Abstract
Objective
To find antagonistic strains in the respiratory tract having bacteriostatic properties against common pathogens.
Methods
The oropharyngeal microbiota of five healthy children aged 4–6 years were collected and α‐hemolytic bacteria screened on 15% sheep blood agar. Bacteriostatic effects of the isolated α‐hemolytic bacteria on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pyogenes were evaluated by the Oxford cup method. Antagonistic strains were identified by mass spectrometry, and the16S rDNAs were sequenced, and their best bacteriostatic concentrations and antagonistic spectra for Klebsiella pneumoniae, Proteus vulgaris, Enterobacter cloacae, Acinetobacter Baumanii, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pyogenes were evaluated.
Results
Of 300 isolated α‐hemolytic bacterial clones, four exhibited bacteriostatic activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pyogenes. Mass spectrometric analyses revealed that two of them were Streptococcus mitis and two others were Streptococcus parasanguinis strains. Further tests showed that all 4 antagonistic strains also had bacteriostatic effects on Klebsiella pneumoniae, Proteus vulgaris, Enterobacter cloacae, and Acinetobacter Baumanii, and the mode of action was not mediated by lactic acid production.
Conclusion
Four antagonistic Streptococcus strains derived from oropharyngeal microbiotas showed bacteriostatic effects on pathogens and may be involved in pharyngeal microbiome homeostasis.
Keywords: a‐hemolytic streptococcus, antagonistic, broad‐spectrum resistance, probiotics, respiratory strains
In the present study, we screened 300 bacterial strains from the oropharyngeal microbiota of five healthy children and found four antagonistic Streptococcus strains, which exhibited broad‐spectrum antagonistic activities for Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Enterobacter cloacae and Acinetobacter baumanii. We suggest, that the growth of this Streptococcus strains may prevent pathogenic bacterial expansion.
![]()
1. INTRODUCTION
A recent report states that mortality associated with respiratory tract infections (RTIs) is the fifth‐leading cause of death overall ("Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013," 2015). In children less than 5 years old, the health effects are serious since nearly 1 million of these children died from RTIs in 2015 (Gao, Kang, Yu, & Ren, 2014; Lelieveld, Haines, & Pozzer, 2018). There are many causes of RTIS, mainly indoor and outdoor environmental pollution (Korzeniewski, Nitsch‐Osuch, Chciałowski, & Korsak, 2013; Kovesi et al., 2006), and RTIs are often accompanied by improper use of antibiotics (Schroeck et al., 2015). In previous studies, it has been reported that the pharyngeal microbiome may have a protective function for RTIs. Microbial succession in the respiratory tract is determined in infancy and is linked to microbiota stability and respiratory health characteristics (Biesbroek et al., 2014; Bosch et al., 2017; Gao et al., 2014). According to the Food and Agriculture Organization of the United Nations and the WHO, probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (Hill et al., 2014), with the focus being mainly on the gut bacterial composition (Carolina Maldonado, Ivanna Novotny, Esteban, Alejandra de Moreno de, & Gabriela, 2015; Didari, Mozaffari, Nikfar, & Abdollahi, 2015; Martinez, Bedani, & Saad, 2015; Quigley & Shanahan, 2014). However, a role for probiotics in reducing nasal colonization by multidrug‐resistant bacteria (Warrack, Panjikar, Duster, & Safdar, 2014) and respiratory tract infections (Gao et al., 2014) has been proposed. For symbiotic microbiota of the upper respiratory tract, research has mainly focused on antagonistic strains against Streptococcus pyogenes (S. pyogenes) (Humphreys & McBain, 2019) (Guglielmetti et al., 2010), but also on Streptococcus pneumoniae (S. pneumoniae) (Roos, Hakansson, & Holm, 2001) and Staphylococcus aureus (S. aureus) (Popova et al., 2012).
In the present study, we analyzed the oropharyngeal microbiota in order to find probiotic strains that can antagonize pathogenic bacteria in healthy children.
2. MATERIALS AND METHODS
2.1. Pathogenic bacterial strains
The bacterial strains S. aureus (CICC 10201, China), Escherichia coli (E. coli) (CICC 10003, China), and Pseudomonas aeruginosa (P. aeruginosa) (CICC 20152, China) were purchased from the China Center of Industrial Culture Collection (CICC) and Klebsiella pneumonia (K. pneumonia) CGMCC 1.0839, Proteus vulgaris (P. vulgaris) CGMCC 1.1651, Enterobacter cloacae (E. cloacae) CGMCC 1.8726, and Acinetobacter Baumannii (A. Baumannii) CGMCC 1.10395 from the China General Microbiological Culture Collection Center. S. pyogenes is a clinical isolate found in our hospital.
2.2. Antagonistic bacterial strain collection
Bacterial microbiota taken from the posterior pharyngeal walls of 5 healthy children (4–6 years old, Shenyang area) were collected at least 2 hr after meals using disposable sterile throat swab tubes (Chuangxin Medical Instrument Factory). After collection, the throat cotton swabs were placed in 1 ml of brain heart infusion (BHI) medium (BHI, Hopebio, China). After the suspension was diluted 1,000‐fold, 200 µl of bacterial solution was applied to a medium containing 15% sheep blood agar (Hopebio, China) and cultured at 37°C for 24 hr. Single colonies with green hemolytic rings were picked up with a sterile inoculating loop, streaked on 15% sheep blood agar medium, and cultured in an incubator at 37°C for 24 hr. As negative controls, swabs without throat contact but otherwise using the same protocol were used.
2.3. Screening of antagonistic bacterial strains for bacteriostatic effects on pathogenic bacteria
2.3.1. Primary screening
Single α‐hemolytic strains were isolated (about 300 strains from 5 pharyngeal swabs were detected in the initial screen), streaked on 15% sheep blood agar medium, and incubated at 37°C overnight. Then, a perforator (a sterile 1,000 μl pipette tip, Eppendorf) was used to perforate the cultured plate in order to obtain a bacterial cake with a diameter of about 8 mm. Then, the cakes were placed in nutrient agar medium coated with 200 µl of S. aureus (1 × 105 cfu/ml) and cultured at 37°C for 24 hr to look for any S. aureus growth retardation.
2.3.2. Secondary screening
Colonies of the antagonistic strains were selected with a sterile inoculating loop, streaked onto 15% sheep blood agar medium plates, and incubated at 37°C overnight. Then, colonies from the entire plate were scraped off and dissolved in 800 µl of BHI medium after which 200 µl of bacterial suspensions (1–2 × 1010/ml) was placed in Oxford cups on a nutrient agar medium coated with 100 µl of pathogenic bacteria solutions (5–6 × 105 cfu/ml) and cultured at 37°C for 24 hr to look for any bacteriostatic effects (Qian & Huang, 2000).
2.3.3. Screening for antagonistic/pathogen bactericidal ratios
One hundred microliter of a pathogen stock solution (5–6 × 105 cfu/ml) was mixed with 100 µl of an antagonistic strain stock solution (1–2 × 1010/ml) or 10, 100 and 1,000 fold dilutions, and streaked on a 15% sheep blood agar plate. α‐hemolytic antagonistic and β‐hemolytic pathogenic bacterial strains were then counted after 24 hr incubation at 37°C.
2.4. Identification of antagonistic strains
Antagonistic strains were identified using mass spectrometry (MALDITOF MS, Vitek MS, BioMérieux, France) according to the manufacturer's instruction. 16S rDNA sequencing was performed with a bacterial 16S rDNA PCR Kit fast (800) (Takara, Japan) according to the manufacturer's protocol. Briefly, the colonies were selected and dissolved in 50 μl of sterile distilled water. After centrifugation at 8,000× g for 5 min, the supernatant was discarded and the pellets were dissolved in 50 μl of sterile distilled water again. After the subsequent addition of 50 μl 100 mM NaOH, the mixed suspension was heated at 95°C for 15 min. After adding 11 μl 1M Tris‐HCl (pH 7.0) and mixing, the suspension was centrifuged again and 2 μl supernatant was used for the PCR amplification of the bacterial 16S rDNA region, which consisted of 25 cycles at 94°C for 5 s, 55°C for 1 s and 68°C for 4 s in a ready to mix solution according to the manufacturer's instructions. After aliquots of the PCR products were analyzed with a 2% agarose gel (≈0.8 Kb), with a positive control (E. coli DNA) and a negative control without DNA, 100 ng DNA was sequenced with the sequencing primer 10F supplied with the kit (Sangon Biotech (Shanghai) Co., Ltd).
3. RESULTS
First, the 300 α‐hemolytic bacterial colonies, collected from the initial screening on 15% sheep blood agar medium plates, were used for secondary screening using the Oxford cup method. As a result, 36 strains with growth inhibition effects on S. aureus were selected. Next, we used the Oxford cup method to determine the intensity of S. aureus growth inhibition of the 36 antagonistic strains. As a result, seven strains were selected, which produced S. aureus growth inhibition rings on the agar of >10 mm (Table 1).
Table 1.
Result of antibacterial diameter screening of 36 antagonistic respiratory probiotics derived from the first screening against Staphylococcus aureus (only strains with an inhibitory zone of >10 mm were selected)
| Antagonistic bacterial strain number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Bacteriostasis diameter (mm) | 12 | 16 | 16 | 23 | 15 | 15 | 21 |
Next, the growth inhibition of the seven screened antagonistic throat bacterial strains on S. aureus, E. coli, P. aeruginosa, and S. pyogenes was tested with the Oxford cup method, which led to the identification of four strains effective against the growth of the four pathogens (Figure 1).
Figure 1.
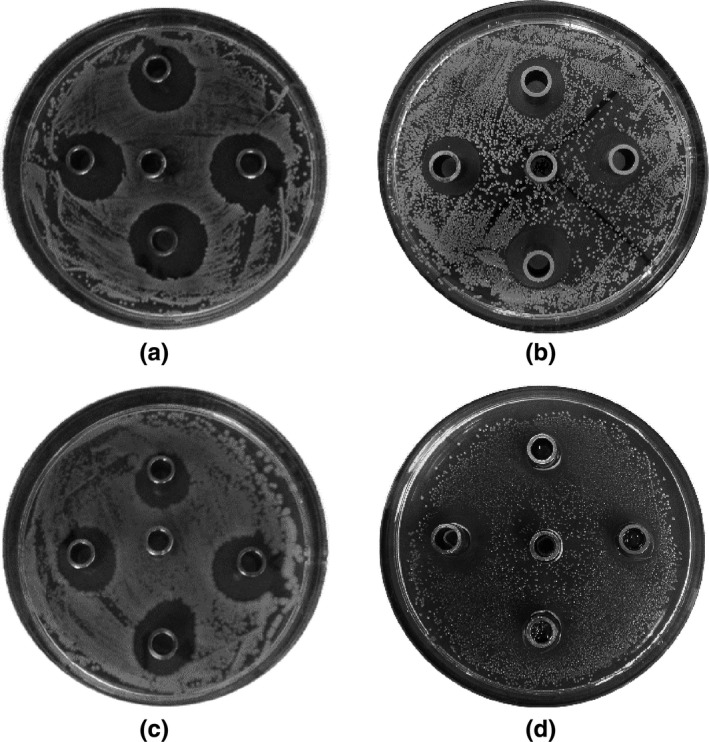
Results of antibacterial Oxford cup analyses of antagonistic strains initially screened only for S. aureus against four common pathogenic bacteria. (a) Antagonistic activity against S. aureus, (b) Antagonistic activity against E. coli, (c) Antagonistic activity against P. aeruginosa, (d) Antagonistic activity against S. pyogenes. (Top: strain number: 1, left: strain number 2, bottom: strain number 4, right: strain number 7, middle: saline)
MALDITOF MS and 16S rDNA analyses identified strain number 1 as S. parasanguinis with the closest 16S rDNA match to S. mitis NCTC 12,261 (pairwise similarity of 96.8%) further named S. parasanguinis I; strain number 2 as S. mitis with a pairwise 16S rDNA similarity of 99.21% to NCTC 12,261 (further named S. mitis I); strain number 4 as S. mitis with a pairwise 16S rDNA similarity of 96.42% to S. dentisani DSM 27,088 (further named S. mitis II); and strain number 7 as S. parasanguinis with a pairwise 16S rDNA similarity of 96.99% to NCTC 12,261 (further named S. parasanguinis II).
Determination of the optimum antibacterial concentration of antagonistic strains against four common pathogens (S. aureus, E. coli, P. aeruginosa, and S. pyogenes) revealed that antagonistic stock solutions (100 µl 1–2 × 109) had the most bactericidal activity against the pathogenic bacteria (100 µl 5–6 × 104), with decreasing efficacy when diluted 10 times (100 µl 1–2 × 108), 100 times (100 µl 1–2 × 107), and 1,000 times (100 µl 1–2 × 106) (Table 2).
Table 2.
Quantitative determination of effective bacterial ratios of four antagonistic strains against Staphylococcus aureus, Escherichia coli, and “+,−”indicates the resulting ratio of antagonistic strains to pathogens; ++++: ≥100:1, +++: ~10:1, ++: ~1:1, +: ~1:10, ±: ≤1:100,: none
| S. parasanguinis I | S. mitis I | S. mitis II | S. parasanguinis II | S. parasanguinis I | S. mitis I | S. mitis II | S. parasanguinis II | |
|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | |||||||
| x1 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| x10 | ++ | ++ | ++++ | ++++ | ++ | ++ | +++ | +++ |
| x100 | + | + | +/− | + | + | + | − | +++ |
| x1000 | − | − | − | +/− | − | − | − | +++ |
| P. aeruginosa | S. pyogenes | |||||||
| x1 | ++++ | ++++ | ++++ | ++++ | +++ | ++++ | ++++ | +++ |
| x10 | ++ | ++ | +++ | +++ | ++ | +++ | ++ | ++ |
| x100 | + | + | − | + | +/− | + | + | + |
| x1000 | + | − | − | − | − | − | − | − |
As evident in Table 3, all four antagonistic strains had bacteriostatic effects on the common pathogens S. aureus, P. aeruginosa, E. coli, K. pneumonia, P. vulgaris, E. cloacae, A. baumannii, and S. pyogenes. In addition, bromcresol purple assays revealed that the mode of action was not mediated by lactic acid production (Figure A1 in Appendix).
Table 3.
Determination of bactericidal effects of the 4 derived antagonistic strains against common pathogens
| Pathogens | S. aureus | P. aeruginosa | E. coli | K. pneumoniae | P. vulgaris | E. cloacae | A. baumannii | S. pyogenes |
|---|---|---|---|---|---|---|---|---|
| Antagonistic strains | ||||||||
| S. parasanguinis I | +++ | ++ | +++ | ++ | +++ | ++ | ++ | ++ |
| S. mitis I | +++ | ++ | +++ | ++ | ++ | ++ | ++ | +++ |
| S. mitis II | +++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ |
| S. parasanguinis II | +++ | ++ | +++ | ++ | ++ | +++ | +++ | ++ |
+++, Bacteriostasis diameter ≥20 mm; ++, 15 mm ≤ Bacteriostasis diameter <20 mm.
4. DISCUSSION
As result of screening in the present study, two S. mitis and two S. parasanguinis strains, derived from the oropharyngeal microbiota of five healthy children were isolated and showed antagonistic effects against S. aureus, P. aeruginosa, E. coli, K. pneumonia, P. vulgaris, E. cloacae and A. baumannii, and S. pyogenes. It has been suggested that in order to prevent respiratory tract infections microbiome homeostasis with beneficial species abundance should be maintained and that infections result from a disturbed balance between pathogenic expansion and microbiome homeostasis (Faden et al., 1997; Gao et al., 2014). Therefore, we suggest that the four antagonistic Streptococcus strains might lead to a balance between pathogens and probiotics in the respiratory tract, thereby preventing expansion of the pathogens and concomitant respiratory infections. In a previous study, administration of the Streptococcus salivarius K15 strain to children with recurrent oral streptococcal pathology reduced streptococcal pharyngeal infections and acute otitis media episodes (Di Pierro et al., 2012). Other examples of probiotic treatment for limiting the overgrowth of potential pathogens include nasal sprays containing streptococci strains, and in previous trials, S. mitis has been shown to be beneficial for decreasing the recurrence rates of otitis media, tonsillitis or pharyngotonsillitis, and group A streptococci infections (Falck, Grahn‐Hakansson, Holm, Roos, & Lagergren, 1999; Roos, Grahn, Holm, Johansson, & Lind, 1993; Roos et al., 2001; Roos, Holm, Grahn, & Lind, 1993; Roos, Holm, Grahn‐Hakansson, & Lagergren, 1996; Santagati et al., 2015). However, whether one of the antagonistic S. mitis or S. parasanguinis strains from this study had similar effects needs to be further investigated.
5. CONCLUSIONS
In this study, we isolated from the oropharyngeal microbiota of five healthy children two antagonistic S. mitis and one S. parasanguinis strain, which exhibited broad‐spectrum antagonistic activity against S. aureus, P. aeruginosa, E. coli, K. pneumonia, P. vulgaris, E. cloacae, A. baumannii, and S. pyogenes. We suggest that the growth of the mentioned streptococci strains may prevent pathogenic bacterial expansion.
CONFLICTS OF INTEREST
None declared.
AUTHORS CONTRIBUTION
XL, SL, and CX: conceptualized. XL, BY, YS, SL, DL, YZ, and CX: collected the data. XL, BY, YS, SL, and CX: analyzed the data. XL, YS, and CX: wrote the original draft. XL, BY, YS, SL, and CX: involved in writing review and editing. All authors have read and approved the manuscript. The authors were solely responsible for the conception and conduction of the study and for writing the manuscript.
ETHICAL APPROVAL
The study was conducted in accordance with the “Declaration of Helsinki” guidelines and approved by the Ethics Committee of Shenyang Medical College (approval number: No. 2015052902). Written informed consent was obtained from the legal representatives of the participating children.
ACKNOWLEDGMENTS
This work was supported by the Liaoning Province Natural Science Foundation of China [20180551234], Research Foundation of Shenyang Science and Technology Bureau [18‐400‐4‐09] and the Research Foundation of Shenyang Medical College [20183051]. The funding organizations had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
APPENDIX 1.
Figure A1.
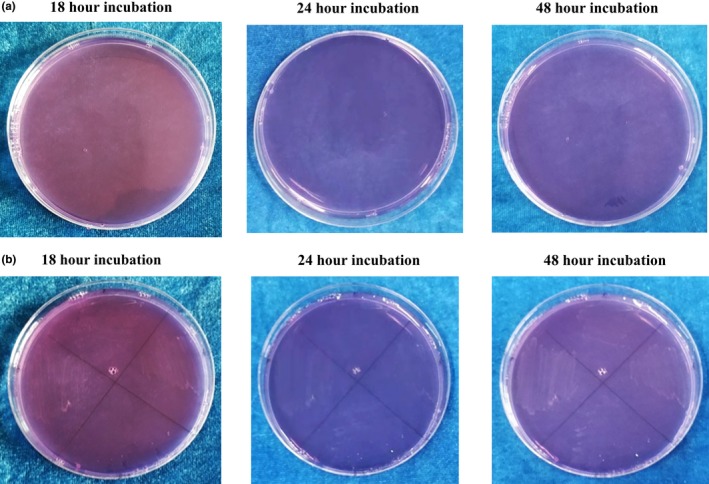
Test for detection of lactic acid production with agar containing 0.04 g/L Bromcresol Purple (Cat No. 36408, Millipore). (a) Control agar plates without bacterial colonies at 18, 24 and 48 hr incubation. (b) S. parasanguinis I, S. mitis I, S. mitis II, and S. parasanguinis II after 18, 24, and 48 hr incubation. No lactic acid production took place
Li X, Yang B, Sun Y, et al. Screening of antagonistic strains of respiratory origin and analysis of their bacteriostatic effects on pathogens. MicrobiologyOpen. 2019;8:e940 10.1002/mbo3.940
DATA AVAILABILITY STATEMENT
The data will be available on request from the corresponding author. The 16S rDNA sequences are available on NCBI: S. parasanguinis I (accession number MN068913), S. mitis I (accession number MN061052), S. mitis II (accession number MN061052), and S. parasanguinis II (accession number MN12046468).
REFERENCES
- Biesbroek, G. , Tsivtsivadze, E. , Sanders, E. A. M. , Montijn, R. , Veenhoven, R. H. , Keijser, B. J. F. , & Bogaert, D. (2014). Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. American Journal of Respiratory and Critical Care Medicine, 190(11), 1283–1292. 10.1164/rccm.201407-1240OC [DOI] [PubMed] [Google Scholar]
- Bosch, A. A. T. M. , Piters, W. A. A. , & d van Houten, M. A. , Chu, M. L. J. N. , Biesbroek, G. , Kool, J. , … Bogaert, D. (2017). Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. a prospective cohort study. American Journal of Respiratory and Critical Care Medicine, 196(12), 1582–1590. 10.1164/rccm.201703-0554OC [DOI] [PubMed] [Google Scholar]
- Carolina Maldonado, G. , Ivanna Novotny, N. , Esteban, C. , Alejandra de Moreno de, L. , & Gabriela, P. (2015). Role of probiotics and functional foods in health: Gut immune stimulation by two probiotic strains and a potential probiotic yoghurt. Endocrine, Metabolic & Immune Disorders ‐ Drug Targets, 15(1), 37–45. 10.2174/1871530314666141216121349 [DOI] [PubMed] [Google Scholar]
- Di Pierro, F. , Donato, G. , Fomia, F. , Adami, T. , Careddu, D. , Cassandro, C. , & Albera, R. (2012). Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. International Journal of General Medicine, 5, 991–997. 10.2147/IJGM.S38859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didari, T. , Mozaffari, S. , Nikfar, S. , & Abdollahi, M. (2015). Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta‐analysis. World Journal of Gastroenterology, 21(10), 3072–3084. 10.3748/wjg.v21.i10.3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden, H. , Duffy, L. , Wasielewski, R. , Wolf, J. , Krystofik, D. , & Tung, Y. (1997). Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. The Journal of Infectious Diseases, 175(6), 1440–1445. 10.1086/516477 [DOI] [PubMed] [Google Scholar]
- Falck, G. , Grahn‐Hakansson, E. , Holm, S. E. , Roos, K. , & Lagergren, L. (1999). Tolerance and efficacy of interfering alpha‐streptococci in recurrence of streptococcal pharyngotonsillitis: A placebo‐controlled study. Acta Oto‐Laryngologica, 119(8), 944–948. 10.1080/00016489950180333 [DOI] [PubMed] [Google Scholar]
- Gao, Z. , Kang, Y. , Yu, J. , & Ren, L. (2014). Human pharyngeal microbiome may play a protective role in respiratory tract infections. Genomics, Proteomics & Bioinformatics, 12(3), 144–150. 10.1016/j.gpb.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators . (2015) Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 385(9963), 117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti, S. , Taverniti, V. , Minuzzo, M. , Arioli, S. , Stuknyte, M. , Karp, M. , & Mora, D. (2010). Oral bacteria as potential probiotics for the pharyngeal mucosa. Applied and Environmental Microbiology, 76(12), 3948–3958. 10.1128/AEM.00109-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , Pot, B. , … Sanders, M. E. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Humphreys, G. J. , & McBain, A. J. (2019). Antagonistic effects of Streptococcus and Lactobacillus probiotics in pharyngeal biofilms. Letters to Applied Microbiology, 68(4), 303–312. 10.1111/lam.13133 [DOI] [PubMed] [Google Scholar]
- Korzeniewski, K. , Nitsch‐Osuch, A. , Chciałowski, A. , & Korsak, J. (2013). Environmental factors, immune changes and respiratory diseases in troops during military activities. Respiratory Physiology & Neurobiology, 187(1), 118–122. 10.1016/j.resp.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesi, T. , Creery, D. , Gilbert, N. L. , Dales, R. , Fugler, D. , Thompson, B. , … Miller, J. D. (2006). Indoor air quality risk factors for severe lower respiratory tract infections in Inuit infants in Baffin Region, Nunavut: A pilot study. Indoor Air, 16(4), 266–275. 10.1111/j.1600-0668.2006.00423.x [DOI] [PubMed] [Google Scholar]
- Lelieveld, J. , Haines, A. , & Pozzer, A. (2018). Age‐dependent health risk from ambient air pollution: A modelling and data analysis of childhood mortality in middle‐income and low‐income countries. The Lancet Planetary Health, 2(7), e292–e300. 10.1016/S2542-5196(18)30147-5 [DOI] [PubMed] [Google Scholar]
- Martinez, R. C. R. , Bedani, R. , & Saad, S. M. I. (2015). Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: An update for current perspectives and future challenges. British Journal of Nutrition, 114(12), 1993–2015. 10.1017/S0007114515003864 [DOI] [PubMed] [Google Scholar]
- Popova, M. , Molimard, P. , Courau, S. , Crociani, J. , Dufour, C. , Le Vacon, F. , & Carton, T. (2012). Beneficial effects of probiotics in upper respiratory tract infections and their mechanical actions to antagonize pathogens. Journal of Applied Microbiology, 113(6), 1305–1318. 10.1111/j.1365-2672.2012.05394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, C. R. , & Huang, Y. X. (2000). Laboratory Experiments In Microbiology (p. 207–208). Beijing, China: Peking University Press. [Google Scholar]
- Quigley, E. M. , & Shanahan, F. (2014). The future of probiotics for disorders of the brain‐gut axis. Advances in Experimental Medicine and Biology, 817, 417–432. 10.1007/978-1-4939-0897-4_19 [DOI] [PubMed] [Google Scholar]
- Roos, K. , Grahn, E. , Holm, S. E. , Johansson, H. , & Lind, L. (1993). Interfering alpha‐streptococci as a protection against recurrent streptococcal tonsillitis in children. International Journal of Pediatric Otorhinolaryngology, 25(1–3), 141–148. [DOI] [PubMed] [Google Scholar]
- Roos, K. , Hakansson, E. G. , & Holm, S. (2001). Effect of recolonisation with "interfering" alpha streptococci on recurrences of acute and secretory otitis media in children: Randomised placebo controlled trial. BMJ, 322(7280), 210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, K. , Holm, S. E. , Grahn, E. , & Lind, L. (1993). Alpha‐streptococci as supplementary treatment of recurrent streptococcal tonsillitis: A randomized placebo‐controlled study. Scandinavian Journal of Infectious Diseases, 25(1), 31–35. 10.1080/00365549309169666 [DOI] [PubMed] [Google Scholar]
- Roos, K. , Holm, S. E. , Grahn‐Hakansson, E. , & Lagergren, L. (1996). Recolonization with selected alpha‐streptococci for prophylaxis of recurrent streptococcal pharyngotonsillitis–a randomized placebo‐controlled multicentre study. Scandinavian Journal of Infectious Diseases, 28(5), 459–462. [DOI] [PubMed] [Google Scholar]
- Santagati, M. , Scillato, M. , Muscaridola, N. , Metoldo, V. , La Mantia, I. , & Stefani, S. (2015). Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. European Journal of Clinical Microbiology & Infectious Diseases, 34(10), 2075–2080. 10.1007/s10096-015-2454-2 [DOI] [PubMed] [Google Scholar]
- Schroeck, J. L. , Ruh, C. A. , Sellick, J. A. Jr , Ott, M. C. , Mattappallil, A. , & Mergenhagen, K. A. (2015). Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrobial Agents and Chemotherapy, 59(7), 3848–3852. 10.1128/AAC.00652-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrack, S. , Panjikar, P. , Duster, M. , & Safdar, N. (2014). Tolerability of a probiotic in subjects with a history of methicillin‐resistant Staphylococcus aureus colonisation. Beneficial Microbes, 5(4), 389–395. 10.3920/BM2013.0062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on request from the corresponding author. The 16S rDNA sequences are available on NCBI: S. parasanguinis I (accession number MN068913), S. mitis I (accession number MN061052), S. mitis II (accession number MN061052), and S. parasanguinis II (accession number MN12046468).


