Abstract
Rationale for review
Young adults of childbearing age and pregnant women are travelling more frequently to tropical areas, exposing them to specific arboviral infections such as dengue, zika and chikungunya viruses, which may impact ongoing and future pregnancies. In this narrative review, we analyse their potential consequences on pregnancy outcomes and discuss current travel recommendations.
Main findings
Dengue virus may be associated with severe maternal complications, particularly post-partum haemorrhage. Its association with adverse fetal outcomes remains unclear, but prematurity, growth retardation and stillbirths may occur, particularly in cases of severe maternal infection. Zika virus is a teratogenic infectious agent associated with severe brain lesions, with similar risks to other well-known TORCH pathogens. Implications of chikungunya virus in pregnancy are mostly related to intrapartum transmission that may be associated with severe neonatal infections and long-term morbidity.
Travel recommendations
Few agencies provide specific travel recommendations for travelling pregnant patients or couples trying to conceive and discrepancies exist, particularly regarding Zika virus prevention. The risks significantly depend on epidemiological factors that may be difficult to predict. Prevention relies principally on mosquito control measures. Couples trying to conceive and pregnant women should receive adequate information about the potential risks. It seems reasonable to advise pregnant women to avoid unnecessary travel to Aedes spp. endemic regions. The current rationale to avoid travel and delay conception is debatable in the absence of any epidemic. Post-travel laboratory testing should be reserved for symptomatic patients.
Keywords: Dengue, Chikungunya, Zika, Pregnancy
Introduction
With the changes brought about by globalization, notably the significant reduction of the cost of flying, travel has become a popular leisure activity, particularly for young adults of childbearing age, such as honeymooners. Tropical regions are popular destinations where there is an increased chance of exposure to tropical infectious agents, such as arboviruses (arthropod-borne viruses). In addition to the routine risks, such infections may impact an ongoing or future pregnancy, adding an additional challenge in pre- and post-travel advice. Over the last decades, the worldwide dissemination of arboviruses, such as dengue virus (DENV), Zika virus (ZIKV) and chikungunya virus (CHIKV) has emerged as an important public health issue. These three viruses share a common vector—Aedes aegypti—and to a lesser extent Aedes albopictus and therefore often co-circulate. DENV is now considered endemic in most tropical regions, with an incidence of over 400 million cases per year.1 ZIKV was associated with a large epidemic reported in 2013 in French Polynesia, before reaching the Americas in the same year and spreading extensively in 2015.2 As of July 2019, evidence of ZIKV transmission has been reported in 87 countries and territories throughout the world.3
Similarly, CHIKV re-emerged in 2005–2006 after more than 30 years of quiescence causing a massive epidemic in the Indian Ocean islands,4 followed by its spread to the American continent in 2013.5
In this narrative review, we will discuss the impact of these three major arboviruses on pregnancy outcomes and discuss the latest travel recommendations for couples trying to conceive and pregnant women in order to improve counselling.
Background: epidemiology and transmission
DENV and ZIKV are arboviruses of the Flavivirus genus (Flaviviridae family), which includes other important pathogens like West Nile, yellow fever and Japanese encephalitis viruses. While there is only one serotype of ZIKV, there are four major serotypes of DENV, which differ phylogenetically and antigenically.6 Flaviviruses are single-stranded positive-sense RNA viruses. Upon translation, the single polyprotein is cleaved into three structural proteins (capsid, precursor membrane and envelope) and seven non-structural proteins (NS1, N2A, N2B, N3, N4A, N4B and N5).7 Non-structural proteins have a role in viral replication and modulation of the cell antiviral response.8
CHIKV is an Alphavirus belonging to the Togaviridae family. Other clinically relevant Alphaviruses include the O’nyong’nyong virus in Africa, Mayaro virus in Latin America and Ross River virus in Australia.9 Their genome consists of a positive-sense RNA molecule encoding four non-structural proteins (nsP1–4) and five structural proteins (C, E3, E2, 6K and E1).10
DENV, ZIKV and CHIKV are mainly transmitted by the Aedes spp. mosquitoes, which are widely present in tropical and subtropical areas.1,10,11 While the primary vector is A. aegypti, transmission has also been documented for A. albopictus, which is also present at more northerly latitudes, including in Southern Europe.12 The large distribution in urban areas and pandemic potential of these viruses is related to their ability to use humans as reservoirs in comparison to other arboviruses.13
Recent epidemics have led to the description of non-vector-borne transmission, including vertical transmission from mother to neonate and transmission through blood products.14–16 Though perinatal transmission was first reported during the French Polynesia outbreak,17 vertical transmission and its fetal/neonatal consequences became a major concern during the recent South American ZIKV epidemic.18,19 Calvet et al. were the first to isolate ZIKV particles in the amniotic fluid of two fetuses presenting with significant cerebral anomalies, demonstrating transplacental transmission.20 Materno-fetal transmission was further confirmed using in vivo models.21,22
During the 2013–14 outbreak in French Polynesia, ZIKV was recovered from semen, suggesting a potential sexual route of transmission, which caused great concern. Such transmission has been previously described in 2011, in a couple returning from Africa, but the potential implications were overlooked.23 Since then, many cases of sexual transmission have been reported: from men to men, men to women and women to men, but only one case of ZIKV congenital syndrome following sexual transmission has been reported.24,25 Interestingly, persistence of ZIKV RNA in semen was reported for several months, generally up to 3 months after exposure to the virus.26,27 Nevertheless, the burden of sexual transmission remains unclear. A recent study demonstrated that a third of male patients have detectable ZIKV RNA in their semen, and 3 out of 46 patients had infective particles, detectable only during the first month post-infection.27 It was later estimated by a mathematical model that sexual transmission may have contributed to 3% of the total case burden in countries experiencing outbreaks in Latin America.28
These observations raised the question regarding clinical relevance of non-vector transmission for other arboviruses. Non-vector transmission has been reported for DENV infections and is mostly related to contact with blood of viremic patients; mucocutaneous and transplant transmissions have also been described.29 Vertical transmission was reported for CHIKV infection and is mostly associated with intrapartum transmission.14 Three cases of transplacental transmission have been reported in the literature, in which CHIKV RNA was detected in the amniotic fluid, placentas or fetal brains.30
While multiple studies have failed to detect DENV in semen, prolonged detection of DENV RNA in semen has been reported in one recent case, although PCR contamination cannot be excluded.31,32 More recently, a case of suspected sexual transmission (female to male) was described in a patient returning from Indonesia.33 CHIKV sexual transmission is plausible, as viral RNA in semen was reported in a patient 30 days after symptoms appeared. Interestingly, this man had a co-infection with DENV, with undetectable DENV RNA in his semen.34 Consequently, sexual transmission of DENV and CHIKV cannot be excluded. Nevertheless, it seems to be extremely rare and should not impact any public health recommendations.35 Although sexual transmission of ZIKV is well documented, its impact on the global burden of disease remains low. The risk of CHIKV, DENV and ZIKV essentially remain by essence related to the presence of competent mosquitoes.
Clinical manifestations and management of infections during pregnancy
Infections during pregnancy have long been known to potentially impair its course. Consequences include (i) increased maternal morbidity, as observed with the influenza virus,36 (ii) preterm labour and/or preterm premature rupture of the membranes, associated with bacterial vaginosis or urinary tract infections,37,37 (iii) stillbirths and low birth weight, as observed in low- and middle-income countries following malaria and syphilis infections,38,39 (iv) in utero fetal infections that may lead to congenital manifestations as observed with the TORCH agents (Toxoplasmosis, Others [including, Syphilis, Varicella Zoster], Rubella, Cytomegalovirus (CMV) and Herpes simplex virus (HSV))40 or severe fetal diseases, such as the parvovirus B19-induced anaemia or congenital HIV41 and (5) neonatal infections, such as group B streptococcal meningitis.
Here, we will review specific outcomes associated with these three emerging arboviral diseases for travelling pregnant patients or couples who are planning for pregnancy. Table 1 provides a simplified and clinically relevant comparison of the pregnancy outcomes associated with these viruses. A systematic review of pregnancy outcomes associated with all arboviral diseases has been recently published and may provide additional information.42
Table 1.
Pregnancy outcomes associated with arboviruses
| Flaviviridae | Togaviridae | ||
|---|---|---|---|
| Flavivirus | Alphavirus | ||
| ZIKV | DENV | CHIKV | |
| Increased maternal complications | No | Yes | No |
| Sexual transmission | Yes | Not of public health significance | Not of public health significance |
| Transplacental transmission | Yes | yes | Yes, rare (3 cases) |
| Adverse pregnancy outcomes | |||
| Fetal malformation | Yes; severe | No | No |
| Premature birth | No | Yes very likely related to severity of maternal disease | No |
| Fetal loss | Yes | Yes very likely related to severity of maternal disease | Yes, rare (3 cases) |
| SGA | Yes | No | No |
| Perinatal transmission | Yes, rare | Yes | Yes |
| Adverse neonatal outcomes | |||
| Mild infection (e.g. rash, hepatitis, thrombocytopenia) | Yes | Yes | Yes |
| Severe disease (e.g. sepsis, encephalitis) | No | Yes, rare | Yes |
| Long-term sequelae | Yes; severe | No | Yes; severe |
*In bold, main complication observed.
Dengue virus
Risk for travellers
In most immunocompetent adult patients, DENV infection will remain unnoticed.1 About 25% of infected patients will develop symptomatic infections, the majority of which will experience dengue fever, which is a self-limiting disease marked by high fever and non-specific flu-like symptoms, while a minority may evolve to severe dengue. The latter is defined by the presence of dengue fever with one of the following complications: severe plasma leakage associated with dengue shock syndrome or pulmonary edema, severe bleeding or severe organ impairment.43 There is an increased risk of developing severe dengue during a secondary infection with a different DENV serotype.44 Neurological complications are not frequent following dengue fever, but cases of Guillain–Barré syndrome (GBS), an acute inflammatory demyelinating polyneuropathy, which manifests as progressive acute paralysis and may lead to respiratory arrest, have been described.45 Though mortality associated with severe dengue remains low, intense management may be required43 and its economic consequences, particularly in low resource settings, are high.1
Maternal outcomes
Pregnant women represent a unique group at risk of severe complications associated with dengue infection; maternal mortality has been estimated to be increased by a factor of three.46 Features of severe dengue (i.e. thrombocytopenia and elevated liver enzymes) overlap pregnancy-specific diseases such as preeclampsia, HELLP syndrome (haemolysis, elevated liver enzymes, low platelets) or gestational thrombocytopenia and may be difficult to diagnose. Serological analysis is therefore mandatory to allow for optimal management.43 Additionally, physiological haemodilution of the pregnant patient may delay the diagnosis of severe dengue.47
Complications of maternal infection that have been suggested in pregnant women include severe hypovolemic shock due to plasma leakage, severe haemorrhages and preeclampsia, which might be favoured by the plasma leakage state.42,48 A recent prospective matched controlled study, however, identified no association with maternal complications like preeclampsia and maternal haemorrhage. Nevertheless, severe dengue infection was associated with an increase in post-partum haemorrhage after adjusting for potential cofounding factors (aRR 8.6 (95% CI 1.2–62)) with an attributable fraction of 31%.48 The severity of the disease might therefore significantly influence the onset of maternal complications.
Fetal and neonatal outcomes
When looking at fetal outcomes in cases of maternal dengue infection, materno-fetal transmission has been documented in several case reports, with detection of DENV particles in placentas of aborted fetuses and specific IgM, NS1 antigen or DENV RNA in newborn sera.49–51 In the most recent prospective cohort study of 54 women, the risk of vertical transmission was estimated at between 18.5 and 22.7% (95% confidence interval 9.25–37.8%) and up to 56.2% when considering only maternal infections within 15 days prior to and 2 days after delivery.49
Although a recent study conducted in Brazil suggested an association of maternal dengue infection with congenital anomalies, only the presence of unspecified congenital malformations of the brain reached statistical significance. This large population-based study (16 103 312 live births) had several flaws. Specifically, conclusions were reached by matching routine birth notification information to dengue notification records; therefore, reports of confounding factors, particularly presence of co-infections, maternal age, medications and recreational drugs, were missing, limiting any definitive conclusions.52 More importantly, a recent large retrospective cohort study on 3898 pregnant patients with symptomatic DENV infection did not find any increased risk of malformations compared to uninfected patients (3165 patients) nor routine control newborns (3738).53 Thus, DENV does not seem to be associated with congenital malformations.42 Some associations with premature birth and growth retardation are reported with DENV infections. A recent meta-analysis including six cohort studies and two case–control studies54 reported a minor association with preterm birth and pregnancy loss less than 22 weeks’ gestation between pregnancies with and without dengue infection. The odd ratios were 1.71 (95% CI 1.06–2.76) for preterm birth and 3.51 (95% CI 1.15–10.77) for pregnancy loss. In contrast, no association was found with low birth weight, defined as birth weight below the 10th centile or birth weight <2500 g (OR 1.41, 95% CI 0.90–2.21.54 Significant heterogeneities were observed between the studies and may have been related to the lack of control for confounding factors in some of the cohort studies and the differences used in the diagnostic criteria of maternal DENV infection (clinical and biological vs biological only).54 Interestingly, a recent prospective matched case–control study did not find such an association with preterm birth or pregnancy loss after adjustment for confounding factors, suggesting an indirect mechanism.48 Such complications might be observed only in cases of severe maternal DENV infection. A study performed in French Guiana included 73 cases of symptomatic DENV infections, among which 27% suffered from severe DENV infection and 219 controls. No associations were found with prematurity, low birth weight, stillbirth or miscarriage.48 Similar findings were also observed in a large Brazilian retrospective study where no association was found with low birth weight <2500 g when compared to uninfected patients or control newborns (aOR 1.17, 95% CI 0.99–1.39, P = 0.07; aOR 1.00, 0.85–1.17, P = 0.97, respectively). In this study, including only symptomatic maternal infections, a minor increase in preterm birth rate was observed by comparison to uninfected pregnant patients (aOR 1.26 (1.06–1.49, P = 0.006), but not with the reference newborn population (aOR 0.98 (0.83–1.16, P = 0.84). This further supports the importance of the severity of the disease in maternal and fetal complications, especially when considering the potential need for iatrogenic preterm delivery in cases of severe disease. This is discussed further later.
Perinatal transmission of DENV during delivery has been associated with severe dengue fever in newborns; such events remain nevertheless rare.49,55 Delivery by caesarean section does not seem to be protective.55
Clinical management of acute dengue infection during pregnancy
Management of acute dengue infection in pregnant women is similar to non-pregnant patients and consists of supportive measures: fluid replacement therapy and analgesia.43 Nevertheless, pregnant patients represent WHO category B patients and should be monitored as inpatients. In case of imminent delivery, transfer to a tertiary centre capable of dealing with major obstetric haemorrhage should be attempted.
Several publications, including from the WHO,43 have suggested that treatment with tocolytics may be beneficial in pregnant women presenting with severe dengue disease in the third trimester. Such treatment would allow sufficient time for platelets to increase, thus reducing the risk of postpartum haemorrhage and allowing for regional anaesthesia. Moreover, it would reduce the rate of neonatal transmission and subsequent complications. Nevertheless, evidence supporting this approach is currently lacking. In a recent retrospective study performed on 33 patients in Colombia, among which 6 received tocolytic agents (magnesium sulphate n = 5, nifedipine n = 2 or atosiban n = 1), pregnancy was prolonged for a median of 1 day (IQR 1–4), allowing platelets to increase in three of five patients, while three patients delivered prematurely. All five newborns required hospitalizations.56 The small number of patients included in this retrospective study, as well as the lack of information on DENV neonatal status, limits any conclusions. Further prospective studies are needed to better evaluate the benefit of such treatment. Therefore, tocolysis should be reserved for specific situations, for example to allow transfer to a tertiary centre or in cases of preterm labour occurring at a gestational age where delaying pregnancy is beneficial to the newborn. It should only be used if preeclampsia, HELLP syndrome or chorioamnionitis have been excluded.
Timing of delivery (i.e. induction of labour) remains an important challenge in severe dengue occurring in the third trimester. Literature remains scarce in terms of active management and is only based on case reports. In one case describing severe maternal secondary dengue infection complicated by encephalitis and severe plasma leakage at 38 weeks’ gestation (WG), caesarean section was performed on Day 2 post-intensive care admission due to fetal distress, despite the mother being sedated and mechanically ventilated since her admission. The mother suffered from recurrent post-operative bleeding complications requiring a second surgery.47
In this case, delivery focused on fetal health and was delayed to avoid unnecessary trauma to the mother that might increase the risk of bleeding. On the other hand, in cases of maternal shock, delivery might significantly improve maternal resuscitation, reducing the oxygen requirement and improving venous return. Such an approach was anticipated by a team in Sri Lanka in a case of a woman with haemorrhagic dengue fever at 38 WG who delivered by caesarean section as soon as warning signs presented.57 At the time of the section, hematocrit remained within normal parameters and platelets were 72 G/L, allowing for spinal anaesthesia. Caesarean section was uneventful. After an initial deterioration due to progression of capillary leakage, she recovered well. The newborn was diagnosed with a congenital dengue infection at Day 5 of life, requiring intensive care unit treatment. Alternatively, expectant management can be achieved as illustrated in another case report of dengue fever at 37 WG complicated by encephalitis and severe thrombocytopenia without any plasma leakage, conservative management was achieved until platelets increased to 70 G/L allowing for induction of labour. She underwent a vaginal delivery, and no perinatal complications were recorded.58
The decision for delivery should be made by experienced obstetricians on the basis of maternal status, severity of the disease, fetal well-being and gestational age. Early delivery during the active phase of the disease might increase the risk of perinatal transmission and the risk of maternal haemorrhage. On the other hand, maintaining the pregnancy might severely compromise the fetus due to placental insufficiency and impair adequate maternal resuscitation measures.
Chikungunya virus
Risk for travellers
Though the vast majority of CHIKV infections is asymptomatic, 50–97% of the infected individuals will present with nonspecific symptoms such as fever, maculopapular rash, non-purulent conjunctivitis and arthralgia.59 Rare complications include GBS, similarly to DENV, hepatitis and myocarditis.60 In 15 to 60% of cases, CHIKV infection might result in chronic sequelae consisting of rheumatism, persistent joint pain and swelling, especially in elderly patients. These symptoms may last for several years and lead to bone erosions.61–63
Maternal outcomes
Acute CHIKV infection is not associated with an increased risk of complications in pregnant women.42 However, pregnant women are, similar to the non-pregnant women, at risk of long-term sequelae and sepsis requiring intensive care unit treatment, especially in cases of infection in the third trimester.64
Fetal and neonatal outcomes
Concerns regarding CHIKV are mostly related to perinatal transmission at the time of labour and their potential consequences on newborns. Indeed, a recent cohort study based on 1400 pregnant patients preformed during the 2006 epidemic in La Réunion showed similar rates of stillbirth, congenital anomalies, low birth weight and preterm labour between infected (n = 658) and uninfected patients (n = 655); asymptomatic patients were excluded from the analysis after controlling for potential confounding factors.65 Fetal loss associated with maternal CHIKV infection has only been reported in three cases.30 In contrast, perinatal transmission may reach up to 50% in case of maternal viremia at the time of delivery (i.e. from 2 days before until 2 days after delivery).42,65,66 Caesarean section delivery does not seem to be protective.30 In the largest cohort study performed so far, all infected newborns were symptomatic, with 52.6% of them presenting with encephalopathy.30 Most affected newborns are asymptomatic at birth, and first signs of infection only develop between 3 to 5 days post-delivery. These include fever, joint swelling, diverse forms of skin rash, including petechiae, biological anomalies such as thrombocytopenia and elevated liver enzymes.30,67 Of most concern are the long-term consequences of neonatal infection. In a prospective cohort study performed during the CHIKV epidemic in La Réunion, infected newborns exhibited significantly reduced developmental quotient scores at 2 years of age in comparison to non-infected exposed controls, among which 12.1% suffered from severe developmental delay.68
Management of acute CHIKV infection during pregnancy
In view of the severity of the neonatal disease and the risk of transmission in cases of maternal viremia close to delivery, attempts should be made to delay delivery. Similar to what has been proposed for DENV management, several publications have suggested that tocolytic therapy may be an option. In a cohort study of 60 patients with an acute CHIKV infection in Colombia, 38 were in their third trimester and 15 delivered around the time of acute infection with a mean latency of 6.3 days ± 1.4.64 Three patients received tocolytic therapy with nifedipine therapy, which allowed mean prolongation of pregnancy of 2.3 days. In this cohort, no clinical neonatal CHIKV was diagnosed, and among the six newborns who had RT-PCR analysis at birth, all were negative. These findings suggest that deliveries beyond 5 days of acute symptoms may reduce the risk of transmission, though the low number of cases limits definitive conclusions. Regarding tocolytic therapy, no evidence currently supports its routine use. Furthermore, although tocolytics are known to efficiently delay delivery in threated pre-term labour for up to 48 h, allowing fetal lung maturation, their efficiency over this time interval is not known.69 Their use should, therefore, be reserved for specific situations. As mentioned, caesarean section does not seem to be associated with a reduction of transmission, and mode of delivery should be guided by obstetric indications only. Efforts should primarily focus on preventing maternal infection near term and adequate monitoring of exposed newborns. These newborns should be hospitalized and benefit from adequate laboratory screening to obtain a definite diagnosis.64
Zika virus
Risk for travellers
Similar to DENV and CHIKV, ZIKV infection is mostly asymptomatic. When present, the symptoms largely mimic DENV or CHIKV infections and clinical differentiation is not possible. These symptoms are self-resolving.
The main risk of ZIKV infection in adults, similar to DENV and CHIKV, is the potential to develop GBS. This association has been confirmed in a matched controlled study performed on 42 GBS cases during the ZIKV epidemic in French Polynesia, all of which presented with high titres of neutralizing antibodies to ZIKV, which significantly differed from the control group (55.7%).70 Though severe, this remains a rare complication. The incidence rate of GBS cases during the French Polynesian outbreak was estimated to be 0.24 per 1000 cases of ZIKV infection. This rate is similar to what is known for other bacterial or viral infections associated with GBS such as C. jejuni infections with a post-infection incidence rate estimated between 0.25 and 0.65/1000 cases.71
Maternal outcomes
Maternal infections are not associated with increased complications, such as sepsis or GBS syndrome.72,73 Similarly to the general population, ZIKV infection is mostly asymptomatic, with symptoms only described in 17 to 38%.73,74
Fetal and neonatal outcomes
Consequences of in utero fetal infection might be severe. Among many other countries, ZIKV caused a large epidemic in Brazil and was associated with a dramatic increase in the incidence of microcephaly in neonates, leading the Brazilian Ministry of Health to declare a national health emergency in November 2015.75,76 Congenital microcephaly is an anatomical deformity in which the head circumference of the fetus is at least two standard deviations below the average population for the same sex, gestational age and ethnicity. It is associated with a reduced brain size and frequently abnormal neurological structural development.77,78 Microcephaly might be caused by several conditions, including chromosomal aberrations or genetic syndromes,79 and maternal viral infections such as cytomegalovirus80 and rubella,81 or by maternal toxin (alcohol) use82 during pregnancy. The association between ZIKV and microcephaly was unexpected, as flaviviruses were not known for causing birth defects in humans.83 It has now been confirmed by several epidemiological and animal model studies.84 More importantly, the consequences of ZIKV congenital infections are now better described and defined as congenital Zika syndrome (CZS).85,86 Aside from microcephaly, features of CZS include thin cerebral cortices with subcortical calcifications, macular scars with pigmented retinal findings, congenital contractures, cerebral atrophy, ventriculomegaly, cerebellar hypoplasia and arthrogryposis, among others.85 Additionally, transient hepatitis, jaundice and mild anaemia have been described.87,88 Indeed, in a recent study performed in French Guiana, the most common symptoms observed among a cohort of newborns with laboratory confirmation of congenital infection were jaundice (25%, 95% CI 16.6–35.8) when compared to uninfected exposed newborns (9%, 95% CI 6.1–13.9%) and mild anaemia observed in 30% of infected newborns (19.5–42.7) compared to 4% (2.1–8.0) of uninfected newborns.86 Similarly, transient hepatitis with spontaneous resolution at 4 months of age was described in peripartum infected newborns in French Polynesia.88 Severe brain anomalies might only be the tip of the iceberg of the CZS; thus, the full spectrum of CZS still remains to be defined. Some infants may only develop subtle brain anomalies or microcephaly in the post-natal life.89 Others may merely manifest additional neurological symptoms, such as swallowing dysfunction90 or abnormal ophthalmologic findings at birth. In some cases, the latter was the only manifestation of the disease without any CNS malformations.86,91 Pomar et al. recently described the major and minor signs linked to ZIKV congenital infection.72
Similar to other congenital infections, not all exposed fetuses will become infected and not all infected fetuses will develop symptoms. Though initial studies reported a 46% rate of adverse outcomes among offspring of ZIKV-positive women vs 11.5% among offspring of ZIKV-negative women (P < 0.001),92 more recent studies described a lower estimate of between 1 and 8%,87,93–95 depending on the definition of adverse neonatal outcome and the timing of maternal infection. Maternal infections in the first trimester seem to be associated with more severe adverse outcomes, similar to what is known for other congenital infections.94–96 Few studies have evaluated the exact risk of materno-fetal transmission. In a recent cohort study of 291 exposed fetuses with a confirmed maternal infection, materno-fetal transmission was confirmed in 26.1% of exposed fetuses (n = 76), among which 32.9% suffered from severe symptoms defined as severe neurological complications or stillbirth. The population attributable fraction was 60.8%, suggesting an overestimation of the risk. Interestingly, such observation is comparable to other congenital infection such as CMV for which materno-fetal transmission is estimated to be around 30–35% and associated with symptoms at birth in 10–15% of cases.97
Peripartum transmission of ZIKV has been reported occasionally and was associated with mild diseases in the affected new-born, such as transient hepatitis.88
Management of acute ZIKV infection in pregnancy
Generally, no specific measures are required during maternal infection. Nevertheless, due to the risk of materno-fetal transmission in the presence of a confirmed maternal infection, a specific and close follow-up should be implemented in a competent facility. Recommendations regarding the management of pregnancy in cases of a confirmed maternal infection have been published.98 Monitoring should include close ultrasound surveillance.98,99 Invasive procedures (i.e. amniocentesis to diagnose a fetal infection) should only be performed in the presence of abnormal ultrasound findings.100 Of note, the sensitivity and specificity of amniocentesis and fetal blood sampling to confirm a fetal infection are not known. The progressive disappearance of ZIKV RNA in the fetal compartment has been described, which may impair its diagnosis and increase the false negative rate.101
Exposed newborns should be tested for ZIKV and monitored closely for any adverse outcomes. In addition to cerebral imaging, investigations at birth should focus on detecting any hearing and vision abnormalities, as well as close monitoring of developmental milestones.
Travel medicine implications
Diagnostic considerations
The clinical presentations of DENV, CHIKV and ZIKV are similar, and therefore, specific diagnosis requires the use of laboratory tools. With frequent co-circulation of these three arboviral diseases, diagnosis can be challenging, though mandatory to allow adequate management and counselling especially in at-risk populations such as pregnant women.
In the viremic state, up to Day 5 post-symptom onset, DENV can be diagnosed by RT-PCR, or NS1 antigen detection in blood samples (in primary infections, NS1 antigen may persist for a longer period).1,102 RT-PCR further allows for DENV serotyping. In the acute phase and up to 2 weeks after symptom onset, ZIKV infection can be diagnosed by RNA isolation in blood or urine. ZIKV RNA is usually detected in plasma up to 5–7 days, and 14 days in urine.103,104 In pregnant women, viremia has been reported for up to 126 days.25 Recently, several studies have demonstrated the prolonged detection of ZIKV RNA in whole blood by comparison to plasma samples.105 Whole blood samples should therefore be considered in asymptomatic patients requiring a confirmed diagnosis, such as pregnant women with fetal anomalies.11
Challenges exist in differentiating ZIKV from DENV infections after the viremic state, when neither virus can be detected in blood, as serological diagnosis might be unreliable, particularly in secondary infections. This represents a significant challenge, particularly in asymptomatic returning travellers due to the co-circulation of DENV and ZIKV. The difficulties are further increased by the co-circulation of the four DENV serotypes in most endemic areas,106 as a previous infection with one DENV serotype does not provide protective immunity against other serotypes and secondary infections may occur.
For ZIKV and DENV infections, specific IgM, as well as neutralizing antibodies, can be detected as early as 4–5 days post-infections and for up to 12 weeks.1,107 In primary infections, a negative IgM ELISA test 12 weeks after exposure is a strong argument for the absence of a recent infection, while a positive or inconclusive IgM test needs to be confirmed by plaque reduction neutralization test (PRNT), which identifies specific neutralizing antibodies. Importantly, patients previously exposed to other flaviviruses may display an important serologic cross-reactivity.108 Though cross-reaction may occur in primary infections, titres of neutralizing antibodies of the infecting agent will be significantly higher.103
During secondary flavivirus infection or in the context of a previous vaccination, re-infection will not stimulate the production of IgM antibodies, but prompt high titres of ZIKV IgG antibodies.109 The absence of a specific IgM response is due to the high antigenic similarities between flaviviruses. Furthermore, this re-stimulation may suppress the production of specific antibodies to the novel infective agent, a phenomenon called the original antigenic sin.110 Though rare in non-endemic areas, the absence of antibody response (IgG, IgM and neutralizing antibodies) has been described in returning patients with a RT-PCR confirming acute ZIKV infection from non-endemic countries, among which two women were pregnant. In that context, a negative IgM result does not necessarily exclude a recent infection, particularly in cases of multiple exposures. Recently, a novel NS1 IgM/IgG ELISA assay has been developed111 with good sensitivity and specificity with combined IgM and IgG detection. Cross-reactions are also described for NS1-based assays, but a recent study has suggested that the combination of E protein-based ELISA IgM assay and NS1 IgG and IgM ELISA assay might help distinguish ZIKV infection in the setting of a past DENV infection from a secondary DENV infection.112
As CHIKV is an alphavirus, serologic diagnosis is not impacted by cross-circulation of ZIKV and DENV. Infection is confirmed by a positive RT-PCR, specific IgM detection or IgG seroconversion.5 The sensitivity of RT-PCR is lower, starting from Days 4 to 7.5 IgM titres increase from Day 5 onwards and may persist for up to 18 months post-infection in some cases, impairing accurate documentation of the timing of infection onset. Cross-reactions with other alphaviruses have also been described.113 Therefore, the CDC recommends confirmation of a positive or inconclusive test with PRNT.113
General pre-travel advice for pregnant women
Currently, there are no vaccines or medicines to prevent ZIKV and CHIKV diseases among travellers. One vaccine, Dengvaxia (developed by Sanofi Pasteur), has been licensed for DENV, but its use was primarily directed for endemic areas, whose population was previously exposed to DENV.114 Due to a shared vector (i.e. Aedes spp. mosquitoes), these three viruses often co-circulate, particularly in South East Asia and South America. Travellers to areas with a high circulataion of A. albopictus should therefore receive general information about the three viruses. Specific emphasis on a particular virus, as stated below, should be made depending on the local and current epidemilogical situation. Several agencies, such as the American and European Centers of Diseases Control and Prevention, provide regular updated information. Prevention for travelers primarily involves protection against mosquito bites by the use of correct insect repellent or protective clothing, while sexual transmission is prevented by protected sexual intercourse.115
In terms of preventive measures against mosquitoes for pregnant travellers, Environmental Protection Agency (EPA)-registered insect repellents, such as diethyl-m-toluamide (DEET), picaridin, lemon eucalyptus oil or para-menthane-diol (PMD) and IR3535 (ethyl butylacetylaminopropionate), as well as permethrin-treated clothing and gear, to prevent mosquito bites are not harmful to use on pregnant and breastfeeding women.116–118 Careful hand and skin washing prior to breastfeeding is advised.
Of note, when counselling pregnant women on travelling to a DENV, ZIKV and CHIKV endemic area, one should not forget routine travel recommendations. Most Aedes spp. endemic areas overlap with regions endemic for Anopheline spp., the vector of malaria, which is responsible for well-established complications in pregnancy.38 Furthermore, additional risks associated with travelling may put pregnant women at increased risk, such as food-borne diseases or thromboembolic diseases associated with prolonged travel.119,120
ZIKV: specific pre- and post-travel advice
The risk for travellers is essentially related to the risk of mosquito-borne transmission and the epidemiologic situation, the risk of infection very likely being high during epidemics, and low to intermediate in countries with past circulation or endemic circulation.121 Though ZIKV is still circulating in some countries,122 as per 2019, no countries report epidemic circulation of ZIKV.123 At present, the Americas and the Caribbean have reported a significant decline in the number of ZIKV cases, while retrospective studies have reported a wide distribution among Asian and African countries. Therefore, at present the global risk to travellers appears low to moderate.121 Nevertheless, current travel recommendations for ZIKV prevention are still a matter of debate and significant discrepancies exist between international recommendations, with certain national and international organizations (i.e. Switzerland and The Netherlands) being more liberal as they consider the risks to be minimal at present, whereas others (i.e. USA and WHO) are more restrictive, due to the limited capacities of surveillance studies and remaining questions regarding the exact risks related to sexual and materno-fetal transmissions. Figure 1 presents the comparison of selected international recommendations presented at the recent 16th Conference of the International Society of Travel Medicine.123–128
Figure 1.
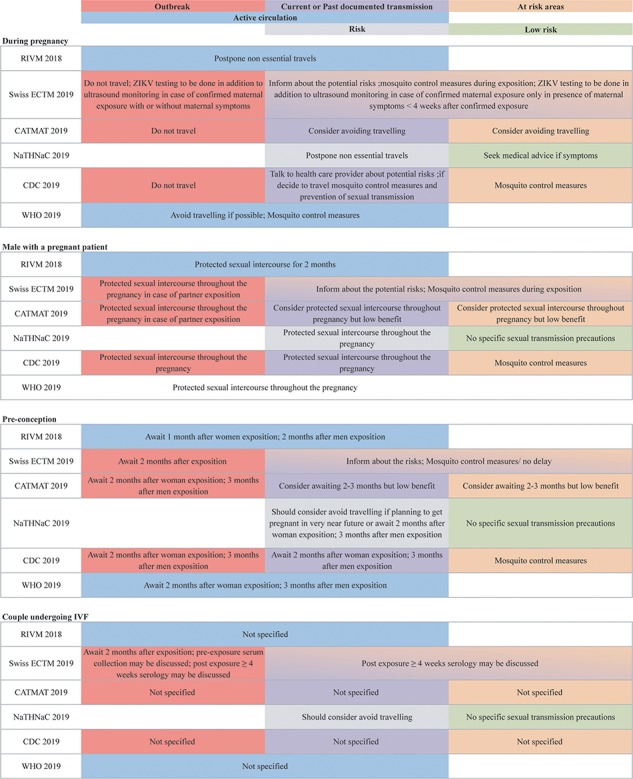
Comparison of the different recommendations for Zika virus. All agencies use different definitions to classify at risk areas. The CDC (USA), CATMAT (Canada) and the ECTM (Switzerland) use the CDC definitions (https://wwwnc.cdc.gov/travel/page/zika-travel-information), while the NaTHNaC (UK) defines the risk for every country (https://travelhealthpro.org.uk/countries)
In terms of ZIKV prevention, we have recently published our concerns regarding overly restrictive recommendations.117 In endemic areas, the initial guidelines stated that pregnancy attempts should be postponed for individuals that were potentially exposed to ZIKV. For returning travellers, women were advised to delay pregnancy for 2 months, while the recommended interval was 6 months for men, due to the persistence of the virus in male reproductive organs.130–132 Infectious viral particles have been detected in semen up to a maximum of 69 days after initial ZIKV symptoms, while this time frame is only 2 days in vaginal fluid. Only a minority (4%) of semen samples positive for ZIKV RNA, however, actually contained infectious particles, and in most cases shedding of these particles was limited to 30 days after the onset of symptoms.27,129 These observations have therefore challenged the recommendations, especially given their potential impact on family planning in the setting of billions of people living and travelling in ZIKV-affected areas.129,133 Consequently, the US Centers for Disease Control and Prevention (CDC) updated its recommendations for men with possible ZIKV exposure who are considering conceiving with their partners. They now suggest a shorter waiting time of 3 months between the possible ZIKV infection and conception. The use of condoms or abstinence from intercourse for the whole pregnancy is also advised. The recommended interval between possible ZIKV infection and conception for women is unchanged (2 months).123,134 In order to avoid different recommendations for men and women and since the couple could be seen as a unit, the Swiss agency recommends 2 months for all for travellers returning from epidemic areas.
Moreover, recent studies have provided a better estimation of the risk of materno-fetal transmission, severe fetal infections and long-term consequences associated with ZIKV infection during pregnancy.87,135 Recent data suggest that both immediate- and long-term sequelae are similar to other well-known congenital infections, such as CMV,86,135 and further argue in favour of less restrictive measures. Furthermore, the risks of adverse pregnancy outcomes depend mainly on the local incidence of ZIKV, which was estimated to range between 1% in Brazil and up to 75% in Yap Island depending on the time of the epidemic.13,136 Risk of infection for travellers remains unknown, but is probably lower than for people living in epidemic areas especially in cases of short stays and is further reduced now due to the declining circulation of ZIKV. In comparison, the risk of CMV infection during pregnancy is estimated at 1–2% in seronegative women, which represents up to 50% of women of childbearing age in most industrialized countries, with an approximate total prevalence of congenital CMV infection of 0.7%.137 This risk is considered completely acceptable by all obstetric agencies and prevention is based only on hygienic measures (hand washing, no sharing of toothbrush, etc.); routine pre-natal screening is not recommended.40,138 CMV is transmitted through all body fluids (urine, saliva and blood) including sexual contact, with viral particles detected up to 14 months in semen.139 Interestingly, no specific recommendations regarding sexual behaviour for pregnant women or couples trying to conceive have ever been elaborated.
All agencies agree that ZIKV laboratory testing should not be performed in asymptomatic patients.140–143 Therefore, laboratory testing before travelling is not recommended for couples trying to conceive or pregnant women.124,143,144 The Swiss Institute has suggested that laboratory testing might be considered in specific situations such as couples undergoing medically assisted procreation or in the case of multiple exposures (e.g. multiple trips to endemic areas).124 Nevertheless, the benefit of such testing in these specific populations remains largely unknown and limitations of the laboratory tests mentioned earlier should be clearly explained.
Similarly, following exposures, testing should only be performed in pregnant women in the presence of maternal symptoms or in the presence of abnormal prenatal ultrasound findings. In pregnant women, these should include RT-PCR analysis performed in whole blood and urine samples, as well as IgM detection.11,140,142,144–146 In the case of an increased risk of exposure, such as during an outbreak, testing might be advisable in asymptomatic pregnant women. Due to the risk of false-negative results, the Swiss agency recommends to add an additional ultrasound 4 weeks post-exposure as well as an additional ultrasound in the third trimester in such situations, independently of the result of the laboratory testing.98,140
Testing post-exposure in asymptomatic couples trying to conceive is not routinely recommended.140–143,147 This recommendation is based on the significant reduction of ZIKV circulation in most parts of the world, reducing the pre-test probability147 and the limited reliability of serological analysis as discussed above. Furthermore, detection of viral RNA can be misleading, as a positive test does not imply the presence of infective viral particles.148 In semen, viral RNA can be detected up to 6 months, long after the decline of infective viral particles.131
DENV and CHIKV: specific pre- and post-travel advice
DENV is endemic in over 100 countries.148 Similarly, since 2013, CHIKV is present in all subtropical and tropical areas.149 At the present, most centres for disease control and prevention do not make specific recommendations for pregnant women and mosquito prevention measures should be taken to avoid DENV or CHIKV infection in pregnant women, as for any other traveller. Nevertheless, we agree with CDC and the Public Health England, which specify that pregnant patients should be informed about the risks of adverse pregnancy outcomes and suggest avoiding unnecessary travel, particularly in the third trimester due to the risk associated with perinatal transmission and maternal consequences.149–151 Other agencies recommend routine measures to protect against mosquito bites.115,141 Similarly to ZIKV, routine laboratory testing prior to travel is not recommended for DENV or CHIKV and post-travel testing should be reserved for symptomatic patients.115,148
Conclusions
The emergence and dissemination of arboviral diseases have led to the recognition of their implications and potential significant complications in pregnancy. Their worldwide distribution, the risk of rapid re-emergence and the difficulties related to diagnostic considerations significantly challenge public health authorities when establishing practical travel recommendations. Current information is mostly provided by cohort studies performed following epidemics in autochthonous populations. Further studies are needed to better understand the specific risk for travellers associated with arboviruses and effort should be made to gather all potential confounding factors. Recommendations are subject to rapid change. Nevertheless, efforts should be made to adequately inform travellers about potential risks in order to make optimal decisions and reassure exposed couples trying to conceive and pregnant women that the risks in cases of exposure remain low, especially regarding ZIKV exposure.
Author contributions
M.V., Y.C.C., S.D.M., S.M., D.B. and M.S. performed literature search and manuscript preparation. L.P., B.G. and D.M. provided critical feedback of the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Department of Obstetrics and Gynecology, Lausanne University Hospital, Switzerland, and by the SNSF grant numbers 310030-156169/1, 320030-169853/1 and 320030-169853/2 attributed to David Baud. David Baud is also supported by the Lausanne University (Interdisciplinary Grant), by the ‘Fondation Leenaards’ through the ‘Bourse pour la relève académique’, by the ‘Fondation Divesa’ and by the ‘Loterie Romande’.
Acknowledgements
None.
Conflict of interest: None declared.
References
- 1. Wilder-Smith A, Ooi E-E, Horstick O, Wills B. Dengue. Lancet Lond Engl 2019; 393:350–63. [DOI] [PubMed] [Google Scholar]
- 2. Faria NR, Azevedo R do S da S, MUG Kraemer, et al. Zika virus in the Americas: early epidemiological and genetic findings. Science 2016; 352:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Zika epidemiology update. 2019; published online July. https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1.
- 4. Paul BJ, Sadanand S. Chikungunya infection: a re-emerging epidemic. Rheumatol Ther 2018; 5:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Natrajan MS, Rojas A, Waggoner JJ. Beyond fever and pain: diagnostic methods for chikungunya virus. J Clin Microbiol 2019; 57. doi: 10.1128/JCM.00350-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uno N, Ross TM. Dengue virus and the host innate immune response. Emerg Microbes Infect 2018; 7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sirohi D, Kuhn RJ. Zika virus structure, maturation, and receptors. J Infect Dis 2017; 216:S935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang A, Thurmond S, Islas L, Hui K, Hai R. Zika virus genome biology and molecular pathogenesis. Emerg Microbes Infect 2017; 6:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levi LI, Vignuzzi M. Arthritogenic alphaviruses: a worldwide emerging threat? Microorganisms 2019; 7. doi: 10.3390/microorganisms7050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015; 372:1231–9. [DOI] [PubMed] [Google Scholar]
- 11. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet Lond Engl 2017; 390:2099–109. [DOI] [PubMed] [Google Scholar]
- 12. Kraemer MU, Sinka ME, Duda KA et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duffy MR, Chen T-H, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 14. Contopoulos-Ioannidis D, Newman-Lindsay S, Chow C, LaBeaud AD. Mother-to-child transmission of Chikungunya virus: a systematic review and meta-analysis. PLoS Negl Trop Dis 2018; 12:e0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Musso D, Stramer SL. AABB Transfusion-Transmitted Diseases Committee, Busch MP, International Society of Blood Transfusion Working Party on Transfusion-Transmitted Infectious Diseases. Zika virus: a new challenge for blood transfusion. Lancet Lond Engl 2016; 387:1993–4. [DOI] [PubMed] [Google Scholar]
- 16. Pouliot SH, Xiong X, Harville E et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv 2010; 65:107–18. [DOI] [PubMed] [Google Scholar]
- 17. Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2014; 19. doi: doi.org/10.2807/1560-7917.ES2014.19.13.20751. [PubMed] [Google Scholar]
- 18. Mlakar J, Korva M, Tul N et al. Zika virus associated with microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 19. Rodrigues LC. Microcephaly and Zika virus infection. Lancet Lond Engl 2016; 387:2070–2. [DOI] [PubMed] [Google Scholar]
- 20. Calvet G, Aguiar RS, Melo ASO et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 2016; 16:653–60. [DOI] [PubMed] [Google Scholar]
- 21. Li C, Xu D, Ye Q et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 2016; 19:120–6. [DOI] [PubMed] [Google Scholar]
- 22. Yockey LJ, Varela L, Rakib T et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 2016; 166:1247–1256.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foy BD, Kobylinski KC, Chilson Foy JL et al. Probable non-vector-borne transmission of Zika virus, Colorado. USA Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakkas H, Bozidis P, Giannakopoulos X, Sofikitis N, Papadopoulou C. An update on sexual transmission of Zika virus. Pathogens 2018; 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desclaux A, de Lamballerie X, Leparc-Goffart I et al. Probable sexually transmitted Zika virus infection in a pregnant woman. N Engl J Med 2018; 378:1458–60. [DOI] [PubMed] [Google Scholar]
- 26. Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de X, Yazdanpanah Y. Long-lasting persistence of Zika virus in semen. Clin Infect Dis 2016; ciw509. [DOI] [PubMed] [Google Scholar]
- 27. Mead PS, Duggal NK, Hook SA et al. Zika Virus shedding in semen of symptomatic infected men. N Engl J Med 2018; 378:1377–85. [DOI] [PubMed] [Google Scholar]
- 28. Gao D, Lou Y, He D et al. Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Rep 2016; 6: 28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen LH, Wilson ME. Update on non-vector transmission of dengue: relevant studies with Zika and other flaviviruses. Trop Dis Travel Med Vaccines 2016; 2. doi: 10.1186/s40794-016-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gérardin P, Barau G, Michault A et al. Multidisciplinary prospective study of mother-to-child Chikungunya virus infections on the island of La Réunion. PLoS Med 2008; 5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lalle E, Colavita F, Iannetta M et al. Prolonged detection of dengue virus RNA in the semen of a man returning from Thailand to Italy, January 2018. Eurosurveillance 2018; 23. doi: 10.2807/1560-7917.ES.2018.23.18.18-00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molton JS, Low I, Choy MMJ et al. Dengue virus not detected in human semen. J Travel Med 2018; 25. doi: 10.1093/jtm/tay023. [DOI] [PubMed] [Google Scholar]
- 33. Lee C, Lee H. Probable female to male sexual transmission of dengue virus infection. Infect Dis 2019; 51:150–2. [DOI] [PubMed] [Google Scholar]
- 34. Bandeira AC, Campos GS, Rocha VFD et al. Prolonged shedding of Chikungunya virus in semen and urine: a new perspective for diagnosis and implications for transmission. IDCases 2016; 6:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilder-Smith A. Can dengue virus be sexually transmitted. J Travel Med . doi: 10.1093/jtm/tay157. [DOI] [PubMed] [Google Scholar]
- 36. Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009–2010 pandemic. Obstet Gynecol 2015; 126:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hillier SL, Nugent RP, Eschenbach DA et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 38. Desai M, ter Kuile FO, Nosten F et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 39. Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 2001; 64:28–35. [DOI] [PubMed] [Google Scholar]
- 40. American College of Obstetricians and Gynecologists Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015; 125:1510–25. [DOI] [PubMed] [Google Scholar]
- 41. de Jong EP, Walther FJ, Kroes ACM, Oepkes D. Parvovirus B19 infection in pregnancy: new insights and management. Prenat Diagn 2011; 31:419–25. [DOI] [PubMed] [Google Scholar]
- 42. Charlier C, Beaudoin M-C, Couderc T, Lortholary O, Lecuit M. Arboviruses and pregnancy: maternal, fetal, and neonatal effects. Lancet Child Adolesc Health 2017; 1:134–46. [DOI] [PubMed] [Google Scholar]
- 43. World Health Organization and Special Programme for Research and Training in Tropical Diseases Handbook for clinical management of dengue. 2012; published online Nov. https://www.who.int/denguecontrol/9789241504713/en/.
- 44. Katzelnick LC, Gresh L, Halloran ME et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358:929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain-Barré syndrome: a case report and review of the literature. J Med Case Reports 2018; 12. doi: 10.1186/s13256-018-1626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paixao ES, Harron K, Campbell O et al. Dengue in pregnancy and maternal mortality: a cohort analysis using routine data. Sci Rep 2018; 8:9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hariyanto H, Yahya CQ, Wibowo P, Tampubolon OE. Management of severe dengue hemorrhagic fever and bleeding complications in a primigravida patient: a case report. J Med Case Reports 2016; 10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basurko C, Everhard S, Matheus S et al. A prospective matched study on symptomatic dengue in pregnancy. PloS One 2018; 13:e0202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Basurko C, Matheus S, Hildéral H et al. Estimating the risk of vertical transmission of dengue: a prospective study. Am J Trop Med Hyg 2018; 98:1826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribeiro CF, Lopes VGS, Brasil P, Coelho J, Muniz AG, Nogueira RMR. Perinatal transmission of dengue: a report of 7 cases. J Pediatr 2013; 163:1514–6. [DOI] [PubMed] [Google Scholar]
- 51. Yang J, Zhang J, Deng Q et al. Investigation on prenatal dengue infections in a dengue outbreak in Guangzhou City, China. Infect Dis 2017; 49:315–7. [DOI] [PubMed] [Google Scholar]
- 52. Paixão ES, Teixeira MG, Costa M da CN, Barreto ML, Rodrigues LC. Symptomatic dengue during pregnancy and congenital neurologic malformations. Emerg Infect Dis 2018; 24:1748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nascimento LB, Siqueira CM, Coelho GE, Siqueira JB. Symptomatic dengue infection during pregnancy and livebirth outcomes in Brazil, 2007–13: a retrospective observational cohort study. Lancet Infect Dis 2017; 17:949–56. [DOI] [PubMed] [Google Scholar]
- 54. Paixão ES, Teixeira MG, Costa M da CN, Rodrigues LC. Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16:857–65. [DOI] [PubMed] [Google Scholar]
- 55. Sirinavin S, Nuntnarumit P, Supapannachart S, Boonkasidecha S, Techasaensiri C, Yoksarn S. Vertical dengue infection: case reports and review. Pediatr Infect Dis J 2004; 23:1042–7. [DOI] [PubMed] [Google Scholar]
- 56. Escobar MF, Mora BL, Cedano JA, Loaiza S, Rosso F. Comprehensive treatment in severe dengue during preterm and term labor: could tocolysis be useful? J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2019; 1–6. [DOI] [PubMed] [Google Scholar]
- 57. Bopeththa BVKM, Hemapriya S, Gayan Niranga KK, Kotigala DSK. A case report of dengue haemorrhagic fever during the peripartum period: challenges in management and a case of vertical dengue transmission. BMC Infect Dis 2018; 18:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rajagopala L, Satharasinghe RL, Karunarathna M. A rare case of dengue encephalopathy complicating a term pregnancy. BMC Res Notes 2017; 10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burt FJ, Chen W, Miner JJ et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis 2017; 17:e107–17. [DOI] [PubMed] [Google Scholar]
- 60. Lebrun G, Chadda K, Reboux A-H, Martinet O, Gaüzère B-A. Guillain-Barré syndrome after Chikungunya infection. Emerg Infect Dis 2009; 15:495–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brighton SW, Simson IW. A destructive arthropathy following Chikungunya virus arthritis--a possible association. Clin Rheumatol 1984; 3:253–8. [DOI] [PubMed] [Google Scholar]
- 62. Malvy D, Ezzedine K, Mamani-Matsuda M et al. Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect Dis 2009; 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manimunda SP, Vijayachari P, Uppoor R et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg 2010; 104:392–9. [DOI] [PubMed] [Google Scholar]
- 64. Escobar M, Nieto AJ, Loaiza-Osorio S, Barona JS, Rosso F. Pregnant women hospitalized with Chikungunya virus infection, Colombia, 2015. Emerg Infect Dis 2017; 23. doi: 10.3201/eid2311.170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fritel X, Rollot O, Gerardin P et al. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis 2010; 16:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Torres JR, Falleiros-Arlant LH, Dueñas L, Pleitez-Navarrete J, Salgado DM, JB-D C. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis 2016; 51:85–8. [DOI] [PubMed] [Google Scholar]
- 67. Duarte M do CMB, Oliveira Neto AF de, Bezerra PG de M et al. Infecção por Chikungunya em lactentes. Rev Bras Saúde Materno Infant 2016; 16:S63–71. [Google Scholar]
- 68. Gérardin P, Sampériz S, Ramful D et al. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis 2014; 8:e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ 2012; 345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cao-Lormeau V, Blake A, Mons S et al. Guillain-Barré Syndrome outbreak caused by ZIKA virus infection in French Polynesia. Lancet Lond Engl 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yuki N, Hartung H-P. Guillain-Barré syndrome. N Engl J Med 2012; 366:2294–304. [DOI] [PubMed] [Google Scholar]
- 72. Pomar L, Musso D, Malinger G, Vouga M, Panchaud A, Baud D. Zika virus during pregnancy: from maternal exposure to congenital Zika virus syndrome. Prenat Diagn 2019published online March 13. doi: 10.1002/pd.5446. [DOI] [PubMed] [Google Scholar]
- 73. Flamand C, Fritzell C, Matheus S et al. The proportion of asymptomatic infections and spectrum of disease among pregnant women infected by Zika virus: systematic monitoring in French Guiana, 2016. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2017; 22. doi: 10.2807/1560-7917.ES.2017.22.44.17-00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Honein MA, Dawson AL, Petersen EE et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317:59–68. [DOI] [PubMed] [Google Scholar]
- 75. de Oliveira WK, Carmo EH, Henriques CM et al. Zika virus infection and associated neurologic disorders in Brazil. N Engl J Med 2017; 376:1591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Secretaria de Vigilância em Saúde Ministério da Saúde. Situação epidemiológica de ocorrência de microcefalias no Brasil 2015; 46:1–3. [Google Scholar]
- 77. DeSilva M, Munoz FM, Sell E et al. Congenital microcephaly: case definition & guidelines for data collection, analysis, and presentation of safety data after maternal immunisation. Vaccine 2017; 35:6472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ashwal S, Michelson D, Plawner L, Dobyns WB. Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Abuelo D. Microcephaly syndromes. Semin Pediatr Neurol 2007; 14:118–27. [DOI] [PubMed] [Google Scholar]
- 80. Noyola DE, Demmler GJ, Nelson CT et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 2001; 138:325–31. [DOI] [PubMed] [Google Scholar]
- 81. Santis MD, Cavaliere AF, Straface G, Caruso A. Rubella infection in pregnancy. Reprod Toxicol 2006; 21:390–8. [DOI] [PubMed] [Google Scholar]
- 82. Krauss MJ, Morrissey AE, Winn HN, Amon E, Leet TL. Microcephaly: an epidemiologic analysis. Am J Obstet Gynecol 2003; 188:1484–9discussion 1489-1490. [DOI] [PubMed] [Google Scholar]
- 83. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects — reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 84. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects--reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 85. Moore CA, Staples JE, Dobyns WB et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pomar L, Vouga M, Lambert V et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ 2018; 363: k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pomar L, Vouga M, Lambert V et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ 2018; k4431:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Besnard M, Dub T, Gérardin P. Outcomes for 2 children after peripartum acquisition of Zika virus infection, French Polynesia, 2013-2014. Emerg Infect Dis 2017; 23:1421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van LV. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb Mortal Wkly Rep 2016; 65. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 90. Subissi L, Dub T, Besnard M et al. Zika virus infection during pregnancy and effects on early childhood development, French Polynesia, 2013-2016. Emerg Infect Dis 2018; 24:1850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zin AA, Tsui I, Rossetto J et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr 2017; 171:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brasil P, Pereira JP, Moreira ME et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cauchemez S, Besnard M, Bompard P et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. The Lancet 2016; 387:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shapiro-Mendoza CK, Rice ME, Galang RR et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy - U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hoen B, Schaub B, Funk AL et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018 published online March 14 . doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 96. Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev 2016; 29:659–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 98. Vouga M, Musso D, Panchaud A, Baud D. Clinical management of pregnant women exposed to Zika virus. Lancet Infect Dis 2016; 16:773. [DOI] [PubMed] [Google Scholar]
- 99. Schaub B, Gueneret M, Jolivet E et al. Cerebral damage in congenital Zika virus syndrome: an ultrasound case series In: Lancet Child Adolesc Health. in Press, 2017. [DOI] [PubMed] [Google Scholar]
- 100. Vouga M, Musso D, Van T, Baud D. CDC guidelines for pregnant women during the Zika virus outbreak. Lancet Lond Engl 2016; 387:843–4. [DOI] [PubMed] [Google Scholar]
- 101. Schaub B, Vouga M, Najioullah F et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect Dis 2017published online Feb 10. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 102. Muller DA, Depelsenaire ACI, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis 2017; 215:S89–95. [DOI] [PubMed] [Google Scholar]
- 103. Landry ML, St George K. Laboratory diagnosis of Zika virus infection. Arch Pathol Lab Med 2017; 141:60–7. [DOI] [PubMed] [Google Scholar]
- 104. Joguet G, Mansuy J-M, Matusali G et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017; 17:1200–8. [DOI] [PubMed] [Google Scholar]
- 105. Voermans JJC, Pas SD, van der A et al. Whole-blood testing for diagnosis of acute Zika virus infections in routine diagnostic setting. Emerg Infect Dis J 2019; 25. doi: 10.3201/eid2507.182000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Masyeni S, Yohan B, Somia IKA, Myint KSA, Sasmono RT. Dengue infection in international travellers visiting Bali, Indonesia. J Travel Med 2018; 25. doi: 10.1093/jtm/tay061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet Lond Engl 2017; 390:2099–109. [DOI] [PubMed] [Google Scholar]
- 108. Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barzon L, Percivalle E, Pacenti M et al. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am 2018; 66:1173–80. [DOI] [PubMed] [Google Scholar]
- 110. Vatti A, Monsalve DM, Pacheco Y, Chang C, Anaya J-M, Gershwin ME. Original antigenic sin: a comprehensive review. J Autoimmun 2017; 83:12–21. [DOI] [PubMed] [Google Scholar]
- 111. Steinhagen K, Probst C, Radzimski C et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2016; 21. doi: 10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tsai W-Y, Youn HH, Brites C et al. Distinguishing secondary dengue virus infection from Zika virus infection with previous dengue by a combination of 3 simple serological tests. Clin Infect Dis Off Publ Infect Dis Soc Am 2017; 65:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis 2016; 214:S471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 2017; 17:e101–6. [DOI] [PubMed] [Google Scholar]
- 115. Centers for Disease Control and Prevention. Chikungunya Virus - Prevention Published online. Dec 2018; 17: https://www.cdc.gov/chikungunya/prevention/index.html. [Google Scholar]
- 116. Centre de Référence sur les Agents Tératogènes Répulsifs - Grossesse et Allaitement. 2018; Published online Dec 7 http://lecrat.fr/spip.php?page=article&id_article=444. [Google Scholar]
- 117. Koren G, Matsui D, Bailey B. DEET-based insect repellents: safety implications for children and pregnant and lactating women. CMAJ Can Med Assoc J J Assoc Medicale Can 2003; 169:209–12. [PMC free article] [PubMed] [Google Scholar]
- 118. NaTHNaC Travel Health Pro - Pregnancy. 2016; published online June 29. https://travelhealthpro.org.uk/factsheet/45/pregnancy.
- 119. An Advisory CATMAT. Committee Statement (ACS) Committee to Advise on Tropical Medicine and Travel*†. 2010; published online Aug 3. https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2010-36/canada-communicable-disease-report-7.html#c9.
- 120. European Centre for Disease Prevention and Control Zika virus transmission worldwide - 9 April. 2019; published online Sept 4. https://ecdc.europa.eu/sites/portal/files/documents/zika-risk-assessment-9-april-2019.pdf.
- 121. Hamer DH, Chen LH. Zika in Angola and India. J Travel Med 2019; 26. doi: 10.1093/jtm/taz012. [DOI] [PubMed] [Google Scholar]
- 122. Centers for Disease Control and Prevention Zika Travel Information (Update April 2019). https://wwwnc.cdc.gov/travel/page/zika-travel-information(accessed March 5, 2019).
- 123. Swiss Tropical and Public Health Institue Zika Virus Information and recommendations of the Swiss Expert Committee for Travel Medicine (ECTM) * (Update April 2019). 2019; published online April 23. https://www.swisstph.ch/en/travelclinic/zika-info/.
- 124. WHO WHO guidelines for the prevention of sexual transmission of Zika virus: executive summary. 2019. https://apps.who.int/iris/bitstream/handle/10665/311026/WHO-RHR-19.4-eng.pdf?ua=1. [PubMed]
- 125. Zika Virus CATMAT. Prevention and Treatment Recommendations. 2019; published online May 2. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/zika-virus-prevention-treatment-recommendations.html#tb1.
- 126. Public Health England Zika virus: preventing infection by sexual transmission. 2019; published online Feb 27. https://www.gov.uk/guidance/zika-virus-preventing-infection-by-sexual-transmission.
- 127. RIVM Zika Virus. 2018; published online Nov 20. https://www.rivm.nl/en/zika-virus.
- 128. Vouga M, Musso D, Goorhuis A, Freedman DO, Baud D. Updated Zika virus recommendations are needed. The Lancet 2018; 392:818–9. [DOI] [PubMed] [Google Scholar]
- 129. Atkinson B, Hearn P, Afrough B et al. Detection of Zika virus in semen. Emerg Infect Dis 2016; 22:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barzon L, Pacenti M, Franchin E et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2016; 21. doi: 10.2807/1560-7917.ES.2016.21.32.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Turmel JM, Abgueguen P, Hubert B et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet Lond Engl 2016; 387:2501. [DOI] [PubMed] [Google Scholar]
- 132. Chen LH, Hamer DH. Zika virus and sexual transmission: updated preconception guidance. J Travel Med 2018; 25. doi: 10.1093/jtm/tay095. [DOI] [PubMed] [Google Scholar]
- 133. Centers for Disease Control and Prevention. Sexual Transmission and Prevention Published online. May 2019; 21: https://www.cdc.gov/zika/prevention/sexual-transmission-prevention.html. [Google Scholar]
- 134. Nielsen-Saines K, Brasil P, Kerin T et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019; 25:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Luna E, Romano C, Araujo E et al. Low prevalence after the first Zika virus epidemic wave in Southeastern Brazil. Int J Infect Dis 2018; 73:17. [Google Scholar]
- 136. Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol 2017; 38:97–107. [DOI] [PubMed] [Google Scholar]
- 137. The royal college of obstetricians and gynecologists. Congenital Cytomegalovirus Infection Update on Treatment. Scientific Impact Paper No. 56 In: BJOG Int J Obstet Gynaecol, 2018, https://www.rcog.org.uk/en/guidelines-research-services/guidelines/sip56/. [DOI] [PubMed]
- 138. Lang DJ, Kummer JF, Hartley DP. Cytomegalovirus in semen. Persistence and demonstration in extracellular fluids. N Engl J Med 1974; 291:121–3. [DOI] [PubMed] [Google Scholar]
- 139. Eperon G, Veit O, Baud D, Eperon I. Zika virus: update of the practical guidelines. Rev Med Suisse 2019; 15:911–6. [PubMed] [Google Scholar]
- 140. NaTHNaC Zika - risk assessment. 2019; published online Feb 27. https://travelhealthpro.org.uk/factsheet/5/zika--risk-assessment.
- 141. CATMAT Zika virus: for health professionals. 2019; published online Jan 18. https://www.canada.ca/en/public-health/services/diseases/zika-virus/health-professionals.html.
- 142. Centers for Disease Control and Prevention Zika virus for health care providers - testing guidance. 2019; published online May 21. https://www.cdc.gov/zika/hc-providers/testing-guidance.html.
- 143. Centers for Disease Control and Prevention Pregnant women - testing & diagnosis for zika virus. 2019; published online Feb 26. https://www.cdc.gov/pregnancy/zika/testing-follow-up/testing-and-diagnosis.html.
- 144. Public Health England Revised algorithm1for assessing pregnant women with a history of travel during pregnancy to countries or areas with risk for Zika virus (ZIKV) transmission2. 2019; published online Feb 27. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/780782/Zika_testing_algorithm_for_assessing_pregnant_women.pdf.
- 145. Centers for Disease Control and Prevention Pregnant women - prenatal care. https://www.cdc.gov/pregnancy/zika/testing-follow-up/prenatal-care.html.
- 146. Oduyebo T. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure — United States (including U.S. territories), July 2017. MMWR Morb Mortal Wkly Rep 2017; 66. doi: 10.15585/mmwr.mm6629e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Centers for Disease Control and Prevention. Dengue Virus Published online. Jan 2019; 15 https://www.cdc.gov/dengue/index.html . [Google Scholar]
- 149. Staples JE, Hills SL, Powers AM. Chapter 3 infectious diseases related to travel - Chikungunya. CDC Yellow Book 2017; published online May 31. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/chikungunya; . [Google Scholar]
- 150. CDC Women trying to become pregnant|Zika and pregnancy|CDC. Cent Dis Control Prev 2018; published online Aug 21. https://www.cdc.gov/pregnancy/zika/women-and-their-partners.html [Google Scholar]
- 151. Public Health England Zika virus: travel advice. GOVUK 2019; published online Feb 27. https://www.gov.uk/guidance/zika-virus-travel-advice [Google Scholar]


