Summary
Efficient motility requires polarized cells, with pseudopods at the front and a retracting rear. Polarization is maintained by restricting the pseudopod catalyst, active Rac, to the front. Here, we show that the actin nucleation-promoting factor Wiskott-Aldrich syndrome protein (WASP) contributes to maintenance of front-rear polarity by controlling localization and cellular levels of active Rac. Dictyostelium cells lacking WASP inappropriately activate Rac at the rear, which affects their polarity and speed. WASP’s Cdc42 and Rac interacting binding (“CRIB”) motif has been thought to be essential for its activation. However, we show that the CRIB motif’s biological role is unexpectedly complex. WASP CRIB mutants are no longer able to restrict Rac activity to the front, and cannot generate new pseudopods when SCAR/WAVE is absent. Overall levels of Rac activity also increase when WASP is unable to bind to Rac. However, WASP without a functional CRIB domain localizes normally at clathrin pits during endocytosis, and activates Arp2/3 complex. Similarly, chemical inhibition of Rac does not affect WASP localization or activation at sites of endocytosis. Thus, the interaction between small GTPases and WASP is more complex than previously thought—Rac regulates a subset of WASP functions, but WASP reciprocally restricts active Rac through its CRIB motif.
Keywords: actin polymerization, Arp2/3 complex, cell polarity, uropod, small GTPases, CRIB motif, actin cytoskeleton
Graphical Abstract
Highlights
-
•
WASP exploits its CRIB motif to remove active Rac from the membrane via endocytosis
-
•
WASP is recruited to clathrin-coated pits (CCPs) independently of small GTPases
-
•
WASP triggers actin polymerization at CCPs independently of small GTPase activation
-
•
WASP maintains homeostasis in Dictyostelium by controlling the level of active Rac
Amato et al. have discovered a mechanism that contributes to front-rear polarization in migrating cells. During clathrin-mediated endocytosis, Dictyostelium WASP interacts with active Rac via its CRIB motif. This interaction leads to incorporation of active Rac within endocytic vesicles, which facilitates its removal from inappropriate locations.
Introduction
Filamentous actin (F-actin) fulfils numerous functions in migrating cells. One crucial role is the generation of protrusions, such as pseudopods and lamellipods. A second is maintenance of intracellular trafficking, by driving endocytosis and vesicle sorting. These diverse functions depend upon the highly conserved Arp2/3 complex, which drives branching and growth of the actin network [1].
Cells rely on the Wiskott-Aldrich syndrome protein (WASP) family of nucleation-promoting factors (NPFs) to control the Arp2/3 complex spatially and temporally. The founding member of the family, WASP [2], is specific to hematopoietic lineages. Vertebrates possess a ubiquitous WASP paralog, N-WASP, which was originally described as a neural-specific gene [3] although expressed in nearly all cell types [4]. Other eukaryotes, including Dictyostelium, yeasts, and Drosophila, express a single WASP, which is the ortholog of vertebrates’ ubiquitous isoform [5]. WASPs’ principal role is to facilitate clathrin-mediated endocytosis (CME) [6, 7, 8, 9].
Other members of the WASP family recruit and activate the Arp2/3 complex to other structures and for other purposes. For instance, the SCAR/WAVE complex drives formation of actin-rich protrusions [10, 11, 12, 13] and is therefore an established regulator of cell migration and polarization.
Protrusions and clathrin-coated pits (CCPs) tend to occur in different regions of the cell. Protrusions can be generated almost anywhere on the membrane, but those that cause locomotion are usually initiated at the cell front, where SCAR/WAVE localizes. Whether CCPs are internalized in defined areas of the cell appears cell type dependent. In highly motile cells, including lymphocytes [14], leukocytes [15], and Dictyostelium [16], CME tends to occur at the rear.
WASP and SCAR/WAVE are normally spatially and functionally segregated. Nevertheless, they are plastic. In Dictyostelium and Caenorhabditis, WASP can compensate for loss of SCAR/WAVE [12, 17], generating actin protrusions at the cell front. Thus, WASP can respond to the upstream signals that drive pseudopod extension but does not normally do so. What determines segregation of WASP and SCAR/WAVE remains an important open question.
One prediction is that upstream regulators are crucial—distinct sub-cellular localization of activators may account for WASP’s and SCAR/WAVE’s distinct sub-cellular distribution and function. However, some of the upstream regulators appear to be shared between WASPs and SCAR/WAVE, for example, Nck [18, 19]. Small guanosine triphosphatases (GTPases) are also known crucial activators of both WASPs and SCAR/WAVE. However, while SCAR/WAVE has consistently been linked to Rac1 [20, 21, 22], there is no coherent model of how WASPs’ behavior is regulated by small guanosine triphosphatases (GTPases).
WASPs from most organisms contain a “CRIB” (Cdc42/Rac interacting binding) motif, which binds selectively and specifically to the active form of Rac and Cdc42 [23] in many different proteins [23, 24]. Early in vitro measurements concluded that Cdc42 had a major role in activating N-WASP [4, 25]. However, more recent and precise biochemical analysis suggests that Cdc42 activates hematopoietic WASP, while Rac1 also interacts with N-WASP [26]. For Dictyostelium WASP, most attention has focused on the unusual RacC [27], although WASP also efficiently interacts with members of the Rac1 subfamily [27], which are more abundant (http://dictyexpress.biolab.si) and more closely related to mammalian Cdc42 and Racs. The Dictyostelium genome contains genes for many Rac relatives but no Cdc42 [28].
Understanding spatial and functional segregation of WASPs and SCAR/WAVE requires an improved comprehension of interactions with small GTPases. In fact, a model whereby Rac mediates the activation of both NPFs fits poorly with their distinct sub-cellular localization and functionality.
Recent work offers a fresh perspective on how cells maintain spatial and functional separation of WASP and SCAR/WAVE [6]. Loss of WASP in Dictyostelium causes aberrant accumulation of SCAR/WAVE at the rear, leading to defective retraction [6] and compromised cell polarity. Here, we dissect the role for WASP in maintenance of front-rear polarization.
We demonstrate that WASP exploits its CRIB-mediated interaction with active Rac to limit where the active GTPase is found. Furthermore, this work clarifies the importance of GTPases for WASP’s function: a direct interaction with active GTPases is not needed for WASP to trigger actin polymerization during CME, but is required for WASP to generate pseudopods in SCAR/WAVE’s absence. More provocatively, our study suggests a reversed role for the interaction between WASP and GTPases: the presence of a CRIB motif does not only mean that WASP activity requires GTPase regulation, but that WASP modulates the distribution of GTPases after they are activated.
Results
Loss of WASP Causes Accumulation of SCAR/WAVE and Active Rac at the Rear
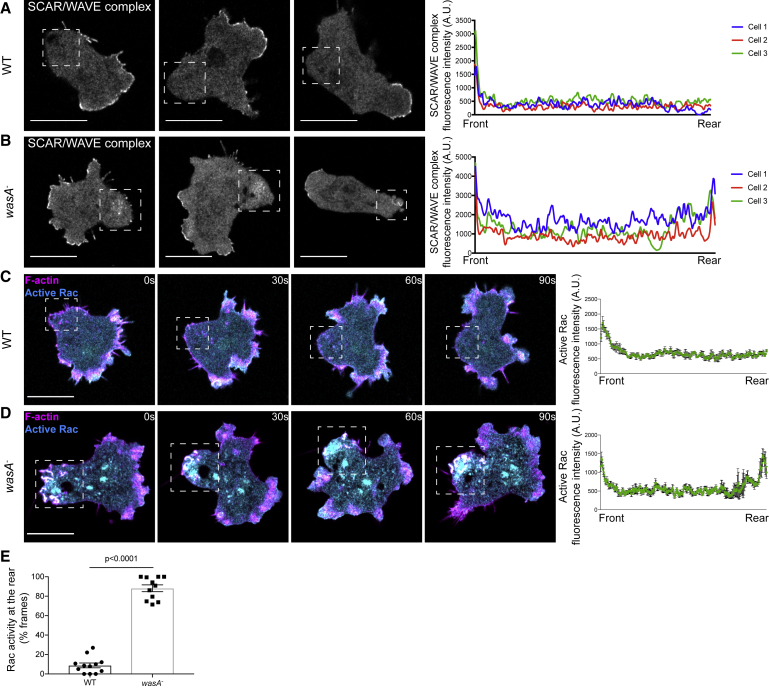
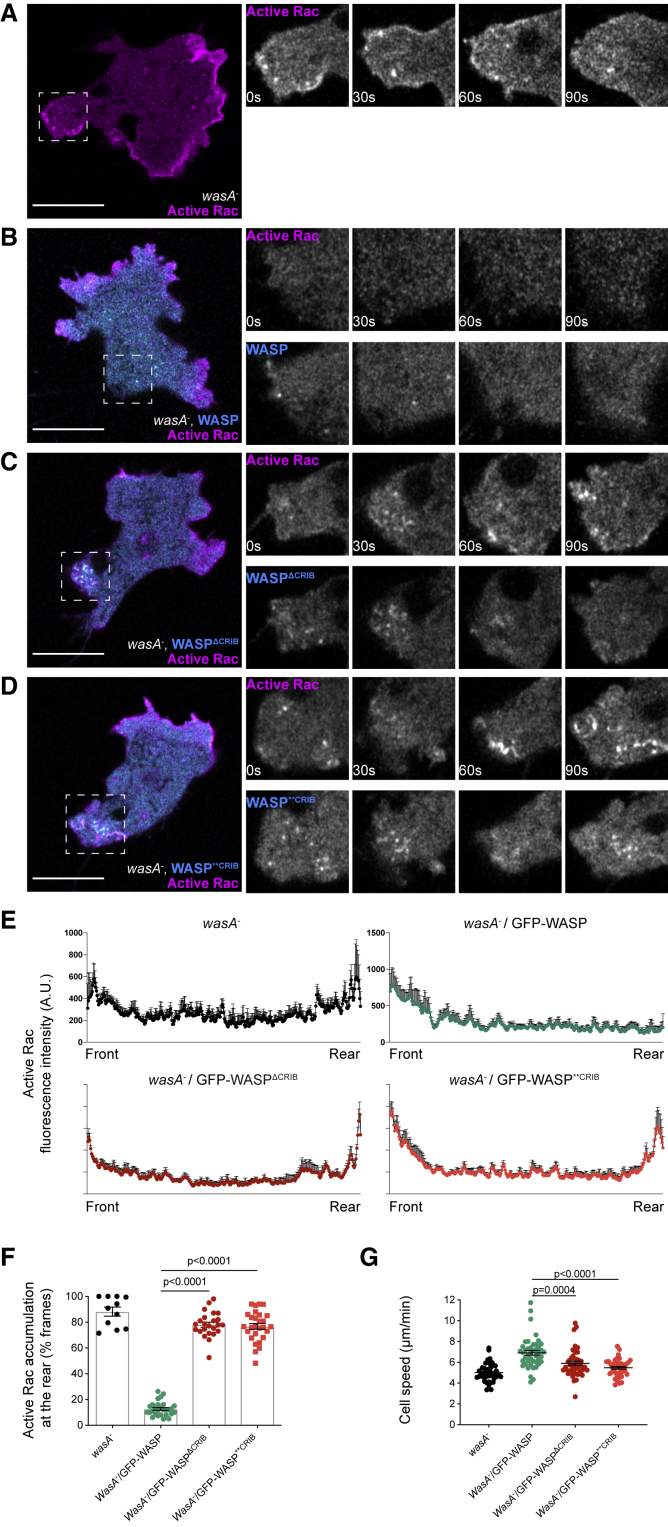
Previous work shows that knockout mutants in the Dictyostelium gene encoding WASP, wasA, migrate slower than their wild-type counterparts, due to a defect in rear retraction [6]. Detailed analysis revealed that, although wild-type cells confine SCAR/WAVE at the extending protrusions (Figure 1A), wasA− cells also accumulate it within an enlarged rear (Figure 1B). Mutants fail to confine SCAR/WAVE to a single region of their plasma membrane, resulting in a nearly bipolar appearance. Here, we examined the drivers of wasA− cells’ inability to exclude SCAR/WAVE from their rear.
Figure 1.
wasA− Cells Accumulate SCAR/WAVE and Active Rac at the Rear
(A and B) SCAR/WAVE (HSPC300-GFP) localizes at the front but not at the rear (squares) of migrating wild-type (WT) cells (A) but is present at both ends in wasA− cells (B). Fluorescence plots on the right-hand side report the SCAR/WAVE's intensity along a line connecting front to rear of representative cells at a given time point. Scale bars represent 10 μm.
(C and D) Active Rac (PakB CRIB-GFP) is confined to the front and excluded from the rear (squares) of migrating WT cells (C) but is present at the enlarged rear (squares) of wasA− cells (D); see also Figure S1. Related to Video S1. Fluorescence plots show active Rac enrichment along a line connecting front to rear of individual cells at four time points. F-actin (LifeAct-mRFP) is detected at the front and on transient spots at the rear (squares) of WT cells (C) and at the front but also on persistent structures at the rear (squares) of wasA− cells (D). Scale bars represent 10 μm.
(E) Frequency (% frames; yes/no scoring) of active Rac enrichment at the rear of migrating WT and wasA− cells. WT (n = 12 cells): 8.9 ± 2.5; wasA− (n = 11 cells): 88.2 ± 3.5; means ± SEM. Mann-Whitney test; p < 0.0001.
One intriguing possibility was that WASP may be responsible for the spatial restriction of SCAR/WAVE’s activators, preventing actin polymerization in unwanted areas of the cell. Because active Rac is one of the major SCAR/WAVE regulators [20, 21, 29], we asked whether localization or dynamics of the GTP-bound GTPase was affected in wasA− cells. We imaged living cells expressing fluorescently tagged CRIB motif of PakB, which binds to many Dictyostelium Racs, including Rac1A-C and RacC, with high-affinity in vitro [30] and is thus an effective reporter for active Rac [12]. Similar constructs have been used to monitor endogenous active Rac in mammalian cells [31, 32, 33]. As expected, wild-type cells accumulate active Rac at the leading edge (Figures 1C and S1A), where it co-localizes with F-actin. wasA− cells also accumulate active Rac at the enlarged rear (Figures 1D and S1B; Video S1), leading to the formation of F-actin-rich structures at both ends. Quantification revealed that, although wild-type cells occasionally show active Rac at the rear, mostly when pseudopods are swept from the front of the cell as it moves, wasA− cells more frequently accumulate active Rac at their backs (Figure 1E). Because the localization of both active Rac and SCAR/WAVE is aberrant in wasA− cells and Rac directly regulates SCAR/WAVE, the simplest conclusion would be that WASP limits the amount of membrane with active Rac.
Wild-type cells (WT, top panel) accumulate active Rac (active Rac marker: PakB CRIB-GFP) at their front. No active Rac is generally detected at their rear (asterisk). WASP knockout cells (wasA−, bottom panel) accumulate active Rac at their front, where protrusions are generated, as well as at persistent structures at their enlarged rears (asterisk). Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
Generation of WASP CRIB Mutants
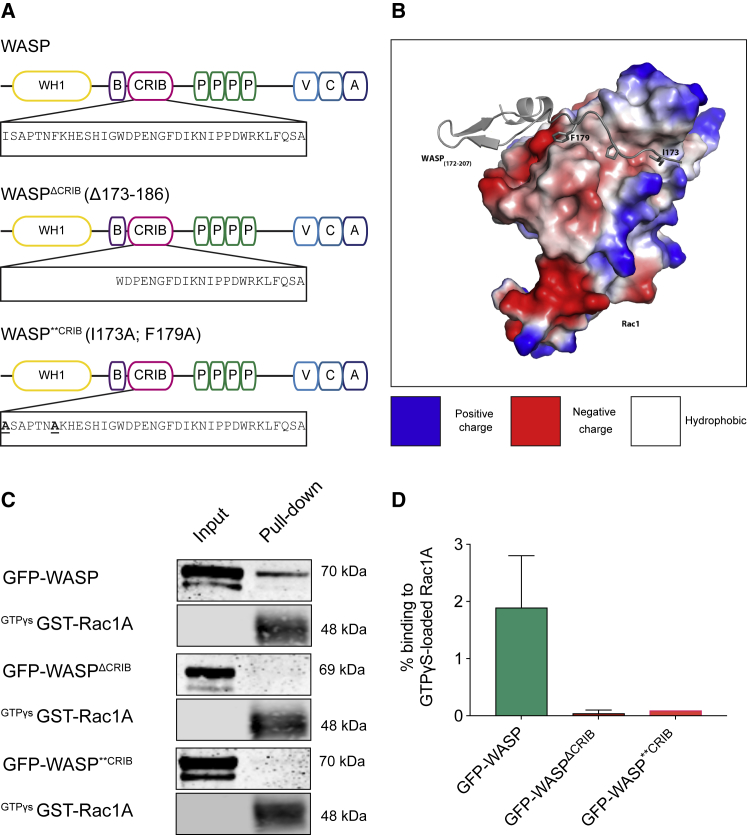
We tested possible molecular mechanisms underlying WASP-mediated confinement of active Rac and SCAR/WAVE. We focused on WASP’s CRIB motif, which enables WASP to bind active GTPases, by introducing mutations (Figure 2A). The first mutant, WASPΔCRIB, harbors a deletion of 14 residues (173–186) of the CRIB’s core [23]. A related mutant has been described in Drosophila [34]. To ensure this substantial deletion did not affect function, we designed a second mutant (WASP∗∗CRIB), containing only two conservative amino acidic changes (I173A; F179A), chosen for their position in the WASP/Rac interface (Figure 2B), inferred from the structure of the complex between Cdc42 and WASP’s minimal p21 binding domain, which includes the CRIB motif [35]. Changing them to alternative hydrophobic amino acids should maximally diminish the binding energy, with minimal change to the CRIB motif’s structure. Importantly, both changes affect the N terminus of the CRIB motif, which is not primarily involved in maintenance of the autoinhibited conformation [36]. We therefore do not expect these mutations to steer WASP to an inappropriately active conformation.
Figure 2.
Mutations in the WASP CRIB Motif Abrogate Binding to Active Rac1
(A) WASP domain composition and mutations introduced within the CRIB motif. From top to bottom: WASP; WASPΔCRIB; and WASP∗∗CRIB are shown.
(B) 3D representation of WASP/Rac1 interface. WASP (gray) residues I173 and F179 establish contacts with a hydrophobic (white) region of Rac1.
(C) GFP-WASP (first panel) interacts with active (GTPγs-bound) Rac1A, GFP-WASPΔCRIB and GFP-ASP∗∗CRIB (third and fifth panels) do not (IB = anti-GFP). Anti-GST immunoblot was performed (second, fourth, and sixth panels) to verify the expression of GST-Rac1A. Related to Figure S2.
(D) Immunoblot quantification shows no binding of GFP-WASPΔCRIB and GFP-WASP∗∗CRIB to active Rac1 (GFP-WASP: 1.9% ± 0.9%; GFP-WASPΔCRIB: 0.05% ± 0.05%; GFP-WASP∗∗CRIB: 0.1; mean ± SEM; n = 3).
We tested the two WASP CRIB mutants’ interaction with active Rac using pull-down assays. We examined Rac1A, highly expressed and closely related to canonical Rac1 of other species, and RacC, which has been associated with Dictyostelium WASP [27]. Bacterially purified glutathione S-transferase (GST)-Racs were activated by loading with GTPγs, a non-hydrolyzable analog of GTP. Wild-type WASP binds to active Racs, but neither CRIB mutant is able to bind either GTPase (Figures 2C, 2D, and S2).
Rac Binding Is Not Required to Recruit WASP to CCPs
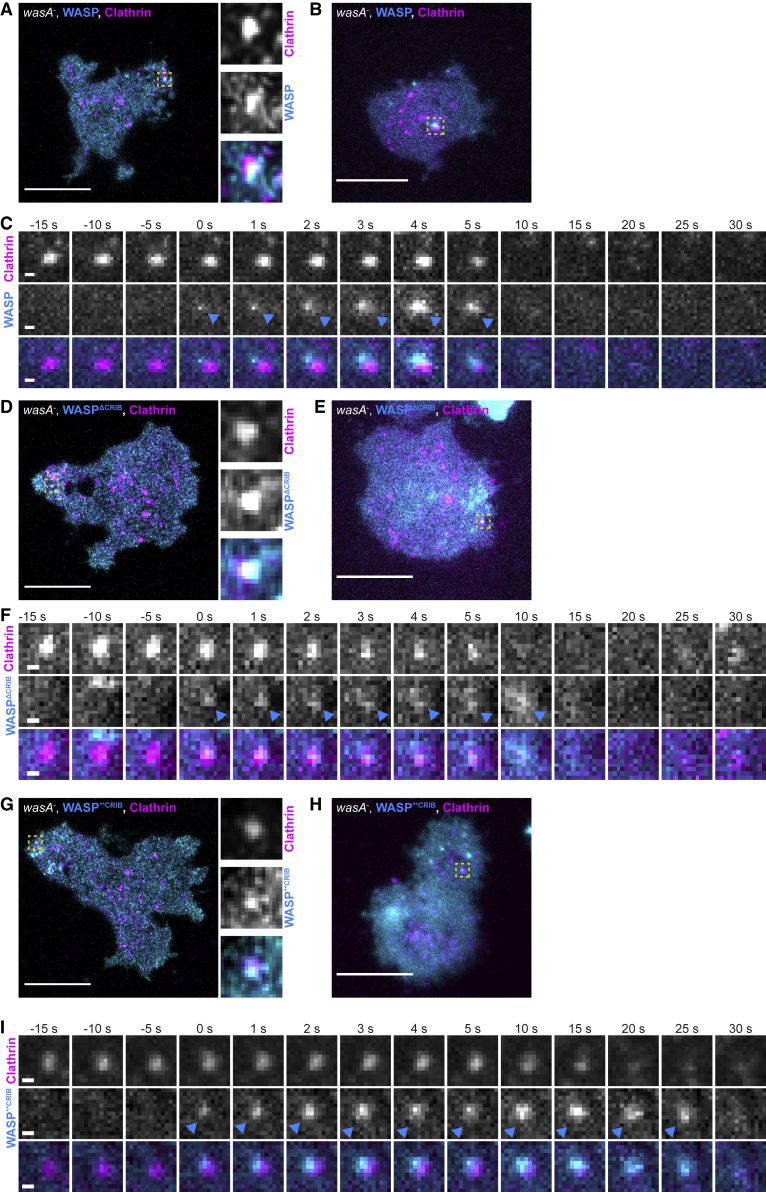
The most widely accepted model of WASP regulation asserts that Rac or Cdc42 have an essential role in controlling WASP’s activity [4, 25, 26, 27], implying WASP mutants that cannot interact with active Rac should lose function. To test which physiological functions of WASP depend on Rac regulation and a functional CRIB domain, we imaged wasA− cells co-expressing fluorescently tagged WASP mutants and clathrin.
As shown in Figures 3A and 3B, wild-type WASP generates puncta that overlap with CCPs. At any time, the number of clathrin puncta that are WASP positive is low; this is because WASP appears in a brief burst while CCPs persist on the plasma membrane for a longer period of time. Figure 3C shows a representative example of clathrin/WASP dynamics visualized using TIRF (total internal reflection fluorescence) microscopy of cells gently compressed under agarose, which diminishes front-rear polarity. WASP is recruited on a pre-existing CCP, where it sits for a few seconds before disappearing along with clathrin. Clearance of WASP and clathrin from the TIRF field is considered as a bona fide sign of CCP internalization [37]. To our surprise, both WASP CRIB mutants generate puncta that coincide with CCPs (Figures 3D, 3E, 3G, and 3H). Live TIRF imaging confirmed that WASPΔCRIB and WASP∗∗CRIB are recruited to pre-existing clathrin puncta (Figures 3F and 3I).
Figure 3.
WASP Does Not Require a Direct Interaction with Active Rac to Localize to CCPs
(A) Live imaging of migrating wasA− cells co-expressing WASP (GFP-WASP, rescue) and clathrin light chain (clc)-mRFPmars. Example of clathrin/WASP co-localization is indicated (square) and highlighted (insets). Scale bar represents 10 μm.
(B) TIRF microscopy of wasA− cells co-expressing GFP-WASP (rescue) and clc-mRFPmars gently compressed under agarose. Example of clathrin/WASP co-localization is indicated (square). Scale bar represents 10 μm.
(C) TIRF imaging shows GFP-WASP appearing (t = 0 s, cyan arrowhead) on a pre-existing CCP. Clathrin and WASP disappear synchronously (t = 10 s). Scale bars represent 0.5 μm.
(D) Live imaging of migrating wasA− cells co-expressing GFP-WASPΔCRIB and clc-mRFPmars. Example of clathrin/WASPΔCRIB co-localization is indicated (square) and highlighted (insets). Scale bar represents 10 μm.
(E) TIRF microscopy of wasA− cells co-expressing GFP-WASPΔCRIB and clc-mRFPmars gently compressed under agarose. Example of clathrin/WASPΔCRIB co-localization is indicated (square). Scale bar represents 10 μm.
(F) TIRF imaging shows GFP-WASPΔCRIB appearing on a pre-existing CCP (t = 0 s, cyan arrowhead). Clathrin and GFP-WASPΔCRIB disappear synchronously (t = 15 s). Scale bars represent 0.5 μm.
(G) Live imaging of migrating wasA− cells co-expressing GFP-WASP∗∗CRIB and clc-mRFPmars. Example of clathrin/WASP∗∗CRIB co-localization is indicated (square) and highlighted (insets). Scale bar represents 10 μm.
(H) TIRF microscopy of wasA− cells co-expressing GFP-WASP∗∗CRIB and clc-mRFPmars gently compressed under agarose. Example of clathrin/WASP∗∗CRIB co-localization is indicated (square). Scale bar represents 10 μm.
(I) TIRF imaging shows GFP-WASP∗∗CRIB appearing on a pre-existing CCP (t = 0 s, cyan arrowhead). Clathrin and GFP-WASP∗∗CRIB disappear from the TIRF field synchronously (t = 25 s). Scale bars represent 0.5 μm.
This clearly demonstrates that a direct interaction with active Rac is not required for WASP to be recruited to CCPs, suggesting that factors other than GTPases control WASP’s sub-cellular localization in living cells during endocytosis.
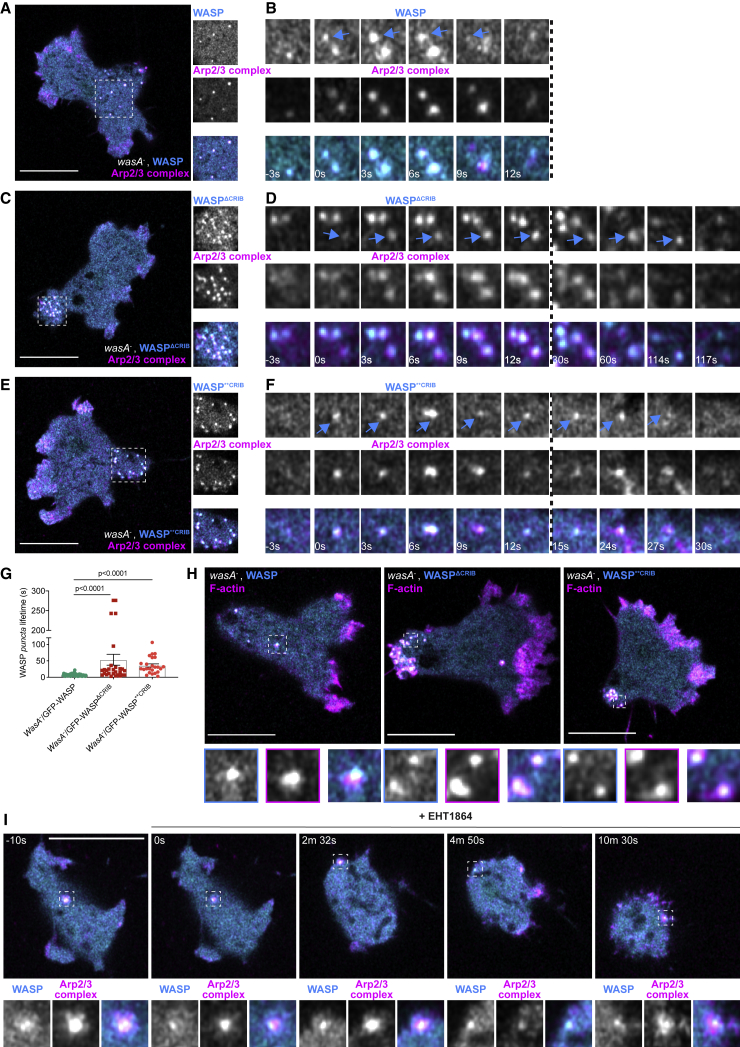
WASP Does Not Require Rac to Recruit Arp2/3 Complex and Actin
We noticed that the WASP CRIB mutants co-localize with clathrin for longer than wild-type WASP. This could be caused by their inability to trigger actin polymerization on CCPs, as F-actin is thought to provide the force required for CME [38, 39] and is mandatory in yeast and Dictyostelium [6, 40], but not all mammalian cells [41, 42]. Given our demonstration that loss of WASP causes increased CCP lifetime due to the lack of actin (Figure 2E in [6]), we tested whether WASP CRIB mutants are able to trigger actin polymerization once recruited to CCPs. In essentially 100% of cases, WASP spots (which tend to be distributed over the rear half of polarized cells) are Arp2/3 complex positive, presumably reflecting the fact that WASP is activated as it is targeted to CCPs, so the Arp2/3 complex is recruited synchronously (Figures 4A and 4B). Unexpectedly, 100% of puncta generated by mutated WASPs are also Arp2/3 complex positive (Figures 4C–4F). Spots generated by WASPΔCRIB and WASP∗∗CRIB were more likely to be seen within the enlarged rear of the cell, with increased lifetimes (Figure 4G). Consistent with the Arp2/3 complex data, F-actin was observed on all WASP puncta irrespective of the presence of a functional CRIB domain (Figure 4H; Video S2). WASP’s ability to drive actin polymerization at CCPs is therefore independent of small GTPase binding.
Figure 4.
WASP Does Not Require Active Rac to Recruit the Arp2/3 Complex and Trigger Actin Polymerization on Puncta
(A) Live imaging of migrating wasA− cells co-expressing GFP-WASP (rescue) and mRFPmars2-ArpC4. WASP puncta are evenly distributed at the rear and are all Arp2/3 complex positive. The area indicated by the square is highlighted within insets. Scale bar represents 10 μm.
(B) GFP-WASP appears on a punctum (t = 0 s, cyan arrow) alongside mRFPmars2-ArpC4 and then synchronously disappears (t = 9 s).
(C) Live imaging of migrating wasA− cells co-expressing GFP-WASPΔCRIB and mRFPmars2-ArpC4. All WASPΔCRIBpuncta cluster within the enlarged rear (square). As highlighted (insets), all GFP-WASPΔCRIB spots are Arp2/3 complex positive. Scale bar represents 10 μm.
(D) GFP-WASPΔCRIB appears as a punctum (t = 0 s, cyan arrow) along with mRFPmars2-ArpC4. WASPΔCRIB remains within spots (alongside the Arp2/3 complex) and then synchronously disappears (t = 117 s).
(E) Live imaging of migrating wasA− cells co-expressing GFP-WASP∗∗CRIB and mRFPmars2-ArpC4. All WASP∗∗CRIBpuncta cluster within the enlarged rear (square). As shown in insets, all WASPΔCRIB spots are Arp2/3 complex positive. Scale bar represents 10 μm.
(F) GFP-WASP∗∗CRIB appears as a punctum (t = 0 s, cyan arrow) alongside mRFPmars2-ArpC4. They disappear synchronously (t = 27 s).
(G) Lifetime of wild-type and WASP CRIB mutants’ puncta. wasA−/GFP-WASP (n = 52 puncta): 7.2 ± 0.5 s; wasA−/GFP-WASPΔCRIB (n = 28 puncta): 53.4 ± 16.6 s; wasA−/GFP-WASP∗∗CRIB (n = 24 puncta): 35.8 ± 5.1 s; means ± SEM. Kruskal-Wallis test, wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB = p < 0.0001; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB = p < 0.0001).
(H) Live imaging of migrating wasA− cells expressing LifeAct-mRFPmars2 and GFP-WASP (rescue, left), GFP-WASPΔCRIB (center), and GFP-WASP∗∗CRIB (right). All puncta generated by wild-type WASP or WASP CRIB are F-actin positive. Examples indicated by squares are highlighted within insets. Scale bars represent 10 μm. Related to Video S2.
(I) Live imaging of wasA− cells expressing GFP-WASP and mRFPmars2-ArpC4 treated with Rac inhibitor (EHT1864). Before EHT1864 addition (t = −10 s), cells are polarized and WASP generates Arp2/3-complex-positive puncta (square). Shortly after treatment (t = 0 s), cells become rounder. As highlighted by squares, WASP generates Arp2/3-complex-positive puncta even after 10 min from treatment. Scale bar represents 10 μm.
See also Figure S3.
wasA− cells expressing LifeAct (F-actin reporter) along with GFP-WASP (rescue, top panel), GFP-WASPΔCRIB (middle panel) or GFP- WASP∗∗CRIB (bottom panel) were imaged during directed migration. Rescued cells generate transient puncta that appear evenly distributed away from the front (asterisk). Cells expressing either WASP CRIB mutant generate longer-lived puncta that cluster within the enlarged uropods (asterisk). At all timepoints, WASP spots are F-actin positive. Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
For further experimental support, we tested WASP’s activity upon chemical inhibition of Rac. Because no Rac inhibitor had been verified for Dictyostelium, we tested candidates using wild-type cells expressing an active Rac marker. NSC23766 [43] failed to show any effect (not shown). However, EHT1864, which blocks Rac’s loading with GTP in mammalian cells [44, 45], was very effective in Dictyostelium (Figure S3A). A minimal dose of 3 μM causes rapid loss of membrane localization of the active Rac marker, leading to progressive cell rounding.
In agreement with our data highlighting the ability of CRIB-mutated WASP to be recruited to CCPs, we found that WASP is able to generate Arp2/3-complex- and F-actin-positive puncta after Rac inhibition (Figures 4I, S3B, and S3C). Quantitative analysis (Figures S3D and S3E) shows that EHT1864 does not alter the rate at which WASP puncta are generated or their lifetime. This result reinforces our conclusion that WASP does not need active GTPases to localize correctly during CME or to recruit Arp2/3 complex and generate F-actin.
WASP Interaction with Active Rac Is Essential for Front-Rear Polarity
The generation of WASP CRIB mutants allowed us to test whether WASP requires a direct interaction with active Rac to maintain front-rear polarization.
wasA− cells accumulate active Rac at the leading edge, but also within the enlarged rear (Figures 5A and 1D). wasA− cells expressing GFP-tagged WASP (rescue) behave normally, accumulating active Rac exclusively at the front (Figure 5B). CRIB-mutated WASPs do not suppress the accumulation of active Rac at the rear—both mutants generate discrete puncta that accumulate within the enlarged rear, together with persistent active Rac-marker-positive structures like those seen in unrescued wasA− cells (Figures 5C and 5D; Video S3). At a given time, a subset of puncta in WASP CRIB mutants is also enriched in active Rac. WASP-CRIB-mutants and active Rac appear to have distinct dynamics: active Rac appears to remain at the plasma membrane for longer than CRIB-mutated WASPs. Therefore, although some active Rac-positive structures are not enriched in CRIB-mutated WASPs, we cannot exclude that they were enriched in CRIB-mutated WASP at some stage. Representative plots showing active Rac marker localization from front to rear are shown in Figure 5E. Quantification (Figure 5F) reveals that cells expressing CRIB-mutated WASPs accumulate active Rac at the rear to the same degree as wasA− cells, while wild-type WASP rescues the spatial confinement of active Rac at the leading edge. In line with previous reports from wasA− cells [6], cells expressing either WASP CRIB mutant are significantly slower than those expressing wild-type WASP (Figure 5G).
Figure 5.
WASP Requires a Functional CRIB Motif to Confine Active Rac at the Leading Edge during Migration
Live imaging of migrating wasA− cells expressing active Rac marker (PakB CRIB-mRFPmars2). Insets highlight the cells’ rear (squares) at different time points.
(A) Active Rac accumulates at the rear of wasA− cells at all time points. Scale bar represents 10 μm.
(B) In cells expressing GFP-WASP, no sign of active Rac enrichment at the rear can be detected. Scale bar represents 10 μm.
(C and D) In cells expressing GFP-WASPΔCRIB (C) or GFP-WASP∗∗CRIB (D), active Rac can be detected within the enlarged rear. Puncta generated by WASP CRIB mutants cluster at the enlarged rear. Scale bars represent 10 μm. Related to Video S3.
(E) Fluorescence plots reporting the active Rac marker’s intensity at four time points along a line connecting front to rear of wasA− cells (top left), and wasA− cells expressing either WASP (wasA−/GFP-WASP, top right), WASPΔCRIB (wasA−/GFP-WASPΔCRIB, bottom left), or WASP∗∗CRIB (wasA−/GFP-WASP∗∗CRIB, bottom right). Error bars represent SEM.
(F) Frequency of active Rac accumulation at the back of wasA− cells and wasA− cells expressing wild-type WASP or WASP CRIB mutants. wasA− (n = 11 cells): 88.2% ± 3.5%; wasA−/GFP-WASP (n = 28 cells): 12.7% ± 1.0%; wasA−/GFP-WASPΔCRIB (n = 24 cells): 78.1% ± 2.0%; wasA−/GFP-WASP∗∗CRIB (n = 28 cells): 76.7% ± 2.2%; means ± SEM. One-way ANOVA; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p < 0.0001; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p < 0.0001.
(G) Migratory speed of wasA− cells and wasA− cells expressing wild-type WASP or WASP CRIB mutants. wasA− (n = 45 cells): 5.0 ± 0.1 μm/min; wasA−/GFP-WASP (n = 44 cells): 6.9 ± 0.2 μm/min; wasA−/GFP-WASPΔCRIB (n = 44 cells): 5.9 ± 0.2 μm/min; wasA−/GFP-WASP∗∗CRIB (n = 41 cells): 5.5 ± 0.1 μm/min; means ± SEM. Kruskal-Wallis test; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p = 0.0004; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p < 0.0001.
wasA− cells expressing active Rac marker (PakB CRIB-mRFPmars2) along with GFP-WASP (rescue, left panel), GFP-WASPΔCRIB (central panel) or GFP- WASP∗∗CRIB (right panel) were imaged during directed migration. Rescued cells do not accumulate active Rac at their rears (asterisk), where WASP generates transient puncta. Cell expressing either CRIB-mutated WASP accumulate active Rac at the front, as well as within the enlarged rears (asterisk), where it can be detected on persistent structures that are also occasionally mutant WASP-positive. Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
Our results show that WASP requires an intact CRIB motif to exclude active Rac from the cell rear during migration.
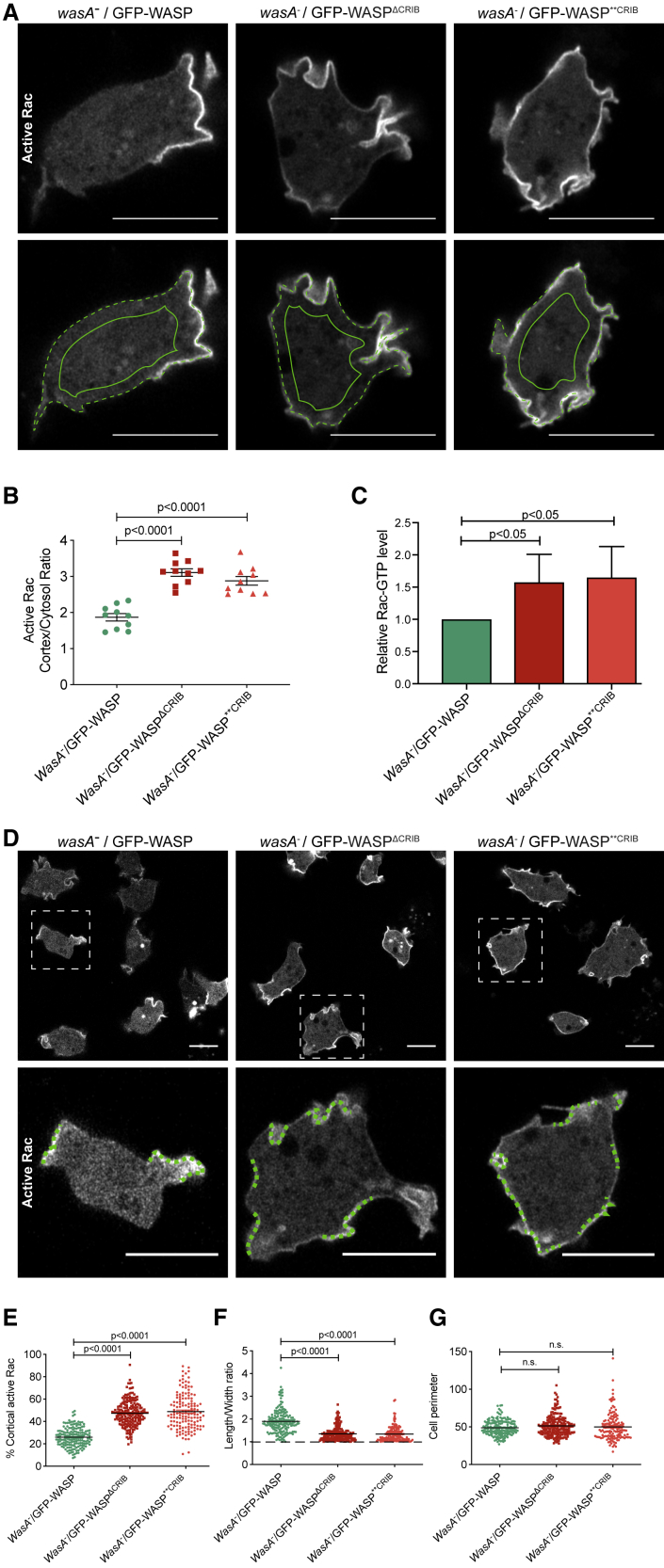
WASP Contributes to Homeostasis of Active Rac Levels
We examined whether WASP works locally, to remove active Rac from inappropriate locations, or has a more global role in Rac regulation.
Taking into account that active Rac is largely membrane bound [46], and that the CRIB-based marker interacts selectively with GTP-bound Rac [30] but is cytosolic when unbound, then the ratio of active Rac marker at the membrane and in the cytosol offers a measure of the total levels of active Rac in the cell [12]. We therefore measured the relative quantities of active Rac marker at the membrane and within the cytosol in cells expressing CRIB-mutated WASPs. As shown in Figure 6A (quantified in Figure 6B), the membrane:cytosol ratio of the active Rac marker is consistently and significantly higher in cells expressing a CRIB-mutated WASP in comparison with cells expressing wild-type WASP. We confirmed this result by pulling down the active Rac from cell lysates using GST-CRIB beads (Figure 6C). Again, we observed a higher level of active Rac in cell expressing WASP CRIB mutants.
Figure 6.
WASP Requires a Functional CRIB Motif to Maintain Homeostatic Levels of Active Rac
(A) Airyscan confocal imaging of wasA− cells co-expressing active Rac marker (PakB CRIB-mRFPmars2) and GFP-tagged WASP (wild-type, ΔCRIB, or ∗∗CRIB; green channel not shown). Images were acquired consistently through the mid-point of cells. The ratio between the intensity of the active Rac marker along the plasma membrane (dashed line) and the cytosol (solid line) was measured. Scale bars represent 10 μm.
(B) Quantification of active Rac marker membrane:cytosol intensity ratio from multiple cells as in (A) wasA−/GFP-WASP (n = 10 cells): 1.9 ± 0.1; wasA−/GFP-WASPΔCRIB (n = 10 cells): 3.1 ± 0.1; wasA−/GFP-WASP∗∗CRIB (n = 10 cells): 2.9 ± 0.1; means ± SEM. One-way ANOVA; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p < 0.0001; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p < 0.0001.
(C) Pull-down assay for active Rac levels. Active Rac was selectively precipitated from wasA− cells expressing wild-type or CRIB mutant WASPs, using GST-PAK-CRIB as bait. Active Rac was measured by western blot using an anti-Rac antibody and a fluorescent secondary antibody (n = 3 independent experiments; single-tailed t test).
(D) Airyscan confocal imaging of wasA− cells co-expressing active Rac marker and GFP-tagged WASP (wild-type, ΔCRIB, or ∗∗CRIB; green channel not shown). Cells expressing wild-type WASP (left) accumulate active Rac on a small proportion of their plasma membrane (dashed lines within inset). Cells expressing WASPΔCRIB or WASP∗∗CRIB (center and right, respectively) accumulate active Rac in a larger proportion of their plasma membrane (dashed lines within inset). Scale bars represent 10 μm.
(E) Percentage of plasma membrane enriched in active Rac in cells expressing wild-type or CRIB-mutated WASPs. Multiple cells from an experiment as in (C) wasA−/GFP-WASP (n = 168 cells): 25.9% ± 0.7%; wasA−/GFP-WASPΔCRIB (n = 204 cells): 47.3% ± 0.8%; wasA−/GFP-WASP∗∗CRIB (n = 145 cells): 48.8% ± 1.3%; means ± SEM. Mann-Whitney test; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p < 0.0001; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p < 0.0001. Related to Figure S4.
(F) Length-to-width ratio of cells expressing wild-type or CRIB-mutated WASP. Multiple cells from an experiment as in (C) wasA−/GFP-WASP (n = 168 cells): 1.9 ± 0.04; wasA−/GFP-WASPΔCRIB (n = 204 cells): 1.4 ± 0.02; wasA−/GFP-WASP∗∗CRIB (n = 145 cells): 1.3 ± 0.03; means ± SEM. Mann-Whitney test; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p < 0.0001; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p < 0.0001.
(G) Perimeter of cells expressing wild-type or CRIB-mutated WASP. Multiple cells from an experiment as in (C) wasA−/GFP-WASP (n = 168 cells): 48.8 ± 0.8; wasA−/GFP-WASPΔCRIB (n = 204 cells): 51.3 ± 0.9; wasA−/GFP-WASP∗∗CRIB (n = 145 cells): 49.9 ± 1.5; means ± SEM. Kruskal-Wallis test; wasA−/GFP-WASP versus wasA−/GFP-WASPΔCRIB: p = 0.56; wasA−/GFP-WASP versus wasA−/GFP-WASP∗∗CRIB: p = 0.84.
We also examined the extent of active Rac patches. Cells expressing CRIB-mutated WASPs accumulate the active Rac marker in a significantly larger portion of their plasma membrane than cells expressing wild-type WASP (Figures 6D and 6E). This increase in active Rac localization is accompanied by a reduction in cell polarity (Figure 6F). Cells expressing wild-type WASP are elongated even when migrating randomly, while those expressing either WASP CRIB mutant are more rounded, with a length:width ratio of nearly one. No significant difference between the average perimeter of cells expressing wild-type or CRIB-mutated WASPs was seen (Figure 6G), so the more widespread localization of the active Rac marker in cells expressing WASP CRIB mutants is not due to the availability of more plasma membrane. We confirmed the correlation between active Rac levels and the proportion of plasma membrane labeled by the active Rac marker using tetracycline-inducible dominant-active (G12V) Rac1A. Induction with tetracycline causes cells to accumulate the active Rac marker on a higher proportion of the plasma membrane, often close to 100% (Figures S4A and S4B).
Altogether, our data show that cells whose WASP lacks a functional CRIB motif, and thus cannot bind Rac, accumulate more active Rac on a higher portion of the plasma membrane than cells with an intact WASP.
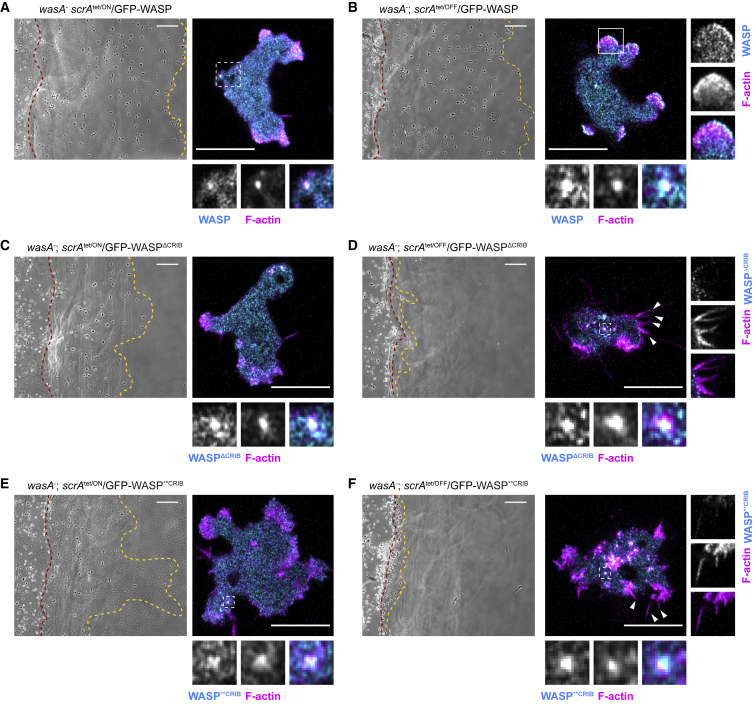
WASP Requires Active Rac Interaction to Replace SCAR/WAVE at Pseudopods
Internalization of CCPs is by far the clearest and evolutionarily conserved role of WASP; however, WASP can contextually fulfil other biological roles. For instance, WASP can drive protrusion extension in cells that have lost SCAR/WAVE [12]. We therefore asked whether WASP can drive pseudopods extension independently of Rac1, using a cell line in which wasA is knocked out and SCAR expressed under a tetracycline-inducible promoter (wasA− scrAtet). We expressed CRIB-mutated GFP-WASP in wasA− scrAtet cells and examined their ability to migrate toward chemoattractant in the presence of tetracycline (when SCAR/WAVE is expressed) or without (when the only pseudopod inducer was the exogenously expressed WASP).
As shown in Figure 7A, cells expressing SCAR/WAVE and wild-type WASP (wasA− scrAtet/ON/GFP-WASP) migrate efficiently in a chemotactic gradient. In these cells, WASP localizes to F-actin-rich puncta. Once the expression of SCAR/WAVE is turned off (Figure 7B), cells expressing wild-type WASP (wasA− scrAtet/OFF/GFP-WASP) are still able to move effectively. In these cells, WASP is additionally localized to the leading edge, where it drives formation of pseudopods, as previously described [12]. Cells expressing WASPΔCRIB or WASP∗∗CRIB migrate fairly efficiently toward a chemoattractant as long as the expression of SCAR/WAVE is maintained (Figures 7C and 7E; wasA− scrAtet/ON/GFP-WASPΔCRIB and wasA− scrAtet/ON/GFP-WASP∗∗CRIB, respectively). In each case, the mutated WASPs localize to puncta clustered within the enlarged rear. When expression of SCAR/WAVE is turned off, cells expressing either WASP CRIB mutant lose the ability to move, highly resembling cells lacking SCAR and WASP [6] (Figures 7D and 7F; wasA− scrAtet/OFF/GFP-WASPΔCRIB and wasA− scrAtet/OFF/GFP-WASP∗∗CRIB, respectively). Under these circumstances, pseudopods are no longer generated but replaced by inefficient spiky protrusions, which cannot translocate the cell.
Figure 7.
WASP Requires a Direct Interaction with Active Rac to Drive Pseudopod Extension in the Absence of SCAR/WAVE
(A) Inducible double null cells expressing GFP-WASP kept in the presence of tetracycline to maintain SCAR/WAVE expression (wasA−scrAtet/ON/GFP-WASP) were allowed to chemotax and imaged by phase-contrast microscopy. Cells migrate efficiently from start (red line) to end (yellow line). Scale bar represents 100 μm. Live imaging (right-hand panel) shows GFP-WASP localizing to actin-rich puncta at the cell rear (square). Scale bar represents 10 μm.
(B) Inducible double-null cells expressing GFP-WASP kept in the absence of tetracycline to suppress SCAR/WAVE expression (wasA−; scrAtet/OFF/GFP-WASP) were allowed to chemotax and imaged by phase-contrast microscopy. Cells migrate efficiently from start (red line) to end (yellow line). Scale bar represents 100 μm. Live imaging (right-hand panel) shows GFP-WASP localizing to actin-rich puncta at the cell rear (dashed square) and at pseudopods generated at the front (solid square). Scale bar represents 10 μm.
(C) Inducible double-null cells expressing GFP-WASPΔCRIB, kept in the presence of tetracycline to maintain SCAR/WAVE expression (wasA−; scrAtet/ON/GFP-WASPΔCRIB), were allowed to chemotax and imaged by phase-contrast microscopy. Cells are able to migrate from start (red line) to end (yellow line), although less efficiently than cells shown in (A). Scale bars represent 100 μm. Live imaging (right-hand panel) shows GFP-WASPΔCRIB localizing at actin-rich puncta accumulated at the enlarged rear (squares). Scale bars represent 10 μm.
(D) Inducible double-null cells expressing GFP-WASPΔCRIB, deprived of tetracycline to suppress SCAR/WAVE expression (wasA−; scrAtet/OFF/GFP-WASPΔCRIB), were allowed to chemotax and imaged using phase-contrast microscopy. These cells do not migrate under agarose (proximity of the red and yellow lines, start and end point, respectively). Scale bars represent 100 μm. Live imaging (right-hand panels) reveals that cells expressing GFP-WASPΔCRIB WASP do not generate pseudopods and form spiky protrusions instead (arrowheads). The ability of GFP-WASPΔCRIB to generate actin-rich puncta is not affected (dashed squares). Scale bars represent 10 μm.
(E) Inducible double-null cells expressing GFP-WASP∗∗CRIB, kept in the presence of tetracycline to maintain SCAR/WAVE expression (wasA−; scrAtet/ON/GFP-WASP∗∗CRIB), were allowed to chemotax and imaged by phase-contrast microscopy. Cells are able to migrate from start (red line) to end (yellow line), although less efficiently than cells shown in (A). Scale bars represent 100 μm. Live imaging (right-hand panel) shows GFP-WASP**CRIB localizing at actin-rich puncta accumulated at the enlarged rear (squares). Scale bars represent 10μm.
(F) Inducible double-null cells expressing GFP-WASP∗∗CRIB, deprived of tetracycline to suppress SCAR/WAVE expression (wasA−; scrAtet/OFF/GFP-WASP∗∗CRIB), were allowed to chemotax and imaged using phase-contrast microscopy. These cells do not migrate under agarose (proximity of the red and yellow lines, start and end point, respectively). Live imaging (right-hand panels) reveals that cells expressing a GFP-WASP∗∗CRIB do not generate pseudopods and form spiky protrusions instead (arrowheads). The ability of GFP-WASP∗∗CRIB to generate actin-rich puncta is not affected (dashed squares). Scale bars represent 10 μm.
Thus, a functional CRIB motif—and therefore a direct activation by Rac—is essential for WASP to stimulate pseudopod formation in the absence of SCAR/WAVE.
Discussion
WASP’s Interaction with Small GTPases: Implications for WASP Regulation
Considering how important actin nucleation promoting factors are, for processes as diverse as cell movement [10, 11, 12], macropinocytosis [47], vesicular sorting [48, 49], mitosis [50], meiosis [51], and DNA repair [52], their control is exceptionally poorly understood. The SCAR/WAVE complex contains five proteins [21] and harbors multiple potential phosphorylation and protein-protein interaction sites [53], but the relevance of most is largely unknown. WASPs, the family that includes mammalian WASP and N-WASP, the singular Dictyostelium and Drosophila WASP, were thought to be an exception. Largely based on biochemical data, the interaction with active GTPases (Cdc42 or Rac) has long been considered essential for WASP activation. Other factors, such as PIP2 and adaptor proteins, may contribute to the NPFs activation [3, 4, 25, 26, 54, 55, 56]. But in vitro settings do not recapitulate the complexity of a living cell, and early data implying a key role for small GTPases in WASP activation have not been corroborated in living cells. Studies in Drosophila suggest that CRIB-mutated WASP can still fulfil its developmental roles [34], and a CRIB-deleted N-WASP still triggers actin comet tails formation in PIP5K-overexpressing cultured fibroblasts [57]. Evolutionary arguments point in the same direction: yeasts’ WASPs have lost their Rac-binding CRIB motif during evolution but facilitate CME by a remarkably similar mechanism to higher eukaryotes’ WASPs [8].
In other words, although a number of reviews describe GTPase binding as an essential step toward WASP localization and activation [58, 59], our understanding of this interaction’s importance to living cells is still poor. Our work adds to this complex jigsaw. By introducing a deletion within the WASP CRIB motif or—importantly—a finer mutation that abrogates interaction with Rac using only two conservative substitutions, we show that WASP does not rely on GTPases to localize to CCPs nor to induce Arp2/3-complex-mediated actin polymerization once there.
WASP’s Role in Front-Rear Polarity
If WASP does not need GTPases to localize to CCPs or to trigger actin polymerization on puncta, why does it interact with them? CRIB motifs are the best-understood GTPase interactors, with high specificity for the active forms of Rac and Cdc42 [23]. Dictyostelium lacks a Cdc42 [28], which first appears just before fungal and metazoan evolutionary lineages divide, but its WASP has a well-conserved CRIB motif that gives strong binding specificity to active Rac1A and C [27]. What are its physiological roles?
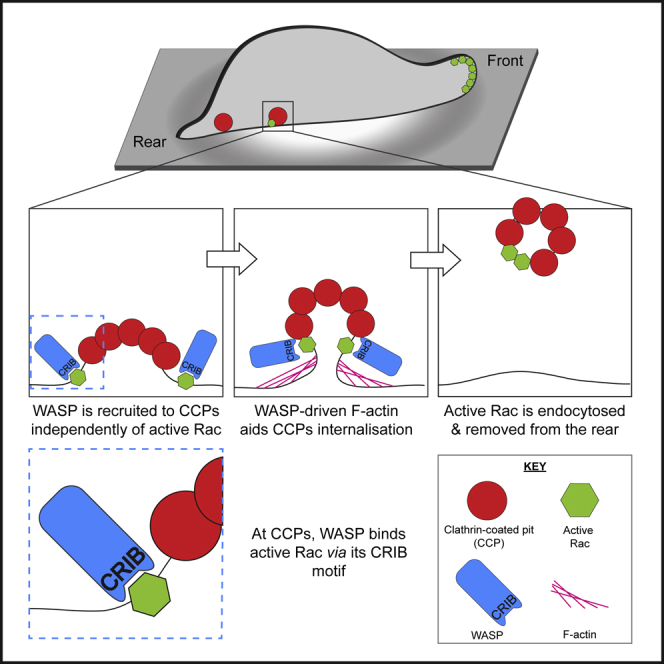
We provide two related answers. First, WASP needs a direct interaction with active Rac to compensate for loss of SCAR/WAVE. WASPs are not normally involved in pseudopod formation [60] but take over when SCAR/WAVE is absent [17]. Because active Rac is the key specifier of the leading edge, it is unsurprising that WASP requires Rac binding to do so.
The second role for Rac binding to WASP is more subtle. WASP exploits its CRIB motif to prevent aberrant accumulation of the GTPase at the cell rear. When there is no WASP, cells lose polarity in an idiosyncratic way—they generate pseudopods at the front but also carry a substantial, complex assembly at the rear that slows and depolarizes them. This aggregate contains active Rac and therefore continuously recruits SCAR/WAVE and Arp2/3 complex, inducing actin polymerization. This raises two main considerations. It highlights our poor understanding of the uropod, which may simplistically be seen as a black hole where unnecessary migratory components are passively drawn, and it underscores our incomplete knowledge of how cells achieve spatial and functional segregation of WASP and SCAR/WAVE.
We propose a novel role for WASP as a homeostatic regulator of active Rac. We envision a model whereby WASP at CCPs, in addition to triggering Arp2/3-complex-mediated actin polymerization, also removes active Rac from the membrane through its CRIB motif. In wild-type cells, we cannot detect the fluorescent active Rac marker at endocytic puncta, primarily for two reasons. First, active Rac that is recruited to a CCP is WASP bound, and thus unable to also bind our active Rac marker; second, the high membrane background masks the few active Rac molecules that, we predict, are recruited by each CCP. WASP-mediated clearance of active Rac will obviously occur faster when GTPases are excessively activated, and slower when there is little GTPase activation, so cellular levels of active Rac are maintained. Spatially, given that CME occurs mostly at the rear of migrating cells, active Rac will be removed faster there. Thus, WASP will ensure that active Rac is removed from the cell rear, maintaining front-rear polarity.
Our proposed role of WASP does not exclude other models of cell polarity. Rather, WASP-mediated clearance of active Rac may collaborate with other mechanisms underpinning spatial confinement of active Rac, such as membrane tension [61], in maintaining a dominant front.
WASP and CRIB Motifs: “The Cowl Does Not Make the Monk”
When WASP was first discovered, the presence of a CRIB motif led researchers to conclude that its activity was dependent on GTPases [3]. This now seems an oversimplification. We have shown here that WASP does not rely on the availability of (nor on the interaction with) active Rac to recruit Arp2/3 complex, or drive actin polymerization, during CME. On the other hand, WASP depends on a direct, CRIB-mediated, interaction with active Rac to drive pseudopod extension in the absence of SCAR/WAVE. Therefore, WASP’s requirement for active Rac appears to be context dependent, not an absolute requirement as initially thought. In the broader context, this means that inhibiting Rac may not of itself compromise WASP functionality. WASP has many physiological roles (e.g., invadopodia formation), and its CRIB motif is likely to be important for a subset of other processes that we have not explored.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat monoclonal [3H9] Anti-GFP | Chromotek | Cat# 3h9-100; RRID: AB_10773374 |
| Mouse monoclonal [GST.B6] Anti-GST | Abcam | Cat# ab18183; RRID: AB_444305 |
| Mouse monoclonal [23A8] Anti-Rac1 | MERCK | Cat# 05-389; RRID: AB_309712 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Rac inhibitor | Tocris | No. #3872 |
| Guanosine 5′-[β,γ-imido]triphosphate trisodium salt hydrate (GTPγs) | Sigma-Aldrich | No. #G0635 |
| Critical Commercial Assays | ||
| Active Rac pull-down assay | Cytoskeleton | No. #BK035 |
| Experimental Models: Cell Lines | ||
| AX3, wasA::bsrR | Insall Lab [6] | AD7_1 |
| AX3, wasA::bsrR parent | Insall Lab [6] | AD7_6 |
| JH8, scrA:pyr5-6, tet-onscrA; G418R, wasA::bsrR | Insall Lab [6] | AD12_21 |
| Oligonucleotides | ||
| CATTCTCTGGATCCCATTCCAAATCTTTCTCTTCCGATGAA | This manuscript | oSB5 |
| AGAGAAAGATTTGGAATGGGATCCAGAGAATGG TTTTGATA |
This manuscript | oSB6 |
| TGCGTTGGTTGGAGCTGATGCTTCCAAA TCTTTCTCTTCCGAT |
This manuscript | oSB15 |
| GCATCAGCTCCAACCAACGCAAAACATG AAAGTCATATTGGTTGG |
This manuscript | oSB16 |
| Recombinant DNA | ||
| pakB CRIB-GFP/LifeAct-mRFP co-expression vector | This manuscript | pAD149 |
| GFP-WASP/pakB CRIB-mRFPmars2 co-expression vector | This manuscript | pCA37 |
| GFP-WASPΔCRIB/pakB CRIB-mRFPmars2 co-expression vector | This manuscript | pCA44 |
| GFP-WASP∗∗CRIB/pakB CRIB-mRFPmars2 co-expression vector | This manuscript | pCA46 |
| GFP- WASPΔCRIB/LifeAct-mRFPmars2 co-expression vector | This manuscript | pCA73 |
| GFP-WASP∗∗CRIB /LifeAct-mRFPmars2 co-expression vector | This manuscript | pCA74 |
| GFP- WASP/LifeAct-mRFPmars2 co-expression vector | This manuscript | pCA75 |
| HSPC300-GFP/mRFPmars2-ArpC4 co-expression vector | Insall Lab [50] | pDM604 |
| GFP-WASP/CLC-mRFPmars co-expression vector | Insall Lab [6] | pDM656 |
| Dox/onG12V Rac1 inducible expression vector | Insall Lab [12] | pDM987 |
| GFP-WASPΔCRIB expression vector | This manuscript | pSBZ9 |
| GFP-WASP∗∗CRIB expression vector | This manuscript | pSBZ13 |
| GFP-WASP expression vector | This manuscript | pSBZ14 |
| GFP- WASPΔCRIB/mRFPmars2-ArpC4 co-expression vector | This manuscript | pSBZ16 |
| GFP- WASP∗∗CRIB/mRFPmars2-ArpC4 co-expression vector | This manuscript | pSBZ18 |
| GFP- WASP/mRFPmars2-ArpC4 co-expression vector | This manuscript | pSBZ19 |
| GFP- WASPΔCRIB/CLC-mRFPmars co-expression vector | This manuscript | pSBZ21 |
| GFP- WASP∗∗CRIB/CLC-mRFPmars co-expression vector | This manuscript | pSBZ23 |
| GST-Rac1A bacterial expressing vector | Prof. Arjan Kortholt, University of Groningen | pGEXRac1A |
| GST-RacC bacterial expression vector | Prof. Arjan Kortholt, University of Groningen | PGEXRacC |
| Software and Algorithms | ||
| Fiji (image handling) | National Institutes of Health | https://fiji.sc |
| Prism (statistics) | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
Lead Contact and Materials Availability
Further information and requests for reagents should be directed to and will be fulfilled by the Lead Contact, Clelia Amato (clelia.amato@ed.ac.uk). Recombinant DNA generated during this study has not been deposited in a public repository but is available from the Lead Contact on request.
Experimental Model and Subject Details
Cell culture
Dictyostelium wild-type, wasA knockout, and inducible double null cells were grown at 22°C on Petri dishes in HL5 supplemented with vitamins and micro-elements (Formedium).
Biochemistry
E. coli BL21 cells transformed with bacterial expression vectors were plated on ampicillin-containing SM plates, or kept in LB medium plus ampicillin in shaking conditions (100 or 200 rpm) at room temperature or 37°C as needed.
Method Details
Cell transfection
Prior to transfection, Dictyostelium cells were resuspended in E-buffer (10 mM KNaPO4, pH 6.1, 50 mM sucrose), incubated with DNA of interest, and elecroporated at 500V using the ECM399 system (Harvard Apparatus UK). Transfected cells were then transferred in Petri dishes containing medium, and selected 24 hour later by addition of 50 μg/ml hygromycin.
Protein purification
E. coli BL21 cells were transformed with a pGEXRac1A-encoding vector by heat-shock at 42°C, and plated overnight on ampicillin-containing SM plates. One colony was transferred to a tube containing LB medium (1% Bacto-tryptone, 0.5% Bacto-yeast extract, 17 mM NaCl, pH 7) plus ampicillin, and kept in shaking condition (200 rpm) at 37°C until it reached the OD600 of 2. The bacterial suspension was then transferred in a larger volume of LB medium plus ampicillin, and kept overnight in shaking conditions (100 rpm) at room temperature. Once the bacterial suspension reached the OD600 of 0.4, 500 μΜ IPTG (Isopropyl β-D-1 thiogalactopyranoside) was added to yield the expression of GST-Rac1A, and bacteria were kept overnight in shaking conditions (100 rpm) at room temperature. Bacteria were then spun down using the Avanti J6-MI centrifuge (Beckman Coulter) at 4°C 3000 rpm for 20 minutes, and lysed using a detergent-based lysis buffer (1% Triton X-100, Halt Protease Inhibitor Cocktail, 1mM DTT). The lysate was sonicated 10 times at the maximum power with 10 s interval between cycles using a Soniprep 150 (MSE), and spun down at 4°C for 10 minutes at 12096 × g using an Avanti J-25 centrifuge (Beckman Coulter). The sonicated lysate was incubated for 2 hours at 4°C with beads (Glutathione High Capacity Magnetic Agarose Beads, Sigma-Aldrich) previously washed in Rac buffer (50 mM Tris HCl pH 7.5, 100 mM NaCl, Halt Protease Inhibitor Cocktail, 1mM DTT). The beads were then washed in Rac buffer and incubated with 1 mM GTPγs (Guanosine 5′-[β,γ-imido]triphosphate trisodium salt hydrate, Sigma-Aldrich) overnight at 4°C in the presence of 15 mM EDTA and 100 units of CIP (Alkaline Phosphatase, Calf Intestinal, NEB). MgCl2 was ultimately added to the tube at the final concentration of 60 mM in order to close the Rac’s nucleotide-binding pocket.
GST-Rac pull-down assay and immunoblotting
Dictyostelium cells were resuspended in lysis buffer (50 mM Tris HCl pH 8, 100 mM NaCl, 30 mM MgCl2, 0.1% Triton X-100, Halt Protease Inhibitor Cocktail, 1mM DTT) and the resulting lysate added to the tube containing GTPγs-Rac1A-loaded beads for 1 hour at 4°C. The tubes were placed on a magnet and washed twice in washing buffer (50 mM Tris HCl pH 8, 100 mM NaCl, 30 mM MgCl2, Halt Protease Inhibitor Cocktail, 1mM DTT). Beads were then washed twice with 1 mL of washing buffer, resuspended in NuPAGE LDS Sample Buffer (ThermoFisher) and incubated at 100°C for 5 minutes. Proteins contained in the eluted fraction were separated using NuPAGE 4%–12% Bis-Tris protein gels (ThermoFisher). Protein were transferred on nitrocellulose membrane (Amersham Protran®, Merck) at 80V for 1 hour. The membrane was blocked for 1-hour in the presence of 5% semi-skimmed milk diluted in TBS. The blocked membrane was incubated overnight at 4°C with the desired primary antibody. The membrane was washed three times with TBST and incubated with the appropriate secondary antibody diluted in 5% BSA in TBST. The membrane was washed three times with TBST, scanned using Odyssey CLx (LI-COR) and analyzed using Image Studio Lite™ software (LI-COR).
Active Rac pull-down assay
Active Rac levels were determined by pull-down assay using GST-PAK-CRIB immobilised to Glutathione beads according to manufacturer’s instructions (Cytoskeleton, Inc, Active Rac Kit). Briefly, wasA- cells expressing GFP-WASP (wasA-/GFP-WASP) or respective CRIB mutants (wasA-/GFP-WASP∗∗CRIB and wasA-/GFP-WASPΔCRIB) were collected from a confluent 30 cm Petri dish and resuspended in lysis buffer supplemented with HALT™ EDTA-free Protease Inhibitor Cocktail at 4°C. After a brief clarification at 10,000 × g for a minute, the supernatant is loaded into GST-PAK-CRIB beads in Eppendorf tubes and incubated for 1 hour at 4°C with gentle rotation. The washed beads were then resuspended in 2x sample buffer and boiled for 3 minutes at 95°C. Proteins contained in the eluted fractions were separated using NuPAGE 12% Bis-Tris protein gels (ThermoFisher) and transferred into nitrocellulose membrane as described previously. The membrane is then blocked for 30 minutes in the presence of 5% semi-skimmed milk diluted in TBS. The blocked membrane was incubated overnight at 4°C with a mouse monoclonal anti-Rac antibody (1:500 dilution). The membrane was washed once with TBST and incubated with appropriate secondary antibody diluted in 5% BSA in TBST. After three washes, the membrane was scanned using Odyssey CLx (LI-COR) and analyzed using Image Studio Lite™ software (LI-COR). Wild-type cells expressing G12V Rac1A under a tetracycline-inducible promoter was used a positive control in the above experiments. In short, cells were treated with tetracycline to the final concentration of 10 μg/ml to induce Rac G12V expression. Cells were harvested 2-3 hours post-induction and processed as described above. The levels of active Rac is quantified using Image Studio Lite™.
Microscopy
Super-resolution microscopy was adopted to investigate the ability of WASP CRIB mutants to localize to clathrin-coated pits and to recruit the Arp2/3 complex. Images were acquires using a Zeiss LSM880 equipped with a 63x/1.40 NA objective. GFP was excited at 488 nm, RFP at 561 nm. Images were acquired using the ZEN imaging software every 1 or 2 s. TIRF microscopy was utilized to monitor the dynamics of clathrin, WASPs, Arp2/3 complex and actin on the ventral surface of the cells. Images were acquired using a modified Nikon Eclipse TE 2000-U microscope equipped with a photometrics Evolve 512 camera and a DualView DV2 emission splitter. GFP and RFP were excited using 473 nm and 561 nm wavelengths respectively. A 100x/1.40 NA TIRF objective was used. Images were acquired every 1 or 2 s using the MetaMorph software. Phase-contrast microscopy was performed to test the ability of cells expressing WASP CRIB mutants to migrate following loss of SCAR/WAVE. Images were acquired using a Nikon ECLIPSE TE2000-E inverted microscope with a 10x/0.30 NA objective and equipped with a QImaging Retiga EXi digital camera. Spinning disk confocal microscopy was utilized to monitor WASP dynamics upon expression of dominant active Rac. Images were acquired using a Nikon Ti-E inverted microscope equipped with a Yokogawa CSU-X spinning disc confocal unit in combination with a High resolution Andor Neo sCMOS camera. A 100x/1.4 NA objective was used. GFP was excited at 488 nm, RFP at 561 nm. Images were acquired using the Andor IQ 2 software.
Chemotaxis assay
The under-agarose chemotaxis assay [62] was used to investigate the ability of cells expressing no WASP or WASP CRIB mutants to confine active Rac at the leading edge during migration, as well as the ability of inducible double null cells expressing WASP CRIB mutants to generate pseudopods following loss of SCAR/WAVE. A 50mm glass bottom dish (MatTek) was first treated with 10 mg/ml BSA to reduce the resistance encountered by cells crawling under agarose. Seakem GTG agarose was dissolved to the final concentration of 0.4% in LoFlo (Formedium), a low fluorescence medium that improves the quality of imaging. Dissolved agarose was cast on a pre-treated glass bottom dish and allowed to set. Once set, the agarose was cut using a scalpel so to create two wells separated by a 5 mm bridge; this was gently wriggled loose to facilitate cells’ crawling. Cells of interest were resuspended in LoFlo, counted using CASY® Model TT Cell Counter (Innovatis), and diluted to a final concentration of 5 × 105 cells/ml. 200 μL of cell suspension was seeded on the left well created within the agarose layer. The other well was filled with 100 μM folic acid diluted in LoFlo. A square coverslip was carefully lowered down in order to cover both wells and prevent evaporation; the glass bottom dish was thereafter incubated at 22°C. Imaging was performed 3-10 hours after setting up the assay.
Rac inhibitor treatment
Cells expressing a red-tagged active Rac marker (PakB CRIB-mRFPmars2) or a GFP-tagged WASP alongside re-tagged LifeAct or Arp2/3 complex were resuspended in LoFlo, and 105 cells seeded on a borosilicate glass 8 well chamber (ThermoFisher). Cells were allowed to adhere and then treated with the Rac inhibitor EHT1864. Images were acquired prior to addition of the inhibitor (to monitor the dynamics of the probe in the presence of active Rac), during addition of the drug (to visualize its most acute effects) and for 10-12 minutes after treatment (to verify whether cells could recover).
Induction of G12V Rac1 expression
Cells expressing G12V Rac1A under a tetracycline-inducible promoter along with GFP-WASP or RFP-tagged active Rac marker were resuspended in LoFlo and seeded to a final concentration of 5 × 105 cells/ml on a borosilicate glass 8 well chamber (ThermoFisher) or on a 35 mm glass bottom dish (MatTek). Once adhering, cells were treated with tetracycline to the final concentration of 10 μg/ml to yield G12V Rac1A expression. Cells were images from the moment of tetracycline addition up to 2-3 hours post-induction.
Image processing
Images acquired using phase-contrast, TIRF or confocal microscopes were exported as. TIFF files, while those acquired using the super-resolution confocal were exported as. CZI files. Images were all imported into Fiji software [63] and processed as required, including cropping and linear brightness/contrast adjustment. TIRF images were processed using the Windowed-Sinc Filter (Kunito Yoshida, Department of Biological Sciences, Imperial College London). Clathrin, WASP and Arp2/3 complex puncta dynamics was monitored using the MTrackJ plugin [64]. The same plugin was utilized along with a Chemotaxis Tool plugin (Gerhard Trapp and Elias Horn, ibidi GmbH) to measure speed and directionality of migrating cells. Images acquired using the super-resolution confocal microscope were subjected to Airyscan processing and to a 2D automatic deconvolution.
Quantification and Statistical Analysis
Unpaired t test, Mann-Whitney test, one-way ANOVA with a Kruskal-Wallis multiple comparison test or a Tukey’s multiple comparisons test were performed to generate p values and test for statistical significance using GraphPad Prism 7 or 8. Plots were generated using the same software. Statistical details of experiments, including n, mean, SEM and p values, are indicated in figure legends.
Data and Code Availability
This study did not generate any unique datasets or code.
Acknowledgments
We thank Margaret O’Prey and Beatson Advanced Imaging Resource (BAIR) staff for assistance with microscopy, Stephen Barratt for assistance with cloning of the CRIB mutants, and Douwe Veltman for providing plasmids. Funding was provided by Cancer Research UK grants A15672, A24450, and multidisciplinary grant A20017. Sequences and gene expression data were provided by Dictybase. We are very grateful to members of the Insall and Machesky Labs for helpful comments on the manuscript.
Author Contributions
Conceptualization, C.A. and R.H.I.; Methodology, C.A. and S.I.; Investigation, C.A., P.A.T., A.J.D., and K.S.; Review and Editing, C.A., R.H.I., and L.M.M.; Visualization, C.A. and R.H.I.; Supervision, C.A. and R.H.I.
Declaration of Interests
The authors declare no competing interests.
Published: November 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cub.2019.10.036.
Supplemental Information
References
- 1.Mullins R.D., Heuser J.A., Pollard T.D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derry J.M., Ochs H.D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 3.Miki H., Miura K., Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 4.Miki H., Sasaki T., Takai Y., Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 5.Veltman D.M., Insall R.H. WASP family proteins: their evolution and its physiological implications. Mol. Biol. Cell. 2010;21:2880–2893. doi: 10.1091/mbc.E10-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson A.J., Amato C., Thomason P.A., Insall R.H. WASP family proteins and formins compete in pseudopod- and bleb-based migration. J. Cell Biol. 2018;217:701–714. doi: 10.1083/jcb.201705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochubey O., Majumdar A., Klingauf J. Imaging clathrin dynamics in Drosophila melanogaster hemocytes reveals a role for actin in vesicle fission. Traffic. 2006;7:1614–1627. doi: 10.1111/j.1600-0854.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 8.Madania A., Dumoulin P., Grava S., Kitamoto H., Schärer-Brodbeck C., Soulard A., Moreau V., Winsor B. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell. 1999;10:3521–3538. doi: 10.1091/mbc.10.10.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrifield C.J., Qualmann B., Kessels M.M., Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 2004;83:13–18. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- 10.Evans I.R., Ghai P.A., Urbančič V., Tan K.L., Wood W. SCAR/WAVE-mediated processing of engulfed apoptotic corpses is essential for effective macrophage migration in Drosophila. Cell Death Differ. 2013;20:709–720. doi: 10.1038/cdd.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunda P., Craig G., Dominguez V., Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr. Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Veltman D.M., King J.S., Machesky L.M., Insall R.H. SCAR knockouts in Dictyostelium: WASP assumes SCAR’s position and upstream regulators in pseudopods. J. Cell Biol. 2012;198:501–508. doi: 10.1083/jcb.201205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner O.D., Rentel M.C., Ott A., Brown G.E., Jedrychowski M., Yaffe M.B., Gygi S.P., Cantley L.C., Bourne H.R., Kirschner M.W. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samaniego R., Sánchez-Martín L., Estecha A., Sánchez-Mateos P. Rho/ROCK and myosin II control the polarized distribution of endocytic clathrin structures at the uropod of moving T lymphocytes. J. Cell Sci. 2007;120:3534–3543. doi: 10.1242/jcs.006296. [DOI] [PubMed] [Google Scholar]
- 15.Davis B.H., Walter R.J., Pearson C.B., Becker E.L., Oliver J.M. Membrane activity and topography of F-Met-Leu-Phe-Treated polymorphonuclear leukocytes. Acute and sustained responses to chemotactic peptide. Am. J. Pathol. 1982;108:206–216. [PMC free article] [PubMed] [Google Scholar]
- 16.Damer C.K., O’Halloran T.J. Spatially regulated recruitment of clathrin to the plasma membrane during capping and cell translocation. Mol. Biol. Cell. 2000;11:2151–2159. doi: 10.1091/mbc.11.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z., Chai Y., Jiang Y., Li W., Hu H., Li W., Wu J.W., Wang Z.X., Huang S., Ou G. Functional coordination of WAVE and WASP in C. elegans neuroblast migration. Dev. Cell. 2016;39:224–238. doi: 10.1016/j.devcel.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Banjade S., Wu Q., Mittal A., Peeples W.B., Pappu R.V., Rosen M.K. Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proc. Natl. Acad. Sci. USA. 2015;112:E6426–E6435. doi: 10.1073/pnas.1508778112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pils S., Kopp K., Peterson L., Delgado Tascón J., Nyffenegger-Jann N.J., Hauck C.R. The adaptor molecule Nck localizes the WAVE complex to promote actin polymerization during CEACAM3-mediated phagocytosis of bacteria. PLoS ONE. 2012;7:e32808. doi: 10.1371/journal.pone.0032808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Borek D., Padrick S.B., Gomez T.S., Metlagel Z., Ismail A.M., Umetani J., Billadeau D.D., Otwinowski Z., Rosen M.K. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eden S., Rohatgi R., Podtelejnikov A.V., Mann M., Kirschner M.W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 22.Rohn J.L., Sims D., Liu T., Fedorova M., Schöck F., Dopie J., Vartiainen M.K., Kiger A.A., Perrimon N., Baum B. Comparative RNAi screening identifies a conserved core metazoan actinome by phenotype. J. Cell Biol. 2011;194:789–805. doi: 10.1083/jcb.201103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burbelo P.D., Drechsel D., Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 24.Pirone D.M., Carter D.E., Burbelo P.D. Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet. 2001;17:370–373. doi: 10.1016/s0168-9525(01)02311-3. [DOI] [PubMed] [Google Scholar]
- 25.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M.W. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 26.Tomasevic N., Jia Z., Russell A., Fujii T., Hartman J.J., Clancy S., Wang M., Beraud C., Wood K.W., Sakowicz R. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry. 2007;46:3494–3502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- 27.Han J.W., Leeper L., Rivero F., Chung C.Y. Role of RacC for the regulation of WASP and phosphatidylinositol 3-kinase during chemotaxis of Dictyostelium. J. Biol. Chem. 2006;281:35224–35234. doi: 10.1074/jbc.M605997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivero F., Dislich H., Glöckner G., Noegel A.A. The Dictyostelium discoideum family of Rho-related proteins. Nucleic Acids Res. 2001;29:1068–1079. doi: 10.1093/nar/29.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebensohn A.M., Kirschner M.W. Activation of the WAVE complex by coincident signals controls actin assembly. Mol. Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Roche M., Mahasneh A., Lee S.F., Rivero F., Côté G.P. Cellular distribution and functions of wild-type and constitutively activated Dictyostelium PakB. Mol. Biol. Cell. 2005;16:238–247. doi: 10.1091/mbc.E04-06-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraynov V.S., Chamberlain C., Bokoch G.M., Schwartz M.A., Slabaugh S., Hahn K.M. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 32.Weiner O.D., Marganski W.A., Wu L.F., Altschuler S.J., Kirschner M.W. An actin-based wave generator organizes cell motility. PLoS Biol. 2007;5:e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., Bourne H.R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 34.Tal T., Vaizel-Ohayon D., Schejter E.D. Conserved interactions with cytoskeletal but not signaling elements are an essential aspect of Drosophila WASp function. Dev. Biol. 2002;243:260–271. doi: 10.1006/dbio.2002.0571. [DOI] [PubMed] [Google Scholar]
- 35.Abdul-Manan N., Aghazadeh B., Liu G.A., Majumdar A., Ouerfelli O., Siminovitch K.A., Rosen M.K. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 36.Kim A.S., Kakalis L.T., Abdul-Manan N., Liu G.A., Rosen M.K. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 37.Merrifield C.J., Feldman M.E., Wan L., Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 38.Aghamohammadzadeh S., Ayscough K.R. Differential requirements for actin during yeast and mammalian endocytosis. Nat. Cell Biol. 2009;11:1039–1042. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulant S., Kural C., Zeeh J.C., Ubelmann F., Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 2011;13:1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaksonen M., Sun Y., Drubin D.G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto L.M., Roth R., Heuser J.E., Schmid S.L. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb T.A., Ivanov I.E., Adesnik M., Sabatini D.D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J. Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y., Dickerson J.B., Guo F., Zheng J., Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onesto C., Shutes A., Picard V., Schweighoffer F., Der C.J. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- 45.Shutes A., Onesto C., Picard V., Leblond B., Schweighoffer F., Der C.J. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- 46.Moissoglu K., Slepchenko B.M., Meller N., Horwitz A.F., Schwartz M.A. In vivo dynamics of Rac-membrane interactions. Mol. Biol. Cell. 2006;17:2770–2779. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veltman D.M., Lemieux M.G., Knecht D.A., Insall R.H. PIP3-dependent macropinocytosis is incompatible with chemotaxis. J. Cell Biol. 2014;204:497–505. doi: 10.1083/jcb.201309081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carnell M., Zech T., Calaminus S.D., Ura S., Hagedorn M., Johnston S.A., May R.C., Soldati T., Machesky L.M., Insall R.H. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J. Cell Biol. 2011;193:831–839. doi: 10.1083/jcb.201009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derivery E., Sousa C., Gautier J.J., Lombard B., Loew D., Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 50.King J.S., Veltman D.M., Georgiou M., Baum B., Insall R.H. SCAR/WAVE is activated at mitosis and drives myosin-independent cytokinesis. J. Cell Sci. 2010;123:2246–2255. doi: 10.1242/jcs.063735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burdyniuk M., Callegari A., Mori M., Nédélec F., Lénárt P. F-Actin nucleated on chromosomes coordinates their capture by microtubules in oocyte meiosis. J. Cell Biol. 2018;217:2661–2674. doi: 10.1083/jcb.201802080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caridi C.P., D’Agostino C., Ryu T., Zapotoczny G., Delabaere L., Li X., Khodaverdian V.Y., Amaral N., Lin E., Rau A.R., Chiolo I. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature. 2018;559:54–60. doi: 10.1038/s41586-018-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pocha S.M., Cory G.O. WAVE2 is regulated by multiple phosphorylation events within its VCA domain. Cell Motil. Cytoskeleton. 2009;66:36–47. doi: 10.1002/cm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlier M.F., Nioche P., Broutin-L’Hermite I., Boujemaa R., Le Clainche C., Egile C., Garbay C., Ducruix A., Sansonetti P., Pantaloni D. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J. Biol. Chem. 2000;275:21946–21952. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- 55.Papayannopoulos V., Co C., Prehoda K.E., Snapper S., Taunton J., Lim W.A. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol. Cell. 2005;17:181–191. doi: 10.1016/j.molcel.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 56.Prehoda K.E., Scott J.A., Mullins R.D., Lim W.A. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 57.Benesch S., Lommel S., Steffen A., Stradal T.E., Scaplehorn N., Way M., Wehland J., Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J. Biol. Chem. 2002;277:37771–37776. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- 58.Kessels M.M., Schwintzer L., Schlobinski D., Qualmann B. Controlling actin cytoskeletal organization and dynamics during neuronal morphogenesis. Eur. J. Cell Biol. 2011;90:926–933. doi: 10.1016/j.ejcb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Prehoda K.E., Lim W.A. How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2. Curr. Opin. Cell Biol. 2002;14:149–154. doi: 10.1016/s0955-0674(02)00307-1. [DOI] [PubMed] [Google Scholar]
- 60.Sarmiento C., Wang W., Dovas A., Yamaguchi H., Sidani M., El-Sibai M., Desmarais V., Holman H.A., Kitchen S., Backer J.M. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J. Cell Biol. 2008;180:1245–1260. doi: 10.1083/jcb.200708123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houk A.R., Jilkine A., Mejean C.O., Boltyanskiy R., Dufresne E.R., Angenent S.B., Altschuler S.J., Wu L.F., Weiner O.D. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laevsky G., Knecht D.A. Under-agarose folate chemotaxis of Dictyostelium discoideum amoebae in permissive and mechanically inhibited conditions. Biotechniques. 2001;31:1140–1142, 1144, 1146–1149. doi: 10.2144/01315rr03. [DOI] [PubMed] [Google Scholar]
- 63.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meijering E., Dzyubachyk O., Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type cells (WT, top panel) accumulate active Rac (active Rac marker: PakB CRIB-GFP) at their front. No active Rac is generally detected at their rear (asterisk). WASP knockout cells (wasA−, bottom panel) accumulate active Rac at their front, where protrusions are generated, as well as at persistent structures at their enlarged rears (asterisk). Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
wasA− cells expressing LifeAct (F-actin reporter) along with GFP-WASP (rescue, top panel), GFP-WASPΔCRIB (middle panel) or GFP- WASP∗∗CRIB (bottom panel) were imaged during directed migration. Rescued cells generate transient puncta that appear evenly distributed away from the front (asterisk). Cells expressing either WASP CRIB mutant generate longer-lived puncta that cluster within the enlarged uropods (asterisk). At all timepoints, WASP spots are F-actin positive. Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
wasA− cells expressing active Rac marker (PakB CRIB-mRFPmars2) along with GFP-WASP (rescue, left panel), GFP-WASPΔCRIB (central panel) or GFP- WASP∗∗CRIB (right panel) were imaged during directed migration. Rescued cells do not accumulate active Rac at their rears (asterisk), where WASP generates transient puncta. Cell expressing either CRIB-mutated WASP accumulate active Rac at the front, as well as within the enlarged rears (asterisk), where it can be detected on persistent structures that are also occasionally mutant WASP-positive. Scale bar, 10 μm. Images were taken every 3 s, video plays at 5 frames/second.
Data Availability Statement
This study did not generate any unique datasets or code.