Abstract
Radiation sensitizers that can selectively act on cancer cells hold great promise to patients who receive radiation therapy. We developed a novel targeted therapy and radiation sensitizer for non-small cell lung cancer (NSCLC) based on cetuximab conjugated nanoparticle that targets epidermal growth factor receptor (EGFR) and delivers small interfering RNA (siRNA) against polo-like kinase (PLK1). EGFR is overexpressed in 50% of lung cancer patients and a mediator of DNA repair, while PLK1 is a key mitotic regulator whose inhibition enhances radiation sensitivity. The nanoparticle construct (C-siPLK1-NP) effectively targets EGFR+ NSCLC cells and reduces PLK1 expression, leading to G2/M arrest and cell death. Furthermore, we show a synergistic combination between C-siPLK1-NP and radiation, which was confirmed in vivo in A549 flank tumors. We also demonstrate the translational potential of C-siPLK1-NP as a systemic therapeutic in orthotopic lung tumor model, where administration of C-siPLK1-NP reduced tumor growth and led to prolonged survival. Our findings demonstrate that C-siPLK1-NP is effective as a targeted therapy and as a potent radiation sensitizer for NSCLC. Potential application to other EGFR+ cancer types such as colorectal and breast cancer is also demonstrated.
1. INTRODUCTION
Non-small cell lung cancer (NSCLC), which makes up 85% of lung cancers, is the leading cause of cancer mortality, resulting in more deaths than colon, breast, and prostate cancers combined, and represents nearly a fourth of total cancer deaths [1]. Radiation therapy remains a cornerstone in lung cancer treatment that is administered to over half of all patients as part of their treatment paradigm [2]. Advances in medical imaging and radiation technology have allowed for more precise and accurate delivery of ionizing radiation (IR); however, outcomes for lung cancer patients have not improved [3] as the five year survival remains 18% [1]. To improve radiation therapy and patient outcomes, traditional efforts focused on the use of chemotherapy, oxygen mimics, or metallic nanoparticles in combination with IR. However, lack of tumor specificity of these approaches results in a greater toxicity to patients and ultimately limits the therapeutic benefit [4]. More recently, advancements in precision medicine have motivated the use of molecularly targeted therapies to improve radiation therapy by selectively acting on cancer cells. The radiation therapy oncology group clinical trial RTOG 0617 for stage III NSCLC, which aimed to improve local tumor control and prolong survival by increasing the radiation dose (from 60 Gy to 74 Gy) in a chemoradiation regimen, did not result in better outcomes but rather caused higher toxicity to patients leading to reduced survival [5]. In the same trial however, the addition of cetuximab (an EGFR-directed monoclonal antibody believed to inhibit DNA repair) led to modest improvements in patients with high EGFR expression [5]. This highlights the potential of molecularly targeted agents to improve the therapeutic ratio of IR leading to better outcomes for patients. Despite this promise, the only FDA approved targeted therapy for combination with radiation is cetuximab for head and neck cancer [6]. For NSCLC, several targets other than EGFR have been investigated in clinical trials including HDAC [7], PI3K/AKT [8], mTOR [9], and VEGF [10]. However, these trials have not led to significant improvement for patients and still often associated with higher grades of toxic side effects. The most promising results were observed with the PI3K/AKT inhibitor Nelfinavir which led to a 5-year survival of 37% for stage III NSCLC patients, albeit with a population size of just 35 patients [8]. Thus, identifying new targeted therapy and IR combinations to improve NSCLC treatment is needed.
The goal of this research is to develop a targeted therapeutic to enhance radiation sensitivity for NSCLC. We previously reported on a human epidermal growth factor receptor 2 (HER2) antibody conjugated mesoporous silica nanoparticle (MSNP) that could target cancer cells in multiple HER2+ breast tumor mouse models and deliver small interfering RNA (siRNA) to impart gene silencing efficacy [11–13]. Herein, we developed the MSNP platform for lung cancer, where effective targeted therapies are an urgent need. By conjugating an EGFR monoclonal antibody on MSNPs and delivering siRNA against polo-like kinase 1 (PLK1), we show that the nanoparticle is effective as both a single agent therapy and as a radiation sensitizer for NSCLC.
We target PLK1, a key mitotic regulator, which is overexpressed in lung cancer and other various types of cancer [14]. Previous studies have shown that high PLK1 expression is correlated with reduced survival for cancer patients [15–17]. Inhibition of PLK1 results in failure to complete mitosis, which leads to G2/M cell cycle arrest and apoptotic cell death. As G2/M is the most IR sensitive cell cycle phase, PLK1 inhibition also sensitizes cancer cells to IR [18]. Furthermore, PLK1 has been shown to contribute to resistance of cancer cells to several drugs including taxanes, doxorubicin, gemcitabine [19], and EGFR inhibitors [20]. In addition, PLK1 has been identified as a target to kill various cancer stem cells [21–23], which are resistant to standard therapies including radiation and chemotherapy, and therefore lead to cancer relapse. Collectively, these observations suggest that inhibition of PLK1 may have promising therapeutic potential for cancer treatment. However, a major limitation is that current PLK1 small molecule inhibitors are ineffective for solid tumors due to their low tumor bioavailability and toxic side effects to healthy cells. PLK1 inhibitors must have long half-lives to achieve sufficient intra-tumor concentrations, but this results in sustained exposure to hematopoietic precursor cells in blood and bone marrow, leading to hematologic dose-limiting toxicities (neutropenia and thrombocytopenia) [24–27]. Of all PLK1 inhibitors, volasertib has shown the most promise having reached phase III clinical trial but only for acute myeloid leukemia (blood cancer) [28] and eventually failed to meet primary endpoint of objective response [29]. For lung cancer, volasertib was terminated as a monotherapy early in a phase II clinical trial due to a lack of response [30]. Therefore, an effective PLK1 therapeutic remains an unmet clinical need for solid tumors, including lung cancers. We hypothesize that PLK1 siRNA delivered by our MSNP platform can circumvent the issues associated with low tumor bioavailability and toxic side effects of current PLK1 inhibitors.
To deliver the nanoparticle platform specifically to lung cancer cells, we conjugate the EGFR monoclonal antibody, cetuximab, to the nanoparticles. EGFR is overexpressed in several cancers, and its high expression correlates positively with poor prognosis [31–34]. In NSCLC, EGFR is overexpressed in about 50% of patients [35] with higher EGFR expression in more advanced stages [31, 36]. Thus, the receptor is an appropriate homing target. Furthermore, following IR damage, EGFR is phosphorylated and translocates to the nucleus where it plays a role in mediating DNA repair [37]. In this regard, addition of cetuximab may have therapeutic benefit as it has been shown to block the translocation of EGFR to the nucleus following IR [38]. Therefore, cetuximab on the nanoparticle may also provide a therapeutic effect, in addition to mediating the targeting to EGFR+ lung cancer cells. We hypothesize that the combination of the EGFR antibody cetuximab and PLK1 siRNA on the nanoparticles (C-siPLK1-NP) would serve as potent radiation sensitizer for NSCLC, as illustrated in Fig. 1A. An effective radiation sensitizer would render cancer cells more susceptible to death by IR, thereby improving treatment efficacy and reducing adverse effects of radiation therapy. Thus, our study highlights a novel strategy that may significantly improve the outcomes and quality of life for lung cancer patients.
Figure 1. EGFR-targeted (cetuximab) mesoporous silica nanoparticle (NP) platform for PLK1 siRNA (siPLK1) delivery or C-siPLK1-NP.
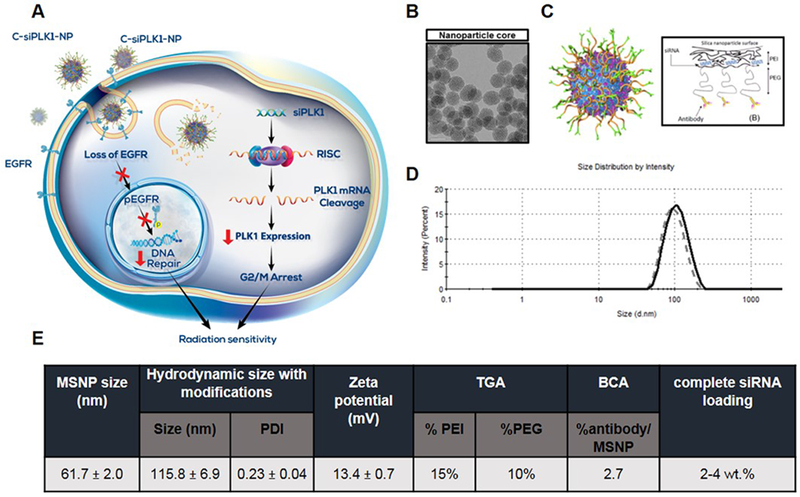
(A) Scheme of central hypothesis illustrating the proposed combination effect of EGFR antibody and siPLK1 on our nanoparticle platform as a novel radiation sensitizer. C-siPLK1-NPs bind to EGFR receptors and are internalized, resulting in the loss of EGFR and phosphorylated EGFR, which can normally reach the nucleus to repair DNA. This reduces the cell’s ability to repair the damage caused by radiation. Simultaneously, siPLK1 on the nanoparticles is released in the cytosol and incorporated in the RNA induced silencing complex (RISC) to mediate PLK1 mRNA cleavage, which reduces PLK1 protein expression and arrests the cells in G2/M where they are most sensitive to radiation damage. Therefore, the platform serves a dual role (by targeting PLK1 and EGFR) to sensitize NSCLC cells to radiation. (B) TEM image of 50-nm MSNP (scale bar = 50 nm). (C) Schematic of the nanoparticle construct with layer-by-layer surface modifications. (D) Representative hydrodynamic size of C-NP with (solid) and without siRNA (dotted) loading by Zetasizer. (E) Characterization of C-siRNA-NP. Hydrodynamic size of bare MSNP and C-siRNA-NP determined by Zetasizer. Data expressed as mean ± SD. Polymer loading (PEI and PEG) determined by thermal gravimetric analysis (TGA). Antibody (cetuximab) loading determined by BCA assay. Complete siRNA binding at 2 wt.% and 4 wt.% assessed by loading a fluorescent labeled siRNA (Dy677-siRNA) on C-NP.
2. MATERIALS AND METHODS
2.1. Nanoparticle synthesis and characterization
Sol-gel synthesis of bare MSNPs and layer-by-layer surface coating of MSNPs was carried out in the same manner as in our previous report [11, 39]. For conjugation of cetuximab to PEG of the nanoparticles, cetuximab (2 mg/ml, OHSU pharmacy) was buffer-exchanged to PBS pH 8 using Zeba Spin columns (Thermo Fisher Scientific) and thiolated with Traut’s reagent (50-fold molar excess) for 2 hr (350 rpm). Thiolated cetuximab was then exchanged to buffer PBS pH 7.2 and added to MSNP-PEI-PEG at 10% w/w for shaking (300 rpm) overnight at 4°C. The next day, nanoparticles were washed 2x with PBS pH 7.2. SiRNA is loaded last by quick mixing with NP (~5 minutes). Nanoparticles size and charge were determined by Zetasizer (Malvern). To quantify polymer loading, 1 mg nanoparticles (MSNP, MSNP-PEI, or MSNP-PEI-PEG) were heated to 950 °C (20 °C/min) with TGA Q50 (TA Instruments). Weight/temperature profiles of MSNP, MSNP-PEI, and MSNP-PEI-PEG were compared to determine percent loading of each polymer and final silica yield. Amount of antibody on NP was determined by Pierce BCA protein assay kit (Thermo Fisher Scientific). SiRNA loading extent on NP was determined by fluorescence using a fluorescent tagged siRNA (Dy677-siSCR), as in our previous report [11].
2.2. Cell culture and reagents
Non-small cell lung cancer cells A549 (CCL-185) and H460 (HTB-177) were obtained from ATCC and cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS). A549 cells with stable expression of red-shifted firefly luciferase gene (Bioware® Brite Cell line A549-Red-Fluc) were purchased from Perkin Elmer and maintained under the same conditions as parental A549 cells. Normal lung epithelial cells NL20 (CRL-2503) were purchased from ATCC and maintained in the recommended complete growth medium. In vivo grade siRNA was purchased from Dharmacon. The siRNA sequences used were: PLK1 (antisense 5’-UAUUCAUUCUUCUUGAUCCGG-3’); scrambled SCR (antisense 5’-UUAGUCGACAUGUAAACCA-3’). Scrambled siRNA with dyes (DyLight 677 or Alexa Fluor 488) attached to the sense strand were purchased from Dharmacon.
2.3. Nanoparticle cellular internalization and EGFR surface expression
Nanoparticle internalization in cells was performed in suspension as we have previously reported [11]. Briefly, cells (1×106) were harvested and incubated with Alexa Fluor 488 dye tagged siSCR nanoparticles (100 μg NP) for one hr. Cells were then washed 3x with FACS buffer and resuspended in 0.5 mL FACS buffer. Trypan blue (0.4% in PBS, 0.5 mL) was added to each suspension to exclude signal from non-internalized nanoparticles. Cells were analyzed on a Guava easyCyte (Millipore Sigma) flow cytometer (10,000 events per sample). For EGFR cell surface expression of cancer and normal cells, human EGFR antibody (cetuximab) was used as primary antibody followed by washing 3x with FACS buffer, before staining with anti-human Alexa Fluor 488 secondary antibody (Life Technologies) for one hr. Cells were then washed 3x with FACS and analyzed with flow cytometer. For EGFR cell surface expression post treatments, 1 million cells were treated with non-target NP, C-NP (3 μg cetuximab), or cetuximab (100 μg) for two hr. Cells were washed with FACS buffer before staining with an Alexa Fluor 647 labeled EGFR antibody (BD Biosciences), washing, and analyzing with flow (10,000 events per sample, biological replicates).
2.4. Animal Studies
For evaluation of the therapeutic as a radiation sensitizer, A549 cells (5 million) were subcutaneously injected into left and right flank of 6-week old male SCID mice (NCI SCID/NCr; Charles River Laboratories) in matrigel (Corning). Tumor growth was monitored using a Vernier caliper and volume calculated by 0.5 x length x width2. When tumor sizes reached average of 120 mm3, mice were grouped to receive saline, C-siSCR-NP, or C-siPLK1-NP intra-tumoral injections to both left and right tumors. Three days following each NP injection (0.3 nmol siRNA per tumor), the left tumors of mice were irradiated at 2 Gy using a small animal x-ray irradiator. Mice were anesthetized and a lead shield (Braintree Scientific) that exposes only the left flank of mice was used. NP and IR were administered once a week for 6 consecutive weeks. Two weeks after last radiation dose, mice were sacrificed and all tumors were harvested, weighed, and prepared for RNA analysis.
To establish orthotopic tumors in lungs of mice, we adopted an intra-tracheal instillation procedure [40] (detailed in Supplementary Methods). Three weeks following intratracheal instillation, mice were injected intravenously with 300 μl of saline, C-siSCR-NP, or C-siPLK1-NP (0.5 mg siRNA/kg animal). Luminescence in lungs (tumor burden) was monitored with IVIS. For IVIS, mice were i.p. injected with 150 mg/kg luciferin (Gold Biotechnology) 20 minutes prior to imaging. Tumor burden was determined by averaging photon flux of mice in prone and supine positions. Mice were monitored daily and weighed once a week during the course of study. All studies were reviewed and approved by Institutional Animal Care and Use Committee (IACUC) at Oregon Health and Science University (OHSU).
2.5. Statistical analysis
Comparison between two groups was performed with Student’s t test. Comparisons among 3 or more groups were performed using one-way ANOVA with Bonferroni’s correction for multiple comparisons, or two-way ANOVA with Tukey’s correction when comparing treatments across IR doses (i.e. γH2ax). Tumor burden over the course of treatment was analyzed using two-way ANOVA with Tukey’s correction for multiple comparisons. Kaplan Meier survival curve was analyzed using the log-rank (Mantel-Cox) method. Significance was set at p < 0.05. In vitro data are expressed as mean ± SD; in vivo data are expressed as mean ± SEM. GraphPad Prism 8.0 (GraphPad Software Inc.) was used for all statistical analysis.
Procedures of western blot, RT-PCR, cell viability, cell cycle arrest, clonogenic survival, γH2ax staining, and apoptosis are provided in Supplementary Methods.
3. RESULTS
3.1. Nanoparticle characteristics
In comparison to other nanoparticle drug carriers, MSNPs offer several advantages such as being biologically benign, having large surface area and high porosity, ease of controlling size and modifying surface chemistry, and high scalability. Fig. 1A depicts a schematic representation of the proposed combination effect of EGFR antibody and siPLK1 on our nanoparticle platform. Our platform consists of an MSNP core (~50 nm by TEM – Fig. 1B) coated layer-by-layer with: 1) bio-reducible crosslinked polyethylene imine (PEI) which allows the use of low MW PEI as a cationic polymer for siRNA binding and effective endosomal escape, 2) polyethylene glycol (PEG) to prevent aggregation, opsonization, and immune response, and 3) antibody to target specific cell type. To target EGFR+ cells, cetuximab was conjugated to the nanoparticle platform (Fig. 1C) to obtain a final particle size of 110 nm (Fig. 1D) with a slightly cationic charge of +13 mV in 10 mM NaCl (Fig. 1E). We achieved excellent batch to batch nanoparticle synthesis as measured in terms of core particle size, final size after surface modification, siRNA loading, and knock-down efficacy using luciferase as a model gene as shown in Supplementary Fig. S1. The composition of the final construct contains 15% PEI and 10% PEG (quantified by thermal gravimetric analysis, TGA), and 2.7% antibody/MSNP (quantified by BCA assay) (Fig. 1E). SiRNA (2 wt. %) is loaded last onto the nanoparticle via electrostatic interactions between the negatively charged siRNA and the cationic polymer PEI. As the loading of siRNA on the nanoparticle is sequence nonspecific, any siRNA (or a set of siRNAs) can be loaded in under 5 minutes [39]. This flexibility in changing siRNAs offers potential for future personalized medicine approaches.
3.2. Cetuximab conjugated nanoparticles are internalized in EGFR+ NSCLC cells
To determine whether cetuximab conjugated nanoparticles (C-NP) target EGFR+ cancer cells, we performed flow cytometry on EGFR+ cells incubated with siRNA-loaded C-NP. EGFR was chosen as the homing target for NSCLC due to its overexpression, as well as its role in DNA damage repair following IR. Dye tagged (Alexa Fluor 488) siRNA was loaded to C-NP and incubated with two high EGFR expressing NSCLC cell lines (A549 and H460) and a low EGFR normal lung epithelial cell line, NL20 (see EGFR expression in Fig. 2A). After quenching cells with Trypan blue (to exclude non-internalized particles), uptake in the cell lines was quantified by flow cytometry. The EGFR+ cancer cells internalized the nanoparticles more than 8-fold over the EGFR-low normal lung cell line, illustrating the preferential targeting of nanoparticles to EGFR+ cells (Fig. 2B). To confirm the engagement of C-NP to EGFR, cancer cells were treated with C-NP, non-targeted nanoparticles, or free cetuximab antibody. Following two hr incubation, cells were washed and analyzed with flow cytometry for cell surface EGFR level. As shown in Fig. 2C–D, the targeted nanoparticles effectively reduced cell surface EGFR level by over 50% in both cell lines when compared with cells treated with non-targeted nanoparticles or non-treated cells. Moreover, C-NP was more effective than free cetuximab antibody despite much lower dose of cetuximab on the nanoparticles (3 μg cetuximab) than free cetuximab (100 μg). This owes to the high density of cetuximab on the nanoparticles (i.e., at 2.7 wt.% and 8.8 × 1013 nanoparticles per gram, there are 1.3×103 antibodies per one nanoparticle) that the cell surfaces were exposed to.
Figure 2. Specific cellular uptake of C-siRNA-NP to EGFR+ cells.
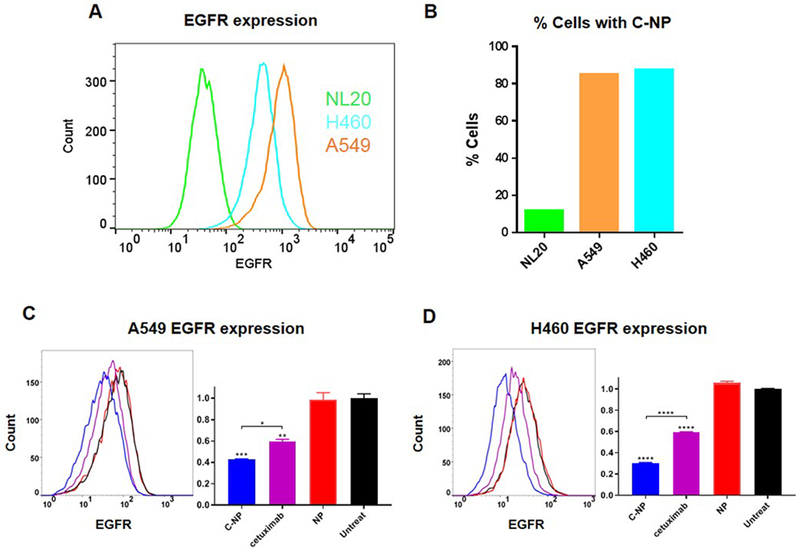
(A) EGFR levels of NSCLC (A549, H460) and normal (NL20) cells by flow cytometry. (B) A fluorescent labeled siRNA (Alexa Fluor 488 siRNA) on C-NP shows higher internalization in EGFR+ NSCLC cells (A549, H460) over normal NL20 lung cells by 8-fold. EGFR surface labeling in (C) A549 and (D) H460 upon incubation with C-NP, cetuximab, or NP in NSCLC cell lines. 100 μg NP or C-NP doses (2.7 μg cetuximab), and 100 μg free cetuximab, were treated; all with 2 hr contact time. Data presented as mean ± SD from independent duplicates (10,000 events per sample); *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
3.3. Efficacy of PLK1 knockdown with C-siPLK1-NP in NSCLC
PLK1 is a key target to treat lung cancer and other cancers [14]. However, effective PLK1 inhibition in the clinics remains elusive. To examine PLK1 silencing efficacy by C-NP in vitro, NSCLC cells were treated with C-NP loaded with siRNA against PLK1 (C-siPLK1-NP) or scrambled siRNA (C-siSCR-NP). The selected siPLK1 sequence was previously screened from four potential sequences and identified to have the best PLK1 knockdown efficacy [13]. As shown in Fig. 3, C-siPLK1-NP effectively knocked down >80% of PLK1 mRNA (Fig. 3A) and reduced > 90% of PLK1 protein expression (Fig. 3B) in both EGFR+ lung cancer cells, while the scrambled siRNA nanoparticle had no effect. Knockdown of PLK1 also led to reduction of other key cancer genes including PI3K, phospho-AKT, and phospho-STAT3, in agreement with previous reports studying PLK1 inhibition [41–43] (Supplementary Fig. S2). The consequence of PLK1 knockdown in the NSCLC cells resulted in significant loss of cell viability (Fig. 3C). The viability of cells treated with C-siPLK1-NP was also significantly lower than cells treated with the non-targeted siPLK1-NP (Supplementary Fig. S3A–B). Furthermore, PLK1 knockdown resulted in the accumulation of cells in G2/M phase of cell cycle, similar to the effect of the PLK1 inhibitor BI2536 (Fig. 3D–E). Inducing G2/M arrest increases the cell’s sensitivity to IR damage, as cells in the G2/M phase are more sensitive to IR than cells in G1 or S phase [44]. Based on this, we determined the time point in which PLK1 knockdown resulted in the highest accumulation of cells in G2/M (Supplementary Fig. S3C–D). G2/M arrest induced by PLK1 knockdown was first observed at 24 hr and increased up to 72 hr post treatment. We used the 72 hr time point in subsequent studies to assess the efficacy of C-siPLK1-NP as a radiation sensitizer.
Figure 3. Effects of C-siPLK1-NP treatment on NSCLC (A549, H460) cell lines.

(A) 48-hr PLK1 mRNA knockdown (HPRT used as house-keeping gene) and (B) 72-hr PLK1 protein reduction at 50 nM siRNA dose in A549 and H460. (C) 4-day cell viability at 30 nM siRNA dose in A549 and H460. Data presented as mean ± SD from 3–4 independent samples; ***P<0.001, ****P<0.0001 vs. siSCR control. Cell cycle arrest increase in G2/M phase in (D) A549 and (E) H460 72 hr post treatment of C-siPLK1-NP (50 nM as siRNA) or BI2536 (PLK1 inhibitor, 10 nM). Data presented as mean ± SD from independent duplicates (10,000 events per sample); ****P<0.0001 vs. untreat control.
3.4. Specificity and efficacy of cetuximab conjugated nanoparticles in other EGFR+ cancers
In addition to NSCLC, EGFR and PLK1 overexpression are observed in other cancers including breast, colorectal, and head and neck cancers. In this regard, C-siPLK1-NP also has great promise to serve as a therapeutic and radiation sensitizer for these cancers. To further investigate targeting specificity of C-NP, we used a panel of breast cancer cells with low to high EGFR expression (Supplementary Fig. S4A). As reported in [45] and shown in Supplementary Fig. S4B, low EGFR expressing cancer cells (KPL4 and MCF7) still take up C-NP but not to the same extent as the medium (BT549 and MDAMB231) or high (MDAMB468) EGFR expressing cancer cells. The phenotype specificity of C-siPLK1-NP was also assessed using a panel of colorectal cancer cells with varying EGFR and PLK1 expression levels (Supplementary Fig. S5A). As shown in Supplementary Fig. S5B, both EGFR and PLK1 expression levels play a role in phenotype specificity of C-siPLK1-NP. When both EGFR and PLK1 expression are similar, a similar response is observed (see HCT29 and HCT116). While, when PLK1 expression is similar, higher EGFR expression leads to superior responses (see SW48 vs. SW480). Importantly, C-siPLK1-NP significantly reduced cell viability in all EGFR+ cancer cell lines tested, which illustrates the broad applicability of C-siPLK1-NP to treat various types of EGFR+ cancer as well as cancer with heterogeneous levels of EGFR such as NSCLC.
3.5. Targeted nano-therapeutic enhances IR damage in vitro
The efficacy of the nanoparticles as a radiation sensitizer was assessed in vitro by established assays: clonogenic survival, γH2ax induction, and apoptosis (Fig. 4). A549 cells were treated with C-siPLK1-NP, C-siSCR-NP, or PBS for 72 hr and irradiated at 2, 4, and 6 Gy. As shown in Fig. 4A, C-siPLK1-NP alone reduced colony formation by 60% and when combined with 2 Gy was more effective than 2 Gy and 4 Gy IR alone and resulted in just 10% survival. The radiosensitizing effect was also enhanced with higher IR doses (Fig. 4B). We also determined the synergy of the combination using the Chou-Talalay method [46]. The clonogenic survival dose response curves of C-siPLK1-NP alone, IR alone, and their combination are shown in Supplementary Fig. S6A–B. The combination index (CI) of C-siPLK1-NP (50 nM as siPLK1) and IR indicates a strong synergistic effect (CI ranged from 0.3-0.5) at all doses tested (2-6 Gy) (Supplementary Fig. 6C). Complementary to the clonogenic survival, pre-treated cells were irradiated and plated for 7 days to assess cell viability by CellTiter-Glo (CTG) assay. As shown in Supplementary Fig. S6D, cells that received a combination of IR and either nanoparticle (C-siSCR-NP or C-siPLK1-NP) were significantly less viable than those exposed to the single treatments (nanoparticles or IR alone). Similar results were obtained with H460 cell line, which has moderate EGFR level compared to high-EGFR A549 cell line (Supplementary Fig. S7). Additionally, we assessed γH2ax foci induction and apoptosis (24 hr post IR or 4 days post NP treatment). H2ax is phosphorylated in response to DNA damaging agents (e.g. chemo or IR) and thus can be used as a marker to assess DNA damage caused by treatments, in particular double strand breaks [47]. As shown in Fig. 4C, IR, C-siSCR-NP, siPLK1-NP, or C-siPLK1-NP alone induced γH2ax foci, indicating that all have intrinsic DNA damage ability. However, the highest foci induction was achieved with C-siPLK1-NP in combination with 6 Gy IR. Moreover, by comparing initial DNA damage (at 1 hr post IR) and repair kinetics (at 24 hrs), we find that both siPLK1 and cetuximab on the nanoparticles hindered the cancer’s ability to repair DNA damage induced by IR (Supplementary Figure S6E). This illustrates the therapeutic benefit of cetuximab (reducing DNA repair capacity) on the nanoparticles in addition to its targeting to EGFR+ cells (shown in Fig. 2B). In addition, Annexin V/PI staining was used to confirm apoptotic cell death in response to treatment. The combination of C-siPLK1-NP and IR resulted in over 50% of cells in late apoptosis (Annexin+/PI+), compared with 33% and 7% for C-siPLK1-NP or IR alone, respectively (Fig. 4D).
Figure 4. C-siPLK1-NP sensitizes A549 lung cancer cells to radiation.
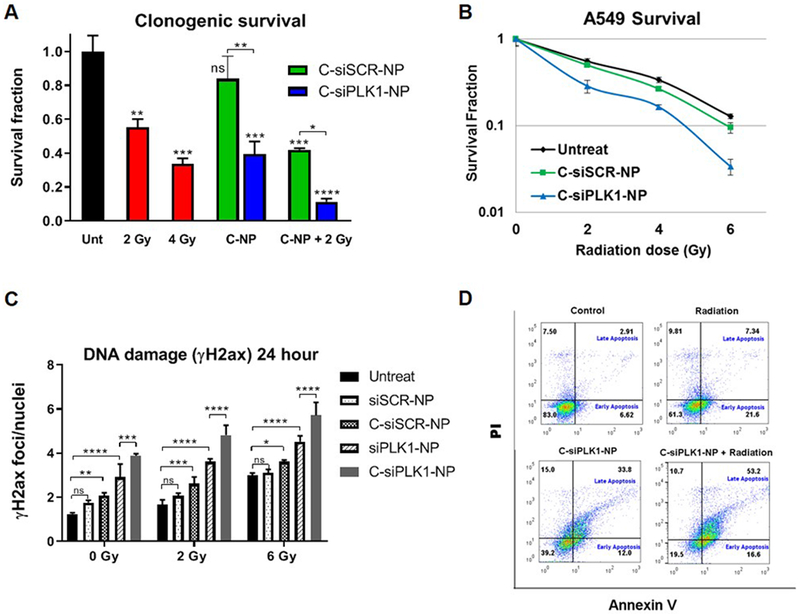
Cells were treated with C-siSCR-NP or C-siPLK1-NP (50 nM as siRNA) for 72 hrs followed by 2-6 Gy irradiation and re-plated for clonogenic survival assay (A-B). (C) γH2ax foci induction at 24 hr post irradiation (0, 2, or 6 Gy). Non-target NP or C-NP with siPLK1 or siSCR (30 nM) were treated 72 hr prior to irradiation. Data presented as mean ± SD from 3–4 independent samples (9 images per sample); *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. (G) Annexin/PI staining 24 hr post 6 Gy irradiation (10,000 events per sample).
3.6. C-siPLK1-NP enhances IR sensitivity in vivo
To investigate the combination of C-siPLK1-NP with IR in vivo, we chose a well-controlled easy-to-irradiate mouse model in which A549 lung cancer cells (5 million) were inoculated in both flanks of Nude SCID mice (two tumors per mouse). When tumors reached ~120 mm3, we intratumorally injected saline, C-siSCR-NP, or C-siPLK1-NP to both tumors on each mouse (at 0.3 nmol siRNA per tumor, once a week). At 72 hr post treatment, 2 Gy IR was administered to the left tumor (see Fig. 5A) using a small animal x-ray irradiator with a lead shield that exposes only the left flank of the mouse. The treatments were administered for 6 consecutive weeks. As shown in Fig. 5, treatments with C-siPLK1-NP (Fig. 5B) or IR alone (Fig. 5C) slowed down the tumor growth after multiple doses of NP or IR, while the combination of IR and C-siPLK1-NP (Fig. 5C) resulted in immediate tumor control and eventual regression of the tumors. Furthermore, tumors that received the combination of C-siSCR-NP and IR had superior tumor control than IR alone (Fig. 5B–C), owing to the IR sensitizing effects of cetuximab, as previously discussed. Two weeks post last IR dose, mice were sacrificed and tumors were weighed and harvested for mRNA analysis. A significant reduction in tumor weight was observed for mice treated with either nanoparticle (C-siSCR-NP or C-siPLK1-NP) in combination with IR (Fig. 5D). As shown in Fig. 5E, tumors treated with C-siPLK1-NP and IR had significantly less PLK1 mRNA than saline treated or IR treated tumors, confirming that tumor reduction was due to PLK1 knockdown. In all, our in vitro and in vivo findings demonstrate the potential of C-siPLK1-NP as a radiosensitizer.
Figure 5. C-siPLK1-NP enhances radiation effects in vivo.
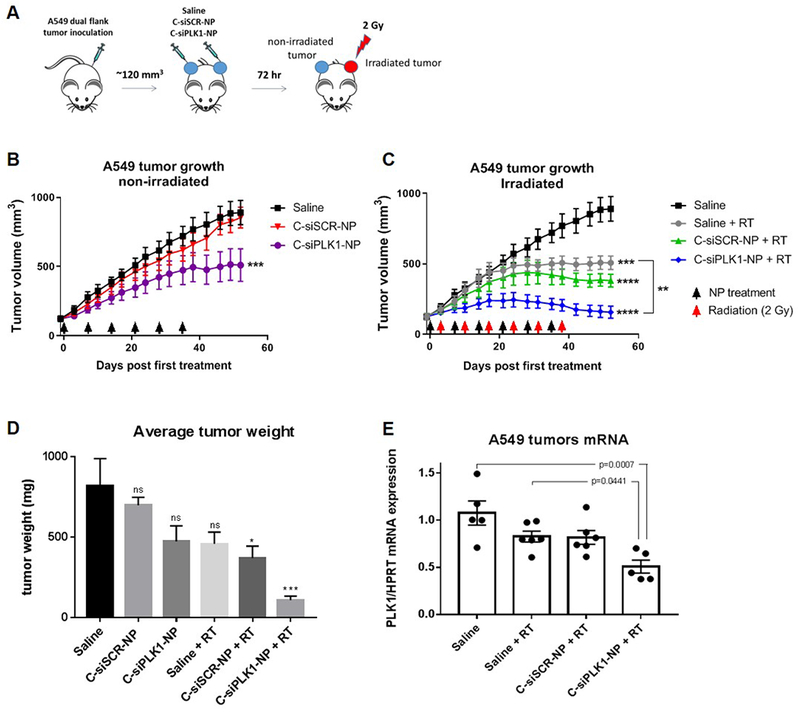
(A) 5 million A549 tumor cells were inoculated in both flanks of SCID mice. Treatments (0.3 nmol siRNA per tumor, once a week) and radiation (2 Gy to the left tumor only; 72 hrs post treatments with nanoparticles) were administered for 6 weeks (n=7). Growth of (B) non-irradiated tumors and (C) irradiated tumors in A549 tumor bearing mice treated with saline, C-siSCR-NP, or C-siPLK1-NP. (D) Average tumor weight at sacrifice (day 52 post first treatment; two weeks post last radiation dose). (E) PLK1 mRNA expression of the tumors from (D). Arrows indicate treatment dates. Data presented as mean ± SEM; *P<0.05, **P<0.01, ***P<0.001, ****p<0.0001 .
3.7. Efficacy of C-siPLK1-NP in an orthotopic lung tumor model
To assess the translational potential of the nano-therapeutic as a systemic therapy, we developed an orthotopic lung tumor model by a non-surgical intratracheal instillation procedure (Fig. 6). A549 cancer cells expressing luciferase (5 million) were injected through the trachea in anesthesized mice using gavage needles with rounded tips. Tumor growth signal was monitored by bioluminescence using in vivo imaging system (IVIS), and upon sacrifice, large tumor nodes are macroscopically visible confirming the presence of tumor in lungs (Fig. 6A). Three weeks after tumor inoculation, mice were grouped and injected weekly with saline, C-siSCR-NP, or C-siPLK1-NP intravenously (Fig. 6B). Tumor growth was monitored weekly by luminescent signal of mice in prone and supine positions using IVIS. As shown in Fig. 6C–D, C-siPLK1-NP significantly reduced the growth of the orthotopic tumors after 8 administrations at a dose of 0.5 mg siRNA/kg animal once per week. Furthermore, mice exhibited no weight loss during treatments (Fig. 6E), indicating the safety of the nanoparticle platform, which is in agreement with our prior work. Extended tumor control after the last treatment was also observed for mice treated with C-siPLK1-NP (Fig. 6F), which led to prolonged survival compared with mice treated with C-siSCR-NP or saline (Fig. 6G). This confirms our in vitro findings that C-siPLK1-NP is effective as a single agent therapeutic for NSCLC, and demonstrates the safety and efficacy of the platform, and its potential to serve as a targeted therapy for lung cancer. As previously demonstrated, such efficacy is expected to signficantly increase when combining with radiation therapy. The follow-on work will combine C-siPLK1-NP and IR using this orthotopic lung tumor model with a clinically relevant scheme, such as 1 injection of C-siPLK1-NP followed by 5 fractions of 2 Gy given over 5 consecutive days.
Figure 6. C-siPLK1-NP reduces orthotopic lung tumor growth.
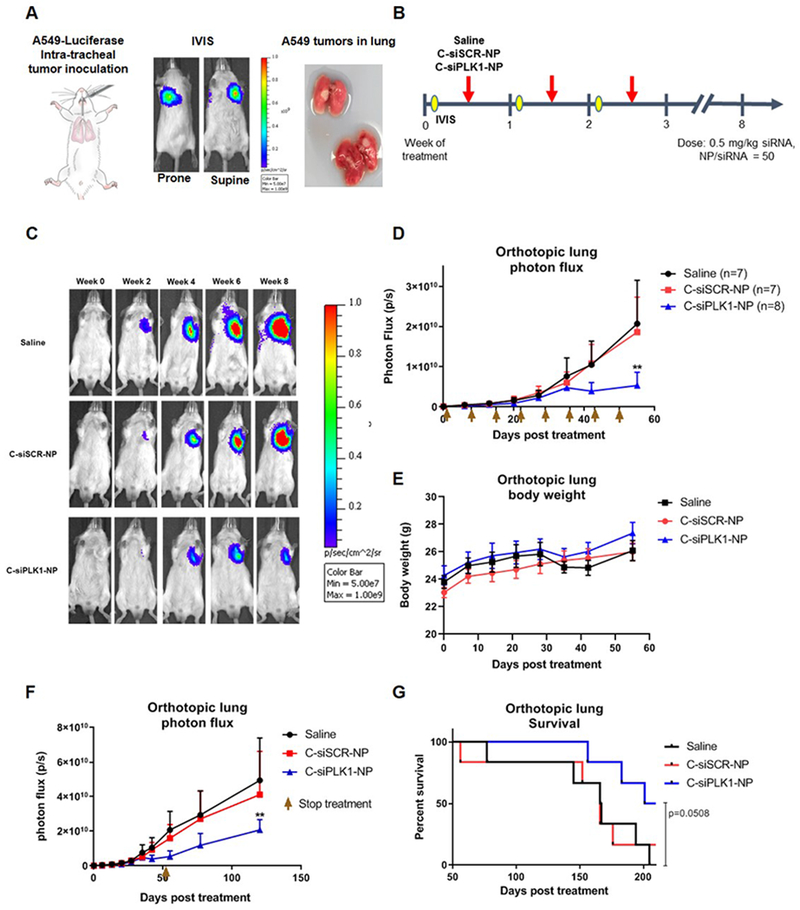
(A) In vivo images showing luminescent signal of A549-Luc tumors from intratracheal inoculation, which was confirmed with the presence of tumor nodes in the lungs. (B) Once tumors were established (3 weeks post inoculation), mice were treated with saline, C-siSCR-NP, or C-siPLK1-NP once per week at 0.5 mg/kg siRNA dose for 8 weeks. (C) IVIS imaging for C-siPLK1-NP, C-siSCR-NP, or saline treated mouse in supine position over the course of treatment. (D) Lung tumor growth determined by average photon flux of prone and supine position for each mouse (n=7–8). Arrows indicate treatment days. (E) Body weight of mice during NP treatment administration. (F) Orthotopic lung tumor growth during and after completion of treatments (marked by arrow). Data presented as mean + SEM, *P<0.05, **P<0.01 vs. saline. (G) Kaplan-Meier Survival curve showing extended survival for mice treated with C-siPLK1-NP (n=6), P=0.0508 vs. saline. Copyright permission obtained from Encapsula NanoSciences for mouse cartoon in (A).
4. DISCUSSION
Molecularly targeted therapeutics that can enhance the effects of IR have potential to benefit millions of cancer patients who receive radiation therapy. Herein, we have developed a novel radiation sensitizer based on a mesoporous silica nanoparticle (MSNP) platform. By conjugating an EGFR-antibody to MSNP and delivering PLK1 siRNA, we show that the nano-therapeutic can effectively target NSCLC cells to initiate cell death and sensitize tumor cells to IR.
The majority of lung cancer patients are diagnosed at advanced disease stages and require systemic therapy to relieve symptoms and prolong survival [2]. Platinum based chemotherapeutics remain the standard of care, but have limited efficacy and carry significant side effects [48]. For patients who harbor a mutated epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) translocation, targeted therapy with EGFR or ALK inhibitors may be administered. While such targeted therapies have dramatically improved outcomes for some patients, one drawback is that they are prone to resistance [49]. Furthermore, most NSCLC patients do not harbor EGFR or ALK abnormalities and there are no targeted therapies for KRAS and many other identified or unknown oncogenic drivers. Immunotherapy, targeting programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1), has shown promising results but still benefits just a minority of patients [50, 51]. Effective systemic therapy with minimal side effects is consequently an area of unmet clinical need.
In this research, we focused on wild type EGFR and KRAS mutant NSCLC (e.g. A549, H460) since there are no current targeted therapies for this patient subgroup (over 30% of lung adenocarcinomas) [52]. However, we anticipate the therapeutic to be applicable to any cancer patient whose tumors have high EGFR expression such as lung, breast, colon, glioblastoma, and head and neck cancers [53], and in particular for patients receiving radiation therapy. Radiation therapy is currently administered to the majority of lung cancer patients in various stages of disease. For unresectable locally advanced NSCLC, the standard of care consists of conventional external beam IR (30 fractions of 2 Gy each) with concurrent chemotherapy – which carries significant toxicity. Furthermore, the role of radiation therapy for lung cancer patients continues to expand with new technologies and techniques. For example, stereotactic body radiotherapy, which allows for the delivery of high doses of IR per fraction (e.g. 5 fractions of 10 Gy each), has shown promise as an alternative approach to surgical resection for early stage lung cancer patients [54]. Thus, the targeted radiation sensitizer we have developed here will potentially benefit lung cancer patients in all disease stages.
While EGFR antibodies and inhibitors are established drugs for patients with EGFR mutations, PLK1 inhibitors have been plagued by low tumor bioavailability and dose limiting toxicities. We expect that C-siPLK1-NP can overcome these current limitations of PLK1 inhibitors in the clinics. SiRNA knockdown of PLK1 may be advantageous over antibodies and inhibitors because it orchestrates its effect at the mRNA level instead of the protein level, which may overcome certain resistance mechanisms. For instance, we have previously reported that siRNA can overcome both intrinsic and acquired resistance of HER2+ cancer cells to small molecules or antibodies targeting the same protein [12]. In addition we also found that cancer was not prone to develop resistance to siRNA as they would to small molecule inhibitors or antibodies [55]. The nanoparticle construct can also improve tumor bioavailability via the enhanced permeability and retention effect [56]. Furthermore, cancer cell targeting by cetuximab on the nanoparticles would reduce off-target effects to healthy cells.
Ultimately, we envision that the application of EGFR-antibody conjugated nanoparticle for delivering siPLK1 will be impactful as a lung cancer treatment in 1) patients with KRAS or other mutations for which there are currently no targeted therapies, 2) combination with radiation therapy to increase sensitivity and as a result, reduce doses and toxic side effects, and 3) overcoming cancer resistance and relapse by effectively targeting PLK1 of cancer stem cells as shown in previous reports [21–23]. In our prior work, we reported on the MSNP platform’s overall safety, biocompatibility, long-term storage and stability [39], as well as efficacy in multiple breast cancer models [11–13]. Thus, the platform is already well positioned to advance to clinical trials. Our findings herein illustrate that C-siPLK1-NP has great potential to serve as a potent radiation sensitizer and to meet the clinical need of an effective therapeutic against PLK1, which is a key target to defeat cancer. In addition, we show that co-targeting both EGFR and PLK1 is a highly effective strategy to enhance IR sensitivity, which warrants further investigation for all EGFR expressing cancers as aforementioned.
Supplementary Material
HIGHLIGHTS.
We report novel cetuximab conjugated nanoparticle delivering PLK1 siRNA (C-siPLK1-NP)
C-siPLK1-NP can treat NSCLC as a single agent and sensitize the cancer to radiation
Systemic treatment reduces orthotopic lung tumor growth in mice and prolongs survival
C-siPLK1-NP also has potential application in EGFR+ breast and colorectal cancer
ACKNOWLEDGEMENTS
We thank Dr. Monica Hinds and Dr. Sudarshan Anand for their inputs and recommendations on experiments and reviewing of the data. We thank Dr. Charles Thomas and Dr. Jerry Jaboin for providing clinical perspective which aided formulation of studies. Thanks to Amy Wells for her help with intratracheal injection to develop orthotopic lung tumors. We thank Dr. Tania Vu for her independent review of data.
FINANCIAL SUPPORT: This work was funded by NIH/NCI grant# R44CA217534 (Ngamcherdtrakul/Yantasee/Gray), NIH/NCATS grant# R43TR001906 (Ngamcherdtrakul/Yantasee), Gordon Moore foundation (Gray), the Prospect Creek Foundation (Gray/Yantasee), Hillcrest Committee Pilot Award (Yantasee), and OHSU/OSU Cancer Prevention and Control Initiative (Horizon) Pilot (Yantasee).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: OHSU, WN, DSB, JWG, and WY have a significant financial interest in PDX Pharmaceuticals, LLC, a company that may have a commercial interest in the results of this research and technology. This potential personal and institutional conflict of interest has been reviewed and managed by OHSU.
5 REFERENCES
- [1].American Cancer Society. Cancer Facts & Figures, (2018).
- [2].Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A, Cancer treatment and survivorship statistics, 2016, CA: A Cancer Journal for Clinicians, 66 (2016) 271–289. [DOI] [PubMed] [Google Scholar]
- [3].Baker S, Dahele M, Lagerwaard FJ, Senan S, A critical review of recent developments in radiotherapy for non-small cell lung cancer, Radiation Oncology, 11 (2016) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schaue D, McBride WH, Opportunities and challenges of radiotherapy for treating cancer, Nat Rev Clin Oncol, 12 (2015) 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomized, two-by-two factorial phase 3 study, The Lancet. Oncology, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ahmad SS, Crittenden MR, Tran PT, Kluetz PG, Blumenthal GM, Bulbeck H, Baird RD, Williams KJ, Illidge T, Hahn S, Lawrence TS, Spears PA, Walker AJ, Sharma RA, Clinical Development of Novel Drug-Radiotherapy Combinations, Clinical Cancer Research, (2018) clincanres.2466.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Decker RH, Gettinger SN, Glazer PM, Wilson LD, Vorinostat, a Histone Deacetylase Inhibitor, in Combination with Thoracic Radiotherapy in Advanced Non-small Cell Lung Cancer: A Dose Escalation Study, International Journal of Radiation Oncology • Biology • Physics, 81 (2011) S574–S575. [Google Scholar]
- [8].Rengan R, Mick R, Pryma DA, Lin LL, Plastaras J, Simone CB II, Gupta A, Evans TL, Stevenson J, Langer C, Kucharczuk J, Friedberg JS, Lam S, Patsch D, Hahn SM, Maity A, Long-term Results of a Phase I/II Trial of Nelfinavir with Concurrent Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer, International Journal of Radiation Oncology • Biology • Physics, 102 (2018) S19. [Google Scholar]
- [9].Deutsch E, Le Pechoux C, Faivre L, Rivera S, Tao Y, Pignon JP, Angokai M, Bahleda R, Deandreis D, Angevin E, Hennequin C, Besse B, Levy A, Soria JC, Phase I trial of everolimus in combination with thoracic radiotherapy in non-small-cell lung cancer, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 26 (2015) 1223–1229. [DOI] [PubMed] [Google Scholar]
- [10].Wozniak AJ, Moon J, Thomas CR Jr., Kelly K, Mack PC, Gaspar LE, Raben D, Fitzgerald TJ, Pandya KJ, Gandara DR, A Pilot Trial of Cisplatin/Etoposide/Radiotherapy Followed by Consolidation Docetaxel and the Combination of Bevacizumab (NSC-704865) in Patients With Inoperable Locally Advanced Stage III Non-Small-Cell Lung Cancer: SWOG S0533, Clinical lung cancer, 16 (2015) 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ngamcherdtrakul W, Morry J, Gu S, Castro DJ, Goodyear SM, Sangvanich T, Reda MM, Lee R, Mihelic SA, Beckman BL, Hu Z, Gray JW, Yantasee W, Cationic Polymer Modified Mesoporous Silica Nanoparticles for Targeted SiRNA Delivery to HER2+ Breast Cancer, Advanced functional materials, 25 (2015) 2646–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gu S, Hu Z, Ngamcherdtrakul W, Castro DJ, Morry J, Reda MM, Gray JW, Yantasee W, Therapeutic siRNA for drug-resistant HER2-positive breast cancer, Oncotarget, 7 (2016) 14727–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morry J, Ngamcherdtrakul W, Gu S, Reda M, Castro DJ, Sangvanich T, Gray JW, Yantasee W, Targeted treatment of metastatic breast cancer by PLK1 siRNA delivered by an antioxidant nanoparticle platform, Molecular cancer therapeutics, (2017) DOI: 10.1158/1535-7163.mct-1116-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu Z, Sun Q, Wang X, PLK1, A Potential Target for Cancer Therapy, Translational oncology, 10 (2016) 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang ZX, Xue D, Liu ZL, Lu BB, Bian HB, Pan X, Yin YM, Overexpression of polo-like kinase 1 and its clinical significance in human non-small cell lung cancer, The international journal of biochemistry & cell biology, 44 (2012) 200–210. [DOI] [PubMed] [Google Scholar]
- [16].Cheng MW, Wang BC, Weng ZQ, Zhu XW, Clinicopathological significance of Polo-like kinase 1 (PLK1) expression in human malignant glioma, Acta histochemica, 114 (2012) 503–509. [DOI] [PubMed] [Google Scholar]
- [17].Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K, Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck, Cancer research, 59 (1999) 2794–2797. [PubMed] [Google Scholar]
- [18].Inoue M, Yoshimura M, Kobayashi M, Morinibu A, Itasaka S, Hiraoka M, Harada H, PLK1 blockade enhances therapeutic effects of radiation by inducing cell cycle arrest at the mitotic phase, Scientific reports, 5 (2015) 15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gutteridge RE, Ndiaye MA, Liu X, Ahmad N, Plk1 Inhibitors in Cancer Therapy: From Laboratory to Clinics, Molecular cancer therapeutics, 15 (2016) 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, Singh R, Wang L, Nilsson M, Goonatilake R, Tong P, Li L, Giri U, Villalobos P, Mino B, Rodriguez-Canales J, Wistuba I, Wang J, Heymach JV, Johnson FM, Polo-like kinase 1 inhibition diminishes acquired resistance to epidermal growth factor receptor inhibition in non-small cell lung cancer with T790M mutations, Oncotarget, 7 (2016) 47998–48010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Francescangeli F, Patrizii M, Signore M, Federici G, Di Franco S, Pagliuca A, Baiocchi M, Biffoni M, Ricci Vitiani L, Todaro M, De Maria R, Zeuner A, Proliferation state and polo-like kinase1 dependence of tumorigenic colon cancer cells, Stem cells (Dayton, Ohio), 30 (2012) 1819–1830. [DOI] [PubMed] [Google Scholar]
- [22].Danovi D, Folarin A, Gogolok S, Ender C, Elbatsh AM, Engstrom PG, Stricker SH, Gagrica S, Georgian A, Yu D, U KP, Harvey KJ, Ferretti P, Paddison PJ, Preston JE, Abbott NJ, Bertone P, Smith A, Pollard SM, A high-content small molecule screen identifies sensitivity of glioblastoma stem cells to inhibition of polo-like kinase 1, PloS one, 8 (2013) e77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu K, Law JH, Fotovati A, Dunn SE, Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor-initiating cells, Breast cancer research : BCR, 14 (2012) R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Braud F, Cascinu S, Spitaleri G, Pilz K, Clementi L, Liu D, Sikken P, De Pas T, A phase I, dose-escalation study of volasertib combined with nintedanib in advanced solid tumors, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 26 (2015) 2341–2346. [DOI] [PubMed] [Google Scholar]
- [25].Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P, Munzert G, A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours, European journal of cancer (Oxford, England : 1990), 48 (2012) 179–186. [DOI] [PubMed] [Google Scholar]
- [26].Lin CC, Su WC, Yen CJ, Hsu CH, Su WP, Yeh KH, Lu YS, Cheng AL, Huang DC, Fritsch H, Voss F, Taube T, Yang JC, A phase I study of two dosing schedules of volasertib (BI 6727), an intravenous polo-like kinase inhibitor, in patients with advanced solid malignancies, British journal of cancer, 110 (2014) 2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Frost A, Mross K, Steinbild S, Hedbom S, Unger C, Kaiser R, Trommeshauser D, Munzert G, Phase i study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours, Current oncology (Toronto, Ont.), 19 (2012) e28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gjertsen BT, Schoffski P, Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy, Leukemia, 29 (2015) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ingelheim B, Results of Phase III study of volasertib for the treatment of acute myeloid leukemia presented at European Hematology Association Annual Meeting, Ridgefield, Conn., 2016. [Google Scholar]
- [30].Ellis PM, Leighl NB, Hirsh V, Reaume MN, Blais N, Wierzbicki R, Sadrolhefazi B, Gu Y, Liu D, Pilz K, Chu Q, Randomized A, Open-Label Phase II Trial of Volasertib as Monotherapy and in Combination With Standard-Dose Pemetrexed Compared With Pemetrexed Monotherapy in Second-Line Treatment for Non-Small-Cell Lung Cancer, Clinical lung cancer, 16 (2015) 457–465. [DOI] [PubMed] [Google Scholar]
- [31].Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, Mossetti C, Ardissone F, Lausi P, Scagliotti GV, Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 15 (2004) 28–32. [DOI] [PubMed] [Google Scholar]
- [32].Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, Gonzalez-Guerrero JF, Rodriguez-Fernandez IA, Verdines-Perez A, Templeton AJ, Ocana A, Seruga B, Tannock IF, Amir E, Vera-Badillo FE, Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis, Cancer treatment reviews, 62 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [33].Alterio D, Marvaso G, Maffini F, Gandini S, Chiocca S, Ferrari A, Preda L, Rocca MC, Lepanto D, Fodor C, Volpe S, Dicuonzo S, Laudati A, Giugliano G, Ansarin M, Jereczek-Fossa BA, Role of EGFR as prognostic factor in head and neck cancer patients treated with surgery and postoperative radiotherapy: proposal of a new approach behind the EGFR overexpression, Medical oncology (Northwood, London, England), 34 (2017) 107. [DOI] [PubMed] [Google Scholar]
- [34].Galizia G, Lieto E, Ferraraccio F, De Vita F, Castellano P, Orditura M, Imperatore V, La Mura A, La Manna G, Pinto M, Catalano G, Pignatelli C, Ciardiello F, Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery, Annals of surgical oncology, 13 (2006) 823–835. [DOI] [PubMed] [Google Scholar]
- [35].Nakamura H, Kawasaki N, Taguchi M, Kabasawa K, Survival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: a meta-analysis, Thorax, 61 (2006) 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fujino S, Enokibori T, Tezuka N, Asada Y, Inoue S, Kato H, Mori A, A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer, European Journal of Cancer, 32 (1996) 2070–2074. [DOI] [PubMed] [Google Scholar]
- [37].Chen DJ, Nirodi CS, The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage, Clin Cancer Res, 13 (2007) 6555–6560. [DOI] [PubMed] [Google Scholar]
- [38].Nyati MK, Morgan MA, Feng FY, Lawrence TS, Integration of EGFR inhibitors with radiochemotherapy, Nature reviews. Cancer, 6 (2006) 876–885. [DOI] [PubMed] [Google Scholar]
- [39].Ngamcherdtrakul W, Sangvanich T, Reda M, Gu S, Bejan D, Yantasee W, Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery, Int J Nanomedicine, 13 (2018) 4015–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC, Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation, Journal of immunology (Baltimore, Md. : 1950), 175 (2005) 3369–3376. [DOI] [PubMed] [Google Scholar]
- [41].Li Z, Li J, Bi P, Lu Y, Burcham G, Elzey BD, Ratliff T, Konieczny SF, Ahmad N, Kuang S, Liu X, Plk1 phosphorylation of PTEN causes a tumor-promoting metabolic state, Molecular and cellular biology, 34 (2014) 3642–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abdelfatah S, Berg A, Huang Q, Yang LJ, Hamdoun S, Klinger A, Greten HJ, Fleischer E, Berg T, Wong VKW, Efferth T, MCC1019, a selective inhibitor of the Polo-box domain of Polo-like kinase 1 as novel, potent anticancer candidate, Acta Pharmaceutica Sinica B, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yan W, Yu H, Li W, Li F, Wang S, Yu N, Jiang Q, Plk1 promotes the migration of human lung adenocarcinoma epithelial cells via STAT3 signaling, Oncology letters, 16 (2018) 6801–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pawlik TM, Keyomarsi K, Role of cell cycle in mediating sensitivity to radiotherapy, Int J Radiat Oncol Biol Phys, 59 (2004) 928–942. [DOI] [PubMed] [Google Scholar]
- [45].Ngamcherdtrakul W, Sangvanich T, Goodyear S, Reda M, Gu S, Castro DJ, Punnakitikashem P, Yantasee W, Lanthanide-Loaded Nanoparticles as Potential Fluorescent and Mass Probes for High-Content Protein Analysis, Bioengineering (Basel), 6 (2019) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chou TC, Drug combination studies and their synergy quantification using the Chou-Talalay method, Cancer Res, 70 (2010) 440–446. [DOI] [PubMed] [Google Scholar]
- [47].Sharma A, Singh K, Almasan A, Histone H2AX phosphorylation: a marker for DNA damage, Methods in molecular biology (Clifton, N.J.), 920 (2012) 613–626. [DOI] [PubMed] [Google Scholar]
- [48].Fennell DA, Summers Y, Cadranel J, Benepal T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C, Ferry D, Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer, Cancer treatment reviews, 44 (2016) 42–50. [DOI] [PubMed] [Google Scholar]
- [49].Maione P, Sacco PC, Sgambato A, Casaluce F, Rossi A, Gridelli C, Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application, Therapeutic advances in medical oncology, 7 (2015) 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr., Wu YL, Paz-Ares L, Lung cancer: current therapies and new targeted treatments, Lancet, 389 (2017) 299–311. [DOI] [PubMed] [Google Scholar]
- [51].Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer, 375 (2016) 1823–1833. [DOI] [PubMed] [Google Scholar]
- [52].Cancer N Genome Atlas Research, Comprehensive molecular profiling of lung adenocarcinoma, Nature, 511 (2014) 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yewale C, Baradia D, Vhora I, Patil S, Misra A, Epidermal growth factor receptor targeting in cancer: A review of trends and strategies, Biomaterials, 34 (2013) 8690–8707. [DOI] [PubMed] [Google Scholar]
- [54].Ceniceros L, Aristu J, Castanon E, Rolfo C, Legaspi J, Olarte A, Valtuena G, Moreno M, Gil-Bazo I, Stereotactic body radiotherapy (SBRT) for the treatment of inoperable stage I non-small cell lung cancer patients, Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 18 (2016) 259–268. [DOI] [PubMed] [Google Scholar]
- [55].Gu S, Ngamcherdtrakul W, Reda M, Hu Z, Gray JW, Yantasee W, Lack of acquired resistance in HER2-positive breast cancer cells after long-term HER2 siRNA nanoparticle treatment, PloS one, 13 (2018) e0198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nature biotechnology, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


