Abstract
Cardiovascular disease (CVD) is the leading cause of death internationally. We aimed to model the impact of CVD preventive double therapy (a statin and anti-hypertensive) by clinician-assessed absolute risk level. An established and validated multi-state life-table model for the national New Zealand (NZ) population was adapted. The new version of the model specifically considered the 60–64-year-old male population which was stratified by risk using a published NZ-specific CVD risk equation. The intervention period of treatment was for five years, but a lifetime horizon was used for measuring benefits and costs (a five-year horizon was also implemented). We found that for this group offering double therapy was highly cost-effective in all absolute risk categories (eg, NZ$1580 per QALY gained in the >20% in 5 years risk stratum; 95%UI: Dominant to NZ$3990). Even in the lowest risk stratum (≤5% risk in 5 years), the cost per QALY was only NZ$25,500 (NZ$28,200 and US$19,100 in 2018). At an individual level, the gain for those who responded to the screening offer and commenced preventive treatment ranged from 0.6 to 4.9 months of quality-adjusted life gained (or less than a month gain with a five-year horizon). Nevertheless, at the individual level, patient considerations are critical as some people may decide that this amount of average health gain does not justify taking daily medication.
Subject terms: Health care economics, Epidemiology, Patient education
Introduction
Cardiovascular disease (CVD) is the single largest disease category causing death globally in 2017 at an estimated 17·8 million deaths, followed by neoplasms at 9·56 million deaths1. Furthermore, this Global Burden of Disease study noted that “the increasing prevalence of obesity might explain why death rates for cardiovascular disease are no longer declining in Australia, Austria, Brazil, Germany, Netherlands, the UK, and the USA”1.
Fortunately, CVD is cost-effectively preventable using such means as tobacco control but also preventive pharmacotherapy2. Indeed, international work has reported that statins are cost-effective for the primary prevention of CVD (eg, a Cochrane Review3 and a review by Kazi et al.4). A systematic review of economic evaluations in low- and middle-income countries has also reported that both lipid-lowering and blood pressure lowering medication is typically cost-effective or even cost-saving when used for primary prevention of CVD (including in combination)2.
Furthermore, when intensive lipid lowering treatment (relative to standard lipid lowering) is considered, it has been reported that this treatment is cost-effective in all groups (ie, in a study from the Netherlands – albeit this being for patients with established CVD5). Similarly, an Australian study reported that “recommending blood pressure-lowering drugs to everyone with at least 5% absolute risk and statin drugs to everyone with at least 10% absolute risk” would generate health gain and save the Australian Government $5.4 billion over the lifetime of the population (or $7.1 billion if New Zealand statin prices were matched, 2008 prices)6.
Nevertheless, uncertainties remain with a report that industry-funded studies of statins provide more favourable cost-effectiveness estimates7. Also due to country variation in disease epidemiology and costs, policy-makers need jurisdiction-specific analyses on health gain, costs and cost-effectiveness. There also needs to be better individual level information for patients on how much quality-adjusted life that they might gain from taking daily medication.
New Zealand is an ideal case study country to consider such issues, given that consideration of absolute CVD risk has been promoted to clinicians for a long time8, albeit with this approach still not always dominating in clinical practice9. There is also evidence for successful campaigns to increase preventive pharmacotherapy eg, statin use in Māori10, the Indigenous population. Furthermore, net health sector cost-savings from further CVD prevention are also potentially large eg, NZ$1.1 billion from a single sodium reduction intervention modelled over the remaining lifetime of New Zealand adults (n = 2.3 million 35+ year olds)11. Another study of the impact of tobacco tax increases in this country also estimated very large health sector cost-savings, although this model also included impacts on reducing cancers and respiratory diseases along with CVD12.
Given this background, we aimed to model the impact of CVD preventive pharmacotherapy by clinician-assessed absolute risk level and identify the associated health gain, impact on health system costs and cost-effectiveness for 60–64 year old males. We selected this age-group as just an initial starting point for future work on assessing this approach of considering absolute CVD risk. In addition, this age-group of men is of particular interest as it is the working age with the highest rate of CVD and improving health in this age-group may enhance productivity of the economy (including for those citizens who continue in the paid workforce after age 65 years). We focused on double therapy (a combination of a statin and an anti-hypertensive) as these preventive medicines are already widely used in this way in New Zealand and aspirin as a preventive pharmacotherapy is more controversial.
Methods
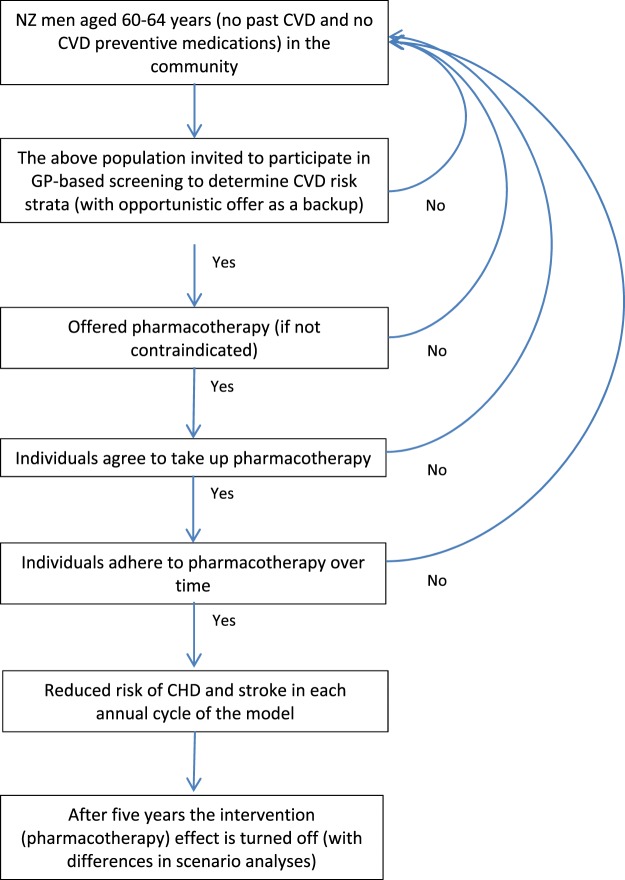
We developed a CVD multi-state life-table (MSLT) model from an established MSLT (used for tobacco control) to model the whole New Zealand population throughout their lifetime and estimated health gains and health system costs. Figure 1 provides an overview of the modelled intervention process. This is then followed by more detailed descriptions of the new CVD multi-state life-table (MSLT) model and key parameters.
Figure 1.
Intervention flowchart for CVD risk screening and provision of CVD preventive pharmacotherapy in this new CVD MSLT Model.
Multi-state life-table model
Our study built on the BODE3 Tobacco Control multi-state life-table (TC-MSLT) Model from which we have published results from previously12–19. This original model benefits from rich national epidemiological data by sex, age, and ethnicity (Māori and non-Māori), as well as costing data. Results from the CVD component of this model have also been validated via a head-to-head comparison with a separate model with a different structure ie, a CVD model built in TreeAge and used for dietary salt interventions11,20,21. The data in this TC-MSLT Model are used to estimate quality-adjusted life-years (QALYs) gained and net health system costs over the remaining life of the 2011 New Zealand population. The specific enhancements made for the current study are outlined in more detail below.
Study population
Integrating CVD risk data from a synthetic national population
As the TC-MSLT Model lacked data on grouping individuals by level of absolute CVD risk, we stratified the cohorts into categories of absolute CVD risk. We utilised previous work using New Zealand-specific CVD risk prediction equations to create a synthetic simulation popoulation22. The variables required for the risk equation predictions were: age, sex, ethnicity, social deprivation, smoking status, diabetes status, personal history of CVD, blood pressure and lipid-lowering medication treatment, systolic blood pressure, the total cholesterol to high density lipoprotein cholesterol ratio (TC:HDL), and family history of premature CVD (with these from the PREDICT dataset, Auckland University). These risk equations were then applied to a synthetic population of 2.45 million New Zealand adults to estimate numbers and rates of CVD events. This population was formed by extracting all anonymised 30–84-year-old respondents to the 2013 census, with such variables on age, sex, ethnicity, social deprivation and smoking status. Uncertainty was generated by sampling from 100 synthetic populations. A more detailed description of this synthetic data generation is provided elsewhere22.
We focused on the male population aged 60–64 years who were not on CVD preventive medication (with standard deviations of the sampled means) and who had no previous diagnoses of CVD in the TC-MSLT Model. Similarly, they also had no previous diagnoses of the following: chronic kidney disease, rheumatic heart disease, congestive heart failure and atrial fibrillation22. Table 1 provides an example of the data for men aged 60–64 years.
Table 1.
Example data for the predicted five-year risk of a CVD event for the synthetic national population for non-Māori and Māori men (60–64 years, with no past CVD events and on no CVD medication22).
| Five-year cumulative absolute risk (%) strata for CVD events (fatal and non-fatal) | Non-Māori | Māori | ||
|---|---|---|---|---|
| Population (N) | Average risk within each risk stratum | Population (N) | Average risk within each risk stratum | |
| >20% | 56 | 22.9 | 44 | 22.9 |
| >15, ≤20% | 265 | 16.8 | 169 | 16.9 |
| >10, ≤15% | 1882 | 11.7 | 772 | 11.9 |
| >5, ≤10% | 19,577 | 6.5 | 2611 | 7.1 |
| >0, ≤5% | 36,319 | 3.6 | 875 | 4.1 |
| Total | 58,099 | — | 4470 | — |
| Average risk* | — | 5.0 | — | 7.9 |
*Calculated as the population weighted average risk across strata.
Building CVD risk stratification to create the CVD MSLT Model
We then took the TC-MSLT Model and modified it to create the CVD MSLT Model. This involved splitting the modelled population into three separate components (with replication for each ethnicity grouping in the age 60–64-year-group of men).
Population A: This was the group who were not on any CVD medications and did not have prevalent CVD in 2011. This group was then divided into five strata of differing levels of five-year absolute risk of a CVD event as per the proportions in the synthetic population work22. It is this population that was the intervention population in the model ie, those potentially offered CVD preventive pharmacotherapy.
Population B: This was the group with no prevalent CVD in 2011 but who were already on CVD preventive medication. This group were given incidence rates of CVD based on the estimated five-year absolute risk of a CVD event as per the synthetic population work22.
Population C: This was the group who had prevalent CVD in 2011, regardless of medication status. Again, the proportion in this group was derived from the synthetic population distribution.
Collectively these three groups cover all New Zealand men in this selected age-group. In addition, we needed to provide unique case fatality rates for the strata in Group A. There were no published New Zealand data for this (the case fatality data exist by age-group only23) so we considered the results from the meta-analysis by Zambon et al.24 and used the regression equation for CVD mortality by CVD incidence (Figure 2(c) in Zambon et al.) to mathematically disaggregate the case fatality risks by absolute risk strata – ensuring the case fatality over all strata combined was preserved.
There is also evidence that those with elevated CVD incidence also have relatively elevated non-CVD mortality rates (eg, data abstracted from the meta-analysis by Thomopoulos et al.25 albeit without age-standardisation). Based on this evidence from Thomopoulos et al., we specified a two-fold increase in non-CVD mortality rates for the highest compared to lowest absolute risk strata, with a linear trend over intervening risk strata in Population A (with wide uncertainty around this 2.0 value included in our modelling [confidence intervals: 1.0 to 3.0 times]).
Model calibration
The MSLT is dynamic, meaning that our initial data from the TC-MSLT model and parameters from external literature inputs (above) once simulated through strata of CVD risk may not generate the same number of disease events, life years lived, etc, as the un-stratified population in the TC-MSLT. To be consistent with the TC-MSLT, we therefore required that the outputs summed or averaged across absolute CVD risk had to be equal to those in the non-stratified TC-MSLT at a five-year time horizon. Accordingly, we ran optimisation routines (in the R programming language) for case fatality rates (separately for CHD and stroke) and non-CVD background mortality rates (BMR). That is the weighted sum of the BMRs by absolute risk had to return the BMR for both the non-Māori and Māori populations in this age-group, ie, the BMR used in the TC-MSLT Model. This process allowed us to achieve complete matching (with the original model) of the cumulative count of CVD and non-CVD deaths after five years only. Fig. S1 in the Supplementary Material further details this process.
Modelling approach and key parameters
For each ethnicity grouping we used the adjusted five-year CVD risk estimates (see above for an example) and ran the intervention of offering and providing (if accepted) double therapy for a five-year period. Further details on the epidemiological and intervention parameters are below and in Tables 2–4.
Table 3.
Selected additional description of intervention-specific cost parameter details (see Supplementary Material Table S2 for the full details).
| Input Parameter | Source | Expected Value and 95% UI | Distri-bution |
|---|---|---|---|
| Costs for CVD assessment and being prescribed CVD preventive pharmacotherapy | |||
|
GP visits for initial CVD risk assessment and on-going prescriptions and check-ups (same for double therapy and single medications) |
PHARMAC cost resource manual38 | NZ$218 (in 2011 dollars) per annum over the five year intervention period | Gamma, SD ± 20% |
| Fasting lipid test on first consultation (required for all CVD risk assessment, both double therapy and single medications) | HealthTracker data for 2011 |
$28.29 (in first year of intervention period only) |
Gamma, SD ± 10% |
| Two annual prescriptions via telephone from GP (same for double therapy and single medications) | PHARMAC cost resource manual38 | $28.93 per annum (2011 dollars) | Gamma, SD ± 10% |
| Pharmacist payments for double therapy (2 medicines at 4 times year) | PHARMAC cost resource manual38 and pricing data for an agreement with community pharmacies39 | $41.57 per annum (2011 dollars) | Gamma, SD ± 10% |
| Pharmacist payments for dispensing single medications (1 dispensing 4 times year) | As above | $20.78 per annum | Gamma, SD ± 10% |
| Pharmaceuticals | |||
| Lipid-lowering medication (same for double therapy and single medications) | PHARMAC Online Schedule in 2017 (https://www.pharmac.govt.nz/Schedule) | $10.97 (2011 dollars) | Gamma, SD ± 10% |
| Anti-hypertensive (same for double therapy and single medications) | As above | $6.21 (2011 dollars) | Gamma, SD ± 10% |
| Total annual cost of double therapy | See above | $17.18 per annum (2011 dollars) | See for individual medicines |
Table 2.
Summary of epidemiological and cost parameters used in the modelling (see Supplementary Material Table S1 for the full details).
| Input parameter/s | Source | Heterogeneity | Expected Value and 95% UI | Distribution |
|---|---|---|---|---|
| Intervention parameters | ||||
| General practitioner (GP) level screening for CVD risk: provision of offer and with the GP asking at opportunistic consultations as a backup (for both double therapy and single medications) | National District Health Board (DHB) data | Variation by Māori/non-Māori | Māori: 86% Non-Māori: 92% (using median values for all DHBs, no mean values available) | Beta, SD = ±5% |
| Intervention uptake by patients when recommended by a GP (for both double therapy and single medications) | NZ data33 | No variation (see details in the column to the left) | 77% overall | Normal, SD = ±10% |
| Decline in adherence to pharmacotherapy throughout the 5-year intervention period (for double therapy and single medications) | NZ data34 and authors’ assumptions | No variation (see details in the column to the left) | Over the whole 5 y period a 22.5% linear decline in adherence | Beta distribution (SD +/−5% of the cumulative reduction value) |
| Effect of CVD preventive pharmacotherapy on risk of CHD and stroke events | See Table 4 | No variation | See Table 4 | Log-normal |
| Sensitivity and scenario analyses (for both double therapy and single medications) | ||||
| Varying the discount rate | We used 0% and 6% in sensitivity analyses (as per our BODE3 modelling protocol26). | |||
| Equity analysis | In this analysis we gave the Māori population the same potential envelope of health gain as per non-Māori, ie, the same morbidity and mortality rates as non-Māori35. This prevents Māori in the analysis from effectively being penalised due to poorer existing health relative to non-Māori. | |||
| Halving of effect sizes for risk reduction (ie, treatment effect by CVD risk strata) | This scenario was considered given that the trial data might not be fully generalisable to the adult population in this target age-group (eg, trials tend to involve patients with elevated risk levels). | |||
| 5-year time horizon | As per the base-case analysis, but where the benefits (QALYs gained) and health costs were tallied up at 5 years. | |||
| 10-year time horizon | As per the base-case analysis, but where the benefits (QALYs gained) and health costs were tallied up at 10 years. | |||
| 20-year time horizon | As above but for the 20-year point. | |||
| Continuing use of therapy for 10 years (ie, extending intervention duration in base model from 5 years to 10 years) | We assumed that after the initial 5-year decline in adherence, that adherence would then plateau (as per above in the 50% to 70% range). Of note is that for those in NZ with a known history of CVD, the use of two CVD medication categories (BP-lowering and lipid-lowering) was 70% in the older 65–74 year old age-group36. | |||
| Continuing use of therapy for 20 years | As in the row above but for 20-years. | |||
| Costs | ||||
| Background health system costs for all citizens (adjusted for CHD and stroke costs) | As per BODE3 costing methods37 | Nil | Uncertainty: ±10% SD. | Log-normal |
| GP visits, prescriptions, pharmaceutical costs | See Table 3 | Nil | See Table 3 | See Table 3 |
Table 4.
Relative risks for preventing CVD events from preventive CVD pharmacotherapy versus no medication (95% CI) (applying to all CVD risk strata).
| Outcome | Statin* | Anti-hypertensive** | Double therapy (as calculated for this study)# |
|---|---|---|---|
| Total CHD events (non-fatal and fatal) |
0.73 (0.67 to 0.80) Cochrane Review3 |
0.8140 (0.73 to 0.89) (Using SD = 5% of the point estimate) |
0.59 (0.50 to 0.69) (used in this modelling) |
| Total stroke events (non-fatal and fatal) |
0.71 (0.62 to 0.82) USPSTF Review29 |
0.7540 (0.68 to 0.82) (Using SD = 5% of the point estimate) |
0.53 (0.42 to 0.66) (used in this modelling) |
*These results are consistent with a long-term trial that found that among individuals with LDL-C ≥ 190 mg/dL, pravastatin reduced the risk of CHD death, cardiovascular death and all-cause mortality by 28% (p = 0.020), 25% (p = 0.009) and 18% (p = 0.004), respectively, over a total of 20-years of follow-up41. USPSTF: US Preventive Services Task Force.
**Results from Law et al. for those aged 60–69 years for one medication at the standard dose in the range for the mean systolic BP for those in this age-group in NZ (based on NZ survey data42), ie, 138 mmHg for men and 132 mmHg for women.
#The effects from each medication are assumed to be independent. To calculate the aggregate effect of double therapy, the relative risks from each monotherapy were multiplied together. The Ersatz Excel plugin was used to generate the 95% CI by running 2000 iterations of a log-normal distribution.
The impact of the intervention in terms of health gain in QALYs were accumulated over the remainder of the cohort’s life. Of note, we assumed that the intervention period was only for five years, with treatment ending at this point for those who took up the intervention at the start (with 10 and 20 years in sensitivity analysis). Furthermore, we also assumed that CVD risks for the intervention groups all returned to average within strata risks after the five year intervention period.
Likewise, net health system costs were tallied up, including the cost of the intervention, the costs of averted health care (from preventing CVD; by including disease costs in the MSLT), and the costs of additional health care from any extended lifespan (due to health system costs assigned to all alive citizens, which with increased longevity from the intervention contribute to additional health expenditure in later life).
Sensitivity and scenario analyses were run to encompass differing discount rates (0% and 6% as per our BODE3 Research Protocol26) and the impact of taking double therapy for longer periods (10 and 20 years). Monotherapy of either a statin or an anti-hypertensive alone, were also examined.
Ethical approval
Approval for use of anonymised administrative data as part of the BODE3 Programme has been granted by the Health and Disability Ethics Committees (reference number H13/049).
Results
For men in this 60–64-year age-group, the potential offer of double therapy (a statin and an anti-hypertensive) was found to be highly cost-effective in all absolute risk strata (when using the threshold of <NZ$45,000 per QALY as being cost-effective, ie, approximately real GDP per capita in New Zealand in 2011) (Table 5). Indeed, it was extremely cost-effective in the highest risk stratum (incremental cost-effectiveness ratio (ICER): NZ$1580 per QALY gained for >20% risk) and in the lowest risk stratum (≤5%) it was still very cost-effective with an ICER of NZ$25,500 (NZ$28,200 and US$19,100 in 2018) per QALY gained (95%UI: NZ$12,300 to NZ$41,500 (NZ$13,600 to 45,900 in 2018)).
Table 5.
Health gains (QALYs) and net health system cost impacts for 60–64-year-old men (Māori and non-Māori) from the offer of five-years of double therapy involving a statin and an anti-hypertensive, 3% discount rate, and a lifetime horizon*.
| Five-year cumulative absolute risk strata | Total QALYs gained (non-Māori) | QALYs gained per 1000 people (non-Māori) | Total QALYs gained (Māori) | QALYs gained per 1000 people (Māori) | Total QALYs gained (ethnic groupings combined) | Net costs in NZ$ million (ethnic groupings combined) | ICER (NZ$ per QALY gained)** |
|---|---|---|---|---|---|---|---|
| >20% |
16.7 (13.0 to 20.5) |
289 (225 to 355) |
10.2 (7.70 to 12.7) |
243 (183 to 302) |
26.9 (20.8 to 33.0) |
$0.04 ($−0.02 to $0.1) |
1580 (Dominant to $3990) |
| >15, ≤20% |
55.4 (43.9 to 67.9) |
203 (160 to 248) |
28.7 (22.3 to 35.4) |
177 (138 to 218) |
84.0 (66.3 to 103) |
$0.16 ($−0.09 to $0.39) |
1930 (Dominant to $4960) |
| >10, ≤15% |
263 (205 to 319) |
135 (106 to 164) |
91.7 (70.0 to 112) |
124 (94.7 to 152) |
354 (276 to 430) |
$1.18 ($−1.5 to $2.55) |
$3430 (Dominant to $7860) |
| >5, ≤10% |
1410 (1110 to 1720) |
70.0 (54.9 to 85.3) |
179 (139 to 220) |
71.6 (55.7 to 87.9) |
1590 (1250 to 1940) |
$14.8 ($4.8 to $25.3) |
$9510 ($2740 to $18,000) |
| >0, ≤5% |
1330 (1060 to 1610) |
35.5 (28.3 to 43.1) |
32.3 (25.6 to 39.2) |
38.6 (30.6 to 46.9) |
1360 (1090 to 1650) |
$34.1 ($18.5 to $51.4 |
$25,500 ($12,300 to $41,500) |
*For those starting with no past CVD events and no past CVD medication; using 92% screened, 77% uptake and an overall 22.5% decline in adherence over time; life-time QALYs and life-time costs but for a 5-year treatment period only, 3% discount rate, with 95% uncertainty intervals.
**In this context, a “Dominant” ICER means that the intervention leads to a population health gain at a net cost-saving to society, in comparison with no treatment.
The highest absolute population level health gains were not from treating men in the highest two risk categories (gaining only 26.9 and 84.0 QALYs respectively), but from the lowest two risk categories (1590 QALYs for the >5, ≤10% stratum; 1360 QALYs for the ≤5% stratum).
The per capita QALY gain for Māori men (Indigenous) was similar to non-Māori, albeit slightly greater for Māori in the lowest two risk groups (Table 5). The health gains for Māori were further increased with an “equity analysis” scenario, in which non-Māori background mortality and morbidity were used for Māori (Table S3 in the Supplementary Material). All the other sensitivity and scenario analyses produced net health gain and were cost-effective, except for the half effect size scenario, where the double therapy intervention was no longer cost-effective for the lowest risk stratum (ICERs: NZ$66,100 (NZ$73,100 in 2018), Table S3 in the Supplementary Material). As expected, the smallest health gains were seen with the base-case five-year intervention period and the largest when the intervention period was extended to either 10 or 20 years in scenario analyses (Table S4 in the Supplementary Material).
At an individual level, the health gain for those who responded to the screening offer and commenced treatment with double therapy ranged from 0.6 to 4.9 months of quality-adjusted life gained, depending on risk strata (Table 6). Slightly higher values for per capita gain were apparent when these health gains were not discounted (Table 6).
Table 6.
Average individual level health gain associated with five-years of double therapy with (3%) and without (0%) discounting to potentially facilitate more informed patient-clinician discussions around medication use (the values at 0% discount rate are in brackets)
| Five-year cumulative absolute risk strata | Quality-adjusted healthy months of life gained for the average cohort member from the offer of screening (intention-to-treat style of analysis) | Quality-adjusted healthy months of life gained for those who respond to the screening offer and commence treatment* | ||
|---|---|---|---|---|
| Non-Māori | Māori | Non-Māori | Māori | |
| >20% | 3.5 (5.2) | 2.9 (4.1) | 4.9 (7.3) | 4.1 (5.8) |
| >15, ≤20% | 2.4 (3.7) | 2.1 (3.1) | 3.4 (5.3) | 3.0 (4.4) |
| >10, ≤15% | 1.6 (2.6) | 1.5 (2.2) | 2.3 (3.6) | 2.1 (3.1) |
| >5, ≤10% | 0.8 (1.4) | 0.9 (1.3) | 1.2 (1.9) | 1.2 (1.9) |
| >0, ≤5% | 0.4 (0.7) | 0.5 (0.7) | 0.6 (1.0) | 0.7 (1.0) |
*Albeit with the reduction in adherence as in the base-case model of 22.5% over the five-year period of treatment.
In terms of monotherapy treatments, there was greater health gain in each risk strata for statin treatment than for anti-hypertensive treatment (eg, 34% higher for the second to lowest risk group [1030/771] Table S5 in the Supplementary Material). Similarly, for cost-effectiveness where treatment in all risk categories was cost-effective for only a statin (ICER range: $3740 to $43,500 (NZ$4140 to 48,100 in 2018)) but not in the lowest risk stratum for only an anti-hypertensive (ICER range: $6470 to $62,400 (NZ$7160 to 69,000 in 2018)).
Discussion
Main findings and interpretation
In the selected population group of middle-aged men aged 60–64, the potential offer of CVD preventive double therapy was highly cost-effective from a lifetime time horizon perspective at a 3% discount rate, for all levels of absolute risk. Nevertheless, in this case study we have performed this analysis for only one age-group of men and so we plan on further work to cover both sexes and for a much wider range of adult-age-groups; including among older age-groups where the benefits and risks of preventive pharmacotherapy may be more finely balanced.
The results of double therapy being cost-effective in all risk categories was not surprising given that these two medications (statins and anti-hypertensives) are effective, are relatively low cost, and previous international work on cost-effectiveness is favourable (see Introduction). The latter is especially the case for New Zealand, which has a central government agency (PHARMAC) that negotiates hard with the pharmaceutical industry for low prices – including for generics which all modelled statins and anti-hypertensives typically are.
Even so, there are likely to be even better value for money investments for preventing CVD such as advancing tobacco control, reducing sodium in processed foods, and modifying the obesogenic environment2. For example, our modelling of tobacco control interventions suggest that these are strongly cost-saving in New Zealand12,14, as are nearly all dietary salt reduction interventions (eg, in the processed food supply)11,20,21.
The individual level results in our study are not strictly comparable with other work. Even so, in a Dutch study of people with established CVD, the estimated per patient lifetime gain from taking a statin was 1.7 months (ie, 0.14 QALY at a 1.5% discount rate for a population with mean age of 61 years)5. From Australian work6, which did not specifically provide per capita results, we have estimated an average per person gain of taking a statin at 1.4 months (ie, for those with a ≥5% five year risk over their remaining life course, median age in late 60 s for men and early 70 s for women, and a discount rate of 3%).
Study strengths and limitations
Strengths of this study included that it was built on a well-established original model (the TC-MSLT Model) that captured downward CVD incidence and case-fatality trends. The model also had a rich level of parameterisation with very detailed epidemiological and health cost data for New Zealand. The enhanced model had the benefits of using CVD risk data from a synthetic national population that used a New Zealand-specific CVD risk equation that took into account ethnicity (albeit an equation that has been further refined subsequently27). Furthermore, the CVD health problem being addressed is a major one for all high-income countries and it is well-defined in the sense that doctors regularly assess absolute CVD risk in their patients in the New Zealand setting, and low-cost preventive medications are available to prescribe. This work is also novel in terms of the level of modelling sophistication for determining health gain and cost-effectiveness within absolute risk strata.
Limitations that may mean we have underestimated health gains (and therefore underestimated cost-effectiveness), include assuming a future downward trend in CVD incidence and background mortality; this might not hold given the obesity epidemic (see Introduction). Further under-estimation may occur due to not including benefits around: preventing peripheral vascular disease and chronic kidney disease; from better controlling high blood pressure itself (eg, headaches from hypertension); and possibly the psychological reassurance or anxiety reduction provided by being on preventive medication. There is also some evidence that statins are associated with “lower risks of dementia and cognitive impairment, venous thrombo-embolism, fractures and pneumonia” – but also possibly increased risks of myopathy and diabetes28. However, in the systematic review by the US Preventive Services Task Force29, statins were not clearly associated with either myalgias (RR, 0.96 [95% CI, 0.79 to 1.16]), or increased risk of diabetes (RR, 1.05 [95% CI, 0.91 to 1.20]).
We may have also underestimated benefits for the higher risk strata and over-estimated those for the lowest risk strata. That is the benefit of statins appears to be probably disproportionately greater for those at highest risk given that those at higher risk will typically have higher cholesterol levels and there is evidence from a systematic review that statin treatment benefits will be greater for those with higher baseline cholesterol levels30.
Offsetting the above likely underestimation of health gains, is failure to adequately capture likely higher morbidity and mortality among those initially benefitting from treatment. In our modelling the fraction of the simulated cohort that has a CVD event prevented in the five years of treatment is assumed to have the same future CVD event rates as all surviving members of the cohort. Yet if people who have a CVD event prevented due to treatment have a higher risk of future CVD events than the remainder of people in the nominally same CVD risk strata (as seems plausible), then we will have overestimated survivorship and health gains. However, we are not aware of data on the magnitude of any such effect to allow its inclusion in the modelling.
Our base-case parameters used the best available New Zealand evidence (ie, 92% screened, 77% uptake of pharmacotherapy and an overall 22.5% decline in adherence over the five-year intervention period). But in some other countries the uptake and adherence may be improved with the current availability of fixed dose combinations that combine a statin and anti-hypertensive into a single tablet rather than two (which are not yet available in New Zealand31).
We also lacked adequate data to capture the potential adverse effects of preventive pharmacotherapy (eg, the possible association with muscle pain from statins as mentioned above) prior to discontinuation of treatment. That is, our model assumed those developing significant adverse effects became immediately non-adherent. Nevertheless, our model still did not capture the disutility for those who remain adherent but experience medication disutility simply from having to take daily medicine.
Finally, our results were only for men aged 60–64, yet the cost-effectiveness of the intervention will undoubtedly vary with different age groups, an area we are researching further. We would also be open to model comparison exercises with other research groups (ie, to evaluate model structure uncertainty) as has been successfully done with diabetes models via the Mount Hood Challenge process.
Potential implications for future research
There is a need to extend this research to a wider range of adult age-groups (as referred to above), especially to the very elderly where harms from preventive medication may be greater. A more sophisticated analysis could compare the impact of packages of pharmacotherapy with various lifestyle interventions eg, smoking cessation, dietary changes (eg, sodium reduction), and increased physical activity.
Potential implications for policy-makers
Policy-makers should consider these results alongside many other estimates for health gain, cost impacts and cost-effectiveness from CVD preventive interventions, as per an Australian and New Zealand online interactive league table32. System-level interventions for preventing CVD (eg, tobacco control, changing the obesogenic food environment etc) will typically have larger impact and be more likely to actually save costs. Nevertheless, offering double therapy as assessed in this study seems to be a cost-effective use of public health resources. Given that reducing health inequalities is also a health sector goal in many countries, then specifically promoting preventive pharmacotherapy for select population groups could be prioritised (eg, as per successful past New Zealand work on increasing statin use by Māori10).
In terms of more informed decision-making between patients and clinicians around taking preventive pharmacotherapy, our estimates around average extra months of quality-adjusted life gained (Table 6) are of potential value. These could be included in online tools that patients could use when making decisions around taking daily medication.
Conclusions
In the selected population group of middle-aged men aged 60–64, the offer of CVD preventive double therapy (a statin and an anti-hypertensive) was highly cost-effective by conventional criteria for all levels of absolute CVD risk. Even so, more population-level interventions such as advancing tobacco control are more likely to generate large health gains and are more likely to be cost-saving. But at the individual level, patient considerations are critical as some people may decide that the average gain of 0.6 to 4.9 months of extra life (or less than a month with a five-year time horizon) does not justify the taking of daily medication.
Supplementary information
Acknowledgements
The authors thank Professor Rod Jackson and Dr. Romana Pylypchuk at the University of Auckland for their work on the PREDICT model and for data sharing. The PREDICT research project at the University of Auckland was supported by the Health Research Council (grants 03/183 and 08/121). Work on the synthetic population development by JK was supported by a PhD Scholarship associated with HRC support for the PREDICT work and the Centre of Excellence in Population Ageing Research, Australian Research Council (CEPAR) (CE170100005). The authors also thank: Professor Philip Clarke (Oxford University) and Dr. Wing Cheuk Chan of Counties Manukau District Health Board for helpful comments on the parameters and modelling, along with BODE3 colleagues who helped develop the initial TC-MSLT Model (Dr. Cristina Cleghorn, Dr. Giorgi Kvizhinadze, Dr. Linda Cobiac and June Atkinson). This modelling work was funded by the Ministry of Business, Innovation and Employment (MBIE) (grant: UOOX1406), and supported by additional modelling development work funded by the Health Research Council of New Zealand (grants: 10/248 and 16/443).
Author contributions
T.B. and N.W. organised the funding support. The study was designed by N.W., T.B. and N.N. N.W. led the data collection and parameter specification. N.N. build the new CVD version of the model and ran the analyses. A.M. contributed to model revisions and calibration work. J.K. created the simulated New Zealand population. N.W. led the manuscript writing. All authors edited the manuscript and all reviewed the final manuscript.
Data availability
Supplemental information with additional methods and results is attached. Sharing of anonymised cohort data with other researchers or official agencies of the other epidemiological and costing data will generally be possible on request from the authors (pending approval of the relevant official agencies).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55372-8.
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminde LN, Takah NF, Zapata-Diomedi B, Veerman JL. Primary and secondary prevention interventions for cardiovascular disease in low-income and middle-income countries: a systematic review of economic evaluations. Cost Eff Resour Alloc. 2018;16:22. doi: 10.1186/s12962-018-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor, F. et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev1 (2013). [DOI] [PMC free article] [PubMed]
- 4.Kazi DS, Penko JM, Bibbins-Domingo K. Statins for primary prevention of cardiovascular disease: Review of evidence and recommendations for clinical practice. Med Clin North Am. 2017;101:689–699. doi: 10.1016/j.mcna.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Stam-Slob, M. C., van der Graaf, Y., Greving, J. P., Dorresteijn, J. A. & Visseren, F. L. Cost-effectiveness of intensifying lipid-lowering therapy with statins based on individual absolute benefit in coronary artery disease patients. J Am Heart Assoc6, 10.1161/JAHA.116.004648 (2017). [DOI] [PMC free article] [PubMed]
- 6.Cobiac LJ, Magnus A, Barendregt JJ, Carter R, Vos T. Improving the cost-effectiveness of cardiovascular disease prevention in Australia: a modelling study. BMC Public Health. 2012;12:398. doi: 10.1186/1471-2458-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catala-Lopez F, Sanfelix-Gimeno G, Ridao M, Peiro S. When are statins cost-effective in cardiovascular prevention? A systematic review of sponsorship bias and conclusions in economic evaluations of statins. PLoS One. 2013;8:e69462. doi: 10.1371/journal.pone.0069462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson R, et al. Management of raised blood pressure in New Zealand: a discussion document. BMJ. 1993;307:107–110. doi: 10.1136/bmj.307.6896.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson T, Jackson R, Wells S, Kerr A, Marshall R. An observational study of how clinicians use cardiovascular risk assessment to inform statin prescribing decisions. N Z Med J. 2017;130:28–38. [PubMed] [Google Scholar]
- 10.Norris P, et al. Equity in statin use in New Zealand. J Prim Health Care. 2014;6:17–22. doi: 10.1071/HC14017. [DOI] [PubMed] [Google Scholar]
- 11.Nghiem N, Blakely T, Cobiac LJ, Pearson AL, Wilson N. Health and economic impacts of eight different dietary salt reduction interventions. PLoS One. 2015;10:e0123915. doi: 10.1371/journal.pone.0123915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakely T, et al. Health, health inequality, and cost impacts of annual increases in tobacco tax: Multistate life table modeling in New Zealand. PLoS Med. 2015;12:e1001856. doi: 10.1371/journal.pmed.1001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson Amber L, Cleghorn Christine L, van der Deen Frederieke S, Cobiac Linda J, Kvizhinadze Giorgi, Nghiem Nhung, Blakely Tony, Wilson Nick. Tobacco retail outlet restrictions: health and cost impacts from multistate life-table modelling in a national population. Tobacco Control. 2016;26(5):579–585. doi: 10.1136/tobaccocontrol-2015-052846. [DOI] [PubMed] [Google Scholar]
- 14.van der Deen, F. S. et al. Impact of five tobacco endgame strategies on future smoking prevalence, population health and health system costs: two modelling studies to inform the tobacco endgame. Tob Control (E-publication 24 June) (2017). [DOI] [PubMed]
- 15.Cleghorn Christine L, Blakely Tony, Kvizhinadze Giorgi, van der Deen Frederieke S, Nghiem Nhung, Cobiac Linda J, Wilson Nick. Impact of increasing tobacco taxes on working-age adults: short-term health gain, health equity and cost savings. Tobacco Control. 2017;27(e2):e167–e170. doi: 10.1136/tobaccocontrol-2017-053914. [DOI] [PubMed] [Google Scholar]
- 16.Nghiem N, et al. A national quitline service and its promotion in the mass media: modelling the health gain, health equity and cost-utility. Tob Control. 2018;27:434–441. doi: 10.1136/tobaccocontrol-2017-053660. [DOI] [PubMed] [Google Scholar]
- 17.Petrović-van der Deen Frederieke S, Blakely Tony, Kvizhinadze Giorgi, Cleghorn Christine L, Cobiac Linda J, Wilson Nick. Restricting tobacco sales to only pharmacies combined with cessation advice: a modelling study of the future smoking prevalence, health and cost impacts. Tobacco Control. 2018;28(6):643–650. doi: 10.1136/tobaccocontrol-2018-054600. [DOI] [PubMed] [Google Scholar]
- 18.Petrović-van der Deen Frederieke S., Wilson Nick, Crothers Anna, Cleghorn Christine L., Gartner Coral, Blakely Tony. Potential Country-level Health and Cost Impacts of Legalizing Domestic Sale of Vaporized Nicotine Products. Epidemiology. 2019;30(3):396–404. doi: 10.1097/EDE.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 19.Singh, A., Petrović-van der Deen, F. S., Carvalho, N., Lopez, A. D. & Blakely, T. Impact of tax and tobacco-free generation on health-adjusted life years in the Solomon Islands: a multistate life table simulation. Tob Control, tobaccocontrol-2018-054861, 10.1136/tobaccocontrol-2018-054861 (2019). [DOI] [PubMed]
- 20.Nghiem N, Blakely T, Cobiac LJ, Cleghorn CL, Wilson N. The health gains and cost savings of dietary salt reduction interventions, with equity and age distributional aspects. BMC Public Health. 2016;16:423. doi: 10.1186/s12889-016-3102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson N, et al. Modeling health gains and cost savings for ten dietary salt reduction targets. Nutr J. 2016;15:44. doi: 10.1186/s12937-016-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight J, Wells S, Marshall R, Exeter D, Jackson R. Developing a synthetic national population to investigate the impact of different cardiovascular disease risk management strategies: A derivation and validation study. PLoS One. 2017;12:e0173170. doi: 10.1371/journal.pone.0173170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grey C, et al. One in four major ischaemic heart disease events are fatal and 60% are pre-hospital deaths: a national data-linkage study (ANZACS-QI 8) Eur Heart J. 2017;38:172–180. doi: 10.1093/eurheartj/ehv524. [DOI] [PubMed] [Google Scholar]
- 24.Zambon A, Arfe A, Corrao G, Zanchetti A. Relationships of different types of event to cardiovascular death in trials of antihypertensive treatment: an aid to definition of total cardiovascular disease risk in hypertension. J Hypertens. 2014;32:495–508. doi: 10.1097/HJH.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 25.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk–overview and meta-analyses of randomized trials. J Hypertens. 2014;32:2305–2314. doi: 10.1097/HJH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 26.Blakely, T., Foster, R., Wilson, N. & BODE3 Team. Burden of Disease Epidemiology, Equity and Cost-Effectiveness (BODE3) Study Protocol. Version 2.1. Technical Report No.3. Wellington: Department of Public Health, University of Otago, Wellington, http://www.otago.ac.nz/wellington/otago042986.pdf (December 2012).
- 27.Pylypchuk R, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391:1897–1907. doi: 10.1016/S0140-6736(18)30664-0. [DOI] [PubMed] [Google Scholar]
- 28.Macedo AF, et al. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. doi: 10.1186/1741-7015-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 30.Navarese EP, et al. Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis. JAMA. 2018;319:1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson N, Jones AC, Nghiem N, Blakely T. Preventing cardiovascular disease in New Zealand: making better use of statins but also tobacco control, changing the food supply and other strategies. N Z Med J. 2018;131:61–67. [PubMed] [Google Scholar]
- 32.University of Otago & University of Melbourne. ANZ-HILT: Australia and New Zealand Health Intervention League Table (Vers 2.0). [Available from, https://league-table.shinyapps.io/bode3/] (2019).
- 33.Wells S, et al. Cohort Profile: The PREDICT Cardiovascular Disease Cohort in New Zealand Primary Care (PREDICT-CVD 19) Int J Epidemiol. 2017;46:22. doi: 10.1093/ije/dyv312. [DOI] [PubMed] [Google Scholar]
- 34.Mehta S, et al. Initiation and maintenance of cardiovascular medications following cardiovascular risk assessment in a large primary care cohort: PREDICT CVD-16. Eur J Prev Cardiol. 2014;21:192–202. doi: 10.1177/2047487312462150. [DOI] [PubMed] [Google Scholar]
- 35.McLeod M, Blakely T, Kvizhinadze G, Harris R. Why equal treatment is not always equitable: the impact of existing ethnic health inequalities in cost-effectiveness modeling. Popul Health Metr. 2014;12:15. doi: 10.1186/1478-7954-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta S, et al. Under-utilisation of preventive medication in patients with cardiovascular disease is greatest in younger age groups (PREDICT-CVD 15) J Prim Health Care. 2011;3:93–101. doi: 10.1071/HC11093. [DOI] [PubMed] [Google Scholar]
- 37.Kvizhinadze, G., Nghiem, N., Atkinson, J. & Blakely, T. Cost off-sets used in BODE³ multistate lifetable models. Burden of Disease Epidemiology, Equity and Cost-Effectiveness Programme. Technical Report no. 15. Wellington, Department of Public Health, University of Otago, Wellington (2016).
- 38.PHARMAC. Cost Resource Manual. (PHARMAC, Wellington, New Zealand, 2015).
- 39.Technical Advisory Services (TAS). Community Pharmacy Services Agreement (template for agreements with specific DHBs), https://tas.health.nz/assets/Publications/Pharmacy-Documents/The-Agreement/CPSA-12-contracts/Consolidated-version-2017/21081836-CPS022CPSAExtensionConsolidated-20170706.pdf (Accessed 10 September 2017).
- 40.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallejo-Vaz Antonio J., Robertson Michele, Catapano Alberico L., Watts Gerald F., Kastelein John J., Packard Chris J., Ford Ian, Ray Kausik K. Low-Density Lipoprotein Cholesterol Lowering for the Primary Prevention of Cardiovascular Disease Among Men With Primary Elevations of Low-Density Lipoprotein Cholesterol Levels of 190 mg/dL or Above. Circulation. 2017;136(20):1878–1891. doi: 10.1161/CIRCULATIONAHA.117.027966. [DOI] [PubMed] [Google Scholar]
- 42.McLean RM, Williams S, Mann JI, Miller JC, Parnell WR. Blood pressure and hypertension in New Zealand: results from the 2008/09 Adult Nutrition Survey. N Z Med J. 2013;126:1–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental information with additional methods and results is attached. Sharing of anonymised cohort data with other researchers or official agencies of the other epidemiological and costing data will generally be possible on request from the authors (pending approval of the relevant official agencies).