Abstract
Ecological factors, host characteristics and/or interactions among microbes may all shape the occurrence of microbes and the structure of microbial communities within organisms. In the past, disentangling these factors and determining their relative importance in shaping within-host microbiota communities has been hampered by analytical limitations to account for (dis)similar environmental preferences (‘environmental filtering’). Here we used a joint species distribution modelling (JSDM) approach to characterize the bacterial microbiota of one of the most important disease vectors in Europe, the sheep tick Ixodes ricinus, along ecological gradients in the Swiss Alps. Although our study captured extensive environmental variation along elevational clines, the explanatory power of such large-scale ecological factors was comparably weak, suggesting that tick-specific traits and behaviours, microhabitat and -climate experienced by ticks, and interactions among microbes play an important role in shaping tick microbial communities. Indeed, when accounting for shared environmental preferences, evidence for significant patterns of positive or negative co-occurrence among microbes was found, which is indicative of competition or facilitation processes. Signals of facilitation were observed primarily among human pathogens, leading to co-infection within ticks, whereas signals of competition were observed between the tick endosymbiont Spiroplasma and human pathogens. These findings highlight the important role of small-scale ecological variation and microbe-microbe interactions in shaping tick microbial communities and the dynamics of tick-borne disease.
Keywords: Tick-borne pathogens, Species distribution modelling, Community composition, Borrelia burgdorferi, Lyme disease
Introduction
Microbial communities within organisms consist of symbionts, commensals, mutualists and pathogens that co-occur simultaneously and potentially influence each other (Petney & Andrews, 1998; Rigaud, Perrot-Minnot & Brown, 2010; Sofonea, Alizon & Michalakis, 2015). These microbial communities may be shaped by a range of factors and processes, including the environment, host and microbe genetics and the occurrence and abundance of other microbial species (Adair & Douglas, 2017). For example, certain microbial species might tolerate only specific abiotic conditions, which makes it more likely that species with similar requirements co-occur within a host (‘environmental filtering’, Dallas & Presley, 2014). Similarly, the host’s immune system can influence colonization success of microbes (Hawley & Altizer, 2011), with cross-immunity preventing the colonization of different microbes with similar antigenic properties (Durand et al., 2015). Furthermore, mutualistic interactions between hosts and microbes can influence the structure of bacterial communities within host individuals (Chu & Mazmanian, 2013; Lee et al., 2013). Finally, direct interactions among microbes might affect colonization, or replication success after colonization, through competition or facilitation processes. Competition may occur when different microbes use the same, limited resources within a host (Lello et al., 2004), whereas facilitation may occur directly through the production of public goods (West & Buckling, 2003) or indirectly through the modification of the host’s physiology (Abraham et al., 2017) or immune defense (Rodríguez et al., 1999).
Ixodes ricinus is the most common tick species in Europe and an important vector for a range of human, domestic animal and wildlife pathogens (Medlock et al., 2013). Its distribution and abundance are strongly influenced by environmental conditions, in particular temperature and humidity (Cortinas et al., 2002; Gatewood et al., 2009). Previous studies that characterized the bacterial community composition of I. ricinus ticks have found mostly environmental and free-living bacteria but also several endosymbionts and human, domestic animal or wildlife pathogens, including Borrelia (Mannelli et al., 2012), Rickettsia (Venclikova et al., 2014), Anaplasma (Jahfari et al., 2014) and Candidatus Neoehrlichia (Kawahara et al., 2004).
Differences in the bacterial community structure and composition of ticks across habitats (Estrada-Peña et al., 2018), geographical sites (Carpi et al., 2011), and tick life stages and sexes (Carpi et al., 2011; Vayssier-Taussat et al., 2013) have been documented. Large-scale biotic or abiotic factors such as vegetation structure, elevation, temperature or rainfall may influence tick microbial communities directly, or indirectly through effects on tick physiology or activity patterns (Van Treuren et al., 2015) or via influencing the distribution and abundance of tick hosts species (Randolph et al., 1999; MacDonald et al., 2017). Small-scale and/or tick-specific effects on microbial communities may be explained by microhabitat or microclimatic conditions experienced by individual ticks (Gern, Morán Cadenas & Burri, 2008), individual tick behavior or genetics (Hawlena et al., 2013), direct biotic interactions among microbes (Moutailler et al., 2016) or parallel acquisition from a host during a bloodmeal (Andersson, Scherman & Råberg, 2014; Belli et al., 2017; Swei & Kwan, 2017).
Currently, the relative importance of these factors in shaping tick microbial communities is not well understood, which hampers progress in our understanding of the processes shaping microbial communities in nature and predicting the occurrence of specific microbes (e.g., human pathogens). Elevational gradients are excellently suited to quantify the importance of large-scale ecological variation in shaping tick bacterial microbiota because they cover a large range of environmental conditions within a small geographical area. Furthermore, including replicated transects along gradients allow us to quantify the robustness of ecological associations within sites and along elevational clines on tick microbial communities.
Ixodes ticks are commonly found to be co-infected with several (human, domestic animal and/or wildlife) pathogens (Andersson, Scherman & Råberg, 2013; Michelet et al., 2014; Diuk-Wasser, Vannier & Krause, 2016; Moutailler et al., 2016). Currently, it is unknown whether these co-infection patterns are caused by similar environmental preferences of pathogens, parallel acquisition from host communities or direct microbe-microbe interactions within ticks. Yet, previous studies suggest that the latter process, (i.e., facilitation and competition processes among microbes) may play a role in shaping microbial communities (Haine, 2008; Bonnet et al., 2017). For example, it has been found that pathogenic Rickettsia species prevent co-infection with other Rickettsia species in Dermacentor variabilis ticks (Macaluso et al., 2002), whereas the presence of Francisella sp. endosymbionts increases the colonization success of pathogenic Francisella novicida in D. andersoni ticks (Gall et al., 2016). Facilitation has also been suggested to promote co-infection with different Borrelia afzelii strains in Ixodes ricinus ticks (Andersson, Scherman & Råberg, 2013). Most strikingly, dysbiosis in I. scapularis ticks (i.e., ticks with low microbiotal diversity) leads to a defective peritrophic matrix which decreases the colonization success of B. burgdorferi s.s., suggesting that the pathogen requires the presence of an intact microbiota to be able to invade ticks (Narasimhan et al., 2014). Thus, the microbial community may have a crucial impact on vector competence of ticks and thereby on disease dynamics.
Yet, as outlined above, co-occurrence of microbes can be due to environmental filtering or direct microbial interactions, and distinguishing between these processes is non-trivial. Indeed, previous studies that have documented pathogen co-occurrence in ticks have not accounted for potential confounding variables such as shared ecological requirements, and are thus limited in their ability to differentiate between co-occurrences due to shared environmental niches, and co-occurrence shaped by facilitation or competition among microbes.
To address these gaps, we exploited the substantial environmental heterogeneity along replicated elevational gradients in the Swiss Alps to quantify the relative importance of environmental factors, tick characteristics and direct microbial interactions in influencing the structure of bacterial communities in I. ricinus ticks in general, and the (co-)occurrence of pathogens in particular, using a combination of 16S sequencing and joint species distribution modelling (JSDM) (Warton et al., 2015). By taking shared environmental preferences into account, JSDMs allows to identify residual co-occurrence patterns among microbes that can result from unaccounted environmental effects or direct microbial interactions. However, the correct spatial scale with regards to the focal biological processes is of importance, as well as the type of the hypothesized biotic interaction (Araújo & Rozenfeld, 2014; Zurell, Pollock & Thuiller, 2018) when interpreting JSDM patterns (Dormann et al., 2018).
Specifically, we ask (i) how do large-scale abiotic factors and small scale tick-level variables affect tick microbiota composition, (ii) which large-scale abiotic and small-scale tick-level variables predict pathogen occurrence, and (iii) are there patterns of non-random microbial co-occurrence that cannot be explained by environmental responses and might be due to unmeasured variables, such as microbial interactions.
Material and Methods
Tick sampling
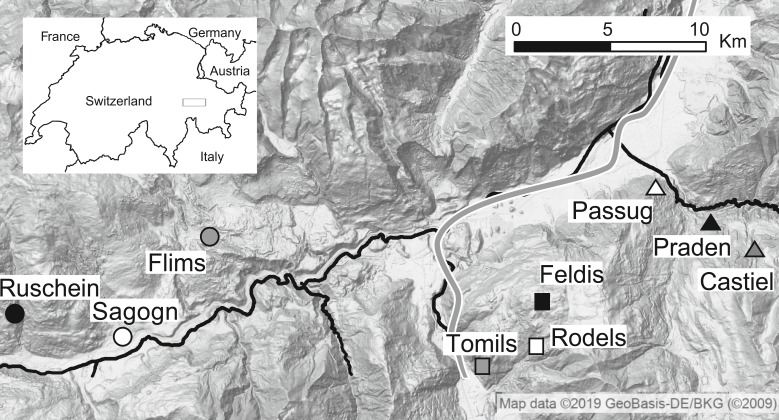
Questing Ixodes ricinus ticks were collected at three locations in the Swiss Alps (Kanton Graubünden). At each location, one site at low (630–732 m above sea level, masl), one at medium (1,094–1,138 masl) and one at high (1,454–1 673 masl) elevation were identified (Fig. 1, Table 1, N = 9 sampling sites). At each site, questing ticks were sampled thrice, once in June, once in July, and once in August 2014 by dragging a white blanket (1 m × 1 m) over the ground vegetation as described previously (Lemoine, Cornetti & Tschirren, 2018). Ticks were collected from the blanket and stored in 95% ethanol. Tick species, sex and life stage were verified by morphological features following (Hillyard, 1996) using a stereomicroscope.
Figure 1. Location of tick sampling sites in the Swiss Alps.
Different shapes (i.e., circle, square and triangle) represent the different locations and different colours represent elevation (white: low, grey: middle, black: high). Rivers and motorway are shown in black. Map data ©2019 Google, GeoBasis-DE/BKG.
Table 1. Tick sampling sites in the Swiss Alps.
| Location | Site | Coordinates | Elevation | Succesfully sequenced Ixodes ricinus ticks | |||
|---|---|---|---|---|---|---|---|
| North | East | (masl) | nymphs | males | females | ||
| 1 | Sagogn | 46.783 | 9.233 | 693 | 0 | 9 | 15 |
| Flims | 46.827 | 9.280 | 1138 | 3 | 5 | 3 | |
| Ruschein | 46.795 | 9.169 | 1454 | 0 | 1 | 1 | |
| 2 | Rodels | 46.760 | 9.425 | 630 | 2 | 5 | 4 |
| Tomils | 46.772 | 9.453 | 1144 | 3 | 6 | 4 | |
| Feldis | 46.789 | 9.453 | 1673 | 1 | 1 | 0 | |
| 3 | Passug | 46.840 | 9.538 | 732 | 0 | 5 | 6 |
| Castiel | 46.826 | 9.569 | 1094 | 0 | 3 | 3 | |
| Praden | 46.817 | 9.589 | 1582 | 1 | 0 | 1 | |
Environmental variables
For each sampling site, we compiled information on large-scale, site-level ecological variables by obtaining data on elevation, slope and aspect using DHM25, land use data from swissTLM3D (both from Federal Office of Topography swisstopo) and data on temperature and precipitation from Landscape Dynamics (Swiss Federal Research Institute for Water, Snow and Landscape Research WSL and Federal Office of Meteorology and Climatology MeteoSwiss; Thornton, Running & White, 1997). Data on I. ricinus abundance and the abundance of a key tick host, the bank vole (Myodes glareolus), as well as the ratio of bank vole to other rodents at our sampling sites were obtained from Cornetti et al. (2016) and Cornetti et al. (2018). Details on the different variables and a justification why they were included to characterise large-scale ecological conditions is provided in the Supplemental Information.
Tick DNA isolation and quantification of neutral genetic diversity
The number of analysed ticks per site is presented in Table 1. Although we aimed to include similar numbers of I. ricinus ticks from each sampling site and month, variation in the number of ticks per site (Table 1) was unavoidable because of variation in tick abundance across sites (Lemoine, Cornetti & Tschirren, 2018). To avoid contamination, we performed DNA isolation and amplifications in a laminar flow cabinet. Each tick was washed thrice with sterile water before sterilizing it with 3% hydrogen peroxide. Ticks were then cut in half with a sterilized blade to facilitate DNA isolation. DNA was extracted using DNeasy Blood & Tissue kit (Qiagen; Hilden, Germany).
Host genetics may affect pathogen and endosymbiont colonisation and replication success (Archie & Ezenwa, 2011). In order to quantify individual and population-level genetic diversity, we genotyped ticks at 11 microsatellite markers in two multiplexed amplifications (see Supplementary Material for details). Not all markers were successfully amplified in all samples, but none of the samples contained more than two failed markers. We used package poppr (Kamvar, Tabima & Grünwald, 2014) in R 3.4.1 (R Core Team, 2013) to test for linkage disequilibrium and deviation from Hardy-Weinberg equilibrium. Individual observed heterozygosity was determined for each tick as a proportion of heterozygous markers to all successfully amplified markers. Expected population level heterozygosity was determined with poppr. The former was used as a tick-level explanatory variable (together with tick sex and life stage), the latter was used as a site-level explanatory variable.
Tick microbiota sequencing
We characterized tick bacterial community composition by sequencing the hypervariable V4 region of the 16S rRNA (16S) gene. Negative controls (extraction reagent blank, N = 2 and PCR controls, N = 3) were processed alongside the tick samples. Sequencing libraries were prepared following the Earth Microbiome 16S Illumina Amplicon protocol, using the primers 515FB and 806RB (Carey, Walters & Knight, 2013) (see Supplemental Information for details). Samples and negative controls were randomized across two plates. The libraries were sequenced on Illumina MiSeq at the Functional Genomic Center Zurich with a target length of 250 bp following the manufacturer’s protocol. The obtained sequence data were analyzed following the mothur pipeline with MiSeq standard operation procedures (Kozich et al., 2013). Sequences have been deposited to the Sequence Read Archive under BioProject PRJNA506875. The complete metadata of the samples and their matching sequence accession numbers have been submitted to FigShare (doi:10.6084/m9.figshare.7380767).
As we are not able to assess whether individual OTUs are resident or not, and we do not know their transmission routes, a special focus of our analysis was on tick endosymbionts and tick-borne human, domestic animal or wildlife pathogens (Table 2), which are obligate residents. This approach does not mean that the other OTUs would not have a substantial effects on ticks and other tick symbionts. Identification of endosymbionts and pathogens is described in the Supplemental Information.
Table 2. Common tick endosymbionts and/or putative human pathogens observed in I. ricinus ticks.
See Supplemental Information for information on OTU assignment.
| OTU | Label | Human pathogen/ tick endosymbiont | Occurrence (% of analyzed ticks) |
|---|---|---|---|
| Otu0001 | Midichloria | endosymbiont | 100 |
| Otu0003 | Spiroplasma | endosymbiont | 41 |
| Otu0005 | Rickettsiella | endosymbiont | 63 |
| Otu0021 | Lariskella | endosymbiont | 49 |
| Otu0031 | Rickettsia helvetica | both | 16 |
| R. monacensis | both | 6 | |
| Otu0067 | Rickettsia sp. | both | 25 |
| Otu0076 | Anaplasma | both | 33 |
| Otu0086 | Candidatus Neoehrlichia | both | 22 |
| Otu0088 | Borrelia afzelii | pathogen | 9 |
| B. miyamotoi | pathogen | 10 | |
| B. garinii | pathogen | 6 | |
| B. valaisiana | pathogen | 2 |
Joint species distribution modelling of microbiota composition
Only samples with >500 reads and OTUs which were present in at least two samples were included in the analyses (Table 1). As the most common OTU, the intra-mitochondrial endosymbiont Candidatus Midichloria (Lo et al., 2006), was present in all samples, it was not included in the modelling. For the occurrence matrix, an OTU was determined to be present in a tick if >5 reads were identified in a sample (following Aivelo & Norberg, 2017).
We used a JSDM framework called Hierarchical Modelling of Species Communities (HMSC, (Ovaskainen et al., 2017a) to examine how environmental variables correlate with pathogen and tick endosymbiont occurrence in ticks, and whether there are non-random residual associations among different OTUs and/or oligotypes, implying direct facilitation or competition effects among microbes. This approach combines information on environmental covariates, bacterial species traits, spatiotemporal context and sampling design to explain the presence or absence of OTUs (Fig. S2). The associations among OTUs are captured with the latent part of the framework, modelling the residual variance after accounting for the effects of the environment. The estimates for these latent variables can be then translated into residual correlations among response variables, i.e., OTUs and/or oligotypes. These correlations thus reflect (dis)associations which cannot be explained by shared responses to the environment.
We compiled occurrence matrices for OTUs for each individual tick as a response variable. For each sampling unit, i.e., a row in our response variable matrix, we included information on the identity of the sampling unit (tick ID), its location, sampling site (for which we included also the spatial structure as coordinates) and month, describing the study design. To reach a better resolution within specific OTUs, we analyzed known human, domestic animal or wildlife pathogens, tick endosymbionts and their close relatives within the 100 most common OTUs with oligotyping pipeline (Eren et al., 2014). Oligotyping uses all the sequences, which form an OTU, and performs Shannon Entropy Analysis to regroup sequences based on within-OTU variation. This results in higher-resolution grouping than OTUs as the different oligotypes might differ only by a single nucleotide (Eren et al., 2014). We used the standard operation procedures of the oligotyping pipeline software (http://oligotyping.org; Eren et al., 2013). We labelled the resulting oligotypes through BLAST search (Camacho et al., 2009). For some species, such as Rickettsia spp., the V4 region of 16S might not have enough resolution (Greay et al., 2018), and thus, the labels should not be considered as definite identifications.
Including a large number of explanatory variables in statistical models is inherently challenging. To reduce the number of variables, while maintaining their information value, we used two variable sets in the model: (a) a set of full-effect explanatory variables, and (b) explanatory variables under variable selection (Ovaskainen et al., 2017b). The full-effect variable set included an intercept, two tick-level variables (tick sex or life stage and individual heterozygosity) and two site-level variables (tick abundance and elevation of the sampling site). Additionally, we included information whether a specific OTU is an endosymbiont and/or a human, domestic animal or wildlife pathogen (Abrego, Norberg & Ovaskainen, 2016). This allowed us to test if endosymbionts and/or pathogens respond differentially to environmental conditions than other OTUs. The set of explanatory variables under variable selection included additional information on the environmental conditions of the sites (namely the number of days above 7 °C during the year, monthly precipitation, mean monthly temperature, forest coverage, slope, aspect, bank vole abundance, the proportion of voles to other rodents and expected tick heterozygosity) (Table S1). We considered all parameter estimates, including associations among bacterial OTUs, having strong statistical support and thus being ’significant‘ if the 90% central credible interval of the parameter did not overlap with zero (see Supplemental Information for additional model details). The model was run in Matlab R2017 (The MathWorks, Natick, MA, USA).
Results
Ixodes ricinus microbiota composition
We 16S sequenced the bacterial community of 92 Ixodes ricinus ticks which resulted in 13,214,477 reads. No amplification was observed in the five negative controls (i.e., their sequencing did not result in any reads) and one tick was sequenced twice. After contig assembly and quality control 1,656,287 reads were retained. Most of the discarded reads were either shorter than 250 bp or chimeras. There was a median of 1,562 quality-controlled reads per sample, with an interquartile range of 6319. 82 samples with more than 500 reads per sample, a plateauing accumulation curve and a Good’s coverage estimator ≥0.95 were included in the subsequent analyses (Fig. S1). In total, 5,181 bacterial OTUs were identified. The median number of OTUs when rarified to 500 reads per sample was 89 OTUs, with a 95% confidence interval of 78.3–98.5 OTUs.
Six OTUs were present in at least 90% of the samples: Ca. Midichloria (Otu0001), Sphingomonas (Otu0002, 0006 and 0007), Pseudomonas (Otu0011) and Delftia (Otu0012). Together, they represented 50.2% of all reads. We used oligotyping to further divide OTU0031 ‘Rickettsia’ into two oligotypes labelled as ‘R. helvetica’ and ‘R. monacensis’, and OTU0086 ‘Borrelia’ into four oligotypes labelled as ‘B. afzelii’, ‘B. valaisian a’ and ‘B. garinii’ and ‘B. miyamotoi’. 635 OTUs and oligotypes were used in subsequent analyses, including 14 endosymbionts and / or human, domestic animal or wildlife pathogens (Table 2).
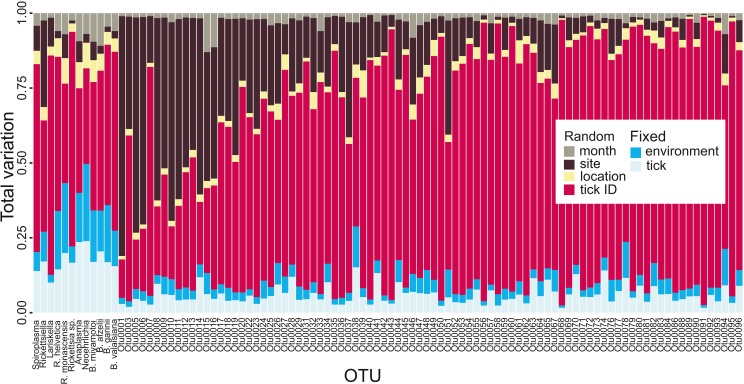
Tick microbiota variance partitioning
Variance partitioning revealed that most of the variation in tick microbiota composition explained by our model related to tick ID: for the hundred most common OTUs, tick ID accounted for 64.1% of the variation explained by the model. Fixed effects (e.g., tick life stage, elevation; see Table S1) accounted for 12.5% (tick-level: 7.3%, site-level: 5.2%) and spatial and temporal random effects (i.e., location, site and month) explained 23.3% (Fig. 2). This suggests that there is extensive tick-level variation which cannot be accounted for by tick-specific characteristics included in our model (i.e., sex, life stage, genetic diversity) or site-level environmental factors. The pattern differed slightly for endosymbionts and human, domestic animal or wildlife pathogens: while tick ID was still the most important variable explaining 49.9%, fixed effects explained 31.8% (tick-level: 17.5%, site-level: 14.3%) and spatial and temporal random effects explained 18.3% of the total variation explained by the model, when averaged over all pathogens and endosymbionts (Fig. 2). Thus, tick- and site-level fixed effects explained a larger proportion of the variation in the occurrence of obligate resident pathogens and endosymbionts than the occurrence of other (potentially non-resident) OTUs.
Figure 2. Tick microbial community variance partitioning for different fixed and random effects.
The first three columns represent tick endosymbionts, the next three columns are OTUs which are both tick endosymbionts and human pathogens and the subsequent six columns represent human pathogens. The other columns represent the 88 most common OTUs found in I. ricinus, ordered by read frequency. Month, sampling site, location and tick ID were included in the model as random effects, whereas fixed effects were divided into environmental (elevation, temperature, precipitation, forest coverage, slope, aspect, vole abundance and vole-to-other-rodents ratio) and tick-specific variables (life stage or sex, individual heterozygosity, abundance, expected population heterozygosity). See raw data in Figshare for information on OTU labels (DOI: 10.6084/m9.figshare.7380767.v3).
Tick-specific and environmental factors related to OTU occurrence
The occurrence of tick endosymbionts and pathogens was strongly associated with specific explanatory variables, yet associations were typically microbe-specific rather than universal (Table 3). The two most important variables explaining the presence or absence of tick endosymbionts and human, domestic animal or wildlife pathogens were tick sex and elevation of the sampling site: adult female ticks were less likely to harbour the endosymbionts Spiroplasma, Rickettsiella, Lariskella and Rickettsia spp. (Table 3), and ticks at higher elevations had higher probability to harbour R. helvetica and R. monacensis, but were less probable to harbour B. garinii (Table 3). Slope and aspect were also significant predictors of tick endosymbionts and pathogen occurrence, with ticks from sites facing northwards having a higher probability of harbouring Spiroplasma and B. afzelii, and ticks from sites on steeper slopes having a higher probability of harbouring Rickettsia sp. (Table 3). Furthermore, a higher tick abundance was associated with a higher probability of Rickettsiella and Ca. Neoehrlichia occurrence (Table 3). Relationships between tick life stage, mean temperature, the number of days >7 °C or forest cover and the occurrence of specific OTUs were not strongly statistically supported.
Table 3. Associations between tick-specific and environmental variables and the occurrence of endosymbionts and human pathogens in I. ricinus ticks.
A positive sign indicates that higher variable values are associated with a higher probability of OTU occurrence. A higher aspect value means that a site is facing northwards. Only associations with strong statistical support (based on the 90% central credible interval) are presented.
| Full variable set | Variable selection set | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tick sex (Female) | Tick life stage (Nymph) | Tick abundance | Tick heterozygosity | Elevation | Tick population expected heterozygosity | Number of days >7 °C | Precipitation | Mean temperature | Forest cover | Slope | Aspect | Vole abundance | Vole/other rodents ratio | ||
| Otu0003 | Spiroplasma | − | + | − | |||||||||||
| Otu0005 | Rickettsiella | − | + | − | − | + | − | ||||||||
| Otu0022 | Lariskella | − | − | ||||||||||||
| Otu0031 | Rickettsia helvetica | + | − | ||||||||||||
| R. monacensis | + | − | − | ||||||||||||
| Otu0067 | Rickettsia sp. | − | + | ||||||||||||
| Otu0076 | Anaplasma | ||||||||||||||
| Otu0086 | Ca. Neoehrlichia | + | |||||||||||||
| Otu0088 | Borrelia afzelii | + | |||||||||||||
| B. miyamotoi | |||||||||||||||
| B. garinii | − | ||||||||||||||
| B. valaisiana | |||||||||||||||
The effect sizes of strongly statistically supported associations varied substantially (Figs. S4A–S4I). For example, threefold increase in vole abundance corresponded to less than one percentage point decrease of R. monacensis prevalence (Fig. S4B), whereas a threefold increase in tick abundance corresponded to a threefold increase in Neoehrlichia prevalence from 8% to 27% (Fig. S4E).
Patterns of microbial association within ticks
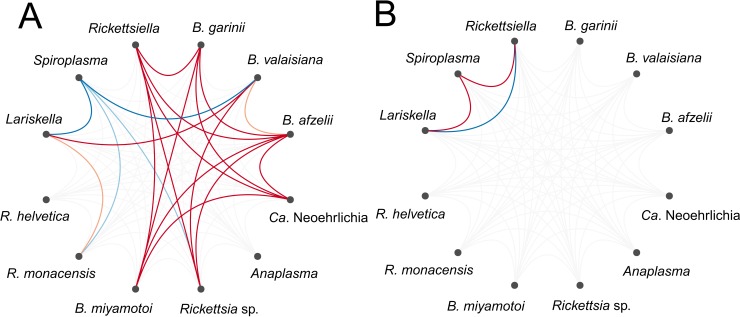
Numerous bacterial OTUs were either significantly more or less likely to co-occur within a tick than expected by chance after accounting for shared environmental preferences (Fig. 3A; Table S2). At the level of the individual tick, the occurrence of the tick endosymbiont Spiroplasma was negatively associated with the occurrence of the endosymbiont Lariskella and several tick-borne pathogens, namely Rickettsia sp., Ca. Neoehrlichia and B. miyamotoi (Fig. 3A). Associations among pathogens, if they occurred, were all positive (Fig. 3A), suggesting that ticks are more likely to be co-infected with several human, domestic animal or wildlife pathogens simultaneously than expected by chance or based on shared environmental preferences. Borrelia oligotypes showed positive co-occurrence patterns among each other, except for B. miyamotoi, which was not associated with other Borrelia sp., but negatively with Spiroplasma and positively with Lariskella. At the level of the sampling site, significant associations were sparser. Spiroplasma was more likely to co-occur with Lariskella and Rickettsiella across sites, whereas the latter two were less likely to co-occur across sites than expected by chance after accounting for shared environmental preferences (Fig. 3B). At the level of month or location, there were no significant associations.
Figure 3. Residual association patterns among endosymbionts and human pathogens within ticks on (A) individual tick-level and (B) on site-level after accounting for shared environmental preference.
Red lines represent positive associations and blue lines negative associations. Only associations with strong statistical support (i.e., based on the 90% central credible interval) are presented. Darker colors indicate stronger associations.
Discussion
We used a JSDM framework to quantify the relative importance of large scale, site-level environmental variables, tick-level characteristics and interactions among microbes in shaping tick microbiota composition along elevational gradients in the Swiss Alps. We show that although our study captured extensive environmental variation, with sampling sites spanning across an elevational gradient from 630–1,580 masl, and a large number of ecological variables was considered in our models, the explanatory power of such large-scale ecological factors was comparably weak. In contrast, individual tick ID explained over 60% of the variation in microbiota composition. This substantial microbiota variation across individual ticks may be partly explained by some of the bacteria present in ticks being non-resident (i.e., bacteria that were by chance obtained from the environment through the mouth, the anal pore or spiracles or during blood-feeding; Horner-Devine & Bohannan, 2006; Engel & Moran, 2013; Zolnik et al., 2016; Zolnik et al., 2018; Ross et al., 2018). Indeed, there has been a debate whether ticks have a stable microbiota (Ross et al., 2018), mirroring the wider debate on how common resident microbiota is in arthropod hosts (Hammer et al., 2017).
However, also for endosymbionts and human, domestic animal or wildlife pathogens, which are obligate resident, tick ID accounted for half of the variation in occurrence, suggesting that microhabitat or -climatic conditions experienced by individual ticks, tick-specific traits and behaviors not included in our models, as well as microbial interactions within ticks such as facilitation and competition (Abraham et al., 2017; Gurfield et al., 2017), play a crucial role in shaping microbiota composition and the occurrence of endosymbionts and human or wildlife pathogens in I. ricinus. Focusing on such small-scale variables, rather than large-scale climatic or environmental factors as is usually done when modelling tick-borne pathogen prevalence (Norman, Worton & Gilbert, 2016; Rosà et al., 2018), is thus likely a more fruitful approach to advance our understanding of microbiota composition of natural populations as well as (tick-borne) disease dynamics.
Co-occurrence of human, domestic animal or wildlife pathogens in ticks has been documented previously, both in I. ricinus (Lommano et al., 2012; Michelet et al., 2014) and other tick species (Gurfield et al., 2017; Laaksonen et al., 2018). Yet, previous studies did not control for environmental filtering, which limited their ability to disentangle shared responses to the environment from direct microbe-microbe interactions. Our study revealed that when accounting for shared environmental preferences, associations among human or wildlife pathogens were often pronounced and mostly positive. These positive associations may result from direct facilitation among microbes or parallel colonization from co-infected tick hosts. Because our sampling unit was the whole tick, whereas bacteria inhabiting a tick can be situated in different organs, co-occurrence at the tick-level does not necessarily mean that there is direct interaction between co-occurring OTUs, although indirect interactions, via, e.g., host immune system, can still occur.
Within ticks, the significant positive associations among the Lyme disease-causing Borrelia genospecies (B. afzelii, B. garinii and B. valaisiana) were particularly striking. This positive co-occurrence is surprising because B. garinii and B. valaisiana are bird specialists (Hanincova et al., 2003b; Comstedt, Jakobsson & Bergström, 2011), whereas B. afzelii is a rodent specialist (Hanincova et al., 2003a). Thus, the parallel colonization from co-infected tick hosts cannot explain this pattern. Rather the positive co-occurrence is indicative of facilitation processes among Borrelia genospecies, as has been suggested previously (Andersson, Scherman & Råberg, 2013). Such facilitation, and the resulting co-infection of ticks with several Borrelia genospecies has implications for the severity, diagnosis, treatment and control of Lyme disease. Finally, the co-occurrence of these different Borrelia genospecies suggests that I. ricinus feeds on multiple, phylogenetically diverse host species during its life cycle and does not show pathogen-mediated host specialization as has been suggested previously (McCoy et al., 2005; McCoy, Léger & Dietrich, 2013).
Although associations among microbes were mostly positive, there were negative associations between the tick endosymbiont Spiroplasma and several human or wildlife pathogens, which may be explained by competition. The most common infection route for Spiroplasma is maternal (i.e., vertical) transmission (Herren & Lemaitre, 2011), indicating that horizontal or environmental transfer plays a minor role in its transmission. Protective effects of Spiroplasma have been previously described in Drosophila spp., where Spiroplasma is associated with a decreased probability of nematode and parasitoids infections (Xie, Vilchez & Mateos, 2010; Jaenike et al., 2013). Although the exact mechanisms mediating Spiroplasma-induced competition effects are currently unknown, this finding may stimulate further research into the potential of tick endosymbionts to manage tick-borne pathogens.
In contrast to the numerous positive or negative associations among microbes at the tick-level, little statistical support for positive or negative microbial co-occurrence was found at the site-level, with the exception of the associations among three endosymbionts. Interestingly, the pattern of co-occurrence of Spiroplasma and Lariskella at the site-level was opposite from what was observed at the tick-level. It suggests that Spiroplasma and Lariskella are more likely to co-occur at the same sites but not within the same tick. Co-occurrence at the site-level can be due to an environmental variable not included in our model, for which the three OTUs had similar responses. It has also been suggested that negative associations generate checkerboard patterns of co-occurrence that can be captured at finer spatial scales but that are lost with increasing scales, but positive associations can be captured across scales (Araújo & Rozenfeld, 2014).
Despite the large among-tick variation in microbiota composition, we identified a range of environmental variables that significantly predicted the occurrence of specific tick endosymbionts and human, domestic animal or wildlife pathogens. However, the predictor variables as well as their effect were typically OTU-specific rather than universal. For example, B. garinii was less likely to occur at higher elevations, whereas R. helvetica and R. monascensis were more likely to occur at higher elevations. Generally, the environmental factors shaping Rickettsia spp. distribution are poorly understood, as is their range of host species (Halos et al., 2010; Eremeeva & Dasch, 2015). Yet, it has previously been found that spotted fever incidence in humans, caused by R. ricketsii, is highest in areas or regions, where ticks are less common (Atkinson et al., 2013). This is in line with our findings and suggests that Rickettsia spp. are more likely to colonize ticks living under suboptimal conditions (e.g., at range edges).
The finding that B. garinii is less likely to occur at higher elevations is in line with previous observations (Jouda, Perret & Gern, 2004b; Cornetti et al., 2018) and may be explained by changes in vegetation structure and associated changes in host communities (Halos et al., 2010), in particular changes in the diversity and/or abundance of birds, the natural hosts of B. garinii (Comstedt, Jakobsson & Bergström, 2011). In contrast, the occurrence of the mammal specialist B. afzelii was not related to elevation, potentially because elevational clines in mammal diversity and/or abundance are less pronounced (McCain, 2005). Indeed, we did not observe an association between elevation and bank vole abundance across our study sites (ANOVA: F1,8 = 0.357, p = 0.57, R2 = 0.05).
Interestingly, temperature and precipitation, which vary strongly across elevational gradients (average temperature and precipitation: high elevation sites: 11.8 °C and 17.8 mm per month; in low sites: 16.5 °C and 12.1 mm per month), were not significant predictors of the occurrence of endosymbionts or human or wildlife pathogens, with the exception of precipitation correlating positively with the probability of Rickettsiella occurrence. This may be partly explained by the temperature and precipitation measures included in our models not fully capturing the microclimatic variation across sites and along elevational clines. Indeed, slope and aspect, which are important determinants of the topography, and thus microclimate (Bennie et al., 2008), were significant predictors of pathogen and endosymbiont occurrence. The probability of Rickettsia sp. occurrence was higher on steeper slopes. Furthermore, the probability of occurrence was higher on north-facing slopes for B. afzelii and Spiroplasma and higher on south-facing slopes for Rickettsiella (see also Stuen, Granquist & Silaghi, 2013). Microclimatic conditions may affect microbial occurrence directly, or indirectly via affecting tick behavior or host community composition (Swei, Meentemeyer & Briggs, 2011; Lawson et al., 2014). Furthermore, topography can affect population connectivity and dispersal in metapopulation networks (Swei & Kwan, 2017).
Previous work has found that tick abundance is a strong predictor of Borrelia spp. prevalence, potentially because larger tick populations facilitate co-feeding transmission (Jouda, Perret & Gern, 2004a). No relationship between Borrelia spp. occurrence and tick abundance was observed in our study. However, both Ca. Neoehrlichia and Rickettsiella were more common at sites where ticks were more abundant, suggesting that co-feeding transmission may also play a role in the life cycle of these microbes.
Finally, differences in host competence can lead to dilution effects and thus affect the prevalence of tick-borne pathogens (Keesing, Holt & Ostfeld, 2006). Whereas for some tick-borne pathogens the vertebrate hosts are known or suspected (e.g., small mammals for B. afzelii (Hanincova et al., 2003a) and Ca. Neoehrlichia (Jahfari et al., 2012), birds for B. garinii and B. valaisiana (Hanincova et al., 2003b), both for Anaplasma (Keesing et al., 2012) and R. helvetica (Sprong et al., 2009), for others the host species range is less well understood (e.g., B. miyamotoi; Wagemakers et al., 2015). The bank vole is a common tick host at our study sites and their abundance was a significant negative predictor of R. monacensis and R. helvetica occurrence. Interestingly, bank voles are not known hosts for either (Burri et al., 2014). Most likely, the relation is thus indirect, explained by an unmeasured biotic or abiotic variable that correlates with bank vole abundance. No evidence was found that the proportion of bank voles to other rodents affects the prevalence of tick-borne pathogens.
A limitation of our sampling design is the uneven sample distribution across sites. We collected ticks up to the upper elevational limit of tick distribution, which leads to a large variation in environmental variables included in our models, but at the same times means that we have a limited number of samples from the high elevation sites. Yet, adequate model fit suggests that this uneven sample distribution did not compromise model performance. Furthermore, although JSDM is a powerful approach to model community structure, it has a number of limitations. First, it assumes that interactions among microbes are similar across environments (but see Tikhonov et al., 2017). This is not necessarily the case as both environmental factors and tick host community may shape microbial interactions (Elliot, Blanford & Thomas, 2002). Second, the model assumes that the explanatory variables affect the microbial community composition (or rather, the presence or absence of individual OTUs), but not vice versa. However, this is a valid assumption for most environmental (e.g., elevation and temperature) and tick-related variables (e.g., tick sex, life stage) included in our models. Thirdly, covariation among explanatory variables poses a problem to any correlative modelling approach. Our model is built on two distinct variable sets to aid in handling such covariation: the full variable set includes elevation, whereas the variables with the strongest covariation (i.e., temperature and precipitation) are included in the variable selection set. Fourthly, the inferred residual associations between focal taxa are assumed to be symmetrical. If there are asymmetric interactions (e.g., predator–prey-relationships), the sum outcome can be seen as either positive or negative correlation (Zurell, Pollock & Thuiller, 2018). However, in our study, the expectation was facilitation or competition, which are symmetric positive or negative interactions, respectively. Thus, given sufficient signal, we expect that the focal interactions can be captured by our modelling approach.
Conclusions
Our study demonstrates that a JSDM framework can contribute to a better understanding of the factors shaping bacterial communities in natural populations as well as patterns of co-occurrence among microbes. Overall, our study highlights the role of small-scale, tick-level characteristics rather than large-scale ecological variation in shaping microbial communities of I. ricinus. We identified a number of ecological variables that predict the occurrence of specific tick endosymbionts and human, domestic animal or wildlife pathogens with strong statistical support, but these effects were typically microbe-specific rather than universal. This highlights that environmental change can have different, even opposite effects on different human pathogens, and thus disease risk. Furthermore, by accounting for shared environmental preferences, our approach identified patterns of microbial co-occurrence that are consistent with microbe-microbe interactions, which result in pathogen co-infections within ticks, as well as competition between Spiroplasma and a number of human, domestic animal or wildlife pathogens. The latter opens up new and exciting avenues for the control and management of tick-borne diseases in regions with high human disease incidence.
Supplemental Information
Acknowledgments
We thank Mélissa Lemoine for providing ticks, the numerous people who helped collecting ticks in the field, Glauco Camenisch, Elisa Granato, Jennifer Morger and Alessia Pennachia for help with laboratory work, Lucy Poveda for help with MiSeq sequencing, Frédéric Guillaume for providing access to IT infrastructure, Janine Bolliger and Dirk Schmatz from Swiss Federal Institute for Forest, Snow and Landscape Research WSL for providing spatial data and for help with spatial analyses, and Otso Ovaskainen for discussions on the modelling and reviewers for constructive comments on the manuscript.
Funding Statement
This study was funded by Finnish Cultural Foundation Postdoc Pool grant (to Tuomas Aivelo), the Stiftung für wissenschaftliche Forschung an der Universität Zürich (17_027), the Swiss National Science Foundation (PP00P3_128386 and PP00P3_157455), the University of Zurich Research Priority Program “Evolution in Action: from Genomes to Ecosystems”, the Faculty of Science of the University of Zurich, and the Baugarten Stiftung (all to Barbara Tschirren). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Tuomas Aivelo conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Anna Norberg analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Barbara Tschirren conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The field collection was approved by the Amt for Lebensmittelsicherheit und Tiergesundheit des Kantons Graubünden (Dr. T. Bürge and Dr R. Hannimann; permit 2012_17) and the Gemeindeschreiber of the municipalities of Klosters, Grüsch, Rothenbrunnen / Tomils, Flims, Chur / Churwalden and Malans.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
Sequences are available at the Sequence Read Archive: BioProject PRJNA506875.
Data Availability
The following information was supplied regarding data availability:
The complete metadata of the samples and their matching sequence accession numbers area available at Figshare:Aivelo, Tuomas; Tschirren, Barbara; Norberg, Anna (2019): Tick data used in “Human pathogen co-occurrence in Ixodes ricinus ticks: effects of landscape topography, climatic factors and microbiota interactions”. figshare. Dataset. DOI: 10.6084/m9.figshare.7380767.v3.
References
- Abraham et al. (2017).Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, Ansari JM, Jefferson KK, Cava F, Jacobs-Wagner C, Fikrig E. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E781–E790. doi: 10.1073/pnas.1613422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrego, Norberg & Ovaskainen (2016).Abrego N, Norberg A, Ovaskainen O. Measuring and predicting the influence of traits on the assembly processes of wood-inhabiting fungi. Journal of Ecology. 2016;105:1070–1081. doi: 10.1111/1365-2745.12722. [DOI] [Google Scholar]
- Adair & Douglas (2017).Adair KL, Douglas AE. Making a microbiome: the many determinants of host-associated microbial community composition. Current Opinion in Microbiology. 2017;35:23–29. doi: 10.1016/j.mib.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Aivelo & Norberg (2017).Aivelo T, Norberg A. Parasite-microbiota interactions potentially affect intestinal communities in wild mammals. Journal of Animal Ecology. 2017;87:438–447. doi: 10.1111/1365-2656.12708. [DOI] [PubMed] [Google Scholar]
- Andersson, Scherman & Råberg (2013).Andersson M, Scherman K, Råberg L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? The American Naturalist. 2013;181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- Andersson, Scherman & Råberg (2014).Andersson M, Scherman K, Råberg L. Infection dynamics of the tick-borne pathogen “Candidatus Neoehrlichia mikurensis” and coinfections with Borrelia afzelii in bank voles in Southern Sweden. Applied and Environmental Microbiology. 2014;80:1645–1649. doi: 10.1128/AEM.03469-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo & Rozenfeld (2014).Araújo MB, Rozenfeld A. The geographic scaling of biotic interactions. Ecography. 2014;37:406–415. doi: 10.1111/j.1600-0587.2013.00643.x. [DOI] [Google Scholar]
- Archie & Ezenwa (2011).Archie EA, Ezenwa VO. Population genetic structure and history of a generalist parasite infecting multiple sympatric host species. International Journal for Parasitology. 2011;41:89–98. doi: 10.1016/j.ijpara.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Atkinson et al. (2013).Atkinson SF, Sarkar S, Aviña A, Schuermann JA, Williamson P. Modelling spatial concordance between Rocky Mountain spotted fever disease incidence and habitat probability of its vector Dermacentor variabilis (American dog tick) Geospatial Health. 2013;7:91–100. doi: 10.4081/gh.2012.108. [DOI] [PubMed] [Google Scholar]
- Belli et al. (2017).Belli A, Sarr A, Rais O, Rego ROM, Voordouw MJ. Ticks infected via co-feeding transmission can transmit Lyme borreliosis to vertebrate hosts. Scientific Reports. 2017;7:1–13. doi: 10.1038/s41598-017-05231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennie et al. (2008).Bennie J, Huntley B, Wiltshire A, Hill MO, Baxter R, Wood CEHM, Ripton A, Pe C. Slope, aspect and climate: spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecological Modelling. 2008;6:47–59. doi: 10.1016/j.ecolmodel.2008.04.010. [DOI] [Google Scholar]
- Bonnet et al. (2017).Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Frontiers in Cellular and Infection Microbiology. 2017;7 doi: 10.3389/fcimb.2017.00236. Article 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri et al. (2014).Burri C, Schumann O, Schumann C, Gern L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks and Tick-borne Diseases. 2014;5:245–251. doi: 10.1016/j.ttbdis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Camacho et al. (2009).Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, Walters & Knight (2013).Carey HV, Walters WA, Knight R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2013;304:R33–R42. doi: 10.1152/ajpregu.00387.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi et al. (2011).Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP, Drautz DI, Rizzoli A, Schuster SC. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLOS ONE. 2011;6:e25604. doi: 10.1371/journal.pone.0025604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu & Mazmanian (2013).Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nature Immunology. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstedt, Jakobsson & Bergström (2011).Comstedt P, Jakobsson T, Bergström S. Global ecology and epidemiology of Borrelia garinii spirochetes. Infection Ecology Epidemiology. 2011;1 doi: 10.3402/iee.v1i0.9545. Article 9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetti et al. (2018).Cornetti L, Hilfiker D, Lemoine M, Tschirren B. Small-scale spatial variation in infection risk shapes the evolution of a Borrelia resistance gene in wild rodents. Molecular Ecology. 2018:3515–3524. doi: 10.1111/mec.14812. [DOI] [PubMed] [Google Scholar]
- Cornetti et al. (2016).Cornetti L, Lemoine M, Hilfiker D, Morger J, Reeh K, Tschirren B. Higher genetic diversity on mountain tops: The role of historical and contemporary processes in shaping genetic variation in the bank vole. Biological Journal of the Linnean Society. 2016;118:233–244. doi: 10.1111/bij.12723. [DOI] [Google Scholar]
- Cortinas et al. (2002).Cortinas MR, Guerra MA, Jones CJ, Kitron U. Detection, characterization, and prediction of tick-borne disease foci. International Journal of Medical Microbiology. 2002;291:11–20. doi: 10.1016/S1438-4221(02)80003-0. [DOI] [PubMed] [Google Scholar]
- Dallas & Presley (2014).Dallas T, Presley SJ. Relative importance of host environment, transmission potential and host phylogeny to the structure of parasite metacommunities. Oikos. 2014;123:866–874. doi: 10.1111/oik.00707. [DOI] [Google Scholar]
- Diuk-Wasser, Vannier & Krause (2016).Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends in Parasitology. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann et al. (2018).Dormann CF, Bobrowski M, Dehling DM, Harris DJ, Hartig F, Lischke H, Moretti MD, Pagel J, Pinkert S, Schleuning M, Schmidt SI, Sheppard CS, Steinbauer MJ, Zeuss D, Kraan C. Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Global Ecology and Biogeography. 2018;27:1004–1016. doi: 10.1111/geb.12759. [DOI] [Google Scholar]
- Durand et al. (2015).Durand J, Jacquet M, Paillard L, Rais O, Gern L, Voordouw MJ. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Applied and Environmental Microbiology. 2015;81:7740–7752. doi: 10.1128/AEM.02296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot, Blanford & Thomas (2002).Elliot SL, Blanford S, Thomas MB. Host-pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proceedings of the Royal Society B: Biological Sciences. 2002;269:1599–1607. doi: 10.1098/rspb.2002.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel & Moran (2013).Engel P, Moran NA. The gut microbiota of insects—diversity in structure and function. FEMS Microbiology Reviews. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Eremeeva & Dasch (2015).Eremeeva ME, Dasch GA. Challenges Posed by tick-borne rickettsiae: eco-epidemiology and public health implications. Frontiers in Public Health. 2015;3:1–17. doi: 10.3389/fpubh.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2014).Eren AM, Borisy GG, Huse SM, Mark Welch JL. Oligotyping analysis of the human oral microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2875–E2884. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2013).Eren AM, Maignien L, Sul WJ, Murphy LG, Grim SL, Morrison HG, Sogin ML. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Methods in Ecology and Evolution. 2013;4:1111–1119. doi: 10.1111/2041-210X.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña et al. (2018).Estrada-Peña A, Cabezas-Cruz A, Pollet T, Vayssier-Taussat M, Cosson J-F. High throughput sequencing and network analysis disentangle the microbial communities of ticks and hosts within and between ecosystems. Frontiers in Cellular and Infection Microbiology. 2018;8 doi: 10.3389/fcimb.2018.00236. Article 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall et al. (2016).Gall CA, Reif KE, Scoles GA, Mason KL, Mousel M, Noh SM, Brayton KA. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME Journal. 2016;10:1846–1855. doi: 10.1038/ismej.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood et al. (2009).Gatewood AG, Liebman K, Vourc’h G, Bunikis J, Hamer S, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser M. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Applied and Environmental Microbiology. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern, Morán Cadenas & Burri (2008).Gern L, Morán Cadenas F, Burri C. Influence of some climatic factors on Ixodes ricinus ticks studied along altitudinal gradients in two geographic regions in Switzerland. International Journal of Medical Microbiology. 2008;298:55–59. doi: 10.1016/j.ijmm.2008.01.005. [DOI] [Google Scholar]
- Greay et al. (2018).Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, Irwin PJ. Recent insights into the tick microbiome gained through next-generation sequencing. Parasites and Vectors. 2018;11 doi: 10.1186/s13071-017-2550-5. Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfield et al. (2017).Gurfield N, Grewal S, Cua LS, Torres PJ, Kelley ST. Endosymbiont interference and microbial diversity of the Pacific coast tick, Dermacentor occidentalis, in San Diego County, California. PeerJ. 2017;5:e3202. doi: 10.7717/peerj.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haine (2008).Haine ER. Symbiont-mediated protection. Proceedings of the Royal Society B: Biological Sciences. 2008;275:353–361. doi: 10.1098/rspb.2007.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halos et al. (2010).Halos L, Bord S, Cotté V, Gasqui P, Abrial D, Barnouin J, Boulouis HJ, Vayssier-Taussat M, Vourc’H G. Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Applied and Environmental Microbiology. 2010;76:4413–4420. doi: 10.1128/AEM.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer et al. (2017).Hammer TJ, Janzen DH, Hallwachs W, Jaffe SP, Fierer N. Caterpillars lack a resident gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:9641–9646. doi: 10.1073/pnas.1707186114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova et al. (2003a).Hanincova K, Schäfer SM, Etti S, Sewell HS, Taragelová V, Ziak D, Labuda M, Kurtenbach K. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003a;126:11–20. doi: 10.1017/S0031182002002548. [DOI] [PubMed] [Google Scholar]
- Hanincova et al. (2003b).Hanincova K, Taragelova V, Koci J, Schafer SM, Hails R, Ullmann AJ, Piesman J, Labuda M, Kurtenbach K. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Applied and Environmental Microbiology. 2003b;69:2825–2830. doi: 10.1128/AEM.69.5.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlena et al. (2013).Hawlena H, Rynkiewicz E, Toh E, Alfred A, Durden LA, Hastriter MW, Nelson DE, Rong R, Munro D, Dong Q, Fuqua C, Clay K. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. The ISME Journal. 2013;7:221–223. doi: 10.1038/ismej.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley & Altizer (2011).Hawley DM, Altizer SM. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology. 2011;25:48–60. doi: 10.1111/j.1365-2435.2010.01753.x. [DOI] [Google Scholar]
- Herren & Lemaitre (2011).Herren JK, Lemaitre B. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cellular Microbiology. 2011;13:1385–1396. doi: 10.1111/j.1462-5822.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- Hillyard (1996).Hillyard PD. Ticks of North-West Europe. Field Studies Council for the Linnean Society of London and the Estuarine and Coastal Sciences Association; London: 1996. [Google Scholar]
- Horner-Devine & Bohannan (2006).Horner-Devine MC, Bohannan BJM. Phylogenetic clustering and overdispersion in bacterial communities. Ecology. 2006;87:100–108. doi: 10.1890/0012-9658(2006)87[100:pcaoib]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jaenike et al. (2013).Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2013;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- Jahfari et al. (2012).Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte E, Takken W, Heyman P, Medlock JM, Heylen D, Kleve J, Sprong H. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasites & Vectors. 2012;5 doi: 10.1186/1756-3305-5-74. Article 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari et al. (2014).Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MPG, Hovius JWR, Sprong H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes and New Infections. 2014;2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouda, Perret & Gern (2004a).Jouda F, Perret J, Gern L. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. Journal of Medical Entomology. 2004a;41:162–169. doi: 10.1603/0022-2585-41.2.162. [DOI] [PubMed] [Google Scholar]
- Jouda, Perret & Gern (2004b).Jouda F, Perret J-L, Gern L. Density of questing Ixodes ricinus nymphs and adults infected by Borrelia burgdorferi sensu lato in Switzerland: spatiotemporal pattern at a regional scale. Vector-borne and Zoonotic Diseases. 2004b;4:23–32. doi: 10.1089/153036604773082960. [DOI] [PubMed] [Google Scholar]
- Kamvar, Tabima & Grünwald (2014).Kamvar ZN, Tabima JF, Grünwald NJ. Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ. 2014;2:e281. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara et al. (2004).Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, Shibata S, Zhang C, Tsuji M. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. International Journal of Systematic and Evolutionary Microbiology. 2004;54:1837–1843. doi: 10.1099/ijs.0.63260-0. [DOI] [PubMed] [Google Scholar]
- Keesing et al. (2012).Keesing F, Hersh MH, Tibbetts M, McHenry DJ, Duerr S, Brunner J, Killilea M, LoGiudice K, Schmidt KA, Ostfeld RS. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerging Infectious Diseases. 2012;18:2013–2016. doi: 10.3201/eid1812.120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, Holt & Ostfeld (2006).Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecology Letters. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Kozich et al. (2013).Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen et al. (2018).Laaksonen M, Klemola T, Feuth E, Sormunen JJ, Puisto A, Mäkelä S, Penttinen R, Ruohomäki K, Hänninen J, Sääksjärvi IE, Vuorinen I, Sprong H, Hytönen J, Vesterinen EJ. Tick-borne pathogens in Finland: comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasites & Vectors. 2018;11 doi: 10.1186/s13071-018-3131-y. Article 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson et al. (2014).Lawson CR, Bennie J, Hodgson JA, Thomas CD, Wilson RJ. Topographic microclimates drive microhabitat associations at the range margin of a butterfly. Ecography. 2014;37:732–740. doi: 10.1111/ecog.00535. [DOI] [Google Scholar]
- Lee et al. (2013).Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–429. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lello et al. (2004).Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. Competition and mutualism among the gut helminths of a mammalian host. Nature. 2004;428:20–24. doi: 10.1038/nature02472.1. [DOI] [PubMed] [Google Scholar]
- Lemoine, Cornetti & Tschirren (2018).Lemoine M, Cornetti L, Tschirren B. Does Borrelia burgdorferi sensu lato facilitate the colonisation of marginal habitats by Ixodes ricinus? A correlative study in the Swiss Alps. BioRxiv. 2018 doi: 10.1101/273490. [DOI] [PMC free article] [PubMed]
- Lo et al. (2006).Lo N, Beninati T, Sassera D, Bouman EAP, Santagati S, Gern L, Sambri V, Masuzawa T, Gray JS, Jaenson TGT, Bouattour A, Kenny MJ, Guner ES, Kharitonenkov IG, Bitam I, Bandi C. Widespread distribution and high prevalence of an alpha-proteobacterial symbiont in the tick Ixodes ricinus. Environmental Microbiology. 2006;8:1280–1287. doi: 10.1111/j.1462-2920.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- Lommano et al. (2012).Lommano E, Bertaiola L, Dupasquier C, Gern L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Applied and Environmental Microbiology. 2012;78:4606–4612. doi: 10.1128/AEM.07961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso et al. (2002).Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. Journal of Medical Entomology. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- MacDonald et al. (2017).MacDonald AJ, Hyon DW, Iii JBB, Connor KEO, Swei A, Briggs CJ. Lyme disease risk in southern California: abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasites & Vectors. 2017;10:7. doi: 10.1186/s13071-016-1938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli et al. (2012).Mannelli A, Bertolotti L, Gern L, Gray J. Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiological Reviews. 2012;36:837–861. doi: 10.1111/j.1574-6976.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- McCain (2005).McCain CM. Elevational gradients in diversity of small mammals. Ecology. 2005;86:366–372. [Google Scholar]
- McCoy et al. (2005).McCoy KD, Chapuis E, Tirard C, Boulinier T, Michalakis Y, Le Bohec C, Le Maho Y, Gauthier-Clerc M. Recurrent evolution of host-specialized races in a globally distributed parasite. Proceedings of the Royal Society B: Biological Sciences. 2005;272:2389–2395. doi: 10.1098/rspb.2005.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy, Léger & Dietrich (2013).McCoy KD, Léger E, Dietrich M. Host specialization in ticks and transmission of tick-borne diseases: a review. Frontiers in Cellular and Infection Microbiology. 2013;3 doi: 10.3389/fcimb.2013.00057. Article 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock et al. (2013).Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, Golovljova I, Jaenson TGT, Jensen J-K, Jensen PM, Kazimirova M, Oteo J, Papa A, Pfister K, Plantard O, Randolph SE, Rizzoli A, Santos-Silva MM, Sprong H, Vial L, Hendrickx G, Zeller H, Van Bortel W. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites & vectors. 2013;6 doi: 10.1186/1756-3305-6-1. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet et al. (2014).Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, Van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bødker R, Fach P, Moutailler S. High-throughput screening of tick-borne pathogens in Europe. Frontiers in Cellular and Infection Microbiology. 2014;4 doi: 10.3389/fcimb.2014.00103. Article 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutailler et al. (2016).Moutailler S, Valiente Moro C, Vaumourin E, Michelet L, Tran FH, Devillers E, Cosson JF, Gasqui P, Van VT, Mavingui P, Vourc’h G, Vayssier-Taussat M. Co-infection of ticks: the rule rather than the exception. PLOS Neglected Tropical Diseases. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan et al. (2014).Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, Eppler-Epstein R, Deponte K, Fish D, Fikrig E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host and Microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, Worton & Gilbert (2016).Norman RA, Worton AJ, Gilbert L. Past and future perspectives on mathematical models of tick-borne pathogens. Parasitology. 2016;143:850–859. doi: 10.1017/S0031182015001523. [DOI] [PubMed] [Google Scholar]
- Ovaskainen et al. (2017b).Ovaskainen O, Tikhonov G, Dunson D, Grøtan V, Engen S, Sæther B-E, Abrego N. How are species interactions structured in species-rich communities? A new method for analysing time-series data. Proceedings of the Royal Society B: Biological Sciences. 2017b;284:20170768. doi: 10.1098/rspb.2017.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovaskainen et al. (2017a).Ovaskainen O, Tikhonov G, Norberg A, Blanchet FG, Duan L, Dunson D, Roslin T, Abrego N. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecology Letters. 2017a;20:561–576. doi: 10.1111/ele.12757. [DOI] [PubMed] [Google Scholar]
- Petney & Andrews (1998).Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team . R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- Randolph et al. (1999).Randolph SE, Miklisová D, Lysy J, Rogers DJ, Labuda M. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177–186. doi: 10.1017/S0031182098003643. [DOI] [PubMed] [Google Scholar]
- Rigaud, Perrot-Minnot & Brown (2010).Rigaud T, Perrot-Minnot M-J, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez et al. (1999).Rodríguez M, Terrazas LI, Márquez R, Bojalil R. Susceptibility to Trypanosoma cruzi is modified by a previous non-related infection. Parasite Immunology. 1999;21:177–185. doi: 10.1046/j.1365-3024.1999.00218.x. [DOI] [PubMed] [Google Scholar]
- Rosà et al. (2018).Rosà R, Andreo V, Tagliapietra V, Baráková I, Arnoldi D, Hauffe H, Manica M, Rosso F, Blaňarová L, Bona M, Derdáková M, Hamšíková Z, Kazimírová M, Kraljik J, Kocianová E, Mahríková L, Minichová L, Mošanský L, Slovák M, Stanko M, Špitalská E, Ducheyne E, Neteler M, Hubálek Z, Rudolf I, Venclikova K, Silaghi C, Overzier E, Farkas R, Földvári G, Hornok S, Takács N, Rizzoli A. Effect of climate and land use on the spatiotemporal variability of tick-borne bacteria in Europe. International Journal of Environmental Research and Public Health. 2018;15 doi: 10.3390/ijerph15040732. Article 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross et al. (2018).Ross BD, Hayes B, Radey MC, Lee X, Josek T, Bjork J, Neitzel D, Paskewitz S, Chou S, Mougous JD. Ixodes scapularis does not harbor a stable midgut microbiome. ISME Journal. 2018;12:2596–2607. doi: 10.1038/s41396-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofonea, Alizon & Michalakis (2015).Sofonea MT, Alizon S, Michalakis Y. From within-host interactions to epidemiological competition: a general model for multiple infections. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370 doi: 10.1098/rstb.2014.0303. Article 201403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong et al. (2009).Sprong H, Wielinga PR, Fonville M, Reusken C, Brandenburg AH, Borgsteede F, Gaasenbeek C, Van Der Giessen JW. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasites and Vectors. 2009;2 doi: 10.1186/1756-3305-2-41. Article 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuen, Granquist & Silaghi (2013).Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Frontiers in Cellular and Infection Microbiology. 2013;3 doi: 10.3389/fcimb.2013.00031. Article 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei & Kwan (2017).Swei A, Kwan JY. Tick microbiome and pathogen acquisition altered by host blood meal. ISME Journal. 2017;11:813–816. doi: 10.1038/ismej.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei, Meentemeyer & Briggs (2011).Swei A, Meentemeyer R, Briggs CJ. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (Acari: Ixodidae) with Borrelia burgdorferi. Journal of Medical Entomology. 2011;48:20–28. doi: 10.1603/ME10131. [DOI] [PubMed] [Google Scholar]
- Thornton, Running & White (1997).Thornton PE, Running SW, White MA. Generating surfaces of daily meteorological variables over large regions of complex terrain. Journal of Hydrology. 1997;190:214–251. [Google Scholar]
- Tikhonov et al. (2017).Tikhonov G, Abrego N, Dunson D, Ovaskainen O. Using joint species distribution models for evaluating how species-to-species associations depend on the environmental context. Methods in Ecology and Evolution. 2017;8:443–452. doi: 10.1111/2041-210X.12723. [DOI] [Google Scholar]
- Van Treuren et al. (2015).Van Treuren W, Ponnusamy L, Brinkerhoff RJ, Gonzalez A, Parobek CM, Juliano JJ, Andreadis TG, Falco RC, Ziegler LB, Hathaway N, Keeler C, Emch M, Bailey JA, Roe RM, Apperson CS, Knight R, Meshnick SR. Variation in the microbiota of Ixodes ticks with regard to geography, species, and sex. Applied and Environmental Microbiology. 2015;81:6200–6209. doi: 10.1128/AEM.01562-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssier-Taussat et al. (2013).Vayssier-Taussat M, Moutailler S, Michelet L, Devillers E, Bonnet S, Cheval J, Hebert C, Eloit M. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLOS ONE. 2013;8:e81439. doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venclikova et al. (2014).Venclikova K, Rudolf I, Mendel J, Betasova L, Hubalek Z. Rickettsiae in questing Ixodes ricinus ticks in the Czech Republic. Ticks and Tick-borne Diseases. 2014;5:135–138. doi: 10.1016/j.ttbdis.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Wagemakers et al. (2015).Wagemakers A, Staarink PJ, Sprong H, Hovius JWR. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends in Parasitology. 2015;31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Warton et al. (2015).Warton DI, Blanchet FG, O’Hara RB, Ovaskainen O, Taskinen S, Walker SC, Hui FKC. So many variables: joint modeling in community ecology. Trends in Ecology & Evolution. 2015;30:766–779. doi: 10.1016/j.tree.2015.09.007. [DOI] [PubMed] [Google Scholar]
- West & Buckling (2003).West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proceedings of the Royal Society B: Biological Sciences. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Vilchez & Mateos (2010).Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLOS ONE. 2010;5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik et al. (2018).Zolnik CP, Falco RC, Daniels TJ, Kolokotronis SO. Transient influence of blood meal and natural environment on blacklegged tick bacterial communities. Ticks and Tick-borne Diseases. 2018;9:563–572. doi: 10.1016/j.ttbdis.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Zolnik et al. (2016).Zolnik CP, Prill RJ, Falco RC, Daniels TJ, Kolokotronis SO. Microbiome changes through ontogeny of a tick pathogen vector. Molecular Ecology. 2016;25:4963–4977. doi: 10.1111/mec.13832. [DOI] [PubMed] [Google Scholar]
- Zurell, Pollock & Thuiller (2018).Zurell D, Pollock LJ, Thuiller W. Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments? Ecography. 2018;41:1812–1819. doi: 10.1111/ecog.03315. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The complete metadata of the samples and their matching sequence accession numbers area available at Figshare:Aivelo, Tuomas; Tschirren, Barbara; Norberg, Anna (2019): Tick data used in “Human pathogen co-occurrence in Ixodes ricinus ticks: effects of landscape topography, climatic factors and microbiota interactions”. figshare. Dataset. DOI: 10.6084/m9.figshare.7380767.v3.