Abstract
Many different adjuvants are currently being developed for subunit vaccines against a number of pathogens and diseases. Rational design is increasingly used to develop novel vaccine adjuvants, which requires extensive knowledge of, for example, the desired immune responses, target antigen-presenting cell subsets, their localization, and expression of relevant pattern-recognition receptors. The adjuvant mechanism of action and efficacy are usually evaluated in animal models, where mice are by far the most used. In this review, we present methods for assessing adjuvant efficacy and function in animal models: (1) whole-body biodistribution evaluated by using fluorescently and radioactively labeled vaccine components; (2) association and activation of immune cell subsets at the injection site, in the draining lymph node, and the spleen; (4) adaptive immune responses, such as cytotoxic T-lymphocytes, various T-helper cell subsets, and antibody responses, which may be quantitatively evaluated using ELISA, ELISPOT, and immunoplex assays and qualitatively evaluated using flow cytometric and single cell sequencing assays; and (5) effector responses, for example, antigen-specific cytotoxic potential of CD8+ T cells and antibody neutralization assays. While the vaccine-induced immune responses in mice often correlate with the responses induced in humans, there are instances where immune responses detected in mice are not translated to the human situation. We discuss some examples of correlation and discrepancy between mouse and human immune responses and how to understand them.
Keywords: vaccine, adjuvant, immunogenicity assessment, biodistribution, antibody responses, cell mediated immune responses, cytotoxic T cell responses, in vivo tracking
Introduction: Adjuvants for Subunit Vaccines
Many vaccines currently licensed for human use are based on whole, inactivated, or attenuated pathogens, of which some have additionally been adjuvanted with aluminum salts. These vaccines are very effective for prevention of disease with a number of pathogens, for example, measles, mumps, and diphtheria.1 Traditional vaccines mainly induce strong neutralizing antibody responses, and the target pathogens do not change their surface structure over time.1,2 However, novel vaccine formulations are necessary to prevent or treat a number of difficult pathogen and disease targets, requiring complex immune responses. Possible targets include pandemic influenza, chlamydia, tuberculosis, HIV, and cancers.1,2 For these, vaccines inducing concomitant humoral and cell-mediated immune responses or cytotoxic T-lymphocytes are necessary. Such vaccines can be prepared by including appropriate vaccine adjuvants designed to induce and control immune responses against co-administered antigens.
The term adjuvant covers delivery systems and immunostimulators, while some adjuvants possess both properties.3–5 Adjuvants can be designed based on the characteristics and localization of the identified target cells and the immunostimulators required to induce the desired immune responses. Several types of delivery systems are applied in vaccines for humans or are being evaluated in preclinical and clinical studies, typically with the common feature of being particles, for example, aluminum salts, emulsions, liposomes, and virosomes.6,7 The immunostimulators are introduced to induce the required immune responses by acting as ligands for pattern recognition receptors (PRRs), for example, Toll-like receptors (TLRs), C-type lectin receptors, retinoic acid-inducible gene-I-like receptors, and nucleotide-binding oligomerization domain (NOD)-like receptors.8–11 Due to the diverse nature of the receptors, the ligands also arise from diverse classes of molecules, for example, lipids, proteins, peptides, sugars, DNA, and RNA, and different formulation approaches are required to incorporate them into the delivery systems.12 The functionality and development of both delivery systems and immunostimulators as adjuvants are reviewed elsewhere.6,13–19
Aluminum-based adjuvants have been extensively used in human vaccines for almost a century, and for most of that period no other adjuvants were approved for human use.20 The adjuvant effects of aluminum-based adjuvants were empirically discovered, while the exact mechanisms of action have remained relatively obscure until recently.21,22 However, novel vaccine adjuvants are increasingly tailored to induce specific immune responses, which have been identified as critical for the prevention of target diseases. We have at our laboratory designed a palette of liposomal vaccine adjuvants capable of inducing various immune response profiles. The ones most advanced were made specifically to induce strong T-cell-mediated immune responses; Th1-skewed CD4+ T-cell responses induced by CAF01 (dimethyldioctadecylammonium bromide [DDA] and trehalose-6,6´-dibehenate) and CD8+ T-cell responses induced by CAF09 (DDA, synthetic monomycoloyl glycerol [MMG], and polyinosinic:polycytidylic acid [poly(I:C)]).23,24 The adjuvants have been evaluated in vivo in different animal models using a variety of immunization routes.24–26 Our experience with these vaccines, including the evaluation of their immunostimulatory and mechanistic profile, will be the basis for the present review.
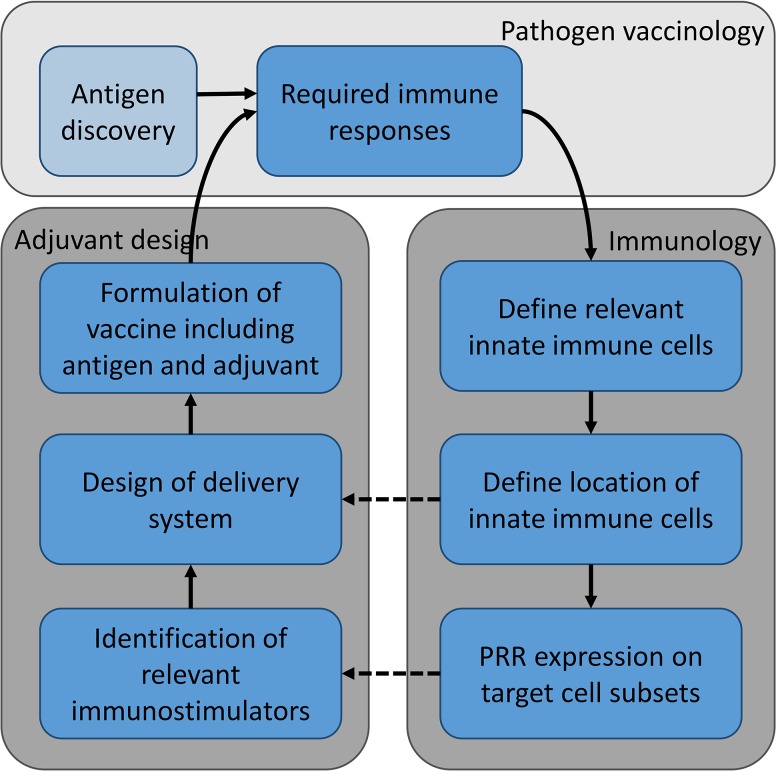
Rational Design of Vaccine Adjuvants
Progress has been made in guiding immunity through a detailed mechanistic understanding of innate immune cell biology and the response of professional antigen-presenting cells (APCs) to various stimuli. Based on this, rational design can be applied, taking into account antigen type, target cell subsets and phenotype, and immunization routes, which guide the choice of delivery system and immunostimulators. Rational design basically means designing the vaccine to present sufficient amounts of the right antigen in the right conformation to the appropriate cell populations while supplying the right co-stimuli for a sufficient amount of time. Choice of conformation and dose of antigen are often handled by antigen discovery programs with focus on specific disease targets. In this review, we will focus on rational design of adjuvants, and thus immunogen design will not be further discussed here. Targeting of appropriate cell populations with the right co-stimuli and timing serve as guidance for rational design of novel adjuvants and require knowledge of numerous aspects: (1) what is the required immune response to prevent disease from a given pathogen, (2) which innate immune cells are relevant to induce said immune response, (3) where are these innate cell subsets located, and (4) which PRRs do the cells express (Figure 1). The questions can be answered by using animal models, as they enable mapping the whole-body effects of vaccination. Knowledge of the required immune responses, and the innate cells and cytokines involved, and the localization in the body can be acquired by evaluating stimulation and proliferation of innate and effector cells upon administration of the vaccine via a number of different assays as described below.
Figure 1.
Rational design of vaccine adjuvants. The required immune responses are identified based on pathogen vaccinology and defined by the type of vaccine; different immune responses might be required for a prophylactic vaccine preventing disease, and a therapeutic vaccine treating disease or preventing clinical symptoms. Based on the required immune responses, the relevant innate immune cells must be identified along with evaluation of their localization within the body and PRR expression on target cells. Knowledge of these factors can be used to design the delivery system and identify relevant immunostimulatory molecules, respectively. The delivery systems are often nanoparticle-based structures of diverse origin, for example, liposomes, emulsion, virosomes, or aluminum salts. The design choices of delivery systems depend, amongst other things, on the location of the target innate cells, the route of administration, the chosen immunostimulators, and association mode of antigen. Relevant immunostimulators are often identified based on the PRR expression of target innate cells subsets. These may be antigen-presenting cells (eg, DCs and macrophages) or cells with bystander function. Antigen discovery programs can, independently of adjuvant design programs, identify immunogenic antigens for a given pathogen and be used to develop recombinant antigens that in combination with suitable adjuvants induce pathogen-specific immune responses. The vaccine formulation (adjuvant + antigen) is tested in vivo in relevant animal models to characterize the induced immune responses and, possibly, the response to a pathogen challenge.
Evaluation of the Biodistribution and Cellular Association of Adjuvanted Vaccines
Many vaccines have been developed without detailed knowledge of the targeted cell populations. However, the biodistribution and cellular association patterns of adjuvanted vaccines are of utmost importance for the induction of specific immune responses. One such example is the CAF09 adjuvant that induces strong CD8+ T-cell responses when given intraperitoneally (i.p.), but not upon subcutaneous (s.c.) or intramuscular (i.m.) immunization. This is presumably due to the formation of a persistent depot at the site of injection (SOI), which prevents targeting of the innate immune cell subsets specialized in CD8+ T-cell induction.24,27 The adjuvanted vaccines will predominantly be actively transported or drain via the lymphatics, and it is therefore of importance to know the draining lymph nodes (LNs) from a given injection site.
In vivo evaluation of the draining pattern from various injection sites can be performed by injection of fluorescently labelled particles followed by noninvasive imaging of the animal, allowing for assessment of the biodistribution pattern of the injected particles in the same animal over time (Figure 2a). For example, the draining LNs following i.p. administration were evaluated in rats by injection of near infra-red fluorescent quantum dots and human serum albumin conjugated with IR-Dye800.28 The draining LNs and lymph flow was identified by imaging the rats in intervals up to 24 hours after i.p. administration, revealing primary and secondary draining LNs.28 Alternatively, lymphatic mapping can be performed by using a visible dye such as Evans Blue dye, which was used to visualize the draining LNs following hind leg and lateral tail vein administration in mice.29 Mapping the biodistribution pattern of a vaccine administered via a certain route provides important information about which compartments are affected by the vaccine. Thus, it may be possible to assess if the correct organs and cell types are targeted to induce the desired immune response.
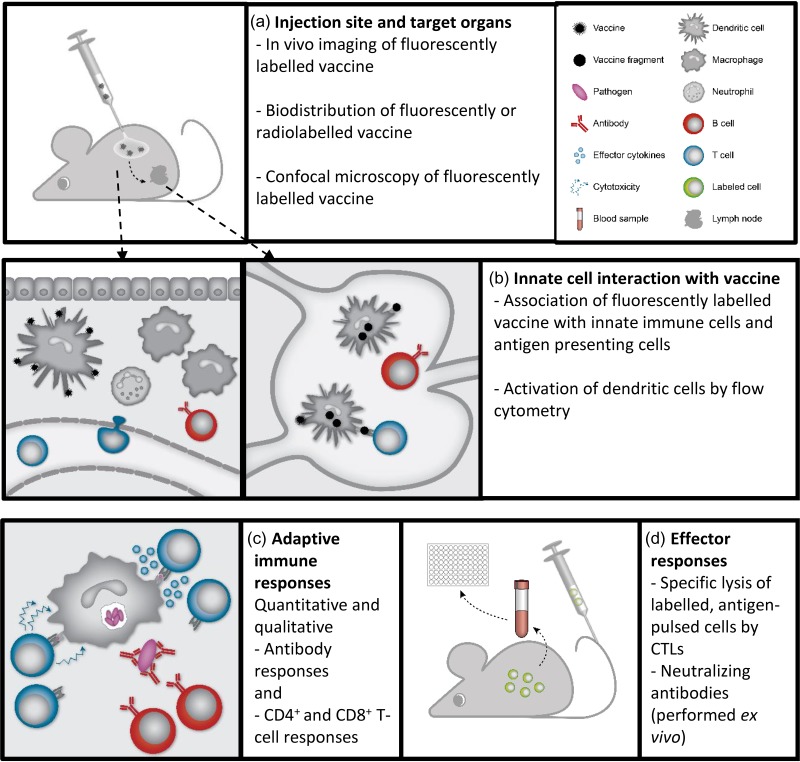
Figure 2.
Evaluation of vaccines—from identification of target cells to desired immune response profile. A number of different assays can be utilized to assess adjuvanted subunit vaccine function and efficacy. (A) Assays evaluating biodistribution and organ localization of vaccine components on a whole-body level are often based on detection of fluorescently or radiolabeled adjuvants and antigens. (B) In target organs such as the injection site and draining lymph nodes, association of vaccine components with innate immune cells and antigen-presenting cells can be evaluated using flow cytometry-based assays. These assays can be used to elucidate the mechanism of action for vaccine adjuvants and which antigen presenting cells that are activated by the adjuvant. (C) Qualitative and quantitative evaluation of the adaptive immune responses to subunit vaccines is used to assess vaccine efficacy. Quantitative responses can be measured by ELISA, ELISPOT, and immunoplex assays, whereas flow cytometry-based assays and antibody avidity assays can be used to evaluate the qualitative immune responses. (D) Vaccine efficacy can be assessed using alternative assays to pathogen challenge models. The cytotoxic potential of CD8+ T cells can be evaluated in antigen-specific lysis assays, while antibody functionality may be evaluated in neutralization assays or with methods to assess antibody Fc-dependent functionalities such as cytotoxicity, complement activation or phagocytosis.
Vaccine Interaction with Innate Immune Cells
Localization of vaccine components in the organs, particularly the LNs, has been illuminated by confocal microscopy (Figure 2a). The spatial localization of the vaccine components in the draining LNs was evaluated following s.c. immunization with the emulsion-based adjuvant MF59 fluorescently labeled with the lipid tracer dioctadecyl-tetramethylindodicarbocyanine perchlorate and intrinsically fluorescent PE-antigen.30 The LNs were stained for relevant expression markers (eg, the macrophage marker F4/80 and the germinal center [GC] marker GL7), which made it possible to assess the co-localization of the vaccine components with specific LN compartments.30 In a study evaluating the dependence of particle size on LN entry, red fluorescent 20-nm and green fluorescent 1000-nm particles were co-administered in the footpad of mice.31 The results showed that the small particles likely entered the LNs freely, whereas the large particles required trafficking by dendritic cells (DCs).31 In another study, fluorescently labelled chicken egg ovalbumin (OVA) formulated in nanoparticles based on poly(lactide-co-hydroxymethylglycolic acid) was used to perform in vivo tracking over 13 days following s.c. administration with concomitant assessment of the levels of OVA at the SOI and in the draining LNs.32
Cellular association of fluorescently labeled vaccine components can be evaluated by flow cytometry, where cell subsets are detected by staining with appropriate fluorescently labeled antibodies.27,33 This approach was used to identify targeting of DCs in the draining LNs by liposomal adjuvants (labeled with 7-nitro-2-1,3-benzoxadiazol-4-yl or 3,3′-dioctadecyloxacarbocyanine perchlorate) administered via different administration routes.27,33 This allows for concomitant evaluation of the target lymphoid tissues the antigen drains to and the phenotype and activation of the targeted cell subsets (Figure 2b). Furthermore, the localization of fluorescently labeled antigen and adjuvant can be investigated by immunofluorescent staining and microscopy.
Using CAF09, we tracked fluorescently labeled antigen and noticed that i.p. administration targeted the CD8+ T-cell priming CD8α+ DCs in LNs and spleen while s.c. and i.m. administration did not.27 Thus, s.c./i.m. administration of CAF09-adjuvanted vaccines prevented efficient vaccine drainage to the intended target cells. Based on this information, as an example of rational vaccine design, we have recently demonstrated that reformulating the CAF09 adjuvant to limit the depot effect can be an effective means to obtain CD8+ T-cell responses after s.c./i.m. immunization.34
Characterization of the association of the vaccine adjuvant and antigen with specific innate cell subsets at the injection site and in the lymphoid organs can be used to identify cell-mediated transport to the injection site and give an insight into the APC subsets priming the resulting immune response.35–37 The cellular association of the emulsion-based adjuvant MF59 was evaluated in the injected muscle and the draining LNs using a flow cytometry panel of fluorescently labeled anti-Ly6C, -Ly6G, -CD11b, -CD11c, -F4/80, and -MHC-II antibodies, the expression patterns of which could be combined to identify neutrophils, eosinophils, inflammatory monocytes, macrophages, and different DC subsets.35 MF59 was found to be mainly associated with neutrophils and inflammatory monocytes in the muscle tissue, which were thought to facilitate rapid cell-mediated transport to the draining LNs.35 In another study, the liposome-based adjuvant AS01 was shown to induce a transient influx of neutrophils (SSChighCD11b+Ly6Ghigh) and monocytes (Ly6ChighCD11b+Ly6G-) into the muscle injection site.36 Similar innate cell reactions were observed in rhesus macaques immunized with HIV-1 envelope protein adjuvanted with an aluminum-TLR7-ligand complex or MF59. In this study, fluorescently labelled antigen was found to associate with neutrophils, monocytes, and myeloid DCs at the muscle injection site. Furthermore, the study showed that priming of antigen-specific CD4+ T cells happened exclusively in the draining LNs.37 Flow cytometry coupled with high-throughput imaging of immunofluorescent staining, such as imagestream, represents a novel promising tool to investigate innate immune cell targeting, uptake, and subcellular location. For example, it was shown that the experimental adjuvant carbomer carbopol was located intracellularly in a number of different innate immune cells and that many cells had taken up multiple carbopol particles.38
Activation of DCs can be evaluated by assessing the increase in expression of activation markers such as CD40, CD80, CD86, and MHC-II using flow cytometry.33,39,40 Thus, it is the change of surface marker expression on a single cell level that is evaluated, typically measured as the mean fluorescence intensity, rather than the number of cells expressing a certain cell marker. Mice immunized with the 2-component adjuvant IC31 showed significantly increased levels of CD40, CD80, and CD86 expression specifically on adjuvant-associated DCs in the LNs compared with control mice and mice immunized with the TLR-9-ligand CpG.40 To investigate the heterogeneity of innate immune cells responding to vaccination, single cell sorting followed by RNA sequencing is a powerful technique. It enables genome-wide profiling of mRNA expression and has the potential to reveal the heterogeneity of APCs, otherwise masked at the bulk cell level.41
Quantitative Assessment of Vaccine Biodistribution
Injection of radiolabeled vaccine particles is also used for qualitative and quantitative assessment of the biodistribution on both organ and cellular levels (Figure 2a). Quantitative evaluation of the biodistribution of an adjuvanted vaccine can be performed by injection of radioactively labelled particles.27,42–46 The method enables quantitative assessment of injected vaccine particles in separate excised organs, for example, the draining LNs, the spleen, and the SOI, typically calculated as the ratio of the initial vaccine dose. One benefit of using radiolabeling of the vaccine is the possibility of performing concomitant evaluation of the levels of different components of the vaccine in various organs. Individual labeling of a liposomal adjuvant and a protein antigen with 1H-cholesterol and covalent linkage with 125I, respectively, enabled separate assessment of the relative antigen and adjuvant levels at the SOI and in the draining LNs.43,46 This approach was used to evaluate the co-localization of antigen and adjuvant at the SOI and in the draining LNs as a consequence of protein and particle charge.43 After i.m. administration, a negatively charged antigen adjuvanted with a cationic liposome remained at the SOI for a longer time than a positively charged antigen (lysozyme) adjuvanted with the same cationic liposome, while neutral liposomes also caused rapid drainage of the negatively charged antigen from the SOI.43 In a study using similar techniques, the lipid bilayer fluidity of the cationic liposomal adjuvant was shown to be critical for the biodistribution pattern.47 Thus, liposomes, which are in a rigid gel state at body temperature, form a depot at the SOI over the 15-day study period, whereas fluid liposomes do not form a depot at a SOI but enter the draining LNs in appreciable amounts already 1 day after administration.47 Evaluation of how vaccine adjuvants associate with immune cells, locally and systemically, and how these cells are activated can provide valuable knowledge of the mechanism of action of the adjuvants. Furthermore, this knowledge can aid the design of novel vaccine adjuvants as the connection between activation of innate immune cells and the induced immune responses may be determined.
Characterization of Immunostimulators That Activate Target Cells
Identification of the optimal combination of immunostimulators to be used for activating specific subsets of immune cells requires knowledge of the expression of PRRs on the cells in question. As an example, TLR3 is expressed on both LN-resident CD8α+ and migratory CD103+ cross-presenting DCs, and activation of TLR3 is required to induce cross-priming of CD8+ T cells.48 Thus, TLR3 ligands are often used in adjuvant formulations intended for induction of CD8+ T-cell responses.49 Advances in sequencing as well as systems and computational immunology have provided the field with online databases such as the Immunological Genome Project (Immgen), where the PRR gene expression on a large number of immune cells can be found for mouse and human (www.immgen.org). Such databases can be used as a tool to identify possible immunostimulators in the design phase of novel adjuvant formulations. It should be noted that dependent on the activation signals, the PRR expression profile may change. Thus, whereas Immgen displays PRR expression in the unpertubed steady state, initial activation by adjuvants may change the PRR profile of target cells, possibly providing access to additional PRRs. Importantly, gene expression analyses do not necessarily reflect actual protein expression. Protein expression of target PRRs should therefore be confirmed by such means as flow cytometry or by proteomic approaches.
Due to the complexities of the immune system, which requires interactions of several different cell subsets to induce and maintain antigen-specific immune responses, functional evaluations of vaccine candidates are preferably performed in animal models. For example, antibody responses to T-cell-dependent antigens require that antigen is complexed, for example, via complement deposition, is transported/drained to lymphoid tissue, and then taken up by specialized macrophage and DC subsets and delivered to follicular B cells. At the same time, T cells must be activated by DCs and form contact with the B cells in the draining LN.
Immunostimulators are often evaluated in vitro to identify the activation pathways in the target cells. Furthermore, in vitro evaluation of the immunogenic effects of an immunostimulator on the chosen cell strain can indicate the effects achieved upon in vivo administration. Correlation between in vivo and in vitro studies has been shown for the TLR7/8-ligand R848, which produced cytokines corresponding to a Th1-skewed CD4+ T-cell response both in human-derived leukocytes and following s.c. immunization in an o/w-emulsion.50 A synergistic effect of co-administration of MMG and the TLR9-ligand CpG was observed in vitro in J774 macrophages measured as secretion of IL-6.51 A similar synergistic effect was observed following immunization of mice with H56-adjuvanted CpG/DDA/MMG-liposomes with respect to IFN-γ and IL-17 secretion.51 The TLR3-ligand poly(I:C) formulated in poly-(L-lysine)-microspheres stimulated in vitro CD8+ T-cell proliferation by monocyte-derived DCs and secretion of IL-6, IL-12p70, and TNF-α.52 In vivo administration to mice of poly(I:C) formulated with CAF01 similarly induced strong CD8+ T-cell responses but low levels of IL-6 and TNF-α.53 Thus, in vitro studies can provide insights into early stimulation patterns by immunostimulators but should not replace in vivo evaluation of the complete vaccine adjuvant.
The complex interplay between the different cells of the immune system may be the cause of the vastly different results obtained with the synthetic MMG analogue MMG-6 in both in vitro and in vivo studies, respectively.54,55 In the in vitro studies, neat MMG-6 failed to stimulate monocyte-derived DCs, whereas MMG-6 incorporated into DDA-based liposomes were capable of inducing robust Th1-skewed CD4+ T-cell and total IgG antibody responses in vivo.54,55 This illustrates the importance of evaluating the immunostimulators as part of the final adjuvant, as the formulation might alter the configuration of the molecules and the mode of presentation to the receptors. Furthermore, in vitro studies often rely on the function of a single cell subset, whereas in vivo studies enable simultaneous activation of several types of cell subsets. Further, in vitro evaluation of the Mycobacterium tuberculosis (M.tb)-derived MMG showed stimulation of the human Mincle receptor, which was not induced in the murine Mincle receptor.56 However, MMG induces strong immune responses when administered in combination with DDA-based liposomes in murine studies,57 indicating that it either stimulates the immune response through unknown receptors or adopts a conformation within the liposome, which enables interaction with the Mincle receptor.
The signaling pathway of trehalose-6,6´-dibehenate has been Investigated via both in vitro stimulation of bone marrow macrophages and in vivo administration of CAF01.58,59 In vitro stimulation of macrophages, measured as release of nitrites and G-CSF, is dependent on cellular expression of the Mincle receptor and independent on MyD88 expression.58 In contrast, antigen-specific secretion of IFN-γ and IL-17 by draining LN-isolated cells required expression of both MyD88 and Mincle.59 This illustrates that the signaling pathways in the in vivo situation may be different from those found in in vitro studies, possibly due to the interaction between several different immune cell subsets in vivo. Furthermore, the cell lines or peripheral blood mononuclear cells tested in in vitro studies may be vastly different in composition compared with the cellular populations at the injection site.
Evaluation of Vaccine-Induced Adaptive Immune Responses
Evaluation of Antibody Responses
The best-established correlate of protection against a number of diseases is antibody responses.60 Antibody responses can be measured as antibody titer by standard enzyme-linked immunosorbent assay (ELISA)-based approaches or by more disease-specific approaches, such as virus or bacterial neutralization (Figure 2, c and d). Vaccination elicits B-cell activation in the draining LNs, followed by formation of GCs in which affinity maturation and antibody class-switching occurs.61 Adjuvants may affect the magnitude of GC responses, which can be measured by flow cytometry or immunofluorescent staining and confocal microscopy. Some spontaneous GC formation may occur in naïve animals, and it may therefore be beneficial to include a fluorescently labeled antigenic probe in the analysis,62 thus enabling detection of antigen-specific GC responses. It should be noted that the kinetics of GC responses might vary with properties of the antigen and adjuvant used, thus requiring kinetic studies to define the peak response for a given vaccine. The affinity maturation of the antibody response can also be followed, using ELISA-based methods, such as limiting antigen dilution or chaotrope methods. In the latter, the resistance of the antigen-antibody complex to urea or NaSCN is evaluated as a measure for antibody affinity. Antibody avidity may correlate with protection. For example, for a meningococcal vaccine, serum antibody avidity significantly correlated with bactericidal titres.63 Other methods to measure the strength of antigen-antibody interactions include surface plasmon resonance.64 Ultimately, the GC B cells may undergo 1 of 2 productive fates, which are desired for all infections requiring antibody-dependent protection: memory B cells or plasma cells. It has recently become appreciated that early GCs have a preponderance for generating memory B cells, while plasma cell formation requires more progressed GCs.65 An interesting possibility would be to modify immunization protocols or adjuvants to change GC persistence and thereby possibly alter the plasma cell to memory B cell output ratio. Memory B cells are circulatory and can be followed using flow cytometry. The phenotype of memory B cells is quite diverse and both class-switched and IgM positive memory B cells exist.66,67 The best way to quantify memory B cells is therefore to use a probe (fluorescently labeled antigen) in combination with standard memory B cell markers (eg, B220+, IgD+, CD38+ in mice). Antigen-specific memory B cells can further be sorted by FACS and used to provide information on B-cell receptor heavy- and light-chain gene usage (variable, diversity, and joining genes) after vaccination, or single-cell sorted and used for production of antigen-specific monoclonal antibodies.68 For example, in macaques immunized with HIV-1 Env, memory B cells were single-sorted for CD4 binding site-reactivity using 2 fluorescent probes and used to produce monoclonal CD4 binding site reactive antibodies. This allowed for the further studies of vaccine-induced antibody recombination events and CD4 binding site specificities.69 B cell receptor sequencing may also be performed to investigate how immunoglobulin sequence repertoire changes following vaccination70 or how the type of vaccine or adjuvants may potentially influence B cell receptor variable gene usage. For example, upon immunization with a Plasmodium vivax antigen, including a TLR agonist expanded the diversity of the variable region sequences in comparison with the use of an oil-in-water emulsion adjuvant alone.71 It is also possible to stimulate plasma cell formation from memory B cells using B cell mitogens, such as the TLR7 agonist R848 or the TLR9 agonist CpG B.72 The number of antibody secreting cells can then be evaluated by enzyme-linked immunospot (ELISPOT) or secreted antibodies can be measured by ELISA. Dependent on the vaccine, plasma cells can be maintained for lifetime. Long-lived plasma cells home to the bone marrow and can be phenotypically characterized by flow cytometry and their antibody secretion can be followed by ELISPOT.73 Tetanus-specific plasma cells were evaluated 10 years post-vaccination by ELISPOT from bone marrow samples.74
Standard evaluation of vaccine-induced antibody responses include determination of antigen-specific serum IgG levels. Dependent on the properties of the vaccine antigen, or the type of adjuvant, different antibody isotypes may be elicited. For example, in response to protein antigens, the CAF01 adjuvant elicits a balanced Th1/Th2 profile, characterized by both IgG1 and IgG2a/c antibodies, while aluminum hydroxide induces mainly IgG1 antibody responses to the same antigens in mice.75 These antibody subclasses may (due to the structural properties of the Fc region) differentially bind to Fc receptors (FcR), which in turn may affect FcR-mediated antibody functions such as antibody-dependent cellular cytotoxicity, complement activation, and phagocytosis.76,77 In humans, an ENV GP120 vaccine (VAX003) elicited IgG4 antibodies that may have outcompeted more functional Ig subclasses (IgG1 and IgG3), and depletion of IgG4 gave higher antibody functional responses.78 An intriguing possibility is also that vaccines may influence the antibody Fc region glycosylation patterns, which may also affect Fc receptor binding and thus antibody FcR-mediated functions.79 For example, it was found that an aluminum-adjuvanted recombinant gp120 vaccine induced a different antibody FC region glycan profile compared with an adenovirus based HIV-1 envelope A vaccine.79 Dependent on the disease target, it may therefore be important to broaden the evaluation of antibody responses to include antibody avidity as well as antibody isotypes and functional attributes. To probe correlates of vaccine-induced immunity in more detail, transcriptomics and metabolomics show great promise. For example, evaluation of innate and adaptive immunity to Herpes zoster vaccination in humans was supplemented with metabolomics to reveal an interconnected immune network of metabolic pathways that correlated with adaptive immune responses.80
Evaluation of CD4+ and CD8+ T-cell Responses
Antigen-specific CD4+ and CD8+ T cells are important to prevent or combat infectious diseases. Therefore, evaluation of antigen-specific CD4+ and CD8+ T cells induced by novel vaccine formulations is an important measure of vaccine efficacy (Figure 2c).
A well-established method for evaluating antigen-specific CD4+ and CD8+ T-cell responses is stimulation of single-cell suspensions from target organs with the subunit antigen and minimal CD8 epitope peptides, respectively. Intracellular flow cytometry can be applied to single cell suspensions stimulated for a short amount of time to assess the production of cytokines on a cellular level. Furthermore, harvested supernatants of single cell suspensions stimulated for a longer time (typically 3–5 days) may be used to quantify the cytokine production on a cell population level using ELISAs and multiplex assays such as Luminex and Meso Scale Discovery.72,81,82
The CD8+ T-cell responses induced by immunization with CAF09-adjuvanted M.tb.-antigen TB10.3 (as the whole protein or the CD8 epitope-containing peptide, P1) were evaluated by stimulating single cell suspensions of splenocytes with the minimal CD8 epitope, IMYNYPAM.24 Subsequent fluorescent antibody staining of the splenocytes permitted evaluation of IFN-γ, IL-2, and TNF-α expression by CD4+ and CD8+ T cells using flow cytometry.24 A similar flow cytrometry panel was used to evaluate the CD4+ and CD8+ T-cell responses in splenocytes of rhesus macaques immunized with the influenza vaccine Fluzone adjuvanted with the cationic lipid/DNA complex-adjuvant JVRS-100. Immunization with the adjuvanted vaccine resulted in higher levels of multifunctional CD4+ and CD8+ T cells compared with macaques immunized with unadjuvanted Fluzone.83 This assay evaluates the quantitative functionality of the CD8+ T cells as the level of cytokine producing cells in response to the stimulus. Furthermore, the levels of polyfunctionality in the stimulated cells can be used to assess the potential of a vaccine to induce lasting immune responses. Thus, IFN-γ+,IL-2+,TNF-α+ CD8+ T cells are considered memory T cells, which give rise to a long-lived immune response, whereas a short-lived effector response may be defined as IFN-γ+,TNF-α+ and IFN-γ+ CD8+ T cells.84
The repertoire of induced CD4+ T cells is critical for the induced functional immune responses; Th1 responses induce proinflammatory responses and help the induction and sustaining of CD8+ T-cell responses, whereas Th2 responses help promote antibody class switching, and Th17 CD4+ T cells are thought to be important for establishing mucosal immune responses.85 Intracellular staining and flow cytometry on single cell suspensions stimulated with the antigen can also be used for evaluation of CD4+ Th1-cell responses utilizing the IFN-γ+, IL-2+, and TNF-α+ intracellular staining assay,83,84 which may also include anti-IL-17-antibodies to assess the Th17-skewed CD4+ T-cell response.85
Flow cytometry has limitations to the number of antibodies that can be analyzed at one time due to spectral overlap of the fluorophores conjugated to the antibodies. An alternative method for analysis of T-cell populations is cytometry by time-of-flight (CyTOF), where the antibodies are conjugated to heavy metal isotopes by metal chelating polymers rather than the fluorophores used for flow cytometry assays.86 Staining of stimulated cells with heavy metal isotope-conjugated antibodies enables detection of the cells by using mass spectroscopy. Due to little overlap between the heavy metal isotopes, the number of antibodies used for each assay can be increased compared with flow cytometry. However, the data acquisition rate is low at 300 to 500 events/s compared with the acquisition rates of flow cytometry at orders of magnitude at 103 to 105 events/s.86,87 Thus, 36 different antibodies were used to identify subpopulations of human CD8+ memory and effector T cells. The functionality of subpopulations of CD8+ T cells were identified by principal component analysis and combinatorial diversity achieved by Boolean gating, which were distinct for different virus-specific CD8+ T cells.88 These data analysis approaches are suitable for simultaneous assessment of different immune cell populations due the large amounts of data generated using CyTOF.87 Thus, differences in cytokine and receptor expression patterns of immune cell subsets (CD4+ and CD8+ T cells, B cells and monocytes) were assessed for naïve and influenza-vaccinated mice after influenza challenge.87 CyTOF has potential for assessing changes in type and functionality of immune cell subsets after immunization with different subunit vaccine adjuvants. It may be possible to identify correlates of protection in disease challenge models or show differences between different adjuvants. Furthermore, the method requires low sample volumes enabling longitudinal studies using blood samples.87
The cytokine levels in response to stimulation with antigen can also be assessed by using ELISPOT, where the released cytokines are captured by cytokine-specific antibodies adsorbed to the well.89,90 This approach was used to quantify the expression of IFN-γ, IL-4, and IL-2 in mice immunized with virus-like particles, which showed that concomitant co-stimulation with poly(I:C) increased the cytokine responses.89 ELISPOT is also very useful in other animal models where flow cytometry antibodies are scarce. For example, to assess the IFN-γ responses in splenocytes to different tetanus toxoid doses adjuvanted with CAF09 in a study in Göttingen minipigs. The study showed that low doses of tetanus toxoid (1 and 10 μg/dose) resulted in the induction of IFN-γ responses, which were diminished when the dose was increased to 100 μg.90 In one study, Luminex, ELISPOT, and intracellular flow cytometry were used with antibody ELISA assays to compare different adjuvants in a DNA plasmid prime/adjuvanted protein boost regimen.91 The combination of assays allowed the identification of adjuvants capable of robustly boosting the primed immune responses, while providing detailed information on the differences in induced cytokine levels and T-cell responses induced by the different adjuvants. Thus, the results showed that MPLA, ISCOMATRIX, and QS-21-based adjuvants were capable of inducing antibody responses towards the antigen, though the cytokine profiles differed.91
Identification of CD4 and CD8 epitopes in novel protein- or peptide-based antigens can be achieved by epitope-mapping, where splenocytes from immunized mice are stimulated with individual peptides spanning the entire protein. Assessment of cytokine-producing T cells by intracellular flow cytometry in response to the individual peptides serve to identify CD4+ and CD8+ T-cell epitopes. This approach was used to elucidate the induction of CD4+ and CD8+ T-cell responses following immunization with recombinant NS3 protein antigen or the corresponding peptide mix both adjuvanted with CAF09.92 The epitope mapping revealed that immunization with the peptide mix resulted in recognition of more CD4 epitopes compared with the recombinant protein, whereas 2 CD8 epitopes were induced by the peptide mix, while none were observed with the NS3 protein.92
Pentamer/tetramer/dextramer-conjugated CD8 epitope-loaded MHC-I molecules are used to assess the level of antigen-specific CD8+ T cells in the relevant organs using flow cytometry. In a DC-based vaccine pulsed with the antigen TRP2 and adjuvanted with soluble poly(I:C), the percentage of TRP2-specific CD8+ T cells was assessed using a Kb/TRP2 tetramer and an anti-CD8 antibody.93 The induction of CD8+ T-cell responses against the antigens TB10.3-P1, OVA, Gag p24, and E7 adjuvanted with CAF09, compared with using CAF01 as adjuvant, were evaluated using the specific minimal CD8-epitopes loaded onto the appropriate pentamer/dextramer-conjugated MHC-I molecules. The cell subsets were identified by co-staining with anti-CD8, -CD4, -CD19, and -CD44 antibodies.24 While assessment of the number of antigen-specific CD8+ T cells give a good indication of the efficacy of the administered vaccine, the results should be evaluated in combination with functionality assays, for example, production of cytokines or antigen-specific cytotoxicity assays as described below.
The proliferation of antigen-specific CD4+ and CD8+ T cells upon therapeutic vaccination can be used as a measure of how well the cells respond to vaccination. In the bromodeoxyuridine (BrdU) assay, mice are fed BrdU in the drinking water, or by i.v. or i.p. administration, for a few days prior to euthanization. BrdU is incorporated into the DNA of proliferating cells and can be imaged by fluorescent anti-BrdU antibodies in flow cytometry assays.94,95 In a study of therapeutic vaccination of mice infected with chronic lymphocytic choriomeningitis virus, it was shown that a low amount of antigen-specific CD8+ T cells proliferated in presence of a chronic infection compared with noninfected, preimmunized control mice.94 In another study, mice were vaccinated with recombinant M.tb. antigen adjuvanted with cationic liposomes for the prime and boosted as an adenovector. Following M.tb. pulmonary challenge, proliferative antigen-specific CD4+ T cells were recruited to the lungs to a higher degree than antigen-specific CD8+ T cells.95
It may be of interest to investigate where vaccine-induced, antigen-specific CD4+ and CD8+ T cells localize upon pathogen challenge. Evaluation of tissue and circulatory localization of immune cells can be performed by i.v. injection of fluorescently labelled anti-CD45 antibodies a few minutes before killing.96 The antibodies bind to CD45-expressing lymphocytes in the blood, thus enabling sorting of circulatory immune cells (CD45+) from tissue resident immune cells (CD45−) in highly perfused organs, such as the lungs.96,97 In a study of a M.tb. subunit vaccine, fluorescently labelled antigen-specific CD4+ T cells were adoptively transferred from donor mice immunized with low (5 μg) and high (50 μg) doses of adjuvanted antigen into M.tb-infected mice. One day after adoptive transfer, the CD45-labelling assay was used to evaluate the level of transferred antigen-specific CD4+ T cells that homed to the lung parenchyma. It was shown that CD4+ T cells from mice immunized with a low dose of antigen homed most efficiently to the lung parenchyma.97 In another study, the assay was used to evaluate how the immunization routes affected the levels of IgA+ B cells levels in the lungs and vasculature.82 It was shown that a s.c. priming followed by an intranasal booster vaccination with adjuvanted ScpA antigen induced higher levels of homing to the lung parenchyma of IgA+ B cells compared with a subcutaneous booster vaccination.82 There are several assays to assess the CD4+ and CD8+ T-cell responses in animal models. The choice of assay may depend on the animal model, as the use of flow cytometry requires the access to antibodies, which organs are being assayed, and whether information on an individual cellular, organ, or systemic level is required.
Evaluation of Antigen-Specific Cytotoxic Potential for CD8+ T Cells
When assessing the efficacy of a subunit vaccine, it may be desirable to use alternatives to disease challenge models for evaluation of antigen-specific cytotoxicity of CD8+ T cells. The study animals are spared from experiencing the target disease, which may cause discomfort and pain. Furthermore, it enables separation of adjuvant function and efficacy from immunity preventing disease. Specifically, the latter may not be completely elucidated, as is the case for M.tb infection, where for example a strong pathogen-derived antigen-specific CD8+ T-cell response was not preventive of disease in a mouse model.98
Several different assays exist to measure cell-mediated cytotoxicity, where the 51Cr release assay is regarded as the “golden standard.”99 Cell-mediated cytotoxicity is detected when radioactive 51Cr is released from target cells, which were initially pulsed with sodium chromate.99 The assay is performed ex vivo, which enables selection of specific target cell populations at different effector to target cell ratios.100 In a mouse study of a cell-based vaccine against renal cell carcinoma, this approach was used to show that the vaccine induced tumor-specific cytotoxicity, with little lysis of tissue control cells.100
One assay is measuring the specific lysis of i.v.-injected, fluorescently labeled, minimal CD8 epitope-pulsed splenocytes into immunized animals. A weakness of the assay is that the transfer of epitope peptide-pulsed splenocytes to immunized mice limits the results to encompass only the chosen epitopes. Thus, synergistic (or opposing) immune responses involving simultaneous antibody, CD4+, and CD8+ T-cell responses cannot be evaluated using this method alone but must be done in combination with ex vivo stimulation of target cells.
In the specific lysis assay, single cell suspensions of splenocytes from naïve mice are pulsed with different concentrations of the cellular dye carboxyfluorescein succinimidyl ester (CFSE) resulting in distinct populations, which can be further pulsed with the minimal CD8+ epitopes of interest, always leaving one population unpulsed. The pooled populations are injected i.v. into recipient mice, and the specific lysis of the pulsed splenocytes is determined typically after 24 hours by calculating the ratio of peptide-pulsed to unpulsed splenocytes in relevant organs in the recipient mice. In a study evaluating a CAF09-adjuvanted pepmix vaccine against hepatitis C virus, the level of specific lysis to 2 different peptides containing CD8 epitopes was compared by i.v. injection of splenocytes labeled with 3 different concentrations of CFSE and 10 μg/mL of each peptide.92
A complex protocol involving up to 216 separately fluorescently stained splenocyte populations was developed by Quah et al., intended for detailed in vivo assessment of CD8+ T-cell avidity and concomitant evaluation of several CD8 epitopes.101 Splenocyte populations derived from naïve mice were stained with 4-6 concentrations of the fluorescent dyes CFSE, celltrace violet, and cell proliferation dye, including a nonstained population, followed by pulsing with different concentrations of minimal CD8 epitopes prior to injection into immunized mice. Separation of donor and recipient cells was achieved by using B6.CD45.1 donor mice, thus allowing selective fluorescent antibody staining of CD45.1 in the B6.CD45.2 recipient mice. The avidity of induced antigen-specific CD8+ T cells was shown to depend on the type of antigen, as SIINFEKL-specific CD8+ T cells showed a high level of specific killing even at low peptide concentrations on donor cells. In contrast, the epitopes GP33 and NP68 resulted in lower avidities, with distinctly peptide-concentration dependent specific lysis levels by antigen-specific CD8+ T cells.101
Consideration for Use of Animal Models to Predict Immunity in Humans
One big hurdle in vaccine development is to transfer novel vaccines and adjuvants from preclinical studies into clinical trials. An important aspect here is obviously the need for animal models that optimally reflect human (or target animal) vaccine-induced immunity, toxicology, and prevention of disease against the pathogens in question. The choice of animal model requires that the relevant parts of the immune system are comparable with the target species in receptor expression and cellular responses. By far, most in vivo vaccine efficacy studies are performed on inbred mice. The structure of the immune system in mice and humans is overall highly similar, but some characteristics are different and should be taken into consideration when using mouse models. Covering this issue in detail is not our scope with this review, although it deserves some attention. We have focused on a few important topics.
Innate Sensing
The innate immune system is conserved between all multicellular organisms in some form in contrast to the adaptive immune system, which is found in vertebrates only.102 There are vast differences in the types, numbers, and functions of TLRs, C-type lectin receptors, retinoic acid-inducible gene-I-like receptors, and NOD-like receptors between species, which has been reviewed elsewhere.102 Furthermore, there are differences in the immune cell compositions and functions (eg, the ratio of leukocytes and the responses to IFN-γ), and, importantly, resistance is favored in humans, whereas tolerance is favored in mice.103
TLR7 and TLR8 are often grouped as they are both activated by single-stranded RNA and imidazoquinolines, such as R848 and 3M-052. However, the 2 TLRs respond very differently to stimulation in mice and humans, with human TLR8 responding to stimulation with single-stranded RNA, while no response is raised by murine TLR8.104,105 It has been suggested that murine TLR8 has no function, but it has been shown that TLR8-deficient mice have an increased expression of TLR7 and develop autoimmune diseases.106 Thus, preclinical testing of R848 and 3M-052 as vaccine adjuvants in mice likely evaluates the activation of TLR7, whereas in humans, both TLR7 and TLR8 have a function in the induction of an immune response.107,108
Humoral Immune Responses
In all higher vertebrates, the initial antibody response to immunization is mainly produced by GC-independent plasmablasts or early plasma cells in the draining LN and constitutes primarily of IgM antibodies. This is followed by formation of GCs.109,110 The kinetics of the GC responses, and the overall phenotype of the main cellular subsets involved (follicular B cells, DCs, and T follicular helper cells [Tfh]) are similar between species commonly used in vaccine research. However, there are well-known differences in the Ig isotypes between the species. Mice produce IgM, IgA, IgD, IgE, and 4 subtypes of IgG: IgG1, IgG2a/c, IgG2b, and IgG3.111 The same applies to rat, but rat IgG2b corresponds to mouse IgG2a/c and rat IgG2c to mouse IgG3. Pigs have up to 6 different IgG subclasses and rabbits have only one. Humans also express IgM and have 2 subtypes of IgA, IgA1 and IgA2, in addition to IgD and IgE. In humans there are also 4 subtypes of IgG (IgG1, IgG2, IgG3, and IgG4), but these do not correspond directly to those found in the rodents.111 A particularly important aspect related to vaccine research is production of IgG1. While this is related to an IL-4-driven Th2 response in mice and is often paralleled by concomitant IgE production, the same does not apply to humans, where IL-4 can instead drive IgG4 production. Vaccination with protein antigens generally induces IgG1, IgG2b, and IgG2a/c in mice, while IgG3 is mostly produced in response to T-independent antigens, such as TLR ligands or polysaccharides.112,113 In humans, protein antigens stimulate mostly IgG1 and IgG3, and polysaccharides stimulate IgG2.114 IgG4 is typically produced in chronic infections and may be stimulated with repeated immunizations using high antigen doses, which is utilized in allergen immunotherapy.115 Notably, there is no mouse equivalent to human IgG4,116 and the mice may therefore be suboptimal as a model for potential IgG4-mediated allergen immunotherapy.
Mucosal immune responses show some additional distinct features between mouse and man. Thus, while the primary mucosal antibody produced is IgA in both species, mice mainly produce dimeric IgA both in serum and in mucosal sites. In humans the secretory IgA is mainly dimeric or polymeric, whereas serum IgA is mainly monomeric, making it easy to distinguish between locally produced and serum IgA.117,118 Secretory IgA is transported across epithelial cells via the polymeric Ig receptor (pIgR). In mice, large amounts of pIgA are cleared from plasma and transported to bile by pIgR-expressing hepatocytes. In contrast, in humans, biliary epithelial cells express pIgR and perform the pIgA secretion into bile. This means that in humans there is much less circulating pIgA transported into bile and that most IgA in bile is secretory IgA produced by local plasma cells.117 Pigs may be a better model for elucidating mucosal IgA responses, as porcine IgA is more homologous to human IgA than mouse or rat IgA.117 Mucosal immune responses can also be evaluated in pigs by measuring mucosal pIgR levels. Another limitation of mouse models in mucosal immune responses is the lack of FcαR, which is otherwise conserved in mammals.119
The functional attributes of antibodies are largely determined by their Fc properties and, similar to the differences in antibody classes and subclasses, FcR expression varies between species used for vaccine evaluation. Both mice and humans express the FcR for IgM (FcμR or TOSO).120 However, in contrast to humans, mice lack FcαRI. Humans express the Fc receptors for IgG, FcγRI, FcγRIIA, FcγRIIC, and hFcγRIIIA, which are activating, and FcγRIIB, which is inhibitory.111 Human IgGs can bind to all the FcγR receptors, except IgG2, which cannot bind FcγRI. FcRn, which is used for transport of Igs, can also bind to all IgG subclasses111 and also exists in mice.121 In addition, mice express FcγRI, FcγIIB, FcγRIII, and FcγRIV.122 Similar to humans, FcγRIIB is inhibitory, whereas the rest of the FcγRs are activating.122 Notably, mice, but not humans, express FcγRIV, which can only bind mouse IgG2a/b/c and not IgG1.111,123 Since FcγRIV may function by mediating ADCC,124 IgG2 antibodies may be more efficient to perform this function in mice. It should also be noted that great differences in expression pattern between mouse and human FcγR exist. For example, the expression of human FcγRIIIA is restricted to NK and monocytic cells, whereas this is not the case in mice.111
Cell-Mediated Immune Responses
The biggest challenges when it comes to correlating vaccine-induced, cell-mediated immune responses between species is that these responses are most often measured in cells derived from lymphoid organs or tissues, whereas the same analysis in humans is almost exclusively derived from blood samples. The two most well-described CD4+ T-cell subsets, Th1 and Th2, are well characterized in humans, and a clinical trial with CAF01 showed good correlation between mouse and man regarding these subsets.125 Correlates of induction of other subsets like Th17, Treg, and Tfh cells on the other hand are still lacking behind.
Th17 CD4+ T cells are thought to be critical for mucosal protection against pathogen entry and are identified by their ability to produce the cytokine IL-17. For example, the populations of Th17 CD4+ T cells vary with M.tb infection status in humans, that is, recently infected, latently infected, and active disease.126 It has also been shown that people with impaired IL-17 function often suffer from chronic mucocutaneous candidiasis, recurrent or persistent symptomatic infection of the nails, skin, and mucosae by Candida albicans.127–129 Subunit vaccines adjuvanted with CAF01 have been shown to induce robust Th17 CD4+ T-cell responses in both spleen and lungs in mice.82,85 However, attempts to detect IL-17 induction in human blood samples after vaccinations using CAF01 have so far failed.125 The reasons for this can be multiple. The mechanism of induction of Th17 CD4+ T-cell responses have not been completely elucidated. Thus, though there are similarities between human and murine Th17 CD4+ T cells, it is not certain that they are induced via similar pathways and that Th17 is in fact induced by CAF01 in humans. Maybe more likely, the amount of Th17 cells in blood samples is below detection level with the commonly used techniques like IL-17 cytokine ELISA/ELISPOT and flow cytometry. Th17 is induced and detectable but not with the biomarkers currently used for detection. IL-17-producing cells were detected in the blood in a recent study evaluating an oral enterotoxigenic Eschericia coli vaccine, ETVAX, with or without dmLT adjuvant.130 This vaccine was found to induce the appearance of activated T cells with a Th17 and gut-homing phenotype in peripheral blood.130 So far, similarly to Th17 cells, detection of Tfh responses in humans has been hampered by the difficulty to obtain the relevant tissue. However, a subset of circulating Tfh cells has been identified in mice and humans, which shares functional properties with GC Tfh cells.130–132
The primary function of Treg is to maintain immunological homeostasis and prevent excessive inflammation. Consequently, Treg might also interfere with vaccine-induced immunity. In a recent clinical study, the ability of 4 commonly used antiviral vaccines to induce human CD4+ Treg responses was investigated. Peripheral blood mononuclear cells obtained from healthy volunteers that had been vaccinated with either trivalent influenza vaccine with or without the addition of adjuvant MF59 (Fluad or Agrippal), a HBV subunit vaccine (Engerix-B) or a live attenuated yellow fever vaccine (Stamaril).133 At several days post vaccination, the frequency and phenotype of CD4+ Treg subpopulations in peripheral blood was examined by flow cytometry. For comparison, mice were vaccinated with influenza and hepatitis B vaccines and the Treg frequency was analyzed in draining LNs and spleen at several days post vaccination. Overall, the study showed that vaccination with vaccines with an already established safe profile have only minimal impact on frequencies and characteristics of Treg over time. However, it also showed that the systemic changes in Treg frequency found in mice were not identical to the human data. The authors suggest that this may be caused by the fact that the human systemic Treg frequency was determined in blood and that of mice in the spleen, or that there are differences in Treg definitions between species.133
The induction and evaluation of CD8+ T-cell responses in humans is relatively well described. However, most successful CD8 T-cell-inducing vaccines are based on viral vectors, the reason most probably being that priming of antigen-specific CD8+ T-cell responses by adjuvanted peptide-/protein-based vaccines requires presentation of a CD8 epitope on MHC-I on specialized cross-priming DCs.134 In humans, the CD141+CLEC9A+ DCs have been identified as a superior cross-priming DC subset compared with other DC subsets,135 which correspond to the cross-priming CD8α+ and CD103+ DC subsets characterized in mice.136,137 These DC subsets are genetically closely related between the species138 and share expression of the receptors TLR3 and XCR1, which are important for the cross-priming functionality.135,139 Thus, the mouse can generally be considered a suitable animal model for evaluating adjuvants for their ability to induce cross-priming and subsequent CD8+ T-cell responses.
Conclusion
The use of mice to evaluate vaccine immunogenicity, and especially adjuvant mechanism, is highly relevant, albeit one has to take a few aspects into consideration when trying to predict the function in humans. Does the target receptor specificity, cell distribution, and functionality in mice reflect that in humans, and does the injection route commonly used in mice result in the same immune responses as the one intended to be used in humans? In addition, one has to reflect on whether the immune responses evaluated in mouse organs like LNs, spleen, lungs, intestines, skin, genital tract, etc. can also be detected in blood samples, which is often the only accessible sample material from humans. Therefore, when performing preclinical studies, it should be considered to do the same analysis in blood as done on other tissues to counteract setbacks in clinical development.
Acknowledgments
We thank EC-H2020 project TBVAC2020 (grant agreement #643381), EC-FP7 project UNISEC (grant agreement #602012), Innovative Medicines Initiative Joint Undertaking project BIOVACSAFE (grant agreement #115308), TRANSVAC2 (grant agreement #730964), the Innovationfund Denmark project NeoPepVac (case #7051-00010A), and NIH project U01AI124284 for financial support. We furthermore thank Karen Smith Korsholm for providing illustrations for Figure 2.
References
- 1. Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25(12):1361–1366. [DOI] [PubMed] [Google Scholar]
- 2. Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56(10):1458–1465. [DOI] [PubMed] [Google Scholar]
- 3. Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):505–517. [DOI] [PubMed] [Google Scholar]
- 4. Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–S68. [DOI] [PubMed] [Google Scholar]
- 5. Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Hagan DT, Fox CB. New generation adjuvants—from empiricism to rational design. Vaccine. 2015;33(Supplement 2):B14–B20. [DOI] [PubMed] [Google Scholar]
- 7. Chauhan N, Tiwari S, Iype T, Jain U. An overview of adjuvants utilized in prophylactic vaccine formulation as immunomodulators. Expert Rev Vaccines. 2017;16(5):491–502. [DOI] [PubMed] [Google Scholar]
- 8. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3):pii: a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117(5):979–987. [DOI] [PubMed] [Google Scholar]
- 10. Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci USA. 2014;111(34):12294–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tandrup Schmidt S, Foged C, Smith Korsholm K, Rades T, Christensen D. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics. 2016;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srivastava A, Gowda DV, Madhunapantula SV, Shinde CG, Iyer M. Mucosal vaccines: a paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS. 2015;123(4):275–288. [DOI] [PubMed] [Google Scholar]
- 15. Amorij J-P, Kersten GFA, Saluja V, et al. Towards tailored vaccine delivery: needs, challenges and perspectives. J Control Release. 2012;161(2):363–376. [DOI] [PubMed] [Google Scholar]
- 16. Bonam SR, Partidos CD, Halmuthur SKM, Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol Sci. 2017;38(9):771–793. [DOI] [PubMed] [Google Scholar]
- 17. O’Hagan DT, Friedland LR, Hanon E, Didierlaurent AM. Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017;47:93–102. [DOI] [PubMed] [Google Scholar]
- 18. Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2(6):159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28:Suppl. 3:C25–C36. [DOI] [PubMed] [Google Scholar]
- 20. De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol 2008;38(8):2068–2071. [DOI] [PubMed] [Google Scholar]
- 21. Kool M, Soullié T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. [DOI] [PubMed] [Google Scholar]
- 23. Davidsen J, Rosenkrands I, Christensen D, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718(1–2):22–31. [DOI] [PubMed] [Google Scholar]
- 24. Korsholm KS, Hansen J, Karlsen K, et al. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32(31):3927–3935. [DOI] [PubMed] [Google Scholar]
- 25. Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the major outer membrane protein. J Infect Dis. 2015;212(6):978–989. [DOI] [PubMed] [Google Scholar]
- 26. Lorenzen E, Follmann F, Bøje S, et al. Intramuscular priming and intranasal boosting induce strong genital immunity through secretory IgA in minipigs infected with Chlamydia trachomatis. Front Immunol. 2015;6:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt ST, Khadke S, Korsholm KS, et al. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8+ T-cell responses: the immunological consequences of the biodistribution profile. J Control Release. 2016;239:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parungo C, Soybel D, Colson Y, et al. Lymphatic drainage of the peritoneal space: a pattern dependent on bowel lymphatics. Ann Surg Oncol. 2007;14(2):286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332(1–2):170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cantisani R, Pezzicoli A, Cioncada R, et al. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J Immunol. 2015;194(4):1717–1725. [DOI] [PubMed] [Google Scholar]
- 31. Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. [DOI] [PubMed] [Google Scholar]
- 32. Rahimian S, Kleinovink JW, Fransen MF, et al. Near-infrared labeled, ovalbumin loaded polymeric nanoparticles based on a hydrophilic polyester as model vaccine: in vivo tracking and evaluation of antigen-specific CD8+ T cell immune response. Biomaterials. 2015;37(0):469–477. [DOI] [PubMed] [Google Scholar]
- 33. Kamath AT, Mastelic B, Christensen D, et al. Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses. J Immunol. 2012;188(10):4828–4837. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt ST, Pedersen GK, Neustrup MA, et al. Induction of cytotoxic T-lymphocyte responses upon subcutaneous administration of a subunit vaccine adjuvanted with an emulsion containing the toll-like receptor 3 ligand poly(I:C). Front Immunol. 2018;9:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calabro S, Tortoli M, Baudner BC, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. [DOI] [PubMed] [Google Scholar]
- 36. Didierlaurent AM, Collignon C, Bourguignon P, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol. 2014;193(4):1920–1930. [DOI] [PubMed] [Google Scholar]
- 37. Liang F, Lindgren G, Sandgren KJ, et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci Transl Med. 2017;9(393):pii: eaal2094. [DOI] [PubMed] [Google Scholar]
- 38. Gartlan KH, Krashias G, Wegmann F, et al. Sterile inflammation induced by Carbopol elicits robust adaptive immune responses in the absence of pathogen-associated molecular patterns. Vaccine. 2016;34(19):2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan Y, Sahdev P, Ochyl LJ, Akerberg J, Moon JJ. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamath AT, Valenti MP, Rochat A-F, et al. Protective anti-mycobacterial T cell responses through exquisite in vivo activation of vaccine-targeted dendritic cells. Eur J Immunol. 2008;38(5):1247–1256. [DOI] [PubMed] [Google Scholar]
- 41. Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15(2):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–156. [DOI] [PubMed] [Google Scholar]
- 43. Henriksen-Lacey M, Christensen D, Bramwell VW, et al. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release. 2010;145(2):102–108. [DOI] [PubMed] [Google Scholar]
- 44. Moghimi SM, Moghimi M. Enhanced lymph node retention of subcutaneously injected IgG1-PEG2000-liposomes through pentameric IgM antibody-mediated vesicular aggregation. Biochim Biophys Acta. 2008;1778(1):51–55. [DOI] [PubMed] [Google Scholar]
- 45. Allen TM, Hansen CB, Guo LSS. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochim Biophys Acta. 1993;1150(1):9–16. [DOI] [PubMed] [Google Scholar]
- 46. Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J Control Release. 2012;158(1):72–77. [DOI] [PubMed] [Google Scholar]
- 47. Christensen D, Henriksen-Lacey M, Kamath AT, et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 2012;160(3):468–476. [DOI] [PubMed] [Google Scholar]
- 48. Jelinek I, Leonard JN, Price GE, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 2011;186(4):2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hafner AM, Corthésy B, Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Deliv Rev. 2013;65(10):1386–1399. [DOI] [PubMed] [Google Scholar]
- 50. Dowling DJ, van Haren SD, Scheid A, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight. 2017;2(6):e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karlsen K, Korsholm KS, Mortensen R, et al. A stable nanoparticulate DDA/MMG formulation acts synergistically with CpG ODN 1826 to enhance the CD4+ T-cell response. Nanomedicine. 2014;9(17):2625–2638. [DOI] [PubMed] [Google Scholar]
- 52. Hafner AM, Corthésy B, Textor M, Merkle HP. Tuning the immune response of dendritic cells to surface-assembled poly(I:C) on microspheres through synergistic interactions between phagocytic and TLR3 signaling. Biomaterials. 2011;32(10):2651–2661. [DOI] [PubMed] [Google Scholar]
- 53. Nordly P, Rose F, Christensen D, et al. Immunity by formulation design: Induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Control Release. 2011;150(3):307–317. [DOI] [PubMed] [Google Scholar]
- 54. Martin-Bertelsen B, Korsholm KS, Rose F, et al. The supramolecular structure is decisive for the immunostimulatory properties of synthetic analogues of a mycobacterial lipid in vitro. RSC Adv. 2013;3(43):20673–20683. [Google Scholar]
- 55. Martin-Bertelsen B, Korsholm KS, Roces CB, et al. Nano-self-assemblies based on synthetic analogues of mycobacterial monomycoloyl glycerol and DDA: supramolecular structure and adjuvant efficacy. Mol Pharm. 2016;13(8):2771–2781. [DOI] [PubMed] [Google Scholar]
- 56. Hattori Y, Morita D, Fujiwara N, et al. Glycerol monomycolate is a novel ligand for the human, but not mouse macrophage inducible C-type lectin, Mincle. J Biol Chem. 2014;289(22):15405–15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nordly P, Korsholm KS, Pedersen EA, et al. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur J Pharm Biopharm. 2011;77(1):89–98. [DOI] [PubMed] [Google Scholar]
- 58. Huber A, Kallerup RS, Korsholm KS, et al. Trehalose diester glycolipids are superior to the monoesters in binding to Mincle, activation of macrophages in vitro and adjuvant activity in vivo. Innate Immun. 2016;22(6):405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Desel C, Werninghaus K, Ritter M, et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. 2013;8(1):e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45(3):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zuccarino-Catania GV, Sadanand S, Weisel FJ, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15(7):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vermont CL, van Dijken HH, van Limpt CJ et al. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect Immun. 2002;70(2):584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pedersen GK, Hoschler K, Oie Solbak SM, et al. Serum IgG titres, but not avidity, correlates with neutralizing antibody response after H5N1 vaccination. Vaccine. 2014;32(35):4550–4557. [DOI] [PubMed] [Google Scholar]
- 65. Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. 2016;44(1):116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanz I, Wei C, Lee FE-H, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20(1):67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weisel F, Shlomchik M. Memory B cells of mice and humans. Annu Rev Immunol. 2017;35:255–284. [DOI] [PubMed] [Google Scholar]
- 68. Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329(1–2):112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sundling C, Li Y, Huynh N, et al. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci Transl Med. 2012;4(142):142ra196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Galson JD, Truck J, Fowler A, et al. Analysis of B cell repertoire dynamics following hepatitis B vaccination in humans, and enrichment of vaccine-specific antibody sequences. EBioMedicine. 2015;2(12):2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wiley SR, Raman VS, Desbien A, et al. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci Transl Med. 2011;3(93):93ra69. [DOI] [PubMed] [Google Scholar]
- 72. Pedersen GK, Ebensen T, Gjeraker IH, et al. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One. 2011;6(11):e26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med. 2011;208(13):2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hammarlund E, Thomas A, Amanna IJ, et al. Plasma cell survival in the absence of B cell memory. Nat Commun. 2017;8(1):1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Knudsen NPH, Olsen A, Buonsanti C, et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci Rep. 2016;6:19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bournazos S, Ravetch JV. Fcgamma receptor function and the design of vaccination strategies. Immunity. 2017;47(2):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chung AW, Ghebremichael M, Robinson H, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra238. [DOI] [PubMed] [Google Scholar]
- 79. Mahan AE, Jennewein MF, Suscovich T, et al. Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog. 2016;12(3):e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li S, Sullivan NL, Rouphael N, et al. Metabolic phenotypes of response to vaccination in humans. Cell. 2017;169(5):862–877.e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schmidt ST, Neustrup MA, Harloff-Helleberg S, et al. Systematic investigation of the role of surfactant composition and choice of oil: design of a nanoemulsion-based adjuvant inducing concomitant humoral and CD4+ T-cell responses. Pharm Res. 2017;34(8):1716–1727. [DOI] [PubMed] [Google Scholar]
- 82. Christensen D, Mortensen R, Rosenkrands I, Dietrich J, Andersen P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2016;10(1):260–270. [DOI] [PubMed] [Google Scholar]
- 83. Lay M, Callejo B, Chang S, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone®) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009;27(29):3811–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. [DOI] [PubMed] [Google Scholar]
- 85. Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 2012;80(10):3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lin D, Maecker HT. Mass cytometry assays for antigen-specific T cells using CyTOF. Methods Mol Biol. 2018;1678:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reeves PM, Sluder AE, Paul SR, Scholzen A, Kashiwagi S, Poznansky MC. Application and utility of mass cytometry in vaccine development. FASEB J 2018;32(1):5–15. [DOI] [PubMed] [Google Scholar]
- 88. Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36(1):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang C, Zheng X, Gai W, et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Overgaard NH, Frøsig TM, Jakobsen JT, Buus S, Andersen MH, Jungersen G. Low antigen dose formulated in CAF09 adjuvant favours a cytotoxic T-cell response following intraperitoneal immunization in Göttingen minipigs. Vaccine. 2017;35(42):5629–5636. [DOI] [PubMed] [Google Scholar]
- 91. Buglione-Corbett R, Pouliot K, Marty-Roix R, et al. Serum cytokine profiles associated with specific adjuvants used in a DNA prime-protein boost vaccination strategy. PLoS One. 2013;8(9):e74820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Filskov J, Mikkelsen M, Hansen PR, et al. Broadening CD4+ and CD8+ T cell responses against hepatitis C virus by vaccination with NS3 overlapping peptide panels in cross-priming liposomes. J Virol. 2017;91(14):pii: e00130-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28(3):9. [DOI] [PubMed] [Google Scholar]
- 94. Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79(14):8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Elvang T, Christensen JP, Billeskov R, et al. CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PLoS One. 2009;4(4):e5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Anderson KG, Mayer-Barber K, Sung H, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Billeskov R, Lindenstrøm T, Woodworth J, et al. High antigen dose is detrimental to post-exposure vaccine protection against Tuberculosis. Front Immunol. 2018;8:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lindenstrøm T, Aagaard C, Christensen D, Agger EM, Andersen P. High-frequency vaccine-induced CD8+ T cells specific for an epitope naturally processed during infection with M. tuberculosis do not confer protection. Eur J Immunol. 2014;44(6):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines. 2010;9(6):601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Doehn C, Esser N, Pauels H-G, et al. Mode-of-action, efficacy, and safety of a homologous multi-epitope vaccine in a murine model for adjuvant treatment of renal cell carcinoma. Eur Urol. 2009;56(1):123–133. [DOI] [PubMed] [Google Scholar]
- 101. Quah BJC, Wijesundara DK, Ranasinghe C, Parish CR. Fluorescent target array killing assay: a multiplex cytotoxic T-cell assay to measure detailed T-cell antigen specificity and avidity in vivo. Cytometry A. 2012;81A(8):679–690. [DOI] [PubMed] [Google Scholar]
- 102. Bryant CE, Monie TP. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2012;2(4):120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34(5):433–454. [PubMed] [Google Scholar]
- 104. Kastenmüller K, Wille-Reece U, Lindsay RWB, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest 2011;121(5):1782–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. [DOI] [PubMed] [Google Scholar]
- 106. Demaria O, Pagni PP, Traub S, et al. TLR8 deficiency leads to autoimmunity in mice. J Clin Invest 2010;120(10):3651–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Van Hoeven N, Fox CB, Granger B, et al. A formulated TLR7/8 agonist is a flexible, highly potent and effective adjuvant for pandemic influenza vaccines. Sci Rep. 2017;7:46426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ilyinskii PO, Roy CJ, O’Neil CP, et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32(24):2882–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ellebedy A, Jackson K, Boyd S, Ahmed R. Defining antigen specific plasmablast and memory B cell subsets in blood following viral infection and vaccination of humans. Eur J Immunol. 2016;46:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15(3):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119(24):5640–5649. [DOI] [PubMed] [Google Scholar]
- 112. Vinuesa CG, Sze DMY, Cook MC, et al. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur J Immunol. 2003;33(2):297–305. [DOI] [PubMed] [Google Scholar]
- 113. Pedersen GK, Adori M, Khoenkhoen S, Dosenovic P, Beutler B, Karlsson Hedestam GB. B-1a transitional cells are phenotypically distinct and are lacking in mice deficient in IkappaBNS. Proc Natl Acad Sci USA. 2014;111(39):E4119–E4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hjelholt A, Christiansen G, Sorensen US, Birkelund S. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog Dis. 2013;67(3):206–213. [DOI] [PubMed] [Google Scholar]
- 115. van de Veen W, Akdis M. Role of IgG4 in IgE-mediated allergic responses. J Allergy Clin Immunol. 2016;138(5):1434–1435. [DOI] [PubMed] [Google Scholar]
- 116. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Snoeck V, Peters IR, Cox E. The IgA system: a comparison of structure and function in different species. Vet Res. 2006;37(3):455–467. [DOI] [PubMed] [Google Scholar]
- 118. Brandtzaeg P. Secretory IgA: designed for anti-microbial defense. Front Immunol. 2013;4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Decot V, Woerly G, Loyens M, et al. Heterogeneity of expression of IgA receptors by human, mouse, and rat eosinophils. J Immunol. 2005;174(2):628–635. [DOI] [PubMed] [Google Scholar]
- 120. Kubagawa H, Kubagawa Y, Jones D, Nasti TH, Walter MR, Honjo K. The old but new IgM Fc receptor (Fc mu R). Curr Top Microbiol Immunol. 2014;382:3–28. [DOI] [PubMed] [Google Scholar]
- 121. Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem. 2010;285(7):4826–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268(1):25–51. [DOI] [PubMed] [Google Scholar]
- 123. Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23(1):41–51. [DOI] [PubMed] [Google Scholar]
- 124. Nimmerjahn F, Lux A, Albert H, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci USA. 2010;107(45):19396–19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. van Dissel JT, Joosten SA, Hoff ST, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–7107. [DOI] [PubMed] [Google Scholar]
- 126. Coulter F, Parrish A, Manning D, et al. IL-17 production from T helper 17, mucosal-associated invariant T, and γδ cells in tuberculosis infection and disease. Front Immunol. 2017;8:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova J-L. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tasaki S, Cho T, Nagao J-I, et al. Th17 cells differentiated with mycelial membranes of Candida albicans prevent oral candidiasis. FEMS Yeast Res. 2018:18(3). doi: 10.1093/femsyr/foy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cárdeno A, Magnusson MK, Quiding-Järbrink M, Lundgren A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Sci Rep. 2018;8(1):2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morita R, Schmitt N, Bentebibel S-E, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schmitt N, Bentebibel S-E, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. de Wolf ACMT, van Aalst S, Ludwig IS, et al. Regulatory T cell frequencies and phenotypes following anti-viral vaccination. PLoS One. 2017;12(6):e0179942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kurts C, Robinson BWS, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10(6):403–414. [DOI] [PubMed] [Google Scholar]
- 135. Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207(6):1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van der Aa E, van Montfoort N, Woltman AM. BDCA3+CLEC9A+ human dendritic cell function and development. Semin Cell Dev Biol. 2015;41:39–48. [DOI] [PubMed] [Google Scholar]
- 137. Lauterbach H, Bathke B, Gilles S, et al. Mouse CD8α(+) DCs and human BDCA3(+) DCs are major producers of IFN-λ in response to poly IC. J Exp Med. 2010;207(12):2703–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Robbins SH, Walzer T, Dembélé D, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9(1):R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bachem A, Güttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010;207(6):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]