Abstract
Purpose of review:
Recently published studies have provided new evidence for a role of oral health on risk of cancer. This review summarizes the latest research on this topic, including several new cohort studies that have examined associations on periodontal disease and cancer risk.
Recent findings:
The most consistent findings for associations with periodontal disease have been observed for lung cancer; five out of seven studies have reported statistically significant increases in risk of lung cancer. For pancreatic, colorectal and head and neck cancers, the associations are less consistent across studies, and the overall summary relative risk estimates are not statistically significant. However, these associations remain of interest, given the limitations of existing data (i.e., measurement error in periodontal disease assessment and small sample sizes), and growing support for biological mechanisms on how bacteria previously linked to periodontal disease may play a role in carcinogenesis.
Summary:
Future studies need improved assessment of periodontal disease in population- based studies to determine if heterogeneity of current studies resides with measurement error. Periodontal disease treatment and prevention may turn out to be important targetable cancer prevention strategies.
Keywords: periodontal disease, lung cancer, pancreatic cancer, colorectal cancer, head and neck cancer, Fusobacterium nucleatum, Porphyromonas gingivalis
1. Introduction
The most significant reductions in cancer rates that have occurred over the past century have been tied to changes in prevalence of major risk factors, or to progress made to detect premalignant lesions. The most striking example was the dramatic reduction in lung cancer rates that followed the decrease in smoking prevalence in the U.S. Reductions in cervical and colorectal cancer have also been observed with improved detection of pre-cancer lesions and with population screening. In Taiwan, reductions in liver cancer rates are being reported among cohorts of children vaccinated for hepatitis B virus [1]. Targeting early detection and prevention strategies to reduce cancer burden is critical, and oral health is emerging as a field that may have the potential to contribute to cancer prevention [2]. To evaluate the role of oral health on cancer burden and determine whether oral health prevention strategies may be effective, it is critical to determine whether associations observed in population studies are causal. To date, no randomized clinical trials have been conducted to evaluate whether prevention or treatment of periodontal disease can decrease cancer risk as these studies are not feasible to implement. Consequently, causality can only be evaluated using observational data and by confirming biological mechanisms with animal models; if the evidence is sufficiently strong, recommendations can be made to advocated for prevention through improvements in dental coverage and improved awareness of the risk. This chapter will provide a summary of the current evidence on periodontal disease and cancer risk from observational data, as well as a discussion on biological plausibility for these associations.
2. Observational Studies Linking Periodontal Disease to Cancer
a. Head and Neck Cancers
The first observational studies on oral health and cancer risk focused on oral cancers, based on the physical proximity of the conditions. Numerous case-control studies have reported positive associations between tooth loss and oral cancers, with the earliest studies dating back to the early 1990s [3]. Interest in oral health and cancer subsequently extended to other head and neck sites, including pharyngeal and laryngeal cancers. Overall, findings on periodontal disease and head and neck cancer (HNC) have been inconsistent, with associations ranging from inverse to strongly positive. Inconsistencies in results across studies have raised concerns about study internal validity, that can be affected by measurement errors, selection bias and confounding. Associations from cohort studies, which are less prone to selection bias than retrospective case-control studies, have been weak, with statistically non-significant relative risks ranging from 1.10 to 1.60 [4–7]. However, cohort studies examining cancer with low incidence rates, such as HNC, often have small numbers of cases available and have limited ability to detect lower risks. Furthermore, large cohort studies often have poor measures of periodontal disease, relying on self-reported history of periodontal disease, or reported tooth mobility.
Some of the strongest associations between periodontal disease and HNC were observed in case-control studies that had accurate assessment of periodontal disease status. Using clinical measurements of bone loss [8, 9] and periodontal pocket depth [10] to determine extent of disease, these studies have reported 4–10 fold higher risks of HNC with severe periodontitis [8–10]. While these studies are retrospective and susceptible to selection bias, the strong dose-response associations between exposure and cancer risk are noteworthy, and more research is necessary to determine whether prevention could reduce incidence of HNC.
There is growing interest in the relationship between periodontal disease and human papillomavirus (HPV) infections in the oral cavity, and whether these conditions could interact to impact oral cancer. It has been suggested that periodontal disease may play a role in persistence of HPV infection because about one quarter of gingival biopsies of subjects with periodontal pockets contain HPV [11]. Additionally, higher HPV-positive oral tumors have been observed among individuals with periodontal disease [12], and a recent study reported that Hispanic individuals (with no history of cancer) who had severe periodontal disease were at almost 3-fold higher risk of having HPV-positive oral saliva than those without periodontal disease [13]. Together, these findings highlight the need to investigate the relationship between periodontal disease and HNC by HPV status to further clarify whether these two factors interact to increase the risk of HNC.
b. Lung Cancer
The associations between periodontal disease and lung cancer have been consistent across seven unique prospective cohorts (published from 2003 to 2019) after controlling for potential confounding factors including smoking– the National Health and Nutrition Examination Survey Follow-up Study (NHANES FS)[14], Health Professionals Follow-up Study (HPFS)[15], Swedish twin study [16], Women’s Health Initiative - Observational Study (WHI OS)[17, 4], Buffalo Osteoporosis and Periodontal Disease (OsteoPerio) study[18], Atherosclerosis Risk in Communities (ARIC) study[19] and the Southern Community Cohort Study (SCCS)[20]. It should be noted that the Swedish twin study defined “periodontal disease” using a tooth mobility measure [16]. Figure 1 provides a random-effects meta-analysis of results from these cohort studies; overall, periodontal disease was associated with 40% higher risk of lung cancer (pooled smoking adjusted RR = 1.42, 95% CI 1.27, 1.59) with no significant statistical heterogeneity (I2 = 22%, P = 0.26) (See Technical Appendix for the meta-analysis methods). Given the real concern for confounding by smoking for lung cancer associations, cohort studies that did not adjust for smoking were not included in the meta-analysis (i.e., [21, 6, 7]). The strongest associations have been reported in three cohort studies with detailed dental examinations (NHANES, OsteoPerio and ARIC), highlighting the need for accurate measurement of periodontal disease status to evaluate risk.
Figure 1.
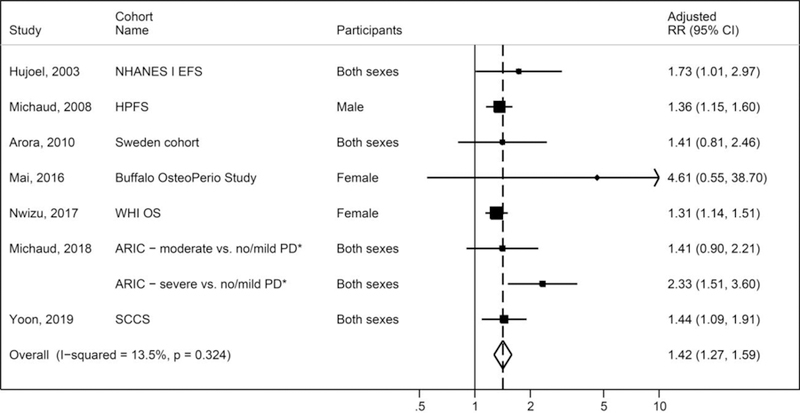
Meta-analysis of associations between periodontal disease and lung cancer in cohort studies
Legends: PD = periodontal disease; RR = relative risks; *ARIC study reported compared moderate to no/mild PD and severe to no/mild PD. The random-effects meta-analysis did not adjust for the correlation between the two adjusted RRs in ARIC study; and thus, the pooled estimate was slightly inflated.
Although a statistically significant 4-fold increase in lung cancer risk was reported among never smokers in the ARIC cohort study [19], no statistically significant associations were reported for never smokers in two other cohort studies [5, 17]. An interesting finding is the possibility that smokers may be at greater risk of developing lung cancer if they have periodontal disease. In the WHI OS cohort, women with high smoking intensity and periodontal disease were at higher risk than those smoking the same amount but with no periodontal disease (HR = 1.29, 95% CI 1.95, 3.87) [17]. In the SCCS cohort, a 2-fold higher risk was reported in heavy smokers with a history of periodontal disease, compared to heavy smokers without periodontal disease (OR = 2.05, 95% CI = 1.38–3.05) [20]. The overall evidence on periodontal disease and lung cancer is considered strong, yet questions remain on causality, and further research is needed to evaluate potential mechanisms and opportunities for cancer prevention through risk stratification.
c. Colorectal Cancer
Findings from seven prospective cohorts (HPFS [15], Swedish twin cohort [16], NHANES III FS [22], Buffalo OsteoPerio study [18], WHI OS [4], Nurses’ Health Study [NHS] [23], and ARIC [19]) showed less consistent, and weaker associations between periodontal disease and colorectal cancer, than those for lung cancer. While results from the Swedish twin study and ARIC study cohorts were of similar magnitude for colorectal cancer (HR = 1.61, 95% CI 1.13, 2.33 in the Sweden cohort; RR = 1.51, 95% CI 0.95, 2.42 for severe periodontitis, compared to no periodontitis in the ARIC cohort) no association was observed in the HPFS (HR = 1.05; 95% 0.90, 1.23), WHI OS cohort study (HR = 0.98, 95% CI 0.82, 1.17) [4] and in the NHS cohort (RR = 0.89, 95% CI 0.72, 1.10) [23]. The NHANES FS and Buffalo OsteoPerio cohorts reported highly uncertain results (wide confidence intervals) due to small number of colorectal cancer cases [22, 18]. In the meta-analysis, the smoking-adjusted results from these cohort studies showed a small elevated risk of colorectal cancer associated with periodontal disease (pooled adjusted RR = 1.11; 95% CI 0.93, 1.33), although the result was not statistically significant with large heterogeneity (I2 = 53.8%, P = 0.027) (Figure 2).
Figure 2.
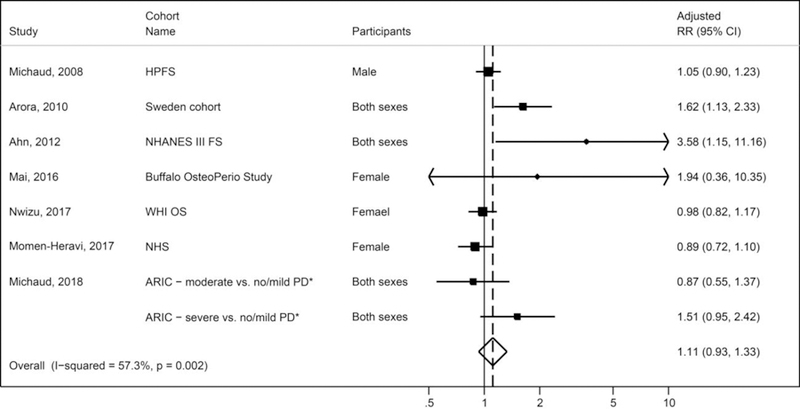
Meta-analysis of associations between periodontal disease and colorectal cancer in cohort studies
Legends: PD = periodontal disease; RR = relative risks; *ARIC study reported compared mild, moderate, or severe PD to no PD. The random-effects meta-analysis did not adjust for the correlations among the three adjusted RRs in ARIC study; and thus, the pooled estimate was slightly inflated
A retrospective cohort study conducted in Taiwan using insurance records of periodontal treatment also reported a higher risk of colorectal cancer (HR = 1.64, 95% CI 1.50, 1.80) [24]; however, this study was not included in the meta-analysis because many known confounding factors such as smoking, alcohol consumption or body mass index were not adjusted in the analyses [25]. Despite variable findings across cohort studies, the associations between periodontal disease and colorectal cancer deserve attention because of the known link between inflammation and colorectal cancer, as well as accumulating data demonstrating a potential role for Fusobacterium nucleatum in colorectal carcinogenesis [26–28]. Importantly, Fusobacterium nucleatum has been associated with periodontal disease; studies have shown that the prevalence of F. nucleatum increases with the severity of disease, progression of inflammation and pocket depth, and animal studies support a causal role in periodontal disease, with added virulence when combined with other periodontal disease pathogens [29]. Furthermore, F. nucleatum can disseminate from the mouth to colonize different body sites, including the colon, cause local inflammation and activate pathways that may impact carcinogenesis [29]. (Discussed also in 3. Biological Plausibility for Periodontal Disease and Cancer section).
d. Pancreatic Cancer
The associations between periodontal disease and pancreatic cancer risk have been examined in seven prospective cohort studies [16, 14, 7, 30, 22, 4, 15]. However, only four of these cohorts (HPFS [15], Sweden twin study [16], NHANES III FS [22], and WHI OS [4]) adjusted for smoking in the analyses. Random-effects meta-analysis combining the smoking-adjusted results from these cohort studies indicates that periodontal disease is associated with a higher risk of pancreatic cancer with similar magnitude as risk of lung cancer (pooled adjusted RR = 1.48; 95% CI 0.92, 1.30), although the result is not statistically significant with large heterogeneity (I2 = 76.7%, P = 0.005) (Figure 3). The three cohort studies that were not included in the meta-analysis (due to lack of adjustment for smoking in their analyses) also consistently showed significant associations between periodontal disease and risk of pancreatic cancer, with relative risks ranging from 1.55 to 2.28 [14, 30, 7], however for these studies, confounding by smoking cannot be ruled out. Only one cohort study (Nwizu et al. 2017), using self-report for periodontal disease assessment, has reported no significant association between self-reported periodontal disease and pancreatic cancer (RR = 0.89, 95% CI 0.68, 1.18) [4]. In contrast to reports for periodontal disease and pancreatic cancer, findings on tooth loss (a possible marker of past severe periodontitis) and pancreatic cancer have been inconsistent, but largely null [3].
Figure 3.
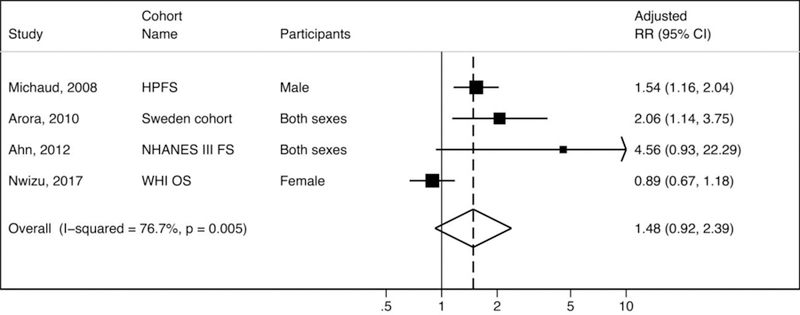
Meta-analysis of associations between periodontal disease and pancreatic cancer in cohort studies
Legends: RR = relative risks
One possible mechanism linking periodontal disease to pancreatic cancer is via oral microbes passing through the oral mucosal barrier and entering into the blood circulation, or translocating to the pancreas by migrating through the stomach and into the duodenum. (Biological mechanisms discussed in section 3. Biological Plausibility for Periodontal Disease and Cancer). Several studies have examined the relation between oral bacteria previously associated with periodontal disease and risk of pancreatic cancer. The first study examined the association between antibodies to periodontal pathogens and risk of pancreatic cancer using blood samples collected prior to cancer diagnosis in a large prospective cohort study [31]. The results showed a greater than 2-fold increase in risk of pancreatic cancer among those subjects with high levels of antibodies to a pathogenic strain of P. gingivalis (OR 2.38, 95% CI 1.16, 4.90, comparing >200 ng/ml vs. <200 ng/ml) after adjusting for known risk factors, including smoking [31]. Another cohort study found a 3-fold higher risk of orodigestive cancer mortality (RR 3.03, 95% CI 0.99, 9.31) among individuals with antibodies to P. gingivalis (>69.1 EU, compared to less than 69.1); unfortunately, small case numbers did not allow for a separate analysis on pancreatic cancer [22]. The largest cohort analysis to date (combining data from two large prospective cohort studies) observed a 1.6-fold increased risk of pancreatic cancer for presence (versus absence) of P. gingivalis taxa measured directly in saliva using 16S RNA genes and a 2-fold increase risk for higher mean of Aggregatibacter actinomycetemcomitans taxa, another periodontal pathogen [32]. This cohort analysis was unique in that saliva samples had been obtained up to 10 years prior to cancer diagnosis, unlike other studies measuring bacterial DNA in saliva in cancer patients [33–35] [32].
e. All Cancers
To date, findings from eight unique cohort studies evaluating the associations between periodontal disease and risk for all cancers combined have been surprisingly consistent [15, 16, 18, 7, 14, 6, 4, 19], with relative risks ranging between 1.14 and 1.55. All individual study results, except one [18], were statistically significant. Unfortunately, three of these studies did not adjust for smoking status [14, 7, 6]; after removing these studies, the highest relative risk is 1.24 [19]. The overall associations between periodontal disease and total cancer risk among never smokers were either weak [4, 5] or null [18]. The differences in the observed relationship between smokers and never smokers (for periodontal disease) may also be due to heterogeneity of types of cancers represented in the smoking groups; smoking-related cancers (e.g., lung and pancreatic cancers) are associated with periodontal disease even among never smokers [5], whereas breast and prostate cancers, which contribute a large proportion of total cancers, are not associated with periodontal disease.
3. Biological Plausibility for Periodontal Disease and Cancer
Biological plausibility for the role of periodontal disease as a causal factor in carcinogenesis, has solidified with mechanistic studies demonstrating how bacteria can impact the immune response and interact with human cells to activate signalling pathways that can lead to carcinogenesis [36]. To date, two key microbial pathobionts, Fusobacterium nucleatum and Porphyromonas gingivalis, appear to play important roles in tumorigenesis and in the development of a cancer-promoting environment. F. nucleatum and P. gingivalis both contribute to molecular and biochemical changes associated with malignancy through similar and distinct mechanisms.
Both F. nucleatum and P. gingivalis are gram-negative bacteria that possess the lipopolysaccharide (LPS) structures on cell surfaces. Gram-negative organisms have been shown to stimulate the innate immune system, leading to increases in nuclear factor kappa B (NF- κB), IL-6, IL-8, and TNF-α, key inflammatory markers that have been implicated in carcinogenesis [37].
F. nucleatum bacteria have been identified in human colorectal cancer (CRC) tissues and studies have demonstrated that patients with higher tumor F. nucleatum load have poorer survival [38]. In experimental studies, mouse xenografts of human primary colorectal adenocarcinomas retained viable Fusobacterium, and antibiotic treatment in these mice resulted in reduction of Fusobacterium load, cancer cell proliferation and overall tumor growth [38]. Similarly, in the ApcMin/+ mouse model of intestinal tumorigenesis, Fusobacterium nucleatum (introduced in food) resulted in higher tumor multiplicity and in the recruitment of tumor-infitrating myeloid cells [27].
Numerous experimental studies have explored how these bacteria alter mechanistic pathways. F. nucleatum expresses an adhesion molecule FadA that contributes to F. nucleatum’s ability to bind endothelial and epithelial cells [37]. This binding has been shown to stimulate growth in human colon cancer cells through the activation of the beta-catenin pathway, which upregulates a multitude of genes associated with cell growth and inflammation [39]. Moreover, FadA gene levels are significantly increased in colon adenoma and carcinoma tissues compared with normal controls [39]. F. nucleatum has also been shown to upregulate a number of intracellular kinases, the majority of which are involved in cell proliferation, cell survival, and DNA repair pathways [40].
Human colon cancer tissues have been found to express higher levels of Gal-GalNAc moieties on their surfaces compared with non-malignant cells [41]. F. nucleatum possesses a Fap2 surface protein that binds to galactose domains, indicating a potential mechanism for invasion in colon tissues. Indeed, compared to P. gingivalis, F. nucleatum exhibits a much greater degree of tumor localization.
Once bound and localized, F. nucleatum promotes tumorigenesis in a variety of ways. Presence of F. nucleatum infection in malignant masses in humans has been previously associated with DNA malfunction in the form of microsatellite instability, CpG island methylation, and mutations in the BRAF and KRAS genes known to promote colorectal cancer [40]. These genetic alterations are thought to be a downstream result of F. nucleatum-mediated pro-inflammatory pathways, including the generation of reactive oxygen species (ROS) and the cytokine cascade mentioned above. Proenca et al. describe microRNA (miRNA) gene products that are upregulated or downregulated upon infection with F. nucleatum, implicating these miRNA mediators in the initiation of the inflammatory cascade [42].
While F. nucleatum is thought to play a role in periodontal disease, P. gingivalis has been characterised as a keystone pathogen for chronic periodontitis, given the strong evidence for pathogenicity [43]. P. gingivalis has been associated with malignancies of the oral cavity, particularly oral squamous cell carcinoma (OSCC) and esophageal squamous cell carcinoma (ESCC)[44, 45]. In the oral cavity, P. gingivalis infection is more prevalent in cancerous tissue than in non-cancerous tissue, and bacterial load is positively correlated with stage of differentiation, lymph node metastasis, and TNM staging of ESCC [45]. Furthermore, in a chemically-induced oral carcinoma mouse model, oral infections with P. gingivalis or F. nucleatum were shown to promote OSCC proliferation [46].
P. gingivalis promotes carcinogenesis primarily through alterations in the cell cycle and immune modulation [40] [47]. P. gingivalis has been shown to prevent apoptosis in gingival epithelial cells through enhancement of the JAK1/STAT pathway and suppression of the Bcl2 family of proteins that normally regulate cell death through the mitochondria [40]. Moreover, P. gingivalis accelerates the progression of oral endothelial cells through the S and G2 phases of the cell cycle by upregulating cyclins A, D, and E, key players in the p53 tumor suppressor pathway [40]. To access epithelial cells, P. gingivalis upregulates expression of several matrix metalloproteinase enzymes, which aid to break down collagen and further enhance the inflammatory, pro-malignant environment [40]. DC-SIGN is a key signaling receptor and immune-modulator for pathogens, particularly through the apoptotic pathway [47]. P. gingivalis induces FOXO silencing through the DC-SIGN pathway, resulting in further inhibition of cellular apoptosis and enhances cell proliferation in the gingival epithelium [47].
Subtypes of P. gingivalis that express fimbriae on their surface are particularly immunosuppressive [48]. P. gingivalis is able to bypass autophagic destruction of immune dendritic cells using its Mfa1 fimbriae, which has also been implicated in changes in gene expression for over 70 dendritic cell genes [48]. P. gingivalis has also been shown to infect monocytes and alter their differentiation, thereby reducing cytotoxic T cell formation [47].
Bacterial DNA from the oral cavity, including bacteria associated with periodontal disease, have been identified in tissue of patients with colorectal cancer [26, 49, 38] and pancreatic cancer [50], and could be directly involved in carcinogenesis, through the mechanisms described above. The biological plausibility of the associations reported provide additional evidence to support a causal link between periodontal disease and cancer risk.
4. Conclusions
Despite the large number of studies that have been published on oral health and cancer in the past decade, many questions remain, and it remains unclear whether periodontal disease prevention and/or treatment can reduce cancer incidence and mortality. Observational studies with strong study designs are urgently needed to address the critical question of whether prevention or treatment for periodontal disease will impact cancer development, recurrence or mortality.
Cancer development takes place over several decades, and exposures that are known to cause cancer often occur decades prior to diagnosis. In animal models, oral bacterial infections with F. nucleatum have been shown to promote the growth of xenografts of human colorectal cancers [38], accelerate tumorigenesis in ApcMin/+ mice [27], and increase proliferation of chemically-induced oral tumors [46], suggesting that these exposures may have a late effect in carcinogenesis. More research is needed to determine how these experiments might translate to carcinogenesis in human. At this time, it is difficult to predict whether treatment of periodontitis would translate to a reduction in cancer burden.
If the associations with oral infections are indeed causal, prevention of periodontal disease will likely have a more pronounced impact on cancer than treatment given the long latency periods seen with cancer. Improving our understanding of the relationship between periodontal disease and other risk factors, as they relate to cancer risk, as well as the identification of potential bacteria that may be involved in carcinogenesis, may also provide new opportunities for early cancer detection (through biomarker discovery) and inform on if active treatment for periodontal disease will reduce cancer burden.
5. Technical Appendix
In this chapter, three random-effects model meta-analyses were performed to combine the smoking-adjusted relative risks of lung, colorectal, and pancreatic cancers comparing individuals with periodontal disease (as defined within the original studies) and those without [51]. Statistical heterogeneity was quantified using both the Q statistic (considered significant when the P value was less than 0.10) and the I2 index. All meta- analyses were conducted using Stata metan package [52], and two-tailed P values less than 0.05 were considered statistically significant.
Acknowledgments
Research supported by the 2018 AACR-Johnson & Johnson Lung Cancer Innovation Science Grants Number 18–90-52-MICH.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Mei Chung, Benjamin R. York, and Dominique S. Michaud each declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016;151(3):472–80 e1. doi: 10.1053/j.gastro.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA. Reducing Cancer Burden in the Population: An Overview of Epidemiologic Evidence to Support Policies, Systems, and Environmental Changes. Epidemiol Rev 2017;39(1):1–10. doi: 10.1093/epirev/mxx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud DS, Fu Z, Shi J, Chung M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol Rev 2017;39(1):49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nwizu NN, Marshall JR, Moysich K, Genco RJ, Hovey KM, Mai X et al. Periodontal Disease and Incident Cancer Risk among Postmenopausal Women: Results from the Women’s Health Initiative Observational Cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2017;26(8):1255–65. doi: 10.1158/1055-9965.EPI-17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2016;27(5):941–7. doi: 10.1093/annonc/mdw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SD, Tsai MC, Huang CC, Kao LT, Chen CH. A population-based study on the associations between chronic periodontitis and the risk of cancer. International journal of clinical oncology 2016;21(2):219–23. doi: 10.1007/s10147-015-0884-6. [DOI] [PubMed] [Google Scholar]
- 7.Heikkila P, But A, Sorsa T, Haukka J. Periodontitis and cancer mortality: Register-based cohort study of 68,273 adults in 10-year follow-up. International journal of cancer Journal international du cancer 2018. doi: 10.1002/ijc.31254. [DOI] [PubMed] [Google Scholar]
- 8.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(9):2406–12. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 9.Moergel M, Kammerer P, Kasaj A, Armouti E, Alshihri A, Weyer V et al. Chronic periodontitis and its possible association with oral squamous cell carcinoma - a retrospective case control study. Head & face medicine 2013;9:39. doi: 10.1186/1746-160X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraes RC, Dias FL, Figueredo CM, Fischer RG. Association between Chronic Periodontitis and Oral/Oropharyngeal Cancer. Brazilian dental journal 2016;27(3):261–6. doi: 10.1590/0103-6440201600754. [DOI] [PubMed] [Google Scholar]
- 11.Hormia M, Willberg J, Ruokonen H, Syrjanen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. Journal of periodontology 2005;76(3):358–63. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Tezal M, Sullivan Nasca M, Stoler DL, Melendy T, Hyland A, Smaldino PJ et al. Chronic periodontitis- human papillomavirus synergy in base of tongue cancers. Archives of otolaryngology--head & neck surgery 2009;135(4):391–6. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz AP, Gonzalez D, Vivaldi-Oliver J, Castaneda M, Rivera V, Diaz E et al. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus research 2018. doi: 10.1016/j.pvr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Annals of epidemiology 2003;13(5):312–6. [DOI] [PubMed] [Google Scholar]
- 15.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. The Lancet Oncology 2008;9(6):550–8. 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA. An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. American journal of epidemiology 2010;171(2):253–9. doi:kwp340 [pii] 10.1093/aje/kwp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mai X, LaMonte MJ, Hovey KM, Nwizu N, Freudenheim JL, Tezal M et al. History of periodontal disease diagnosis and lung cancer incidence in the Women’s Health Initiative Observational Study. Cancer causes & control : CCC 2014;25(8):1045–53. doi: 10.1007/s10552-014-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai X, LaMonte MJ, Hovey KM, Freudenheim JL, Andrews CA, Genco RJ et al. Periodontal disease severity and cancer risk in postmenopausal women: the Buffalo OsteoPerio Study. Cancer causes & control : CCC 2016;27(2):217–28. doi: 10.1007/s10552-015-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud DS, Lu J, Peacock-Villada AY, Barber JR, Joshu CE, Prizment AE et al. Periodontal Disease Assessed Using Clinical Dental Measurements and Cancer Risk in the ARIC Study. Journal of the National Cancer Institute 2018. doi: 10.1093/jnci/djx278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon HS, Wen W, Long J, Zheng W, Blot WJ, Cai Q. Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung Cancer 2019;127:90–5. doi: 10.1016/j.lungcan.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, Hsu CH et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM : monthly journal of the Association of Physicians 2014;107(4):283–90. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 22.Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012;33(5):1055–8. doi:bgs112 [pii] 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momen-Heravi F, Babic A, Tworoger SS, Zhang L, Wu K, Smith-Warner SA et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. International journal of cancer Journal international du cancer 2017;140(3):646–52. doi: 10.1002/ijc.30486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu JM, Shen CJ, Chou YC, Hung CF, Tian YF, You SL et al. Risk of colorectal cancer in patients with periodontal disease severity: a nationwide, population-based cohort study. International journal of colorectal disease 2018;33(3):349–52. doi: 10.1007/s00384-018-2965-2. [DOI] [PubMed] [Google Scholar]
- 25.Chou SH, Tung YC, Wu LS, Chang CJ, Kung S, Chu PH. Severity of chronic periodontitis and risk of gastrointestinal cancers: A population-based follow-up study from Taiwan. Medicine 2018;97(27):e11386. doi: 10.1097/MD.0000000000011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome research 2012;22(2):292–8. doi:gr.126573.111 [pii] 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe 2013;14(2):207–15. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt RA, Cochrane K. Tumor Potentiating Mechanisms of Fusobacterium nucleatum, A Multifaceted Microbe. Gastroenterology 2017;152(4):694–6. doi: 10.1053/j.gastro.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol 2015;23:141–7. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JS, Tsai CR, Chen LT, Shan YS. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas 2016;45(1):134–41. doi: 10.1097/MPA.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 31.Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013;62(12):1764–70. doi:gutjnl-2012–303006 [pii] 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 2018;67(1):120–7. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson SH, Satagopan J, Xu Y, Ling L, Leong S, Orlow I et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: a pilot study. Cancer causes & control : CCC 2017;28(9):959–69. doi: 10.1007/s10552-017-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012;61(4):582–8. doi:gutjnl-2011– 300784 [pii] 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres PJ, Fletcher EM, Gibbons SM, Bouvet M, Doran KS, Kelley ST. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015;3:e1373. doi: 10.7717/peerj.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagnaire A, Nadel B, Raoult D, Neefjes J, Gorvel JP. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nature reviews Microbiology 2017;15(2):109–28. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 37.Baba Y, Iwatsuki M, Yoshida N, Watanabe M, Baba H. Review of the gut microbiome and esophageal cancer: Pathogenesis and potential clinical implications. Ann Gastroenterol Surg 2017;1(2):99–104. doi: 10.1002/ags3.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017;358(6369):1443–8. doi: 10.1126/science.aal5240.** Role of oral bacteria in colorectal carcinogenesis.
- 39.Lauritano D, Sbordone L, Nardone M, Iapichino A, Scapoli L, Carinci F. Focus on periodontal disease and colorectal carcinoma. Oral Implantol (Rome) 2017;10(3):229–33. doi: 10.11138/orl/2017.10.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. Journal of oral microbiology 2016;8:32762. doi: 10.3402/jom.v8.32762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abed J, Emgard JE, Zamir G, Faroja M, Almogy G, Grenov A et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell host & microbe 2016;20(2):215–25. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proenca MA, Biselli JM, Succi M, Severino FE, Berardinelli GN, Caetano A et al. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World journal of gastroenterology 2018;24(47):5351–65. doi: 10.3748/wjg.v24.i47.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussan H, Clinton SK, Roberts K, Bailey MT. Fusobacterium’s link to colorectal neoplasia sequenced: A systematic review and future insights. World journal of gastroenterology 2017;23(48):8626–50. doi: 10.3748/wjg.v23.i48.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng F, Liu J, Guo Y, Li C, Wang H, Wang H et al. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Front Cell Infect Microbiol 2017;7:57. doi: 10.3389/fcimb.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao S, Li S, Ma Z, Liang S, Shan T, Zhang M et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infectious agents and cancer 2016;11:3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015;6(26):22613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arjunan P, Meghil MM, Pi W, Xu J, Lang L, El-Awady A et al. Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic Cell Proliferation. Scientific reports 2018;8(1):16607. doi: 10.1038/s41598-018-35126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arjunan P, El-Awady A, Dannebaum RO, Kunde-Ramamoorthy G, Cutler CW. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Molecular oral microbiology 2016;31(1):78–93. doi: 10.1111/omi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome research 2012;22(2):299–306. doi:gr.126516.111 [pii] 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Castillo E, Meier R, Chung M, Koestler DC, Chen T, Paster BJ et al. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2018. doi: 10.1158/1055-9965.EPI-18-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DerSimonian R, Laird N. Meta-analysis in clincial trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 52.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Steichen T et al. METAN: Stata module for fixed and random effects meta-analysis. Statistical Software Components: Boston College Department of Economics; 2006.


