Abstract
Background:
Maternal thyroid hormones are essential for fetal brain development in early gestation. Perfluoroalkyl substances (PFASs)—widespread and persistent pollutants—have been suggested to interfere with maternal thyroid hormones in the second or third trimesters, but evidence for an association in the early pregnancy period is sparse.
Objectives:
Our goal was to evaluate the gestational-week specific associations of maternal thyroid-stimulating hormone (TSH) and free thyroxine (fT4) levels with plasma concentrations of six PFAS chemicals in the first and second pregnancy trimester.
Methods:
A cross-sectional analysis was conducted using 1,366 maternal blood samples collected between gestational weeks (GWs) 5 and 19 (median, 8 gestational weeks) in the Danish National Birth Cohort (DNBC) during 1996–2002. We estimated the percentage changes of serum TSH and fT4 levels according to concentrations (in nanograms per milliliter) of six PFAS chemicals modeled as per interquartile range (IQR) increase or by exposure quartiles. Moreover, we contrasted the estimated week-specific TSH or fT4 levels by PFAS quartile and estimated ORs for binary high or low TSH and fT4 status based on the week-specific distribution according to IQR increase of PFAS.
Results:
TSH levels followed a U-curve trend in early pregnancy with a nadir at GW10, whereas fT4 levels were less fluctuated in the samples. There were no apparent associations between any of the PFASs and changes of average TSH or fT4 levels in total samples. In gestational-week–specific analyses, we found that the estimated TSH values were higher among the highest perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonate (PFHxS), and perfluoroheptane sulfonate (PFHpS) quartiles compared with the lower quartiles from GW5 to GW10, but the difference became null or even reversed after GW10. For binary outcomes, perfluorodecanoic acid (PFDA) was associated with high fT4 status before GW10 [ (95% CI: 1.04, 2.05)].
Conclusions:
We observed some gestational-week–specific associations between high exposure to several PFAS and TSH level in early gestations. Further research of the biology and the potential clinical impact regarding thyroid hormones disruptions in early pregnancy is needed. https://doi.org/10.1289/EHP5482
Introduction
Perfluoroalkyl substances (PFASs) are synthetic chemicals manufactured since the 1950s that have been used to water- and stain proof various industrial and consumer products including clothing, carpets, food packing material, and kitchenware (Bergman et al. 2013; Lindstrom et al. 2011). PFASs are extremely resistant to biotransformation and environmental degradation. The two most commonly used PFASs, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have estimated biological half-lives of 3–5 y in humans (Houde et al. 2006; Olsen et al. 2007). Humans are nearly ubiquitously exposed to PFASs from bioaccumulation in the food chain or contamination of food packaging material, indoor air and household environments, or drinking water (Sun et al. 2016). Production of PFOS and PFOA has been decreasing in the United States and Europe since 2000 (Bergman et al. 2013), but they are still widely detectable (Bjerregaard-Olesen et al. 2016; Chu et al. 2016; Kato et al. 2011). At the same time, exposures to other types of PFASs such as perfluorononanoic acid (PFNA) have found to be increasing (Bjerregaard-Olesen et al. 2016; Kato et al. 2011). Newer types of fluorinated compounds used as substitutes for PFOA, such as Gen-X [also named perfluoro-2-propoxypropanoic acid (PFPrOPrA) or hexafluoropropylene oxide–dimer acid (HFPO-DA)], have also been recently detected in biota (Chu et al. 2016; Gebbink et al. 2017; Sun et al. 2016).
Thyroid hormones (THs) are critical for normal brain development of the fetus during pregnancy and in early postnatal life (Burrow et al. 1994; Greenhill 2017). The fetus begins producing its own supply of THs around the end of the first trimester, and the fetal thyroid gland may be functionally mature at approximately 18 to 20 weeks of gestation (Greenhill 2017; Morreale de Escobar et al. 2004). Thus, in the first to mid-second trimester, the fetus depends largely on maternal THs (Burrow et al. 1994). Recently, evidence has indicated that even subtle changes in maternal thyroid function during pregnancy may impair neurodevelopment in the child (Korevaar et al. 2016; Henrichs et al. 2010; Haddow et al. 1999; Andersen et al. 2017). Maternal TH levels in early gestations change during the course of pregnancy, posing challenges for the detection of anomalies of thyroid function in this early, important period of development (Laurberg et al. 2016).
Biological studies have demonstrated that PFASs have a rather strong ability to interfere with thyroid function, possibly by affecting enzymes in the thyroid gland or disturbing hypothalamic–pituitary–thyroid (HPT) axis responsiveness or binding to transthyretin (Jensen and Leffers 2008; Long et al. 2013; Weiss et al. 2009; Yu et al. 2009). A previous review showed that higher levels of PFOS have been associated with higher levels of thyroid-stimulating hormone (TSH) in the second trimester of pregnancy in four epidemiological studies, but associations between other types of PFAS and THs were inconclusive (Ballesteros et al. 2017). The majority of previous studies were smaller in size and have evaluated associations between PFASs and THs only in the second and third trimester (Ballesteros et al. 2017). Only one study evaluated PFASs and THs in early pregnancy [around gestational week (GW) 10] among 732 mothers in Boston, Massachusetts, and found no associations between six types of PFAS and maternal total thyroxine (T4); however, PFOA, perfluorohexane sulfonate (PFHxS), and 2-(N-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA) were inversely associated with the maternal free T4 (fT4) index, calculated from total T4 and level of triiodothyronine (T3) resin uptake (T3U) to estimate the circulating fT4 levels after accounting for thyroid-binding protein levels (Preston et al. 2018).
In this study, we evaluated the associations between six types of PFAS and maternal thyroid function (determined by TSH and fT4) in early pregnancy among 1,366 pregnant women enrolled in the Danish National Birth Cohort (DNBC). We also investigated whether the possible PFAS and TSH or fT4 relationships might vary by gestational week in early pregnancy.
Materials and Methods
Study Participants
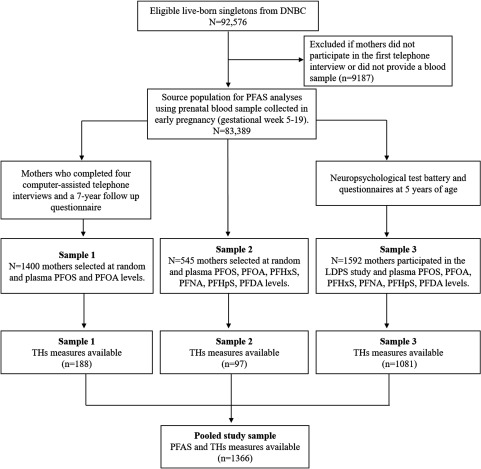
The DNBC is a national pregnancy cohort study established during 1996–2002 with 100,417 pregnant women originally enrolled by their general practitioners at their first antenatal visit (Olsen et al. 2001). In the present study, we analyzed data from 1,366 pregnancies in the DNBC for which both PFAS and TH levels were measured in the same maternal plasma samples all collected in the same time period (i.e., 1996–2002) at study enrollment and during GW5 to GW19 {. Specifically, prenatal PFAS measures were generated in three subcohort studies nested within the DNBC (termed Study Samples 1, 2, and 3 according to the chronological order of the time of measure) (Fei et al. 2007; Liew et al. 2014, 2018b). TH levels were measured in two samples, with one selected about 12% random subset of the DNBC to establish TH screening reference limits in early pregnancy (Laurberg et al. 2016). The second-sample included mothers and children participated in the nested Lifestyle During Pregnancy Study (LDPS) cohort designed to evaluate prenatal alcohol intake and neuropsychological outcomes in children at 5 years of age (Andersen et al. 2018b; Kesmodel et al. 2010).
Details of the sampling criteria and flowchart of selection for the three study samples are presented in Figure 1. Briefly, Study Sample 1 measured PFOA and PFOS for 1,400 mothers selected at random from among those who completed all four baseline interviews and a 7-y follow-up questionnaire (Fei et al. 2007); among these women, 188 had TH measures available. Study Sample 2 included 545 children randomly selected at birth (boy-to-girl ratio of 4:1) as population controls for a case–cohort study (Liew et al. 2015); among these children, 97 had TH measures available. Study Sample 3 contained the majority of all samples used in this study () from mothers and children who participated in the nested LDPS cohort at 5 years of age (Kesmodel et al. 2010). Study Sample 3 had a higher proportion of women with alcohol intake during pregnancy due to the over-sampling strategy. Measures of 16 types of PFAS were available in Study Samples 2 and 3.
Figure 1.
Sample selection for the three substudy samples. Note: DNBC, Danish National Birth Cohort; LDPS, Lifestyle During Pregnancy Study; PFAS, perfluoroalkyl substance; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; TH, thyroid hormone.
All participants provided written informed consent at the time of inclusion in the DNBC. The research protocol for this study was approved by the Danish data inspectorate (journal no. 2016-051-000001, serial no. 1343), and the institutional review boards at UCLA (16-001849) and Yale University (2000024089).
PFAS Measurements
All blood samples collected in the DNBC were sent by mail to the Statens Serum Institute in Copenhagen and stored in or freezers. Samples in the Study Samples 2 and 3 were analyzed at the Department of Environmental Science at Aarhus University, Denmark (Liew et al. 2014), and samples in Study Sample 1 were analyzed at the Toxicology Laboratory (San Paul, Minnesota, USA) (Ehresman et al. 2007). Details about the analytic methods for PFAS measures have been described elsewhere (Fei et al. 2007; Liew et al. 2014, 2015). Briefly, stored maternal plasma retrieved from the biobank for each participants were sorted in random order. Both laboratories employed similar analytic methods using a solid-phase extraction technique for sample extraction and purification, and liquid chromatography–tandem mass spectrometry to measure PFAS concentrations. Only PFOS and PFOA were measured in Study Sample 1 because only these two PFAS compounds could be measured in 2007. Sixteen PFASs were measured in Study Samples 2 and 3 between 2011 and 2014. For Study Samples 2 and 3, our analyses focused on six types of PFAS that were quantifiable in of all measured samples and included PFOS at 100%; PFOA, 100%; PFHxS, 98%; perfluoroheptane sulfonate (PFHpS), 96%; PFNA, 92%; and perfluorodecanoic acid (PFDA), 90% (Liew et al. 2014, 2015). Values below the lower limit of quantitation were replaced by a multiple imputation algorithm that included all PFASs and some demographic variables (Liew et al. 2014, 2018b). Details on detection and quantitation limits for each PFAS can be found in Table S1.
Comparisons of PFAS measurements in the two laboratories have been performed previously (Liew et al. 2014). We found that the absolute PFOS and PFOA value read-outs at the laboratory were slightly higher than at the Aarhus University laboratory, but the correlations of PFOS and PFOA concentrations measured in the same samples () by the two laboratories were very high (Pearson correlation for PFOS and for PFOA). Pooled analyses combining PFAS measures across the study samples generated in the DNBC have been conducted (Bach et al. 2015; Ernst et al. 2019; Meng et al. 2018).
Thyroid Hormone Measurements
TSH and fT4 were measured in 2015 using the Dimension Vista® automated immunoassay (Siemens Healthcare Diagnostics) as described in detail elsewhere (Laurberg et al. 2016). Most of the women included in the studies did not have thyroid disease (i.e., no diagnosis or treatment) nor did they receive treatment for thyroid disease at the time of blood sampling (Andersen et al. 2018a). The gestational-week–specific distributions of TSH and fT4 from GW5 to GW19 in the DNBC have been described previously; TSH exhibited a U-curved trend with a nadir at GW10, and fT4 exhibited an inverted U-curved trend with a peak also at GW10 (Laurberg et al. 2016).
Covariates
Potential confounding factors were selected a priori according to a directed acyclic graph (see Figure S1). Our models included maternal age at delivery (, 25–29, 30–34, ), parity (0, 1, ), socio-occupational status (a four-level variable created based on the highest maternal or paternal education and occupation as 1 for a high-grade professional with long-term education; 2 for a medium-grade professional with medium-term education; 3 for a skilled worker or work in position that required a shorter education; and 4 for unskilled workers, unemployment, or those on financial assistance), maternal prepregnancy body mass index (BMI; , 18.5 to , 25 to , ), gestational week of blood sampling measure (continuous and square transformed), maternal smoking during pregnancy (yes, no), and child’s birth year (, ). Information regarding these demographic variables were collected from maternal interveiws conducted during pregnancy. The gestational week of blood sampling was calculated using the date of sampling registered in the DNBC biobank, and the date of birth and the gestational age at birth of the child were obtained from the Danish Medical Birth Registry (Laurberg et al. 2016). In Denmark, gestational age is recorded based on the date of last menstrual period (on average 2 weeks before conception) in combination with ultrasound verification. We calculated the first day from the last menstrual period by subtracting the registered gestational age (in days) from the date the child was born, and then estimated the gestational week of blood sampling from the date of sampling. Gestational week of blood sampling ranged from GW5 to GW19.
Statistical Analysis
TSH and fT4 levels were first analyzed as continuous values after a natural-log transformation to address normality assumptions in linear regression models and improve model fit. We used multivariable linear regression models to estimate the relationships between ln-TSH or ln-fT4 and PFAS exposures. To increase the interpretability of the results, the coefficient for the exposure variable in this model was exponentiated and reported as the relative change (or ratio) of the geometric mean of TSH (in milli-international units per liter) and fT4 (in picomoles per liter) according to exposures. Moreover, we also calculated the percent difference using the formula to facilitate comparisons with a previous study (Preston et al. 2018). PFASs were analyzed as continuous [per interquartile range (IQR) increase] or categorical (quartiles with the lowest quartile as reference) variables. The IQR for each PFAS was calculated using the total sample distributions (see Table S2). All potential covariates mentioned above were also included in the model.
We conducted stratified analyses using binary categories for socio-occupational status (Levels 1 and 2 vs. Levels 3 and 4), parity (nulliparous vs. multiparous), maternal smoking (smoker vs. nonsmoker), child’s birth year ( vs. ), geographical residence (East vs. West Denmark), maternal age at birth ( vs. ), and alcohol intake during pregnancy (drinker vs. nondrinker) because these factors had previously been suggested to be associated with maternal TH function in the DNBC (Andersen et al. 2018a, 2018b; Laurberg et al. 2016) and they could potentially modify the effect estimates of PFAS exposures (Ballesteros et al. 2017; Liew et al. 2018a; Rappazzo et al. 2017). Tests of heterogeneity were also performed by assessing the p-value of the interaction term for each PFAS and potential modifying factors in the regression models.
Because maternal TSH or fT4 values may follow (inverted) U-shapes in early pregnancy, we included a linear and a quadratic term for the continuous gestational week variable in the models. To account for possible laboratory differences or batch effect, an indicator variable for study (1, 2, or 3) was entered for all continuous PFAS analyses (Bach et al. 2015; Meng et al. 2018), and a study sample–specific cutoff was used to generate PFAS quartiles. PFAS levels below the lower limit of quantitation and missing covariate values were replaced using a multiple imputation algorithm that included six PFAS and all of the abovementioned covariates in the model (Lubin et al. 2004).
We also fitted a more flexible model to study whether the PFAS and TSH or fT4 relationships might vary by gestational week. We included interaction terms for the gestational week (continuous and square transformed) and binary exposure variables (the lowest three quartiles vs. the highest quartile) into multivariable linear regression models. We then estimated the expected TSH and fT4 values for each PFAS exposure group in each gestational week while adjusting for potential confounders using the marginal standardization form of the predictive margins (Williams 2012). Considering the dimensionality of this model, all PFAS values were dichotomized as low or high. First, we compared the three lower PFAS quartiles to the highest quartile, and second, we compared only the lowest to the highest quartile. We furthermore performed analyses for binary PFAS and continuous TSH and fT4 values according to whether the samples were collected before or after GW10. GW10 was suggested as the nadir point for the dynamic changes of TSH and fT4 during early pregnancy, as previously reported for this cohort (Laurberg et al. 2016).
Moreover, we studied possible TH abnormalities based on the gestational-week–specific distribution of TSH and fT4 (Laurberg et al. 2016). Because the number of women with clinical or subclinical hyper- or hypothyroidism (Cooper and Biondi 2012) was very small and we focused on the dynamic changes in THs by gestational week (Laurberg et al. 2016) rather than thyroid disease status, we used 10th and 90th percentile week–specific cutoffs to classify women as having a relatively high or low TSH or fT4 hormone status; for example, low TSH (), high TSH (), low fT4 (), or high fT4 () status. Multinomial logistic regression models were used to estimate odds ratios (ORs) for each outcome and continuous PFAS exposures. We used normal [10th–90th] TH levels as the reference group to which we compared low () or high () TH–level groups. We also performed the multinomial logistic regression models using only Study Sample 3 or excluding 37 women who self-reported ever receiving a diagnosis of thyroid disease, including those currently having the conditions in early gestations.
In sensitivities analyses, we additionally adjusted for geographical residence (East vs. West Denmark) as a proxy variable for iodine intake. There are geographical differences in iodine intake levels due to different iodine contents in Danish groundwater (Laurberg et al. 2010). Moreover, we also controlled for fish intake (less than once per month, less than once per week, more than once per week) and maternal alcohol intake (yes, no) during early pregnancy. To assess the influence of co-exposure to different PFASs, we contrasted multi-pollutant models for continuous TH levels after including one PFAS at a time according to their prevalence. Our first model included only PFOS and PFOA, and we then added—one by one—PFHxS, PFNA, PFHpS, and PFDA (in this order). Analyses that co-adjusted for all six PFASs were also performed with gestational-week–specific TH values and binary PFAS exposures.
Stabilized inverse-probability-weights (IPWs) were used to account for subject selections into each of the subsamples (Liew et al. 2018b; Meng et al. 2018). Briefly, the IPWs first accounted for the disproportionate infant’s sex ratios in Study Sample 2 and the oversampling of alcohol drinkers in Study Sample 3. The selection criteria of Study Samples 1 and 3 conditioned on follow-up at 5 years of age in the nested LDPS cohort (among those invited, participated), or at 7 years of age among mothers who returned an online questionnaire (among those invited, participated), which could induce selection bias. Therefore, the IPWs also incorporated the modeled probabilities of follow-up using a range of baseline factors that were available for all eligible women. Some of the main predictors for participation included maternal age, parental social class, prepregnancy BMI, maternal smoking and alcohol intake during pregnancy, organic food intake during pregnancy, child’s birth year, and child’s birth outcome such as preterm birth and low birth weight (Liew et al. 2018b; Meng et al. 2018). Robust variance estimators were used to compute 95% confidence intervals (CIs) in all weighted regression analyses. All statistical analyses were conducted using SAS (version 9.4; SAS Institute Inc.) and STATA (version 15; StataCorp LLC).
Results
Table 1 presents the baseline demographic characteristics of the study participants (unweighted) by the study sample. The PFOS and PFOA values in Study Sample 1 were slightly higher because of laboratory difference, whereas PFAS levels in Study Samples 2 and 3 were rather comparable. A Pearson correlation matrix for the six PFASs is presented in Table S3. The gestational-week–specific distribution of maternal TSH and fT4 in all samples is presented in Table S4.
Table 1.
Study characteristics of the three study samples (unweighted) from the Danish National Birth Cohort.
| Characteristics | Total | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|---|
| () | () | () | () | |
| Thyroid hormones [GM (95% CI)] | ||||
| TSH (mIU/L) | 1.13 (1.08, 1.19) | 1.20 (1.06, 1.37) | 1.01 (0.86, 1.18) | 1.13 (1.08, 1.19) |
| fT4 (pmol/L) | 14.2 (14.1, 14.3) | 14.6 (14.3, 14.9) | 14.9 (14.6, 15.2) | 14.1 (14.0, 14.2) |
| PFAS {ng/mL [median (IQR)]} | ||||
| PFOS | 29.5 (22.6–37.7) | 34.2 (27.5–43.5) | 29.6 (19.3–37.0) | 28.6 (22.2–36.5) |
| PFOA | 4.52 (3.38–5.80) | 5.41 (3.39–7.10) | 4.14 (2.88–5.84) | 4.43 (3.34–5.62) |
| PFHxS | 1.11 (0.83–1.39) | NAa | 0.94 (0.58–1.23) | 1.13 (0.84–1.41) |
| PFNA | 0.45 (0.36–0.57) | NAa | 0.42 (0.34–0.52) | 0.46 (0.36–0.57) |
| PFHpS | 0.37 (0.27–0.49) | NAa | 0.30 (0.21–0.43) | 0.38 (0.28–0.50) |
| PFDA | 0.17 (0.13–0.22) | NAa | 0.16 (0.11–0.24) | 0.17 (0.14–0.22) |
| Mother’s age {y [ (%)]} | ||||
| 19–29 | 607 (44.4) | 82 (43.6) | 51 (52.6) | 474 (43.9) |
| 30–34 | 534 (39.1) | 74 (39.4) | 34 (35.0) | 426 (39.4) |
| 35–45 | 225 (16.5) | 32 (17.0) | 12 (12.4) | 181 (16.7) |
| Gestational week at time of blood collection [mean (min, max)] | 8.3 (5, 19) | 8.0 (5, 13) | 7.8 (5, 14) | 8.4 (5, 19) |
| Parity [ (%)] | ||||
| 0 | 716 (52.4) | 85 (45.2) | 49 (50.5) | 582 (53.8) |
| 1 | 410 (30.0) | 68 (36.2) | 34 (35.1) | 308 (28.5) |
| 240 (17.6) | 35 (18.6) | 14 (14.4) | 191 (17.7) | |
| Parental socio-occupational status [ (%)] | ||||
| 1 (the highest) | 491 (35.9) | 61 (32.5) | 28 (28.9) | 402 (37.2) |
| 2 | 450 (32.9) | 59 (43.6) | 28 (28.9) | 363 (33.6) |
| 3 | 388 (28.4) | 62 (33.0) | 36 (37.1) | 290 (26.8) |
| 4 (the lowest) | 37 (2.7) | 6 (3.2) | 5 (5.2) | 26 (2.4) |
| Maternal smoking during early pregnancy [ (%)] | 432 (31.6) | 52 (27.7) | 26 (26.8) | 354 (32.8) |
| Mother’s prepregnancy BMI { [ (%)]} | ||||
| 79 (5.8) | 8 (4.3) | 8 (8.3) | 63 (5.8) | |
| 923 (67.6) | 113 (60.1) | 59 (60.8) | 751 (69.5) | |
| 262 (19.2) | 47 (25.0) | 21 (21.7) | 194 (18.0) | |
| 102 (7.5) | 20 (10.6) | 9 (9.3) | 73 (6.8) | |
| Birth year {y [ (%)]} | ||||
| 402 (29.4) | 56 (29.6) | 35 (35.7) | 311 (28.8) | |
| 966 (70.6) | 133 (70.4) | 63 (64.3) | 770 (71.2) | |
| Fish intake | ||||
| No | 52 (3.8) | 6 (3.2) | 5 (5.1) | 41 (3.8) |
| Low | 220 (16.1) | 34 (18.0) | 18 (18.4) | 168 (15.5) |
| Medium | 673 (49.2) | 95 (50.3) | 45 (45.9) | 533 (49.3) |
| High | 423 (30.9) | 54 (28.6) | 30 (30.6) | 339 (31.4) |
| Geographical residence [ (%)] | ||||
| East | 484 (35.4) | 53 (28.2) | 43 (44.3) | 388 (35.9) |
| West | 882 (64.6) | 135 (71.8) | 54 (55.7) | 693 (64.1) |
Note: BMI, body mass index; CI, confidence interval; fT4, free thyroxine; GM, geometric mean; IQR, interquartile range; max, maximum; min, minimum; NA, not available; PFAS, perfluoroalkyl substance; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; Ref, reference; TSH, thyroid-stimulating hormone.
PFHxS, PFNA, PFHpS, and PFDA were not measured in Sample 1.
PFAS Exposure and Average Changes of Thyroid Hormone Levels in Early Pregnancy
There were no apparent associations between higher PFASs in a linear model or exposure quartiles and changes in average TSH or fT4 levels in all samples (Table 2). These findings did not change when additionally adjusting for fish intake in pregnancy, geographical residence, and alcohol intake (see Table S5) or in models that mutually adjusted for different types of PFAS (see Table S6). There was also no evidence for effect measure modifications with any of the PFASs by strata of socio-occupational status, parity, maternal smoking, child’s birth year, geographical residence, maternal age at birth, or alcohol intake during pregnancy (see Table S7).
Table 2.
Associations between maternal thyroid hormone levels and plasma PFAS levels (ng/mL) in all samples.
| PFAS | TSH (mIU/L) | fT4 (pmol/L) | ||
|---|---|---|---|---|
| Relative percentage difference (95% CI) | Absolute percentage difference (95% CI) | Relative percentage difference (95% CI) | Absolute percentage difference (95% CI) | |
| PFOS | ||||
| Per IQR increase | 1.04 (0.96, 1.14) | 4.3 (, 13.8) | 1.00 (0.99, 1.02) | 0.4 (, 1.8) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.86 (0.69, 1.06) | (, 5.7) | 1.03 (0.99, 1.06) | 2.5 (, 5.8) |
| Quartile 3 | 0.96 (0.78, 1.17) | (, 17.4) | 1.02 (0.99, 1.05) | 2.1 (, 5.0) |
| Quartile 4 | 1.01 (0.83, 1.22) | 0.8 (, 22.0) | 1.01 (0.98, 1.04) | 0.8 (, 3.9) |
| PFOA | ||||
| Per IQR increase | 1.01 (0.93, 1.1) | 1.5 (, 10.3) | 1.01 (0.99, 1.02) | 0.6 (, 1.9) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.96 (0.78, 1.19) | (, 18.8) | 0.99 (0.97, 1.02) | (, 2.4) |
| Quartile 3 | 1.02 (0.83, 1.25) | 1.6 (, 24.7) | 1.01 (0.98, 1.04) | 1.2 (, 4.4) |
| Quartile 4 | 1.08 (0.86, 1.36) | 8.2 (, 35.9) | 1.01 (0.98, 1.04) | 0.7 (, 3.9) |
| PFHxS | ||||
| Per IQR increase | 1.02 (0.96, 1.08) | 1.7 (, 8.1) | 1.00 (0.98, 1.01) | (, 1.0) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.98 (0.79, 1.21) | (, 20.8) | 0.99 (0.96, 1.02) | (, 2.3) |
| Quartile 3 | 0.92 (0.68, 1.24) | (, 24.2) | 1.01 (0.97, 1.05) | 1.3 (, 5.4) |
| Quartile 4 | 1.03 (0.84, 1.27) | 3.0 (, 27.0) | 1.02 (0.99, 1.06) | 2.5 (, 6.1) |
| PFNA | ||||
| Per IQR increase | 1.01 (0.95, 1.08) | 1.3 (, 7.7) | 1.00 (0.99, 1.01) | (, 0.9) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.97 (0.75, 1.26) | (, 25.9) | 1.00 (0.96, 1.04) | (, 3.7) |
| Quartile 3 | 1.01 (0.81, 1.27) | 1.4 (, 27.1) | 1.01 (0.97, 1.04) | 0.6 (, 4.3) |
| Quartile 4 | 1.03 (0.82, 1.29) | 3.1 (, 29.0) | 1.00 (0.96, 1.03) | (, 3.1) |
| PFHpS | ||||
| Per IQR increase | 1.03 (0.94, 1.13) | 3.4 (, 13.4) | 1.00 (0.99, 1.02) | 0.1 (, 1.6) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.95 (0.75, 1.22) | (, 22.1) | 0.99 (0.96, 1.03) | (, 2.9) |
| Quartile 3 | 1.01 (0.82, 1.23) | 0.6 (, 23.5) | 1.02 (0.99, 1.05) | 1.8 (, 5.1) |
| Quartile 4 | 1.07 (0.86, 1.33) | 7.1 (, 33.0) | 1.00 (0.97, 1.03) | (, 3.1) |
| PFDA | ||||
| Per IQR increase | 0.99 (0.93, 1.05) | (, 4.9) | 1.01 (1.00, 1.02) | 0.8 (, 1.9) |
| Quartile 1 | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.97 (0.79, 1.21) | (, 20.6) | 1.01 (0.98, 1.04) | 1.0 (, 4.3) |
| Quartile 3 | 1.00 (0.78, 1.29) | 0.4 (, 28.5) | 1.02 (0.98, 1.05) | 1.5 (, 5.4) |
| Quartile 4 | 1.04 (0.83, 1.29) | 3.6 (, 28.8) | 1.00 (0.96, 1.03) | (, 2.7) |
Note: The model was adjusted for maternal age, parental socio-occupational status, prepregnancy BMI, parity, maternal smoking, and birth year. Study sample indicators (1, 2, 3) were included in the model for continuous exposure and study-specific distributions were used to define PFAS quartiles. Relative percentage difference was calculated as exp(beta). Absolute percentage difference was calculated as . BMI, body mass index; CI, confidence interval; fT4, free thyroxine; IQR, interquartile range; PFAS, perfluoroalkyl substance; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; Ref, reference; TSH, thyroid-stimulating hormone.
Potential Modifying Effects of PFAS on Thyroid Hormones by Gestational Week
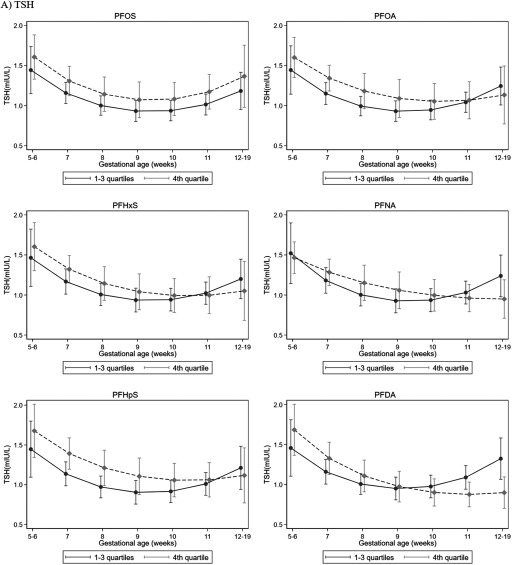
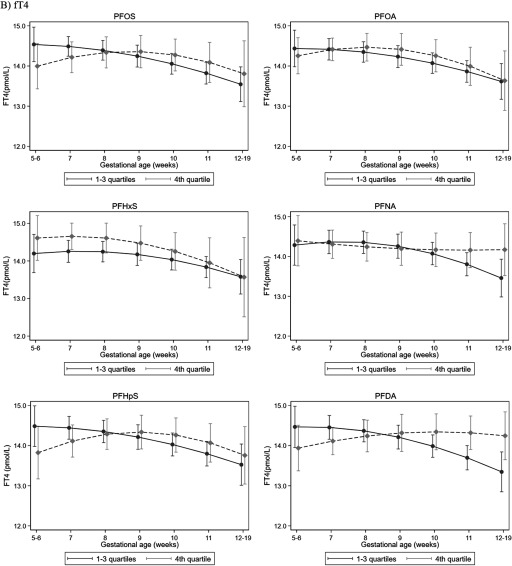
In models that allowed gestational-week–specific comparisons of the expected TSH or fT4 values by PFAS exposure, we found that the estimated TSH levels were higher in the top exposure quartile compared with the lower three quartiles for all PFASs from GW5 through GW9 or 10, but this trend became null and reversed after GW10; that is, after GW10, the highest quartile of exposure appeared to be associated with a lower estimated TSH level except for PFOS (Figure 2; see also Table S8). Less apparent differences in trend were found for fT4. There was also a possible reversing of trends for the top quartiles of PFOS, PFHpS, and PFDA that had lower expected fT4 levels prior to GW8 but higher thereafter. These reversing trends (i.e., higher estimated TSH prior to GW10 but lower after) were found for PFOA, PFNA, and PFHpS when we compared the highest to the lowest quartiles (see Figure S2). Trends did not change after simultaneously adjusting for all six PFASs (see Figure S3). In analyses stratified by gestational week (, ) at which samples were collected (see Table S9), the highest PFDA quartile was associated with higher TSH levels before GW10 but with lower TSH levels at or after GW10 (). Similar directions were observed for other PFAS and TSH levels, but the 95% CIs of the gestational-week–stratified estimates overlapped and included the null.
Figure 2.
Adjusted (A) TSH and (B) fT4 levels in each gestational week according to binary PFAS exposure. We estimated the expected TSH and fT4 value for each gestational week, comparing the top PFAS quartile to the lower three quartiles while adjusting for maternal age, socio-occupational status, BMI, parity, smoking, and birth year. Study-specific distribution was used to define PFAS quartiles. The model also included interaction terms between PFAS (binary) and gestational week (continuous value and a square term). Whiskers and bars represent 95% CIs in each gestation week. Detailed numeric data for the figure is presented in Table S8. Note: BMI, body mass index; CI, confidence interval; fT4, free thyroxine; PFAS, perfluoroalkyl substance; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; TSH, thyroid-stimulating hormone.
PFAS Exposures on Thyroid Status (Based on Gestational-Week–specific Cutoffs)
There were no apparent associations between PFAS exposures and week-specific high or low TSH or fT4 status in the analysis restricted to samples taken before GW10 except for PFDA, which appeared to be correlated with high fT4 levels in the early pregnancy weeks [ (95% CI: 1.04, 1.80)] (Table 3). Results were similarly null in the analysis that included all gestational weeks (GW5 to GW19) but only using Study Sample 3 and in the analysis excluding women with thyroid diseases (see Tables S10 and S11).
Table 3.
High or low week–specific thyroid hormones status according plasma PFAS levels from GW5 to GW10.
| Per IQR increase of each PFAS (ng/mL) | OR and 95% CIb,c | |||
|---|---|---|---|---|
| Low TSH ()a | High TSH ()a | Low fT4 ()a | High fT4 ()a | |
| PFOS | 1.10 (0.70, 1.74) | 0.82 (0.51, 1.31) | 0.77 (0.51, 1.17) | 1.13 (0.75, 1.68) |
| PFOA | 0.85 (0.59, 1.24) | 0.73 (0.43, 1.25) | 0.70 (0.36, 1.39) | 1.05 (0.85, 1.31) |
| PFHxS | 1.12 (0.85, 1.47) | 1.15 (0.90, 1.47) | 1.07 (0.71, 1.63) | 1.18 (0.91, 1.53) |
| PFNA | 0.96 (0.70, 1.32) | 0.81 (0.57, 1.15) | 0.87 (0.63, 1.18) | 1.09 (0.84, 1.43) |
| PFHpS | 1.31 (0.80, 2.15) | 0.87 (0.53, 1.42) | 0.82 (0.53, 1.26) | 1.19 (0.74, 1.92) |
| PFDA | 1.15 (0.89, 1.50) | 0.86 (0.61, 1.23) | 0.87 (0.60, 1.27) | 1.37 (1.04, 1.80) |
Note: BMI, body mass index; CI, confidence interval; fT4, free thyroxine; GW, gestational week; IQR, interquartile range; OR, odds ratio; PFAS, perfluoroalkyl substance; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; TSH, thyroid-stimulating hormone.
Low or high TSH and fT4 status were defined using the week-specific lowest 10th or highest 90th percentile.
ORs for each thyroid status were calculated per IQR increase in continuous variables.
Adjusted for maternal age, parental socio-occupational status, BMI, parity, maternal smoking, birth year, and an indicator for study sample.
Discussion
In this study, we found no strong association between six types of prenatal PFASs and average changes of TSH or fT4 values in prenatal maternal samples collected from GW5 to GW19 in the DNBC. However, gestational-week–specific analyses suggested a possible crossover trend with top exposure quartiles for several PFASs being associated with higher TSH values up to GW10 and lower values thereafter, and the opposite pattern was observed for fT4 and the highest quartiles of three types of PFAS. These results suggest that PFASs may affect the natural U- or inverted U-shape trends for TSH or fT4 levels in the first trimester of pregnancy with a turning point at GW9 or GW10 (Greenhill 2017; Laurberg et al. 2016). Nevertheless, these gestational-week–specific comparisons in our data were not precise enough to allow us to draw strong conclusions and reevaluation is needed in larger samples. The associations found between PFDA and high fT4 status in early pregnancy could reflect chance findings, but they warrant further investigation.
Even a small disruption in maternal THs may have considerable implications for fetal neurodevelopment (Lazarus et al. 2012), but little is currently known about the possible influence that PFASs may have on these maternal hormones in early gestation. Several pregnancy cohort studies have investigated associations between PFASs and THs in the second or third pregnancy trimesters, but most had relatively small sample sizes with fewer than 400 subjects (Ballesteros et al. 2017; Jensen and Leffers 2008; Wang et al. 2014). Different types of PFASs have been suggested to be either positively or negatively correlated with THs in these studies, with the most consistent finding being a positive association between PFOS and TSH status in the second trimester (Ballesteros et al. 2017). One recent study from Boston that analyzed 732 early pregnancy samples collected around GW10 reported that PFOA, PFOS, and PFNA were inversely associated with TSH levels but only among the subset of women with positive thyroid peroxidase antibody and that PFOA, PFHxS, and MeFOSAA were inversely associated with a maternal fT4 index calculated from total T4 and T3 uptake (Preston et al. 2018). Our study examined fT4 levels measured via immunoassay instead of using an fT4 index. Although fT4 immunoassay methods could be sensitive to alterations in thyroxine-binding protein in pregnancy (Alexander et al. 2017; Lee et al. 2009), previous studies in the DNBC found that fT4 measures, as well as TSH measures during early pregnancy, were predictive of adverse neurodevelopmental disorders and impaired psychological function in the offspring (Andersen et al. 2018a, b). In addition, our large sample size enabled us to evaluate whether each of the PFAS and TSH or fT4 associations varied by gestational week.
Our findings indicate that PFASs possibly disrupt the normal dynamic TH response in early pregnancy, but whether these minor changes have any developmental implications for the fetus is unknown. Toxicological studies have indicated that PFASs, mainly PFOS and PFOA, have thyroid-disrupting effects that result in hypothyroid function, possibly by regulating hepatic glucuronidation enzymes and deiodinases in the thyroid gland (Yu et al. 2009) or reducing HPT axis responsiveness (Long et al. 2013) or binding to transthyretin (Ren et al. 2015; Weiss et al. 2009). Moreover, PFHxS was reported to induce the expression of type 3 deiodinase (Vongphachan et al. 2011), which is the physiological inactivator of THs (Huang 2005). The dynamic changes in thyroid homeostasis in early pregnancy are mainly due to the surge in human chorionic gonadotropin (hCG) produced by the placenta (i.e., the alpha subunit of the hCG protein, nearly identical to the alpha subunit of TSH), thus TH activities may be altered if PFASs disrupt optimal placenta development and subsequently influence hCG levels (Kim et al. 2011; Yoshimura and Hershman 1995). The null findings from our main analysis need to be further elaborated. A possible explanation is that such an analysis does not consider gestational-week–specific variations; for example, if PFASs affect TH levels differently in early versus mid-gestation, this would be expected to result in an overall null association when measures are averaged across all weeks.
Our study has several limitations. First, our analyses did not account for other hemodynamic factors such as albumin and the estimated glomerular filtration rate (eGFR) that could have influenced our results. Albumin is a minor thyroid-binding protein that also binds to PFASs (Loccisano et al. 2013; Zoeller 2010), and the eGFR might affect the renal clearance of PFASs and of iodine—needed for TH production—in pregnancy (Loccisano et al. 2013; Sagiv et al. 2015; Zoeller 2010). Nevertheless, the Boston study showed no evidence for confounding of associations between PFASs and THs in early pregnancy when adjusting for albumin or eGFR in their analyses (Preston et al. 2018). Second, thyroid peroxidase antibody status was not measured in the DNBC; thus, we were not able to evaluate possible thyroid-altering effects of PFASs through autoimmune damage (Preston et al. 2018; Webster et al. 2014). We did not have sufficient numbers to evaluate associations in women with overt or subclinical thyroid dysfunction, and few women in these samples reported thyroid diseases and treatment during early gestations (Andersen et al. 2018a, 2018b). Therefore, the findings of this study are not generalizable to women with thyroid diseases. In addition, we did not have information on iodine intake in the women, and iodine is an essential micronutrient necessary for the synthesis of THs and preventing long-term adverse health outcomes (Inoue et al. 2018; Zimmermann and Boelaert 2015). Main sources of iodine in Denmark are milk, dairy products, iodized salt (used in the commercial production of bread), seafood, and other iodine-containing supplements (Rasmussen et al. 2002). In Denmark, the policy of compulsory iodine supplementation was established on a national level in 2000 (Blomberg et al. 2012). Denmark was previously iodine deficient, with regional differences (Andersen et al. 2015). Thus, we used residency as a proxy for iodine levels in our study population, but the results of the stratified analyses were similar. Future studies should address the potential modifying effect of iodine in the association between PFASs and THs in early pregnancy. We cannot rule out influences from residual uncontrolled confounding, especially dietary habits and lifestyles. Although our analyses were cross sectional, the six evaluated PFASs have long biological half-lives, and thus our measures likely represent cumulative exposures from before pregnancy until the blood samples were taken. Our findings from gestational-week–specific analyses lack statistical precision, especially in early and later weeks when we had fewer samples available. Most of the women who enrolled in the DNBC were healthy and did not have thyroid disorders, and our study did not have sufficient numbers to evaluate associations in women with clinical or subclinical thyroid dysfunction. Finally, TH levels in each gestational week were from different women, and further investigation with repeated samples for each woman is warranted.
In conclusion, we did not find strong evidence to suggest that the evaluated PFASs were associated with overall TSH and fT4 values in the DNBC. Our gestational-week–specific analyses, however, suggested that at the highest PFAS levels, TH levels may be impacted in early pregnancy.
Supplementary Material
Acknowledgments
Z.L., J.O., and S.L.A. conceptualized and designed the study. Z.L., J.O., and B.R. supervised the research activities. Z.L. and K.I. performed data analyses. Z.L. and K.I. drafted the manuscript. All authors contributed to writing the manuscript and interpretation of results.
The Danish National Research Foundation established the Danish Epidemiology Science Centre, which initiated and created the Danish National Birth Cohort. Additional support for the Danish National Birth Cohort was obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation and The Danish Council for Strategic Research.
This work is supported by the National Institutes of Health/National Institute of Environmental Health Sciences Pathway to Independence Award (K99ES026729/R00ES026729) and the Danish Council for Strategic Research (10-092818; http://www.fetotox.au.dk). K.I. was supported by the Burroughs Wellcome Fund Interschool Training Program in Chronic Diseases (BWF-CHIP) and Fellowship in Epidemiology at UCLA.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5482).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389, PMID: 28056690, 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Andersen S, Liew Z, Vestergaard P, Olsen J. 2018a. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab 103(2):660–670, PMID: 29220528, 10.1210/jc.2017-02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Andersen S, Vestergaard P, Olsen J. 2018b. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish nationwide case-cohort study. Thyroid 28(4):537–546, PMID: 29584590, 10.1089/thy.2017.0425. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Carlé A, Karmisholt J, Pedersen IB, Andersen S. 2017. Mechanisms in endocrinology: neurodevelopmental disorders in children born to mothers with thyroid dysfunction: evidence of fetal programming? Eur J Endocrinol 177(1):R27–R36, PMID: 28377377, 10.1530/EJE-16-0947. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Sørensen LK, Krejbjerg A, Møller M, Klitbo DM, Nøhr SB, et al. 2015. Iodine status in Danish pregnant and breastfeeding women including studies of some challenges in urinary iodine status evaluation. J Trace Elem Med Biol 31:285–289, PMID: 25535149, 10.1016/j.jtemb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC, et al. 2015. Perfluoroalkyl acids and time to pregnancy revisited: an update from the Danish National Birth Cohort. Environ Health 14:59, PMID: 26148742, 10.1186/s12940-015-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa M-J. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28, PMID: 27884404, 10.1016/j.envint.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. 2013. State of the Science of Endocrine Disrupting Chemicals—2012. Geneva, Switzerland: United Nations Environment Programme and World Health Organization; http://apps.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf [accessed 24 April 2018]. [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, et al. 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environ Int 91:14–21, PMID: 26891270, 10.1016/j.envint.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. 2012. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer 131(10):2360–2366, PMID: 22337133, 10.1002/ijc.27497. [DOI] [PubMed] [Google Scholar]
- Burrow GN, Fisher DA, Larsen PR. 1994. Maternal and fetal thyroid function. N Engl J Med 331(16):1072–1078, PMID: 8090169, 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Chu S, Letcher RJ, McGoldrick DJ, Backus SM. 2016. A new fluorinated surfactant contaminant in biota: perfluorobutane sulfonamide in several fish species. Environ Sci Technol 50(2):669–675, PMID: 26649981, 10.1021/acs.est.5b05058. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Biondi B. 2012. Subclinical thyroid disease. Lancet 379(9821):1142–1154, PMID: 22273398, 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang S-C, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 103(2):176–184, PMID: 16893538, 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect 127(1):17004, PMID: 30628845, 10.1289/EHP3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: 18008003, 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L, van Leeuwen S. 2017. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands. Environ Sci Technol 51(19):11057–11065, PMID: 28853567, 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill C. 2017. Thyroid function: thyroid hypofunction in pregnancy. Nat Rev Endocrinol 13(10):562, PMID: 28799552, 10.1038/nrendo.2017.106. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555, PMID: 10451459, 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. 2010. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the Generation R Study. J Clin Endocrinol Metab 95(9):4227–4234, PMID: 20534757, 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. 2006. Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40(11):3463–3473, PMID: 16786681, 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Huang SA. 2005. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15(8):875–881, PMID: 16131330, 10.1089/thy.2005.15.875. [DOI] [PubMed] [Google Scholar]
- Inoue K, Leung AM, Sugiyama T, Tsujimoto T, Makita N, Nangaku M, et al. 2018. Urinary iodine concentrations and mortality among U.S. adults. Thyroid 28(7):913–920, PMID: 29882490, 10.1089/thy.2018.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. 2008. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31(2):161–169, PMID: 18315716, 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: 21469664, 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kesmodel US, Underbjerg M, Kilburn TR, Bakketeig L, Mortensen EL, Landrø NI, et al. 2010. Lifestyle during pregnancy: neurodevelopmental effects at 5 years of age. The design and implementation of a prospective follow-up study. Scand J Public Health 38(2):208–219, PMID: 20064917, 10.1177/1403494809357093. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, et al. 2011. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol 45(17):7465–7472, PMID: 21805959, 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 4(1):35–43, PMID: 26497402, 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Andersen SL, Hindersson P, Nohr EA, Olsen J. 2016. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: a study within the Danish National Birth Cohort. J Clin Endocrinol Metab 101(6):2484–2492, PMID: 27115059, 10.1210/jc.2016-1387. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. 2010. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 24(1):13–27, PMID: 20172467, 10.1016/j.beem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. 2012. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366(6):493–501, PMID: 22316443, 10.1056/NEJMoa1106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, et al. 2009. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 200(3):260.e1–260.e6, PMID: 19114271, 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y. 2018a. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: 29556975, 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr EA, et al. 2018b. Prenatal exposure to perfluoroalkyl substances and IQ scores at age 5; a study in the Danish National Birth Cohort. Environ Health Perspect 126(6):067004, PMID: 29897723, 10.1289/EHP2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bonefeld-Jørgensen EC, Henriksen TB, Nohr EA, Bech BH, et al. 2014. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am J Epidemiol 180(6):574–581, PMID: 25139206, 10.1093/aje/kwu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case–control study in the Danish National Birth Cohort. Environ Health Perspect 123(4):367–373, PMID: 25616253, 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Longnecker MP, Campbell JL Jr, Andersen ME, Clewell HJ III.. 2013. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxicol Environ Health A 76(1):25–57, PMID: 23151209, 10.1080/15287394.2012.722523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Ghisari M, Bonefeld-Jørgensen EC. 2013. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20(11):8045–8056, PMID: 23539207, 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. 2018. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health 15(9):E1832, PMID: 30149566, 10.3390/ijerph15091832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151(Suppl 3):U25–U37, PMID: 15554884, 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, et al. 2001. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health 29(4):300–307, PMID: 11775787, 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Oken E, Claus Henn B, McClean MD, Rifas-Shiman SL, et al. 2018. Maternal plasma per- and polyfluoroalkyl substance concentrations in early pregnancy and maternal and neonatal thyroid function in a prospective birth cohort: Project Viva (USA). Environ Health Perspect 126(2):027013, PMID: 29488882, 10.1289/EHP2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14(7):E691, PMID: 28654008, 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LB, Ovesen L, Bülow I, Jørgensen T, Knudsen N, Laurberg P, et al. 2002. Dietary iodine intake and urinary iodine excretion in a Danish population: effect of geography, supplements and food choice. Br J Nutr 87(1):61–69, PMID: 11895314, 10.1079/BJN2001474. [DOI] [PubMed] [Google Scholar]
- Ren X-M, Zhang Y-F, Guo L-H, Qin Z-F, Lv Q-Y, Zhang L-Y. 2015. Structure-activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Arch Toxicol 89(2):233–242, PMID: 24819616, 10.1007/s00204-014-1258-y. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. 2015. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 49(19):11849–11858, PMID: 26333069, 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River watershed of North Carolina. Environ Sci Technol Lett 3(12):415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Vongphachan V, Cassone CG, Wu D, Chiu S, Crump D, Kennedy SW. 2011. Effects of perfluoroalkyl compounds on mRNA expression levels of thyroid hormone-responsive genes in primary cultures of avian neuronal cells. Toxicol Sci 120(2):392–402, PMID: 21212296, 10.1093/toxsci/kfq395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen P-C, Lien G-W, Chen H-Y, Tseng Y-C, et al. 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 122(5):529–534, PMID: 24577800, 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 133:338–347, PMID: 25019470, 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PEG, van Leeuwen SPJ, Hamers T. 2009. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci 109(2):206–216, PMID: 19293372, 10.1093/toxsci/kfp055. [DOI] [PubMed] [Google Scholar]
- Williams R. 2012. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 12(2):308–331, 10.1177/1536867X1201200209. [DOI] [Google Scholar]
- Yoshimura M, Hershman JM. 1995. Thyrotropic action of human chorionic gonadotropin. Thyroid 5(5):425–434, PMID: 8563483, 10.1089/thy.1995.5.425. [DOI] [PubMed] [Google Scholar]
- Yu W-G, Liu W, Jin Y-H. 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ Toxicol Chem 28(5):990–996, PMID: 19045937, 10.1897/08-345.1. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Boelaert K. 2015. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3(4):286–295, PMID: 25591468, 10.1016/S2213-8587(14)70225-6. [DOI] [PubMed] [Google Scholar]
- Zoeller TR. 2010. Environmental chemicals targeting thyroid. Hormones (Athens) 9(1):28–40, PMID: 20363719, 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.