Significance
Phytochrome B (phyB) is the predominant red light photoreceptor that transduces red light signals to downstream signaling. On red light exposure, photoactivated phyB interacts with a transcription factor termed PHYTOCHROME INTERACTING FACTOR 3 (PIF3), a repressor of red light signaling, triggering its rapid phosphorylation and subsequent degradation. Thus, phyB-PIF3 defines a critical regulatory hub for red light-mediated seedling development. In this study, we show that B-BOX CONTAINING PROTEIN 4 (BBX4) is a key component involved in the phyB-PIF3 regulatory module. phyB directly interacts with BBX4 and positively controls the abundance of BBX4 in red light. Accumulated BBX4 directly interacts with PIF3 to inhibit its transcriptional activation activity toward target genes, thereby promoting photomorphogenesis.
Keywords: phyB, COP1, BBX4, photomorphogenesis, light signaling
Abstract
Phytochrome B (phyB) absorbs red light signals and subsequently initiates a set of molecular events in plant cells to promote photomorphogenesis. Here we show that phyB directly interacts with B-BOX CONTAINING PROTEIN 4 (BBX4), a positive regulator of red light signaling, and positively controls its abundance in red light. BBX4 associates with PHYTOCHROME INTERACTING FACTOR 3 (PIF3) and represses PIF3 transcriptional activation activity and PIF3-controlled gene expression. The degradation of BBX4 in darkness is dependent on CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) and the 26S proteasome system. Collectively, BBX4 acts as a key component of the phyB-PIF3–mediated signaling module and fine tunes the red light action. phyB promotes the accumulation of BBX4, which in turn serves to repress PIF3 action through direct physical interaction to promote photomorphogenic development in red light.
Light signals are perceived by a variety of wavelength-specific photoreceptors, including phytochromes (phys), cryptochromes, phototripins, and UV-B resistance locus 8 (1–5). Of these, phys sense red (R) and far-red (FR) light signals to mediate various developmental processes in plants, including seed germination, photomorphogenesis, shade avoidance, flowering, and senescence (6, 7). The phys can be photoconverted between 2 states, an inactive Pr form absorbing R light and a biologically active Pfr form sensing FR light. phyA-phyE have both unique and overlapping functions, among which phyB is the primary R light photoreceptor in Arabidopsis (8, 9).
Photoactivated phyB translocates from the cytoplasm to the nucleus, where it interacts directly with a subset of basic helix-loop-helix (bHLH) transcription factors, termed phytochrome-interacting factors (PIFs) including PIF1, PIF3, PIF4, and PIF5. Subsequently, this molecular event leads to the promotion of their degradation and their sequestration from their target promoters (10). PIF3, the founding member of the PIFs, is identified by a yeast two-hybrid screen using phyB as the bait (11). phyB interacts with and recruits PIF3 into nuclear bodies during the dark-to-light transition before its degradation (12). PIF3 is a key repressor of phyB-mediated signaling, which regulates hypocotyl elongation, cotyledon expansion of seedlings, and chloroplast development (13, 14). In the dark, 2 E3 ubiquitin ligases defined by CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) and DE-ETIOLATED 1, stabilize the abundance of PIF3 (15, 16). Thus, PIF3 is enriched in the nucleus and mediates the expression levels of a large group of target genes to maintain the state of skotomorphogenesis in plants. On light illumination, photoactivated phyA and phyB interact directly with PIF3 and rapidly trigger its phosphorylation on multiple residues and subsequent degradation through the 26S proteasome system (17, 18). The phosphorylation of PIF3 is directly mediated by phy and photoregulatory protein kinases (19, 20). The SCFEBF1/2 E3 ligase complex targets phosphorylated PIF3 for ubiquitination and degradation under a wide range of light intensity conditions (21), while under high light conditions, the CUL3LRBs E3 ligase complex simultaneously targets both phyB and phosphorylated PIF3 for ubiquitination and concurrent degradation to reduce the sensitivity of plant cells to red light (22). In addition, it has been shown that PIF3 promotes the degradation of phyB to attenuate plant light responses (18, 23, 24).
Recent work has revealed that a subset of B box-containing proteins (BBXs) play critical roles in light-dependent development in plants. Multiple BBXs, acting downstream of various photoreceptors, function in COP1- and HY5-mediated light signaling pathways in promoting or repressing seedling development (25–29). BBX4 (also known as CONSTANS-LIKE 3 [COL3]) contains 2 tandem B-box domains in its N-terminal half and a conserved CCT (CO, COL, TOC1) domain in its C-terminal region (30). BBX4 directly associates with FLOWERING LOCUS T (FT) promoter through its CCT domain in the presence of BBX32 to repress FT expression and flowering (31). In addition to repressing flowering, BBX4 is also involved in various physiological and developmental processes, including photomorphogenesis, formation of lateral root and shoot branching, shoot elongation, and accumulation of anthocyanin (32). A loss-of-function bbx4 mutant specifically displays elongated hypocotyls in R light, but not in blue (B) and FR light (32). This indicates that BBX4 acts as a positive regulator of phyB-mediated signaling. However, the molecular mechanism underlying BBX4 in the regulation of R light-mediated inhibition of hypocotyl elongation has remained largely unknown.
In this study, we demonstrated that 2 key regulators of R light signaling, phyB and PIF3, both physically interact with BBX4 in response to R light. BBX4 protein level accumulated to high abundance in R light in a phyB-dependent manner. BBX4 genetically acts upstream of PIF3 and represses its transcriptional activation activity. In short, on R light illumination, photoactivated phyB directly associates with BBX4 and promotes its accumulation. Thus, accumulated BBX4 interacts with PIF3 to inhibit its transcriptional activation activity, thereby promoting photomorphogenic development.
Results
BBX4 Is a Positive Regulator of Red Light Signaling.
BBX4 acts as a positive regulator of the phyB-mediated inhibition of hypocotyl elongation (32). Consistently, 2 independent bbx4 single mutants, bbx4-1 and bbx4-2, which were generated by the clustered regulatory interspaced short palindromic repeats (CRISPR)/Cas9 technique (ref. 33 and SI Appendix, Fig. S1A), showed similar hypocotyl phenotypes with Col (wild-type [WT]) when grown in the dark (D), B, and FR light conditions (SI Appendix, Fig. S1). However, they displayed significantly elongated hypocotyls compared with Col grown in white (W) and R light conditions (SI Appendix, Fig. S1 D–G). These data further confirm BBX4 as a positive regulator of R light signaling.
To verify these genetic results, we generated 2 independent YFP-tagged BBX4 (YFP-BBX4) transgenic lines, in which the expression of BBX4 was overexpressed and YFP-BBX4 protein was clearly detectable (SI Appendix, Fig. S2 A and B). They showed a similar etiolated phenotype as Col (WT) when grown in darkness (SI Appendix, Fig. S2 C and D); however, these 2 transgenic lines overexpressing BBX4 displayed markedly shortened hypocotyls in the W, B, R, and FR light conditions tested (SI Appendix, Fig. S2 E–L), indicating that overexpression of BBX4 confers hypersensitivity to inhibition of hypocotyl elongation in response to various wavelength-specific light signals in Arabidopsis.
phyB Genetically and Physically Interacts with BBX4.
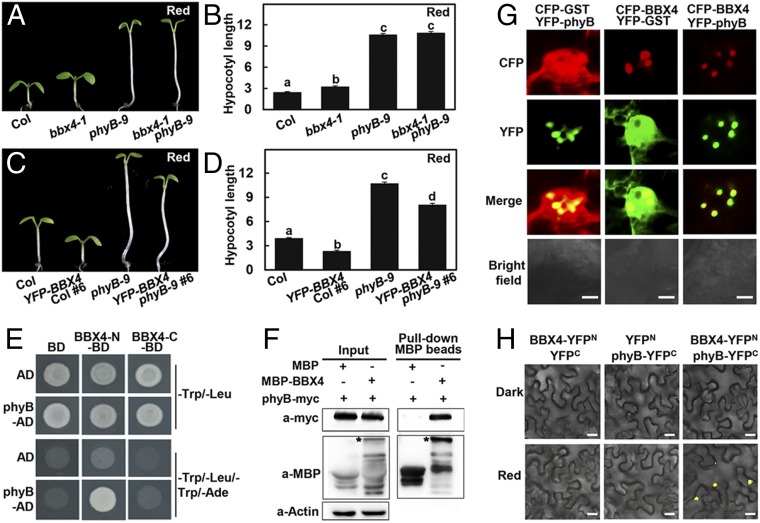
Considering that phyB is the predominant R light photoreceptor and BBX4 functions in R light signaling (9, 32), we investigated the genetic interplay between phyB and BBX4. Both bbx4-1 and phyB-9 showed longer hypocotyls than Col, and hypocotyl length was obviously longer in phyB-9 mutant seedlings compared with bbx4-1 seedlings in R light (Fig. 1 A and B). The double-mutant phyB-9 bbx4-1 was indistinguishable from phyB-9 grown in R light (Fig. 1 A and B). YFP-BBX4 showed shortened hypocotyls compared with Col and phyB-9. The hypocotyl length of YFP-BBX4 phyB-9 was shorter than that of phyB-9, but longer than that of Col and YFP-BBX4 (Fig. 1 C and D). In addition, the PBC (phyB-CFP phyB-9) transgenic line showed shorter hypocotyls than Col and bbx4-1, and PBC bbx4-1 exhibited similar hypocotyl phenotypes as PBC grown in R light (SI Appendix, Fig. S3 A and B). These genetic results suggest that BBX4 likely acts downstream of phyB in mediating part of R light signaling.
Fig. 1.
phyB genetically and physically interacts with BBX4. (A and B) Hypocotyl phenotype (A) and length (B) of 4-d-old Col, bbx4-1, phyB-9, and bbx4-1 phyB-9 seedlings grown in R (115.8 μmol/m2/s) light. The unit of hypocotyl length is millimeters. The experiments were performed 3 times with similar results. The graphs depict one of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (C and D) Hypocotyl phenotype (C) and length (D) of 4-d-old Col, YFP-BBX4 #6, phyB-9, and YFP-BBX4 phyB-9 #6 seedlings grown in R light (115.8 μmol/m2/s). The unit of hypocotyl length is millimeters. The experiments were performed 3 times, with similar results. The graphs depict 1 of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (E) Yeast two-hybrid interactions between the BBX4 and phyB. (F) Semi-in vivo pull-down assay of BBX4 with phyB. Total plant protein was extracted from 4-d-old phyB-myc transgenic seedlings grown in R light (115.8 μmol/m2/s). Equal amounts of MBP and MBP-BBX4 proteins were added to total plant protein extracts. The asterisk indicates MBP-BBX4. Actin served as a negative control. (G) BBX4 and phyB colocalize to the nuclear bodies in tobacco cells. CFP-BBX4 and YFP-phyB were transiently coexpressed in tobacco leaves. CFP-GST and YFP-GST served as negative controls. (Scale bars: 5 µm.) (H) BiFC assay showing the interaction of BBX4 with phyB in R light. BBX4 and phyB were fused to the N- and C-terminal fragments of YFP (YFPN and YFPC, respectively). Unfused YFPN and YFPC fragments served as negative controls. (Scale bars: 20 μm.)
We next examined whether BBX4 interacts with phyB at the protein level. As full-length and middle portion of BBX4 (103 to 201) showed self-activation activity in yeast cells (SI Appendix, Fig. S4), we fused binding domain (BD) with the BBX4 N-terminal half (BBX4-N, 1 to 102) containing 2 conserved B-box domains or the C-terminal region (BBX4-C, 202 to 294) carrying an intact CCT domain for yeast two-hybrid assays. Both of these BBX4 truncation proteins were expressed at comparable levels in yeast cells when coexpressed with phyB (SI Appendix, Fig. S5). BBX4-N, but not BBX4-C, was able to interact with phyB in yeast cells (Fig. 1E).
Next, purified MBP-fused BBX4 (MBP-BBX4) recombinant protein and cell extracts from the transgenic seedlings expressing myc-phyB grown in R light were used for pull-down assays. MBP-BBX4, but not the MBP (negative control) could pull down the myc-phyB protein as detected on immunoblot assays (Fig. 1F). In addition, phyB-YFP and CFP-BBX4 colocalized in the nuclear bodies when transiently coexpressed in Arabidopsis protoplasts. The negative controls GST-CFP and phyB-YFP, or CFP-BBX4 and GST-YFP, did not exhibit any colocalization in the same experiments (Fig. 1G).
We next used a bimolecular fluorescence complementation (BiFC) assay and fused BBX4 with a split N-terminal of YFP (YFPN) and phyB with a split C-terminal of YFP (YFPC). YFP signals could not be observed when transiently coexpressed BBX4-YFPN and phyB-YFPC in Nicotiana benthamiana leaves were incubated in darkness; however, strong YFP signals were clearly detected on transference to R light (Fig. 1H). These data suggest that phyB physically interacts with BBX4 likely in an R light-dependent manner.
phyB Stabilizes the Abundance of BBX4 in Red Light.
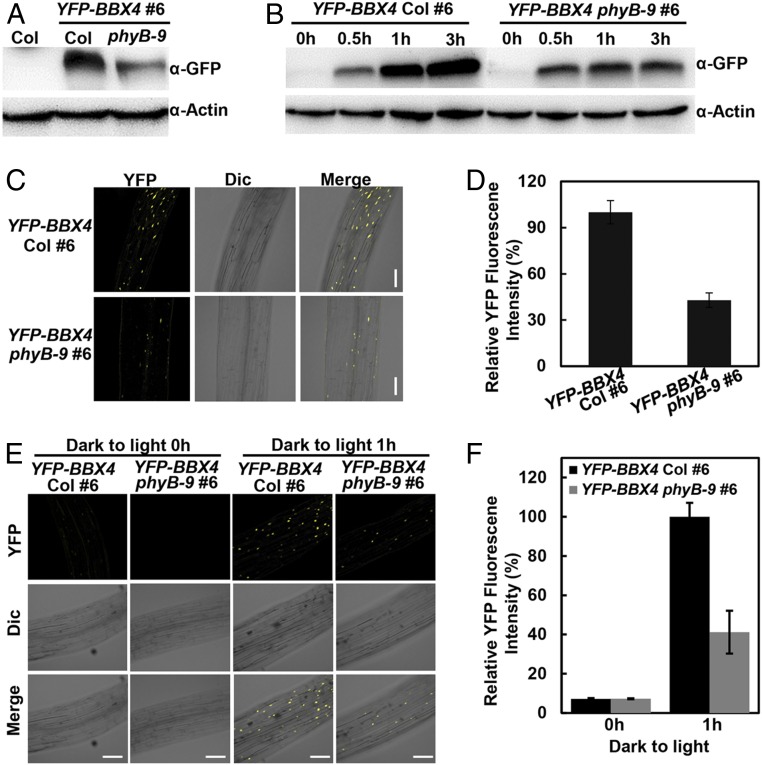
Photoactivated phyB interacts with PIF3 and subsequently promotes its phosphorylation and degradation (11, 17, 34). Therefore, to examine the functional consequence of phyB–BBX4 interaction, we introduced a phyB-9 mutation into the YFP-BBX4 transgenic line by genetic crossing and examined whether phyB affects the abundance of BBX4 in R light. YFP-BBX4 was abundant in YFP-BBX4 seedlings but obviously decreased in YFP-BBX4 phyB-9 seedlings grown in constant R light (Fig. 2A). In addition, YFP-BBX4 accumulated markedly more YFP-BBX4 protein compared with YFP-BBX4 phyB-9 on transference to R light for 1 h and 3 h (Fig. 2B). Consistently, the YFP signals in YFP-BBX4 phyB seedlings were clearly reduced compared with those in YFP-BBX4 grown in constant R light or on 1 h of R light irradiation (Fig. 2 C and D). The transcript levels of BBX4 in YFP-BBX4 were only slightly reduced compared with those in YFP-BBX4 phyB-9 when grown in darkness for 4 d on transference to red light for 3 h or grown in red light, respectively (SI Appendix, Fig. S6). These results suggest that phyB positively modulates BBX4 abundance in R light.
Fig. 2.
phyB stabilizes BBX4 in R light. (A) YFP-BBX4 protein levels in YFP-BBX4 Col #6 and YFP-BBX4 phyB-9 #6 grown in R light (115.8 μmol/m2/s) for 4 d. Col served as a negative control. (B) Immunoblot analysis of YFP-BBX4 protein levels in YFP-BBX4 Col #6 and YFP-BBX4 phyB-9 #6 grown in the dark for 4 d and then transferred to R light (115.8 μmol/m2/s) for 0, 0.5, 1, and 3 h, as indicated. Actin served as a loading control. (C and D) Analysis of YFP fluorescence signals in hypocotyls of YFP-BBX4 Col #6 and YFP-BBX4 phyB-9 #6 seedlings grown in R light (115.8 μmol/m2/s) for 4 d. The corresponding fluorescence intensity was measured using ImageJ and was compared between the overall signals from the images, as shown in D. Data are mean ± SE (n ≥ 10). (Scale bars: 100 μm.) (E and F) Analysis of YFP fluorescence signals in hypocotyls of YFP-BBX4 Col #6 and YFP-BBX4 phyB-9 #6 seedlings grown in the dark for 4 d, then transferred to R light (115.8 μmol/m2/s) for 0 and 1 h. The corresponding fluorescence intensity was measured using ImageJ software and compared between the overall signals from the images, as shown in F. Data are mean ± SE (n ≥ 10). (Scale bars: 100 μm.)
Because phyB rapidly forms nuclear bodies on R light exposure (35), we examined whether BBX4 has any effect on the formation of phyB nuclear bodies. Nuclear bodies were clearly detectable in PBC, consistent with previous studies (35). Nuclear bodies observed in the nucleus of PBC bbx4-1 were similar to those in PBC (SI Appendix, Fig. S7), implying that BBX4 might not affect the formation of phyB nuclear bodies.
BBX4 Undergoes COP1-Mediated Degradation in Darkness.
A previous study showed that COP1 physically interacts with BBX4 (32), and thus we examined whether COP1 mediates the degradation of BBX4. We first examined whether BBX4 is degraded via the 26S proteasome system. For this, 4-d-old dark-grown YFP-BBX4 transgenic seedlings were applied with DMSO or various concentrations of MG132 (a proteasome inhibitor) for 3 h. YFP-BBX4 protein levels were clearly increased when the transgenic seedlings were treated with 200 μM MG132 (SI Appendix, Fig. S8A).
Next, we introduced cop1-4 and cop1-6 mutations into YFP-BBX4 transgenic lines by genetic crossing. Etiolated YFP-BBX4 cop1-4 and YFP-BBX4 cop1-6 transgenic lines displayed clearly stronger YFP signals compared with YFP-BBX4 transgenic seedlings (SI Appendix, Fig. S8B). These observations indicate that COP1 promotes the degradation of BBX4 via the 26S proteasome system in etiolated seedlings.
BBX4 Genetically and Physically Interacts with PIF3.
PIF3, acting directly downstream of phyB, represses R light-mediated seedling development (11). We thus investigated the genetic interaction between BBX4 and PIF3. As reported, bbx4-1 displayed longer hypocotyls but pif3-1 had shorter hypocotyls compared with Col when grown in R light. The hypocotyl length of bbx4-1 pif3-1 was indistinguishable from that of pif3-1, indicating that BBX4 and PIF3 function in the same pathway in the regulation of R light-mediated hypocotyl growth (Fig. 3 A and B).
Fig. 3.
BBX4 genetically and physically interacts with PIF3. (A and B) Hypocotyl phenotype (A) and length (B) of 4-d-old Col, bbx4-1, pif3-1 and bbx4-1 pif3-1 seedlings grown in R light (115.8 μmol/m2/s). The unit of hypocotyl length is millimeters. The experiments were performed 3 times, with similar results. The graphs depict 1 of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (C) Yeast two-hybrid interactions between the BBX4 and PIF3. (D) FRET between CFP-PIF3 and YFP-BBX4 analyzed by acceptor bleaching in nuclei. (Top) Representative prebleach nuclei coexpressing YFP-BBX4 and CFP-PIF3 excited with a 514-nm or 405-nm laser, resulting in emission from YFP or CFP, respectively. (Bottom) The same nuclei after bleaching excited with a 514-nm or 405-nm laser. (E) The relative intensities of both YFP and CFP inside the nuclei were measured once before and twice after the bleaching, as indicated in D. (F) BiFC assay showing the interaction of BBX4 with PIF3 in red light. BBX4 and PIF3 were fused to the N- and C-terminal fragments of YFP (YFPN and YFPC, respectively). Unfused YFPN and YFPC fragments served as negative controls. (Scale bars: 40 μm.)
We next tested whether BBX4 interacts with PIF3. Both BBX4-N and BBX4-C proteins were expressed at similar levels in yeast cells when coexpressed with PIF3 (SI Appendix, Fig. S5). BBX4-N, but not BBX4-C, could interact with PIF3 in yeast cells, indicating that B-box domain of BBX4 might mediate its interaction with PIF3 (Fig. 3C). We carried out fluorescence resonance energy transfer (FRET) experiments to verify these results. CFP-PIF3 with YFP-BBX4 were coexpressed in onion epidermal cells. After excitation with 405- and 514-nm wavelength light sources, emission of YFP-BBX4 was reduced dramatically, whereas emission from CFP-PIF3 increased (Fig. 3 D and E), indicating that FRET had occurred between CFP-PIF3 and YFP-BBX4 proteins before the bleach in living plant cells. Furthermore, we performed BiFC analysis and transiently coexpressed BBX4-YFPN and PIF3-YFPC in N. benthamiana leaves. We did not observe any YFP signals when N. benthamiana leaves incubated in darkness; however, YFP signals were clearly detectable on R light exposure. The negative controls BBX4-YFPN and YFPC, or YFPN and PIF3-YFPC could not produce any detectable YFP signals (Fig. 3F). Taken together, these data support a conclusion that BBX4 physically interacts with PIF3 likely in an R light-dependent manner.
BBX4 Represses PIF3 Transcriptional Activation Activity.
To assess the biological significance for BBX4 and PIF3 interaction, we first tested whether BBX4 affects the transcriptional activity of PIF3. We found that PIF3 could activate the proBBX23:LacZ reporter in yeast cells, consistent with a previous study (36). BBX4 could not activate proBBX23:LacZ; however, the activation of PIF3 on proBBX23:LacZ was dramatically decreased in the presence of BBX4, as revealed by analysis of β-galactosidase activity (Fig. 4A). Moreover, PIF3 could activate proBBX29:LUC reporter (a PIF3-regulated gene; ref. 37) when transiently expressed in Arabidopsis protoplasts. BBX4 alone had no effect on the proBBX29:LUC reporter in the same system; however, the activation of proBBX29:LUC by PIF3 was significantly decreased when PIF3 was transiently coexpressed together with BBX4 (Fig. 4 B and C). These results indicate that BBX4 can repress the transcriptional activation activity of PIF3 toward its targets. In addition, the expression of 6 PIF3-regulated genes tested—BBX23, BBX29, XTR7, SNRK2.5, SDR, and ARF18 (37)—were down-regulated in pif3-1 but up-regulated in bbx4-1. The transcript levels of these 6 genes tested in the pif3-1 bbx4-1 double mutant were comparable to those in pif3-1 (Fig. 4D), suggesting that BBX4 regulates gene expression, at least in part, in a PIF3-dependent manner.
Fig. 4.
BBX4 inhibits the transcriptional activation activity of PIF3. (A) Yeast one-hybrid analysis showing that BBX4 inhibits the ability of PIF3 to bind to BBX23 promoter. Error bars represent SD (n =3). **P < 0.01, Student’s t test. (B) Schematic representation of constructs used in the transient transfection assay in Arabidopsis protoplasts. Arrows after the 35S promoters indicate that the transcriptional start site BBX29 promoter was fused to the firefly luciferase to create the reporter construct. (C) Transient dual LUC reporter gene assay showing that BBX4 represses the transcriptional activity of PIF3 on proBBX29:LUC reporter. Error bars represent SD (n = 3). **P < 0.01, Student’s t test. (D) Expression levels of BBX23, BBX29, XTR7, SNRK2.5, SDR, and ARF18 in Col, pif3-1, bbx4-1, and bbx4-1pif3-1 mutants. All seedlings were grown in darkness for 3 d and then transferred to R light for 30 min. Error bars represent SD (n = 3).
Discussion
R light is predominantly perceived by phyB, which mediates a variety of light-dependent physiological and developmental processes in plants (38). phyB maintains a biologically inactive form in the cytoplasm of plant cells in darkness. On R light irradiation, phyB is converted to a biologically active state and translocates into the nucleus, in which it interacts with multiple families of transcription factors, such as PIF3 and EIN3, to induce their degradation (11, 17, 34, 39). Here we show that BBX4 is a phyB-interacting protein, but phyB mediates a high level of BBX4 accumulation in R light. Accumulated BBX4 associates with PIF3 to repress its transcriptional activation activity and PIF3-controlled gene expression, consequently promoting phyB-mediated photomorphogenic development.
Photoactivated phyB promptly shifts into the nucleus and interacts with the COP1-SPA1 E3 ubiquitin ligase complex to interfere with its biochemical activity (40). COP1 interacts and colocalizes with BBX4 in yeast and living plant cells, respectively. Mutation in BBX4 partially suppresses the constitutively photomorphogenic phenotype of cop1 in darkness (32). The degradation of BBX4 in dark-grown seedlings was dependent on COP1 as well as on the 26S proteasome system (SI Appendix, Fig. S8). These facts imply that phyB-mediated inhibition of COP1 activity might lead to the accumulation of BBX4 in response to R light (Fig. 2). phyB was seen to directly interact with BBX4 (Fig. 1), and this molecular event might also contribute to the stabilization of BBX4 in R light. The robust increase of BBX4 is a necessary step for phyB-mediated seedling development. Although phyB rapidly induces the phosphorylation and degradation of the majority of PIF3 on R light exposure, the abnormal hypocotyl phenotypes of pif3 mutant and transgenic seedlings overexpressing PIF3 grown in R light support the conclusion that the remaining pool of PIF3 also has a negative role on photomorphogenesis in R light (41). BBX4 physically associates with PIF3 (Fig. 3), thereby inhibiting PIF3 transcriptional activation activity to affect PIF3-regulated gene expression (Fig. 4). Consequently, these molecular events result in the promotion of photomorphogenic development in R light. BBX4 could directly bind to the FT promoter and repress its expression in the presence of BBX32 (31), suggesting that BBX4 functions as a transcription factor in regulating physiological and developmental processes. Therefore, light induced-BBX4 could also directly associate with a number of target gene promoters to control their transcription and mediate photomorphogenesis.
phyB was found to stabilize BBX4 (Fig. 2) but to promote the degradation of PIF3 (17, 18), demonstrating that phyB has opposite effects on the stability of these 2 types of transcription factors. BBX4 and PIF3 antagonistically regulate phyB-mediated signaling, and BBX4 acts as a positive regulator of R light signaling (ref. 32 and SI Appendix, Figs. S1 and S2), while PIF3 represses R light-dependent seedling development (11). Taken together, this evidence supports the conclusion that phyB promotes photomorphogenesis in R light not only by promoting degradation of negative regulators like PIF3, but also by facilitating the accumulation of positive regulators like BBX4.
Global transcriptomic analysis has revealed that phyB is able to associate with a large number of gene promoter regions and affect massive gene expression on R light irradiation (42, 43). phyB lacks any recognizable DNA-binding domain (9), indicating that binding of phyB to DNA requires additional transcription factors. Consistently, an increasing number of studies have shown that phyB regulates downstream gene expression by interacting with multiple transcription factors. phyB interacts with PHOTOPERIODIC CONTROL OF HYPOCOTYL 1 (PCH1) to repress LONG HYPOCOTYL IN FAR-RED (HFR1), ARABIDOPSIS THALIANA HOMEOBOX PROTEIN 2 (ATHB2), and PIF4 transcription (44). phyB can form a tripartite complex with PHYTOCHROME-DEPENDENT LATEFLOWERING (PHL) and CONSTANS (CO) to activate the expression of FT (45). Previous studies (12) and the present study have revealed that phyB, BBX4, and PIF3 can physically interact with one another (Figs. 1 and 3), implying that they might coexist in a protein complex in some circumstances, most likely early after R light exposure. Formation of a phyB-BBX4-PIF3 tripartite complex also might be required for R light- and phyB-mediated transcriptional reprogramming. phyB inhibits PIF3 action not only by triggering its degradation, but also by sequestering it from its target sites (46, 47). phyB promoted the accumulation of BBX4 that could inhibit PIF3 biochemical activity (Figs. 2 and 4), indicating that phyB might also repress PIF3 action by increasing the abundance of BBX4.
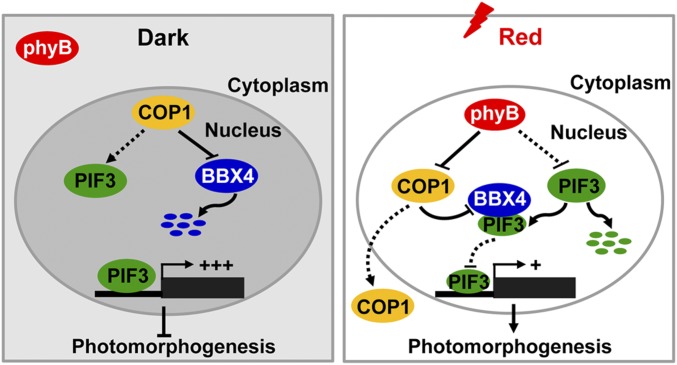
In conclusion, we demonstrate that BBX4 is a previously unidentified phyB- interacting protein whose abundance positively modulated by phyB in R light. It appears that phyB, BBX4, and PIF3 function coordinately in R light signaling. On one hand, photoactivated phyB promotes the degradation of PIF3. On the other hand, phyB interferes with the biochemical activity of COP1-SPA1 complex, thus leading to the accumulation of BBX4 on R light irradiation. Accumulated BBX4 physically interacts with the remaining pool of PIF3 and inhibit its transcriptional activity, strengthening the phyB-mediated R light inactivation of PIF3 activity. Consequently, phyB-BBX4-PIF3 might be a critical regulatory module that fine tunes photomorphogenic development in responsiveness of R light (Fig. 5).
Fig. 5.
A proposed working model depicting the mechanism of BBX4 in phyB-PIF3–mediated light signaling. In darkness, phyB is in inactive form in the cytoplasm. COP1 stabilizes PIF3 and interacts with BBX4 to promote its degradation via the 26S proteasome system. Highly accumulated PIF3 induces the expression of its direct-target genes to repress photomorphogenesis. On R light illumination, phyB is converted to a biologically active form and translocated into the nucleus. Photoactivated phyB promotes PIF3 protein degradation and induces the accumulation of BBX4 protein, likely by inhibiting the COP1–BBX4 association. In addition, accumulated BBX4 interacts with the remaining PIF3 to inhibit the transcription of PIF3 direct-target genes and promote photomorphogenesis.
Materials and Methods
Plant Materials and Growth Conditions.
The Arabidopsis thaliana phyB-9 (48) and pif3-1 (40) mutants, PHYB-CFP (PBC; ref. 35) and myc-phyB (49) transgenic lines were of the Col-0 ecotype. Double-mutant/transgenic plants were generated by genetic crossing, and homozygous lines were verified by PCR genotyping or antibiotic screen. Seeds were surface-sterilized with 30% commercial Clorox bleach and sown on 1× Murashige and Skoog (MS) medium containing 1% sucrose and 0.8% agar. The seeds were stratified in darkness for 3 d at 4 °C, then transferred to light chambers maintained at 22 °C. The fluence rates of the light growth chambers were 13.24 μmol/m2/s for W light, 3.88 μmol/m2/s for B light, 115.8 μmol/m2/s for R light, and 4.3 μmol/m2/s for FR light.
Plasmid Construction.
The full-length BBX4, phyB, or PIF3 coding sequence (CDS) were cloned into the pDONR223 vector using Gateway BP Clonase enzyme mix (Invitrogen) and introduced into the plant binary vector pEarlyGate 102, pEarlyGate 104, pSPYNE, or pSPYCE (50) using Gateway LR Clonase enzyme mix (Invitrogen). To produce constructs for yeast two-hybrid assays, full-length BBX4, BBX4-N (1–102) and BBX4-C (202-294) fragments were cloned into the EcoRI/BamHI sites of pGBKT7 vector (Clontech). Full-length phyB and PIF3 were cloned into the EcoRI/BamHI sites of pGADT7 vector (Clontech). To produce constructs for purification of MBP-BBX4 recombinant protein, full-length BBX4 was cloned into EcoRI/SalI site of pMAL-c2x vector. The primers used for plasmids construction were listed in SI Appendix, Table S1.
Generation of bbx4 Mutants Using CRISPR/Cas9 Technique.
The bbx4 mutants were generated by CRISPR/Cas9 technique as described previously (51), and 23-bp target sites (5′-N20NGG-3′) were searched on the web site of CRISPR-GE (http://skl.scau.edu.cn/) (33). The sgRNA target sites of BBX4 were subcloned into a pHEE401E vector. After transformation into Agrobacterium tumefaciens GV3101 by the freeze-thaw method, the binary constructs were introduced into Col via the floral dip method. The T0 plants were sowed on the MS plates containing 50 mg/L hygromycin. The resistant seedlings (T1) were transferred to soil. The mutations in BBX4 were identified by PCR amplification and sequencing. Homozygous mutants were crossed with Col to remove the T-DNA insertion including CRISPER/Cas9.
Transgenic Plants.
The pEarlyGate104-BBX4 construct was transformed into A. tumefaciens GV3101 by the freeze-thaw method. The floral dip method was used to generate transgenic plants (52). Transgenic plants were selected on MS medium containing 20 mg/L Basta. Homozygous lines were used for genetic and biochemical studies.
Measurement of Hypocotyl Length.
To measure the hypocotyl length of seedlings, seeds were surface-sterilized and sown on MS plates. After stratification at 4 °C in darkness for 3 d, the seeds were placed in continuous white light for 8 h to induce uniform germination. The seeds were then transferred to dark or different light conditions and incubated at 22 °C for 4 d. The hypocotyl length of seedlings was measured using ImageJ software.
Immunoblot Analysis.
For immunoblot analysis, Arabidopsis seedlings were homogenized in protein extraction buffer containing 100 mM NaH2PO4, 10 mM Tris⋅HCl pH 8.0, 200 mM NaCl, 8 M urea, 1 mM PMSF, and 1× complete protease inhibitor mixture (Roche). The primary antibodies used in this study were anti-GFP (Abmart; catalog no. M20004M), anti-myc (Sigma-Aldrich; catalog no. M4439), and anti-actin (Sigma-Aldrich; catalog no. A0480).
Yeast Two-Hybrid Assays.
For the GAL4 two-hybrid assays, the respective combinations of pGAD-T7 and pGBK-T7 fusion plasmids were cotransformed into yeast strain Y2HGold via the lithium acetate transformation procedure, as described in the Yeast Protocols Handbook (Clontech). The empty pGAD-T7 and pGBKT7 vectors were cotransformed in parallel as negative controls. The interactions were examined on SD/-Trp/-Leu/-His/-Ade medium (Clontech).
BiFC Assay.
The YFPN and YFPC fused plasmids were transformed into Agrobacterium strain GV3101, and the indicated transformants pairs were infiltrated into N. benthamiana leaves. After incubation in darkness or exposure to the red light for 36 h, the YFP fluorescence signals were observed and imaged under a Carl Zeiss LSM510 Meta confocal laser scanning microscope. YFP fluorescence was excited by a 514-nm laser and detected between 517 and 589 nm.
FRET Assay.
The FRET experiments were carried out as described previously (32, 53). In brief, the 35S:CFP-PIF3 and 35S:YFP-BBX4 constructs were introduced into onion (Allium cepa) epidermal cells by particle bombardment and incubated, and live cell images were acquired using a Zeiss Axiovert 200 microscope equipped with a laser scanning confocal imaging LSM 510 Meta system. Cells were visualized at 24 h after particle bombardment using the confocal microscope through a Plan-Neofluor 403/1.3 oil (differential interference contrast) objective. The multitracking mode was used to eliminate spillover between fluorescence channels. The CFP was excited by a 405-nm laser diode and the YFP was excited by an argon-ion laser, both at low intensities. Regions of interest were selected and bleached over 100 iterations using the argon-ion laser at 100%.
Semi-in Vivo Pull-Down Assay.
The total protein of 4-d-old red light-grown phyB-myc seedlings was extracted with extraction buffer containing 150 mM NaCl, 10 mM Tris·HCl pH 7.5, 2 mM EDTA, 0.5% Nonidet P-40, and 1× protease inhibitor mixture (Roche). MBP-BBX4 or MBP purified recombinant proteins were mixed with total proteins and then incubated with anti-MBP beads (Sigma-Aldrich) for 4 h at 4 °C. The anti-MBP (New England BioLabs; catalog no. E8032S), anti-myc (Sigma-Aldrich; catalog no. M4439), and anti-actin (Sigma-Aldrich; catalog no. A0480) antibodies were used for immunoblotting detection.
Total RNA Isolation and qRT-PCR.
Four-d-old Arabidopsis seedlings grown in R light (115.8 μmol/m2/s) were used to isolate total RNA with the Qiagen RNeasy Plant Mini Kit. cDNA synthesis reactions were performed with 5× All-In-One RT MasterMix (Applied Biological Materials) according to the manufacturer’s instructions. cDNA templates and primer sets were mixed with Hieff qPCR SYBR Green Master Mix (Yeasen), and real-time PCR was performed on a StepOnePlus Real-Time PCR system (Applied Biosystems). Each experiment was performed at least 3 times with similar results, and 3 technical replicates were performed for each sample. The expression levels were normalized to that of housekeeping gene PP2A. The primers used in the qRT-PCR analyses are listed in SI Appendix, Table S1.
Statistical Analysis.
Statistical analyses were performed using Microsoft Excel, GraphPad Prism version 5.0, and an online program (https://astatsa.com/OneWay_Anova_with_TukeyHSD/).
Data Availability.
Sequence data from this article are available in the Arabidopsis Genome Initiative database libraries (https://www.arabidopsis.org/) under the following accession nos.: AT2G18790 for phyB, AT1G09530 for PIF3, AT2G24790 for BBX4, and AT2G32950 for COP1.
Supplementary Material
Acknowledgments
We thank Dr. Magnus Holm (deceased) for help with the FRET experiments and in initiating this study, and Dr. Meng Chen for the phyB-CFP phyB-9 seeds. This work was supported by grants from National Key R&D Program of China (2017YFA0503800), National Natural Science Foundation of China (31621001, 31900210, and 31970258), Peking-Tsinghua Center for Life Sciences, Southern University of Science and Technology, and Nanjing Agricultural University.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915149116/-/DCSupplemental.
References
- 1.Sharrock R. A., Quail P. H., Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3, 1745–1757 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Gallagher S., Short T. W., Ray P. M., Pratt L. H., Briggs W. R., Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc. Natl. Acad. Sci. U.S.A. 85, 8003–8007 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C., et al. , Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269, 968–970 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Guo H., Yang H., Mockler T. C., Lin C., Regulation of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Rizzini L., et al. , Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Kami C., Lorrain S., Hornitschek P., Fankhauser C., Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91, 29–66 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Franklin K. A., Quail P. H., Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61, 11–24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae G., Choi G., Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59, 281–311 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Chen M., Chory J., Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21, 664–671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham V. N., Kathare P. K., Huq E., Phytochromes and phytochrome interacting factors. Plant Physiol. 176, 1025–1038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni M., Tepperman J. M., Quail P. H., PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Ni M., Tepperman J. M., Quail P. H., Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Park E., et al. , Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45, 968–975 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Stephenson P. G., Fankhauser C., Terry M. J., PIF3 is a repressor of chloroplast development. Proc. Natl. Acad. Sci. U.S.A. 106, 7654–7659 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer D., et al. , Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16, 1433–1445 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J., et al. , Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell 26, 3630–3645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P. H., Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23, 439–446 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Ni W., et al. , Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell 25, 2679–2698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin A. Y., et al. , Evidence that phytochrome functions as a protein kinase in plant light signalling. Nat. Commun. 7, 11545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni W., et al. , PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3. Nat. Commun. 8, 15236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J., et al. , Light-dependent degradation of PIF3 by SCFEBF1/2 promotes a photomorphogenic response in Arabidopsis. Curr. Biol. 27, 2420–2430.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni W., et al. , A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344, 1160–1164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sady B., Kikis E. A., Monte E., Quail P. H., Mechanistic duality of transcription factor function in phytochrome signaling. Proc. Natl. Acad. Sci. U.S.A. 105, 2232–2237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang I. C., Henriques R., Seo H. S., Nagatani A., Chua N. H., Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22, 2370–2383 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D., et al. , BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 113, 7655–7660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu D., Jiang Y., Li J., Holm M., Deng X. W., The B-box domain protein BBX21 promotes photomorphogenesis. Plant Physiol. 176, 2365–2375 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin F., et al. , B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell 30, 2006–2019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heng Y., et al. , B-Box containing proteins BBX30 and BBX31, acting downstream of HY5, negatively regulate photomorphogenesis in Arabidopsis. Plant Physiol. 180, 497–508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav A., et al. , The B-Box-Containing microProtein miP1a/BBX31 regulates photomorphogenesis and UV-B protection. Plant Physiol. 179, 1876–1892 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna R., et al. , The Arabidopsis B-box zinc finger family. Plant Cell 21, 3416–3420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi P., Carvallo M., Hamilton E. E., Preuss S., Kay S. A., Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proc. Natl. Acad. Sci. U.S.A. 114, 172–177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta S., Hettiarachchi G. H., Deng X. W., Holm M., Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18, 70–84 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z. P., et al. , Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanna R., et al. , A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16, 3033–3044 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M., Tao Y., Lim J., Shaw A., Chory J., Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15, 637–642 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., et al. , A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiol. 174, 2487–2500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., et al. , A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 9, e1003244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockwell N. C., Su Y. S., Lagarias J. C., Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57, 837–858 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi H., et al. , The red light receptor phytochrome B directly enhances substrate-E3 ligase interactions to attenuate ethylene responses. Dev. Cell 39, 597–610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X. D., et al. , Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol. Plant 8, 467–478 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Kim J., et al. , Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15, 2399–2407 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tepperman J. M., et al. , Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 38, 725–739 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Jung J. H., et al. , Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Huang H., et al. , PCH1 integrates circadian and light-signaling pathways to control photoperiod-responsive growth in Arabidopsis. eLife 5, e13292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endo M., Tanigawa Y., Murakami T., Araki T., Nagatani A., PHYTOCHROME-DEPENDENT LATE-FLOWERING accelerates flowering through physical interactions with phytochrome B and CONSTANS. Proc. Natl. Acad. Sci. U.S.A. 110, 18017–18022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park E., et al. , Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J. 72, 537–546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park E., Kim Y., Choi G., Phytochrome B requires PIF degradation and sequestration to induce light responses across a wide range of light conditions. Plant Cell 30, 1277–1292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neff M. M., Chory J., Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118, 27–35 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu P., et al. , Phytochrome B and AGB1 coordinately regulate photomorphogenesis by antagonistically modulating PIF3 stability in Arabidopsis. Mol. Plant 12, 229–247 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Earley K. W., et al. , Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Xie X., et al. , CRISPR-GE: A convenient software toolkit for CRISPR-based genome editing. Mol. Plant 10, 1246–1249 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Clough S. J., Bent A. F., Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Datta S., Hettiarachchi C., Johansson H., Holm M., SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 19, 3242–3255 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data from this article are available in the Arabidopsis Genome Initiative database libraries (https://www.arabidopsis.org/) under the following accession nos.: AT2G18790 for phyB, AT1G09530 for PIF3, AT2G24790 for BBX4, and AT2G32950 for COP1.