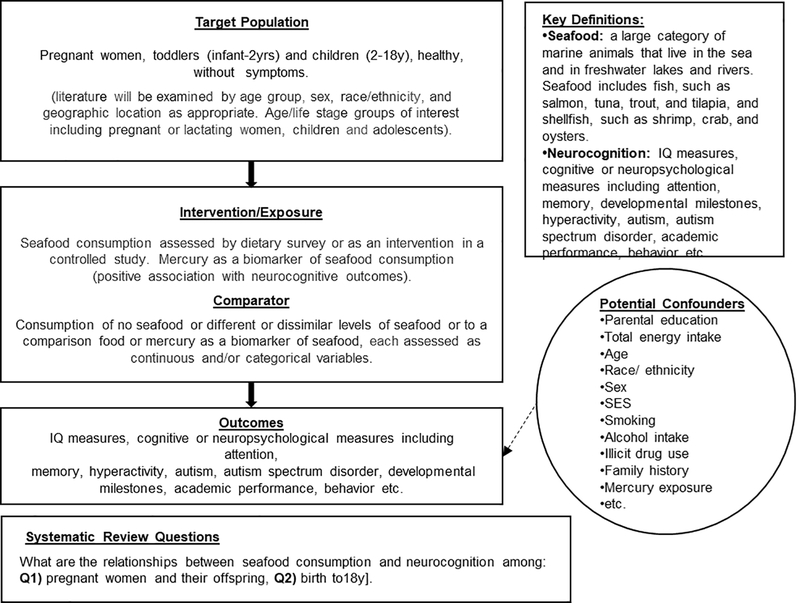
Systematic reviews of the evidence relating to the two questions developed by the USDA and HHS for the 2020 Dietary Guidelines Advisory Committee were conducted by a Technical Expert Collaborative (TEC) group, an interdisciplinary team including content matter experts holding advanced degrees in nutrition, medicine, chemistry, library science, or a related field and with experience serving on US and international science-based policy committees (see supplementary materials). All work described in this document was done by members of the TEC; no unnamed staff were involved. The TEC followed the methodology (https://nesr.usda.gov) of the USDA’s NESR team (formerly known as the Nutrition Evidence Library), and as described in detail by Obbagy et al. [11]. All TEC members were trained in systematic review methodology as detailed on the 2020 Dietary Guidelines Advisory Committee /NESR website https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews. Search methodologies including databases and search terms conformed to NESR methodologies. A representative of the TEC clarified questions about the implementation of the systematic methodology and use of the Risk of Bias rating instruments with the NESR team. The TEC identified both questions to be addressed by the systematic review directly from the 2020 Dietary Guidelines Advisory Committee website https://www.dietaryguidelines.gov/work-underway/review-science/topics-and-questions-under-review. An analytic framework was developed which was applicable to both questions. This analytic framework defined the target population, described seafood exposures and interventions, outcomes, primary confounders and specified key definitions. (Figure 1)
Figure 1.
Analytical Framework
Study criteria
The TEC developed a priori criteria for inclusion and exclusion for each of the two systematic methodology questions. To be included, studies needed to be published in English and conducted in very high or high Human Development Index countries [11]. In addition, included studies were required to have one of the following study designs: randomized controlled trial (RCT), prospective cohort study, or case control studies, in which cases were defined as having “neurocognitive disorders and were compared to matched healthy controls”, as described by the NESR team at https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews (accessed June 18, 2019). Eligible participants were (for question #40) pregnant women and their offspring, and (for question #41) children who ate seafood between birth to 18 years of all genders. Question #40 was stated so as to evaluate the relationship between maternal seafood consumption and neurocognition in the infant we interpreted “infant” as “offspring” and thus we assessed neurocognitive impacts both in infancy and throughout child development. Included studies were required to have assessed either women who were primarily healthy (i.e., some subjects, but not all, may have had a chronic or pregnancy-related condition) at baseline or children who were primarily healthy.
Neurocognition was defined as a large category of neurodevelopmental and neuropsychiatric outcomes including IQ measures, cognitive or neuropsychological measures including attention, memory, developmental milestones, hyperactivity, autism, autism spectrum disorder, academic performance, behavior, psychiatric diagnostic category (Diagnostic and Statistical Manual (DSM), etc. This definition of neurocognition was consistent with that described during the 2020 Dietary Guidelines Advisory Committee public meeting of March 28–29, 2019. Studies were required to have reported on the relationship between at least one independent variable (seafood consumption) and with at least one dependent variable ((neurocognition).
Mercury itself (or Hg chemical forms, e.g. methyl-mercury) has no known beneficial effects on neurodevelopment. Despite this, some studies reported a positive relationship between mercury levels on neurocognitive outcomes. Greater seafood consumption is often associated with higher mercury exposure. Therefore, when higher mercury levels were associated with cognitive benefits, these mercury levels were highly likely to be reflecting the nutritional effects of seafood and as such, these studies were also included. Comparators included the consumption of either no seafood or higher vs. lower intakes of seafood. Whenever possible, available data were evaluated to assess “oily” seafood species (e.g. tuna, mackerel, swordfish, salmon, sardines etc.) as compared to “white fish” (e.g. tilapia, cod, pollock, haddock, etc.) We included neurocognitive outcome measures that were age-appropriate, valid and widely accepted for both systematic review questions.
Literature search, screening, and selection
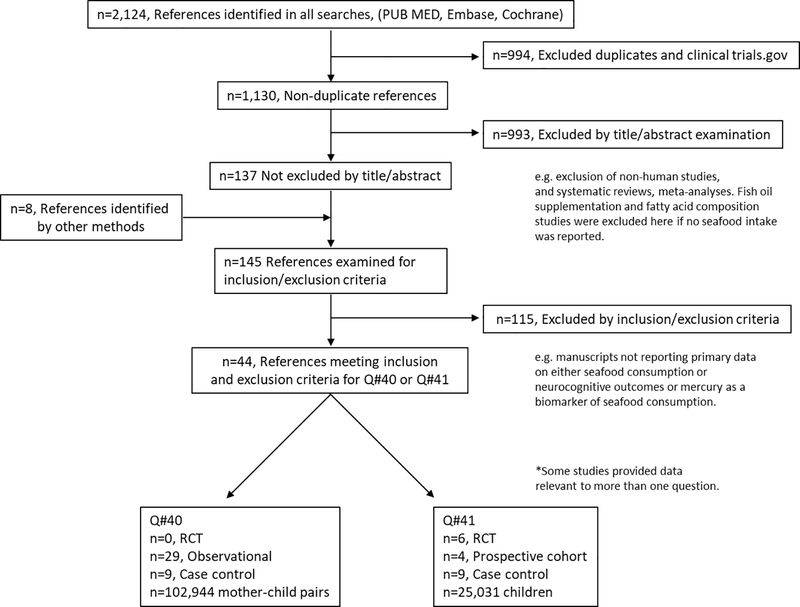
TEC members conducted searches of peer reviewed published literature with date ranges of January 1980–April 2019 in three databases (Cochrane, EMBASE, and PubMed). Search terms defining seafood included seafood, fish, and dietary patterns enriched in seafood. Search patterns for neurocognitive outcomes included developmental milestones, IQ, attention, behavior, social and emotional development, and diagnostic category (e.g. attention deficit hyperactivity disorder) customized for each database. Figure 2 presents the study selection process. The search plan including the full list of databases and search strategies is available (supplementary materials). To ensure that all relevant articles were identified, a manual search was conducted to find articles that may not have been discovered by our electronic database search. Recommendations were solicited from content matter experts to identify additional articles of potential relevance. Two TEC members independently screened each article’s title and/or abstract for relevance using the a priori inclusion and exclusion criteria. Relevant articles were independently screened by two other TEC members at the full-text level. Any disagreements regarding inclusion or exclusion were discussed and resolved among TEC members. The excluded articles and reasons for exclusion are available (supplementary materials). No studies published earlier than 2000 were included, per the DGAC methodology.
Figure 2.
Manuscript Search and Selection
Data extraction and risk of bias assessment
The following domains were extracted for articles: study characteristics, participant characteristics, information on the exposure/independent variables and outcome/dependent variables, confounding variables, statistical adjustments, mercury exposure (if available), results and limitations. At least one additional TEC member verified the completeness and accuracy of the extracted data for quality control. Reported outcomes had to be statistically significant. Studies that reported “weak”, “trend” or similarly characterized outcomes, whether trending beneficial or adverse, that were not statistically significant, were defined in this review as being null. TEC members independently assessed the risk of selection, performance, detection, and attrition biases using the Revised Cochrane risk-of-bias Tool for Randomized Trials https://www.riskofbias.info/welcome/rob-2–0-tool/current-version-of-rob-2 or the Risk of Bias-Nutritional Observational Scale (ROB-NOS) adapted by the NESR team for use in nutritional observational studies from the ROBINS-1 https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews. Differences in judgements about risk of bias for each article were reconciled by discussion among raters and verification by an independent third rater. Seafood amounts were standardized to ounces/week (oz/wk) with one meal assumed to be 4 oz unless otherwise defined by the study. Mercury exposures were standardized from blood concentrations to hair mercury using the conversion table in the FDA quantitative assessment of net effects (Table V3 p. 92) [9], with mercury in maternal blood (ug/L) = 3.59* hair mercury (ppm). Cord blood mercury concentrations were standardized to maternal hair mercury using data from Grandjean et al. (1992) [12]; cord blood mercury (nmol/L) = 5.0* mercury maternal hair (nmol/g).
Evidence synthesis, conclusion statements, evidence grading and research recommendations
For each systematic review question, the evidence was synthesized qualitatively and graded, conclusion statements were developed, and research recommendations were developed as per NESR methodologies [11]. Briefly, TEC members independently reviewed the extracted data, full-text articles and a description of the body of evidence. Based on the inputs from TEC members, primary TEC members drafted the evidence synthesis including overarching themes and the similarities and differences in findings. A conclusion statement was written to answer each systematic review question, reflecting the synthesis and grading of the available evidence. The TEC used NESR’s grading rubric to assign a grade of strong, moderate, limited, or grade not assignable to the evidence underlying each conclusion statement [11]. The grading rubric evaluates internal validity, adequacy, and consistency of the evidence, as well as impact (including clinical impact) and generalizability [11]. TEC members identified research recommendations throughout the process. The conclusion statements developed here were not formulated to make policy recommendations and do not reflect the policy or position of the USDA and HHS, the 2020 Dietary Guidelines Advisory Committee or any Federal or State or private institution. They should not be interpreted to be dietary guidance or advice.
RESULTS
The initial search yielded 2,124 articles across neurocognitive outcomes including IQ, verbal development, scholastic achievement, behavior, attention (including risk of attention deficit hyperactivity disorder (ADHD)), autistic phenotypes, cerebral palsy, stereopsis and infant development, including milestones. 994 articles were excluded as duplicates or clinicaltrials.gov citations. 993 articles were excluded based on review of their titles and abstracts and 8 articles were identified by hand search. TEC analysts examined 145 full text articles in detail for inclusion/exclusion and excluded an additional 115 articles (figure 2). Common reasons for exclusion were ineligible study design (e.g. cross-sectional studies) and failure to utilize seafood consumption as an independent variable or a parameter of neurodevelopment as a dependent variable (see supplementary materials). For question #40 this process yielded 29 articles comprising 102,944 mother-child pairs, (29 prospective cohort studies) (Table 1). For question #41 this process yielded 15 articles, comprising 25,031 children (6 RCTs, 4 prospective cohorts, and 9 case control) (Table 2). These studies were published between 2001 and 2019.
Table 1.
Maternal seafood consumption in pregnancy and neurocognitive development in their children
| Year Author Study Design Location Number of mother child pairs Risk of Bias1 |
Neurocognitive outcomes Clinically meaningful?2 |
Beneficial/adverse/null and Size of effects (as compared to: e.g. no/highest seafood, or continuous) |
Child’s age at effect | Amount of seafood consumed -Mean+ SD (oz/wk) - (range) - Amount assoc. with largest beneficial or adverse effect - Outcomes categorized by seafood type? (e.g. oily/white/species) |
Mercury exposure (if provided, standardized to hair, ppm3) | Comments: |
|---|---|---|---|---|---|---|
| 2007 Budtz-Jørgensen et al. Prospective Cohort Faroe Islands n=1,022 Moderate Risk of Bias |
Verbal Boston Naming Test (BNT) WISC-R similarities; California Verbal Learning Test (CVLT) Motor Neuropsychological Examination System (NES2) Finger tapping (CATYSYS) CM=PY |
Beneficial Greater seafood consumption was beneficial for motor function outcomes, both at 7 and 14 yr and spatial functioning at 14 yr, increasing from 0 to 4 oz/wk and from 4 to ≥12 oz/wk |
14 yr 7 yr |
Mean and SD of seafood consumption not reported. Greatest benefits ≥ 12 oz/wk Seafood consumption was distinguished from consumption of pilot whale meat and blubber that occurred in this cohort. Outcomes not categorized by white/oily or by species. |
Geometric mean 4.27 pm (interquart range 2.6–7.7) (as reported in Grandjean et al., 1997) Most mercury exposure in this cohort originated from pilot whale. Greater seafood consumption was not associated with higher mercury levels (Grandjean et al., 1995). |
Maternal seafood consumption was beneficially assoc. with offspring test scores, while mercury, mostly from pilot whale, was independently associated with adverse neurocognitive outcomes. |
| 2008 Gale et al. Prospective Cohort United Kingdom n=217 Moderate Risk of Bias |
Strengths and Difficulties Questionnaire (SDQ) Wechsler Abbreviated Scale of Intelligence (WISC) CM=Y |
Beneficial Compared to mothers who ate no seafood, verbal IQ was higher among children: ≤ 4 oz/wk, 7.66 points (95% CI −.1 to 15.4); 4–8 oz/wk, 7.32 points (95% CI .26 to 14.4); ≥12 oz/wk, 8.07 points (95% CI .28 to 15.9). Children of mothers not eating oily seafood in early pregnancy had greater risk of hyperactivity: OR 2.94, (95% CI 1.28 to 6.7) |
9 yr | Mean and SD of seafood consumption not reported. (range 0–≥12 oz/wk) Greatest benefit ≥12 oz/wk Outcomes not categorized by white/oily or by species for IQ but categorized as oily for hyperactivity. |
Not reported | Greater seafood consumption was associated with greater verbal IQ in a dose response relationship. Children of mothers not eating oily seafood in early pregnancy had a nearly 3 times greater risk of hyperactivity. |
| 2018 Golding et al. Prospective Cohort United Kingdom n=3840 Moderate Risk of Bias |
Autism and autistic traits scale derived from Social and Communication Disorders Checklist (SCDC), Child Communication Checklist) 9 yr, Emotionality, Activity, Sociability temperament traits (EAS) temperament scale) 3 yr, repetitive behavior 5 yr. CM=Y |
Beneficial When the mother ate no seafood, the adjusted odds ratio (AOR) for poor social cognition was 1.63 [95% CI 1.02, 2.62] per SD of mercury (p= 0.041). This result was significantly different from the association among the offspring of seafood eaters (AOR = 0.74 [95% CI 0.41, 1.35]). |
9 yr | Not reported for this subgroup analysis. [Cohort characterized Hibbeln et al. 2007, Mean 8.3 SD 7.2 oz/wk) range (0 to 115 oz/wk) Outcomes not categorized by white/oily or by species. |
Mean 0.60 (SD 0.26) hair ppm | Increasing exposure to mercury did not increase risk of autism or autistic traits so long as mother ate seafood. When mother did not eat seafood, there was increased risk of poor social cognition. |
| 2017 Furlong et al. Prospective Cohort USA n=210 Moderate Risk of Bias |
Wechsler Intelligence Scales-IV (WISC-IV) and Behavior rating inventory of executive functioning (BRIEF). CM=Y |
Beneficial Children scored 7.71 higher points on the WISC perceptual reasoning factor of IQ p = 0.0422, 95%CI = 0.36, 15.06 (??=0.50, 95% CI 0.03, 0.97, SE = 3.752) per can of seafood (4 oz)/wk of maternal consumption.5 |
7–9 yr | Canned seafood consumption during pregnancy, per one can (4 oz/wk) Greatest benefit ≥ 8 oz/wk5 Outcomes not categorized by white/oily or by species. |
Not reported | Canned seafood consumption was associated with improved perceptual reasoning component of IQ, but results may have been due to testing multiple outcomes. |
| 2007 Hibbeln et al. Prospective Cohort United Kingdom n=8801 |
Denver Developmental Screening Test (DDST) Strength and Difficulties |
Beneficial In 9 of 23 outcomes the greatest risk of low outcomes, e.g. Greater risk of low verbal IQ was among offspring of |
DDST 6 mo 18 mo 30 mo 42 mo |
Mean 8.3 oz/wk; (SD 7.2 oz/wk) Range 0 to 115 oz/wk Greatest benefit, i.e., least risk of low outcomes, was with consumption ≥12 pz/wk (range |
Not reported in this subgroup analysis. [Characterized in Golding et al. (2017): |
Children were more likely to have a variety of neurocognitive benefits, including improved verbal IQ, |
|
Moderate Risk of Bias |
Questionnaire (SDQ) Weschler Intelligence Scale for Children III UK (WISC-III UK) CM=Y |
mothers consuming none vs. >12 oz/wk OR = 1·48, 95% CI 1·16–1·90 vs. > 0–12 oz/wk OR= 1·09, 95% CI 0·92–1·29; overall trend, p=0·004) |
SDQ 7 yr WISC-III 8y |
12–115 oz/wk) Outcomes not categorized by white/oily or by species. |
Median=0.52 hair ppm, range 0.07– 3.55 ppm. | when their mothers ate >12 oz/wk as compared to those eating <12 oz/wk. |
| 2018 Hibbeln et al. Prospective Cohort United Kingdom n=2224 Moderate Risk of Bias |
15 scholastic achievement tests including, reading, spelling, phoneme awareness, mathematics and science. CM=PN |
Null Each test score (except for arithmetic) was higher for children whose mothers ate seafood compared to children whose mothers ate no seafood, in unadjusted (but not in adjusted) analyses. |
7 yr 8 yr 9 yr |
Not reported for this subgroup analysis. [Cohort characterized Hibbeln et al. (2007), Mean 8.3 oz/wk (SD 7.2 oz/wk) range 0 to 115 oz/wk Outcomes not categorized by white/oily or by species. |
Not reported for this subgroup analysis. [characterized in Golding et al. (2017) Median =0.52 hair ppm (range 0.07–3.55) ppm] |
There was an indication of beneficial effects from seafood on scholastic tests but only in unadjusted analysis. There was no indication of any adverse effects of maternal mercury levels on scholastic abilities of the offspring whether the mother ate seafood or not. |
| 2017 Golding et al. Prospective Cohort United Kingdom n=4134 Moderate Risk of Bias |
Wechsler Intelligence Scale for Children (WISC-III UK) CM=Y |
Beneficial Total IQ 9.5 points higher in the highest decile of mercury, as compared to the lowest decile, among seafood eating mothers. Among mothers not eating seafood, full-scale IQ trended to decrease (but not statistically significant) with higher mercury. |
8 yr | Mean 8.3 oz/wk (SD 7.2) range (0 to 115] [Cohort characterized Hibbeln et al. 2007] Greatest benefit was assoc. with highest decile of mercury (0.94–3.55 ppm), among seafood consuming mothers. Among seafood-consuming mothers full-scale IQ increased +0.84 (IQ points): 95%CI +0.13, +1.56; with increasing mercury (per 1SD of mercury) p= 0.021) No differences found when comparing oily to white seafood. |
Median =0.52 hair ppm Range: (0.07– 3.55 ppm) Mercury exposures did not differ comparing mothers who ate seafood to those who did not. |
Among children of seafood eating mothers, those with the highest mercury levels (> 3.39 ppm) had 9.5 points higher IQ as compared to those wIth the lowest levels (<1.28 ppm) (table 2). Among offspring of seafood-consumers, increasing mercury, was assoc. with higher IQ in a dose response pattern that extended to the highest levels of mercury. In contrast, when women who did not eat seafood, mean IQ levels among offspring stayed roughly the same (although trended downward) as mercury increased. The maternal mercury levels were the essentially the same in both groups. |
| 2012 Sagiv et al. Prospective Cohort USA n = 515 Moderate Risk of Bias |
Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) for ADHD Neurobehavioral Evaluation System 2 (NES2) Continuous performance test (CPT). CM=Y |
Beneficial Higher seafood consumption (>8 vs. ≤8 oz/wk) was protective for adverse ADHD-related outcomes. Lower seafood consumption (≤8 vs. >8 oz/wk) was associated with greater risk of ADHD-diagnoses (e.g. impulsive/hyperactive [RR = 2.5; (95% CI,1.6– 5.0)]. |
8 yr | Mean 3.7oz/wk (3.9) Median 2.3 oz/wk (range 0.0–22.6) 52% of mothers consumed more than 8 oz/wk. Greatest benefit 8–22.6 oz/wk Outcomes not categorized by white/oily or by species. |
Mean 0.45 hair ppm (range, 0.03–5.14 ppm) Protective associations between higher mercury and better CPT reaction times were found in girls (whose mother had <1 ppm). |
Maternal seafood consumption > 8 oz/wk was associated with neurocognitive benefits for ADHD-related outcomes as compared to <8 oz/wk. Seafood was protective despite adverse effects of mercury, (comparing ≥ 1ppm to < 1ppm. |
| 2013 Deroma et al. Prospective Cohort Northern Italy n=242 Serious Risk of Bias |
Weschler Intelligence Scale for Children-III (Verbal and Full IQ, (WISC-III) CM=N |
Null Neither maternal consumption of canned nor fresh seafood were significantly associated with full scale, verbal or performance IQ. |
7.7 yr | Mean 3.2 oz/wk (SD and range not reported) Categorized by fresh or canned. Canned seafood trended toward negative associations with all the outcome variables but were not statistically significant in all adjusted analyses. |
Mean 1.33 hair ppm, Median 0.93 ppm (range 0.06–8.03) (area contaminated by mercury spills) |
Although maternal seafood and mercury were positively correlated, the effects of mercury and seafood on neurological outcomes trended in opposite directions (seafood beneficial, mercury adverse). |
| 2016 Oken et al. Prospective Cohort USA n=1068 Moderate Risk of Bias |
Kauffman Brief Intelligence Test (KBIT) WRAVMA drawing, WRAML design memory, WRAML picture memory, and WRAML summary score). CM=PY |
Null Maternal seafood intake examined continuously and categorized as 0, 0–12, and ≥12 oz/wk. |
7.7 yr | Mean 6.8 oz/wk SD 6.0 range (0–48 oz/wk) Not assoc. with benefit or harm Outcomes not categorized by white/oily or by species. |
Erythrocyte mercury mean 4.0 (SD 3.6) ppb range (0, 38.2) ppb (not convertible to hair ppm) | This study reported no benefit from seafood or harm from mercury, up to 48 oz/wk and 38.2 ppb (erythrocyte) on an abbreviated test of IQ and other tests. Exceeding 12 oz/wk of seafood was not associated with harm, despite reported exposure to mercury. |
| 2016 Steenweg-de Graaff Prospective Cohort n=3,802 Netherlands Serious Risk of Bias |
Autistic Traits: The Social Responsiveness Scale (SRS) and Child Behavior Checklist, Pervasive Developmental Problems subscale (PDS); and Non-verbal IQ: 2 subtests of “Snijders-Oomen Niet-verbale Intelligentietest–Revisie” (SON-R 2½–7) CM=N |
Null No relationship between maternal seafood consumption and SRC, PDS and non-verbal IQ scores (SON-R 2½–7). |
6 yr | Median 2.65 oz/wk (range 0–21.2) Reported in Heppe et al 2011 (PMID: 21266095) Outcomes not categorized by white/oily or by species. |
Not reported | Seafood consumption was measured against only non-verbal components of IQ, (verbal IQ not measured) with null results for performance components. Uncertain if critical confounding variables were assessed. |
| 2018 Vejrup et al. Prospective Cohort Norway n=38,581 Moderate Risk of Bias |
Speech and Language Assessment Scale (SLAS) Ages and Stages Questionnaire (ASQ), and Twenty Statements about Language-Related Difficulties (language 20). CM=Y |
Beneficial Positive associations for mothers consuming > 14.1 oz/wk vs. 0–3.5 oz/wk for all child outcomes (SLAS, ASQ, language-20) in adjusted analyses. |
5 yr | Median 7.6 oz/ wk (range 0–65 oz/wk). Greatest benefits (14.1–65 oz/wk) (mean not reported) Outcomes not categorized by white/oily or by species. |
Mean 0.29 hair ppm (range 0–3.8 ppm), (n=2,239) Increased dietary mercury exposure was associated with improved SLAS scores when mothers had a seafood intake ≤14.1 oz/wk in the adjusted analysis. Mercury exposure was null, however, in the group >14.1 oz/wk (n=210). |
Maternal seafood consumption was beneficially assoc. with offspring language and communication skills. |
| 2016 Julvez et al. Prospective Cohort Spain n=1,892 (14 mo) n=1,589 (5 yr) Moderate Risk of Bias |
Bailey Scales of Infant Development (BSID) McCarthy Scales of Children’s Abilities (MSCA) Childhood Asperger Syndrome Test (CAST) CM=PY |
Beneficial Julvez et al. divided their cohort into quartiles for fatty seafood and quintiles for all seafood. The results for all seafood are in the 5th column. Offspring of mothers within the highest quartile for consumption of large fatty seafood (>10 oz/wk) had an adjusted increase of 2.29 points in McCarthy general cognitive score (95% CI: 0.42, 4.16). For MSCA outcomes, large fatty seafood (but not other subtypes), showed beneficial relationships across all quartiles. For CAST outcomes, large fatty seafood had beneficial relationships in quartile 4, lean seafood in quartiles, 2,3 and 5 and shellfish in quartile 3. |
14 mo 5 yr |
Mean 17.5 oz/wk Median 16.6 oz/wk Quintile means (all seafood) Q1, 6.9 oz/wk Q2, 11.9 oz/wk Q3, 16.2 oz/wk Q4, 21.2 oz/wk Q5, 30.2 oz/wk For all seafood, maximum benefits for BSID, CAST, MSCA total score, verbal and memory were in Q4 (mean 21.2 oz/wk); and MSCA executive function, and motor were in Q3/Q4 followed by an attenuation of a positive association in Q 5 (mean 30.2 oz/wk, but still trending beneficial). |
(Mercury exposures were as reported in Llop et al., 2012) Definitions as provided by the authors: 1) large fatty seafood, (such as tuna, swordfish, albacore) 2) smaller fatty seafood, (such as mackerel, sardines, anchovies, salmon” and “tinned sardines/mackerel”) 3) lean seafood, (such as hake, sole, or bream”; and “tinned tuna,” which has similar levels of DHA and mercury as lean seafood) 4) shellfish, (“such as shrimp, prawns, lobster, or crab”; “clams, mussels, oysters”; and “squid, octopus, cuttlefish”) |
Beneficial associations remained positive through >30.2 oz/wk of maternal seafood consumption for child neurodevelopment, among a population characterized by high seafood consumption. Intake of small fatty seafood was a predominant predictor of neurocognitive development at 14 mo. and lean and large fatty seafood were predominant predictors of neurocognitive benefits at 5 yrs. Lower risk of autism-spectrum traits were also observed with total, lean, and large fatty seafood consumption. |
| 2008 Lederman et al. Prospective Cohort USA n=329 Moderate Risk of Bias |
i) Psychomotor development index, (BSID-II) ii) Weschler Intelligence Scale for Children-III (Verbal and Full IQ, (WISC-III) CM=Y |
i) Beneficial 8.7 point increase, any vs. no seafood ii) Beneficial 5.6 point increase, any vs. no seafood, on verbal and full IQ |
i) 3yr ii) 4yr |
Amounts not reported Groups were defined as any vs. no consumption Greatest benefit - any Outcomes not categorized by white/oily or by species. |
All subjects Mean=0.64 ppm range (0.002–4.4) Seafood eaters Mean=0.72 ppm (range -unknown) |
Maternal seafood consumption was beneficially assoc. with offspring psychomotor and IQ test scores despite the reported exposures to mercury. The IQ benefit was for verbal and full IQ but not for performance IQ. Conversely, when measured independent from seafood, mercury exposures were adversely associated with scores on the same tests. |
| 2008 Mendez et al. Prospective Cohort Menorca, Spain n=392 Moderate Risk of Bias |
McCarthy Scales of Children’s Abilities (MCSA) CM=PY |
Beneficial Among children breast-fed for 6 mo., maternal seafood intakes of 8–12 oz/wk was associated with significantly higher scores of 5.9 to 8.6 points on MCSA subscales compared ≤ 4 oz/wk. |
4 yr | Mean= 6.76 oz/wk (SD 6) (range not reported) Greatest benefits 8–12 oz/wk. No benefits or adverse effects for ≥12 oz /wk [Mean= 23.12 oz/wk (SD 15.44)] Outcomes differentiated by seafood and “other” seafood with lower DHA content (squid and shellfish). |
Not reported | Beneficial effects of seafood consumption other than shellfish and squid were apparent among women breastfeeding <6 mo. These benefits were not seen >12 oz/wk but there were only 20 individuals in that category. |
| 2001 Williams et al. Prospective Cohort United Kingdom n=435 Moderate Risk of Bias |
High-grade stereopsis, (stereoscopic vision) CM= PY |
Beneficial Children whose mothers ate oily seafood achieved high-grade stereopsis sooner than those whose mothers did not (adj OR: 1.57; 95% CI: 1.00, 2.45). |
3.5 yr | Any seafood consumption (<2 oz/ wk or ≥2 oz/wk) (range not reported) Greatest benefit ≥2 oz/wk oily seafood Oily fish - beneficial White fish - null Shellfish - null |
Not reported | Children whose mothers ate oily seafood were 57% more likely to achieve high-grade stereopsis by age 3.5 yr. |
| 2008 Oken et al. Prospective Cohort USA n=341 Moderate Risk of Bias |
Wide Range Assessment of Visual Motor Abilities (WRAVMA) Peabody Picture Vocabulary Test (PPVT) CM=PY |
Beneficial WRAVMA drawing β=6.4, (95 % CI: 2.1, 10.7) WRAVMA total β=6.4, (95 % CI: 2.0, 10.8) >8 oz/wk vs. none PPVT-no association |
3 yr | Mean 6.0 (5.6) oz/wk range, 0–30) oz/wk Canned tuna Beneficial outcomes seen at ≥8 oz/wk (range, >8–30 oz/wk) on the WRAVMA for canned tuna and for all seafood as follows: Compared to eating no canned tuna, mothers eating canned tuna ≥ 8 oz/wk had children with higher scores on the WRAVMA (total 5.6, 95% CI: 1.4, 9.8). Seafood ≥8 oz/wk of all seafood with mercury below the 90th percentile was beneficial on the WRAVMA. Outcome for ≥8 oz/wk with mercury above the 90th percentile trended somewhat less beneficial, but those results was not statistically significant. Outcomes comparing >8 oz/wk vs. <8 oz/wk of seafood other than canned tuna were null. Outcomes categorized by canned tuna, seafood other than canned tuna and all seafood. |
Mean 0.53 hair ppm (SD, 0.47) range (0–2.3) ppm n=98 |
This study reported a beneficial effect from canned tuna on one test. It also attempted to measure beneficial effects from nutrients and adverse effects from mercury acting simultaneously for all seafood. Most of the results were not statistically significant, but trended toward greater than ≥ 8 oz/wk with less mercury being more beneficial than ≥ 8 oz/wk with more mercury and both being more beneficial than <8 oz/wk. None of the results <8 were statistically significant. |
| 2008 Davidson et al. Prospective Cohort Republic of the Seychelles n=229 Moderate Risk of Bias |
Bayley Scales Infant Development-II (BSID-II) Mental Developmental Index (MDI) Psychomotor Developmental Index (PDI) CM=PY |
Null Maternal seafood not associated with 16 outcomes; higher mercury associated with adverse scores on one outcome (PDI), but not in models adjusting for dietary nutrient intakes. |
5.9 mo 30 mo |
Mean 36 oz/wk Outcomes not categorized by white/oily or by species. |
Mean 5.7 ppm (SD 3.7) (range 0.2–8.5) |
No beneficial or adverse effects from high levels of seafood consumption and high levels of mercury exposure (mean of 5.7 ppm is between 99.5th and 99.9th percentiles of U.S. exposure). It has been hypothesized that beneficial effects were not consistently seen in this study because the high consumption effectively saturated the participants so no further gains could be seen by the researchers. |
| 2010 Lynch et al. Prospective Cohort Republic of the Seychelles n=229 Moderate Risk of Bias |
Bayley Scales Infant Development-II (BSID-II) Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI) CM=PY |
Beneficial A beneficial relationship was reported between docosahexaenoic acid (DHA) and the PDI outcome at 30 mo. modified by increasing mercury levels such that at 11 ppm exposure, the beneficial effect of DHA was eliminated. For MDI at 30 mo and PDI at 9 mo the relationship to DHA was similarly modified at 9 ppm, trending negative. |
9 mo 30 mo |
Mean 36 oz/wk Here, DHA was considered as a biomarker of seafood consumption. Outcomes not categorized by white/oily or by species. |
Mean 5.7 ppm (SD 3.7) (range 0.2–8.5) (previously reported in Davidson et al. 2008) |
DHA had beneficial effects on the BSID-II PDI that were reduced or eliminate at higher mercury exposures (past 11 ppm maternal hair). The change occurred at mercury exposure levels that were nearly twice the 99.9th percentiles of exposure for U.S. women of childbearing age. |
| 2013 Valent et al. Prospective Cohort Italy n=606 Moderate Risk of Bias |
Bailey Scale of Infant Development (BSID-III) including cognitive, language, motor, social-emotional, and adaptive functioning subscales CM=PN |
Beneficial Positive associations between maternal seafood intake and social-emotional scores in a fully adjusted model that included mercury in cord blood (β = 1.84, p = 0.03). |
18 mo | Mean 9.3 oz/wk (SD 6.8) (range 0–44 oz/wk). Greatest benefit up to 44 oz/wk Outcomes not categorized by white/oily or by species. |
Arithmetic mean 1.061 ppm (SD 1.028) range (0.017–13.52) ppm |
Maternal seafood intake beneficially associated with social-emotional scores but not with the others. No association between maternal hair mercury and any BSID-III scores. |
| 2008 Oken et al Prospective Cohort Denmark n=25,446 Moderate Risk of Bias |
Developmental milestone scores including motor social or cognitive development. CM=Y |
Beneficial OR 1.29 (95% CI; 1.20, 1.38) comparing Highest quintile to (14 oz/wk, range 9.7–121) the lowest quartile (1.3 oz/wk, range: 0–2.6) |
6 mo 18 mo |
Mean = 6.6 oz/wk. (range: 0–121) Dose response benefits were continuous through to 121 oz/wk. For each additional 4 oz/ wk of seafood, the OR for higher development was 1.49 (95% CI: 1.33, 1.66) Outcomes not categorized by white/oily or by species. |
Not reported | The magnitude of improvement in neurocognition was similar comparing breastfeeding >10 mo. to mothers consuming seafood >12 oz/wk. The highest quintile of seafood consumption in this cohort (mean of 14.5 oz/wk, range of 9.75 to 121 oz/wk) was associated with better attainment of developmental milestones at 18 months of age as compared to the lowest quintile of consumption (mean of 1.3 oz/wk, range of 0 – 2.6 oz/wk. |
| 2019 Barbone Prospective Cohort Italy, Slovenia, Croatia, and Greece n=1086 Moderate Risk of Bias |
Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III). CM=Y |
Beneficial Beneficial associations between increasing mercury in maternal hair and lower risk of suboptimal language scores (β=0.55; 95%CI: 0.05–1.05) and receptive communication scores (β=0.12; 95%CI: 0.02–0.22). |
18 mo | All seafood 5.6 (SD 4.8) oz/wk (range not reported) Range not provided [if this is true] |
Mean 0.997 ppm (SD 1.035) (range 0.017–13.52) |
Although seafood consumption was measured, this study measured the associations between maternal mercury and neurocognition. Higher mercury was associated with better language and receptive communication scores in linear models across the range of mercury levels. |
| 2004 Daniels et al. n=7,421 United Kingdom Prospective Cohort Moderate Risk Bias |
MacArthur Communicative Development Inventory (MCDI) Denver Developmental Screening Test (DDST) CM=PY |
Beneficial For children whose mothers consumed ≥18 oz/wk, the adjusted MCDI score was higher,72 (95% CI; 71–74), as compared to when mothers ate no seafood 68 (95% CI; 66 – 71). The DDST total was 2% higher among children whose mothers ate seafood 4.5–13.5 oz/wk as compared to none. |
15 mo 18 mo |
Greatest benefits at 4.5–13.5 oz/wk for vocab. comprehension (MCDI) and total DDSI, benefits continue through ≥18 oz/wk. Greatest benefit ≥18 oz/wk for social activity (MCDI) No difference when comparing outcomes to consumption of white seafood and to oily seafood |
Total cord mercury (geometric mean ng/g 0.01 SD (0.4) (not convertible to hair ppm) | Maternal seafood consumption was beneficially assoc. with offspring test scores, despite quantified mercury exposure. |
| 2016 Hu et al. Prospective Cohort China n=410 Moderate Risk of Bias |
Gesell developmental schedules (GDS) CM=PY |
Beneficial Greater seafood consumption was assoc. with better developmental quotient (DQ) scores of the adaptive domain (r=0.11, p =0.04). At the same time, a log-unit increase in umbilical blood mercury levels was associated with a 4.22-point (95 %CI 0.77 to 7.67) increase in the adaptive domain and a 4.06-point (95 %CI 0.51 to 7.62) increase in the social domain. |
1 yr | Mean 3.5 oz/wk (est. from frequencies ≥ 4 oz/wk = 32.7% > 2 oz/wk < 4 oz/wk =39.5% ≤ 2 oz/wk = 27.8%) Greatest benefit unknown Outcomes not categorized by white/oily or by species. |
Geomean 0.20 hair ppm (range <LOD-0.74) A-mean 0.23 hair ppm (SD 0.11) |
Both maternal seafood consumption and maternal mercury were associated with neurocognitive benefits. The authors attributed the positive associations to the beneficial nutrients in seafood despite presence of mercury from seafood. |
| 2005 Oken et al. Prospective Cohort US n=135 Moderate Risk of Bias |
Visual recognition memory (VRM) CM=PY |
Beneficial For each additional weekly seafood serving (4 oz), offspring VRM score was 4.0 points higher [95% CI), 1.3 to 6.7] |
6 mo | Mean = 4.8 oz/wk (range, 0–22) Dose response benefits were continuous through to 22 oz/wk. Outcomes not categorized by white/oily or by species. |
Mean 0.55 ppm, Range (0.02–2.38) Geometric mean 0.45 ppm with 10% of samples > 1.2 ppm |
Maternal seafood consumption was beneficially assoc. higher with offspring test scores while mercury was independently associated with lower test scores. The gain per each additional serving of seafood was greater than the reduction per each additional serving from mercury. |
| 2016 Xu et al. Prospective Cohort USA n=344 Moderate Risk of Bias |
NICU Network Neurobehavioral Scale (NNNS), CM=PN |
Beneficial Greater seafood consumption was associated with less need for special handling (β = −0.0027, SE = 0.0009, p = 0.002) and among girls, higher asymmetry scores (β=0.007, SE=0.003, p=0.02). |
5 wk | Median: 1.3 oz/wk (total of 52 oz per woman during entire pregnancy (interquartile range: 6–17). Outcomes not categorized by white/oily or by species. |
Geomean 0.18 hair ppm (95% CI; 0.16–0.21) (range 0.004–1.78) ppm Greater mercury associated with less need for special handing. |
Mothers had very low levels of seafood consumption. Nonetheless, infants whose mothers consumed more seafood had better attention and needed less special handling. Seafood was not associated with harm, despite exposure to mercury. |
| 2010 Suzuki et al. Prospective Cohort Japan n=498 Moderate Risk of Bias |
Neonatal Behavioral Assessment Scale (NBAS) CM=PN |
Beneficial Total seafood intake assoc. with beneficial motor scores R2=0.102, p<0.05, in one but not all adjusted models. (Continuous) |
3 days | Mean 12.6 (SD 8.6) oz/wk Range (0.01–77.2) oz/wk Beneficial up to 77.2 oz/wk Outcomes not categorized by white/oily or by species. |
Median 1.96 Mean 2.22 (SD 1.16) (range 0.29–9.35) |
Maternal seafood consumption was associated with beneficial outcomes while mercury was independently associated with adverse outcomes. Per the authors, the data suggested that prenatal mercury adversely affects neonatal neurobehavioral function while maternal seafood intake appears to be beneficial. |
| 2011 Davidson et al. Prospective Cohort Republic of the Seychelles n= 462 Moderate Risk of Bias |
California Verbal Learning Test (CVLT), Wisconsin Card Sorting Test (WCST), the Woodcock-Johnson (W-J-II) Achievement Test, Subtests of the Cambridge Neuropsychological Test Automated Battery (CANTAB), and measures of problematic behaviors. CM=Y |
Beneficial Increasing prenatal mercury was null for 21 endpoints but associated with better scores on four endpoints (higher W-J-II math calculation scores, reduced numbers of trials on the Intra-Extradimensional Shift Set of the CANTAB), fewer reports of substance use and incidents of and referrals for problematic behaviors in school. However, increasing prenatal mercury was adversely associated with one level of referrals to a school counselor. |
17 yr | Mean 48 oz/wk (as previously reported. Shamlaye, et al. 1995) Outcomes not categorized by white/oily or by species. |
Mean 6.9 ppm (SD 4.4) (range 0.54–22.74) Relationships to beneficial neurocognitive outcomes reported through the highest levels of mercury, in linear models. |
Although seafood consumption was measured, the results were in terms of mercury associations with test scores. We include this study because it is the only study that reported outcomes out to 17 years of age. The oldest in other studies was nine years of age. Ocean seafood appeared to benefit neurocognitive development despite contributing an increasing exposure to mercury. Since mercury has no known benefit to neurocognitive development, the mercury in this study was likely to reflect the seafood consumed in this cohort. |
| 2016 Llop et al. Prospective Cohort Spain n=1362 Moderate Risk of Bias |
McCarthy Scales of Children’s Abilities (MSCA). CM=PY |
Beneficial A doubling in mercury was associated with higher scores in most of the MSCA scales (β=1.29; 95% CI 0.28–2.31 general cognitive scale). |
4–5 yr | Categories of seafood consumption oz/wk <12 12–20 >20–32 Greatest benefits >32 |
and corresponding Hair-Hg ppm 0.98 (0.24–1.2) 1.44 (1.32–1.56) 1.89 (1.76–1.98) 2.04 (1.9–2.2) |
Statistically significant beneficial effects started to emerge at ≥ 12 oz/wk (MSCA verbal, numerical and memory), which was roughly at or above the EPA Reference Dose for mercury in this cohort. |
RoB NOS scale. Summary and number of bias domains rated as low/ moderate/serious
Clinically meaningful (CM) Y= Yes, PY= Probably Yes, PY= Probably No, N=No, NI= not enough information
Standardization from blood to hair mercury concentration as per the Net Effects Assessment 2014 (Table V3 p. 92) mercury in maternal blood (ug/L) = 3.59*[10] hair mercury (ppm). The cord blood mercury concentrations were standardized to maternal hair using data from Grandjean et al. (1992) [12]; cord blood mercury nmol/L = 5.0* mercury maternal hair (nmol/g) [12]
one seafood meal is estimated to be 4 oz across all studies, unless otherwise defined by the study.
data from corresponding author
Table 2.
Seafood consumption during childhood and adolescence (up to 18 years of age) and neurocognitive development.
| Year, Author Study Design Location Number children/adolescents Risk of Bias1 |
Neurocognitive outcomes Clinically meaningful?2 |
Beneficial/adverse/null and Size of effects (as compared to: e.g. no/highest seafood, or continuous) |
Child’s age at consumption and at effect | Amount of seafood consumed -Mean+ SD (oz/wk) - (range) - Amount assoc. with largest beneficial or adverse effect - Outcomes categorized by seafood type? (e.g. oily/ white/species) |
Mercury exposure (if provided, standardized to hair, ppm3) | Comments: |
|---|---|---|---|---|---|---|
| 2009 Kim et al. Prosp. cohort Sweden n=9,448 Moderate Risk of Bias |
Total school grades (sum of grades in 16 subjects, max of 320 total grades. CM=Y |
Beneficial Mean of 225.5 total grades (SD 58.3) when consumption was > one meal/wk vs. Mean of 196.6 (SD 63.4) when consumption was <one meal/wk |
15 – 16 yr | - > one meal/wk = >4 oz/wk4 Amounts measured. ≥4 oz/wk1 = 4 oz/wk ≤4 oz/wk Greatest benefit ≥ 4 oz/wk Outcomes not categorized by white/oily or by species. |
Not reported | Seafood consumption of >one meal/wk at 15 yr. was associated with the higher academic grades at 16 yr. |
| 2008 Åberg et al. Prosp Cohort Sweden n=4,792 Moderate Risk of Bias |
Standardized intelligence tests for the Swedish Military. CM = Y |
Beneficial Seafood consumption ≥4 oz/wk vs. <4 oz/wk was associated with higher combined intelligence per stanine (0.58 units; 95% CI 0.39, 0.76), verbal performance (0.45; 95% CI 0.27, 0.63) and visuospatial performance (0.50; 95% CI 0.31, 0.69). |
15 – 18 yr. | Mean and SD not reported ≥4 oz/wk (20.2%) vs. 4 oz/wk (56.6%) vs. <4 oz/wk (22.7%) Greatest benefit at ≥ 4 oz/wk Outcomes not categorized by white/oily or by species. |
Not reported | In this study of adolescent males there was a beneficial association between number of seafood meals per week at age 15 and intelligence test performance at age 18. |
| 2017 Liu et al. Prospective Cohort China n=541 Moderate Risk of Bias |
Wechsler Intelligence Scale for Children-Revised (WISC-R) CM=Y |
Beneficial Improvement comparing ≥ 4 oz/wk vs. < 2 oz/wk Verbal IQ 4.75 pts improvement p< 0.002 Cohens d= 0.6 Performance IQ 3.39 pts improvement p<0.026 Cohens d= 0.416 Full scale improvement 4.80 pts, p<0.003 Cohens d=0.57 |
Seafood consumption at 9–11 yr. IQ at 12 yr. |
Mean and SD not reported Greatest benefit at ≥ 4 oz/wk Outcomes not categorized by white/oily or by species. |
Not reported | Greater seafood consumption was associated with higher IQ and fewer sleep disturbance problems. Effects of seafood on IQ appeared to be mediated by sleep quality. Children with fewer sleep disturbance problems were more likely to have higher cognitive function. |
| 2004 Daniels et al. Prospective cohort UK n= 7421 Moderate Risk of Bias |
MacArthur Communicative Development Inventory (MCDI) Denver Developmental Screening Test (DDST) CM=PN |
Beneficial All mean MCDI scores were slightly higher among children who ate seafood at least once per week at 6 months and at 12 months of age. Relations between the infants’ seafood intake and DDST scores followed a similar pattern but were of smaller magnitude. |
Consumption at 6 & 12 mo. Testing at 15 mo on the MCDI and at 18 mo on the DDST |
. Comparison of rarely/ never to >1 meal (4 oz)/wk. Outcomes not categorized by white/oily or by species. |
Not reported | Positive associations with seafood were consistent but small in magnitude. |
| Attention Deficit Hyperactivity Disorder (ADHD) outcomes | ||||||
| 2017 Rios-Hernández Case control Madrid, Spain n=120 Moderate Risk of Bias |
Diagnostic and Statistical Manual of Mental Disorders, (ADHD RS-IV) Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-PL) |
Beneficial Fatty seafood consumption was significantly greater in healthy children than in children with ADHD. (tertiles of intakes) Highest (reference) Middle OR 1.84 (95 % CI 0.75–4.49) Lowest OR 2.50 (95 % CI 1.02–6.15) P for linear trend .046 |
Consumption and determining what was being consumed occurred among children who were 6–16 yr | Mean and SD not reported (amounts in each tertile not reported) Greatest benefit from fatty seafood ≥ 8–12 oz/wk Fatty seafood (blue fish) vs. white seafood. |
Not reported | This study compared adherence to a “Mediterranean diet” that included fatty seafood by children diagnosed with ADHD to healthy children. Fatty seafood was the only food that appeared to be protective for ADHD within the Mediterranean dietary pattern. A source of uncertainty is that the findings may be due to differing dietary choices among children with ADHD rather than due to a cause of the ADHD. |
| 2016 Zhou et al. Case control China n=592 Moderate Risk of Bias |
Diagnostic and Statistical Manual of Mental Disorders, 4th ed., revised (DSM-IV-R). Criteria for ADHD CM=Y |
Beneficial Highest tertial (Ref) 1.00 Mid. OR =1.6 (95 % CI 0.94–2.6) Low OR= 2.3 (95 % CI 1.36–3.70) p< 0.006 |
6–14 yr | Mean and SD not reported The “fish-white meat” dietary pattern was rich in shellfish, deep water seafood, white meat, freshwater seafood, organ meat and fungi and algae. Outcomes not categorized by white/oily or by species. |
Not reported | This study compared dietary patterns of children with and without ADHD. The “fish-white meat” was the only dietary pattern assoc. with lower risk of AHDH. A source of uncertainty is that the findings may be due to dietary choices among children with ADHD rather than due to a cause of ADHD. |
| 2014 Woo et al. Case control Korea n=192 Moderate Risk of Bias |
Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) for ADHD CM=Y |
Beneficial Children with the lowest adherence to a “traditional-healthy dietary pattern” that included seafood had greater risk of ADHD diagnosis OR= 3.2 (95% CI: −0.83–1.26) than children with the greatest adherence to the “traditional-healthy pattern.” |
7–12 yr | Mean and SD not reported Outcomes were categorized by fatty (i.e., oily) fish and bone fish. |
Not reported | Fatty and bone fish were predominant characteristics of the “traditional-healthy” dietary pattern. A source of uncertainty is that the findings may be due to differing dietary choices among children with ADHD rather than due a cause of ADHD. |
| 2018 San Mauro Martin et al. Case control Spain n=89 Moderate Risk of Bias |
Diagnostic and Statistical Manual of Mental Disorders, (ADHD RS-IV) CM=PN |
Beneficial Lower adherence to a Mediterranean diet containing seafood was associated with a greater likelihood of an ADHD diagnosis. 95% of children without ADHD regularly consumed ≥8–12 oz/wk of seafood while 78% of children with ADHD regularly consumed >8–12 oz/wk of seafood (p=.003) Regular seafood consumption (≥8–12 /wk); Cases 78 % vs. controls 95% p< 0.003 |
9–10 yr | Mean and SD not reported ≥8–12 oz/wk vs. <8–12 oz/wk Greatest benefit ≥8–12 oz/wk Outcomes not categorized by white/oily or by species. |
Not reported | Within the Mediterranean dietary pattern regular and greater seafood consumption appeared to be protective for ADHD. A source of uncertainty is that the findings may be due to differing dietary choices among children with ADHD rather than that due to a cause of ADHD. |
| 2010 Hertz-Picciotto et al. Case Control USA n=452 Serious Risk of Bias |
Risk of autism/autism spectrum disorder (AU/ASD) vs. Typical development (TD) vs. Delayed development CM = Y |
Beneficial TD children were more likely to consume seafood (TD vs. AU/ASD) any seafood (76% vs. 43%) tuna (44% vs. 18 %) ocean (58% vs. 36%) freshwater (20% vs. 6%) all (p < 0.0001) |
2–5y for both | Comparison of any seafood vs. no seafood, Not able to determine amounts Outcomes categorized by “any fish,” “ocean fish,” “tuna,” and “freshwater fish”. |
TD= 0.17 SD 0.29 ppm AU/SUD = mean 0.14 SD 0.30 ppm p=ns |
Sources of uncertainty included: Differences between groups were not controlled for confounders; and Findings may be due to differing dietary choices among children with AU/ASD rather than due to a cause of AU/ASD |
| Randomized controlled trials | ||||||
| 2018 Øyen et al. RCT Norway n=232 Moderate Risk of Bias |
Wechsler Preschool and Primary Scale of Intelligence, 3rd edition (WPPSI-III) and The 9-Hole Peg Test (9-HPT) CM=Y |
Beneficial In the fully adjusted analysis, (including compliance, i.e., whether the children fully ate the meals provided to them), mean WPPSI-III total score improved more in the seafood (20.4, 95% CI 17.5–23.3) than in the meat group (15.2, 95% CI 12.4–18.0, p = 0.006) The seafood group gained 1.2 more IQ points per 3.5 oz of food eaten than the meat group (p < 0.0001) |
FINS-KIDS study |
Mean of 44.0 (SD 4.0) total study meals (5.4–8.5 oz/wk) were provided over 16 wks. 3 hot lunches/wk for 16 wk Each meal contained 1.8–2.8 oz fatty seafood (herring/mackerel) or meat (chicken/lamb/beef) |
Reported in Kvestad et al. (2018) Seafood group Baseline: mean 0.373 (SD 0.211) ppm |
WPPSI-III total scores and processing speeds over 16 wks, assigned to consume 3 fatty seafood lunches/wk as compared to 3 meat lunches/wk. |
| 2018 Hysing et al. RCT Norway n=232 Moderate Risk of Bias |
Strengths and Difficulties Questionnaire (SDQ) Sleep by parental questionnaire CM=N |
Null No impact comparing assignment to seafood or meat lunches on SDQ scores or sleep. |
4–6 yr | Children in the seafood group had an increase in exposure to mercury (change of +0.162, 95% CI 0.111, 0.213 ppm), whereas children in the meat group had decreased exposure to mercury (change of −0.053, 95% CI −0.103, −0.002 ppm). | Post: mean 0.529 (SD 0.259) ppm p<0.001 Meat group Baseline: Mean 0.374 (SD 0.198) ppm Post: Mean 0.315 (SD 0.182) ppm <0.001 |
No evidence of improvement in mental health measures on the SDQ or for sleep. |
| 2018 Kvestad et al. RCT Norway n=232 Moderate Risk of Bbias |
Wechsler Preschool and Primary Scale of Intelligence -III (WPPSI-III) CM=Y |
Beneficial After adjusting for mercury levels, assignment to consume 3 fatty seafood meals/wk improved total scores 164.5 (95% CI; 160.9– 168.1) as compared to assignment to meat lunches. 159.0 (95% CI; 155.6, 162.4) p<0.008 There were no notable associations between mercury and the WPPSI-III raw scores at baseline or after 16 weeks of the fish/meat. |
Fatty seafood lunches increased WPPSI-III total scores in comparison to meat lunches. The fatty lunches increased mercury exposures without harm being detected. | |||
| 2017 Handeland et al. RCT Norway n=426 Moderate Risk of Bias |
Attention performance (d2 test of attention) CM=PN |
Beneficial Improvement in processing speed was significantly less in the meat (−11.8; 95% CI: −23.3, −0.4) and supplement (−13.4; 95% CI: −24.9, −1.8) group compared to the seafood group (reference). The supplement group showed less improvement in total performance (−10.4; 95% CI: −20.0, −0.7) compared to the food group (reference). |
FINS-TEENS Study 14–15yr |
Randomized to receive for 12 weeks: Fatty seafood; 9.6 oz/wk or Meat; 9.6 oz/wk or Capsules; 5.7 gm/wk of omega-3 HUFA (estimated to be the same amount of omega-3 highly unsaturated fatty acids (HUFAs) in the fatty seafood group) |
Not reported | A small beneficial effect on attention from lunches of fatty seafood, compared to meat and supplements on processing speed. No evidence of improvement in mental health measures on the SDQ. But not sufficiently powered (by author report). A significant source of uncertainty is low dietary compliance. Children consuming at least half of the meals/capsules: 38% seafood, 56% meat, 87% capsules. |
| 2017 Skotheim et al. RCT Norway n=425 Moderate Risk of Bias FINS-TEENS study |
Strengths and Difficulties Questionnaire (SDQ) CM=N |
Null No significant differences among the seafood, meat, and capsule groups. Per author report “The results should be seen as preliminary however as the dietary compliance in the seafood group was low and the analyses in the high score group (high SDQ) were under powered”. |
||||
| 2015 Sørensen et al. RCT Denmark n=726 Moderate Risk of Bias |
Cognitive performance, the d2-test of attention and Danish standard tests in reading and math. CM=Y |
Beneficial The intervention improved ‘school performance’ (p =0·015), ‘reading comprehension’ (p =0·043). The dose–response relationship suggested that approximately 20 % of the intervention effect on ‘school performance’ could be related to the increase in omega-3 fatty acid status. Difficult to determine if clinically meaningful, size of effect obscure. |
10 yr | Seafood served 2x/wk, in school lunches 1 fatty and 2 lean seafood types/ 3 wk menu Among those eating seafood the median fish intake was 1.7 oz/wk (95 % CI 1.1, 12.2; P< 0·001) higher during the intervention period and the proportion of children eating seafood was higher in the intervention period than in the control period (91 v. 73%; P< 0·001), Outcomes not categorized by white/oily or by species. |
Not reported | Effects not solely attributable to seafood intake. However, assoc. of increases in a biomarker of seafood intake (EPA+ DHA) with better cognitive performance give greater confidence in a contribution from seafood. |
RoB NOS scale. Number of bias domains rated low/ moderate/serious
Clinically meaningful (CM) Y= Yes, PY= Probably Yes, PY= Probably NO, N=NO, NI= not enough information
Standardization from blood to hair mercury concentration as per the Net Effects Assessment 2014 (Table V3 p. 92) [10]
one seafood meal is estimated to be 4 oz across all studies, unless otherwise defined by the study.
Question #40, What is the relationship between maternal seafood consumption during pregnancy and lactation and the neurocognitive development of the infant?
A total of 29 studies were identified for this question [13–41]. They represent 24 unique cohorts that met the criteria for inclusion and did not meet criteria for exclusion for this question. Of these 29 studies, seven were conducted in the United States [15, 20, 22, 27, 32, 34, 37]; seven in the United Kingdom [13, 14, 17, 19, 35, 38, 39]; three in Spain [23, 31, 36]; two in Italy [28, 29] three in the Republic of the Seychelles [18, 25, 26]; and one each in Denmark [21], Norway [40], the Netherlands [33], China [30], Japan [24], the Faroe Islands [16] and another in a consortium of European countries [41]. The sample sizes ranged from 135 [15] to 38,581 mother-child pairs [40] with a median sample size of 498 mother child pairs [24].
Overall, the study subjects were healthy and had access to health care. Most of the studies included women between 20 and 40 years of age, with an average age of 29.3 yrs., although many studies included adolescent pregnancies. Fifteen studies described race/ethnicity with an average of 75.5% of the individuals in those cohorts reported being white (range 0–100%). In China [30] and Japan [24] 100% were Asian. Twenty-three studies described maternal education with an average of 58.7% having finished high school (range 15–100%). Thirteen studies reported prevalence of low socioeconomic status with an average of 24% having finished high school (range 8–64%). Twenty studies described any smoking during pregnancy with an average prevalence of 18% (range 4.5–35%). Thirteen studies described drinking alcohol during pregnancy with an average prevalence of 44.6% (range 0–76%). Twelve studies reported “any breastfeeding” with an average of 74.4% (range 10–100%).
Amounts and/or types of seafood consumed were assessed by a food frequency questionnaire (FFQ) for 27 of 29 studies. Lederman et al. (2008) [20] did not use an FFQ but asked about seafood consumption habits. Lynch et al. (2011) and Davidson et al. (2011) [25, 26], both in the Republic of the Seychelles, reported results from FFQs conducted previously in that cohort [42, 43]. Of the studies that used FFQs, 16 studies reported that the FFQ prompted responses by asking about specific species [13–15, 17, 19, 22–24, 27–29, 31, 32, 34, 36, 41]. Eleven studies reported that the FFQ asked about canned tuna or canned fish [15, 22, 24, 27–29, 31, 32, 36, 37, 41]. Ten studies reported that the FFQ provided prompts for frequency, e.g. “less than once per week,” or “more than once per day” [15, 17, 19, 22, 29, 31, 32, 35, 37, 39]. Two studies reported administering an FFQ during first trimester [31, 36]. Eight studies reported administering an FFQ during the second trimester [15, 18, 21, 22, 32–34, 40]. Eight studies reported administering an FFQ during the third trimester [13, 14, 17, 19, 31, 35, 37, 38]. Ten studies reported administering an FFQ after delivery [16, 23, 24, 27–30, 32, 34, 41]. Two studies, in the Faroe Islands and Japan [16, 24], also reported collecting information on consumption of whale in addition to seafood.
Six studies used the FFQ information to report neurocognitive outcomes for oily, or white, or shellfish separately [13, 14, 19, 23, 31, 39]. Since oily fish are higher in omega-3 fatty acids, differences in outcomes, or lack of differences, between oily and white seafood, could be germane to the contribution that these fatty acids may make to neurocognitive effects. Two studies provided neurocognitive outcomes for canned seafood separately without stating the specific type or species of seafood [28, 37]. One study reported neurocognitive outcomes for canned tuna [22]. This was the only study involving maternal consumption that reported outcomes for a specific seafood. Twenty four studies provided neurocognitive outcomes for seafood intake without differentiation among species [13–24, 27–36, 38, 40]. One study used other categorizations of seafood [31].
Of these 29 studies, 24 reported that seafood consumption among mothers was associated with beneficial outcomes to neurocognition on some or all of the tests administered to their children [13–17, 19–27, 29–31, 34–37, 39–41]. The beneficial outcomes appeared on tests administered as early as three days of age and as late as 17 years in age, although nearly all of the testing occurred through age nine (see Table 1).
The five remaining studies reported no significant associations and thus were null for all tests administered [18, 28, 32, 33, 38]. Of the five studies reporting completely null results, one was rated as having serious risk of bias on the ROB-NOS due to uncertainty that critical confounding variables were assessed [33] and another one was similarly rated due to reporting unadjusted results as being significant [28] when adjusted analyses were not all statistically significant. Dietary assessment in that study [28] was also problematic due to difficulties in distinguishing prenatal and child intakes None of the studies reported adverse associations between seafood consumption and neurocognitive development.
Higher offspring IQ scores were associated with greater maternal seafood consumption in five studies that measured IQ on a Wechsler Scale of Intelligence (WISC). Gale et al. (2008) [19] reported 8.07 points higher verbal IQ scores (95% CI 0.28 to 15.9) among children when comparing maternal consumption of ≥12 oz/wk vs. none. Golding et al. (2017) [35] reported that among mothers who ate fish, their children’s total IQ averaged 109.3 (SD 1.095) and 99.8 (SD 1.095) in the highest and lowest deciles of mercury exposure respectively. This 9.5 point difference in IQ indicated that greater mercury exposure was not net adverse, provided the mothers ate seafood, and likely indicated that greater seafood consumption increased child IQ. Furlong et al. (2018) [37] reported 7.71 higher points on the perceptual reasoning component of IQ, comparing > 8 oz/wk to none. Comparing any seafood vs. no maternal seafood, Lederman et al. (2008) [20] reported a 5.6-point increase in verbal and total IQ. Hibbeln et al. (2007) [17] reported lower risk of suboptimal verbal and total IQ (OR = 1·48, 95% CI 1·16–1·90) with >12 oz/wk vs. none. Gains in verbal IQ appear to have provided most of the contribution to total IQ in at least three of these studies [17, 19, 20]. Consistent with the findings in improved verbal development, Vejrup et al. (2018) [40] reported more favorable scores on the Speech and Language Assessment Scale (SLAS), the Ages and Stages Questionnaire (ASQ), and on Twenty Statements about Language-Related Difficulties (language 20) scores when comparing >14 oz/wk vs. none. These findings were consistent across all levels of fish intake.
Other studies reported improvements in other neurodevelopmental domains but did not find improvements in verbal development. Oken et al. (2008) reported beneficial associations to offspring on the Wide Range Assessment of Visual Motor Abilities (WRAVMA) at three years [22] and null associations on the Peabody Picture Vocabulary test at age three and the Kauffman Brief Intelligence Test (KBIT) and WRAVMA scores at age 7.7. Budtz-Jørgensen et al. (2007) [16] reported maternal seafood benefits to offspring visual and motor skills, but not to verbal development at 7 and 14 yrs. Steenweg-de Graaff et al. (2016) [33] reported null associations with IQ, but did not evaluate verbal IQ or verbal development parameters. Sagiv et al. [27] reported that lower maternal seafood consumption (≤8 oz/wk) as compared to higher consumption (>8 oz/wk) was associated with greater risk of offspring ADHD diagnoses (e.g. impulsive reactive phenotype, RR= 2.5 95% CI 1.6, 5.0), this despite reporting adverse effects of mercury, (comparing > 1ppm to < 1ppm) when assessed as an independent variable separately from seafood. Gale et al. (2008) [19] reported that children of mothers not eating oily seafood had nearly three times greater risks of hyperactivity (OR=2.94, 95% CI 1.28, 6.7) as compared to children whose mothers had eaten oily seafood.
Improvements in early childhood neurodevelopment were consistently reported on the McCarthy Scales of Children’s Abilities (MSCA) by Mendez et al. (2009) [23] (5.9 to 8.6 points, 8–12 oz/wk vs. <4 oz/wk), Julvez et al. (2016) [31] (2.29 points, >10 oz/wk) and by LLop et al. (2016) [36]. Daniels et al. (2004)[14] reported scores on the MacArthur Communicative Development Inventory (MCDI)four points higher comparing offspring of mothers consuming >18 oz/ wk vs. none. Additionally, in that study scores were higher on the Denver Developmental Screening Test (DDST) with higher seafood consumption (4.5–13.5 oz/wk vs. none).
Improvements were reported on the BSID by Julvez et al. (2016) [31] for lean and small fish, by Lederman et al. (2008) [20] at 36 and 48 mo. and by Lynch et al. (2011) [26], Valent et al. (2013) [29], and Barbone et al. (2019) [41]. Comparing any seafood vs. no maternal seafood, Lederman et al. (2008) [20] reported 8.7 higher points in the Bayley Scales of Infant Development Psychomotor Development Index, (PDI). Oken et al. (2008) compared the highest quintile of maternal intake (14 oz/wk) to the lowest quintile (1.3 oz/wk) and found greater likelihood of attaining developmental milestones at both six and 18 months (OR=1.29, 95% CI 1.20, 1.38). Hu et al. (2016) [30] found greater maternal seafood to be correlated with better Gesell Developmental Scores in the adaptive domain. Among neonates, Xu et al. (2016) [34] reported that greater maternal seafood consumption was associated with “less need for special handling” and “higher asymmetry” scores at five weeks of age while Suzuki et al. (2010) [24] reported beneficial associations with motor scores at three days of age on the Neonatal Behavioral Assessment Scale.
Relationships to types of seafood
Regarding oily vs. white or lean seafood, two studies found no differences in outcomes [14, 39], while one study found a beneficial association with oily seafood, but not with white [13]. In that study, children of mothers who ate oily seafood were more likely to achieve high grade stereopsis (stereoscopic vision) by 3.5 years of age (adj OR=1.57, 95% CI 1.00, 2.45) as compared to children whose mothers did not. Another study reported benefits associated with oily seafood, but it is not clear whether oily was more beneficial than white [19]. One study found beneficial associations with oily seafood and with all seafood [31]. Another reported that all seafood (minus squid and shellfish low in the omega-3 fatty acid docosahexaenoic acid (DHA) was beneficial as compared to consumption of squid and shellfish [23]. Two studies that examined relationships between eating canned seafood (without specifying the species contents) were null and beneficial respectively [28, 37], while another reported benefits associated with eating eight or more ounces of canned tuna per week as compared to eating no canned tuna [22].
Relationships to neurocognitive development of seafood through lowest to highest intakes
The lowest and highest levels of seafood consumption in the 29 studies ranged from none to 121 oz/wk [21]. Benefits to neurocognitive development were found at the lowest levels of seafood consumption (i.e., 1.3 oz/wk [34], two oz/wk [13, 30], and four oz/wk [21, 37] as compared to no consumption). Maternal seafood consumption in a category characterized as ≥12 oz/wk, as compared to lower amounts, was evaluated in nine studies [16, 17, 19, 21–23, 31, 36, 40]. Seven of these reported neurocognitive benefits and two reported neither benefit or harm [23, 32]. Oken at al. (2008) [22], Llop et al. (2016) [36] and Vejrup et al. (2018) [40] reported benefits of consumption ≥12 oz/wk despite this intake being associated with higher mercury exposures. Vejrup et al. (2018) [40] reported that over the entire range (0–56 oz/wk) of intake, higher maternal seafood consumption was associated with more favorable language and communication scores. Greater seafood intake was highly correlated with higher mercury exposures and this study also found that higher maternal mercury blood levels were associated with greater benefits in three scales of language development. No study reported any adverse effects from maternal consumption of ≥12 oz/wk.Two studies directly reported that the greatest benefits in their cohorts were in consumption categories characterized as ≥ 12 oz/wk [17, 19]. One study reported beneficial associations in its highest consumption category with a mean of 14.5 oz/wk [21] while another study reported benefits above 14.1 oz/wk [40]. One study reported beneficial associations >18 oz/wk [14].. Two studies reported benefits above 30 oz/wk [31, 36].
No adverse effects on neurocognitive outcomes were reported from maternal seafood consumption despite very high reported levels of seafood intake, e.g., up to 121 oz/wk [21], 115 oz/wk [13, 14, 17, 35, 38, 39], 77 oz/wk [24], 65 oz/wk [40], 120 oz/wk [25], 44 oz/wk [29] 32 oz/wk (mean) [36 ] and 30 oz/wk (mean) [22, 31]. Three studies reported that data consistent with a plateau level or asymptotic flattening of the beneficial dose-response relationship whereby after a certain amount, further increases of maternal seafood intake resulted in smaller neurodevelopmental gains. Julvez et al. (2016) [31] reported that the highest scores on most tests were in the fourth quintile of seafood consumption in that cohort (mean of 21.2 oz/wk) or in the third/fourth quintiles followed by an attenuation of a positive association in the fifth (highest) quintile (mean 30.2 oz/wk), but still beneficial. In the Avon Longitudinal Study of Parents and Children cohort in the United Kingdom, Daniels et al. (2004) [14] and Hibbeln et al. (2007) [17] described asymptotic flattening of the beneficial dose response relationship in the highest levels of intake (>12 oz/wk) for early developmental measures.
Mercury results
Mercury levels from blood or hair obtained from mothers during pregnancy or from umbilical cord blood were reported in 23 of 29 cohorts [14–18, 20, 22, 24–32, 34–36, 38–41]. With the exception of cord [14] and erythrocyte mercury levels [32], we converted results to the equivalent of parts per million (ppm) of maternal hair mercury as described in Table 1. Hu et al. (2016) [30] reported the lowest mean mercury exposure (geometric mean 0.2 hair ppm (range <LOD-0.74), arithmetic-mean 0.23 hair ppm (SD 0.11). Davidson et al. (2011) [25] reported the highest mean maternal mercury exposure (hair: mean 6.9 ppm, SD 4.4, range 0.54–22.74). For context, 0.2 ppm is just above the 50th percentile of exposure for women of childbearing age in the United States while 6.9 ppm corresponds to exposure above the 99.9th percentile [9]. Higher mercury levels were associated with improved neurocognitive outcomes in both of these studies [25, 30].
Nearly all of the studies that measured mercury exposure treated it as an independent variable and reported associations with neurocognition separately from the associations they reported between seafood and neurocognition. Several studies reported that higher maternal mercury levels were adversely associated with neurocognition when examined independently of seafood. However, in each case seafood consumption itself was beneficially associated with cognitive development [15, 16, 20, 24, 39]. In 12 studies, greater maternal seafood consumption had overall beneficial effects for child neurocognitive development despite also measuring exposure to mercury [15, 16, 18, 20, 22, 24–27, 39–41]. One study attempted to measure the extent to which maternal exposure to mercury affected the association between seafood consumption and neurocognition [22]. That study reported trend data that ≥ 8 oz/wk with less mercury was more beneficial than >8 ounces per week with more mercury, however these relationships were not statistically significant. One study developed a model suggesting that the benefits DHA (an essential fatty acid rich in seafood) decreased as exposure to mercury approached nine and 11 ppm in hair depending on the neurocognitive test. There was no benefit beyond these levels. Both levels are substantially above the 99.9th percentile of exposure in U.S. women of childbearing age [26]. We mention this study because it is the only one we identified that provided evidence for a mitigating effect of mercury on the benefits associated with a nutrient found in seafood (DHA).
Six studies reported null associations between maternal mercury levels and neurocognition [28–30, 32, 33, 38]. Seven studies reported seemingly paradoxical findings, that higher levels of mercury had beneficial relationships to neurocognitive development[25, 27, 34–36, 40, 41]. For example, in one study a doubling in mercury was associated with higher scores in most of the McCarthy Scales of Child Abilities scales [36]. As noted earlier, this result is likely due to higher maternal mercury levels acting as an indicator of greater seafood consumption.
The United States Environmental Protection Agency (EPA) Reference Dose (RfD) of 1.1 ppm in maternal hair was exceeded in 22 studies [14–18, 20, 22, 24–29, 31, 32, 34–36, 38–41]. Eight of these studies had mean exposures above 1.1 ppm [16, 18, 24, 25, 31, 36], and all the studies had participants with exposures that were many times higher than the RfD. None of these studies reported net adverse effects on neurocognitive development seafood consumption in any amount.
Evaluation of criteria for the conclusion statement, question #40
The conclusion statement below was developed based on the following evaluation criteria of elements in the USDA NESR conclusion statement (https://nesr.usda.gov).
Element 1, Risk of Bias – Grade II, Moderate
Nearly all studies met the criteria for having “moderate” risk of bias utilizing the instruments and procedures described by the NESR [11]. The most common reason for failure to meet criteria for low risk of bias was the inability to be completely certain that no residual confounding was present, an inherent characteristic of all observational studies. Assessment of evidence as moderate rather than strong reflected the fact that that the evidence is based on observational studies, rather than well designed RCTs. An RCT that randomized pregnant women to a no-fish control group over an entire pregnancy would be contrary to the current Dietary Guidelines for Americans recommendation for fish intake.
Element 2, Quantity - Grade I: Strong
Several excellent and many good quality studies were included in this systematic review of 29 articles comprising 102,944 mother-child pairs [13–27, 29–32, 34–41].
Element 3, Consistency - Grade 1: Strong
Twenty- five of the 29 studies reported beneficial associations between seafood and neurocognitive development with good consistency throughout neurocognitive development utilizing well validated, age appropriate instruments. None of the 25 studies reporting benefits were judged to have serious risk of bias using the ROB-NOS. In contrast, two of the five studies reporting null effects on all outcomes were judged to have serious risk of bias. No studies reported net adverse effects.
Element 4, Impact - Grade 1: Strong
In all cases the studied outcomes related directly to the systematic review question and in most cases the size of effects was clinically meaningful or probably clinically meaningful. For example, the gains in IQ in these studies ranged from 5.6 to 9.5 IQ points. In comparison, breastfeeding results in benefit for full term infants of 2.66 higher IQ points after adjusting for maternal intelligence [44].
Element 5, Generalizability- Grade 1: Strong
The studies provided data directly from seven U.S. cohorts and 18 European cohorts generalizable to U.S. populations. The characteristics of the study populations with regards to age, education, occupational status, family characteristics, etc. closely aligned with U.S. characteristics. The cohorts in the Republic of the Seychelles consumed large amounts of seafood (i.e., a mean of 48 oz/wk), resulting in high mercury exposures (averages of 5.9 and 6.9 ppm maternal hair). The mercury concentrations of seafood consumed in the Seychelles were similar to that of seafood available in the United States and thus generalizable to U.S. commercial species in that respect, while sea mammals such as pilot whales were not consumed [18]. In addition, the amounts of seafood consumed and the lower exposures to mercury in the Seychelles overlap with high end amounts and exposures in the United States [9]. Consequently, these findings can be generalized to high consumption populations within the U.S. However, overall generalizability of the 29 studies would have been improved with greater inclusion of more low income or socially vulnerable populations and populations that depend on recreational and subsistence catch.
Results question #41: What is the relationship between seafood consumption during childhood and adolescence (up to 18 years of age) and neurocognitive development?”
A total of 15 studies were identified describing seafood consumption among children and their neurocognitive outcomes [14, 45–58]. Four studies were prospective cohort trials [14, 45, 49, 51], and five were case control studies [47, 53, 54, 57, 58]. Seven were reports from three RCTs: the FINS-KIDS study produced three manuscripts [48, 50, 52] and the FINS-TEENS study produced three manuscripts [46, 55, 56]. Six studies were reported from Norway [46, 48, 50, 52, 55, 56]. There were two each from Sweden [45, 49], China [51, 58], and Spain [53, 54]; and there was one each from Korea [57], the U.S. [47] and the United Kingdom [14]. One United Kingdom study also reported seafood consumption during pregnancy and is included in the systematic review for Question #40 [14].
Overall, these studies were conducted with subjects who were all healthy, or healthy subjects without ADHD or developmental disabilities were used as comparators. All participants had access to health care. Rates of obesity among participants was generally very low, typically <2 %. Parent education was high with an average of 34.8% terminating with high school and 46.7% with completing higher education degrees. Two studies conducted in China [51, 58] and one in Korea [57] had no Caucasians. Among the remainder of the studies, were 89% white and non-immigrant. Auberg et al. (2009) [45] studied Swedish military conscripts, 100% of which were male. The remainder of the studies had an average of 54% males. Hertz-Picciotto et al. (2010) [47] studied children with autism or autism spectrum disorder of whom 75% were male, reflecting the normal sex distribution of the disorder. Ages averaged 9.3 years across all studies with a range of 18 mo. [14] to 16 years [49].
All 15 studies used FFQs or similar instruments to determine amounts or types of seafood consumed. [14, 45–58]. Six RCT studies used FFQs to provide information on seafood consumption outside of an intervention involving fish meals at school [46, 48, 50, 52, 55, 56, 59]. Six studies provided neurocognitive outcomes for oily seafood separately [46, 48, 50, 52, 53, 57]. Two studies reported that their FFQs prompted responses by asking about specific species of seafood [47, 58].
Neurocognitive outcomes question #41
Thirteen of the 15 manuscripts reported beneficial neurocognitive outcomes associated with consumption of seafood among children [14, 45–47, 49–54, 56–58]. Two reported findings that were null [48, 55] and none reported adverse outcomes. Beneficial relationships with measures of intelligence were reported in the two largest prospective cohort trials (school grades at 16 years of age [49] and standardized intelligence tests [45]) and a smaller prospective cohort (WISC-III [51]) among children 12 years old. In that study, verbal IQ gained 4.75 points while full scale IQ scores gained 4.8 points when seafood consumption was ≥4 oz/wk compared to less seafood. Seafood consumption at 15 mo. was associated with better MCDI scores at 18 mo. [14]. In four studies, greater seafood consumption [53, 58] or dietary patterns characterized by seafood consumption [54, 57] were associated with lower risks for a diagnosis of Attention Deficit Hyperactivity Disorder using Diagnostic Statistical Manual criteria. One case-control study reported that seafood consumption was associated with lower risk of autism or autism spectrum disorder [47], but the study was judged to have a serious risk of bias due to inadequate control of confounding variables and the possible influence of diagnosis on dietary choices.
Relationships to types of seafood
Two studies suggested that oily seafood as opposed to white seafood may be somewhat protective against risk of ADHD-related disorders, although the results might simply represent dietary preferences among children with and without ADHD-related disorders [53, 57]. In three RCTs, children eating oily seafood meals had greater improvement on test scores as compared to meat meals [46, 50, 52] and in one of these, oily seafood meals were more beneficial in that regard than omega-3 supplements [46]. These studies did not compare oily seafood to white seafood, however. No other study reported outcomes for specific species. No studies provided neurocognitive outcomes for canned fish separately.
Relationships to neurocognitive development of seafood at lowest to highest intakes
Five studies reported benefits [14, 45, 47, 49, 51] when comparing seafood consumption above one meal/wk (4 oz/wk). Two studies [53, 54] reported that an intake of > 8–12 oz/wk was associated with the greatest benefits. Insufficient data were reported on ranges, gradients and upper quantities of seafood consumed to make any assessment of dose response relationships or conclusions regarding maximal levels of benefit. Only one study characterized results by any differentiation of oily/fatty seafood and white seafood [53] finding that fatty seafood was more protective than white seafood.
Mercury results
Two studies reported measurements of mercury exposure in the children [47, 50]. In Kvestad et al. 2018 consumption for seafood in an RCT caused mercury to increase from 0.373 ppm to 0.520 ppm [50]. Despite this elevation in mercury, neurocognitive benefits were reported. Hertz-Picciotto et al. 2010, reported that seafood consumption was associated with higher mercury levels, which were nearly twice as high in children with “typical development” (0.29 ppm) as compared to children with symptoms of autism spectrum disorder (0.14 ppm). Although mercury levels were not reported in the remainder of the studies, it is highly likely that greater seafood consumption in those studies resulted in higher mercury exposures. Since no study reported net adverse outcomes from seafood consumption in children, it is unlikely that mercury exposure from seafood was associated with substantive neurocognitive harms.
Evaluation of criteria for the conclusion statement, question #41
The conclusion statement below was developed based on the following evaluation criteria of elements in the USDA NESR conclusion statement (https://nesr.usda.gov).
Element 1, Risk of Bias – Grade II, Moderate
Nearly all studies met criteria for having “moderate” risk of bias utilizing the instruments and procedures described by the NESR [11]. The most common reason for failure to meet criteria for low risk of bias was inability to be certain of no residual confounding, an inherent characteristic of all observational studies. Seven reports from four RCTs were published, These RCTS were moderately sized (n=183, 232, 426 and 726). Three [48, 50, 52] reported results from the FINS-KIDS Study and two [46, 55] from the FINS-TEENS Study.
Element 2, Quantity - Grade I: Strong
All of the 15 studies in this systematic review were either of excellent or good quality, comprising 25,031 children [14, 45–58].
Element 3, Consistency - Grade 1: Strong
Thirteen of the 15 papers reported beneficial neurocognitive outcomes associated with consumption of seafood among children using age appropriate well validated instruments [14, 45–47, 49–58] The two studies that reported gains in IQ are in addition to the five that reported IQ gains in association with maternal consumption [45, 51]. Two studies reported null findings on some outcomes [48, 55] although beneficial results for other aspects of neurocognitive development were reported in studies of the same cohorts. No studies reported adverse outcomes.
Element 4, Impact - Grade 1: Strong
In all cases the studied outcomes related directly to the systematic review question and in most cases the size of effect was clinically meaningful (e.g., the gains in IQ and lower risks of ADHD).
Element 5, Generalizability- Grade 1: Moderate
The studies provided data from cohorts generalizable to U.S. populations, e.g., one U.S. and 10 European cohorts. Children were healthy and had good access to medical care. The characteristics of the study populations with regards to age, education, occupational status, family characteristics, etc. aligned with U.S. characteristics. However, non-white populations were under-represented as compared to the U.S. population.
Discussion
Here we followed the systematic review methodology detailed by the USDA’s NESR team (https://nesr.usda.gov) to evaluate two questions to be addressed by the 2020 SAC-Dietary Guidelines for Americans and contribute perspectives from a technical expert committee with extensive topical experience. We conclude that there is moderate and consistent evidence that seafood consumption both during pregnancy and childhood benefits neurocognitive development. The magnitude of effects was clinically significant. In the seven studies that reported gains in IQ, the gains ranged from 4.8 points [51] to 9.5 IQ points [35] when seafood was consumed in the highest consumption categories in those cohorts. Such gains could be significant on an individual and population-wide basis. Thirteen studies reported as beneficial the consumption of ≥12 oz/wk or categories that included consumption >12 oz/wk [14–17, 19, 21, 22, 24, 25, 27, 31, 36, 40]. Two studies reported the consumption of >12 oz// wk was not associated with harm [23, 32]. Llop et al. (2016) [36] and Vejrup et al. (2018) [40] also reported benefits of consuming ≥12 oz/wk despite also finding associations between greater seafood consumption and higher mercury levels. In 10 of these studies, consuming ≥12 oz/wk was more beneficial that consuming <12 oz/wk for at least some outcomes [14, 16, 17, 19, 21, 22, 27, 31, 36, 40].
One of the most significant outcomes of this systematic review is the absence of evidence for any net adverse effects of seafood on neurocognitive development even at the highest levels of intake. We found no evidence to support an upper limit of 12 oz/wk of commercial seafood (i.e., evidence that exceeding this intake was associated with harm). Three studies that provide evidence that consuming between 12 and 20 oz/wk amounts provide maximum benefits [14, 17, 31]. The United States Environmental Protection Agency (EPA) Reference Dose (RfD) of 1.1 ppm in maternal hair was exceeded in 22 studies, often by many times [14–18, 20, 22, 24–29, 31, 32, 34–36, 38–41]. Despite this, we found that consuming seafood during pregnancy and childhood was likely beneficial and clearly not adverse to neurocognition. Higher mercury levels were associated with benefits to neurocognitive outcomes in seven studies with 45,957 mother infant pairs, likely indicating greater seafood consumption [25, 27, 34–36, 40, 41]. These studies corroborate that the US EPA RfD is a level of exposure deemed to be without appreciable risk [10].
The published studies typically did not qualify species of seafood consumed making it impossible to evaluate effects of specific species or types of seafood on neurocognition. We could find no data regarding effects on neurocognitive development from consuming species of seafood distinguished by varying amounts of mercury by any quantitative definition (e.g., “low”, “moderate” and “high” mercury seafood). There was insufficient direct data to form any conclusions regarding freshwater non-commercial species; none of the studies we reviewed distinguished freshwater non-commercial catch from commercially available seafood or wild caught from aquacultured species , however, a reasonable assumption is that the majority of the seafood consumed was commercially available and included a substantial amount of aquacultured seafood. Aquacultured seafood tend to be toward the low end in terms of mercury because they are grown rapidly without much opportunity to accumulate mercury [9]. Finally, most studies did not report seafood preparation, however, one study did report an adverse association between fried seafood and neurocognition [31].
Research recommendations
TEC members identified research gaps and offered the following recommendations:
Conduct more prospective cohort research in diverse populations within and outside of the United States.
Use standardized outcome measures when possible (e.g. IQ or IQ equivalent scores, especially for verbal development), so that outcomes can be consistently scored, compared, and reproduced across studies.
More detailed recording of species and preparation.
Conduct studies to develop a better understanding of whether seafood containing greater quantities of omega-3 fatty acids, i.e., oily or fatty seafood, is more beneficial than seafood containing less, i.e., white seafood.
Assess contributions of genetic variants and their interactions with dietary intakes.
Conduct adequately powered RCTs when possible to better support causal inferences.
Conduct more studies on effects from maternal seafood consumption beyond nine years of age.
Conduct studies on the effects on neurocognition from non-commercial catch from rivers, lakes, and streams.
Conclusion Statement Question #40
After reviewing the evidence, the TEC members developed the following conclusion statement to answer the question #40 assessing the relationship of seafood consumption during pregnancy and lactation to neurocognitive development of the infant:
“Moderate and consistent evidence indicates that consumption of a wide range of amounts and types of commercially available seafood during pregnancy is associated with improved neurocognitive development of offspring as compared to eating no seafood”. This evidence does not meet the criteria for “strong” evidence only due to the absence of randomized controlled trials that may not be ethical or feasible to conduct. Overall, benefits to neurocognitive development began to appear at the lowest amounts of seafood consumed (~4 oz/wk) and continued into the highest categories of consumption in those cohorts (>100 oz/wk). Benefits consistently increased from no seafood consumption upwards through approximately >12–30 oz/wk. After those levels of consumption, benefits continued to be present. The TEC could find no upper limit of seafood intake that resulted in adverse outcomes for any measure of neurocognitive development as compared to eating no seafood or less seafood even though the highest amounts exceeded current average intake of Americans (< 5 oz/wk) by more than 20-fold (>100 oz/wk). Seafood provided overall benefits to neurocognitive development even when mercury exposures in the same study populations were high by U.S. standards. In some studies, oily fish (tuna, salmon, swordfish, sardines, etc.) showed benefits when white fish and shellfish did not, however data are insufficient from these studies overall for a conclusive statement regarding types of seafood or specific species that convey the greatest benefits. Most of the research was conducted in healthy women and offspring consistent with U.S. population demographics. Evidence is insufficient to assess the relationship between seafood consumption during lactation and neurocognitive development.”
Conclusion Statement Question #41
After reviewing the evidence, the TEC members developed the following conclusion statement to answer the question #41 assessing the relationship of seafood consumption during childhood and adolescence (up to 18 years of age) and neurocognitive development:
“Moderate and consistent evidence indicates that consumption of >4 oz/wk and likely >12 oz/wk of a wide range of commercially available seafood during childhood through adolescence has beneficial associations to a wide spectrum of neurocognitive outcomes as compared to consuming no seafood. The evidence does not meet the criteria for “strong evidence because of an insufficient number of randomized controlled trials. The TEC could find no amount of seafood in these studies that resulted in net adverse outcomes for any measures of neurocognitive development in children; however upper ranges of consumption were not well described. Seafood provided overall benefits to neurocognitive development and exceeded potential harms from mercury in seafood. However, specific levels of mercury exposure were often not reported. Data were insufficient for a conclusive statement regarding neurocognitive effects from types of seafood or specific species. Most of the research was conducted in healthy children reasonably consistent with U.S. population demographics.”
Supplementary Material
Highlights.
We conducted two systematic reviews, evaluating the relationship between seafood consumption in pregnancies and in childhood on neurocognitive development using methodologies detailed by the Dietary Guidelines for Americans Scientific Advisory Committee 2020–2025.
This evaluation of seafood inherently integrates any adverse effects from neurotoxicants, if any, and benefits to neurocognition from omega-3 fats, as well as other nutrients critical to optimal neurological development.
No adverse effects of seafood consumption on neurocognition were found in 44 publications reporting on 102,944 mother-offspring pairs and 25,031 children.
Benefits to neurocognitive development began at the lowest amounts of seafood consumed in pregnancy (~4 oz/wk) and up to >100 oz/wk, with benefits to age appropriate measures of neurocognitive development including an average increase of 7.7 IQ points.
Consumption of >4 oz/wk and likely >12 oz/wk of seafood during childhood had beneficial associations with neurocognitive outcomes.
A clear understanding of the effects of seafood consumption on neurocognition can have significant public health implications.
Acknowledgements
The Intramural Research program of the National Institute on Alcohol Abuse and Alcoholism provided support for this work, however this publication should not be considered to be a policy or position of NIAAA, the National Institutes of Health or any U.S. Federal Agency.
Footnotes
Conflicts of interest
The following authors have no conflicts to declare, JRH, PS, JTB, JG, BJH, PK-E, BL, SLC, GM, JJS, MAC, SEC. WS Harris is the President of OmegaQuant Analytics, LLC a laboratory that offers fatty acid testing for researchers, clinicians and consumers. The corresponding author does not consider this to be a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
CAPT Joseph R. Hibbeln, Acting Chief Section on Nutritional Neurosciences, National Institute on Alcohol Abuse and Alcoholism, NIH, USA..
Philip Spiller, Former Director of the Office of Seafood, Center for Food Safety and Applied Nutrition, U.S., Food and Drug Administration (retired).
J. Thomas Brenna, Dell Pediatric Research Institute, Depts of Pediatrics, of Chemistry, and of Nutrition, University of Texas at Austin, Texas, USA.
Jean Golding, Emeritus Professor of Pediatric and Perinatal Epidemiology, Population Health Sciences, University of Bristol. Bristol, UK.
Bruce J. Holub, Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada
William S. Harris, Department of Internal Medicine, University of South Dakota School of Medicine and OmegaQuant Analytics, LLC; Sioux Falls, SD, USA.
Penny Kris-Etherton, Distinguished Professor of Nutrition, Department of Nutritional Sciences, Penn State University, University Park, Pennsylvania, USA..
Bill Lands, Fellow, American Society for Nutrition, College Park, Maryland, USA.
Sonja L. Connor, Research Associate Professor, Endocrinology, Diabetes and Clinical Nutrition, Oregon Health and Science University, Portland, Oregon, USA..
Gary Myers, Professor of Neurology, Pediatrics, and Environmental Medicine, University of Rochester Medical Center, Rochester, New York, USA..
J. J. Strain, Emeritus Professor of Human Nutrition, Nutrition Innovation Centre for Food & Health, (NICHE), Ulster University, Coleraine, Northern Ireland, UK
Michael A Crawford, FSB, FRCPATH, Visiting Professor, Division of Obstetrics and Gynaecology, Department of Surgery and Cancer, Imperial College London, UK.
Susan E. Carlson, University Distinguished Professor, Department of Dietetics and Nutrition, University of Kansas Medical Center, Kansas City, Kansas, USA.
References
- [1].Schwarzenberg SJ, Georgieff MK, Committee On N, Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health, Pediatrics 141(2) (2018). [DOI] [PubMed] [Google Scholar]
- [2].Bonham MP, Duffy EM, Robson PJ, Wallace JM, Myers GJ, Davidson PW, Clarkson TW, Shamlaye CF, Strain JJ, Livingstone MB, Contribution of fish to intakes of micronutrients important for fetal development: a dietary survey of pregnant women in the Republic of Seychelles, Public Health Nutr 12(9) (2009) 1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dietary Guidelines Advisory Committee 2010, Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2010 to the Secretary of Agriculture and the Secretary of Health and Human Services, 2010.
- [4].Dietary Guideline Advisory Committee on the Dietary Guidelines for Americans 2015–2020, Report of the Dietary Guideline Advisory Committee on the Dietary Guidelines for Americans 2015–2020 2015, p. Part D Chapter 2 p. 3.
- [5].H. Canada, Prenatal Nutrition Guidlines for Health Professionals, 2009.
- [6].EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies), Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury, EFSA Journal 12(7) ( 2014) 3761. [Google Scholar]
- [7].FAO/WHO, Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption. Rome, 25–29 January 2010., 2011. [Google Scholar]
- [8].S.P.e. al, An abundance of seafood consumption studies presents new opportunities to evaluate effects on neurocognitive development, Prostaglandins Leukot Essent Fatty Acids (In preparation). [DOI] [PMC free article] [PubMed]
- [9].Food and Drug Administration USA, Quantitative Assessment of the Net Effects on Fetal Neurodevelopment from Eating Commercial Fish (As Measured by IQ and also by Early Age Verbal Development in Children), 2014.
- [10].National Research Council, Toxicological Effects of Methylmercury, National Academy Press; 2000. [PubMed] [Google Scholar]
- [11].Obbagy JE, Spahn JM, Wong YP, Psota TL, Spill MK, Dreibelbis C, Gungor DE, Nadaud P, Raghavan R, Callahan EH, English LK, Kingshipp BL, LaPergola CC, Shapiro MJ, Stoody EE, Systematic review methods for the Pregnancy and Birth to 24 Months Project, Am J Clin Nutr 109(Supplement_7) (2019) 698S–704S. [DOI] [PubMed] [Google Scholar]
- [12].Grandjean P, Weihe P, Jorgensen PJ, Clarkson T, Cernichiari E, Videro T, Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead, Arch Environ Health 47(3) (1992) 185–95. [DOI] [PubMed] [Google Scholar]
- [13].Williams C, Birch EE, Emmett PM, Northstone K, Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study, Am J Clin Nutr 73(2) (2001) 316–22. [DOI] [PubMed] [Google Scholar]
- [14].Daniels JL, Longnecker MP, Rowland AS, Golding J, Fish intake during pregnancy and early cognitive development of offspring, Epidemiology 15(4) (2004) 394–402. [DOI] [PubMed] [Google Scholar]
- [15].Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW, Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort, Environmental health perspectives 113(10) (2005) 1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Budtz-Jorgensen E, Grandjean P, Weihe P, Separation of risks and benefits of seafood intake, Environmental health perspectives 115(3) (2007) 323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J, Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study, Lancet 369(9561) (2007) 578–85. [DOI] [PubMed] [Google Scholar]
- [18].Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JM, Robson PJ, Duffy EM, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Clarkson TW, Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy, Neurotoxicology 29(5) (2008) 767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gale CR, Robinson SM, Godfrey KM, Law CM, Schlotz W, O’Callaghan FJ, Oily fish intake during pregnancy--association with lower hyperactivity but not with higher full-scale IQ in offspring, J Child Psychol Psychiatry 49(10) (2008) 1061–8. [DOI] [PubMed] [Google Scholar]
- [20].Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, Viswanathan S, Becker M, Stein JL, Wang RY, Perera FP, Relation between cord blood mercury levels and early child development in a World Trade Center cohort, Environmental health perspectives 116(8) (2008) 1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M, Bellinger DC, Hadders-Algra M, Michaelsen KF, Olsen SF, Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort, Am J Clin Nutr 88(3) (2008) 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW, Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort, American journal of epidemiology 167(10) (2008) 1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mendez MA, Torrent M, Julvez J, Ribas-Fito N, Kogevinas M, Sunyer J, Maternal fish and other seafood intakes during pregnancy and child neurodevelopment at age 4 years, Public Health Nutr 12(10) (2009) 1702–10. [DOI] [PubMed] [Google Scholar]
- [24].Suzuki K, Nakai K, Sugawara T, Nakamura T, Ohba T, Shimada M, Hosokawa T, Okamura K, Sakai T, Kurokawa N, Murata K, Satoh C, Satoh H, Neurobehavioral effects of prenatal exposure to methylmercury and PCBs, and seafood intake: neonatal behavioral assessment scale results of Tohoku study of child development, Environmental research 110(7) (2010) 699–704. [DOI] [PubMed] [Google Scholar]
- [25].Davidson PW, Cory-Slechta DA, Thurston SW, Huang LS, Shamlaye CF, Gunzler D, Watson G, van Wijngaarden E, Zareba G, Klein JD, Clarkson TW, Strain JJ, Myers GJ, Fish consumption and prenatal methylmercury exposure: cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study, Neurotoxicology 32(6) (2011) 711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lynch ML, Huang LS, Cox C, Strain JJ, Myers GJ, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JM, Duffy EM, Clarkson TW, Davidson PW, Varying coefficient function models to explore interactions between maternal nutritional status and prenatal methylmercury toxicity in the Seychelles Child Development Nutrition Study, Environmental research 111(1) (2011) 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sagiv SK, Thurston SW, Bellinger DC, Amarasiriwardena C, Korrick SA, Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children, Arch Pediatr Adolesc Med 166(12) (2012) 1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deroma L, Parpinel M, Tognin V, Channoufi L, Tratnik J, Horvat M, Valent F, Barbone F, Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site, International journal of hygiene and environmental health 216(4) (2013) 486–93. [DOI] [PubMed] [Google Scholar]
- [29].Valent F, Mariuz M, Bin M, Little D, Mazej D, Tognin V, Tratnik J, McAfee AJ, Mulhern MS, Parpinel M, Carrozzi M, Horvat M, Tamburlini G, Barbone F, Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: a prospective cohort study in Italy, Journal of epidemiology 23(5) (2013) 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu Y, Chen L, Wang C, Zhou Y, Zhang Y, Wang Y, Shi R, Gao Y, Tian Y, Prenatal low-level mercury exposure and infant neurodevelopment at 12 months in rural northern China, Environmental science and pollution research international 23(12) (2016) 12050–9. [DOI] [PubMed] [Google Scholar]
- [31].Julvez J, Mendez M, Fernandez-Barres S, Romaguera D, Vioque J, Llop S, Ibarluzea J, Guxens M, Avella-Garcia C, Tardon A, Riano I, Andiarena A, Robinson O, Arija V, Esnaola M, Ballester F, Sunyer J, Maternal Consumption of Seafood in Pregnancy and Child Neuropsychological Development: A Longitudinal Study Based on a Population With High Consumption Levels, American journal of epidemiology 183(3) (2016) 169–82. [DOI] [PubMed] [Google Scholar]
- [32].Oken E, Rifas-Shiman SL, Amarasiriwardena C, Jayawardene I, Bellinger DC, Hibbeln JR, Wright RO, Gillman MW, Maternal prenatal fish consumption and cognition in mid childhood: Mercury, fatty acids, and selenium, Neurotoxicology and teratology 57 (2016) 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Steenweg-de Graaff J, Tiemeier H, Ghassabian A, Rijlaarsdam J, Jaddoe VW, Verhulst FC, Roza SJ, Maternal Fatty Acid Status During Pregnancy and Child Autistic Traits: The Generation R Study, American journal of epidemiology 183(9) (2016) 792–9. [DOI] [PubMed] [Google Scholar]
- [34].Xu Y, Khoury JC, Sucharew H, Dietrich K, Yolton K, Low-level gestational exposure to mercury and maternal fish consumption: Associations with neurobehavior in early infancy, Neurotoxicology and teratology 54 (2016) 61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Golding J, Hibbeln JR, Gregory SM, Iles-Caven Y, Emond A, Taylor CM, Maternal prenatal blood mercury is not adversely associated with offspring IQ at 8 years provided the mother eats fish: A British prebirth cohort study, International journal of hygiene and environmental health 220(7) (2017) 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Llop S, Ballester F, Murcia M, Forns J, Tardon A, Andiarena A, Vioque J, Ibarluzea J, Fernandez-Somoano A, Sunyer J, Julvez J, Rebagliato M, Lopez-Espinosa MJ, Prenatal exposure to mercury and neuropsychological development in young children: the role of fish consumption, Int J Epidemiol 46(3) (2017) 827–838. [DOI] [PubMed] [Google Scholar]
- [37].Furlong M, Herring AH, Goldman BD, Daniels JL, Wolff MS, Engel LS, Engel SM, Early Life Characteristics and Neurodevelopmental Phenotypes in the Mount Sinai Children’s Environmental Health Center, Child psychiatry and human development 49(4) (2018) 534–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hibbeln J, Gregory S, Iles-Caven Y, Taylor CM, Emond A, Golding J, Total mercury exposure in early pregnancy has no adverse association with scholastic ability of the offspring particularly if the mother eats fish, Environment international 116 (2018) 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Golding J, Rai D, Gregory S, Ellis G, Emond A, Iles-Caven Y, Hibbeln J, Taylor C, Prenatal mercury exposure and features of autism: a prospective population study, Mol Autism 9 (2018) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vejrup K, Brandlistuen RE, Brantsaeter AL, Knutsen HK, Caspersen IH, Alexander J, Lundh T, Meltzer HM, Magnus P, Haugen M, Prenatal mercury exposure, maternal seafood consumption and associations with child language at five years, Environment international 110 (2018) 71–79. [DOI] [PubMed] [Google Scholar]
- [41].Barbone F, Rosolen V, Mariuz M, Parpinel M, Casetta A, Sammartano F, Ronfani L, Vecchi Brumatti L, Bin M, Castriotta L, Valent F, Little DL, Mazej D, Snoj Tratnik J, Miklavčič Višnjevec A, Sofianou K, Špirić Z, Krsnik M, Osredkar J, Neubauer D, Kodrič J, Stropnik S, Prpić I, Petrović O, Vlašić-Cicvarić I, Horvat M, Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: Results from the Mediterranean PHIME cohort, International journal of hygiene and environmental health 222(1) (2019) 9–21. [DOI] [PubMed] [Google Scholar]
- [42].Shamlaye CF, Marsh DO, Myers GJ, Cox C, Davidson PW, Choisy O, Cernichiari E, Choi A, Tanner MA, Clarkson TW, The Seychelles child development study on neurodevelopmental outcomes in children following in utero exposure to methylmercury from a maternal fish diet: background and demographics, Neurotoxicology 16(4) (1995) 597–612. [PubMed] [Google Scholar]
- [43].Conway MC, Mulhern MS, McSorley EM, van Wijngaarden E, Strain JJ, Myers GJ, Davidson PW, Shamlaye CF, Yeates AJ, Dietary Determinants of Polyunsaturated Fatty Acid (PUFA) Status in a High Fish-Eating Cohort during Pregnancy, Nutrients 10(7) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Anderson JW, Johnstone BM, Remley DT, Breast-feeding and cognitive development: a meta-analysis, Am J Clin Nutr 70(4) (1999) 525–35. [DOI] [PubMed] [Google Scholar]
- [45].Aberg MA, Aberg N, Brisman J, Sundberg R, Winkvist A, Toren K, Fish intake of Swedish male adolescents is a predictor of cognitive performance, Acta paediatrica (Oslo, Norway : 1992) 98(3) (2009) 555–60. [DOI] [PubMed] [Google Scholar]
- [46].Handeland K, Skotheim S, Baste V, Graff IE, Froyland L, Lie O, Kjellevold M, Markhus MW, Stormark KM, Oyen J, et al. , The effects of fatty fish intake on adolescents’ nutritional status and associations with attention performance: results from the FINS-TEENS randomized controlled trial, Nutrition journal 17(1) (no pagination) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hertz-Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah IN, Blood mercury concentrations in CHARGE Study children with and without autism, Environmental health perspectives 118(1) (2010) 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hysing M, Kvestad I, Kjellevold M, Kolden Midtbo L, Graff IE, Lie O, Hurum H, Stormark KM, Oyen J , Fatty Fish Intake and the Effect on Mental Health and Sleep in Preschool Children in FINS-KIDS, a Randomized Controlled Trial, Nutrients 10(10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim JL, Winkvist A, Aberg MA, Aberg N, Sundberg R, Toren K, Brisman J, Fish consumption and school grades in Swedish adolescents: a study of the large general population, Acta paediatrica (Oslo, Norway : 1992) 99(1) (2010) 72–7. [DOI] [PubMed] [Google Scholar]
- [50].Kvestad I, Vabo S, Kjellevold M, Nostbakken OJ, Midtbo LK, Hysing M, Markhus MW, Madsen L, Handeland K, Graff IE, Lie O, Froyland L, Stormark KM, Dahl L, Oyen J, Fatty fish, hair mercury and cognitive function in Norwegian preschool children: Results from the randomized controlled trial FINS-KIDS, Environment international 121(Pt 2) (2018) 1098–1105. [DOI] [PubMed] [Google Scholar]
- [51].Liu J, Cui Y, Li L, Wu L, Hanlon A, Pinto-Martin J, Raine A, Hibbeln JR, The mediating role of sleep in the fish consumption - cognitive functioning relationship: a cohort study, Sci Rep 7(1) (2017) 17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Oyen J, Kvestad I, Midtbo LK, Graff IE, Hysing M, Stormark KM, Markhus MW, Baste V, Froyland L, Koletzko B, Demmelmair H, Dahl L, Lie O, Kjellevold M, Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children, BMC Med 16(1) (2018) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rios-Hernandez A, Alda JA, Farran-Codina A, Ferreira-Garcia E, Izquierdo-Pulido M, The Mediterranean Diet and ADHD in Children and Adolescents, Pediatrics 139(2) (2017). [DOI] [PubMed] [Google Scholar]
- [54].San Mauro Martin I, Blumenfeld Olivares JA, Garicano Vilar E, Echeverry Lopez M, Garcia Bernat M, Quevedo Santos Y, Blanco Lopez M, Elortegui Pascual P, Borregon Rivilla E, Rincon Barrado M, Nutritional and environmental factors in attention-deficit hyperactivity disorder (ADHD): A cross-sectional study, Nutritional neuroscience 21(9) (2018) 641–647. [DOI] [PubMed] [Google Scholar]
- [55].Skotheim S, Handeland K, Kjellevold M, Oyen J, Froyland L, Lie O, Eide Graff I, Baste V, Stormark KM, Dahl L, The effect of school meals with fatty fish on adolescents’ self-reported symptoms for mental health: FINS-TEENS - a randomized controlled intervention trial, Food Nutr Res 61(1) (2017) 1383818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sorensen LB, Damsgaard CT, Dalskov SM, Petersen RA, Egelund N, Dyssegaard CB, Stark KD, Andersen R, Tetens I, Astrup A, Michaelsen KF, Lauritzen L, Diet-induced changes in iron and n-3 fatty acid status and associations with cognitive performance in 8–11-year-old Danish children: secondary analyses of the Optimal Well-Being, Development and Health for Danish Children through a Healthy New Nordic Diet School Meal Study, Br J Nutr 114(10) (2015) 1623–37. [DOI] [PubMed] [Google Scholar]
- [57].Woo HD, Kim DW, Hong YS, Kim YM, Seo JH, Choe BM, Park JH, Kang JW, Yoo JH, Chueh HW, Lee JH, Kwak MJ, Kim J, Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD), Nutrients 6(4) (2014) 1539–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhou F, Wu F, Zou S, Chen Y, Feng C, Fan G, Dietary, Nutrient Patterns and Blood Essential Elements in Chinese Children with ADHD, Nutrients 8(6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dalton A, Wolmarans P, Witthuhn RC, van Stuijvenberg ME, Swanevelder SA, Smuts CM, A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source, Prostaglandins, leukotrienes, and essential fatty acids 80(2– 3) (2009) 143– 149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.