Abstract
Chronic inflammation in many infectious and metabolic diseases, and some cancers, is accompanied by the presence of foam cells. These cells form when the intracellular lipid content of macrophages exceeds their capacity to maintain lipid homeostasis. Concurrently, critical macrophage immune functions are diminished. Current paradigms of foam cell formation derive from studies of atherosclerosis. However, recent studies indicate that the mechanisms of foam cell biogenesis during tuberculosis differ from those operating during atherogenesis. Here, we review how foam cell formation and function vary with disease context. Since foam cells are therapeutic targets in atherosclerosis, further research on the disease-specific mechanisms of foam cell biogenesis and function is needed to explore the therapeutic consequences of targeting these cells in other diseases.
Keywords: foam cells, maladaptive immune response, lipid droplets, chronic inflammation
Foam cells: similar functions but disease-specific biogenesis
Foam cells form through dysregulated lipid metabolism in mammalian macrophages: lipid accumulation that exceeds the homeostatic capacity of macrophages triggers lipid droplet formation, which results in the foamy appearance of these macrophages (Box 1). Foam cells are associated with chronic inflammation in certain cancers and in metabolic, infectious, and autoimmune diseases (Table 1 and Box 2). Formation of the foam cell can impair macrophage immune function and contribute to pathogenesis. For example, in atherosclerosis, foam cells are critical in the initial formation, development, and instability of the atherosclerotic plaque [1]. During tuberculosis, foam cell death by necrosis enlarges tuberculous lesions in lung parenchyma, causing progressive lung tissue destruction and loss of pulmonary function in infected rabbits and marmosets, and in individuals with active tuberculosis [2]. In multiple sclerosis (MS), myelin-laden foam cells in brain lesions have been associated with demyelinating active and chronic active lesions, but not with inactive lesions [3]. In cancer, the association between tumor-associated macrophages and cancer-promoting inflammation suggests that foam cells might aid tumor initiation and progression [4]. Thus, studies across a variety of disease contexts indicate that foam cells contribute to maladaptive immune responses. Consequently, the recent finding of lipid-laden macrophages in broncho-alveolar lavages from patients presenting with severe lung injury associated with e-cigarette smoking (vaping) (for example, [5]) strongly suggests a role for these cells in this unfavorable outcome. Still unclear is foam cell biogenesis. The current paradigm derives from studies of atherosclerosis in which foam cell formation has been associated with dysregulated cholesterol metabolism. The abundance of atherosclerosis work and the relative paucity of foam cell studies in other pathologies have perpetuated a view of foam cells as cholesterol-rich macrophages [6]. However, recent work in tuberculosis challenges this paradigm, since tuberculous foam cells are enriched in triglycerides rather than cholesterol derivatives [2]. Thus, tuberculous foam cell biogenesis must differ from that of the atherogenic foam cell, strongly implying that the mechanism of foam cell formation is disease-specific.
Box 1. Lipid droplets.
Lipid droplets are cytosolic quasi-organelles involved in cellular metabolism and regulation of immune responses [11]. They comprise a phospholipid monolayer surrounding a core of neutral lipids, primarily cholesteryl esters (CE) and/or triglycerides (TAG). The phospholipid monolayer contains hundreds of proteins, including enzymes of lipid metabolism, membrane-trafficking GTPases, and immunological mediators [11]. Lipid droplets originate from the endoplasmic reticulum: neutral lipids accumulate at specific sites within the endoplasmic reticulum lipid bilayer to form the budding, initial structure. Nascent lipid droplets grow by local synthesis of neutral lipids or via fusion of small droplets.
When energy is needed, lipid droplets can be broken down by lipolysis or lipophagy [121]. When cellular lipid homeostasis is perturbed by pathological events, such as chronic inflammatory conditions, lipid droplets accumulate in the cytoplasm. Lipid droplets have been reported to vary in size, number, and protein and lipid composition in different cell types and in response to different stimuli, suggesting specialized functions [11].
In human white adipocytes, which are highly adapted for lipid storage, lipid droplets are almost exclusively TAG-rich [122]. In contrast, in human steroidogenic cells, which are the sites of steroid hormone biosynthesis, lipid droplets are enriched in CE [123]. Lipid droplets induced by infection also differ in lipid composition, depending on pathogen and host cell type [124]. For example, human and murine liver cell lines and murine hepatocytes infected with hepatitis C virus accumulate TAG [125], while human Schwann cells infected with Mycobacterium leprae contain increased levels of cholesterol and CE [126].
Macrophages, which are highly plastic cells, change their physiology in response to particular environmental cues [127]: in vitro they can accumulate either TAG- or CE-rich droplets depending on stress conditions. For example, when human macrophages are exposed to hypoxia, or infected with Mycobacterium tuberculosis, and murine macrophages are incubated with fatty acids, they accumulate TAG-rich lipid droplets [2, 128, 129]. In contrast, when murine macrophages are cultured in the presence of cholesterol or human macrophages are infected with Mycobacterium leprae, they accumulate CE-rich lipid droplets [60, 129].
Lipid droplets are intimately connected with immune functions [11]. For example, they relate to eicosanoid production and antimicrobial properties of macrophages, as discussed for foam cells. In another example, lipid droplets in cancer-associated murine dendritic cells reduce antigen presentation by major histocompatibility complex class I [130]. Thus, changes in lipid droplet number, size, and composition may reflect immune cell functional status in ways that are poorly understood.
Table 1.
Examples of foam cells in disease
| Diseases | References | Species |
|---|---|---|
| Metabolic diseases | ||
| Hyperlipidemia-associated atherosclerosis | [1, 94, 95] | Human, mouse, rabbit |
| Diabetes-associated atherosclerosis and diabetic nephropathy | [71, 96, 97] | Human, mouse |
| Insulin-resistance-associated and hyperglycemia-associated atherosclerosis | [73, 98] | Human, mouse |
| Obesity-associated atherosclerosis and adipose tissue foam cells | [73, 99] | Human, mouse |
| Niemann-Pick Disease | [100, 101] | Human, mouse |
| Infectious pathogens | ||
| Bacteria | ||
| Mycobacterium tuberculosis | [2, 7, 61] | Human, mouse, rabbit |
| Mycobacterium bovis BCG | [14, 33] | Mouse |
| Mycobacterium avium | [102] | Mouse |
| Mycobacterium leprae | [60] | Human |
| Mycobacterium fortuitum | [103] | Human |
| Mycobacterium marinum | [104] | Zebrafish |
| Salmonella typhimurium | [105] | Mouse |
| Nocardia brasiliensis | [106] | Mouse |
| Coxiella burnetii | [107, 108] | Human, mouse |
| Chlamydia pneumoniae-associated atherosclerosis | [109] | Mouse |
| Parasites | ||
| Leishmania major | [58] | Mouse |
| Toxoplasma gondii | [9] | Mouse |
| Trypanosoma cruzi | [12, 110] | Mouse |
| Fungi | ||
| Histoplasma capsulatum | [13] | Mouse |
| Viruses | ||
| HIV-associated atherosclerosis | [111] | Human |
| Autoimmune diseases | ||
| Multiple sclerosis | [3, 112, 113] | Human |
| Systemic Lupus Erythematosus-associated atherosclerosis | [114] | Human |
| Rheumatoid arthritis | [115] | Human |
| Cancer | ||
| Papillary renal cell carcinoma | [116] | Human |
| Esophageal xanthoma | [117] | Human |
| Non-small cell lung carcinoma | [118] | Human |
| Others | ||
| Vaping-induced acute respiratory distress syndrome | [5] | Human |
Box 2. Novel disease contexts for foam cells.
In addition to the most studied examples, such as atherosclerosis and tuberculosis, foam cells have been reported in novel disease contexts, in which the nature of the inducing signals, the composition of the storage lipids, and the molecular pathways of foam cell biogenesis still remain to be elucidated.
One example is multiple sclerosis (MS), where myelin-laden foam cells are found in lesions of the central nervous system [3]. While initially thought to promote lesion progression by producing inflammatory cytokines, foam cells within MS lesions appear to exhibit considerable phenotypic variation, including intermediate activation status and anti-inflammatory programs, as demonstrated by immunohistochemistry analysis of M1 and M2 marker expression in MS lesions [112, 113].
Recent studies have also reported foam cells in cancer. Papillary renal cell carcinoma, a prevalent renal cell carcinoma, features a papillary growth pattern with focal aggregation of foam cells [116]. Foam cells can also be found in human esophageal xanthoma [117] and in non-small cell lung carcinoma [118]. The presence of foam cells in cancerous lesions does not imply a causative role for these cells in tumor progression. However, it is tempting to speculate that foam cells may have a tumor-promoting activity since tumor-associated macrophages can aid tumor growth by promoting angiogenesis and tissue remodeling, and by suppressing adaptive immunity [4].
Foam cells have also been reported in the adipose tissue of obese humans and in mouse models of obesity [99, 131]. Diet-induced obesity features immune cells infiltrating fat tissue and low-grade inflammation associated with insulin-resistance [132]. Among these are adipose tissue macrophages, which may serve both beneficial and detrimental functions [131]. Silencing macrophage lipoprotein lipase in ob/ob obese mice decreased foam cell formation in fat tissue and caused a marked impairment in glucose tolerance, suggesting that foam cells might contribute to beneficial lipid storage within adipose tissues [133]. However, adipose foam cells obtained from C57BL/6 obese mice (fed with a high-fat diet) and co-cultured with fat explants were found to attenuate insulin responsiveness of adipose tissue (measured as Akt activation status) relative to fat explants co-cultured with non-foamy macrophages, pointing to a putative detrimental role for adipose foam cells [99]. Moreover, foam cells might contribute to chronic low-grade inflammation of adipose tissue in metabolic disorders [134]; however this remains to be further investigated.
In this review, we discuss how foam cells contribute to the pathogenesis of several infectious and non-infectious diseases. We contrast the molecular features of atherogenic and tuberculous foam cells, encompassing the idea that the immunopathological context drives foam cell biogenesis, yielding distinct foam cell subtypes (“one size does not fit all”) that however bear seemingly similar functions. These differences are relevant, as they suggest that different pathways of foam cell formation might bear potential as novel therapeutic targets to treat a variety of conditions characterized by the presence of foam cells (Box 3).
Box 3. Foam cells as novel putative therapeutic targets.
Recent immunometabolism studies have demonstrated that altered metabolic profiles in macrophages modulate the activation state and function of these cells [135]. Therefore, the metabolic reprogramming of macrophages has attracted attention as a novel therapeutic approach, particularly when dysfunctional macrophages contribute to the pathogenesis in chronic inflammatory diseases [135]. Since foam cells represent a type of dysfunctional macrophage, they have emerged as putative therapeutic targets for metabolic and infectious diseases [60, 82].
Several pharmacological approaches that modulate macrophage functions, such as monocyte/macrophage migration and adhesion, cholesterol handling, efferocytosis, cell death, and regulation of inflammation, have been considered for the prevention or treatment of atherosclerosis [82]. For example, transgenic and pharmacological clearing of senescent foam cells in Ldlr−/− mice resulted in marked lesion regression and inhibited plaque growth, fibrous cap thinning, and elastic fiber degeneration relative to controls [1]. In another approach, genetic or antibody-mediated inactivation of CD146 (adhesion surface receptor expressed by foam cells in murine atheromas which controls foam cell formation in murine macrophages in vitro [136]) decreased macrophage plaque retention and alleviated atherosclerosis in ApoE−/− mice relative to controls [136]. Moreover, relative to controls, autophagy-enhancing compounds, such as Ginsenoside Rb1, decreased foam cell formation and increased atherosclerotic plaque stability in mice [137], pointing at autophagy of macrophages as a potential therapeutic target [138]. Furthermore, immunization of ApoE−/− mice with foam cells reduced the number and size of atherosclerotic lesions and the proportion of foam cells in the lesions, and increased the numbers of CD4+ and CD8+ T cells in the spleen and foam-cell-specific IgG antibodies in plasma, relative to WT mice [139]. Thus, whole-cell vaccination utilizing foam cells might hold promise as a putative prevention and treatment strategy for atherosclerosis, pending further studies.
Increasing our knowledge on the foam-cell-inducing pathways operating in infectious diseases holds promise for host-directed therapies targeting foam cells. For example, inhibition of de novo cholesterol biosynthesis by statins can decrease intracellular M. leprae survival in human blood monocytes relative to controls [60]. Moreover, factors regulating triglyceride accumulation in M. tuberculosis-infected macrophages [2] have chemical inhibitors that are currently used in the clinic (for example, mTORC1 inhibitors and cancer therapy [140]). Thus, important scenarios exist for repurposing lipid-powering drugs for host-directed therapy against infectious diseases.
Foam cells can facilitate pathogenesis
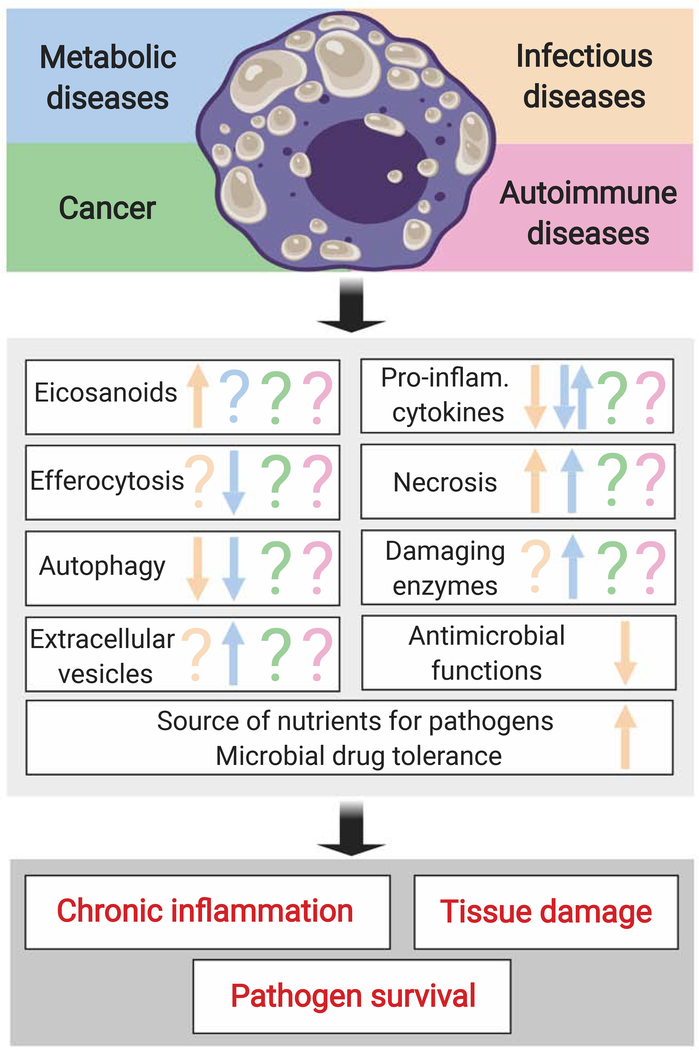
The notion that foam cells contribute to maladaptive responses derives from findings that foam cells tend to lose immune functions, induce tissue damage, and sustain survival of intracellular pathogens (Figure 1) [7–10]. We discuss the main functional phenotypes of foam cells below.
Figure 1. Foam cells can contribute to disease pathogenesis.
The top panel shows certain types of human diseases associated with the presence of foam cells. The middle panel lists the macrophage functions that have been studied in foam cells. The bottom panel indicates the major, disease-promoting outcomes associated with the maladaptive, foam-cell responses. Arrow up, upregulation; arrow down, downregulation; question mark, unknown. Inflam: inflammatory.
Foam cells produce eicosanoids
In addition to contributing to lipid homeostasis, lipid droplets are also sites of production of eicosanoids (see Glossary) (e.g., prostaglandins, leukotrienes, and lipoxins), synthesized from arachidonic acid stored in phospholipids and neutral lipids within lipid droplets [11]. Eicosanoid release by foam cells has been reported only in the context of infectious diseases. One example is seen with murine macrophages infected with Trypanosoma cruzi (the causative agent of Chagas disease). These macrophages accumulate lipid droplets containing cycloxygenase-2 (COX-2), which synthesizes prostaglandin E2 (PGE2) (for example, [12]). Inhibiting lipid droplet biogenesis with the nonsteroidal anti-inflammatory drugs aspirin and NS-398 decreases PGE2 synthesis in T. cruzi-infected macrophages in vitro [12]. Moreover, murine macrophages infected with Histoplasma capsulatum (the causative agent of histoplasmosis) accumulate lipid droplets and release PGE2 and leukotriene B4 (LTB4) in vitro [13]. In addition, pleural leukocytes and macrophages from mice infected with Mycobacterium bovis Bacillus Calmette-Guérin (BCG) accumulate lipid droplets bearing COX-2 and 5-lipoxygenase (5-LO), an enzyme involved in leukotriene biosynthesis [14]. Blocking in vitro accumulation of lipid droplets in BCG-infected murine peritoneal macrophages decreases the production of PGE2 [15].
The eicosanoid microenvironment affects infection outcome in vitro and in vivo. For example, M. tuberculosis-infected macrophages from Alox5−/− mice, deficient in lipoxin 4 (LXA4), undergo twice more apoptosis, and present half the bacillary burden than wild type (WT) cells [16]. In contrast, M. tuberculosis-infected macrophages from Ptges−/− mice, deficient in PGE2, exhibit three times more necrosis and bacillary burden than their WT counterparts [16, 17]. Similar effects are observed in vivo, as M. tuberculosis-infected Alox5−/− mice have ~100-times lower lung bacterial burden than Ptges−/− mice at 28 days post-infection (dpi) [16]. Alox5−/− mice also show decreased lung inflammation and necrosis, plus higher survival, relative to WT animals (50% mutant mice and no WT mice survived at 300 dpi) [18]. Thus, eicosanoid production may be a mechanism by which foam cells can interfere with infection control.
Foam cells exhibit altered production of pro-inflammatory cytokines
The inflammatory phenotype of foam cells is an area of ongoing investigation. Most data are currently derived from in vitro studies on atherogenic foam cells. They delineate complex, at times contrasting, situations, since both enhanced and decreased production of pro-inflammatory cytokines by atherogenic foam cells have been reported.
Specifically, studies have shown that lipid-loaded macrophages produce pro-inflammatory cytokines in multiple ways. In one mechanism, in vitro binding of oxidized low-density lipoproteins (LDL) to the CD36/TLR4/TLR6 complex induced pro-inflammatory gene expression and pro-inflammatory cytokine production in C57BL/6 murine macrophages [19]. In another, experiments utilizing knock-out Tlr4−/− C57BL/6 mice showed that long-chain saturated fatty acids induced inflammatory pathways in bone-marrow-derived macrophages (BMDM) by TLR4-dependent priming that altered cellular metabolism, gene expression, lipid metabolic pathways, and membrane lipid composition [20]. In a third mechanism, NLRP3 inflammasome with consequent IL-1β release was activated by (i) cholesterol crystal accumulation in human peripheral blood cells and murine macrophages following treatment with cholesterol crystals in vitro and in vivo (In mice) [21]; (ii) lipid-induced ER stress in human and murine macrophages in vitro [22]; lysosome dysfunction and mitochondrial dysfunction in murine macrophages [23, 24]; and defective autophagy in murine macrophages in vitro and in vivo [25]. Intracellular lipid accumulation, inflammasome activation, and IL-1β production have also been observed in human monocyte-derived macrophages incubated in vitro with plasma lipoproteins or with extracellular lipoprotein particles from human atheromas [26].
However, evidence also exists that cholesterol accumulation may dampen the production of pro-inflammatory cytokines secreted by macrophages. For example, lipid-loaded peritoneal macrophages, isolated from LDL receptor-deficient (Ldlr−/−) mice fed a high-fat, high cholesterol diet, exhibited diminished expression of TLR4-responsive genes relative to Ldlr−/− mice fed with a normal-fat diet [27], as did murine macrophages loaded in vitro with cholesterol, or modified LDL [27, 28]. Moreover, the conversion of cultured human macrophages into foam cells induced by acetylated LDL can suppress NF-κB activation, TNFα secretion, and the expression of genes encoding TNFα, IL-1β, CXCL8, CCL19, and COX-2 in response to M1-polarizing factors [29]. Additionally, accelerated atherosclerosis due to hypercholesterolemia does not appear to be accompanied by changes in the expression of genes encoding inflammatory mediators in lesional foam cells isolated from ApoE−/− mice (a model of atherosclerosis) fed with a Western-type diet, relative to foam cells isolated from ApoE−/− mice fed conventional mouse chow [30].
Furthermore, comparative analysis of transcriptomes of foamy and non-foamy macrophages isolated from atherosclerotic lesions in mice showed that intimal non-foamy macrophages were enriched in inflammation-related genes such as Il1b, Nfkbia, Nlrp3, and Tnf. In contrast, foamy macrophages showed higher expression of lipid metabolism genes and reduced expression of inflammation-related genes relative to non-foamy cells [31]. Thus, based on the above evidence, one can envision several, non-mutually exclusive, hypothetical scenarios, at least in the context of atherosclerotic lesions: i) foam cells might change over time; in the early phases of plaque formation, they might initially clear intimal lipoproteins in an attempt to block lesion progression. As the lesion progresses, however, they might become engulfed with lipids and undergo apoptotic cell death, worsening the atherosclerotic lesion; ii) multiple foam cell subpopulations might exist; iii) foam cells might contribute to chronic inflammation in atheromas by mechanisms other than pro-inflammatory cytokine production [31]. Further research is warranted to evaluate these possibilities.
No data are available concerning the inflammatory phenotype of foam cells in other immunopathological contexts. In vitro work in tuberculosis models, showed that infection-induced lipid-droplet-filled macrophages exhibit anti-inflammatory properties regulated by the peroxisome proliferator-activated receptor γ (PPARγ) and the testicular nuclear receptor 4 (TR4) [32, 33]. Specifically, M. tuberculosis-infected THP-1 cells defective in either receptor showed increased expression of IL-6 and TNFα and decreased expression of alternative polarization markers, such as IL-10, arginase, Dectin-1, mannose receptor, and inducible nitric oxide synthase relative to control THP-1 cells [32]. These results suggested that M. tuberculosis might divert host-response signaling to promote lipid accumulation and down-modulate macrophage responses, thereby favoring pathogen survival. Collectively, the observations derived from atherogenesis and tuberculosis studies suggest that the inflammatory phenotype of foam cells might vary with the immunopathological context. Identifying such phenotypes in different disease models may help determine how foam cells can contribute to clinical outcomes and ideally inform intervention strategies.
Foam cells release tissue-damaging enzymes and extracellular vesicles
The release of tissue-damaging enzymes and extracellular vesicles by foam cells has been studied only in the context of atherosclerosis. Evidence collected in rabbit and mouse models of atherosclerosis demonstrates that lesional foam cells can produce matrix metalloproteinases (MMP) [34, 35], enzymes that have been implicated in plaque destabilization and rupture in MMP-deficient ApoE−/− mice [36] and in humans [10]. In rabbit and human lesions, foam cells express higher amounts of MMP-14 and lower amounts of TIMP-3 (MMP-3 inhibitor) relative to non-foamy macrophages, and are typically located in rupture-prone atherosclerotic plaques [10, 37]. Moreover, treating patients with symptomatic carotid artery stenosis with pravastatin (a lipid-lowering agent), can decrease MMP content and increase the concentrations of MMP inhibitor, TIMP-1, and collagen content in carotid plaques [38]. These observations collectively suggest that lipid accumulation and abundance of tissue damaging enzymes might be linked to plaque stability.
From another angle, extracellular vesicles (bearing proteins, lipids, and RNA) can contribute to physiological functions as diverse as immunosurveillance, blood coagulation, stem cell maintenance, and tissue repair [39]. Murine macrophage cell lines treated with oxidized LDL to induce foam cell formation, produce more than twice the number of extracellular vesicles relative to untreated macrophages [40]. Moreover, these LDL-induced vesicles can promote vascular smooth-cell adhesion and migration in vitro[40]; they can also inhibit naïve murine macrophage migration in vitro and efflux of macrophages from the peritoneum in a mouse model of peritonitis[41]. This study proposed that these effects might result from vesicle-mediated transferring of microRNAs (miRNAs; in particular miR-146a) which were bioinformatically predicted to target genes involved in cell migration and adhesion pathways [41]. Together, these findings suggested that the production of extracellular vesicles might be one of the ways by which foam cells can accelerate atherosclerosis. Further work is necessary to robustly establish this mechanism.
Foam cells can contribute to necrosis
Advanced atherosclerotic and necrotizing tuberculous lesions are characterized by a necrotic core, which is associated with non-resolving inflammation and tissue damage [42, 43]. Relationships between foam cells and necrosis are well documented in both pathologies.
In atherosclerotic lesions, excessive cholesterol loading triggers apoptosis in foam cells [44]. Apoptotic foam cells are not efficiently cleared from the lesional tissue, and they undergo secondary necrosis, which induces inflammation [45]. The insufficient clearance of apoptotic cells is due to the progressive impairment of efferocytosis [45], associated with the inability to produce pro-reparative and anti-inflammatory mediators, thus impairing tissue homeostasis and contributing to tissue damage [45, 46]. Foam cell formation and defective efferocytosis by macrophages might be linked [45]. For example, since expression of the antiphagocytic marker CD47 is high in necrotizing areas of human atherosclerotic plaques (where foam cells are also located), one possibility is that foam cells highly express “do not eat me” signals and are inefficiently cleared [47]. Another possibility is that increased expression of ADAM MMPs during atherogenesis might result in proteolytic removal from the macrophage surface of apoptotic receptors, such as CD36 (scavenger receptor of class B) and MerTK (macrophage efferocytosis receptor c-Mer tyrosine kinase) [48, 49]. Moreover, foam cells might themselves exhibit defective efferocytosis due, for example, to competition between lipid uptake and apoptotic cell uptake, as observed in vitro with LDL-treated macrophages [50]. Furthermore, in ATG5-deficient fat-fed Ldlr−/− mice, defective autophagy in macrophages (associated with atherogenic foam cell formation [51]) promoted apoptosis and decreased efferocytosis relative to controls, thereby contributing to plaque necrosis [52]. By utilizing one or more of the above-described mechanisms, foam cells might drive pro-inflammatory processes, formation of the necrotic core of advanced lesions, as well as plaque instability.
In tuberculosis, foam cells are associated with necrotic granulomas in rabbits, marmosets and humans, localizing in the lesional areas that surround the lipid-rich necrotic core called caseum [2, 7]. While the mechanisms leading to foam cell death have not been detailed in tuberculosis, it is clear that foam cells can contribute to necrotic core formation by releasing their triglyceride-rich content into the caseum, since the same triglyceride species are found both in caseum and in the surrounding foam cell-rich regions in animal and human lesions [2]. A causative link between foam cells and necrosis was mechanistically demonstrated in human monocytic cell lines by showing that chemical inhibition of lipolysis by Mepenzolate bromide could increase necrosis relative to controls, while chemical inhibition of triglyceride and fatty acid biosynthesis with Triacsin C could prevent cells from undergoing necrosis [53]. In addition, foam cells can contribute to chronic inflammation, since the intracellular content they release during necrosis is pro-inflammatory [45]. By favoring caseum formation and sustaining chronic inflammation, foam cells might enable disease progression: clinical and histopathological parameters indicate that granuloma caseation and enlargement lead to progressive destruction of lung tissue and loss of pulmonary function [54]. Moreover, it is known that as granulomas cavitate and release their content into the respiratory airways, extracellular tubercle bacilli can be released into the external environment. Furthermore, ex vivo measurements of the antimycobacterial activity of anti-TB drugs in caseum obtained from tuberculous rabbit granulomas have shown a link between host cell necrosis and loss of drug susceptibility of Mycobacterium tuberculosis [55]. Thus, by favoring caseum formation, tuberculous foam cells can contribute to chronic inflammation, tissue damage, reduced susceptibility to antibiotic treatment, and transmission of infection.
Foam cells exhibit impaired antimicrobial activity
Storage-lipid accumulation correlates with reduced antimicrobial functions of macrophages. For example, human monocyte-derived macrophages incubated with mycolic acids from M. tuberculosis become foamy and display impaired respiratory burst (measured by nitroblue tetrazolium staining); when infected with fluorophore-labeled mycobacteria, these cells exhibit defective phagocytosis [7]. Moreover, lipid droplet content inversely correlates with autophagy and M. tuberculosis killing in murine and human monocyte-derived macrophages ex vivo [2, 8]. Furthermore, experiments in TR4- or PPARγ- deficient THP-1 cells show that phagosomal maturation (as shown via co-localization of GFP-expressing bacteria and lysosomes with confocal microscopy) and production of reactive oxygen species (measured with a specific probe using flow cytometry) are impaired relative to WT cells, implicating TR4 and PPARγ signaling in these processes [32]. In particular, PPARγ links lipid metabolism with downregulation of pro-inflammatory responses, mycobacterial killing, and vitamin D-dependent antimicrobial mechanisms in murine and human macrophages [15, 56]. In addition, murine studies indicate that foam cells from M. tuberculosis-infected murine lungs are characterized by downregulation of CD40 and major histocompatibility (MHC) class II markers and increased expression of anti-apoptotic markers [57]. Thus, foam cell formation can result in impaired macrophage antimicrobial activity, at least in tuberculosis models.
Foam cells act as nutrient source
The storage lipids accumulated in foam cells infected with intracellular pathogens might constitute a source of nutrients for the pathogen. The intracellular parasites Toxoplasma gondii [9] and Leishmania major [58] induce the fusion between host lipid droplets and the parasitophorous vacuole of murine macrophages and fibroblast cell lines. Specifically, intracellular growth of Toxoplasma gondii is defective in fibroblast cell lines deficient for the diglyceride acyltransferase (DGAT) enzyme (which is required for the accumulation of triglyceride-containing lipid droplets) and in WT fibroblasts treated with chemical inhibitors of host triglyceride lipolysis and fatty acid oxidation [9]. Among bacteria, Chlamydia trachomatis, an obligate intracellular bacterial species, induces translocation of host lipid droplets into the chlamydial inclusion (the vacuole containing the replicative form of the bacterium) in HeLa cells [59]. Similarly, microscopic analysis of human leprosy skin biopsies shows co-localization between cholesterol-rich lipid droplets and M. leprae-containing phagosomes in foam cells [60]. Treatment with statins (inhibitors of de novo cholesterol biosynthesis) decreases bacterial viability in human monocytes, indicating that intracellular survival of M. leprae relies on cholesterol accumulation in infected cells [60]. Additionally, transmission electron microscopy of M. tuberculosis-infected human monocyte-derived macrophages shows that bacilli-containing phagosomes migrate toward the host cell lipid droplets and the bacilli are ultimately engulfed by the lipid droplets [7]. M. tuberculosis can then acquire free fatty acids from host lipid droplet triglycerides and use them for biosynthesis of its triglyceride-rich lipid inclusions [61]. When mycobacteria accumulate lipid inclusions in lipid droplet-rich macrophages, they enter a dormancy state, change the composition of their cell wall, and become less readily killed by drugs [61]. Thus, foam cells may contribute to tuberculosis pathogenesis by promoting M. tuberculosis persistence and drug tolerance. Collectively, these findings suggest that intracellular pathogens might alter organelle trafficking in host cells to acquire lipids stored in host droplets and use them as an energy source for replication or survival.
Foam cells in tuberculosis vs. atherosclerosis
Similarities in lesional architecture
Necrotizing tuberculous granulomas and fibroatheromas (advanced intimal lesions with a necrotic core) are structurally similar at the histopathological level (Key Figure, Figure 2). Both lesion types are characterized by aggregates of various immune cells distributed around a lipid-rich necrotic center [43, 62]. The fibroblasts surrounding fibrocaseous tuberculous granulomas and the myofibroblasts in the arterial intima during atherogenesis both produce an extracellular matrix, which most likely represents a “scar” response to inflammation [63]. Foam cells are present in regions immediately adjacent to the necrotic core both in tuberculous granulomas and in atheromas of humans [2, 64]. The chronic and non-resolving inflammatory response to disease-specific stimuli (infection in tuberculosis, and lipoproteins in atherosclerosis) associated with foam cell accumulation and death generates progressive tissue damage. Indeed, the proportion of foam cells correlates with the extent of lesional necrosis and tissue damage in tuberculous granulomas and atheromas [7, 42]. In tuberculosis, damage of the lung parenchyma results in loss of pulmonary function, while granuloma liquefaction and cavitation into the airway ultimately leads to release of the extracellular bacilli into the external environment and the transmission of infection [7]. In advanced atherosclerosis, fibrous cap thinning and plaque rupture in the arterial wall may result in occlusion of the lumen, thrombosis, and thrombosis-induced organ infarction [45].
Key Figure, Figure 2. Human tuberculous granulomas and atheromas show similar architecture but different foam cell biogenesis.
(A, B) Lesional structure and cellular composition of human necrotizing tuberculous granulomas (left panels) and advanced atherosclerotic lesions (right panels) (haematoxylin and eosin (H&E) staining). (A) Left: Necrotic core (caseum, C) and cellular (CR) regions of granuloma. Higher magnification (black box inset) shows the foam-cell-rich area (arrows). Right: Atherosclerotic plaque in coronary artery. The bluish discoloration (*) within the necrotic core (NC) is due to inflammatory cells. At higher magnification, the foam-cell-rich area (arrows) is located near necrotic areas; the inflamed plaque shows surface erosion and luminal thrombus (Th). In both panels, the presence of foam cells shows as vacuole-rich areas due to lipid loss during H&E staining. Scale bars are shown as available. Images were reproduced with permission [2, 119]. (B) The two lesions have similar architectures and share immune and stromal cell types, albeit triggered by different stimuli, located in different anatomical compartments, and having different geometry (quasi-symmetric lesion with central necrotic core in tuberculosis; asymmetric lesion with lateral necrotic core in atherosclerosis). Mtb, M. tuberculosis. (C) Tuberculous and atherogenic foam cell biogenesis. Left: Bacteria-activated TLR2 signaling induces the transcription factor PPARγ. Macrophages secrete TNFα and 3-hydroxy butyrate (3HB). TNF-receptor signaling triggers caspase and mTORC1 signaling [2], inducing triglyceride (TAG) synthesis and blocking TAG degradation [76]. 3HB binds the G-protein-coupled receptor GPR109A and prevents TAG hydrolysis by stabilizing perilipins [70]. TAG accumulation is also induced by micro-RNA33 (miRNA33) (hydrolysis inhibition) and the transcription factor TR4 (unknown mechanism). Right: Atherogenic foam cells are generated by inducing cholesterol-rich lipoprotein uptake, subverting cholesterol trafficking, and reducing cholesterol efflux [62]. The uncontrolled uptake of native LDL and modified LDL (mLDL) (mediated by scavenger receptors CD36, SR-A, LOX-1) disrupts cholesterol homeostasis [83, 84, 120]. Excess free cholesterol (FC) is accumulated as cholesteryl esters (CE) in lipid droplets [85]. CE mobilization through lipophagy and lypolysis is impaired and FC efflux through ABCA1 and ABCG1 transporters is decreased [51, 89, 92]. Arrowheads vs Barheads = positive vs negative regulation.
Dissimilarities in foam cell content
The key difference between tuberculous foam cells and atherogenic foam cells is the type of storage lipid they accumulate. Using mass spectrometry-based quantification of triglycerides, free cholesterol, and cholesteryl esters in tuberculous granuloma foam cell-rich areas, and in lipid-rich necrotic areas sampled by laser-capture microdissection, our work showed triglycerides as the dominant storage lipids in necrotizing lung granulomas from M. tuberculosis-infected rabbits and marmosets [2]. Moreover, the same triglyceride species profile was observed in granulomas from the two animal models and from humans with active tuberculosis, suggesting a conserved mechanism for accumulating storage lipids in macrophages during M. tuberculosis infection [2]. Our work also showed that lipid droplets accumulated in M. tuberculosis-infected human monocyte-derived macrophages were triglyceride-rich. Specifically, inhibiting triglyceride biosynthesis with A-922500 (a DGAT1 inhibitor) prevented lipid droplet accumulation in infected human macrophages, while treatment with cholesterol synthesis inhibitors had no effect on lipid droplet content [2].
In contrast, extensive research has established that atherogenic foam cells are cholesteryl ester-rich (for example, [6]). Over 40 years ago, cholesteryl esters were reported to account for 94% of the lipid content of lipid droplets present in fatty streaks of human aortas [65]. Free cholesterol and cholesteryl ester species were subsequently imaged in human atherosclerotic plaques [66]. Among recent in vivo studies, three-dimensional (3D) electron microscopy indicated that extracellular lipids accumulated in human carotid plaques as distinct 3D structures; these structures included aggregated and fused lipoprotein particles and cholesterol crystals [26]. Those images were interpreted as showing foam cells in the process of engulfing cholesterol crystals [26]. Moreover, isolation and molecular characterization of extracellular lipoprotein particles present in plaques identified free cholesterol and cholesteryl esters as their main components, while triglyceride amounts were low [26]. Lipidomic profiling of human atherosclerotic plaques also revealed that cholesteryl esters were the most enriched lipid class in diseased arteries, relative to healthy ones, and that cholesteryl ester species differed between vulnerable and stable plaque areas [67].
The stark differences in foam-cell lipid content in tuberculous and atherosclerotic lesions suggest that different foam cell subsets exist between these two diseases.
Dissimilarities in foam cell biogenesis
The different lipid content of foam cells in tuberculosis vs atherosclerosis most likely reflects the nature of specific pro-lipogenic stimuli and downstream lipid metabolism pathways.
With regard to the nature of the stimuli, bacterial constituents might interact with specific macrophage pattern recognition receptors in tuberculosis and trigger an intracellular signaling cascade that ultimately perturbs triglyceride homeostasis. In support of this possibility, it has been observed that mycobacterial cell wall components (e.g. mycolic acids) and secreted proteins (e.g. ESAT-6), which bind to the macrophage receptors TR4 and TLR2, respectively [68, 69], are pro-lipogenic [7, 68, 70]. In contrast, macrophages can become cholesteryl ester-laden in atherosclerosis as they attempt to remove lipoproteins from the blood vessel intima [62]. However, in addition to hyperlipidemia, atherosclerotic lesions can be found in the context of various chronic inflammatory diseases (Table 1) in which cholesterol homeostasis is disrupted and foam cells form. The foam-cell-inducing factors described in atherosclerosis include glucose, insulin, pro-inflammatory cytokines, and monocytosis (inflammatory monocytes -- Ly6Chi in mice and CD14++ in humans -- are the major subpopulation of monocytes that contribute to atherosclerosis progression; they can be found in myeloproliferative diseases, post-myocardial infarction, and hypercholesterolemia) (examples are [63, 71–73]).
The molecular mechanisms that contribute to foam cell biogenesis during tuberculosis are depicted in Key Figure, Figure 2. In vitro mechanistic studies have elucidated pathogen-activated macrophage pathways that participate in both de novo triglyceride biosynthesis and lipolysis inhibition. For instance, TLR2-deficient murine peritoneal macrophages infected with BCG in vitro do not express the lipid sensing nuclear receptor PPARγ nor do they accumulate lipid droplets, suggesting that the TLR2/PPARγ axis might aid tuberculous foam cell formation [15]. These findings are consistent with the well documented roles of PPARγ in regulating lipid metabolism in health and disease [74]. In particular, evidence exists for PPARγ induction of lipid droplet accumulation in monocytic cell lines (THP-1 cells) infected with M. tuberculosis: PPARγ-deficient cells accumulate fewer lipid droplets than PPARγ-sufficient cells following infection, and treatment with PPARγ agonists (GW1929 and rosiglitazone) reverses the anti-lipogenic activity of vitamin D on the infected cells [32, 56]. Furthermore, in vitro experiments with PPARγ- or TR4-deficient THP-1 cells showed that PPARγ and TR4 can synergistically induce lipid droplet accumulation and decrease antimicrobial functions following M. tuberculosis infection [32]. In addition, interaction between bacterial keto-mycolic acids and TR4 seems to be essential for mycolic acid-mediated induction of foam cells in vitro, since foam cell formation is decreased in keto-mycolic-acid-treated TR4-deficient human monocyte-derived macrophages relative to TR4-sufficient cells [68].
By measuring the effect of treatment with specific chemical inhibitors on lipid droplet content of M. tuberculosis infected human monocyte-derived macrophages, triglyceride accumulation in these infected cells was recently shown to require TNF receptor (TNFR) signaling, activation of the downstream caspase cascade, and activation of the mechanistic target of rapamycin complex 1 (mTORC1) [2]. mTORC1 and caspases might contribute to triglyceride accumulation by regulating several cellular functions. For example, mTORC1 positively regulates PPARγ during lipogenesis in murine hepatocytes ex vivo and in vivo, and induces the expression of the sterol regulatory element binding proteins-1c (SREBP-1c) -- the master regulator of triglyceride biosynthesis-- in rat hepatocytes in vivo and ex vivo [75, 76]. Caspase activation induces mitochondrial dysfunction [76], which is associated with reduced fatty acid utilization and consequent lipid accumulation [77]. Both mTORC1 and caspases inhibit autophagy [78, 79], which promotes lipid catabolism by delivering lipid droplet-stored triglycerides to lysosomes (lipophagy) [80]. Further evidence for a role for autophagy in tuberculous foam cell formation showed that induced expression of the microRNA miR-33 and its passenger strand miR-33* in M. tuberculosis-infected murine and human macrophages concurrently inhibited autophagy and increased fatty acid storage in lipid droplets relative to controls[8].
Lipid droplet accumulation in M. tuberculosis-infected macrophages can also result from impaired host lipolysis by a mechanism in which 3-hydroxybutyrate (3HB), secreted by macrophages, binds G protein-coupled receptor GPCR109A, which modulates the cAMP-dependent signaling pathway in THP-1 cells [70]. Reduced cellular cAMP concentrations result in decreased perilipin 1 phosphorylation by protein kinase A (PKA) and consequent perilipin 1 stabilization. Non-phosphorylated perilipin 1 resides on the surface of lipid droplets and protects against lipolysis by triglyceride hormone-sensitive lipase (HSL), thereby favoring lipid droplet accumulation in the macrophage [70]. An axis involving interferon (IFN) γ and hypoxia-inducible factor 1a (IFNγ/HIF-1α) has also been implicated in M. tuberculosis-induced foam cell formation, since much fewer IFNγ-induced lipid droplets form in HIF-1α-deficient murine BDMDs in vitro [81].
The molecular mechanisms underlying the formation of foam cells during atherogenesis (Key Figure, Figure 2), have been detailed elsewhere [6, 62, 63, 82] (Box 4). Briefly, cholesteryl ester-laden foam cells arise from uncontrolled macrophage internalization and processing of cholesterol-rich native and modified low-density lipoproteins (LDL). Native LDL enters macrophages by macro-pinocytosis and phagocytosis, while modified LDL uptake requires scavenger receptors, including CD36 (scavenger receptor of class B), scavenger receptor class A (SR-A), and the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) [83, 84]. After LDL internalization, cholesteryl esters of lipoproteins are hydrolyzed to free cholesterol and fatty acids in the late endolysosomal compartment [6]. Free cholesterol is then trafficked to the endoplasmic reticulum, where it is re-esterified by acetyl-coenzyme A:cholesterol acetyltransferase 1 (ACAT1) to cholesteryl esters, subsequently accumulating in lipid droplets [85].
Box 4. Atherogenesis.
Atherogenesis initiates when cholesterol-rich apolipoprotein-B-containing lipoproteins are retained in the arterial subendothelial space at regions of disturbed blood flow in medium-sized arteries. When lipoproteins bind to subendothelial proteoglycans, the lipid and protein components of lipoproteins undergo modifications, mainly oxidation and hydrolysis, causing lipoprotein aggregation and exacerbating lipoprotein retention [62]. Modified lipoproteins induce an inflammatory response characterized by cytokine and chemokine secretion plus altered expression of adhesion molecules by endothelial cells. The inflammatory signals lead to monocyte recruitment into the intima, where they differentiate into macrophages and dendritic cells, which interact with atherogenic lipoproteins [62].
The turn-over of cholesterol-rich droplets is physiologically ensured by lipolytic mechanisms that include (i) cholesteryl ester hydrolysis to free cholesterol by neutral cholesteryl ester hydrolase 1 (Nceh1) and hormone sensitive lipase [86] and (ii) degradation of cholesteryl esters by lysosomal acid lipase during lipophagy [87]. Efflux of the resulting free cholesterol may occur by passive diffusion from the plasma membrane or by the energy-requiring reverse cholesterol transport system. The latter comprises the ATP-binding cassette transporter 1 (ABCA1), the ATP-binding cassette sub-family G member-1 (ABCG1), and the scavenger receptor B1 (SR-B1) [6, 88]. Cholesterol in plasma becomes complexed with Apolipoprotein A (ApoA1) and high-density lipoproteins (HDL) and targeted to the liver for excretion [6]. During atherogenesis, cholesterol efflux is impaired by the combined effect of dampened lipolysis, lysosomal dysfunction caused by free cholesterol accumulation, altered cholesterol trafficking, impaired lipophagy, and downregulated reverse cholesterol transport [51, 89–93].
In summary, different mechanisms connect pro-lipogenic stimuli, lipid metabolism pathways, and lipid content of foam cells during atherosclerosis and tuberculosis. They thus define the existence of two subsets of foam cells.
Concluding remarks
Macrophages develop into foam cells under various pathological contexts. In some cases, foam cell biology has been well studied, such as the atherogenic process; in others, the presence of foam cells has been long known but only recently investigated mechanistically, such as in tuberculosis; in yet other pathologies, foam cells have been only recently discovered, such as in certain cancers and autoimmune diseases (Table 1). It seems reasonable to interpret the body of data -- small or large - generated for each of these diseases as indicating that, regardless of pathological context, foam cells are macrophages with impaired immune functions. Thus, the study of foam cells emerges as a novel area of immunometabolism research. In contrast with the loss of immune functions, common to various pathological conditions, it is now clear that biogenesis of foam cells may occur through a variety of mechanisms that depend on immunopathological context (“one size does not fit all”). For example, tuberculous and atherogenic foam cells are generated in response to different stimuli that result in subverted triglyceride or cholesterol homeostasis, respectively. Two interrelated conclusions derive from this recent realization. One is that the cholesterol-rich, atherogenic foam cells cannot be used as the sole paradigm of foam cell biology. The second is that there exist at least two subsets of foam cells, one being cholesterol-rich (the atherosclerosis paradigm) and the other being triglyceride-rich (the tuberculosis paradigm). Future work will have to test the latter scenario and identify the mechanisms that underlie the phenotype and function of triglyceride-rich foam cells (see Outstanding questions).
OUTSTANDING QUESTION BOX.
What are the mechanisms of biogenesis and lipid content of foam cells generated during metabolic and infectious diseases (other than atherosclerosis and tuberculosis), in some autoimmune diseases and cancers? Are there additional foam cells subsets besides those defined by atherogenic and tuberculous foam cells?
Can cross-comparisons among immunopathological conditions associated with cholesterol-rich or triglyceride-rich foam cells lead to the identification of subset-specific pro-lipogenic stimuli? Pro-lipogenic stimuli triggering a particular foam cell subset may derive from a specific combination of microenvironmental factors encountered by macrophages, including tissue composition and/or the nature of the inflammatory process.
Is the inflammatory phenotype of foam cells context-specific? Do foam cell subpopulations exist in the same disease context? While it is clear that foam cells contribute to pathogenesis and disease progression, whether the inflammatory phenotype of foam cells is conserved in different microenvironments and among different immunopathological contexts remains to be determined.
Do triglyceride-rich and cholesterol-rich foam cells produce the same species of eicosanoids?
Are extracellular vesicles a general mechanism of inter-cell communication used by foam cells? Extracellular vesicles produced by foam cells may help accelerate development of atherosclerosis. Since extracellular vesicles have been increasingly found in many metabolic disorders, the question arises as to whether foam cells generated in various disease contexts all produce these vesicles to modulate the activities of neighboring cells.
Revealing the mechanisms of foam cell generation specific to particular diseases may be clinically relevant, given that foam cells are already potential targets of pharmacological intervention against atherosclerosis (Box 3) [82]. Thus, foam cells might offer a novel point of attack against the diseases in which they are found. Moreover, re-classifying a number of diseases based on foam cell characteristics and performing cross-comparisons among different immunopathological conditions might help identify the nature of pro-lipogenic stimuli and their role in these conditions. This may be particularly relevant for newly recognized or understudied foam-cell-associated diseases, where identifying trigger stimuli might inform therapeutic approaches. It will be exciting to define in the future how foam cell characteristics and functions might be harnessed for therapeutic purposes to potentially treat a variety of maladies.
Highlights.
Foam cells can exhibit impaired immune functions and contribute to the pathogenesis of various diseases by inducing inflammation and tissue damage, regardless of pathological context. They also facilitate pathogen survival in infectious diseases.
Biogenesis and storage lipid composition of foam cells depend on immunopathological context and are disease-specific.
The cholesterol-rich foam cell formed during atherosclerosis and the triglyceride-rich foam cell found in tuberculosis can be taken to represent two different paradigms of foam cell formation.
Foam cells offer a novel putative target of pharmacological attack against disease, since they have been often implicated in pathogenesis and disease progression.
Acknowledgments
We are grateful to Karl Drlica for critical comments on the manuscript. Funding to the Gennaro laboratory was provided by the New Jersey Health Foundation and the National Institutes of Health.
GLOSSARY
- Atheromas
Lesions of the arterial intima that occur during atherosclerosis
- Autophagy
A series of regulated processes for the transfer of intracellular components (molecules and organelles) to lysosomes for degradation
- Carotid artery stenosis
Atherosclerotic narrowing of the carotid artery
- Caseum
Lipid-rich necrotic material of “cheese-like” appearance that occupies the center of the necrotizing tuberculous granuloma
- Chagas disease
Infectious disease caused by the parasite Trypanosoma cruzi that is transmitted to animals and humans by insect vectors
- Efferocytosis
Highly regulated clearance of apoptotic cells by phagocytes that maintains homeostasis, prevents autoimmune diseases, and resolves inflammatory insults
- Eicosanoids
Bioactive signaling lipids derived from arachidonic acid and related polyunsaturated fatty acids; they act locally to regulate a variety of homeostatic and inflammatory processes
- Extracellular vesicles
Cell-derived membranous structures originating from the endosomal system (exosomes) or shed from the plasma membrane (microvesicles); they represent a mechanism for intercellular communication
- Fibrous cap thinning
Progressive decrease in thickness of the atheroma fibrous cap in advanced lesions; it may lead to plaque rupture and thrombosis
- Granulomas
Clusters of immune cells forming in response to an infectious or noninfectious (foreign) agent
- Cavitation
Release into an adjacent airway of the liquefying necrotic material at the center of a necrotic tuberculous granuloma; it facilitates infection transmission
- Histoplasmosis
Infection caused by the inhalation of spores produced by the fungus Histoplasma capsulatum
- Laser capture-microdissection
Sample preparation technique that enables isolation of subpopulations of tissue cells by using microscopic visualization and laser-based dissection
- Lipophagy
Form of autophagy in which intracellular lipid droplets are degraded following the fusion of lipid droplet-containing autophagosomes with lysosomes
- M1-polarizing
Inducing the classically activated M1 pro-inflammatory phenotype of macrophages
- mTORC1
Protein kinase complex that links nutrient sensing to regulation of cellular metabolism
- Parasitophorous vacuole
Vacuole derived from the host plasma membrane within which parasites of the phylum Apicomplexa reside and replicate
- Pattern recognition receptors
recognize conserved pathogen associated molecular structures (PAMPs), and play key roles in innate immunity
- Perilipin 1
Protein located on the surface of lipid droplets in eukaryotic cells; it is the key regulator of storage lipid lipolysis
- PPARγ
Member of the lipid-sensing nuclear receptor family that acts as a transcriptional regulator of cellular lipid and glucose metabolism, cell proliferation and differentiation, and inflammation. It forms heterodimers with the retinoid X receptor and binds to PPAR response elements located in the promoter region of target genes
- Pro-lipogenic
Induces the accumulation of cytoplasmic lipid droplets
- Scavenger receptors
Receptors that bind and internalize a variety of ligands, including endogenous and modified host-derived molecules and microbial pathogens. They are involved in the clearance of modified lipoproteins by phagocytes during atherosclerosis and in the regulation of innate immune responses through the recognition of pathogen-associated molecular patterns
- Sterol regulatory element binding proteins-1c (SREBP-1c)
transcription factor that regulates cellular lipogenesis and lipid homeostasis
- Testicular nuclear receptor 4 (TR4)
nuclear receptor that transcriptionally regulates cell metabolism, replication, and death. It is transactivated by fatty acid metabolites and thiazolidinedione compounds. It binds hormone-response elements located in the promoter region of target genes
- TLR
Microbial-sensing proteins expressed by immune cells. Various families of TLRs recognize specific pathogen-associated molecular patterns (PAMPs) and trigger intracellular signaling events that regulate activation of innate and adaptive immunity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Childs BG et al. (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354 (6311), 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrini V et al. (2018) Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog 14 (8), e1007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grajchen E et al. (2018) The physiology of foamy phagocytes in multiple sclerosis. Acta Neuropathol Commun 6 (1), 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye H et al. (2018) Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis 9 (5), 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiani DC (2019) Vaping-Induced Lung Injury. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 6.Moore KJ et al. (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13 (10), 709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyron P et al. (2008) Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4 (11), e1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouimet M et al. (2016) Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol 17 (6), 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan SJ et al. (2017) Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog 13 (6), e1006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JL et al. (2014) Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediators Inflamm 2014, 276457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Brok MH et al. (2018) Lipid Droplets as Immune Modulators in Myeloid Cells. Trends Immunol 39 (5), 380–392. [DOI] [PubMed] [Google Scholar]
- 12.D’Avila H et al. (2011) Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E(2) generation and increased parasite growth. J Infect Dis 204 (6), 951–61. [DOI] [PubMed] [Google Scholar]
- 13.Sorgi CA et al. (2009) Histoplasma capsulatum cell wall {beta}-glucan induces lipid body formation through CD18, TLR2, and dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection. J Immunol 182 (7), 4025–35. [DOI] [PubMed] [Google Scholar]
- 14.D’Avila H et al. (2006) Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol 176 (5), 3087–97. [DOI] [PubMed] [Google Scholar]
- 15.Almeida PE et al. (2009) Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol 183 (2), 1337–45. [DOI] [PubMed] [Google Scholar]
- 16.Divangahi M et al. (2009) Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 10 (8), 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M et al. (2008) Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205 (12), 2791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bafica A et al. (2005) Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest 115 (6), 1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart CR et al. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11 (2), 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster GI et al. (2018) Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab 27 (5), 1096–1110 e5. [DOI] [PubMed] [Google Scholar]
- 21.Duewell P et al. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464 (7293), 1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robblee MM et al. (2016) Saturated Fatty Acids Engage an IRE1alpha-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep 14 (11), 2611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emanuel R et al. (2014) Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol 34 (9), 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang EV et al. (2017) Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 171 (5), 1057–1071 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razani B et al. (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15 (4), 534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehti S et al. (2018) Extracellular Lipids Accumulate in Human Carotid Arteries as Distinct Three-Dimensional Structures and Have Proinflammatory Properties. Am J Pathol 188 (2), 525–538. [DOI] [PubMed] [Google Scholar]
- 27.Spann NJ et al. (2012) Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151 (1), 138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jongstra-Bilen J et al. (2017) Oxidized Low-Density Lipoprotein Loading of Macrophages Downregulates TLR-Induced Proinflammatory Responses in a Gene-Specific and Temporal Manner through Transcriptional Control. J Immunol 199 (6), 2149–2157. [DOI] [PubMed] [Google Scholar]
- 29.da Silva RF et al. (2016) Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 248, 170–8. [DOI] [PubMed] [Google Scholar]
- 30.Goo YH et al. (2016) Transcriptional Profiling of Foam Cells Reveals Induction of Guanylate-Binding Proteins Following Western Diet Acceleration of Atherosclerosis in the Absence of Global Changes in Inflammation. J Am Heart Assoc 5 (4), e002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K et al. (2018) Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res 123 (10), 1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan S et al. (2012) Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol 188 (11), 5593–603. [DOI] [PubMed] [Google Scholar]
- 33.Almeida PE et al. (2014) Differential TLR2 downstream signaling regulates lipid metabolism and cytokine production triggered by Mycobacterium bovis BCG infection. Biochim Biophys Acta 1841 (1), 97–107. [DOI] [PubMed] [Google Scholar]
- 34.Galis ZS et al. (1995) Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A 92 (2), 402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes EM et al. (2014) Classical and Alternative Activation and Metalloproteinase Expression Occurs in Foam Cell Macrophages in Male and Female ApoE Null Mice in the Absence of T and B Lymphocytes. Front Immunol 5, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JL et al. (2005) Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A 102 (43), 15575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JL et al. (2008) Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages. Arterioscler Thromb Vasc Biol 28 (9), 1647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crisby M et al. (2001) Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103 (7), 926–33. [DOI] [PubMed] [Google Scholar]
- 39.E.L.A S. et al. (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12 (5), 347–57. [DOI] [PubMed] [Google Scholar]
- 40.Niu C et al. (2016) Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J Am Heart Assoc 5 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen MA et al. (2018) Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol 38 (1), 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otsuka F et al. (2016) Pathology of coronary atherosclerosis and thrombosis. Cardiovasc Diagn Ther 6 (4), 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagan AJ and Ramakrishnan L (2014) Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb Perspect Med 5 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotamisligil GS (2010) Endoplasmic reticulum stress and atherosclerosis. Nat Med 16 (4), 396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brophy ML et al. (2017) Eating the Dead to Keep Atherosclerosis at Bay. Front Cardiovasc Med 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S et al. (2019) Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab 29 (2), 443–456 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kojima Y et al. (2016) CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536 (7614), 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Driscoll WS et al. (2013) Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ Res 113 (1), 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai B et al. (2017) MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest 127 (2), 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulanco MC et al. (2017) Complement Protein C1q Enhances Macrophage Foam Cell Survival and Efferocytosis. J Immunol 198 (1), 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong SJ et al. (2018) Prdx1 (peroxiredoxin 1) deficiency reduces cholesterol efflux via impaired macrophage lipophagic flux. Autophagy 14 (1), 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao X et al. (2012) Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15 (4), 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehrotra P et al. (2014) Pathogenicity of Mycobacterium tuberculosis is expressed by regulating metabolic thresholds of the host macrophage. PLoS Pathog 10 (7), e1004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravimohan S et al. (2018) Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 27 (147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarathy JP et al. (2018) Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum. Antimicrob Agents Chemother 62 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salamon H et al. (2014) Cutting Edge: Vitamin D Regulates Lipid Metabolism in Mycobacterium tuberculosis Infection. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ordway D et al. (2005) Foamy macrophages within lung granulomas of mice infected with Mycobacterium tuberculosis express molecules characteristic of dendritic cells and antiapoptotic markers of the TNF receptor-associated factor family. J Immunol 175 (6), 3873–81. [DOI] [PubMed] [Google Scholar]
- 58.Rabhi S et al. (2016) Lipid Droplet Formation, Their Localization and Dynamics during Leishmania major Macrophage Infection. PLoS One 11 (2), e0148640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cocchiaro JL et al. (2008) Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A 105 (27), 9379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattos KA et al. (2014) Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cell Microbiol 16 (6), 797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniel J et al. (2011) Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7 (6), e1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore KJ and Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145 (3), 341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabas I and Lichtman AH (2017) Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 47 (4), 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bentzon JF et al. (2014) Mechanisms of plaque formation and rupture. Circ Res 114 (12), 1852–66. [DOI] [PubMed] [Google Scholar]
- 65.Lang PD and Insull W Jr. (1970) Lipid droplets in atherosclerotic fatty streaks of human aorta. J Clin Invest 49 (8), 1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uchida Y et al. (2010) Two-dimensional visualization of cholesterol and cholesteryl esters within human coronary plaques by near-infrared fluorescence angioscopy. Clin Cardiol 33 (12), 775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stegemann C et al. (2011) Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet 4 (3), 232–42. [DOI] [PubMed] [Google Scholar]
- 68.Dkhar HK et al. (2014) Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granulomas: a case of a heterologous and noncanonical ligand-receptor pair. J Immunol 193 (1), 295–305. [DOI] [PubMed] [Google Scholar]
- 69.Pathak SK et al. (2007) Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8 (6), 610–8. [DOI] [PubMed] [Google Scholar]
- 70.Singh V et al. (2012) Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 12 (5), 669–81. [DOI] [PubMed] [Google Scholar]
- 71.Mauldin JP et al. (2006) Reduction in ABCG1 in Type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem 281 (30), 21216–24. [DOI] [PubMed] [Google Scholar]
- 72.O’Rourke L et al. (2002) Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem 277 (45), 42557–62. [DOI] [PubMed] [Google Scholar]
- 73.Reardon CA et al. (2018) Obesity and Insulin Resistance Promote Atherosclerosis through an IFNgamma-Regulated Macrophage Protein Network. Cell Rep 23 (10), 3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gross B et al. (2017) PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol 13 (1), 36–49. [DOI] [PubMed] [Google Scholar]
- 75.Li S et al. (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A 107 (8), 3441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z et al. (2014) Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway. Proc Natl Acad Sci U S A 111 (36), 13163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boren J and Brindle KM (2012) Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ 19 (9), 1561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J et al. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13 (2), 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsapras P and Nezis IP (2017) Caspase involvement in autophagy. Cell Death Differ 24 (8), 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh R et al. (2009) Autophagy regulates lipid metabolism. Nature 458 (7242), 1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knight M et al. (2018) Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-gamma/HIF-1αlpha signaling and supports host defense. PLoS Pathog 14 (1), e1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maguire EM et al. (2019) Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vascul Pharmacol 112, 54–71. [DOI] [PubMed] [Google Scholar]
- 83.Mehta JL et al. (2007) Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100 (11), 1634–42. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Z et al. (2005) Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler Thromb Vasc Biol 25 (1), 168–73. [DOI] [PubMed] [Google Scholar]
- 85.Rong JX et al. (2013) ACAT inhibition reduces the progression of preexisting, advanced atherosclerotic mouse lesions without plaque or systemic toxicity. Arterioscler Thromb Vasc Biol 33 (1), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakai K et al. (2014) Critical role of neutral cholesteryl ester hydrolase 1 in cholesteryl ester hydrolysis in murine macrophages. J Lipid Res 55 (10), 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ouimet M et al. (2011) Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 13 (6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgerald ML et al. (2010) ABC transporters, atherosclerosis and inflammation. Atherosclerosis 211 (2), 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sekiya M et al. (2009) Ablation of neutral cholesterol ester hydrolase 1 accelerates atherosclerosis. Cell Metab 10 (3), 219–28. [DOI] [PubMed] [Google Scholar]
- 90.Harte RA et al. (2000) Low level expression of hormone-sensitive lipase in arterial macrophage-derived foam cells: potential explanation for low rates of cholesteryl ester hydrolysis. Atherosclerosis 149 (2), 343–50. [DOI] [PubMed] [Google Scholar]
- 91.Xu X et al. (2016) Lysosomal cholesterol accumulation in macrophages leading to coronary atherosclerosis in CD38(−/−) mice. J Cell Mol Med 20 (6), 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Westerterp M et al. (2013) Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res 112 (11), 1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L et al. (2016) Pdcd4 deficiency enhances macrophage lipoautophagy and attenuates foam cell formation and atherosclerosis in mice. Cell Death Dis 7, e2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakagawa T et al. (2019) Distribution of atherosclerotic lesions in various arteries of WHHLMI rabbits, an animal model of familial hypercholesterolemia. Exp Anim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rallidis LS et al. (2019) Prevalence of heterozygous familial hypercholesterolemia and combined hyperlipidemia phenotype in very young survivors of myocardial infarction and their association with the severity of atheromatous burden. J Clin Lipidol. [DOI] [PubMed] [Google Scholar]
- 96.Eom M et al. (2015) Foam cells and the pathogenesis of kidney disease. Curr Opin Nephrol Hypertens 24 (3), 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaplan M et al. (2012) Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther 136 (2), 175–85. [DOI] [PubMed] [Google Scholar]
- 98.Bornfeldt KE and Tabas I (2011) Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 14 (5), 575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shapiro H et al. (2013) Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab 98 (3), 1173–81. [DOI] [PubMed] [Google Scholar]
- 100.Dhami R et al. (2001) Analysis of the lung pathology and alveolar macrophage function in the acid sphingomyelinase--deficient mouse model of Niemann-Pick disease. Lab Invest 81 (7), 987–99. [DOI] [PubMed] [Google Scholar]
- 101.Thurberg BL et al. (2012) Liver and skin histopathology in adults with acid sphingomyelinase deficiency (Niemann-Pick disease type B ). Am J Surg Pathol 36 (8), 1234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johansen MD et al. (2019) Mycobacterium avium subspecies paratuberculosis is able to manipulate host lipid metabolism and accumulate cholesterol within macrophages. Microb Pathog 130, 44–53. [DOI] [PubMed] [Google Scholar]
- 103.Okamori S et al. (2018) Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: a case report with microbiological analysis. BMC Infect Dis 18 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johansen MD et al. (2018) Mycobacterium marinum infection drives foam cell differentiation in zebrafish infection models. Dev Comp Immunol 88, 169–172. [DOI] [PubMed] [Google Scholar]
- 105.Nawabi P et al. (2008) Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol 68 (1), 173–85. [DOI] [PubMed] [Google Scholar]
- 106.Meester I et al. (2014) Nocardia brasiliensis induces formation of foamy macrophages and dendritic cells in vitro and in vivo. PLoS One 9 (6), e100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mulye M et al. (2018) Altering lipid droplet homeostasis affects Coxiella burnetii intracellular growth. PLoS One 13 (2), e0192215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brouqui P et al. (1994) Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med 97 (5), 451–8. [DOI] [PubMed] [Google Scholar]
- 109.Tumurkhuu G et al. (2018) Chlamydia pneumoniae Hijacks a Host Autoregulatory IL-1beta Loop to Drive Foam Cell Formation and Accelerate Atherosclerosis. Cell Metab 28 (3), 432–448 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lovo-Martins MI et al. (2018) Extracellular Vesicles Shed By Trypanosoma cruzi Potentiate Infection and Elicit Lipid Body Formation and PGE2 Production in Murine Macrophages. Front Immunol 9, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernard MA et al. (2014) HIV-derived ssRNA binds to TLR8 to induce inflammation-driven macrophage foam cell formation. PLoS One 9 (8), e104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boven LA et al. (2006) Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129 (Pt 2), 517–26. [DOI] [PubMed] [Google Scholar]
- 113.Vogel DY et al. (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 10, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Teixeira V and Tam LS (2017) Novel Insights in Systemic Lupus Erythematosus and Atherosclerosis. Front Med (Lausanne) 4, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winyard PG et al. (1993) Presence of foam cells containing oxidised low density lipoprotein in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis 52 (9), 677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krawczyk KM et al. (2017) Papillary renal cell carcinoma-derived chemerin, IL-8, and CXCL16 promote monocyte recruitment and differentiation into foam-cell macrophages. Lab Invest 97 (11), 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uehara K et al. (2017) Esophageal Xanthoma: Presence of M2 Macrophages Suggests Association with Late Inflammatory and Reparative Processes. Open Med (Wars) 12, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu-Jarin X et al. (2003) Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol 16 (11), 1102–8. [DOI] [PubMed] [Google Scholar]
- 119.Stone JR (2012) Pathology of myocardial infarction, coronary artery disease, plaque disruption, and the vulnerable atherosclerotic plaque. Diagnostic Histopathology 18 (11), 478–483. [Google Scholar]
- 120.Kunjathoor VV et al. (2002) Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277 (51), 49982–8. [DOI] [PubMed] [Google Scholar]
- 121.Wang CW (2016) Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta 1861 (8 Pt B), 793–805. [DOI] [PubMed] [Google Scholar]
- 122.Spalding KL et al. (2017) Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat Commun 8, 15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen WJ et al. (2016) Lipid droplets and steroidogenic cells. Exp Cell Res 340 (2), 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vallochi AL et al. (2018) Lipid Droplet, a Key Player in Host-Parasite Interactions. Front Immunol 9, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Camus G et al. (2014) The hepatitis C virus core protein inhibits adipose triglyceride lipase (ATGL)-mediated lipid mobilization and enhances the ATGL interaction with comparative gene identification 58 (CGI-58) and lipid droplets. J Biol Chem 289 (52), 35770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mattos KA et al. (2011) Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol 13 (2), 259–73. [DOI] [PubMed] [Google Scholar]
- 127.Mosser DM and Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8 (12), 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bostrom P et al. (2006) Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol 26 (8), 1871–6. [DOI] [PubMed] [Google Scholar]
- 129.Bautista G et al. (2014) Polarized THG microscopy identifies compositionally different lipid droplets in mammalian cells. Biophys J 107 (10), 2230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Veglia F et al. (2017) Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun 8 (1), 2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coats BR et al. (2017) Metabolically Activated Adipose Tissue Macrophages Perform Detrimental and Beneficial Functions during Diet-Induced Obesity. Cell Rep 20 (13), 3149–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asghar A and Sheikh N (2017) Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol 315, 18–26. [DOI] [PubMed] [Google Scholar]
- 133.Aouadi M et al. (2014) Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab 307 (4), E374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villarroya F et al. (2018) Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med 284 (5), 492–504. [DOI] [PubMed] [Google Scholar]
- 135.Hotamisligil GS (2017) Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 47 (3), 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo Y et al. (2017) Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res 27 (3), 352–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qiao L et al. (2017) Ginsenoside Rb1 Enhances Atherosclerotic Plaque Stability by Improving Autophagy and Lipid Metabolism in Macrophage Foam Cells. Front Pharmacol 8, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sergin I and Razani B (2014) Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis. Trends Endocrinol Metab 25 (5), 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang F et al. (2018) Macrophage Foam Cell-Targeting Immunization Attenuates Atherosclerosis. Front Immunol 9, 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hua H et al. (2019) Targeting mTOR for cancer therapy. J Hematol Oncol 12 (1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]