Abstract
Stroboscopic luminance flicker has been found to prevent the increase in eye growth normally associated with form deprivation through the release of retinal dopamine. In this study, we sought to investigate whether dopamine plays a role in the decreased growth observed with 2 Hz sine-wave luminance flicker and increased growth with color flicker. Starting 5-7 days after hatching, chicks were exposed to 2 Hz sinusoidally modulated illumination (Mean: 680 lux) for 4 days and were otherwise in the dark. Chicks were exposed to color-modulated red and green (RG) light, to luminance modulated RGB components (LUM), or to a no-flicker (NF) control. Chicks received daily 10 uL intravitreal injections of apomorphine, spiperone, or saline. Fellow eyes received no injection. Spiperone injections prevented the decrease in eye growth typically seen with LUM flicker, with a relative increase in eye length, but no other significant effects compared with saline controls. Apomorphine injections prevented the increase in eye growth typically seen with RG flicker, with a relative decrease in eye length compared to saline controls. These results indicate a role for the activation of D2- receptor types in the inhibition of eye growth in response to luminance flicker, and a lack of dopamine receptor activation associated with the increase in eye growth with color flicker.
1. Introduction
Theoretically, longitudinal chromatic aberration (LCA) could be of value in inferring the sign of defocus (Flitcroft 1990). Indeed, recent studies have explored how LCA may be used to guide eye growth, providing evidence that during emmetropization the eye compares retinal cone contrast, and can monitor the change in color contrast over time, to determine the sign of blur and guide emmetropization (Rucker and Wallman 2009, Rucker and Wallman 2012, Rucker 2013). The purpose of this experiment is to determine the role of dopamine in this signaling mechanism.
As a result of LCA, short-wavelength light is refracted more than long-wavelength light when passing through the eye (Bedford and Wyszecki 1957). In an emmetropic eye, the dispersion of wavelengths by LCA in front and behind the retina creates image blur. As a result, LCA creates higher cone contrast for long-wavelength sensitive cones compared to short-wavelength sensitive cones when the eye is myopically defocused (blue/green wavelengths focused in front of the retina), because long-wavelengths are focused closest to the retina. Likewise, the cone contrast is higher for short-wavelength sensitive cones when the eye is hyperopically defocused (green/red wavelengths focused behind the retina), because short-wavelengths are focused closest to the retina.
Rucker and Wallman (2012) describe three possible methods by which the eye may use LCA to interpret the visual environment and guide emmetropization. The first method involves the eye adjusting its focus through a trial-and-error method to maximize image contrast and clarity, which we will refer to as a luminance cue. Detection of the sign of defocus involves a process of sampling the image at multiple focal planes.
The second method involves retinal circuitry comparing the color of the retinal image through a comparison of retinal cone contrast of at least two different cone types, which we will refer to as a chromatic cue. As a result of the relative defocus of the different wavelengths by LCA, the bright components of the retinal image will appear reddish with myopic defocus and bluish with hyperopic defocus. Detection of these color differences can provide an instantaneous cue for defocus in chicks (Rucker and Wallman 2009).
The third method incorporates both of the above methods and involves recognition of how contrast in the retinal image changes over time as the eye grows or changes focus, which we will refer to as a temporal cue. An analysis of how retinal cone contrast changes with defocus, indicates that color contrast of the retinal image changes when the eye is hyperopically defocused (chromatic cue). However, with increasing myopic defocus there is an increase in blur (luminance cue) but no change in the chromatic cue. Hence the ability to detect the change in color contrast provides a cue for hyperopic defocus. A change in luminance contrast without a color change provides a cue for myopic defocus.
An experiment by Rucker and Wallman (2012) using 2 Hz sinusoidal flicker confirmed that the chick eye can use this temporal cue to determine the sign of defocus, producing increased growth with exposure to color flicker and decreased growth with exposure to luminance flicker. Subsequent experiments have indicated that the chick emmetropization mechanism is most sensitive to changes in luminance contrast at high temporal frequencies (5 and 10 Hz) and that high contrast (70-80%) is necessary for the response (Rucker, Britton et al. 2015, Rucker, Henriksen et al. 2018). The greatest growth response occurs with red/green color flicker at low temporal frequencies (Rucker, Britton et al. 2018). Because high contrast stroboscopic flicker has been associated with an increase in retinal dopamine release (Rohrer, Iuvone et al. 1995), we are interested in whether or not dopaminergic mechanisms play a role in these temporal growth responses.
Evidence that retinal dopamine plays a pivotal role in modulating emmetropization is evidenced by the action of the non-selective dopamine receptor agonist apomorphine in inhibiting form deprivation myopia in chicks (Stone, Lin et al. 1989, Rohrer, Spira et al. 1993, Schmid and Wildsoet 2004), primates (Iuvone, Tigges et al. 1991), and guinea pigs (Dong, Zhi et al. 2011). Dopamine agonists have also been reported to inhibit lens-induced myopia in chicks (Schmid and Wildsoet 2004), but species differences exist (Dong, Zhi et al. 2011). The role of dopamine in myopia has been recently reviewed by Feldkaemper and Schaeffel (2013) and Zhou, Pardue, Iuvone, and Qu (2017). Although there is no available pharmacological characterization of dopamine receptors specifically in the chick, it has been reported that the pharmacological properties of D1- and D2-like receptors in quail (closely related to chicken) are very similar to those in other vertebrates (Kubikova, Vyboh et al. 2009).
Dopamine receptors fall into two family types, D1-like receptors that stimulate adenylate cyclase and cAMP production (D1 and D5), and D2-like receptors that decrease cAMP production (D2, D3, D4). Although apomorphine is a non-selective dopamine receptor agonist, it reportedly has a greater affinity for D2 receptor types than D1 receptor types (Baldessarini, Kula et al. 1994). Spiperone is a selective D2-receptor type antagonist (Quail: Ki for D2 vs D3, D4, D1, D5 = 0.06 vs 0.6, 0.08, 350, 3500) (Seeman and Van Tol 1994). These values do not necessarily indicate that D2-receptors are responsible for the ocular effects that we observe in chicks but provide the best estimate available. Stimulation of D2-receptors has been implicated in antagonizing the anti-myopia effect of a number of different drug treatments and paradigms noted below. Spiperone antagonizes the anti-myopia effects of apomorphine, di-isoproylfluorophosphate (a parasympathomimetic drug), and mamba toxin-3 (MT3; ligand for muscarinic receptor M4) in form deprivation myopia (Stone, Lin et al. 1989, Cottriall, Brew et al. 2001). Spiperone also acts to antagonize the anti-myopia effect of unrestricted vision from short-term diffuser-removal in form deprivation myopia (McCarthy, Megaw et al. 2007, Nickla and Totonelly 2011).
The aim of this experiment was to determine whether dopamine is involved in the growth responses seen with 2 Hz color and luminance flicker that provide chromatic and luminance cues, respectively. Given the results of the form deprivation and lens experiments, our hypothesis was that apomorphine would inhibit the growth response to color flicker, and spiperone would inhibit the growth reduction seen with luminance flicker.
2. Materials and methods
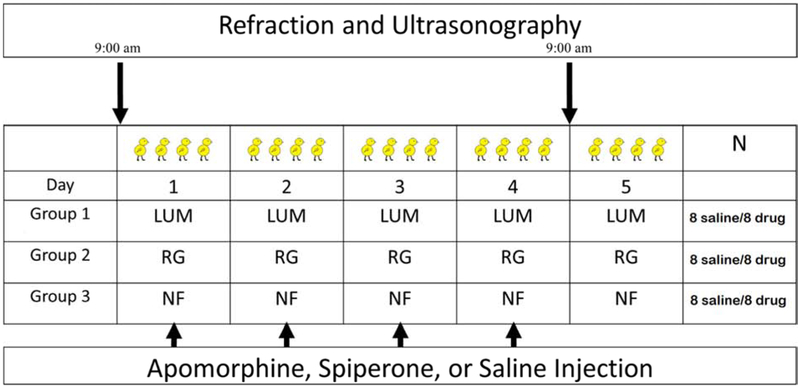
C-strain White Leghorn chicks (Gallus gallus domesticus) were obtained as eggs from Cornell University, Ithaca, NY. After hatching, the chicks were reared under fluorescent lighting in a 12 hour light, 12 hour dark cycle (8am to 8pm) and given food and water ad libitum. Experiments were started when chicks were 5-7 days old. Measurements were performed at the start and end days of the experiment at 9 am with A-scan ultrasonography (Nickla, Wildsoet et al. 1998) using a 30 MHz transducer sampled at 100 Hz and gain of 59 dB on Olympus NDT equipment. A Hartinger refractometer (Zeiss, Jena, Germany) was calibrated using a Heine artificial eye and used to measure refractive errors (Wallman and Adams 1987). All measurements were taken on chicks anesthetized with 1.5% – 2.0% isoflurane in oxygen. Animal use followed ARVO procedures for the Use of Animals in Ophthalmic and Vision Research, and was approved by the New England College of Optometry Institutional Animal Care and Use Committee.
2.1. Illumination Conditions
Three illumination conditions were tested: sinusoidal 2 Hz luminance flicker (LUM), sinusoidal 2 Hz color flicker (RG), and No Flicker (NF). Luminance flicker was produced with in-phase sinusoidal modulation (2 Hz) of the red (615 nm, half-bandwidth 20 nm), green (515 nm, half-bandwidth 35nm), and blue (465 nm, half-bandwidth 25 nm) LEDs. RG color modulation was produced with the red and green LED flickering in counterphase. Sinusoidal, counterphase, modulation of the light source produces a gradual change of color of the light from red to green with an intermediate change to yellow. Sinusoidal modulation of a single temporal frequency prevents interference from higher temporal frequency harmonics and the associated response non-linearities. “No flicker” was set at the mean illuminance level of both flickering conditions and was produced by combining the red, green, and blue components without modulation. All three illumination conditions were used separately in each of the experimental drug groups. The mean irradiances of the individual components of the light source were set to 50 μW cm2 for red, green and blue, which is equivalent to 214 “chick lux” for red, 191 “chick lux” for green, and 64 “chick lux” for blue as in an earlier experiment (Rucker, Britton et al. 2015, Rucker, Henriksen et al. 2018). Since chicks have different wavelength sensitivities to humans we refer to illuminance corrected for the chick photopic sensitivity function (Chen and Goldsmith 1984) as “chick lux”, which differs from “human lux” as a function of wavelength. Neutral density filters were used to refine control of the mean intensity of the lighting conditions. All three conditions had a mean illuminance equivalent to 680 human lux. The cage was illuminated by an LED Titan RGB bulb (Lamina Ceramics: Atlas Light Engine) controlled by an eight channel, 12-bit Access I/O, USB-DA-8A digital to analog converter with waveform generator functionality connected to BuckPucks (LuxDrive: 3021 D-E-500) that provided a linear current output over a range of 1.6 - 4.3 V. Light output was calibrated and a sinusoidal output produced digitally using lookup tables, and confirmed by measurement of illuminance output (Newport Model 818-SL serial number: 6915). Both eyes of the chicks were equally exposed. 2 Hz was chosen because it has been previously shown to cause hyperopic or myopic shifts with LUM or RG flicker, respectively (Rucker and Wallman 2012), and is well within the range of flicker sensitivity of chickens (Jarvis, Taylor et al. 2002). When chicks were not in the cage, they were housed in the dark in a light-and-sound proof chamber.
2.2. Drug conditions
Three drug conditions were used: dopamine agonist (Tocris Bioscience) apomorphine (non-specific), the dopamine antagonist (Tocris Bioscience) spiperone (5-HT serotonin and D2-dopamine specific) and saline. A separate group of saline-injected control chicks was used to control for any “yoking effect” on the control eye which has previously been shown in animals with form deprivation (Bradley, Fernandes et al. 1999, Smith, Bradley et al. 1999, Smith and Hung 2000, Schaeffel, Burkhardt et al. 2004, Howlett and McFadden 2006) and with negative lens wear (Hung, Crawford et al. 1995, Troilo, Totonelly et al. 2009). Following published protocols (Nickla, Totonelly et al. 2010, Nickla and Totonelly 2011), anesthetized chicks received 10μL intravitreal injections of apomorphine (10 nmoles), spiperone (5 nmoles) or saline (0.75%) in either the left or right eye, at 1 pm. The fellow eyes were not given injections and were used as controls. Apomorphine was dissolved in saline, while spiperone was dissolved in 1mg/mL ascorbic acid solution heated to 30°for 10 min while stirring (Ashby and Schaeffel 2011). The apomorphine drug concentration used here has been shown to be within the range needed to create a statistically significant difference in vitreous chamber depth between apomorphine injected and control eyes (Schmid and Wildsoet 2004).
After injection, each chick was immediately returned to the cage. Injections were conducted using a 30G needle, piercing the skin of the lids over the superior temporal sclera after removing the feathers. The same injection site was used for all subsequent injections. The needle was slowly removed while the skin around the injection site was squeezed together using small forceps.
2.3. Procedure
The chicks were allowed to roam freely in a 32 × 20 inch diameter cage 8 hours each day from 9 am to 5 pm. In total, 48 chicks were used in the Apomorphine Experiment and 48 in the Spiperone Experiment. Of these, 16 chicks were used in each of the three lighting conditions (LUM, RG, Steady), with 8 chicks given saline (vehicle) injections and 8 chicks given drug injections. Two birds were removed from the apomorphine (RG) and (NF) conditions because of cataract formation following intravitreal injection.
2.4. Analysis
The goal of the analysis was to isolate the effect of the drug in each of the illumination conditions. To achieve this goal, we followed the following analysis procedure:
The injection effect under different illuminance conditions was calculated as the “Mean Difference in Change” between the two eyes in the saline condition for each illumination condition. This can be represented as: ((Σ(ΔX-ΔN))/n)c = (ΔXm-ΔNm) Salinec, where m is the mean of each factor, c is the specific illumination condition, and n is the number of birds in the illumination condition.
The drug effect was calculated as the “Relative Difference in Change” by subtracting the “Mean Difference in Change” in saline in a particular illumination condition from each of the drug injected animals exposed to the same illumination condition. The “Relative Difference in Change” in each drug and illumination condition was compared to zero with t-tests. This can be represented as: (ΔX-ΔN) Drugc - (ΔXm-ΔNm) Salinec.
Eye length was calculated as the distance from the anterior cornea to the posterior sclera. In saline injected eyes, this value was compared to zero with t-tests. Analysis between illumination groups was conducted using a multi-factorial ANOVA to test for statistical significance. If there was statistical significance, then comparisons were made between illumination conditions using two-way unpaired t-tests. To test for normality, the Kolmogorov Smirnov Test was performed in SPSS software.
3. Results
3.1. Effects of Saline Injections in the Control Birds
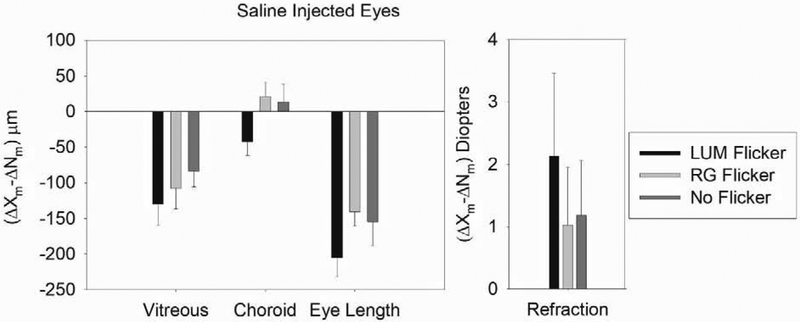
The effects of saline injections are shown in Figure 2. There was no significant difference in refraction or ocular dimension between the saline injected birds in any illumination condition (ANOVA: Axial: p > 0.05; Vitreous: p > 0.05; Choroid: p > 0.05; RE: p > 0.05). Confirming the results of previous experiments, binocular eye growth was greater with RG than with LUM flicker (p<0.05).
Figure 2. The Effect of Monocular Saline Injections in the Spiperone and Apomorphine Experiments.
Figure 2 shows the mean difference in change between the saline injected and fellow eyes in the control birds in the two experiments. There was no significant difference in refraction or ocular dimension between saline injected birds in any illumination condition. Error bars indicate standard errors of the mean.
3.2. Drug Effects on Eye Length
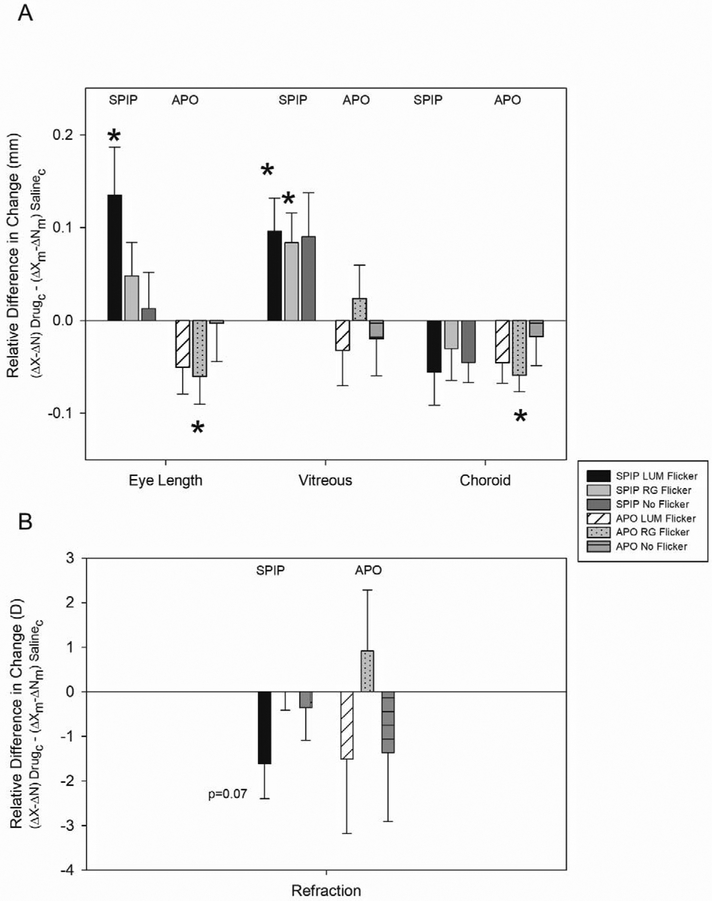
As shown in Figure 3, spiperone injections produced a relative increase in eye length compared to apomorphine injections, when compared to their saline injected controls (ANOVA: p = 0.009).
Figure 3. Relative Difference in Change in Eye Components with Monocular Spiperone and Apomorphine Injections.
A) Spiperone injections prevented the decrease in eye growth typically seen with luminance flicker. Eyes injected with spiperone and exposed to LUM flicker showed a relative increase in eye length, a corresponding increase in vitreal chamber growth, and myopic progression (p < 0.07) relative to saline controls. Eyes injected with apomorphine and exposed to LUM showed no effect on any ocular component or refraction relative to saline controls. Error bars indicate standard errors of the mean. *p < 0.05.
B) Apomorphine injections prevented the increase in eye growth typically seen with color flicker. Eyes injected with apomorphine showed a relative decrease in eye growth and thinning of the choroid relative to the saline controls. Eyes injected with spiperone and exposed to RG showed a relative increase in vitreal chamber depth compared to saline controls. Comparison of relative change compared to zero made using two-tailed student’s t-tests. Error bars indicate standard errors of the mean. *p < 0.05; **p < 0.01
C) Neither of the drugs affected refraction or ocular dimensions in the No Flicker condition.
As our hypothesis predicted, spiperone injections prevented the decrease in eye growth typically seen with LUM flicker. Spiperone injected eyes grew more with exposure to LUM (Figure 3A). With exposure to LUM, eye length in spiperone injected eyes grew more (135 ± 52 μm) than in saline injected eyes (p = 0.027). With exposure to RG and No Flicker, there was no difference in eye length changes in saline and spiperone injected eyes (RG: 48 ± 36 μm; No Flicker: 13 ± 39 μm).
As our hypothesis predicted, apomorphine injections prevented the increase in eye growth typically seen with RG flicker (Figure 3A). We observed a reduction in eye growth when eyes exposed to RG were injected with apomorphine. With RG there was a relative decrease in eye length of −63 ± 37μm (p = 0.03) in apomorphine injected eyes compared to the saline controls. However, in LUM and No Flicker there was no significant difference between saline and apomorphine injected eyes (LUM: −42 ± 33 μm; p = 0.19; No Flicker: −3 ± 41 μm; p = 0.54).
3.3. Drug Effects on Vitreous Length
The relatively greater eye growth associated with spiperone injections, compared to apomorphine injections, are reflected in the change in vitreous chamber depth (ANOVA: p = 0.005).
As our hypothesis predicted, spiperone prevented the vitreal growth inhibition typically seen in LUM exposed eyes and enhanced vitreal growth in both LUM and RG-exposed eyes (Figure 3A). In LUM and RG exposed eyes, spiperone injections produced greater vitreous chamber growth than saline injections (LUM: 96 ± 36 μm; p = 0.03; RG: 84 ± 32 μm, p = 0.03). However, consistent with the lack of effect of spiperone on eye length in the No Flicker condition, spiperone injections had no significant effect on the vitreous chamber depth in the No Flicker condition (90 ± 47 μm; p = 0.10).
Despite the reduction in eye growth in apomorphine injected eyes with exposure to RG flicker, apomorphine injections had no effect on the vitreous chamber depth in any illumination condition (Figure 3A). Apomorphine injections, relative to saline injections, produced only small changes in vitreal growth in LUM (−20 ± 43 μm), RG (23 ± 36 μm) and No Flicker (−20 ± 40 Pμm).
3.4. Drug Effects on Choroidal Thickness
Spiperone had no effect on choroidal thickness in any illumination condition (Figure 3A: LUM (−25 ± 22 μm), RG (−30 ± 34 μm) or No Flicker (−45 ± 22 μm)) compared to the injection effect in saline controls (LUM: p = 0.15; RG: p = 0.39; No Flicker: p = 0.08).
Since there was a reduction in eye length, but not vitreous chamber depth, with apomorphine injections when the eye was exposed to RG, we might expect the choroid to have thinned in this condition. Indeed, choroids thinned in apomorphine injected chicks exposed to RG flicker (Apo: −59 ±18 μm; p = 0.006) but not in chicks exposed to LUM (−45 ± 22 μm; p = 0.31) or No Flicker (−17 ± 31 μm; p = 0.28) as can be seen in Figure 3A. The choroidal thinning compensated to some extent for the apomorphine-induced decrease in eye length in RG.
3.5. Drug Effects on Refraction
With exposure to LUM flicker, spiperone injection caused no significant change in refraction compared to the saline control (−1.6 D ± 0.8 D; p = 0.07) (Figure 3B). Similarly, with RG and No Flicker, there was no relative refractive shift in the spiperone injected birds when compared to the saline controls (RG: p = 0.99; No Flicker: p = 0.64).
With exposure to RG flicker, the apormorphine injection caused no significant change in refraction compared to the saline control (0.91 ± 1.37 D; p = 0.53). With exposure to LUM and No Flicker there was no additional effect of the drug on refraction. The relative myopic refractive shift in the apomorphine injected birds exposed to LUM and No Flicker was not significantly different to that of the saline controls (LUM: −1.51 ± 1.80 D, p = 0.44; No Flicker: −1.36 ± 1.54 D, p = 0.41).
4. Discussion
The hypothesis that the non-specific dopamine agonist, apomorphine, will inhibit red/green color (RG) flicker-induced axial eye growth, and the D2-like, dopamine antagonist, spiperone, will prevent luminance (LUM) flicker-induced axial eye growth inhibition is supported. Eyes grew less with apomorphine injections when exposed to RG flicker, and more with spiperone when exposed to LUM flicker. The results show that spiperone can prevent LUM flicker-induced hyperopia in chicks through eye growth and vitreal growth changes, implicating the D2-receptor type in LUM flicker-induced growth changes. In contrast, apomorphine inhibits RG flicker-induced eye growth, but does not enhance LUM flicker-induced growth inhibition, suggesting that RG-induced eye growth occurs through a lack of dopamine receptor stimulation. The D2-like dopamine antagonist did not have any significant effect on choroidal thickness in any illumination condition, while the non-specific dopamine agonist caused significant choroidal thinning in the RG-exposed eyes, suggesting non-D2 receptor control of choroidal thickness.
4.1. Significance of the Results
Increased eye growth in spiperone injected eyes exposed to LUM suggests that LUM flicker-induced hyperopia in the fellow eye may be due to D2 receptor activation. D2 receptor activation with quinpirole has been shown to inhibit eye growth in form-deprivation myopia (McCarthy, Megaw et al. 2007) and lens-induced myopia (Feldkaemper and Schaeffel 2013). Additionally, the D2 receptor antagonists, spiperone and sulpiride, have been shown to suppress the protective effects of normal vision on myopic eye growth resulting from form deprivation (McCarthy, Megaw et al. 2007) and lens-induced myopia (Feldkaemper and Schaeffel 2013). While the D1 receptor antagonist SCH23390 does not suppress the protective effect of diffuser-removal on form deprivation (McCarthy, Megaw et al. 2007, Nickla and Totonelly 2011). A D1 receptor antagonist was not tested in this experiment, therefore, it is possible, though unlikely, that a D1 receptor antagonist would inhibit hyperopic eye growth under LUM flicker. Thus we propose that D2 receptors are implicated in growth inhibition in response to changes in luminance contrast even at the low temporal frequency of 2 Hz.
Decreased eye growth in apomorphine injected eyes exposed to RG suggests that RG flicker induced myopia in the fellow eye may be induced by a lack of D1 or D2 receptor activation. Although apomorphine caused a significant reduction in eye growth in RG-exposed animals, there was no significant change in refraction. It is possible that the myopiagenic effects of RG flicker are not of sufficient dioptric power for apomorphine to show an appreciable effect on refraction with our measurement techniques. A study on guinea pigs found no significant inhibition of lens-induced eye growth in guinea pigs treated with apomorphine, which the authors postulate is due to their use of lower magnitude −4.00 D defocusing lenses compared to those used in other lens-induced myopia studies (Nickla, Totonelly et al. 2010).
With spiperone injections there was a myopic shift with LUM that did not reach significance, but there was no refractive effect with RG, despite an increase in vitreous chamber depth in both conditions. This lack of refractive effect may have arisen from changes in the anterior eye, but unfortunately we did not measure corneal changes in this experiment. However, a previous experiment demonstrated a lack of corneal emmetropization in the absence of a blue light component (Rucker, Britton et al. 2015).
Ascorbic acid in the vehicle solution has been associated with a reduced form deprivation response in tree shrew (Ward, Siegwart et al. 2016) raising concerns about its effect in other species. Following earlier experimental protocols, ascorbic acid was present as a vehicle in the spiperone condition (Rohrer, Spira et al. 1993, McCarthy, Megaw et al. 2007, Ashby and Schaeffel 2011, Nickla and Totonelly 2011), but not in the apomorphine (Iuvone, Tigges et al. 1991, Nickla, Totonelly et al. 2010) or saline (Nickla, Totonelly et al. 2010, Nickla and Totonelly 2011) conditions. Ascorbic acid acts to stabilize apomorphine, so its absence would tend to reduce the effectiveness of the drug and reduce the likelihood of finding a significant difference relative to the saline controls. Ascorbic acid can also inhibit the binding of spiperone to receptor sites (Tolbert, Morris et al. 1992) again reducing the effectiveness of the drug and reducing the likelihood of finding a significant difference relative to the saline controls.
As noted in other experiments (Rucker and Wallman 2009, Rucker and Wallman 2012, Nickla and Totonelly 2015, Rucker, Britton et al. 2015, Rucker, Britton et al. 2018) choroidal changes were contrary to what has become expected with regards to changes in eye length in lens wearing animals. In eyes exposed to RG flicker, apomorphine caused choroidal thinning that counteracted the decrease in eye length—a feature noted in a previous study (Rucker and Wallman 2012). In this present study, the finding that apomorphine caused thinning of the choroid is in contrast to the findings by Nickla, Totonelly, and Dhillon (2010), which showed that growth inhibition by apomorphine elicited transient choroidal thickening in response to the intravitreal injection, followed by thinning in response to monocular negative lens wear in steady light. However, the current study used younger birds (5-7 days) and did not include monocular negative lens wear, nor did it examine early time points to determine whether transient effects were seen.
This study showed that significant choroidal thinning due to the dopamine agonist was seen only in the RG condition. Changes in luminance modulation that are present in the LUM condition are known to stimulate retinal dopamine release (Kramer 1971, Iuvone, Galli et al. 1978). The choroidal thinning seen with RG flicker may occur through vasoconstriction of the choroidal blood vessels through inhibition of a nitric oxide pathway (Nickla, Lee et al. 2013, Carr and Stell 2016). Previous research has shown that dopamine acts upstream of nitric oxide release, which causes choroid thickening and growth inhibition in both form deprived and negative lens-wearing chicks (Kramer 1971, Iuvone, Galli et al. 1978, Nickla, Lee et al. 2013). Nitric oxide is also implicated in the reduction in eye growth associated with atropine treatment in form deprived animals (Carr and Stell 2016). If the interaction between the RG lighting condition and apomorphine inhibits this nitric oxide pathway, it may cause relative choroidal thinning. Thinning may also occur through the activation of receptors on the RPE, which in turn decrease ion and fluid transport to the choroid (Zhang and Wildsoet 2015).
4.2. Clinical Relevance
Clinically, the results of this study show that dopamine plays an important role in reducing eye growth but only when the eye is temporally stimulated. More studies are needed to determine whether or not these results can be applied to develop future visual environments that reduce the risk for myopia development in humans.
5. Conclusions
Apomorphine inhibited the increase in eye growth that is typically seen in the (RG) color flicker condition, while spiperone inhibited the reduction in eye growth that is typically seen with the (LUM) luminance flicker condition. The major finding of this study is that it is likely that D2-specific dopaminergic receptor activation underlies the reduction in growth associated with luminance flicker, while the enhancement of eye growth typically seen with (RG) color flicker is likely due to the lack of D2-specific dopaminergic receptor activation. Future experiments should confirm these findings by measuring retinal and vitreal dopamine or 3,4 dihydroxyphenylacetic (DOPAC) levels in chicks exposed to these illumination conditions.
Figure 1.
Chicks were allowed to roam freely in a 32 × 20 inch diameter cage 8 hours each day from 9am to 5pm. Both eyes of the chicks were equally exposed to one of three conditions: a sinusoidal 2Hz luminance flicker (LUM), 2Hz color flicker (RG), or No Flicker (NF). All three conditions had a mean illuminance of 680 lux. Chicks were injected daily with either a dopamine agonist (apomorphine), a dopamine antagonist (spiperone: D2 receptor specific), or saline. Measurements were made with A-scan ultrasonography and a Hartinger Refractometer on anesthetized chicks at 9am at the beginning and end of the experiment.
Highlights.
Spiperone injections prevented growth inhibition seen with luminance flicker
Apomorphine injections prevented eye growth typically seen with color flicker
These results indicate a role for D2- receptor types in eye growth regulation
Acknowledgements
The authors thank Dr. Debora Nickla for her advice on drug dosing and administration and Dr. Li Deng who provided statistical guidance on experimental design and analysis. The research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under Award Number R01EY023281 and by the NIH T35 program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant information: National Eye Institute of the National Institutes of Health under Award Number R01EY023281 and the NIH T35 program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby RS and Schaeffel F (2011). "The effect of bright light on lens compensation in chicks." Invest Ophthalmol Vis Sci 51(10): 5247–5253. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Kula NS, Zong R and Neumeyer JL (1994). "Receptor affinities of aporphine enantiomers in rat brain tissue." Eur J Pharmacol 254(1-2): 199–203. [DOI] [PubMed] [Google Scholar]
- Bedford RE and Wyszecki G (1957). "Axial chromatic aberration of the human eye." J Opt Soc Am 47(6): 564–565. [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A and Boothe RG (1999). "The refractive development of untreated eyes of rhesus monkeys varies according to the treatment received by their fellow eyes." Vision Res 39(10): 1749–1757. [DOI] [PubMed] [Google Scholar]
- Carr BJ and Stell WK (2016). "Nitric Oxide (NO) Mediates the Inhibition of Form-Deprivation Myopia by Atropine in Chicks." Sci Rep 6(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DM and Goldsmith TH (1984). "Appearance of a Purkinje shift in the developing retina of the chick." J Exp Zool 229(2): 265–271. [DOI] [PubMed] [Google Scholar]
- Cottriall CL, Brew J, Vessey KA and McBrien NA (2001). "Diisopropylfluorophosphate alters retinal neurotransmitter levels and reduces experimentally-induced myopia." Naunyn Schmiedebergs Arch Pharmacol 364(4): 372–382. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J and Zhou X (2011). "Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs." Mol Vis 17: 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- Feldkaemper M and Schaeffel F (2013). "An updated view on the role of dopamine in myopia." Exp Eye Res 114: 106–119. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI (1990). "A neural and computational model for the chromatic control of accommodation." Vis Neurosci 5(6): 547–555. [DOI] [PubMed] [Google Scholar]
- Howlett MH and McFadden SA (2006). "Form-deprivation myopia in the guinea pig (Cavia porcellus)." Vision Res 46(1-2): 267–283. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML and Smith EL (1995). "Spectacle lenses alter eye growth and the refractive status of young monkeys." Nat Med 1(8): 761–765. [DOI] [PubMed] [Google Scholar]
- luvone PM, Galli CL, Garrison-Gund CK and Neff NH (1978). "Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons." Science 202(4370): 901–902. [DOI] [PubMed] [Google Scholar]
- luvone PM, Tigges M, Stone RA, Lambert S and Laties AM (1991). "Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia." Invest Ophthalmol Vis Sci 32(5): 1674–1677. [PubMed] [Google Scholar]
- Jarvis JR, Taylor NR, Prescott NB, Meeks I and Wathes CM (2002). "Measuring and modelling the photopic flicker sensitivity of the chicken (Gallus g. domesticus)." Vision Res 42(1): 99–106. [DOI] [PubMed] [Google Scholar]
- Kramer SG (1971). "Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo." Invest Ophthalmol 10(6): 438–452. [PubMed] [Google Scholar]
- Kubikova L, Vyboh P and Kostal L (2009). "Kinetics and pharmacology of the D1- and D2-like dopamine receptors in Japanese quail brain." Cell Mol Neurobiol 29(6-7): 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M and Morgan IG (2007). "Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia." Exp Eye Res 84y1): 100–107. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Lee L and Totonelly K (2013). "Nitric oxide synthase inhibitors prevent the growth-inhibiting effects of quinpirole." Optom Vis Sci 90(11): 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL and Totonelly K (2011). "Dopamine antagonists and brief vision distinguish lens-induced-and form-deprivation-induced myopia." Exp Eye Res 93(5): 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL and Totonelly K (2015). "Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth." Clin Exp Optom 98(6): 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K and Dhillon B (2010). "Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks." Exp Eye Res 91(5): 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C and Wallman J (1998). "Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes." Exp Eye Res 66(2): 163–181. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Iuvone PM and Stell WK (1995). "Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick." Brain Res 686(2): 169–181. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Spira AW and Stell WK (1993). "Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium." Vis Neurosci 10(3): 447–453. [DOI] [PubMed] [Google Scholar]
- Rucker F, Britton S, Spatcher M and Hanowsky S (2015). "Blue Light Protects Against Temporal Frequency Sensitive Refractive Changes." Invest Ophthalmol Vis Sci 56(10): 6121–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F, Britton S and Taylor C (2018). "Color and Temporal Frequency Sensitive Eye Growth in Chick." Invest Ophthalmol Vis Sci 59(15): 6003–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F, Henriksen M, Yanase T and Taylor C (2018). "The role of temporal contrast and blue light in emmetropization." Vision Res 151: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F and Wallman J (2012). "Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus." J Vis 12(6): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ (2013). "The role of luminance and chromatic cues in emmetropisation." Ophthalmic Physiol Opt 33(3): 196–214. [DOI] [PubMed] [Google Scholar]
- Rucker FJ and Wallman J 2009). "Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth." Vision Res 49(14): 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC and Williams RW (2004). "Measurement of refractive state and deprivation myopia in two strains of mice." Optom Vis Sci 81(2): 99–110. [DOI] [PubMed] [Google Scholar]
- Schmid KL and Wildsoet CF (2004). "Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks." Optom Vis Sci 81(2): 137–147. [DOI] [PubMed] [Google Scholar]
- Seeman P and Van Tol HH (1994). "Dopamine receptor pharmacology." Trends Pharmacol Sci 15(7): 264–270. [DOI] [PubMed] [Google Scholar]
- Smith EL, Bradley DV, Fernandes A and Boothe RG (1999). "Form deprivation myopia in adolescent monkeys." Optom Vis Sci 76(6 428–432. [DOI] [PubMed] [Google Scholar]
- Smith EL and Hung LF (2000). "Form-deprivation myopia in monkeys is a graded phenomenon." Vision Res 40(4): 371–381. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM and Iuvone PM (1989). "Retinal dopamine and form-deprivation myopia." Proc Natl Acad Sci U S A 86(2 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LC, Morris PE Jr., Spollen JJ and Ashe SC (1992). "Stereospecific effects of ascorbic acid and analogues on D1 and D2 agonist binding." Life Sci 51(12): 921–930. [DOI] [PubMed] [Google Scholar]
- Troilo D, Totonelly K and Harb E (2009). "Imposed anisometropia, accommodation, and regulation of refractive state." Optom Vis Sci 86(1): E31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J and Adams JI (1987). "Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization." Vision Res 27(7): 1139–1163. [DOI] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT Jr., Frost MR and Norton TT (2016). "The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews." Exp Eye Res 145: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y and Wildsoet CF (2015). "RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia." Prog Mol Biol Transl Sci 134: 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone PM and Qu J (2017). "Dopamine signaling and myopia development: What are the key challenges." Prog Retin Eye Res 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]