Abstract
Stress is an important risk factor for the development of substance use disorder (SUD). Exposure to both stress and drugs abuse lead to changes in synaptic plasticity and stress-induced alterations in synaptic plasticity may contribute to later vulnerability to SUD. Recent developmental neuroscience studies have identified microglia as regulators of synaptic plasticity. As both stress and drugs of abuse lead to microglial activation, we propose this as a potential mechanism underlying their ability to change synaptic plasticity. This review focuses on three components of synaptic plasticity: spine density, brain-derived neurotrophic factor (BDNF) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression. Their roles in addiction, stress, and development will be reviewed, as well as possible mechanisms by which microglia could regulate their function. Potential links between stress, vulnerability to addiction, and microglial activity will be explored.
Keywords: Stress, Drugs of Abuse, Substance Use Disorder, microglia, AMPA, BDNF, spine density
Introduction
Addiction is a major health crisis in the United States. Overdose deaths have doubled in the past decade, with over 64,000 deaths in 2016 (Hedegaard et al., 2017; Kopec et al., 2018). Exposure to stressful life events contributes to vulnerability to substance use disorder (SUD, see Table 1 for acronym definitions) (Breslau et al., 2003; Dembo et al., 1988; Harrison et al., 1997; Widom et al., 1999). A history of trauma is associated with increased drug use in adolescents (Clark et al., 1997; Harrison et al., 1997). Similarly, patients with post-traumatic stress disorder have an increased risk of developing a SUD (Breslau et al., 2003; Snider et al., 2013). Since stress is such an important risk factor for the development of SUD, understanding the biology behind the interaction of stress and drugs of abuse could aid in the development of novel therapeutics.
Table 1.
List of acronyms and definitions.
| Acronym | Definition |
|---|---|
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazo- lepropionic acid receptor |
| BDNF | brain-derived neurotrophic factor |
| CRF | corticotropin-releasing factor |
| CX3CL1 | fractalkine |
| CX3CR1 | fractalkine receptor |
| DAMP | danger-associated molecular pattern |
| MSN | medium spiny neuron |
| NLRP3 | nucleotide-binding oligomerization domain, leucine-rich repeat and pyrine domain protein 3 |
| NMDAR | N-methyl-D-aspartate receptor |
| PFC | prefrontal cortex |
| SUD | substance use disorder |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor- alpha |
| VTA | ventral tegmental area |
Lately there has been increased interest in the role of microglia in both substance use and stress (Brown et al., 2018; Crews et al., 2017b; Snider et al., 2013; Weber et al., 2015; Wohleb et al., 2018). Much of this research has focused on the pro-inflammatory response induced by drugs of abuse (Brown et al., 2018; Crews et al., 2017a) and stress (Weber et al., 2015; Wohleb et al., 2018). However, little is known about how microglia modulate neuronal function. Research from the developmental literature suggests that microglia may be contributing to changes in synaptic plasticity (Paolicelli et al., 2011). Changes in synaptic plasticity are a hallmark of both SUD and responses to stress. Drugs of abuse cause long lasting neuroadaptations in reward-related brain structures, including electrophysiological changes and structural plasticity (Dong and Nestler, 2014). Similarly, stress causes neuroadaptations in a variety of brain regions, such as the amygdala, the hippocampus, and the prefrontal cortex (PFC). In the nucleus accumbens, stress leads to neuroadaptations in the medium spiny neurons which are believed to enhance vulnerability to addiction (Taylor et al., 2014). In the case of both stress and drugs of abuse, synaptic plasticity changes are maladaptive and correlate with depression and drug craving, respectively (Bessa et al., 2013; Loweth et al., 2014).
Along with these forms of stimulus-induced plasticity, normal brain development is also characterized by enhanced synaptic plasticity. Microglia are necessary for these developmental processes, acting as mediators of synaptic plasticity (Miyamoto et al., 2016; Paolicelli et al., 2011). Given that many of the biological mechanisms underpinning normal development are similar to what researchers see in preclinical models of cocaine use disorder, perhaps microglia are also contributing to drug-induced plasticity changes (Dong and Nestler, 2014; see Table 2 for summary). There is also evidence that microglia can influence synaptic plasticity in adulthood, suggesting that microglia do not lose this ability to modulate synaptic plasticity after development (Parkhurst et al., 2013).
Table 2.
Summary of alterations to synaptic plasticity caused by cocaine, stress and microglia.
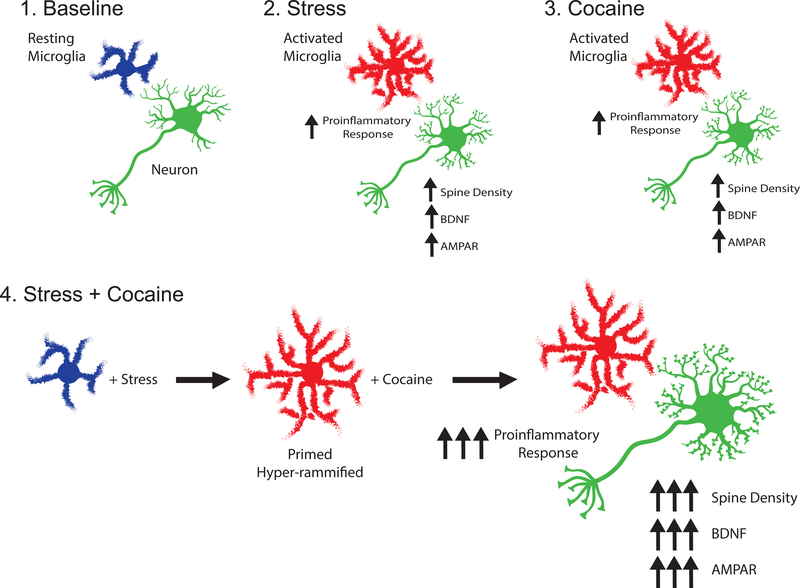
In this review, we will discuss the role of microglia in synaptic plasticity, BDNF release, and AMPA receptor expression and the ability of stress and drugs of abuse to activate microglia. Although traditionally research has focused on the role of microglia in development, we will discuss our hypothesis that glial cells also participate in synaptic plasticity following exposure to stress and cocaine. Further, we propose that stress may prime microglia to potentiate their response to addictive drugs, thus contributing to stress-induced increases in vulnerability to substance use (Fig. 1).
Figure 1. Proposed mechanism for a role of microglia in stress-induced vulnerability to cocaine addiction.
Microglia at rest transition into a “primed” state following exposure to stress. The presence of a second immune challenge, in this case cocaine, leads to enhanced spines, BDNF, and AMPA receptor expression in the nucleus accumbens.
The Interplay between Stress and Substance Use.
Epidemiological studies have identified stress as an important risk factor for the development of addiction. People exposed to traumatic events, particularly during childhood are more likely to meet criteria for a SUD later in life (Breslau et al., 2003; Clark et al., 1997; Dembo et al., 1988; Harrison et al., 1997; Widom et al., 1999). It is difficult to determine in clinical populations whether the stress was causal in the increased vulnerability to SUD or if there is a common mediating factor. However, studies in animals have demonstrated that following stress, animals will self-administer more nicotine, cocaine, and ethanol (Fosnocht et al., 2018; Sperling et al., 2010; Yu et al., 2014). Additionally, stress will induce drug seeking after forced withdrawal (see Mantsch et al., 2016).
This enhanced vulnerability to addiction-like behaviors following stress may be due, in part, to the ability of stress to modulate drug-induced synaptic plasticity in the nucleus accumbens. For example, male rats that undergo chronic variable stress show increased dendritic complexity of medium spiny neurons (MSNs) in the accumbens core (Taylor et al., 2014). Because activity within the core of the accumbens is associated with compulsive drug consumption, it has been theorized that stress may facilitate the formation of habitual drug-taking behavior (Taylor et al., 2014). Increases in the spine density of MSNs in the accumbens following social defeat or maternal separation stress have also been reported (Christoffel et al., 2011; Warren et al., 2014). It has also been shown that stress alters gene expression of proteins associated with synaptogenesis in male mice (Warren et al., 2014). Taken together, these data suggest that stress is able to alter the structure of neurons in reward-related brain regions such as the nucleus accumbens.
In addition to structural plasticity, stress alters the physiology of neurons in the accumbens and the ventral tegmental area (VTA). Male rats that undergo sleep deprivation stress show both increases in alcohol consumption and ΔFosB, a transcription factor involved in drug-related neuronal plasticity (Reséndiz-Flores and Escobar, 2018). In male mice, cold water stress enhances glutamatergic transmission in the accumbens and in dopaminergic neurons in the VTA (Campioni et al., 2009; Saal et al., 2003). Repeated social stress has also been shown to enhance N-methyl-D-aspartate receptor (NMDAR)-dependent long term potentiation in the VTA, which correlates with an enhanced conditioned place preference score upon later exposure to cocaine (Stelly et al., 2016). Sub-chronic variable stress alters vesicular glutamate transporters in the nucleus accumbens of female mice but not male mice, suggesting that there are likely sex-differences in the effects of stress on plasticity in reward related brain regions (Brancato et al., 2017).
Together these data suggest that stress increases vulnerability to the development of addictive behaviors in part by enhancing synaptic plasticity in the nucleus accumbens. However, more work is needed to elucidate the mechanisms that create this enhanced synaptic plasticity, and how it leads to more severe addictive phenotypes. We propose microglia partially mediate this stress-induced vulnerability to addiction (Brown and Bachtell, 2018; Lo Iacono et al., 2018).
Microglial Activation Following Exposure to Stress and Drug of Abuse.
Exposure to either stress or drugs of abuse leads to activation of microglia. For example, chronic restraint stress increases Iba1 expression in the PFC and this increase can be reversed by the microglia inhibitor minocycline (Hinwood et al., 2012). Further, it is known that stress activates microglia via the production of danger-associated molecular patterns (DAMPs; Maslanik et al., 2013). DAMPs in turn bind to toll-like receptor 4 (TLR4) on microglia, leading to the activation of the inflammasome nucleotide-binding oligomerization domain, leucine-rich repeat and pyrine domain protein 3 (NLRP3). By engaging TLRs and NLRP3, stress-induced DAMPs cause microglia to produce a variety of pro-inflammatory cytokines (Fleshner et al., 2017). Although the majority of studies on the influence of stress on microglia have been done only in male animals, there is evidence for sex differences in stress-induced microglial response. In contrast to the stress-induced increases in activated microglia in male rats, stress decreases the number of activated microglia in female rats (Bollinger et al., 2016).
Drugs of abuse can activate microglia through similar mechanisms. Minocycline inhibits cocaine-induced locomotor sensitization, suggesting that microglia are responsive to stimulants and contribute to behavioral drug responses (Chen et al., 2009). Additionally, much work has been done examining the involvement of TLR4 in the development of addictive behaviors. Both cocaine and morphine are capable of binding directly to TLR4 (Northcutt et al., 2015; Wang et al., 2012a). Through TLR4 signaling, cocaine and morphine cause microglia to produce pro-inflammatory cytokines (Northcutt et al., 2015; Wang et al., 2012a). Further, inhibiting TLR4 activation in the VTA leads to a decrease in cocaine seeking (Brown et al., 2018). Similarly, VTA inhibition of the interleukin-1 receptor, which binds the pro-inflammatory cytokine interleukin-1 beta also decreases cocaine seeking (Brown et al., 2018). Together this suggests that drug-induced activation of microglia induced by TLR4 activation in the VTA plays a role in cocaine relapse behaviors (Brown et al., 2018). Consistent with this, TLR4 knockout mice have dampened cocaine-induced conditioned place preference relative to wild types (Kashima and Grueter, 2017). Given that TLR4 is expressed primarily on microglia, these TLR4 data suggest that microglia activation is necessary for the development of addiction-like behavior (Kashima and Grueter, 2017).
Microglia and Synaptic Plasticity
While it has been established that exposure to both stress and drugs of abuse elicit a pro-inflammatory response from microglia, the impact of this response on neuronal function is still unclear. Microglia have been shown to survey the brain and transition from a neutral state to a pro-inflammatory state upon detection of danger, similar to macrophages in the periphery (Franco and Fernández-Suárez, 2015). However, microglia may also play a role in processes beyond the typical innate immune response (Paolicelli et al., 2011). For instance, non-activated microglia are still extremely mobile and have constant, dynamic contact with neurons (Nimmerjahn et al., 2005). Much of the research on the role of microglia in neuronal processes has been conducted in animal models of brain development. Microglia have been implicated in a variety of developmental processes, including neurogenesis (Erblich et al., 2011), axon support (Chamak et al., 1994), and synaptic pruning (Paolicelli et al., 2011). Here we focus on several aspects of synaptic plasticity during development and how microglia influence these processes.
Spine Elimination and Formation.
Dendritic spines are protrusions from dendrites that contain the majority of excitatory synapses in the brain (Harris, 1999; Lai et al., 2016). On spiny neurons, dendritic spine measurements represent an approximation for the number of synapses on a neuron (Alvarez and Sabatini, 2007; Lai et al., 2016). By adulthood the majority of spines are stable, however during development spines are more labile (i.e. heightened spine formation and elimination) (Alvarez and Sabatini, 2007). Microglia have been implicated in both the elimination and formation of synapses/dendritic spines (Paolicelli et al., 2011; Parkhurst et al., 2013).
Spine elimination is due in part to the limited availability of neurotrophic survival signals needed to maintain synapses (Lu and Figurov, 1997). However, there is a growing body of literature that suggests that erroneous synapses are also actively pruned by microglia (Ji et al., 2013; Paolicelli et al., 2011; Schafer et al., 2012; Stevens et al., 2007). The chemokine fractalkine (CX3CL1) and its receptor, CX3CR1, are essential components of microglial migration (Liang et al., 2009). Neurons release CX3CL1 which binds to CX3CR1 expressed solely on microglia (Harrison et al., 1998; Jung et al., 2000). CX3CR1 knockout mice show a decrease in the overall number of microglia in the developing brain and a corresponding increase in post-synaptic density 95 staining (a synaptic marker), as well as increased immature synapses and spine density. This suggests that fractalkine signaling and microglial migration are necessary for synaptic pruning in normal developing brains (Paolicelli et al., 2011). This pruning is not random, as microglia preferentially engulf weak synapses. This marking of weak synapses is mediated by complement signaling (Schafer et al., 2012; Stevens et al., 2007). The primary role of complement signaling is to improve phagocytosis of antigens, but a similar mechanism seems to be in place to tag synapses to be phagocytosed by microglia (Stevens et al., 2007). Furthermore, genetic variation of the complement component 4 is associated with risk for schizophrenia, suggesting that alterations in complement signaling lead to inappropriate synaptic pruning in development. This indicates that maladaptive synaptic pruning by microglia can lead to pathological outcomes in adulthood (Sekar et al., 2016). Additionally, microglia eliminate D1 receptors tagged by complement proteins in the nucleus accumbens during adolescence in males but not females (Kopec et al., 2018). This suggests that microglia may regulate developmental synaptic plasticity in a sex-dependent manor.
Along with this role in spine elimination, microglia also seem to be important for the formation of new spines. In vitro experiments have shown that higher concentrations of microglia lead to increased spine density in cultured hippocampal neurons (Lim et al., 2013). Mice with a genetic ablation of microglia show a decrease in both the elimination and formation of dendritic spines (Parkhurst et al., 2013). These mice also show deficits in spine formation as adults that correspond with diminished performance on a motor learning task (Parkhurst et al., 2013). Further, contact between microglia and neurons stimulates the formation of dendritic spines early in development but not in later development (Miyamoto et al., 2016). Taken together, these data suggest that microglia exercise bidirectional regulation of spine density.
Brain-derived Neurotrophic Factor
Neurotrophins are growth factors that act as survival signals to neurons and promote cell survival and growth (Cohen-Cory et al., 2010). BDNF is one such neurotrophin and it has been shown to regulate neuron survival, neurite growth, and synaptogenesis during development and learning (Huang and Reichardt, 2001; Parkhurst et al., 2013). Microglia have been shown to assist in learning-related synaptic plasticity through the secretion of BDNF (Parkhurst et al., 2013). Mice with a microglial-specific knockout of BDNF show impaired spine formation following motor task training (Parkhurst et al., 2013). Interestingly, these mice also show impaired learning of the task relative to controls, suggesting that learning-induced spine formation on neurons is dependent on microglial BDNF production (Parkhurst et al., 2013).
AMPA Receptor Expression
Microglia may also be able to alter AMPA receptors (AMPARs) in developing brains. AMPARs are glutamate receptors that are critical to the development of long-term potentiation and their trafficking in and out of the synapse is an important measure of synaptic plasticity (see Huganir and Nicoll, 2013 for review). During development, the brain has increased levels of a unique type of glutamatergic synapse that does not contain AMPARs, known as silent synapses. Silent synapses are characterized as synapses that contain NMDARs but no AMPARs (Huang et al., 2015b). NMDARs contain magnesium plugs that are only jettisoned when the cell is depolarized (Blanke and VanDongen, 2009). To achieve that depolarization, AMPARs must be present to bind glutamate and allow positive ions to enter the cell (Blanke and VanDongen, 2009). In a silent synapse, there are no AMPARs therefore the NMDARs cannot be activated and the synapse is “silent” (Huang et al., 2015b). AMPARs are extremely dynamic and are trafficked quickly as a result of experience (Zhu, 2009). Therefore, it is theorized that silent synapses serve to prime the brain for periods of heightened synaptic plasticity (Dong and Nestler, 2014). These silent synapses are present in high levels in developing brains and are greatly reduced following puberty (Hanse et al., 2013). The transition of silent synapses to functional synapses is necessary to ending critical periods of heightened plasticity in the visual cortex (Huang et al., 2015a). Prevention of the maturation of silent synapses extended the critical period of visual development, known as juvenile ocular dominance plasticity, into late adulthood (Huang et al., 2015a). The enhanced learning seen in children compared to adults could be due in part to the concentration of silent synapses (Dong and Nestler, 2014).
Silent synapses become functional upon the insertion of AMPARs into the synapse (Dong and Nestler, 2014). Interestingly, it seems microglia are capable of regulating AMPA receptor expression on neurons via the secretion of the pro-inflammatory cytokine tumor necrosis factor- alpha (TNF-α) (Beattie et al., 2002; He et al., 2012; Leonoudakis et al., 2004; Leonoudakis et al., 2008; Lewitus et al., 2016; Yin et al., 2012). The literature is somewhat mixed, with some researchers finding that TNF-α enhances AMPAR signaling through the insertion of calcium permeable AMPARs (He et al., 2012; Leonoudakis et al., 2008); and other studies finding decreased AMPAR signaling in response to TNF-α (Lewitus et al., 2016). Therefore, it is possible that TNF-α bidirectionally regulates AMPAR expression on neurons. The studies that found that TNF-α enhances AMPAR were both conducted in the hippocampus, while the study that found TNF-α decreases AMPAR was examining the nucleus accumbens, so there may be brain region specific responses of AMPAR to TNF-α (He et al., 2012; Leonoudakis et al., 2008; Lewitus et al., 2016).
Synaptic Plasticity and Cocaine Addiction
Due to the many observations that drugs of abuse cause heightened synaptic plasticity in reward-related brain regions, Dong and Nestler (2014) have conceptualized addiction as reward pathways reverting back to a developmental-like state. One measure in particular of synaptic plasticity has received a lot of attention in the addiction literature: spine density. On MSNs in the nucleus accumbens, spine density is altered upon exposure to drugs of abuse. Spines are dynamic and can appear rapidly but are also very long lasting (Lai et al., 2016). These long lasting changes to the neuron morphology are believed to contribute to long-lasting craving in addicts (Christian et al., 2017). However, different drugs of abuse modulate spine density in the accumbens differently (Spiga et al., 2014). While cocaine causes an increase in MSN spine density (Cahill et al., 2017; Kim et al., 2011b; Lee et al., 2006; Robinson and Kolb, 1999; Toda et al., 2010), opioids have been shown to decrease MSN spine density (Miller et al., 2012; Spiga et al., 2014).
Typically, neurotrophins are prevalent in developing brains but present in much lower levels in adulthood (for review see Huang and Reichardt, 2001). In the case of cocaine addiction, adult brains once again have increased levels of neurotrophins, particularly BDNF (McGinty et al., 2010). Increased BDNF levels (both mRNA and protein) have been observed in multiple brain regions related to reward processing such as the medial PFC, VTA, and the nucleus accumbens (Anderson et al., 2017; Bahi et al., 2008; Giannotti et al., 2014; Grimm et al., 2003; Li et al., 2013). In the accumbens, BDNF protein levels correlate with cocaine craving (Li et al., 2013). Following periods of forced abstinence, animals exhibit an increase in drug seeking coined incubation of craving (see Pickens et al., 2011 for review). This same increase in drug craving following periods of abstinence has also been demonstrated in humans with SUD (Bedi et al., 2011; Li et al., 2015; Parvaz et al., 2016)). This incubation of craving is dependent on changes in synaptic plasticity (Christian et al., 2017; Li et al., 2013; Loweth et al., 2014). In the case of cocaine, this includes increases in dendritic spine density (Christian et al., 2017). Interestingly, cocaine exposure increases BDNF protein levels in the accumbens on a similar timescale, with levels elevated compared to controls at around withdrawal day 45 and 90 (Li et al., 2013). While the relationship between incubation of craving, spine density, and BDNF is still being elucidated, some researchers hypothesize that BDNF is upregulated following prolonged glutamatergic transmission and contributes to the maintenance of craving (Loweth et al., 2014).
Incubation of craving also correlates with an increased number of silent synapses in the nucleus accumbens following exposure to drugs of abuse (Huang et al., 2015b). In models of cocaine use disorder, silent synapse levels peak during the first days of withdrawal (Huang et al., 2015b). As the withdrawal period continues, AMPARs are inserted into the silent synapses, thereby “un-silencing” them (Huang et al., 2015b). AMPARs can be divided into two subgroups: GluA2-lacking calcium permeable-AMPARs and GluA2-containing, calcium impermeable AMPAR (Ma et al., 2016). This distinction is important because calcium permeable-AMPARs have increased conductance compared to calcium impermeable-AMPARs (Ma et al., 2016). In the case of cocaine withdrawal, it is specifically calcium permeable-AMPARs that are inserted into the silent synapses and correlate with enhanced cocaine craving (Loweth et al., 2014). In fact, methods that promote the insertion of calcium impermeable-AMPARs in the synapse over calcium permeable-AMPAR actually reduce cocaine-seeking behaviors (Briand et al., 2014; Famous et al., 2008; Ma et al., 2016). Taken together, these data suggest that the development of silent synapses and un-silencing with calcium permeable-AMPARs, are an important component of cocaine-related neuroplasticity.
Exposure to cocaine leads to heightened synaptic plasticity in the nucleus accumbens, including increases in spine density, levels of the neurotrophic factor BDNF, and silent synapses. On a molecular level, these synaptic changes resemble normal developmental plasticity. This supports the hypothesis that cocaine inappropriately turns on mechanisms for synaptic plasticity that are supposed to be turned off once the brain is fully developed, leading to addictive phenotypes (Dong and Nestler, 2014). Given the recent discoveries of the role of microglia in developmental synaptic plasticity, it’s possible that microglia are also assisting in the drug-related changes in the nucleus accumbens.
Possible Role for Microglia in Cocaine Addiction-Related Plasticity
Microglia are potential contributors to the changes in spine density seen following exposure to drugs of abuse, given their established role in regulating spine density during development. Upon exposure to cocaine, microglia may increase contact with MSNs and promote formation of more spines. In support of this possibility, microglia have been shown to modify spine density in the nucleus accumbens of adult mice in an experience-dependent manner. One study has shown that male mice exposed to a high fat/high sugar diet show a decrease in spine density in the nucleus accumbens shell. This decrease in spine density was rescued by minocycline, a microglia inhibitor, suggesting that microglial activation by the high fat/high sugar diet led to altered spine density in the accumbens (Gutiérrez-Martos et al., 2018). Given that microglia have been implicated in the restructuring of neurons in the accumbens in response to rewarding stimuli, their role in addiction-related spine density changes should be investigated.
Multiple studies have shown that incubation of cocaine craving correlates with increased BDNF levels in the accumbens (Christian et al., 2017; Li et al., 2013; Loweth et al., 2014), however most of these experiments have not assessed the origins of this increased BDNF. Given that microglial BDNF facilitates spine growth during the learning of a new task (Parkhurst et al., 2013), it is possible that microglia are responsible for the observed increase in BDNF. That is, following exposure to cocaine, microglial BDNF levels may gradually increase as microglia facilitate the formation of new spines, causing incubation of craving. In support of this, microglial cells incubated with cocaine for 72 hours in vitro produce extremely high levels of BDNF compared to those incubated without cocaine in the medium (Cotto et al., 2018). Neurons incubated with cocaine produced much lower levels of BDNF in comparison, suggesting that microglia may be the primary producers of BDNF in response to cocaine (Cotto et al., 2018). Further studies are needed to determine the role of microglial BDNF in vivo, including the importance of microglial BDNF in the incubation of craving. A mouse line generated by Parkhurst et al. (2013) that allows for the knockout of BDNF specifically from microglial cells would be a useful tool in this exploration. One could theorize that mice without microglial BDNF would show fewer addictive behaviors following cocaine exposure.
Microglia could be contributing to the incubation of craving by regulating AMPAR expression on neurons following cocaine exposure. As mentioned above, cocaine is capable of activating microglia through DAMPs which in turn bind to TLRs (Crews et al., 2017b). This sets off a signaling cascade that culminates with the production of pro-inflammatory cytokines, including TNF-α, which regulates AMPA expression on neurons (Beattie et al., 2002; Crews et al., 2017b). Repeated experimenter administered cocaine leads to activation of striatal microglia and increased TNF-α production, which in turn reduces glutamatergic transmission in the nucleus accumbens (Lewitus et al., 2016). Under these conditions, activating microglia following cocaine exposure leads to a reduction in AMPA/NMDA ratios in the accumbens of male mice, while also reducing locomotor sensitization to cocaine. It appears, at least in these experimental conditions, that TNF-α plays a protective role, dampening the pathological synaptic plasticity (Lewitus et al., 2016). Incubation of craving could be mediated by microglia activation. Cocaine self-administration protocols that elicit incubation of cocaine craving result in a prolonged pro-inflammatory response (Loweth et al., 2014). During this time, microglia are producing TNF-α, which may act to prevent the unsilencing of silent synapses by reducing the expression of calcium permeable-AMPARs. During withdrawal, microglia are no longer being activated by cocaine, so gradually TNF-α levels will decrease. As TNF-α levels are reduced, more calcium permeable-AMPAR will be able to be expressed, leading to unsilencing of synapses, enhanced glutamatergic transmission and increased cocaine craving. It is important to point out that this only one hypothesis. The Lewitus et al. (2016) study uses researcher-administered cocaine injections, which would likely not induce incubation of craving (Loweth et al., 2014). Also, the behavioral output they measured was locomotor sensitization and craving was never assessed (Lewitus et al., 2016). However, given the importance of silent synapses and AMPAR trafficking in cocaine-related plasticity (Dong and Nestler, 2014; Huang et al., 2015b), and the evidence that TNF-α is able to regulate AMPAR expression on neurons (Beattie et al., 2002; He et al., 2012; Lewitus et al., 2016), further exploration into the relationship between microglial TNF-α and cocaine-induced silent synapses is merited. One approach would be to artificially increase TNF-α levels during withdrawal, as the inflammatory response is waning. This may delay the unsilencing of glutamatergic synapses, and reduce craving levels.
Synaptic Plasticity and Stress
The relationship between stress and synaptic changes in the brain is complex, with most of the data suggesting that stress has opposing effects on many measures of synaptic plasticity in different brain regions (see Bennett and Lagopoulos, 2014 for review). For example, the effects of stress on spine density vary depending on the brain region of interest. Generally speaking, chronic stressors tend to increase spine density in the amygdala (Cohen et al., 2014; Skrzypiec et al., 2013), while decreasing spine density in the PFC (Hains et al., 2015; Luczynski et al., 2015; Radley et al., 2006; Wang et al., 2012b).
While less research has been done on the effects of stress on spine density in the nucleus accumbens, several studies indicate that stress increases spine density in the accumbens (Christoffel et al., 2011; Muhammad et al., 2012; Warren et al., 2014; Yang et al., 2015). As stated above, these stress-induced changes in the synaptic plasticity of the accumbens may contribute to the high co-morbidity rates seen between stress disorders and addiction. However, one similar study found no effect of social isolation following weaning on spine density in the nucleus accumbens (Wang et al., 2012b). The other studies used social defeat and foot shock stress paradigms rather than social isolation, which may indicate that the type of stressor is an important factor in determining how stress impacts accumbens spine density. Further research will help elucidate the conditions in which these changes in spine density occur. Like spine density, several studies have found that stress increases BDNF levels in the nucleus accumbens (Bessa et al., 2013; Nikulina et al., 2012; Walsh et al., 2014; Yang et al., 2015). Male rats show elevated levels of BDNF mRNA following chronic mild stress (Bessa et al., 2013).
Stress has also been shown to alter glutamatergic transmission. Stress alters AMPA/NMDA ratios and AMPA-mediated EPSCs in the nucleus accumbens and the hippocampus (Campioni et al., 2009; Liu et al., 2015). Stress also appears to reduce the phosphorylation of the AMPAR subunit GluA1 at both the S831 and S845 phosphorylation sites in hippocampal neurons (Liu et al., 2015). As the levels of phosphorylation of GluA1 are related to their expression at the membrane (Man et al., 2007), these findings support a stress-induced decrease in AMPAR signaling. Further, animals who have undergone social defeat stress show decreased levels in the GluA1 subunit in the PFC and hippocampus (Yang et al., 2016). However, the same stressor seems to elevate GluA1 levels in the nucleus accumbens (Yang et al., 2016).
To our knowledge, there are no published studies examining whether stress leads to the formation of silent synapses in the nucleus accumbens. However, stressful experiences lead to the generation of silent synapses in the amygdala (Ito et al., 2015; Suvrathan et al., 2014). Interestingly, these silent synapses corresponded with increased fear responses, suggesting that the stressful events led to heightened synaptic plasticity which in turn led to enhanced fear learning (Ito et al., 2015; Suvrathan et al., 2014). When using other measures of synaptic plasticity, such as dendritic spine density, researchers generally see hypertrophy in both the amygdala and the accumbens (Christoffel et al., 2011; Cohen et al., 2014; Skrzypiec et al., 2013; Warren et al., 2014). Since similar stress-induced changes are seen in this measure of plasticity in the amygdala and accumbens, it is possible that stress also causes silent synapses to be formed in the nucleus accumbens. Electrophysiological studies of the accumbens in stressed vs naïve animals could be used to test this hypothesis.
Possible Roles of Microglia in Stress Plasticity
Microglia are involved in spine elimination and formation during normal development (Miyamoto et al., 2016; Paolicelli et al., 2011). It is possible that microglia also contribute to the increases in spine density and BDNF seen in the nucleus accumbens following stress. Stress leads to the production of glucocorticoid, mineralcorticoid and corticotropin-releasing factor (CRF) (see Joëls and Baram, 2009). It should be noted that all three of these receptors, glucocorticoid receptors, mineralcorticoid receptors, and CRF receptors, are expressed on microglia (Chantong et al., 2012; Kim et al., 2011a). Given that the hormones that contribute to the stress response also bind to microglia, and that stress can activate microglia via DAMPs, it is possible that microglia are also contributing to stress-related neuroadaptations. Chronic unpredictable stress decreases spine density in the PFC of male mice, and this decreased spine density could be prevented by blocking colony stimulating factor 1, which is released by neurons and activates microglia (Wohleb et al., 2018). This indicates that microglial activation is needed for stress-induced changes in spine density (Wohleb et al., 2018). Whether a similar mechanism is happening in the nucleus accumbens remains to be seen.
Microglia also mediate stress-induced changes in AMPAR expression and function. Inhibition of microglia reverses reduction in GluA1 phosphorylation following chronic unpredictable stress (Liu et al., 2015). Again, these data are from hippocampal neurons, and to our knowledge the role of microglia in accumbens AMPAR expression has not been explored. However, this research indicates that microglia actively participate in synaptic plasticity responses to stress.
Stress Primes Microglia: Implications for Stress-induced Vulnerability to Cocaine Addiction
Stress activates microglia via the production of DAMPs and signaling through TLR4. However it is important to point out that stress also primes microglia for a second immune challenge, meaning that once the cells are activated by stress, they show enhanced release of pro-inflammatory cytokines when activated a second time (Weber et al., 2015). This enhanced activation is due in part to glucocorticoids, as antagonism of the glucocorticoid receptor attenuates the microglia response in stressed animals to a second pro-inflammatory challenge (Frank et al., 2012). Stress priming of microglia also elevates levels of the DAMP high motility group box-1, which in turn leads to increased inflammasome NLRP3 protein levels (Weber et al., 2015). Additionally, stress-primed microglia are morphologically distinct from control microglia (Hinwood et al., 2013). They exhibit hyper-ramification, or more complex branching than the non-stressed control microglia, which can be prevented by minocycline (Hinwood et al., 2013). It should be noted that many of these studies only examined male animals. A recent study found that both male and female rats undergo stress priming of the immune system, but it appears to be dependent on microglia in males but not females (Fonken et al., 2018). As more studies examine sex differences in stress and immune interactions, the underlying mechanisms will be elucidated.
In many stress-priming studies, the second challenge is the endotoxin lipopolysaccharide, which elicits a strong pro-inflammatory response from microglia (Frank et al., 2012; Weber et al., 2013; Weber et al., 2015). However, mice exposed to early life stress have enhanced pro-inflammatory responses to cocaine later in adulthood (Lo Iacono et al., 2018). This suggests that stress can prime microglia to cocaine as well. The stressed animals also exhibited less dopaminergic transmission in the VTA, which was reversed by the inhibition of microglia (Lo Iacono et al., 2018). Interestingly, this study also investigated the effects of childhood maltreatment in human cocaine users, finding that childhood trauma correlated with higher levels of circulating pro-inflammatory cytokines in response to cocaine (Lo Iacono et al., 2018). Thus, it appears that this animal model for studying early stress and addiction has some translational validity. Another recent study has demonstrated that TNF-α induced by early life stress alters AMPAR subunit expression and enhances cocaine conditioning in male rats. By blocking TNF-α, researchers were able to normalize both AMPAR subunit expression and cocaine response (Ganguly et al., 2019). Given that microglia are known to produce TNF-α in response to stress, it’s possible that this stress-enhanced cocaine conditioning is mediated by microglia.
Stress is a major risk factor for the development of addiction in humans (Breslau et al., 2003; Clark et al., 1997; Dembo et al., 1988; Harrison et al., 1997; Widom et al., 1999). Perhaps this is due, in part, to stress altering subsequent microglial activation by drugs of abuse. If in fact microglia contribute to addiction related plasticity, stressed-primed microglia may have greater capacity to alter synaptic plasticity in reward-related brain regions in response to drugs of abuse (Fig. 1). Given the vast literature of synaptic changes following exposure to stress and drugs of abuse, and the role of microglia in similar processes, it seems probable that microglia are important mediators of this synaptic plasticity and could possibly contribute the stress-induced vulnerability to addiction. Recent work by Lo Iacono et al., (2018) and Ganguly et al. (2019) support this hypothesis.
Conclusion
While microglia have historically been studied for their role in innate immunity, recent work has focused on the role of microglia in the regulation of synaptic plasticity during development (Paolicelli et al., 2011; Tremblay et al., 2010). Given the similarities in plasticity during development and plasticity during addiction and stress, it seems likely that microglia would also influence plasticity following exposure to stressors and drugs of abuse (Table 2). Exposure to both drugs of abuse and stress lead to increases in circulating pro-inflammatory cytokines (Fox et al., 2012; Levandowski et al., 2016; Nabati et al., 2013) (Weber et al., 2015; Wohleb et al., 2018). In animal models, multiple drugs of abuse have been shown to elicit a pro-inflammatory response in the brain (Chen et al., 2012; Coller and Hutchinson, 2012; Crawford et al., 2006; Crews et al., 2017a; Hutchinson et al., 2012; Hutchinson and Watkins, 2014; Jacobsen et al., 2014; Lopez-Pedrajas et al., 2015). Given that microglia play such an active role in synaptic changes during normal development and learning, there is potential for microglia to not only influence stress and drug responses through inflammation, but also via regulation of synaptic plasticity.
During development, microglia can regulate synaptic plasticity bidirectionally (see Ueno and Yamashita, 2014 for review). This could be of particular interest in the context of addiction. While this review has focused primarily on cocaine neuroadaptations, different drugs of abuse elicit varied neuroadaptations (Miller et al., 2012). For instance, cocaine and opioids often have polar opposite effects on the brain (Miller et al., 2012). While cocaine increases spine density in the accumbens, opioids decrease it (Miller et al., 2012). Cocaine causes increases in BDNF levels (McGinty et al., 2010), opioids decrease it (Chen et al., 2012). And while incubation of opioid craving has been demonstrated in rodents (Li et al., 2008; Theberge et al., 2012), the molecular underpinnings of that process are not as well characterized as cocaine craving. It is possible that the molecular mechanisms of incubation of cocaine craving look very different from incubation of opioid craving. As microglia have bidirectional control of synaptic plasticity (i.e. can eliminate or form new spines (Paolicelli et al., 2011; Parkhurst et al., 2013) and can upregulate or downregulate calcium permeable AMPAR via cytokine production (Beattie et al., 2002; Lewitus et al., 2016; Lewitus et al., 2014), they could represent a common mechanism leading to opposing effects on plasticity seen with these different classes of drugs. Further testing is needed to determine the role of microglia on the divergent neuroadaptations of cocaine and opioids. Stress also leads to opposing synaptic changes, with plasticity being enhanced in some brain regions, and inhibited in others (Cohen et al., 2014; Hains et al., 2015; Luczynski et al., 2015; Radley et al., 2006; Skrzypiec et al., 2013; Wang et al., 2012b). Perhaps this is due in part to the bidirectional regulation of synaptic plasticity exerted by microglia.
Of note, the model proposed in this review (Fig. 1) is influenced by the timing of the stressor. When animals are exposed to stress during early development (Lo Iacono et al., 2018), microglia may not only be primed for future cocaine-induced plasticity, but normal developmental processes may be disrupted. Given the stated importance of microglia to healthy development, exposure to stress during sensitive developmental windows may prevent microglia from effectively pruning synapses or sculpting circuits (Brown and Bachtell, 2018). This combined with the primed immune response could make stress during important developmental windows more damaging than stress during adulthood. More experiments are needed to determine the importance of the timing of the stressor.
This review largely does not discuss sex-differences in the immune responses to stress or drugs of abuse. While the data that is available certainly suggests that sex is a major factor in the immune response to stress, there are far fewer studies done in females than males. Generally, the findings of these studies suggest that male immune systems are activated more readily by stress, that microglia play a more active role in the development of reward circuitry in males, and males are more vulnerable to subsequent development of addictive behaviors (Bollinger et al., 2016; Ganguly et al., 2019; Kopec et al., 2018). This line of research is in its infancy, but there is enough evidence of sex-differences in these processes and the importance of using both males and females when studying stress and the immune system should not be overlooked.
While this review focuses on pre-clinical studies, the role of the innate immune system in addiction and stress has also been examined in humans (Chan et al., 2015; Fox et al., 2012; Pedraz et al., 2015). In these studies, drugs of abuse modulate circulating pro-inflammatory cytokines in the periphery (Chan et al., 2015; de Timary et al., 2017; Fox et al., 2012; Pedraz et al., 2015). There have also been advances in technology that allow researchers to study microglia in vivo in the human brain (Cagnin et al., 2002). For instance, positron emission topography (PET) scans can be used to detect activated microglia (Cagnin et al., 2002). Results from these studies have been mixed, with studies showing increases of microglial activation (methamphetamine; Sekine et al., 2008)), no difference in microglia activation (cocaine; Narendran et al., 2014) and decreased microglia activation (alcohol; Hillmer et al., 2017; Kalk et al., 2017) in response to drugs of abuse. This could possibly be explained by the differences in the abused drugs. Inflammation has also been shown to correlate with stress-related disorders in humans, such as post-traumatic stress disorder and depression (Haapakoski et al., 2015; Passos et al., 2015). PET scans examining microglia activation in depressed patients have been inconsistent, with some finding increases in activation, while other found no differences between depressed patients and healthy controls (Hannestad et al., 2013; Setiawan et al., 2015). These inconsistencies could be explained in part by differences in patient populations, and technological limitations (see van der Doef et al., 2015 for review). However, a recent paper from Lo Iacono et al. (2018) discovered evidence of an immunological link between stress and addiction in humans. They found that cocaine addicts with a history of childhood trauma had elevated levels of circulating pro-inflammatory cytokines compared to cocaine addicts without childhood trauma. This finding is the first evidence seen in humans that supports the model proposed in Fig. 1.
Stress and addiction are both characterized by changes in synaptic plasticity. Recently there have been new insights into the role of microglia in synaptic plasticity during development and learning, however the role of microglia in stress and addiction-induced plasticity remains understudied. Perhaps by studying microglia not only for their inflammatory processes but also as synaptic regulators, we will get a clearer understanding of the link between stress and addiction.
Highlights.
Stress and drugs of abuse both alter synaptic plasticity, specifically spine density, brain-derived neurotrophic factor (BDNF) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression.
Microglia are activated by both stress and drugs of abuse, and have been shown to regulate synaptic plasticity during brain development.
Microglia may mediate stress- and drug-induced synaptic plasticity.
Primed microglia may account for stress-induced vulnerability to substance use disorders.
Acknowledgments
Funding
This work was supported by National Institute on Drug Abuse (NIDA) Grant R00 DA033372 (L.A.B.) and a Brain & Behavior Research Foundation NARSAD award (L.A.B.).
Footnotes
Disclosure
The authors declare no conflict of interest.
Bottom of Form
- Alvarez VA, Sabatini BL, 2007. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30, 79–97. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Wissman AM, Chemplanikal J, Buzin N, Guzman D, Larson EB, Neve RL, Nestler EJ, Cowan CW, Self DW, 2017. BDNF-TrkB controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proc Natl Acad Sci U S A 114, 9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL, 2008. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 199, 169–182. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC, 2002. Control of synaptic strength by glial TNFalpha. Science 295, 2282–2285. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H, 2011. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69, 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J, 2014. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol 112, 80–99. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N, 2013. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Translational psychiatry 3, e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke ML, VanDongen AMJ, 2009. Activation Mechanisms of the NMDA Receptor, in: Van Dongen AM (Ed.), Biology of the NMDA Receptor, Boca Raton (FL). [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM, Wellman CL, 2016. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE, 2017. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR, 2003. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of general psychiatry 60, 289–294. [DOI] [PubMed] [Google Scholar]
- Briand LA, Kimmey BA, Ortinski PI, Huganir RL, Pierce RC, 2014. Disruption of glutamate receptor-interacting protein in nucleus accumbens enhances vulnerability to cocaine relapse. Neuropsychopharmacology 39, 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KT, Bachtell RK, 2018. Activation of the Immune System During a Developmental Window May Provide a Link Between Early Life Stress and Future Susceptibility to Cocaine Abuse. Biol Psychiatry 84, 865–866. [DOI] [PubMed] [Google Scholar]
- Brown KT, Levis SC, O’Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR, Bachtell RK, 2018. Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav Immun 67, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Gerhard A, Banati RB, 2002. In vivo imaging of neuroinflammation. Eur Neuropsychopharmacol 12, 581–586. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Walker DM, Gancarz AM, Wang ZJ, Lardner CK, Bagot RC, Neve RL, Dietz DM, Nestler EJ, 2017. The dendritic spine morphogenic effects of repeated cocaine use occur through the regulation of serum response factor signaling. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS, 2009. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol 101, 3192–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamak B, Morandi V, Mallat M, 1994. Brain macrophages stimulate neurite growth and regeneration by secreting thrombospondin. Journal of neuroscience research 38, 221–233. [DOI] [PubMed] [Google Scholar]
- Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY, 2015. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res 226, 230–234. [DOI] [PubMed] [Google Scholar]
- Chantong B, Kratschmar DV, Nashev LG, Balazs Z, Odermatt A, 2012. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J Neuroinflammation 9, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H, 2009. Minocycline affects cocaine sensitization in mice. Neurosci Lett 452, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Tao PL, Chu CH, Chen SH, Wu HE, Tseng LF, Hong JS, Lu RB, 2012. Low-dose memantine attenuated morphine addictive behavior through its anti-inflammation and neurotrophic effects in rats. J Neuroimmune Pharmacol 7, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ, Estepanian D, Araghi CH, Stutzmann GE, Wolf ME, 2017. Dynamic Alterations of Rat Nucleus Accumbens Dendritic Spines over 2 Months of Abstinence from Extended-Access Cocaine Self-Administration. Neuropsychopharmacology 42, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ, 2011. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Lesnick L, Hegedus AM, 1997. Traumas and other adverse life events in adolescents with alcohol abuse and dependence. J Am Acad Child Adolesc Psychiatry 36, 1744–1751. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z, 2014. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur Neuropsychopharmacol 24, 1925–1944. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S, 2010. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70, 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR, 2012. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134, 219–245. [DOI] [PubMed] [Google Scholar]
- Cotto B, Li H, Tuma RF, Ward SJ, Langford D, 2018. Cocaine-mediated activation of microglia and microglial MeCP2 and BDNF production. Neurobiol Dis 117, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford FC, Wood ML, Wilson SE, Mathura VS, Hollen TR, Geall F, Kolippakkam DN, Mullan MJ, 2006. Cocaine induced inflammatory response in human neuronal progenitor cells. J Neurochem 97, 662–674. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG, 2017a. The role of neuroimmune signaling in alcoholism. Neuropharmacology 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG, Vetreno RP, 2017b. Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234, 1483–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Timary P, Starkel P, Delzenne NM, Leclercq S, 2017. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 122, 148–160. [DOI] [PubMed] [Google Scholar]
- Dembo R, Dertke M, Borders S, Washburn M, Schmeidler J, 1988. The relationship between physical and sexual abuse and tobacco, alcohol, and illicit drug use among youths in a juvenile detention center. Int J Addict 23, 351–378. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ, 2014. The neural rejuvenation hypothesis of cocaine addiction. Trends in pharmacological sciences 35, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW, 2011. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6, e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC, 2008. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci 28, 11061–11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, Maier SF, 2017. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 42, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, D’Angelo HM, Daut RA, Hampson EC, Ayala MT, Watkins LR, Maier SF, 2018. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun 70, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA, 2018. Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R, 2012. Immune system inflammation in cocaine dependent individuals: implications for medications development. Human psychopharmacology 27, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Fernández-Suárez D, 2015. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131, 65–86. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF, 2012. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun 26, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, Honeycutt JA, Rowe JR, Demaestri C, Brenhouse HC, 2019. Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F, 2014. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int J Neuropsychopharmacol 17, 625–634. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y, 2003. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23, 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Martos M, Girard B, Mendonça-Netto S, Perroy J, Valjent E, Maldonado R, Martin M, 2018. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict Biol 23, 735–749. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M, 2015. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Yabe Y, Arnsten AF, 2015. Chronic Stimulation of Alpha-2A-Adrenoceptors With Guanfacine Protects Rodent Prefrontal Cortex Dendritic Spines and Cognition From the Effects of Chronic Stress. Neurobiol Stress 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee JY, O’Connor KC, Pelletier D, Carson RE, 2013. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Seth H, Riebe I, 2013. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci 14, 839–850. [DOI] [PubMed] [Google Scholar]
- Harris KM, 1999. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol 9, 343–348. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L, 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95, 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PA, Fulkerson JA, Beebe TJ, 1997. Multiple substance use among adolescent physical and sexual abuse victims. Child Abuse Negl 21, 529–539. [DOI] [PubMed] [Google Scholar]
- He P, Liu Q, Wu J, Shen Y, 2012. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. FASEB J 26, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Miniño AM, 2017. Drug Overdose Deaths in the United States, 1999–2016. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Hillmer AT, Sandiego CM, Hannestad J, Angarita GA, Kumar A, McGovern EM, Huang Y, O’Connor KC, Carson RE, O’Malley SS, Cosgrove KP, 2017. In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry 22, 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR, 2012. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22, 1442–1454. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR, 2013. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 23, 1784–1797. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF, 2001. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosang L, Dong Y, Löwel S, Schlüter OM, 2015a. Progressive maturation of silent synapses governs the duration of a critical period. Proc Natl Acad Sci U S A 112, E3131–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schlüter OM, Dong Y, 2015b. Silent Synapses Speak Up: Updates of the Neural Rejuvenation Hypothesis of Drug Addiction. Neuroscientist 21, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA, 2013. AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Watkins LR, 2014. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76 Pt B, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A, 2015. Observation of Distressed Conspecific as a Model of Emotional Trauma Generates Silent Synapses in the Prefrontal-Amygdala Pathway and Enhances Fear Learning, but Ketamine Abolishes those Effects. Neuropsychopharmacology 40, 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JH, Watkins LR, Hutchinson MR, 2014. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. International review of neurobiology 118, 129–163. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE, 2013. Microglia actively regulate the number of functional synapses. PLoS One 8, e56293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ, 2009. The neuro-symphony of stress. Nat Rev Neurosci 10, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR, 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Guo Q, Owen D, Cherian R, Erritzoe D, Gilmour A, Ribeiro AS, McGonigle J, Waldman A, Matthews P, Cavanagh J, McInnes I, Dar K, Gunn R, Rabiner EA, Lingford-Hughes AR, 2017. Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: a [(11)C]PBR28 PET study. Translational psychiatry 7, e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima DT, Grueter BA, 2017. Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc Natl Acad Sci U S A 114, 8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Ryu DH, Hwang S, 2011a. The expression of corticotropin-releasing factor and its receptors in the spinal cord and dorsal root ganglion in a rat model of neuropathic pain. Anat Cell Biol 44, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park BH, Lee JH, Park SK, Kim JH, 2011b. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry 69, 1026–1034. [DOI] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, Bilbo SD, 2018. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun 9, 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Jordan BA, Ma XM, Srivastava DP, Tolias KF, 2016. Molecular Mechanisms of Dendritic Spine Development and Plasticity. Neural Plast 2016, 2078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P, 2006. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A 103, 3399–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC, 2004. TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol 1, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Zhao P, Beattie EC, 2008. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J Neurosci 28, 2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandowski ML, Viola TW, Prado CH, Wieck A, Bauer ME, Brietzke E, Grassi-Oliveira R, 2016. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend 167, 140–148. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D, 2016. Microglial TNF-α Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron 90, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D, 2014. An adaptive role of TNFα in the regulation of striatal synapses. J Neurosci 34, 6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L, 2015. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol 20, 513–522. [DOI] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME, 2013. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci 33, 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L, 2008. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28, 13248–13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT, 2009. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci 50, 4444–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Park E, You B, Jung Y, Park AR, Park SG, Lee JR, 2013. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One 8, e81218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li J, Dai P, Zhao F, Zheng G, Jing J, Wang J, Luo W, Chen J, 2015. Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress-induced cognitive dysfunction. Stress 18, 96–106. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Catale C, Martini A, Valzania A, Viscomi MT, Chiurchiu V, Guatteo E, Bussone S, Perrone F, Di Sabato P, Arico E, D’Argenio A, Troisi A, Mercuri NB, Maccarrone M, Puglisi-Allegra S, Casella P, Carola V, 2018. From Traumatic Childhood to Cocaine Abuse: The Critical Function of the Immune System. Biol Psychiatry 84, 905–916. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrajas R, Ramirez-Lamelas DT, Muriach B, Sanchez-Villarejo MV, Almansa I, Vidal-Gil L, Romero FJ, Barcia JM, Muriach M, 2015. Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front Cell Neurosci 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME, 2014. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology 76 Pt B, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Figurov A, 1997. Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8, 1–12. [DOI] [PubMed] [Google Scholar]
- Luczynski P, Moquin L, Gratton A, 2015. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress 18, 654–667. [DOI] [PubMed] [Google Scholar]
- Ma YY, Wang X, Huang Y, Marie H, Nestler EJ, Schlüter OM, Dong Y, 2016. Re-silencing of silent synapses unmasks anti-relapse effects of environmental enrichment. Proc Natl Acad Sci U S A 113, 5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL, 2007. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A 104, 3579–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y, 2016. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41, 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M, 2013. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun 28, 54–62. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW Jr., Berglind WJ, 2010. Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC, Zhang L, Dummer BW, Cariveau DR, Loh H, Law PY, Liao D, 2012. Differential modulation of drug-induced structural and functional plasticity of dendritic spines. Mol Pharmacol 82, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J, 2016. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun 7, 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B, 2012. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 216, 103–109. [DOI] [PubMed] [Google Scholar]
- Nabati S, Asadikaram G, Arababadi MK, Shahabinejad G, Rezaeian M, Mahmoodi M, Kennedy D, 2013. The plasma levels of the cytokines in opium-addicts and the effects of opium on the cytokines secretion by their lymphocytes. Immunol Lett 152, 42–46. [DOI] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML, Kodavali CV, Nimgaonkar VL, 2014. Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci 34, 9945–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP, 2012. Intermittent social defeat stress enhances mesocorticolimbic ΔFosB/BDNF co-expression and persistently activates corticotegmental neurons: implication for vulnerability to psychostimulants. Neuroscience 212, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR, 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 20, 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan WB, 2013. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ, 2016. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry 73, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV, Kapczinski F, Kauer-Sant’Anna M, 2015. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Pedraz M, Martin-Velasco AI, Garcia-Marchena N, Araos P, Serrano A, Romero-Sanchiz P, Suarez J, Castilla-Ortega E, Barrios V, Campos-Cloute R, Ruiz JJ, Torrens M, Chowen JA, Argente J, de la Torre R, Santin LJ, Villanua MA, Rodriguez de Fonseca F, Pavon FJ, 2015. Plasma concentrations of BDNF and IGF-1 in abstinent cocaine users with high prevalence of substance use disorders: relationship to psychiatric comorbidity. PLoS One 10, e0118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y, 2011. Neurobiology of the incubation of drug craving. Trends Neurosci 34, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH, 2006. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16, 313–320. [DOI] [PubMed] [Google Scholar]
- Reséndiz-Flores M, Escobar C, 2018. Circadian disruption favors alcohol consumption and differential ΔFosB accumulation in Corticolimbic structures. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B, 1999. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11, 1598–1604. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC, 2003. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA, Consortium, S.W.G.o.t.P.G., 2016. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL, 2008. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28, 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH, 2015. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec AE, Shah RS, Schiavon E, Baker E, Skene N, Pawlak R, Mucha M, 2013. Stress-induced lipocalin-2 controls dendritic spine formation and neuronal activity in the amygdala. PLoS One 8, e61046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM, 2013. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol 701, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP, 2010. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 210, 199–209. [DOI] [PubMed] [Google Scholar]
- Spiga S, Mulas G, Piras F, Diana M, 2014. The “addicted” spine. Front Neuroanat 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelly CE, Pomrenze MB, Cook JB, Morikawa H, 2016. Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA, 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S, 2014. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci 369, 20130151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Anglin JM, Paode PR, Riggert AG, Olive MF, Conrad CD, 2014. Chronic stress may facilitate the recruitment of habit- and addiction-related neurocircuitries through neuronal restructuring of the striatum. Neuroscience 280, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, Shaham Y, 2012. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 224, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen H, Kalivas PW, 2010. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox Res 18, 410–415. [DOI] [PubMed] [Google Scholar]
- Tremblay M, Lowery RL, Majewska AK, 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Yamashita T, 2014. Bidirectional tuning of microglia in the developing brain: from neurogenesis to neural circuit formation. Curr Opin Neurobiol 27, 8–15. [DOI] [PubMed] [Google Scholar]
- van der Doef TF, Doorduin J, van Berckel BNM, Cervenka S, 2015. Assessing brain immune activation in psychiatric disorders: clinical and preclinical PET imaging studies of the 18-kDa translocator protein. Clin Transl Imaging 3, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH, 2014. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17, 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H, 2012a. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 109, 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ, 2012b. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain structure & function 217, 337–351. [DOI] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA, 2014. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci 36, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF, 2013. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav Immun 32, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF, 2015. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 35, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Weiler BL, Cottler LB, 1999. Childhood victimization and drug abuse: a comparison of prospective and retrospective findings. Journal of consulting and clinical psychology 67, 867–880. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, Duman RS, 2018. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol Psychiatry 83, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K, 2016. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 233, 3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K, 2015. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol 18, pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yin HZ, Hsu CI, Yu S, Rao SD, Sorkin LS, Weiss JH, 2012. TNF-α triggers rapid membrane insertion of Ca(2+) permeable AMPA receptors into adult motor neurons and enhances their susceptibility to slow excitotoxic injury. Experimental neurology 238, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Chen H, Sharp BM, 2014. Amplified reacquisition of nicotine self-administration in rats by repeated stress during abstinence. Psychopharmacology (Berl) 231, 3189–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, 2009. Activity level-dependent synapse-specific AMPA receptor trafficking regulates transmission kinetics. J Neurosci 29, 6320–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]