Abstract
Background
Shared decision-making at end of life (eol) requires discussions about goals of care and prioritization of length of life compared with quality of life. The purpose of the present study was to describe patient and oncologist discordance with respect to goals of care and to explore possible predictors of discordance.
Methods
Patients with metastatic cancer and their oncologists completed an interview at study enrolment and every 3 months thereafter until the death of the patient or the end of the study period (15 months). All interviewees used a 100-point visual analog scale to represent their current goals of care, with quality of life (scored as 0) and survival (scored as 100) serving as anchors. Discordance was defined as an absolute difference between patient and oncologist goals of care of 40 points or more.
Results
The study enrolled 378 patients and 11 oncologists. At baseline, 24% discordance was observed, and for patients who survived, discordance was 24% at their last interview. For patients who died, discordance was 28% at the last interview before death, with discordance having been 70% at enrolment. Dissatisfaction with eol care was reported by 23% of the caregivers for patients with discordance at baseline and by 8% of the caregivers for patients who had no discordance (p = 0.049; ϕ = 0.20).
Conclusions
The data indicate the presence of significant ongoing oncologist–patient discordance with respect to goals of care. Early use of a simple visual analog scale to assess goals of care can inform the oncologist about the patient’s goals and lead to delivery of care that is aligned with patient goals.
Keywords: Goals of care, quality of life, decision-making, oncologists, patients
INTRODUCTION
The need for high-quality communication and shared decision-making between oncologists and patients with respect to goals of care has been well documented1–6 and endorsed by major health care organizations. When discussions between oncologists and patients about goals of care are held, patients are more likely to receive care that is consistent with their preferences7,8 and to have a better quality of life near death1,9–11. Best clinical practices include eliciting decision-making preferences and understanding the patient’s goals of care1. In addition, professional guidelines recommend that goals of care and end-of-life (eol) discussions begin during periods of relative medical stability12—preferably when the patient is initially diagnosed with metastatic cancer13–18.
A model commonly used to guide treatment (and goals of care) discussions is shared decision-making. These are the key components in shared decision-making19,20:
■ At a minimum, the patient and physician should be involved.
■ The patient and physician share information relevant to treatment decisions being made.
■ The patient and physician discuss their goals for treatment and attempt to build consensus with respect to the preferred treatment.
■ The agreed-upon treatment is delivered.
That model19 served as the foundation for our examination of concordance in oncologist and patient goals of care, and our assessment of the extent to which oncologists understand the goals of care for patients—a key indicator of shared decision-making and high-quality care.
Research has shown that early discussions about goals of care are associated with better quality of life1, reduced use of non-beneficial medical care near death21, and positive family outcomes after death10. In addition, work has shown that conversations about goals of care often fail to provide patients with sufficient information about prognosis to allow for appropriate decisions1,22 and usually occur so late in the patient’s illness that their effect on care processes is reduced1,23. Intervention studies have aimed to incorporate best practices for goals-of-care discussions and have focused on more education for physicians (communication, eol discussions)24, systematizing goals-of-care discussions such that they occur earlier in the trajectory of care for the patient25, and implementing structured formats to guide goals-of-care discussions26. Although some interventions show promise, many interventions require either or both of extensive training (of physicians or health care providers) and restructuring of an already complex system of health care monitoring and delivery.
To date, little work has examined the goals of care the oncologist has for the patient and the oncologist’s perceptions of the patient’s goals of care, including how those perceptions align with patient-identified goals of care. Alignment is important because shared decision-making relies on treatment decisions being made based on a consensus between the patient and the oncologist about the preferred treatment19,20. For true shared decision-making to take place, the oncologist and the patient must therefore both be involved in determining the treatment plan. To date, however, no longitudinal study has examined the extent to which an oncologist’s understanding of the values and goals of patients direct care or how shared decision-making influences the process of establishing goals of care1,3,5,8,27.
The effect of shared decision-making has the potential to last past the patient’s death for caregivers and family members28. When patients and physicians do not engage in a shared decision-making process that leads to consensus about treatment (especially at eol), caregivers can face issues of regret and dissatisfaction with respect to the eol experience29. The role of patient–oncologist discordance about goals of care is one measure that can provide insight into whether the oncologist understands the patient’s goals of care for treatment at eol—a factor that can mitigate feelings of regret and dissatisfaction in the family.
Our study had two purposes:
■ to evaluate the extent of patient–oncologist discordance with respect to goals of care over time for patients with metastatic lung, gastrointestinal, and pancreatic cancer; and
■ to examine whether predictors of discordance in goals of care were evident.
A secondary purpose was to examine the relationship between patient–oncologist discordance about goals of care and caregiver satisfaction with eol care for those patients who died.
METHODS
Patients and Oncologists
This descriptive, longitudinal analysis enrolled 11 oncologists and 378 patients with metastatic cancer from outpatient clinics at Seidman Cancer Center, University Hospitals Cleveland Medical Center, Cleveland, OH, U.S.A. The Seidman Cancer Center is a tertiary care cancer program, and a member of the U.S. National Cancer Institute–designated Case Comprehensive Cancer Center. All clinicians provided verbal consent, and all patients provided written informed consent. The study site’s institutional review board approved the study.
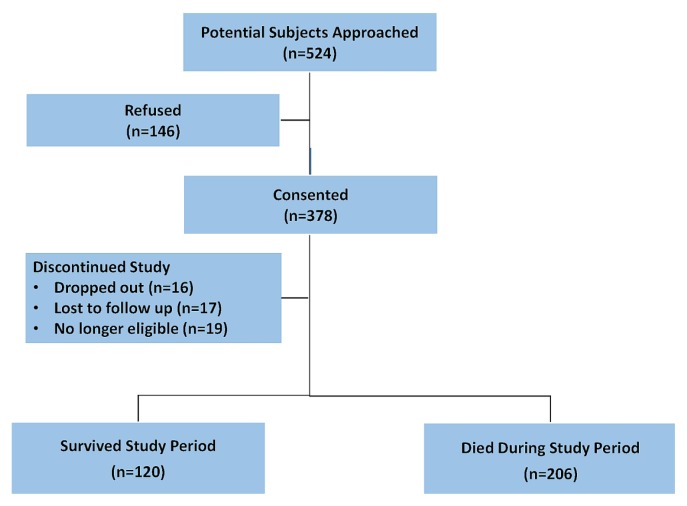
A convenience sample was enrolled from January 2015 to March 2017, with follow-up until November 2018. Patients were eligible to participate if they were 18 years of age or older, had a diagnosis of stage iv lung or gastrointestinal cancer, or of stage iib or greater pancreatic cancer, and were able to speak and comprehend English. Of the 524 eligible patients who were approached, 378 (72.1%) consented to participate, and 146 (27.9%) declined participation. No differences between consenters and refusers in age, sex, or race were observed. Oncologists were eligible to participate if they were the enrolled patient’s primary oncologist.
Caregivers
Of 237 caregivers who participated in the study, 101 (42.6%) were caregivers of patients who died; those caregivers provided data about satisfaction with eol care. On average, caregivers were predominantly women (68.3%), white (85.1%), and the spouse of the patient who died (63.4%). All caregiver participants provided informed consent to participate.
Procedures
Potential patient participants were approached in the outpatient oncology clinic setting. After written informed consent had been obtained, patients were given an iPad loaded with all items associated with the study. Each patient and oncologist was surveyed at study enrolment and every 3 months for 15 months or until the patient died or was transferred to hospice care.
In addition to demographic and clinical data collected at baseline, patients were asked a single goals-of-care question and a single question evaluating treatment effectiveness at each data collection interview. In addition to demographic data provided at baseline, oncologists provided responses to two goals-of-care questions, a single question evaluating treatment effectiveness, and a survival expectation question at each data collection interview. Baseline data collection took approximately 10 minutes, and each follow-up data collection took about 2 minutes.
Patient-Reported and Oncologist-Reported Measures
Goals of care were measured using a approach similar to that in prior work that had a reported test–retest reliability of 0.803. Patients were asked “Regarding your care, what is most important to you right now?” Oncologists were asked “Regarding the care of this patient, what is most important to you right now?” and “Regarding the care of this patient, what do you think is most important to the patient right now?” The latter two questions were asked to assess, respectively, what the oncologist felt was best for the patient with respect to goals of care and what the oncologist assumed (understood) their patient wanted with respect to goals of care. The response scale for all questions was a visual analog scale (vas) with “quality of life is all that matters” serving as one anchor (scored as 0) and “length of life is all that matters” serving as the other anchor (scored as 100). The decision to use a single-item vas was based on research demonstrating that vas items are less vulnerable to bias from confounding factors, better avoid the ceiling effect, and require less time to complete than Likert-scaled items30,31. Compared with multi-item questionnaires, instruments using a vas have been shown to be psychometrically sound with respect to reliability, validity, and anchor-based responsiveness32.
Goals-of-care discordance (the dependent variable) was defined as an absolute difference on the vas of 40 points or more between the patient’s goals-of-care answer and the oncologist’s goals-of-care answer about what they thought was best for the patient. Research has shown that patients and health professionals often have different preferences for treatment, and that recognition of those preferences is an important first step to reaching consensus about an appropriate treatment choice33. We based the definition of “discordance” on several factors. First, we wanted to differentiate clinically significant differences from minor variation (where differences tend to cluster around the mean), and we felt that capturing a difference of 40 points or more would be large enough to capture agreement (or not) by patients and oncologists about goals of care. Second, we felt that a difference of 40 points or more would most likely capture goals-of-care priorities that were placed by patients and oncologists on different sides of the scale (or heading in different directions).
Predictor variables were measured at all time points and were included in the logistic regression analysis to determine whether a model that predicted discordance could be derived. All predictor variables had been shown in empirical work to relate to eol decisions or goals-of-care discussions.
Survival expectation (predictor variable completed by oncologists) was measured by asking the oncologist “Given your patient’s current condition, what do you expect in terms of survival 6 months from now?” The response scale was a vas with the anchors “Very unlikely” (scored as 0) and “Very likely” (scored as 100).
Strength of religious faith (predictor variable completed by patients) was measured using the validated Santa Clara Strength of Religious Faith Brief Questionnaire, with total scores ranging from 5 (low strength of faith) to 20 (strong strength of faith)34.
End-of-life values (predictor variable completed by patients) were measured by the validated 8-item End of Life Values Scale, which assesses values relevant to eol care, treatments, or goals of care. The scale for each item ranges from 0 (not at all important) to 5 (extremely important)35.
Symptoms (predictor variable completed by patients) were measured using the validated Edmonton Symptom Assessment System, which assesses the presence and severity of 9 symptoms commonly seen in palliative care patients. The scale for answers ranges from 0 (symptom not present) to 10 (worst possible symptom)36.
Caregiver satisfaction with eol care (completed by caregivers) was measured using the validated Family Satisfaction with End-of-Life Care instrument. This 13-item survey has a Likert response scale ranging from 1 (very dissatisfied) to 5 (very satisfied)37.
Attrition and Missing Data
At study enrolment, data for goals of care were missing in 3 of 378 cases, and those cases were not included in the analyses. In addition, as seen in Figure 1, 52 individuals did not complete the study, and their end-of-study data were not included in the study analyses. As a result, 375 cases were included in the enrolment analyses, and 326, in the end-of-study (or death) analyses. For cases included in the analyses, complete data had been provided by patients and oncologists alike for all variables included in the analyses.
FIGURE 1.
Study flow diagram.
Data Analysis
For unadjusted analyses comparing patient and oncologist characteristics in the discordance groups (potential confounders), we used analyses of variance (continuous variables) and chi-square (Fisher exact) tests. For continuous variables that violated the assumption of homogeneity of variance, we used the Welch analysis of variance for interpretation. Correlations were used to assess the relationships between patient goals of care, goals of care that the oncologist felt were best for the patient, and goals of care that the oncologist assumed the patient wanted. Discordance was described using chi-square (Fisher exact) tests.
Logistic regression was used to evaluate the effect of predictor variables on the dichotomous criterion variable of oncologist–patient discordance with respect to goals of care. Multicollinearity was tested by examining bivariate correlations for all predictor variables (to assess for moderate-to-strong correlations) and assessing variation inflation factors (to assess for factors greater than 4)38,39. All variables met those criteria and were included. In addition, large sample sizes (>10 events per variable) are required to provide sufficient numbers in both categories of the response variable38,39. The number of events per variable was 14, which was sufficient for conducting the logistic regression.
In the logistic regression model, marital status and income level were entered as covariates (statistically significant difference by discordance). Next, all predictor variables were entered into the model. We used Wald tests to evaluate the null hypothesis that the coefficient associated with each predictor variable was zero. The goodness of fit of the model was tested using the Hosmer–Lemeshow test, which indicates how well the model fits the data by comparing observed and predicted outcomes38,39.
RESULTS
Tables I and II present the characteristics of the patients and oncologists. All patients had metastatic disease, and more than half (54.5%) died during the study period. Data for 58% of the patients were provided by 4 oncologists; the remaining 7 oncologists provided data for 42% of the patients. The average time from study enrolment to death was 7.1 months. Table III presents the oncologist data for assessment of goals, treatment, and survival by discordance group.
TABLE I.
Baseline demographic and clinical characteristics of 378 patients by discordance status
| Variable | Oncologist–patient discordance | p Value | Effect size | |
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
| Patients (n) | 90 | 285 | ||
|
| ||||
| Mean age (years) | 64.3±10.4 | 63.2±11.2 | 0.38 | 0.10 |
|
| ||||
| Mean ESAS score | 26.5±19.5 | 23.9±17.8 | 0.26 | 0.14 |
|
| ||||
| Mean evaluation of Tx effectiveness | 71.7±24.9 | 74.3±22.0 | 0.37 | 0.11 |
|
| ||||
| Mean goals of care | 42.7±41.8 | 52.0±22.8 | 0.05 | 0.28 |
|
| ||||
| Mean religious faith scorea | 14.9±4.8 | 15.3±4.0 | 0.46 | 0.09 |
|
| ||||
| Sex [n (%) women] | 51 (56.7) | 140 (49.1) | 0.21 | 0.06 |
|
| ||||
| Ethnicity [n (%) white] | 61 (67.8) | 218 (76.5) | 0.10 | 0.09 |
|
| ||||
| Married [n (%) yes] | 44 (48.9) | 188 (66.0) | 0.004 | 0.15 |
|
| ||||
| ECOG PSb [n (%)] | ||||
| 0 | 25 (27.8) | 70 (24.6) | 0.73 | 0.06 |
| 1 | 51 (56.7) | 179 (63.0) | ||
| 2 | 11 (12.2) | 27 (9.5) | ||
| 3 | 3 (3.3) | 8 (2.8) | ||
|
| ||||
| Cancer type [n (%)] | ||||
| Gastrointestinal | 43 (47.8) | 132 (46.3) | 0.97 | 0.01 |
| Lung | 31 (34.4) | 100 (35.1) | ||
| Pancreatic | 16 (17.8) | 53 (18.6) | ||
|
| ||||
| Annual household incomec [n (%)] | ||||
| ≤$20,000 | 28 (34.1) | 49 (19.4) | 0.02 | 0.16 |
| $21,000–$49,999 | 19 (23.2) | 83 (32.8) | ||
| ≥$50,000 | 35 (42.7) | 121 (47.8) | ||
|
| ||||
| Living willb [n (%) yes] | 55 (61.1) | 185 (65.1) | 0.49 | 0.04 |
|
| ||||
| During study period … [n (%) yes] | ||||
| Received palliative care | 32 (35.6) | 117 (41.1) | 0.35 | 0.05 |
| Died | 46 (51.1) | 158 (55.4) | 0.47 | 0.04 |
On the Santa Clara Strength of Religious Faith Brief Questionnaire.
Percentages in the “No discordance” group are based on 284 patients for whom these data were available.
Percentages based on 82 (yes group) and 253 (no group) responses.
ESAS = Edmonton Symptom Assessment System; Tx = treatment; ECOG PS = Eastern Cooperative Oncology Group performance status.
TABLE II.
Baseline characteristics of 11 oncologists
| Variable | Value |
|---|---|
| Age (years) | |
| Mean | 43.9±5.4 |
| Range | 37–54 |
|
| |
| Oncology practice (years) | |
| Mean | 12.8±14.0 |
| Range | 4–26 |
|
| |
| Religious faith scorea | |
| Mean | 11.5±4.8 |
| Range | 5–18 |
|
| |
| Ethnicity [n (%) white] | 7 (70.0) |
|
| |
| Sex [n (%) women] | 4 (40.0) |
On the Santa Clara Strength of Religious Faith Brief Questionnaire.
TABLE III.
Oncologist assessment of goals, treatment, and survival by discordance status
| Assessment item | Oncologist–patient discordance | p Value | Effect size | |
|---|---|---|---|---|
| Yes | No | |||
| Goals of care that the oncologist thought were best for the patient | 52.9±25.9 | 54.7±20.9 | 0.55 | 0.08 |
| Goals of care that the oncologist assumed the patient wanted | 56.2±28.2 | 57.8±22.5 | 0.62 | 0.06 |
| Evaluation of treatment effectiveness | 57.5±32.3 | 62.4±25.4 | 0.24 | 0.17 |
| Survival expectation | 66.9±25.9 | 71.9±23.3 | 0.10 | 0.20 |
Goals-of-Care Discordance
At study enrolment, 90 of the patient–oncologist dyads (24.0%; 95% confidence interval: 19.8% to 28.7%) had a goals-of-care discordance (difference between the patient’s goals of care and the goals of care the oncologist thought were best for the patient). Statistically significant differences on two baseline variables were evident between dyads with and without a baseline discordance (Table I): patient marital status (p = 0.004, ϕ = 0.15) and patient income level (p = 0.02; Cramér V = 0.16). Compared with patients without discordance, those with discordance were less likely to be married and more likely to have an annual income less than $20,000.
We were also interested in evaluating the discordance between a patient’s goals and the oncologist’s assumption about the patient’s goals. There were 93 patient–oncologist dyads with that type of discordance (24.8%; 95% confidence interval: 20.7% to 29.4%). No statistically significant differences were evident between the discordance groups for any descriptive variables.
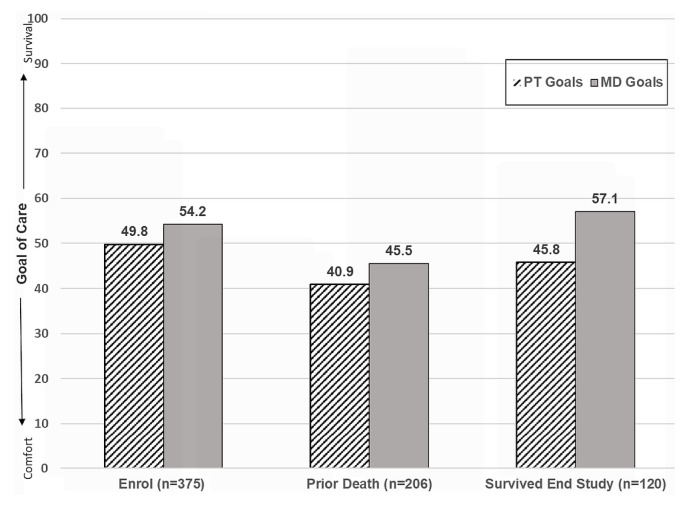
As seen in Figure 2, the difference between the goals of care expressed by patients and the goals of care that oncologists thought were best for their patients at study enrolment was nonsignificant (p = 0.41). The difference was significant at the last interview before death (p = 0.018) and at the last study interview for patients who survived throughout the study period (p = 0.003). For all comparisons, goals of care that the oncologists thought were best for their patients tended more toward survival than did the goals of care expressed by the patients.
FIGURE 2.
Patient (PT) and oncologist (MD) goals of care at study enrolment and at last interview before death or last interview during the study period.
Goals-of-Care Discordance for Patients Who Died
In shared decision-making, one of the major reasons that patients and physicians share information and build a consensus about treatment is the importance of delivering care that is consistent with the patient’s goals and values—especially at eol10. To describe the degree to which there was (or was not) consensus between oncologists and patients, we examined, for patients who died, discordance between the patient’s goals of care and goals of care that the oncologist thought were best for the patient from enrolment until the last interview before death.
Of the 204 patients who died and had complete dyadic data, 46 (22.5%) had patient–oncologist goals of care discordance at study enrolment, and 57 (27.9%; 95% confidence interval: 22.0% to 34.2%) had discordance at the last interview before death. Of the 46 with discordance at study enrolment, 32 (69.6%) also had discordance before death, with discordance at enrolment being a strong predictor of discordance before death (r = 0.62, p < 0.001).
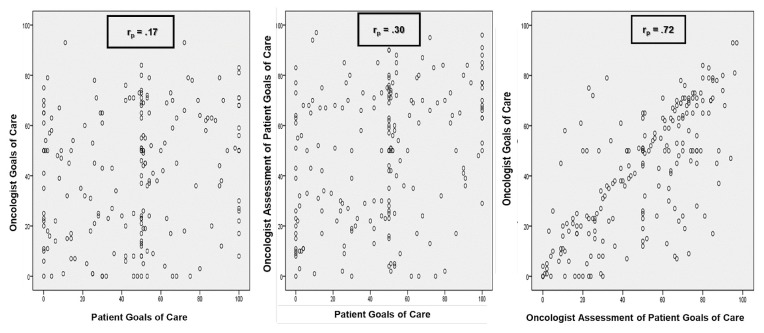
As seen in Figure 3, the relationship between the patient’s goals of care and the goals of care that the oncologist thought were best for the patient was weak at the last interview before death (r = 0.17). The relationship between the patient’s goals and the goals that the oncologist assumed the patient wanted was slightly stronger (r = 0.30). However, the strongest relationship was observed between the goals of care oncologists thought were best for their patients and the goals of care oncologists assumed their patients wanted (r = 0.72).
FIGURE 3.
Scatterplots of oncologist and patient goals of care at last interview before death (n=206). rP = Pearson correlation coefficient.
Of the 206 patients who died, 137 had caregivers. Of those 137 caregivers, 101 (73.7%) consented to interviews using the Family Satisfaction with End-of-Life Care tool 2 months after the patient’s death. To increase the sample size per cell, caregiver responses were dichotomized into “dissatisfied or very dissatisfied with eol care” and “satisfied or very satisfied with eol care.” For this subsample, the rate of oncologist–patient discordance at enrolment was 22.2%. Of caregivers for patients in dyads with discordance, 22.7% reported being dissatisfied with eol care; caregivers for patients in dyads without discordance had a reported dissatisfaction rate of 7.8% (p = 0.049, ϕ = 0.20).
Predictors of Goals-of-Care Discordance
Next, we tested a model that included predictor variables shown to relate to patient goals of care (or eol decisions) in prior empirical research or to significantly relate to discordance in our bivariate analyses3,5,11,40–43. The criterion variable was oncologist–patient discordance for goals of care at baseline. Predictor variables—patient characteristics (age, sex, race, living will, palliative care use)3,40,41, symptoms40, religious faith42, and eol values42,43; and oncologist age, sex, race, and survival expectation44 for the patient—were then tested as a single group in a binary logistic regression model. Patient marital status and patient income level (variables found to be statistically significant by discordance status) were entered as covariates.
After the covariates had been added, 19 predictor variables were added to the model (χ2 = 35.8, p = 0.03). The Nagelkerke R2 was 0.17, and the Homer-Lemeshow χ2 indicated that the variables were an adequate fit for the model [χ2(8) = 12.3, p = 0.14]. The overall correct classification for the model was 75.9%, with a 95.4% correct classification for no discordance and a 18.9% correct classification for discordance. One significant predictor variable emerged in the model: patient age (coefficient = 0.04; Wald χ2 = 5.83; odds ratio: 1.04; 95% confidence interval: 1.01 to 1.07; p = 0.016). Thus, for every year increase in patient age, a 4% increase in the odds of oncologist–patient discordance was expected.
DISCUSSION
Our study produced several findings of note. First, we observed a moderate (but not strong) relationship between the goals of care oncologists assumed that patients wanted and the goals of care expressed by patients. That observation indicates a lack of oncologist understanding of a patient’s goals of care—reflecting a lack of high-quality communication and decision-making19,20. That finding is supported by previous research reporting that limited knowledge of patient goals by physicians and proxies is a barrier to eol discussions and advance care planning45,46. However, previous research has not also considered the extent to which a physician might believe they have knowledge about their patient’s goals when that knowledge might be unwarranted or based on flawed assumptions or understanding. Our research specifically examined the relationships between the goals that the oncologist thinks are best for the patient, the goals that the oncologist assumes the patient wants, and the patient’s own goals for care, thus providing a more robust understanding of the issue.
Lack of understanding by oncologists of the goals of care preferred by patients could be reduced with the use of a simple tool (such as our vas). Our tool prompts oncologists to consider the goals of care preferred by patients, potentially leading to enhanced communication and shared decision-making with the patient that translates into appropriate treatment decisions incorporating the goals of the patient and oncologist alike.
Second, the strong relationship between the goals of care that oncologists thought were best for their patients and the goals of care that oncologists assumed their patients wanted speaks to the strong possibility of false consensus bias on the part of oncologists. It also reinforces our finding of misunderstanding on the part of oncologists about the goals of care preferred by patients and highlights the need for oncologists to regularly ask their patients about goals of care. The need to facilitate an understanding on the part of oncologists about patient-preferred goals of care has been the focus of research that has identified facilitators and barriers to goals-of-care discussions17,46,47. Intervention research has examined eol discussion formats28,48, early palliative care discussions23, and strategies for locations and type of discussions48,49, aiming to enhance the number and quality of those discussions. However, none of the interventions has focused on the use of a short and simple tool that could be useful for beginning a discussion about goals of care—a discussion that could lead to more in-depth conversations over time.
Third, almost a quarter of all patients had goals-of-care discordance with their oncologist at baseline. The presence of discordance at baseline was a strong predictor of discordance before death. The fact that discordance was relatively stable over time demonstrates that, even in the presence of disease progression, oncologists did not understand the goals and preferences of their patients. According to shared decision-making and quality care indices, that lack of understanding signals a quality-of-care issue specific to eol care in the cancer population.
Discordance was also found to affect caregivers months after the patient’s death. Caregiver dissatisfaction with eol care in the discordance group was about triple that in the group without discordance. The effect size was small-to-medium, but it is important to note that the effect of the discordance extended beyond the patient’s experience and had negative effects on caregivers as well. To date, evidence linking the understanding of oncologists about patient-preferred goals of care with post-death caregiver outcomes has not been reported. That finding adds to the empirical work related to cancer caregiving.
Finally, we found no robust predictive factors of discordance. Although patient age was a significant predictor of discordance, the effect size was weak. Oncologists therefore cannot make assumptions about goals of care based on suppositions about sex, race, religiosity, and so on, but must ask patients directly about their goals of care. As demonstrated in the work here, the risk is that oncologists might make flawed assumptions about patient goals based partly on the goals of care that the oncologists think are best for their patients. Empirical research has identified several factors that relate to eol decision-making (goals-of-care discussions, advance care planning) that were included in our analytic model (oncologist years of experience46, patient’s poor functional status or symptom burden1,46, and patient religiosity46,50, among others). None of those variables related to our outcome variable (goals of care discordance). One explanation for that finding might be that our outcome measure was tapping into a phenomenon different from that in prior work: whether oncologist goals of care align with patient goals of care.
Given the collective findings in the present study, additional strategies for obtaining information about a patient’s goals of care are needed. First, we recommend the use of a single question with an easy-to-interpret response scale (such as our vas) to assess the patient’s goals of care over time. In our study, the vas was feasible, easy to administer, and well received by oncologists and patients. By evaluating oncologist and patient goals of care over time, oncologists can ensure that they understand each patient’s goals of care throughout the trajectory of care. Asking that question can serve as an opportunity to easily identify opportunities for additional discussion, goal clarification, and treatment consensus building. Second, given the stability of discordance over time, we recommend conducting such assessments early in the treatment phase and then routinely throughout the patient’s trajectory of care. That recommendation has been substantiated by other work23,47,49 in which patients have identified a desire for ongoing goals-of-care discussions that allow for processing of information over time and opportunities to revise goals as the situation changes. By routinely assessing and re-evaluating goals of care, oncologists can be better informed about patient goals, which can potentially increase the likelihood that patients will receive eol care consistent with their preferred goals of care.
Limitations
Several limitations of this study should be noted. First, convenience sampling was used, and while we found no differences between the groups of consenters and refusers, there is a risk of limited generalizability of results. Second, the study was conducted at a single tertiary care medical system, which limits the generalizability of the findings. Third, the study design was descriptive in nature, and therefore no cause-and-effect implications can be assumed. Fourth, we did not ascertain whether explicit goals-of-care discussions had occurred between oncologists and patients during the study period. Although the inaccuracy of the assessments by oncologists of patient-preferred goals of care suggests that explicit goals-of-care discussions did not take place, we have no data in that regard. Fifth, 5 oncologists provided data for 66% of the patients who participated in the study. Although the contributions of each of those oncologists were similar, and none had a goals-of-care points average for their patients that was significantly different from the averages of the other oncologists, it is possible that the results might have been biased in some unforeseen way, thus limiting study generalizability.
Finally, the vas is a self-report measure, and the 40-point threshold for the definition of discord was not pilot-tested before study initiation. Self-report measures carry general concerns about biased estimates of self-assessed behaviour and particular concerns when used in intervention research51. To mitigate those effects, patients and oncologists were blinded to each other’s vas assessments, which were submitted privately (on an iPad) with no individual seeing the responses. Although we did not pilot-test the 40-point threshold for the definition of discordance, we did conduct post-study analyses using other measures for concurrent validity, finding that the vas performed well.
CONCLUSIONS
Ongoing efforts are needed to attain best clinical practices in the discussion of goals of care—particularly between oncologists and their patients. A single question about goals of care in a vas format can be useful in obtaining a response that informs ongoing communication so that the oncologist understands their patient’s preferred goals of care and that shared decision-making is promoted.
ACKNOWLEDGMENTS
We thank the patients and oncologists who participated in our study and the hospital staff who facilitated data collection.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Bernacki R, Block SD on behalf of the American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994–2003. doi: 10.1001/jamainternmed.2014.5271. [DOI] [PubMed] [Google Scholar]
- 2.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomized controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meropol NJ, Egleston BL, Buzaglo JS, et al. on behalf of the connect study research group. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–66. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fallowfield L, Jenkins V. Effective communication skills are the key to good cancer care. Eur J Cancer. 1999;35:1592–7. doi: 10.1016/S0959-8049(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 5.Tulsky JA, Beach MC, Butow PN, et al. A research agenda for communication between health care professionals and patients living with serious illnesses. JAMA Intern Med. 2017;177:1361–6. doi: 10.1001/jamainternmed.2017.2005. [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Burnett CB, Rowland JH, et al. Communication between physicians and older women with localized breast cancer: implications for treatment and patient satisfaction. J Clin Oncol. 2002;20:1008–16. doi: 10.1200/JCO.20.4.1008. [DOI] [PubMed] [Google Scholar]
- 7.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–84. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–8. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright AA, Mack JW, Kritek PA, et al. Influence of patients’ preferences and treatment site on cancer patients’ end-of-life care. Cancer. 2010;116:4656–63. doi: 10.1002/cncr.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack JW, Chen K, Boscoe FP, et al. Underuse of hospice care by Medicaid-insured patients with stage iv lung cancer in New York and California. J Clin Oncol. 2013;31:2569–79. doi: 10.1200/JCO.2012.45.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med. 2000;172:310–13. doi: 10.1136/ewjm.172.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The AM, Hak T, Koëter G, van Der Wal G. Collusion in doctor–patient communication about imminent death: an ethnographic study. BMJ. 2000;321:1376–81. doi: 10.1136/bmj.321.7273.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christakis NA. Predicting patient survival before and after hospice enrollment. Hosp J. 1998;13:71–87. doi: 10.1080/0742-969X.1998.11882889. [DOI] [PubMed] [Google Scholar]
- 15.Raskin W, Harle I, Hopman WM, Booth CM. Prognosis, treatment benefit and goals of care: what do oncologists discuss with patients who have incurable cancer? Clin Oncol (R Coll Radiol) 2016;28:209–14. doi: 10.1016/j.clon.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Hamid AA, Ha FJ, Das O, Weickhardt AJ. Communicating prognosis of patients with advanced cancer between health care providers: a tertiary cancer center review of written correspondence. Ann Palliat Med. 2018;7:404–10. doi: 10.21037/apm.2018.06.02. [DOI] [PubMed] [Google Scholar]
- 17.Ethier JL, Paramsothy T, You JJ, Fowler R, Gandhi S. Perceived barriers to goals of care discussions with patients with advanced cancer and their families in the ambulatory setting: a multicenter survey of oncologists. J Palliat Care. 2018;33:125–42. doi: 10.1177/0825859718762287. [DOI] [PubMed] [Google Scholar]
- 18.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30:4387–95. doi: 10.1200/JCO.2012.43.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (Or it takes at least two to tango) Soc Sci Med. 1997;44:681–92. doi: 10.1016/S0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 20.Kane HL, Halpern MT, Squiers LB, Treiman KA, McCormack LA. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin. 2014;64:377–88. doi: 10.3322/caac.21245. [DOI] [PubMed] [Google Scholar]
- 21.Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869–84. doi: 10.1177/0269216315586659. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000–25. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 23.Davis MP, Temel JS, Balboni T, Glare P. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med. 2015;4:99–121. doi: 10.3978/j.issn.2224-5820.2015.04.04. [DOI] [PubMed] [Google Scholar]
- 24.Bergman J, Ballon-Landa E, Lerman SE, Kwan L, Bennett CJ, Litwin MS. Engaging physician learners through a Web-based platform: individualized end-of-life education. Am J Hosp Palliat Care. 2016;33:748–54. doi: 10.1177/1049909115598741. [DOI] [PubMed] [Google Scholar]
- 25.Mori M, Shimizu C, Ogawa A, Okusaka T, Yoshida S, Morita T. A national survey to systematically identify factors associated with oncologists’ attitudes toward end-of-life discussions: what determines timing of end-of-life discussions? Oncologist. 2015;20:1304–11. doi: 10.1634/theoncologist.2015-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oczkowski SJ, Chung HO, Hanvey L, Mbuagbaw L, You JJ. Communication tools for end-of-life decision-making in ambulatory care settings: a systematic review and meta-analysis. PLoS One. 2016;11:e0150671. doi: 10.1371/journal.pone.0150671. [Erratum in: PLoS One 2018;13:e0203911] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421–6. doi: 10.1001/jamaoncol.2016.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giguere AMC, Lawani MA, Fortier-Brochu É, et al. Tailoring and evaluating an intervention to improve shared decision-making among seniors with dementia, their caregivers, and healthcare providers: study protocol for a randomized controlled trial. Trials. 2018;19:332. doi: 10.1186/s13063-018-2697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otani H, Yoshida S, Morita T, et al. Meaningful communication before death, but not present at the time of death itself, is associated with better outcomes on measures of depression and complicated grief among bereaved family members of cancer patients. J Pain Symptom Manage. 2017;54:273–9. doi: 10.1016/j.jpainsymman.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Voutilainen A, Pitkäaho T, Kvist T, Vehviläinen-Julkunen K. How to ask about patient satisfaction? The visual analogue scale is less vulnerable to confounding factors and ceiling effect than a symmetric Likert scale. J Adv Nurs. 2016;72:946–57. doi: 10.1111/jan.12875. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlmann T, Dantlgraber M, Reips UD. Investigating measurement equivalence of visual analogue scales and Likert-type scales in Internet-based personality questionnaires. Behav Res Methods. 2017;49:2173–81. doi: 10.3758/s13428-016-0850-x. [Erratum in: Behav Res Methods 2017;49:2182] [DOI] [PubMed] [Google Scholar]
- 32.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–20. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery AA, Fahey T. How do patients’ treatment preferences compare with those of clinicians? Qual Health Care. 2001;10(suppl 1):i39–43. doi: 10.1136/qhc.0100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plante TG. The Santa Clara Strength of Religious Faith questionnaire: assessing faith engagement in a brief and nondenominational manner. Religions. 2010;1:3–8. doi: 10.3390/rel1010003. [DOI] [Google Scholar]
- 35.Winter L. Patient values and preferences for end-of-life treatments: are values better predictors than a living will? J Palliat Med. 2013;16:362–8. doi: 10.1089/jpm.2012.0303. [DOI] [PubMed] [Google Scholar]
- 36.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present and future developments. J Pain Symptom Manage. 2017;53:630–43. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teresi JA, Ornstein K, Ocepek-Welikson K, Ramirez M, Siu A. Performance of the Family Satisfaction with the End-of-Life Care (famcare) measure in an ethnically diverse cohort: psychometric analyses using item response theory. Support Care Cancer. 2014;22:399–408. doi: 10.1007/s00520-013-1988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–85. doi: 10.1016/S0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 39.Bewick V, Cheek L, Ball J. Statistics review: logistic regression. Crit Care. 2005;9:112–18. doi: 10.1186/cc3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50:496–500. doi: 10.1046/j.1532-5415.2002.50116.x. [DOI] [PubMed] [Google Scholar]
- 41.Cosgriff JA, Pisani M, Bradley EH, O’Leary JR, Fried TR. The association between treatment preferences and trajectories of care at the end-of-life. J Gen Intern Med. 2007;22:1566–71. doi: 10.1007/s11606-007-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parks SM, Winter L. End of life decision-making for cancer patients. Prim Care. 2009;36:811–23. doi: 10.1016/j.pop.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Berlin A. Goals of care and end of life in the icu. Surg Clin North Am. 2017;97:1275–90. doi: 10.1016/j.suc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Daly BJ, Douglas SL, O’Toole E, et al. Complexity analysis of decision-making in the critically ill. J Intensive Care Med. 2018;33:557–66. doi: 10.1177/0885066616678394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saraiya B, Bodnar-Deren S, Leventhal E, Leventhal H. End-of-life planning and its relevance for patients’ and oncologists’ decisions in choosing cancer therapy. Cancer. 2008;113(suppl):3540–7. doi: 10.1002/cncr.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulman-Green D, Lin JJ, Smith CB, Feder S, Bickell NA. Facilitators and barriers to oncologists’ conduct of goals of care conversations. J Palliat Care. 2018;33:143–8. doi: 10.1177/0825859718777361. [DOI] [PubMed] [Google Scholar]
- 47.Schulman-Green D, Smith CB, Lin JJ, Feder S, Bickell NA. Oncologists’ and patients’ perceptions of initial, intermediate, and final goals of care conversations. J Pain Symptom Manage. 2018;55:890–6. doi: 10.1016/j.jpainsymman.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Solomon R, Smith C, Kallio J, et al. Speaking up: how patient and physician voices shaped a trial to improve goals-of-care discussions. Patient. 2017;10:489–501. doi: 10.1007/s40271-017-0226-z. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto S, Arao H, Masutani E, et al. Decision making regarding the place of end-of-life cancer care: the burden on bereaved families and related factors. J Pain Symptom Manage. 2017;53:862–70. doi: 10.1016/j.jpainsymman.2016.12.348. [DOI] [PubMed] [Google Scholar]
- 50.Karches KE, Chung GS, Arora V, Meltzer DO, Curlin FA. Religiosity, spirituality, and end-of-life planning: a single-site survey of medical inpatients. J Pain Symptom Manage. 2012;44:843–51. doi: 10.1016/j.jpainsymman.2011.12.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res. 2011;2:320–32. doi: 10.1504/IJBHR.2011.043414. [DOI] [PMC free article] [PubMed] [Google Scholar]