Abstract
Systemic therapy of advanced hepatocellular carcinoma (HCC) with the small molecule multi-kinase inhibitor sorafenib is associated with large inter-individual pharmacokinetic variability and unpredictable side effects potentially requiring dose reduction or treatment termination. Organic cation transporter OCT1 (gene SLC22A1) has been proposed as clinical biomarker of HCC response. Because proof is lacking that OCT1 transports sorafenib, we used a combinatorial approach to define how OCT1 contributes to sorafenib transport. Overexpression of functional OCT1 protein in Xenopus laevis oocytes and mammalian cell lines did not facilitate sorafenib transport. Otherwise, sorafenib considerably accumulated in liver cancer cell lines despite negligible OCT1 mRNA and protein levels. Sorafenib pharmacokinetics was independent of OCT1 genotype in mice. Finally, SLC22A1 mRNA expression was significantly reduced by DNA methylation in The Cancer Genome Atlas HCC cohort. These results clearly demonstrate OCT1-independent cellular sorafenib uptake indicating that OCT1 is apparently not a valid biomarker of sorafenib response in HCC.
Keywords: Sorafenib, Multi-kinase inhibitor, Hepatocellular carcinoma, Transporters, OCT1
Introduction
Hepatocellular carcinoma (HCC) is the third highest cause of cancer-related death and each year, more than half of a million people are diagnosed with HCC. Early-stage HCC can be potentially cured by surgical resection or liver transplantation while advanced or progressed HCC is still detrimental.1,2 In the U.S., sorafenib (Nexavar) remains the only US Food & Drug Administration (FDA)-approved small molecule for the first-line treatment of advanced HCC, although its therapeutic benefits are modest.2,3 Sorafenib is a multikinase inhibitor that targets the Raf/Ras pathway, VEGFR1, 2, and 3, and PDGFRβ.2 It inhibits tumor cell proliferation and growth through reducing tumor angiogenesis and signaling as well as increasing tumor cell apoptosis due to this dual function.4 After its first FDA-approval in 2007, sorafenib was subsequently approved for treatment of advanced thyroid and renal cell carcinomas, and has shown activity in acute myeloid leukemia and ovarian cancer.5 However, like other kinase inhibitors, sorafenib is afflicted by large inter-individual pharmacokinetic variability, a relatively narrow therapeutic window, and the occurrence of debilitating side effects that require dose-reduction or even discontinuation of treatment.6-8 Exposure-toxicity relationships have been established for sorafenib9 and although the metabolic pathways of sorafenib are reasonably well understood and involve the formation of Phase I and Phase II metabolites,10-12 the primary cause of its large inter-individual pharmacokinetic variability remains unknown.
We previously reported that the cellular uptake of certain sorafenib metabolites is regulated by organic anion transporting polypeptide 1B (OATP1B)-type transporters11 and that their efflux can be mediated by ATP-binding cassette (ABC) transporters such as ABCC2 and ABCC3.5 The major mechanism of cellular uptake of sorafenib itself, however, remains uncertain. Recent studies have suggested that (i) hepatocellular carcinoma cells transfected with reduced- or loss-of-function variants of organic cation transporter 1 (OCT1) are potentially less sensitive to sorafenib-mediated cytotoxicity,13 that (ii) cellular accumulation of sorafenib may be increased in Chinese hamster ovary (CHO) cells overexpressing OCT1,14 and that (iii) sorafenib is a poor inhibitor of the transport of known OCT1 substrates.15,16 Based on these observations, OCT1, an organic cation transporter that is highly expressed on the basolateral membrane of hepatocytes,17,18 has been proposed as biomarker for sorafenib response in HCC therapy13,19 although proof is lacking that OCT1 indeed transports sorafenib. This knowledge is essential for evaluation of OCT1 as a valid biomarker. For instance we could previously show that imatinib is not a OCT1 substrate, thus questioning OCT1 as clinical relevant response biomarker for imatinib in treatment of chronic myeloid leukemia (CML).20 Therefore, to address the critical question whether OCT1 transports sorafenib, we rigorously re-examined the transport of sorafenib using various in vitro and in vivo model systems. Integrating the results from these complementary studies, we conclude that the cellular uptake of sorafenib occurs independently of OCT1.
Results
OCT1 does not transport sorafenib in vitro
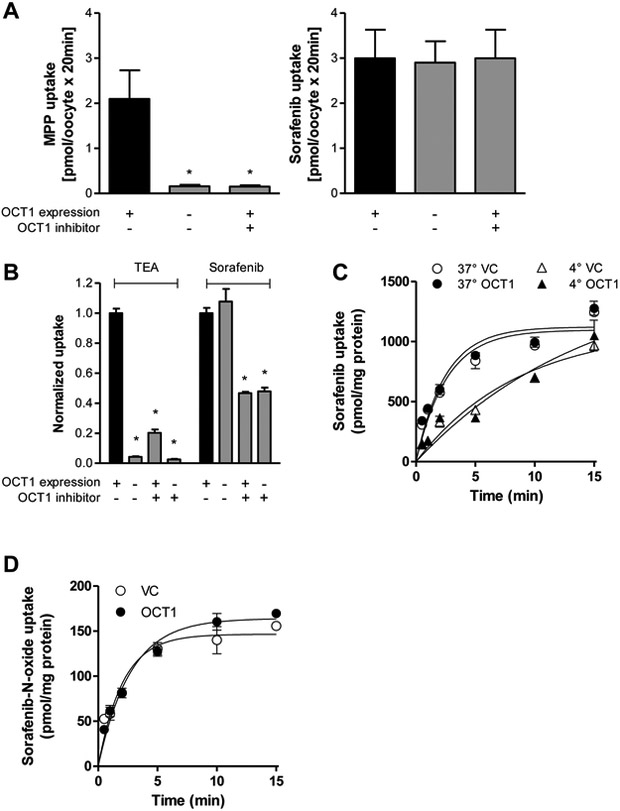
In order to evaluate the contribution of OCT1 to the cellular uptake of sorafenib, we initially used OCT1-expressing oocytes as well as an OCT1-overexpressing model generated in HEK293 cells. Uptake of the OCT1 probe substrate MPP increased 13-fold from 0.16 pmol/oocyte/20 min in the absence of OCT1 to 2.1 pmol/oocyte/20 min in the presence of OCT1 and was completely blocked by 2.5 mM MPP (Fig. 1A). In contrast, sorafenib uptake was not different between OCT1-expressing and nonexpressing oocytes in the absence or presence of MPP. Moreover, we analyzed sorafenib transport in HEK293 cells expressing OCT1 (HEK293-OCT1). Overexpression of the transporter was confirmed by increased uptake by 23.5±1.2-fold of the OCT1 probe substrate TEA in HEK293-OCT1 cells compared to cells carrying an empty vector (HEK293-VC) (Fig. 1B). In contrast to TEA, no difference in sorafenib uptake was observed between HEK293-OCT1 and HEK293-VC cells (Fig. 1B). The OCT1 inhibitor decynium22 decreased sorafenib uptake in these cells suggesting that an OCT1-independent, decynium22-sensitive transport mechanism for sorafenib is in operation in HEK293 cells. This conclusion is consistent with the notion that the uptake of sorafenib (Fig. 1C) is time-dependent and reaches a plateau after about 10 min in both HEK293-OCT1 and HEK293-VC cells. Moreover, the transport of sorafenib in HEK293 cells was reduced when experiments were performed at 4 °C compared with 37 °C (Fig. 1C) independently of OCT1, further suggesting that the uptake mechanism is transporter mediated. Sorafenib-N-oxide is the main metabolite of sorafenib and accounts for 9-16% of sorafenib analytes in plasma in adult healthy volunteers and cancer patients10,21 and for 27-33% in children with AML.21 Because sorafenib-N-oxide has similar potency as the parent compound sorafenib,22 cellular uptake of sorafenib-N-oxide may also contribute to antitumor activity of sorafenib. However, sorafenib-N-oxide was also not transported by OCT1 (Fig. 1D).
Figure 1.
Transport of sorafenib in cells overexpressing human OCT1. (A) Uptake of the OCT1 substrate MPP (1 μM) and sorafenib (2 μM) in the absence or presence of MPP (2.5 mM), which is also an OCT1 inhibitor, into OCT1-expressing or nonexpressing oocytes after 20 min. Data are means and SD of 10 observations. The star (*) represents statistical significance (P<0.05) vs oocytes expressing OCT1. (B) Comparison between uptake of the OCT1 probe substrate TEA (100 μM) and sorafenib (0.2 μM) into HEK293 cells expressing an empty pcDNA3 vector (VC) or human OCT1 in the presence or absence of the OCT1 inhibitor decynium22 (5 μM) after 10-min incubations. (C) Time and temperature dependence of sorafenib (7.5 μM) uptake into HEK293 cells expressing VC or OCT1. (D) Time-dependent uptake of sorafenib-N-oxide (1 μM) into HEK293 cells expressing VC or OCT1. Data are presented as mean (bars or symbols) and SD (error bars) of 3 observations. The star (*) represents statistical significance (P<0.05) vs HEK293 cells expressing OCT1 in the absence of decynium22.
OCT2 and OCT3 were also analyzed whether they are able to transport sorafenib because they may be important for sorafenib pharmacokinetics thereby potentially contributing to antitumor activity. OCT2 is a renal transporter,23 which could play a role in the uptake of sorafenib into the kidney cells, where sorafenib is glucuronidated by UGT1A924 and then excreted into urine. About 19% of a dose of sorafenib is found in urine.10 Like OCT1, OCT3 is also expressed in the sinusoidal membrane of hepatocytes18 and might thereby act as an alternative transporter to OCT1. The results (Figure S1) show that both OCT1-related transporters do not transport sorafenib excluding both as alternative sorafenib uptake transporters.
Lack of sorafenib transport by OCT1 in vivo
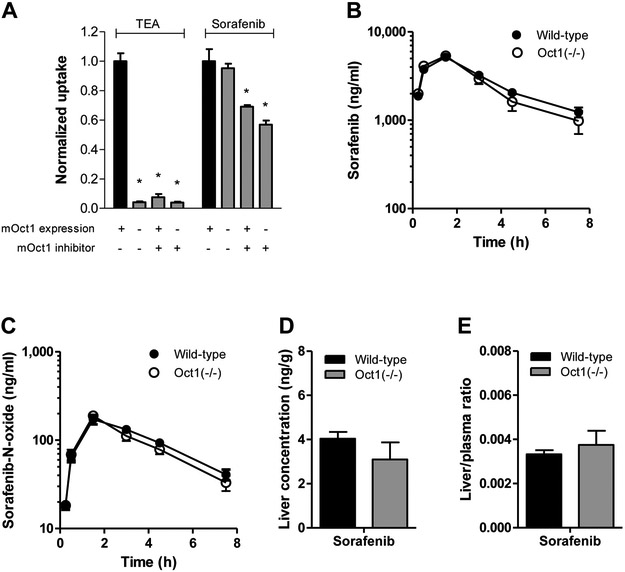
To evaluate potential species dependence, sorafenib uptake studies were also performed in HEK293 cells transfected with the ortholog mouse transporter mOct1 (HEK293-mOct1). The results were comparable to those for human OCT1 in that no difference in sorafenib uptake was observed between HEK293-mOct1 and HEK293-VC cells, regardless of pre-incubation with decynium22 (Fig. 2A) or sorafenib concentrations, and similar observations were made for the related transporter mOct2 (Figure S2). Next, we used Oct1-deficient mice20 to assess its contribution to pharmacokinetics of sorafenib based on the expectation that a lack of Oct1 would result in decreased hepatic uptake of substrate drugs and subsequently cause delayed elimination. In line with the in vitro uptake studies, however, we observed minimal difference in pharmacokinetic parameters of sorafenib and the hepatic metabolite sorafenib-N-oxide in Oct1-deficient mice compared with wild-type mice (Fig. 2B-C; Table S1). Likewise, no significant differences were observed in the liver concentrations or the liver-to-plasma ratio of sorafenib (Fig. 2D,E) and sorafenib-glucuronide. Hepatic levels of sorafenib-N-oxide were below the lower limit of quantification of the analytical method (data not shown). In addition to oxidative metabolism, sorafenib also undergoes glucuronidation by UGT1A9, primarily in the liver.10 Because it has been shown that hepatic transporters are able to transport sorafenib-glucuronide,11 we evaluated for a comprehensive analysis, whether OCT1 affects the pharmacokinetics of sorafenib-glucuronide. However, a lack of Oct1 had no effect on sorafenib-glucuronide pharmacokinetics, liver concentration and liver-to-plasma ratio (Figure S3).
Figure 2.
Evaluation of transport of sorafenib by mouse OCT1 (mOCT1) in vitro and in vivo. (A) Comparison between uptake of the mOCT1 probe substrate TEA (100 μM) and sorafenib (0.2 μM) into HEK293 cells expressing an empty pcDNA3 vector (VC) or mouse OCT1 in the presence or absence of the OCT1 inhibitor decynium22 (5 μM) after 10-min incubations. Data are presented as mean (bars) and SD (error bars) of 6 observations. The star (*) represents statistical significance (P<0.05) vs cells expressing OCT1 in the absence of decynium22. (B-D) Influence of OCT1-deficiency on the plasma pharmacokinetics of sorafenib (B) and its metabolite sorafenib-N-oxide (C). Corresponding liver levels (D) and liver-to-plasma ratios (E) are shown for sorafenib at the end of the experimental sampling period (7.5 h) are presented as mean (bars) and SE (error bars) of 9 observations. All animals received sorafenib as a single oral dose of 10 mg/kg.
Cellular sorafenib uptake in cancer cells
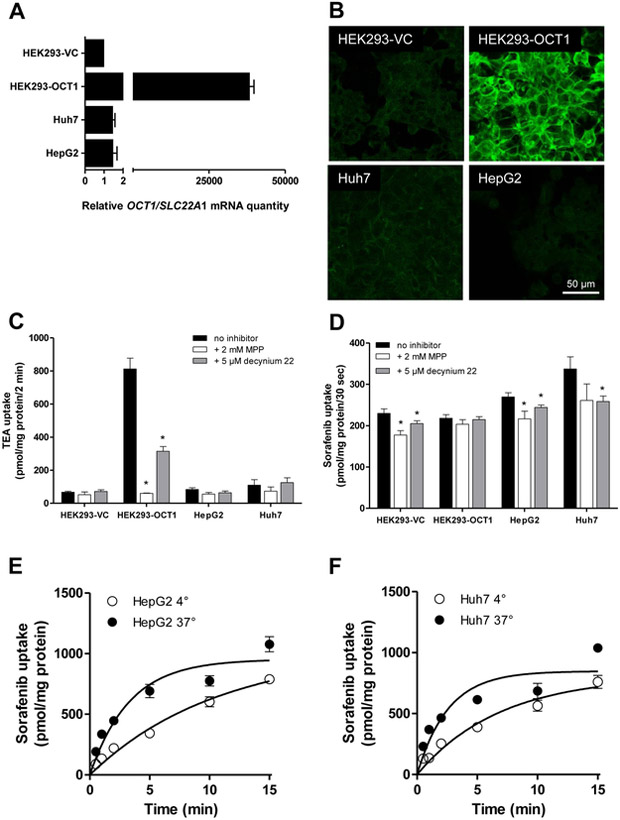
In order to evaluate the potential contribution of OCT1 to sorafenib uptake into cancer cells, we first determined the OCT1 mRNA level in the commonly-used liver cancer cell lines, HepG2 and Huh7. While HEK293-OCT1 cells showed a high level of OCT1 mRNA expression, levels were approximately 35,000-fold lower in the liver cancer cell lines (Fig. 3A). This observation was confirmed by the lack of substantial OCT1 protein expression in both the HepG2 and Huh7 cell lines compared with HEK293-OCT1 cells, as determined by immuno-staining and confocal laser scanning microscopy (Fig. 3B). Compared to HEK293-OCT1 cells, the liver cancer cells did not take up appreciable levels of the OCT1 substrate, TEA (Fig. 3C). The uptake of sorafenib in HepG2 and Huh7 cells was comparable to that observed in HEK293-OCT1 and HEK293-VC cells although this process was sensitive to inhibition by decynium22 (Fig. 3D). As observed in HEK293 cells, the intracellular accumulation of sorafenib in HepG2 and Huh7 cells was saturable, increased over time, and was found to be temperature-dependent even after a 30-s incubation period (Fig. 3E-F). This observation is consistent with previously published data for sorafenib uptake into primary hepatocytes.14 However, since HepG2 and Huh7 do not express OCT1, the time- and temperature-dependence of sorafenib uptake in the liver cells is likely mediated by other, currently unknown transporters.
Figure 3.
Relative expression and function of OCT1 in liver cancer cells. (A) Relative expression of OCT1 (SLC22A1) mRNA in HEK293 cells expressing an empty pcDNA3 vector (VC; set to 1) or human OCT1, and the human liver cancer cell line Huh7 and HepG2. (B) Confocal laser scanning micrographs of OCT1 in the same cell lines. Bars: 40 μm. (C, D) Uptake of TEA (100 μM; C) TEA and sorafenib (0.2 μM; D) in the same cell lines in the presence or absence of the OCT1 inhibitors MPP or decynium22 after 10-min incubations. Data are presented as mean (bars) and SD (error bars) of 3 observations. The star (*) represents statistical significance (P<0.05) vs cells in the absence of inhibitors. (E, F) Time- and temperature dependence of sorafenib transport in the human liver cancer cells HepG2 (E) and Huh7 (F). Data are presented as mean (symbols) and SD (error bars) of 3 observations.
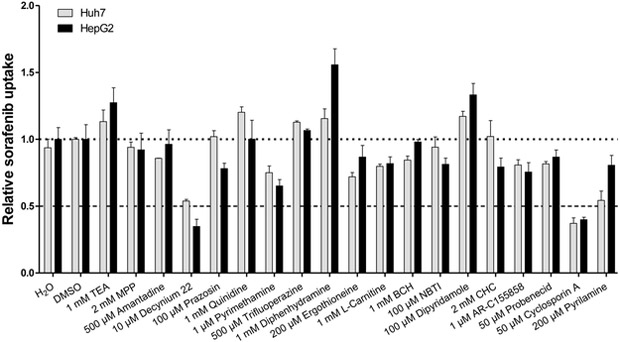
To shed light on the identity of this unknown transport mechanism, we next evaluated the uptake of sorafenib in liver cancer cell lines in the presence of inhibitors of organic cation transporters (OCTs), novel organic cation transporters (OCTNs), multidrug and toxin extrusion proteins (MATEs), amino-acid transporters, monocarboxylate transporters (MCTs), nucleoside transporters, organic anion transporters (OATs) and organic anion transporting polypeptides (OATPs). The transport inhibitors are listed in Table S2. Using an arbitrary cutoff of 50% inhibition, we found that decynium22 and cyclosporin A were the only compounds that inhibited sorafenib uptake (Fig. 4).
Figure 4.
Influence of transporter inhibitors on sorafenib uptake in liver cancer cells. HepG2 or Huh7 cells were co-incubated with designated amount of inhibitors and sorafenib (0.2 μM) for 10 min. Uptake values were normalized to that observed for the DMSO group. Data are presented as mean (bars) and SD (error bars) of 3 observations. The star (*) represents statistical significance (P<0.05) vs cells in the absence of inhibitors.
Membrane binding of sorafenib
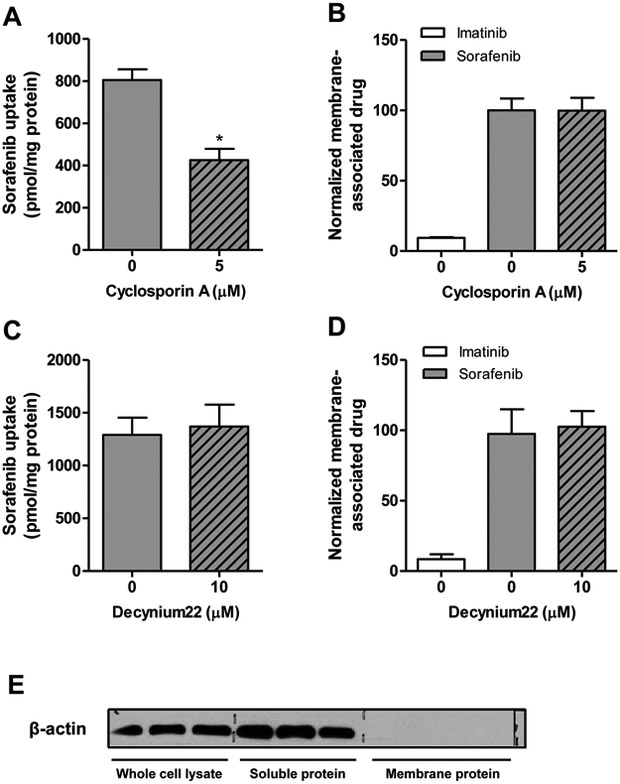
One concern of cellular in vitro experiments with sorafenib is that the compound can bind non-specifically, resulting in artificially high uptake values in certain cell types and potentially leading to false positive results. Consistent with the hydrophobic nature of sorafenib, it was previously reported that sorafenib inserts into the lipid-water interface of the cell membrane bilayer.25 Based on this observation, we next evaluated whether the observed inhibitory effects of decynium22 and cyclosporin A can be explained by the interference with non-specific membrane binding of sorafenib. In HeLa cells, sorafenib showed a high level of apparent cellular uptake (>800 pmol/mg protein) after a 10-min incubation, and sorafenib uptake in HeLa cells was also inhibited by co-incubation with cyclosporin A but not decynium22 (Fig. 5A-D). We found that neither inhibitor interfered with the non-specific membrane binding of sorafenib, suggesting that these agents can directly block sorafenib uptake. The purity of membrane fraction isolated was confirmed by the absence of beta-actin through western blot (Fig. 5E).
Figure 5.
Membrane binding of sorafenib. (A, B) Sorafenib (0.2 μM) uptake in HeLa cells in the presence and absence of transporter inhibitor cyclosporin A (A) and membrane-associated-drug (B) with or without cycloporin A after 10 min incubations. (C, D) Sorafenib uptake (C) and levels of membrane-associated drug (D) were also evaluated in the presence or absence of decynium22. Imatinib was used as a negative control compound. Data are presented as mean (bars) and SD (error bars) of 3 observations. (E) Expression of β-actin expression in whole cell lysate without membrane isolation, and in soluble protein and membrane protein fractions after membrane isolation as determined by immunoblotting.
Intracellular target engagement of p38α by sorafenib
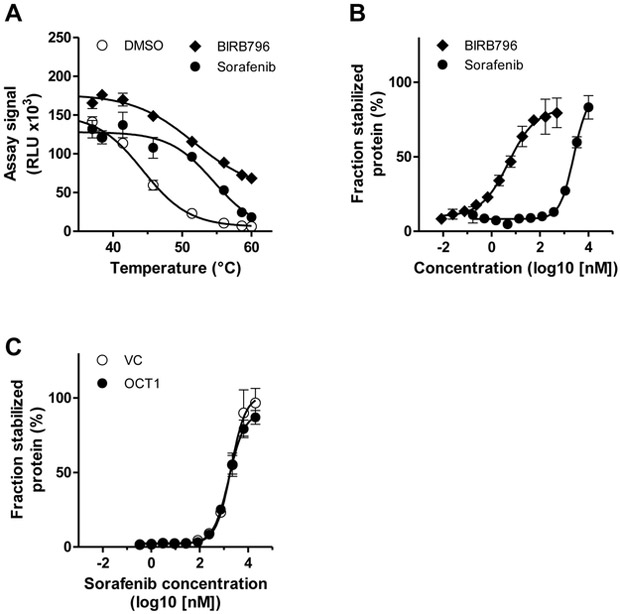
To further support that sorafenib is taken up into cells rather than binding to the membrane, we established a cellular thermal shift assay (CETSA) for p38α, with which ligand-induced changes of p38α can be monitored in live cells. Sorafenib is not only a B-Raf kinase inhibitor (in vitro IC50 value of 25 nM), but also inhibits p38α with a similar IC50 value of 38 nM.26 In initial experiments, we determined the thermostability of p38α in the absence or presence of BIRB796, a known inhibitor of p38α,27 and sorafenib. Apparent aggregation temperatures increased from 44.3 °C (DMSO) to 51.7 °C and 54.5 °C by incubation of live HEK293 cells with BIRB796 and sorafenib, respectively (Fig. 6A). Initial experiments also showed a concentration-dependent increase in thermostability resulting in characteristic fingerprints of the p38α target engagement for both compounds (Fig. 6B). These results indicated that BIRB796 and sorafenib both interacted with intracellular p38α leading to its stabilization in intact cells. Finally, we compared engagement of p38α by sorafenib in HEK-OCT1 and HEK-VC cells and observed similar p38α engagement levels for both cell lines (Fig. 6C) supporting the notion that cellular sorafenib uptake is independent of OCT1.
Figure 6.
Cellular thermal shift assays with intact HEK293 cells. (A) Melting curves of p38α-ePL measured after 60 min of incubation of cells with DMSO (1%), the positive control inhibitor BIRB796 (5 nM) or sorafenib (5 μM). Data are presented as mean (symbols) and SD (error bars) of 2 determinations. (B) Isothermal inhibitor dose-response curves at 51.4 °C after incubation of cells expressing p38α-ePL for 60 min with BIRB796 and sorafenib. Data are presented as mean (symbols) and SD (error bars) of 2 determinations. (C) Isothermal inhibitor dose-response curves at 51.4 °C after incubation of HEK-VC and HEK-OCT1 cells expressing p38α-ePL with sorafenib for 10 min. Data are presented as mean (symbols) and SD (error bars) of 6 determinations. RLU, relative light units.
Discussion
The impact of the uptake transporter OCT1 in determining response to sorafenib treatment is a topic of ongoing debate, and inherited genetic variation in OCT1,13 differential effects of tumoral mRNA levels13,19 or protein levels,28 and variability in cellular uptake of sorafenib29,30 have all been speculated to contribute to treatment outcome in some but not all studies. However, the pertinent question as to whether OCT1 can actually transport sorafenib remains unanswered. The current study therefore sought to systematically investigate the role of OCT1 in sorafenib transport by using complementary experimental strategies to ultimately demonstrate that (i) sorafenib is not transported by OCT1 in vitro or in vivo in mice; (ii) commonly employed liver cancer cell lines appear to lack functional expression of the OCT1 protein; and (iii) extensive cellular uptake of sorafenib into cancer cell lines occurs via OCT1-independent transport mechanisms.
The notion that OCT1 may act as an important uptake transporter for sorafenib was first derived from in vitro studies showing that certain agents known to inhibit OCT1, including quinine, also inhibited sorafenib uptake into hepatocellular carcinoma and cholangiocarcinoma cells.13 Because these kind of experiments do not definitively demonstrate that sorafenib is actually transported by OCT1, we used well-established OCT1-expressing cell models to study OCT1-mediated transport.20 Our finding that OCT1 overexpression in HEK293 cells or oocytes does not promote sorafenib uptake despite a significant uptake of an OCT1 probe substrate confirms a previous report,31 and indicates that OCT1 is unlikely to be involved in sorafenib transport. Moreover, although our CETSA experiments confirmed cellular uptake of sorafenib, the intracellular target engagement of p38α was independent of OCT1. These observations were further corroborated by our studies with mammalian cells engineered to overexpress the mouse ortholog transporter mOCT1. As we recently demonstrated,20 and also validated in the present study, these OCT1 transfectants show substantial uptake of known OCT1 substrates such as TEA and importantly, express high levels of OCT1 protein. Moreover, uptake of the probe substrate TEA is almost completely abolished by the established OCT1 inhibitor decynium22. Yet, sorafenib uptake into the OCT1-transfected HEK293 cells did not differ from that into vector-transfected control cells and could be inhibited by decynium22 in the absence of OCT1. These results demonstrate that substantial overexpression of functional OCT1 protein does not result in sorafenib uptake.
Our data seem at odds with two previous studies showing increases in the cellular accumulation of sorafenib in OCT1-transfected Xenopus laevis oocytes and CHO cells, respectively. While Herraez et al.13 discuss that their results support the conclusion that decreased OCT1 expression may affect the ability of sorafenib to reach active intracellular concentrations in tumors, Swift et al.14 rather conclude that sorafenib transport is predominantly occurring in a transporter-independent manner. Of note, in both studies OCT1 expression was only confirmed at the mRNA level, sorafenib uptake was not consistently measured in the presence of OCT1 inhibitors, and experiments did not always include proper controls, including cells transfected with the empty vector. It is therefore ambiguous whether the marginal increases observed for the intracellular accumulation of sorafenib are actually due to OCT1 function or to differential expression of other transporters of putative relevance to sorafenib in OCT1-transfected cells compared with the vector-transfected cells.
It was previously suggested that the ability of decynium22 to inhibit sorafenib uptake in suspended human hepatocytes is likely due to OCT1.14 Interestingly, in that study, sorafenib uptake in non-transfected mock CHO cells was also partially inhibited by MPP, a known OCT1 inhibitor, suggesting that other MPP-sensitive endogenous transport proteins in the CHO cell line are involved in sorafenib uptake. This observation is consistent with our finding that decynium22 inhibited sorafenib uptake in mock HEK293 cells in the absence of OCT1. The unknown transport protein that is sensitive to MPP or decynium22 in mock cells may also be present in human hepatocytes. These findings further highlight the general limitation when using inhibitors in that they may not be as “specific” as expected and that inhibition of presently unidentified transporters may occur. Ideally, uptake studies are performed in a cell line with negligible background activity, which can then be used for overexpression. However, this is the case for neither Xenopus laevis oocytes nor for CHO or HEK293 cell lines that are all showing high sorafenib uptake already in the absence of OCT1. It is therefore necessary to use different complementary approaches, as we did in our present work, to assess the role of a given transporter in substrate uptake.
In addition to the studies with OCT1-expressing cells, we also determined the effect of OCT1 deficiency on sorafenib pharmacokinetics in vivo using knockout mice. OCT1 is highly expressed in human and murine liver and a major determinant of hepatic accumulation of certain organic cations. However, hepatic accumulation of sorafenib was occurring independently of OCT1, further supporting the notion that OCT1 does not mediate sorafenib transport. This thesis was further corroborated by the demonstration that sorafenib is taken up extensively into liver cancer cell lines although OCT1 is barely detectable at the mRNA and protein level. This finding is in agreement with previous studies in which Huh7 cells failed to display functional activity of OCT1,32 and where basal mRNA levels of OCT1 were found to be low in HepG2 cells33 and hepatocellular carcinoma samples compared with corresponding non-tumor tissue.34,35 Moreover, it has been suggested that, when expression of membrane transporter proteins was determined, there were no significant differences in expression levels of OCT1 between sorafenib-resistant hepatocellular carcinoma clones and parent cells.36
To gain further insight into alternative transporter proteins involved in the cellular uptake of sorafenib, several additional accumulation studies were performed in HepG2 and Huh7 cells. In line with studies reported by Swift et al.,14 we found that sorafenib uptake was relatively fast, temperature-dependent, and saturable, suggesting carrier-mediated transport. Although a high degree of drug uptake was observed at a reduced temperature of 4 ˚C, this does not necessarily reflect a passive mechanism. Indeed, even at ice-cold temperatures (0.5-0.7 °C), the function of uptake transporters such as ENT1 and GLUT1 can be substantially retained in isolated membrane vesicles, suggesting that a low-temperature tolerance might be operational for some transporter proteins.37 Alternatively, our findings confirm an earlier observation25 that sorafenib can bind extensively (up to 70% of total cell-associated drug) and non-specifically to cell membranes.
This study also suggested that the intracellular uptake of sorafenib might be associated with an organic anion transporting polypeptide, given that drug uptake, but not the non-specific membrane binding, into HepG2 and Huh7 cells was sensitive to cyclosporin A. This compound is a known inhibitor of several known organic anion transporting polypeptides, including OATP1B1, OATP1B3 and OATP2B1,38 and of these uptake transporters, OATP2B1 is functionally expressed in both HepG233 and Huh732 cells. However, we did not detect a different sorafenib uptake by HEK-VC cells or HEK cells overexpressing mOATP2B1 or hOATP2B1 or an effect of OATP2B1 deficiency in mice on sorafenib pharmacokinetics (Figure S4) indicating the involvement of other transporters in the HepG2 and Huh7 cells, which may be inhibited by cyclosporin A. These might be OATP1B1 in the HepG2 cells33 or OATP1B3 in the Huh7 cells,32 both of which are also expressed in hepatocytes.38 While the role of OATP1B1 and OATP1B3 in uptake of sorafenib is incompletely defined11,14,31 both are certainly involved in the hepatocellular uptake of sorafenib glucuronide.11
The apparent discrepancy between our current findings and the previously reported associations of OCT1 mRNA or membrane protein levels with clinical outcome of systemic therapy with sorafenib warrants further investigation. We therefore extended our previous studies on OCT1 expression and regulation in HCC34 using the publicly available TCGA data set. Our analysis confirmed previous findings34,39 that OCT1 mRNA expression is significantly downregulated in HCC (Figure. S5A) and that the large interindividual variability of OCT1 mRNA levels in HCC is mostly explained by methylation of specific CpG sites, i.e. cg13466809 and cg27292431 in the 5’-UTR and exon 1 of the OCT1 gene, respectively (Fig. S5B-C), which was also confirmed in a recent independent analysis of the TCGA data set.40 In line with the strong decrease of OCT1 mRNA levels, a concomitant decrease of OCT1 protein in HCC tissue in comparison to non-tumor tissue was consistently observed in several studies by antibody-based techniques.28,34,35,39 In a recent targeted proteomics-based analysis, reduction of OCT1 protein in HCC tissue was quantified to be >65% compared with adjacent non-tumor tissue.41 This indicates that OCT1 is not a major factor of drug disposition in HCC.
It is possible that the clinical correlations of OCT1 mRNA or protein levels are spurious or that OCT1 expression levels merely represent a prognostic rather than a sorafenib-specific predictive biomarker of efficacy in HCC.19,28 Alternatively, it is possible that OCT1 expression serves as a composite surrogate for the expression and function of several transporters that are relevant to the intracellular uptake and retention of sorafenib. In similar fashion, genetic variants in the OCT1 gene need not be causative for poor response to sorafenib-based therapy but could rather denote linkage to variants in other genes of relevance to mechanisms of sorafenib action. Based on our present findings that cellular uptake of sorafenib and its active metabolite sorafenib-N-oxide is independent of OCT1, the reliability of OCT1 as a biomarker for sorafenib response in HCC appears questionable. We previously obtained a similar result for the tyrosine kinase inhibitor imatinib and OCT1, which had been suggested as biomarker for therapy resistance in CML, but which does not transport imatinib either.20
Conclusion
This study indicates that sorafenib is not a transported substrate of OCT1 and that this transporter by itself is unlikely to contribute to the disposition and activity profiles of sorafenib in HCC. Further study is warranted to determine the contribution of other as yet unknown transporters to the pharmacokinetics and pharmacodynamics of sorafenib.
Methods
See Supplementary Material for details on chemicals, transport studies using OCT1-expressing oocytes and statistical analysis.
Mammalian cell culture and immunolabeling of cells
Generation of the vector with the SLC22A1 reference sequence NM_003057 and of OCT1-overexpressing human embryonic kidney (HEK293) cells and the corresponding cells transfected with an empty vector as well as cultivation of the cells has been previously described.20,42 HepG2 and HeLa cell lines were purchased from ATCC and the Huh7 cell line was purchased from the Japanese Collection of Research Bioresources (Osaka, Japan). For further details see Supplementary Material.
Transport and inhibition studies
Uptake and inhibition experiments with probe substrates, sorafenib and sorafenib-N-oxide into cells was performed as described previously.20,42 Uptake studies were generally done with sorafenib concentrations of 0.2 μM in the presence or absence of inhibitors for a period of 10 min, unless stated otherwise. In some cases, sorafenib uptake was studied at 7.5 μM in the presence of 10% FCS, which is equivalent to a free sorafenib concentration of 0.2 μM.6 Drug uptake was stopped by washing the cells with ice-cold buffer and lysis with 0.2% SDS. Intracellular drug accumulation was quantified by liquid scintillation counting, and results were normalized to total protein concentration as measured using a Pierce BCA Protein Assay Kit (ThermoFisher Scientific).
Membrane binding studies
HeLa cells were plated into a 6-well plate 24 h before experiment. Upon confluence, the cells were washed with phosphate-buffered (PBS), and uptake was initiated by adding 1 ml of 0.2 μM sorafenib in the presence or absence of inhibitors. The water-soluble kinase inhibitor imatinib was used as a control compound. After 10 min, the uptake assay was terminated by washing the cells 3 times with ice-cold PBS; cells were then dissociated with 200 μl TrypLE (ThermoFisher Scientific) for 3-5 min and suspended with 800 μl ice-cold washing buffer (ProteoExtract® Native Membrane Protein Extraction Kit, EMD Millipore). The suspended cells were transferred to a 1.5 ml Eppendorf tube individually. Three of them were assigned as control that, after 2 times washing with washing buffer, were lysed with 400 μl of 1N NaOH overnight on an orbital shaker and neutralized with 200 μl of 2N HCl (whole cell lysate). Membrane fractions of the other three were isolated following the manufactory protocol, which is based on a differential extraction procedure under non-denaturing conditions. Radioactivity was measured through a liquid scintillation analyzer (Tri-Carb 4810TR, PerkinElmer). Immunoblotting for β-actin was performed as described.43
Intracellular target engagement of p38α kinase
Intracellular engagement of p38α (MAPK14) kinase was measured using the InCell Pulse Target Engagement Assay Kit (Eurofins DiscoverX). This assay is based on the enzyme fragment complementation technology44 and monitors ligand-induced changes in thermal stability of proteins in live cells, a method recently introduced as cellular thermal shift assay (CETSA).45
An expression vector encoding p38α with a C-terminal fusion of the enhanced ProLabel® (ePL) peptide was generated. For this, p38α was amplified from p38α-encoding vector SC126560 (Origene) using forward primer 5’-ACGCGTCACCATGTCTCAGGAGA GGCCCACGTTC and reverse primer 5’-CCCGGGTAGGACTCCATCTCTTC TTGGCAAGGG, which introduced a Kozak sequence at the 5’ end upstream of the Start codon and removed the Stop codon at the 3’ end of p38α, respectively. The PCR fragment was subcloned into pCR2.1-TOPO (ThermoFisher Scientific) and then into pICP-ePL-C (Eurofins DiscoverX) using restriction sites MluI and SmaI resulting in vector pICP-ePL-C-p38α, which encodes fusion protein p38α-ePL.
HEK293 cells were transiently transfected with vector pICP-ePL-C-p38α and Metafectene Pro (Biontex) and used 48 hours after start of transfection. For generating melting curves, pulse denaturation (3 min) was performed at 8 different temperatures within a range of 37 °C to 60 °C using a PCR cycler (C1000 Touch Thermal Cycler, BioRad). For generating isothermal inhibitor dose-response curves, cells were incubated with different compound concentrations and pulse denaturation (3 min) was carried out at 51.4 °C. BIRB796 (500 nM) and DMSO were included as positive and negative controls, respectively. All reactions were incubated for 60 min with the detection reagent and luminescence signals were subsequently measured for 0.5 sec/well using a plate reader (EnSpire, PerkinElmer). The fraction of stabilized protein was calculated as described46 as [((signal – negative control)/positive control)*100].
Pharmacokinetics experiments
Female Oct1-deficient mice (Oct1/2(−/−) knockout mice, Taconic)20 and wild-type littermates, all on an FVB background, between 9-14 weeks old were bred in-house and used in all experiments. Experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institution of Health and were approved by the Institutional Animal Care and Use Committee of the Ohio State University. Animals were housed in a temperature-controlled environment with a 12-hour light cycle and given a standard diet and water. Pharmacokinetic studies were performed as described previously.5,11 Briefly, sorafenib (10 mg/kg) was formulated in 50% Cremophor EL and 50% ethanol, then diluted 1:4 with deionized water and given orally after 3-hour fasting. Serial blood samples were taken from individual mice at 0.25, 0.5 and 1h from submandibular vein, at 2 and 4 h from the retro-orbital sinus, and at 7.5 h by a terminal cardiac puncture, and livers were collected at the final time point. All blood samples were centrifuged at 3,000×g for 5 min, and tissues were homogenized in 10 volumes (w/v) of water and stored at −80°C until analysis by a validated method based on high-performance liquid chromatography and tandem mass spectrometry detection, as described.12 Pharmacokinetic parameters were calculated using non-compartmental analysis with the software package WinNonlin 7.0 (Phoenix).
Analysis of OCT1 gene methylation and expression in a hepatocellular carcinoma patient cohort
Transcriptome profiling data (“HTSeq - FPKM-UQ”) of The Cancer Genome Atlas (TCGA) liver cohort (“LIHC”; 371 cases, 50 normal) were obtained from the Genomic Data Commons Portal (https://gdc-portal.nci.nih.gov/) on December 9, 2016. Transcriptome data were transformed to log2(FPKM+1)-values for all analyses in this study. DNA methylation data (Illumina Infinium HumanMethylation 450K BeadChip; level 3 data) was downloaded from https://tcga.xenahubs.net on February 16, 2017.
Supplementary Material
Figure S1: Time dependence of sorafenib transport in HEK293 cells overexpressing human OCT2 or human OCT3
Figure S2: Evaluation of mouse OCT1 and mouse OCT2 as transporters of sorafenib
Figure S3: Influence of OCT1-deficiency on the plasma pharmacokinetics of sorafenib-glucuronide
Figure S4: Evaluation of mouse Oatp2b1 and human OATP2B1 as transporters of sorafenib
Figure S5: Expression and DNA methylation of OCT1/SLC22A1 in HCC and non-tumor tissue of the TCGA cohort
Table S1: Sorafenib pharmacokinetic parameters in wild-type and OCT1(−/−) mice after a single oral dose of 10 mg/kg
Table S2: Drug transporter inhibitors for chemical screening to characterize sorafenib uptake by HepG2 and Huh7 cells
Study Highlights.
What is the current knowledge on the subject?
Sorafenib is approved for systemic treatment of advanced hepatocellular carcinoma (HCC), yet therapeutic benefits are modest. Organic cation transporter 1 (OCT1) has been suggested as biomarker of sorafenib response in HCC although data are conflicting whether OCT1 mediates cellular sorafenib uptake.
What question did this study address?
This study addresses for the first time the critical question whether OCT1 transports sorafenib. A combination of complementary in vitro and in vivo methods involving heterologous expression models overexpressing OCT1 (Xenopus laevis oocytes, HEK293 cells), transcriptomic and proteomic profiling of liver cancer cell lines, and in vivo pharmacokinetic studies in OCT1-deficient mice was used to unambiguously define the contribution of OCT1 to the transport of sorafenib.
What does this study add to our knowledge?
These data demonstrate that cellular sorafenib uptake is independent of OCT1 but rather depends on alternative mechanisms.
How might this change clinical pharmacology or translational science?
These results show that OCT1 is not relevant for sorafenib transport and should not be clinically implemented as biomarker of sorafenib response in HCC.
Acknowledgements
We gratefully acknowledge the excellent technical assistance of Silvia Hübner and Alice Gibson. The results shown here are in part based upon data generated by the TCGA Research Network. We would like to thank The Cancer Genome Atlas initiative, all tissue donors, and investigators who contributed to the acquisition and analyses of the samples used in our study. Information about TCGA and the investigators and institutions who constitute the TCGA research network can be found at http://cancergenome.nih.gov/.
Financial support
This work was in part supported by the Robert-Bosch Foundation, Stuttgart, Germany, the Interfaculty Centre for Pharmacogenomics and Pharma Research (ICEPHA) Grant Tübingen–Stuttgart, Germany, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy - EXC 2180 – 390900677, National Institutes of Health (NIH) grants R01CA138744 and R01CA215802, and Pelotonia, Ohio, USA.
Footnotes
Conflict of interest: The authors declared no competing interests for this work.
References
- 1.El-Serag HB Hepatocellular carcinoma. N.Engl.J.Med. 365, 1118–1127 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM et al. Sorafenib in advanced hepatocellular carcinoma. N.Engl.J.Med. 359, 378–390 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Cheng A-L, Meinhardt G, Nakajima K, de Sanctis Y & Llovet J Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma. Analysis of two phase III studies. J.Hepatol. 67, 999–1008 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Liu L et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Vasilyeva A et al. Hepatocellular shuttling and recirculation of sorafenib-glucuronide is dependent on Abcc2, Abcc3, and Oatp1a/1b. Cancer Res. 75, 2729–2736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker SD & Hu S Pharmacokinetic considerations for new targeted therapies. Clin.Pharmacol.Ther. 85, 208–211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drenberg CD, Baker SD & Sparreboom A Integrating clinical pharmacology concepts in individualized therapy with tyrosine kinase inhibitors. Clin.Pharmacol.Ther. 93, 215–219 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Strumberg D et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12, 426–437 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Boudou-Rouquette P et al. Variability of sorafenib toxicity and exposure over time. A pharmacokinetic/pharmacodynamic analysis. Oncologist 17, 1204–1212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M & Sundaresan P Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother.Pharmacol. 57, 685–692 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman EI et al. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin.Cancer Res. 19, 1458–1466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bins S et al. Influence of OATP1B1 function on the disposition of sorafenib-β-D-glucuronide. Clin.Transl.Sci. 10, 271–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herraez E et al. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 58, 1065–1073 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Swift B et al. Sorafenib hepatobiliary disposition: mechanisms of hepatic uptake and disposition of generated metabolites. Drug Metab.Dispos. 41, 1179–1186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minematsu T & Giacomini KM Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol.Cancer Ther. 10, 531–539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston RA, Rawling T, Chan T, Zhou F & Murray M Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug Metab.Dispos. 42, 1851–1857 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Nies AT, Herrmann E, Brom M & Keppler D Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1). Naunyn Schmiedebergs Arch.Pharmacol. 376, 449–461 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Nies AT et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50, 1227–1240 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Grimm D et al. Organic Cation Transporter 1 (OCT1) mRNA expression in hepatocellular carcinoma as a biomarker for sorafenib treatment. BMC Cancer 16, 94–2150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nies AT et al. Cellular uptake of imatinib into leukemic cells is independent of human organic cation transporter 1 (OCT1). Clin.Cancer Res. 20, 985–994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman EI et al. Ontogeny and sorafenib metabolism. Clin.Cancer Res. 18, 5788–5795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba H et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J.Clin.Oncol. 29, 3293–3300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nies AT, Koepsell H, Damme K, Schwab M, Fromm MF & Kim RB Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb.Exp.Pharmacol. 201, 105–167 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Margaillan G et al. Quantitative profiling of human renal UDP-glucuronosyltransferases and glucuronidation activity: a comparison of normal and tumoral kidney tissues. Drug Metab.Dispos. 43, 611–619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haralampiev I et al. The interaction of sorafenib and regorafenib with membranes is modulated by their lipid composition. Biochim.Biophys.Acta 1858, 2871–2881 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm S et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat.Rev.Drug Discov. 5, 835–844 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Pargellis C et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat.Struct.Biol. 9, 268–272 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Geier A et al. The lack of the organic cation transporter OCT1 at the plasma membrane of tumor cells precludes a positive response to sorafenib in patients with hepatocellular carcinoma. Oncotarget 8, 15846–15857 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano E, Briz O, Macias RIR, Serrano MA, Marin JJG & Herraez E Genetic heterogeneity of SLC22 family of transporters in drug disposition. J.Pers.Med. 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macias RIR et al. Role of drug transporters in the sensitivity of acute myeloid leukemia to sorafenib. Oncotarget 9, 28474–28485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu S et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin.Cancer Res. 15, 6062–6069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jouan E, Le Vee M, Denizot C, Parmentier Y & Fardel O Drug transporter expression and activity in human hepatoma HuH-7 cells. Pharmaceutics 9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues AC, Curi R, Genvigir FD, Hirata MH & Hirata RD The expression of efflux and uptake transporters are regulated by statins in Caco-2 and HepG2 cells. Acta Pharmacol.Sin. 30, 956–964 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaeffeler E et al. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 3, 82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namisaki T et al. Differential expression of drug uptake and efflux transporters in Japanese patients with hepatocellular carcinoma. Drug Metab.Dispos. 42, 2033–2040 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Tomonari T et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget 7, 7207–7215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takano M, Kimura E, Suzuki S, Nagai J & Yumoto R Human erythrocyte nucleoside transporter ENT1 functions at ice-cold temperatures. Drug Metab.Pharmacokinet. 25, 351–360 (2010). [DOI] [PubMed] [Google Scholar]
- 38.König J, Müller F & Fromm MF Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol.Rev. 65, 944–966 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Heise M et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 12, 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Abdulla R et al. Epigenetic events involved in OCT1-dependent impaired response of hepatocellular carcinoma to sorafenib. Br.J.Pharmacol. 176, 787–800 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billington S et al. Transporter expression in noncancerous and cancerous liver tissue from donors with hepatocellular carcinoma and chronic hepatitis C infection quantified by LC-MS/MS proteomics. Drug Metab.Dispos. 46, 189–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M & Schwab M Proton pump inhibitors inhibit metformin uptake by organic cation uptake transporters (OCTs). PLoS One 6, e22163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprowl JA et al. A phosphotyrosine switch regulates organic cation transporters. Nat.Commun. 7, 10880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson KR & Eglen RM Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev.Technol. 5, 137–144 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Martinez Molina D et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Mateus A et al. Prediction of intracellular exposure bridges the gap between target- and cell-based drug discovery. Proc.Natl.Acad.Sci. U S A 114, E6231–E6239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Time dependence of sorafenib transport in HEK293 cells overexpressing human OCT2 or human OCT3
Figure S2: Evaluation of mouse OCT1 and mouse OCT2 as transporters of sorafenib
Figure S3: Influence of OCT1-deficiency on the plasma pharmacokinetics of sorafenib-glucuronide
Figure S4: Evaluation of mouse Oatp2b1 and human OATP2B1 as transporters of sorafenib
Figure S5: Expression and DNA methylation of OCT1/SLC22A1 in HCC and non-tumor tissue of the TCGA cohort
Table S1: Sorafenib pharmacokinetic parameters in wild-type and OCT1(−/−) mice after a single oral dose of 10 mg/kg
Table S2: Drug transporter inhibitors for chemical screening to characterize sorafenib uptake by HepG2 and Huh7 cells