Abstract
Background:
Exposure to excessive heat, which will continue to increase with climate change, is associated with increased morbidity due to a range of noncommunicable diseases (NCDs). Whether this is true for diabetes is unknown.
Objectives:
We aimed to quantify the relationship between heat exposure and risk of hospitalization due to diabetes in Brazil.
Methods:
Data on hospitalizations and weather conditions were collected from 1,814 cities during the hot seasons from 2000 to 2015. A time-stratified case-crossover design was used to quantify the association between hospitalization for diabetes and heat exposure. Region-specific odds ratios (ORs) were used to calculate the attributable fractions (AFs).
Results:
A total of 553,351 hospitalizations associated with diabetes were recorded during 2000–2015. Every 5°C increase in daily mean temperature was associated with 6% [; 95% confidence interval (CI): 1.04, 1.07] increase in hospitalization due to diabetes with lag 0–3 d. The association was greatest (; 95% CI: 1.13, 1.23) in those of age, but did not vary by sex, and was generally consistent by region and type of diabetes. Assuming a causal association, we estimated that 7.3% (95% CI: 3.5, 10.9) of all hospitalizations due to diabetes in the hot season could be attributed to heat exposure during the study period.
Discussion:
Short-term heat exposure may increase the burden of diabetes-related hospitalization, especially among the very elderly. As global temperatures continue to rise, this burden is likely to increase. https://doi.org/10.1289/EHP5688
Introduction
Diabetes mellitus is an important public health concern affecting an estimated 422 million (including both diagnosed and undiagnosed) people worldwide (Zhou et al. 2016). It is a heterogeneous condition that is comprised mainly of type 2 diabetes (75–85%), with type 1 diabetes constituting 5–10% of all diabetes (WHO 2016a). In recent years, numerous studies have suggested that climate change—especially global warming—is associated with morbidity from many noncommunicable diseases (NCDs) (Watts et al. 2018). The impact of an increase in mean temperature on diabetes-related morbidity is unknown. Compared to the general population, individuals with diabetes are more sensitive to extreme temperatures, especially heat, because of impaired thermoregulatory control, regardless of their diabetes type (Kenny et al. 2016). Several time-series studies have suggested that short-term heat exposure is associated with an increase in mortality due to diabetes (Li et al. 2014; Luan et al. 2018; Schwartz 2005; Seposo et al. 2017; Yang et al. 2016). Similarly, a previous study from the United Kingdom reported 10% increased odds of seeking medical consultation for every 1°C increase above 22°C among individuals with type 2 diabetes (Hajat et al. 2017). The effect of heat exposure on risk of diabetes-related hospitalization in the United States has also been examined but with conflicting results: one study (Semenza et al. 1999) reported that heatwaves were associated with a 30% increase in hospital admissions related to diabetes, whereas a second study showed no association (Knowlton et al. 2009).
About 75% of the burden of diabetes occurs in low- and middle-income countries, especially those constituting the BRIC (Brazil, Russia, India, and China) nations (Dagenais et al. 2016). From 2000 to 2015, the prevalence of diabetes in Brazil rose from 3.6% to 6.1% (Duncan et al. 2017), roughly the equivalent of 12 million people, placing Brazil as the fourth-highest country in the world in the number of people living with diabetes (Zhou et al. 2016). Brazil is also one of the countries most affected by global warming. It has reported a 2°C increase in surface temperature from 1901 to 2012, considerably higher than most other countries (Myhre et al. 2013).
In this study, we characterize the association between short-term heat exposure and risk of hospitalization associated with diabetes in the Brazilian population, using a national hospitalization data set spanning from 2000 to 2015. Further, we explored whether the association was consistent across types of diabetes and within subgroups of the population based on age, sex, and region. Finally, assuming a causal association, we estimated the fraction of all hospitalizations associated with diabetes that could be attributable to heat exposure.
Methods
Data Collection
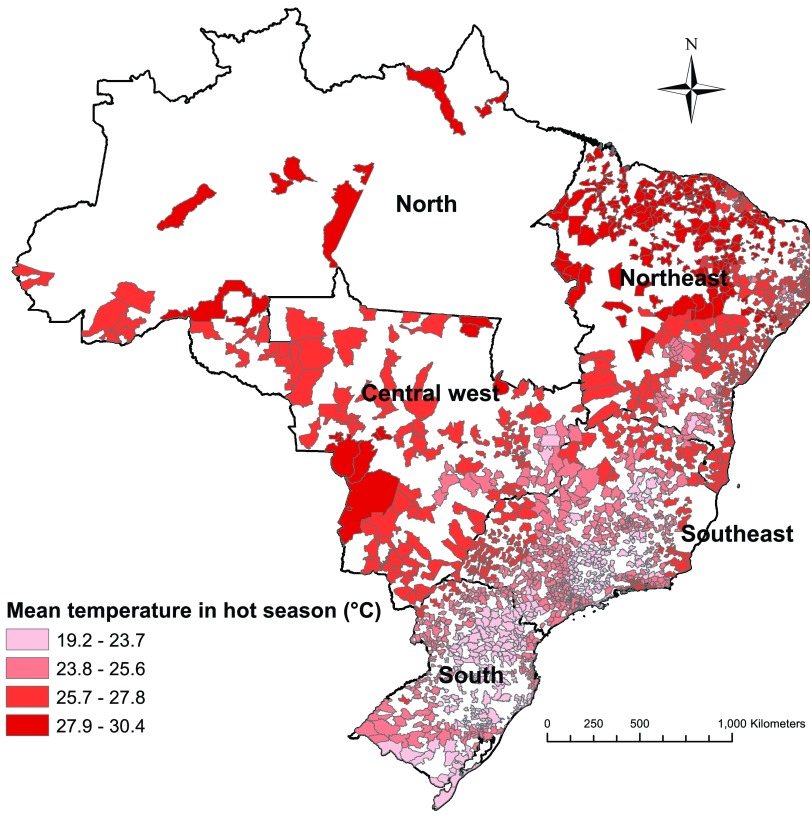
Data on hospitalization for diabetes between 1 January 2000 and 31 December 2015 were collected from 1,814 Brazilian cities, which comprised 78.4% of the national population. These cities were located in five regions (north, northeast, central west, southeast, and south) that are officially defined by the Brazilian government based on physical, political, social, and economic similarities (Duran 2013) (Figure 1). Individual data for each patient were collected from the Brazilian Unified Health System (BUHS). The data included information on the date of admission, primary diagnosis, sex, and age. The primary diagnosis was coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). We only extracted hospitalization data with ICD-10 codes (E10, E11, E12, E13, and E14) for diabetes mellitus. The four-character subdivisions of E10−E14 were used to define the complications of diabetes. For example, E10.0 and E10.7 refer to type 1 diabetes with coma and multiple complications, respectively (see Table S1 or WHO 2016b). To be consistent with previous studies (Hajat et al. 2017; Knowlton et al. 2009; Li et al. 2014; Luan et al. 2018; Schwartz 2005; Semenza et al. 1999; Seposo et al. 2017; Yang et al. 2016), we did not include gestational diabetes, which is a condition of pregnancy.
Figure 1.
Location of 1,814 cities in Brazil enrolled in the study and their mean temperatures in hot seasons from 2000 to 2015. Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus).
The daily minimum and maximum temperatures were sourced from a national meteorological data set ( resolution). This data set was interpolated by Xavier et al. (2016) using the inverse distance weighting approach with data from 735 weather stations. City-specific weather conditions were represented using the center for each city. In this study, we used daily mean temperature, calculated by the average of daily minimum and maximum temperatures, to estimate the effect of heat exposure on risk of hospitalization due to diabetes, because it reflects the general thermal characteristics for each day (WMO 2011).
Data on daily relative humidity were collected from city-specific weather stations through the Brazilian National Institute of Meteorology. However, humidity data were only available for 193 cities during 2000–2012. We did not collect data on daily air pollution because of the lack of air quality–monitoring stations in Brazilian cities. Further, air pollution is more likely to be a mediator than a true confounder of the association between temperature and health, and thus should not be adjusted for (Buckley et al. 2014).
This study was approved by the Monash University Human Research Ethics Committee. The Brazilian Ministry of Health did not require ethical approval or informed consent for secondary analysis of aggregated anonymized data from the BUHS.
Statistical Analyses
Assessing the temperature–hospitalization association.
As this study focused on the effect of heat exposure (high temperature), we restricted our analyses to the hot season to be consistent with previous studies (Alessandrini et al. 2011; Basu et al. 2012; Bhaskaran et al. 2012; Isaksen et al. 2015). We defined the hot season as the adjacent 4 hottest months for each city in Brazil (Zhao et al. 2019). According to this definition, the exact calendar months of hot season varied by city. For example, the hot season of São Paulo was December to March, while the hot season of Manaus was August to November. The association between hospitalization for diabetes and heat exposure was evaluated by a time-stratified case-crossover design with conditional logistic regression models (Levy et al. 2001; Li et al. 2016). For each admission, the daily mean temperature during the risk period was compared with those in the same city during the control periods. Control periods comprised the same days of the week in the same calendar month for each diabetes mellitus admission as the risk periods. This design adjusts for time-dependent confounders (e.g., temporal trend and day of the week) and time-constant confounders (e.g., sex, age, individual lifestyle, or behavior) (Janes et al. 2005a, 2005b).
The relationship between diabetes mellitus admission and heat exposure was fitted by a cross-basis function (Guo 2017). Our initial analyses showed that the association was linear and persisted for 3 d. Therefore, we used a linear function for the exposure–response dimension. We used a natural cubic spline (Chen et al. 2018; Gasparrini et al. 2010, 2015) with three degrees of freedom (df) for the lag–response dimension (lag 0–3 d). The holiday effect was controlled for by adding a dichotomous variable (whether that date was a holiday) to the model.
Stratified analyses were conducted by types of diabetes mellitus, regions, sex, five age groups (0–19, 20–39, 40–59, 60–79, and ), and complications. The heat exposure–diabetes hospitalization association was presented as the odds ratio (OR) and 95% confidence interval (CI) of diabetes hospitalization for every 5°C increase in daily mean temperature. The OR obtained from our case-crossover analysis is interpretable as a relative risk (RR), as the control selection scheme based on density sampling leads to control times that represent the average exposure in the study population (Greenland and Thomas 1982; Hogue et al. 1983). Meta-regression was applied to check the statistical differences in the ORs between subgroups or different models in sensitivity analyses. Specifically, the effect estimates of different subgroups with standard error (e.g., five region-specific effect estimates; two sex-specific effect estimates) were modeled against the meta-predictors (e.g., region as a categorical variable with five levels; sex as a categorical variable with two levels).
Several sensitivity analyses were applied to check the robustness of our results. First, we altered the maximum lag days from 3 to 7 d, and changed the df of lag days from 3 to 4. Second, using the data set of 193 cities that included information on relative humidity, we adjusted for the average relative humidity in lag 0–3 d as a natural cubic spline with 3 df, to test the potential confounding effect of humidity. Third, we altered the definition of a hot season to the 5 or 6 city-specific adjacent hottest months. Finally, we repeated the main model analyses in cold season (city-specific 4 coldest months) and moderate season (city-specific months other than cold and hot season). The linearity of the heat exposure–diabetes hospitalization association was examined by distributed lag nonlinear models (Gasparrini et al. 2010; Gasparrini 2014).
Calculating the attributable burden of diabetes hospitalization related to heat exposure.
Assuming a causal relationship, we estimated the attributable burden of hospitalization for diabetes due to heat exposure for each city using the formula , where is the city-specific cumulative lag 0–3 RR associated with the temperature on day i vs. the reference temperature, and is the city-specific average diabetes hospitalization on day i, , , and (Gasparrini and Leone 2014; Hu et al. 2018). was calculated as , where is the region-specific cumulative lag 0–3 OR (interpretable as the RR) for diabetes hospitalization with a 5°C increase in temperature in the region where the city is located, is the city-specific daily mean temperature on day i, and is the city-specific reference temperature. Since our preliminary analyses had shown the association between temperature- and diabetes-related hospitalization was linear, the reference temperatures for each city were selected as the city-specific minimum temperatures in hot season during the study period (Zhao et al. 2019). Total attributable cases (ACs) were generated by summing the for all included cities during the study period. The corresponding attributable fractions (AFs) of hospitalizations associated with diabetes were calculated by dividing the total AC by total diabetes hospitalization cases. We repeated the above procedures based on subgroup samples to obtain AFs of subgroups by sex, age, and type of diabetes.
R software (version 3.3.2; R Development Core Team) was used to perform all data analyses. The packages survival, dlnm, and mvmeta were used to fit conditional logistic regression, distributed lag linear or nonlinear models, and meta-regression, respectively (Gasparrini et al. 2012). A two-sided p-value of less than 0.05 was considered to be statistically significant.
Results
The daily mean [ (SD)] temperature was during the hot seasons in all cities included, ranging from in the south to in the north during 2000 to 2015 (Table 1). Overall, there was a total of 553,351 (57.5% female) hospitalizations for diabetes with a median age of 60.5 y (interquartile range: 47.7–71.3 y). Among hospitalizations with a specific primary diagnosis, those associated with type 1 diabetes were most frequent (30.9%; 56.8% female; median age: 59.5 y) followed by type 2 diabetes (6.9%; 56.6% female; median age: 61.7 y). A large proportion of hospitalizations was associated with unspecified diabetes (54.4%; 58.0% female; median age: 60.8 y).
Table 1.
Summary of hospitalizations for diabetes mellitus and daily mean temperature (with standard deviations) by region in 1,814 Brazilian cities during the 2000–2015 hot seasons.
| Region | Enrolled cities | Population coverage (%) | Cases of different types of diabetes mellitus | Temperature () (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Type 1 (E10) | Type 2 (E11) | Malnutrition related (E12) | Other specified (E13) | Unspecified (E14) | Total (E10–E14) | ||||
| National | 1,814 | 78.4% | 171,520 | 37,912 | 7,504 | 35,350 | 301,065 | 553,351 | |
| North | 28 | 26.3% | 3,310 | 1,256 | 75 | 1,847 | 7,569 | 14,057 | |
| Northeast | 662 | 78.0% | 46,801 | 8,311 | 2,170 | 8,836 | 90,888 | 157,006 | |
| Central west | 128 | 80.7% | 16,796 | 4,140 | 712 | 5,227 | 22,666 | 49,541 | |
| Southeast | 622 | 87.0% | 74,052 | 17,910 | 2,449 | 10,132 | 126,680 | 231,223 | |
| South | 374 | 83.2% | 30,561 | 6,295 | 2,098 | 9,308 | 53,262 | 101,524 | |
| Female (%) | — | — | 56.8 | 56.6 | 58.6 | 58.1 | 58.0 | 57.5 | — |
| Age, median (IQR) | — | — | 59.5 | 61.7 | 61.5 | 60.9 | 60.8 | 60.5 | — |
| — | — | (45.4–70.7) | (50.3–72.2) | (49.4–72.0) | (49.1–71.3) | (48.3–71.6) | (47.7–71.3) | — | |
Note: Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus). Population coverage was calculated as the total population in included cities divided by the national (or regional) total population, according to population data from Brazilian Census 2010 (BIGS 2010). E10–E14 are ICD-10 codes of diabetes. The bottom row in Table represents interquartile range (IQR) of age. —, no data; IQR, interquartile range; SD, standard deviation.
In general, daily mean temperatures on case days were slightly (an average of 0.02–0.07°C) higher than control days in all regions, and the differences were statistically significant () in all regions except for the north (Table S2). The nonsignificant result in the north could be explained by its smaller population coverage (only 26% vs. 78–87% in other regions) and smaller sample size compared to other regions. This could also explain the lower precision of its region-specific effect estimates compared to other regions. The time-series plot of daily mean temperature in a selected city (São Joaquim, the city with the median SD of daily mean temperature during the study period) is shown in Figure S1.
Association between temperature and hospitalizations for diabetes
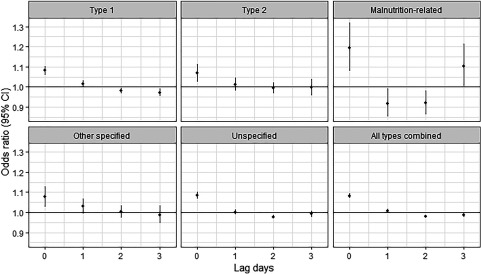
The association between temperature and hospitalization for diabetes was linear, and the lag patterns were generally similar across the different types of diabetes (Figure S2; Figure 2; Table S3). Positive associations between heat exposure and hospitalization were evident on the same day (lag 0), but associations were inverse after a 1- to 3-d lag. This pattern is consistent with temporal displacement or harvesting effect, whereby events that would have occurred in the absence of an exposure occur at an earlier point in time because of the exposure so that acute positive associations are followed by inverse associations due to a temporary reduction in the susceptible population (Hajat et al. 2005). This lag pattern was consistent across sex, regions, complications, and age groups with the exception of those of age, for whom the associations were null or positive for all lags (Figures S3–S5; Table S3).
Figure 2.
The associations between heat exposure (every 5°C increase in daily mean temperature during the hot season) and hospitalization for diabetes mellitus [odds ratios with 95% confidence intervals (CIs)] across lag 0–3 d by diabetes subtype. The estimates are for lag 0–3 d and came from time-stratified case-crossover analyses modeled by conditional logistic regression with a cross-basis function for daily mean temperature. The model was adjusted for public holidays. Corresponding numeric data are provided in Table S3. Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus).
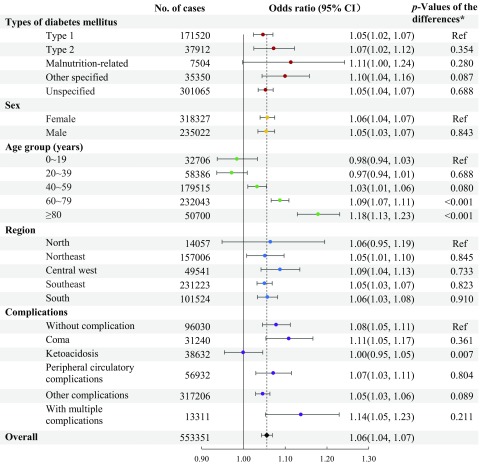
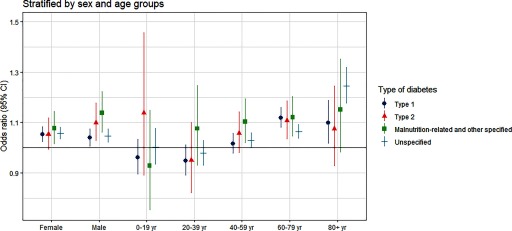
Figure 3 shows that every 5°C increase in daily mean temperature was associated with a 6% (; 95% CI: 1.04, 1.07) greater risk of hospitalization for diabetes hospitalization for lag 0–3 d at the national level. Associations were similar for men and women. Although there was some variation by diabetes type (they seem to be stronger in malnutrition-related and other specified diabetes) and region, the differences were not statistically significant, and all associations were positive. There was, however, interaction with age such that the effect size increased across successive age groups above 40 y and was maximal in those of age (; 95% CI: 1.13, 1.23). Associations were positive among subgroups defined by the presence or absence of diabetes complications, except among individuals with ketoacidosis, for whom the association was null. When the subgroup analyses were further stratified by different types of diabetes, no clear patterns emerged (Figure 4; Figures S6–S7; Table S4).
Figure 3.
The association between heat exposure (every 5°C increase in daily mean temperature during the hot season) and hospitalization for diabetes mellitus [odds ratios with 95% confidence intervals (CIs)] over lag 0–3 d. The odds ratios represent the cumulative association over lag 0–3 d. They came from time-stratified case-crossover analyses modeled by conditional logistic regression with a cross-basis function for daily mean temperature. The model was adjusted for public holidays. Note: p-Values of the differences were estimated by meta-regression to test the difference in effect estimates between subgroups. Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus).
Figure 4.
The association between heat exposure (every 5°C increase in daily mean temperature during the hot season) and diabetes hospitalization [odds ratios with 95% confidence intervals (CIs)], stratified by diabetes subtype and by sex and age group. The odds ratios represent the cumulative association over lag 0–3 d. They came from time-stratified case-crossover analyses modeled by conditional logistic regression with a cross-basis function for daily mean temperature. The model adjusted for public holidays. Corresponding numeric data are provided in Table S4. Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus).
Attributable burden of hospitalizations for diabetes due to heat exposure
Assuming causality, we estimated that 7.3% (95% CI: 3.5, 10.9) of all hospitalizations related to diabetes during the hot seasons [equivalent to 40,543 (95% CI: 19,533; 60,389) hospitalization cases] could be attributed to heat exposure during the study period (Table 2). This fraction was especially high in the elderly, with nearly one in five hospital admissions for diabetes (19.2%, 95% CI: 6.5, 29.5) related to heat exposure.
Table 2.
The fraction and cases of hospitalization for diabetes mellitus attributable to heat exposure during the hot seasons from 2000 to 2015 in Brazil.
| No. of attributable cases (95% CI) | Attributable fraction (95% CI) (%) | |
|---|---|---|
| Types of diabetes mellitus | ||
| Type 1 | 11,380 (3,399; 34,168) | 6.6 (2.0, 19.9) |
| Type 2 | 4,368 (2,328; 14,607) | 11.5 (6.1, 38.5) |
| Malnutrition related | 1,454 (, 2,784) | 19.4 (, 37.1) |
| Other specified | 4,576 (, 8,301) | 12.9 (, 23.5) |
| Unspecified | 18,670 (4,962; 52,286) | 6.2 (1.6, 17.4) |
| Sex | ||
| Female | 24,448 (14,802; 58,626) | 7.7 (4.6, 18.4) |
| Male | 16,125 (4,374; 42,755) | 6.9 (1.9, 18.2) |
| Age group (years) | ||
| N/A | N/A | |
| N/A | N/A | |
| 8,277 (2,317; 34,487) | 4.6 (1.3, 19.2) | |
| 24,973 (15,484; 53,044) | 10.8 (6.7, 22.9) | |
| 9,734 (3,301; 14,935) | 19.2 (6.5, 29.5) | |
| Region | ||
| North | 1,050 (, 2,757) | 7.5 (, 19.6) |
| Northeast | 7,786 (1,210; 14,062) | 5.0 (0.8, 9.0) |
| Central west | 6,531 (3,332; 9,489) | 13.2 (6.7, 19.2) |
| Southeast | 14,866 (9,751; 19,851) | 6.4 (4.2, 8.6) |
| South | 10,310 (6,200; 14,230) | 10.2 (6.1, 14.0) |
| Overall | 40,543 (19,533; 60,389) | 7.3 (3.5, 10.9) |
Note: Attributable fractions and attributable cases were not calculated in 0- to 19- and 20- to 39-year-old people because the associations between temperature and diabetes hospitalization were nonsignificant, and the odds ratios (ORs) were less than 1 in these two age groups. Hot season was defined as the city-specific adjacent 4 hottest months and varied by city (e.g., December to March for São Paulo, August to November for Manaus). The attributable fractions were estimated based on region-specific ORs for cumulative lags (0–3 days) relative to city-specific minimum daily mean temperatures during hot seasons from 2000 to 2015. CI, confidence interval; N/A, not applicable.
Results of sensitivity analyses
Sensitivity analyses indicated that our results were robust by changing the df of lag days from 3 to 4 (Table S5). When adding the maximum lag of daily mean temperature from 3 to 7 d, the cumulative ORs decreased except for cases of other specific diabetes, which is consistent with expectations based on individual lags of 0–3 d. However, the decreases in cumulative ORs in longer lags were not statistically significant except for overall and unspecified diabetes at lag 0–7 d. Adjusting for relative humidity in a subsample of 193 cities had minimal influence on the results as estimated by the primary models (Table S6). The results also remained largely unchanged when defining 5 or 6 adjacent hottest months as the hot season (Table S7). Every 5°C increase in daily mean temperature was associated with a smaller increase in hospitalization for diabetes in the cold season (; 95% CI: 1.01, 1.03; p-value for the difference between cold and hot ) and moderate season (; 95% CI: 1.03, 1.05; p-value for the difference between moderate and hot ) compared to the hot season (; 95% CI: 1.04, 1.07) (Table S8).
Discussion
To our knowledge, this is the first nationwide study to quantify the association between heat exposure and risk of hospitalization for diabetes over a 16-y period. Our findings indicate that in the Brazilian population, short-term heat exposure during the hot season was significantly associated with greater risk of hospitalization related to diabetes. Overall, assuming a cause–effect relationship, we estimated that 7.3% (95% CI: 3.5, 10.9) of all hospitalizations associated with diabetes in the hot season could be attributable to heat exposure. The nature of the relationship was consistent in women and men but was stronger among older age groups. The magnitude of the association was similar, irrespective of diabetes subtype, or in the presence of diabetes-related complications (with the possible exception of those with ketoacidosis).
Our findings are generally consistent with reported observations from the few previous studies that have examined the relationship between heat exposure with either diabetes-related morbidity or mortality. In particular, the greater susceptibility of the elderly to heat exposure has been consistently documented (Li et al. 2014; Luan et al. 2018; Schwartz 2005; Seposo et al. 2017; Yang et al. 2016).
The mechanistic pathways underlying the observed association between heat exposure and increased risk of hospitalization for diabetes are not well understood but have been speculated upon. For example, compared with unaffected individuals, those with diabetes have impaired thermoregulatory capacity, including impaired functioning of the sweat glands and low blood flow reduction during heat exposure, which can reduce the capacity to dissipate heat effectively (Petrofsky et al. 2005). In addition, some medications used in the treatment of diabetes (particularly those affecting fluid balance) may exacerbate the risk of heat-related illness (e.g., dehydration, heat exhaustion) by decreasing skin blood flow and sweating (Yardley et al. 2013). High ambient temperatures may also result in a greater insulin peak effect, thereby increasing the risk of a hypoglycemic episode (Al-Qaissi et al. 2019; Dumke et al. 2015). This may explain our finding that there was no association between heat exposure and diabetic hospitalization among subjects with ketoacidosis (which is caused by hypoinsulinemia and hyperglycemia). These mechanisms also suggest why elderly individuals with diabetes are particularly susceptible to heat exposure, as they generally have poorer thermoregulatory function and worse glucose homeostasis than younger adults.
Strategies that may ameliorate the diabetes morbidity burden associated with heat exposure are mainly concerned with modifying behavior, which may be particularly difficult for the very elderly and those in the lowest socioeconomic groups. For example, increasing fluid consumption, staying indoors in an air-conditioned environment, and reducing normal activity levels are all effective strategies but may not be feasible for the most disadvantaged members of society. Brazil is one of the fastest-aging countries worldwide. Owing to the decline in both mortality and fertility, the proportion of people aged 65 y or above in Brazil will increase to about 35% by 2040 (Cuevas et al. 2017). In addition, it is predicted that the diabetes prevalence in South and Central America will increase by 65% from 2015 to 2040 (Ogurtsova et al. 2017). Combined, both factors will increase the size of the vulnerable population—elderly people with diabetes. This will pose additional challenges for Brazil’s health care system to cope with heat-related diabetes morbidity burden in the future.
Global warming is a threat to public health in the 21st century. It has been estimated that the global mean temperature will increase 2.7°C by 2100, even if all mitigation strategies in the Paris Agreement are fully implemented (International Energy Agency 2015; Watts et al. 2017). Heat exposures and heatwaves have been shown to increase morbidity due to NCDs, particularly cardiovascular and respiratory diseases (Phung et al. 2016; Turner et al. 2012; Watts et al. 2018), and thus, the burden of these diseases will increase with global warming. We estimated an RR of 1.06 for diabetes hospitalizations with a 5°C increase in daily mean temperature. This corresponds to an RR of 1.032 () for a 2.7°C increase, or a 3.2% increase in hospital admissions for diabetes as a result of climate change, assuming all other risk factors remain unchanged. This underscores the potential for the diabetes morbidity burden to increase due to climate change.
The present study has several strengths. First, this is by far the largest study that has evaluated the association between temperature and diabetes-related hospitalization. Second, with access to a national data set covering nearly 80% of the Brazilian population and spanning 16 y, this study is representative both geographically and temporally. Moreover, our findings may also be relevant to other middle-income countries (e.g., China, India). Finally, as Brazil is a large country with significant diversity in temperatures, our results are also likely to be relevant to populations in other South American countries.
However, several limitations of this study should be acknowledged. First, we only had access to grid city-level temperature data rather than individual-level data, which may have underestimated the association between heat exposure and diabetes-related hospitalization (Guo et al. 2013). Second, consistent with a recent survey of diabetes hospitalizations in Brazil (Rosa et al. 2018), over half of the cases (54%) were hospital admissions due to unspecified diabetes, and another 6.4% were for other specified diabetes. Because the BUHS covers the cost of all diabetes care, regardless of ICD-10 subtype, hospitals might lack a financial incentive to report a specific diagnosis code, and thus, our ability to assess potential variation in heat-related risks of hospitalization according to specific subtypes of disease was limited. The estimated heat-related risk of hospitalization due to unspecified diabetes (; 95% CI: 1.04, 1.07) was similar to estimates for both type 1 and type 2 diabetes (; 95% CI: 1.02, 1.07 and ; 95% CI: 1.02, 1.12, respectively), while the association with other specified diabetes was stronger (; 95% CI 1.04, 1.16; for the difference from type 1 diabetes hospitalizations). Third, we were unable to adjust for relative humidity in the main model due to limited access to relevant data. High levels of relative humidly may have exacerbated the impact of high temperature by impeding the evaporation of sweat and heat dissipation (Chandler 2001). However, our sensitivity analyses indicated that adjustment for relative humidity in the data set from 193 cities had minimal effects on the overall results. Fourth, due to the unavailability of relevant data, we could not evaluate whether the heat effects could be explained or modified by diabetes-related comorbidities (e.g., cardiovascular diseases, kidney diseases) or medication use. Finally, due to the nonavailability of data, this study was restricted to hospitalizations as the measure of morbidity; future studies using other measures of diabetes-related morbidity such as emergency room visits, outpatient visits, and general practice consultations are warranted in order to ascertain a more comprehensive understanding of the burden of diabetes due to heat exposure.
In conclusion, our nationwide analysis indicated that heat exposure was associated with increased risk of diabetes-related hospitalization in Brazil, especially among the very elderly. These findings add to the expanding evidence base that climate change, and global warming in particular, is likely to have an increasingly important and detrimental role in human health over the coming decades.
Supplementary Material
Acknowledgments
R.X. was supported by the China Scholarship Council (201806010405). Q.Z. was supported by a Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship. S.L. was supported by an Early Career Fellowship of the Australian National Health and Medical Research Council (APP1109193). Y.G. was supported by a Career Development Fellowship of the Australian National Health and Medical Research Council (APP1107107). The funding bodies did not play any role in the study design, data collection, data analyses, results interpretation, and writing of this manuscript. The authors thank the Brazilian Ministry of Health for providing the hospitalization data and also thank the Brazilian National Institute of Meteorology for providing the meteorological data.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5688).
These authors contributed equally to this work.
M.J.A. holds investigator-initiated grants from Pfizer and Boehringer-Ingelheim for unrelated research and an unrelated consultancy from Sanofi. All other authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alessandrini E, Zauli Sajani S, Scotto F, Miglio R, Marchesi S, Lauriola P. 2011. Emergency ambulance dispatches and apparent temperature: a time series analysis in Emilia-Romagna, Italy. Environ Res 111(8):1192–1200, PMID: 21816396, 10.1016/j.envres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Al-Qaissi A, Papageorgiou M, Javed Z, Heise T, Rigby AS, Garrett AT, et al. 2019. Environmental effects of ambient temperature and relative humidity on insulin pharmacodynamics in adults with type 1 diabetes mellitus. Diabetes Obes Metab 21(3):569–574, PMID: 30311402, 10.1111/dom.13555. [DOI] [PubMed] [Google Scholar]
- Basu R, Pearson D, Malig B, Broadwin R, Green R. 2012. The effect of high ambient temperature on emergency room visits. Epidemiology 23(6):813–820, PMID: 23007039, 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K, Armstrong B, Hajat S, Haines A, Wilkinson P, Smeeth L. 2012. Heat and risk of myocardial infarction: hourly level case-crossover analysis of MINAP database. BMJ 345:e8050, PMID: 23243290, 10.1136/bmj.e8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIGS (Brazilian Institute of Geography and Statistics). 2010. 2010 Population Census. https://www.ibge.gov.br/en/statistics/social/population/18391-2010-population-census.html?edicao=19263&t=resultados [accessed 18 October 2018].
- Buckley JP, Samet JM, Richardson DB. 2014. Commentary: does air pollution confound studies of temperature? Epidemiology 25(2):242–245, PMID: 24487206, 10.1097/EDE.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Chandler N. 2001. What is relative humidity and how does it affect how I feel outside? https://science.howstuffworks.com/nature/climate-weather/atmospheric/question651.htm [accessed 2 September 2019].
- Chen RJ, Yin P, Wang LJ, Liu C, Niu Y, Wang WD, et al. 2018. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ 363:k4306, PMID: 30381293, 10.1136/bmj.k4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas MA, Karpowicz MI, Mulas-Granados MC, Soto M. 2017. Fiscal challenges of population aging in Brazil. IMF Work Paper 2017(99):29, 10.5089/9781475595550.001. [DOI] [Google Scholar]
- Dagenais GR, Gerstein HC, Zhang X, McQueen M, Lear S, Lopez-Jaramillo P, et al. 2016. Variations in diabetes prevalence in low-, middle-, and high-income countries: results from the prospective urban and rural epidemiological study. Diabetes Care 39(5):780–787, PMID: 26965719, 10.2337/dc15-2338. [DOI] [PubMed] [Google Scholar]
- Dumke CL, Slivka DR, Cuddy JS, Hailes WS, Rose SM, Ruby BC. 2015. The effect of environmental temperature on glucose and insulin after an oral glucose tolerance test in healthy young men. Wilderness Environ Med 26(3):335–342, PMID: 25937547, 10.1016/j.wem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Cousin E, Moradi-Lakeh M, Passos VMA, Franca EB, et al. 2017. The burden of diabetes and hyperglycemia in Brazil-past and present: findings from the Global Burden of Disease Study 2015. Diabetol Metab Syndr 9:18, PMID: 28293304, 10.1186/s13098-017-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran R. 2013. Brazilian regions. https://thebrazilbusiness.com/article/brazilian-regions [accessed 18 July 2019].
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2012. Multivariate meta‐analysis for non‐linear and other multi‐parameter associations. Stat Med 31(29):3821–3839, PMID: 22807043, 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386(9991):369–375, PMID: 26003380, 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Leone M. 2014. Attributable risk from distributed lag models. BMC Med Res Methodol 14:55, PMID: 24758509, 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2014. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 33(5):881–899, PMID: 24027094, 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Thomas DC. 1982. On the need for the rare disease assumption in case-control studies. Am J Epidemiol 116(3):547–553, PMID: 7124721, 10.1093/oxfordjournals.aje.a113439. [DOI] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Tong S. 2013. Spatiotemporal model or time series model for assessing city-wide temperature effects on mortality? Environ Res 120:55–62, PMID: 23026801, 10.1016/j.envres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Guo Y. 2017. Hourly associations between heat and ambulance calls. Environ Pollut 220(Pt B):1424–1428, PMID: 27825842, 10.1016/j.envpol.2016.10.091. [DOI] [PubMed] [Google Scholar]
- Hajat S, Armstrong BG, Gouveia N, Wilkinson P. 2005. Mortality displacement of heat-related deaths: a comparison of Delhi, São Paulo, and London. Epidemiology 16(5):613–620, PMID: 16135936, 10.1097/01.ede.0000164559.41092.2a. [DOI] [PubMed] [Google Scholar]
- Hajat S, Haines A, Sarran C, Sharma A, Bates C, Fleming LE. 2017. The effect of ambient temperature on type-2-diabetes: case-crossover analysis of 4+ million GP consultations across England. Environ Health 16(1):73, PMID: 28701216, 10.1186/s12940-017-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue CJ, Gaylor DW, Schulz KF. 1983. Estimators of relative risk for case-control studies. Am J Epidemiol 118(3):396–407, PMID: 6613982, 10.1093/oxfordjournals.aje.a113646. [DOI] [PubMed] [Google Scholar]
- Hu K, Guo Y, Hu D, Du R, Yang X, Zhong J, et al. 2018. Mortality burden attributable to PM1 in Zhejiang province, China. Environ Int 121(Pt 1):515–522, PMID: 30292144, 10.1016/j.envint.2018.09.033. [DOI] [PubMed] [Google Scholar]
- International Energy Agency. 2015. Energy and Climate Change: World Energy Outlook Special Briefing for COP21. Paris, France: International Energy Agency. [Google Scholar]
- Isaksen TB, Yost MG, Hom EK, Ren Y, Lyons H, Fenske RA. 2015. Increased hospital admissions associated with extreme-heat exposure in King County, Washington, 1990–2010. Rev Environ Health 30(1):51–64, PMID: 25719287, 10.1515/reveh-2014-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. 2005a. Case–crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 16(6):717–726, PMID: 16222160, 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. 2005b. Overlap bias in the case‐crossover design, with application to air pollution exposures. Stat Med 24(2):285–300, PMID: 15546133, 10.1002/sim.1889. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Sigal RJ, McGinn R. 2016. Body temperature regulation in diabetes. Temperature (Austin) 3(1):119–145, PMID: 27227101, 10.1080/23328940.2015.1131506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, et al. 2009. The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect 117(1):61–67, PMID: 19165388, 10.1289/ehp.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Sheppard L, Checkoway H, Kaufman J, Lumley T, Koenig J, et al. 2001. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology 12(2):193–199, PMID: 11246580, 10.1097/00001648-200103000-00011. [DOI] [PubMed] [Google Scholar]
- Li S, Guo Y, Williams G. 2016. Acute impact of hourly ambient air pollution on preterm birth. Environ Health Perspect 124(10):1623–1629, PMID: 27128028, 10.1289/EHP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lan L, Wang Y, Yang C, Tang W, Cui G, et al. 2014. Extremely cold and hot temperatures increase the risk of diabetes mortality in metropolitan areas of two Chinese cities. Environ Res 134:91–97, PMID: 25086705, 10.1016/j.envres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Luan GJ, Yin P, Wang LJ, Zhou MG. 2018. An observational study of high air temperature on diabetes mortality in six cities in China. Zhonghua Liu Xing Bing Xue Za Zhi 39(5):646–650, PMID: 29860810, 10.3760/cma.j.issn.0254-6450.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Myhre G, Shindell D, Bréon F, Collins W, Fuglestvedt J, Huang J, et al. 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, et al., eds. Cambridge, UK and New York, NY: Cambridge University Press. [Google Scholar]
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. 2017. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50, PMID: 28437734, 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Petrofsky J, Lee S, Cuneo-Libarona M. 2005. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monitor 11(12):Cr562–Cr569, PMID: 16319786. [PubMed] [Google Scholar]
- Phung D, Thai PK, Guo Y, Morawska L, Rutherford S, Chu C. 2016. Ambient temperature and risk of cardiovascular hospitalization: an updated systematic review and meta-analysis. Sci Total Environ 550:1084–1102, PMID: 26871555, 10.1016/j.scitotenv.2016.01.154. [DOI] [PubMed] [Google Scholar]
- Rosa MQM, Rosa RDS, Correia MG, Araujo DV, Bahia LR, Toscano CM. 2018. Disease and economic burden of hospitalizations attributable to diabetes mellitus and its complications: a nationwide study in brazil. Int J Environ Res Public Health 15:(2):E294, PMID: 29419786, 10.3390/ijerph15020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. 2005. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology 16(1):67–72, PMID: 15613947, 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. 1999. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16(4):269–277, PMID: 10493281, 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Seposo XT, Dang TN, Honda Y. 2017. How does ambient air temperature affect diabetes mortality in tropical cities? Int J Environ Res Public Health 14(4):E385, PMID: 28379204, 10.3390/ijerph14040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LR, Barnett AG, Connell D, Tong S. 2012. Ambient temperature and cardiorespiratory morbidity: a systematic review and meta-analysis. Epidemiology 23(4):594–606, PMID: 22531668, 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- Watts N, Adger WN, Ayeb-Karlsson S, Bai YQ, Byass P, Campbell-Lendrum D, et al. 2017. The Lancet Countdown: tracking progress on health and climate change. Lancet 389(10074):1151–1164, PMID: 27856085, 10.1016/S0140-6736(16)32124-9. [DOI] [PubMed] [Google Scholar]
- Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Berry H, et al. 2018. The 2018 report of the Lancet Countdown on health and climate change: shaping the health of nations for centuries to come. Lancet 392(10163):2479–2514, PMID: 30503045, 10.1016/S0140-6736(18)32594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WMO (World Meteorological Organization). 2011. Guide to Climatological Practices. WMO‐No. 100, 3rd ed Geneva, Switzerland: World Meteorological Organization. [Google Scholar]
- WHO (World Health Organization). 2016a. Global Report on Diabetes. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO. 2016b. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. https://icd.who.int/browse10/2016/en [accessed 18 October 2018].
- Xavier AC, King CW, Scanlon BR. 2016. Daily gridded meteorological variables in Brazil (1980–2013). Int J Climatol 36(6):2644–2659, 10.1002/joc.4518. [DOI] [Google Scholar]
- Yang J, Yin P, Zhou M, Ou CQ, Li M, Liu Y, et al. 2016. The effect of ambient temperature on diabetes mortality in China: a multi-city time series study. Sci Total Environ 543(Pt A):75–82, PMID: 26580729, 10.1016/j.scitotenv.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Yardley JE, Stapleton JM, Sigal RJ, Kenny GP. 2013. Do heat events pose a greater health risk for individuals with type 2 diabetes? Diabetes Technol Ther 15(6):520–529, PMID: 23530578, 10.1089/dia.2012.0324. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Li S, Coelho MSZS, Saldiva PHN, Hu K, Arblaster JM, et al. 2019. Geographic, demographic, and temporal variations in the association between heat exposure and hospitalization in Brazil: a nationwide study between 2000 and 2015. Environ Health Perspect 127(1):17001, PMID: 30620212, 10.1289/EHP3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Lu Y, Hajifathalian K, Bentham J, Di Cesare M, Danaei G, et al. 2016. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387(10027):1513–1530, PMID: 27061677, 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.