Summary
Life on earth has evolved under constant environmental changes; in response to these changes, most organisms have developed an endogenous clock that allows them to anticipate daily and seasonal changes and adapt their biology accordingly. Light cycles synchronize biological rhythms and are controlled by an endogenous clock that is entrained by environmental cues. Light is known to play a key role in the biology of symbiotic corals as they exhibit many biological processes entrained by daily light patterns. In this study, we aimed at determining the effect of constant dim light on coral's perception of diel and monthly cycles. Our results show that under constant dim light corals display a loss of rhythmic processes and constant stimuli by light, which initiates signal transduction that results in an abnormal cell cycle, cell proliferation, and protein synthesis. The results emphasize how constant dim light can mask the biological clock of Acropora digitifera.
Subject Areas: Ecology, Biological Sciences, Chronobiology, Transcriptomics
Graphical Abstract
Highlights
-
•
Light entrains many biological processes governed by the endogenous clock
-
•
Constant dim light overrides the biological clock of A. digitifera corals
-
•
Artificial light impacts the processes that allow corals to thrive in our oceans
-
•
The increase of artificial light in coastal areas is a growing threat to coral reefs
Ecology; Biological Sciences; Chronobiology; Transcriptomics
Introduction
Most organisms, from cyanobacteria to mammals, exhibit biological rhythms by synchronizing their behavioral and physiological activities with cyclic changes in the environment (Aschoff, 1960). Among these rhythms of varying lengths, twenty-four-hour periodicity is widely observed in most organisms (Raible et al., 2017). In the absence of environmental cues, the periodicity drifts away from the natural phase and is referred to as “free running.” For this reason, synchronization by environmental time cues is required (Harmer et al., 2001). Studies attempting to understand the mechanisms of endogenous clocks have focused mainly on circadian clocks showing it is based on positive and negative molecules interacting in feedback loops (Raible et al., 2017, Roenneberg and Merrow, 2005, Zantke et al., 2013). However, many organisms exhibit biological rhythms with longer (infradian) periodicity. One of these longer rhythms is the circalunar clock that oscillates with a period of approximately 1 month (~29.5 days) (Aschoff, 1981). External environmental cues (light, temperature) are used to entrain the oscillators of other biological clocks, with longer and shorter periodicities, in adaptation to the local conditions (Dupré and Loudon, 2007, Franke, 1985, Kaiser et al., 2011, Naylor, 2010, Schnytzer et al., 2018).
The lunar cycle refers to the 29.5 days (lunar month) required for the moon to orbit around the Earth and the 24.8 h (lunar day) required for the moon to reach the same spot on the Earth. Several recurring events are linked to the position of the moon relative to the Earth and the sun. Among these events, there are a few causing environmental changes, such as the tidal forces causing changes in water level and current, moonlight intensity, and time of moonrise (Gibson, 1992, Schnytzer et al., 2018).
Solar light is a well-known synchronizer of the circadian clock; yet, moonlight, which is incident sunlight reflected from the lunar surface, is known to affect periodic activities occurring in a variety of animals (Bentley et al., 2001, Kaniewska et al., 2015, Kronfeld-Schor et al., 2013, Rosenberg et al., 2017, Schnytzer et al., 2018, Takemura et al., 2004).
One of the earliest phenomena to be tied to the lunar cycle is the reproduction of marine invertebrates. In many species, including reef corals (Richmond and Hunter, 1990), fishes (Takemura et al., 2004), echinoderms (Mercier and Hamel, 2014), mollusks (Counihan et al., 2001), and crustaceans (Skov et al., 2005), the annual breeding events are synchronized with the lunar phase. This synchronization is commonly thought to be mediated by external cues that may act directly or indirectly to reset or maintain the biological clocks. Successful fertilization of marine invertebrates relies on the tight synchronization between the release of male and female germ products. The moon cycle provides these organisms with a predictable time frame that can be used for synchronization even across wide-spread populations (Babcock et al., 1986).
Coral reproductive timing is characterized by strong lunar rhythmicity (Boch et al., 2011, Hanafy et al., 2010, Jokiel et al., 1985, Kaniewska et al., 2015, Richmond and Hunter, 1990, Shlesinger and Loya, 1985). A common example is the mass spawning in the Great Barrier Reef, where changes in moonlight intensity can act as a final cue triggering the spawning of many different species of scleractinian corals as well as hundreds of other invertebrates, over a couple of nights (Harrison et al., 1984).
Light plays a key role in the growth, reproduction, and physiology of scleractinian corals that host phototrophic symbionts and reside in shallow water (Cohen et al., 2016, Kaniewska et al., 2015, Levy et al., 2007, Rosenberg et al., 2017, Wijgerde et al., 2014). The action spectra of coral photoreception reveal a maximum sensitivity in the blue region, at 480 nm (Gorbunov and Falkowski, 2002), which is also known to entrain the circadian clocks of insects and mammals via cryptochromes (CRYs), which are DNA photolyase-like photoreceptor proteins (Falciatore and Bowler, 2005). This makes corals capable of sensing low levels of blue light consistent with the detection of lunar irradiation (Gorbunov and Falkowski, 2002, Levy et al., 2007, Wuitchik et al., 2019).
Corals lack specialized light-sensing organs, yet display photosensitive behavior (Babcock et al., 1986, Gorbunov and Falkowski, 2002, Harrison et al., 1984, Jokiel et al., 1985, Levy et al., 2003, Levy et al., 2001, Oldach et al., 2017). A survey of Acropora digitifera photoreceptors revealed seven opsin and three cryptochrome genes (Shoguchi et al., 2013). A previous study on the coral Acropora millepora showed that the known light receptor and core clock gene cryptochromes displayed rhythmic expression in response to light:dark treatment and are influenced by lunar light (Levy et al., 2007). Photoreceptors are also responsible for the photoentrainment of circadian rhythms, and their threshold of photoreception sensitivity is low enough for corals to sense natural moonlight (Gorbunov and Falkowski, 2002, Levy et al., 2007, Reitzel et al., 2010). Photoentrainment of rhythmic processes in corals is highly important as it regulates metabolism, photosynthesis, synchronized spawning, and calcification (Sorek et al., 2014).
Here, we explore the complexities of gene expression according to the lunar phase changes in a reef-building coral, A. digitifera (Figure 1), using RNA sequencing (RNA-seq) analysis.
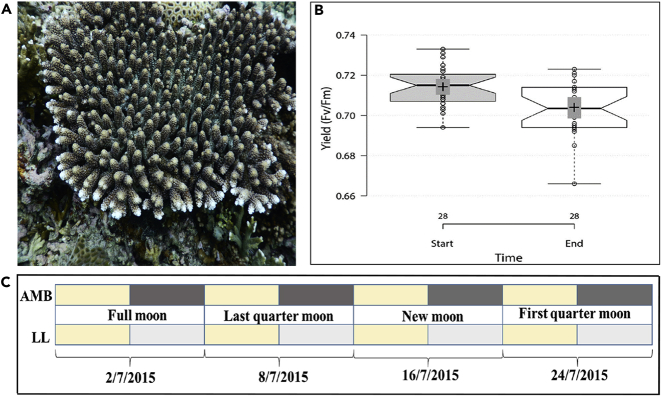
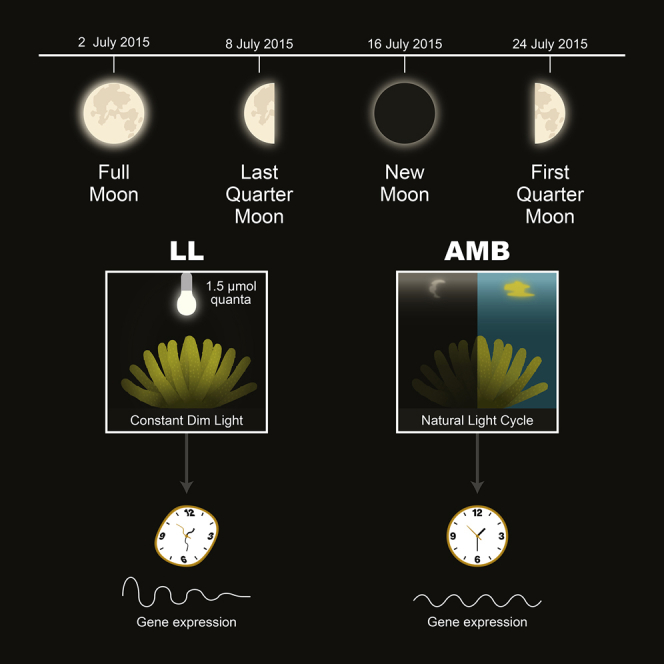
Figure 1.
Experimental Setup
(A) Acropora digitifera colony prior to collection from Sesoko Island Reef, Okinawa, Japan.
(B) Box plot showing the maximum quantum yield (Fv/Fm) for LL colonies (n = 5) from the first sampling day (Start) and at the last sampling day (End). Centerlines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend to a minimum and maximum values; crosses represent sample means; bars indicate 95% confidence intervals of the means. Median values are 0.71 and 0.70 at the start and end, respectively.
(C) Sampling regime indicating the two light treatments, dates of sampling, and moon phases. AMB, Ambient conditions; LL, Light: Light treatment, constant light. Yellow bars represent day time, black bars represent objective night time, and gray bars represent subjective night.
Results
Photosynthetic Yield
By measuring photosynthetic yield at the beginning and end of the experiment for each treatment (AMB and LL), we confirmed that coral colonies had no significant decrease in maximal quantum yield; median values of maximal quantum yield (Fv/Fm) were 0.71 for all colonies at the start of the experiment and 0.70 and 0.71 for LL and AMB colonies, respectively, at the end of the experiment (Figure 1B). Thus, we can conclude that coral bleaching and/or damage did not have an impact on the results reported here.
Differential Expression and Pathway Analysis
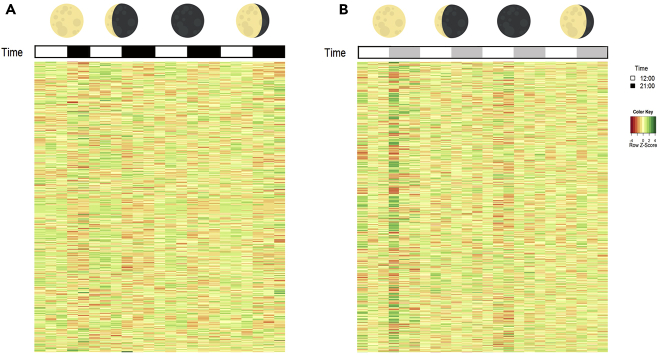
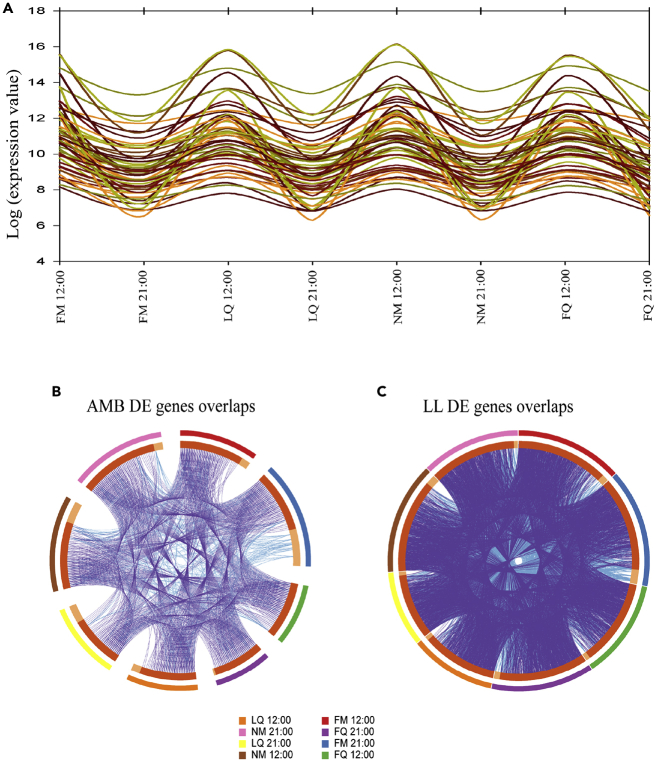
To discover biologically important changes in gene expression between conditions, we performed differential expression analysis of our RNA-seq data. Each of the 48 samples sent for RNA sequencing yielded an average of 16,062,693 paired-end sequences (Table S1 Sequencing metrics). Samples were aligned to the A. digitifera transcriptome with an average of 40% sequences from each sample mapped to the known transcriptome (Supplemental Information) and 23,642 gene models were found. We started with comparing all AMB with LL samples and arraying them by sampling time and moon phase to understand how the time of day and the moon phase causes differences in expression level between the experimental groups (Figures 2A and 2B). Our results showed a substantial difference in the expression levels of differentially expressed (DE) genes between the experimental groups; LL samples had 4,472 genes up-regulated (19% of total genes counts) and 4,691 genes down-regulated (20% of total genes counts) when compared with the AMB samples. In the AMB samples, we could recognize gene patterns responding to both moon phase and time of day with levels changing accordingly, with a specific pattern for the day time and night time samples (Figure 2A). As opposed to the AMB samples, the LL samples showed different expression levels at each sampling point (Figure 2B). The LL full moon samples (first sampling day) showed strong variation in expression levels between day and night that did not repeat in the following sampling points. Interestingly, after almost 3 weeks of sampling and 4 weeks under LL conditions, the new moon samples showed a strong variation between day and night time samples. Although we observed the strong peak in expression during the new moon day time samples, the patterns had different expression than the AMB new moon day time samples. We then continued with the MetaCycle analysis to evaluate periodicity in the expressed transcripts in both conditions. AMB samples showed 58 synexpression clusters that were rhythmic across all sampling time points (Figure 3A) and had specific gene networks for every time point as well as unique genes that corresponded with only one sampling point (Figure 3B). The LL samples did not show any rhythmic gene clusters across the different sampling points and had more gene overlaps and less unique genes for specific time points than the AMB samples (Figure 3C).
Figure 2.
Gene Expression across the Moon Phases
(A) Corals under Ambient conditions (AMB).
(B) Corals under constant light conditions (LL). Moon phases are presented by chronological sampling days: starting from the left side: full moon, third quarter, new moon, and first quarter. White bars indicate 12:00 p.m. sampling; black and gray bars indicate 21:00 p.m. sampling. All bars represent three samples; AMB night sample from the full moon represents two samples. Gene expression values are represented by Z score.
Figure 3.
Rhythmic Analysis and Gene Overlap
(A) AMB sample rhythmic gene expression of top 58 gene clusters across all sampling points.
(B) Circos plot showing the overlaps of DE genes of AMB samples between all sampling points.
(C) Circos plot showing the overlaps of DE genes of LL samples between all sampling points. Outer circles represent sample time, inner circles show in light orange the unique genes and dark orange shared genes, purple lines are gene overlap, and blue lines are shared enriched terms. FM, full moon; LQ, last quarter moon; NM, new moon; FQ, first quarter moon.
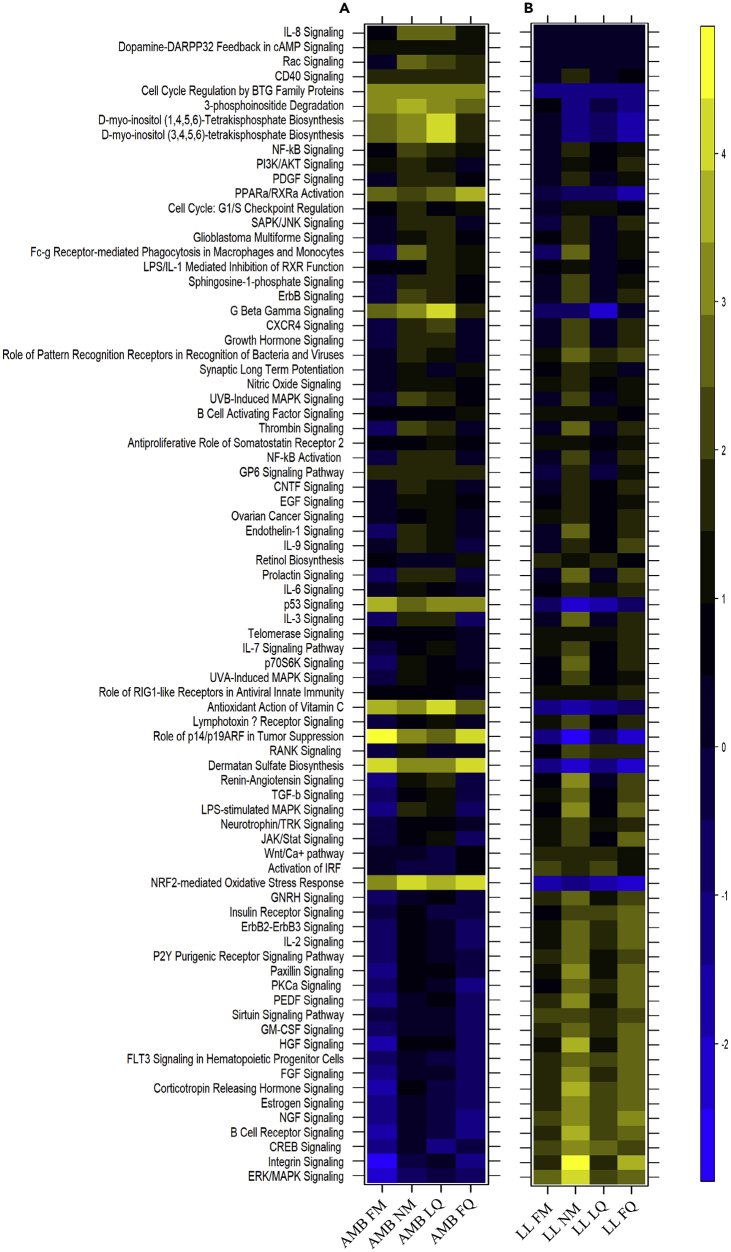
We were able to identify the different clusters that are DE in each moon phase and arrange them according to specific pathways (Figure 4). Many of the pathways show variation across the moon phases in both groups but have the opposite expression pattern. For example, ERK/MAPK, Integrin, NGF, and CREB signaling in the LL samples are up-regulated or not DE in the different moon phases, whereas the same signaling pathways are down-regulated or not DE in the AMB samples. The main pathways that were down-regulated in the LL samples and up-regulated in the AMB samples include the D-myo-inositol (3,4,5,6)-tetrakisphosphate Biosynthesis, D-myo-inositol (1,4,5,6)-Tetrakisphosphate Biosynthesis, 3-phosphoinositide Degradation, G Beta Gamma Signaling, Cell Cycle Regulation by BTG Family Proteins, p53 Signaling, Role of p14/p19ARF in Tumor Suppression, NRF2-Mediated Oxidative Stress Response, and PPARα/RXRα Activation (Figure 4). We could also find pathways that are DE between the moon phases only in the LL samples and not in the AMB samples such as CD40 and GP6 signaling pathways. Some of the pathways found to be DE under AMB conditions and not under LL conditions include Rac, Dopamine, and IL-8 signaling. The AMB pathway cluster represents the natural variance coral express throughout the lunar phase of a single month.
Figure 4.
Gene Ontology Enrichment Analysis of Differentially Expressed (DE) Genes
(A) Canonical pathways oscillation enriched in corals under Ambient conditions (AMB).
(B) Canonical pathways oscillation enriched in corals under constant light conditions (LL). The color scale indicates the process enrichment based on Z score. Moon phases are indicated at the bottom. FM, full moon; LQ, last quarter moon; NM, new moon; FQ, first quarter moon.
Cyclic Gene Expression
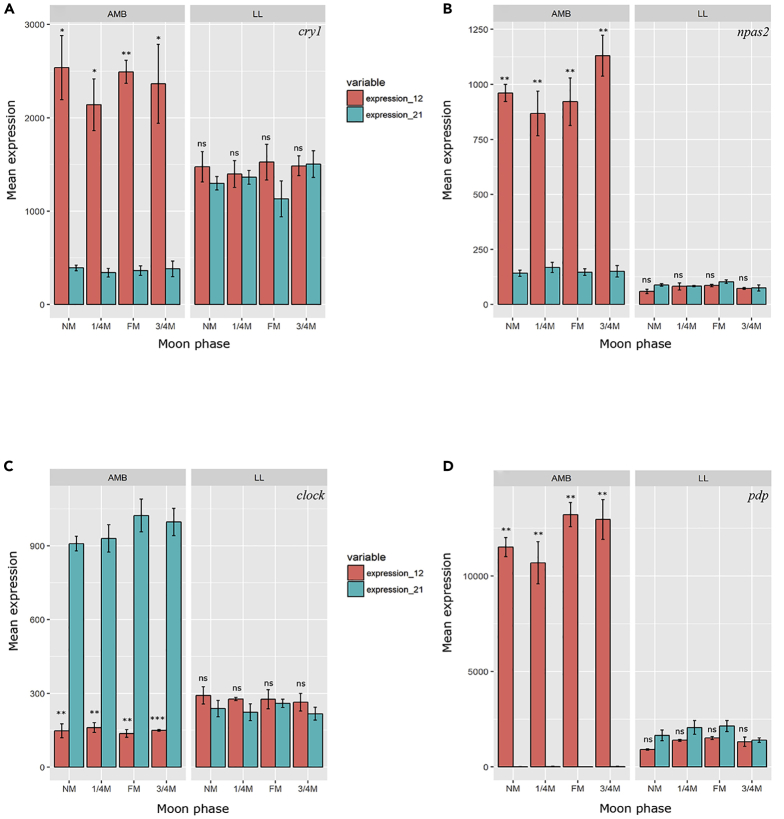
Next, the expression levels of known clock-related genes of A. digitifera in the different lunar phases under the two light conditions were established. In the AMB samples three of the genes, cry1, npas2, and pdp, showed the synexpression pattern (Figures 5A, 5B, and 5D), high levels during day time sampling and lower levels during night time with a slight difference between the moon phases. The clock gene showed the opposite expression pattern (Figure 5C) with high levels during the night and lower levels during the day. All of the above clock-related genes expressed in the LL samples showed no significant variance between night and day and moon phases.
Figure 5.
Mean RNA Expression of Clock-Related Genes in the Coral Acropora digitifera along the Moon Phases under Natural and Constant Light Conditions
(A) cry1; (B) npas2; (C) clock; (D) pdp. NM, new moon; 1/4M, first quarter moon; FM, full moon; 3/4M, third quarter moon. The left panel in every graph represents ambient conditions corals (AMB); the right panel represents corals under constant light conditions (LL). Red bars represent gene expression at 12:00 p.m., light blue bars represent gene expression at 21:00 p.m. Represented p value is for comparison between AMB of day and night samples and LL samples of day and night. *p < 0.05, **p < 0.01, ***p < 0.001, ns, p > 0.05.
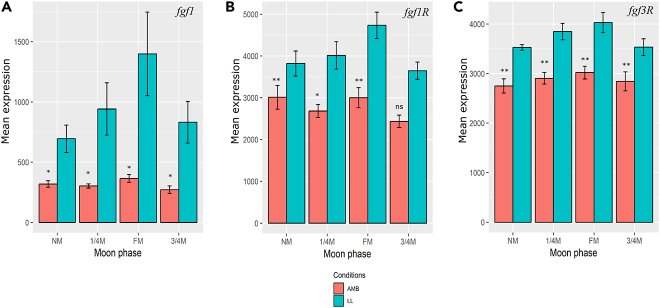
Last, we tested another group of genes in relation to light exposer, the Fibroblast growth factors (FGFs). Three annotated FGF genes in the A. digitifera transcriptome were found, FGF1R, FGF3R, and FGF1, that were highly expressed in the LL samples during all moon phases as opposed to the AMB samples where levels were constantly lower (Figures 6A–6C). In addition, no significant variance between day and night was observed.
Figure 6.
Mean Expression of Fibroblast Growth Factors (FGFs) in the Coral Acropora digitifera along the Moon Phases under Natural and Constant Light Conditions
(A) fgf1; (B) fgf1r; (C) fgf3r. NM, new moon; 1/4M, first quarter moon; FM, full moon; 3/4M, third-quarter moon; AMB, corals under ambient conditions; LL, corals under constant light conditions. Red bars represent gene expression in AMB samples; light blue bars represent gene expression in LL samples. The represented p value is for comparison between AMB samples and LL samples. *p < 0.05, **p < 0.01, ns, p > 0.05.
By comparing all AMB genes with each other we were interested in genes that react solely to the lunar phase and not to the time of day. We found a group of DE genes (n = 23 genes with annotations) that are involved in initiating many signaling pathways via GPCRs activation and resulting in DNA binding and RNA transcription. Additional pathways that were enriched in response to lunar phase are microtubule binding and extracellular matrix organization.
Discussion
Only a few studies up to date have focused on the coral response to lunar cycle (Jokiel et al., 1985, Kaniewska et al., 2015, Levy et al., 2007, Rosenberg et al., 2017) and coral circadian gene expression across the lunar phase (Brady et al., 2016, Levy et al., 2007); yet, none of them have addressed the aspect of low dim light on the complete gene expression of a candidate coral over an entire lunar cycle. Continuous and intermittent low lighting both have been shown to have effects; short pulses of light during the night are sufficient to disrupt the circadian clocks. Artificial light may be difficult to distinguish from natural changes in light intensity and duration and could override the endogenous clock (Dominoni, 2015, Gaston et al., 2013). This study demonstrates the potential impacts that modern lighting can have on coral reefs.
Our results show that corals under ambient conditions, perceiving natural light:dark and lunar cycles, express diel and monthly variation in gene expression (Figure 2A) as a response to the environmental changes. Our AMB samples present the natural variation in gene expression that occurs in A. digitifera during the month of July and was used as a control reference in the analysis. In the LL samples, there was no continuous cyclic variation in gene expression in response to the time of day or the lunar phase (Figure 2B). In many organisms, rhythmicity loss as a result of constant conditions is well described for both gene expression and behavior, specifically under constant dim light (Granados-Fuentes, 2004, Njus et al., 1977, Steinlechner et al., 2002). The absence of entrainment cues causes the loss of these rhythmic patterns. Previous studies have shown that, in case of returning to natural light and dark conditions, the rhythmicity will be restored (Granados-Fuentes, 2004, Njus et al., 1977, Steinlechner et al., 2002). LL samples collected on the day of the full moon, after a few days of constant dim light, did have a significant difference in gene expression between the day and the night. We postulate that these differences are natural fluctuations in gene expression, which were still under the regulation of an endogenous clock that was able to overcome the masking effect of the continuous dim light. Many studies have shown that the biological clock of an organism can maintain a rhythm in gene expression or behavior after being kept in constant conditions for a few days (Chabot et al., 2016, Griffin et al., 1999, Levy et al., 2011, Oren et al., 2015, Peres et al., 2014, Sorek et al., 2013, Sorek and Levy, 2012). Therefore, we assume the oscillations in gene expression under LL conditions at the first sampling day are still governed by the clock machinery. Daytime new moon samples under LL conditions expressed down-regulation of gene clusters that are surprising not only in appearance but also in expression pattern when compared with the equivalent AMB samples. Observing the DE genes temporal pattern during the four moon phases under natural conditions shows that corals present diel and monthly variations in gene expression, whereas after a few days under constant conditions rhythmicity is lost.
When focusing on enriched pathways constituted by the DE genes, we found many pathways that act in contrary mode between the AMB and LL samples (Figures 4A and 4B). The ERK/MAPK signaling pathway causes signal transduction from a receptor on the cell's surface to the cell's nucleus (Seger and Krebs, 1995) and was found to be up-regulated in the LL samples (Figure 4B) and down-regulated in the AMB samples (Figure 4A). This signaling pathway initiates cellular processes such as proliferation, differentiation, and development as a result of an external signal (Seger and Krebs, 1995). The ERK/MAPK signaling pathway together with the Integrin and NGF signaling pathways, which were also up-regulated in the LL samples, regulate the cell cycle, cell proliferation, and cell growth (Howe et al., 1998, Howe et al., 2001, Howe and Mobley, 2004, Niederhauser et al., 2000, Schwartz, 2001). We propose that constant dim light can cause a continuous signal for the initiation of these pathways and therefore known rhythmic processes, such as cell cycle, are no longer under tight regulation that is probably masked (Armbrust et al., 1989, Hunt and Sassone-Corsi, 2007, Jacquet et al., 2001, Matsuo et al., 2003).
An additional up-regulated pathway that was enriched in the LL samples, while down-regulated in the AMB samples, is the CREB signaling pathway. CREB is a transcription factor, which binds to DNA sequences called cAMP response elements (CRE) and causes an increase or decrease in the transcription of these genes (Bourtchuladze et al., 1994, Purves, 2004). Circadian rhythm entrainment by light and clock gene transcription is partly under CREB phosphorylation regulation (Riccio et al., 2006, Tischkau et al., 2003, Zhang et al., 1996). The CREB signaling pathway reacts to light and provides information essential for normal clock function as described previously (Obrietan et al., 1999). Therefore, it is not surprising that the CREB signaling pathway was up-regulated under dim light as it reacted to the constant stimulation of light. Other pathways enriched in the LL samples such as CD40 and GP6 signaling pathways are linked to cell regulation process, immune system, and phosphorylation (Faris, 1994, p. 40; Jones et al., 2007, Sokol et al., 2012). Pathways that were only DE under AMB conditions can all be found to have circadian regulation or modulating rhythmic processes by dynamically regulating the concentration of intracellular second messengers. These pathways include the Rac, Dopamine, and IL-8 signaling pathways (Beaulieu and Gainetdinov, 2011, Duffield et al., 2002, Ghasemi et al., 2011, Hermann et al., 2006, Hwang et al., 2013, Norman et al., 2005, Yujnovsky et al., 2006). Finding these rhythmic processes to be enriched only in the AMB samples gives an additional proof that under LL conditions the rhythmicity is lost and most known genes that have a diel or monthly fluctuation in expression would not be enriched in the LL samples.
To determine the loss of rhythmicity at the mechanism level and not only on gene expression level, we compared the gene expression of known clock genes of A. digitifera between the two light conditions (Figure 5). All four clock-related genes, cry1, npas2, clock, and pdp, exhibited diel variation in expression in AMB samples across all moon phases, whereas under LL conditions there was no significant diel variation in gene expression and levels were constantly lower than in the AMB samples. Loss of expression, “dampening,” in related clock genes is a known phenomenon after a few days under constant conditions with no entrainment cue (Chabot et al., 2016, Dominoni, 2015, Dupré and Loudon, 2007, Griffin et al., 1999, Raible et al., 2017, Reitzel et al., 2010, Roenneberg and Merrow, 2005, Sorek et al., 2014, Sorek et al., 2013). The coordination of many biological processes is facilitated by the biological clock; therefore, disruptions in cueing or masking the clock machinery have the potential to influence a range of downstream pathways (Ceriani et al., 2002, Kondratov et al., 2006, Somers et al., 1998, Takahashi et al., 2008, Zheng et al., 2001).
When looking at an organism under constant light it is important to examine the effect not only on rhythmic biological processes but also on cells that perceive light, photoreceptors. Studies attempting to understand this effect of light on photoreceptors have found that constant light causes photoreceptor degradation and upregulation of Fibroblast Growth Factors (FGFs) (Campochiaro et al., 1996, Hicks and Courtois, 1992, Hicks and Courtois, 1988, Hisatomi et al., 2002, Hochmann et al., 2012, Qin et al., 2011). FGFs are the largest family of growth factors involved in soft-tissue growth and regeneration (Basilico and Moscatelli, 1992). It was found that, under constant light, FGFs cause regeneration of photoreceptors that were degraded owing to the effect of the light treatment (Campochiaro et al., 1996, Hisatomi et al., 2002, Hochmann et al., 2012, Qin et al., 2011). In our data, we found that three FGFs were upregulated in the LL samples compared with the AMB samples (Figures 6A–6C). These results correspond with previous studies showing that FGFs protect photoreceptors from the damaging effect of constant light (Gao and Hollyfield, 1996, LaVail et al., 1992).
Genes that were found to be responsive to the lunar cycle and not to the diel cycle revealed pathways altered between the lunar phases. Most of these genes use signaling pathways initiated by GPCRs, pathways known to be environmentally responsive, mainly to light. Many genes show changes in expression levels based on the lunar phase (Brady et al., 2016, Fukushiro et al., 2011, Levy et al., 2007, Numata and Helm, 2014), but most of them show changes between day and night time. The observed genes create specific pathways that react solely to the lunar cycle and are most likely responsive to the moonlight since they are connected to the GPCR signaling pathway. These pathways result in DNA binding and RNA transcription, and we postulate the moon phase has a role in determining gene expression, which regulates the organization and formation of extracellular matrix assisted by microtubules.
To conclude, this study shows that constant dim light conditions could cause loss of rhythmic processes in the coral A. digitifera. The results demonstrate that even low levels of illumination could override the changes in the moon phase and cause loss of circadian and circalunar regulation. The diel light cycle controls many vital processes; the connection between artificial light and clock disorders is well known, and difficulties with adjusting the circadian clock in corals could impact the natural processes that allow them to thrive and populate the oceans. Since the lunar phase controls oogenesis in many corals, the masking of moonlight could affect the synchronized spawning phenomenon and cause reproduction failure in heavily lit areas. Therefore, the increase of artificial light in coastal areas is a growing threat to coral reefs around the world.
Limitations of the Study
From a clock point of view, one of the challenging tasks is to plan the sampling regime according to the turnover of the RNA molecules to understand the daily and monthly oscillations of gene expression. Future studies should focus, at a molecular level, on the obstruction of constant light on a proposed circalunar oscillator, by diel sampling across an entire lunar month, to test whether the above processes are controlled by one circadian clock or by two separate circadian and circalunar clocks.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Lee Eyal-Shaham, Gal Eyal, and Tal Eyal for all the help during the experiment. In addition, we thank the entire staff and students of the “Tropical Biosphere Research Center” in Sesoko Island, especially the Harii lab members. The research leading to this paper has received funding from the Israel Science Foundation (ISF), grant number 3928 to O.L. This study represents partial fulfillment of the requirements for a Ph.D. thesis for Y.R. at Faculty of Life Sciences Bar-Ilan University, Israel.
Author Contributions
Y.R. and O.L. designed the research; the experiment was carried out by Y.R., O.L., S.H., and F.S. T.D. performed data analysis. Y.R. and O.L. wrote the first draft of the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare they have no competing interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.040.
Data and Code Availability
The sequencing data reported in this study have been deposited to the Sequence Read Archive “SRA, PRJNA526391”: Characterizing circalunar and diel rhythmicity in the model coral Acropora digitifera, a molecular perspective. Correspondence and request for materials should be addressed to Y.R. yaelirose@gmail.com or O.L. oren.levy@biu.ac.il.
Supplemental Information
References
- Armbrust E.V., Bowen J.D., Olson R.J., Chisholm S.W. Effect of light on the cell cycle of a marine synechococcus strain. Appl. Environ. Microbiol. 1989;55:425–432. doi: 10.1128/aem.55.2.425-432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. A survey on biological rhythms. In: Aschoff J., editor. Biological Rhythms. Springer US; 1981. pp. 3–10. [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Babcock R.C., Bull G.D., Harrison P.L., Heyward A.J., Oliver J.K., Wallace C.C., Willis B.L. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 1986;90:379–394. [Google Scholar]
- Basilico C., Moscatelli D. The Fgf family of growth factors and oncogenes. In: Vande Woude G.F., Klein G., editors. Advances in Cancer Research. Academic Press; 1992. pp. 115–165. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.-M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bentley M.G., Olive P.J.W., Last K. Sexual satellites, moonlight and the nuptial dances of worms: the influence of the moon on the reproduction of marine animals. In: Dordrecht, editor. Earth-Moon Relationships. Springer; 2001. pp. 67–84. [Google Scholar]
- Boch C.A., Ananthasubramaniam B., Sweeney A.M., Doyle F.J., Morse D.E. Effects of light dynamics on coral spawning synchrony. Biol. Bull. 2011;220:161–173. doi: 10.1086/BBLv220n3p161. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brady A.K., Willis B.L., Harder L.D., Vize P.D. Lunar phase modulates circadian gene expression cycles in the broadcast spawning coral Acropora millepora. Biol. Bull. 2016;230:130–142. doi: 10.1086/BBLv230n2p130. [DOI] [PubMed] [Google Scholar]
- Campochiaro P.A., Chang M., Ohsato M., Vinores S.A., Nie Z., Hjelmeland L., Mansukhani A., Basilico C., Zack D.J. Retinal degeneration in transgenic mice with photoreceptor-specific expression of a dominant-negative fibroblast growth factor receptor. J. Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani M.F., Hogenesch J.B., Yanovsky M., Panda S., Straume M., Kay S.A. Genome-wide expression analysis in Drosophila Reveals genes controlling circadian behavior. J. Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot C.C., Ramberg-Pihl N.C., Watson W.H. Circalunidian clocks control tidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. Mar. Freshw. Behav. Physiol. 2016;49:75–91. doi: 10.1080/10236244.2015.1127679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Dubinsky Z., Erez J. Light enhanced calcification in hermatypic corals: new insights from light spectral responses. Front. Mar. Sci. 2016;2 [Google Scholar]
- Counihan R.T., McNamara D.C., Souter D.C., Jebreen E.J., Preston N.P., Johnson C.R., Degnan B.M. Pattern, synchrony and predictability of spawning of the tropical abalone Haliotis asinina from Heron Reef, Australia. Mar. Ecol. Prog. Ser. 2001;213:193–202. [Google Scholar]
- Dominoni D.M. The effects of light pollution on biological rhythms of birds: an integrated, mechanistic perspective. J. Ornithol. 2015;156:409–418. [Google Scholar]
- Duffield G.E., Best J.D., Meurers B.H., Bittner A., Loros J.J., Dunlap J.C. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr. Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- Dupré S.M., Loudon A.S.I. Circannual clocks: annual timers unraveled in sheep. Curr. Biol. 2007;17:R216–R217. doi: 10.1016/j.cub.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Falciatore A., Bowler C. The evolution and function of blue and red light photoreceptors. In: Jan, editor. Current Topics in Developmental Biology. Academic Press; 2005. pp. 317–350. [DOI] [PubMed] [Google Scholar]
- Faris M. CD40 signaling pathway: anti-CD40 monoclonal antibody induces rapid dephosphorylation and phosphorylation of tyrosine-phosphorylated proteins including protein tyrosine kinase Lyn, Fyn, and Syk and the appearance of a 28-kD tyrosine phosphorylated protein. J. Exp. Med. 1994;179:1923–1931. doi: 10.1084/jem.179.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H.-D. On a clocklike mechanism timing lunar-rhythmic reproduction in Typosyllis prolifera (Polychaeta) J. Comp. Physiol. A. 1985;156:553–561. [Google Scholar]
- Fukushiro M., Takeuchi T., Takeuchi Y., Hur S.-P., Sugama N., Takemura A., Kubo Y., Okano K., Okano T. Lunar phase-dependent expression of cryptochrome and a photoperiodic mechanism for lunar phase-recognition in a reef fish, goldlined spinefoot. PLoS One. 2011;6:e28643. doi: 10.1371/journal.pone.0028643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Hollyfield J.G. Basic fibroblast growth factor: increased gene expression in inherited and light-induced photoreceptor degeneration. Exp. Eye Res. 1996;62:181–190. doi: 10.1006/exer.1996.0022. [DOI] [PubMed] [Google Scholar]
- Gaston K.J., Bennie J., Davies T.W., Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 2013;88:912–927. doi: 10.1111/brv.12036. [DOI] [PubMed] [Google Scholar]
- Ghasemi H., Ghazanfari T., Yaraee R., Faghihzadeh S., Hassan Z.M. Roles of IL-8 in ocular inflammations: a review. Ocul. Immunol. Inflamm. 2011;19:401–412. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- Gibson R.N. Tidally-synchronised behaviour in marine fishes. In: Ali, editor. Rhythms in Fishes, NATO ASI Series. Springer; 1992. pp. 63–81. [Google Scholar]
- Gorbunov M.Y., Falkowski P.G. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr. 2002;47:309–315. [Google Scholar]
- Granados-Fuentes D. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J. Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin E.A., Staknis D., Weitz C.J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Hanafy M.H., Aamer M.A., Habib M., Rouphael A.B., Baird A.H. Synchronous reproduction of corals in the red sea. Coral Reefs. 2010;29:119–124. [Google Scholar]
- Harmer S.L., Panda S., Kay S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Harrison P.L., Babcock R.C., Bull G.D., Oliver J.K., Wallace C.C., Willis B.L. Mass spawning in tropical reef corals. Science. 1984;223:1186–1189. doi: 10.1126/science.223.4641.1186. [DOI] [PubMed] [Google Scholar]
- Hermann C., von Aulock S., Dehus O., Keller M., Okigami H., Gantner F., Wendel A., Hartung T. Endogenous cortisol determines the circadian rhythm of lipopolysaccharide- but not lipoteichoic acid-inducible cytokine release. Eur. J. Immunol. 2006;36:371–379. doi: 10.1002/eji.200535470. [DOI] [PubMed] [Google Scholar]
- Hicks D., Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J. Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D., Courtois Y. Acidic fibroblast growth factor stimulates opsin levels in retinal photoreceptor cells in vitro. FEBS Lett. 1988;234:475–479. doi: 10.1016/0014-5793(88)80141-8. [DOI] [PubMed] [Google Scholar]
- Hisatomi T., Sakamoto T., Goto Y., Yamanaka I., Oshima Y., Hata Y., Ishibashi T., Inomata H., Susin S.A., Kroemer G. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr. Eye Res. 2002;24:161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- Hochmann S., Kaslin J., Hans S., Weber A., Machate A., Geffarth M., Funk R.H.W., Brand M. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS One. 2012;7:e30365. doi: 10.1371/journal.pone.0030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A., Aplin A.E., Alahari S.K., Juliano R. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Howe C.L., Mobley W.C. Signaling endosome hypothesis: a cellular mechanism for long distance communication. J. Neurobiol. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- Howe C.L., Valletta J.S., Rusnak A.S., Mobley W.C. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- Hunt T., Sassone-Corsi P. Riding tandem: circadian clocks and the cell cycle. Cell. 2007;129:461–464. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hwang C.K., Chaurasia S.S., Jackson C.R., Chan G.C.-K., Storm D.R., Iuvone P.M. Circadian rhythm of contrast sensitivity is regulated by a dopamine–neuronal PAS-domain protein 2–adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J. Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet S., Partensky F., Marie D., Casotti R., Vaulot D. Cell cycle regulation by light in Prochlorococcus strains. Appl. Environ. Microbiol. 2001;67:782–790. doi: 10.1128/AEM.67.2.782-790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel P.L., Ito R.Y., Liu P.M. Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Mar. Biol. 1985;88:167–174. [Google Scholar]
- Jones C.I., Garner S.F., Angenent W., Bernard A., Berzuini C., Burns P., Farndale R.W., Hogwood J., Rankin A., Stephens J.C. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J. Thromb. Haemost. 2007;5:1756–1765. doi: 10.1111/j.1538-7836.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- Kaiser T.S., Neumann D., Heckel D.G. Timing the tides: genetic control of diurnal and lunar emergence times is correlated in the marine midge Clunio marinus. BMC Genet. 2011;12:49. doi: 10.1186/1471-2156-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniewska P., Alon S., Karako-Lampert S., Hoegh-Guldberg O., Levy O. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. Elife. 2015;4 doi: 10.7554/eLife.09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov R.V., Kondratova A.A., Gorbacheva V.Y., Vykhovanets O.V., Antoch M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld-Schor N., Dominoni D., Iglesia H., de la Levy O., Herzog E.D., Dayan T., Helfrich-Forster C. Chronobiology by moonlight. Proc. R. Soc. B. 2013;280:20123088. doi: 10.1098/rspb.2012.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail M.M., Unoki K., Yasumura D., Matthes M.T., Yancopoulos G.D., Steinberg R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl. Acad. Sci. U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Appelbaum L., Leggat W., Gothlif Y., Hayward D.C., Miller D.J., Hoegh-Guldberg O. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science. 2007;318:467–470. doi: 10.1126/science.1145432. [DOI] [PubMed] [Google Scholar]
- Levy O., Dubinsky Z., Achituv Y. Photobehavior of stony corals: responses to light spectra and intensity. J. Exp. Biol. 2003;206:4041–4049. doi: 10.1242/jeb.00622. [DOI] [PubMed] [Google Scholar]
- Levy O., Kaniewska P., Alon S., Eisenberg E., Karako-Lampert S., Bay L.K., Reef R., Rodriguez-Lanetty M., Miller D.J., Hoegh-Guldberg O. Complex diel cycles of gene expression in coral-algal symbiosis. Science. 2011;331:175. doi: 10.1126/science.1196419. [DOI] [PubMed] [Google Scholar]
- Levy O., Mizrahi L., Chadwick-Furman N.E., Achituv Y. Factors controlling the expansion behavior of Favia favus (Cnidaria: scleractinia): effects of light, flow, and planktonic prey. Biol. Bull. 2001;200:118–126. doi: 10.2307/1543305. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Yamaguchi S., Mitsui S., Emi A., Shimoda F., Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- Mercier A., Hamel J.-F. Lunar periods in the annual reproductive cycles of marine invertebrates from cold subtidal and deep-sea environments. In: Numata, Helm, editors. Annual, Lunar, and Tidal Clocks. Springer; 2014. pp. 99–120. [Google Scholar]
- Naylor E. Cambridge University Press; 2010. Chronobiology of Marine Organisms. [Google Scholar]
- Niederhauser O., Mangold M., Schubenel R., Kusznir E.A., Schmidt D., Hertel C. NGF ligand alters NGF signaling via p75NTR and TrkA. J. Neurosci. Res. 2000;61:263–272. doi: 10.1002/1097-4547(20000801)61:3<263::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Njus D., McMurry L., Hastings J.W. Conditionality of circadian rhythmicity: synergistic action of light and temperature. J. Comp. Physiol. 1977;117:335–344. [Google Scholar]
- Norman K.R., Fazzio R.T., Mellem J.E., Espelt M.V., Strange K., Beckerle M.C., Maricq A.V. The Rho/Rac-family guanine nucleotide exchange factor VAV-1 regulates rhythmic behaviors in C. elegans. Cell. 2005;123:119–132. doi: 10.1016/j.cell.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Numata H., Helm B., editors. Annual, Lunar, and Tidal Clocks. Springer; 2014. [Google Scholar]
- Obrietan K., Impey S., Smith D., Athos J., Storm D.R. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J. Biol. Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Oldach M.J., Workentine M., Matz M.V., Fan T.-Y., Vize P.D. Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol. Ecol. 2017;26:2514–2526. doi: 10.1111/mec.14043. [DOI] [PubMed] [Google Scholar]
- Oren M., Tarrant A.M., Alon S., Simon-Blecher N., Elbaz I., Appelbaum L., Levy O. Profiling molecular and behavioral circadian rhythms in the non-symbiotic sea anemone Nematostella vectensis. Sci. Rep. 2015;5:11418. doi: 10.1038/srep11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres R., Reitzel A.M., Passamaneck Y., Afeche S.C., Cipolla-Neto J., Marques A.C., Martindale M.Q. Developmental and light-entrained expression of melatonin and its relationship to the circadian clock in the sea anemone Nematostella vectensis. Evodevo. 2014;5:26. doi: 10.1186/2041-9139-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., editor. Neuroscience. Third Edition. Sinauer Associates, Publishers; 2004. [Google Scholar]
- Qin Z., Kidd A.R., Thomas J.L., Poss K.D., Hyde D.R., Raymond P.A., Thummel R. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp. Eye Res. 2011;93:726–734. doi: 10.1016/j.exer.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F., Takekata H., Tessmar-Raible K. An overview of monthly rhythms and clocks. Front. Neurol. 2017;8 doi: 10.3389/fneur.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel A.M., Behrendt L., Tarrant A.M. Light entrained rhythmic gene expression in the sea anemone Nematostella vectensis: the evolution of the animal circadian clock. PLoS One. 2010;5:e12805. doi: 10.1371/journal.pone.0012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A., Alvania R.S., Lonze B.E., Ramanan N., Kim T., Huang Y., Dawson T.M., Snyder S.H., Ginty D.D. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Richmond R., Hunter C. Reproduction and recruitment of corals: comparisons among the caribbean, the tropical pacific, and the red sea. Mar. Ecol. Prog. Ser. 1990;60:185–203. [Google Scholar]
- Roenneberg T., Merrow M. Circadian clocks — the fall and rise of physiology. Nat. Rev. Mol. Cell Biol. 2005;6:965–971. doi: 10.1038/nrm1766. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y., Doniger T., Harii S., Sinniger F., Levy O. Canonical and cellular pathways timing gamete release in Acropora digitifera, Okinawa. Jpn. Mol. Ecol. 2017;26:2698–2710. doi: 10.1111/mec.14062. [DOI] [PubMed] [Google Scholar]
- Schnytzer Y., Simon-Blecher N., Li J., Ben-Asher H.W., Salmon-Divon M., Achituv Y., Hughes M.E., Levy O. Tidal and diel orchestration of behaviour and gene expression in an intertidal mollusc. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-23167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A. Integrin signaling revisited. Trends Cell Biol. 2001;11:466–470. doi: 10.1016/s0962-8924(01)02152-3. [DOI] [PubMed] [Google Scholar]
- Seger R., Krebs E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shlesinger Y., Loya Y. Coral community reproductive patterns: red sea versus the Great barrier reef. Science. 1985;228:1333–1335. doi: 10.1126/science.228.4705.1333. [DOI] [PubMed] [Google Scholar]
- Shoguchi E., Tanaka M., Shinzato C., Kawashima T., Satoh N. A genome-wide survey of photoreceptor and circadian genes in the coral, Acropora digitifera. Gene. 2013;515:426–431. doi: 10.1016/j.gene.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Skov M.W., Hartnoll R.G., Ruwa R.K., Shunula J.P., Vannini M., Cannicci S. Marching to a different drummer: crabs synchronize reproduction to a 14-month lunar-tidal cycle. Ecology. 2005;86:1164–1171. [Google Scholar]
- Sokol J., Biringer K., Skerenova M., Hasko M., Bartosova L., Stasko J., Danko J., Kubisz P. Platelet aggregation abnormalities in patients with fetal losses: the GP6 gene polymorphism. Fertil. Steril. 2012;98:1170–1174. doi: 10.1016/j.fertnstert.2012.07.1108. [DOI] [PubMed] [Google Scholar]
- Somers D.E., Webb A.A., Pearson M., Kay S.A. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- Sorek M., Díaz-Almeyda E.M., Medina M., Levy O. Circadian clocks in symbiotic corals: the duet between Symbiodinium algae and their coral host. Mar. Genomics. 2014;14:47–57. doi: 10.1016/j.margen.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Sorek M., Levy O. Influence of the quantity and quality of light on photosynthetic periodicity in coral endosymbiotic algae. PLoS One. 2012;7:e43264. doi: 10.1371/journal.pone.0043264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek M., Yacobi Y.Z., Roopin M., Berman-Frank I., Levy O. Photosynthetic circadian rhythmicity patterns of Symbiodium, the coral endosymbiotic algae. Proc. R. Soc. B Biol. Sci. 2013;280:20122942. doi: 10.1098/rspb.2012.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlechner S., Jacobmeier B., Scherbarth F., Dernbach H., Kruse F., Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J. Biol. Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- Takahashi J.S., Hong H.-K., Ko C.H., McDearmon E.L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura A., Rahman M.S., Nakamura S., Park Y.J., Takano K. Lunar cycles and reproductive activity in reef fishes with particular attention to rabbitfishes. Fish Fish. (Oxf.) 2004;5:317–328. [Google Scholar]
- Tischkau S.A., Mitchell J.W., Tyan S.-H., Buchanan G.F., Gillette M.U. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J. Biol. Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- Wijgerde T., van Melis A., Silva C.I.F., Leal M.C., Vogels L., Mutter C., Osinga R. Red light represses the photophysiology of the scleractinian coral stylophora pistillata. PLoS One. 2014;9:e92781. doi: 10.1371/journal.pone.0092781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuitchik D.M., Wang D., Pells T.J., Karimi K., Ward S., Vize P.D. Seasonal temperature, the lunar cycle and diurnal rhythms interact in a combinatorial manner to modulate genomic responses to the environment in a reef-building coral. Mol. Ecol. 2019;28:3629–3641. doi: 10.1111/mec.15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujnovsky I., Hirayama J., Doi M., Borrelli E., Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. Proc. Natl. Acad. Sci. U S A. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantke J., Ishikawa-Fujiwara T., Arboleda E., Lohs C., Schipany K., Hallay N., Straw A.D., Todo T., Tessmar-Raible K. Circadian and circalunar clock interactions in a marine annelid. Cell Rep. 2013;5:99–113. doi: 10.1016/j.celrep.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kornhauser J.M., Zee P.C., Mayo K.E., Takahashi J.S., Turek F.W. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and creb phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z.S., Eichele G., Bradley A., Lee C.C. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data reported in this study have been deposited to the Sequence Read Archive “SRA, PRJNA526391”: Characterizing circalunar and diel rhythmicity in the model coral Acropora digitifera, a molecular perspective. Correspondence and request for materials should be addressed to Y.R. yaelirose@gmail.com or O.L. oren.levy@biu.ac.il.