Abstract
The clinical diagnosis of new-onset type 1 diabetes has, for many years, been considered relatively straightforward. Recently, however, there is increasing awareness that within this single clinical phenotype exists considerable heterogeneity: disease onset spans the complete age range; genetic susceptibility is complex; rates of progression differ markedly, as does insulin secretory capacity; and complication rates, glycemic control, and therapeutic intervention efficacy vary widely. Mechanistic and immunopathological studies typically show considerable patchiness across subjects, undermining conclusions regarding disease pathways. Without better understanding, type 1 diabetes heterogeneity represents a major barrier both to deciphering pathogenesis and to the translational effort of designing, conducting, and interpreting clinical trials of disease-modifying agents. This realization comes during a period of unprecedented change in clinical medicine, with increasing emphasis on greater individualization and precision. For complex disorders such as type 1 diabetes, the option of maintaining the “single disease” approach appears untenable, as does the notion of individualizing each single patient’s care, obliging us to conceptualize type 1 diabetes less in terms of phenotypes (observable characteristics) and more in terms of disease endotypes (underlying biological mechanisms). Here, we provide our view on an approach to dissect heterogeneity in type 1 diabetes. Using lessons from other diseases and the data gathered to date, we aim to delineate a roadmap through which the field can incorporate the endotype concept into laboratory and clinical practice. We predict that such an effort will accelerate the implementation of precision medicine and has the potential for impact on our approach to translational research, trial design, and clinical management.
Introduction
Describing aspects of biology as “heterogeneous” often has a negative connotation. It is a term that is used when we do not understand a measured or observed aspect of disease or when we need to explain data that are not consistent. However, it is evident that recognizing that there are “different kinds” of cells, genes, types of response, and severity of disease could offer a set of opportunities for therapies to work and biomarkers to be meaningful. Thus, it may be time to exploit heterogeneity rather than curse it and to use the opportunity to carve out endotypes of type 1 diabetes that have traction both in the clinic and in the laboratory.
The introduction of the term “endotype” can largely be attributed to developments in the field of asthma (1) when it became apparent in the late 1990s that different pathogenic mechanisms induce a similar symptom cluster and manifest as a phenotype, the implications being 1) that there are multiple pathways to disease and 2) that pathway-specific therapeutic strategies will appear to have limited success if applied to a population of subjects in which the pathway is only active in a subgroup. From this new thinking, the term “endotype” was coined; in this case, the term reflects a subtype of type 1 diabetes that can be defined by a distinct functional or pathobiological mechanism (that is also tractable therapeutically).
Here, we focus on gaining a better understanding of heterogeneity in type 1 diabetes and how the endotype concept might be introduced to the field in order to bring about a sea change in clinical practice and research activity. As the number of targeted immunotherapy treatments under development continues to grow and associated clinical trial activity proceeds unabated, this is a propitious moment in which to evaluate whether, and how, a strategic approach to disease heterogeneity could unlock the power of disease-modifying drugs designed to arrest β-cell decline. Since the existence of heterogeneity in disease traits is a critical component of the rationale for studying endotypes, it is valuable to begin by reflecting on the nature of type 1 diabetes diversity. Cataloguing all reported aspects of heterogeneity in detail is beyond the scope of this article, and therefore some key examples are highlighted in Table 1, and others are expanded below in endotype definition led by observations and hypotheses. Examples include continuous as well as qualitative variables and span the different stages of disease. Of note, traits are often linked (e.g., age and HLA-specific autoimmunity) in such a way that suggests associations that could be built into distinct pathobiological entities (endotypes).
Table 1.
Examples of heterogeneity in type 1 diabetes–associated traits
| Early disease (stages 0–2) | Clinical disease onset (stage 3) | Established disease (stage 4) | |
|---|---|---|---|
| Phenotypic | • BMI: Children <12 years old have higher rate of type 1 diabetes progression if overweight/obese (25); disease course in overweight/obese is modified by presence of polymorphism in TCF7L2 (26). | • Age of diagnosis: Typically younger age associates with lower number of insulin-containing islets, serum C-peptide concentrations, duration of remission period; higher frequency of diabetic ketoacidosis; hyper-immune CD20hi insulitis; HLA-DR3/DR4 haplotypes; gene polymorphisms in PTPRK, THEMIS, and IAA; and higher overall number of AAbs, excess mortality, and frequency of cardiovascular disease in stage 4 (reviewed in 28). Adult onset more frequently GADA only, in association with other autoimmune diseases; gene PFKFB3 (29). | • Emergence of other autoimmune diseases (reviewed in 30). |
| • Ethnicity: Risk of type 1 diabetes development differs according to ethnic group (27). | • Coexistence of other autoimmune diseases (reviewed in 30). | ||
| Genetic | • HLA: Typically at age <2 years, IAA emerges first and associates with HLA-DRB1*0401/DQA1*0301/DQB1*0302 genotype; at age >6 years, GADA emerges first and associates with HLA-DRB1*0301/DQA1*0501/DQB1*0201 genotype (34). Specific haplotypes with high-risk DQ genes associate with rapid progression to stage 3 (35,36); low-risk DQ genes (e.g., DQB1*0602) reduce type 1 diabetes development (37). | • CTSH polymorphism associates with higher daily insulin dose and faster disease progression (61). | • Chromosome 1 gene variants associate with severity of β-cell loss (60). |
| • Genetic risk score: Specific constellations of gene polymorphisms associate with faster progression to stage 3 (38–40). | • TCF7L2 polymorphism associates with milder immunologic and metabolic phenotype (62). | ||
| Immune | • Autoantibodies: type, affinity, titer, spreading, and tendency to revert (43–46). | • Autoantigen-specific reactivities (41,42). | • IL-2 signaling defect (55–57), Treg activity (reviewed in 58), CD8 antigen experience, and exhaustion (59) |
| • Type I interferon signature: detected in peripheral whole blood and purified neutrophils (47–49). | |||
| • Activation of innate immunity: detected in circulation (22,50). | |||
| • T-cell signatures: CD4 proinflammatory (IFN-γ), regulated (IL-10) (51,52), T follicular helper cells (53,54). | |||
| Metabolic | • Proinsulin/insulin processing: dysregulated, residual insulin staining, proinsulin staining pattern (reviewed in 33). | • Insulin secretory pattern: early insulin response associates with faster rate of loss of insulin secretion (32). | • Insulin secretion: long-term sustainers/nonsustainers (31) |
| • Pathology: cellular constituents and extent of insulitis (17,18); evidence of viruses. | |||
| • Metabolic: glucose and Index60 in relation to age and C-peptide (31,32). |
AAbs, autoantibodies; Treg, regulatory T cell.
The Impact of Type 1 Diabetes Heterogeneity on Clinical Trials and Research
An overarching goal of type 1 diabetes research has been to bring forward disease-modifying therapies that preserve β-cell function (2). This has been allied with progress made in the design and conduct of intervention (stage 3) and prevention (stages 1 and 2) trials. Despite some successes and considerable knowledge gain, no agent has progressed beyond phase III clinical trials and into clinical practice, and as such, type 1 diabetes remains an outlier among the autoimmune diseases. Numerous factors account for this, but it is our contention that disease heterogeneity contributes to this impasse in the field (3,4).
Hitherto, the potential confounding effect of heterogeneity has been insufficiently addressed in the design of type 1 diabetes clinical trials, which typically adopt very basic and standard inclusion criteria (e.g., short time from disease onset, wide age range, single autoantibody) to assure consistency, enable cross-trial analysis, and, at a practical level, facilitate recruitment. Yet, one can imagine that factors such as disease severity, age, and underlying genetic predisposition could each influence trial outcomes and treatment responsiveness (“theratypes”) (5). These are rarely, if ever, used as stratifiers and when prespecified as covariates their utility is often limited by insufficient statistical power. In practice, whether a trial succeeds or fails in meeting its primary objective(s), there are often subgroups of subjects who appear to benefit from the therapy. One such example is monoclonal anti-CD3 antibody (2). Despite promising results in phase II trials, a phase III trial with this agent did not meet its primary composite outcome of insulin use (<0.5 units/kg per day) and glycated hemoglobin A1c (<6.5%) at 1 year. However, some patients appear to have responded rather well (i.e., younger subjects with higher C-peptide at study entry and patients from North America and Europe) (6). More recently, this approach has shown efficacy in prevention of diabetes progression in high-risk subjects without diabetes; intriguingly, subgroups defined by HLA and zinc transporter 8 autoantibodies appear to be differentially responsive to the drug (7). Similarly, while oral insulin did not realize its primary objective to delay progression from stage 1 to stage 3 type 1 diabetes, an independently randomized subgroup (distinguished by having first-phase insulin release lower than a specified threshold) had a 31-month delay in disease progression (8). These observations should be an incentive for stratification to be built into trial design and for the field to contemplate development of drugs that work for the few rather than the many. Moreover, and importantly for this discussion, this kind of observation is a major learning opportunity (9) and could emerge as being a critical path to endotype definition (see below).
In sum, there are opportunities to conduct smarter clinical and laboratory studies. The risk of continuing with current trends is an excess of nonproductive science, poor utilization of resources, and disillusion among our patients and constituencies.
Learning From Other Diseases
One way to start integrating endotypes into type 1 diabetes studies and appreciate their potential impact is to draw upon experiences in other complex diseases in which they have been an important part of the development of precision medicine approaches. Asthma is the prototypical example, in which the delineation of a subset of patients whose airway disease is driven by type 2 cytokines (the T2-high/low paradigm) led to use of the term “endotyping” to describe subpopulations in which the underlying disease is caused by a uniform pathobiologic or molecular mechanism (1). The endotyping paradigm proved critical for advancing a new age of asthma medications and has invoked the use of a more stringent definition of the term “endotype” that incorporates successful disease modification by a therapeutic agent that targets the putative pathobiological mechanism. The essential requirements for success of the endotype model in asthma were a robust understanding of at least one pathobiological pathway, a therapeutic intervention that interdicts this, and a robust biomarker to identify the disease subtype (10).
An obvious question, therefore, is, “Can we simply transfer these principles over to type 1 diabetes?” This is not so easy, as type 1 diabetes has several complicating features. As examples: the target organ is inaccessible for scientific interrogation in living individuals, there is uncertainty about the canonical immunological pathways that are responsible for β-cell death (and probably there is heterogeneity), and the disease burden is greatest in children and adolescents, restricting some types of experimentation. Indeed, age is clearly such a major driver of heterogeneity in type 1 diabetes (Table 1) and other diseases that it merits further discussion (see below). Taken together, these disease features militate against easy solutions to the endotype question.
From Phenotypes to Endotypes in Type 1 Diabetes: A Roadmap to Maximizing Opportunities
Several approaches are beginning to emerge that could assist in defining endotypes, including greater accessibility to tools for sophisticated human immunophenotyping; specific, targeted immune therapies; and the application of systems immunology and new statistical tools. These offer opportunities that are based on 1) observation/hypothesis-driven approaches, as well as 2) unsupervised/data-driven methodologies and 3) response to therapy. In each case these approaches will benefit from the opportunities that arise to study natural history cohorts such as TrialNet (11), The Environmental Determinants of Diabetes in the Young (TEDDY) (12), and INNODIA (13) as well as responder/nonresponder subgroups in clinical trials (8).
Endotype Definition Led by Observations and Hypotheses
A clear recognition of the heterogeneous traits present in type 1 diabetes has given rise to numerous examples of possible pathophysiological processes that could be considered to be compatible with the definition of an endotype, and focused study addressing one or two of these seems a reasonable place to start. With this in mind, one of the more obvious examples relates to a phenotype (e.g., development of a specific islet cell autoantibody) and its link to a genotype (e.g., HLA) that would strongly infer that a distinct pathophysiological process is in operation. Birth cohort studies of subjects with high risk of type 1 diabetes that examine the timing of emergence of specific autoantibodies indicate an early peak of incidence of insulin autoantibody (IAA) as the first marker of autoimmunity, strongly linked to the HLA-DR4 haplotype; in contrast, GAD autoantibodies (GADA) emerge as the sole marker of autoimmunity later, and with a strong link to the HLA-DR3 haplotype (Table 1). This example raises an important question, namely, whether a specific endotype represents a discrete, etiological event and pathway or whether it is a distinct outcome and pathological track that arises on the background of causal mechanisms that are the same for all disease cases. It is probably too soon to be definitive on this aspect of type 1 diabetes endotypes, and this important concept will require careful teasing apart using cohort studies and a better knowledge of causality. For example, one can hypothesize a pathway in which tolerance to (pro)insulin is breached early following presentation of (pro)insulin or related peptides by class II HLA molecules on the HLA-DR4 haplotype, leading to T- and B-cell activation and autoantibody production; and tolerance to GAD is similarly broken—perhaps at a slower pace or following different precipitants—by presentation of GAD peptides by HLA-DR3 haplotype–linked molecules. In both situations, the underlying causative event could be shared (e.g., virus-mediated damage to islets) or distinct (e.g., molecular mimicry for proinsulin or GAD), and in both there is a common pathogenesis involving progression to multiple autoantibodies, signifying increased risk of disease, as well as progression to diabetes (14). These processes could be termed the “proinsulin autoimmune-DR4” (PADR4) and “GAD autoimmune-DR3” (GADR3) endotypes. Going forward, the field could focus on defining the related but distinct pathophysiological pathways more precisely, as well as using these two markers (autoantibody and HLA) as stratifiers for any therapeutic that emerges as being particularly efficacious in limiting or reversing specific autoantigen presentation and loss of tolerance (15). One of the challenges in this context is that the reliable identification of these two endotypes currently requires sampling close to first seroconversion. Indeed, findings from the Type 1 Diabetes Prediction and Prevention (DIPP) study suggest that perhaps only half of those testing positive for GADA at diagnosis had GADA as the first detectable autoantibody, making the case that better biomarkers of PADR4 and GADR3 will be required (16).
A second example is given by the demonstration that in the pancreas, two distinct types of insulitic lesions are present in subjects with recent-onset type 1 diabetes, distinguishable by the degree of cellular infiltrate and presence of CD20+ B cells (termed hyperimmune CD20hi and pauci-immune CD20lo) (17,18). This phenotype carries important implications for endotype definition and treatment strategies. For example, the hyperimmune CD20hi status, which appears to be most overt in the younger age-group, could be responsive to B-cell depletion therapies.
In both of these examples, age could be a confounding influence. For the putative PADR4 and GADR3 endotypes, it will be important to examine the role of age and whether this is a proxy for different gene-environment interactions (e.g., diet, infection) or immunological maturity. It is plausible that what appears as a pathobiological phenomenon (e.g., a greater preponderance of B lymphocytes in islet immune infiltrates in young children with type 1 diabetes) is actually a reflection, at least in part, of an age-related physiological difference in immune responsiveness (e.g., B-lymphocyte number and percentage are higher in the blood in young children compared with later childhood) (19). Age thus functions as a proxy for changes in immune and metabolic function. A much better understanding of the maturation of the key physiological systems in childhood would undoubtedly help here, and perhaps endotypes in which the pathobiology diverges from the physiology would be of particular interest. At the very least, mechanistic and discovery science studies aiming to uncover endotypes should be careful to select participants a priori (for example, according to age, sex, autoantibody status, and HLA) or as bins according to these features post hoc. An emphasis on age relatedness is thus an important part of the roadmap and will undoubtedly help yield clearer data and uncover the nature of physiology/pathobiology relationships and their proxies.
Data-Driven Endotype Discovery
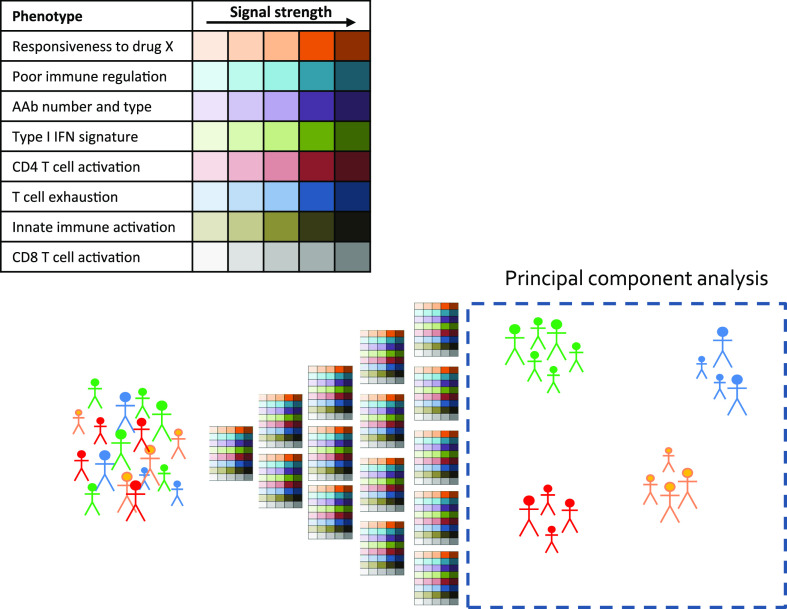
Beyond these clear and somewhat binary examples, one innovative approach that could be adopted in type 1 diabetes for defining more complex endotypes is the palette model, proposed by McCarthy (20). The principle is that several selected major traits (i.e., palette colors) are assigned as present/absent across a scale (i.e., color shades) for each given subject. Given a sufficient number of subjects, there would be the potential to identify subgroups of subjects whose disease is reflected in palettes with a similar color/shade composition. In addition, the palette colors could go beyond the measurement of known traits but also include system approaches (such as immunomics by mass cytometry, whole blood transcriptomics, metabolomics, and proteomics) as in the design of the INNODIA consortium studies (13). There is insufficient space here to do justice to the many studies that have defined potentially important immune and metabolic phenotypes that could contribute to complex endotype definitions in type 1 diabetes; therefore, as a means to illustrate the palette as a potential part of the roadmap, several of the more prominent examples are shown in Fig. 1, along with a strategy for discovering how they could be used going forward. For example, subjects with multiple dominant phenotypes indicative of immune hyperresponsiveness (e.g., multiple autoantibodies, high antigen-specific T-cell proliferation, activated CD8 T cells) will cluster together (Fig. 1). McCarthy describes several advantageous features of this model, including: implicit acknowledgment of the multifactorial nature of type 1 diabetes, potential to reflect progression rates and response to therapy, enablement of targeted therapies (e.g., for T cell, B cell, interferon), focusing of research efforts onto therapeutics and encouraging identification of the extremes (“archetypes”), and the potential to identify surrogates that are more facile to measure than multiple different phenotypes. Developing this model could be envisaged as a collaborative effort across the key type 1 diabetes research networks to achieve sufficient data points for clusters to appear.
Figure 1.
The palette model for defining endotypes. A series of characteristics are defined and graded using immunoassays and the data analyzed for evidence of clustering to reveal complex endotypes.
Endotype Definition Led by Responders Versus Nonresponders Analysis
Further insights into disease pathways that could lead us to endotypes follow a reversed discovery track; these are learnings from the study of clinical responses in the setting of intervention trials, in which a therapeutic agent appears to be most effective in a subgroup of patients. Examples for anti-CD3 and oral insulin are given above; further indications of such theratypes include the analysis of the effects of costimulation blockade on immune compartments in the setting of the TrialNet intervention study with the costimulation blocking agent CTLA4-Ig (Abatacept) (21). A plasma-induced transcription assay showed that the patients exhibiting high innate inflammatory bias at baseline exhibited more rapid disease progression as well as a greater therapeutic response to CTLA4-Ig (22). In another study, a treatment-induced change in the configuration of memory/naïve compartments of CD4+ T lymphocytes was reported (23). These findings further support the existence of discrete endotypes of type 1 diabetes that exhibit distinct immunoregulatory profiles at clinical onset and that these may be useful for design and analysis of clinical trials.
These examples, in addition to many others (24), provide support for a strategy that is being increasingly adopted to understand drug mechanisms of action, human physiology, and disease, namely, the use of experimental medicine studies (for example, a drug or intervention is used to examine hypothetical changes in the immune system as the primary end point) rather than clinical trials (efficacy or safety is the outcome). One could also contemplate the use of combinations of therapies (each with distinct mechansims of actions) across a diverse population to highlight drugs with distinct, subgroup-dependent effects.
Conclusions: Moving From Phenotypes to Endotypes in Type 1 Diabetes
Ultimately, the considerable effort required to establish robust endotypes of type 1 diabetes must be justified in terms of its importance for, and impact upon, clinical management, clinical trial design, and research studies on disease pathogenesis. Examples of the bearing this might have are therefore worth considering. In relation to new patients being seen for the first time in the type 1 diabetes clinic, for example, the identification of endotypes with rapid and unrelenting progression to a state of minimal C-peptide secretion, as opposed to prolonged honeymooning with limited insulin requirement, could guide management decisions such as pump adoption or other advanced technologies and the intensity with which education programs are pursued. A greater impact might be seen in the design of immunotherapy trials in the short-term and adoption of disease-modifying therapies into clinical practice in the longer term. In trials, the clear definition of type 1 diabetes endotypes that associate with responsiveness to specific therapies could provide sufficiently compelling early-phase outcome data so that drugs make a faster transition to market and are explicitly earmarked for use in a disease subset. To arrive at these advances will take sustained, high-quality research that must be conducted with cognizance of the potential positive/negative impact of heterogeneous traits and phenotypes. Performing experiments with human samples, and taking into consideration the possibility that, for example, males and females have different immunological behavior depending on age, hormonal status, BMI, and other factors, is likely to yield data of higher quality, with lower variance, and thus make a more incisive contribution to knowledge and understanding. If these “codes of practice” are widely adopted and studies and clinics are conducted against a background of wide awareness of the endotype concept, then there is the definite potential for significant advances in practice to be made.
During an era that is unprecedented in the application of immune and biologic therapies to disorders as diverse as cancer, hypercholesterolemia, and psoriasis, type 1 diabetes remains an outlier in terms of not having a disease-modifying therapy beyond single hormone replacement. This means that despite representing a major unmet need, it stands to miss out on the benefits of precision medicine. One of the barriers to overcome in order to address this current, parlous status is the impact of disease heterogeneity. We propose that defining, understanding, and applying disease endotypes in type 1 diabetes are steps that warrant keen attention as we design new laboratory and clinical studies. A revised model for disease investigation and management, entailing categorization of patients by biology, should replace the “one size fits all” approach and would be transformational.
Article Information
Acknowledgments. This manuscript is the result of a 2-day meeting, held with the generous support of the Leona M. and Harry B. Helmsley Charitable Trust, focused on understanding heterogeneity and defining endotypes in type 1 diabetes. The authors thank the following for helpful discussion of the topics aired in this manuscript: Tee Bahnson (Benaroya Research Institute), Susan Geyer (TrialNet Coordinating Center [University of South Florida]), Georg Hollander (University of Oxford), and Edward Wakeland (University of Texas Southwestern Medical Center).
Funding. The Leona M. and Harry B. Helmsley Charitable Trust sponsored the 2-day meeting that was held on this topic in January 2018.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. The following authors contributed to the idea conceptualization and 2-day meeting preparation, attended the meeting in person, and delineated the manuscript backbone: M.B., S.A., M.A.A., T.M.B., C.E.-M., C.J.G., P.A.G., M.J.H., J.P.K., S.A.L., E.F.M., N.G.M., R.A.O., T.P., M.C.P., X.Q., M.J.R., B.O.R., D.Sc., D.Sk., and M.P. In addition, the following authors (being part of the TrialNet Steering Committee) significantly contributed to the discussion and brainstorming that followed the abovementioned meeting: M.S.A., D.B., P.J.B., E.B., L.A.D.M., S.E.G., K.C.H., M.K., L.J., M.L., A.P., D.Sc., and D.Sk. Thus, all authors made substantial contributions to conception and design of the manuscript, participated in drafting the manuscript or revising it critically for important intellectual content, and gave final approval of the version to be submitted. M.B. and M.P. wrote the manuscript.
Footnotes
S.A., M.S.A., M.A.A., D.B., P.J.B., E.B., T.M.B., L.A.D.M., C.E.-M., S.E.G., C.J.G., P.A.G., K.C.H., M.J.H., M.K., L.J., J.P.K., S.A.L., M.L., E.F.M., N.G.M., R.A.O., T.P., M.C.P., A.P., X.Q., M.J.R., B.O.R., D.Sc., and D.Sk. contributed equally to this work.
M.B. is currently affiliated with the Telethon Foundation, Milan, Italy.
References
- 1.Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011;127:355–360 [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum C, VanBuecken D, Lord S. Disease-modifying therapies in type 1 diabetes: a look into the future of diabetes practice. Drugs 2019;79:43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvigsson J Time to leave rigid traditions in type 1 diabetes research. Immunotherapy 2017;9:619–621 [DOI] [PubMed] [Google Scholar]
- 4.Woittiez NJC, Roep BO. Impact of disease heterogeneity on treatment efficacy of immunotherapy in type 1 diabetes: different shades of gray. Immunotherapy 2015;7:163–174 [DOI] [PubMed] [Google Scholar]
- 5.Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest 2019;130:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherry N, Hagopian W, Ludvigsson J, et al.; Protégé Trial Investigators . Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group, Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017;318:1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 2019;56:219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglia M, Anderson MS, Buckner JH, et al. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia 2017;60:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rewers M, Hyöty H, Lernmark Å, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) study: 2018 update. Curr Diab Rep 2018;18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu C, Lahesmaa R, Bonifacio E, Achenbach P, Tree T. Immunological biomarkers for the development and progression of type 1 diabetes. Diabetologia 2018;61:2252–2258 [DOI] [PubMed] [Google Scholar]
- 14.Ziegler R, Alper CA, Awdeh ZL, et al. Specific association of HLA-DR4 with increased prevalence and level of insulin autoantibodies in first-degree relatives of patients with type I diabetes. Diabetes 1991;40:709–714 [DOI] [PubMed] [Google Scholar]
- 15.Roep BO, Wheeler DCS, Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 2019;7:65–74 [DOI] [PubMed] [Google Scholar]
- 16.Ilonen J, Lempainen J, Hammais A, et al.; Finnish Pediatric Diabetes Register . Primary islet autoantibody at initial seroconversion and autoantibodies at diagnosis of type 1 diabetes as markers of disease heterogeneity. Pediatr Diabetes 2018;19:284–292 [DOI] [PubMed] [Google Scholar]
- 17.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016;65:1362–1369 [DOI] [PubMed] [Google Scholar]
- 19.Tosato F, Bucciol G, Pantano G, et al. Lymphocytes subsets reference values in childhood. Cytometry A 2015;87:81–85 [DOI] [PubMed] [Google Scholar]
- 20.McCarthy MI Painting a new picture of personalised medicine for diabetes. Diabetologia 2017;60:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orban T, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet Abatacept Study Group . Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera SM, Engle S, Kaldunski M, et al.; Type 1 Diabetes TrialNet CTLA4-Ig (Abatacept) Study Group . Innate immune activity as a predictor of persistent insulin secretion and association with responsiveness to CTLA4-Ig treatment in recent-onset type 1 diabetes. Diabetologia 2018;61:2356–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orban T, Beam CA, Xu P, et al.; Type 1 Diabetes TrialNet Abatacept Study Group . Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes 2014;63:3449–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmegrim KCR, de Azevedo JTC, Arruda LCM, et al. Immunological balance is associated with clinical outcome after autologous hematopoietic stem cell transplantation in type 1 diabetes. Front Immunol 2017;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redondo MJ, Steck AK, Sosenko J, et al.; Type 1 Diabetes TrialNet Study Group . Transcription factor 7-like 2 (TCF7L2) gene polymorphism and progression from single to multiple autoantibody positivity in individuals at risk for type 1 diabetes. Diabetes Care 2018;41:2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tosur M, Geyer SM, Rodriguez H, Libman I, Baidal DA, Redondo MJ; Type 1 Diabetes TrialNet Study Group . Ethnic differences in progression of islet autoimmunity and type 1 diabetes in relatives at risk. Diabetologia 2018;61:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leete P, Mallone R, Richardson SJ, Sosenko JM, Redondo MJ, Evans-Molina C. The effect of age on the progression and severity of type 1 diabetes: potential effects on disease mechanisms. Curr Diab Rep 2018;18:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cousminer DL, Ahlqvist E, Mishra R, et al.; Bone Mineral Density in Childhood Study . First genome-wide association study of latent autoimmune diabetes in adults reveals novel insights linking immune and metabolic diabetes. Diabetes Care 2018;41:2396–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev 2016;15:644–648 [DOI] [PubMed] [Google Scholar]
- 31.Oram RA, Jones AG, Besser REJ, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Calvo T, Richardson SJ, Pugliese A. Pancreas pathology during the natural history of type 1 diabetes. Curr Diab Rep 2018;18:124. [DOI] [PubMed] [Google Scholar]
- 34.Simell S, Hoppu S, Simell T, et al. Age at development of type 1 diabetes– and celiac disease–associated antibodies and clinical disease in genetically susceptible children observed from birth. Diabetes Care 2010;33:774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyerlein A, Bonifacio E, Vehik K, et al.; TEDDY Study Group . Progression from islet autoimmunity to clinical type 1 diabetes is influenced by genetic factors: results from the prospective TEDDY study. J Med Genet 2019;56:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krischer JP, Liu X, Lernmark Å, et al.; TEDDY Study Group . The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes 2017;66:3122–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugliese A, Boulware D, Yu L, et al.; Type 1 Diabetes TrialNet Study Group . HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes 2016;65:1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steck AK, Dong F, Waugh K, et al. Predictors of slow progression to diabetes in children with multiple islet autoantibodies. J Autoimmun 2016;72:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonifacio E, Beyerlein A, Hippich M, et al.; TEDDY Study Group . Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med 2018;15:e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redondo MJ, Geyer S, Steck AK, et al.; Type 1 Diabetes TrialNet Study Group . A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 2018;41:1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.So M, Elso CM, Tresoldi E, et al. Proinsulin C-peptide is an autoantigen in people with type 1 diabetes. Proc Natl Acad Sci U S A 2018;115:10732–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arif S, Moore F, Marks K, et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death. Diabetes 2011;60:2112–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 45.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler A-G, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004;114:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler A-G, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vecchio F, Lo Buono N, Stabilini A, et al.; DRI_Biorepository Group; Type 1 Diabetes TrialNet Study Group . Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018;3:122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira RC, Guo H, Coulson RMR, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 2014;63:2538–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallionpää H, Elo LL, Laajala E, et al. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes 2014;63:2402–2414 [DOI] [PubMed] [Google Scholar]
- 50.Cabrera SM, Chen Y-G, Hagopian WA, Hessner MJ. Blood-based signatures in type 1 diabetes. Diabetologia 2016;59:414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenefeck R, Wang CJ, Kapadi T, et al. Follicular helper T cell signature in type 1 diabetes. J Clin Invest 2015;125:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viisanen T, Ihantola E-L, Näntö-Salonen K, et al. Circulating CXCR5+PD-1+ICOS+ follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies. Diabetes 2017;66:437–447 [DOI] [PubMed] [Google Scholar]
- 55.Long SA, Cerosaletti K, Bollyky PL, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwedhelm K, Thorpe J, Murray SA, et al. Attenuated IL-2R signaling in CD4 memory T cells of T1D subjects is intrinsic and dependent on activation state. Clin Immunol 2017;181:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang JHM, Cutler AJ, Ferreira RC, et al. Natural variation in interleukin-2 sensitivity influences regulatory T-cell frequency and function in individuals with long-standing type 1 diabetes. Diabetes 2015;64:3891–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia 2017;60:1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeo L, Woodwyk A, Sood S, et al. Autoreactive T effector memory differentiation mirrors β cell function in type 1 diabetes. J Clin Invest 2018;128:3460–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roshandel D, Gubitosi-Klug R, Bull SB, et al.; DCCT/EDIC Research Group . Meta-genome-wide association studies identify a locus on chromosome 1 and multiple variants in the MHC region for serum C-peptide in type 1 diabetes. Diabetologia 2018;61:1098–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fløyel T, Brorsson C, Nielsen LB, et al. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci U S A 2014;111:10305–10310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Redondo MJ, Geyer S, Steck AK, et al.; Type 1 Diabetes TrialNet Study Group . TCF7L2 genetic variants contribute to phenotypic heterogeneity of type 1 diabetes. Diabetes Care 2018;41:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]